Abstract
Characterization of virus-specific immune responses to human immunodeficiency virus type 1 (HIV-1) and simian immunodeficiency virus (SIV) is important to understanding the early virus-host interactions that may determine the course of virus infection and disease. Using a comprehensive panel of serological assays, we have previously demonstrated a complex and lengthy maturation of virus-specific antibody responses elicited by attenuated strains of SIV that was closely associated with the development of protective immunity. In the present study, we expand these analyses to address several questions regarding the nature of the virus-specific antibody responses to pathogenic SIV, SIV/HIV-1 (SHIV), and HIV-1 infections. The results demonstrate for the first time a common theme of antibody maturation to SIV, SHIV, and HIV-1 infections that is characterized by ongoing changes in antibody titer, conformational dependence, and antibody avidity during the first 6 to 10 months following virus infection. We demonstrate that this gradual evolution of virus-specific antibody responses is independent of the levels of virus replication and the pathogenicity of the infection viral strain. While the serological assays used in these studies were useful in discriminating between protective and nonprotective antibody responses during evaluation of vaccine efficacy with attenuated SIV, these same assays do not distinguish the clinical outcome of infection in pathogenic SIV, SHIV, or HIV-1 infections. These results likely reflect differences in the immune mechanisms involved in mediating protection from virus challenge compared to those that control an established viral infection, and they suggest that additional characteristics of both humoral and cellular responses evolve during this early immune maturation.
Immune responses to infections with human immunodeficiency virus (HIV) and the closely related simian immunodeficiency virus (SIV) are detected within the first several weeks following infection (25, 26). These responses include the production of virus-specific antibodies and the expansion of virus-specific populations of both CD4+ and CD8+ T cells. While cytotoxic T lymphocytes have been proposed to play an important role in controlling the initial primary viremia (4, 23), vigorous humoral immune responses to several viral antigens are also generated during this primary viremic episode (14, 31). Following this acute stage of infection, HIV type 1 (HIV-1)-infected patients then enter a period of asymptomatic clinical latency during which time the quantitative levels of virus-specific antibodies in the plasma remain high (32). It is during this asymptomatic period that virus-specific immune responses appear to effectively control virus replication (17, 45).
Characterization of the specific immune responses involved in controlling and limiting HIV-1 and SIV virus replication in vivo is important to understanding the early virus-host interactions that may determine the course of virus infection and disease. In addition, these studies can identify the nature of protective immune responses for vaccine development. To date, the most successful vaccines have resulted from experimental inoculation of macaques with naturally or genetically engineered attenuated strains of SIV that establish infection without resulting in clinical signs of disease (10, 12, 27, 35, 46). Infection of monkeys with attenuated virus strains was capable of eliciting immune responses necessary to limit virus infection and disease progression; however, broadly protective immunity was found to be highly dependent on the length of time postinfection, suggesting a necessary maturation of immune responses. This time-dependent ability of monkeys infected with attenuated SIV to control virus replication and disease following experimental challenge with pathogenic SIV provides an ideal model in which to elucidate the protective parameters involved in this immunologic control.
We have previously used a comprehensive panel of serological assays to define a complex and lengthy maturation of viral envelope-specific antibody responses in macaques inoculated with attenuated strains of SIV (11). These studies identified discriminating differences in both the quantitative and qualitative properties of the envelope-specific antibody that in general paralleled the development of protective immunity. During the first 6 to 8 months postinfection, we identified a gradual evolution of envelope-specific antibody responses that was characterized by progressive changes in antibody titer, conformational dependence, and antibody avidity (immature immunity). These virus-specific antibody responses eventually achieved a relatively consistent antibody titer, conformational dependence, and antibody avidity that were maintained indefinitely (mature immunity). In addition to defining a maturation of virus-specific antibody responses, these serological studies described for the first time an association between the effectiveness of an attenuated vaccine and its capacity to produce a mature antibody response, and they further indicated that a combination of several antibody parameters was superior to a single antibody parameter as a prognostic indicator to evaluate candidate vaccines. To complement our studies demonstrating that this lengthy maturation of virus-specific antibodies was common to several different attenuated strains of SIV (including both macrophage-tropic and lymphocyte-tropic attenuated strains), we have also reported a similar maturation of antibody responses in equine infectious anemia virus (EIAV) infection of ponies (16), determined by using these same serological assays. These results suggest that this complex and lengthy maturation of virus-specific antibody responses may be a common property of all lentivirus infections that has profound implications for the design and evaluation of future immunization strategies.
Thus, this study was designed to address several questions: Is the evolution of envelope-specific antibody in a pathogenic SIV infection similar to that observed with an attenuated SIV infection? Are there differences in the nature of the antibody response in a pathogenic SIV infection that can be associated with (or predictive of) the progression to disease? Do we observe a similar maturation of envelope-specific antibody to an HIV-1 envelope when it is expressed in an SIV background (i.e., in an SIV/HIV-1 [SHIV] infection)? Does this complex and lengthy antibody maturation observed in SIV infection of monkeys also occur in HIV-1-infected patients?
MATERIALS AND METHODS
Sources of serum antibody. (i) SIV/B670Tulane-infected macaques.
Rhesus macaques born at the Tulane Regional Primate Research Center were infected intravenously with 10 animal infectious doses of the heterogeneous primary isolate SIV/B670Tulane (34). Following experimental infection, all monkeys were monitored for clinical signs of infection and disease progression as previously described (10). While all monkeys infected with SIV/B670Tulane remained chronically infected (as determined by detectable levels of viral RNA by reverse transcription-PCR), there was a wide spectrum of disease progression evident (32a). In general, the majority of the monkeys experimentally infected with pathogenic SIV/B670Tulane developed clinical signs of disease (determined by high viral loads with consistent virus recovery, CD4+ T-cell decline, and the development of clinical disease) during the first 2 years postinfection and are classified as progressors (N245, M835, N415, and M834). While a small percentage of monkeys infected with SIV/B670Tulane are classified as rapid progressors, developing fatal disease within a few months after infection, these monkeys had no detectable virus-specific antibody responses at any time postinfection and therefore could not be included in the study. In addition, a small percentage of monkeys infected with SIV/B670Tulane remained healthy and asymptomatic for more than 2 years postinfection and are classified as nonprogressors (N033, N129, and N395).
(ii) SHIV-infected macaques.
SHIV DNA encoding the env, tat, rev, and vpu genes of HIV-1/HXBc2 on a background of SIVmac239 was transfected into CEMx174 cells to produce a virus stock that was passaged in macaques as previously described (21, 22). Pigtailed macaques inoculated with passages 2 and 3 (SHIV-P2 and SHIV-P3) of this virus developed simian AIDS. As previously described, virus from a pigtailed macaque inoculated with SHIV-P3 was propagated in peripheral blood mononuclear cells from a naive macaque in vitro and designated SHIVKU-1; SHIVKU-2 was obtained by the in vivo passage of SHIVKU-1 in rhesus macaques. The SHIV-infected monkeys were also divided into two groups: those that progressed to disease as determined by repeated virus recovery and high viral loads, CD4+ T-cell decline, and clinical signs of disease (monkeys 14A, 16C, 19G, and PQc) and those that remained healthy and asymptomatic (monkeys 14B, 19A, and Pfb). As described above for SIV/B670Tulane-infected monkeys, a small percentage of rapid progressors were also observed in this panel of SHIV-infected monkeys. However, since they also failed to make detectable virus-specific antibody responses, they could not be included in this study.
(iii) HIV-1-infected patients.
Longitudinal serum samples from four HIV-1-infected patients were obtained from the University of Alabama at Birmingham (4, 9, 36). FASH and WEAU progressed to disease more rapidly (determined by rapid CD4+ T-cell decline, high viral loads, and the early onset of opportunistic infection following seroconversion), while HOBR and SUMA experienced a more gradual immune decline and onset of disease. These patients did not receive antiviral therapy at any time during the study, and the final time points used in this study do not represent terminal samples for each patient.
For the serological studies described herein, all serum samples were derived from peripheral blood and were heat inactivated at 56°C for 30 min prior to use to inactivate any infectious virus.
Envelope glycoprotein antigens.
The uncloned SIV/B7 is a noninfectious virus produced by a CEMx174 cell line stably infected with a reverse transcriptase-defective variant of SIVsmH3 (24). HIV-1/IIIB was kindly provided by Larry Arthur (National Cancer Institute-Frederick Cancer Research and Development Center, Frederick, Md.) (37). Preparations of SIV/B7 or HIV-1/IIIB were purified by gradient centrifugation as previously described (33), and the protein concentrations were determined by using the Bio-Rad DC protein assay.
Gradient-purified preparations of SIV/B7 or HIV-1/IIIB were disrupted with 1% Triton X-100 (TX) and used as sources of native viral envelope glycoproteins in the concanavalin A (ConA) enzyme-linked immunosorbent assay (ELISA) (38). To produce denatured viral envelope glycoproteins, TX-disrupted gradient-purified virus (SIV/B7 or HIV-1/IIIB) was treated with 0.12 M β-mercaptoethanol and 8 M urea to reduce disulfide bonds and then with iodoacetic acid to irreversibly carboxymethylate the reduced sulfhydryl groups (28). The denatured virus was then dialyzed against phosphate-buffered saline (PBS) and concentrated by spinning through a Centricon filter (Millipore). The final protein concentration was determined using the Bio-Rad DC protein assay.
Measurement of viral envelope glycoprotein-specific antibody endpoint titers by ConA ELISA.
Serum samples from SIV/B670Tulane-infected macaques, SHIV-infected macaques, or HIV-1-infected patients were analyzed for their reactivity to native viral envelope glycoproteins in a ConA ELISA as previously described (11). Briefly, Immulon 2HB plates (Dynex Corporation) coated with ConA (5 μg/well; Vector Laboratories) were used to adsorb the viral envelope glycoproteins from TX-disrupted virus (1 to 3 μg of total viral protein represents approximately 30 to 90 ng of envelope glycoprotein per well). After blocking of the wells with 5% (wt/vol) nonfat dry milk diluted in PBS (BLOTTO), serial twofold-diluted monkey or human serum was added to each well and incubated for 1 h at room temperature. Following extensive washing with PBS, the wells were incubated with peroxidase-conjugated anti-monkey or anti-human immunoglobulin G (IgG; Sigma) for 1 h at room temperature. The wells were again extensively washed with PBS and incubated with TM Blue substrate (Intergen), and color development was achieved by the addition of 1 N sulfuric acid. Antibody reactivity to the ConA-anchored glycoprotein substrate was then determined by measuring the optical density (OD) at 450 nm, using an automated plate reader (Dynatech Laboratories). Endpoint titers are reported as the last serial twofold dilution whose OD was twice that of normal monkey or normal human serum or as an OD of 0.1, whichever value was greater. All endpoint titer values represent at least two independent experiments.
Measurement of viral envelope glycoprotein-specific conformational dependence.
Serum samples were analyzed for conformational dependence by comparing the serum antibody reactivities to native and denatured glycoprotein substrates in a ConA ELISA as previously described (11). Briefly, native or denatured viral envelope glycoproteins were captured onto microtiter plates by using ConA as described above for the endpoint titer ELISA. Test sera, diluted in BLOTTO to produce an OD at 450 nm of 1.0 to 1.5 in the endpoint titer ConA ELISA procedure, were reacted in triplicate wells with either the ConA-anchored native or denatured envelope glycoproteins. Following extensive washing with PBS, the wells were incubated with peroxidase-conjugated anti-monkey or anti-human IgG and developed as described above for the endpoint titer ConA ELISA. A conformation ratio was then calculated from the antibody reactivities to native versus denatured envelope glycoprotein substrates. Thus, the conformation ratio is a direct measure of the conformational dependence of a particular antibody sample (i.e., the larger the conformation ratio above 1.0 the greater the requirement for native envelope glycoprotein structure, while conformation ratios below 1.0 reflect predominant specificity for linear envelope determinants).
Measurement of viral envelope glycoprotein-specific antibody avidity.
The avidity index values of serum antibodies to the native viral envelope were determined by measuring the resistance of serum antibody-envelope glycoprotein complexes in the ConA ELISA to 8 M urea as previously described (11). Briefly, native viral envelope glycoproteins were captured onto microtiter plates by using ConA as described above for the endpoint titer ELISA. All test sera were again diluted in BLOTTO to produce an OD at 450 nm of about 1.0 to 1.5 in the endpoint titer ConA ELISA procedure and were plated in two sets of triplicate wells. Following the serum incubation, triplicate wells were treated in parallel with either PBS alone or a solution of 8 M urea in PBS (three times for 5 min each). Following extensive washing with PBS, the wells were incubated with peroxidase-conjugated anti-monkey or anti-human IgG and developed as described above for the endpoint titer ConA ELISA. The avidity index was then calculated from the ratio of the absorbance value obtained with urea treatment to that observed with PBS treatment multiplied by 100%. Antibodies with avidity index values of <30% are designated low avidity, those with index values between 30 and 50% are designated intermediate avidity, and those with index values >50% are designated high avidity (18).
While measurements of antibody conformational dependence and antibody avidity were performed at the dilution producing an OD at 450 nm of about 1.0 to 1.5 in the endpoint titer ELISA procedure, at least three independent experiments using several different dilutions within this linear range were performed to ensure that the variation in actual values was within 10%.
RESULTS
Evolution of envelope-specific antibody responses in monkeys experimentally infected with pathogenic SIV/B670Tulane.
Previous studies of rhesus macaques infected with attenuated strains of SIV revealed a lengthy maturation of virus-specific antibody responses following infection (11). To determine whether the length of time required to achieve antibody maturation was due to the low levels of virus replication associated with attenuated SIV infections, we sought to characterize the evolution of virus-specific antibody responses in rhesus macaques following infection with a pathogenic virus that replicates to much higher levels in vivo. In general, we were interested in determining whether the time required for antibody maturation following pathogenic SIV infection would occur more rapidly in the presence of higher levels of virus replication in vivo. In addition, we sought to determine whether the evolution of antibody responses differed in progressor and nonprogressor monkeys infected with a pathogenic strain of SIV. For these studies, we used a longitudinal panel of serum samples taken at monthly intervals from rhesus macaques infected with a pathogenic primary isolate SIV/B670Tulane that were determined to be progressors or nonprogressors as defined in Materials and Methods. These serum samples were analyzed for antibody titer, conformational dependence, and antibody avidity by the ConA ELISA procedure.
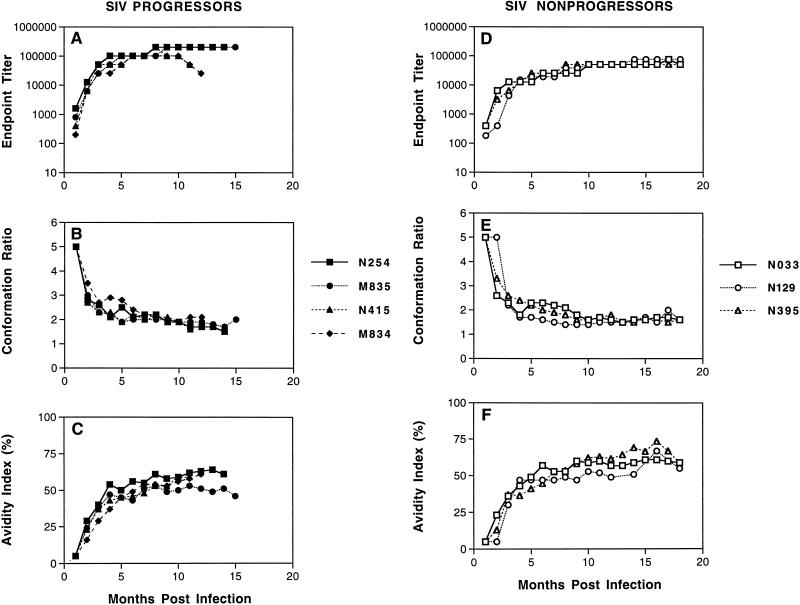
As shown in Fig. 1A, envelope-specific antibodies were detected in SIV/B670Tulane progressors by 1 month postinfection and continued to gradually increase over the first several months, reaching maximum titers ranging from 1:51,200 to 1:102,400 by about 6 months postinfection. Once these maximum endpoint titers were achieved, they remained relatively constant for at least 18 months postinfection. In addition to the quantitative increase observed in serum antibody (endpoint titers), qualitative properties of this SIV-specific antibody response also evolved during the first few months postinfection, as reflected by changes in conformational dependence (Fig. 1B) and antibody avidity (Fig. 1C). While the serum antibody response was preferentially conformational at all time points after infection (indicated by conformation ratios of >1), the conformational dependence of the envelope-specific antibodies rapidly decreased from a high of 5 at 1 month postinfection to an average ratio of about 2 by 6 months postinfection, where it was maintained for at least 18 months postinfection. These changes in conformational dependence were accompanied by changes in antibody avidity, determined by the stability of the envelope antigen-serum antibody complexes to treatment with 8 M urea. Early postinfection, the envelope antigen-serum antibody complexes were predominantly sensitive to 8 M urea, as reflected by the avidity index values of ≤5% at 1 to 2 months postinfection. However, during the first several months postinfection, the avidity index values gradually increased, reaching high values averaging 57% by 6 months postinfection. Once achieved, these levels of high-avidity antibodies were maintained for at least 18 months postinfection. Thus, these results reveal similar maturation of envelope-specific antibody responses during infections with pathogenic and attenuated SIV during the first 6 months postinfection, indicating that the observed immune maturation is evidently not accelerated by higher levels of virus replication and associated higher levels of antigen exposure.
FIG. 1.
Maturation of envelope-specific antibody responses in monkeys infected with SIV/B670Tulane. Longitudinal serum samples were obtained from rhesus macaques at the indicated times after infection with SIV/B670Tulane. (A and C) The reciprocal endpoint titer was determined by measuring the serum reactivity to native viral envelope glycoproteins in the ConA ELISA. (B and D) The conformational dependence of serum antibodies to SIV envelope glycoproteins was determined by measuring the serum reactivity to native viral glycoproteins compared to that of denatured viral glycoproteins in the ConA ELISA. (C and F) The avidity of serum antibodies for SIV envelope glycoproteins was determined by measuring the resistance of serum antibody-envelope glycoprotein immune complexes to disruption by treatment with 8 M urea in the ConA ELISA. All three techniques are described in Materials and Methods.
We next determined whether the maturation of virus-specific antibody to pathogenic SIV/B670Tulane infection differed between monkeys that progressed to disease and those that remained asymptomatic. To characterize the evolution of virus-specific antibody in the small percentage of nonprogressor monkeys infected with SIV/B670Tulane, endpoint titer, conformational dependence, and antibody avidity were analyzed by the ConA ELISA. As with progressor sera, we observed detectable envelope-specific antibodies by 1 month postinfection that continued to gradually increase over the first several months, reaching maximum titers ranging from 1:51,200 to 1:102,400 by 4 to 6 months postinfection (Fig. 1D). Once these maximum endpoint titers were achieved, they also remained relatively constant for at least 18 months postinfection. In addition to changes in the quantitative levels of virus-specific antibody, changes in conformational dependence (Fig. 1E) and antibody avidity (Fig. 1F) were also evident during the first several months postinfection. While the serum antibody response again remained preferentially conformational at all time points after infection, the conformational dependence of the envelope-specific antibodies rapidly decreased from a high of 5 at 1 month postinfection to an average ratio of 1.8 by 4 to 6 months postinfection, where it was maintained for at least 18 months postinfection. Finally, antibody avidity gradually increased from extremely low avidity index values of ≤5% at 1 to 2 months postinfection to high values averaging 53% by 6 months postinfection. Once achieved, levels of these high-avidity antibodies were also maintained for at least 18 months postinfection.
In general, the pattern of antibody maturation observed in the panel of nonprogressors was identical to that observed in progressors, indicating that these serological assays do not predict disease progression. The lack of correlation between immune maturation and disease progression is in distinct contrast to the close association previously reported between immune maturation and protection from virus exposure in monkeys inoculated with attenuated SIV strains (11). This difference may reflect an important distinction between immune control of an existing SIV infection and virus exposure.
Evolution of envelope-specific antibody responses in monkeys infected with pathogenic SHIV.
To determine whether the relatively slow antibody maturation observed in both attenuated and pathogenic SIV infections was unique to SIV envelope antigens, we characterized the evolution of virus-specific antibody responses to the HIV-1 envelope expressed in the context of a SHIV chimeric virus infection of monkeys. For these studies, we used a panel of monkeys infected with various strains of pathogenic SHIV (22). Once again we analyzed longitudinal serum samples from monkeys infected with pathogenic SHIV that were determined to be progressors or nonprogressors as defined in Materials and Methods.
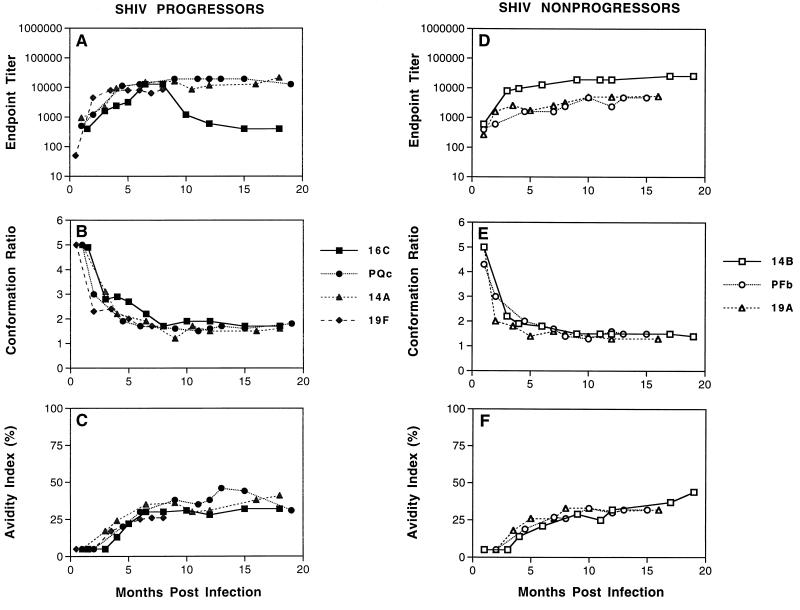
As shown in Fig. 2, quantitative and qualitative changes in HIV-1 envelope-specific serum antibody were evident during the first several months postinfection in both groups of SHIV-infected monkeys. Serum antibodies directed against the HIV-1 envelope were detectable in all monkeys by 1 month postinfection, and these antibody titers gradually increased during the first 6 months postinfection, reaching maximum titers averaging from 1:6,000 to 1:10,000. Once maximum titers were achieved, they were maintained at relatively constant levels for at least 18 months postinfection for both progressors (Fig. 2A) and nonprogressors (Fig. 2D). Similar to the antibody maturation observed in SIV-infected monkeys, qualitative changes in antibody conformational dependence (Fig. 2B and E) and antibody avidity (Fig. 2C and F) were also observed during this period. All SHIV-infected monkeys displayed serum antibodies that were predominantly conformational at all time points postinfection (conformation ratios of >1). However, the conformational dependence of the envelope-specific antibodies rapidly decreased from a high of 5 in both groups of SHIV-infected monkeys at 1 month postinfection to an average steady state ratio of about 1.5 both for progressors and nonprogressors by 4 to 6 months postinfection. In addition, changes in antibody avidity were also evident during the first several months postinfection. While extremely low avidity index values of ≤5% were evident at 1 to 2 months postinfection, antibody avidity values gradually increased during the first 10 months postinfection, reaching maximum intermediate values of 30 and 35% for the progressors and nonprogressors, respectively.
FIG. 2.
Maturation of envelope-specific antibody responses in monkeys infected with SHIV. Longitudinal serum samples were obtained from rhesus macaques at the indicated times after infection with recombinant SHIV. (A and C) The reciprocal endpoint titer was determined by measuring the serum reactivity to native viral envelope glycoproteins in the ConA ELISA. (B and D) The conformational dependence of serum antibodies to HIV-1 envelope glycoproteins was determined by measuring the serum reactivity to native viral glycoproteins compared to that of denatured viral glycoproteins in the ConA ELISA. (C and F) The avidity of serum antibodies for HIV-1 envelope glycoproteins was determined by measuring the resistance of serum antibody-envelope glycoprotein immune complexes to disruption by treatment with 8 M urea in the ConA ELISA. All three techniques are described in Materials and Methods.
These data demonstrate a gradual maturation of HIV-1 envelope-specific serum antibodies in response to SHIV infection during the first several months postinfection characterized by increasing antibody endpoint titer, decreasing conformational dependence, and increasing antibody avidity. While the absolute values and length of time postinfection to reach antibody maturation were slightly different from those observed in attenuated or pathogenic SIV infections, the proposed model that virus-specific antibody requires about 6 months postinfection to fully mature is strikingly similar between the two viruses despite different origins of the viral envelope. Also as observed in SIV infections, the evolution of HIV-1 envelope-specific serum antibodies in SHIV infections appears to be independent of the clinical course, as evidenced by similar antibody maturation in SHIV-infected monkeys that progressed to disease compared and those that remained asymptomatic during the first 18 months postinfection.
Evolution of envelope-specific antibody responses in HIV-1-infected patients.
Having defined similar maturation of envelope-specific antibodies to several SIV strains and to the HIV-1 envelope in the context of a SHIV infection, it was of particular interest to further these studies of antibody maturation by looking at the evolution of viral envelope-specific serum antibodies in patients infected with HIV-1. In particular, we were interested in determining whether this model of antibody maturation would hold true for the human immune system as it encountered a diverse quasispecies of primary HIV-1 strains present in the natural infection. For these studies, we obtained longitudinal serum samples from four HIV-1-infected patients. Once again, analyses of antibody endpoint titer, conformational dependence, and antibody avidity were performed by using the ConA ELISA.
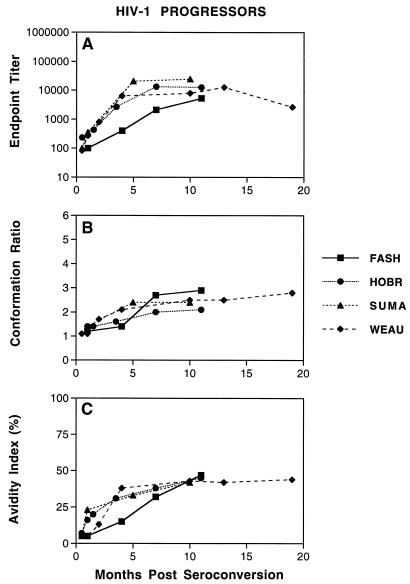
Similar to the observations described for attenuated and pathogenic SIV and SHIV infections, we observed a gradual maturation of envelope-specific antibody responses in serum from HIV-1-infected patients that was characterized by ongoing changes in antibody endpoint titers, conformational dependence, and antibody avidity. As shown in Fig. 3A, serum antibodies to the HIV-1 envelope were detected in all patients by 1 to 2 months following seroconversion. These antibody titers gradually increased, reaching average maximum titers of about 1:6,400 by 6 months after seroconversion. These changes in the quantitative measure of antibody endpoint titers were accompanied by changes in conformational dependence (Fig. 3B) and antibody avidity (Fig. 3C) during the first several months following seroconversion. Once again, serum antibody directed against the HIV-1 envelope remained predominantly conformational at all times, as evidenced by conformation ratios of >1. All of the patients demonstrated a gradual increase in the conformational dependence during the first 6 months after seroconversion; conformation ratios averaged 1.3 at 1 to 2 months after seroconversion, increasing to an average of 2.4 by 6 months after seroconversion. The changes in conformational dependence were accompanied by changes in antibody avidity (Fig. 3C). Low avidity index values of ≤5% were evident during the first month after seroconversion but increased to maximum intermediate values averaging 44% by about 10 months after seroconversion. It is interesting that all four patients demonstrated sequential increases in antibody avidity accompanied by increases in antibody conformational dependence over the same time period. These data indicate the absence of a simple inverse correlation between serum antibody avidity and conformational dependence, as suggested by Binley et al. (3) using a different avidity assay (2).
FIG. 3.
Maturation of envelope-specific antibody responses in HIV-1-infected patients. Longitudinal serum samples were obtained from HIV-1-infected patients at the indicated times following seroconversion. (A) The reciprocal endpoint titer was determined by measuring the serum reactivity to native viral envelope glycoproteins in the ConA ELISA. (B) The conformational dependence of serum antibodies to HIV-1 envelope glycoproteins was determined by measuring the serum reactivity to native viral glycoproteins compared to that of denatured viral glycoproteins in the ConA ELISA. (C and F) The avidity of serum antibodies for HIV-1 envelope glycoproteins was determined by measuring the resistance of serum antibody-envelope glycoprotein immune complexes to disruption by treatment with 8 M urea in the ConA ELISA. All three techniques are described in Materials and Methods.
In general, the serological analyses demonstrate a gradual maturation of HIV-1 envelope-specific serum antibodies in response to HIV-1 infection during the first several months postinfection (reflected by ongoing changes in antibody endpoint titer, conformational dependence, and antibody avidity) that is similar, but not identical, with the progression of serum antibodies from SIV- or SHIV-infected monkeys. While a maturation of antibody responses was observed, these results suggest that this complex and lengthy maturation of virus-specific antibody responses observed in SIV, SHIV, and HIV-1 infections may be common to all lentivirus infections.
DISCUSSION
We previously described a complex and lengthy maturation of envelope-specific antibody responses to attenuated SIV infection that is characterized by progressive changes in antibody endpoint titers, conformational dependence, and antibody avidity during the first 6 to 8 months postinfection (11). Once achieved, these mature antibody responses are maintained indefinitely and, together with cellular immune responses to the same, are associated with the development of protective immunity to experimental virus challenge. To further these studies, the present study was designed to address several specific questions regarding the nature of the virus-specific antibody responses to SIV, SHIV, and HIV-1 infections. In particular, we were interested in determining whether this complex maturation of virus-specific antibody responses was unique to attenuated SIV infections of monkeys or a common property of all lentivirus infections. The results of these studies identified several new important findings: (i) the gradual evolution of envelope-specific antibodies to SIV in infected rhesus macaques is similar, regardless of the replication properties of the infecting viral strain; (ii) the lengthy maturation of envelope-specific antibodies in rhesus macaques is similar, despite the origin of the envelope (i.e., SIV or HIV-1); (iii) antibody maturation of envelope-specific antibodies is common to SIV or SHIV infections of monkeys and HIV-1 infection of humans, although the time and pattern of antibody evolution may differ; and (iv) the pattern of early antibody maturation does not appear to distinguish the long-term clinical outcome of virus infection (progressors versus nonprogressors).
Our initial studies focused on the characterization of envelope-specific antibody responses to pathogenic SIV infection. The relatively gradual maturation of envelope-specific antibody responses to SIV infection despite the presence of constant immune stimulation is in distinct contrast to the rapid maturation of the antibody response observed in other viral infections (39, 47) and raises several questions regarding the capacity of the immune system to mount an effective response to lentiviruses. Previous studies from our lab indicated that SIV envelope sequence variation was not required for maturation of the antibody response (10). We previously hypothesized that the gradual maturation of virus-specific antibody responses to attenuated SIV infections may reflect the extremely low levels of virus replication in vivo (11). However, the results presented here demonstrate that the increased levels of virus replication did not accelerate the antibody maturation process, since strikingly similar antibody maturation to SIV/B670Tulane infection was observed in monkeys irrespective of whether the rate of virus replication in the animals was rapid or slow. Instead, these results suggest a requirement only for continuous presence of viral antigens to achieve maturation of antibody responses. Since maturation of the antiviral antibody response is one of the factors associated with the development of immune protection against virulent virus (10, 11), these findings indicate that effective vaccines to SIV or HIV may require strategies that facilitate prolonged presentation of viral antigen to ensure sufficient maturation of antibody responses. In this regard, vaccines consisting of attenuated live viruses or viral DNA appear to be optimum candidates.
Several immunological parameters, including differences in the quantitative amounts of antibodies to various viral proteins (3, 14, 19, 41, 44, 46) and the qualitative differences in virus-specific antibodies, such as neutralizing antibodies (1, 6, 15, 20, 29, 30, 44, 46), and in the binding avidities of the antibodies (5, 7, 8, 13, 40, 42) have been reported to be useful indicators for disease progression in HIV-1 and SIV infections. We have previously reported that the combination of serological antibody parameters (e.g., antibody conformation and avidity) used in the present study were useful in discriminating between protective and nonprotective antibody responses when one is evaluating candidate subunit and attenuated SIV vaccines (10, 11). In contrast to the correlation between immune maturation and protection from virus challenge, results of the present study indicate that the serological parameters that define immune maturation do not discriminate between progressors and nonprogressors in either the SIV or the SHIV system. These differences likely reflect critical differences in immune mechanisms that mediate protection from virus challenge and those that control an established viral infection. For example, immune responses in monkeys inoculated with attenuated SIV strains provide protection from virus challenge but do not completely suppress or eliminate replication of the vaccine strain. Therefore, it is apparently much easier to achieve immune protection against challenge virus than to accomplish immune control of an established lentivirus infection. In light of these intrinsic differences, the antibody responses found in nonprogressors may be an inappropriate and perhaps unnecessarily rigorous marker for vaccine efficacy against HIV-1.
In addition to the maturation of virus-specific antibodies to SIV envelope antigens, this study revealed for the first time similar patterns of evolution of envelope-specific antibodies in response to SHIV infection during the first several months postinfection. While the antibody maturation observed in SHIV-infected monkeys was in general similar to that of SIV-infected monkeys, some interesting differences in the progression of antibody avidity were observed. First, the maturation of antibody avidity was a longer process for SHIV-infected monkeys, averaging 10 months compared to only 6 months for SIV-infected monkeys. This delay was particularly interesting since the antibody endpoint titer and conformational dependence reached mature levels by 6 months postinfection in these same SHIV-infected monkeys, 4 months ahead of avidity maturation. A second difference between SIV and SHIV antibody maturation patterns is that mature SHIV-specific antibody reached only intermediate avidity values compared to the high-avidity antibody observed with SIV-infected monkeys. Since the serological measurement of antibody avidity differentiates polyclonal serum antibody populations based on the relative stability of the antigen-antibody interactions to a particular chemical treatment, avidity values represent an average of numerous variables, including antibody affinity, conformational dependence, and the molecular nature of the antigen-antibody interaction (43). Thus, the absolute avidity value associated with completed maturation of antibody responses may reflect differences in viral envelope antigenicity in vitro or immunogenicity in vivo. Additional studies are required to distinguish between these two possibilities.
Finally, we demonstrate here for the first time an evolution of envelope-specific antibody responses in HIV-1-infected patients that in general closely resembles the maturation process observed in SIV and SHIV infections of monkeys. While all three lentivirus infections were characterized by increases in antibody titer and antibody avidity during immune maturation, it is interesting that all four HIV-1-infected patients displayed increasing antibody conformational dependence, in contrast to the decreasing conformational dependence associated with SIV and SHIV infections of monkeys. This pattern of increasing antibody conformational dependence prevalent in HIV-1 infections was previously observed with immune maturation to EIAV infection of horses (16). Taken together, the results of our studies in the EIAV, SIV, SHIV, and HIV-1 infection systems indicate that a relatively complex and lengthy maturation of antibody responses is a common feature of lentivirus infections.
As with all serological assays, the results of this study need to be interpreted with the understanding that we are measuring only a very small number of antibody properties. It is likely that many other, and perhaps more important, changes in antibody populations as well as cellular immune responses occur during the initial maturation of immune responses to a lentivirus infection. Novel humoral and cellular immunoassays are needed to more thoroughly define the evolution of responses to lentivirus infections, to identify immune mediators of protection, and to define immune correlates of protection.
ACKNOWLEDGMENTS
K.S.C., R.C.M., and M.M.-C. were supported by NIH grant UO1 AI28243; K.S.C. was supported by NIH grant T32 AI07487; O.N. and S.V.J. were supported by NIH grants RO1 AI40372, RO1 AI38492, and RR06753.
REFERENCES
- 1.Beer B, Norley S, Cichutek K, Kurth R. Control of initial viremia coincides with neutralizing antibody response after SIVmac infection of rhesus macaques. AIDS. 1996;10:681–682. doi: 10.1097/00002030-199606000-00018. [DOI] [PubMed] [Google Scholar]
- 2.Binley J M, Arshad H, Fouts T R, Moore J P. An investigation of the high-avidity antibody response to glycoprotein 120 of human immunodeficiency virus type 1. AIDS Res Hum Retroviruses. 1997;13:1007–1015. doi: 10.1089/aid.1997.13.1007. [DOI] [PubMed] [Google Scholar]
- 3.Binley J M, Klasse P J, Cao Y, Jones I, Markowitz M, Ho D D, Moore J P. Differential regulation of the antibody responses to Gag and Env proteins of human immunodeficiency virus type 1. J Virol. 1997;71:2799–2809. doi: 10.1128/jvi.71.4.2799-2809.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borrow O, Lewicki H, Hahn B H, Shaw G M, Oldstone M B A. Virus-specific CD8+ T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol. 1994;68:6103–6110. doi: 10.1128/jvi.68.9.6103-6110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brostrom C, Sonnerberg A, Sallberg M. Human immunodeficiency virus type-1 infected patients with no disease progression display high avidity antibody to autologous V3 sequences. J Infect Dis. 1995;171:509–511. doi: 10.1093/infdis/171.2.509. [DOI] [PubMed] [Google Scholar]
- 6.Cao Y, Qin L, Zhang L, Safrit J, Ho D D. Virologic and immunologic characterization of long-term survivors of human immunodeficiency virus type 1 infection. N Engl J Med. 1995;332:201–208. doi: 10.1056/NEJM199501263320401. [DOI] [PubMed] [Google Scholar]
- 7.Chargelegue D, O’Toole C M, Colvin B T. A longitudinal study of the IgG antibody response to HIV-1 p17 gag protein in HIV-1+ patients with haemophilia: titre and avidity. Clin Exp Immunol. 1993;93:331–336. doi: 10.1111/j.1365-2249.1993.tb08181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chargelegue D, Stanley C M, O’Toole C M, Colvin B T, Steward M W. The absence or loss of antibodies of high affinity to human immunodeficiency virus (HIV) is associated with disease progression in HIV-1 infected patients. J Infect Dis. 1995;172:897. doi: 10.1093/infdis/172.3.897. [DOI] [PubMed] [Google Scholar]
- 9.Clark S J, Saag M S, Decker W D, Campbell-Hill S, Roberson J L, Veldkamp P J, Kappes J C, Hahn B H, Shaw G M. High titres of cytopathic virus in plasma of patients with symptomatic primary HIV-1 infection. N Engl J Med. 1991;324:954–960. doi: 10.1056/NEJM199104043241404. [DOI] [PubMed] [Google Scholar]
- 10.Clements J E, Montelaro R C, Zink M C, Amadee A M, Miller S, Trichel A M, Jagerski B, Hauer D, Martin L N, Bohm R P, Murphey-Corb M. Cross-protective immune responses induced in rhesus macaques by immunization with an attenuated macrophage-tropic simian immunodeficiency virus. J Virol. 1995;69:2737–2744. doi: 10.1128/jvi.69.5.2737-2744.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cole K S, Rowles J L, Murphey-Corb M, Clements J E, Unangst T, Robinson J, Desrosiers R C, Wyand M S, Montelaro R C. Evolution of envelope-specific antibody responses in monkeys experimentally infected or immunized with SIV and its association with the development of protective immunity. J Virol. 1997;71:5069–5079. doi: 10.1128/jvi.71.7.5069-5079.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daniel M D, Kirchhoff F, Czajak S C, Sehgal P K, Desrosiers R C. Protective effects of a live attenuated SIV vaccine with a deletion in the nef gene. Science. 1992;258:1938–1941. doi: 10.1126/science.1470917. [DOI] [PubMed] [Google Scholar]
- 13.Devash Y, Calvelli T, Wood D, Reagan K, Rubinstein A. Vertical transmission of HIV-1 is correlated with the absence of high affinity/avidity maternal antibodies to the gp120 principal neutralizing domain. Proc Natl Acad Sci USA. 1990;87:3445–3449. doi: 10.1073/pnas.87.9.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fenouillet E, Blanes N, Coutellier A, Gluckman J C. Monitoring of antibodies against human immunodeficiency virus type 1 p25 core protein as a prognostic indicator. J Infect Dis. 1993;166:1011–1018. doi: 10.1093/infdis/166.3.611. [DOI] [PubMed] [Google Scholar]
- 15.Haigwood N L, Watson A, Sutton W F, McClure J, Lewis A, Ranchalis J, Travis B, Voss G, Letvin N L, Hu S-L, Hirsch V M, Johnson P R. Passive immune globulin therapy in the SIV/macaque model: early intervention can alter disease profile. Immunol Lett. 1996;51:107–114. doi: 10.1016/0165-2478(96)02563-1. [DOI] [PubMed] [Google Scholar]
- 16.Hammond S A, Cook S J, Lichtenstein D L, Issel C J, Montelaro R C. Maturation of the cellular and humoral immune responses to persistent infection in horses by equine infectious anemia virus is a complex and lengthy process. J Virol. 1997;71:3840–3852. doi: 10.1128/jvi.71.5.3840-3852.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haynes B F G, Pantaleo G, Fauci A S. Toward an understanding of the correlates of protective immunity to HIV infection. Science. 1996;271:324–328. doi: 10.1126/science.271.5247.324. [DOI] [PubMed] [Google Scholar]
- 18.Hedman K, Hietala J, Tiilikainen A, Hartikainen-Sorri A-L, Raiha K, Suni J, Vaananen P, Pietilainen M. Maturation of immunoglobulin G avidity after rubella vaccination studied by an enzyme linked immunosorbent assay (avidity-ELISA) and by haemolysis typing. J Med Virol. 1989;27:293–298. doi: 10.1002/jmv.1890270407. [DOI] [PubMed] [Google Scholar]
- 19.Hogervorst E, Jurrians S, deWolf E, vanWijk A, Wiersma A, Valk M, Roos M, vanGemen B, Coutinho R, Miedema F, Goudsmit J. Predictors for non- and slow-progression in human immunodeficiency virus (HIV) type 1 infection: low viral RNA copy numbers in serum and maintenance of high HIV-1 p24-specific but not V3-specific antibody levels. J Infect Dis. 1995;171:811–821. doi: 10.1093/infdis/171.4.811. [DOI] [PubMed] [Google Scholar]
- 20.Homsey J, Meyer M, Levy J A. Serum enhancement of human immunodeficiency virus (HIV) correlates with disease in HIV-infected individuals. J Virol. 1990;64:1437–1440. doi: 10.1128/jvi.64.4.1437-1440.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joag S V, Li Z, Foresman L, Stephens E B, Zhao L J, Adany I, Pinson D M, McClure H M, Narayan O. Chimeric simian/human immunodeficiency virus that causes progressive loss of CD4+ T cells and AIDS in pig-tailed macaques. J Virol. 1996;70:3189–3197. doi: 10.1128/jvi.70.5.3189-3197.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joag S V, Li Z, Foresman L, Pinson D M, Raghavan R, Zhuge W, Adany I, Wang C, Jia F, Sheffer D, Ranchalis J, Watson A, Narayan O. Characterization of the pathogenic KU-SHIV model of acquired immunodeficiency syndrome in macaques. AIDS Res Hum Retroviruses. 1997;13:635–645. doi: 10.1089/aid.1997.13.635. [DOI] [PubMed] [Google Scholar]
- 23.Koup R A, Safrit J T, Cao Y, Andrews C A, McLeod G, Borkowsky W, Farthing C, Ho D D. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kraiselburd, E. N., and J. V. Torres. 1995. Properties of virus-like particles produced by SIV-chronically infected human cell clones. Cell. Mol. Biol. 41(Suppl. 1):S41–S52. [PubMed]
- 25.Levy J A. HIV and the pathogenesis of AIDS. Washington, D.C: ASM Press; 1998. pp. 253–284. [Google Scholar]
- 26.Levy J A. HIV and the pathogenesis of AIDS. Washington, D.C: ASM Press; 1998. pp. 229–252. [Google Scholar]
- 27.Lohman B L, McChesney M B, Miller C J, McGowan E, Joye S M, Van Rompay K A, Reay E, Antipa L, Pedersen N C, Marthas M L. A partially attenuated simian immunodeficiency virus induces host immunity that correlates with resistance to pathogenic virus challenge. J Virol. 1994;68:7021–7029. doi: 10.1128/jvi.68.11.7021-7029.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Means G E, Feeney R E. Chemical modifications of proteins. San Francisco, Calif: Holden-Day, Inc.; 1971. pp. 216–222. [Google Scholar]
- 29.Montefiori D C, Baba T W, Li A, Bilska M, Ruprecht R M. Neutralizing and infection-enhancing antibody responses do not correlate with the differential pathogenicity of SIV mac239delta3 in adult and infant rhesus monkeys. J Immunol. 1996;157:5528–5535. [PubMed] [Google Scholar]
- 30.Moog C, Fleury H J A, Pellegrin I, Kirn A, Aubertin A M. Autologous and heterologous neutralizing antibody responses following initial seroconversion in human immunodeficiency virus type 1-infected individuals. J Virol. 1997;71:3734–3741. doi: 10.1128/jvi.71.5.3734-3741.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moore J P, Cao Y, Ho D D, Koup R A. Development of the anti-gp120 antibody response during seroconversion to human immunodeficiency virus type 1. J Virol. 1994;68:5142–5155. doi: 10.1128/jvi.68.8.5142-5155.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moore J P, Ho D D. Antibodies to discontinuous or conformationally sensitive epitopes on the gp120 glycoprotein of human immunodeficiency virus type 1 are highly prevalent in sera of infected humans. J Virol. 1993;67:863–875. doi: 10.1128/jvi.67.2.863-875.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32a.Murphey-Corb, M. Unpublished data.
- 33.Murphey-Corb M, Martin L N, Davison-Fairburn B, Montelaro R C, Miller M, West M, Ohkawa S, Baskin G B, Zhang J-Y, Putney S D, Allison A C, Eppstein D A. A formalin-inactivated whole SIV vaccine confers protection in macaques. Science. 1989;246:1293–1297. doi: 10.1126/science.2555923. [DOI] [PubMed] [Google Scholar]
- 34.Murphey-Corb M, Martin L N, Rangan S R S, Baskin G B, Gormus B J, Wolf R H, West W A, Amdes M, Montelaro R C. Isolation of an HTLV-III-related retrovirus from macaques with simian AIDS and its possible origin in asymptomatic mangabeys. Nature. 1986;321:435–437. doi: 10.1038/321435a0. [DOI] [PubMed] [Google Scholar]
- 35.Norley S, Beer B, Binninger-Schinzel D, Cosma C, Kurth R. Protection from pathogenic SIVmac challenge following short-term infection with a nef-deficient attenuated virus. Virology. 1996;219:195–205. doi: 10.1006/viro.1996.0237. [DOI] [PubMed] [Google Scholar]
- 36.Piatak M, Saag M S, Yang L C, Clark S J, Kappes J C, Luk K C, Hahn B H, Shaw G M, Lifson J D. High levels of HIV-1 in plasma during all stages of infection determined by competitive PCR. Science. 1993;259:1749–1754. doi: 10.1126/science.8096089. [DOI] [PubMed] [Google Scholar]
- 37.Popovic M, Sarngadharan M G, Read E, Gallo R C. Detection, isolation and continuous production of cytopathic retroviruses (HTLV III) from patients with AIDS and pre-AIDS. Science. 1984;224:497–500. doi: 10.1126/science.6200935. [DOI] [PubMed] [Google Scholar]
- 38.Robinson J E, Holton D, Liu J, McMurdo H, Murciano A, Gohd R. A novel enzyme-linked immunosorbent assay (ELISA) for the detection of antibodies to HIV-1 envelope glycoproteins based on immobilization of viral glycoproteins in microtiter wells coated with concanavalin A. J Immunol Methods. 1990;132:63–71. doi: 10.1016/0022-1759(90)90399-g. [DOI] [PubMed] [Google Scholar]
- 39.Salmi A A. Antibody affinity and protection in virus infections. Curr Opin Immunol. 1991;3:503–506. doi: 10.1016/0952-7915(91)90011-o. [DOI] [PubMed] [Google Scholar]
- 40.Simon F, Rahimy C, Krivine A, Levine M, Pepin J M, Lapierre D, Denamur E, Vernoux L, DeCrepy A, Blot P, Vilmer E, Brun-Vezinet F. Antibody avidity measurement and immune complex dissociation for serological diagnosis of vertically acquired HIV-1 infection. J Acquired Immune Defic Syndr. 1993;6:201–207. [PubMed] [Google Scholar]
- 41.Strathdee S A, Frank J W, McLaughlin J, Leblanc M, Major C, O’Shaughnessy M V, Read S E. Quantitative measures of human immunodeficiency virus-specific antibodies predict progression to AIDS. J Infect Dis. 1995;172:1375–1379. doi: 10.1093/infdis/172.5.1375. [DOI] [PubMed] [Google Scholar]
- 42.Thomas H I J, Wilson S, O’Toole C M, Lister C M, Saeed A M, Watkins R P F, Morgan-Capner P. Differential maturation of avidity of IgG antibodies to gp41, p24 and p17 following infection with HIV-1. Clin Exp Immunol. 1996;103:185–191. doi: 10.1046/j.1365-2249.1996.951642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Underwood P A. Measurement of the affinity of antiviral antibodies. Adv Virus Res. 1988;34:283–309. doi: 10.1016/s0065-3527(08)60521-7. [DOI] [PubMed] [Google Scholar]
- 44.Weber J N, Clapham P R, Weiss R A, Parker D, Roberts C, Duncan J, Weller I, Carne C, Tedder R S, Pinching A J, Cheingsong-Popov R. Human immunodeficiency virus infection in two cohorts of homosexual men: neutralizing sera and association of anti-GAG antibody with prognosis. Lancet. 1987;i:119–121. doi: 10.1016/s0140-6736(87)91964-7. [DOI] [PubMed] [Google Scholar]
- 45.Wei X, Ghosh S K, Taylor M E, Johnson V A, Emini E A, Deutsch P, Lifson J D, Bonhoeffer S, Nowak M A, Hahn B H, Saag M S, Shaw G M. Viral dynamics in human immunodeficiency virus type 1 infection. Nature. 1995;373:117–122. doi: 10.1038/373117a0. [DOI] [PubMed] [Google Scholar]
- 46.Wyand M S, Manson K H, Garcia-Moll M, Montefiori D, Desrosiers R C. Vaccine protection by a triple deletion mutant of simian immunodeficiency virus. J Virol. 1996;70:3724–3733. doi: 10.1128/jvi.70.6.3724-3733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zinkernagel R M. Immunology taught by viruses. Science. 1996;271:173–178. doi: 10.1126/science.271.5246.173. [DOI] [PubMed] [Google Scholar]