Abstract
Background:
Pragmatic trials are increasingly recognized for providing real-world evidence on treatment choices.
Objective:
The objective of this study is to investigate the use and characteristics of pragmatic trials in multiple sclerosis (MS).
Methods:
Systematic literature search and analysis of pragmatic trials on any intervention published up to 2022. The assessment of pragmatism with PRECIS-2 (PRagmatic Explanatory Continuum Indicator Summary-2) is performed.
Results:
We identified 48 pragmatic trials published 1967–2022 that included a median of 82 participants (interquartile range (IQR) = 42–160) to assess typically supportive care interventions (n = 41; 85%). Only seven trials assessed drugs (15%). Only three trials (6%) included >500 participants. Trials were mostly from the United Kingdom (n = 18; 38%), Italy (n = 6; 13%), the United States and Denmark (each n = 5; 10%). Primary outcomes were diverse, for example, quality-of-life, physical functioning, or disease activity. Only 1 trial (2%) used routinely collected data for outcome ascertainment. No trial was very pragmatic in all design aspects, but 14 trials (29%) were widely pragmatic (i.e. PRECIS-2 score ⩾ 4/5 in all domains).
Conclusion:
Only few and mostly small pragmatic trials exist in MS which rarely assess drugs. Despite the widely available routine data infrastructures, very few trials utilize them. There is an urgent need to leverage the potential of this pioneering study design to provide useful randomized real-world evidence.
Keywords: Multiple sclerosis, pragmatic clinical trial, randomized controlled trial, routinely collected health data
Background
With the emergence of new treatment options in routine care, persons with multiple sclerosis (pwMS) and their caregivers increasingly have a choice between various therapeutic strategies. This requires solid evidence to inform decisions in daily routine. Pragmatic trials are randomized clinical trials designed to directly inform treatment decisions in practice. 1 They are a key element of real-world evidence 2 by leveraging the increasingly available sources of routinely collected data and overcoming the traditional shortcomings of non-randomized real-world evidence.3,4 Conversely to traditional trials, they compare real-world care alternatives rather than testing experimental interventions under development, they deliver interventions such as drugs without measures that would never occur in practice to increase the adherence to them, and they use comparators that do exist in routine care, not such as placebo controls. They primarily consider health-related, patient-relevant outcomes, as these are critical to decision-making, rather than focusing on more mechanistic outcomes such as biomarkers which are more relevant for research and development of treatments. Pragmatic trials aim to answer the question what the best treatment choice is, conversely to traditional trials that investigate the interventions themselves.
Most recently, the COVID-19 pandemic has brought back memories of the great medical progress made in the 1980s with large pragmatic trials in cardiology.1,5,6 Such “large simple trials”7,8 were the blueprint for the now legendary RECOVERY trial, 9 which rapidly delivered the first drug with proven survival benefit for COVID-19 during the pandemic. This trial, designed in a few days and conducted with unprecedented speed, recruiting more than 10,000 patients in 3 months, has reinvigorated the idea of pragmatic trials.
These lessons and opportunities raise the question of where we stand in other areas—including in MS. The ability to leverage real-world data infrastructure and overcoming laborious, costly, and often slow data collection procedures has been identified as key to generate “randomized real-world evidence.” 10 MS is carefully studied in numerous international observational studies based on excellent routine data infrastructures11,12 making this field another prime candidate for pragmatic trials. We aimed to systematically investigate to what extent such conditions may have led to pragmatic trials being conducted in MS, what characteristics they have, and which clinical questions they aim to answer.
Methods
Design
We generated a systematic overview of the pragmatic randomized trials research landscape in MS and followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. 13
Eligibility criteria and assessment of pragmatism
We included any pragmatic randomized trial assessing any intervention and comparator on any health-related outcome in pwMS that reports on trial findings and was published as a journal article in English language with no restriction of the publication year. We considered trials that randomized participants individually (i.e. individuals were randomly assigned to an intervention) or per cluster (i.e. a group of individuals, e.g. communities or hospital ward, was randomly assigned to an intervention).
To determine if trials are “pragmatic,” we assessed the pragmatism of potentially eligible trials with the PRragmatic–Explanatory Continuum Indicator Summary-2 (PRECIS-2). PRECIS-2 has nine domains that can impact the pragmatism (eligibility criteria, recruitment, setting, organization, flexibility in delivery of the intervention, flexibility in adherence of the intervention, follow-up, primary outcome, and primary analysis; Table 1). 14 Each domain was independently assessed by two reviewers (J.H. and P.J., G.J.N., K.D., T.V.T.N., or L.G.H.) based on a 5-point scale (according to PRECIS-2 categories, i.e. 1 = very explanatory, 2 = rather explanatory, 3 = equally pragmatic and explanatory, 4 = rather pragmatic, 5 = very pragmatic, or no information). Trials were considered “pragmatic” if none of the PRECIS-2 domains was assessed below 3. We skipped the PRECIS-2 assessment and directly excluded trials with a clearly explanatory design feature (i.e. blinding, placebo-controlled), trials with a crossover design (i.e. the way how interventions are delivered may not be true care options), and self-labeled feasibility trials when the intervention was under development and the trials primarily intended to assess process-related outcomes (e.g. intervention acceptance).
Table 1.
PRECIS-2 domains with examples.
| Domain | Pragmatic approach (derived from) 15 with notional examples for MS research |
|---|---|
| Eligibility criteria | Include anyone of the target population of interest (e.g. the persons with MS who in usual care would have to choose between the alternatives that the trial randomly allocates) |
| Recruitment | Recruitment from usual care (e.g. without extra resources or incentives) |
| Setting | Conduct the trial in usual care (e.g. MS centers or participants’ home) |
| Organization | Identical organization as usual care (e.g. involving clinicians or nurses with same qualification and experience as in routine) |
| Flexibility in delivery of the intervention | Full flexibility in how the intervention is delivered (e.g. follow standard clinical routine) |
| Flexibility in adherence of the intervention | Full flexibility in how participants engage with the intervention (e.g. no special/artificial measures to enforce adherence to drug use, such as pill-count) |
| Follow-up | Identical intensity of measurement and follow-up as in usual care (e.g. embedded in routine clinic appointments) |
| Primary outcome | Primary outcome(s) with decision-relevance to participants (e.g. relapses, quality-of-life) |
| Primary analysis | Include all participants in the analysis (e.g. no per-protocol analysis) |
PRECIS-2: PRragmatic–Explanatory Continuum Indicator Summary-2.
Trials that were self-labeled as “pragmatic” and met the other eligibility criteria were assessed by PRECIS-2 but included irrespective of their individual domain PRECIS-2 scores (we considered these to be most inclusive in our approach, acknowledging that self-labeling of pragmatism is a poor predictor of pragmatism). 16
Information sources and search strategy
We searched for relevant trials via MEDLINE/Ovid, using the, to our knowledge, the only available validated search strategy for pragmatic trials 15 in combination with a search strategy for MS 17 (date of last search 1 December 2022; Appendix 1). Given the limited sensitivity of the pragmatic trial search strategy (46%), we additionally searched Scopus for references that cited the CONSORT-extension reporting guideline for pragmatic trials 18 or the seminal article describing the concept of pragmatic trials (published in 1967; 19 republished in 2009) 20 and mentioned “Multiple Sclerosis” in their title, abstract, journal name, keyword, or any other database field (date of last search on 28 March 2023).
To quantify the sensitivity of the pragmatic trial search component in our search strategy, we manually screened a random sample of 100 articles identified in MEDLINE/Ovid (last search 19 December 2022) using our search strategy but removing all pragmatic trial-related search terms (i.e. the same keywords and index terms related to randomized trials and MS as above, but not restricted to keywords that indicate pragmatic design features; Appendix 1).
Study selection
One reviewer (J.H.) screened titles, abstracts, and full texts for eligibility. A second reviewer (P.J. or L.G.H.) confirmed the assessment, if necessary.
Data extraction
For data extraction, we used the PragMeta database infrastructure that is described in detail elsewhere. 21 Briefly, one reviewer (J.H.) extracted information on trial topic (i.e. treatment, supportive care, etc.), if the trial was self-labeled as “pragmatic,” type of MS, number of participants randomized, type of intervention and comparison, primary or co-primary outcome(s) (if not specified, all outcomes mentioned), outcome assessor, length of follow-up, unit of randomization, number of trial sites, number of trial arms, use of routinely collected data, trial registration and protocol availability, country of conduct, funding, patient and public involvement, and bibliographic information (i.e. authors, publication year, and journal).
Data analysis
We used descriptive statistics (range, interquartile range (IQR), and total/sum for continuous variables; number and percentages for categorical variables). We used R (version 4.2.2) for data analysis.
Results
Our search retrieved 420 records with 53 eligible publications reporting on 48 trials22–69 (Appendices 1 and 2).
The 48 trials were published between 1967 and 2022 (median 2015; using the first publication if there were multiple ones; Figure 1), of which 12 (25%) were self-labeled as “pragmatic.” The trials were published in 25 different journals, with 19 trials (40%) being published in Multiple Sclerosis Journal and Clinical Rehabilitation. Twenty-nine trials (60%) referred to a trial registration and 19 (40%) to a protocol. Most of the trials (n = 33; 69%) were publicly funded without any support by the industry (Table 2; Appendices 3 and 5).
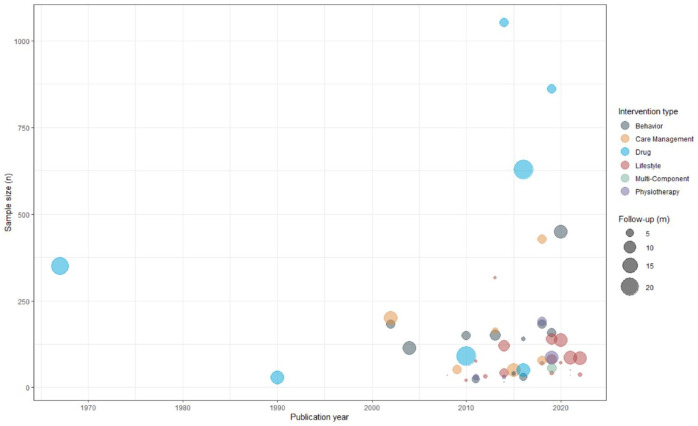
Figure 1.
Time trend of trials showing sample size, intervention type, and length of follow-up.
m: months; n: number of randomized participants.
Table 2.
Trial characteristics (n = 48).
| n (%) | |
|---|---|
| Clinical characteristics | |
| Number of randomized patients, median (range; IQR; overall) | 82 (15–1053; 42–160; 7534) |
| Type of MS a | |
| Relapsing-remitting MS | 36 (75) |
| Secondary progressive MS | 31 (65) |
| Primary progressive MS | 28 (58) |
| Not specified | 7 (15) |
| Intervention type | |
| Pharmacological/treatment | 7 (15) |
| Non-pharmacological/supportive care a | 41 (86) |
| Lifestyle | 18 (38) |
| Behavior | 13 (27) |
| Care management | 6 (13) |
| Physiotherapy | 6 (13) |
| Comparison type | |
| Usual care | 25 (52) |
| Wait-list | 8 (17) |
| Lifestyle | 4 (8) |
| Drug | 7 (15) |
| No intervention | 2 (4) |
| Behavior | 1 (2) |
| Physiotherapy | 1 (2) |
| Design characteristics | |
| Multinational | 4 (8) |
| Multicenter | 25 (52) |
| Involvement of patient representatives | |
| Yes | 4 (8) |
| Unclear | 11 (23) |
| Not reported | 33 (69) |
| Use of routinely collected data | |
| Yes a | 10 (21) |
| Recruitment purpose | 10 (21) |
| Type of data: registry | 5 (10) |
| Type of data: health records | 1 (2) |
| Type of data: not reported | 3 (6) |
| Type of data: other | 1 (2) |
| Outcome collection | 1 (2) |
| Type of data: health records | 1 (2) |
| Unclear | 2 (4) |
| Not reported | 36 (75) |
| Reporting characteristics | |
| Self-labeled as “pragmatic” | 12 (25) |
| Referred to a trial registry entry | 29 (60) |
| Referred to a trial protocol | 19 (40) |
| Funding | |
| Public | 33 (69) |
| Public, supported by industry | 4 (8) |
| Industry | 4 (8) |
| No funding received | 1 (2) |
| Not reported | 6 (13) |
IQR: interquartile range; MS: multiple sclerosis.
More than one category possible.
Clinical questions of the included trials
Trials included participants with relapsing-remitting MS (n = 36; 75%), and/or secondary progressive MS (n = 31; 65%), and/or primary progressive MS (n = 28; 58%) or did not specify the type of MS (n = 7; 15%) (Tables 2 and 4; Appendices 3 and 5).
Table 4.
Individual trial description (n = 48).
| Authors and reference | Country of conduct | Type of MS | N | Intervention, comparison, and main outcome b | Follow-up (m) |
|---|---|---|---|---|---|
| Non-pharmacological interventions (n = 41) | |||||
| Riemenschneider et al. 62 | Denmark | RRMS | 84 | Aerobic exercise intervention vs usual care on relapse and the global brain atrophy rate | 12 |
| Straudi et al. 63 | Italy | RRMS, SPMS, PPMS | 36 | Physical exercises vs wait-list on walking endurance | 3 |
| Langeskov-Christensen et al. 66 | Denmark | RRMS, SPMS, PPMS | 86 | Aerobic exercise intervention vs wait-list on cognitive performance, depression, and cardiorespiratory fitness b | 12 |
| Torkhani et al. 56 | France | RRMS, SPMS, PPMS | 35 | Mindfulness-based intervention and physical exercises vs lifestyle intervention on fatigue, mobility, and quality of life | 2 |
| Williams et al. 2021 61 | Australia | RRMS, SPMS, PPMS | 50 | In-person physical exercises vs home-based physical exercises on gait speed | 2 |
| Callesen et al. 25 | Denmark | RRMS, SPMS, PPMS | 71 | Physical exercises vs wait-list on walking speed | 2.5 |
| Lincoln et al. 44 and Janiaud et al. 71 a | The United Kingdom | RRMS, SPMS, PPMS | 449 | Cognitive rehabilitation vs usual care on quality of life | 12 |
| Mayo et al. 45 a | Canada | RRMS, SPMS, PPMS | 137 | Personally adapted physical exercises vs guideline-based physical exercises on peak oxygen consumption | 12 |
| Arntzen et al.22,72 | Norway | RRMS, SPMS, PPMS | 80 | Physical exercises vs usual care on balance | 7.5 |
| Feys et al. 30 a | Belgium | Not specified | 42 | Remote physical exercises vs wait-list on physical fitness, walking capacity and perceived ability, functional mobility, and quality of life | 3 |
| Freeman et al. 26 a | The United Kingdom | SPMS, PPMS | 140 | Physical exercises vs usual care on motor function | 9 |
| Gunn et al. 36 a | The United Kingdom | SPMS | 56 | Self-management program vs usual care on walking ability and quality of life b | 7 |
| Jongen et al. 40 | The Netherlands | RRMS | 158 | Cognitive theory–based intervention vs usual care on self-efficacy | 6 |
| Renfrew et al. 52 | The United Kingdom | RRMS, SPMS, PPMS | 85 | Ankle-foot orthoses vs functional electrical stimulation on walking speed | 12 |
| Boesen et al. 23 a | Denmark | RRMS, SPMS, PPMS | 427 | Multidisciplinary rehabilitation vs wait-list on quality of life | 6 |
| McClurg et al. 46 a | The United Kingdom | RRMS, SPMS, PPMS | 191 | Massage vs usual care on bowel dysfunction | 6 |
| Solari et al. 53 | Italy | SPMS, PPMS | 78 | Palliative care management vs usual care on quality of life and burden | 6 |
| Stuifbergen et al. 54 a | The United States | RRMS, SPMS, PPMS | 183 | Computer-assisted cognitive rehabilitation vs usual care on neurocognitive competence | 6 |
| Vermöhlen et al. 58 | Germany | Not specified | 70 | Hippotherapy vs usual care on balance | 3 |
| Khayeri et al. 41 | Iran | Not specified | 140 | Happiness principle–based cognitive and behavioral intervention vs usual care on depression, stress, anxiety, and fatigue b | 3 |
| Kiropoulos et al. | Australia | RRMS | 30 | Cognitive behavioral therapy vs usual care on depressive symptoms | 5 |
| Bogosian et al. 24 | The United Kingdom | SPMS, PPMS | 40 | Remote mindfulness intervention vs wait-list on distress | 3 |
| Papeix et al. 49 | France | RRMS, SPMS, PPMS | 50 | Integrated multidisciplinary care approach vs usual care on quality of life | 12 |
| Carter et al. 27 | The United Kingdom | RRMS, SPMS, PPMS | 120 | Physical exercises vs usual care on exercise behavior | 9 |
| Finch et al. 31 a | Canada | RRMS, SPMS, PPMS | 15 | Massage vs wait-list on self-efficacy b | 2 |
| Medina-Perez et al. 68 | Spain | RRMS | 42 | Physical exercises vs usual care on maximal contraction, muscle power, and muscle endurance b | 6 |
| Paul et al. 50 a | The United Kingdom | RRMS, SPMS, PPMS | 30 | Web-based physiotherapy vs usual care on walking speed | 3 |
| Garrett et al. 33 b | Ireland | RRMS, SPMS, PPMS | 316 | Physical exercises vs no intervention on physical impact | 2.5 |
| Humphreys et al. 39 | The United Kingdom | RRMS, SPMS, PPMS | 151 | Cognitive behavioral–based intervention vs wait-list on global health | 8 |
| Thomas et al. 55 b | The United Kingdom | RRMS, SPMS, PPMS | 164 | Fatigue management program vs usual care on fatigue, self-efficacy, and quality of life | 4 |
| Learmonth et al. 43 | The United Kingdom | Not specified | 32 | Physical exercises vs usual care on walking speed | 3 |
| Cooper et al. 26 | The United Kingdom | RRMS, SPMS | 24 | Computerized cognitive behavioral therapy vs usual care on depressive symptoms | 5.25 |
| Dodd et al. 29 | Australia | RRMS | 76 | Physical exercises vs usual care on walking endurance | 2.5 |
| Miller et al. 47 | The United Kingdom | SPMS, PPMS | 30 | Physiotherapy vs usual care on physical and psychological impact | 4 |
| Grossman et al. 35 | Switzerland | RRMS, SPMS | 150 | Mindfulness intervention vs usual care on quality of life, depression, and fatigue | 6 |
| Velikonja et al. 57 | Slovenia | RRMS, SPMS, PPMS | 20 | Sports climbing vs yoga on spasticity, cognitive function, mood/depressive symptoms, attention, and fatigue b | 2.5 |
| Higginson et al. 38 | The United Kingdom | RRMS, SPMS, PPMS | 52 | Palliative care vs usual care on anxiety and palliative care concerns/needs, pain, caregiver burden, and costs b | 6 |
| Hermens et al. 37 | Italy; Spain; Belgium | Not specified | 35 | Technology-supported physical exercises intervention vs usual care on motor function | 2 |
| Ward et al. 59 | The United Kingdom | Not specified | 114 | Personalized education and information vs standardized information on falls or skin sores | 12 |
| O’Hara et al. 48 | The United Kingdom | RRMS, SPMS, PPMS | 183 | Self-management program vs no intervention on mobility, activities of daily living, quality of life, and need for assistance b | 6 |
| Pozzilli et al. 51 | Italy | RRMS, SPMS, PPMS | 201 | Home care vs usual care on disability, fatigue, mood/depressive symptoms, quality of life, resource use, and costs b | 12 |
| Pharmacological interventions (n = 7) | |||||
| Cutter et al. 28 | Russia; Poland; Italy; France; Croatia; the United States; Mexico; Spain; Austria; Turkey; Belgium; Argentina; Germany; Finland | RRMS | 861 | Glatiramer acetate lower vs higher dose on medication satisfaction | 6 |
| Giovannoni et al. 34 | Australia; Belgium; Brazil; Canada; Croatia; Czech Republic; Denmark; France; Germany; Israel; Italy; Mexico; Netherlands; Poland; Russia; Serbia; Spain; Sweden; Ukraine; the United Kingdom; the United States | RRMS | 628 | Alemtuzumab vs interferon beta-1a (Rebif) on disability b | 24 |
| Weinstock-Guttman et al. 60 | The United States | RRMS | 50 | Immediate discontinuation natalizumab and initiated another DMT vs two more natalizumab infusions on lesion activity and relapse | 12 |
| Calkwood et al. 64 | The United States; Canada | RRMS | 1053 | Switch to fingolimod vs standard DMT (Avonex, Copaxone, Rebif, Betaseron, or Extavia) on treatment satisfaction | 6 |
| Mazdeh et al. 67 | Iran | RRMS, SPMS, PPMS | 90 | Interferon beta-1a (Avonex or Rebif) vs interferon beta-1b (Betaferon) on disability and relapse b | 24 |
| Hoogstraten et al. 65 | The Netherlands | RRMS | 29 | Corticotrophin vs vitamin B on disability, functioning, mobility, and activities of daily living b | 12 |
| Millar et al. 69 | The United Kingdom | Not specified | 350 | Corticotrophin and vitamin B vs vitamin B alone on disability and relapse b | 19 |
DMT: disease-modifying therapy; MS: multiple sclerosis; N: number of randomized participants; RRMS: relapsing-remitting multiple sclerosis; SPMS: secondary progressive multiple sclerosis; PRMS: primary progressive multiple sclerosis.
Trials self-labeled as “pragmatic.”
Main outcome(s) extracted (i.e. primary or co-primary); if not specified as such, we extracted all outcomes mentioned (indicated byb).
Most trials assessed non-pharmacological interventions (n = 41; 85%) with primarily lifestyle (n = 18; 38%) and/or behavioral (n = 13; 27%) components (Figure 1 and Table 3). Only seven trials assessed drugs (15%; alemtuzumab, 34 corticotrophin,65,69 glatiramer acetate, 28 natalizumab, 60 interferon beta-1a, 67 and fingolimod); 64 these were published between 1967 and 2019 (median 2014) and had a median of 350 participants (IQR = 70–745; overall 3601). The most frequent comparator was usual care (n = 25; 52%).
Table 3.
PRECIS-2 assessment.
| PRECIS-2 domain | Total (48 trials) | Self-labeled as “pragmatic” only (12 trials) | Drugs only (7 trials) | Use of RCD only (10 trials) |
|---|---|---|---|---|
| Median (IQR, range); n | Median (IQR, range) | Median (IQR, range) | Median (IQR, range) | |
| Eligibility criteria a | 4 (4–5, 2–5); 48 | 4.5 (4–5, 2–5) | 4 (3–4, 3–5) | 4.5 (4–5, 2–5) |
| Recruitment a | 4 (4–5, 3–5); 35 | 4 (4–5, 3–5) | 5 (5–5, 5–5) | 4.5 (4–5, 3–5) |
| Setting | 4.5 (4–5, 3–5); 48 | 5 (4–5, 4–5) | 5 (4–5, 4.5–5) | 5 (4–5, 4–5) |
| Organization | 4.5 (4–5, 2–5); 44 | 4 (3–4, 2–5) | 5 (5–5, 3–5) | 4.5 (3.25–5, 3–5) |
| Flexibility—intervention delivery | 5 (4–5, 3–5); 37 | 5 (3–5, 3–5) | 5 (5–5, 5–5) | 5 (4–5, 3–5) |
| Flexibility—intervention adherence | 3.5 (3–4, 2–5); 26 | 3 (2.25–4, 2–5) | 4 (4–4, 4–4) | 3.5 (3–4, 3–4) |
| Follow-up | 4 (3–4, 1–5); 47 | 3 (3–4, 1–5) | 4 (3.5–4, 3–4) | 4 (3–4, 3–5) |
| Primary outcome | 5 (5–5, 1–5); 6 | 5 (5–5, 1–5) | 5 (4.75–5, 4–5) | 4 (3–4, 3–5) |
| Primary analysis | 5 (4–5, 2–5); 37 | 4 (3–4, 2–5) | 5 (5–5, 5–5) | 5 (4.25–5, 3–5) |
IQR: interquartile range; n: number of trials with sufficient information to assess individual PRECIS-2 domains; PRECIS-2: PRagmatic Explanatory Continuum Indicator Summary 2; RCD: routinely collected data.
Individual trial assessment per domain is provided in Appendix 4; 1 = very explanatory; 2 = rather explanatory; 3 = equally pragmatic/explanatory; 4 = rather pragmatic; 5 = very pragmatic. 70
For the one cluster-randomized trial, we could not identify sufficient information to assess the domains eligibility criteria and recruitment on cluster level.
Primary outcomes were diverse, assessing or example, quality of life, physical functioning, and disease activity. The median length of follow-up was 6 months (range = 2–24; IQR = 3–12) (Tables 2 and 4 and Figure 1; Appendix 5).
Design characteristics
The 48 trials included a median of 82 participants (range = 15–1053; IQR = 42–160; overall = 7534; Figure 1). Only three trials (6%) included >500 participants. All trials randomized participants individually; there was only one cluster-randomized trial. Trials had a median of two arms (range = 2–4; IQR = 2–3) and a median of two trial centers (range = 1–191; IQR = 1–5). They were conducted mostly in the United Kingdom (n = 18; 38%), Italy (n = 6; 13%), the United States, and Denmark (each n = 5; 10%); only four trials (8%) were conducted internationally. Outcomes were mostly assessed in a non-blinded fashion (n = 28; 58%). Only four trials (8%) clearly stated information on patient and public involvement. The 10 trials (21%) that clearly reported the use of routinely collected data (mostly registry-based data; n = 5; 10%) leveraged these data for recruitment, and only 1 trial (2%) used routinely collected data for outcome ascertainment (Tables 2 and 4; Appendices and 5).
Pragmatic trial features
None of the identified trials was very pragmatic (i.e. scoring 5) in all domains of the PRECIS-2 tool, but 14 trials (29%) scored rather pragmatic or very pragmatic (i.e. 4 or higher) in all domains (Appendices 4 and 5).
Most trials were very pragmatic (median PRECIS-2 score 4.5–5) for the domains setting (IQR = 4–5), organization (IQR = 4–5), flexibility in intervention delivery (IQR = 4–5), primary outcome (IQR = 5–5), and primary analysis (IQR = 4–5). For example, they were conducted in a similar setting to which the results were intended to be applied (e.g. a home-delivered intervention), 26 the expertise and resources needed for the organization of the trial did not much deviate from usual care (e.g. trained staff who is not available in usual care), 33 the delivery of the intervention was as in usual care (e.g. the way of how to deliver the intervention was left to the discretion of the therapist), 22 the primary outcome was very relevant for decision-making of patients (e.g. quality of life), 39 and the primary analysis was according to the intention to treat (i.e. not only considering those that strictly adhered to a protocol). 62
With a median PRECIS-2 score of 3.5–4 in the domains eligibility criteria (IQR = 4–5), recruitment (IQR = 4–5), flexibility in intervention adherence (IQR = 3–4), and follow-up (IQR = 3–4), the pragmatic design features were less pronounced. For example, the eligibility criteria excluded persons likely to receive the intervention in routine care (e.g. pregnant women 22 or elderly 45 ). Other factors limiting pragmatism included an artificially intensive resource use for participant recruitment (e.g. advertisement 42 ), that unusual measures were used to monitor and promote intervention adherence (e.g. monitored through phone calls 30 ), or that there were artificially intensive (long and frequent) follow-up visits deviating from usual care (e.g. daily diary completion for 6 weeks 46 ) (Table 3 and Figure 2; Appendices 4 and 5).
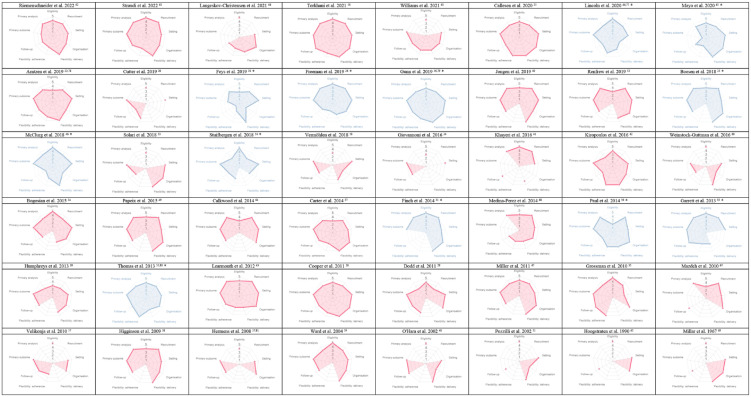
Figure 2.
PRECIS-2 assessment wheels of individual trials (n = 48).
PRECIS-2: PRagmatic Explanatory Continuum Indicator Summary-2.
Trials self-labeled as “pragmatic” (PRECIS-2 wheels highlighted in blue); missing dot indicates insufficient information to assess a PRECIS-2 domain.
The PRECIS-2 assessment results for trials self-labeled as “pragmatic” (n = 12; 25%), trials assessing drugs (n = 7; 15%), and trials using routinely collected data (n = 10; 21%) were not clearly different to the overall sample (Table 3).
Discussion
We identified 48 pragmatic trials in the field of MS, most of them with small sample sizes, more recently published, and very few assessing drugs. The spectrum of their research questions and primary outcomes is very wide and design features that may indicate pragmatism, such as the use of routinely collected data, were rare.
The first pragmatic trial in MS was published in 1967 comparing a treatment with corticotrophin with usual care, 69 the same year as the seminal article by Schwartz and Lellouch introduced the concept of pragmatism for clinical trials.19,20 Until about 10 years ago, there were almost no pragmatic trials in MS, and when they finally became a bit more common, they remained relatively small, almost always with fewer than 100 patients. There are no such large pragmatic trials in MS as the famous GISSI trial (Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico) from Italy, which enrolled more than 11,000 patients from February 1984 to June 1985 and demonstrated the mortality benefit of early thrombolytic treatment with streptokinase in acute myocardial infarction. 73 Large simple trials have the advantage that they can uncover the typically small to modest average treatment effects that we can unfortunately only expect with most modern treatments, 74 and they can also reveal subgroup effects that allow us to better personalize therapies and identify those patients who may expect larger benefits. This requires a heterogeneous study population and not a population homogenized according to strict eligibility criteria that lacks diversity. Too restricted samples have been also highlighted in rehabilitation trials in MS as the main hurdle to evidence-based decision-making. 75
While the high incidence of myocardial infarction (GISSI) or COVID-19 (RECOVERY) certainly facilitated the conduct of these large trials to some degree, MS has a higher prevalence as a chronic condition and pwMS are often treated in structured care settings with scheduled regular follow-up visits, structured assessment of the most decision-relevant outcomes (such as the Expanded Disability Status Scale (EDSS)), and systematic data collection embedded in routine care. The MSBase registry alone includes systematic follow-up of more than 92,000 patients with routinely collected data. 12 Using such data infrastructures for randomized trials could provide directly applicable real-world evidence with pragmatic trials, saving costs and resources, among other benefits.
A classic example of this approach comes (again) from cardiology, the Thrombus Aspiration in ST-Elevation Myocardial Infarction (TASTE) trial in Sweden, which was based on a quality control registry and showed that patients with myocardial infarction did not benefit from thrombus aspiration as coronary intervention. In this trial with more than 7000 randomized patients, the data quality was excellent (perfect completeness, zero losses to follow-up) and the costs were minimal (US$50 per patient). 76
In MS, the trials that we identified were rather small compared to the examples from cardiology outlined above. Among the largest ones was the CONFIDENCE study 28 with almost 1000 participants from 88 study centers in 14 countries that answered the question which of two doses of glatiramer acetate provided more medication satisfaction (in this study, pwMS preferred glatiramer acetate 40 mg three times weekly to 20 mg once daily). The CONFIDENCE study still had features that were not perfectly in line with a pragmatic intend (e.g. it had an intense follow-up assessment which deviates from usual care). An example of a trial assessed as pragmatic among all PRECIS-2 domains was from Boesen et al., who determined in more than 400 pwMS whether a 4-week inpatient multidisciplinary rehabilitation resulted in a better quality of life compared to 6 months on a wait-list to receive the rehabilitation afterwards 23 (the scheduled rehabilitation was favorable for several quality of life-related measures). Pragmatism was facilitated by direct recruitment of participants from usual care, the provision of commonly available rehabilitation measures, and clearly decision-relevant outcomes. These two studies present characteristics which are typical for the designs of the other pragmatic trials we found. Unfortunately, these were often not only small, but more than half of them also lacked a blinded outcome assessment, which indicates another area for improvement.
Overall, the PRECIS-2 domains that were often (very) pragmatic across the 48 trials in MS were related to the setting, recruitment, and primary outcome. This may be a direct consequence of having the structured care settings with regular follow-up and the structured assessment of decision-relevant outcomes that are typical for MS care. In this regard, the specific conditions of MS care appear to indeed facilitate pragmatic trials. On the contrary, despite the widely available and often excellent routinely collected data infrastructures (e.g. registries), very few trials seem to utilize this source. Only 10 trials clearly reported the use of routinely collected data, mostly for recruitment, and only 1 trial (2%) used these data for outcome ascertainment. There is an urgent need to leverage the potential of this pioneering study design to provide useful randomized real-world evidence.
Trial teams wishing to use pragmatic designs may encounter external barriers. Clinical trials are increasingly encompassed by an extensive regulatory framework 70 aiming to uphold ethical, legal, and quality standards. Existing guidance and procedures have been developed with a focus on traditional drug-oriented trials such as those for drug development and approval, but they do not focus on trials with a pragmatic intend that compare treatment choices; however, these same systems can be a barrier against reform, if regulatory personnel is unfamiliar with pragmatic trial design. 77 The domains for which we found lower overall pragmatism scores (i.e. eligibility criteria, recruitment procedures, adherence, and follow-up) may all have structural barriers against pragmatic design features, for example, ethical concerns about including vulnerable populations or using electronic consent forms, and requirements for close attention to adherence and extensive follow-up measures.
Our study has several limitations. First, we aimed to provide an overview of the research agenda with pragmatic trials in MS and cannot ensure completeness—we will have missed some pragmatic trials. However, we included every trial published in a PubMed-indexed journal that was labeled as “pragmatic” by the authors. We also searched for studies using a validated search filter specifically for pragmatic trials and used established search components for MS. The screening of studies was done by one reviewer alone and we used only one literature database, but we supplemented our search by screening all articles that cited two key articles on pragmatic trials, regardless of whether they were indexed in PubMed or not. Overall, it is unlikely that the trials we may have missed would have changed the overall interpretation of our findings.
Second, the PRECIS-2 assessment was performed in different teams involving four different second reviewers. However, with one consistent first reviewer, who was experienced in the assessment of pragmatism and following strictly the PRECIS-2 guidance provided by the PRECIS-2 team, 78 we ensured to have very consistent PRECIS-2 ratings.
Third, retrospective assessment of pragmatism is challenging because it is sometimes difficult to judge whether the trial situation is similar to the target situation in which the healthcare decision is to be made. The main criteria we used to exclude most of the trials were, however, objective and independent of the target situation (e.g. use of placebo controls), which limits the impact of this aspect.
Forth, the evaluation of pragmatism depends substantially on the reporting quality. While we were not able to assess all PRECIS-2 domains in all trials due to insufficient information, the pragmatism of some trials will be seen differently by those who were involved in the trials or had closer insights in the original setting and circumstances. While this may have changed some domain assessments, we do not believe that this would substantially change the overall impression of a trial and thus the main interpretation of our findings.
Overall, we found pragmatic features that have been commonly applied in MS trials, illustrating a promising development. Lessons learned from COVID-19 for clinical trials include that “Pragmatism, integration in clinical care, efficient administration, promotion of collaborative structures, and enhanced integration of existing data and facilities might be several of the legacies of COVID-19 on future randomized trials.” 71 In Switzerland, the publicly funded, investigator-initiated MultiSCRIPT project was launched most recently by our group. 72 It is a continuous series of pragmatic trials evaluating healthcare innovations for MS, fully embedded in the Swiss Multiple Sclerosis Cohort (SMSC) and based entirely on routinely collected data from more than 1000 pwMS. 11 MultiSCRIPT aims to resemble these lessons, and we hope it inspires others. The rise of the pragmatic approach is promising; there is still a long way to go in MS research, but the first steps have been taken.
Conclusion
There are only very few and mostly small pragmatic trials in MS and rarely assessing drug treatments. Despite the widely available and often excellent routinely collected data infrastructures, very few trials seem to utilize this source. There is an urgent need to leverage the potential of this pioneering study design to provide useful randomized real-world evidence.
Supplemental Material
Supplemental material, sj-pdf-1-msj-10.1177_13524585231221938 for Use of pragmatic randomized trials in multiple sclerosis: A systematic overview by Julian Hirt, Perrine Janiaud, Pascal Düblin, Giovanni Jacopo Nicoletti, Kinga Dembowska, Thao Vy Thi Nguyen, Tim Woelfle, Cathrine Axfors, Özgür Yaldizli, Cristina Granziera, Jens Kuhle, Ludwig Kappos and Lars G Hemkens in Multiple Sclerosis Journal
Footnotes
Author Contributions: J.H., P.J., and L.G.H. made substantial contributions to the conception and design of the work. J.H., P.J., and L.G.H. have drafted the work or substantively revised it; all authors have approved the submitted version; all authors have agreed both to be personally accountable for the author’s own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
Availability of Data and Materials: All data generated and analyzed in this study are provided on www.PragMeta.org (download data set and filter for module PragMS).
Consent for Publication: Not applicable.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: J.H. declares no competing interests. RC2NB (Research Center for Clinical Neuroimmunology and Neuroscience Basel) is supported by Foundation Clinical Neuroimmunology and Neuroscience Basel. P.J. declares no competing interests. RC2NB (Research Center for Clinical Neuroimmunology and Neuroscience Basel) is supported by Foundation Clinical Neuroimmunology and Neuroscience Basel. P.D. declares no competing interests. G.J.N. declares no competing interests. K.D. declares no competing interests. RC2NB (Research Center for Clinical Neuroimmunology and Neuroscience Basel) is supported by Foundation Clinical Neuroimmunology and Neuroscience Basel. T.V.T.N. declares no competing interests. RC2NB (Research Center for Clinical Neuroimmunology and Neuroscience Basel) is supported by Foundation Clinical Neuroimmunology and Neuroscience Basel. T.W. declares no competing interests. RC2NB (Research Center for Clinical Neuroimmunology and Neuroscience Basel) is supported by Foundation Clinical Neuroimmunology and Neuroscience Basel. C.A. declares no competing interests. RC2NB (Research Center for Clinical Neuroimmunology and Neuroscience Basel) is supported by Foundation Clinical Neuroimmunology and Neuroscience Basel. Ö.Y. received grants from ECTRIMS/MAGNIMS, University of Basel, Pro Patient Stiftung University Hospital Basel, Free Academy Basel, Swiss Multiple Sclerosis Society, Swiss National Science Foundation and advisory board/lecture and consultancy fees from Roche, Sanofi and Biogen. C.G. as the employer of the University Hospital Basel (USB) has received the following fees which were used exclusively for research support: (1) advisory board and consultancy fees from Actelion, Novartis, Genzyme, and F. Hoffmann-La Roche Ltd; (2) speaker fees from Biogen and Genzyme-Sanofi; and (3) research support by F. Hoffmann-La Roche Ltd. Before my employment at USB, I have also received speaker honoraria and travel funding by Novartis. J.K. received speaker fees, research support, travel support, and/or served on advisory boards by the Progressive MS Alliance, Swiss MS Society, Swiss National Research Foundation (320030_189140/1), University of Basel, Biogen, Celgene, Merck, Novartis, Octave Bioscience, Roche, and Sanofi. L.K. has received no personal compensation. His institutions (University Hospital Basel/Stiftung Neuroimmunology and Neuroscience Basel) have received and used exclusively for research support payments for steering committee and advisory board participation, consultancy services, and participation in educational activities from: Actelion, Bayer, BMS, df-mp Molnia & Pohlmann, Celgene, Eli Lilly, EMD Serono, Genentech, Glaxo Smith Kline, Janssen, Japan Tobacco, Merck, MH Consulting, Minoryx, Novartis, F. Hoffmann-La Roche Ltd, Senda Biosciences Inc., Sanofi, Santhera, Shionogi BV, TG Therapeutics, and Wellmera, and license fees for Neurostatus-UHB products; grants from Novartis, InnoSuisse, and Roche. L.G.H. declares no competing interests. RC2NB (Research Center for Clinical Neuroimmunology and Neuroscience Basel) is supported by Foundation Clinical Neuroimmunology and Neuroscience Basel.
Ethical Approval and Consent to Participate: Not applicable.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The PragMeta project is supported by the Swiss National Science Foundation, project ID 188675. The funder had no role in the design of the project; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
ORCID iDs: Julian Hirt  https://orcid.org/0000-0001-6589-3936
https://orcid.org/0000-0001-6589-3936
Tim Woelfle  https://orcid.org/0000-0001-6279-4158
https://orcid.org/0000-0001-6279-4158
Cristina Granziera  https://orcid.org/0000-0002-4917-8761
https://orcid.org/0000-0002-4917-8761
Ludwig Kappos  https://orcid.org/0000-0003-4175-5509
https://orcid.org/0000-0003-4175-5509
Supplemental Material: Supplemental material for this article is available online.
Contributor Information
Julian Hirt, Research Center for Clinical Neuroimmunology and Neuroscience Basel (RC2NB), University Hospital Basel and University of Basel, Basel, Switzerland/Department of Clinical Research, University Hospital Basel and University of Basel, Basel, Switzerland/Department of Health, Eastern Switzerland University of Applied Sciences, St. Gallen, Switzerland.
Perrine Janiaud, Research Center for Clinical Neuroimmunology and Neuroscience Basel (RC2NB), University Hospital Basel and University of Basel, Basel, Switzerland/Department of Clinical Research, University Hospital Basel and University of Basel, Basel, Switzerland.
Pascal Düblin, Department of Clinical Research, University Hospital Basel and University of Basel, Basel, Switzerland.
Giovanni Jacopo Nicoletti, Department of Emergency Medicine, University Hospital Basel, Basel, Switzerland.
Kinga Dembowska, Research Center for Clinical Neuroimmunology and Neuroscience Basel (RC2NB), University Hospital Basel and University of Basel, Basel, Switzerland/MSc program in epidemiology, Swiss TPH, University of Basel, Basel, Switzerland.
Thao Vy Thi Nguyen, Research Center for Clinical Neuroimmunology and Neuroscience Basel (RC2NB), University Hospital Basel and University of Basel, Basel, Switzerland/MSc program in epidemiology, Swiss TPH, University of Basel, Basel, Switzerland.
Tim Woelfle, Research Center for Clinical Neuroimmunology and Neuroscience Basel (RC2NB), University Hospital Basel and University of Basel, Basel, Switzerland.
Cathrine Axfors, Research Center for Clinical Neuroimmunology and Neuroscience Basel (RC2NB), University Hospital Basel and University of Basel, Basel, Switzerland/Department of Clinical Research, University Hospital Basel and University of Basel, Basel, Switzerland.
Özgür Yaldizli, Research Center for Clinical Neuroimmunology and Neuroscience Basel (RC2NB), University Hospital Basel and University of Basel, Basel, Switzerland/Department of Clinical Research, University Hospital Basel and University of Basel, Basel, Switzerland.
Cristina Granziera, Research Center for Clinical Neuroimmunology and Neuroscience Basel (RC2NB), University Hospital Basel and University of Basel, Basel, Switzerland/Department of Clinical Research, University Hospital Basel and University of Basel, Basel, Switzerland.
Jens Kuhle, Research Center for Clinical Neuroimmunology and Neuroscience Basel (RC2NB), University Hospital Basel and University of Basel, Basel, Switzerland/Department of Clinical Research, University Hospital Basel and University of Basel, Basel, Switzerland.
Ludwig Kappos, Research Center for Clinical Neuroimmunology and Neuroscience Basel (RC2NB), University Hospital Basel and University of Basel, Basel, Switzerland/Department of Clinical Research, University Hospital Basel and University of Basel, Basel, Switzerland.
Lars G Hemkens, Research Center for Clinical Neuroimmunology and Neuroscience Basel (RC2NB), University Hospital Basel and University of Basel, Basel, Switzerland/Department of Clinical Research, University Hospital Basel and University of Basel, Basel, Switzerland.
References
- 1. Zwarenstein M. “Pragmatic” and “Explanatory” attitudes to randomized trials. JLL Bulletin, 2016, https://www.jameslindlibrary.org/articles/pragmatic-and-explanatory-attitudes-to-randomized-trials/ (2016, accessed 24 September 2023). [DOI] [PMC free article] [PubMed]
- 2. Food Drug Administration (FDA). Framework for FDA’s Real-World Evidence Program, https://www.fda.gov/media/120060/download (2018, accessed 24 September 2023).
- 3. Hemkens LG, Contopoulos-Ioannidis DG, Ioannidis JPA. Routinely collected data and comparative effectiveness evidence: Promises and limitations. CMAJ 2016; 188: E158–E164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McCord KA, Al-Shahi Salman R, Treweek S, et al. Routinely collected data for randomized trials: Promises, barriers, and implications. Trials 2018; 19: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baigent C, Collins R, Appleby P, et al. ISIS-2: 10 year survival among patients with suspected acute myocardial infarction in randomised comparison of intravenous streptokinase, oral aspirin, both, or neither. The ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. BMJ 1998; 316: 1337–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rovelli F, De Vita C, Feruglio GA, et al. GISSI trial: Early results and late follow-up. J Am Coll Cardiol 1987; 10(5 Suppl. B): 33B–39B. [DOI] [PubMed] [Google Scholar]
- 7. Eapen ZJ, Lauer MS, Temple RJ. The imperative of overcoming barriers to the conduct of large, simple trials. JAMA 2014; 311: 1397–1398. [DOI] [PubMed] [Google Scholar]
- 8. Ioannidis JPA. Why most clinical research is not useful. PLoS Med 2016; 13: e1002049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wise J, Coombes R. Covid-19: The inside story of the RECOVERY trial. BMJ 2020; 370: m2670. [DOI] [PubMed] [Google Scholar]
- 10. Hemkens LG. How routinely collected data for randomized trials provide long-term randomized real-world evidence. JAMA Netw Open 2018; 1: e186014. [DOI] [PubMed] [Google Scholar]
- 11. University of Basel. SMSC—swiss multiple sclerosis cohort, https://smsc.ch/ (2023, accessed 24 October 2023).
- 12. The Alfred Centre. MSBase, https://www.msbase.org (accessed 23 September 2023).
- 13. Page MJ, McKenzie J, Bossuyt PM, et al. Updating guidance for reporting systematic reviews: Development of the PRISMA 2020 statement. J Clin Epidemiol 2021; 134: 103–112. [DOI] [PubMed] [Google Scholar]
- 14. Loudon K, Treweek S, Sullivan F, et al. The PRECIS-2 tool: Designing trials that are fit for purpose. BMJ 2015; 350: h2147. [DOI] [PubMed] [Google Scholar]
- 15. Taljaard M, McDonald S, Nicholls SG, et al. A search filter to identify pragmatic trials in MEDLINE was highly specific but lacked sensitivity. J Clin Epidemiol 2020; 124: 75–84. [DOI] [PubMed] [Google Scholar]
- 16. Dal-Ré R, Janiaud P, Ioannidis JPA. Real-world evidence: How pragmatic are randomized controlled trials labeled as pragmatic? BMC Medicine 2018; 16: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Canadian Agency for Drugs Technologies in Health. Comparative clinical and cost-effectiveness of drug therapies for relapsing-remitting multiple Sclerosis, 2013, https://pubmed.ncbi.nlm.nih.gov/24279002/ [PubMed]
- 18. Zwarenstein M, Treweek S, Gagnier JJ, et al. Improving the reporting of pragmatic trials: An extension of the CONSORT statement. BMJ 2008; 337: a2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schwartz D, Lellouch J. Explanatory and pragmatic attitudes in therapeutical trials. J Chronic Dis 1967; 20: 637–648. [DOI] [PubMed] [Google Scholar]
- 20. Schwartz D, Lellouch J. Explanatory and pragmatic attitudes in therapeutical trials. J Clin Epidemiol 2009; 62: 499–505. [DOI] [PubMed] [Google Scholar]
- 21. Hirt J, Janiaud P, Düblin P, et al. Meta-research on pragmatism of randomized trials: Rationale and design of the PragMeta database. Trials 2023; 24: 437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Arntzen EC, Straume BK, Odeh F, et al. Group-based individualized comprehensive core stability intervention improves balance in persons with multiple sclerosis: A randomized controlled trial. Phys Ther 2019; 99: 1027–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Boesen F, Nørgaard M, Trénel P, et al. Longer term effectiveness of inpatient multidisciplinary rehabilitation on health-related quality of life in MS patients: A pragmatic randomized controlled trial—the Danish MS hospitals rehabilitation study. Mult Scler 2018; 24(3): 340–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bogosian A, Chadwick P, Windgassen S, et al. Distress improves after mindfulness training for progressive MS: A pilot randomised trial. Mult Scler 2015; 21: 1184–1194. [DOI] [PubMed] [Google Scholar]
- 25. Callesen J, Cattaneo D, Brincks J, et al. How do resistance training and balance and motor control training affect gait performance and fatigue impact in people with multiple sclerosis? A randomized controlled multi-center study. Mult Scler 2020; 26: 1420–1432. [DOI] [PubMed] [Google Scholar]
- 26. Cooper CL, Hind D, Parry GD, et al. Computerised cognitive behavioural therapy for the treatment of depression in people with multiple sclerosis: External pilot trial. Trials 2011; 12: 259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Carter A, Daley A, Humphreys L, et al. Pragmatic intervention for increasing self-directed exercise behaviour and improving important health outcomes in people with multiple sclerosis: A randomised controlled trial. Mult Scler 2014; 20(8): 1112–1122. [DOI] [PubMed] [Google Scholar]
- 28. Cutter G, Veneziano A, Grinspan A, et al. Higher satisfaction and adherence with glatiramer acetate 40 mg/mL TIW vs 20 mg/mL QD in RRMS. Mult Scler Relat Disord 2019; 33: 13–21. [DOI] [PubMed] [Google Scholar]
- 29. Dodd KJ, Taylor NF, Shields N, et al. Progressive resistance training did not improve walking but can improve muscle performance, quality of life and fatigue in adults with multiple sclerosis: A randomized controlled trial. Mult Scler 2011; 17: 1362–1374. [DOI] [PubMed] [Google Scholar]
- 30. Feys P, Moumdjian L, Van Halewyck F, et al. Effects of an individual 12-week community-located “start-to-run” program on physical capacity, walking, fatigue, cognitive function, brain volumes, and structures in persons with multiple sclerosis. Mult Scler 2019; 25(1): 92–103. [DOI] [PubMed] [Google Scholar]
- 31. Finch P, Bessonnette S. A pragmatic investigation into the effects of massage therapy on the self efficacy of multiple sclerosis clients. J Bodyw Mov Ther 2014; 18(1): 11–16. [DOI] [PubMed] [Google Scholar]
- 32. Freeman J, Hendrie W, Jarrett L, et al. Assessment of a home-based standing frame programme in people with progressive multiple sclerosis (SUMS): A pragmatic, multi-centre, randomised, controlled trial and cost-effectiveness analysis. Lancet Neurol 2019; 18: 736–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Garrett M, Hogan N, Larkin A, et al. Exercise in the community for people with minimal gait impairment due to MS: An assessor-blind randomized controlled trial. Mult Scler 2013; 19: 782–789. [DOI] [PubMed] [Google Scholar]
- 34. Giovannoni G, Cohen JA, Coles AJ, et al. Alemtuzumab improves preexisting disability in active relapsing-remitting MS patients. Neurology 2016; 87: 1985–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Grossman P, Kappos L, Gensicke H, et al. MS quality of life, depression, and fatigue improve after mindfulness training: A randomized trial. Neurology 2010; 75: 1141–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gunn H, Andrade J, Paul L, et al. A self-management programme to reduce falls and improve safe mobility in people with secondary progressive MS: The BRiMS feasibility RCT. Health Technol Assess 2019; 23(27): 1–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hermens H, Huijgen B, Giacomozzi C, et al. Clinical assessment of the HELLODOC tele-rehabilitation service. Ann Ist Super Sanita 2008; 44(2): 154–163. [PubMed] [Google Scholar]
- 38. Higginson IJ, McCrone P, Hart SR, et al. Is short-term palliative care cost-effective in multiple sclerosis? A randomized phase II trial. J Pain Symptom Manage 2009; 38(6): 816–826. [DOI] [PubMed] [Google Scholar]
- 39. Humphreys I, Drummond AE, Phillips C, et al. Cost-effectiveness of an adjustment group for people with multiple sclerosis and low mood: A randomized trial. Clin Rehabil 2013; 27(11): 963–971. [DOI] [PubMed] [Google Scholar]
- 40. Jongen PJ, van Mastrigt GA, Heerings M, et al. Effect of an intensive 3-day social cognitive treatment (can do treatment) on control self-efficacy in patients with relapsing remitting multiple sclerosis and low disability: A single-centre randomized controlled trial. PLoS ONE 2019; 14(10): e0223482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Khayeri F, Rabiei L, Shamsalinia A, et al. Effect of Fordyce Happiness Model on depression, stress, anxiety, and fatigue in patients with multiple sclerosis. Complement Ther Clin Pract 2016; 25: 130–135. [DOI] [PubMed] [Google Scholar]
- 42. Kiropoulos LA, Kilpatrick T, Holmes A, et al. A pilot randomized controlled trial of a tailored cognitive behavioural therapy based intervention for depressive symptoms in those newly diagnosed with multiple sclerosis. BMC Psychiatry 2016; 16: 435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Learmonth YC, Paul L, Miller L, et al. The effects of a 12-week leisure centre-based, group exercise intervention for people moderately affected with multiple sclerosis: A randomized controlled pilot study. Clin Rehabil 2012; 26(7): 579–593. [DOI] [PubMed] [Google Scholar]
- 44. Lincoln NB, Bradshaw LE, Constantinescu CS, et al. Group cognitive rehabilitation to reduce the psychological impact of multiple sclerosis on quality of life: The CRAMMS RCT. Health Technol Assess 2020; 24(4): 1–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mayo NE, Mate KK, Reid R, et al. Participation in and outcomes from a 12-month tailored exercise programme for people with multiple sclerosis (MSTEP©): A randomized trial. Clin Rehabil 2020; 34(7): 927–937. [DOI] [PubMed] [Google Scholar]
- 46. McClurg D, Harris F, Goodman K, et al. Abdominal massage plus advice, compared with advice only, for neurogenic bowel dysfunction in MS: A RCT. Health Technol Assess 2018; 22: 1–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Miller L, Paul L, Mattison P, et al. Evaluation of a home-based physiotherapy programme for those with moderate to severe multiple sclerosis: A randomized controlled pilot study. Clin Rehabil 2011; 25: 720–730. [DOI] [PubMed] [Google Scholar]
- 48. O’Hara L, Cadbury H, De SL, et al. Evaluation of the effectiveness of professionally guided self-care for people with multiple sclerosis living in the community: A randomized controlled trial. Clin Rehabil 2002; 16(2): 119–128. [DOI] [PubMed] [Google Scholar]
- 49. Papeix C, Gambotti L, Assouad R, et al. Evaluation of an integrated multidisciplinary approach in multiple sclerosis care: A prospective, randomized, controlled study. Mult Scler J Exp Transl Clin 2015; 1: 2055217315608864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Paul L, Coulter EH, Miller L, et al. Web-based physiotherapy for people moderately affected with Multiple Sclerosis; quantitative and qualitative data from a randomized, controlled pilot study. Clin Rehabil 2014; 28(9): 924–935. [DOI] [PubMed] [Google Scholar]
- 51. Pozzilli C, Brunetti M, Amicosante AM, et al. Home based management in multiple sclerosis: Results of a randomised controlled trial. J Neurol Neurosurg Psychiatry 2002; 73(3): 250–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Renfrew LM, Paul L, McFadyen A, et al. The clinical- and cost-effectiveness of functional electrical stimulation and ankle-foot orthoses for foot drop in multiple sclerosis: A multicentre randomized trial. Clin Rehabil 2019; 33(7): 1150–1162. [DOI] [PubMed] [Google Scholar]
- 53. Solari A, Giordano A, Patti F, et al. Randomized controlled trial of a home-based palliative approach for people with severe multiple sclerosis. Mult Scler 2018; 24: 663–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Stuifbergen AK, Becker H, Perez F, et al. Computer-assisted cognitive rehabilitation in persons with multiple sclerosis: Results of a multi-site randomized controlled trial with six month follow-up. Disabil Health J 2018; 11(3): 427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Thomas S, Thomas PW, Kersten P, et al. A pragmatic parallel arm multi-centre randomised controlled trial to assess the effectiveness and cost-effectiveness of a group-based fatigue management programme (FACETS) for people with multiple sclerosis. J Neurol Neurosurg Psychiatry 2013; 84(10): 1092–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Torkhani E, Dematte E, Slawinski J, et al. Improving health of people with multiple sclerosis from a multicenter randomized controlled study in parallel groups: Preliminary results on the efficacy of a mindfulness intervention and intention implementation associated with a physical activity program. Front Psychol 2021; 12: 767784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Velikonja O, Curić K, Ozura A, et al. Influence of sports climbing and yoga on spasticity, cognitive function, mood and fatigue in patients with multiple sclerosis. Clin Neurol Neurosurg 2010; 112(7): 597–601. [DOI] [PubMed] [Google Scholar]
- 58. Vermöhlen V, Schiller P, Schickendantz S, et al. Hippotherapy for patients with multiple sclerosis: A multicenter randomized controlled trial (MS-HIPPO). Mult Scler 2018; 24(10): 1375–1382. [DOI] [PubMed] [Google Scholar]
- 59. Ward CD, Turpin G, Dewey ME, et al. Education for people with progressive neurological conditions can have negative effects: Evidence from a randomized controlled trial. Clin Rehabil 2004; 18(7): 717–725. [DOI] [PubMed] [Google Scholar]
- 60. Weinstock-Guttman B, Hagemeier J, Kavak KS, et al. Randomised natalizumab discontinuation study: Taper protocol may prevent disease reactivation. J Neurol Neurosurg Psychiatry 2016; 87(9): 937–943. [DOI] [PubMed] [Google Scholar]
- 61. Williams KL, Low Choy NL, Brauer SG. Center-based group and home-based individual exercise programs have similar impacts on gait and balance in people with multiple sclerosis: A randomized trial. PM R 2021; 13(1): 9–18. [DOI] [PubMed] [Google Scholar]
- 62. Riemenschneider M, Hvid LG, Ringgaard S, et al. Investigating the potential disease-modifying and neuroprotective efficacy of exercise therapy early in the disease course of multiple sclerosis: The Early Multiple Sclerosis Exercise Study (EMSES). Mult Scler 2022; 28(10): 1620–1629. [DOI] [PubMed] [Google Scholar]
- 63. Straudi S, De Marco G, Martinuzzi C, et al. Combining a supervised and home-based task-oriented circuit training improves walking endurance in patients with multiple sclerosis. Mult Scler Relat Disord 2022; 60: 103721. [DOI] [PubMed] [Google Scholar]
- 64. Calkwood J, Cree B, Crayton H, et al. Impact of a switch to fingolimod versus staying on glatiramer acetate or beta interferons on patient- and physician-reported outcomes in relapsing multiple sclerosis: Post hoc analyses of the EPOC trial. BMC Neurol 2014; 14: 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hoogstraten MC, Minderhoud JM. Long-term effect of ACTH treatment of relapse in multiple sclerosis. Acta Neurologica Scandinavica 1990; 82: 74–77. [DOI] [PubMed] [Google Scholar]
- 66. Langeskov-Christensen M, Hvid LG, Jensen HB, et al. Efficacy of high-intensity aerobic exercise on cognitive performance in people with multiple sclerosis: A randomized controlled trial. Mult Scler 2021; 27(10): 1585–1596. [DOI] [PubMed] [Google Scholar]
- 67. Mazdeh M, Afzali S, Jaafari MR. The therapeutic effect of Avonex, Rebif and Betaferon on EDSS and relapse in multiple sclerosis: A comparative study. Acta Medica Iranica 2010; 48: 83–88. [PubMed] [Google Scholar]
- 68. Medina-Perez C, de Souza-Teixeira F, Fernandez-Gonzalo R, et al. Effects of a resistance training program and subsequent detraining on muscle strength and muscle power in multiple sclerosis patients. Neurorehabilitation 2014; 34(3): 523–530. [DOI] [PubMed] [Google Scholar]
- 69. Millar JH, Vas CJ, Noronha MJ, et al. Long-term treatment of multiple sclerosis with corticotrophin. Lancet 1967; 2: 429–431. [DOI] [PubMed] [Google Scholar]
- 70. National Institutes of Health (NIH). ClinRegs: Aggregating clinical research regulations from around the globe, https://clinregs.niaid.nih.gov/country/united-states# (2023).
- 71. Janiaud P, Hemkens LG, Ioannidis JPA. Challenges and lessons learned from Covid-19 trials—should we be doing clinical trials differently? Can J Cardiol 2021; 37(9): 1353–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. University of Basel . MultiSCRIPT—Treatment Optimization in Multiple Sclerosis. https://dkf.unibas.ch/en/competencies/iict/multiscript/ (2022, accessed 24 September 2023).
- 73. GISSI. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico, https://www.gissi.org/ (accessed 23 September 2023).
- 74. Djulbegovic B, Ioannidis JPA. Precision medicine for individual patients should use population group averages and larger, not smaller, groups. Europ J Clin Invest 2019; 49: e13031. [DOI] [PubMed] [Google Scholar]
- 75. Finlayson M, Al-Mashita L, Sandhu R. Participant diversity in clinical trials of rehabilitation interventions for people with multiple sclerosis: A scoping review. Mult Scler 2023; 29(9): 1149–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Institute of Medicine. Large simple trials and knowledge generation in a learning health system: Workshop summary, https://www.ncbi.nlm.nih.gov/books/NBK201275/#sec_037 (2013, accessed 24 September 2023). [PubMed]
- 77. Rogers A, Paoli G, de Subbarayan S, et al. A systematic review of methods used to conduct decentralised clinical trials. Br J Clin Pharmacol 2022; 88: 2843–2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. University of Dundee. PRECIS-2 toolkit, https://www.precis-2.org/Help/Documentation/Toolkit (2016, accessed 3 October 2023).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-msj-10.1177_13524585231221938 for Use of pragmatic randomized trials in multiple sclerosis: A systematic overview by Julian Hirt, Perrine Janiaud, Pascal Düblin, Giovanni Jacopo Nicoletti, Kinga Dembowska, Thao Vy Thi Nguyen, Tim Woelfle, Cathrine Axfors, Özgür Yaldizli, Cristina Granziera, Jens Kuhle, Ludwig Kappos and Lars G Hemkens in Multiple Sclerosis Journal