Key words: High latitude regions, larval stages, life cycle, parasite, transmission strategies
Abstract
Cercarial activity and survival are crucial traits for the transmission of trematodes. Temperature is particularly important, as faster depletion of limited cercarial energy reserves occurs at high temperatures. Seasonal climate conditions in high latitude regions may be challenging to complete trematode life cycle during the 6-month ice-free period, but temperature effects on the activity and survival of freshwater cercariae have not been previously identified. After experimentally simulating natural subarctic conditions during warmer and colder months (13 and 6°C), a statistical approach identifying changes in the tendency of cercarial activity loss and mortality data was used to detect differences in three trematode genera, represented by four taxa (Diplostomum spp., Apatemon spp., small- and large-sized Plagiorchis spp.). A strong temperature-dependent response was identified in both activity loss and mortality in all taxa, with Diplostomum spp. cercariae showing the most gradual changes compared to other taxa. Furthermore, whilst activity loss and mortality dynamics could not be divided into ‘fish- vs invertebrate-infecting cercariae’ groups, the detected taxa-specific responses in relation to life-history traits indicate the swimming behaviour of cercariae and energy allocation among larvae individuals as the main drivers. Cercariae exploit the short transmission window that allows a stable continuance of trematodes’ life cycles in high-latitude freshwater ecosystems.
Introduction
Many parasite taxa such as trematodes have complex life cycles that involve several developmental stages and subsequent hosts (Galaktionov and Dobrovolskij, 2003). The transmission of trematodes from their first intermediate molluscan hosts to the next invertebrate or vertebrate hosts is typically undertaken by free-living larval stages (cercariae). These cercariae are non-feeding motile stages that need to effectively disperse, localize and infect the next suitable host during their short lifespan (typically 24–72 h; Combes et al., 1994; Morley, 2012). Depending on host type and environmental conditions, transmission strategies involving a great variety of morphological and behavioural features (body size and structure, emergence patterns, swimming activity or vertical distribution in the water column) have evolved making trematodes successful in completing their life cycle (Combes et al., 1994; Morley, 2012).
Cercarial lifespan is generally species-specific, with cercarial activity and survival continuously decreasing over time. The active period of cercariae usually represents the functional lifespan, when infectivity is maximal within a few hours post-emergence from the molluscan host (Ginetsinskaya, 1988; Karvonen et al., 2003). When activity starts to decrease with cercarial ageing, survival starts to decrease as well so that a reduction of longevity is usually linked to a reduction of the active infective period (Lowenberger and Rau, 1994; Pechenik and Fried, 1995). Both cercarial activity and survival are crucial traits for the transmission success, being influenced by several mutually interacting factors under changing environmental conditions (Evans, 1985; Rea and Irwin, 1995; McCarthy, 1999a; Pietrock and Marcogliese, 2003; Thieltges et al., 2008; Koprivnikar et al., 2010; Studer et al., 2010). Temperature is a particularly important factor (e.g. Lawson and Wilson, 1980; Evans, 1985; Rea and Irwin, 1995; McCarthy, 1999b; Mouritsen, 2002), as the depletion of the limited cercarial glycogen energy reserves (Anderson and Whitfield, 1975; Ginetsinskaya, 1988) occurs at higher rates at elevated temperatures, usually resulting in a decrease of cercarial activity and survival (Pechenik and Fried, 1995; McCarthy, 1999b; Karvonen et al., 2003; Thieltges and Rick, 2006; Studer et al., 2010; Studer and Poulin, 2013). However, constant cercarial activity and survival over a larger range of temperatures, as well as increased activity at warmer conditions, have also previously been reported (e.g. Koprivnikar et al., 2010; Morley, 2011; Selbach and Poulin, 2020).
While extensive literature exists on the effects of temperature on the activity and survival of cercariae in temperate regions (e.g. Fingerut et al., 2003; Thieltges and Rick, 2006; Studer and Poulin, 2013; Selbach and Poulin, 2020), research from high latitudes (>60 °N) is so far limited to marine snail-trematode systems (Prokofiev, 1999, 2001). At subarctic and arctic latitudes, the seasonal climate may be challenging for lacustrine trematodes to complete their life cycle during the short ice-free period (<6 months). It has been proposed that trematode taxa can have different survival adaptations to the thermal range experienced in their aquatic habitats, depending on particular geographical environmental conditions (Morley, 2011; Studer and Poulin, 2014). There may also be temperature adaptations in trematode transmission strategies such as cercarial activity and survival to the highly seasonal and cold environments at high latitudes. However, whilst cercarial emergence patterns at low temperatures have been investigated in both temperate and high latitude regions (e.g. 4–20°C, Lyholt and Buchmann, 1996; 4–18°C, Brassard et al., 1982; 6–27°C, Prokofiev et al., 2016; 8°C, Nikolaev et al., 2020), temperature effects on the activity and survival of freshwater cercariae under these conditions still remain to be identified. Investigating general patterns of these important aspects of cercarial biology in relation to seasonal conditions is essential for a better understanding of trematode population and transmission dynamics at high latitudes. Furthermore, this knowledge will be pivotal evaluating the implications of global warming on parasite transmission in colder northern latitudes that are considered most vulnerable to climate change (Kutz et al., 2009; Mas-Coma et al., 2009).
In this study, a series of laboratory experiments simulating natural subarctic conditions in warmer and colder months (two temperature scenarios) were conducted to characterize cercarial activity and survival of different trematode taxa parasitizing a freshwater snail species, the lymnaeid Radix balthica (Soldánová et al., 2017). We used a statistical approach standardly applied in other research areas different to parasitology such as ichthyology and clinical fields (e.g. Ganna et al., 2013; Halttunen et al., 2017, but see van Beest et al., 2019), where activity loss and mortality of cercariae are analysed as a proxy for activity and survival. We further detected different time sections that represent a change in the tendency of data, expecting the first time section to represent the most infective period of the cercariae. We investigated four trematode taxa that differ in terms of morphology, life history and transmission strategy with the aim (1) to quantify the effect of the two temperature scenarios on cercarial activity loss and mortality in the different trematode taxa, and (2) to compare cercarial activity loss and mortality among the four trematode taxa and to relate them to specific morphology and behaviour of the cercariae. We hypothesized that cercarial activity loss and mortality are strongly temperature-dependent, likely showing reduced activity loss and higher mortality at higher temperature. Furthermore, we expect that taxa-specific responses will be related to different life-history traits and transmission strategies to localize and infect their next target hosts, with fish-infecting cercariae (as opposed to invertebrate-infecting) showing a faster increase in activity loss and mortality due to the costly swimming activity.
Materials and methods
Parasite and host material
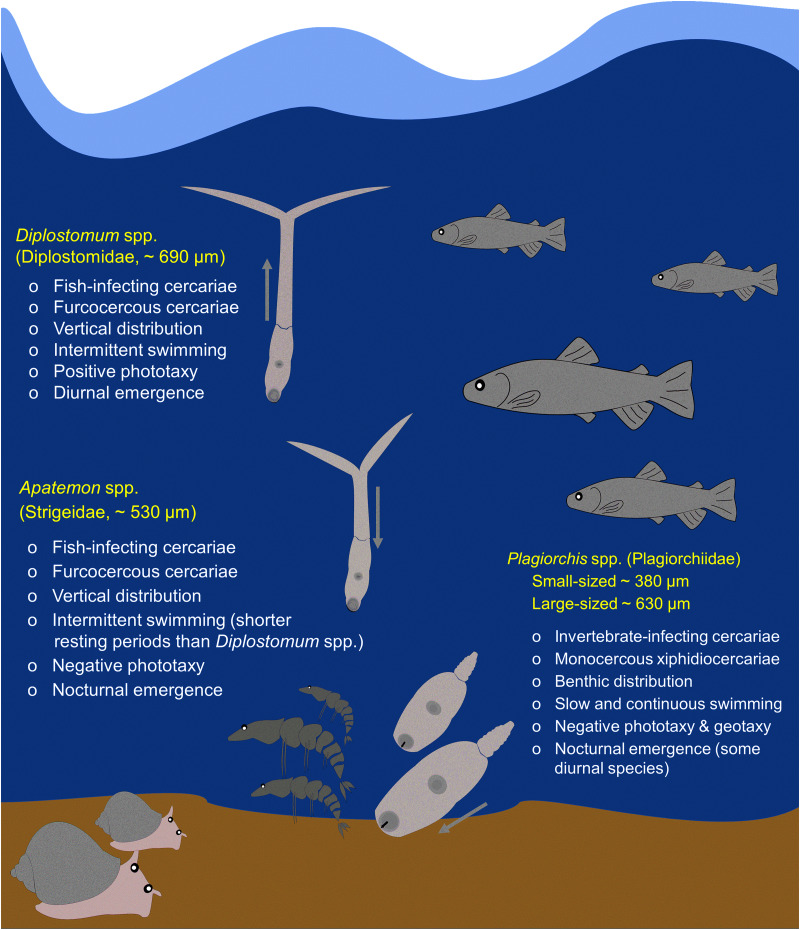
Radix balthica snails were collected by hand from the littoral zone of the subarctic lake Takvatn, northern Norway (69°07′N, 19°05′E) during August and October 2018. Snails were individually incubated in beakers containing 30 mL of lake water under a light source for 24 h, and emerging cercariae were identified in vivo according to previous research (Soldánová et al., 2017). Three genera, represented by four trematode taxa, exhibiting different cercarial morphological features, emergence and swimming behaviours, were selected for experimental trials of cercarial activity loss and mortality, including Diplostomum spp. (Diplostomidae; total length: ~690 μm), Apatemon spp. (Strigeidae; ~530 μm) and Plagiorchis spp. (Plagiorchiidae), in which two taxa were distinguished as small-sized Plagiorchis spp. (~380 μm) and large-sized Plagiorchis spp. (~630 μm) due to overlapping morphological features within this genus, encompassing seven species/lineages in subarctic areas (Soldánová et al., 2017; Kudlai et al., 2021). These genera utilize a three-host life cycle involving bird definitive hosts, but two types of second intermediate hosts, with cercariae either infecting fish (Diplostomum spp. and Apatemon spp.) or benthic amphipods or aquatic insects (Plagiorchis spp.) (Fig. 1). Diplostomum spp. and Apatemon spp. have furcocercous cercariae (i.e. bifurcated tail) that display vertical distribution within the water column and intermittent swimming periods, with Apatemon resting for much shorter periods. Furthermore, while Diplostomum spp. shows a positive photo-orientation, Apatemon shows negative phototaxy (Bell, 1996; Haas et al., 2008). Benthic monocercous xiphidiocercariae (i.e. body with stylet and simple tail) exhibit negative phototaxy and geotaxy, swimming close to the bottom with a continuous and rather slow movement (Lowenberger and Rau, 1994). Cercariae of Diplostomum spp. show diurnal emergence, whereas Apatemon and Plagiorchis show nocturnal emergence (Bell, 1996; McCarthy, 1999a; Karvonen et al., 2004; Haas et al., 2008, but see diurnal-emerging Plagiorchis species in Gorman, 1980). All snails infected with one of the model trematodes were maintained at experimental temperatures, replacing daily the filtered lake water and regularly fed with lettuce (Lactuca sativa).
Fig. 1.
Summary of the life-history traits and transmission strategies (i.e. next target hosts, morphological features, distribution in the water column, swimming behaviours and emergence patterns) of cercariae of the three genera selected for the present study, represented by four trematode taxa: Diplostomum spp., Apatemon spp., small- and large-sized Plagiorchis spp. (see Materials and methods for references). Arrows indicate the direction of phototaxy. The size of cercariae is in proportion to their original size.
Experimental design
To record cercarial activity loss and mortality of the four trematode taxa, laboratory-controlled experiments simulating field water temperature and photoperiod conditions in lake Takvatn were conducted. These scenarios represented subarctic warmer (August; 13°C and 20:4 h light:dark photoperiod) and colder (October; 6°C and 10:14 h) months from the ice-free period, based on data recorded in the field by data loggers (Onset HOBO UA-002-64 Pendant 64 K). A pool of live and active cercariae of the same age (<2 h) was collected from different snail individuals to account for potential variability, using the same group of snails throughout the experiments for each temperature setting/field collection. Cercariae were pipetted into six-well plates, placing approximately five cercariae into 6 mL of filtered lake water (i.e. ~30 cercariae per plate). Plates were then placed in climatic rooms (13 and 6°C) adjusted to reflect natural conditions including sunrise and sunset. Different number of plates with incubated cercariae for each trematode taxon and temperature were used, depending on the availability of infected snails, with a minimum of 200 monitored cercariae for most of the trials (Supplementary Table S1). Once the experiment started, cercarial activity loss and mortality were visually checked and recorded under the stereo microscope after 4 h, and thereafter every 2 h until cercarial death. Live cercariae were classified as active (swimming cercariae) or not active (cercariae barely swimming or crawling, showing erratic movements and spontaneous spasms). Furthermore, cercariae were considered dead when not showing any spasmodic movements and not responding to mechanical stimuli with a fine needle (Koprivnikar et al., 2010; van Beest et al., 2019).
To minimize snail mortality in captivity and ensure a sufficient amount of cercariae for experiments, two climatic rooms were used indistinctly for each temperature scenario (13 and 6°C). To be able to do this, a possible confounding effect of climatic rooms on activity loss and mortality of small-sized Plagiorchis spp. was tested prior to experiments with other trematodes by running trials before and after inverting the temperature in both rooms. Results of a regression Weibull model (package survival, using survreg; Therneau, 2020) revealed differences in both activity loss and mortality of cercariae between some individual plates within the same room (RWM, P < 0.05) and no significant differences between some individual plates from different rooms (RWM, P > 0.05). Furthermore, activity and survival curves followed the same dynamics in both rooms, suggesting that the observed differences are related to the individual plates and therefore not affected by the climatic rooms themselves.
Statistical analysis
To assess the variation in activity loss and mortality rates of cercariae as well as to investigate differences between temperatures and cercariae of different trematode taxa, data were first transformed into individual observations for each cercaria. Data were tested by a Cox proportional hazards regression model (survival::CoxPH, Therneau, 2020). This type of analysis aims to model time to event data, considering death as the event. In this study, we also considered the death of a cercaria as the event to monitor the end of its survival, whereas for activity analyses, we considered the loss of activity of a cercaria as the event to monitor the end of its activity. Therefore, the statistical analyses investigate differences in cercarial activity loss and mortality, as proxies for cercarial activity and survival (i.e. increased activity loss implies lower activity, whereas increased mortality implies lower survival). Similarly, activity loss or mortality represents the probability of cercariae being not active or dead at a given time point, i.e. increased activity loss at higher temperature implies an increased probability of cercariae not being active at that temperature. When analysing the data, if they did not show a constant proportional hazard between levels (using survival::cox.zph, validated at P > 0.05), step functions were used, creating time sections based on the model's residual plots (using survival::survSplit). Different time sections imply a change in the tendency of the data, i.e. when the activity loss or mortality pattern of cercaria changes over time, this is detected and data are split into different sections. Thus, the number of time sections depends on the used dataset, i.e. selected trematode taxa and temperature. This newly applied analytical approach including time sections helps to uncover changes in the activity loss and mortality patterns of cercariae that otherwise would remain overlooked if considering only the total duration of the active periods (e.g. potential periods when the majority of cercariae are active and thus potentially infective).
First, the effect of temperature on cercarial activity loss and mortality was evaluated for each trematode separately (Diplostomum spp., Apatemon spp., and small- and large-sized Plagiorchis spp.), using CoxPH analyses with temperature as a factor (6 vs 13°C, using 6°C as the baseline to which 13°C is compared), and plates as random effect. When experiments could not be run simultaneously due to insufficient amount of cercariae of the same age, the trial was also included as a random effect (i.e. Diplostomum spp., small-sized Plagiorchis spp.). Second, the activity loss and mortality among cercariae taxa at the same temperature (either 6 or 13°C) were compared. CoxPH analyses were used for each temperature separately with trematode as a factor (Diplostomum spp. vs Apatemon spp. vs small-sized Plagiorchis spp. vs large-sized Plagiorchis spp., each used in different analyses as the baseline to which one of the other taxa is compared), and plates and trial as random effects. Survival function curves were created using the Kaplan–Meier estimator (survival::survfit). Censored data (i.e. when a cercaria survives or remains active unexpectedly longer than the experimental period) were included in statistical analyses according to Crawley (2013) for Diplostomum spp. and Apatemon spp. All analyses were performed in R (R Core Team, 2017; version 3.0.1).
Results
Our analyses revealed significant differences in cercarial activity loss and mortality of the four trematode taxa belonging to three genera in relation to temperature (6 and 13°C). Depending on the trematode identity and/or temperature combination, our results suggested different time sections according to the proportionality of the Hazard Ratio, implying a change in the activity loss (section ‘i’ in all Tables) or mortality (section ‘ii’ in all Tables) of cercariae (see below). A temperature-dependent response was detected for most of the taxa and, when comparing different trematodes, differences were larger between Diplostomum spp. and the other three taxa.
Effect of temperature on cercarial activity loss and mortality within trematode taxa
Differences in activity loss rates between temperatures were similar in all tremadode taxa, showing Diplostomum spp. the most pronounced difference in the last time section (Table 1i). As for mortality rates, cercariae of Diplostomum spp. and large-sized Plagiorchis spp. showed the smallest differences between temperatures, whereas differences in mortality of Apatemon spp., and especially small-sized Plagiorchis spp., were substantial (Table 1ii).
Table 1.
Evaluation of the differences in (i) activity loss and (ii) mortality of cercariae of four trematode taxa between temperatures (6 vs 13°C)
| Trematode taxa | (i) Activity loss | (ii) Mortality | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (N, number cercariae) | Time section | exp(β)a | se(β)b | z-valuec | P value | Interpretation of activity loss | Time section | exp(β) | se(β) | z-value | P value | Interpretation of mortality |
|
Diplostomum spp. 6°C, N = 415 13°C, N = 298 |
0–9 h | 0.581 | 0.137 | −3.973 | <0.001 | 1.72 times ▾ | 0–22 h | 1.354 | 0.1015 | 2.985 | 0.003 | 1.35 times ▴ |
| 9–22 h | 2.764 | 0.207 | 4.916 | <0.001 | 2.76 times ▴ | 22–76 h | 1.673 | 0.2398 | 2.145 | 0.032 | 1.67 times ▴ | |
| 22–76 h | 5.3506 | 0.307 | 5.459 | <0.001 | 5.35 times ▴ | |||||||
|
Apatemon spp. 6°C, N = 215 13°C, N = 214 |
0–38 h | 0.669 | 0.113 | −3.558 | <0.001 | 1.49 times ▾ | 0–68 h | 7.888 | 0.1462 | 14.120 | <0.001 | 7.89 times ▴ |
| Small-sized Plagiorchis spp. 6°C, N = 426 13°C, N = 423 |
0–15 h | 3.649 | 0.119 | 10.923 | <0.001 | 3.65 times ▴ | 0–72 h | 11.668 | 0.120 | 20.470 | <0.001 | 11.67 times ▴ |
| 15–20 h | 0.505 | 0.177 | −3.851 | <0.001 | 1.98 times ▾ | |||||||
| 20–48 h | 0.594 | 0.262 | −1.990 | 0.047 | 1.68 times ▾ | |||||||
| Large-sized Plagiorchis spp. 6°C, N = 62 13°C, N = 65 |
0–33 h | 1.510 | 0.373 | 1.105 | 0.269 | 0–44 h | 1.641 | 0.2492 | 1.988 | 0.047 | 1.64 times ▴ | |
| 33–44 h | 0.762 | 0.468 | −0.582 | 0.561 | 44–86 h | 0.347 | 0.3986 | −2.653 | 0.008 | 2.88 times ▾ | ||
| 44–70 h | 0.299 | 0.564 | −2.142 | 0.032 | 3.35 times ▾ | |||||||
Results evaluating (i) Cox proportional hazards regression (CoxPH) [time active cercariae – temperature + plate (random) + trial (random)], and (ii) Cox proportional hazards regression (CoxPH) [time live cercariae – temperature + plate (random) + trial (random)]. If step function was required to accomplish the model assumptions, selected time sections are indicated in the table. The interpretation of results, comparing 6°C to 13°C, uses arrows to indicate a higher (▴) or lower (▾) activity loss or mortality of cercariae at 13°C. Statistically significant results (at α = 0.05) are indicated in bold.
Hazard ratio (exponentiated coefficients). The hazard rate of cercariae at 6°C in both models (i, ii) is 1, to which the other levels are compared. If exp(β) < 1, activity loss or mortality risk is reduced for the trematode taxa at 13°C.
Standard error of coefficients.
Test criterion value.
The activity loss rate of Diplostomum spp. cercariae was divided into three time sections, showing a 2-fold lower activity loss at 13°C compared to 6°C during the first 9 h of cercarial age (i.e. higher activity, Table 1i). Thereafter cercariae showed higher activity loss at 13°C during the next two time sections. In contrast, mortality was higher at 13°C compared to 6°C during the two detected time sections (1- and 2-fold increased mortality, respectively; Table 1ii). Cercariae of Apatemon spp. showed a decreased activity loss and an 8-fold higher mortality at 13°C, both within the single measured time section (Table 1). Cercarial activity loss patterns of both morphotypes of Plagiorchis spp. were similar to some degree, each being split into three time sections. Small-sized Plagiorchis spp. showed a 4-fold higher activity loss at 13°C compared to 6°C during the first 15 h, which later changed, showing a 2-fold decreased activity loss within the following two sections (Table 1i). The activity loss of large-sized Plagiorchis spp. cercariae remained comparable between temperatures during the first two time sections and differed significantly only in the last section with a 3-fold decreased activity loss after 44 h at 13°C compared to 6°C. Higher mortality of small-sized Plagiorchis spp. was detected at 13°C along their whole lifespan, thus detecting a single time section (12-fold increased mortality, Table 1ii). Cercarial mortality of large-sized Plagiorchis spp. split into two time sections, first with 2-fold higher, and second with 3-fold lower mortality at 13°C. This indicates that although large-sized Plagiorchis spp. cercariae suffer from higher mortality at 13°C at the beginning of their lifespan within the first 44 h, they die at a slower rate during the remaining life period.
Differences among trematode taxa in cercarial activity loss and mortality during warm subarctic conditions
Activity loss rates of all trematode pairwise combinations were divided into three time sections and mortality rates into two time sections (Table 2). Differences in both activity loss and mortality at 13°C were stronger when comparing the cercariae of Diplostomum spp. with the three other trematode taxa, following similar patterns in corresponding time sections with Apatemon spp., and small- and large-sized Plagiorchis spp. That is, a significantly decreased activity loss was recorded during the first 16 h (2- to 19-fold across compared trematode, Table 2i), followed by increased activity loss in the second and third time sections when comparing small-sized Plagiorchis spp. and especially Apatemon spp. to Diplostomum spp. (8- to 72-fold increased activity loss). The activity loss of large-sized Plagiorchis spp. and Diplostomum spp. did not differ in the second time section, but showed a significant 6-fold increased activity loss of large-sized Plagiorchis spp. in the third section (Table 2i). Mortality of Diplostomum spp. at 13°C was significantly higher during the first 27 h compared to the three other trematode taxa (9- to 20-fold lower mortality of compared taxa), thereafter decreasing Diplostomum spp.’ mortality in the second time sections (14- to 39-fold higher mortality of compared taxa, Table 2ii).
Table 2.
Evaluation of the differences in (i) activity loss and (ii) mortality among cercariae of different trematode taxa at 13°C
| Trematode taxa (N, number cercariae) | (i) Activity loss | (ii) Mortality | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference vs compared | Time section | exp(β)a | se(β)b | z-valuec | P value | Interpretation of activity loss | Time section | exp(β) | se(β) | z-value | P value | Interpretation of mortality |
|
Diplostomum spp. – Apatemon spp. N = 298 – N = 215 |
0–16 h | 0.067 | 0.190 | −14.297 | <0.001 | 15.04 times ▾ | 0–27 h | 0.10 | 0.175 | −13.448 | <0.001 | 10.47 times ▾ |
| 16–27 h | 13.333 | 0.343 | 7.552 | <0.001 | 13.33 times ▴ | 27–54 h | 14.714 | 0.316 | 8.509 | <0.001 | 14.71 times ▴ | |
| 27–52 h | 71.751 | 0.667 | 6.406 | <0.001 | 71.75 times ▴ | |||||||
|
Diplostomum spp. – small-sized Plagiorchis spp. N = 298 – N = 423 |
0–16 h | 0.432 | 0.112 | −7.500 | <0.001 | 2.31 times ▾ | 0–27 h | 0.111 | 0.134 | −16.375 | <0.001 | 9.02 times ▾ |
| 16–27 h | 7.756 | 0.212 | 9.665 | <0.001 | 7.75 times ▴ | 27–54 h | 38.964 | 0.264 | 13.897 | <0.001 | 38.96 times ▴ | |
| 27–52 h | 4.420 | 0.413 | 3.599 | <0.001 | 4.42 times ▴ | |||||||
|
Diplostomum spp. – large-sized Plagiorchis spp. N = 298 – N = 65 |
0–16 h | 0.053 | 0.596 | −4.934 | <0.001 | 18.96 times ▾ | 0–27 h | 0.049 | 0.473 | −6.371 | <0.001 | 20.31 times ▾ |
| 16–27 h | 2.683 | 0.774 | 1.274 | 0.203 | 27–86 h | 17.487 | 0.564 | 5.072 | <0.001 | 17.49 times ▴ | ||
| 27–70 h | 5.768 | 0.696 | 2.519 | 0.012 | 5.77 times ▴ | |||||||
|
Apatemon spp. – small-sized Plagiorchis spp. N = 215 – N = 423 |
0–16 h | 6.499 | 0.168 | 11.130 | <0.001 | 6.51 times ▴ | 0–27 h | 1.161 | 0.140 | 1.064 | 0.288 | |
| 16–27 h | 0.582 | 0.334 | −1.620 | 0.105 | 27–48 h | 2.648 | 0.231 | 4.213 | <0.001 | 2.65 times ▴ | ||
| 27–38 h | 0.0616 | 0.772 | −3.612 | <0.001 | 16.23 times ▾ | |||||||
|
Apatemon spp. – large-sized Plagiorchis spp. N = 215 – N = 65 |
0–16 h | 0.793 | 0.619 | −0.374 | 0.708 | 0–27 h | 0.515 | 0.497 | −1.334 | 0.182 | ||
| 16–27 h | 0.201 | 0.829 | −1.935 | 0.053 | 27–86 h | 1.188 | 0.573 | 0.301 | 0.763 | |||
| 27–70 h | 0.080 | 0.960 | −2.627 | 0.009 | 12.44 times ▾ | |||||||
| Small-sized Plagiorchis spp. – large-sized Plagiorchis spp. N = 423 – N = 65 |
0–16 h | 0.122 | 0.598 | −3.516 | <0.001 | 8.19 times ▾ | 0–27 h | 0.444 | 0.483 | −1.682 | 0.093 | |
| 16–27 h | 0.346 | 0.766 | −1.386 | 0.166 | 27–86 h | 0.449 | 0.531 | −1.510 | 0.131 | |||
| 27–70 h | 1.305 | 0.720 | 0.370 | 0.712 | ||||||||
Results evaluating (i) Cox proportional hazards regression (CoxPH) [time active cercariae at 13°C – trematode taxa + plate (random) + trial (random)], and (ii) Cox proportional hazards regression (CoxPH) [time live cercariae at 13°C – trematode taxa + plate (random) + trial (random)]. If step function is required to accomplish the model assumptions, time sections are indicated in the table. The interpretation of results, comparing the different taxa of cercariae at 13°C, use arrows to indicate a higher (▴) or lower (▾) activity loss or mortality of the compared trematode taxa (right place in first column). Statistically significant results (at α = 0.05) are indicated in bold.
Hazard ratio (exponentiated coefficients). The hazard rate (exp(β)) of cercariae of each taxon (located in left side of first column) in both models (i, ii) is 1, to which the other taxa are compared. If exp(β) < 1, activity loss or mortality is reduced for the compared trematode taxa (right place in first column).
Standard error of coefficients.
Test criterion value.
Activity loss and mortality of Apatemon spp. cercariae were different when compared to small- and large-sized Plagiorchis spp. While in the first 16 h small-sized Plagiorchis spp. showed a 7-fold activity loss increase compared to Apatemon spp., the second time section was comparable between both taxa, whereas in the third section both Plagiorchis morphotypes showed a decreased activity loss compared to Apatemon spp. (12- to 16-fold across Plagiorchis spp.). Comparable mortality rates were found between Apatemon spp. and both Plagiorchis morphotypes during the first 27 h, whereas small-sized Plagiorchis spp. showed a 3-fold higher mortality than Apatemon spp. in the second time section (Table 2ii).
The activity loss patterns of both Plagiorchis morphotypes were similar except for the first 16 h, when large-sized Plagiorchis spp. showed an 8-fold lower activity loss than small-sized Plagiorchis spp. (Table 2i). Mortality of both small- and large-sized Plagiorchis at 13°C was similar at both time sections.
Differences among trematode taxa in cercarial activity loss and mortality during cold subarctic conditions
Both activity loss and mortality rates of all trematode pairwise combinations were divided into three time sections (Table 3). Statistically significant differences in activity loss and mortality at 6°C were most pronounced when comparing Diplostomum spp. with the three other taxa, especially in comparison with large-sized Plagiorchis spp. (Table 3, Fig. 2). Cercarial activity loss of Diplostomum spp. followed a similar pattern when compared to the other trematode taxa, all the latter showing lower activity loss during the first 13 h (4- to 43-fold across trematode taxa), followed by increased activity loss compared to Diplostomum spp. in the second time section, and especially pronounced in the third time section (41- to 57-fold across trematode taxa). The only exception was the comparable activity loss rates between Diplostomum spp. and large-sized Plagiorchis spp. during the second time section (Table 3i). As for mortality, Apatemon spp., small- and large-sized Plagiorchis spp. showed lower mortality than Diplostomum spp. cercariae during the first time section within 26 h (i.e. 10- to 38-fold), and thereafter showed higher mortality with different change rates depending on the compared trematode taxa and time section (Table 3), the differences being especially strong when compared to large-sized Plagiorchis spp.
Table 3.
Evaluation of the differences in (i) activity loss and (ii) mortality among cercariae of different trematode taxa at 6°C
| Trematode taxa (N, number cercariae) | (i) Activity loss | (ii) Mortality | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference vs compared | Time section | exp(β)a | se(β)b | z-valuec | P value | Interpretation of activity loss | Time section | exp(β) | se(β) | z-value | P value | Interpretation of mortality |
|
Diplostomum spp. – Apatemon spp. N = 415 – N = 215 |
0–13 h | 0.281 | 0.209 | −6.078 | <0.001 | 3.56 times ▾ | 0–26 h | 0.098 | 0.242 | −9.594 | <0.001 | 10.23 times ▾ |
| 13–22 h | 10.729 | 0.273 | 8.708 | <0.001 | 10.73 times ▴ | 26–44 h | 5.927 | 0.299 | 5.955 | <0.001 | 5.92 times ▴ | |
| 22–76 h | 57.114 | 0.307 | 13.196 | <0.001 | 57.11 times ▴ | 44–76 h | 21.123 | 0.316 | 9.643 | <0.001 | 21.12 times ▴ | |
|
Diplostomum spp. – small-sized Plagiorchis spp. N = 415 – N = 426 |
0–13 h | 0.060 | 0.227 | −12.395 | <0.001 | 16.69 times ▾ | 0–26 h | 0.069 | 0.187 | −14.307 | <0.001 | 14.56 times ▾ |
| 13–22 h | 29.135 | 0.269 | 12.560 | <0.001 | 29.13 times ▴ | 26–44 h | 26.774 | 0.239 | 13.739 | <0.001 | 26.77 times ▴ | |
| 22–76 h | 46.31 | 0.276 | 13.875 | <0.001 | 46.31 times ▴ | 44–76 h | 52.312 | 0.326 | 12.138 | <0.001 | 52.31 times ▴ | |
|
Diplostomum spp. – large-sized Plagiorchis spp. N = 415 – N = 62 |
0–13 h | 0.023 | 1.016 | −3.711 | <0.001 | 43.37 times ▾ | 0–26 h | 0.026 | 0.723 | −5.024 | <0.001 | 37.76 times ▾ |
| 13–22 h | 2.353 | 1.250 | 0.684 | 0.494 | 26–44 h | 67.352 | 0.776 | 5.427 | <0.001 | 67.35 times ▴ | ||
| 22–76 h | 40.694 | 1.045 | 3.547 | <0.001 | 40.69 times ▴ | 44–78 h | 211.540 | 0.800 | 6.691 | <0.001 | 211.54 times ▴ | |
|
Apatemon spp. – small-sized Plagiorchis spp. N = 215 – N = 426 |
0–13 h | 0.213 | 0.296 | −5.225 | <0.001 | 4.69 times ▾ | 0–26 h | 0.703 | 0.293 | −1.204 | 0.229 | |
| 13–22 h | 2.716 | 0.333 | 3.001 | 0.003 | 2.72 times ▴ | 26–44 h | 4.5174 | 0.328 | 4.600 | <0.001 | 4.52 times ▴ | |
| 22–48 h | 0.811 | 0.353 | −0.594 | 0.552 | 44–72 h | 2.4766 | 0.405 | 2.240 | 0.025 | 2.48 times ▴ | ||
|
Apatemon spp. – large-sized Plagiorchis spp. N = 215 – N = 62 |
0–13 h | 0.082 | 1.037 | −2.412 | 0.016 | 12.20 times ▾ | 0–26 h | 0.271 | 0.762 | −1.714 | 0.087 | |
| 13–22 h | 0.219 | 1.272 | −1.193 | 0.233 | 26–44 h | 11.364 | 0.815 | 2.983 | 0.003 | 11.36 times ▴ | ||
| 22–64 h | 0.713 | 1.079 | −0.314 | 0.753 | 44–78 h | 10.015 | 0.843 | 2.733 | 0.006 | 10.01 times ▴ | ||
| Small-sized Plagiorchis spp. – large-sized Plagiorchis spp. N = 426 – N = 62 |
0–13 h | 0.385 | 1.037 | −0.921 | 0.357 | 0–26 h | 0.386 | 0.741 | −1.285 | 0.199 | ||
| 13–22 h | 0.081 | 1.264 | −1.991 | 0.047 | 12.38 times ▾ | 26–44 h | 2.516 | 0.783 | 1.177 | 0.239 | ||
| 22–64 h | 0.879 | 1.064 | −0.121 | 0.903 | 44–78 h | 4.048 | 0.788 | 1.774 | 0.076 | |||
Results evaluating (i) Cox proportional hazards regression (CoxPH) [time active cercariae at 6°C – trematode taxa + plate (random) + trial (random)], and (ii) Cox proportional hazards regression (CoxPH) [time live cercariae at 6°C – trematode taxa + plate (random) + trial (random)]. If step function is required to accomplish the model assumptions, time sections are indicated in the table. The interpretation of results, comparing the different taxa of cercariae at 6°C, use arrows to indicate a higher (▴) or lower (▾) activity loss or mortality of the compared trematode taxa (right place in first column). Statistically significant results (at α = 0.05) are indicated in bold.
Hazard ratio (exponentiated coefficients). The hazard rate (exp(β)) of cercariae of each taxon (located in left side of first column) in both models (i, ii) is 1, to which the other taxa are compared. If exp(β) < 1, activity loss or mortality is reduced for the compared trematode taxa (right place in first column).
Standard error of coefficients.
Test criterion value.
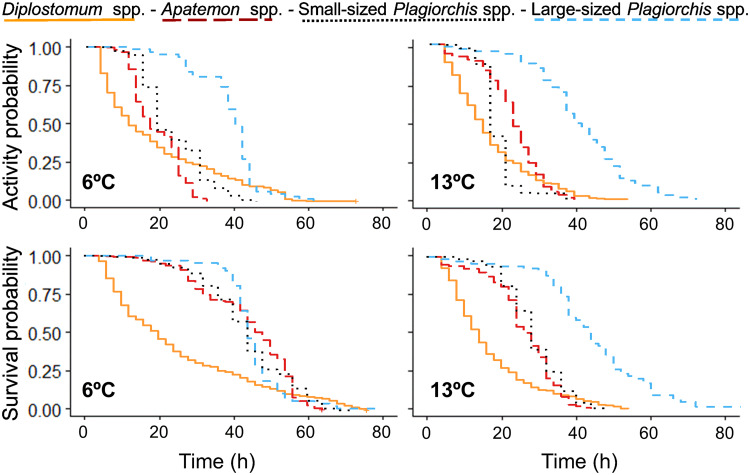
Fig. 2.
Kaplan–Meier activity (top) and survival (bottom) curves for cercariae of four trematode taxa (Diplostomum spp., Apatemon spp., small- and large-sized Plagiorchis spp., differentiated by colours) incubated at 6 and 13°C. Censored data included in the statistical analyses are indicated by a cross at the end of the curve.
The activity loss of Apatemon spp. cercariae was significantly higher in the first time section when compared to both small- and large-sized Plagiorchis spp., the latter showing a 5- to 12-fold decreased activity loss compared to Apatemon spp. during the first 13 h (Table 3i). Thereafter, a significant change in terms of Apatemon spp. decreased activity loss was detected only in the second time section compared to small-sized Plagiorchis spp. Similar mortality rates of Apatemon spp. cercariae and both morphotypes of Plagiorchis were found in the first time sections, but differed significantly after 26 h, showing that both Plagiorchis spp. increased their mortality during the next two time sections (2- to 11-fold, Table 3ii). These differences were more pronounced in comparison to large-sized Plagiorchis spp.
The two Plagiorchis morphotypes were the most similar in both activity loss and mortality, showing significant differences only in their activity loss rates in the second section, as a 12-fold lower activity loss of large-sized Plagiorchis spp. was found compared to small-sized Plagiorchis spp. (Table 3i).
Discussion
Our results demonstrate the first hypothesis that cercarial activity loss and mortality of the four studied trematode taxa from a subarctic freshwater lake are overall strongly temperature-dependent by showing lower activity loss during the first hours of cercarial lifespan and higher mortality at higher temperature for most of the taxa. The lack of a common pattern in terms of similar changes in activity loss and mortality in relation to temperature variation makes it clear that taxa-specific responses exist at each temperature, suggesting an adaptation of trematodes to the short transmission window under the cold and highly seasonal subarctic conditions by having behaviours that allow active periods during the cercarial lifespan to maximize the transmission rates to their next intermediate hosts. Differences were larger between Diplostomum spp. and the other three trematode taxa, not supporting our second hypothesis about the division of activity loss and mortality dynamics into ‘fish- vs invertebrate-infecting cercariae’ groups, as among the fish-infecting cercariae of Diplostomum spp., as opposed to Apatemon spp., exhibited a faster increase in activity loss and mortality during the first hours of their lifespan. Besides, our statistical approach allowed to identify changes in the dynamics of cercarial activity loss and mortality data, thereby recognizing specific time sections. The first time section likely represents the functional lifespan/infective period that cercariae have to infect the next suitable hosts, as shown for numerous species for which the infectivity represents 20–50% of the survival time (Evans and Gordon, 1983; Evans, 1985; Lowenberger and Rau, 1994; Pechenik and Fried, 1995; McCarthy, 1999b; Karvonen et al., 2003; Thieltges and Rick, 2006). Overall, this study highlights that new knowledge on parasite transmission strategies related to the activity loss and mortality of cercariae is highly valuable to understand the ecological dynamics of lacustrine trematodes in high latitude areas.
The strongly temperature-dependent cercarial activity loss and mortality of the studied trematodes, showing taxa-specific responses, is a phenomenon commonly reported in both freshwater and marine trematodes (Poulin, 2006; Thieltges et al., 2008; Morley, 2011). Whilst the life-expectancy of subarctic cercariae was comparable to that reported for other trematodes at a similar temperature range (e.g. 3–15°C; Lo and Lee, 1996; Lyholt and Buchmann, 1996; McCarthy, 1999b; Prokofiev, 1999, 2001; Thieltges and Rick, 2006), it was longer than under warmer conditions (e.g. >15°C; Pechenik and Fried, 1995; Bell, 1996; Mouritsen, 2002; Muñoz-Antolí et al., 2002; Karvonen et al., 2003; Koprivnikar et al., 2010; Studer and Poulin, 2013). Nevertheless, both cercarial activity loss and mortality seem to follow similar trends as taxa inhabiting warmer areas in terms of a generally increased mortality at higher temperatures (with exception of the large-sized Plagiorchis spp. at an advanced age).
Diplostomum spp. cercariae remained active during their early life period at 13°C, thus showing a vigorous swimming behaviour during their most infective period. Thereafter they underwent a loss of activity that drove them to a more passive period until their death. Furthermore, whilst a higher mortality rate of Diplostomum spp. was detected at 13°C, the difference in mortality between the two temperatures was less pronounced than observed for the other taxa, pointing to a higher stability in survival of this trematode across temperatures as suggested for other species (e.g. Koprivnikar et al., 2010; Morley, 2011; Selbach and Poulin, 2020). Apatemon spp. exhibited lower activity loss during its whole lifespan and higher mortality at 13°C, which suggests that despite a lower mortality at 6°C, the activity loss hinders the infection success of cercariae in the subsequent hosts during colder months. Cercariae of both trematodes thus seem to take advantage of the summer months to infect their next intermediate fish hosts. This has been previously described to occur in eye fluke cercariae, which, even though successfully infecting their fish hosts at 7–15°C, were 4–5 times more infective at the warmest temperatures (Lyholt and Buchmann, 1996). Additionally, the cercarial output and transmission of eye flukes to fish in high latitude regions occurs from June to September, peaking in August (e.g. Brassard et al., 1982; Hakalahti et al., 2006). Furthermore, an increase in cercarial productivity to compensate increased mortality at high temperatures has been observed in warmer latitude areas, possibly affecting transmission from the molluscan host (16–20°C, Selbach and Poulin, 2020). Once active infective stages are released from molluscan host, the naturally spatio-temporal heterogeneity in cercarial and host densities might lead to variable encounter rates (Combes et al., 1994; Thieltges and Reise, 2007). To maximize the number of infections, the total time that a group of cercariae remains active (and thus the energy allocation among them) might be different depending on their infection strategy. A random glycogen allocation would allow variable survival periods among cercariae when next hosts availability is unpredictable, resulting in a certain proportion of stages to remain active seeking for potential hosts while others remain latent/quiescent (Fenton and Hudson, 2002). The gradual increase in the activity loss and mortality of Diplostomum spp. in our study, similar to that of Diplostomum spathaceum at 20°C (Karvonen et al., 2003), implies a homogeneous glycogen allocation between cercariae. Karvonen et al. (2003) suggested that since contact between infective stages and fish hosts is likely to be highly aggregated in both time and space due to fish shoaling behaviour, other aspects different to glycogen allocation, such as cercarial seasonal emergence, might be playing a role in cercarial active periods and longevity. Data on the emergence of cercariae would help to explore the existence of seasonal transmission windows in high-latitude areas, if increased emergence, activity and survival of cercariae happen to co-occur under warm or cold subarctic conditions.
Small-sized Plagiorchis spp. showed higher mortality at 13°C, however a large percentage of cercariae losing their activity during the first hours. This indicates that these individuals enter a passive stage very soon. The remaining active cercariae are likely those that will later successfully infect their next intermediate invertebrate hosts, as described for other trematodes (Lawson and Wilson, 1980), suggesting an unequal allocation of the energy reserves exploited during the summer months. Nevertheless, their activity loss was lower at the lower temperature, suggesting that this Plagiorchis morphotype might be overall more active at colder temperatures or in microhabitats with a more stable temperature, such as the bottom of the water column close to the benthic surface, where Plagiorchis spp. are usually distributed (Lowenberger and Rau, 1994).
Cercariae of large-sized Plagiorchis spp. exhibited similar activity loss and mortality patterns at both temperatures. The higher mortality during the first half of their lifespan at 13°C, followed by a later decreased activity loss and mortality, suggests that a higher number of cercariae remain alive and active for longer periods during warmer months. This is in accordance with the findings of Lo and Lee (1996), who showed that some species of cercariae do not follow the general trend of decreased survival at increased temperatures, thus pointing towards mortality as a consequence of the ageing process rather than the temperature itself. The steep changes in cercarial activity and survival curves of large-sized Plagiorchis spp., especially at 6°C, likely suggest an unequal allocation of the energy reserves among the group of cercariae. This may allow some to remain actively host seeking for a longer time, possibly as an adaptation to an unpredictable availability of their next invertebrate hosts (Fenton and Hudson, 2002).
The trematode taxa studied here overall showed a temperature-dependent response well adapted to subarctic conditions, allowing them to complete the life cycle even at low temperatures and thus taking advantage of the transmission window during the 6-month ice-free period. The limited energy reserves, mainly concentrated in the main organ of propulsion (i.e. the tail; Lawson and Wilson, 1980), are likely depleted faster at increased temperatures, and thus is the activity. Hence, this supports the activity/survival trade-off (i.e. the longer active periods, the lower survival) suggested for other trematode species as well (Lawson and Wilson, 1980; Rea and Irwin, 1995; McCarthy, 1999b; Mouritsen, 2002) with the exception of small-sized Plagiorchis spp. during their most infective period.
Regarding the differences in cercarial activity loss and mortality among trematode taxa at warm and cold subarctic conditions, the differences between Diplostomum spp. and the other three trematode taxa remained similar at both temperatures. The changes in Diplostomum spp. activity and survival curves were overall more gradually occurring over time compared to other taxa (see Fig. 2).
The swimming behaviour of the furcocercariae of Diplostomum spp. was very different to that of bottom-dwelling monocercous cercariae of Plagiorchis spp. Therefore, the differences in activity loss and mortality rates may seem obvious, as both cercarial types follow different transmission strategies to locate and invade their next intermediate hosts (i.e. fish vs invertebrates, respectively; Lowenberger and Rau, 1994; Bell, 1996; Haas et al., 2008; Soldánová et al., 2017; see Fig. 1). However, Diplostomum spp. and Apatemon spp. furcocercariae share similar behaviour, alternating between resting and swimming periods that boost the cercariae into the water column, with Diplostomum spp. mostly occupying upper water column positions (Bell, 1996; Haas et al., 2008). The unforeseen results showing differences in activity loss and mortality of both fish-infecting furcocercariae with much more gradual changes observed for Diplostomum spp. cercariae, lead to the assumption that variations in the relative duration of the active swimming and resting periods might be responsible for the inter-taxa differences among these two taxa. A reduction in the duration of active periods with cercarial age (while not active periods increase) has been previously reported, but exceptions have been also described (Chapman, 1974; Whitfield et al., 1977; Rea and Irwin, 1995). The longer resting periods of Diplostomum spp. may allow a more constant utilization of energy reserves per time unit, thus maximizing their active stage until their death. This would allow a gradual successful infection of fish over time, which might contribute to the higher infection intensity of Diplostomum spp. in sticklebacks from Takvatn compared to that of Apatemon spp. (mean intensity 30.4 vs 3.0, respectively; Born-Torrijos et al., 2021). Additionally, the more gradual changes in activity loss and mortality increase of Diplostomum spp. compared to remaining taxa may reflect a dispersal advantage helping to disseminate cercariae and avoiding subsequent heavy parasite-related mortality in next intermediate hosts, as described for various trematode species (e.g. Evans and Gordon, 1983; Lowenberger and Rau, 1994).
Apatemon spp. and Plagiorchis spp. showed more similar activity loss and mortality rates among them, being the minor differences in their patterns likely due to their different swimming behaviour (i.e. intermittent swimming with brief resting periods of Apatemon spp. vs continuous and slow bottom-dwelling of Plagiorchis spp.), given that (i) furcocercariae swim faster than mono-tailed cercariae, and (ii) fish-infecting cercariae swim faster than those infecting invertebrates (Selbach and Poulin, 2018; Morley, 2020). The amount of glycogen reserves is also different between actively swimming and immobile cercariae (Ginetsinskaya, 1988). Whilst Plagiorchis spp. cercariae are not immobile, their displacement speed is much lower (Dixon, 1984; Lowenberger and Rau, 1994; Morley, 2020). This, together with their ability to modulate their swimming speed in close vicinity of their next intermediate hosts to facilitate infection (Morley, 2020), could result in lower energetic requirements.
The lack of the effect of cercarial body size on the response of individuals to temperature changes (Morley, 2011) could explain our observation that the two Plagiorchis spp. morphotypes showed similar activity loss and mortality patterns, despite the only negligible differences in activity loss rates in certain time sections. This could suggest that both morphotypes deplete their energy reserves in a similar way, independently of their size. The overall longer cercarial lifespan and active period of large-sized Plagiorchis spp., already reported for Plagiorchis species (10 days at 4°C and 90 h at 16°C, being infective during 38 and 18 h, respectively, Blankespoor, 1977), could thus simply reflect its larger size and likely larger energy resources, rather than the way it handles the energy. Nevertheless, it cannot be ruled out that the lack of large differences in activity loss and mortality patterns between Plargiochis morphotypes could be related to the existence of several lineages within each morphotype in high latitude areas (Soldánová et al., 2017; Kudlai et al., 2021), as different lineages could utilize divergent life cycles and transmission strategies, and thus activity loss and mortality patterns.
It is speculative whether the time of emergence might also play a role in cercarial mortality, as some cercariae emerge and actively search for their next intermediate hosts during specific times of the day to enhance the contact (Combes et al., 1994). However, although cercariae of the studied genera have been frequently reported as diurnal- (Diplostomum spp., Haas et al., 2008) or nocturnal-emerging (Apatemon spp., Bell, 1996; Plagiorchis spp., Lowenberger and Rau, 1994; but see diurnal-emerging Plagiorchis species in Gorman, 1980), trematodes in high latitude conditions could be highly adapted, similarly to marine species (Prokofiev et al., 2016), by showing different emergence patterns to optimize their transmission to next suitable hosts. The interpretation of cercarial activity loss and mortality in combination with emergence data will enable to better understand the transmission dynamics of lacustrine trematodes in subarctic areas.
Conclusion
Our study shows highly dynamic temperature-dependent activity loss and mortality patterns of trematode transmission stages in subarctic lakes that are likely playing a crucial role in shaping the parasite communities in these systems. Suggestively, these temperature-dependent responses represent adaptations to the short transmission window that allows a stable continuance of trematodes’ life cycles during ice-free periods in high-latitude ecosystems. Whilst activity loss and mortality dynamics could not be divided into ‘fish- vs invertebrate-infecting cercariae’ groups, there is a taxa-specific response to temperature in relation to life-history traits, with swimming behaviour and energy allocation among cercariae likely being the main drivers of activity loss and mortality dynamics also in subarctic conditions. Since trematode population dynamics are influenced by both cercarial production, emergence and survival (Combes and Theron, 1981), further studies on cercarial emergence are needed to allow a more general understanding of the transmission ecology of trematode populations in high-latitude ecosystems, which are considered most vulnerable to climate change. Although further experimental infection studies should be performed to confirm the first sections as the most infective, the suggestion of those periods through the establishment of different time sections is an advantage of the presented activity and survival analyses.
Acknowledgements
We are grateful to Laina Dalsbø, Karin Strand Johannessen, Eirik Haugstvedt Henriksen (UiT The Arctic University of Norway), Hynek Mazanec (Biology Centre, Czech Academy of Sciences; University of South Bohemia in České Budějovice, Czech Republic) and Iveta Sekerášová (Charles University in Prague, Czech Republic) for their assistance during field sampling and experiments.
Author contributions
A.B.-T. and M.S. conceived the ideas and designed methodology. A.B.-T., G.S.B., T.V., R.Kn., R.Kr., P.-A.A. and M.S. participated in samplings. A.B.-T., G.S.B., T.V. and M.S. performed the experimental work. A.B.-T. and G.S.B. statistically analysed the data. A.B.-T. wrote the first draft of the manuscript. All authors contributed critically to the drafts and gave final approval for the publication.
Financial support
Financial support was provided by Czech Science Foundation (A.B.-T., G.S.B., T.V., M.S., grant number 17-20936Y); and UiT The Arctic University of Norway and the Research Council of Norway (R.Kn., R.Kr., P.-A.A., grant number 213610).
Ethical standards
Not applicable.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0031182021002006.
click here to view supplementary material
Data
Data are available from the figshare repository (https://doi.org/10.6084/m9.figshare.17091161.v1, Born-Torrijos et al., 2021).
Conflict of interest
None.
References
- Anderson RM and Whitfield PJ (1975) Survival characteristics of the free-living cercarial population of the ectoparasitic digenean Transversotrema patialense (Soparker, 1924). Parasitology 70, 295–310 [Google Scholar]
- Bell AS (1996) Studies on the Biosystematics and Biology of Strigeids (Digenea) Parasitic in Freshwater Fish (PhD thesis). University of Stirling, Scotland. [Google Scholar]
- Blankespoor HD (1977) Notes on the biology of Plagiorchis noblei Park, 1936 (Trematoda: Plachiorchiidae). Proceedings of the Helminthological Society of Washington 44, 44–50. [Google Scholar]
- Born-Torrijos A, van Beest GS, Vyhlídalová T, Knudsen R, Kristoffersen R, Amundsen PA, Thieltges DW and Soldánová M (2021) Born-Torrijos et al. Data survival and activity analyses. figshare. Dataset. 10.6084/m9.figshare.17091161.v1 [DOI]
- Born-Torrijos A, Paterson R, van Beest GS, Vyhlídalová T, Henriksen EH, Knudsen R, Kristoffersen R, Amundsen P-A and Soldánová M (2021) Cercarial behaviour alters the consumer functional response of three-spined sticklebacks. Journal of Animal Ecology 90, 978–988. [DOI] [PubMed] [Google Scholar]
- Brassard P, Curtis MA and Rau ME (1982) Seasonality of Diplostomum spathaceum (Trematoda: Strigeidae) transmission to brook trout (Salvelinus fontinalis) in northern Quebec, Canada. Canadian Journal of Zoology 60, 2258–2263. [Google Scholar]
- Chapman HD (1974) The behaviour of the cercariae of Cryptocotyle lingua. Zeitschrift für Parasitenkunde 44, 211–226. [DOI] [PubMed] [Google Scholar]
- Combes C and Theron A (1981) Les densites cercariennes. Memoires du Museum National d'Histoire Naturelle, Serie A, Zoologie 119, 186–196. [Google Scholar]
- Combes C, Fournier A, Mone H and Theron A (1994) Behaviours in trematode cercariae that enhance parasite transmission: patterns and processes. Parasitology 109, S3–S13. [DOI] [PubMed] [Google Scholar]
- Crawley MJ (2013) Survival analysis. In John Wiley & Sons Ltd (eds), The R Book. Chichester, UK: John Wiley & Sons Ltd, pp. 883–893. [Google Scholar]
- Dixon MD (1984) Strategies of Host Location Employed by Larval Trematodes (PhD thesis), University of York, UK. [Google Scholar]
- Evans NA (1985) The influence of environmental temperature upon transmission of the cercariae of Echinostoma liei (Digenea: Echinostomatidae). Parasitology 90, 269–275. [Google Scholar]
- Evans NA and Gordon DM (1983) Experimental studies on the transmission dynamics of cercariae of Echinoparyphium recurvatum (Digenea: Echinostomatidae). Parasitology 87, 167–174. [Google Scholar]
- Fenton A and Hudson PJ (2002) Optimal infection strategies: should macroparasites hedge their bets? Oikos 96, 92–101. [Google Scholar]
- Fingerut JT, Zimmer CA and Zimmer RK (2003) Patterns and processes of larval emergence in an estuarine parasite system. Biology Bulletin 205, 110–120. [DOI] [PubMed] [Google Scholar]
- Galaktionov KV and Dobrovolskij AA (2003) The Biology and Evolution of Trematodes: An Essay on the Biology, Morphology, Life Cycles, Transmissions, and Evolution of Digenetic Trematodes. Boston, Dordrecht & London: Kluwer Academic. [Google Scholar]
- Ganna A, Rivadeneira F, Hofman A, Uitterlinden AG, Magnusson PKE, Pedersen NL, Ingelsson E and Tiemeier H (2013) Genetic determinants of mortality. Can findings from genome-wide association studies explain variation in human mortality? Human Genetics 132, 553–561. [DOI] [PubMed] [Google Scholar]
- Ginetsinskaya TA (1988) Trematodes, Their Life Cycles, Biology and Evolution. New Delhi: Amerind. [Google Scholar]
- Gorman AM (1980) Studies on the Biology of Pladiorchis elegans (Rudolphi, 1802), (Trematoda: Digenea) in its Mammalian and Molluscan-Hosts (PhD thesis), University of Leeds, UK. [Google Scholar]
- Haas W, Beran B and Loy C (2008) Selection of the hosts’ habitat by cercariae: from laboratory to the field. Journal of Parasitology 94, 1233–1238. [DOI] [PubMed] [Google Scholar]
- Hakalahti T, Karvonen A and Valtonen ET (2006) Climate warming and disease risks in temperate regions – Argulus coregoni and Diplostomum spathaceum as case studies. Journal of Helminthology 80, 93–98. [DOI] [PubMed] [Google Scholar]
- Halttunen E, Gjelland K-Ø, Hamel S, Serra-Llinares RM, Nilsen R, Arechavala-Lopez P, Skarðhamar J, Johnsen IA, Asplin L, Karlsen Ø, Bjørn P-A and Finstad B (2017) Sea trout adapt their migratory behaviour in response to high salmon lice concentrations. Journal of Fish Diseases 41, 953–967. [DOI] [PubMed] [Google Scholar]
- Karvonen A, Paukku S, Valtonen ET and Hudson PJ (2003) Transmission, infectivity and survival of Diplostomum spathaceum cercariae. Parasitology 127, 217–224. [DOI] [PubMed] [Google Scholar]
- Karvonen A, Kirsi S, Hudson PJ and Valtonen ET (2004) Patterns of cercarial production from Diplostomum spathaceum: terminal investment or bet hedging? Parasitology 129, 87–92. [DOI] [PubMed] [Google Scholar]
- Koprivnikar J, Lim D, Fu C and Brack SHM (2010) Effects of temperature, salinity, and pH on the survival and activity of marine cercariae. Parasitology Research 106, 1167–1177. [DOI] [PubMed] [Google Scholar]
- Kudlai O, Pantoja C, O'Dwyer K, Jouet D, Skírnisson K and Faltýnková A (2021) Diversity of Plagiorchis (Trematoda: Digenea) in high latitudes: species composition and snail host spectrum revealed by integrative taxonomy. Journal of Zoological Systematics and Evolutionary Research 59, 937–962. [Google Scholar]
- Kutz SJ, Jenkins EJ, Veitch AM, Ducrocq J, Polley L, Elkin B and Lair S (2009) The Arctic as a model for anticipating, preventing, and mitigating climate change impacts on host-parasite interactions. Veterinary Parasitology 163, 217–228. [DOI] [PubMed] [Google Scholar]
- Lawson JR and Wilson RA (1980) The survival of the cercariae of Schistosoma mansoni in relation to water temperature and glycogen utilization. Parasitology 81, 337–348. [DOI] [PubMed] [Google Scholar]
- Lo CT and Lee KM (1996) Pattern of emergence and the effects of temperature and light on the emergence and survival of heterophyid cercariae (Centrocestus formosanus and Haplorchis pumilio). Journal of Parasitology 82, 347–350. [PubMed] [Google Scholar]
- Lowenberger CA and Rau ME (1994) Plagiorchis elegans: emergence, longevity and infectivity of cercariae, and host behavioural modifications during cercarial emergence. Parasitology 109, 65–72. [DOI] [PubMed] [Google Scholar]
- Lyholt HCK and Buchmann K (1996) Diplostomum spathaceum: effects of temperature and light on cercarial shedding and infection of rainbow trout. Diseases of Aquatic Organisms 25, 169–173. [Google Scholar]
- Mas-Coma S, Valero MA and Bargues MD (2009) Climate change effects on trematodiases, with emphasis on zoonotic fascioliasis and schistosomiasis. Veterinary Parasitology 163, 264–280. [DOI] [PubMed] [Google Scholar]
- McCarthy AM (1999a) Photoperiodic cercarial emergence patterns of the digeneans Echinoparyphium recurvatum and Plagiorchis sp. from a mixed infection in Lymnaea peregra. Journal of Helminthology 73, 59–62. [Google Scholar]
- McCarthy AM (1999b) The influence of temperature on the survival and infectivity of the cercariae of Echinoparyphium recurvatum (Digenea: Echinostomatidae). Parasitology 118, 383–388. [DOI] [PubMed] [Google Scholar]
- Morley NJ (2011) Thermodynamics of cercarial survival and metabolism in a changing climate. Parasitology 138, 1442–1452. [DOI] [PubMed] [Google Scholar]
- Morley NJ (2012) Cercariae (Platyhelminthes: Trematoda) as neglected components of zooplankton communities in freshwater habitats. Hydrobiologia 691, 7–19. [Google Scholar]
- Morley NJ (2020) Cercarial swimming performance and its potential role as a key variable of trematode transmission. Parasitology 147, 1369–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouritsen KN (2002) The Hydrobia ulvae–Maritrema subdolum association: influence of temperature, salinity, light, water-pressure and secondary host exudates on cercarial emergence and longevity. Journal of Helminthology 76, 341–347. [DOI] [PubMed] [Google Scholar]
- Muñoz-Antolí C, Trelis M, Espert A, Toledo R and Esteban JG (2002) Survival and infectivity of Echinostoma friedi (Trematoda: Echinostomatidae) miracidia and cercariae under experimental conditions. Helminthologia 39, 149–154. [Google Scholar]
- Nikolaev KE, Levakin IA and Galaktionov KV (2020) Seasonal dynamics of trematode infection in the first and the second intermediate hosts: a long-term study at the subarctic marine intertidal. Journal of Sea Research 164, 101931. [Google Scholar]
- Pechenik JA and Fried B (1995) Effect of temperature on survival and infectivity of Echinostoma trivolvis cercariae: a test of the energy limitation hypothesis. Parasitology 111, 373–378. [Google Scholar]
- Pietrock M and Marcogliese DJ (2003) Free-living endohelminth stages: at the mercy of environmental conditions. Trends in Parasitology 19, 293–299. [DOI] [PubMed] [Google Scholar]
- Poulin R (2006) Global warming and temperature-mediated increases in cercarial emergence in trematode parasites. Parasitology 132, 143–151. [DOI] [PubMed] [Google Scholar]
- Prokofiev VV (1999) Influence of temperature and salinity on a life span of cercariae of marine littoral trematodes Cryptocotyle sp. (Heterophyidae), Levinseniella brachysoma and Maritrema subdolum (Microphallidae). Parazitologiya 33, 520–526 (in Russian). [Google Scholar]
- Prokofiev VV (2001) Influence of temperature and salinity on a life span of cercariae of marine littoral trematodes Podocotyle atomon (Opecoealidae) and Renicola thaidus (Renicolidae). Parazitologiya 35, 69–76 (in Russian). [PubMed] [Google Scholar]
- Prokofiev VV, Galaktionov KV and Levakin IA (2016) Patterns of parasite transmission in polar seas: daily rhythms of cercarial emergence from intertidal snails. Journal of Sea Research 113, 85–98. [Google Scholar]
- R Core Team (2017) R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. Available at https://www.r-project.org/. [Google Scholar]
- Rea JG and Irwin SWB (1995) The effects of age, temperature and shadow stimuli on activity patterns of the cercariae of Cryptocotyle lingua (Digenea: Heterophyidae). Parasitology 111, 95–101. [Google Scholar]
- Selbach C and Poulin R (2018) Parasites in space and time: a novel method to assess and illustrate host-searching behaviour of trematode cercariae. Parasitology 145, 1469–1474. [DOI] [PubMed] [Google Scholar]
- Selbach C and Poulin R (2020) Some like it hotter: trematode transmission under changing temperature conditions. Oecologia 194, 745–755. [DOI] [PubMed] [Google Scholar]
- Soldánová M, Georgieva S, Roháčová J, Knudsen R, Kuhn JA, Henriksen EH, Siwertsson A, Shaw JC, Kuris AM, Amundsen P-A, Scholz T, Laffety KD and Kostadinova A (2017) Molecular analyses reveal high species diversity of trematodes in a sub-Arctic lake. International Journal for Parasitology 47, 327–345. [DOI] [PubMed] [Google Scholar]
- Studer A and Poulin R (2013) Cercarial survival in an intertidal trematode: a multifactorial experiment with temperature, salinity and ultraviolet radiation. Parasitology Research 112, 243–249. [DOI] [PubMed] [Google Scholar]
- Studer A and Poulin R (2014) Analysis of trait mean and variability versus temperature in trematode cercariae: is there scope for adaptation to global warming? International Journal for Parasitology 44, 403–413. [DOI] [PubMed] [Google Scholar]
- Studer A, Thieltges DW and Poulin R (2010) Parasites and global warming: net effects of temperature on an intertidal host–parasite system. Marine Ecology Progress Series 415, 11–22. [Google Scholar]
- Therneau T (2020) A package for survival analysis in R. R package version 3.1-11. Available at https://CRAN.R-project.org/package=survival.
- Thieltges DW and Reise K (2007) Spatial heterogeneity in parasite infections at different spatial scales in an intertidal bivalve. Oecologia 150, 569–581. [DOI] [PubMed] [Google Scholar]
- Thieltges DW and Rick J (2006) Effect of temperature on emergence, survival and infectivity of cercariae of the marine trematode Renicola roscovita (Digenea: Renicolidae). Diseases of Aquatic Organisms 73, 63–68. [DOI] [PubMed] [Google Scholar]
- Thieltges DW, Jensen KT and Poulin R (2008) The role of biotic factors in the transmission of free-living endohelminth stages. Parasitology 135, 407–426. [DOI] [PubMed] [Google Scholar]
- van Beest GS, Villar-Torres M, Raga JA, Montero FE and Born-Torrijos A (2019). In vivo fluorescent cercariae reveal the entry portals of Cardiocephaloides longicollis (Rudolphi, 1819) Dubois, 1982 (Strigeidae) into the gilthead seabream Sparus aurata L. Parasites & Vectors 12, 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield PJ, Anderson RM and Bundy DAP (1977) Experimental investigations on the cercariae of an ectoparasitic digenean Transversotrema patialense: general activity patterns. Parasitology 75, 7–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0031182021002006.
click here to view supplementary material
Data Availability Statement
Data are available from the figshare repository (https://doi.org/10.6084/m9.figshare.17091161.v1, Born-Torrijos et al., 2021).