1. Introduction
Identifying the critical isthmus of an intraatrial reentrant tachycardia (IART) circuit in adult congenital heart disease (ACHD) patients is particularly challenging due to widespread areas of low amplitude and scar. Use of Coherent mapping with conduction velocity vectors can aid identification of electrical propagation wavefronts in this cohort of patients.
2. Case presentation
A 38 year old female was referred for an urgent IART ablation, following recent admission for symptoms of palpitations and dyspnoea. A similar admission a month prior had required cardioversion of an IART. She had previously undergone regular follow up in adult congenital heart disease (ACHD) clinic, with a history of transposition of the great arteries, tricuspid atresia and pulmonary stenosis, having undergone a bidirectional Glen at 4 years and an atrio-pulmonary Fontan procedure at 7 years. A subcutaneous implantable cardioverter-defibrillator (SICD) was implanted in 2015 following an out of hospital VF arrest. She had a history of recurrent atrial arrhythmias, having undergone 3 previous right atrial ablation procedures in which radiofrequency (RF) ablation of a cavo-tricuspid isthmus (CTI) line, an intercaval line, a septal micro re-entry circuit and a lateral micro re-entrant circuit were performed.
Informed consent was gained for a repeat IART ablation under general anaesthetic. Warfarin continued uninterrupted, with an INR of 3.2. Right femoral venous access was obtained, with a fixed decapolar catheter positioned in the coronary sinus (CS) and a multielectrode mapping catheter (PentaRay Nav, Biosense Webster, Diamond Bar, CA, USA) introduced to right atrium (RA) via a medium curl Agilis sheath (Abbott). The CARTO 3D mapping system (Biosense Webster) was used.
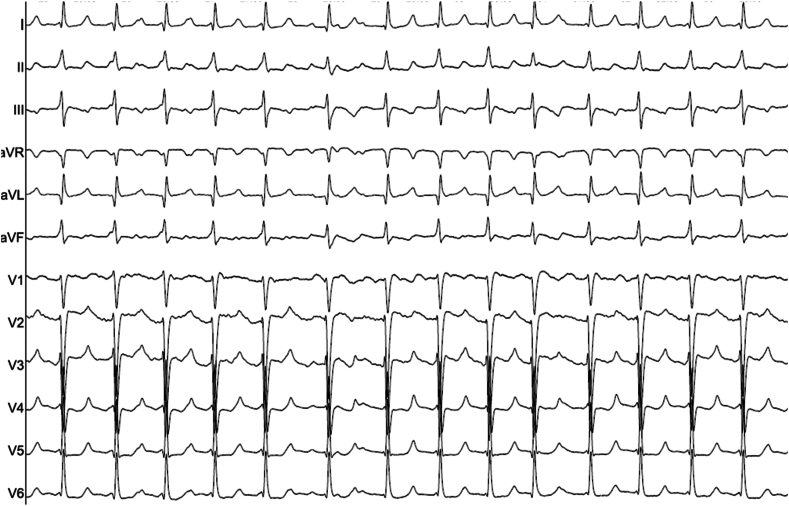
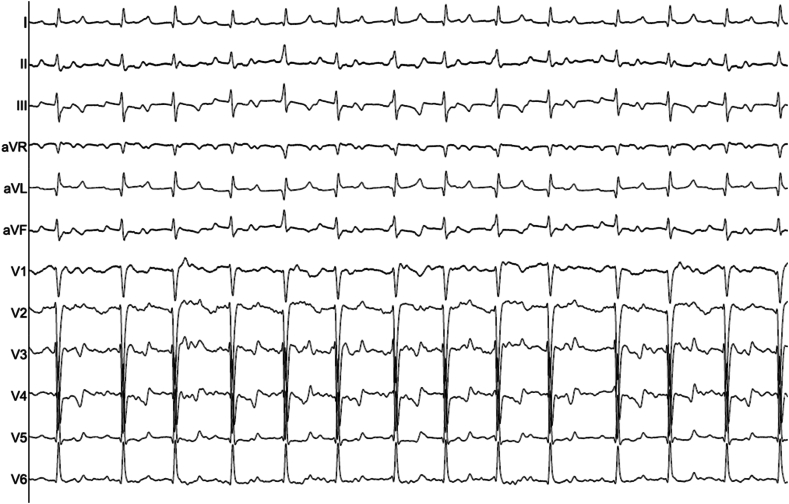
Burst pacing performed from the proximal CS electrodes at 280 ms induced a regular IART (IART1) with a cycle length (CL) of 300 ms (Fig. 1). CS activation was distal to proximal (Fig. 2), however RA origin was suspected due to the aetiology and previous ablation history.
Fig. 1.
12 lead ECG of IART1 (25mm/ms).
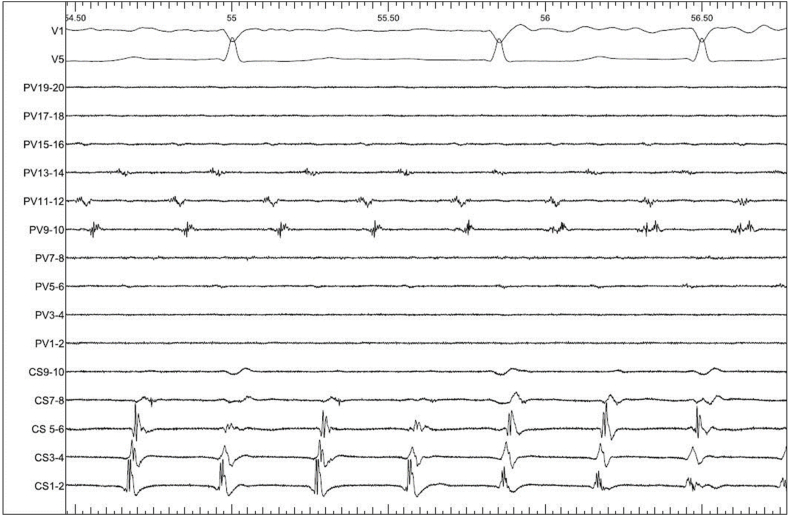
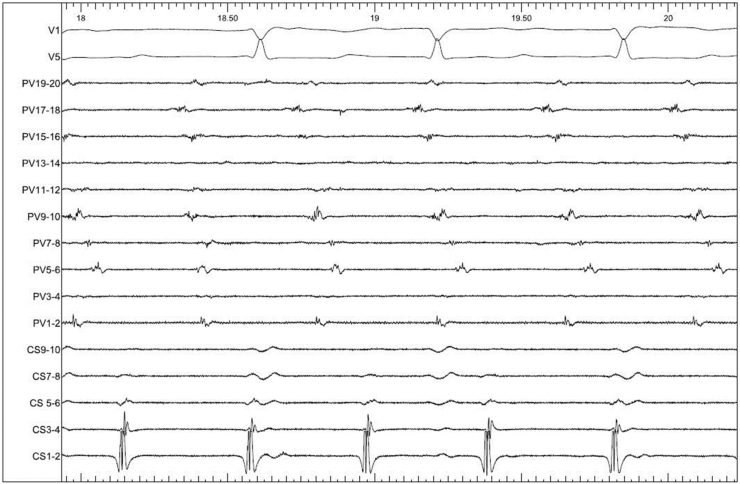
Fig. 2.
Intracardiac EGMs of IART1 (100mm/ms). CS: decapolar catheter in the coronary sinus, PV: Pentaray catheter on the lateral RA wall.
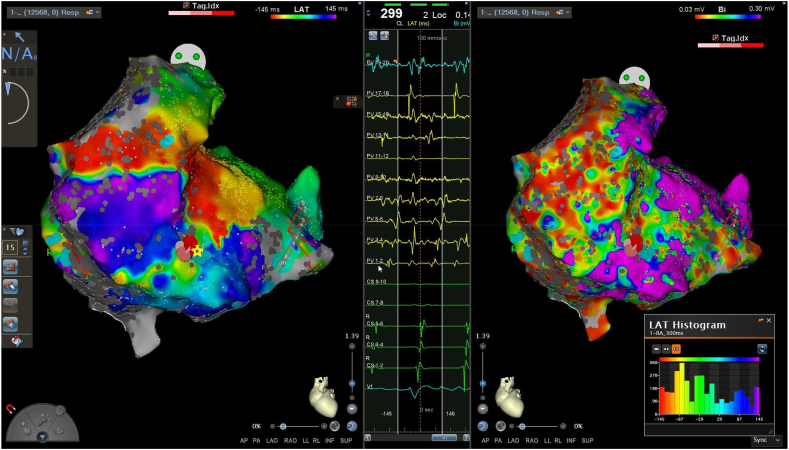
Endocardial electroanatomical (EAM) mapping of the RA with activation mapping of IART1 was performed with the PentaRay catheter, using the CONFIDENSE automatic mapping module to apply predefined filters. 12568 EAM points were collected in a mapping time of 35mins. Bipolar scar and noise floor settings were set to 0.01mV, with a bipolar voltage map showing significant scarring within the RA. LAT histograms confirmed collection of the whole CL within the RA. Analysis of the map was initially performed utilising the local activation time (LAT) map, based on annotation of signals via the greatest negative deflection (-dV/dt) on each unipolar electrogram. This suggested the circuit being intra-atrial, on the low lateral RA wall. A small area of higher voltage tissue (>0.3mV) bordered by scar was defined in this area (Fig. 3, Video 1), however although throughout this area the timing of the LAT signals was similar with propagation therefore showing a broad area of activation within this area (Video 1).
Fig. 3.
LAT map (left) and bipolar voltage map (right) demonstrating IART1. LAT histogram shows the whole IART cycle length is represented within the RA. The central panel showing low voltage fractionated electrograms recorded at the site of the yellow star, where ablation was subsequently performed.
Supplementary video related to this article can be found at https://doi.org/10.1016/j.ipej.2024.01.002
The following is/are the supplementary data related to this article.
LAT map (left) and bipolar voltage map (right) demonstrating IART1, with propagation wavefront displayed. Propagation shows a broad area of simultaneous activation within the channel of healthy tissue delineated by the purple area on the voltage map.1
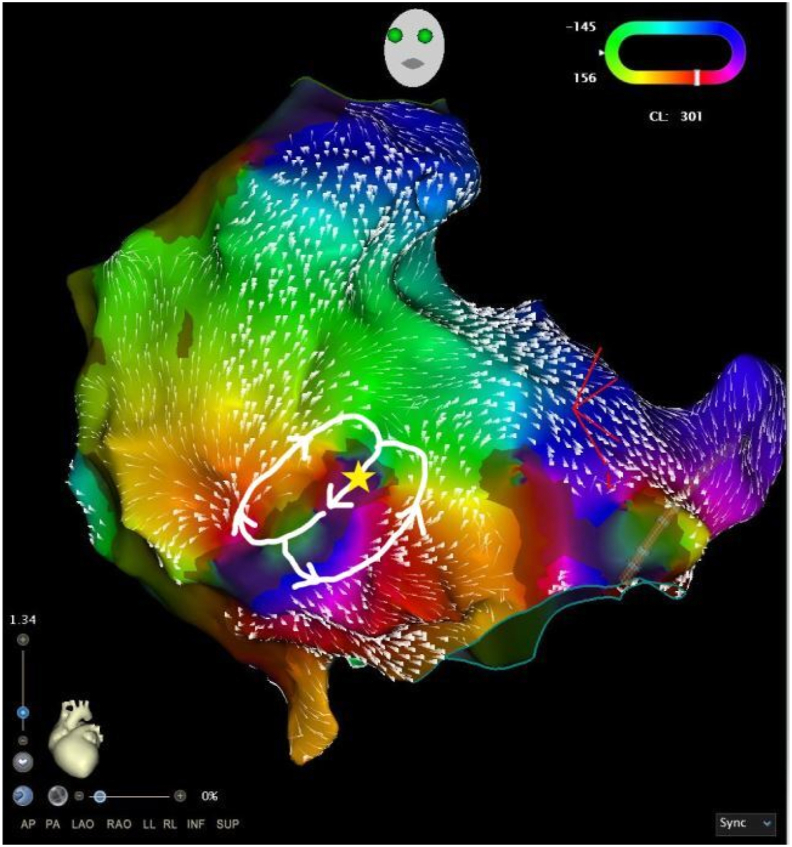
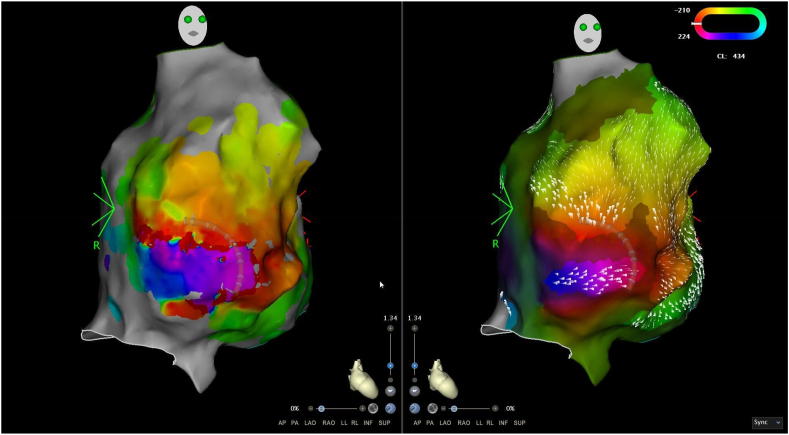
The Coherent CARTO module (Biosense Webster) was applied, displaying colour activation on a cyclic window and thereby removing the concept of early-meets-late. As described previously [1], LAT values are used to display a global best fit solution taking into account areas of slow or non-conduction (SNO) zones. Dynamic conduction velocity vectors projected onto the map indicate areas of slower conduction relative to the conduction velocity of the mapped chamber via thicker conduction velocity vectors. Analysis of the map showed a re-entry figure-of-8 circuit on the low anterior RA wall, medial to the tricuspid valve and centred around a SNO zone (Fig. 4). Dynamic conduction velocity vector arrows were gradually increased from the nominal value until a channel of slow conduction became apparent, indicating slow conduction exiting this channel compared to the vector arrows displayed on adjacent atrial tissue. This was achieved with a threshold value of 21 (Fig. 3, Video 2). Entrainment at this area demonstrated concealed entrainment with a short post-pacing interval (PPI-TCL of <30 ms).
Fig. 4.
Coherent map of LAT demonstrating the tachycardia circuit. Ablation was performed at the site of the star.
Supplementary video related to this article can be found at https://doi.org/10.1016/j.ipej.2024.01.002
The following is/are the supplementary data related to this article.
Coherent map (left) and Bipolar voltage map (right) demonstrating IART1, with propagation wavefront and projected conduction velocity vectors. This shows entry into a narrow channel of healthy tissue within a heavily scarred atrium, with the boundaries marked by the SNO zones defined by Coherent.2
A small cluster of radiofrequency (RF) ablation lesions were performed between 2 areas of scar at the entrance to the isthmus (Fig. 3, Fig. 4) at 40W (ablation time: 111s) using an irrigated catheter (ThermoCool SmartTouch™ Surround Flow, Biosense Webster). Ablation resulted in slowing of IART1 from 300 ms to IART2 with CL of 440 ms, with a change of CS activation to vertical activation suggesting a change in tachycardia circuit (Fig. 5, Fig. 6).
Fig. 5.
12 lead ECG of IART2 (25mm/ms)..
Fig. 6.
Intracardiac EGMs of IART2 (100mm/ms). CS: decapolar catheter in the coronary sinus, PV: Pentaray catheter on the lateral RA wall.).
A remap was performed, with 7716 points collected in 22 mins. LAT and Coherent map analysis showing a figure-of-8 IART circuit at the low-mid lateral RA wall, just lateral to the previous tachycardia circuit (Fig. 7). Entrainment at this area demonstrated concealed entrainment with a short post-pacing interval (PPI-TCL of 30 ms). Dynamic vector arrows set optimised to a threshold of 21 indicated slow conduction through this channel compared to vector arrows displayed on adjacent atrial tissue.
Fig. 7.
LAT map (left) and Coherent map (right) of IART2 demonstrating a re-entry circuit on the low lateral wall, with the Coherent map indicating a more narrow critical isthmus, bounded by SNO zones on either side.
A line of ablation lesions transecting this channel was performed at 40W (652s RF time), anchored to areas of scar at each end, and with termination to sinus rhythm seen on completion of this line. Bidirectional block could not be ascertained due to the heavily scarred atrium, however the tachycardia was non-inducible with atrial burst pacing down to 220 ms. There were no procedural complications.
3. Discussion
Arrhythmias are a well-documented occurrence in adult patients with a Fontan circulation, the incidence of arrhythmia increasing with time following surgery, and reaching up to 75–100 % in patients at 30 years post-surgery [2,3]. Of these the most common has been found to be IART, and adults with an AP connection demonstrate higher occurrence of IART than those with intracardiac lateral tunnel, extracardiac lateral tunnel or extracardiac conduits, and is the only type of Fontan independently associated with IART [4,5].
The occurrence of IART results from pro arrhythmic substrate caused by chronic haemodynamic overload causing fibrotic changes and RA dilation, in conjunction with post surgical atrial incisions and suture lines in these patients [2,3]. Common IART RA circuits in AP Fontan patients are seen around the inferior vena cava (IVC) and near the lateral and septal RA walls and often multiple tachycardia circuits may exist [3]. These resultant IARTs can reduce cardiac output and are associated with clinical decline and increased morbidity and mortality [1,2]. Long-term arrhythmia management is aimed at rhythm control in order to maintain AV synchrony, with catheter ablation playing a significant role in this aim.
High density mapping of IART in scarred atria has shown properties of the critical isthmus to consistently demonstrate lower EGM amplitude and significantly lower conduction velocity than surrounding tissue [6]. This becomes challenging in ACHD patients where widespread areas of low amplitude EGMs due to surgical scar and fibrotic changes makes it challenging to identify the critical isthmus. Long fractionated electrograms can make LAT annotation and identification of diastolic activation direction challenging, and often requires manual review and reannotation of points in this area, increasing procedure time.
The Coherent mapping algorithm has been shown to yield more accurate identification of IART mechanism and define a narrower critical isthmus compared to LAT mapping, with subsequent improved reliability of termination of IART with ablation [1,7]. By comparing the surrounding LAT points with each individual data point, a global pattern of activation is generated leading to reduced uncertainty in annotation of fractionated EGMs when compared to a standard LAT map. The algorithm additionally utilises a mesh of small triangles to reduce spatial interpolation errors that may occur with LAT mapping [7]. In IART1 and IART2 this was demonstrated by a more narrow critical isthmus being defined by Coherent in comparison to that of the LAT map.
The addition of velocity vectors improves ability to identify propagation wavefronts in the context of abnormal electrograms, identifying areas of relative slower conduction with thicker arrows, and relative faster conduction with thinner arrows. In these patients, individual optimisation of the vector threshold value is frequently required in order to differentiate slower conduction velocity through the critical isthmus relative to abnormal conduction velocity already existing throughout the rest of a scarred atrium.
As demonstrated in this case, Coherent mapping of an IART in a patient with complex adult congenital heart disease with an extensively scarred atrium and with previous ablation can be useful for analysis and interpretation by quickly identifying the areas of slow conduction velocity in multiple IARTs that may prove critical in macro re-entry circuit initiation and maintenance.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of Indian Heart Rhythm Society.
References
- 1.Vicera J.J., Lin Y.J., Chang S.L., Lo L.W., Chung F.P., Chao T.F., Chen C.C., Chin C.G., Lugtu I.C., Chen S.A. Identification of critical isthmus using coherent mapping in patients with scar-related atrial tachycardia. J Cardiovasc Electrophysiol. 2020;31:1436–1447. doi: 10.1111/jce.14457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quinton E., Nightingale P., Hudsmith L., Thorne S., Marshall H., Clift P., de Bono J. Prevalence of atrial tachyarrhythmia in adults after Fontan operation. Heart. 2015;101:1672–1677. doi: 10.1136/heartjnl-2015-307514. [DOI] [PubMed] [Google Scholar]
- 3.Laubham M., Blais B., Kamp A.N. Atrial arrhythmias in adults with fontan palliation. Cardiol Ther. 2023;12:473–487. doi: 10.1007/s40119-023-00326-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stephenson EA, Lu M, Berul CI, Etheridge SP, Idriss SF, Margossian R, Reed JH, Prakash A, Sleeper LA, Vetter VL, Blaufox AD. Arrhythmias in a contemporary fontan cohort. JACC (J Am Coll Cardiol); 56 (11): 890-896. [DOI] [PMC free article] [PubMed]
- 5.Song M.K., Bae E.J., Kwon B.S., Kim G.B., Noh C.I., Choi J.Y., Kim W.H., Lee J.R., Kim Y.J. Intra-atrial re-entrant tachycardia in adult patients after Fontan operation. Int J Cardiol. 2015:157–163. doi: 10.1016/j.ijcard.2015.03.157. [DOI] [PubMed] [Google Scholar]
- 6.Latcu D.G., Bun S.S., Viera F., Delassi T., El Jamili M., Al Amoura A., Asoudi N. Selection of critical isthmus in scar-related atrial tachycardia using a new automated ultrahigh resolution mapping system. Circ Arrhythm Electrophysiol. 2017;10 doi: 10.1161/CIRCEP.116.004510. [DOI] [PubMed] [Google Scholar]
- 7.Parameswaran R., Kalman J.M., Lee G. Coherent mapping: a step towards physiological mapping of complex arrhythmias? J Cardiovasc Electrophysiol. 2020;31:1448–1451. doi: 10.1111/jce.14455. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
LAT map (left) and bipolar voltage map (right) demonstrating IART1, with propagation wavefront displayed. Propagation shows a broad area of simultaneous activation within the channel of healthy tissue delineated by the purple area on the voltage map.1
Coherent map (left) and Bipolar voltage map (right) demonstrating IART1, with propagation wavefront and projected conduction velocity vectors. This shows entry into a narrow channel of healthy tissue within a heavily scarred atrium, with the boundaries marked by the SNO zones defined by Coherent.2