Key words: Carvone, nanoemulsion, nematodes, phytotherapy, sodium alginate
Abstract
This work aimed to evaluate the in vitro anthelmintic effect of carvone nanoemulsions on Haemonchus contortus. Three R-carvone nanoemulsions were prepared: uncoated R-carvone nanoemulsions homogenized in a sonicator (UNAlg-son) and homogenized in an ultrahomogenizer (UNAlg-ultra) and sodium alginate-coated R-carvone (CNAlg-ultra). The physicochemical characterizations of the nanoemulsions were carried out. The anthelmintic activity was evaluated using egg hatch test (EHT), larval development test (LDT) and adult worm motility test (AWMT). Changes in cuticle induced in adult H. contortus were evaluated by scanning electron microscopy (SEM). The results were subjected to analysis of variance and compared using the Tukey test (P < 0.05). The effective concentration to inhibit 50% (EC50) of egg hatching and larval development was calculated. The particle sizes were 281.1 nm (UNAlg-son), 152.7 nm (UNAlg-ultra) and 557.8 nm (CNAlg-ultra), and the zeta potentials were −15 mV (UNAlg-son), −10.8 mV (UNAlg-ultra) and −24.2 mV (CNAlg-ultra). The encapsulation efficiency was 99.84 ± 0.01%. SEM of the nanoemulsions showed an increase in size. In EHT, the EC50 values of UNAlg-son, UNAlg-ultra and CNAlg-ultra were 0.19, 0.02 and 0.17 mg mL−1, respectively. In LDT, they were 0.29, 0.31 and 0.95 mg mL−1 for UNAlg-son, UNAlg-ultra and CNAlg-ultra, respectively. The adult motility inhibition was 100% after 12 h of exposure to UNAlg-ultra and CNAlg-ultra, while for UNAlg-son, it was 79.16%. SEM showed changes in the buccal capsule and cuticular damage. It was concluded that R-carvone nanoemulsions showed antiparasitic action demonstrating promise for the control of infections caused by gastrointestinal nematodes in small ruminants.
Introduction
Gastrointestinal nematode infections (GNIs) are one of the main causes of economic losses for sheep and goats worldwide. Among nematodes, Haemonchus contortus is considered the most pathogenic, as it is a haematophagous parasite that causes anaemia, submandibular oedema and, in more severe cases, can lead to death (Wang et al., 2017; Toscano et al., 2018). The control of H. contortus is mainly based on the use of synthetic anthelmintics. However, the intensive use of these drugs and inadequate management have resulted in the selection of resistant and multiresistant nematode populations worldwide (Jackson and Coop, 2000; Bartram et al., 2012; Kaplan and Vidyashankar, 2012; Kotze and Prichard, 2016; Tuersong et al., 2020). Thus, new control strategies are being implemented, such as the use of essential oils from plants and their bioactive compounds (Ribeiro et al., 2015; Ferreira et al., 2016; Araújo-Filho et al., 2019).
Essential oils are complex mixtures of active substances of plant origin that exhibit a variety of properties, such as anthelmintic effects, due to the synergism of their compounds (Zhu et al., 2013; Katiki et al., 2017). Studies evaluating the anthelmintic efficacy of compounds isolated from essential oils, such as monoterpenes e.g. carvacrol, thymol, terpinen-4-ol, limonene and carvone, have demonstrated in vitro and in vivo effects on H. contortus (André et al., 2016; Grando et al., 2016; Katiki et al., 2017, 2019; André et al., 2018; Garbin et al., 2021; Silva et al., 2021). Carvone is a monoterpene that can be found as the main active component of several essential oils, Mentha spicata L., Anethum graveolens L., Carum carvi L. and Lippia alba (De Sousa et al., 2007; Johri, 2011). However, one of the limiting factors for the use of this biocompound is its volatility, instability and low water solubility. Thus, an alternative to improve these characteristics is encapsulation in biopolymer matrices (Lertsutthiwong and Rojsitthisak, 2011).
Nanoemulsions containing bioactive compounds form nanostructured systems that improve physicochemical stability, modulation of release rates and bioavailability of active principles (Abreu et al., 2020). For nanoencapsulation, biopolymers such as sodium alginate are used. It is a natural polysaccharide extracted from brown marine algae, red algae or bacteria. It has been used as an encapsulating matrix because it is a biodegradable polymer that is biocompatible, is sensitivity to pH, has low toxicity and promotes the controlled release of drugs (Wong et al., 2000; Prabaharan, 2015; Martins et al., 2017; Cheng et al., 2020; Hariyadi and Islam, 2020). The composition of formulations, method and preparation conditions are intrinsically related (Lovelyn and Attama, 2011). Thus, the comparison between the preparation methods of nanoemulsions is advantageous, as it allows obtaining more stable nanoemulsions and efficient controlled release systems (Abreu et al., 2020).
Therefore, the objective of this work was to evaluate the anthelmintic activity of R-carvone nanoemulsions produced by different homogenization methods towards H. contortus in vitro.
Materials and methods
Preparation of nanoemulsions
R-(−)-Carvone nanoemulsions (Quinarí®, Ponta Grossa, Paraná, Brazil) were prepared by the high-energy emulsification method with 2 different homogenization techniques (high-pressure homogenization and ultrasound) using the following equipment: an ultrahomogenizer ULTRA380 (Ultrastirrer, Jacareí, São Paulo, Brazil)) and a sonicator Fisherbrand™ Model 50 Sonic Dismembrator (Thermo Fisher Scientific, Waltham, Massachusetts, EUA) with or without the addition of sodium alginate (Dinâmica, Indaiatuba, São Paulo, Brazil) as a polymer matrix. For the preparation of the uncoated R-carvone nanoemulsion with sodium alginate (UNAlg-son), R-carvone and Tween 80 (Dinâmica, Indaiatuba, São Paulo, Bra) were added at a proportion of 1:15. Then, they were homogenized in a sonicator (Fisherbrand™ Model 50 Sonic Dismembrator) for 5 min at an amplitude of 30 μm. For preparation of the uncoated R-carvone nanoemulsion with sodium alginate (UNAlg-ultra), Tween 80 and R-carvone were added at a proportion of 1:2. Then, they were placed in an ultrasonic eco-sonics Q1.8/25 (Indaiatuba, São Paulo, Brazil) bath for 5 min and homogenized in an ultrahomogenizer ULTRA380 (Ultrastirrer) at 18 000 rpm for 2 min. To prepare the sodium alginate-coated R-carvone nanoemulsion (CNAlg-ultra), 4% sodium alginate, Tween 80 and R-carvone were added in proportions of 1:1:4. The solution was subjected to an ultrasonic bath (Q1.8/25, Ultronique) for 5 min and then homogenized in an ultrahomogenizer (ULTRA380, Ultrastirrer) at 22 000 rpm for 5 min.
Stability of nanoparticles
The physical stability of the emulsions homogenized in an ultrahomogenizer (UNAlg-ultra and CNAlg-ultra) was evaluated at 1, 7, 14, 21, 30, 60 and 90 days after preparation. For this procedure, ~10 mL of each of the nanoemulsions was maintained in closed test tubes, without exposure to light, at a temperature of 26°C. Periodic observation was implemented to detect any visual signs of instability, such as creaming, sedimentation or phase separation. Creaming and sedimentation were measured over time with the aid of a caliper. The creaming index (CI) and sedimentation index (SI) were determined by relating the height in centimetres of the precipitates formed in the test tube (Ha) with the total height of the sample (Ht) after the mentioned preparation periods, calculated according to equations (1) and (2), adapted from Mwangi et al. (2016):
| 1 |
| 2 |
Encapsulation efficiency
The encapsulation efficiency (EE) was determined by the difference between the initial amount of carvone in the nanoemulsion and the quantified free amount after ultracentrifugation (8000 rpm for 30 min) (equation (3)) using an Amicon® filter (molecular weight cutoff of 100 kDa, Millipore, UK) (Campelo et al., 2021). In the assay, an aliquot of 1 mL of each nanoemulsion (equivalent to 1.65 mg of carvone) was centrifuged, and the absorbance was determined at 235 nm (λmax of carvone) using a Genesys 6 Spectrophotometers (Thermo Fisher Scientific, Waltham, Massachusetts, EUA). The %EE was determined from a previously prepared carvone standard curve at concentrations ranging from 2 to 18 μg mL−1 (y = 0.0678 − 0.016; R2 = 0.9995). The maximum wavelength obtained in the spectrophotometric scan of carvone was λ = 235 nm. From the constructed standard curve, it was possible to determine the content of encapsulated carvone in the nanoemulsion as well as the EE. The experiment was carried out in triplicate, and the results are expressed as the mean ± standard error:
| 3 |
Physicochemical characterization of nanoemulsions
Particle size and zeta potential were determined by dynamic light scattering using the Malvern Zetasizer/Nanoseries Z590 equipment.
Detection of the main vibrational stretching modes was carried out by means of spectroscopy in the infrared region by Fourier transform (FTIR). Sample readings were performed on a Shimadzu spectrometer from 400 to 4000 cm−1 using KBr inserts.
Scanning electron microscopy (SEM) of nanoemulsions was performed after 42 days of production. A Zeiss 940A microscope was used at an accelerating voltage of 20 kV and a magnification of 100–3000×. Approximately 200 μL of nanoemulsions were placed on rounded metallic structures and dehydrated in a drying oven at 50°C for ~1 h until a thin sample film was obtained on the coin.
Haemonchus contortus isolate
The Kokstad (KOK) isolate of H. contortus multiresistant to commercial synthetic anthelmintics, benzimidazoles, levamisole and macrocyclic lactones was used (Neveu et al., 2007, 2010; Fauvin et al., 2010; Barrere et al., 2014). The isolate was supplied by the Institut National de Recherche pour l'Agriculture, l'Alimentation, et l'Environnement.
Egg hatch test
The egg hatch test (EHT) was performed according to the technique described by Coles et al. (2006). For egg recovery, feces were collected directly from the rectal ampoule of a sheep experimentally infected with the KOK isolate and processed according to Hubert and Kerboeuf (1992). Suspensions (250 μL) containing ~100 fresh eggs were incubated with the same volume of solutions from the following treatments: G1: UNAlg-son and G2: CNAlg-ultra, at concentrations ranging from 0.06 to 2.0 mg mL−1; G3: UNAlg-ultra, at concentrations ranging from 0.125 to 0.007 mg mL−1; G4: 0.025 mg mL−1 of thiabendazole (Sigma-Aldrich®) (positive control); G5: 3% Tween 80 (negative control) and G6: 4% sodium alginate polymer matrix (negative control). The suspension was incubated for 48 h at 25°C, and 5% Lugol's iodine solution was added to stop the hatching of the larvae. For each treatment and control, 5 replicates and 3 repetitions were performed.
Larval development test (LDT)
Eggs of H. contortus were incubated for 24 h to obtain L1. Solutions consisting of 500 μL of L1 (~250 larvae) and equal volumes of the following treatments: G1: UNAlg-son; G2: UNAlg-ultra and G3: CNAlg-ultra, in concentrations ranging from 0.06 to 2.0 mg mL−1; G4: 0.008 mg mL−1 of ivermectin (Sigma-Aldrich®) (positive control); G5: 3% Tween 80 (negative control) and G6: 4% sodium alginate polymer matrix (negative control), were incubated with GNI-free sheep feces for 6 days at room temperature (27°C) (Camurça-Vasconcelos et al., 2007). After the incubation period, L3 were recovered following the technique described by Roberts and O'Sullivan (1950) and counted using a microscope. For each treatment and control, 3 repetitions were performed with 5 replicates for each.
Adult worm motility test
The adult worm motility test (AWMT) was performed according to the method described by Hounzangbe-Adote et al. (2005). Adult females of H. contortus were collected from the abomasum of a sheep with monospecific infection. Soon after the animal was euthanized, the abomasum was removed, and the parasites were washed with saline solution. The mobile females were transferred to a 24-well plate at a density of 3 specimens per well, to which 1 mL phosphate-buffered saline (PBS) with 4% penicillin/streptomycin (Sigma-Aldrich®) was added and maintained at 37°C in a greenhouse with 5% carbon dioxide. After 1 h, 1 mL of treatment was added to the wells: G1: UNAlg-son; G2: UNAlg-ultra and G3: CNAlg-ultra, at concentrations ranging from 800 to 100 μg mL−1; G4: 100 μg mL−1 ivermectin (Sigma-Aldrich®); G5: 3% Tween 80 + PBS (negative control) and G6: 4% sodium alginate polymer matrix (negative control). The plates were maintained in the oven, and after 3, 6 and 12 h of incubation, motility was evaluated using a stereomicroscope. Eight replicates were made for each treatment and control groups.
SEM of adult H. contortus
Nematodes obtained from the AWMT were collected and subjected to SEM. Adult specimens of H. contortus were fixed in a 2.5% glutaraldehyde solution in 0.1 m sodium cacodylate buffer for a period of 72 h. After 3 washes in the same buffer, the parasites were dehydrated in a graded ethanol series (10, 20, 30, 60, 70, 80, 90 and 100%, 5 min each). The samples were dried in an EMSCOPE CPD 750, mounted in metal stubs and coated with gold–palladium for 5 min at 100 Å min−1. Possible changes in the cuticle of the treated parasites were observed with an S450 scanning electron microscope (Hitachi) at an accelerating voltage of 15 kV.
Statistical analysis
In EHT, inhibition of egg hatching was determined according to the following formula: (number of eggs/number of eggs + number of hatched larvae) × 100. In LDT, the effect was determined according to the following formula: (number of L3 in the negative control − number of L3 in the treated group/number of L3 in the negative control) × 100. The percentage of motile adults in AWMT was evaluated as the number of motile worms/total number of worms per well.
In EHT and LDT, the inhibition effects at each concentration of treatment were analysed using analysis of variance (ANOVA) and compared with the Tukey test. To analyse the differences between treatments, the data were subjected to the unpaired t test (2-tailed). In AWMT, the inhibition effects at each concentration of treatments at different times were analysed by 2-way ANOVA and compared with the Tukey test, using the SPSS 22.0 program, considering a significance level of 5% (P < 0.05).
GraphPad Prism® 6.0 software was used considering a significance level of 5% (P < 0.05). Effective concentrations to inhibit 50% (EC50) of egg hatching and larval development were calculated by linear regression using the SPSS 22.0 program.
Results
The macroscopic effects of nanoemulsion stability, such as creaming, sedimentation and phase separation were evaluated, and there was no macroscopic presence of sedimentation phenomena for UNAlg-ultra. For CNAlg-ultra, it was only possible to observe instability phenomena after 60 days, with a sedimentation rate of 6.6%. Creaming formation was observed only for CNAlg-ultra 7 days after storage. The CI values were 18.2% after 7, 14 and 21 days. After 30, 60 and 90 days, the creaming rates varied between 16.1 and 21.5%. In both nanoemulsions, no phase separation phenomenon was observed. The formulations presented nanometric and multimodal particle sizes. The particle size and zeta potential results are shown in Table 1. Samples UNAlg-ultra and UNAlg-son presented an average particle size value of 152.7 and 281.1 nm, respectively; CNAlg-ultra coated with alginate showed the highest particle size value (557.8 nm).
Table 1.
Particle size, zeta potential and polydispersity index of R-carvone nanoemulsions without polymer matrix (NCS-SM and NCT-SM) and R-carvone with polymer matrix (NCT-CM)
| Nanoemulsion | Particle size (nm) | Zeta potential (mV) | Polydispersity index |
|---|---|---|---|
| NCS-SM | 281.1 ± 78.63 | −15 ± 4.25 | 0.909 ± 0.100 |
| NCT-SM | 152.7 ± 13.86 | −10.8 ± 21.43 | 0.605 ± 0.047 |
| NCT-CM | 557.8 ± 21.43 | −24.2 ± 4.65 | 0.576 ± 0.047 |
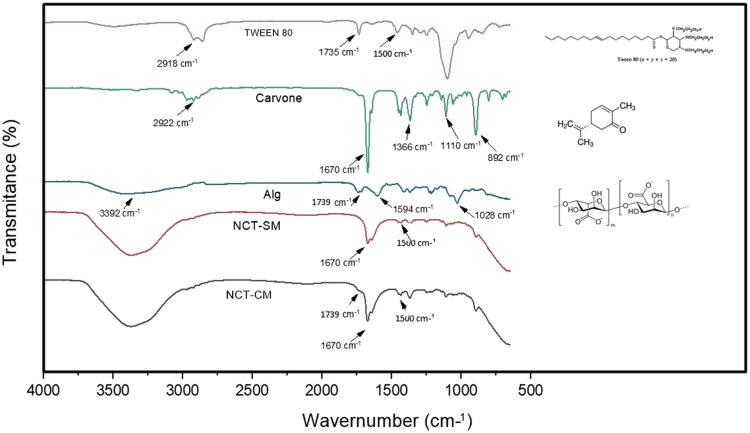
There was an average content of 20.24 ± 1.98% and an average EE of 99.84 ± 0.01%. The FTIR spectra of the compounds carvone, Tween 80 and alginate, as well as UNAlg-ultra and CNAlg-ultra are shown in Fig. 1. Carvone presents the main peaks at 2922 cm−1, which correspond to the stretching of the methyl groups (−CH2), as well as at 1670 cm−1, corresponding to the stretching of the C=C bond, and at 1445 cm−1, due to the C–H strain. Sodium alginate showed bands at 3392 cm−1 due to the stretching of the −OH bond, at 1739 cm−1 corresponding to the stretching of the COO– bond and at 1028 cm−1 attributed to the COH stretch. Tween 80 showed the stretching of CH3 and CH2 groups at 2918 and 2860 cm−1, respectively. Furthermore, it presented an absorption band at 1735 cm−1 due to stretching of the C=O bond, at 1500 cm−1 referring to the angular deformation of the −CH2 methyl groups and at 1095 cm−1 due to stretching of the COC bond. The aqueous nanoemulsions UNAlg-ultra and CNAlg-ultra exhibited a combination of peaks from the constituent materials. The peaks at 1670 and 1336 cm−1 were attributed to C=C and −CH2 stretches of carvone, respectively. The peaks at 1500 cm−1 present in Tween were distinctly found in nanoemulsions. The nanoemulsion with sodium alginate matrix CNAlg-ultra presented a distinction in relation to the nanoemulsion without matrix (UNAlg-ultra) by the discrete presence of a shoulder at 1739 cm, which could be attributed to stretching of the COO− bond of alginate.
Fig. 1.
FTIR spectra of carvone, sodium alginate, Tween 80 and uncoated (UNAlg-ultra) and sodium alginate-coated (CNAlg-ultra) R-carvone nanoemulsions homogenized in an ultrahomogenizer.
The SEM images of the nanoemulsions are shown in Fig. 2. Figure 2A–C shows the 3 types of nanoemulsions at the same magnification (50 000×). Samples UNAlg-Ultra and UNAlg-son showed similar patterns in comparison with the particle size from DLS (Dynamic Light Scattering) results (Table 1). Figure 2A (UNAlg-ultra) shows micelles with spherical morphology and homogeneously arranged in the medium in which they were dispersed. Figure 2B (UNAlg-son) shows particles with similar morphology, but with higher particle size, with uneven spacing between micelles. Figure 2C (CNAlg-ultra) shows the presence of particles with different sizes arranged in a non-uniform manner dispersed along the alginate matrix. Figure 2D–F shows in detail the presence of microspherical inclusions as small particles aggregated to the polymeric matrix network for the sample CNAlg-ultra, in addition to the formation of structures with porous characteristics in the polymeric network. Figure 2C (UNAlg-ultra) shows the presence of particles with different sizes arranged in a non-uniform manner in the dispersion medium.
Fig. 2.
SEM surface images: (a) UNAlg-ultra at 50 000×; (b) UNAlg-son at 50 000×; (c) CNAlg-ultra at 50 000×. Images (d), (e) and (f) show CNAlg-ultra at different magnifications: 1000×, 5000× and 10 000×.
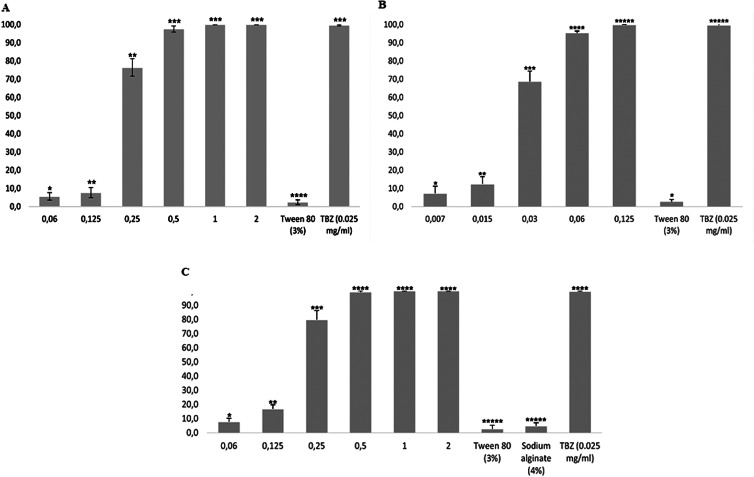
The mean effects of the UNAlg-son, UNAlg-ultra and CNAlg-ultra formulations obtained in the EHT on the H. contortus-resistant isolate to synthetic anthelmintics are shown in Fig. 3. At a concentration of 2 mg mL−1, UNAlg-son, UNAlg-ultra and CNAlg-ultra were 100% effective, showing statistical similarity to the positive control (P > 0.05) and a dose-dependent effect. The EC50 values of UNAlg-son, UNAlg-ultra and CNAlg-ultra were 0.19, 0.02 and 0.17 mg mL−1, respectively.
Fig. 3.
Effects of UNAlg-son (A), UNAlg-ultra (B) and CNAlg-ultra on the hatching of Haemonchus contortus eggs. * Different numbers indicate statistical difference (P < 0.05). UNAlg-son, uncoated R-carvone nanoemulsion with sodium alginate; UNAlg-ultra, uncoated R-carvone nanoemulsion with sodium alginate; CNAlg-ultra, sodium alginate coated R-carvone nanoemulsion; TBZ, thiabendazole (positive control). Sodium alginate solution (4%) was used as a negative control for nanoemulsions.
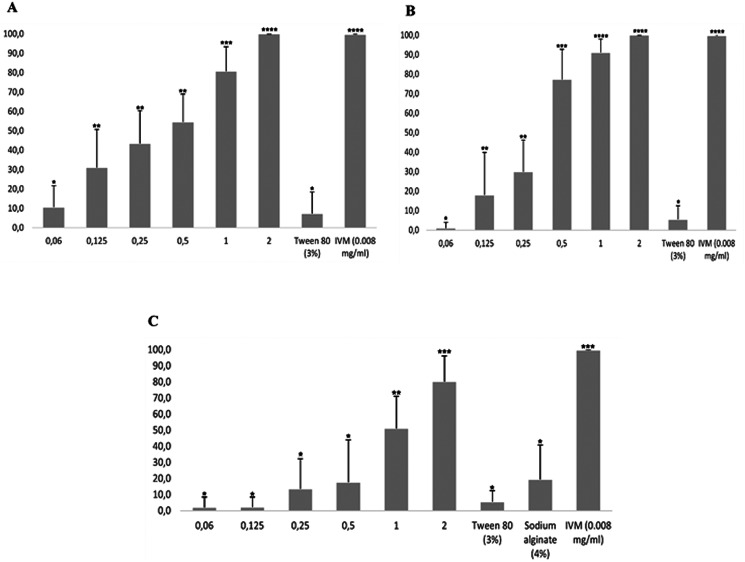
The inhibitory effects of nanoemulsions on LDT are shown in Fig. 4. UNAlg-son and UNAlg-ultra inhibited larval development by 100% at a concentration of 2 mg mL−1, while for CNAlg-ultra, the inhibition was 80.23%. These results did not differ statistically from the positive control (P > 0.05) and showed a dose-dependent effect. The EC50 values in the LDT for UNAlg-son, UNAlg-ultra and CNAlg-ultra were 0.29, 0.31 and 0.95 mg mL−1, respectively.
Fig. 4.
Effects of UNAlg-son (A), UNAlg-ultra (B) and CNAlg-ultra on larval development of H. contortus. *Different numbers indicate statistical difference (P < 0.05). UNAlg-son, uncoated R-carvone nanoemulsion with sodium alginate; UNAlg-ultra, uncoated R-carvone nanoemulsion with sodium alginate; CNAlg-ultra, sodium alginate coated R-carvone nanoemulsion; IVM, ivermectin (positive control). Sodium alginate solution (4%) was used as a negative control for nanoemulsions.
The effects of R-carvone nanoemulsions on the inhibition of H. contortus motility are shown in Fig. 5. UNAlg-ultra and CNAlg-ultra inhibited worm motility by 100% at a concentration of 800 μg mL−1 after 3 h of exposure, while the effectiveness of UNAlg-son was 79.16% after 12 h of exposure. These results did not differ statistically from the positive control (P > 0.05). The motility in the negative (Tween 80® + PBS) and positive controls was 100 and 0%, respectively, at 12 h post-exposure.
Fig. 5.
Effects of UNAlg-son (A), UNAlg-ultra (B) and CNAlg-ultra on adult motility of H. contortus. IVM, ivermectin (positive control); Tween 80 + PBS (negative control).
SE micrographs (Fig. 6) showed changes in the buccal capsule and along the cuticle of female H. contortus after exposure to 800 μg mL−1 nanoemulsions. The alterations consisted of the wrinkling of the longitudinal cuticular ridges (Fig. 3B, E and H). There was wrinkling of the buccal capsule and deformation of the lancet (Fig. 3A and G), similar to the positive control (Fig. 3J). The longitudinal cuticular ridges, buccal capsule and lancet seemed to be well preserved in the negative control group (Fig. 3C, F, I and L).
Fig. 6.
SEM images of ultrastructural changes in the cuticle and cephalic region of adult females of H. contortus: (A) and (B) UNAlg-son; (D) and (E) UNAlg-ultra; (G) and (H) CNAlg-ultra; (C) and (F) Tween 80 + PBS (negative control); (I) and (L) sodium alginate solution 4% (negative control); (J) and (K) ivermectin (positive control).
Discussion
Instability phenomena can limit the period of effective application of emulsified formulations; thus, nanoemulsions based on essential oils require the evaluation of their stability during storage (Guerra-Rosas et al., 2016). The UNAlg-ultra and CNAlg-ultra formulations were more stable for sedimentation and creaming phenomena, respectively, as they presented a percentage of secreted volume lower than 1%, even after 90 days of preparation. The formation of creaming in UNAlg-ultra occurred because the nanoemulsion droplets had a lower density than the surrounding liquid, causing an upward movement of the particles, while the sedimentation of CNAlg-ultra might be due to the larger particle size because of the use of sodium alginate as an encapsulating matrix, in addition to droplets having a higher density than the surrounding liquid, promoting downward movement of the particles (Mcclements, 2010).
The UNAlg-ultra formulation had the smallest particle size (152.7 nm), followed by UNAlg-son (281.1 nm), while the largest size was found for CNAlg-ultra (557.8 nm). UNAlg-Ultra and UNAlg-son showed similar pattern sizes in SEM images from lower to higher sizes. In evaluating the morphology of nanoemulsions by SEM, UNAlg-son presented micelles with higher particle size than UNAlg-Ultra ones, which might be due to the instability process causing a possible aggregation of the droplets and, thus, an increase in their size (Daltin, 2011). The formation of porous structures seen in nanoemulsions, and observed in CNAlg-ultra, is a characteristic of polymeric coating, and has been attributed to the formation of micellar vacuoles in the polymer network (Fernandes et al., 2014; Morais et al., 2016).
The UNAlg-son was homogenized for 5 min, probably because a longer homogenization time could have contributed to obtaining a smaller particle size (Abismail et al., 1999). To prepare the UNAlg-ultra, the high-pressure homogenization method (ultrahomogenizer) was used, producing a nanoemulsion with a smaller particle size due to the homogenization time (2 min) and rotation speed (18 000 rpm). The particle size decreases as the homogenization time or the rotation speed increases (Benavides et al., 2016). In this case, it is evident that UNAlg-ultra sample showed the lowest particle size, where the ultra-stirrer technique showed higher efficiency in the dispersion of the oily phase in nanometric droplets in comparison with the sonication technique. Although CNAlg-ultra was prepared with a higher number of revolutions per minute (22 000 rpm) and a longer homogenization time (5 min), this nanoemulsion presented larger particles. Alginate-coated nanoemulsion showed an increased size due to the external polymer matrix which, because of the size of its molecule, promotes the formation of a larger adsorption interface. The alginate matrix involves the nanoemulsion droplets, and despite the occurrence of an increase in the particle size of the nanoemulsion (Salvia-Trujillo et al., 2014), the external coating may prevent from further coalescence stabilizing nanodroplets size for longer periods of time.
Bortoluzzi et al. (2020) prepared a nanoemulsion of the essential oil of Mentha villosa Hubs (68.8% carvone) using Tween 80 as a surfactant without the addition of a polymer matrix. The sample was first subjected to high-pressure homogenization in an Ultra-Turrax T25 digital IKA (Staufen, Germany) and later in high pressure homogenizer APLAB 10 (Artepeças, São Paulo,Brazil). This nanoemulsion exhibited a larger particle diameter (164 nm) than the CNAlg-ultra nanoemulsion utilized in the present study (152.7 nm), prepared without the addition of a polymer matrix. It has been reported that the primary homogenization of the sample and subsequent use of a high-energy homogenization method, such as microfluidization, promotes a significant reduction in particle size (Santos et al., 2018). However, using only 1 high-energy homogenization technique (ultrahomogenizer), it was possible to obtain a slightly smaller particle size compared with the results obtained by Bortoluzzi et al. (2020) when using 2 homogenization cycles.
The 3 nanoemulsions had negative zeta potentials, which was related to the use of Tween 80, a nonionic surfactant that can generate a negative charge on oil droplets due to the preferential adsorption of hydroxyl ions from the aqueous phase and sodium alginate, which endows it with a negative charge at neutral pH as an encapsulating matrix (Mcclements, 2005; Machado et al., 2012; Gago et al., 2019). The zeta potential value of CNAlg-ultra is close to the recommended stability value, as potentials greater than +30 mV or less than −30 mV are strongly cationic and anionic, respectively, and are classified as stable (Clogston and Patri, 2011).
The R-carvone nanoemulsion was evaluated for its in vitro anthelmintic effect on H. contortus eggs, larvae and adults that were multiresistant. The ovicidal effect of UNAlg-ultra was potentiated (EC50 = 0.02 mg mL−1) with homogenization in an ultrahomogenizer without a polymer matrix in comparison with UNAlg-son (EC50 = 0.18 mg mL−1) and CNAlg-ultra (EC50 = 0.19 mg mL−1). The superior results obtained for the UNAlg-ultra formulation can be explained by its smaller particle size, which possibly allowed greater penetration into the eggshell and, consequently, a better inhibitory effect on larval hatching. The effectiveness of the UNAlg-son and CNAlg-ultra formulations was similar (P > 0.05), indicating that the polymeric system did not enhance the effectiveness of R-carvone on egg hatching. The presence of the sodium alginate matrix as an encapsulating agent in the polymeric system of the UNAlg-ultra may have influenced the controlled release process in the aqueous medium of EHT, prolonging the release of the encapsulated carvone, since the diffusion of drugs retained in the nanoemulsions occurs when the water enters the polymeric system, resulting in swelling and subsequent release of encapsulated drugs (Dash et al., 2011).
UNAlg-son (CE50 = 0.29 mg mL−1) and UNAlg-ultra (CE50 = 0.31 mg mL−1) formulations showed similar effects (P > 0.05), indicating that the type of homogenization did not influence larval development. The CNAlg-ultra (CE50 = 0.95 mg mL−1) formulation was less effective than the other formulations because the LDT uses a solid medium based on feces, hindering the release of the biocompound from the polymeric system. These results are similar to those of Ribeiro et al. (2015), where the nanoemulsion (EC50 = 2.3 mg mL−1) of Eucalyptus staigeriana oil had a lower inhibitory effect than the oil in its free form (EC50 = 1.8 mg mL−1).
UNAlg-ultra and CNAlg-ultra showed a similar effect on the inhibition of adult motility (100%), differing from UNAlg-son, which only attained 16.65% of the inhibitory effect on adult H. contortus motility. This phenomenon may be indicative that high pressurization with an ultrahomogenizer increases the effectiveness of carvone. The use of monoterpenes to inhibit the motility of adult nematodes has been previously reported (André et al., 2016; Araújo-Filho et al., 2018; André et al., 2020). The monoterpenes citronellal and thymol used in the free form completely inhibit the motility of adult H. contortus after 12 and 24 h of exposure, respectively (André et al., 2017; Araújo-Filho et al., 2019). We can conclude that the R-carvone nanoemulsion had a better inhibitory effect on adults, since the inhibition was 100% after 3 h of exposure. Thus, the use of encapsulation can potentiate the anthelmintic effect of bioactive compounds and ensure protection for encapsulated substances, making it a useful alternative for controlling multiresistant populations of gastrointestinal nematodes (Ribeiro et al., 2013; Ribeiro et al., 2014).
SEM allowed the observation of damage in the mouth capsule and cuticle of females of H. contortus exposed to R-carvone nanoemulsions. Cuticle wrinkling in H. contortus caused by exposure to substances with potential anthelmintic effects, such as essential oils, extracts and isolated compounds such as monoterpenes, has been previously described (André et al., 2016, 2017; Ribeiro et al., 2017; Cavalcante et al., 2020). The changes observed in SEM may be related to interactions with the internal structures of nematodes causing physiological disturbances (Brunet et al., 2011), associated with the loss of protective properties of the cuticle, involvement in motility and metabolic changes that occur in the digestive tract of small ruminants (Martínez-Ortíz-de-Montellano et al., 2013).
Some studies suggest that carvone acts as an acetylcholinesterase (AChE) inhibitor (Ryan and Byrne, 1988; Jukic et al., 2007; López and Pascual-Villalobos, 2010; Kurt et al., 2017). Its possible action on AChE receptors observed in vertebrates and invertebrates causes neurotoxic lesions similar to organophosphates, which are potent anthelmintics that are highly toxic to mammals (Miller et al., 1986; Lopez and Pascual-Villalobos, 2010; Ross et al., 2013).
Acknowledgements
The authors acknowledge Central Analítica-UFC/CT-INFRA/MCTI-SISANO/Pró-Equipamentos CAPES for the support. Ms Aguiar has received a master research scholarship from Conselho Nacional de Desenvolvimento Científico e Tecnologico (CNPq). Dra Bevilaqua has received a researcher fellowship from CNPq (310663/2016-4-5).
Author contributions
A. A. R. M. A., C. M. L. B., W. P. P. A. and L. M. B. O.: conceptualization. A. A. R. M. A., W. P. P. A., J. V. A. F., F. O. M. S. A., H. N. P., W. L. C. R.; A. C. F. L. M., M. E. N. P. R.; N. M. P. S. R.; L. O. R. and L. M. B. O.: performed experiments. A. A. R. M. A., C. M. L. B. and W. P. P. A.: data analysis, supervision and project administration. A. A. R. M. A., C. M. L. B., W. P. P. A. and L. M. B. O.: writing the manuscript. All the authors reviewed and approved the manuscript.
Financial support
This study was funded by the Brazilian funding agency Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). Mrs Aguiar received a master's research grant from the National Council for Scientific and Technological Development (130372/2020-0). Dr Bevilaqua is a CNPq fellow (310663/2016-4-5).
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical standards
This study was approved by the Ethics Committee for Animal Use of the State University of Ceará (UECE) and registered under protocol number 390620/2020.
References
- Abismail B, Canselier JP, Wilhelm AM, Delmas H and Gourdon C (1999) Emulsification by ultrasound: drop size distribution and stability. Ultrasonics Sonochemistry 6, 75–83. [DOI] [PubMed] [Google Scholar]
- Abreu FOM, Costa EF, Cardial MRL and André WPP (2020) Polymeric nanoemulsions enriched with Eucalyptus citriodora essential oil. Polímeros 30, e2020024. [Google Scholar]
- André WPP, Ribeiro WLC, Cavalcante GS, Santos JML, Macedo ITF, Paula HCB, Freitas RM, Maia SM, Melo JV and Bevilaqua CML (2016) Comparative efficacy and toxic effects of carvacryl acetate and carvacrol on sheep gastrointestinal nematodes and mice. Veterinary Parasitology 218, 52–58. [DOI] [PubMed] [Google Scholar]
- André WPP, Cavalcante GS, Ribeiro WLC, Santos JML, Macedo ITF, Paula HCB, Morais SM, Melo JV and Bevilaqua CML (2017) Anthelmintic effect of thymol and thymol acetate on sheep gastrointestinal nematodes and their toxicity in mice. Revista Brasileira de Parasitologia Veterinária 26, 323–330. [DOI] [PubMed] [Google Scholar]
- André WPP, Ribeiro WLC, Oliveira LMB, Macedo ITF, Rondon FCM and Bevilaqua CML (2018) Essential oils and their bioactive compounds in the control of gastrointestinal nematodes of small ruminants. Acta Scientiae Veterinariae 46, 552. [Google Scholar]
- André WPP, Paiva JR Jr., Cavalcante GS, Ribeiro WLC, Araújo-Filho JV, Cavalcanti BC, Morais SM, Oliveira LMB, Bevilaqua CML and Abreu FOMS (2020) Chitosan nanoparticles loaded with carvacrol and carvacryl acetate for improved anthelmintic activity. Journal of the Brazilian Chemical Society 31, 1614–1622. [Google Scholar]
- Araújo-Filho JV, Ribeiro WLC, André WPP, Cavalcante GS, Guerra MCM, Muniz CR, Macedo ITF, Rondon FCM, Bevilaqua CML and Oliveira LMB (2018) Effects of Eucalyptus citriodora essential oil and its major component, citronellal, on Haemonchus contortus isolates susceptible and resistant to synthetic anthelmintics. Industrial Crops and Products 124, 294–299. [Google Scholar]
- Araújo-Filho JV, Ribeiro WLC, André WPP, Cavalcante GS, Rios TT, Schwinden GM, Rocha LO, Macedo ITF, Morais SM, Bevilaqua CML and Oliveira LMB (2019) Anthelmintic activity of Eucalyptus citriodora essential oil and its major component, citronellal, on sheep gastrointestinal nematodes. Revista Brasileira de Parasitologia Veterinária 28, 644–651. [DOI] [PubMed] [Google Scholar]
- Barrere V, Beech RN, Charvet CL and Prichard RK (2014) Novel assay for the detection and monitoring of levamisole resistance in Haemonchus contortus. International Journal for Parasitology 44, 235–241. [DOI] [PubMed] [Google Scholar]
- Bartram DJ, Leathwick DM, Taylor MA, Geurden T and Maeder SJ (2012) The role of combination anthelmintic formulations in the sustainable control of sheep nematodes. Veterinary Parasitology 186, 151–158. [DOI] [PubMed] [Google Scholar]
- Benavides S, Cortés P, Parada J and Franco W (2016) Development of alginate microspheres containing thyme essential oil using ionic gelation. Food Chemistry 204, 77–83. [DOI] [PubMed] [Google Scholar]
- Bortoluzzi BB, Buzatti A, Chaaban A, Pritsch IC, Anjos AD, Cipriano RR, Deschamps C and Molento MB (2020) Mentha villosa Hubs., M. x piperita and their bioactives against gastrointestinal nematodes of ruminants and the potential as drug enhancers. Veterinary Parasitology 289, 109317. [DOI] [PubMed] [Google Scholar]
- Brunet S, Fourquaux I and Hoste H (2011) Ultrastructural changes in the third-stage, infective larvae of ruminant nematodes treated with sainfoin (Onobrychis viciifolia) extract. Parasitology International 60, 419–424. [DOI] [PubMed] [Google Scholar]
- Campelo MS, Melo EO, Arrais SP, Nascimento FBSA, Gramosa NV, Soares SA, Ribeiro MENP, Silva CR, Júnior HVN and Ricardo NMPS (2021) Clove essential oil encapsulated on nanocarrier based on polysaccharide: a strategy for the treatment of vaginal candidiasis. Colloids and Surfaces A: Physicochemical and Engineering Aspects 610, 125732. [Google Scholar]
- Camurça-Vasconcelos AL, Bevilaqua CM, Morais SM, Maciel MV, Costa CT, Macedo IT, Oliveira LMB, Braga RR, Silva RA and Vieira LS (2007) Anthelmintic activity of Croton zehntneri and Lippia sidoides essential oils. Veterinary Parasitology 148, 288–294. [DOI] [PubMed] [Google Scholar]
- Cavalcante GS, Morais SM, André WPP, Araújo-Filho JV, Muniz CR, Rocha LO, Ribeiro WLC, Rodrigues ALM, Oliveira LMB, Bevilaqua CML and Ramos MV (2020) Chemical constituents of Calotropis procera latex and ultrastructural effects on Haemonchus contortus. Revista Brasileira de Parasitologia Veterinária 29, 22–25. [Google Scholar]
- Cheng D, Jiang C, Xu J, Liu Z and Mao X (2020) Characteristics and applications of alginate lyases: a review. International Journal of Biological Macromolecules 164, 1304–1320. [DOI] [PubMed] [Google Scholar]
- Clogston JD and Patri AK (2011) Zeta potential measurement. In McNeil S (ed.), Characterization of Nanoparticles Intended for Drug Delivery. Methods in Molecular Biology (Methods and Protocols), 697, 63–70, Humana Press, New York. [DOI] [PubMed] [Google Scholar]
- Coles GC, Jackson F, Pomroy WE, Prichard RK, Von Samsonhimmelstjerna G, Silvestre A, Taylor MA and Vercruysse J (2006) The detection of anthelmintic resistance in nematodes of veterinary importance. Veterinary Parasitology 136, 167–185. [DOI] [PubMed] [Google Scholar]
- Daltin D (2011) Tensoativos: química, propriedades e aplicações. São Paulo: Edgard Blücher Ltda, 330pp. [Google Scholar]
- Dash M, Chiellini F, Ottenbrite RM and Chiellini E (2011) Chitosan – a versatile semi-synthetic polymer in biomedical applications. Progress in Polymer Science 36, 981–1014. [Google Scholar]
- De Sousa DP, Nóbrega FFF and Almeida RN (2007) Influence of the chirality of (R)-(−)- and (S)-(+)-carvone in the central nervous system: a comparative study. Chirality 19, 264–268. [DOI] [PubMed] [Google Scholar]
- Fauvin A, Charvet C, Issouf M, Cortet J and Neveu C (2010) cDNA-AFLP analysis in levamisole-resistant Haemonchus contortus reveals alternative splicing in a nicotinic acetylcholine receptor subunit. Molecular and Biochemical Parasitology 170, 105–107. [DOI] [PubMed] [Google Scholar]
- Fernandes RVDB, Borges SV and Botrel DA (2014) Gum arabic/starch/maltodextrin/inulin as wall materials on the microencapsulation of rosemary essential oil. Carbohydrate Polymers 101, 524–532. [DOI] [PubMed] [Google Scholar]
- Ferreira LE, Benincasa BI, Fachin AL, França SC, Contini SS, Chagas AC and Beleboni RO (2016) Thymus vulgaris L. essential oil and its main component thymol: anthelmintic effects against Haemonchus contortus from sheep. Veterinary Parasitology 228, 70–76. [DOI] [PubMed] [Google Scholar]
- Gago CML, Artiga-Artigas M, Antunes MDC, Faleiro ML, Miguel MG and Martín-Belloso O (2019) Effectiveness of nanoemulsions of clove and lemongrass essential oils and their major components against Escherichia coli and Botrytis cinerea. Journal of Food Science and Technology 56, 2721–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbin VP, Munguía B, Saldaña JC, Deschamps C, Cipriano RR and Molento MB (2021) Chemical characterization and in vitro anthelmintic activity of Citrus bergamia Risso and Citrus X paradisii Macfad essential oil against Haemonchus contortus Kirby isolate. Acta Tropica 217, 105869. [DOI] [PubMed] [Google Scholar]
- Grando TH, De Sá MF, Baldissera MD, Oliveira CB, De Souza ME, Raffin RP, Santos RCV, Domingues R, Minho AP, Leal M and Monteiro SG (2016) In vitro activity of essential oils of free and nanostructured Melaleuca alternifolia and of terpinen-4-ol on eggs and larvae of Haemonchus contortus. Journal of Helminthology 90, 377–382. [DOI] [PubMed] [Google Scholar]
- Guerra-Rosas MI, Morales-Castro J, Ochoa-Martınez LA, Salvia-Trujillo L and Olga Martín-Belloso O (2016) Long-term stability of food-grade nanoemulsions from high methoxyl pectin containing essential oils. Food Hydrocolloids 52, 438–446. [Google Scholar]
- Hariyadi D M and Islam N (2020) Current Status of Alginate in Drug Delivery. Advances in Pharmacological and Pharmaceutical Sciences 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounzangbe-Adote MS, Paolini V, Fouraste I, Moutairou K and Hoste H (2005) In vitro effects of four tropical plants on three life-cycle stages of the parasitic nematode, Haemonchus contortus. Veterinary Science Research 78, 155–160. [DOI] [PubMed] [Google Scholar]
- Hubert J and Kerboeuf DA (1992) Microlarval development assay for the detection of anthelmintic resistance in sheep nematodes. Veterinary Record 130, 442–446. [DOI] [PubMed] [Google Scholar]
- Jackson F and Coop RL (2000) The development of anthelmintic resistance in sheep nematodes. Parasitology 120, 95–107. [DOI] [PubMed] [Google Scholar]
- Johri RK (2011) Cuminum cyminum and Carum carvi: an update. Pharmacognosy Review 5, 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jukic M, Politeo O, Maksimovic M, Milos M and Milos M (2007) In vitro acetylcholinesterase inhibitory properties of thymol, carvacrol and their derivatives thymoquinone and thymohydroquinone. Phytotherapy Research 21, 259–261. [DOI] [PubMed] [Google Scholar]
- Kaplan RM and Vidyashankar AN (2012) An inconvenient truth: global warming and anthelmintic resistance. Veterinary Parasitology 186, 70–78. [DOI] [PubMed] [Google Scholar]
- Katiki LM, Barbieri AME, Araujo RC, Veríssimo CJ, Louvandini H and Ferreira JFS (2017) Synergistic interaction of ten essential oils against Haemonchus contortus in vitro. Veterinary Parasitology 243, 47–51. [DOI] [PubMed] [Google Scholar]
- Katiki LM, Araujo RC, Ziegelmeyer ACP, Gomes G, Gutmanis L, Rodrigues MS, Bueno CJ, Veríssimo H, Louvandini JFS and Ferreira AFT (2019) Evaluation of encapsulated anethole and carvone in lambs artificially- and naturally-infected with Haemonchus contortus. Experimental Parasitology 197, 36–42. [DOI] [PubMed] [Google Scholar]
- Kotze AC and Prichard RK (2016) Anthelmintic resistance in Haemonchus contortus: history, mechanisms and diagnosis. Advances in Parasitology 93, 397–428. [DOI] [PubMed] [Google Scholar]
- Kurt BZ, Gazioglu L, Dag A, Salmas RE, Kaylk G, Durdagi S and Sonmez F (2017) Synthesis, anticholinesterase activity and molecular modeling study of novel carbamate-substituted thymol/carvacrol derivatives. Bioorganic & Medicinal Chemistry 25, 1352–1363. [DOI] [PubMed] [Google Scholar]
- Lertsutthiwong P and Rojsitthisak P (2011) Chitosan-alginate nanocapsules for encapsulation of turmeric oil. Pharmazie 66, 911–915. [PubMed] [Google Scholar]
- López MD and Pascual-Villalobos MJ (2010) Mode of inhibition of acetylcholinesterase by monoterpenoids and implications for pest control. Industrial Crops and Products 31, 284–288. [Google Scholar]
- Lovelyn C and Attama AA (2011) Current state of nanoemulsions in drug delivery. Journal of Biomaterials and Nanobiotechnology 2, 626–639. [Google Scholar]
- Machado AHE, Lundberg D, Ribeiro AJ, Veiga FJ, Lindman B, Miguel MG and Olsson U (2012) Preparation of calcium alginate nanoparticles using water-in-oil (W/O) nanoemulsions. Langmuir 28, 4131–4141. [DOI] [PubMed] [Google Scholar]
- Martins E, Poncelet D, Rodrigues RC and Renard D (2017) Oil encapsulation techniques using alginate as encapsulating agent: applications and drawbacks. Journal of Microencapsulation 4, 754–771. [DOI] [PubMed] [Google Scholar]
- Martínez-Ortíz-de-Montellano C, Arroyo-López C, Fourquaux I, Torres-Costa CA and Hoste H (2013) Scanning electron microscopy of Haemonchus contortus exposed to tannin rich plants under in vivo and in vitro conditions. Experimental Parasitology 133, 281–286. [DOI] [PubMed] [Google Scholar]
- McClements DJ (2005) Food Emulsions: Principles, Practices, and Techniques, Vol. 2. Boca Raton: CRC Press, 714pp. [Google Scholar]
- Mcclements DJ (2010) Emulsion design to improve the delivery of functional lipophilic components. Annual Review of Food Science and Technology 1, 241–269. [DOI] [PubMed] [Google Scholar]
- Miller JE, Baker NF and Farver TB (1986) Anthelmintic treatment of pastured dairy cattle in California. American Journal of Veterinary Research 47, 2036–2040. [PubMed] [Google Scholar]
- Morais ARV, Alencar EN, Xavier FH Jr., Oliveira CM, Marcelino HR, Barratt G, Fessi H, Egito EST and Elaissari A (2016) Freeze-drying of emulsified systems: a review. International Journal of Pharmaceutics 503, 102–114. [DOI] [PubMed] [Google Scholar]
- Mwangi WW, Ho KW, Tey BT and Chan ES (2016) Effects of environmental factors on the physical stability of pickering-emulsions stabilized by chitosan particles. Food Hydrocolloids 60, 543–550. [Google Scholar]
- Neveu C, Charvet C, Fauvin A, Cortet J, Castagnone-Sereno P and Cabaret J (2007) Identification of levamisole resistance markers in the parasitic nematode Haemonchus contortus using a cDNA-AFLP approach. Parasitology 134, 1105–1110. [DOI] [PubMed] [Google Scholar]
- Neveu C, Charvet CL, Fauvin A, Cortet J, Beech RN and Cabaret J (2010) Genetic diversity of levamisole receptor subunits in parasitic nematode species and abbreviated transcripts associated with resistance. Pharmacogenetics and Genomics 20, 414–425. [DOI] [PubMed] [Google Scholar]
- Prabaharan M (2015) Chitosan-based nanoparticles for tumor-targeted drug delivery. International Journal of Biological Macromolecules 72, 1313–1322. [DOI] [PubMed] [Google Scholar]
- Ribeiro WLC, Macedo ITF, Santos JML, Oliveira EF, Camurça-Vasconcelos ALF, Paula HCB and Bevilaqua CML (2013) Activity of chitosan encapsulated Eucalyptus staigeriana essential oil on Haemonchus contortus. Experimental Parasitology 135, 24–29. [DOI] [PubMed] [Google Scholar]
- Ribeiro JC, Ribeiro WLC, Camurça-Vasconcelos ALF, Macedo ITF, Santos JML, Paula HCB, Aráujo-Filho JV, Magalhães RD and Bevilaqua CML (2014) Efficacy of free and nanoencapsulated Eucalyptus citriodora essential oils on sheep gastrointestinal nematodes and toxicity for mice. Veterinary Parasitology 204, 243–248. [DOI] [PubMed] [Google Scholar]
- Ribeiro WLC, Camurça-Vasconcelos ALF, Macedo ITF, Santos JML, Ribeiro JC, Pereira VA, Viana DA, Paula HCB and Bevilaqua CML (2015) In vitro effects of Eucalyptus staigeriana nanoemulsion on Haemonchus contortus and toxicity in rodents. Veterinary Parasitology 212, 444–447. [DOI] [PubMed] [Google Scholar]
- Ribeiro WLC, Camurça-Vasconcelos ALF, Santos JML, Macedo ITF, Ribeiro JC, Oliveira EF, Paula HCB and Bevilaqua CML (2017) The use of Eucalyptus staigeriana nanoemulsion for control of sheep haemonchosis. Pesquisa Veterinária Brasileira 37, 221–226. [Google Scholar]
- Roberts FHS and O'Sullivan JP (1950) Methods for egg counts and larval cultures for strongyles infesting the gastrointestinal tract of cattle. Australian Journal of Agricultural Research 1, 99–102. [Google Scholar]
- Ross SM, McManus IC, Harrison V and Mason O (2013) Neurobehavioral problems following low-level exposure to organophosphate pesticides: a systematic and meta-analytic review. Critical Reviews in Toxicology 43, 21–44. [DOI] [PubMed] [Google Scholar]
- Ryan MF and Byrne O (1988) Plant-insect coevolution and inhibition of acetylcholinesterase. Journal of Chemical Ecology 14, 1965–1975. [DOI] [PubMed] [Google Scholar]
- Salvia-Trujillo L, Rojas-Grau MA, Soliva-Fortuny R and Martın-Belloso O (2014) Formulation of antimicrobial edible nanoemulsions with pseudo-ternary phase experimental design. Food and Bioprocess Technology 7, 3022–3032. [Google Scholar]
- Santos J, Ramírez P, Llinares R, Muñoz J and Trujillo-Cayado LA (2018) Enhancing rosemary oil-in-water microfluidized nanoemulsion properties through formulation optimization by response surface methodology. LWT 97, 370. [Google Scholar]
- Silva CR, Lifschitz AL, Macedo SRD, Campos NRCL, Viana-Filho M, Alcântara ACS, Araújo JG, Alencar LMR and Costa-Junior LM (2021) Combination of synthetic anthelmintics and monoterpenes: assessment of efficacy, and ultrastructural and biophysical properties of Haemonchus contortus using atomic force microscopy. Veterinary Parasitology 290, 109345. [DOI] [PubMed] [Google Scholar]
- Toscano JHB, Lopes LG, Giraldelo LA and Da Silva MH (2018) Identification of appropriate reference genes for local immune-related studies in Morada Nova sheep infected with Haemonchus contortus. Molecular Biology Reports 45, 1253–1262. [DOI] [PubMed] [Google Scholar]
- Tuersong W, He L, Zhu T, Yang X, Zhang Z, Ahmad AA, Di W, Wang C, Zhou C, Liu H, Chen J and Hu M (2020) Development and evaluation of a loop-mediated isothermal amplification (LAMP) assay for the detection of the E198A SNP in the isotype-1 β-tubulin gene of Haemonchus contortus populations in China. Veterinary Parasitology 278, 109040. [DOI] [PubMed] [Google Scholar]
- Wang C, Li F, Zhang Z, Yang X, Ahmad AA, Li X, Du A and Hu M (2017) Recent research progress in China on Haemonchus contortus. Frontiers in Microbiology 8, 1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong TY, Preston LA and Schiller NL (2000) Alginate lyase: review of major sources and enzyme characteristics, structure-function analysis, biological roles, and applications. Annual Review of Microbiology 54, 289–340. [DOI] [PubMed] [Google Scholar]
- Zhu L, Dai J, Li Yang L and Qiu J (2013) Anthelmintic activity of Arisaema franchetianum and Arisaema lobatum essential oils against Haemonchus contortus. Journal of Ethnopharmacology 148, 311–316. [DOI] [PubMed] [Google Scholar]