Key words: Bucephalidae, cercaria, climate change, Digenea, mollusc, parasite phenology, temperature, transmission
Abstract
Global warming is likely to lengthen the seasonal duration of larval release by parasites. We exposed freshwater mussel hosts, Anodonta anatina, from 2 high-latitude populations to high, intermediate and low temperatures throughout the annual cercarial shedding period of the sympatric trematodes Rhipidocotyle fennica and R. campanula, sharing the same transmission pathway. At the individual host level, under warmer conditions, the timing of the cercarial release in both parasite species shifted towards seasonally earlier period while its duration did not change. At the host population level, evidence for the lengthening of larvae shedding period with warming was found for R. fennica. R. campanula started the cercarial release seasonally clearly earlier, and at a lower temperature, than R. fennica. Furthermore, the proportion of mussels shedding cercariae increased, while day-degrees required to start the cercariae shedding decreased in high-temperature treatment in R. fennica. In R. campanula these effects were not found, suggesting that warming can benefit more R. fennica. These results do not completely support the view that climate warming would invariably increase the seasonal duration of larval shedding by parasites, but emphasizes species-specific differences in temperature-dependence and in seasonality of cercarial release.
Introduction
Marked seasonal fluctuation in temperature is a characteristic of high-latitude ecosystems. Such seasonal temperature variation can affect trematode parasites in different ways, including the timing of seasonal production of infective stages (miracidia and cercariae), since their release primarily occurs during the warm summer months in temperate and boreal zones (e.g. Chubb, 1979; Taskinen, 1998a; Karvonen et al., 2004). Indeed, most experimental and field studies (e.g. Fingerut et al., 2003; reviewed in Poulin, 2006; Thieltges and Rick, 2006; Studer et al., 2010; Shim et al., 2013; Rosen et al., 2018; Selbach and Poulin, 2020; Vyhlídalová and Soldánová, 2020) have reported increased release of cercariae with a moderate temperature rise. However, in some cases, this effect can be transient (Paull et al., 2015), absent or even negative, most probably because cercariae emergence rates are tended to decrease at the threshold temperatures and drop with temperature rise (Koprivnikar and Poulin, 2009a, 2009b; Morley and Lewis, 2013). A common expectation is that the predicted climate warming (IPCC, 2014) will increase the seasonal duration of larval release by parasites as a consequence of a longer thermal growing season (longer summer) (Marcogliese, 2001; Harvell et al., 2009; Lõhmus and Björklund, 2015; Prokofiev et al., 2016; Galaktionov, 2017). Such a lengthening of the cercarial release period has been observed in water bodies receiving thermal effluents (e.g. Aho et al., 1982). However, experimental long-term manipulations of temperature conditions over the seasonal cercarial release period are rare. To our knowledge, the only over-season experimental long-term study, by Paull and Johnson (2014), indicates a seasonal shift, rather than lengthening, in the timing of cercariae emergence. This shift to a seasonally clearly earlier occurrence of cercariae emission led to a decrease in parasite transmission and reduced parasite-induced host pathology due to temporal mismatch between cercariae and their target host (Paull and Johnson, 2014). Furthermore, whether such lengthening of the seasonal cercarial shedding period would be a result of a longer cercarial shedding at the individual host level or a result of the seasonal variation between host individuals has remained unexplored. In addition, most experimental studies of cercarial release were conducted at constant – though different – temperatures, while in natural conditions the temperature fluctuates during the cercariae shedding period. Finally, as most of the studies have been performed with snail hosts and marine species, only little is known about temperature effects in freshwater bivalve–trematode associations (however, see Morley and Lewis, 2013; Choo and Taskinen, 2015). This gap demands attention since bivalves are important parts of freshwater ecosystems providing valuable ecosystem services (e.g. Vaughn, 2018), and are very commonly infected by trematodes, frequently with high prevalence of infection (Müller et al., 2015). Bivalves can release a huge amount of cercariae (Taskinen, 1998a) which infect many central prey fish species and are transmitted to the key predatory fishes of freshwater ecosystems (Taskinen et al., 1991; Cribb et al., 2001). Therefore, a long-term study on temperature effects in a freshwater bivalve–trematode association, with temperature treatments mirroring the natural seasonal fluctuations in temperature conditions should be considered necessary.
Cercarial larvae of trematodes emerge over species-specific temperature conditions both in the laboratory and field studies (e.g. Fingerut et al., 2003). However, interspecific comparisons of cercarial production in varying temperature conditions that can reveal species-specific responses have been utilized quite rarely and mainly focus on short-term temporal variability in cercarial emergence (e.g. diurnal rhythms) rather than on large-scale seasonal patterns (de Montaudouin et al., 2016; Prokofiev et al., 2016; Vyhlídalová and Soldánová, 2020; see, however, field observations by Fingerut et al., 2003; Koprivnikar and Poulin, 2009a). In addition, in most previous studies, the influence of temperature on cercariae shedding has been compared between parasite species with different transmission pathways, while species-specific differences in the closely related sympatric parasites sharing the same hosts are rarely studied (Vyhlídalová and Soldánová, 2020). In the present study, we used 2 closely related, sympatric trematodes, Rhipidocotyle campanula and R. fennica, in their shared first intermediate host, freshwater mussel Anodonta anatina. The parasites also have the same second intermediate host, the cyprinid fish Rutilus rutilus (Taskinen et al., 1991; Gibson et al., 1992).
In addition, though the importance of studying temperature-dependent cercariae output in high-latitude areas (>60°) has been highlighted earlier (Morley and Lewis, 2013; Studer and Poulin, 2014; Galaktionov, 2017), experimental investigations are still scarce (Prokofiev et al., 2016). They would be timely since impacts of the climate change are predicted to be the most pronounced at high latitude regions. For example, climate models predict an increase in annual temperature from 2 to 7°C by the 2080s compared to a 1961–1990 baseline period in the current study region Finland at 60–70°N (Jylhä et al., 2004), and in the temperate lakes of the northern hemisphere in general (Sharma et al., 2007).
Production of cercariae is an important component of the complex life cycle of trematodes, underpinning transmission to the next host and hence influencing the fitness of the parasite. Cercariae of trematodes are transmitted to a variety of hosts and, in addition to being an infectious agent, they can serve as a valuable food source for many aquatic organisms (Johnson et al., 2010; Orlofske et al., 2012; Mironova et al., 2019, 2020; McKee et al., 2020). Thus, cercariae play an important role in the functioning of aquatic ecosystems (Kuris et al., 2008; Thieltges et al., 2008; Preston et al., 2013). Therefore, the seasonal timing and duration of the cercarial shedding period can affect different trophic levels in aquatic ecosystems by changing parasite burden and food availability.
In the present long-term (5 months) experiment, we investigated the seasonal cercarial shedding traits using 3 different temperature levels reflecting the natural temperature variation over the distribution range of the bivalve host A. anatina in the current study region, Finland (60–68°N). We suggest that higher temperature will accelerate parasite development, leading to the earlier seasonal start of cercariae emission. This is based on the general view that parasites receive a competitive advantage over the host under high temperature (e.g. see Lõhmus and Björklund, 2015; Marcogliese, 2016 for the discussion) and on the previous observation of the short-term temperature effect on R. fennica cercarial release (Choo and Taskinen, 2015). We were specifically interested in disentangling the seasonal duration of cercarial shedding at the individual host and host population levels. Our hypotheses were that under higher temperatures, mussels will start to emit cercariae of both Rhipidocotyle species seasonally earlier, will emit cercariae for a longer period, and need fewer day-degrees to start cercariae shedding than under lower temperatures.
Materials and methods
Study species
The bivalve mollusc host, A. anatina, is a common European freshwater mussel with a maximum life span >10 years, age of maturation 2–4 years and maximum length of 12 cm (Taskinen and Valtonen, 1995). Anodonta anatina serves as the first intermediate host of the bucephalid trematodes Rhipidocotyle campanula and R. fennica (Taskinen et al., 1991, 1997; Gibson et al., 1992; Müller et al., 2015). Cercariae are produced asexually by sporocysts located in the gonads of the mussel. The prevalence of infection by R. campanula in natural populations is usually less than 10% (Taskinen et al., 1991; Müller et al., 2015), whereas that by R. fennica can be up to 50% (Taskinen et al., 1994). Pronounced seasonality in the developmental stages of cercariae, shedding of cercariae, developmental stages of sporocysts and quantity of sporocysts of Rhipidocotyle species in A. anatina was observed (Taskinen et al., 1994; Taskinen, 1998a). Both parasite species have been linked to decreased growth, survival and reproduction of A. anatina (Taskinen and Valtonen, 1995; Taskinen, 1998b; Jokela et al., 2005; Müller et al., 2015). The second intermediate host of these trematodes is the cyprinid fish R. rutilus, in which R. fennica metacercariae encyst in the fins and R. campanula metacercariae – in the gills (Taskinen et al., 1991; Gibson et al., 1992). The definitive hosts for R. campanula are the percid fishes Perca fluviatilis and Sander lucioperca and the definitive host for R. fennica is the esocid fish Esox lucius (Taskinen et al., 1991; Gibson et al., 1992).
Experimental set-up
Altogether 281 A. anatina mussels were collected from the River Kuusaankoski (17 May 2011; 62°25′N, 26°00′E) and 290 mussels from the River Haajaistenjoki (22 May 2011; 63°63′N, 26°99′E), Finland. These 2 sampling sites were chosen for comparison of cercariae emergence traits in different mussel populations. They were located quite far from each other (distance > 140 km) and differed by environmental conditions, which allowed the collecting of mussels and parasites of various phenotypes and life stories for the experiment. At the Konnevesi Research Station, University of Jyväskylä, the mussels were individually marked and measured. Average shell length ± s.e. for the River Haajaistenjoki mussels was 61.9 ± 0.6 (range 33.0–92.6 mm), and for the River Kuusaankoski mussels 77.7 ± 0.6 (range 38.8–101.7 mm). There was a significant size difference between mussel populations (estimate ± s.e. = 16.9 ± 1.1, t = 16.08, P < 0.0001), while the mussels shedding and non-shedding cercariae did not differ in their size (estimate ± s.e. = 1.83 ± 1.3, t = 1.45, P = 0.15) (Supplementary Fig. S1, Tables S1 and S2). Twelve mussels were infected with both species of parasites and were excluded from the subsequent analyses, except for the analysis of probability of cercariae shedding in different temperature treatments (see section Data analysis). Data about sizes of mussels taking into account parasitic species are presented below.
From the date of collection to 25th June, mussels were kept in the laboratory in 2 tanks (1 population per tank) under flow-through conditions (open tanks that allow a constant flow of new water). The infection status of collected mussels was unknown at this stage (it became clear later during experimental monitoring of cercariae emission), thus infected and uninfected mussels were maintained together. Each 163-l tank was filled with 5 cm of sand at the bottom and supplied with water from the hypolimnetic zone (9 m depth) of Lake Konnevesi at a rate of up to 10 L min−1. Water temperatures in both tanks were similar throughout this period ranging from 10.5°C on 31 May to 11.7°C on 25 June (Fig. 1).
Fig. 1.
The daily (mean ± s.e.) cercarial release of Rhipidocotyle campanula (R. c) and R. fennica (R. f) from the mussel host Anodonta anatina, water temperature profile at mean 3-day intervals and the total duration of cercarial release by mussels from the River Haajaistenjoki (straight horizontal line) and from the River Kuusaankoski (dotted horizontal line), from 31 May to 28 October in the high- (A), intermediate- (B) and low-temperature treatments (C). An asterisk represents the day when the mussels were assigned to the different temperature treatments. Note the different scales on the y-axes.
On 25 June, the mussels were randomly assigned to 1 of the 3 temperature treatments – high, intermediate and low temperature (see below) – with 2 replicate tanks per treatment. Mussels from both populations and from all size groups were distributed evenly to each of the 6 tanks (for mussel numbers per tank, see Table 1). The average water temperatures from 25 June to 28 October, when the experiment was terminated, were 18°C (range 7–24°C), 15°C (range 7–20°C) and 13°C (range 6–18°C) in high, intermediate and low temperatures, respectively. There was no length difference between mussels allocated to temperature treatments (2-way analysis of variance; F2, 553 = 0.056, P = 0.945) and no interaction between population and treatment (F2, 553 = 0.697, P = 0.499). Average shell length of mussels (±s.e.) in high-temperature tanks was 70.0 ± 1.0 mm (range 33.0–101.7 mm), in intermediate temperature tanks 69.4 ± 1.0 mm (range 37.9–100.0 mm) and in low-temperature tanks 69.8 ± 0.9 mm (range 37.4–101.5 mm). See Table S3 for the treatment per population descriptive statistics.
Table 1.
Total numbers of A. anatina mussels (N) and numbers of mussels shedding cercariae (Ns; n shedding R. fennica/n shedding R. campanula) from the River Haajaistenjoki and the River Kuusaankoski kept in high- (HT), intermediate-(IT) and low-temperature (LT) treatments
| N | Ns | Start date | Stop date | Duration | Start °C | Stop °C | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R. fennica | R. campanula | R. fennica | R. campanula | R. f. | R. c. | R. f. | R. c. | R. f. | R. c. | |||
| River Haajaistenjoki | ||||||||||||
| HT | 96 | 33/8 | 12 Jul–8 Aug | 31 May–27 Jun | 27 Jul–18 Sep | 14 Jun–18 Sep | 10 | 16 | 21.5 | 10.5 | 16.0 | 16.0 |
| IT | 97 | 19/11 | 12 Jul–3 Oct | 31 May–4 Sep | 4 Sep–14 Oct | 12 Jul–3 Oct | 14 | 18 | 15.5 | 10.5 | 9.0 | 12.0 |
| LT | 97 | 6/20 | 4 Sep–3 Oct | 31 May–8 Aug | 18 Sep–14 Oct | 14 Jun–3 Oct | 6 | 18 | 16.5 | 10.5 | 11.0 | 11.0 |
| River Kuusaankoski | ||||||||||||
| HT | 93 | 37/3 | 12 Jul–8 Sep | 31 May–14 Jun | 8 Aug–18 Sep | 14 Jun–27 July | 10 | 8 | 21.5 | 10.5 | 16.0 | 23.0 |
| IT | 93 | 13/6 | 12 Jul–14 Oct | 31 May–12 Jul | 4 Sep–28 Oct | 12 Jul–18 Sep | 16 | 16 | 15.5 | 10.5 | 8.0 | 15.7 |
| LT | 95 | 2/8 | 4 Sep–3 Oct | 31 May–8 Aug | 4 Sep–3 Oct | 12 Jul–4 Sep | 4 | 14 | 16.5 | 10.5 | 11.0 | 16.5 |
Start and stop dates represent the range between the earliest and latest observations of cercarial emergence, respectively. Duration indicates the length (weeks) of the cercarial release at the host population level, from the first to the last observation of shedding. Start °C and Stop °C represent the water temperatures (°C) on the dates when the first and last cercariae emerged, respectively.
R. f. and R.c. = Rhipidocotyle fennica and R. campanula, respectively.
The water temperature ranges in the different temperature treatments corresponded to the natural water extreme temperature variations currently occurring throughout the distributional area of A. anatina in Finland, from 60 to 68°N and represent the maximum summer temperatures varying from about 17 to 24°C, respectively (Kuha et al., 2016). The number of days when the average daily water temperature in the different treatments was ⩾15°C, a measure of the length of the warm/growing season, was 74, 71 and 20 days in high, intermediate and low-temperature treatments, respectively.
The temperature treatments were established as follows. (1) High-temperature tanks were placed in outside shelter and supplied with running water – using a pump – from the littoral zone (<2 m depth) of Lake Konnevesi. (2) Intermediate-temperature tanks were kept indoors and supplied with heated hypolimnetic water pumped from Lake Konnevesi. Water was heated in a separate tank with aquarium heaters before delivery to mussel tanks. (3) Low-temperature tanks were kept indoors and supplied with hypolimnetic water pumped from Lake Konnevesi. Anodonta mussels are filter-feeders utilizing phytoplankton, bacteria and fine organic particles (Jorgensen et al., 1984), thus a continuous flow of lake water was necessary to provide the mussels with food. Due to logistic constraints, differences other than temperature existed between the treatments. Mussels in the high-temperature treatment were subject to a larger daily fluctuation of temperature than those in the intermediate- or low-temperature treatments (Fig. 1), as the littoral water and outdoor tanks were used. In addition, the daily/seasonal profile varied such that the daily water temperature in the high-temperature treatment tanks peaked in late July (24°C), while in the intermediate (19°C) and low-temperature tanks (17°C), it peaked in early September (Fig. 1).
The indoor tanks were illuminated by artificial light with the photoperiod set to correspond with the natural rhythm. The outdoor tanks received natural light but the shelter above the tanks provided effective cover against direct sunlight. However, during the 24 h cercarial release monitoring period, similar artificial light was used for all mussels to provide equal light conditions (see below). Water flow into the holding tanks was adjusted such that it was higher in the intermediate and low temperatures (10 L min−1) than in the high-temperature tanks (5 L min−1). This was to compensate for the probable higher food density in the high-temperature tanks that received littoral water, than the intermediate- and low-temperature tanks that received hypolimnetic water. A submersible temperature logger was placed in 1 replicate tank per treatment to measure water temperature every 4 h from 25 June to 28 October (end of the experiment). Temperature in the high-temperature treatment (outdoor tanks) varied much stronger during the day than in other treatments (Fig. 1). However, results by Roushdy (1984) indicate that cercarial release does not differ between constant and diurnally variable temperatures.
Cercarial release from each mussel was followed over a period of 20 weeks by counting cercariae released per A. anatina at roughly 2-week (12–15 days each) intervals between 31 May and 28 October, during a total of 12 monitoring sessions. On each monitoring day, individual mussels were placed in a 4-l transparent plastic box (length 26.5 cm, width 19 cm and height 13.6 cm) filled with 2 L of filtered lake water for 24 h (possible dead mussels were removed at this stage) and then returned to their respective holding tanks. The water temperatures in the monitoring boxes during the 24 h period of cercarial shedding were adjusted to correspond with those in the respective holding tanks and, when necessary, a temperature-controlled room was used. Light conditions during the monitoring were also set to correspond with the natural day length and rhythm because the cercarial release of Rhipidocotyle species is diurnal (Taskinen et al., 1991). The chosen 24 h incubation period allows excluding the effects of circadian rhythms, considerably influencing the results on cercariae shedding (Hannon et al., 2017). The number of cercariae in the box was counted visually (at low densities, <20 cercariae), or microscopically from a 50 mL of the mixed subsample (at high densities, >20 cercariae). The only 1 sample was used for cercarial counts since we were interested in estimating cercariae emergence at different temperatures (but not cercariae emergence per mussel per day), so all mussels from a certain temperature treatment at a certain day can be considered a sample, while each individual mussel is a subsample.
The experiment was terminated on 28 October 2011, when cercarial release approached zero in practically all treatments. For Rhipidocotyle species, the cercarial shedding in the field and in the laboratory has been reported to occur between late May and early October (Taskinen et al., 1994, 1997; Taskinen, 1998a).
Data analysis
Statistical analyses and plots preparation were performed using PASW Statistics 18 and R (R Core Team, 2020). Plots were drawn using ggplot2 (Wickham, 2016), cowplot (Wilke, 2020) and gridExtra packages (Baptiste, 2017), multiple comparisons for GLMs were, when possible, done using multcomp package machinery (Hothorn et al., 2008), while when impossible (e.g. with zero-inflated models) Bonferroni corrections were used.
Fisher test was used to compare the proportion of mussels shedding cercariae in different treatments and independence of infections. In the former case, double-infected mussels were added to both R. campanula and R. fennica columns. When double-infected mussels were excluded, results were similar (see the Supplementary material). For all other analyses, mussels that did not shed cercariae and double-infected mussels were not included in the statistical analyses. Data from replicate tanks were combined, as prior tests revealed no differences between replicates for any measured variable.
To check whether mussels from different treatments and populations differ in their shedding start date, day-degrees required for the start, stop date and the mean duration of cercarial emergence, we used the following strategy. First, we tried generalized linear models (GLMs) with the Gaussian error structure and identity link function. We checked the residuals visually on Q-Q plots and using the Shapiro–Wilks test. Ordinary GLMs (hereinafter GLMs) were preferred due to their easier interpretability. When necessary, response variables were log-transformed. If models' assumptions were still violated, we used negative binomial models since in most cases our data were strictly positive with a heavy right tail. In a few cases, we used zero-inflated models to account for high numbers of zero values (Zeileis et al., 2008). Finally, several times we had to switch to quasi-Poisson models due to the convergence problems with negative binomial models.
We always started with models including mussel length, mussel origin (River Haajaistenjoki or River Kuusaankoski population) and temperature treatment as predictors. Mussel lengths were centred since zero length is not biologically sensible. We considered it biologically relevant to include mussel size since it is often positively correlated with cercariae emergence rate (e.g. Morley et al., 2010). We also included all double interactions, while higher-order interactions were not included due to a lack of a priori hypotheses and interpretation problems.
Initial models were simplified using Akaike's information criterion (AIC) (Symonds and Moussalli, 2011). For quasi-Poisson models, which do not have AIC in the output, nested models were compared using the F-test.
To compare survival of mussels shedding cercariae in different temperature treatments, we started with a logistic regression, where treatment and mussel's length were predictors (interaction was included), and then simplified it.
To check the influence of temperature on the cercariae emission rate, we calculated mean temperature and mean cercariae emission at each sampling day in each treatment. Under temperatures below 9–11°C, mussels rarely emit cercariae, which was predictable since for many species of trematodes such temperatures are likely to be threshold ones for shedding infective stages (Morley and Lewis, 2013). In the subsequent analysis, we used only non-zero emission data. Models, where zero-emission data were included, are presented in the Supplementary material. Qualitatively, they are similar to non-zero ones; however, we suggest that they mainly reflect a large bulk of points with zero-emission at low temperatures rather than a biologically sensible relationship. One point in R. campanula data looked like an obvious outlier (see the Supplementary material) and was excluded from the dataset before the analysis.
We fitted GLMs where temperature and the day of the observation were predictors (interaction included). A seasonality factor (number of days from the beginning of the experiment, which roughly represents the time since the beginning of shedding season) was added since, during the exploratory analysis, we noticed that under similar temperatures, cercariae emissions were lower at the end of shedding period than at the beginning of it. Temperatures were centred since a cercariae emission rate at 0°C has no biological sense, while at mean temperature does have. The response variable (mean shedding rate) was log-transformed.
To check between-species differences in shedding traits, we used a similar analytical strategy; however, instead of the population factor, we added the species factor in our models. We did not include population since our analysis of separate species did not find substantial differences in cercariae shedding traits in mussels from different populations. Since data on the temperature emission start looked very different for different parasitic species and residuals looked ‘fishy’ in any model we tried, a robust regression was used for the data analysis. ‘f.robtest’ function from the sfsmisc (Maechler, 2021) package was used to obtain P values.
Results
Shedding probability
In total, 178 out of 571 A. anatina mussels shed cercariae in the course of observations. Among those, 110 mussels were infected only with R. fennica and 56 only with R. campanula, whereas 12 were infected with both species (excluded from most of data analyses, see above). The empirical probability of co-infection (0.021) was almost equal to the expected one assuming the independence of infections (0.019) (Fisher's exact test: P = 0.53). The proportions of mussels shedding R. fennica significantly differed between temperature treatments with higher share at higher temperatures. The general test on 3 × 2 contingency table and all paired comparisons between treatments were significant even after applying the Bonferroni correction (Fisher's exact test: P < 0.0002, Table S4). For R. campanula, no significant differences between treatments were found (P > 0.2 in all cases, Table S4; Fig. S2). When double infections were included, results were similar (Table S4).
Cercarial release at the total host population level
At the host population level, the total cercarial emergence period (from the first to the last observation of emerged cercariae) of R. fennica ranged from 12 July to 28 October, whereas that of R. campanula ranged from 31 May to 3 October (Table 1; Fig. 1). The total period of cercarial shedding by R. fennica lasted for 10 weeks in the high, and 14–16 weeks in the intermediate, but for only 4–6 weeks in the low-temperature treatment (Table 1; Fig. 1). The total period of cercarial shedding by R. campanula in the low-temperature treatment was clearly longer than that of R. fennica, as it ranged from 14 to 18 weeks (Table 1). In other temperatures, differences in shedding duration between the parasites were not consistent (Table 1). At host population level, duration of cercarial shedding in R. campanula decreased, rather than increased, by temperature (Table 1). Water temperature at the time of the first emergence of R. fennica cercariae varied from 16.5 to 21.5°C, which was clearly higher than 10.5°C observed for R. campanula (Table 1; Fig. 1). The exact timing of the cercariae emergence for each mussel shedding cercariae is given in Figs S7 and S8.
Seasonal cercarial release with respect to temperature
Quantitatively the peak cercarial release by R. fennica co-occurred with the seasonal thermal maximum, but that of R. campanula clearly outran it (Fig. 1). Cercarial shedding by R. fennica increased substantially at temperatures above 15°C (Fig. 2A and B; Fig. S3) but high numbers of R. campanula cercariae were released as soon as the temperature exceeded 10°C (Fig. 2C and D; Fig. S3). For R. fennica a ‘raw’ relationship between temperature and cercariae production (Fig. 2A) shows abnormally low values of the cercariae emission at intermediate temperatures. These values can be explained by the fact that such temperatures could be met twice in a shedding season: in spring and in autumn. The GLM showed that temperature had a strong positive influence on the number of released cercariae (Table 2). The main effect of the season (i.e. day from the start of the experiment) was also positive at the average temperature (Table 2). However, significant interaction suggests that with time the influence of temperature decreased. The amount of variance explained by the model was remarkably high (R2 = 0.83). The residual plot (Fig. 2B) showed that after accounting for the season, the relationship between temperature and cercariae production in R. fennica became almost linear. A similar model (Table S6) for R. campanula did not explain data better than the intercept-only model. However, exclusion of 1 outlier value (see Fig. S4B) resulted in a model where both main effects of seasonality and temperature were significant. With the increase in temperature, the emission of R. campanula also increased; however, the number of emitted cercariae falls with time (Table 2; Fig. 2C and D). There was no interaction between these 2 predictors (F1 = 0.38, P = 0.54). For the similar models including zero values, see the Supplementary material (Tables S5 and S7; Figs S3 and S4A).
Fig. 2.
Relationship between mean temperature during each 3-week monitoring period and release of Rhipidocotyle fennica (A, B) and R. campanula (C, D) cercariae by Anodonta anatina mussels (2 study populations combined). The average cercariae release by mussels from different temperature treatments indicated with dot (high), triangle (intermediate) and square (low temperature). (A, C) ‘Raw’ data. (B, D) Residual plot accounting for the seasonality.
Table 2.
Comparison of cercarial shedding traits between Rhipidocotyle species; 2 populations of the host mussel Anodonta anatina combined
| R. fennica (RF) | R. campanula (RC) | Statistics (estimate ± s.e.) | |
|---|---|---|---|
| Host population level | |||
| n of shedding mussels | 110 | 56 | |
| Shedding period, days | 108 (12 Jun–28 Oct) | 125 (31 May–03 Oct) | |
| Shedding duration (H)a, weeks | 10 | 8–16 | |
| Shedding duration (I)a, weeks | 14–16 | 16–18 | |
| Shedding duration (L)a, weeks | 4–6 | 14–18 | |
| Seasonal peak shedding | During peak T °C | Less tied to T °C | |
| N of released cercariae vs T °C | Positive correlation | Positive correlation | GLM (RF): 1.23 ± 0.20, z = 5.99, P = 0.00002; GLM (RC): 0.162 ± 0.045, z = 3.60, P = 0.001 |
| N of released cercariae vs timeb | Positive correlation | Negative correlationc | GLM (RF): 0.043 ± 0.01, z = 4.54, P = 0.0003; GLM (RC): −0.012 ± 0.004, z = −2.96, P = 0.007 |
| N of released cercariae: T °C × time interaction | Temperature effect decreases with time | Interaction not significant | GLM (RF): −0.006 ± 0.002, z = −3.14, P = 0.006 GLM (RC) (nested models comparison): F1 = 0.38, P = 0.54 |
| Host individual level | |||
| Emission start (time/T °C) | Later (mainly >15–20°C) | Earlier (about 10–12°C) | Zero-inflated model: 1.04 ± 0.10, z = 10.01, P < 0.0001, Table S9d Robust linear model: 10.13 ± 0.44, F = 570.47, P < 0.0001, Table S9d |
| Emission start (effect of T °C) | Earlier at higher T °C | Earlier at higher T °C | See Tables S8a and S8e for all details |
| Proportion of shedding mussels | Higher at high T °C | No effect | Fisher's exact test: P < 0.0002, Table S4 |
| Emission start (effect of host size) | Later for large musselsd | No effect | GLM with log(DV) (RF): 0.011 ± 0.003, z = 3.70, P = 0.0003, Table S8a |
| Emission end (time/temperature) | Later (at similar T °C) | Earlier (at similar T °C) | GLM: 32.6 ± 4.6, t = 7.1, P < 0.0001. Table S9b/ns, Table S9f |
| Emission end (effect of T °C) | Earlier at higher T °C | Earlier at higher T °C | See Table S8b, for all details |
| Emission end (effect of host size) | Later for large mussels | Earlier for large mussels | GLM (RF): 0.34 ± 0.14, t = 2.50, P = 0.014; GLM (RC): −0.58 ± 0.27, t = −2.13, P = 0.038, Tables S8b and S8f |
| Emission duration | Shorter | Longer | Zero-inflated GLM: −0.28 ± 0.0021, z = 0.47, P < 0.0001, Table S9c |
| Emission duration (effect of T °C) | Absent | Absent | |
| Emission duration (host size effect) | No effect | Negative | GLM (RC): −0.66 ± 0.33, t = −2.02, P = 0.048 |
| Emission start (day-degrees) | More | Less | GLM with log(DV): 1.58 ± 0.10, t = 15.7, P < 0.0001 |
| E. start (day-degrees, effect of T °C) | Less needed at higher T | No effect | See Tables S8d and S8g for all treatment comparisons |
H, I, L = high-, intermediate- and low-temperature treatments, respectively.
Time = days from the beginning of experiment.
Exclusion of 1 outlier value (see Fig. S4B) resulted in a model where effects of seasonality were significant.
This relationship did not hold for intermediate temperature (estimate ± s.e. = −0.011 ± 0.002, z = −5.16, P < 0.0001, Table S9a).
Cercarial release at the individual host level at different temperatures
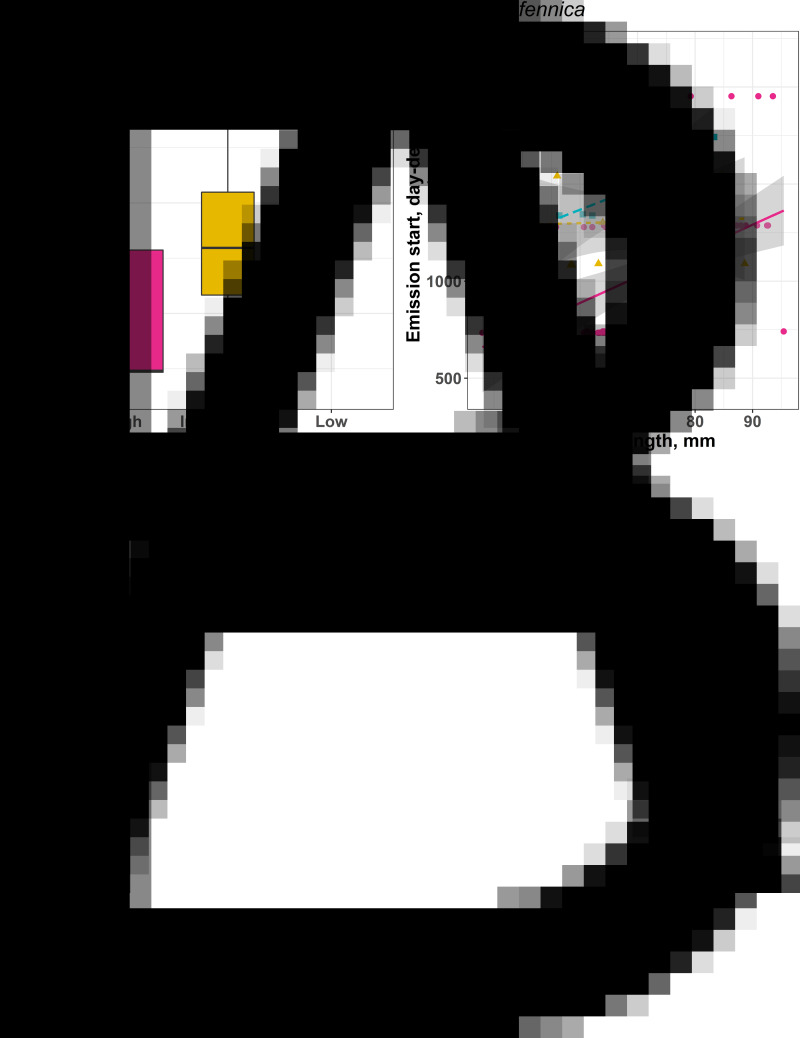
For the sake of brevity, we present only the most important qualitative results here (summarized with statistics in Table 2), while the full statistical models are available in the Supplementary material. At high temperature, emission of R. fennica cercariae started (Table S8a; Fig. 3A and B) and ended (Table S8b; Fig. 3C and D) earlier than at low and intermediate temperatures (Table 2). The differences between low and intermediate temperature treatments were not significant (Tables S8a and S8b, respectively) after correcting for the multiple comparisons. Such a shift in the emission start and end did not result in a substantial emission duration change. Interestingly, in larger mussels, R. fennica cercariae emission started (Table S8a) and stopped (Table S8b) later than in smaller ones. However, although treatment × length interactions were non-significant, the estimates were negative and it seems that the size effect was reliable only in the high-temperature treatment in case of cercariae shedding start (Table S8a; Fig. 3B). Since many mussels appeared to emit cercariae only once during the observations, the emission duration for them was considered a zero, which demands using the zero-inflated model for emission duration analysis (see above). Although R. fennica cercarial release lasted for longer at the host population level in higher temperatures (see above), evidence for this was not sufficient at the individual host level. There was a tendency towards a slightly longer R. fennica cercariae emission duration in low-temperature treatment (Table S8c), but this did not hold after the Bonferroni correction. Importantly, temperature treatment had a substantial effect on the number of day-degrees needed to start the R. fennica cercariae release, with less day-degrees required under high-temperature treatment compared to intermediate and low ones (Fig. 4; Table S8d). Again, the difference between the 2 lower temperature treatments was not significant (Table S8d). The larger mussels needed more day-degrees to start R. fennica cercariae release (Table 2); however, this effect was reliably seen only in the high-temperature treatment.
Fig. 3.
(A, C) Boxplots for number of days needed to start (A) and stop (C) cercariae release in Rhipidocotyle fennica under different temperature treatments (2 study populations combined). Width of box reflects the sample size, height of box denotes limits of upper and lower quartiles, horizontal line is the median of vales, whiskers mark the highest and lowest values within the 1.5 × interquartile range and dots indicate values outside that range. (B, D) Respective plots accounting for size of the mussel host, Anodonta anatina, where high temperature marked with dots and continuous line, intermediate temperature with triangles and dotted line, and low temperature with squares and broken line.
Fig. 4.
Sum of day-degrees needed to start release of Rhipidocotyle fennica cercariae by the mussel host Anodonta anatina (2 study populations combined); boxplot for different temperature treatments (A) and respective plot accounting for size of host (B). For explanation of boxplot details and plot symbols, see Fig. 3.
Twenty-five out of 56 mussels releasing R. campanula cercariae had been already emitting them when the experiment started. Therefore, zero-inflated model was used to evaluate the influence of predictors on the emission start date in R. campanula. After the stepwise removal of non-significant terms, we found that the intercept-only model is the most parsimonious one. However, the AIC of the model, where the temperature is the only predictor, differs from the AIC of the intercept-only one by less than 2 points and, therefore, can be considered informative (Symonds and Moussalli, 2011). Therefore, we decided to present the results of the above-mentioned model (Table S8e) though they should be treated with great care. Essentially, in lower temperatures, R. campanula cercariae emission started later than in high temperature (estimate ± s.e. = 0.70 ± 0.27 and 0.59 ± 0.25, z = 2.65 and 2.34, P = 0.008 and 0.019 for low and intermediate temperature, respectively). The only predictor left in the model explaining the duration of cercariae emission in R. campanula was host length. Larger mussels tended to release cercariae for a shorter time though the relationship was only marginally significant (P = 0.048). This is probably because the larger mussels stopped cercariae emission earlier than smaller ones (Table S8f). Similar to R. fennica, emission of R. campanula cercariae tended to stop later under intermediate and low temperatures; however, only the difference between low- and high-temperature treatments was statistically significant (Table S8f). A substantial share of mussels started to shed R. campanula cercariae simultaneously already in the beginning of the experiment, thus having the same number of day-degrees (115) to start shedding. To account for it, we fitted a zero-inflated model using 115 day-degrees as a zero value to explain variation in day-degrees required for start of R. campanula cercariae shedding. After simplification, the model contained only the host population as a predictor, while no significant effects were found (Table S8g).
Survival of the mussels through the experiment was 64.2% (see Figs S5, S7 and S8). The mortality of mussels shedding cercariae was significantly higher in the high-temperature treatment, while in other 2 treatments mortality was equal (Table S8h). More detailed survival analysis will be published elsewhere.
Cercarial release at the individual host level and differences between parasite species
There was a significant difference between the parasite species with respect to the 5 cercarial shedding traits studied: start date, water temperature and number of day-degrees at the start, stop date and duration of cercarial release (Table S9). However, the temperature effect on these traits was generally similar for both parasite species. First of all, the release of cercariae by R. fennica started seasonally significantly later than that by R. campanula (Table S9a) with the treatment-specific difference varying from 42 to 87 days. In fact, this difference is likely to be even more pronounced since many R. campanula-infected mussels had already started cercariae emission at the beginning of the experiment. Lower temperatures led to later seasonal start of cercariae emission in both species (Table S9a). Rhipidocotyle fennica stopped cercariae release generally later than R. campanula, but lower temperature caused a later stop of cercariae release in both species (Table S9b). Interestingly, the interaction between seasonal timing – and partly also the duration – of cercariae release and the host's length differed in the 2 parasite species. Larger mussels infected with R. campanula ended release earlier (leading to a negative trend between host size and duration of cercariae emission; Table S9c), while those infected with R. fennica both started and ended cercariae release later than the smaller ones, at least in high temperature (leading to mainly positive trend between host size and duration of cercariae emission; Table S9c). The total duration of cercarial release was shorter for R. fennica than for R. campanula (Table S9c) and this difference is likely to be underestimated taking into account that a large share of R. campanula-infected mussels had already started cercariae shedding in the beginning of the experiment.
The water temperature at the start of the seasonal release of cercarial was higher for R. fennica (15–20°C) and lower for R. campanula (10–12°C) (Fig. S6; Table S9d); however, this effect became less pronounced under intermediate and low temperatures. For both species, the number of day-degrees needed to start cercariae shedding was larger in intermediate- and low-temperature treatments comparing to the high-temperature one (Table S9e; Fig. S6). Finally, R. fennica needed much more day-degrees to start the cercariae release but there were no interspecific differences in the temperatures at which cercariae release stopped in any temperature treatments (Fig. S6; Table S9f).
Discussion
Ongoing and predicted increases in global temperatures and in duration of the growing season will have important implications for many host–parasite systems, e.g. changes in the timing of parasite life-cycle stages (Marcogliese, 2001; Mouritsen and Poulin, 2002; Kutz et al., 2005; MacDonald et al., 2021). A common expectation is that the seasonal larval release by parasites will start earlier and become more prolonged as a consequence of increased thermal growing season (Marcogliese, 2001; Harvell et al., 2009; Nikolaev et al., 2020).
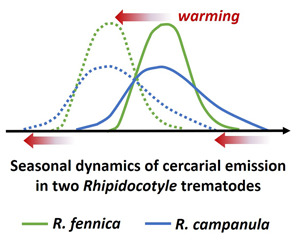
The present study on the variation in the seasonal cercarial shedding patterns of 2 sympatric parasites gives mixed evidence both for and against the aforementioned hypothesis. At the individual host level, high temperature caused a marked shift in the cercariae release period towards earlier start and end of cercariae emission, but – against the expectation – did not result in a longer seasonal duration of cercariae release within an individual host. Such a shift can have important consequences in natural habitats leading to a temporal mismatch in occurrence between hosts and parasites (Lõhmus and Björklund, 2015; Cohen et al., 2017; Gehman et al., 2018), thus, reducing the infection load (Paull and Johnson, 2014; McDevitt-Galles et al., 2020). However, the total period of cercarial shedding by R. fennica at the host population level, from the first to the last observation of emergence, was much longer in high and intermediate-temperature treatments compared with the low-temperature one – as expected by the general hypothesis. Such a population-level effect was not observed in R. campanula probably because about a half of R. campanula-infected mussels had already started shedding cercariae when the experiment begun. In addition, though the temperature did not influence the duration of cercariae release at the individual level, it positively correlated with the numbers of produced cercariae in both species, thus, potentially giving the parasite an advantage in the competition with a host for the host's resources. Importantly, under similar temperatures, fewer cercariae were emitted in the end compared with the beginning of the emission season, which is likely to be a result of exhaustion of the host and/or parasite. Therefore, the present results support the view that climate warming would increase the duration of larval shedding, and lengthen the transmission period by parasites, but that such lengthening is produced by increased variation between host individuals, rather than due to a lengthened individual shedding period.
The results also indicated that even closely related, sympatric parasite species that share the same transmission pathway can respond differently to temperature change (see also Selbach and Poulin, 2020). High variability in cercariae shedding patterns (e.g. daily rhythms) have been found previously both within a single trematode genus (Vyhlídalová and Soldánová, 2020) and species (Théron and Combes, 1988; Riley and Uglem, 1995; Le Clec'h et al., 2021). Rhipidocotyle fennica brought forward the start of seasonal cercarial release and started cercarial release with lower day-degrees in the high-temperature treatment, but for R. campanula this was not found. Furthermore, R. campanula clearly started the seasonal cercarial release earlier, at a lower temperature than R. fennica (10 and 15°C, respectively), with fewer day-degrees, stopped the seasonal cercarial release earlier, and had a markedly longer total seasonal duration of cercarial emission. The peak release of R. fennica cercariae occurred during the warmest weeks in concordance with field observations (Taskinen et al., 1994), and also the proportion of mussels shedding R. fennica cercariae was higher in higher temperature treatments, while in R. campanula this phenomenon was not found. Based on these observations, one might predict that the projected longer summers and higher temperatures associated with climate warming (Tietäväinen et al., 2010; Ruosteenoja et al., 2011) would benefit especially R. fennica, which requires higher temperatures (as well as more day-degrees) to start cercariae release. On the other hand, it can be predicted that R. campanula could thrive better than R. fennica in colder, more northern, short-summer environments, where the early onset of cercarial release is presumably advantageous.
The earlier start of the seasonal cercarial release by R. campanula is difficult to explain by the transmission dynamics, as the 2 species share the same current (the bivalve A. anatina) and next (the fish R. rutilus) host in their life cycles (Taskinen et al., 1991; Gibson et al., 1992). It is also worth noting that both R. fennica and R. campanula are specific only to A. anatina as their first intermediate host in the study area (Taskinen et al., 1991; Gibson et al., 1992). The definitive hosts of R. fennica and R. campanula are the predatory fishes northern pike (E. lucius) and perch/pikeperch (P. fluviatilis/S. lucioperca), respectively (Taskinen et al., 1991; Gibson et al., 1992). Thus, it is also possible that the timing of cercarial shedding could be an adaptation to increase transmission to the final hosts, such as the differential seasonal feeding of the final hosts on roach that we are not aware of. However, it is difficult to believe that the earlier start of cercarial release by R. campanula could be an adaptation only to northern conditions (although it might facilitate occurrence there) because both R. campanula and R. fennica occur as far south as Ukraine (Taskinen et al., 1991; Petkevičiūtė et al., 2014; Stunžėnas et al., 2014; Müller et al., 2015).
We propose that the mechanism enabling the early onset of cercarial release by R. campanula is that they have their cercarial production machinery ‘on standby’ throughout the year (Taskinen et al., 1994). Fully developed, mature cercariae are found in R. campanula sporocysts in high proportions in all seasons, readily available for shedding when a suitable temperature is attained (Taskinen et al., 1994). In R. fennica, mature, ready-to-emerge cercariae are only found during the cercarial shedding period (Taskinen et al., 1994). This probably means that it takes a relatively long time for R. fennica to respond to increasing water temperature in terms of cercarial production, as the growth of sporocyst starts from practically zero in spring (Taskinen et al., 1994). Cercarial release by Rhipidocotyle spp. can also be triggered outside the natural shedding period by transfering infected mussels to high temperature in the laboratory, but also in that case the time needed for R. campanula to start shedding cercariae is much shorter than for R. fennica (Taskinen et al., 1991).
Although our results about the effect of temperature on shedding start and proportion of mussels shedding cercariae predict that warming is likely to benefit R. fennica more than R. campanula, the infection success of parasites may also depend on cercarial output, their survival and infectivity. Temperature effects on these traits can partly compensate for each other, sometimes resulting in similar transmission efficiency at different temperatures (Poulin, 2006). However, it is impossible to account for all these effects in our study.
Two studied parasitic species also differed in their relationships to the host size. Larger R. fennica-infected mussels tended to start cercariae release later and shed them for a longer time, while in R. campanula no clear relationships were seen. Such a difference hints again that even closely related sympatric parasitic species infecting the same host can differ in strategies of host exploitation. Surprisingly, there were almost no differences in cercariae shedding traits in parasites from different populations, which suggests that our study comprises a reasonable amount of generality.
It is important to notice that, although we explained the obtained results by temperature and seasonality effects, there were differences between the treatments also in terms of water flow and water source (littoral vs hypolimnetic), light conditions, temperature fluctuation and seasonal temperature profile. Whereas the high-temperature tanks were kept in an outdoor shelter and were subject to a diurnal temperature fluctuation and natural light, the intermediate- and low-temperature tanks were kept in an indoor tank hall and illuminated with artificial light. However, the photoperiod was equal in all treatments and corresponded to the natural rhythm. In addition, the cercarial release was shown to be similar at constant and diurnally variable temperatures (Roushdy, 1984). According to Choo and Taskinen (2015), a short-term (1 h) temperature increase triggers cercariae emission of R. fennica from A. anodonta, but on the other hand a similar decrease in temperature results in an equivalent decrease in cercariae release. Therefore, the higher daily fluctuation of temperature in the high-temperature treatment probably did not influence the net daily cercariae release. In addition, the monitoring of cercariae release was performed at constant temperature (no variation within a day) in all temperature treatments. Importantly, the mussels in high-temperature treatment supplied with littoral water could receive more food than those in colder treatments, supplied with water from the lake hypolimnion, although we aimed at compensating this by doubling the water flow in the cold- and intermediate-temperature treatments (see section Methods).
Since food deprivation of host can constrain cercariae release (e.g. Seppälä et al., 2015), higher cercariae output in high-temperature treatment obtained in our study could be, e.g. a result of higher food availability for mussel host. However, our data on mussel mortality and weight change during the experiment (i.e. higher mortality in high-temperature treatment, no increase in mussel weight) do not indicate the importance of nutritional differences for the interpretation of the results. Even though we cannot completely rule out confounding factors other than temperature, we do not believe that the difference in water and light source, or temperature fluctuation, could explain the observed contrasting responses in the seasonal cercarial release by R. fennica and R. campanula between the temperature treatments. New infections of mussels during the experiment, via miracidia from unfiltered lake water, were unlikely due to the seasonal maturing of Rhipidocotyle trematodes in late autumn (Taskinen et al., 1991). Thus, the present results should reliably indicate temperature responses in the seasonal timing of cercarial shedding by R. fennica and R. campanula.
Previous studies investigating the seasonal dynamics of trematode cercarial release include field observations showing a significant increase in cercarial emergence during summer months (Taskinen et al., 1994; Taskinen, 1998a; Fingerut et al., 2003), a longer seasonal shedding period in water bodies receiving thermal effluents (Aho et al., 1982) and experimental evidence on the role of temperature in controlling daily cercariae output (Koprivnikar and Poulin, 2009a; Vyhlídalová and Soldánová, 2020), the start and the duration of cercariae emergence (Taskinen et al., 1991; Fingerut et al., 2003; Paull and Johnson, 2014; Prokofiev et al., 2016). Long-term experimental studies like the present one are still scarce (Paull and Johnson, 2014).
The results of this study partly support the idea that climate warming would increase the seasonal duration of larval shedding by parasites, but emphasize species-specific differences in the seasonal cercarial release and transmission with respect to warming (Marcogliese, 2001; Harvell et al., 2009). Research on the geographic distribution of the species is needed to determine whether the observed temperature differences in cercarial shedding traits affect the current distribution and relative abundance of Rhipidocotyle species at the northern boundary of their occurrence. Due to the contrasting species-specific temperature-dependence, the A. anatina–Rhipidocotyle spp. association offers a unique system to study the effects of the ongoing and predicted climate warming on host–parasite relationships at high latitudes.
Acknowledgements
We thank Nebiyu Girgibo and Waidi Alabi for assistance in the field and in the laboratory. Katja Pulkkinen, Anssi Karvonen and Roger Jones provided valuable comments on the manuscript. Roger Jones kindly checked the English of the manuscript. Konnevesi Research Station of JYU provided the facilities and necessary assistance for the experiment.
Author contribution
J. T. conceived the ideas and designed the experiment; J. C. collected the data; J. T. and M. G. analysed the data. All authors contributed to the writing of the manuscript and gave final approval for publication.
Financial support
The research was supported by the JYU Rector's grants for doctoral studies (J. M. C.), the Emil Aaltonen Foundation (J. M. C.), the Biological Interactions Graduate School travel grants for doctorate students, University of Jyväskylä (J. M. C.), the Academy of Finland (J. T., grant number 260704) and the Russian Science Foundation (M. G., grant 19-14-00015).
Ethical standards
None.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0031182022000518.
click here to view supplementary material
Data
Data can be requested from the authors.
Conflict of interest
None.
References
- Aho JM, Camp JW and Esch GW (1982) Long-term studies on the population biology of Diplostomulum scheuringi in a thermally altered reservoir. Journal of Parasitology 68, 695–708. [Google Scholar]
- Baptiste A (2017) gridExtra: miscellaneous functions for ‘Grid’ graphics. R package version 2.3. Available at https://CRAN.R-project.org/package=gridExtra.
- Choo JM and Taskinen J (2015) Effect of short-term temperature change on cercarial release by Rhipidocotyle fennica (Trematoda, Bucephalidae) from the freshwater bivalve host, Anodonta anatina. Ecological Parasitology and Immunology 4, 235932. [Google Scholar]
- Chubb JC (1979) Seasonal occurrence of helminths in freshwater fishes. Part II. Trematoda. Advances in Parasitology 17, 141–313. [DOI] [PubMed] [Google Scholar]
- Cohen JM, Venesky MD, Sauer EL, Civitello DJ, McMahon TA, Roznik EA and Rohr JR (2017) The thermal mismatch hypothesis explains host susceptibility to an emerging infectious disease. Ecology Letters 20, 184–193. [DOI] [PubMed] [Google Scholar]
- Cribb TH, Bray RA and Littlewood TJ (2001) The nature and evolution of the association among digeneans, molluscs and fishes. International Journal for Parasitology 31, 997–1011. [DOI] [PubMed] [Google Scholar]
- De Montaudouin X, Blanchet H, Desclaux-Marchand C, Lavesque N and Bachelet G (2016) Cockle infection by Himasthla quissetensis – I. From cercariae emergence to metacercariae infection. Journal of Sea Research 113, 99–107. [Google Scholar]
- Fingerut JT, Zimmer CA and Zimmer RK (2003) Patterns and processes of larval emergence in an estuarine parasite system. Biological Bulletin 205, 110–120. [DOI] [PubMed] [Google Scholar]
- Galaktionov KV (2017) Patterns and processes influencing helminth parasites of Arctic coastal communities during climate change. Journal of Helminthology 91, 387–408. [DOI] [PubMed] [Google Scholar]
- Gehman AM, Hall RJ and Byers JE (2018) Host and parasite thermal ecology jointly determine the effect of climate warming on epidemic dynamics. PNAS 115, 744–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson DI, Taskinen J and Valtonen ET (1992) Studies on bucephalid digeneans parasitising molluscs and fishes in Finland. II. The description of Rhipidocotyle fennica n. sp. and its discrimination by principal components analysis. Systematic Parasitology 23, 67–79. [Google Scholar]
- Hannon E, Calhoun D, Chadalawada S and Johnson P (2017) Circadian rhythms of trematode parasites: applying mixed models to test underlying patterns. Parasitology 145, 1–9. [DOI] [PubMed] [Google Scholar]
- Harvell D, Altizer S, Cattadori IM, Harrington L and Weil E (2009) Climate change and wildlife diseases: when does the host matter the most? Ecology 90, 912–920. [DOI] [PubMed] [Google Scholar]
- Hothorn T, Bretz F and Westfall P (2008) Simultaneous inference in general parametric models. Biometrical Journal 50, 346–363. [DOI] [PubMed] [Google Scholar]
- IPCC (2014) Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. [Core Writing Team, Pachauri RK and Meyer LA (eds.)]. Geneva, Switzerland: IPCC. [Google Scholar]
- Johnson PT, Dobson A, Lafferty KD, Marcogliese DJ, Orlofske SA, Poulin R and Thieltges DW (2010) When parasites become prey: ecological and epidemiological significance of eating parasites. Trends in Ecology and Evolution 25, 362–371. [DOI] [PubMed] [Google Scholar]
- Jokela J, Taskinen J, Mutikainen P and Kopp K (2005) Virulence of parasites in hosts under environmental stress: experiments with anoxia and starvation. OIKOS 108, 156–164. [Google Scholar]
- Jorgensen CB, Kiorboe T, Mohlenberg F and Riisgard HU (1984) Ciliary and mucus-net filter feeding, with special reference to fluid mechanical characteristics. Marine Ecology Progress Series 15, 283–292. [Google Scholar]
- Jylhä K, Tuomenvirta H and Ruosteenoja K (2004) Climate change projections for Finland during the 21st century. Boreal Environmental Research 9, 127–152. [Google Scholar]
- Karvonen A, Seppälä O and Valtonen ET (2004) Parasite resistance and avoidance behaviour in preventing eye fluke infections in fish. Parasitology 129, 159–164. [DOI] [PubMed] [Google Scholar]
- Koprivnikar J and Poulin R (2009a) Interspecific and intraspecific variation in cercariae release. Journal of Parasitology 95, 14–19. [DOI] [PubMed] [Google Scholar]
- Koprivnikar J and Poulin R (2009b) Effects of temperature, salinity, and water level on the emergence of marine cercariae. Parasitology Research 105, 957–965. [DOI] [PubMed] [Google Scholar]
- Kuha J, Arvola L, Hanson PC, Huotari J, Huttula T, Juntunen J, Järvinen M, Kallio K, Ketola M, Kuoppamäki K, Lepistö A, Lohila A, Paavola R, Vuorenmaa J, Winslow L and Karjalainen J (2016) Response of boreal lakes to episodic weather-induced events. Inland Waters 6, 523–534. [Google Scholar]
- Kuris AM, Hechinger RF, Shaw JC, Whitney KL, Aguirre-Macedo L, Boch CA, Dobson AP, Dunham EJ, Fredensborg BL, Huspeni TC, Lorda J, Mababa L, Mancini FT, Mora AB, Pickering M, Talhouk NL, Torchin ME and Lafferty R (2008) Ecosystem energetic implications of parasite and free-living biomass in three estuaries. Nature 454, 515–518. [DOI] [PubMed] [Google Scholar]
- Kutz SJ, Hoberg EP, Polley L and Jenkins EJ (2005) Global warming is changing the dynamics of Arctic host-parasite systems. Proceedings of the Royal Society Biological Sciences 272, 2571–2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Clec'h W, Chevalier FD, McDew-White M, Menon V, Arya GA and Anderson T (2021) Genetic architecture of transmission stage production and virulence in schistosome parasites. Virulence 12, 1508–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lõhmus M and Björklund M (2015) Climate change: what will it do to fish–parasite interactions? Biological Journal of the Linnean Society 116, 397–411. [Google Scholar]
- MacDonald H, Akçay E and Brisson D (2021) The role of host phenology for parasite transmission. Theoretical Ecology 14, 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maechler M (2021) sfsmisc: Utilities from ‘Seminar fuer Statistik’ ETH Zurich. R package version 1.1-11. Available at https://CRAN.R-project.org/package=sfsmisc.
- Marcogliese DJ (2001) Implications of climate change for parasitism of animals in the aquatic environment. Canadian Journal of Zoology 79, 1331–1352. [Google Scholar]
- Marcogliese DJ (2016) The distribution and abundance of parasites in aquatic ecosystems in a changing climate: more than just temperature. Integrative and Comparative Biology 56, 611–619. [DOI] [PubMed] [Google Scholar]
- McDevitt-Galles T, Moss W, Calhoun D and Johnson P (2020) Phenological synchrony shapes pathology in host–parasite systems. Proceedings of the Royal Society B: Biological Sciences 287, 20192597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee KM, Koprivnikar J, Johnson PTJ and Arts MT (2020) Parasite infectious stages provide essential fatty acids and lipid-rich resources to freshwater consumers. Oecologia 192, 477–488. [DOI] [PubMed] [Google Scholar]
- Mironova E, Gopko M, Pasternak A, Mikheev V and Taskinen J (2019) Trematode cercariae as prey for zooplankton: effect on fitness traits of predators. Parasitology 146, 105–111. [DOI] [PubMed] [Google Scholar]
- Mironova E, Gopko M, Pasternak A, Mikheev V and Taskinen J (2020) Cyclopoids feed selectively on free-living stages of parasites. Freshwater Biology 65, 1450–1459. [Google Scholar]
- Morley NJ and Lewis JW (2013) Thermodynamics of cercarial development and emergence in trematodes. Parasitology 140, 1211–1224. [DOI] [PubMed] [Google Scholar]
- Morley N, Adam M and Lewis J (2010) The effects of host size and temperature on the emergence of Echinoparyphium recurvatum cercariae from Lymnaea peregra under natural light conditions. Journal of Helminthology 84, 317–326. [DOI] [PubMed] [Google Scholar]
- Mouritsen KN and Poulin R (2002) Parasitism, climate oscillations and the structure of natural communities. OIKOS 97, 462–468. [Google Scholar]
- Müller T, Czarnoleski M, Labecka AM, Cichy A, Zając K and Dragosz-Kluska D (2015) Factors affecting trematode infection rates in freshwater mussels. Hydrobiologia 742, 59–70. [Google Scholar]
- Nikolaev K, Levakin I and Galaktionov K (2020) Seasonal dynamics of trematode infection in the first and the second intermediate hosts: a long-term study at the subarctic marine intertidal. Journal of Sea Research 164, 101931. [Google Scholar]
- Orlofske SA, Jadin RC, Preston DL and Johnson PTJ (2012) Parasite transmission in complex communities: predators and alternative hosts alter pathogenic infections in amphibians. Ecology 93, 1247–1253. [DOI] [PubMed] [Google Scholar]
- Paull SH and Johnson PT (2014) Experimental warming drives a seasonal shift in the timing of host-parasite dynamics with consequences for disease risk. Ecology Letters 17, 445–453. [DOI] [PubMed] [Google Scholar]
- Paull SH, Raffel TR, LaFonte BE and Johnson PTJ (2015) How temperature shifts affect parasite production: testing the roles of thermal stress and acclimation. Functional Ecology 29, 941–950. [Google Scholar]
- Petkevičiūtė R, Stunžėnas V and Stanevičiūtė G (2014) Differentiation of European freshwater bucephalids (Digenea: Bucephalidae) based on karyotypes and DNA sequences. Systematic Parasitology 87, 199–212. [DOI] [PubMed] [Google Scholar]
- Poulin R (2006) Global warming and temperature-mediated increases in cercarial emergence in trematode parasites. Parasitology 132, 143–151. [DOI] [PubMed] [Google Scholar]
- Preston DL, Orlofske SA, Lambden JP and Johnson PTJ (2013) Biomass and productivity of trematode parasites in pond ecosystems. Journal of Animal Ecology 82, 509–517. [DOI] [PubMed] [Google Scholar]
- Prokofiev V, Galaktionov KV and Levakin IA (2016) Patterns of parasite transmission in polar seas: daily rhythms of cercarial emergence from intertidal snails. Journal of Sea Research 113, 85–98. [Google Scholar]
- R Core Team (2020) R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. Available at https://www.R-project.org/. [Google Scholar]
- Riley M and Uglem G (1995) Proterometra macrostoma (Digenea: Azygiidae): variations in cercarial morphology and physiology. Parasitology 110, 429–436. [DOI] [PubMed] [Google Scholar]
- Rosen R, Blank S, Akabogu F, Hauschner R, Meneses S, Slater O, Melton A, Tetidrick C and Gosnell W (2018) Effect of temperature on the release of Proterometra macrostoma (Trematoda: Digenea) cercariae from their snail intermediate host, Pleurocera semicarinata (Gastropoda: Pleuroceridae) at North Elkhorn Creek, Kentucky. Comparative Parasitology 85, 202–207. [Google Scholar]
- Roushdy MZ (1984) The effect of diurnal fluctuating temperature on the development of Schistosoma haematobium in Bulinus truncatus. Journal of the Egyptian Society of Parasitology 14, 507–514. [PubMed] [Google Scholar]
- Ruosteenoja K, Räisänen J and Pirinen P (2011) Projected changes in thermal seasons and the growing season in Finland. International Journal of Climatology 31, 1473–1487. [Google Scholar]
- Selbach C and Poulin R (2020) Some like it hotter: trematode transmission under changing temperature conditions. Oecologia 194, 745–755. [DOI] [PubMed] [Google Scholar]
- Seppälä O, Louhi KR, Karvonen A, Rellstab C and Jokela J (2015) Relative reproductive success of co-infecting parasite genotypes under intensified within-host competition. Infection, Genetics and Evolution: Journal of Molecular Epidemiology and Evolutionary Genetics in Infectious Diseases 36, 450–455. [DOI] [PubMed] [Google Scholar]
- Sharma S, Jackson DA, Minns CK and Shuter BJ (2007) Will northern fish populations be in hot water because of climate change? Global Change Biology 13, 2052–2064. [Google Scholar]
- Shim KC, Koprivnikar J and Forbes MR (2013) Variable effects of increased temperature on a trematode parasite and its intertidal hosts. Journal of Experimental Marine Biology and Ecology 439, 61–68. [Google Scholar]
- Studer A and Poulin R (2014) Analysis of trait mean and variability versus temperature in trematode cercariae: is there scope for adaptation to global warming? International Journal for Parasitology 44, 403–413. [DOI] [PubMed] [Google Scholar]
- Studer A, Thieltges DW and Poulin R (2010) Parasites and global warming: net effects of temperature on an intertidal host–parasite system. Marine Ecology Progress Series 415, 11–22. [Google Scholar]
- Stunžėnas V, Petkevičiūtė R, Stanevičiūtė G and Binkienė R (2014) Rhipidocotyle fennica (Digenea: Bucephalidae) from Anodonta anatina and pike Esox lucius in Lithuania. Parasitology Research 113, 3881–3883. [DOI] [PubMed] [Google Scholar]
- Symonds MRE and Moussalli A (2011) A brief guide to model selection, multimodel inference and model averaging in behavioural ecology using Akaike's information criterion. Behavioral Ecology and Sociobiology 65, 13–21. [Google Scholar]
- Taskinen J (1998a) Cercarial production of the trematodes Rhipidocotyle fennica kept in the field. Journal of Parasitology 84, 345–349. [PubMed] [Google Scholar]
- Taskinen J (1998b) Influence of trematode parasitism on the growth of a bivalve host in the field. International Journal of Parasitology 28, 599–602. [DOI] [PubMed] [Google Scholar]
- Taskinen J and Valtonen ET (1995) Age-, size-, and sex-specific infection of Anodonta piscinalis (Bivalvia: Unionidae) with Rhipidocotyle fennica (Digenea: Bucephalidae) and its influence on host reproduction. Canadian Journal of Zoology 73, 887–897. [Google Scholar]
- Taskinen J, Valtonen ET and Gibson DI (1991) Studies on bucephalid digeneans parasitizing molluscs and fishes in Finland I. Ecological data and experimental studies. Systematic Parasitology 19, 81–94. [Google Scholar]
- Taskinen J, Valtonen ET and Mäkelä T (1994) Quantity of sporocysts and seasonality of two Rhipidocotyle species (Digenea: Bucephalidae) in Anodonta piscinalis (Mollusca: Bivalvia). International Journal of Parasitology 24, 877–886. [DOI] [PubMed] [Google Scholar]
- Taskinen J, Mäkelä T and Valtonen ET (1997) Exploitation of Anodonta piscinalis (Bivalvia) by trematodes: parasite tactics and host longevity. Annales Zoologici Fennici 34, 37–46. [Google Scholar]
- Théron A and Combes C (1988) Genetic analysis of cercarial emergence rhythms of Schistosoma mansoni. Behavior Genetics 18, 201–209. [DOI] [PubMed] [Google Scholar]
- Thieltges DW and Rick J (2006) Effects of temperature on emergence, survival and infectivity of cercariae of the marine trematode Renicola roscovita (Digenea: Renicolidae). Diseases of Aquatic Organisms 73, 63–68. [DOI] [PubMed] [Google Scholar]
- Thieltges DW, de Montaudouin X, Fredensborg B, Jensen KT, Koprivnikar J and Poulin R (2008) Production of marine trematode cercariae: a potentially overlooked path of energy flow in benthic systems. Marine Ecology Progress Series 372, 147–155. [Google Scholar]
- Tietäväinen H, Tuomenvirta H and Venäläinen A (2010) Annual and seasonal mean temperatures in Finland during the last 160 years based on gridded temperature data. International Journal of Climatology 30, 2247–2256. [Google Scholar]
- Vaughn CC (2018) Ecosystem services provided by freshwater mussels. Hydrobiologia 810, 15–27. [Google Scholar]
- Vyhlídalová T and Soldánová M (2020) Species-specific patterns in cercarial emergence of Diplostomum spp. from snails Radix lagotis. International Journal for Parasitology 50, 1177–1188. [DOI] [PubMed] [Google Scholar]
- Wickham H (2016) ggplot2: Elegant Graphics for Data Analysis. New York, USA: Springer Verlag. [Google Scholar]
- Wilke C (2020) cowplot: Streamlined Plot Theme and Plot Annotations for ‘ggplot2’. R package version 1.1.1. Available at https://CRAN.R-project.org/package=cowplot.
- Zeileis A, Kleiber C and Jackman S (2008) Regression models for count data in R. Journal of Statistical Software 27, 1–25. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0031182022000518.
click here to view supplementary material
Data Availability Statement
Data can be requested from the authors.