Abstract
A novel packaging strategy combining the salient features of two human parvoviruses, namely the pathogenic parvovirus B19 and the nonpathogenic adeno-associated virus type 2 (AAV), was developed to achieve erythroid cell-specific delivery as well as expression of the transduced gene. The development of such a chimeric vector system was accomplished by packaging heterologous DNA sequences cloned within the inverted terminal repeats of AAV and subsequently packaging the DNA inside the capsid structure of B19 virus. Recombinant B19 virus particles were assembled, as evidenced by electron microscopy as well as DNA slot blot analyses. The hybrid vector failed to transduce nonerythroid human cells, such as 293 cells, as expected. However, MB-02 cells, a human megakaryocytic leukemia cell line which can be infected by B19 virus following erythroid differentiation with erythropoietin (N. C. Munshi, S. Z. Zhou, M. J. Woody, D. A. Morgan, and A. Srivastava, J. Virol. 67:562–566, 1993) but lacks the putative receptor for AAV (S. Ponnazhagan, X.-S. Wang, M. J. Woody, F. Luo, L. Y. Kang, M. L. Nallari, N. C. Munshi, S. Z. Zhou, and A. Srivastava, J. Gen. Virol. 77:1111–1122, 1996), were readily transduced by this vector. The hybrid vector was also found to specifically target the erythroid population in primary human bone marrow cells as well as more immature hematopoietic progenitor cells following erythroid differentiation, as evidenced by selective expression of the transduced gene in these target cells. Preincubation with anticapsid antibodies against B19 virus, but not anticapsid antibodies against AAV, inhibited transduction of primary human erythroid cells. The efficiency of transduction of primary human erythroid cells by the recombinant B19 virus vector was significantly higher than that by the recombinant AAV vector. Further development of the AAV-B19 virus hybrid vector system should prove beneficial in gene therapy protocols aimed at the correction of inherited and acquired human diseases affecting cells of erythroid lineage.
Gene therapy protocols involving recombinant viral vectors have gained attention as a potentially useful modality in molecular medicine. Of the different viral vectors that have been utilized to mediate gene transfer, retrovirus- and adenovirus-based vectors have predominated for over a decade. Recently, adeno-associated virus (AAV)-based vectors have emerged as a useful alternative to the more commonly used retroviral and adenoviral vectors (14). Whereas retroviral and adenoviral vectors may be associated with certain complications, such as the oncogenic properties of the former (6) and the immunogenic problems of the latter (39), AAV has thus far not been shown to be associated with any such pathological situations. In addition, AAV possesses a number of desirable features, including its ability to transduce nondividing cells (7, 19), its broad host range (14), and the ability of the wild-type (wt) AAV genome to integrate site specifically into chromosome 19 in human cells (8–10, 29). Furthermore, wt AAV has also been shown to possess antioncogenic properties (15). Recombinant AAV genomes are constructed by molecularly cloning DNA sequences of interest between the AAV inverted terminal repeats (ITRs), eliminating the entire coding sequence of the wt AAV genome. The recombinant AAV thus produced lacks the viral coding sequences (14, 28) yet retains the properties of stable chromosomal integration and expression of the recombinant genes upon transduction both in vitro and in vivo (2, 3, 14). Until recently, AAV was believed to infect all cell types, transcending the species barrier (14). However, we first suggested that AAV infection is receptor mediated (23), and the identity of the receptor was recently revealed (36).
Parvovirus B19, on the other hand, is a pathogenic virus and is the etiologic agent for a variety of human diseases (1, 5, 18, 26, 30). B19 virus is known to infect human hematopoietic cells in the erythroid lineage (16, 17, 32, 33, 35). It has been suggested that erythrocyte P antigen functions as the receptor for B19 virus infection of target erythroid cells (4). The genomic sequences of both AAV and B19 virus consist of two genes that express proteins (Rep for AAV and NS-1 for B19 virus) involved in replication of the viral genome and generation of the capsid structures (VP1, VP2, and VP3 for AAV and VP1 and VP2 for B19 virus) required for packaging the replicated viral sequences into mature virions. The sequences of both the AAV and B19 virus genomes have been cloned into plasmids that have facilitated detailed analyses of these parvoviruses (27, 31).
In the present studies, we exploited the two unique features of AAV and B19 virus to create a chimeric recombinant vector system to specifically target the primitive erythroid progenitors in human bone marrow cells. Our data indicate that the recombinant B19 virus vectors are significantly more efficient than the recombinant AAV vectors in mediating transduction of primary human erythroid progenitor cells. Further development of this vector system may prove useful in gene therapy applications for diseases affecting the erythroid cell lineage in the human hematopoietic system.
MATERIALS AND METHODS
Cells, plasmids, and viruses.
The human nasopharyngeal carcinoma cell line KB was provided by A. C. Antony (Indiana University School of Medicine, Indianapolis, Ind.), and the human embryonic kidney cell line 293 was obtained from the American Type Culture Collection (Rockville, Md.). These cell lines were maintained as previously described (22–24). The human megakaryocytic leukemia cell line MB-02, a kind gift of D. A. Morgan (Hahnemann Medical College, Philadelphia, Pa.), was maintained and differentiated with erythropoietin (Epo), as previously described (12, 13). Human bone marrow cells were obtained from healthy volunteer donors after informed consent was obtained and approved by the Institutional Review Board for studies involving human subjects. The recombinant AAV helper plasmid pAAV/Ad (28) and the recombinant B19 virus plasmid pYT103 (31) were provided by R. J. Samulski (University of North Carolina, Chapel Hill, N.C.) and P. Tattersall (Yale University School of Medicine, New Haven, Conn.), respectively. The details of construction of the recombinant AAV plasmid containing the bacterial β-galactosidase (β-Gal) gene (lacZ) under control of the cytomegalovirus (CMV) immediate-early promoter (pCMVp-lacZ) have been described previously (20, 21, 23).
Construction of recombinant plasmids and production of recombinant B19 virions.
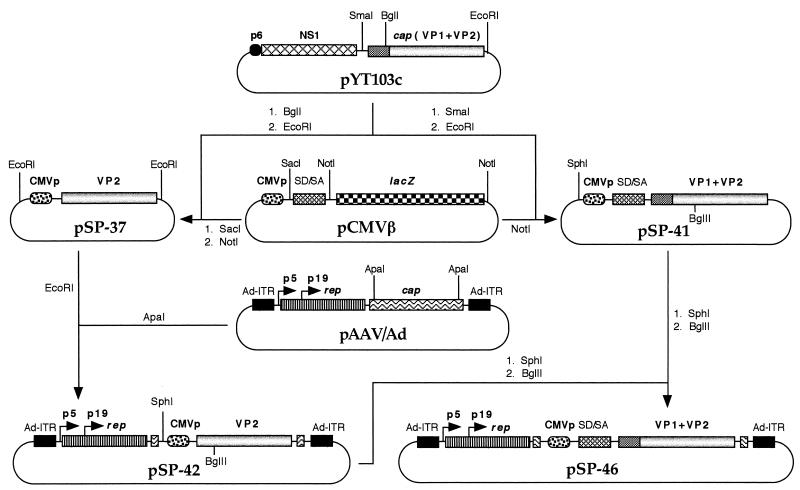
The strategy for constructing recombinant helper plasmids containing the B19 cap genes under control of the CMV promoter is depicted in Fig. 1. Briefly, either the VP2 gene alone or VP1 plus VP2 gene fragments were isolated from plasmid pYT103c (35) and ligated downstream from the CMV promoter in plasmid pCMVβ (Clontech, Palo Alto, Calif.), either with or without the simian virus 40 splice donor-splice acceptor signal, to yield plasmids pSP-37 and pSP-41, respectively. Plasmid pSP-42 was generated by inserting the portion of the B19 cap gene (VP2) with the CMV promoter into plasmid pAAV/Ad, replacing the AAV cap genes. Plasmid pSP-46 was generated by replacing a portion of the CMV promoter-driven B19 VP2 sequence in plasmid pSP-42 with a similar fragment containing the CMV promoter-driven B19 VP1 plus VP2 genes plus the simian virus 40 splice donor-splice acceptor signal derived from plasmid pSP-41. The recombinant helper plasmid pSP-42 or pSP-46 was cotransfected with the recombinant AAV plasmid containing the CMVp-lacZ gene sequences in 293 cells by the calcium phosphate transfection protocol, as previously described (22–24). Rescued and replicated recombinant AAV genomes were subsequently encapsidated in the B19 virus capsid structures. Harvesting and CsCl density gradient purification of the virus were carried out as described previously (20, 21, 24, 37). Quantitative slot blot analysis was performed to determine the physical titers of the virus stocks, as previously described (11, 32, 33).
FIG. 1.
Schematic representation of the experimental strategy to generate recombinant B19-lacZ vectors. The recombinant plasmid pCMVp-lacZ, containing the lacZ gene under control of the CMV promoter inserted between the AAV ITRs, has been previously described (21, 23). Recombinant helper plasmids pSP-42 and pSP-46 contain the AAV rep genes under control of their authentic promoters, but the AAV cap genes have been replaced by the B19 cap genes (VP2 and VP1+VP2, respectively) under control of the CMV promoter and flanked by the adenovirus ITRs. The rest of the steps in generating the recombinant B19-lacZ vectors are described in Materials and Methods.
Electron microscopy.
Recombinant B19-lacZ virions purified on CsCl density gradients were stained with 3% phosphotungstic acid (pH 6.5) and visualized at a magnification of ×80,000 with a Philips 400 electron microscope, as previously described (37).
Isolation of low-density and primitive progenitor human bone marrow cells and cellular differentiation.
Bone marrow aspirates were immediately diluted with an equal volume of Iscove’s modified Dulbecco’s Medium containing 20 U of heparin per ml. Low-density bone marrow (LDBM) cells were obtained by Ficoll-Hypaque (Pharmacia, Piscataway, N.J.) density centrifugation. For isolation of CD34+ cells, mononuclear cells were labeled with anti-CD34 antibodies that were conjugated with magnetic particles. The labeled cells were passed through a magnetic separation column (Miltenyi Biotech, Sunnyvale, Calif.), and CD34− cells were allowed to flow through the column. CD34+ cells were subsequently eluted with MACS buffer (0.5% bovine serum albumin and 5 mM EDTA in 1× phosphate-buffered saline). The purity of the isolated CD34+ cells was determined by fluorescence-activated cell sorting (FACS) and ranged between 90 and 95% (20).
Differentiation of CD34+ cells into erythroid and myeloid lineages in vitro was achieved by the addition of 5 U of Epo per ml and 10 ng of granulocyte colony-stimulating factor, respectively, to cells in liquid cultures supplemented with interleukin-3 (IL-3), IL-6, and stem cell factor (Stem Cell Technologies, Vancouver, British Columbia, Canada), as previously described (20).
Infection of human cells with recombinant AAV and B19 virus vectors and analysis of transduced lacZ gene expression.
Human 293 and MB-02 cells, cultured with or without Epo, were either mock infected or infected at a particle-to-cell ratio of 200:1 at 37°C for 1 h. Cells were washed and incubated in fresh medium at 37°C. Forty-eight hours postinfection, cells were fixed and stained with the X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) substrate and examined under a Nikon inverted microscope for expression of the transduced lacZ gene, as previously described (20, 21, 23). LDBM cells, with or without prior selection with anti-glycophorin A antibodies, and differentiated and undifferentiated CD34+ cells were either mock infected or infected with recombinant B19-lacZ or recombinant AAV-lacZ vectors at particle-to-cell ratios of 200:1 and 100,000:1, respectively, at 37°C for 1 h, following which the cells were grown in the presence of recombinant human IL-3, IL-6, and stem cell factor for 48 h. In some experiments, recombinant vector stocks were preincubated with anticapsid antibodies against AAV (American Research Products, Belmont, Mass.) or B19 virus (Biogenesis, Sandown, N.H.) at 4°C for 90 min prior to infection of glycophorin A-positive LDBM cells. Analysis of expression of the transduced gene was performed with the fluorescent ImaGene Green C12FDG β-Gal substrate (5-dodecanoyl-amino fluorescein di-{β-d-galactopyranoside}) (Molecular Probes Inc., Eugene, Oreg.). After enzymatic hydrolysis by β-Gal, a highly fluorescent fluorescein derivative is generated. Phycoerythrin (PE)-conjugated monoclonal antibodies for CD33, CD34, and glycophorin A were used to select various cell populations. Cells were first incubated with murine monoclonal antibodies specific for CD33, CD34, and glycophorin A. After 30 min on ice, cells were washed, pelleted, and resuspended in culture medium. Cells were then incubated with 300 μM chloroquine for 30 min at 37°C, washed, pelleted, and incubated further with 33 μM ImaGene Green C12FDG β-Gal substrate (Molecular Probes Inc.) for 30 min at 37°C. Following centrifugation, the cells were resuspended in fresh culture medium and analyzed with a Beckton-Dickinson FACScan, as previously described (20, 21). CD34 is expressed on hematopoietic progenitor cells, endothelial cells, and some fibroblasts. CD33 is expressed on monocytes, activated T cells, and some myeloid progenitor cells. Glycophorin A is present on human erythrocytes and erythroid progenitor cells. Cells expressing CD33, CD34, or glycophorin A with a PE fluorescence intensity greater than the highest 3% of the isotype control stained cells were considered positive. In FACS analyses, at least 10,000 events/analysis were recorded for each sample except for some mock-infected controls for which 5,000 events/analysis were recorded. The gates were set based on scattering in mock-transduced controls, and the percentages of cells that fell in the shifted area were used to determine β-Gal-positive cells.
RESULTS
Recombinant AAV genomes can be successfully encapsidated in parvovirus B19 capsids.
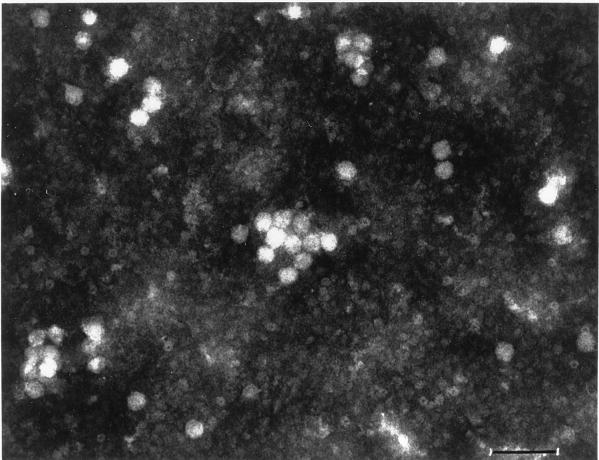
The production of recombinant B19 virus vectors was achieved by generating two helper plasmids, designated pSP-42 and pSP-46, by replacing the AAV cap gene sequences in the AAV helper plasmid pAAV/Ad (28) with the B19 cap gene sequences isolated from plasmid pYT103c (35), leaving the rep gene sequences of AAV intact. The B19 virus sequences were cloned under control of the CMV promoter. The recombinant helper plasmid pSP-42 contains only the VP2 gene, and plasmid pSP-46 contains both the VP1 plus VP2 genes of the B19 cap sequences. Both helper plasmids were initially tested for the ability to mediate efficient rescue and replication of the AAV genome from a recombinant AAV plasmid indicating functional trans-complementation by the AAV rep gene products. The efficiency of rescue and replication of the recombinant AAV CMVp-lacZ genome by plasmids pSP-42 and pSP-46 was nearly the same as that by pAAV/Ad (data not shown). Packaging of the recombinant B19-lacZ vectors was carried out by cotransfecting the pCMVp-lacZ plasmid with either pSP-42 or pSP-46 in 293 cells, as described in Materials and Methods. Recombinant AAV vector stocks containing the same CMVp-lacZ transgene were also prepared, as previously described (20, 21). Based on quantitative DNA slot blot analyses (11), the recombinant B19 viral titers were determined to be approximately 108 particles/ml when pSP-42 was used as a helper plasmid. Interestingly, when pSP-46 was used as a helper plasmid, the recombinant viral titers obtained were approximately 109 particles/ml (data not shown). Subsequently, the presence of virus particles exhibiting icosahedral structures of 25 to 30 nm diameter, similar to the size of parvoviral capsids, was confirmed by electron microscopy (Fig. 2). These particles, purified by centrifugation on CsCl density gradients following exhaustive digestion with DNaseI, banded at a buoyant density of 1.4, characteristic of intact parvoviral particles. Taken together, these results suggest that the recombinant AAV genomes were indeed encapsidated in the B19 virus capsid structures.
FIG. 2.
Electron microscopic image of recombinant B19-lacZ particles. The recombinant virions were purified on CsCl gradients, as described in Materials and Methods. Samples were negatively stained with 3% phosphotungstic acid (pH 6.5), and the particles were visualized at a magnification of ×80,000 with a Philips 400 electron microscope (bar = 80 nm).
Recombinant B19 virions fail to infect nonerythroid human cell lines.
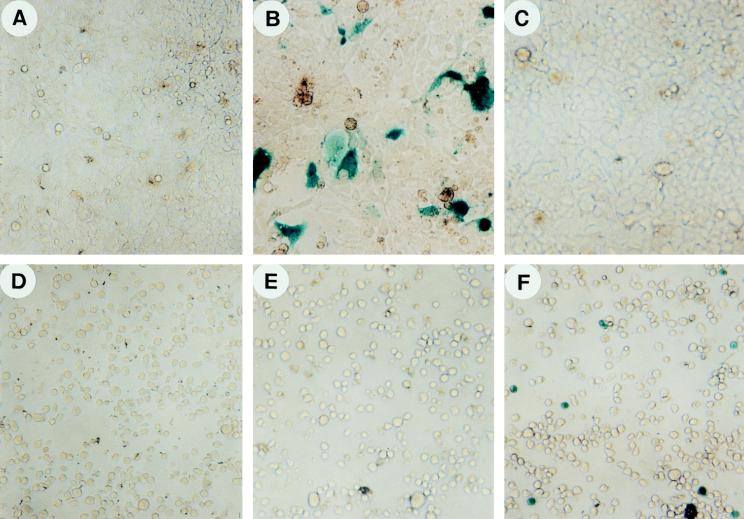
The recombinant B19-lacZ vector was tested for its ability to infect 293 cells, known to be permissive for AAV infection. Erythroid-differentiated MB-02 cells, known to be permissive for B19 virus infection and replication following treatment with Epo (13) but nonpermissive for AAV infection (23), were also used. Undifferentiated MB-02 cells, known to be nonpermissive for infection by both AAV and B19, were included as an appropriate control. Both recombinant vector stocks were used at a virus particle-to-cell ratio of 200:1. Forty-eight hours postinfection, cells were analyzed for transduced lacZ gene expression. The results are depicted in Fig. 3. It is interesting to note that whereas the AAV-lacZ vector was readily able to transduce 293 cells, as evidenced by the appearance of blue cells indicating expression of the transduced gene (Fig. 3B), there was no expression either in the mock-infected or B19-lacZ vector-infected 293 cells (Fig. 3A and C). On the other hand, whereas there was no expression in either mock-infected (Fig. 3D) or AAV-lacZ vector-infected (Fig. 3E) MB-02 cells, as expected, transgene expression was detected in B19-lacZ vector-infected MB-02 cells following erythroid differentiation (Fig. 3F). The transduction efficiency also correlated with the extent of erythroid differentiation (23). Undifferentiated MB-02 cells, which are nonpermissive for B19 infection (13), could not be transduced by the B19-lacZ vector (data not shown), suggesting the ability of this vector to selectively transduce cells following erythroid differentiation.
FIG. 3.
Expression of the transduced lacZ gene mediated by recombinant AAV- and B19-lacZ vectors in human 293 and Epo-differentiated MB-02 cells. Approximately equivalent numbers of 293 cells (A, B, and C) and Epo-differentiated MB-02 cells (D, E, and F) were either mock infected or infected with 200 particles of AAV-lacZ (B and E) and B19-lacZ (C and F) recombinant vectors per cell under identical conditions. Forty-eight hours postinfection, the cells were fixed and stained for analysis of expression of the lacZ gene, as described in Materials and Methods.
Recombinant B19 virus vectors mediate high-efficiency, selective transduction of primary human hematopoietic progenitor cells in the erythroid lineage.
We next wished to establish the specificity of the recombinant B19 virus vectors. To this end, human LDBM mononuclear cells were mock infected or infected with the B19-lacZ vector at a particle-to-cell ratio of 200:1, with or without preincubation of the virus stocks with 1:100 dilutions of anticapsid antibodies against B19 or AAV, as described in Materials and Methods. Forty-eight hours postinfection, cells were sorted for fluorescent β-Gal product and PE-glycophorin A with a FACScan to determine the percentage of cells expressing the lacZ gene. The results indicated that preincubation of the B19-lacZ virions with anti-B19 capsid antibodies inhibited the transduction efficiency in LDBM cells by approximately 36%, whereas anti-AAV capsid antibodies had no effect.
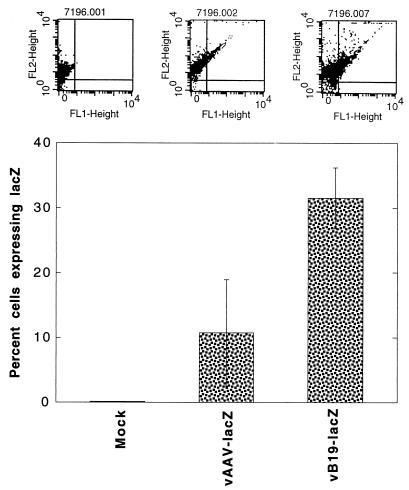
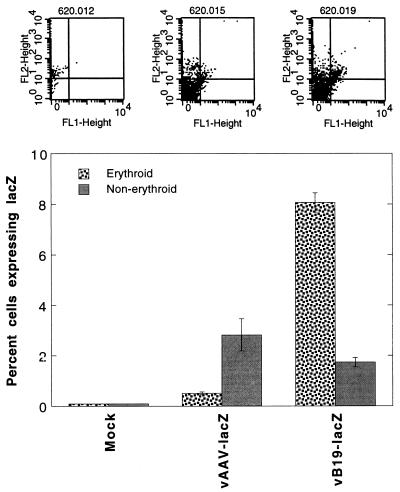
We also carried out a comparative analysis of the efficiency of erythroid cell targeting mediated by recombinant AAV-lacZ and B19-lacZ vectors in primary human bone marrow cells. Human LDBM cells were used in the following three sets of experiments. In the first set, LDBM cells were sorted for glycophorin A positivity to enrich the erythroid fraction (20). Approximately equivalent numbers of cells were either mock infected or infected with the AAV-lacZ vector (105 particles/cell) or with the B19-lacZ vector (2 × 102 particles/cell) under identical conditions. Forty-eight hours postinfection, cells were sorted for fluorescent β-Gal product and PE-glycophorin A, as described above, to determine the percentage of cells expressing the lacZ gene. The results, shown in Fig. 4, indicated that even at 500-fold lower viral titers, recombinant B19 virus vector-mediated delivery of the transduced gene was significantly higher than that by the recombinant AAV vector in the erythroid fraction of primary human bone marrow cells, which strongly suggested, but did not prove, erythroid cell-specific targeting by the recombinant B19 virus vector.
FIG. 4.
FACS analysis of lacZ gene expression in glycophorin A-positive primary human LDBM cells transduced with recombinant AAV- and B19-lacZ vectors. Glycophorin A-positive cells from human LDBM were either mock infected or infected with either 1 × 105 particles of AAV-lacZ vector per cell or 2 × 102 particles of B19-lacZ vector per cell under identical conditions. Forty-eight hours postinfection, cells were harvested and processed for analysis of lacZ gene expression with fluorescent β-Gal product by using a Becton-Dickinson FACScan as described in Materials and Methods. The upper panels are FACS dot plots from a representative experiment indicating cells positive for lacZ (FL1-Height) and glycophorin A (FL2-Height). The results of dual positive cells from three separate experiments are plotted in the bar graph.
In the second set of experiments, CD34+ cells from human LDBM were isolated and either mock infected or infected with the recombinant AAV-lacZ or B19-lacZ vector at a particle-to-cell ratio of 100,000:1 or 200:1, respectively, as described above. Transgene expression was analyzed 48 h postinfection with the fluorescent β-Gal product and the PE-glycophorin A marker, as described above. The results, shown in Fig. 5, indicated specific targeting of the erythroid population of primitive progenitor cells by the B19 virus vector. The AAV vector, on the other hand, showed higher expression in the nonerythroid population of cells, an observation consistent with our previously published studies (37). The low transduction efficiency of the AAV vector in CD34+ cells is also consistent with our recent studies documenting wide variations in the levels of transduction by these vectors (20).
FIG. 5.
FACS analysis of lacZ gene expression in erythroid and nonerythroid populations of primary human bone marrow-derived CD34+ cells transduced with recombinant AAV- and B19-lacZ vectors. CD34+ cells isolated from human LDBM were either mock infected or infected with either 1 × 105 particles of AAV-lacZ vector per cell or 2 × 102 particles of B19-lacZ vector per cell under identical conditions. Forty-eight hours postinfection, cells were harvested and processed for analysis of lacZ gene expression in erythroid and nonerythroid cells by using fluorescent β-Gal product and PE-conjugated glycophorin A antibody, as described in Materials and Methods. The upper panels are FACS dot plots from a representative experiment indicating cells positive for lacZ (FL1-Height) and/or glycophorin A (FL2-Height). Nonerythroid cells expressing the lacZ gene are in the lower right quadrants, and erythroid cells expressing the lacZ gene are in the upper right quadrants. The results of dual positive cells from three separate experiments are plotted in the bar graph.
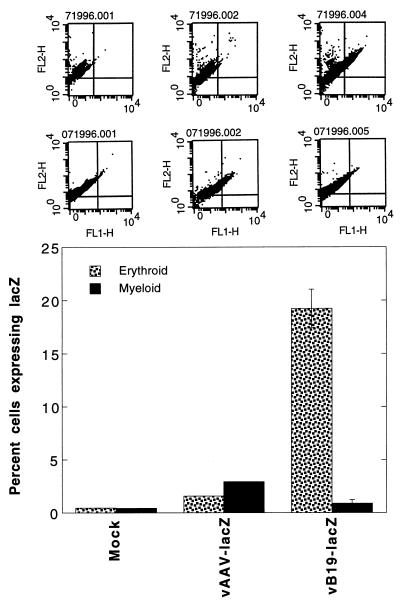
Finally, in the third set of experiments, CD34+ cells were first differentiated into erythroid and myeloid lineages by treatments with Epo and granulocyte colony-stimulating factor, respectively, for 10 days, followed by either mock infection or infection with the recombinant AAV-lacZ or B19-lacZ vector, essentially as described above. Forty-eight hours postinfection, cells were analyzed for transgene expression with fluorescent β-Gal product and PE-glycophorin A for the erythroid population and with fluorescent β-Gal product and PE-CD33 for the myeloid population. The results, shown in Fig. 6, once again indicated that the recombinant B19-lacZ vector was highly efficient in selectively transducing the erythroid-differentiated population of CD34+ cells.
FIG. 6.
FACS analysis of lacZ gene expression in erythroid- and myeloid-differentiated CD34+ primary human bone marrow cells transduced with recombinant AAV- and B19-lacZ vectors. Primary human bone marrow-derived CD34+ cells were allowed to undergo differentiation into erythroid or myeloid lineages with the use of respective cytokine combinations for 10 days in vitro, as described in Materials and Methods. Following differentiation, cells were either mock infected or infected with either 1 × 105 particles of AAV-lacZ vector per cell or 2 × 102 particles of B19-lacZ vector per cell under identical conditions. Forty-eight hours postinfection, cells were harvested and stained with either PE-conjugated glycophorin A antibody (stippled bars) or PE-conjugated CD33 antibody (solid bars) and analyzed for lacZ gene expression in erythroid and myeloid populations by using fluorescent β-Gal product, as described in the legend to Fig. 4. Upper-row panels at the top are FACS dot plots from a representative experiment indicating cells positive for lacZ (FL1-H) and glycophorin A (FL2-H). Lower-row panels at the top represent cells expressing lacZ (FL1-H) and CD33 (FL2-H). The results of dual positive cells from three separate experiments are plotted in the bar graph.
DISCUSSION
One of the long-term interests of our laboratory has been to develop parvovirus-based vectors for the potential treatment of hemoglobinopathies in general and sickle cell anemia and β-thalassemia in particular (34). We have previously reported stable transduction and expression of human globin gene sequences mediated by recombinant AAV vectors both in vitro and in vivo (22, 25, 40). However, AAV vectors may not always be desirable, considering the need to specifically target a selected type of cells in a heterogeneous population, given the broad host range of AAV (14). We sought to exploit the salient feature of parvovirus B19: it possesses a remarkable tropism for erythroid cells in human bone marrow because infection by B19 virus is mediated by the erythrocyte P antigen (4). These studies were prompted by our previously published reports that the B19 viral genome encapsidated within the AAV capsid structure is both infectious and replication competent in cells of erythroid lineage in human bone marrow (35). In addition, we have reported that the B19p6 promoter, the only authentic promoter in the B19 viral genome, is capable of conferring autonomous replication competence and erythroid specificity to AAV in primary human hematopoietic progenitor cells (37). Previous studies utilizing a baculovirus system have indicated that empty capsids of B19 virus could be assembled with VP2 protein alone, since VP2 comprises the majority of the capsid protein (38). In the present studies, although we were able to obtain recombinant B19 encapsidated virions with sequences of VP2 alone using the helper plasmid pSP-42, the addition of VP1 sequences in the helper plasmid pSP-46 increased the packaging efficiency approximately 10-fold. Still, the packaging efficiency of the B19 virus helper plasmid pSP-46 was significantly lower than that of the AAV helper plasmid pAAV/Ad, which is most probably due to the efficiency of different promoters regulating capsid gene expression. Western blot analyses of capsid gene expression corroborated this possibility (data not shown). Replacing the CMV promoter by the authentic AAVp40 promoter or the B19p6 promoter driving expression of the B19 virus capsid gene did not lead to successful production of recombinant B19 virus vectors.
The results of several different experiments with both established and primary human cells clearly document that the recombinant B19 virus vectors can indeed specifically target cells of erythroid lineage in human bone marrow cells. It is perhaps not surprising, therefore, that despite low viral titers, the recombinant B19 virus vector is able to infect cells in the erythroid lineage at a significantly higher efficiency than the recombinant AAV vector. It is intriguing to note, however, that the B19 virus vector is able to transduce CD34+ cells that are positive for glycophorin A on the day of analysis. It is likely that erythroid-differentiated cells which express both glycophorin A and the CD34 marker on their cell surfaces are infected by B19 more readily than are more-undifferentiated CD34+ cells. This is corroborated by the fact that the nonerythroid population of cells that was initially positive for the CD34 marker on the day of sorting did not show any expression of the transduced lacZ gene. However, if multipotential CD34+ cells were transduced by the B19 vector, there should have been transgene expression following differentiation into erythroid, myeloid, and lymphoid lineages; this was not detected. These results indicate that the recombinant B19 virus vector can selectively mediate transduction even in less-differentiated erythroid cells.
The observation that erythroid-differentiated, but not undifferentiated, MB-02 cells showed expression of the transduced lacZ gene suggests that expression of the P antigen receptor correlates with erythroid differentiation of these cells. In this context, it is noteworthy that previous studies from our laboratory have documented that wild-type B19 virus DNA replication and gene expression occur only in erythroid-differentiated MB-02 cells (13). It may now be of interest to investigate whether nonerythroid cells, such as myocardial cells and endothelial cells, that express the P antigen receptor but are nonpermissive for B19 replication can be successfully transduced by the recombinant B19 virus vector. This may help resolve the issue of whether a putative intracellular factor that is present only in cells of erythroid lineage is crucial for successful replication of B19 virus and whether an additional coreceptor(s) is required for successful infection by B19 virus.
The recombinant B19 virus vector system described here offers several potential advantages over the currently used AAV vector system. First, since it remains to be unequivocally established whether stable integration of a recombinant AAV genome in primary cells leads to insertional mutagenesis, it may be desirable to transduce committed erythroid progenitor cells rather than pluripotent hematopoietic stem cells. Second, it may also be desirable to obtain tissue-specific delivery of the therapeutic gene so as not to affect the normal functions of other cell lineages. Third, since approximately 90% of the human population is seropositive for AAV capsid proteins (2, 3), the potential use of AAV vectors in in vivo gene therapy protocols may be limited. The use of B19 capsids composed of VP1 plus VP2 proteins to encapsidate a potentially therapeutic gene can at least partially overcome the problem of neutralizing antibodies against AAV, since only approximately 60% of the human population is seropositive for the B19 capsid proteins (4, 38). It is also tempting to speculate that the use of B19 capsids composed entirely of VP2 proteins may be especially advantageous, since all antigenic epitopes to date have been mapped within the VP1 region (38). Further development of this novel vector system may prove useful in its application for gene therapy of human diseases involving cells of erythroid lineage in general and sickle cell anemia and β-thalassemia in particular.
ACKNOWLEDGMENTS
We thank Richard Samulski and Peter Tattersall for generously providing plasmids pAAV/Ad and pYT103, respectively, and David Williams for supplying human bone marrow cells. We also thank Edward Srour for help with cell sorting and Kelly Hiatt for expert technical assistance.
This research was supported in part by Public Health Service grants (HL-48342, HL-53586, HL-58881, and DK-49218; Centers of Excellence in Molecular Hematology) from the National Institutes of Health and a grant from the Phi Beta Psi sorority. K.A.W. was supported by an “Innovative Medizinische Forschung” Research Fellowship from the University of Münster, Münster, Germany, and A.S. was supported by an Established Investigator Award from the American Heart Association.
REFERENCES
- 1.Anderson M J, Jones S E, Fisher-Hoch S P, Lewis E, Hall S M, Bartlet C L R, Cohen B J, Mortimer P P, Pereira M S. Human parvovirus, the cause of erythema infectiosum (fifth disease)? Lancet. 1983;i:1378. doi: 10.1016/s0140-6736(83)92152-9. [DOI] [PubMed] [Google Scholar]
- 2.Berns K I, Bohenzky R A. Adeno-associated viruses: an update. Adv Virus Res. 1987;32:243–307. doi: 10.1016/s0065-3527(08)60479-0. [DOI] [PubMed] [Google Scholar]
- 3.Berns K I, Giraud C. Biology of adeno-associated virus. Curr Top Microbiol Immunol. 1996;218:1–23. doi: 10.1007/978-3-642-80207-2_1. [DOI] [PubMed] [Google Scholar]
- 4.Brown K E, Anderson S M, Young N S. Erythrocyte P antigen: cellular receptor for B19 parvovirus. Science. 1993;262:114–117. doi: 10.1126/science.8211117. [DOI] [PubMed] [Google Scholar]
- 5.Brown T A, Anand A, Ritchie L D, Clewley J P, Reid T. Intrauterine parvovirus infection associated with hydrops fetalis. Lancet. 1984;ii:1033–1034. doi: 10.1016/s0140-6736(84)91126-7. [DOI] [PubMed] [Google Scholar]
- 6.Donahue R E, Kessler S W, Bodine D, McDonagh K, Dunbar C, Goodman S, Agricola B, Byrne E, Raffeld M, Moen R, Bacher J, Zsebo K M, Nienhuis A W. Helper virus induced T cell lymphoma in non-human primates after retroviral mediated gene transfer. J Exp Med. 1992;176:1125–1135. doi: 10.1084/jem.176.4.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flotte T R, Afione S A, Zeitlin P L. Adeno-associated virus vector gene expression occurs in non-dividing cells in the absence of vector DNA integration. Am J Respir Cell Mol Biol. 1994;11:517–521. doi: 10.1165/ajrcmb.11.5.7946381. [DOI] [PubMed] [Google Scholar]
- 8.Kotin R M, Berns K I. Organization of adeno-associated virus DNA in latently infected Detroit 6 cells. Virology. 1989;170:460–467. doi: 10.1016/0042-6822(89)90437-6. [DOI] [PubMed] [Google Scholar]
- 9.Kotin R M, Menninger J C, Ward D C, Berns K I. Mapping and direct visualization of a region-specific viral DNA integration site on chromosome 19q13-qter. Genomics. 1991;10:831–834. doi: 10.1016/0888-7543(91)90470-y. [DOI] [PubMed] [Google Scholar]
- 10.Kotin R M, Siniscalco M, Samulski R J, Zhu X, Hunter L, Laughlin C A, McLaughlin S, Muzyczka N, Rocchi M, Berns K I. Site-specific integration by adeno-associated virus. Proc Natl Acad Sci USA. 1990;87:2211–2215. doi: 10.1073/pnas.87.6.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kube D M, Srivastava A. Quantitative DNA slot blot analysis: inhibition of DNA binding to membranes by magnesium ions. Nucleic Acids Res. 1997;25:3375–3376. doi: 10.1093/nar/25.16.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morgan D A, Gumuchio D L, Brodsky I. Granulocyte-macrophage colony-stimulating factor dependent growth and erythropoietin-induced differentiation of a human cell line, MB-02. Blood. 1991;78:2860–2871. [PubMed] [Google Scholar]
- 13.Munshi N C, Zhou S Z, Woody M J, Morgan D A, Srivastava A. Successful replication of parvovirus B19 in the human megakaryocytic leukemia cell line MB-02. J Virol. 1993;67:562–566. doi: 10.1128/jvi.67.1.562-566.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muzyczka N. Use of adeno-associated virus as a general transduction vector for mammalian cells. Curr Top Microbiol Immunol. 1992;158:97–129. doi: 10.1007/978-3-642-75608-5_5. [DOI] [PubMed] [Google Scholar]
- 15.Ostrove J M, Duckworth D H, Berns K I. Inhibition of adenovirus-transformed cell oncogenicity by adeno-associated virus. Virology. 1981;113:521–533. doi: 10.1016/0042-6822(81)90180-x. [DOI] [PubMed] [Google Scholar]
- 16.Ozawa K, Kurtzman G J, Young N S. Replication of the B19 parvovirus in human bone marrow cultures. Science. 1986;233:883–886. doi: 10.1126/science.3738514. [DOI] [PubMed] [Google Scholar]
- 17.Ozawa K, Kurtzman G J, Young N S. Productive infection by B19 parvovirus of human erythroid bone marrow cells in vitro. Blood. 1987;70:384–391. [PubMed] [Google Scholar]
- 18.Pattison J R, Jones S E, Hodgson J, Davis L R, White J M, Stroud C E, Murtaza L. Parvovirus infection and hypoplastic crisis in sickle cell disease. Lancet. 1981;i:664–665. doi: 10.1016/s0140-6736(81)91579-8. [DOI] [PubMed] [Google Scholar]
- 19.Podsakoff G, Wong K K, Jr, Chatterjee S. Efficient gene transfer into nondividing cells by adeno-associated virus-based vectors. J Virol. 1994;68:5656–5666. doi: 10.1128/jvi.68.9.5656-5666.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ponnazhagan S, Mukherjee P, Wang X-S, Qing K Y, Kube D M, Mah C, Yoder M C, Srour E F, Srivastava A. Adeno-associated virus type 2-mediated transduction in primary human bone marrow-derived CD34+ hematopoietic progenitor cells: donor variation and correlation of transgene expression with cellular differentiation. J Virol. 1997;71:8262–8267. doi: 10.1128/jvi.71.11.8262-8267.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ponnazhagan S, Mukherjee P, Yoder M C, Wang X-S, Zhou S Z, Kaplan J, Wadsworth S, Srivastava A. Adeno-associated virus 2-mediated gene transfer in vivo: organ-tropism and expression of transduced sequences in mice. Gene. 1997;190:203–210. doi: 10.1016/s0378-1119(96)00576-8. [DOI] [PubMed] [Google Scholar]
- 22.Ponnazhagan S, Nallari M L, Srivastava A. Suppression of human α-globin gene expression mediated by the recombinant adeno-associated virus 2-based antisense vectors. J Exp Med. 1994;179:733–738. doi: 10.1084/jem.179.2.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ponnazhagan S, Wang X-S, Woody M J, Luo F, Kang L Y, Nallari M L, Munshi N C, Zhou S Z, Srivastava A. Differential expression in human cells from the p6 promoter of human parvovirus B19 following plasmid transfection and recombinant adeno-associated virus 2 (AAV) infection: human megakaryocytic leukaemia cells are non-permissive for AAV infection. J Gen Virol. 1996;77:1111–1122. doi: 10.1099/0022-1317-77-6-1111. [DOI] [PubMed] [Google Scholar]
- 24.Ponnazhagan S, Woody M J, Wang X-S, Zhou S Z, Srivastava A. Transcriptional transactivation of parvovirus B19 promoters in nonpermissive human cells by adenovirus type 2. J Virol. 1995;69:8096–8101. doi: 10.1128/jvi.69.12.8096-8101.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ponnazhagan S, Yoder M C, Srivastava A. Adeno-associated virus type 2-mediated transduction of murine hematopoietic cells with long-term repopulating ability and sustained expression of a human globin gene in vivo. J Virol. 1997;71:3098–3104. doi: 10.1128/jvi.71.4.3098-3104.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saarinen U M, Chorba T L, Tattersall P, Young N S, Anderson L J, Palmer E, Coccia P F. Human parvovirus B19 induced epidemic red cell aplasia in patients with hereditary hemolytic anemia. Blood. 1986;67:1411–1417. [PubMed] [Google Scholar]
- 27.Samulski R J, Chang L-S, Shenk T. A recombinant plasmid from which an infectious adeno-associated virus genome can be excised in vitro and its use to study viral replication. J Virol. 1987;61:3096–3101. doi: 10.1128/jvi.61.10.3096-3101.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Samulski R J, Chang L-S, Shenk T. Helper-free stocks of recombinant adeno-associated viruses: normal integration does not require viral gene expression. J Virol. 1989;63:3822–3828. doi: 10.1128/jvi.63.9.3822-3828.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Samulski R J, Zhu X, Xiao X, Brook J, Houseman D E, Epstein N, Hunter L A. Targeted integration of adeno-associated virus (AAV) into human chromosome 19. EMBO J. 1991;10:3941–3950. doi: 10.1002/j.1460-2075.1991.tb04964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Serjeant G R, Mason K, Topley J M, Serjeant B M, Pattison J R, Jones S E, Mohamed R. Outbreak of aplastic crisis in sickle cell anemia associated with parvovirus-like agent. Lancet. 1981;ii:595–597. doi: 10.1016/s0140-6736(81)92739-2. [DOI] [PubMed] [Google Scholar]
- 31.Shade R O, Blundell M C, Cotmore S F, Tattersall P, Astell C R. Nucleotide sequence and genome organization of human parvovirus B19 isolated from the serum of a child during aplastic crisis. J Virol. 1986;58:921–936. doi: 10.1128/jvi.58.3.921-936.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Srivastava A, Bruno E, Briddell R, Cooper R, Srivastava C, van Besien K, Hoffman R. Parvovirus B19-induced perturbation of human megakaryocytopoiesis in vitro. Blood. 1990;76:1997–2004. [PubMed] [Google Scholar]
- 33.Srivastava A, Lu L. Replication of B19 parvovirus in highly enriched hematopoietic progenitor cells from normal human bone marrow. J Virol. 1988;62:3059–3063. doi: 10.1128/jvi.62.8.3059-3063.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Srivastava A, Wang X-S, Ponnazhagan S, Zhou S Z, Yoder M C. Adeno-associated virus 2-mediated transduction and erythroid lineage-specific expression in human hematopoietic progenitor cells. Curr Top Microbiol Immunol. 1996;218:93–117. doi: 10.1007/978-3-642-80207-2_7. [DOI] [PubMed] [Google Scholar]
- 35.Srivastava C H, Samulski R J, Lu L, Larsen S H, Srivastava A. Construction of a recombinant human parvovirus B19: adeno-associated virus 2 (AAV) DNA inverted terminal repeats are functional in an AAV-B19 hybrid virus. Proc Natl Acad Sci USA. 1989;86:8078–8082. doi: 10.1073/pnas.86.20.8078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Summerford C, Samulski R J. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J Virol. 1998;72:1438–1445. doi: 10.1128/jvi.72.2.1438-1445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang X-S, Yoder M C, Zhou S Z, Srivastava A. Parvovirus B19 promoter at map unit 6 confers autonomous replication competence and erythroid specificity to adeno-associated virus 2 in primary human hematopoietic progenitor cells. Proc Natl Acad Sci USA. 1995;92:12416–12420. doi: 10.1073/pnas.92.26.12416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wong S, Momeda M, Field A, Kajigaya S, Young N S. Formation of empty B19 parvovirus capsids by the truncated minor capsid protein. J Virol. 1994;68:4690–4694. doi: 10.1128/jvi.68.7.4690-4694.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang Y, Ertl H C, Wilson J M. MHC class 1-restricted cytotoxic T lymphocytes to viral antigens destroy hepatocytes in mice infected with E1-deleted recombinant adenoviruses. Immunity. 1994;1:433–442. doi: 10.1016/1074-7613(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 40.Zhou S Z, Li Q, Stamatoyannopoulos G, Srivastava A. Adeno-associated virus 2-mediated transduction and erythroid cell-specific expression of a human β-globin gene. Gene Ther. 1996;3:223–229. [PubMed] [Google Scholar]