Abstract
Western Europe boasts advanced health care systems, robust kidney care guidelines, and a well-established health care workforce. Despite this, significant disparities in kidney replacement therapy incidence, prevalence, and transplant access exist. This paper presents the third International Society of Nephrology Global Kidney Health Atlas’s findings on kidney care availability, accessibility, affordability, and quality in 22 Western European countries, representing 99% of the region’s population. The known chronic kidney disease (CKD) prevalence across Western Europe averages 10.6%, slightly above the global median. Cardiovascular diseases account for a substantial portion of CKD-related deaths. Kidney failure incidence varies. Government health expenditure differs; however, most countries offer government-funded acute kidney injury, dialysis, and kidney transplantation care. Hemodialysis and peritoneal dialysis are universally available, with variations in the number of dialysis centers. Kidney transplantation is available in all countries (except for 3 microstates), with variable transplant center prevalence. Conservative kidney management (CKM) is increasingly accessible. The region’s kidney care workforce is substantial, exceeding global averages; however, workforce shortages are reported. Barriers to optimal kidney care include limited workforce capacity, lack of surveillance mechanisms, and suboptimal integration into national noncommunicable disease (NCD) strategies. Policy recognition of CKD as a health priority varies across countries. Although Western Europe exhibits strong kidney care infrastructure, opportunities for improvement exist, particularly in CKD prevention, surveillance, awareness, and policy implementation. Efforts to improve CKD care should include automated detection, educational support, and enhanced workflows. Based on these findings, health care professionals, stakeholders, and policymakers are called to act to enhance kidney care across the region.
Keywords: chronic kidney disease, dialysis, end-stage kidney disease, Europe, kidney registries, kidney transplantation
Western Europe has established functional health care systems, produced numerous kidney guidelines, and achieved a higher prevalence of health care professionals, greater availability of medicines, and more widespread universal health care coverage compared with other world regions.1 For almost 60 years, the European Renal Association Registry has been collecting data on kidney replacement therapy (KRT) modalities (i.e., hemodialysis [HD], peritoneal dialysis [PD], and transplantation) via national and regional kidney registries from 36 countries in Europe.2 Despite this high level of kidney care and KRT surveillance systems, the European Renal Association Registry and the International Society of Nephrology Global Kidney Health Atlas (ISN-GKHA) have highlighted significant disparities in kidney care across Western European countries.1,3, 4, 5, 6, 7 The incidence and prevalence of treated kidney failure and access to kidney transplantation vary considerably across the region without clear explanation.4,7 For people living with kidney failure in Western Europe, HD remains the dominant dialysis modality, with significant variations across countries.8,9 Home therapies, such as home HD and PD, are underused with significant prevalence variations across countries,8 and access to kidney transplantation is not equitable across Western Europe.3
Since the 2019 ISN-GKHA, kidney medicine across Europe has faced new challenges, including recovery from the COVID-19 pandemic, the war in Ukraine,10,11 the Turkey-Syria earthquake, and rising global temperatures,12 with heatwaves seen across Europe. These challenges have had direct effects on the care of people with kidney disease, though the degree to which they have affected kidney care needs further clarification.
The ISN-GKHA is in its third global iteration. The aim of the ISN-GKHA is to understand, compare, and monitor how different countries around the world detect, treat, monitor, and advocate for people with kidney disease.13 In this second Western Europe–specific report, data from the 2023 ISN-GKHA are presented on the availability, accessibility, affordability, and quality of kidney failure care across the ISN Western Europe region. The methodology for this report is described in detail elsewhere.14
Results
The results of this study are presented in tables and figures and broadly summarized into 2 categories: desk research (Table 1,15,16 Table 2,17,18 Table 3,19, 20, 21, 22, 23, 24, 25, 26 Table 4,27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45 and Supplementary Table S146) and survey administration (Table 5, Table 6, Table 7, Figure 1, Figure 2, Figure 3, Supplementary Figure S1, and Supplementary Tables S2–S10).
Table 1.
| Country | Area (square km) | Total population (2022) | GDP (PPP; $ billion) | Total health expenditures (% of GDP) | Total health spending per persona | Government health spending per persona | Out-of-pocket health spending per persona |
|---|---|---|---|---|---|---|---|
| Overall, median [IQR] | 130,483,015 | 7,802,702,984 | 133.8 [39.7–545.0] | 6.2 [4.3–8.2] | 353 [76–1270] | 216 [23–908] | 92 [29–273] |
| ISN Western Europe region, median [IQR] | 3,801,668 | 439,465,153 | 529.3 [351.7–1523.9] | 9.5 [8.2–10.5] | 5088 [3259–5851] | 3798 [2470–4701] | 637 [589–800] |
| Andorra | 468 | 85,560 | 3.3 | 6.7 | 2875 | 1993 | 358 |
| Austria | 83,871 | 8,913,088 | 523.3 | 10.4 | 5721 | 4361 | 907 |
| Belgium | 30,528 | 11,847,338 | 682.9 | 10.7 | 5088 | 3998 | 848 |
| Denmark | 43,094 | 5,920,767 | 378.6 | 10 | 6300 | 5392 | 767 |
| Finland | 357,022 | 5,601,547 | 304.8 | 9.2 | 4553 | 3666 | 755 |
| France | 643,801 | 68,305,148 | 3424.2 | 11.1 | 4838 | 3629 | 403 |
| Germany | 357,022 | 84,316,622 | 4815.5 | 11.7 | 5838 | 4701 | 627 |
| Greece | 131,957 | 10,533,871 | 333.7 | 7.8 | 1599 | 909 | 514 |
| Iceland | 572 | 357,603 | 21.5 | 8.6 | 5670 | 4779 | 800 |
| Ireland | 70,723 | 5,275,004 | 535.3 | 6.7 | 6128 | 4690 | 637 |
| Israel | 20,770 | 8,914,885 | 409.4 | 7.5 | 3259 | 2241 | 627 |
| Italy | 2586 | 61,095,551 | 2713.3 | 8.7 | 3205 | 2491 | 640 |
| Liechtenstein | 160 | 39,711 | 5.0 | N/A | – | – | – |
| Luxembourg | 2586 | 650,364 | 86.1 | 5.4 | 6782 | 5976 | 609 |
| Malta | 316 | 464,186 | 25.2 | 8.2 | 3553 | 2470 | 1013 |
| The Netherlands | 41,543 | 17,400,824 | 1118.1 | 10.1 | 5621 | 3798 | 535 |
| Norway | 323,802 | 5,553,840 | 428.3 | 10.5 | 7254 | 6300 | 929 |
| Portugal | 450,295 | 10,242,081 | 369.6 | 9.5 | 2230 | 1446 | 589 |
| Spain | 505,370 | 47,163,418 | 1929.8 | 9.1 | 3001 | 2246 | 556 |
| Sweden | 450,295 | 10,483,647 | 617.9 | 10.9 | 5851 | 5046 | 740 |
| Switzerland | 41,277 | 8,508,698 | 672.5 | 11.3 | 9801 | 3290 | 2532 |
| United Kingdom | 243,610 | 67,791,400 | 3344.5 | 10.2 | 4903 | 4093 | 625 |
GDP, gross domestic product; IQR, interquartile range; ISN, International Society of Nephrology; N/A, not applicable; PPP, purchasing power parity.
US$ 2021.
Table 2.
| Country | CKD prevalence, % (95% CI) | Death attributed to CKD, % (95% CI) | DALYS attributed to CKD, % (95% CI) | Obesity, % (95% CI) | Increased BP, % (95% CI) | Smoking, % (95% CI) |
|---|---|---|---|---|---|---|
| Overall, median [IQR] | 9.54 [5.87–11.73] | 2.44 [1.58–3.88] | 1.47 [1.07–2.30] | 21.9 [7.8–26.6] | 24.8 [20.8–28.5] | 13.1 [7.8–20.1] |
| ISN Western Europe region, median [IQR] | 10.63 [9.59–11.57] | 2.59 [2.11–3.42] | 1.32 [1.09–1.59] | 24.5 [23.1–26.9] | 19.4 [18.7–20.6] | 18.8 [17.8–21.2] |
| Andorra | 9.17 (8.48–9.80) | 2.59 (2.19–2.94) | 1.19 (1.01–1.36) | 28.0 (22.0–34.0) | 18.7 (13.3–24.8) | 20.6 (18.1–23.0) |
| Austria | 11.57 (10.73–12.38) | 4.08 (3.47–4.51) | 1.85 (1.61–2.09) | 21.9 (17.5–26.8) | 21.0 (15.7–27.1) | 25.2 (22.8–27.8) |
| Belgium | 11.00 (10.28–11.70) | 2.6 (2.2–2.92) | 1.33 (1.15–1.49) | 24.5 (20.7–28.5) | 17.5 (12.9–23.0) | 18.7 (17.0–20.4) |
| Denmark | 10.63 (9.86–11.37) | 2.05 (1.77–2.26) | 1.23 (1.08–1.38) | 21.3 (17.5–25.3) | 20.6 (15.9–25.7) | 17.5 (16.0–19.1) |
| Finland | 10.22 (9.50–10.89) | 1.26 (1.06–1.4) | 0.77 (0.68–0.86) | 24.9 (21.2–28.7) | 19.4 (15.2–24.1) | 16.8 (15.2–18.6) |
| France | 10.49 (9.77–11.28) | 2.11 (1.71–2.42) | 1.09 (0.94–1.24) | 23.2 (18.8–27.8) | 22.0 (16.6–27.8) | 21.4 (19.3–23.4) |
| Germany | 12.27 (11.57–12.96) | 3.67 (3.15–4.06) | 1.8 (1.57–2.02) | 25.7 (21.9–29.8) | 19.9 (14.7–25.1) | 21.2 (19.2–23.3) |
| Greece | 14.67 (12.73–16.91) | 3.58 (3.13–3.97) | 2.1 (1.85–2.36) | 27.4 (22.5–32.7) | 19.1 (13.8–25.3) | 31.2 (28.9–33.5) |
| Iceland | 7.99 (7.44–8.56) | 1.58 (1.32–1.78) | 0.81 (0.7–0.92) | 23.1 (19.1–27.4) | 19.7 (14.1–25.9) | 14.7 (12.9–16.5) |
| Ireland | 9.59 (9.17–10.04) | 2.16 (1.85–2.43) | 1.08 (0.94–1.23) | 26.9 (22.3–31.7) | 19.7 (14.7–25.3) | 21.6 (19.2–24.2) |
| Israel | 8.45 (7.86–9.05) | 5.77 (4.95–6.37) | 2.45 (2.1–2.83) | 26.7 (21.9–31.6) | 16.6 (11.7–22.4) | 17.8 (15.9–20.0) |
| Italy | 12.17 (11.30–13.06) | 2.57 (2.16–2.81) | 1.39 (1.21–1.55) | 22.9 (19.3–26.8) | 21.2 (16.2–26.3) | 19.4 (17.7–21.4) |
| Liechtenstein | – | – | – | – | – | – |
| Luxembourg | 9.84 (9.16–10.53) | 2.87 (2.43–3.22) | 1.32 (1.14–1.49) | 24.2 (19.4–29.4) | 21.9 (16.3–27.9) | 20.9 (18.4–23.6) |
| Malta | 11.91 (11.03–12.77) | 2.79 (2.39–3.12) | 1.59 (1.38–1.8) | 31.0 (25.1–37.0) | 19.4 (13.8–25.5) | 18.8 (16.6–20.9) |
| The Netherlands | 11.26 (10.47–12.05) | 2.41 (2.06–2.69) | 1.24 (1.08–1.4) | 23.1 (19.3–27.0) | 18.7 (14.4–23.7) | 17.9 (16.2–19.5) |
| Norway | 8.82 (8.20–9.41) | 1.87 (1.61–2.03) | 0.97 (0.84–1.08) | 25.0 (20.9–29.2) | 19.7 (14.7–25.0) | 15.3 (13.6–17.2) |
| Portugal | 11.58 (10.63–12.55) | 3.5 (2.98–3.87) | 1.8 (1.56–2.04) | 23.2 (18.8–27.8) | 24.4 (18.2–31.2) | 17.8 (15.9–19.8) |
| Spain | 10.33 (9.57–11.10) | 3.42 (2.82–3.9) | 1.52 (1.3–1.74) | 27.1 (23.2–31.1) | 19.2 (14.5–24.6) | 21.8 (19.7–24.0) |
| Sweden | 11.14 (10.30–11.95) | 2.13 (1.8–2.35) | 1.13 (0.98–1.28) | 22.1 (18.3–26.0) | 19.3 (14.7–24.3) | 11.4 (10.7–12.2) |
| Switzerland | 11.44 (10.63–12.20) | 3.18 (2.63–3.62) | 1.36 (1.15–1.54) | 21.2 (17.3–25.3) | 18.0 (13.5–23.2) | 19.1 (17.1–21.3) |
| United Kingdom | 9.07 (8.43–9.72) | 1.25 (1.1–1.34) | 0.82 (0.72–0.9) | 29.5 (26.6–32.5) | 15.2 (12.2–18.3) | 18.7 (17.0–20.4) |
BP, blood pressure; CI, confidence interval; CKD, chronic kidney disease; DALY, disability-adjusted life year; IQR, interquartile range; ISN, International Society of Nephrology.
Table 3.
Kidney replacement therapy statistics in the ISN Western Europe region19, 20, 21, 22, 23, 24, 25, 26
| Country | Treated KF |
Chronic dialysis (HD + PD) |
Chronic HD |
Chronic PD |
Kidney transplants overall |
Incidence of deceased donor transplants | Incidence of living donor transplants | Incidence of pre-emptive transplants | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Incidence | Prevalence | Incidence | Prevalence | Incidence | Prevalence | Incidence | Prevalence | Incidence | Prevalence | ||||
| Andorra | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Austria | 135 | 1044 | 128.8 | 536.2 | 118.2 | 495.8 | 10.6 | 40.4 | 34 | 507.1 | 29.33 | 4.67 | 6.7 |
| Belgium | 197 | 1337 | 194.2 | 766.15 | 175.85 | 708.65 | 18.35 | 57.5 | 35.95 | 569.65 | 30.86 | 5.09 | 2.9 |
| Denmark | 108 | 952 | 97.9 | 434 | 61.8 | 346.8 | 36.1 | 87.2 | 43.45 | 517.7 | 31.72 | 11.72 | 10 |
| Finland | 94 | 957 | 91.7 | 361 | 69.2 | 306.8 | 22.5 | 54.2 | 48.73 | 601 | 40.36 | 8.36 | 4.3 |
| France | 170 | 1376 | 162.7 | 746.5 | 146.1 | 700.7 | 16.6 | 45.8 | 49.72 | 622.3 | 42.05 | 7.68 | 7.1 |
| Germany | – | 1114 | – | 808 | – | 768.1 | – | 38.8 | 23.74 | – | 18.08 | 5.66 | – |
| Greece | 269 | 1413 | 267.8 | 1164.4 | 255.1 | 1100.5 | 12.7 | 63.9 | 16.54 | 248.9 | 7.98 | 8.56 | 1.1 |
| Iceland | 108 | 810 | 91.5 | 241.3 | 69.3 | 196.9 | 22.2 | 44.4 | 30 | 565.8 | 23.33 | 6.67 | 13.9 |
| Ireland | 88 | 979 | – | 445 | – | 401.39 | – | 39.16 | 27.8 | 533 | 20.8 | 7 | – |
| Israel | 198 | 755 | – | 756 | – | 698.32 | – | 62.35 | 54.09 | 492 | 16.93 | 37.16 | – |
| Italy | 165 | 1276 | – | 800 | – | 701.8 | – | 89.32 | 31.52 | 476 | 26.83 | 4.69 | – |
| Liechtenstein | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Luxembourg | – | 522 | – | 87 | – | 596.7 | – | 4.7 | 12 | – | 12 | 0 | – |
| Malta | – | – | – | – | – | – | – | – | 7.5 | – | 7.5 | 0 | – |
| The Netherlands | 106 | 1034 | 103.4 | 379 | 83.1 | 322.7 | 20.3 | 56.3 | 53.26 | 695.2 | 26.98 | 26.28 | 16.8 |
| Norway | 100 | 1014.5 | 101.6 | 317.3 | 72.2 | 252.2 | 29.4 | 65.1 | 44.7 | 686.4 | 33.52 | 10.93 | 11.8 |
| Portugal | 260 | 2008 | – | 1302 | – | 871.3 | – | 48.1 | 42.06 | 707 | 38.53 | 3.53 | – |
| Spain | 152 | 1368 | 139.2 | 599.6 | 114.7 | 531.4 | 24.5 | 68.2 | 63.17 | 751.2 | 56.25 | 6.92 | 6.8 |
| Sweden | 113 | 996 | 103.5 | 401.2 | 68.4 | 316.1 | 35.1 | 85.1 | 43.63 | 594.7 | 32.06 | 11.57 | 9.7 |
| Switzerland | 99 | 968 | 93.7 | 447.8 | 80.8 | 408.5 | 12.9 | 39.3 | 41.61 | 519.2 | 27.59 | 14.02 | 5.1 |
| United Kingdom | 151 | 1293 | 102.9 | 414.8 | 81.5 | 489 | 21.4 | 69 | 42.77 | 735 | 32.02 | 10.75 | 12.8 |
HD, hemodialysis; ISN, International Society of Nephrology; KF, kidney failure; PD, peritoneal dialysis.
Table 4.
Annual cost of kidney replacement therapy in the ISN Western European region27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45
| Country | HD | PD | Kidney transplant (first year) | Kidney transplant (later years) | HD/PD ratio |
|---|---|---|---|---|---|
| Austria | – | – | – | – | – |
| Belgium | 60,853 | 29,147 | 76,552 | 22,635 | 2.09 |
| Denmark | 71,318 | 63,935 | 35,737 | 9920 | 1.12 |
| Finland | 65,842 | 31,057 | 27,394 | 578 | 2.12 |
| France | 67,830 | 37,269 | 71,627 | 14,480 | 1.82 |
| Germany | 48,256 | 35,465 | 126,993 | 29,533 | 1.36 |
| Greece | 79,499 | 32,622 | 76,629 | 19,924 | 2.44 |
| Iceland | 24,419 | 27,206 | – | – | 0.90 |
| Ireland | 81,031 | 18,959 | – | – | 4.27 |
| Israel | 66,861 | 40,809 | – | – | 1.64 |
| Italy | 62,082 | 72,627 | – | – | 0.85 |
| Liechtenstein | 32,029 | 21,076 | 96,430 | 14,091 | 1.52 |
| Luxembourg | – | – | – | – | – |
| Malta | – | – | – | – | – |
| The Netherlands | – | – | – | – | – |
| Norway | 99,419 | 66,655 | 118,595 | 41,254 | 1.49 |
| Portugal | 71,791 | 50,534 | 41,108 | 25,242 | 1.42 |
| Spain | 30,361 | 30,361 | 116,952 | 12,379 | 1.00 |
| Sweden | 26,937 | 59,854 | 64,822 | 10,630 | 0.45 |
| Switzerland | 80,520 | – | 94,144 | 18,054 | – |
| United Kingdom | 94,085 | 83,070 | 19,255 | – | 1.13 |
HD, hemodialysis; ISN, International Society of Nephrology; PD, peritoneal dialysis.
Costs in US$ in 2021.
Table 5.
Funding structures for kidney care in the ISN Western Europe region
| Country | Acute kidney injury |
CKD no dialysis |
Dialysis |
Transplantation |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Publicly funded by government; free at the point of delivery | Solely private through health insurance providers | Multiple systems | Publicly funded by government; free at the point of delivery | Publicly funded by government but with some fees at the point of delivery | Mix of public and private funding systems | Solely private through health insurance providers | Multiple systems | Publicly funded by government; free at the point of delivery | Publicly funded by government but with some fees at the point of delivery | Mix of public and private funding systems | Solely private through health insurance providers | Multiple systems | Publicly funded by government; free at the point of delivery | Publicly funded by government but with some fees at the point of delivery | Mix of public and private funding systems | Solely private through health insurance providers | Multiple systems | |
| Andorra | X | X | X | |||||||||||||||
| Austria | X | X | X | X | X | |||||||||||||
| Belgium | X | X | X | X | ||||||||||||||
| Denmark | X | X | X | X | X | |||||||||||||
| Finland | X | X | X | X | ||||||||||||||
| France | X | X | X | X | ||||||||||||||
| Germany | X | X | X | X | ||||||||||||||
| Greece | X | X | X | X | X | |||||||||||||
| Iceland | X | X | X | X | X | |||||||||||||
| Ireland | X | X | X | X | ||||||||||||||
| Israel | X | X | X | X | X | |||||||||||||
| Italy | X | X | X | X | X | |||||||||||||
| Liechtenstein | X | X | X | X | ||||||||||||||
| Luxembourg | X | X | X | X | X | |||||||||||||
| Malta | X | X | X | X | X | |||||||||||||
| The Netherlands | X | X | X | X | ||||||||||||||
| Norway | X | X | X | X | ||||||||||||||
| Portugal | X | X | X | X | ||||||||||||||
| Spain | X | X | X | X | ||||||||||||||
| Sweden | X | X | X | X | ||||||||||||||
| Switzerland | X | X | X | X | ||||||||||||||
| United Kingdom | X | X | X | X | ||||||||||||||
CKD, chronic kidney disease; ISN, International Society of Nephrology.
Table 6.
Availability of KRT centers and nephrology workforce in the ISN Western Europe region
| Country | Chronic HD centers pmp | Chronic PD centers pmp | Transplant centers pmp | Nephrologists pmp | Nephrology trainees pmp |
|---|---|---|---|---|---|
| Andorra | 11.69 | 11.69 | – | 0.00 | 0.00 |
| Austria | 5.61 | 1.12 | 0.45 | 33.66 | 5.61 |
| Belgium | 8.44 | – | 0.59 | 21.10 | 4.22 |
| Denmark | 2.70 | 2.70 | 0.51 | 25.33 | 4.05 |
| Finland | 5.18 | 3.93 | 0.18 | 19.64 | 8.93 |
| France | 9.31 | 2.20 | 0.69 | 17.57 | 5.86 |
| Germany | 8.66 | 2.73 | 0.55 | 27.01 | 0.59 |
| Greece | 18.99 | 2.85 | 0.57 | 36.07 | 9.49 |
| Iceland | 13.98 | 2.80 | 2.80 | 30.76 | 0.00 |
| Ireland | 2.46 | 1.90 | 0.19 | 11.75 | 9.48 |
| Israel | 7.74 | 2.24 | 0.67 | 24.68 | 3.93 |
| Italy | 9.00 | 3.27 | 0.65 | – | 49.10 |
| Liechtenstein | 25.18 | 25.18 | N/A | 100.73 | 0.00 |
| Luxembourg | 7.69 | 1.54 | N/A | 12.30 | 6.15 |
| Malta | 4.31 | 2.15 | 2.15 | – | 17.23 |
| The Netherlands | 3.62 | 3.74 | 0.46 | 15.17 | 2.30 |
| Norway | 4.50 | 4.68 | 0.18 | 27.37 | 9.00 |
| Portugal | 12.89 | 1.95 | 0.78 | 34.17 | 14.65 |
| Spain | 4.24 | 1.70 | 0.85 | 53.01 | 10.60 |
| Sweden | 6.68 | 5.25 | 0.38 | 23.85 | 6.68 |
| Switzerland | 10.58 | 7.05 | 0.12 | 41.13 | 5.88 |
| United Kingdom | 1.00 | 1.00 | 0.34 | 12.13 | 6.15 |
HD, hemodialysis; ISN, International Society of Nephrology; KRT, kidney replacement therapy; N/A, not applicable; PD, peritoneal dialysis; pmp, per million population.
Table 7.
Availability of CKD, dialysis, kidney transplant, AKI, and CKM registries in the ISN Western Europe region
| Country | CKD | Dialysis | Transplant | AKI | CKM |
|---|---|---|---|---|---|
| Andorra | |||||
| Austria | X | X | |||
| Belgium | X | X | |||
| Denmark | X | X | |||
| Finland | X | X | |||
| France | X | X | X | ||
| Germany | X | X | |||
| Greece | X | X | |||
| Iceland | X | X | X | X | |
| Ireland | X | X | |||
| Israel | X | X | |||
| Italy | X | X | |||
| Liechtenstein | |||||
| Luxembourg | X | X | X | ||
| Malta | X | X | |||
| The Netherlands | X | X | |||
| Norway | X | X | X | ||
| Portugal | X | X | |||
| Spain | X | X | |||
| Sweden | X | X | X | X | X |
| Switzerland | X | X | |||
| United Kingdom | X | X | X | X |
AKI, acute kidney injury; CKD, chronic kidney disease; CKM, conservative kidney management; ISN, International Society of Nephrology.
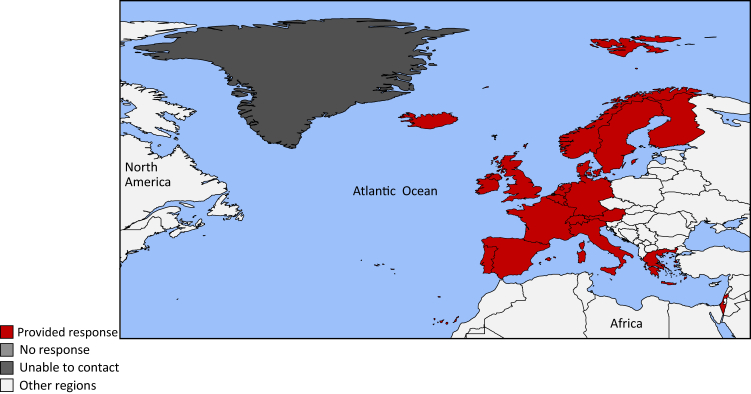
Figure 1.
Countries of the International Society of Nephrology Western Europe region.
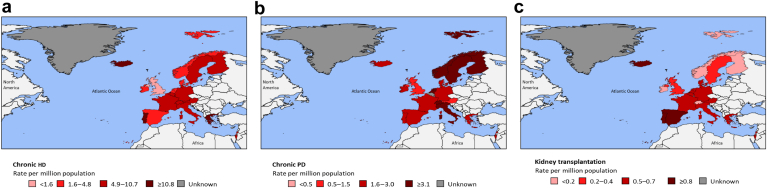
Figure 2.
Availability of kidney replacement therapy centers in the International Society of Nephrology Western Europe region: (a) chronic hemodialysis (HD), (b) peritoneal dialysis (PD), and (c) kidney transplantation.
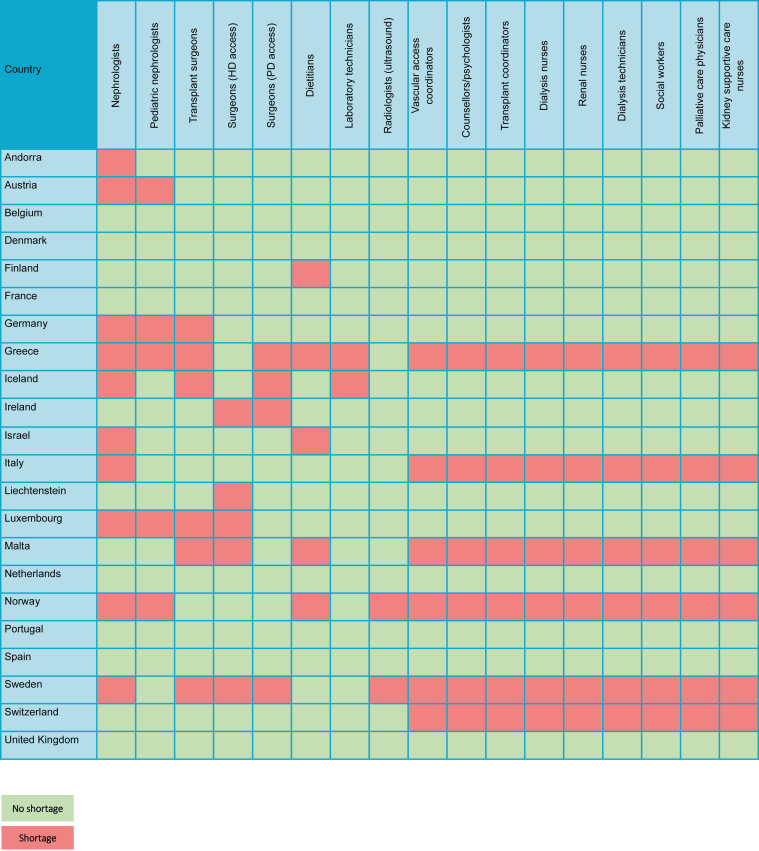
Figure 3.
Workforce shortages for medical kidney care in the International Society of Nephrology Western Europe region. HD, hemodialysis; PD, peritoneal dialysis.
Study setting
For this report, the ISN’s regional classification of countries in Western Europe was used, which includes countries bordering the Mediterranean Sea. Western Europe is one of the richest regions in the world, although variability among countries is observed. Luxembourg, Ireland, Liechtenstein, Switzerland, Norway, and Denmark all appear in the top 10 countries by gross domestic product per capita (Table 1).15 Greenland and Faroe Islands are considered as their own countries by the World Bank, and the data analysis was performed on this basis; however, they are currently part of the Kingdom of Denmark. The Social Progress Index measures the extent to which countries provide for the social and environmental needs of their citizens. Fifteen of the top 20 countries in the Social Progress Index list are in Western Europe,47 whereas 14 of the top 20 countries in the World Happiness Report 2023 are Western European countries.48
The current status of kidney care in the ISN Western Europe
Western Europe is the world region with the highest prevalence of medical doctors (42.3 per million population [pmp]; interquartile range [IQR]: 34.9–50.5 pmp) and nurses (117.8 pmp; IQR: 88.5–167.8 pmp), compared with the median reported worldwide workforce of doctors (17.7 pmp; IQR: 3.8–32.87 pmp) and nurses (36.2 pmp; IQR: 14.4–66.6 pmp; Supplementary Table S1).46 According to the European Renal Association 2020 annual report, the incidence of KRT for the treatment of kidney failure is falling in some Western European countries, while the prevalence continues to grow, currently standing at 1173 pmp.8 Individuals aged over 65 years make up almost half (45%) of the prevalent KRT population.8 Diabetes, hypertension, and renovascular disease account for 26% of the causes of kidney failure in the prevalent population with glomerulonephritis/sclerosis accounting for 18%.8 Almost half of the Western European 2020 prevalent KRT population (47%) were living with a kidney transplant.8 Five-year adjusted patient survival is 46.8% (95% confidence interval [CI]: 46.5–47.1) on dialysis, 92.1% (95% CI: 91.9–92.4) after a first deceased donor transplant, and 95.0% (95% CI: 94.6–95.4) after a first living donor transplant.8
Literature review data for countries in the ISN Western Europe region
The burden of CKD and kidney failure
The prevalence of CKD, defined as stages G1–5/A1–3, ranged from 7.9% (95% CI: 7.4–8.6) in Iceland to 14.7% (95% CI: 12.7–16.9) in Greece with a median of 10.6% (IQR: 9.6%–11.6%; Table 2).17 This was slightly higher than the median for all regions at 9.5% (IQR: 5.9%–11.7%; Table 2).17 The proportion of deaths attributed to CKD was less than 2% in the UK (1.3% [95% CI: 1.1–1.3]), Finland (1.3% [95% CI: 1.1–1.4]), Iceland (1.6% [95% CI: 1.3–1.8]), and Norway (1.9% [95% CI: 1.6–2.0]), whereas it was over 4% in Austria (4.1% [95% CI: 3.5–4.5]) and Israel (5.8% [95% CI: 5.0–6.4]; Table 2).17 The median percentage of deaths worldwide attributed to CKD was 2.4% (IQR: 1.6%–3.9%; Table 2).17
Risk factors associated with CKD also varied substantially across Europe with the proportion of obesity ranging from a fifth of the adult population in Switzerland (21.2% [95% CI: 17.3–25.3]) to almost a third in Malta (31.0% [95% CI: 25.1–37.0]). Hypertension ranged from a sixth of the UK’s adult population (15.2% [95% CI: 12.2–18.3]) to a quarter of Portugal’s adult population (24.4% [95% CI: 18.2–31.2]). One in 10 adults were reported to smoke in Sweden (11.4% [95% CI: 10.7–12.2]), whereas this was almost 1 in 3 adults in Greece (31.2% [95% CI: 28.9–33.5]; Table 2).18
The incidence of the population on KRT failure (dialysis and kidney transplantation) ranged from less than 100 pmp in Ireland, Finland, and Switzerland (88 pmp, 94 pmp, and 99 pmp, respectively) to over 250 pmp in Portugal and Greece (260 pmp and 269 pmp, respectively). Similarly, the prevalence of treated kidney failure ranged from less than 900 pmp in Luxembourg, Israel, and Iceland (522 pmp, 755 pmp, and 810 pmp, respectively) to over 1400 pmp in Greece and Portugal (1413 pmp and 2008 pmp, respectively; Table 3).19, 20, 21, 22, 23, 24, 25, 26 While the median incidence of the population on KRT in the ISN Western Europe region (135.0 pmp [IQR: 106–170 pmp]) was lower than the overall global median (145.5 pmp [IQR: 107.0–212.5 pmp]), the median prevalence of kidney failure was higher (1034.0 pmp [IQR: 957–1337 pmp] for Western Europe and 822.8 pmp [IQR: 556.0–1114.0 pmp] worldwide).19, 20, 21, 22, 23, 24, 25, 26
Most individuals commenced KRT on chronic HD; however, the incidence differed considerably from less than 70 pmp in Denmark, Sweden, Finland, and Iceland (61.8 pmp, 68.4 pmp, 69.2 pmp, and 69.3 pmp, respectively) to over 140 pmp in France, Belgium, and Greece (146.1 pmp, 175.8 pmp, and 255.1 pmp, respectively). Much smaller proportions commenced KRT on PD, with an incidence of less than 15 pmp in Austria, Greece, and Switzerland (10.6 pmp, 12.7 pmp, and 12.9 pmp, respectively). Only Sweden and Denmark had an incidence of greater than 35 pmp (35.1 pmp and 36.1 pmp, respectively). Very few individuals received pre-emptive kidney transplants, which were less than 5 pmp in Greece, Belgium, and Finland (1.1 pmp, 2.9 pmp, and 4.3 pmp, respectively). Pre-emptive kidney transplantation was highest in the UK, Iceland, and the Netherlands (12.8 pmp, 13.9 pmp, and 16.8 pmp, respectively; Table 3).19, 20, 21, 22, 23, 24, 25, 26
The prevalence of chronic HD was lowest in the Nordic countries (Iceland, Norway, Finland, and Sweden) and the Netherlands (196.9 pmp, 252.2 pmp, 306.8 pmp, 316.1 pmp, and 322.7 pmp, respectively) and highest in Belgium, Germany, Portugal, and Greece (708.7 pmp, 768.1 pmp, 871.3 pmp, and 1100.5 pmp, respectively). The prevalence of chronic PD was only 4.7 pmp in Luxembourg, and less than 40 pmp in Germany, Ireland, and Switzerland (38.8 pmp, 39.2 pmp, and 39.3 pmp, respectively), whereas it was over 80 pmp in Sweden, Denmark, and Italy (85.1 pmp, 87.2 pmp, and 89.3 pmp, respectively). Greece, Italy, and Israel had prevalences of individuals living with a kidney transplant of less than 500 pmp (248.9 pmp, 476.0 pmp, and 492.0 pmp, respectively), and Portugal, UK, and Spain had prevalences of over 700 pmp (707.0 pmp, 735.0 pmp, and 751.2 pmp, respectively). While the incidence of living donor kidney transplantation was less than 15 pmp for most countries, it was 26.3 pmp in the Netherlands and 37.2 pmp in Israel (Table 3).19, 20, 21, 22, 23, 24, 25, 26
Overview of gross domestic product and government health expenditure
The total health expenditure as a percentage of gross domestic product ranged from 5.4%, 6.7%, and 6.7% in Luxembourg, Andorra, and Ireland, respectively, to 11.1%, 11.3%, and 11.7% in France, Switzerland, and Germany, respectively, with an average of 9.3%. Total health spending per person ranged 6-fold from US $1599 in Greece to US $9801 in Switzerland, with a median of US $5088 (IQR: US $3259– $5851; Table 1).15 This was considerably higher than the overall health spending of all regions of US $353 (IQR: US $76–$1270; Table 1).15 Government health spending per person ranged 7-fold from US $909 in Greece to US $6300 in Norway, with a median of US $3798 (IQR: US $2470–$4701; Table 1).16 This resulted in an out-of-pocket health spending per person ranging from US $358 in Andorra to US $2532 in Switzerland with a median spend of US $637 (IQR: US $589–$800) per person (Table 1).16 The demographics of the countries in the ISN Western Europe region can be found in Table 1, Table 2, Table 3.15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26
Cost of KRT
The annual cost of HD varied considerably across the region from US $24,419 in Iceland to US $94,085 in the UK. Likewise, the annual cost of PD ranged from US $18,959 in Ireland to US $83,070 in the UK. The first year after kidney transplantation was cheapest in the UK (US $19,255) and most expensive in Germany (US $126,993). Beyond the first year, the differences in the costs of kidney transplantation were striking, ranging from US $578 in Finland to US $41,254 in Norway (Table 4).27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45
Survey response data for the ISN Western Europe region
Characteristics of participating countries
Responses to the survey were received from 22 of 29 countries (75.9%) in the ISN Western Europe region: Andorra, Austria, Belgium, Denmark, Finland, France, Germany, Greece, Iceland, Ireland, Israel, Italy, Liechtenstein, Luxembourg, Malta, the Netherlands, Norway, Portugal, Spain, Sweden, Switzerland, and the UK (Figure 1). These 22 countries had a combined population of 439.5 million people, which represented 99% of the region’s population and covered 3.8 million square kilometers. All countries were classified as high income by the World Bank, with a gross domestic product based on purchasing power parity ranging from US $3.3 billion (2021 US dollars), US $5 billion, and US $21.5 billion in Andorra, Liechtenstein, and Iceland, respectively, to US $3.34 trillion, US $3.42 trillion, and US $4.82 trillion in the UK, France, and Germany, respectively (Table 1).15,49
Health financing for kidney care
In 18 countries (82%), acute kidney injury (AKI), dialysis, and kidney transplantation were publicly funded by the government and free at the point of delivery (Table 5). Liechtenstein relied solely on a private (health insurance) scheme, whereas transplantation in Ireland was publicly funded by the government but included some fees. Sixteen countries (73%) had health care systems that were publicly funded by the government and free at the point of delivery for individuals with non-KRT CKD. Two countries (9%) were publicly funded by the government but included some fees. Two countries (9%) had a mixture of public and private funding systems, and only 1 country (Liechtenstein) had a solely private and out-of-pocket funding system for non-KRT CKD. This was considerably different from the rest of the world where only 27% of non-KRT CKD was publicly funded from the government and 37% was a mixture of public and private financing. Within the ISN Western Europe region, 19 (86%) countries had chronic HD publicly funded by the government and free at the point of delivery, whereas this was the case for only 45% of countries worldwide (Supplementary Table S2).
In the 2023 ISN-GKHA, funding of medications for CKD was free at the point of delivery in Austria, Denmark, Italy, and Malta (18%), whereas funding for medications for dialysis was free at the point of delivery in Austria, Denmark, Greece, Italy, Israel, Malta, and Switzerland (32%; Table 5). Medications for people with CKD and for people receiving dialysis were publicly funded by the government but with some fees in 55% and 45% of countries, respectively. These were much higher rates of government funding of medications than the worldwide median (33% and 28%, respectively). Likewise, 72% of medications for kidney transplant recipients were either publicly funded and free at the point of delivery or with some fees (Table 5), which was higher than the worldwide median of 52%.
Availability of services for the delivery of kidney care
HD and PD were available in all countries of the ISN Western Europe region. The median number of chronic HD centers was 7.7 pmp though this ranged from 1.0 pmp in the UK to 19.0 pmp in Greece (and 25.2 pmp in the microstate of Liechtenstein; Table 6, Figure 2). Home HD for adults and pediatrics was available in 77% of countries (generally not available in Iceland and Israel and not available in Greece, Luxembourg, or Malta). This was higher than the worldwide average of 19%. Chronic PD was available in all countries (2.7 pmp), whereas acute PD was available in only 15 countries (68%; Supplementary Table S3 and Supplementary Figure S1).
Kidney transplantation, both from living and deceased donors, was available in 18 countries (but not in the microstates and small countries of Andorra, Liechtenstein, or Luxembourg; Table 6, Figure 2). These 18 countries all had national waiting lists except Spain, which only had regional waiting lists. The availability of kidney transplantation centers for the region’s countries was 0.69 pmp, ranging from 0.12 pmp in Switzerland to 0.85 pmp in Spain. Kidney transplantation centers were more common in Malta (2.2 pmp) and Iceland (2.8 pmp); however, both countries had populations of fewer than 500,000. Pediatric transplantation was available in 17 countries (77%).
CKM, defined as a planned, holistic, patient-centered care for people living with CKD stage 5, which was a shared decision between the health care worker and the patient, was generally available in 21 (95%) countries This is higher than the worldwide median of 45% (Supplementary Table S4 and Supplementary Figure S1).
Health workforce for the delivery of kidney care
In all countries, bar 2 microstates (91%), nephrologists were primarily responsible for the medical care of individuals with kidney failure. This was slightly higher than the overall global average of 87%. The median number of nephrologists in the ISN Western Europe region was 25.0 pmp (IQR: 16.4–33.9 pmp), which was over double the median number worldwide (11.8 pmp [IQR: 1.8–24.8 pmp]). Within the region, the median prevalence of nephrologists ranged from 11.8 pmp in Ireland to 53.0 pmp in Spain (excluding the microstates). Likewise, the median prevalence of nephrology trainees ranged from 0.59 pmp in Germany to 49.1 pmp in Italy (Table 6). The median prevalence of pediatric nephrologists was 1.6 pmp (IQR: 0.96–2.2 pmp), which was over double the global median prevalence of pediatric nephrologists (0.69 pmp [IQR: 0.03–1.8 pmp]).
There were widespread shortages of health care providers for kidney failure across the region (Figure 3). Only 10 countries (45%) reported sufficient nephrologists, and only 5 countries (23%) reported sufficient pediatric nephrologists. Sixteen countries (73%) reported a shortage of transplant surgeons and HD access surgeons, and 17 countries (77%) reported a shortage of surgeons for PD access. Thirteen countries (59%) required additional counselors or psychologists, and 13 countries (59%) required additional dialysis nurses.
Health information systems, early identification mechanisms for kidney disease, advocacy, and policy for kidney disease
Availability of nephrologists (59%); patient knowledge and attitude (55%); physician availability, access, knowledge, and attitude (50%); and a lack of political will and enabling policies (45%) were the top 4 cited barriers to optimal kidney care in the ISN Western Europe region (Supplementary Table S5).
Country-level national health policies for kidney care were variable in the region, with 14 countries (64%) stating that their country had a national strategy for NCDs (Supplementary Table S6). Only 10 countries (45%) stated that their country had a national strategy for improving the care of people with CKD, with half saying that this strategy was incorporated into an NCD strategy (Supplementary Table S6). Only 5 countries (23%) felt that CKD was a recognized health priority by their country’s government, which was almost half that of the worldwide opinion (48%). This was the same for AKI (23%) though the worldwide opinion was also lower at 19%. The number of countries that felt that their governments recognized kidney failure and/or KRT as a health priority was higher at 64%, but this still left 8 countries (36%) believing that it was not recognized as a health priority.
Dialysis and transplant registries were available in all countries except for the microstates of Andorra and Liechtenstein. CKD registries were less prevalent, being available in only 6 countries (27%), whereas AKI and CKM registries were available in only 2 countries (9%; Table 7).
Causes of hospitalization and death among people living with kidney failure on dialysis
Seventy-three percent of countries reported a mortality of less than 10% in the first year of HD and PD. The commonest cause of death was cardiovascular disease (Supplementary Tables S7 and S8). Approximately half of the countries reported that less than 30% of people receiving HD or PD had at least 1 hospitalization event in the first year of dialysis. The commonest causes of hospitalization among people receiving HD were cardiovascular disease (59% of countries) and access malfunction (14% of countries; Supplementary Table S9). The commonest cases of hospitalization among people receiving PD were cardiovascular disease (32% of countries) and PD-related infection (23% of countries; Supplementary Table S10).
Discussion
The aim of the ISN-GKHA is to understand, compare, and monitor how different countries around the world detect, treat, monitor, and advocate for people with kidney disease.13 In this paper, data from the third ISN-GKHA are presented with a focus on the ISN Western Europe region. Compared with the other regional median values, Western Europe is performing well; however, there are many opportunities for improvement, specifically related to CKD prevention, surveillance, awareness, and policy implementation.
The median prevalence of CKD across the ISN Western Europe region was 10.6%, slightly higher than the median worldwide at 9.5%. Within the region, there was a 2-fold variation between the country with the lowest prevalence, Iceland, and the country with the highest prevalence, Greece. Although caution is required when comparing CKD prevalence due to significant methodological variations in the general population sampling methods and variability in the assessment of kidney function within studies,50 one must still acknowledge that the data are pointing toward 1 in 10 Western Europeans having CKD. CKD is associated with adverse health outcomes, high cardiovascular mortality, and high health care costs. The 2017 Global Burden of Disease study reported a 41.5% increase in the global all-age mortality from CKD between 1990 and 2017.51 The same study reported that most of the burden of CKD was concentrated in the 3 lowest quintiles of the sociodemographic index. Identifying and treating CKD at the earliest stages will not only likely reduce the risk of progression to kidney failure and (cardiovascular) mortality, but it is also an equity imperative.51 A recent Kidney Disease Improving Global Outcomes (KDIGO) Controversies Conference recommended that individuals with hypertension, diabetes, or cardiovascular disease should be screened for CKD.52 Screening the general population for CKD detection is not currently supported by evidence of benefit over costs.53,54 We reported high levels of obesity, hypertension, and smoking across Western Europe, all of which are known risk factors for the development of CKD. Alongside screening for CKD in high-risk populations, preventative measures based around tackling CKD risk factors, such as obesity, hypertension, and smoking, could be an effective CKD preventative measure.
As shown in this study, the ISN Western Europe region has highly developed programs to manage the care of the (relatively small) kidney failure population, with 63% of survey participants reporting that their government recognized kidney failure and KRT as a health priority. Programs to manage the care of the much larger non–KRT-dependent CKD population are less well developed.55 Only 23% felt that CKD was a recognized health priority by their country’s government, which was almost half that of the worldwide opinion (48%). Less than half of the countries surveyed reported that their country had a national strategy for improving the care of people with CKD, with half reporting that this was incorporated into an NCD strategy. Less than a quarter of countries had a national AKI strategy. Even at the European Union level, CKD was not considered in the 2022 Healthier Together European Union NCD Initiative, whereas diabetes, cardiovascular disease, chronic respiratory, mental health, and neurological diseases were.56
Bello et al.55 reported common barriers to the care of people with non–KRT-dependent CKD in 16 Western European countries. These were limited work force capacity, almost a total lack of disease surveillance mechanisms, absence of coordinated CKD care strategies, poor CKD care integration with other NCD control initiatives, and low awareness of the significance of CKD. They suggested potential approaches to improving CKD care by making changes to health systems and policy. These included better models of CKD care, building workforce capacity, incorporating CKD care into (inter)national NCD strategies, and population-based national CKD registries among others.55
Compared with the other regions, the ISN Western Europe region has a much larger medical workforce, typically double the number of adult and pediatric nephrologists compared with the world average and higher government health care expenditure. Despite this, 59% of survey participants reported the insufficient availability of nephrologists as one of the main barriers to optimal kidney care. While increasing the workforce is the obvious solution here, other mechanisms to streamline and improve the delivery of evidence-based CKD care may also play a role. Taylor et al.57 recently reported on the contexts, mechanisms, and outcomes involved in complex interventions to improve CKD care at the primary and secondary care interface. Key intervention components identified were automated identification of higher-risk individuals in primary care with management advice provided to general practitioners, educational support systems, and non–patient-facing nephrologist review. These components had the potential to promote clinician learning while managing individuals with CKD, promote clinician motivation to take steps toward evidence-based CKD management, and integrate dynamically with existing workflows.57
Just under half of countries reported a lack of political will and enabling policies as a barrier to optimal kidney care in the ISN Western Europe region. Bello et al.55 reported low awareness of the importance of CKD among the public, policymakers, and care providers, perpetuating the limitations in optimal kidney care. Surveillance systems such as kidney registries provide data on disease and treatment rates that are needed to advocate for, and support the planning, delivery, and evaluation of kidney services. The data can also highlight discrepancies in available care allowing for the diversion of resources to where they are most needed.58 This region is in a fortunate position of having dialysis and kidney transplant registries available in all countries (except for 2 microstates). In addition, Europe is fortunate to have a European level registry that then collates and presents data allowing for international comparisons and benchmarking.2 Within the ISN Western Europe region, CKD, AKI, and CKM registries were less prevalent, being available in only 6 (27%), 2 (9%), and 2 (9%) countries, respectively. Developing CKD and AKI registries will focus attention on, and effort toward prevention, while data on CKM, be that from patient choice or limited resources, are necessary to advocate for this vulnerable, high-risk group. Although some efforts are being taken to develop these registries,59 collecting both the right data and accurate data,60 especially in the light of limited systematic early CKD detection programs, does hamper their development. The ISN’s SHARing Expertise to support the setup of Renal Registries (SharE-RR) project provides education and support to further establishment and development of kidney registries worldwide.59
Although it is clear that the ISN Western Europe region is performing well in all aspects of kidney care compared with the other 9 ISN regions, improvements are certainly possible and welcome. Western European kidney health care professionals, key stakeholders, and policymakers are called upon to prioritize CKD prevention through awareness campaigns, mitigation of CKD risk factors, and the implementation of surveillance systems, including registries for AKI and non-KRT CKD. These efforts are essential to continue the current positive trend of decreasing the incidence of KRT, a condition that is associated, in both children and adults, with dire clinical and social consequences as well as massive economic costs.61 For people reaching kidney failure, there should be a focus on patient-centered choice for treatments such as CKM and equitable access to home therapies, that is, home HD and PD. Collaborative cooperation is essential to minimize disparities between Western European countries and ensure high standard of treatment for the CKD population living in this region.
There were some limitations to the 2023 ISN-GKHA; these have been discussed.14 One such limitation specific to our region is the lack of more detailed information on pediatric kidney care. Available studies corroborate findings in adults including the availability of pediatric kidney transplantation in 93% of countries, pediatric dialysis facilities for AKI and kidney failure in 95% of countries, and considerable variations in the availability of allied health professionals, high-tech diagnostic methods, and treatment with expensive drugs for children.62 However, this work is important for guiding kidney care policy in the ISN Western Europe region.
Disclosures
GA reports personal fees from Baxter, outside the submitted work. MJS reports grants from Boehringer, Instituto Carlos III, and Marato TV3; personal fees (honoraria) from NovoNordisk, Jansen, Boehringer, Mundipharma, AstraZeneca, Ingelheim Lilly, Vifor, ICU Medical, Fresenius, and Travere Therapeutics; travel support from Travere and Vifor (patent 202131356); and participation on advisory boards for NovoNordisk, Jansen, Boehringer, Mundipharma, AstraZeneca, Ingelheim Lilly, Vifor, ICU Medical, Bayer, GE Healthcare, and Travere Therapeutics, outside the submitted work. SA reports personal fees (salary) from the International Society of Nephrology, outside the submitted work. AKB reports other (consultancy and honoraria) from AMGEN Incorporated and Otsuka, other (consultancy) from Bayer and GSK, and grants from Canadian Institute of Health Research and Heart and Stroke Foundation of Canada, outside the submitted work; he is also an associate editor of the Canadian Journal of Kidney Health and Disease and co-chair of the ISN-Global Kidney Health Atlas. SD reports personal fees (salary) from the International Society of Nephrology, outside the submitted work. J-AD reports personal fees (salary) from the International Society of Nephrology, outside the submitted work. VJ reports personal fees from GSK, AstraZeneca, Baxter Healthcare, Visterra, Biocryst, Chinook, Vera, and Bayer, paid to his institution, outside the submitted work. DWJ reports consultancy fees from Baxter Healthcare, Fresenius Medical Care, AstraZeneca, Bayer, and AWAK; research grants from Baxter Healthcare and Fresenius Medical Care; speaker’s honoraria from Baxter Healthcare, Fresenius Medical Care, ONO, and Boehringer Ingelheim & Lilly; and travel sponsorships from Baxter Healthcare, Fresenius Medical Care, ONO, and Amgen, outside the submitted work. He is also a current recipient of an Australian National Health and Medical Research Council Leadership Investigator Grant, outside the submitted work. CM reports personal fees (salary) from the International Society of Nephrology, outside the submitted work. MN reports grants from KyowaKirin, Boehringer Ingelheim, Chugai, Daiichi Sankyo, Torii, JT, Mitsubishi Tanabe, Takeda, and Bayer, and personal fees from KyowaKirin, Boehringer Ingelheim, Chugai, Daiichi Sankyo, Torii, JT, Mitsubishi Tanabe, Astellas, Akebia, AstraZeneca, and GSK, outside the submitted work. LL reports personal fees from Alexion, AstraZeneca, Biogen Idec, BMS, GSK, Kezar, Novartis, Pfizer, Roche, and Otsuka, outside the submitted work; and is a scientific advisor and investor in Carna Health. All other authors declared no competing interests.
Acknowledgments
The authors appreciate the support from the International Society of Nephrology’s (ISN’s) Executive Committee, regional leadership, and Affiliated Society leaders at the regional and country levels for their help with the ISN–Global Kidney Health Atlas.
Footnotes
Supplementary Table S1. Workforce (specialist physician, doctor, and nurse) prevalences in the International Society of Nephrology (ISN) Western Europe region.∗46
Supplementary Table S2. Health care system funding structure for kidney replacement therapy in the International Society of Nephrology (ISN) Western Europe region, n (100%).
Supplementary Table S3. Availability of in-center hemodialysis, home hemodialysis, and dialysis in the International Society of Nephrology (ISN) Western Europe region, n (100%).
Supplementary Table S4. Availability of choice-restricted conservative kidney management in the International Society of Nephrology (ISN) Western Europe region, n (100%).
Supplementary Table S5. Barriers to optimal kidney care, overall and in the International Society of Nephrology (ISN) Western Europe region, n (100%).
Supplementary Table S6. National health strategies for noncommunicable diseases and chronic kidney disease (CKD)–specific policies in the International Society of Nephrology (ISN) Western Europe region, n (100%).
Supplementary Table S7. Commonest causes of death among people receiving hemodialysis in the International Society of Nephrology (ISN) Western Europe region, n (100%).
Supplementary Table S8. Commonest causes of death among people receiving peritoneal dialysis in the International Society of Nephrology (ISN) Western Europe region, n (100%).
Supplementary Table S9. Commonest causes of hospitalization among people receiving hemodialysis in the International Society of Nephrology (ISN) Western Europe region, n (100%).
Supplementary Table S10. Commonest causes of hospitalization among people receiving peritoneal dialysis in the International Society of Nephrology (ISN) Western Europe region, n (100%).
Supplementary Figure S1. Country-level scorecard on the availability of kidney replacement therapy (KRT), conservative kidney management (CKM), funding for medications, registry, advocacy group, nephrologists, and nephrology trainees in the International Society of Nephrology (ISN) Western Europe region in 2019 and 2023.
Contributor Information
Maria Pippias, Email: maria.pippias@bristol.ac.uk.
Regional Board and ISN-GKHA Team Authors:
Atefeh Amouzegar, Hans-Joachim Anders, Jyoti Baharani, Debasish Banerjee, Boris Bikbov, Edwina A. Brown, Yeoungjee Cho, Kathleen Claes, Naomi Clyne, M. Razeen Davids, Sara N. Davison, Hassane M. Diongole, Smita Divyaveer, Gavin Dreyer, Jan Dudley, Udeme E. Ekrikpo, Isabelle Ethier, Rhys D.R. Evans, Stanley L.S. Fan, Winston Wing-Shing Fung, Maurizio Gallieni, Anukul Ghimire, Ghenette Houston, Htay Htay, Kwaifa Salihu Ibrahim, Georgina Irish, Kailash Jindal, Arif Khwaja, Rowena Lalji, Vassilios Liakopoulos, Valerie A. Luyckx, Manuel Macia, Hans Peter Marti, Piergiorgio Messa, Thomas F. Müller, Aisha M. Nalado, Brendon L. Neuen, Dorothea Nitsch, Fernando Nolasco, Rainer Oberbauer, Mohamed A. Osman, Aikaterini Papagianni, Anna Petrova, Giorgina Barbara Piccoli, Liam Plant, Giuseppe Remuzzi, Parnian Riaz, Joris J. Roelofs, Michael Rudnicki, Syed Saad, Aminu Muhammad Sakajiki, Johannes B. Scheppach, Emily See, Rukshana Shroff, Marit D. Solbu, Stephen M. Sozio, Giovanni FM. Strippoli, Maarten W. Taal, James Tataw Ashu, Sophanny Tiv, Somkanya Tungsanga, Jeroen B. van der Net, Raymond C. Vanholder, Andrea Viecelli, Katie Vinen, Bruno Vogt, Marina Wainstein, Talia Weinstein, David C. Wheeler, Emily K. Yeung, and Deenaz Zaidi
Appendix
The Regional Board and GKHA Team Authors
Atefeh Amouzegar: Division of Nephrology, Department of Medicine, Firoozgar Clinical Research Development Center, Iran University of Medical Sciences, Tehran, Iran
Hans-Joachim Anders: Department of Medicine IV, Hospital of Ludwig Maximilian University of Munich, Munich, Germany
Jyoti Baharani: Department of Renal Medicine, University Hospitals Birmingham, Heartlands Hospital, Birmingham, UK
Debasish Banerjee: Renal and Transplantation Unit, St. George's University Hospitals National Health Service Foundation Trust, Molecular and Clinical Sciences Research Institute, St. George's University of London, London, UK
Boris Bikbov: Scientific-Tools.Org, Global Health Department, Bergamo, Italy
Edwina A. Brown: Imperial College Renal and Transplant Center, Hammersmith Hospital, London, UK
Yeoungjee Cho: Department of Kidney and Transplant Services, Princess Alexandra Hospital, Brisbane, Queensland, Australia; Australasian Kidney Trials Network at the University of Queensland, Brisbane, Queensland, Australia
Kathleen Claes: Department of Nephrology, Dialysis, and Renal Transplantation, University Hospitals Leuven, Leuven, Belgium; Department of Microbiology, Immunology, and Transplantation, Nephrology and Renal Transplantation Research Group, KU Leuven, Leuven, Belgium
Naomi Clyne: Department of Nephrology, Clinical Sciences Lund, Faculty of Medicine, Skåne University Hospital and Lund University, Lund, Sweden
M. Razeen Davids: Division of Nephrology, Department of Medicine, Stellenbosch University and Tygerberg Hospital, Cape Town, South Africa
Sara N. Davison: Division of Nephrology and Immunology, University of Alberta Faculty of Medicine and Dentistry, Edmonton, Alberta, Canada
Hassane M. Diongole: Division of Nephrology, Department of Medicine, National Hospital Zinder, Zinder, Niger; Faculty of Health Sciences, University of Zinder, Zinder, Niger
Smita Divyaveer: Department of Nephrology, Postgraduate Institute of Medical Education and Research, Chandigarh, India
Gavin Dreyer: Department of Nephrology, Barts Health NHS Trust, London, UK
Jan Dudley: Department of Paediatric Nephrology, Bristol Royal Hospital for Children, Bristol, UK
Udeme E. Ekrikpo: Department of Internal Medicine, University of Uyo/University of Uyo Teaching Hospital, Uyo, Nigeria
Isabelle Ethier: Division of Nephrology, Centre Hospitalier de l’Université de Montréal, Montréal, Québec, Canada; Health Innovation and Evaluation hub, Centre de Recherche du Centre Hospitalier de l’Université de Montréal, Montréal, Québec, Canada
Rhys D. R. Evans: University College London Department of Renal Medicine, Royal Free Hospital, London, UK
Stanley L. S. Fan: Department of Renal Medicine and Transplantation, Barts Health NHS Trust, London, UK
Winston Wing-Shing Fung: Department of Medicine & Therapeutics, Prince of Wales Hospital, The Chinese University of Hong Kong, Hong Kong SAR, China
Maurizio Gallieni: Nephrology and Dialysis Unit ASST Fatebenefratelli Sacco, Milano, Italy; Specialty School of Nephrology, Department of Biomedical and Clinical Sciences, University of Milano, Italy
Anukul Ghimire: Division of Nephrology, Department of Medicine, University of Calgary, Calgary, Alberta, Canada
Ghenette Houston: Division of Nephrology and Immunology, Faculty of Medicine and Dentistry, University of Alberta, Edmonton, Alberta, Canada
Htay Htay: Department of Renal Medicine, Singapore General Hospital, Singapore; Duke-NUS Medical School, Singapore
Kwaifa Salihu Ibrahim: Nephrology Unit, Department of Medicine, Wuse District Hospital, Abuja, Nigeria; Department of Internal Medicine, College of Health Sciences, Nile University, Federal Capital Territory, Abuja, Nigeria
Georgina Irish: Australia and New Zealand Dialysis and Transplant (ANZDATA) Registry, South Australia Health and Medical Research Institute, Adelaide, South Australia, Australia; Faculty of Health and Medical Sciences, University of Adelaide, Adelaide, South Australia, Australia; Central and Northern Adelaide Renal and Transplantation Service, Royal Adelaide Hospital, South Australia Health, Adelaide, South Australia, Australia
Kailash Jindal: Division of Nephrology and Immunology, Faculty of Medicine and Dentistry, University of Alberta, Edmonton, Alberta, Canada
Arif Khwaja: Sheffield Kidney Institute, Northern General Hospital, Sheffield, UK
Rowena Lalji: Centre for Kidney Disease Research, University of Queensland, Brisbane, Queensland, Australia; Department of Nephrology, Queensland Children's Hospital, Brisbane, Queensland, Australia; Metro South and Integrated Nephrology and Transplant Services (MINTS), Princess Alexandra Hospital, Brisbane, Queensland, Australia
Vassilios Liakopoulos: 2nd Department of Nephrology, AHEPA University Hospital, Medical School, Aristotle University of Thessaloniki, Thessaloniki, Greece
Valerie A. Luyckx: Department of Public and Global Health, Epidemiology, Biostatistics and Prevention Institute, University of Zurich, Zurich, Switzerland; Renal Division, Brigham and Women's Hospital, Harvard Medical School, Boston, Massachusetts, USA; Department of Paediatrics and Child Health, University of Cape Town, Cape Town, South Africa
Manuel Macia: Department of Nephrology, Hospital Universitario Ntra Sra de Candelaria, Santa Cruz de Tenerife, Spain
Hans Peter Marti: Division of Nephrology, Haukeland University Hospital and University of Bergen, Bergen, Norway
Piergiorgio Messa: Department of Nephrology, Dialysis and Kidney Transplants, IRCCS Ca' Granda Foundation Maggiore Policlinico Hospital, Milan, Italy
Thomas F. Müller: Clinic of Nephrology, University Spital Zürich, Zurich, Switzerland
Aisha M. Nalado: Department of Medicine, Bayero University Kano, Kano, Nigeria
Brendon L. Neuen: Kidney Trials Unit, Royal North Shore Hospital, Sydney, New South Wales, Australia; The George Institute for Global Health, Sydney, New South Wales, Australia
Dorothea Nitsch: Department of Non-communicable Disease Epidemiology, London School of Hygiene & Tropical Medicine, London, UK
Fernando Nolasco: Department of Nephrology, Nova Medical School, Universidade Nova de Lisboa, Lisboa, Portugal
Rainer Oberbauer: Division of Nephrology and Dialysis, Department of Internal Medicine III, Medical University of Vienna, Vienna, Austria
Mohamed A Osman: Department of Family Medicine, University of Ottawa, Ottawa, Ontario, Canada
Aikaterini Papagianni: School of Medicine, Aristotle University of Thessaloniki, Thessaloniki, Greece
Anna Petrova: Department of Propaedeutics of Internal Medicine, Bogomolets National Medical University, Kyiv, Ukraine; Department of Nephrology, “Diavita Institute,” Kyiv, Ukraine
Giorgina Barbara Piccoli: Nephrology, Centre Hospitalier Le Mans, Le Mans, France
Liam Plant: Department of Renal Medicine, Cork University Hospital and University College Cork, Cork, Ireland
Giuseppe Remuzzi: Mario Negri Institute for Pharmacological Research IRCCS, Milan, Italy
Parnian Riaz: Division of Nephrology, Department of Medicine, McMaster University, Hamilton, Ontario, Canada
Joris J. Roelofs: Department of Pathology, Amsterdam University Medical Center, Amsterdam, the Netherlands
Michael Rudnicki: Department of Internal Medicine IV—Nephrology and Hypertension, Medical University Innsbruck, Innsbruck, Austria
Syed Saad: Division of Nephrology and Immunology, Faculty of Medicine and Dentistry, University of Alberta, Edmonton, Alberta, Canada
Aminu Muhammad Sakajiki: Department of Medicine, Usmanu Danfodiyo University and Usmanu Danfodiyo University Teaching Hospital, Sokoto, Nigeria
Johannes B. Scheppach: Department of Nephrology and Medical Intensive Care, Charité—Universitätsmedizin Berlin, Berlin, Germany
Emily See: Department of Nephrology, Royal Melbourne Hospital, Parkville, VIC, Australia; Department of Nephrology, Royal Children's Hospital, Parkville, VIC, Australia; Department of Critical Care, University of Melbourne, Melbourne, Australia
Rukshana Shroff: Paediatric Renal Unit, University College London Great Ormond Street Hospital and Institute of Child Health, London, UK
Marit D. Solbu: Section of Nephrology, University Hospital of North Norway, Tromsø, Norway; Metabolic and Renal Research Group, UiT The Arctic University of Norway, Tromsø, Norway
Stephen M. Sozio: Department of Medicine, Johns Hopkins School of Medicine, Baltimore, Maryland, USA; Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA
Giovanni FM Strippoli: Division of Nephrology, Department of Precision and Regenerative Medicine and Ionian Area (DiMePre-J), University of Bari, Bari, Italy
Maarten W. Taal: Department of Renal Medicine, Royal Derby Hospital, Derby, UK; Centre for Kidney Research and Innovation, School of Medicine, University of Nottingham, Nottingham, UK
James Tataw Ashu: Department of Internal Medicine and Nephrology, Moutier hospital, Moutier, Canton of Berne, Switzerland; Department of Medicine, Service of Nephrology and Hypertension, Geneva University Hospitals, Canton of Geneva, Switzerland
Sophanny Tiv: Division of Nephrology and Immunology, Faculty of Medicine and Dentistry, University of Alberta, Edmonton, Alberta, Canada
Somkanya Tungsanga: Division of Nephrology and Immunology, Faculty of Medicine and Dentistry, University of Alberta, Edmonton, Alberta, Canada; Division of General Internal Medicine-Nephrology, Department of Medicine, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand
Jeroen B. van der Net: Department of Internal Medicine, Albert Schweitzer Hospital, Dordrecht, the Netherlands
Raymond C. Vanholder: Nephrology Section, Department of Internal Medicine and Pediatrics, University Hospital, Ghent, Belgium; European Kidney Health Alliance (EKHA), Brussels, Belgium
Andrea Viecelli: Department of Kidney and Transplant Services, Division of Medicine, Princess Alexandra Hospital, Woolloongabba, Queensland, Australia; University of Queensland, Queensland, Australia; Australasian Kidney Trials Network at the University of Queensland, Brisbane, Queensland, Australia
Katie Vinen: Department of Renal Medicine, King’s College Hospital, Denmark Hill, London, UK
Bruno Vogt: Department of Nephrology and Hypertension, Inselspital, Bern University Hospital, Bern, Switzerland
Marina Wainstein: Faculty of Medicine, University of Queensland, Brisbane, Queensland, Australia; West Moreton Kidney Health Service, Ipswich Hospital, Brisbane, Queensland, Australia
Talia Weinstein: Department of Medicine, Tel Aviv University, Tel Aviv, Israel
David C. Wheeler: Department of Renal Medicine, University College London, London, UK
Emily K. Yeung: Department of Nephrology, Monash Health, Clayton, Victoria, Australia
Deenaz Zaidi: Division of Nephrology and Immunology, Faculty of Medicine and Dentistry, University of Alberta, Edmonton, Alberta, Canada
Regional Board and ISN-GKHA Team Author disclosures
HJA reports personal fees from GSK, Novartis, Bayer, AstraZeneca, Boehringer Ingelheim, Roche, Aptarion, and Otsuka, outside the submitted work. DB reports grants from AstraZeneca ESR and speaker fees from CSL Vifor and Bayer, outside the submitted work. EAB reports grants from Kidney Research UK and personal fees from Baxter Healthcare, Fresenius Medical Care, and Vifor, outside the submitted work. YC reports grants and other from Baxter Healthcare, outside the submitted work. KC reports other from Alexion, AstraZeneca, Astellas, Vifor Pharma, Fresenius Kabi, and Sanofi; and nonfinancial support from GSK, outside the submitted work. MRD reports personal fees (consultancy) from National Renal Care, outside the submitted work, and is the chair of the African Renal Registry and co-chair of the South African Renal Registry. SND reports research funding from Canadian Institutes of Health Research, Alberta Innovates, and Alberta Health services. GD reports personal fees (consulting) from Boerhinger Ingelheim, outside the submitted work. IE reports grants from Fonds de Recherche du Québec—Santé, outside the submitted work. HH reports personal fees from AWAK technology and Baxter Healthcare, and nonfinancial support from Mologic company, outside the submitted work. VL reports personal fees from AstraZeneca, Sanofi, GSK, Alektor, Menarini, and Astellas; and grants from Baxter healthcare, outside the submitted work. VAL reports royalties from Elsevier, consulting fees from the World Health Organization, travel support from the European Renal Association and the International Society of Nephrology (ISN), and a leadership role in Advocacy Working Group of the ISN, outside the submitted work. PM reports unpaid testimony for AIFA, travel support from his organization, and a leadership role in the Italian Society of Nephrology, outside the submitted work. BLN reports personal fees (advisory boards and speaker honoraria) from AstraZeneca and Boehringer Ingelheim; personal fees (advisory boards) from Alexion, Bayer, and Cambridge Healthcare Research; and personal fees (speaker honoraria) from Cornerstone Medical Education, Medscape, and the Limbic, outside the submitted work, with all fees paid to the George Institute for Global Health, outside the submitted work. DN reports grants from the UK Health Foundation, NIHR, MRC, and GSK; and a leadership role in the UKKA, outside the submitted work. RO reports personal fees from Sanofi, CSL Vifor, and Hansa; and other from Alexion, outside the submitted work. AP reports personal fees from Amgen Hellas Ε.P.Ε, Astellas Pharmaceuticals A.Ε.Β.Ε, Baxter Hellas Ε.P.Ε., Genesis Pharma S.A., GlaxoSmithKline A.Ε.Β.Ε., and Menarini Hellas A.E., outside the submitted work. GPB reports personal fees (consultancy honoraria) and other (travel grants) from Fresenius Kabi, outside the submitted work. LP reports personal fees from AstraZeneca, A. Menarini, Boehringer Ingelheim, Servier, MSD, Novatis, Novo Nordisk, and Vifor Pharma; and is President of the Irish Nephrology Society, outside the submitted work. JJR reports personal fees from Novartis, outside the submitted work. MDS reports personal fees from AstraZeneca, Boehringer Ingelheim, Vifor Pharma, and Baxter, outside the submitted work. MWT reports royalties from Elsevier, grants from Fresenius Medical Care, and personal fees from Bayer and Boehringer Ingelheim, outside the submitted work; and is the chair of the International Network of CKD Cohorts. ST reports fellowship grants from the ISN–Salmasi Family and the Kidney Foundation of Thailand, outside the submitted work. RCV reports personal fees from AstraZeneca, GlaxoSmithKline, Fresenius Kabi, Novartis, Kibow, Baxter, Nipro, Fresenius Medical Care, and Nextkidney, outside the submitted work. TW reports consulting fees from GSK, Astellas, Bayer, Boehringer Ingelheim, and AstraZeneca; and personal fees from GSK, Bayer, and AstraZeneca, outside the submitted work; TW also participates on advisory boards for Bayer, Boehringer Ingelheim, and AstraZeneca, outside the submitted work. DCW reports personal fees (consultancy) from AstraZeneca; and personal fees (honoraria) from Astellas, Bayer, Boehringer Ingelheim, Eledon, Galderma, GSK, Jansen, Mundipharma, MSD, Menorini, ProKidney, Pharmacosmos, Tricida, and Vifor, outside the submitted work. All other authors declared no competing interests.
Funding Source
This article is published as part of a supplement sponsored by the International Society of Nephrology with grant funding to the University of Alberta (RES0033080).
Role of the Funder/Sponsor
The International Society of Nephrology provided administrative support for the design and implementation of the study, survey, and data collection activities. The authors were responsible for data management, analysis, and interpretation, as well as manuscript preparation, review, and approval, and the decision to submit the manuscript for publication.
Supplementary Material
Supplementary Table S1. Workforce (specialist physician, doctor, and nurse) prevalences in the International Society of Nephrology (ISN) Western Europe region.∗46
Supplementary Table S2. Health care system funding structure for kidney replacement therapy in the International Society of Nephrology (ISN) Western Europe region, n (100%).
Supplementary Table S3. Availability of in-center hemodialysis, home hemodialysis, and dialysis in the International Society of Nephrology (ISN) Western Europe region, n (100%).
Supplementary Table S4. Availability of choice-restricted conservative kidney management in the International Society of Nephrology (ISN) Western Europe region, n (100%).
Supplementary Table S5. Barriers to optimal kidney care, overall and in the International Society of Nephrology (ISN) Western Europe region, n (100%).
Supplementary Table S6. National health strategies for noncommunicable diseases and chronic kidney disease (CKD)–specific policies in the International Society of Nephrology (ISN) Western Europe region, n (100%).
Supplementary Table S7. Commonest causes of death among people receiving hemodialysis in the International Society of Nephrology (ISN) Western Europe region, n (100%).
Supplementary Table S8. Commonest causes of death among people receiving peritoneal dialysis in the International Society of Nephrology (ISN) Western Europe region, n (100%).
Supplementary Table S9. Commonest causes of hospitalization among people receiving hemodialysis in the International Society of Nephrology (ISN) Western Europe region, n (100%).
Supplementary Table S10. Commonest causes of hospitalization among people receiving peritoneal dialysis in the International Society of Nephrology (ISN) Western Europe region, n (100%).
Supplementary Figure S1. Country-level scorecard on the availability of kidney replacement therapy (KRT), conservative kidney management (CKM), funding for medications, registry, advocacy group, nephrologists, and nephrology trainees in the International Society of Nephrology (ISN) Western Europe region in 2019 and 2023.
References
- 1.Kelly D.M., Anders H.J., Bello A.K., et al. International Society of Nephrology Global Kidney Health Atlas: structures, organization, and services for the management of kidney failure in Western Europe. Kidney Int Suppl. 2021;11:e106–e118. doi: 10.1016/j.kisu.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jager K.J., Wanner C. Fifty years of ERA-EDTA Registry—a registry in transition. Kidney Int Suppl. 2015;5:12–14. doi: 10.1038/kisup.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boenink R., Kramer A., Masoud S., et al. International comparison and time trends of first kidney transplant recipient characteristics across Europe: an ERA Registry study. Nephrol Dial Transplant. Published online August 31, 2023 doi: 10.1093/ndt/gfad189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boenink R., Kramer A., Tuinhout R.E., et al. Trends in kidney transplantation rate across Europe: study from the ERA Registry. Nephrol Dial Transplant. 2023;38:1528–1539. doi: 10.1093/ndt/gfac333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonthuis M., Cuperus L., Chesnaye N.C., et al. Results in the ESPN/ERA-EDTA Registry suggest disparities in access to kidney transplantation but little variation in graft survival of children across Europe. Kidney Int. 2020;98:464–475. doi: 10.1016/j.kint.2020.03.029. [DOI] [PubMed] [Google Scholar]
- 6.Bonthuis M., Vidal E., Bjerre A., et al. Ten-year trends in epidemiology and outcomes of pediatric kidney replacement therapy in Europe: data from the ESPN/ERA-EDTA Registry. Pediatr Nephrol. 2021;36:2337–2348. doi: 10.1007/s00467-021-04928-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pippias M., Jager K.J., Kramer A., et al. The changing trends and outcomes in renal replacement therapy: data from the ERA-EDTA Registry. Nephrol Dial Transplant. 2016;31:831–841. doi: 10.1093/ndt/gfv327. [DOI] [PubMed] [Google Scholar]
- 8.Astley M.E., Boenink R., Abd ElHafeez S., et al. The ERA Registry Annual Report 2020: a summary. Clin Kidney J. 2023;16:1330–1354. doi: 10.1093/ckj/sfad087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Torreggiani M., Piccoli G.B., Moio M.R., et al. Choice of the dialysis modality: practical considerations. J Clin Med. 2023;12:3328. doi: 10.3390/jcm12093328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pawlowicz-Szlarska E., Vanholder R., Sever M.S., et al. Distribution, preparedness and management of Ukrainian adult refugees on dialysis—an international survey by the Renal Disaster Relief Task Force of the European Renal Association. Nephrol Dial Transplant. 2023;38:2407–2415. doi: 10.1093/ndt/gfad073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tuglular S., Luyckx V., Vanholder R., et al. Lessons learned during the war in Ukraine: a report from the Renal Disaster Relief Task Force of the ERA. Nephrol Dial Transplant. 2023;38:1960–1968. doi: 10.1093/ndt/gfad053. [DOI] [PubMed] [Google Scholar]
- 12.Vanholder R., Agar J., Braks M., et al. The European Green Deal and nephrology: a call for action by the European Kidney Health Alliance. Nephrol Dial Transplant. 2023;38:1080–1088. doi: 10.1093/ndt/gfac160. [DOI] [PubMed] [Google Scholar]
- 13.Bello A.K., Johnson D.W., Feehally J., et al. Global Kidney Health Atlas (GKHA): design and methods. Kidney Int Suppl. 2017;7:145–153. doi: 10.1016/j.kisu.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okpechi I.G., Bello A.K., Levin A., Johnson D.W. Update on variability in organization and structures of kidney care across world regions. Kidney Int Suppl. 2024;13:6–11. [Google Scholar]
- 15.Central Intelligence Agency The World Factbook. https://www.cia.gov/the-world-factbook/
- 16.The Institute for Health Metrics and Evaluation Global Health Data Exchange. Global expected health spending 2019-2050. https://ghdx.healthdata.org/record/ihme-data/global-expected-health-spending-2019-2050
- 17.Institute for Health Metrics and Evaluation (IHME) Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2019 (GBD 2019) Results. 2020. https://vizhub.healthdata.org/gbd-results/
- 18.World Health Organization. Global Health Observatory https://www.who.int/data/gho
- 19.European Renal Association ERA Registry Annual Report 2019. https://www.era-online.org/wp-content/uploads/2022/11/ERA-Registry-Annual-Report-2019.pdf
- 20.Finnish Registry for Kidney Diseases Report 2020. https://www.muma.fi/files/5627/Finnish_Registry_for_Kidney_Diseases_Report_2020.pdf
- 21.Jain A.K., Blake P., Cordy P., et al. Global trends in rates of peritoneal dialysis. J Am Soc Nephrol. 2012;23:533–544. doi: 10.1681/ASN.2011060607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nefrovisie, Utrecht, the Netherlands; 2021. Renine Registry annual report 2021. https://www.nefrovisie.nl/wp-content/uploads/2022/02/Jaarrapportage_Renine_2020_web.pdf
- 23.The Norwegian Renal Registry Annual Report 2020. https://www.nephro.no/nnr/AARSRAPPORT_NNR_2020.pdf
- 24.The Renal Association UK Renal Registry 23rd Annual Report. https://ukkidney.org/sites/renal.org/files/23rd_UKRR_ANNUAL_REPORT.pdf
- 25.United States Renal Data System 2019 USRDS Annual Data Report. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD. 2019. https://www.niddk.nih.gov/about-niddk/strategic-plans-reports/usrds/prior-data-reports/2019
- 26.WHO-ONT Global Observatory on Donation and Transplantation. http://www.transplant-observatory.org/data-charts-and-tables/
- 27.Cavallo M.C., Sepe V., Conte F., et al. Cost-effectiveness of kidney transplantation from DCD in Italy. Transplant Proc. 2014;46:3289–3296. doi: 10.1016/j.transproceed.2014.09.146. [DOI] [PubMed] [Google Scholar]
- 28.Ferguson T.W., Harper G.D., Milad J.E., et al. Cost of the quanta SC+ hemodialysis system for self-care in the United Kingdom. Hemodial Int. 2022;26:287–294. doi: 10.1111/hdi.12994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Häckl D., Kossack N., Schoenfelder T. Prävalenz, Kosten der Versorgung und Formen des dialysepflichtigen chronischen Nierenversagens in Deutschland: Vergleich der Dialyseversorgung innerhalb und außerhalb stationärer Pflegeeinrichtungen [Prevalence, costs of medical treatment and modalities of dialysis-dependent chronic renal failure in Germany: comparison of dialysis care of nursing home residents and in outpatient units] Gesundheitswesen. 2021;83:818–828. doi: 10.1055/a-1330-7152. [in German] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haller M., Gutjahr G., Kramar R., et al. Cost-effectiveness analysis of renal replacement therapy in Austria. Nephrol Dial Transplant. 2011;26:2988–2995. doi: 10.1093/ndt/gfq780. [DOI] [PubMed] [Google Scholar]
- 31.Helanterä I., Isola T., Lehtonen T.K., et al. Association of clinical factors with the costs of kidney transplantation in the current era. Ann Transplant. 2019;24:393–400. doi: 10.12659/AOT.915352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jakobsen A., Albrechtsen D., Sødal G., et al. Kostnader ved uremikeromsorg. Hva koster nyretransplantasjon? [Costs of care in uremia. How much does kidney transplantation cost?] Tidsskr Nor Laegeforen. 1990;110:338–341. [in Norwegian] [PubMed] [Google Scholar]
- 33.Jarl J., Desatnik P., Peetz Hansson U., et al. Do kidney transplantations save money? A study using a before-after design and multiple register-based data from Sweden. Clin Kidney J. 2018;11:283–288. doi: 10.1093/ckj/sfx088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jensen C.E., Sørensen P., Petersen K.D. In Denmark kidney transplantation is more cost-effective than dialysis. Dan Med J. 2014;61:A4796. [PubMed] [Google Scholar]
- 35.Jürgensen J.S., Ikenberg R., Greiner R.A., et al. Cost-effectiveness of modern mTOR inhibitor based immunosuppression compared to the standard of care after renal transplantation in Germany. Eur J Health Econ. 2015;16:377–390. doi: 10.1007/s10198-014-0579-3. [DOI] [PubMed] [Google Scholar]
- 36.Kerr M., Bray B., Medcalf J., et al. Estimating the financial cost of chronic kidney disease to the NHS in England. Nephrol Dial Transplant. 2012;27(suppl 3):iii73–iii80. doi: 10.1093/ndt/gfs269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li B., Cairns J.A., Fotheringham J., et al. Understanding cost of care for patients on renal replacement therapy: looking beyond fixed tariffs. Nephrol Dial Transplant. 2015;30:1726–1734. doi: 10.1093/ndt/gfv224. [DOI] [PubMed] [Google Scholar]
- 38.Mohnen S.M., van Oosten M.J.M., Los J., et al. Healthcare costs of patients on different renal replacement modalities—analysis of Dutch health insurance claims data. PLoS One. 2019;14 doi: 10.1371/journal.pone.0220800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roberts G., Holmes J., Williams G., et al. Current costs of dialysis modalities: a comprehensive analysis within the United Kingdom. Perit Dial Int. 2022;42:578–584. doi: 10.1177/08968608211061126. [DOI] [PubMed] [Google Scholar]
- 40.Rocha M.J., Ferreira S., Martins L.S., et al. Cost analysis of renal replacement therapy by transplant in a system of bundled payment of dialysis. Clin Transplant. 2012;26:529–531. doi: 10.1111/j.1399-0012.2011.01571.x. [DOI] [PubMed] [Google Scholar]
- 41.Sandoz M.S., Ess S.M., Keusch G.W., et al. Prevalence and direct medical costs of end-stage renal disease in patients with type 2 diabetes mellitus in Switzerland for 2001. Swiss Med Wkly. 2004;134:448–458. doi: 10.4414/smw.2004.10682. [DOI] [PubMed] [Google Scholar]
- 42.Van Biesen W., Lameire N., Peeters P., et al. Belgium's mixed private/public health care system and its impact on the cost of end-stage renal disease. Int J Health Care Finance Econ. 2007;7:133–148. doi: 10.1007/s10754-007-9013-z. [DOI] [PubMed] [Google Scholar]
- 43.van der Tol A., Lameire N., Morton R.L., et al. An international analysis of dialysis services reimbursement. Clin J Am Soc Nephrol. 2019;14:84–93. doi: 10.2215/CJN.08150718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Villa G., Rodríguez-Carmona A., Fernández-Ortiz L., et al. Cost analysis of the Spanish renal replacement therapy programme. Nephrol Dial Transplant. 2011;26:3709–3714. doi: 10.1093/ndt/gfr088. [DOI] [PubMed] [Google Scholar]
- 45.Zambrowski J.J. Coût de la dialyse [Cost of dialysis in France] Nephrol Ther. 2016;12(suppl 1):S95–S97. doi: 10.1016/j.nephro.2016.02.002. [in French] [DOI] [PubMed] [Google Scholar]
- 46.World Health Organization . Health workforce; 2021. The Global Health Observatory.https://www.who.int/data/gho/data/themes/health-workforce [Google Scholar]
- 47.Social Progressive Imperative Global Index: results. https://www.socialprogress.org/index/global/results/
- 48.Sustainable Development Solutions Network World Happiness Report 2023. https://worldhappiness.report/ed/2023/
- 49.The World Bank World Development Indicators. The world by income and region. https://datatopics.worldbank.org/world-development-indicators/the-world-by-income-and-region.html
- 50.Bruck K., Jager K.J., Dounousi E., et al. Methodology used in studies reporting chronic kidney disease prevalence: a systematic literature review. Nephrol Dial Transplant. 2015;30(suppl 4):iv6–iv16. doi: 10.1093/ndt/gfv131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.GBD Chronic Kidney Disease Collaboration Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395:709–733. doi: 10.1016/S0140-6736(20)30045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shlipak M.G., Tummalapalli S.L., Boulware L.E., et al. The case for early identification and intervention of chronic kidney disease: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2021;99:34–47. doi: 10.1016/j.kint.2020.10.012. [DOI] [PubMed] [Google Scholar]
- 53.Jain V., Sinha S., Shaw C., et al. Re-evaluating national screening for chronic kidney disease in the UK. BMJ. 2023;382 doi: 10.1136/bmj-2022-074265. [DOI] [PubMed] [Google Scholar]
- 54.Bochud M. On the rationale of population screening for chronic kidney disease: a public health perspective. Public Health Rev. 2015;36:11. doi: 10.1186/s40985-015-0009-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bello A.K., Levin A., Manns B.J., et al. Effective CKD care in European countries: challenges and opportunities for health policy. Am J Kidney Dis. 2015;65:15–25. doi: 10.1053/j.ajkd.2014.07.033. [DOI] [PubMed] [Google Scholar]
- 56.Directorate-General for Health and Food Safety. EU Non-Communicable Diseases (NCD) Initiative: guidance document. https://health.ec.europa.eu/publications/eu-non-communicable-diseases-ncds-initiative-guidance-document_en
- 57.Taylor D.M., Nimmo A.M., Caskey F.J., et al. Complex interventions across primary and secondary care to optimize population kidney health: a systematic review and realist synthesis to understand contexts, mechanisms, and outcomes. Clin J Am Soc Nephrol. 2023;18:563–572. doi: 10.2215/CJN.0000000000000136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alwan A., Maclean D.R., Riley L.M., et al. Monitoring and surveillance of chronic non-communicable diseases: progress and capacity in high-burden countries. Lancet. 2010;376:1861–1868. doi: 10.1016/S0140-6736(10)61853-3. [DOI] [PubMed] [Google Scholar]
- 59.Hole B.D., Evans K.M., Pyart R., et al. International collaborative efforts to establish kidney health surveillance systems. Kidney Int. 2020;98:812–816. doi: 10.1016/j.kint.2020.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nistor I., Bolignano D., Haller M.C., et al. Why creating standardized core outcome sets for chronic kidney disease will improve clinical practice. Nephrol Dial Transplant. 2017;32:1268–1273. doi: 10.1093/ndt/gfv365. [DOI] [PubMed] [Google Scholar]
- 61.Kidney Research UK Kidney disease: a UK public health emergency. The health economics of kidney disease to 2033. https://www.kidneyresearchuk.org/wp-content/uploads/2023/06/Economics-of-Kidney-Disease-full-report_accessible.pdf
- 62.Prikhodina L., Ehrich J., Shroff R., et al. The European Society for Paediatric Nephrology study of pediatric renal care in Europe: comparative analysis 1998-2017. Pediatr Nephrol. 2020;35:103–111. doi: 10.1007/s00467-019-04378-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.