Abstract
Background
Facing a surge of COVID-19 cases in late August 2021, the U.S. state of Illinois re-enacted its COVID-19 mask mandate for the general public and issued a requirement for workers in certain professions to be vaccinated against COVID-19 or undergo weekly testing. The mask mandate required any individual, regardless of their vaccination status, to wear a well-fitting mask in an indoor setting.
Methods
We used Illinois Department of Public Health’s COVID-19 confirmed case and vaccination data and investigated scenarios where masking and vaccination would have been reduced to mimic what would have happened had the mask mandate or vaccine requirement not been put in place. The study examined a range of potential reductions in masking and vaccination mimicking potential scenarios had the mask mandate or vaccine requirement not been enacted. We estimated COVID-19 cases and hospitalizations averted by changes in masking and vaccination during the period covering October 20 to December 20, 2021.
Results
We find that the announcement and implementation of a mask mandate are likely to correlate with a strong protective effect at reducing COVID-19 burden and the announcement of a vaccinate-or-test requirement among frontline professionals is likely to correlate with a more modest protective effect at reducing COVID-19 burden. In our most conservative scenario, we estimated that from the period of October 20 to December 20, 2021, the mask mandate likely prevented approximately 58,000 cases and 1,175 hospitalizations, while the vaccinate-or-test requirement may have prevented at most approximately 24,000 cases and 475 hospitalizations.
Conclusion
Our results indicate that mask mandates and vaccine-or-test requirements are vital in mitigating the burden of COVID-19 during surges of the virus.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12889-024-18203-8.
Keywords: Masks, Vaccines, Cases and hospitalizations averted, COVID-19, Modeling
Introduction
Public health mandates or requirements are laws put into place to promote healthy behaviors that mitigate disease burden. The mechanisms through which these policies interact with public health can vary substantially. For instance, a mask mandate aims to reduce disease transmission and a vaccinate-or-test requirement aims to reduce exposure to infectious cases through testing and also reduce the incidence of severe disease and mortality through vaccination. Several studies have estimated the impact of face mask mandates [1–4] and vaccination requirements [5, 6] on COVID-19 transmission.
Facing a surge in COVID-19 cases during the fall of 2021 due to the emergence of the Delta variant [7], the Governor of Illinois issued an executive order on August 26, 2021 [8] that required workers in certain frontline professions (e.g., healthcare workers, school personnel, higher education personnel, and state owned or operated congregate facilities) to get vaccinated against COVID-19 (i.e., at least receive the first dose of a two-dose COVID-19 vaccine series), or undergo, at a minimum, weekly testing for COVID-19 if they remained unvaccinated by September 19, 2021 [9]. At the latest, individuals had to receive their second dose of a two-dose COVID-19 vaccine series 30 days after the September 19th deadline [9]. There was also a mandate that required face mask use for any individual in a public indoor setting, beginning August 30, 2021, regardless of vaccination status. The August 26, 2021 executive order [8] was issued while the rate of cases continued to rise steeply, despite an earlier mask mandate for schools, daycares, and long-term care facilities enacted on August 4, 2021 [10]. The Delta variant represented more than 99% of sampled strains in Illinois at that point [7] and healthcare capacity was strained.
In this paper, we provide estimates of cases and hospitalizations averted by the concomitant mask mandate and vaccinate-or-test requirement in Illinois between October 20 and December 20, 2021. We estimate separately the impact of increases in masking and vaccination. We adjust for a wide range of compliance levels by estimating the impact for various levels of mask-wearing pre- and post-mandate, and for various potential levels of vaccination uptake in the front-line workforce that would follow the announcement of the vaccinate-or-test requirement.
Methods
We estimated the impact of increases in masking and vaccination on COVID-19 incidence in Illinois from October 20 to December 20, 2021. Previous reports have shown that use of face masks reduces SARS-CoV-2 transmission [11–13]. We simplified estimating the impact of mask wearing, hereby referred to as mask effectiveness, by using (i) an average estimate of mask efficacy and (ii) an average percent of population compliant with correct mask wearing (see Technical Appendix for more details).1 For vaccines, the effectiveness of the vaccinate-or-test requirement depends on the difference between the percentage of population that complied with the executive order to vaccinate-or-test, and the percentage that were already vaccinated. This research activity was reviewed by the Centers for Disease Control and Prevention (CDC) and was conducted consistent with applicable federal law and CDC policy.2
We used CDC’s COVIDTracer modeling tool [14] to build an epidemic curve that mimicked the observed one in Illinois over the two-month period (October 20 - December 20, 2021).3 Similar to other studies [15–18] (Castonguay FM, Borah BF, Jeon S, Rainisch G, Kelso P, Adhikari BB, Daltry DJ, Fischer LS, Greening Jr B, Kahn EB, Kahn GJ, Meltzer MI: The Public Health Impact of COVID-19 Variants of Concern on the Effectiveness of Contact Tracing in Vermont, United States, unpublished), the two-month duration balances the need for sufficient time to pass after the start of the mandate to allow for an adequate assessment of the impact of the interventions being studied. Simultaneously, it aims to limit the potential for unknown confounding factors that may alter the impact of the interventions. We assumed that the effectiveness of interventions remained constant over the two-month study period. Those vaccinated following the vaccinate-or-test requirement should have achieved full vaccine-induced immunity4 by mid-October 2021, which matches the start of our analytic timeframe.5 COVIDTracer uses a compartmental Susceptible–Exposed–Infectious–Recovered (SEIR) mathematical model [19]. A user enters location-specific COVID-19 case counts, vaccination levels, a set of parameters describing COVID-19 epidemiology (e.g., basic reproduction number), and estimates of the effectiveness of the interventions (Table 1) (see Technical Appendix for details and Appendix Table A4 for a list of Illinois-specific inputs).
Table 1.
Input table for the COVID-19 epidemiological characteristics, mask mandate, and vaccinate-or-test requirement
| Study Period (Dates covered) | Oct. 20 – Dec. 20, 2021 |
|---|---|
| Assumed COVID-19 Epidemiological Characteristics | |
| Latent period durationa | 2 days |
| Basic reproduction numberb | R0 = 5 |
| % cases asymptomaticc | 40% |
| Infectiousness of asymptomatic casesd | 75% |
| % vaccinated in general populatione | 53.8% |
| Vaccine effectivenessf | 88% |
| Immunity durationg | 180 days |
| Inputs Specific to COVID-19 Mask Mandate | |
| Mask effectivenessh | |
| Pre-mandate | 3.6% – 16.8% |
| Post-mandate | 6.1% – 21.3% |
| Inputs Specific to COVID-19 Vaccinate-or-Test Requirement | |
| Size of the population affected by requirementi | 929,370 |
| % vaccinated in sub-population affected by requirementj | |
| Pre-requirement | 64.8% |
| Post-requirement | 75.9% |
aPer the literature, we used a two-day latent period associated with the SARS-CoV-2 Delta variant [20, 21]
bPer the literature, we used a basic reproduction number of R0 = 5.0 associated with the SARS-CoV-2 Delta variant [22, 23]. The infectivity distribution varies over time and is spread over an 11-day period [24, 25], and the effective reproduction number further depends on the estimated impact of non-pharmaceutical interventions (NPIs) and the size of the susceptible population, see Technical Appendix for more details
cAsymptomatic COVID-19 cases. Patients can be infected, and become infectious, without being symptomatic. They can and likely do contribute to onward transmission of the pathogen [26]
dInfectiousness of asymptomatic COVID-19 cases relative to symptomatic cases [26]
eBased on CDC COVID-19 vaccination data [27]—these data represent overall coverage among all ages at the beginning of the study period
fCOVID-19 vaccine effectiveness is based on the effectiveness of two doses of the monovalent mRNA BNT162b2 (Pfizer-BioNTech, Comirnaty) against the Delta variant [28]
gWe assume SARS-CoV-2 immunity lasts 180 days on average [29], this includes both vaccine-induced and disease-induced immunity
hMask effectiveness is the product of (i) mask efficacy and (ii) mask compliance. The resultant values are the average mask effectiveness in the state of Illinois, for the time-period studied. See Technical Appendix for more details
iBased on Data from the U.S. Bureau of Labor Statistics [30]. See Appendix Table A2
jBased on data from the Centers for Disease Control and Prevention (CDC)’s National Healthcare Safety Network (NHSN) for CMS-certified nursing homes in IL [31]. Note that this data only represents a sub-population of the total population affected by the vaccinate-or-test requirement. See Appendix Table A3
Impact of mask mandate
To model the impact of mandate-induced increased mask effectiveness, we first inputted a selected value from the range provided in Table 1 into COVIDTracer. The pre-mandate mask effectiveness range was 3.6%–16.8% and post-mandate mask effectiveness range of 6.1%–23.3%. For baseline analysis, we used post-mandate mask effectiveness of 14.2%, assuming 20% mask efficacy and 71% compliance. As it is very difficult to measure the degree of compliance with effective mask wearing in any population, we therefore constructed 24 scenarios of combinations of pre-and post-mandate mask effectiveness. We avoided over-estimating the impact of the mask mandate by using pre-mandate mask effectiveness values of less than 20% and only one post-mandate mask effectiveness value of over 20% (see Sensitivity Analyses).
We then “fitted” the curve of cumulative cases modeled by COVIDTracer to the jurisdiction’s reported cases by altering the percentage reduction in transmission ascribed to vaccine and various Non-Pharmaceutical Interventions (NPIs). The estimated percentage reduction in transmission that minimized the difference (i.e., minimized the mean squared error) between the fitted and reported cumulative case curves is the estimate of the effectiveness of non-CICT NPIs (see Appendix for further details). Note that, because there were no measurements of the degree of under-reporting, we had to use the reported cases without any adjustments for possible under-reporting. Correcting for under-reporting may well have increased the estimates of cases and hospitalizations averted, for both mandates. Finally, we simulated what would have occurred without the mask mandate by re-setting the impact of mask effectiveness to one of 2 pre-mandate effectiveness levels (7.2% and 11.2%). The resulting plots are the number of cases that would have occurred without the mask mandate. The difference between the hypothetical plots of cases without mandate-induced increases in mask effectiveness and the plot of the reported cases (which includes the impact of mask mandate) are the cases averted due to the mask mandate. By “fitting the curve” (finding the best match between the SEIR model and the observed data), this methodology prevents over- or under-estimating the combined impact of all interventions.
Sensitivity analyses: mask mandate
Mask wearing compliance depends on several locality factors [32, 33]. As noted earlier, it is very difficult to measure the degree of compliance in large populations. We therefore constructed 24 scenarios of combinations of pre-and post-mandate mask effectiveness (Appendix Table A1).
Impact of vaccinate-or-test requirement
To estimate the impact of the vaccinate-or-test requirement, we followed the same process as described above for masking (Table 1; see the Appendix for further details). The number of cases averted by the vaccinate-or-test requirement depended on (i) the baseline, pre-requirement, vaccination coverage, and (ii) the increase in vaccination coverage attributable to the requirement. We obtained an estimate of 929,370 frontline workers in Illinois who were potentially affected by the vaccinate-or-test requirement (see Appendix Table A2). The National Healthcare Safety Network (NHSN) reported that, for the period analyzed, vaccination coverage of the staff working in certified Centers for Medicare & Medicaid Services (CMS-certified) nursing homes in Illinois increased from 64.8% to 75.9%––an 11.1 percentage point increase in coverage (see Appendix Table A3). We used this 11.1 percentage point increase (equivalent to 103,160 additional persons vaccinated) as a base case scenario for analyzing the impact of the vaccinate-or-test requirement.
Sensitivity analyses: vaccinate-or-test requirement
We do not know what proportion of the 11.1 percentage point increased coverage was due to the vaccinate-or-test requirement. Other factors, such as intent to be vaccinated regardless of the requirement or other requirements/mandates (e.g., the federal mandate for CMS facilities announced during the study period), could have contributed to the increase. To address this uncertainty, we evaluated the impact of an arbitrary assumption that only half of the recorded increase in vaccine coverage could be attributable to the mandate (i.e., a 5.6 percentage point increase, equivalent to 51,580 additional persons vaccinated). Note that the potential indirect impact that the vaccinate-or-test requirement could have had on the general population [6] is not accounted for, which may have increased the overall impact of the requirement.
Results
Impact of mask mandate
In Fig. 1, we present the plot of the cases assuming the post-mandate mask effectiveness of 14.2% (calculated assuming 20% mask efficacy and 71% compliance; represented by the solid black line in Fig. 1). This plot is then compared to the hypothetical plots of increased cases, assuming no mask mandate and a continuation of pre-mask effectiveness of either 7.2% or 11.2% (dotted and dashed lines). The cumulative difference between the dotted or dashed plotted lines and the solid line plot is the estimate of additional cases averted due to the mask mandate.
Fig. 1.
Fitted epidemic curves of COVID-19 case counts showing the impact of the mask mandate
Notes: Fitted epidemic curve of observed COVID-19 case counts, assuming a post-mandate mask effectiveness of 14.2%, and simulated epidemic curves assuming no mandate and continuation of pre-mandate mask effectiveness of either 7.2% or 11.2% (all three for the October 20 – December 20, 2021 period). The solid line is Illinois’s observed (fitted) cumulative COVID-19 case counts, and the broken lines are the simulated curves illustrating the cumulative total COVID-19 cases for the various scenarios that might have occurred if the mask mandate had not been enacted and mask efficacy was 20%. The differences between the solid and broken lines show the benefits of the mask mandate with greater divergence between the solid and broken lines indicating a greater impact. All results assume that the effects of nonpharmaceutical interventions —including masks—were constant over the two months shown
Sensitivity analyses: mask mandate
To calculate a lower-bound estimate of cases averted, we assumed that the mask mandate increased mask effectiveness from 3.6% to 6.1%. This resulted in an estimate of 149,817 additional cases and 3,028 additional hospitalizations averted due to the mask mandate (Table 2). We calculated an upper-bound estimate by increasing the mask effectiveness for pre- and post-mandate to 10.2% and 21.3%, respectively. This resulted in an upper estimate of 1,820,764 additional cases and 36,801 additional hospitalizations averted due to the mask mandate (Table 2). The remainder of the results in Table 1 show the estimates of cases and hospitalizations averted from another 10 scenarios. The results from all 24 scenarios of combinations of pre-and post-mandate mask effectiveness are presented in Appendix Table A1.
Table 2.
Estimated cases and hospitalizations averted due to mask mandate for various levels of mask effectivenessa
| Mask Effectiveness, Pre-mandateb | Mask Effectiveness, Post-mandate | Number of Cases Averted (Number of Hospitalizations Avertedc) |
|---|---|---|
| 3.6% | 6.1% | 149,817 (3,028) |
| 5.0% | 58,233 (1,177) | |
| 3.6% | 7.1% | 232,791 (4,705) |
| 5.6% | 83,250 (1,683) | |
| 7.2% | 12.2% | 415,085 (8,390) |
| 10.0% | 139,155 (2,813) | |
| 7.2% | 14.2% | 734,649 (14,849) |
| 11.2% | 211,125 (4,267) | |
| 10.8% | 18.3% | 901,144 (18,214) |
| 15.0% | 255,426 (5,163) | |
| 10.8% | 21.3% | 1,820,764 (36,801) |
| 16.8% | 417,605 (8,441) |
aSee Appendix Table A1 for the Results from all 24 scenarios of combinations of pre-and post-mandate mask effectiveness
bAssumes the levels of mask-wearing would have remained the same in the absence of the mask mandate
cNumber of hospitalizations averted is calculated by multiplying the estimated number of averted cases by the infection-to-hospitalization ratio, which was assumed to be approximately 2.02% during that period [26]. Infection-to-hospitalization ratio is assumed not to vary with the assumed level of mask efficacy
Impact of vaccinate-or-test requirement
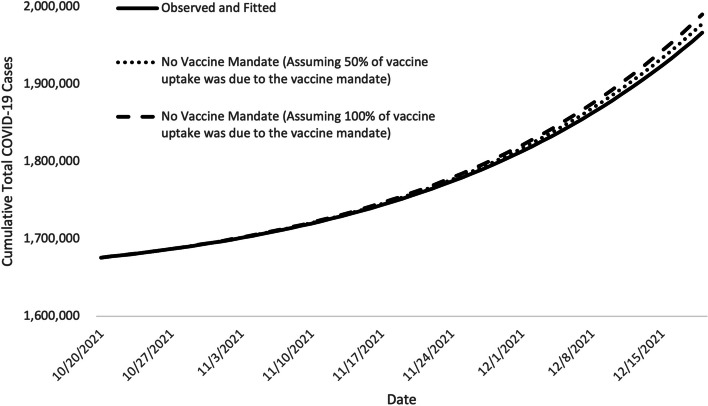
Assuming that the vaccinate-or-test requirement resulted in an 11.1 percentage point increase in persons vaccinated, we estimated that 23,593 cases and 477 hospitalizations were averted (Table 3 and Fig. 2).
Table 3.
Estimated cases and hospitalizations averted by the vaccinate-or-test requirement for different increases in vaccination uptake
| Percentage point increase in vaccination between 8/22 and 10/17 in population due to the vaccinate-or-test requirement (number of people this percentage represents)a | Number Averted | |
|---|---|---|
| 5.6 percentage point (51,580) | Cases | 11,571 |
| Hospitalizationsb | 234 | |
| 11.1 percentage point (103,160) | Cases | 23,593 |
| Hospitalizationsb | 477 |
aNote that we do not know what percentage point can be attributed to the vaccinate-or-test requirement, so the two scenarios here represent examples meant to illustrate what could have happened
bNumber of COVID-19 hospitalizations averted is calculated by multiplying the estimated number of averted cases by the infection-to-hospitalization ratio (factor of approximately 2.02%) [26]. Infection-to-hospitalization ratio is assumed not to vary with the vaccination coverage
Fig. 2.
Fitted epidemic curves of COVID-19 case counts showing the impact of the vaccinate-or-test requirement
Notes: Fitted epidemic curve of observed COVID-19 case counts and of two assumed increases in vaccination coverage attributable to the announcement of the vaccinate-or-test requirement (for October 20 – December 20, 2021). These represent approximately 51,580 and 103,160 individuals vaccinated because of the vaccinate-or-test requirement. The solid line is Illinois’s observed cumulative COVID-19 case counts, and the dashed and dotted lines are the simulated curves illustrating the cumulative total cases for scenarios where there would have been a lower vaccine uptake without the vaccinate-or-test requirement. The differences between the solid and dashed or dotted lines show the number of cases averted by the vaccinate-or-test requirement. All results assume that the effects of other nonpharmaceutical interventions (NPIs) were constant over the two months analyzed
Sensitivity analyses: Vaccinate-or-test Requirement
When we assumed that only half of the increase was attributable to the vaccinate-or-test requirement, an estimated 11,571 cases and 234 hospitalizations were averted by the vaccinate-or-test requirement (Table 3 and Fig. 2).
Discussion
We estimated that increases in masking following the announcement of the mask mandate may have averted at least 58,000 cases in Illinois. The vaccinate-or-test requirement among frontline workers averted up to 24,000 cases during the period studied. The assumed post-mandate mask effectiveness (6.1% - 21.1%) was the most influential variable assessing the impact of mask mandates.
Our results provide data-driven evidence that can inform decision-making regarding public health interventions during times of surge. While there have been concerns regarding the negative impact of such mitigation measures [34], mask mandates are impactful [35] and vaccination requirements have demonstrated the ability to strengthen vaccination intentions across racial and ethnic groups, and even those who may be resistant [36]. Public adherence to these practices due to requirements as opposed to free choice is a more complicated debate. Several studies have demonstrated that while vaccine mandates may result in some vaccine hesitancy, they have been associated with improved vaccination rates [37, 38]. Hospital staff vaccination reports have found that many employees chose vaccination over resignation [37].
Our study has limitations. Several factors made it difficult to use a direct causal identification methodology, such as difference-in-differences. These factors included the absence of a credible control group and several confounding factors due to the important period between the announcement of the intervention and when compliance was required. We had to make several assumptions because the precise impact that the concomitant mask mandate and vaccinate-or-test requirement in Illinois had on COVID-19 burden depended largely on several unobserved factors, namely mask quality, level of mask-wearing pre- and post-mandate, and the proportion of vaccine uptake attributable to mandate. Further, while we estimated the impact of increases in masking and vaccination separately, there may have been unaccounted synergistic effects from combining both interventions [39]. We also assumed that the impact of face masks and vaccination remained constant over the period of study. To reduce the potential impact of such assumption, we limit our study period to two months. We do not account for partial immunity (e.g., if an individual received their first vaccine shot during the study period), and hence assume individuals are either fully susceptible (because the individual was never vaccinated or never infected, or because immunity acquired through vaccination or prior infection was more than 180 days ago [29] and is no longer protective) or fully immune (due to prior infection or vaccination) during the two-month study period. By doing so, we may underestimate the impact of the policies (e.g., because the first dose of a two dose COVID-19 vaccine series may still provide some protection [40] which would increase the impact of the vaccinate-or-test requirement) or overestimate the impact of the policies (e.g., because those protected by the first dose of a two dose COVID-19 vaccine series would not have been protected by masks), with the overall direction of the bias being uncertain. There is also the possibility that the vaccinate-or-test requirement for frontline workers may have had an impact on the general population, as the requirement may have signaled the importance of vaccination for individuals not directly covered by the vaccinate-or-test requirement [6]. Finally, we assumed that there are no differences in disease transmission that are attributable to age, location, or occupation—in other words, every individual in the population is assumed to have the same risk of catching COVID-19 and is assumed to behave in the same way as any other individual. Those affected by the vaccinate-or-test requirement were frontline professionals who could have had potentially very different mixing patterns compared to the general population.
During the two-month study period, almost 2,000 hospitalizations were averted according to our model. Had these hospitalizations occurred, they would have had a significant impact on an already strained healthcare system. These findings can help control viral transmission of diseases other than COVID-19 at both hospital and community levels, and will help refine future decisions on the timing and scale of such public health measures should we find ourselves again in a similar healthcare crisis.
Supplementary Information
Acknowledgements
Nothing to declare.
Disclaimer
The findings and conclusions of this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Authors’ contributions
Conceptualization: FMC, AB, SJ, JF, BBA, LSF, BGJ, AOH, EBK, GJK, JK, SP, SV, MIM. Methodology: FMC, SJ, BBA, LSF, BGJ, EBK, GJK, MIM. Investigation: FMC, SJ. Visualization: FMC. Supervision: SV, MIM. Writing—original draft: FMC, AB, SJ. Writing—review and editing: FMC, AB, SJ, JF, BBA, LSF, BGJ, AOH, EBK, GJK, JK, SP, SV, MIM.
Funding
No funding to report.
Availability of data and materials
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
This research activity was reviewed by the Centers for Disease Control and Prevention (CDC) and was conducted consistent with applicable federal law and CDC policy. See e.g., 45 C.F.R. part 46, 21 C.F.R. part 56; 42 U.S.C. §241(d); 5 U.S.C. §552a; 44 U.S.C. §3501 et seq. The COVID-19 confirmed case and vaccination data used in this study are aggregated at the state level, meaning no personally identifiable information or data were used.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Mask effectiveness is the product of (i) mask efficacy and (ii) mask compliance. Mask efficacy is defined as the extent to which masks reduce the output and uptake of the virus droplets/aerosols (range 10%-30%, see Ueki et al. [11]) and mask compliance is defined as the percentage of the population properly wearing masks.
See e.g., 45 C.F.R. part 46, 21 C.F.R. part 56; 42 U.S.C. §241(d); 5 U.S.C. §552a; 44 U.S.C. §3501 et seq.
This modeling tool has been used by several studies to estimate the impact of CICT [15–18] (Castonguay FM, Borah BF, Jeon S, Rainisch G, Kelso P, Adhikari BB, Daltry DJ, Fischer LS, Greening Jr B, Kahn EB, Kahn GJ, Meltzer MI: The Public Health Impact of COVID-19 Variants of Concern on the Effectiveness of Contact Tracing in Vermont, United States, unpublished) along with instructions provided for replicating this analysis and model use [15, 16].
We defined fully vaccinated as either having received two doses of the monovalent mRNA BNT162b2 (Pfizer-BioNTech, Comirnaty) or monovalent mRNA mRNA-1273 (Moderna, Spikevax) COVID-19 vaccine, or one dose of the single-dose adenovirus vector-based Ad26.COV.S (Janssen [Johnson & Johnson]) COVID-19 vaccine [48].
Recall that the vaccinate-or-test requirement required individuals to get their first dose of a two-dose COVID-19 vaccine series, or undergo, at a minimum, weekly testing for COVID-19 if they remained unvaccinated by September 19, 2021 [9]. CDC recommended at least three weeks between two doses for Pfizer-BioNTech and 28 days between two doses for Moderna any two vaccine [49]. To comply with the requirement, individuals had to receive their second dose of a two-dose COVID-19 vaccine series on October 19, 2021, at the latest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Abaluck J, Kwong LH, Styczynski A, Haque A, Kabir MA, Bates-Jefferys E, Crawford E, Benjamin-Chung J, Raihan S, Rahman S , et al. Impact of community masking on COVID-19: a cluster-randomized trial in Bangladesh, Science. 2022;375(6577):eabi9069. doi: 10.1126/science.abi9069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bundgaard H, Bundgaard JS, Raaschou-Pedersen DET, von Buchwald C, Todsen T, Norsk JB, Pries-Heje MM, Vissing CR, Nielsen PB, Winslow UC , et al. Effectiveness of adding a mask recommendation to other public health measures to prevent SARS-CoV-2 infection in Danish mask wearers: a randomized controlled trial. Annals Internal Med. 2021;174(3):335–343. doi: 10.7326/M20-6817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howard J, Huang A, Li Z, Tufekci Z, Zdimal V, Van Der Westhuizen HM, Von Delft A, Price A, Fridman L , Tang LH, et al. An evidence review of face masks against COVID-19. Proc Nat Acad Sci. 2021;118(4): e2014564118. doi: 10.1073/pnas.2014564118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eikenberry SE, Mancuso M, Iboi E, Phan T, Eikenberry K, Kuang Y, Kostelich E, Gumel AB. To mask or not to mask: Modeling the potential for face mask use by the general public to curtail the COVID-19 pandemic. Infectious disease modelling. 2020;5:293–308. doi: 10.1016/j.idm.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohammed I, Nauman A, Paul P, Ganesan S, Chen KH , Jalil SMS, Jaouni SH, Kawas H, Khan WA, Vattoth AL, et al. The efficacy and effectiveness of the COVID-19 vaccines in reducing infection, severity, hospitalization, and mortality: A systematic review. Hum Vaccin immunother. 2022;18(1):2027160. doi: 10.1080/21645515.2022.2027160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howard-Williams M, Soelaeman RH, Fischer LS, McCord R, Davison R, Dunphy C. Association Between State-Issued COVID-19 Vaccine Mandates and Vaccine Administration Rates in 12 US States and the District of Columbia. JAMA Health Forum. 2022;3(10):e223810–e223810. doi: 10.1001/jamahealthforum.2022.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.E. B. Hodcroft, "CoVariants: SARS-CoV-2 Mutations and Variants of Interest,". Available: https://covariants.org/per-country?region=United+States&country=Illinois. Accessed 29 Aug. 2023.
- 8.JB Pritzker, Governor, "Executive Order 2021-20 (COVID-19 EXECUTIVE ORDER NO. 87)," Governor JB Pritzker © 2023 State of Illinois, August 26, 2021. [online] https://www.illinois.gov/government/executive-orders/executive-order.executive-order-number-20.2021.html.
- 9.JB Pritzker, Governor, "Executive Order 2021-22 (COVID-19 EXECUTIVE ORDER NO. 88)," Governor JB Pritzker © 2023 State of Illinois, September 03, 2021. [online] https://www.illinois.gov/government/executive-orders/executive-order.executive-order-number-22.2021.html.
- 10.JB Pritzker, Governor, "Executive Order Number 18 (COVID-19 EXECUTIVE ORDER NO. 85)," Governor JB Pritzker © 2023 State of Illinois, August 04, 2021. [online] https://www.illinois.gov/government/executive-orders/executive-order.executive-order-number-18.2021.html.
- 11.Ueki H, Furusawa Y, Iwatsuki-Horimoto K, Imai M, Kabata H, Nishimura H, Kawaoka Y. Effectiveness of face masks in preventing airborne transmission of SARS-CoV-2. MSphere. 2020;5(5):e00637–20. doi: 10.1128/mSphere.00637-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng Y, Ma N, Witt C, Rapp S, Wild PS, Andreae MO, Poschl U, Su H. Face masks effectively limit the probability of SARS-CoV-2 transmission. Science. 2021;372(6549):1439–1443. doi: 10.1126/science.abg6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Y, Liang M, Gao L, Ahmed MA, Uy JP, Cheng C, Zhou Q, Sun C. Face masks to prevent transmission of COVID-19: A systematic review and meta-analysis. American journal of infection control. 2021;49(7):900–906. doi: 10.1016/j.ajic.2020.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.U.S. Centers for Disease Control and Prevention , "COVIDTracer and COVIDTracer Advanced," 19 January 2021. Available: https://www.cdc.gov/coronavirus/2019-ncov/php/contact-tracing/COVIDTracerTools.html . Accessed 5 Apr. 2023.
- 15.Jeon S, Rainisch G, Lash RR, Moonan PK, Oeltmann JE , Greening B, Adhikari BB, Meltzer MI, et al. Estimates of cases and hospitalizations averted by COVID-19 case investigation and contact tracing in 14 health jurisdictions in the United States, J Public Health Manag Pract. 2022;28(1):16–24. doi: 10.1097/PHH.0000000000001420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rainisch G, Jeon S, Pappas D, Spencer K, Fischer LS, Adhikari B, Taylor M, Greening B, Moonan P, Oeltmann J, Kahn EB, Washington ML, Meltzer MI. Estimated COVID-19 Cases and Hospitalizations Averted by Case Investigation and Contact Tracing in the US. JAMA Network Open. 2022;5(3):e224042–e224042. doi: 10.1001/jamanetworkopen.2022.4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeon S, Rainisch G, Harris A-M, Shinabery J, Iqbal M, Pallavaram A, Hilton S, Karki S, Moonan PK, Oeltmann JE, Meltzer MI. Estimated Cases Averted by COVID-19 Digital Exposure Notification, Pennsylvania, USA, November 8, 2020–January 2, 2021. Emerging Infectious Diseases. 2023;29(2):426. doi: 10.3201/eid2902.220959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.S. Jeon, L. Watson-Lewis, G. Rainisch, C.-C. Chiu, F. M. Castonguay, L. S. Fischer, P. K. Moonan, J. E. Oeltmann, B. B. Adhikari, H. Lawman and M. I. Meltzer, "Adapting COVID-19 Contact Tracing Protocols to Accommodate Resource Constraints, Philadelphia, Pennsylvania, USA, 2021," Emerging Infectious Diseases, vol. 30, no. 2, 2024. [DOI] [PMC free article] [PubMed]
- 19.IHME COVID-19 Forecasting Team Modeling COVID-19 scenarios for the United States, Nat Med. 2021;27(1):94–105. doi: 10.1038/s41591-020-1132-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, Chen R, Hu F, Lan Y, Yang Z, Zhan C, Shi J, Deng XaJM, Zhong S, et al. Transmission, viral kinetics and clinical characteristics of the emergent SARS-CoV-2 Delta VOC in Guangzhou. EClinicalMedicine. 2021;40:101129. doi: 10.1016/j.eclinm.2021.101129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li, B, Deng A, Li K, Hu Y, Li Z, Shi Y, Xiong Q, Liu Z, Guo Q, Zou L, et al. Viral infection and transmission in a large, well-traced outbreak caused by the SARS-CoV-2 Delta variant, Nat Commun. 2022;13(1):1–9. doi: 10.1038/s41467-022-28089-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Y. Liu and J. Rocklov, "The reproductive number of the Delta variant of SARS-CoV-2 is far higher compared to the ancestral SARS-CoV-2 virus," Journal of travel medicine, vol. 28, no. 7, 2021. [DOI] [PMC free article] [PubMed]
- 23.Alimohamadi Y, Sepandi M, Esmaeilzadeh F. Estimate of the Basic Reproduction Number for Delta variant of SARS-CoV-2: A Systematic Review and Meta-analysis. Journal of Biostatistics and Epidemiology. 2022;8(1):1–7. [Google Scholar]
- 24.He X, Lau EH, Wu P, Deng X, Wang J, Hao X, Lau YC, Wong JY, Guan Y, Tan X, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26(5):672–5. [DOI] [PubMed]
- 25.Ferretti L, Wymant C, Kendall M, Zhao L, Nurtay A, Abeler-Dörner L, Parker M, Bonsall D, Fraser C. "Quantifying SARS-CoV-2 transmission suggests epidemic control with digital contact tracing," Sci. 2020;368(6491). [DOI] [PMC free article] [PubMed]
- 26.U.S. Centers for Disease Control and Prevention, "COVID-19 Pandemic Planning Scenarios," United States Government, 19 March 2021. Online. Available: https://www.cdc.gov/coronavirus/2019-ncov/hcp/planning-scenarios.html#five-scenarios. Accessed 26 Oct. 2022.
- 27.U.S. Centers for Disease Control and Prevention, "COVID-19 Vaccinations in the United States, County,". [online] https://data.cdc.gov/Vaccinations/COVID-19-Vaccinations-in-the-United-States-County/8xkx-amqh. Accessed 8 Mar 2022.
- 28.Lopez Bernal J, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S, Stowe J, Tessier E, Groves N, Dabrera G, et al. "Effectiveness of Covid-19 vaccines against the B. 1.617. 2 (Delta) variant,". N Eng J Med. 2021;385(7):585–94. [DOI] [PMC free article] [PubMed]
- 29.Levin EG, Lustig Y, Cohen C, Fluss R, Indenbaum V, Amit S, Doolman R, Asraf K, Mendelson E, Ziv A, et al. "Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months,". N Eng J Med. 2021;385(24):e84. [DOI] [PMC free article] [PubMed]
- 30.U.S. Bureau of Labor Statistics, "Occupational Employment and Wage Statistics," United States Government, May 2020. Available: https://www.bls.gov/oes/2020/may/oes_il.htm#00-0000. Accessed 10 Mar. 2022.
- 31.U.S. Centers for Disease Control and Prevention, "Weekly HCP & Resident COVID-19 Vaccination," National Healthcare Safety Network (NHSN), 17 April 2023. Available: https://www.cdc.gov/nhsn/ltc/weekly-covid-vac/index.html#anchor_21687. Accessed 22 Feb. 2022.
- 32.Kahane LH. Politicizing the mask: Political, economic and demographic factors affecting mask wearing behavior in the USA. Eastern economic journal. 2021;47(2):163–183. doi: 10.1057/s41302-020-00186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Illinois, Capitol News, "CAPITOL RECAP: Ruling on legal challenge to school mask mandates could come soon," Herald-Whig, 31 01 2022.
- 34.Scheid JL, Lupien SP, Ford GS, West SL. Commentary: physiological and psychological impact of face mask usage during the COVID-19 pandemic. International journal of environmental research and public health. 2020;17(18):6655. doi: 10.3390/ijerph17186655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hansen N-JH, Mano RC. Mask mandates save lives. Journal of Health Economics. 2023;88:102721. doi: 10.1016/j.jhealeco.2022.102721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Albarracin D, Jung H, Song W, Tan A, Fishman J. Rather than inducing psychological reactance, requiring vaccination strengthens intentions to vaccinate in US populations. Scientific Reports. 2021;11(1):20796. doi: 10.1038/s41598-021-00256-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lewandowsky S, Holford D, Schmid P. "Public policy and conspiracies: The case of mandates," Curr Opin Psychol. 2022;47:101427. [DOI] [PMC free article] [PubMed]
- 38.de Figueiredo A, Larson HJ, Reicher SD. The potential impact of vaccine passports on inclination to accept COVID-19 vaccinations in the United Kingdom: Evidence from a large cross-sectional survey and modeling study. EClinicalMedicine. 2021;40:101109. doi: 10.1016/j.eclinm.2021.101109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brussow H, Zuber S. Can a combination of vaccination and face mask wearing contain the COVID-19 pandemic? Microbial Biotechnology. 2022;15(3):721–737. doi: 10.1111/1751-7915.13997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mazzoni A, Di Lauria N, Maggi L, Salvati L, Vanni A, Capone M, Lamacchia G, Mantengoli E, Spinicci M, Zammarchi L, et al. "First-dose mRNA vaccination is sufficient to reactivate immunological memory to SARS-CoV-2 in subjects who have recovered from COVID-19," J Clin Investig.2021;131(12):e149150. 10.1172/JCI149150. [DOI] [PMC free article] [PubMed]
- 41.Xiang Y, Jia Y, Chen L, Guo L, Shu B, Long E. COVID-19 epidemic prediction and the impact of public health interventions: A review of COVID-19 epidemic models. Infectious Disease Modelling. 2021;6:324–342. doi: 10.1016/j.idm.2021.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen M, Li M, Hao Y, Liu Z, Hu L, Wang L. The introduction of population migration to SEIAR for COVID-19 epidemic modeling with an efficient intervention strategy. Information Fusion. 2020;64:252–258. doi: 10.1016/j.inffus.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith LE, Potts HW, Amlot R, Fear NT, Michie S, Rubin GJ. "Adherence to the test, trace, and isolate system in the UK: results from 37 nationally representative surveys,". BMJ. 2021;372(608). [DOI] [PMC free article] [PubMed]
- 44.Park CL, Russell BS, Fendrich M, Finkelstein-Fox L, Hutchison M, Becker J. Americans’ COVID-19 stress, coping, and adherence to CDC guidelines. Journal of general internal medicine. 2020;35(8):2296–2303. doi: 10.1007/s11606-020-05898-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pew Research Center. The Challenges of Contact Tracing as U.S. Battles COVID-19. Washington D.C.; 2020. [online]. https://www.pewresearch.org/internet/2020/10/30/the-challenges-of-contact-tracing-as-u-s-battles-covid-19/.
- 46.Tian L, Li X, Qi F, Tang QY, Tang V, Liu J, Li Z, Cheng X, Li X, Shi Y, et al. Harnessing peak transmission around symptom onset for non-pharmaceutical intervention and containment of the COVID-19 pandemic. Nat Commun. 2021;12(1):1147. doi: 10.1038/s41467-021-21385-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.United States Census Bureau, "Quick Facts," United States Government, 1 July 2022. Available: https://www.census.gov/quickfacts/IL. Accessed 4 May 2023.
- 48.Rosenblum HG, Wallace M, Godfrey M, et al. Interim recommendations from the Advisory Committee on Immunization Practices for the use of bivalent booster doses of COVID-19 vaccines—United States, October 2022. MMWR Morb Mortal Wkly Rep. 2022;71(45):1436–1441. doi: 10.15585/mmwr.mm7145a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kriss JL, Reynolds LE, Wang A, Stokley S, Cole MM, Harris LQ, Shaw LK, Black CL, Singleton JA, Fitter DL. OVID-19 vaccine second-dose completion and interval between first and second doses among vaccinated persons—United States, December 14, 2020- February 14, 2021. Morb Mortal Wkly Rep. 2021;70(11):389. doi: 10.15585/mmwr.mm7011e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.