Abstract
Necroptosis is a caspase-independent form of programmed cell death executed by the receptor interacting protein kinase 1 (RIPK1)-RIPK3-mixed lineage kinase domain-like protein (MLKL) signaling cascade, deregulation of which can cause various human diseases including cancer. Escape from programmed cell death is a hallmark of cancer, leading to uncontrolled growth and drug resistance. Therefore, it is crucial to further understand whether necroptosis plays a key role in therapeutic resistance. In this review, we summarize the recent findings of the link between necroptosis and cancer, and discuss that targeting necroptosis is a new strategy to overcome apoptosis resistance in tumor therapy.
Keywords: Necroptosis, RIPK1, RIPK3, Inflammation, Tumorigenesis
1. Regulation and mechanisms of necroptosis
Apoptosis is the best described programmed cell death [1] induced by various intrinsic and extrinsic factors and is mediated by the activation of caspases. Several types of regulated cell death (RCD), beyond apoptosis, have been characterized, such as pyroptosis, ferroptosis, necroptosis, and cuprotosis [2,3]. Pyroptosis, ferroptosis, and necroptosis are categorized as regulated necrosis. Pyroptosis is an inflammation-induced and highly immunogenic RCD [2]. Ferroptosis is an iron-induced RCD characterized by lipid peroxidation and iron dependence, showing promise of potential antitumor treatment [4,5]. On the other hand, cuprotosis is a recently discovered form of intracellular cupper-dependent RCD driven by copper-induced mitochondrial stress [3].
Necroptosis is best-studied regulated necrotic cell death, which is activated in a caspase-independent manner and characterized as a lytic form of cell death [6–8]. Necroptosis is accompanied by the release of damage-associated molecular patterns (DAMPs) and cytokines, thereby triggering proinflammatory responses. On the other hand, apoptosis is a non-lytic form of cell death in which cellular contents are kept inside apoptotic bodies and thus causes no inflammatory effects. Necroptosis is induced in apoptosis-deficient cells. Therefore, it is regarded as a backup cellular defense mechanism. Necroptosis exerts essential physiological function in development and tissue homeostasis such as antiviral defense and tissue injury repair. Dysregulated necroptosis leads to various pathological conditions, such as chronic intestinal inflammation [9,10], inflammatory skin disease [11,12], non-alcoholic steatohepatitis (NASH) [13,14], multiple sclerosis (MS) [15], and amyotrophic lateral sclerosis (ALS) [16]. In addition, recent findings have revealed a tight correlation between necroptosis and cancer, which has attracted increasing attention. Therefore, understanding the precise molecular mechanism of necroptosis is essential to design possible therapeutic intervention strategies for various necroptosis-mediated human diseases.
Necroptosis is induced by the ligands of the death receptor family, such as tumor necrosis factor (TNF)-α, Fas Ligand (FasL), and TNF-related apoptosis-inducing ligand (TRAIL), by binding to their cognate receptors when caspases are blocked by chemical inhibitors or genetic knockout [17] (Fig. 1). TNF/TNF receptor 1 (TNFR1)-mediated necroptosis is the most well-studied caspase-independent necrosis [6,18,19]. Mechanistically, TNF-α binding to TNFR1 induces the formation of a complex called the TNFR1 signaling complex–complex I, where RIPK1 is ubiquitinated by upstream E3 ligases, including cellular inhibitor of apoptosis (cIAP) 1/2 and linear ubiquitin chain assembly complex (LUBAC) [20–24]. The main downstream pathways of the complex I are NF-κB and mitogen-activated protein kinases (MAPKs), which promote cellular survival [25]. RIPK1 will be deubiquitinated by cylindromatosis protein (CYLD) and A20 when cIAP1/2 is degraded by the small-molecule, second mitochondria-derived activator of caspase (SMAC) mimetics [26–29]. The deubiquitination of RIPK1 triggers RIPK1 dissociation from the complex I and subsequently promotes the formation of complex IIa (RIPK1, FAS-associated death domain protein (FADD), caspase-8, etc.) [30,31], which can cause the activation of caspase-8 and induction of apoptosis. Biologically, activated caspase 8 cleaves RIPK1 and RIPK3 to block the formation of complex IIb (caspase-8, FADD, RIPK1, RIPK3, and MLKL) and the induction of necroptosis. When caspases are blocked by inhibitors (e.g., zVAD) or by the genetic deletion, which prevents the cleavage of the key necroptosis regulators RIPK1 and RIPK3, complex IIb or necrosome will be formed in a RIPK1-kinase-dependent manner [32,33]. In the necrosome, RIPK1 binds to RIPK3 through their respective RIP homotypic interaction motif (RHIM) domains to activate RIPK3, which in turn mediates the phosphorylation and activation of the downstream substrate, MLKL. Subsequently, oligomerization of MLKL disrupts the integrity of plasma membranes and causes cell death [34,35] (Table 1).
Fig. 1.
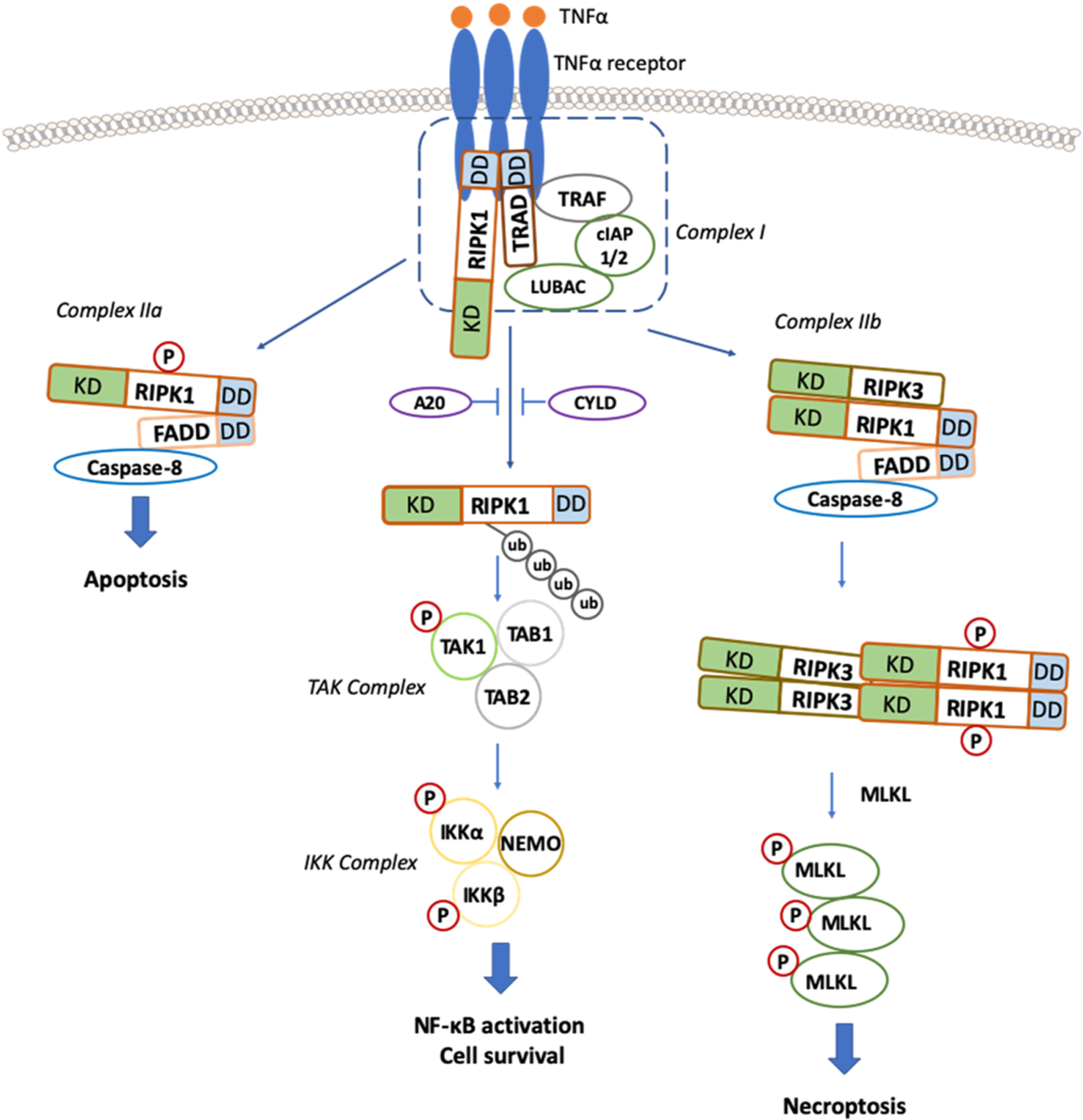
Canonical TNF/TNFR1 dependent Necroptosis Signaling Cascades. Upon TNF-α stimulation, the activated TNF receptor (TNFR) interacts with TNFR1-associated death domain protein (TRADD), TRAFs, and RIPK1 and recruits cIAP1 and cIAP2 to form a plasma membrane-associated complex, resulting in RIPK1 polyubiquitination. Inhibition of cIAPs (by SMAC or SMAC mimetics) leads to deubiquitination of RIPK1 and dissociation of RIPK1 from the complex. RIPK1 then binds to FADD and caspase-8 to form a complex called complex IIa, which activates caspase-8 and leads to apoptosis induction. When caspase-8 activity is blocked, RIPK1 binds to RIPK3 to form a complex IIb or necrosome in a RIPK1-kinase-dependent manner. Then, RIPK3 undergoes auto-phosphorylated and subsequent activation, allowing RIPK3 to recruit and phosphorylate MLKL. The translocation of phosphorylated MLKL to the plasma membrane promotes necroptosis by disrupting plasma and intracellular membrane integrity. KD: kinase domain, DD: death domain.
Table 1.
Necroptotic factors and their roles in necroptosis.
| Necroptotic factors | Key roles in necroptosis | Antagonists | |
|---|---|---|---|
| RIPK1 | Being deubiquitinated under apoptosis-deficient conditions, interacts with RIPK3 to form necrosome | Necrostatin-1 (Nec-1) | [6] |
| RIPK3 | Forms necrosome with RIPK1 and phosphorylates MLKL | GSK843, GSK872 | [36] |
| MLKL | Being phosphorylated by RIPK3, oligomerizes and translocates to plasma membrane to induce membrane permeabilization and necroptosis | Necrosulfonamide (NSA) | [37] |
| cIAP1/2 | Polyubiquitinates RIPK1 via K63-linkage to induce NF-κB signaling | SMAC mimetics | [38] |
| LUBAC | Polyubiquitinates RIPK1via M1-linkage to induce NF-κB signaling | ||
| CYLD | Deubiquitinates RIPK1 to promote complex II formation | ||
| A20 | Deubiquitinates RIPK1 to promote complex II formation |
In addition to these death-receptor ligand-induced necroptosis, lipopolysaccharides (LPS)–toll-like receptor 4 (TLR4) or poly(I:C)–TLR3 treatment with the inhibition of caspases by zVAD-fmk can also induce TIR domain-containing adaptor-inducing interferon-β (TRIF)-mediated necroptosis [39,40] (Fig. 2). TRIF triggers the activation of RIPK3 by the direct RHIM-RHIM interaction to initiate necroptosis [41,42]. On the other hand, z-DNA/RNA binding protein-1 (ZBP1), which was suggested as a cytoplasmic DNA sensor capable of inducing type I interferons (IFNs), and nuclear factor-κB (NF-κB) activation, has been shown to induce RIPK3-mediated necroptosis independently of RIPK1 in response to double-stranded DNA [43–47]. Additionally, interferon has been reported to induce RIPK1/RIPK3-mediated necrosis when caspase-8 or FADD was absent [48,49].
Fig. 2.
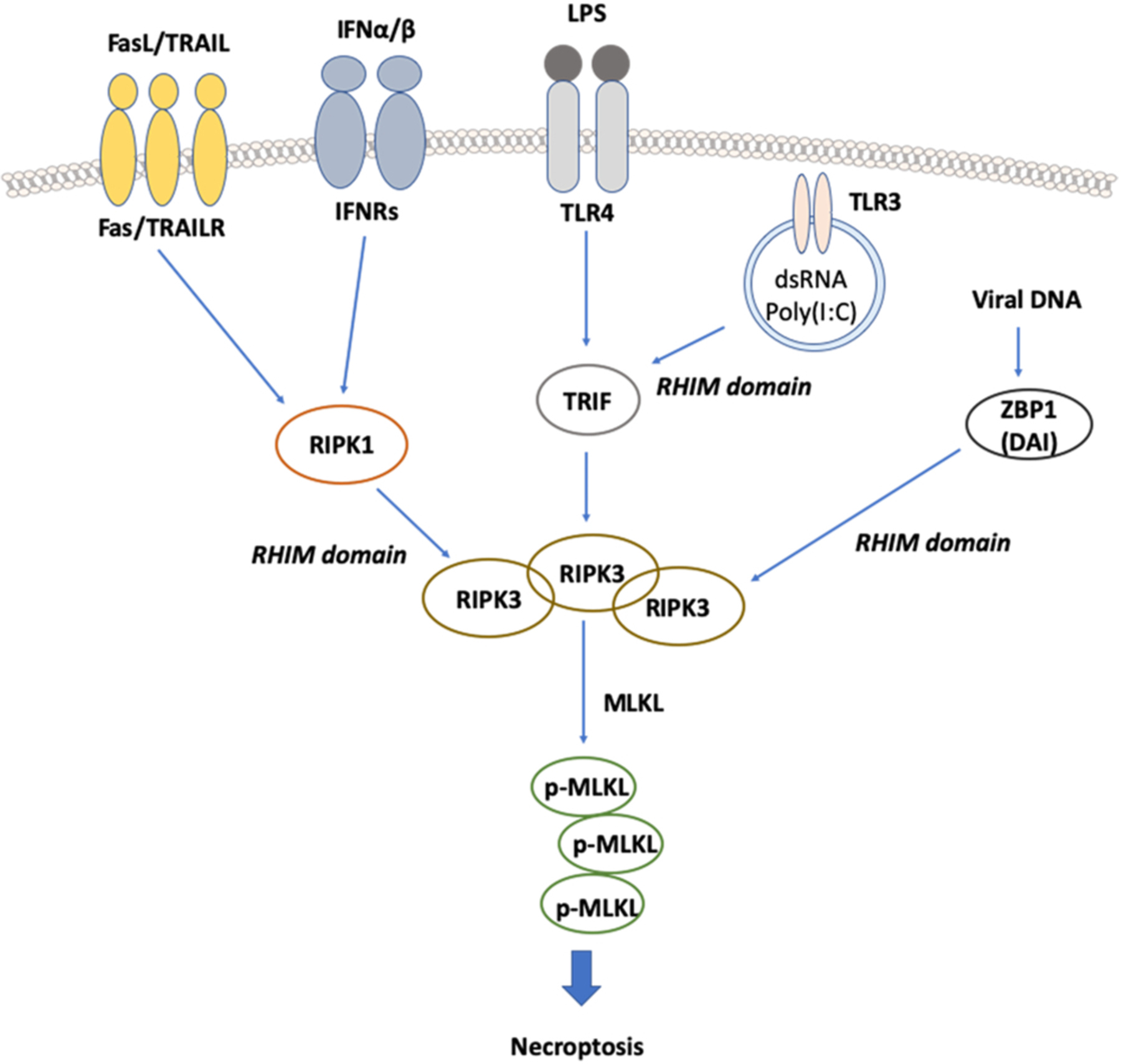
Other stimuli that are capable of inducing necroptosis. FASL, TRAIL, LPS, dsRNA (such as poly (I:C)), and interferon γ (IFNγ), can stimulate their respective receptors to activate TRIF, RIPK1, and subsequent binding to RIPK3 via their RHIM domain to activate RIPK3. Viral infection directly activates RIPK3 through ZBP1/DAI (DNA-dependent activator of interferon regulatory factors) binding to RIPK3. Activated RIPK3 then phosphorylates MLKL and causes the oligomerization of MLKL, membrane insertion of MLKL oligomers, and disruption of plasma and intracellular membrane integrity to induce necroptosis.
2. Downregulation in the core necroptotic factors in cancer
It has been reported that the key mediators of necroptosis, including RIPK3, MLKL, and CYLD, are downregulated in different cancer cells (Table 2). CYLD is a deubiquitinating enzyme identified as a key positive regulator of TNF/TNFR1-mediated necroptosis [26,27,50]. IKKβ-dependent CYLD phosphorylation at Ser568 following TNFR1 stimulation potentiates its deubiquitinase (DUB) activity towards K-63 linked ubiquitin chain on RIPK1 [51]. CYLD was originally demonstrated as a tumor suppressor gene, which was reported to be mutated in Familial Cylindromatosis [52]. These mutations have been found in a variety of human cancers and can lead to resistance to chemotherapy. Lymphoid enhancer-binding factor 1 (LEF1), a downstream effector of the Wnt/β-catenin pathway, is likely responsible for repressing CYLD at the transcriptional levels [53]. Moreover, Snail was found to negatively regulate the transcription levels of CYLD, which promotes tumor progression in malignant melanoma [54]. It was also found that the expression of CYLD was decreased in the colon and hepatocellular carcinoma cells and tumor samples [55].
Table 2.
Downregulations and mutations of necroptotic factors in cancer.
| Cancer | Necroptotic factors downregulated in cancer | |
|---|---|---|
| Acute myeloid leukemia | RIPK3 | [64] |
| Chronic lymphocytic leukemia | CYLD | [53] |
| Melanoma | RIPK3, CYLD | [54] [65] |
| Breast cancer | RIPK3 | [66] |
| Colorectal cancer | RIPK3, MLKL, CYLD | [55] [56] [67] |
| Head and neck squamous cell carcinoma | RIPK1 | [68] |
| Gastric cancer | MLKL | [69] |
| Ovarian cancer | MLKL | [70] |
| Mutation | Expected effect on function | |
| RIPK3 V458M | Identified in RHIM domain: Loss-of-function | [60] |
| MLKL L291P | Identified in pseudokinase domain: Loss-of-function | [63] |
It has been well demonstrated that DNA methylation plays a key role in the silence of the expression of RIPK3. Notably, RIPK3 expression is often silenced in cancer cells in a methylation-dependent mechanism in many cancer cell lines, which may explain why cancer cells can also escape from the caspase-independent necroptosis [56–58]. Consistent with this finding, treatment with the hypomethylating agent decitabine (5-AD) or knockdown of DNMT1 (DNA cytosine-5-methyltransferases) restored RIPK3 expression in multiple cell lines, thereby promoting sensitivity to necroptosis inducers in a RIPK3-dependent manner [58]. There is growing evidence suggesting that RIPK3 silencing in tumor cells is selected during the process of tumor progression, and RIPK3 down-regulation confers cancer cells to chemotherapeutic resistance in cancers [58,59]. Thus, reactivation of RIPK3 expression in cancer cells by using hypomethylating agents might provide an opportunity for triggering chemotherapy-induced cell death. Furthermore, somatic V458M mutations, which resides within RIPK3 RHIM domain, is likely a loss-of-function mutation based on the biochemical analysis showing that V458 is a critical residue for RHIM-mediated protein interaction [60].
The MLKL expression levels were decreased in several cancer cells, including pancreatic adenocarcinoma and primary ovarian cancers, and the downregulation of MLKL was correlated with decreased patient survival [61]. In addition, in human tumor progression, mutations in F398I and L291P have been identified in the pseudokinase domain of MLKL [62]. Ectopic expression of MLKL L291P mutant, but not F398I, facilitates the cells resistant to TNF/TNFR1-mediated necroptosis, suggesting that the L291P is a loss-of-function mutation [63] (Table 2).
3. Necroptosis in tumorigenesis
It has been reported that several necroptosis-related factors exert their function in tumorigenesis of a range of cancers. Reactive oxygen species (ROS) production and cytochrome c release accompanied with mitochondrial dysfunction was induced by TNFα via RIPK1 [71]. Tumor cell necroptosis was induced by Tag7-Hsp70 cytotoxic complex through permeabilization of lysosomes and mitochondria [72]. In prostate adenocarcinoma cell line PC-3, ROS-mediated necroptosis was induced by biogenic selenium nanoparticles through TNF activation, independent of RIPK3 and MLKL, regulated by RIPK1 [73]. In prostate cancer cells under lactic acidosis, necroptosis was induced through mitochondrial dysfunction with the cell communication network factor 1 (CCN1) [74]. In human colon cancer cells, RIPKs collaborated with ROS during necroptosis, which was promoted by z-VAD and cobalt chloride, a reagent inducing hypoxia-inducible factor-1α (HIF1α) expression and mimicking the hypoxic microenvironment of tumor tissue [75]. In human gastric cancer cells, caspase-independent necroptosis was induced by HUHS1015, a newly synthesized naftopidil analog, in association with the accumulation of apoptosis-inducing factor-homologous mitochondrion-associated inducer of death (AMID) in the nucleus [76]. In addition, p53 mutation, SMAC mimetics, and other factors were reported to be involved in the necroptosis of tumors. In colorectal cancer, necroptosis was rare, and p53 mutation might result in autophagy upregulation [77]. Necroptosis was promoted by SIRT3, inhibiting the growth of human small-cell lung cancer cells, and the expression of SIRT3 could regulate the stability of mutant p53 by controlling ubiquitination-mediated proteasomal degradation of the protein [78]. In necroptosis of colon cancer cells, AMP-activated protein kinase (AMPK) played an inhibitory role with p53 null mutation under nutrient starvation [79]. In apoptosis-resistant cancer cells, necroptosis was triggered by IFNγ synergizing with SMAC mimetics [80]. Caspase inhibition combined with SMAC analog, LCL161, induced a necroptosis effect on human breast cancer drug-resistant cells [81,82]. RIPK3 expresses in mouse models of colorectal cancer and a subset of human colorectal cancer cells appears to be the deciding factor of cancer cell susceptibility to SMAC mimetic-induced necroptosis [59].
In many types of cancers, necroptosis is found to be actively involved. In lung cancer, necroptosis was triggered by Betanodavirus B2 protein [83]. Low-level expression of necroptosis factors, such as RIPK3 and PELI1, combined with high-level expression of the DNA damage response factor p53, served as an important indicator in predicting the survival of stage I non-small-cell lung cancer (NSCLC) patients with the squamous cell carcinoma subtype [84]. In colorectal cancer cells, PFK-15 induced genome instability and necroptosis were induced by PFK-15, of which cytotoxicity and genotoxicity were attenuated by deprivation of necroptosis. In this way, a more intimate relationship among PFKFB3, necroptosis, and genome instability, could be revealed, which awaits further in-depth studies [85].
In colon cancer cells, activation of hepatocyte growth factor (HGF) gene was caused by genomic instability, which promoted cancer cell resistance to necroptosis [86]. Besides, adipoRon suppressed tumor growth of pancreatic cancer by inducing necroptosis [87]. In the early stages of prostate cancer, necroptosis was more activated via induced RIPK3 expression, while repressed during the final stages of tumor progression. Moreover, the repression of RIPK3 is related to the increase of both PSA levels and tumor volume, which represents the tumor progression in the final stages [88]. In prostate cancer cells, apoptosis and necroptosis were differentially facilitated by reticulocalbin-1 down-regulation [89]. On the other hand, sensitivity of renal cancer cells to cisplatin-induced necroptosis was regulated by miR-124, targeting the calpain small subunit 1 (Capn4)-CCR4-NOT transcription complex subunit 3 (CNOT3) axis [90]. In low-grade serous, but not serous borderline ovarian tumor cells, necroptosis-like cell death was induced by CD40 [91].
4. Necroptosis promotes inflammation and cancer metastasis
Necroptotic cells could directly induce inflammation by releasing massive damage-associated molecular patterns (DAMPs) into the tissue microenvironment, including interleukin-1 family cytokines, nucleic acids and ribonucleoproteins, histones and high mobility group box 1 protein (HMGB) family members, and ATP [56] (Fig. 3). DAMPs are usually detected by pattern recognition receptors that activate immune responses by inducing the expression of cytokines and chemokines [92, 93]. Metastasis is thought to be an important cause of cancer patients’ mortality. Immune cells and secreted cytokines, chemokines, and growth factors collectively create an inflammatory microenvironment that promotes the invasive and metastatic ability of cancer cells. Necroptosis may facilitate tumor cell metastasis by promoting inflammation. To this end, TNF α has been shown to play a key role in tumor development. It has been demonstrated that elevated expression of TNF-α was elevated in many malignant tumors, which is associated with poor prognosis [94]. As such, inactivation of RIPK1 with the specific inhibitor Nec-1 has been shown to alleviate inflammation and colitis-associated tumorigenesis in a mouse model of the disease [95].
Fig. 3.
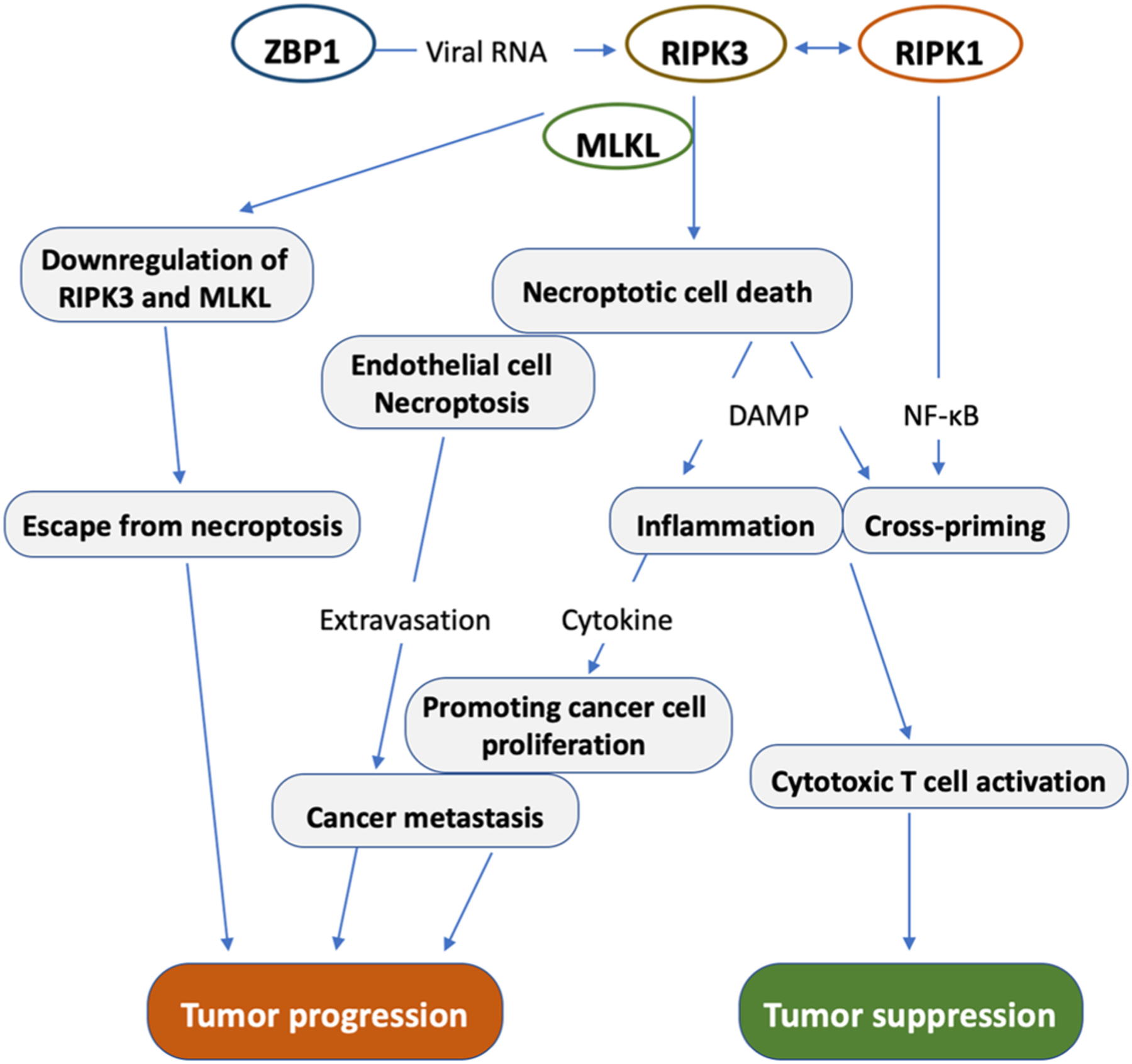
Role of Necroptosis in Cancer.
Tumor metastasis largely depends on the capacity to escape from the bloodstream by passing the endothelial barrier. Tumor cell extravasation through endothelium is a key step in the process of metastasis. Tumor cells were shown to induce necroptosis of endothelial cells by activating death receptor 6 (DR6) to promote tumor cell extravasation and metastasis. Treatment of mice with the RIPK1 inhibitor, Nec-1, or genetically knockout of RIPK3 or MLKL in endothelial cells reduced tumor-cell-induced endothelial necroptosis, tumor cell extravasation, and metastasis [96]. Thus, targeting RIPK1 kinase-dependent necroptotic signaling pathways might be promising anti-metastatic therapies.
Necroptotic cells release damage-associated molecular patterns (DAMPS) to promote inflammation, which leads to cancer growth and metastasis. However, necroptosis can also activate naïve CD8+ T cells for immune defense against cancer. Moreover, tumor cells can induce endothelial cell necroptosis to promote metastasis. On the other hand, many cancer cells can escape from necroptosis by downregulating RIPK3 and MLKL.
5. Necroptosis and mouse cancer model
Mouse models have been used as an efficient tool to evaluate the critical function of necroptosis in regulating tumorigenesis and metastasis. The breast cancer orthotopic mouse model demonstrated that CRISPR-Cas9-mediated MLKL silencing in Mvt-1 mouse mammary tumor cells decreased necroptosis in core tumor necrotic regions, leading to the conversion of necroptosis into apoptotic cell death mode in the tumor. While MLKL KO in the Mvt-1 cells had little effect on tumor growth, the WT Mvt-1-derived tumors were highly metastatic to the lung compared with MLKL-KO cells, suggesting the role of necroptosis in increasing lung metastatic potential of breast cancer cells [97]. In the context of AML, the murine bone marrow transplantation model demonstrated that deletion of RIPK3 or MLKL in myeloid cells in combination with FMS-like tyrosine kinase 3 (FLT3) mutation induces leukemogenesis, confirming the tumor suppressor role of the RIPK3/MLKL signaling [64]. Caspase-8-deficient colorectal cancer (CRC) model mouse displays high sensitivity to SMAC mimetics, which was reversed by RIPK3 deletion, suggesting that RIPK3 may be a critical determinant factor of SMAC mimetic therapy in CRC patients [59]. In the investigation of liver cancer lineage commitment, hepatocyte-specific MLKL knockout mice demonstrate that necroptosis microenvironment promotes transformed hepatocytes into intrahepatic cholangiocarcinoma (ICC), while apoptotic cell surrounded environment facilitates the hepatocytes to develop hepatocellular carcinoma (HCC) [98].
6. Necroptosis and cancer immunity
The activation of naïve cytotoxic CD8+ T cells is critical for immunity against most tumors. Necroptosis has been shown to activate specific types of immune cells and adaptive immunity. Necroptotic cells can provide both antigens and inflammatory stimuli for dendritic cells (DCs), which in turn activate CD8+ T cells through a process called antigen cross-priming [99,100] (Fig. 3). Activation of the NF-κB pathway can provide both antigen and immune stimulation, thereby supporting DC-mediated cross-priming of CD8+ T cells. Cross-priming of CD8+ T cells depends on the RIPK1 scaffold protein function and NF-κB-mediated transcription activity. It has been demonstrated that RIPK3, the key executor of necroptosis, can regulate cytokine expression to mediate the natural killer T (NKT) cell function, and genetic knockout of RIPK3 led to decreased immune responses to metastatic tumor cells by the NKT cells [101]. These findings strongly suggest that necroptosis might play a critical role in anti-tumor immunity by activating CD8+ T cells or NKT cells.
De novo necroptosis created an inflammatory environment mediating tumor susceptibility to immune checkpoint inhibitors [102]. In vivo, Poly(I:C)-induced, TLR3/RIPK3-dependent necroptosis supported immune effector-mediated tumor elimination [103]. In mouse tumor cells, necroptosis was mediated by P. aeruginosa, inducing long-lasting systemic anti-tumor immunity [104]. In human leukocyte antigen (HLA)-negative tumor cells, necroptosis was induced by FasL on the surface of Tag7 (PGRP-S)-activated lymphocytes with the involvement of lysosomes and mitochondria [105]. In adoptively transferred T cells, necroptosis was induced by blocking TCR restimulation, improving tumor control [106].
Tumor cell TLR3 could directly induce necroptosis by poly(I:C), independent of immune effector-mediated tumor shrinkage [107]. In the gut epithelium, the TSC complex subunit 1 (TSC1)/ mechanistic target of rapamycin (mTOR) pathway stood for a metabolic and innate immune checkpoint for intestinal dysfunction and inflammation, and mTOR hyperactivation triggered by western diet or Tsc1 ablation led to epithelium necroptosis [108]. In esophageal squamous cell carcinoma, necroptosis represented as an independent prognostic factor, and it correlated with tumor-infiltrating lymphocytes [109]. In colorectal cancer, oxaliplatin resistance was promoted in part by tumor-associated macrophages via methyltransferase 3, N6-Adenosine-Methyltransferase Complex Catalytic Subunit (METTL3)-mediated m(6)A of TNF receptor-associated factor 5 (TRAF5) and necroptosis [110]. Prostate cancer progression was promoted by SIRT3 and SIRT6 through inhibiting necroptosis-mediated innate immune response [111]. Poor treatment outcome of human papillomavirus positive cervical cancer was predicted by low necroptosis process through decreasing tumor-associated macrophages M1 polarization [112]. A favorable immune cell signature and programmed death-ligand 1 expression in cholangiocarcinoma was correlated with necroptosis [113].
7. Necroptosis and antitumor therapies
Recently, several anti-cancer agents relevant to necroptosis have been reported. Shikonin is widely recognized to play a part in the effect of necroptosis on tumors. Fe(III)-shikonin supramolecular nanomedicine played a role in combined therapy of tumors via ferroptosis and necroptosis [114]. In pancreatic cancer, necroptosis was induced by shikonin, regulating the expression of RIPK1/RIPK3 and synergizing the activity of gemcitabine [115]. Shikonin reduces the growth of docetaxel-resistant prostate cancer cells mainly through inducing necroptosis [116]. In bladder cancer, cisplatin resistance was largely overcome by shikonin, in part through inducing necroptosis [117]. Moreover, in estrogen receptor (ER) positive breast cancer, shikonin has been reported to induce necroptosis and apoptosis [118]. On the other hand, epidermal growth factor receptor (EGFR)-expressing cancer cells were sensitized by amino acid starvation culture condition to gefitinib-mediated cytotoxicity, inducing atypical necroptosis [119].
In multiple cancers, several agents exert an anti-cancer function. In human lung cancer cells, necroptosis was induced by 11-Methoxytabersonine with Autophagy via AMPK/mTOR and c-Jun N-terminal kinase (JNK) signaling pathways [120]. In non-small cell lung cancer, necroptosis was suppressed via RIPK3 promoter methylation, increasing cancer resistance to chemotherapy [121]. 2-Methoxy-6-Acetyl-7-Methyljuglone inhibited lung cancer by inducing necroptosis by targeting RIPK1 [122]. In human non-small cell lung cancer, necroptosis could be triggered by Deoxypodophyllotoxin [123]. In lung cancer cell line A549, cisplatin-triggered cell death was contributed by MLKL–phosphatidylinositol transfer protein alpha (PITPα) signaling-mediated necroptosis [124]. In gastric cancer cells, RIPK1-mediated necroptosis and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activation were induced by Astaxanthin [125]. Furthermore, colorectal cancer growth and metastasis were suppressed by resibufogenin through necroptosis [126]. In addition, in colon cancer cells, necroptosis was induced by dimethyl fumarate [127]. In breast cancer cells, Alysicarpus vaginalis ethyl acetate fraction (AVEAF) exerted anticancer activities associated with ROS-mediated mitochondrial-mediated intrinsic pathway of apoptosis and necroptosis [128]. Bcl-2-associated death promoter (BAD) sensitized breast cancer cells to docetaxel with increased mitotic arrest and necroptosis [129].
Cisplatin-induced necroptosis was also found to be mediated by RIPK1, which could govern proliferation through G2/M checkpoint progression [130]. In androgen‑dependent prostate cancer cells, RIPK1‑dependent necroptosis was induced by Ophiopogonin D’ (OPD’) [131]. Moreover, tumor cells could be sensitized for TRAIL-induced necroptosis by a clinically approved anti-leukemia drug, homoharringtonine [132].
In addition to drug therapy, other tumor therapeutic methods can also be applied to exert necroptosis. Through the RIPK1/RIPK3/MLKL/JNK/interleukin-8 (IL-8) pathway, tumor repopulation after radiotherapy was regulated by necroptosis. Necroptosis was triggered by ROS accumulation and Ca2+ overload, partly through RIPK1/RIPK3/MLKL/JNK/IL-8 pathway. Tumor repopulation after radiotherapy was regulated by necroptosis [133]. Necroptosis triggered by ROS accumulation and Ca2+ overload partly explained the inflammatory responses and anti-cancer effects associated with 1 Hz, 100 mT extremely low frequency-magnetic fields (ELF-MF) [134]. A potent anti-tumor effect was induced by stereotactic body radiation combined with oncolytic vaccinia virus, triggering tumor cell necroptosis and DAMPs [135]. Photothermal/photodynamic therapy of ovarian cancer was guided by CuS-MnS(2) nano-flowers for magnetic resonance imaging via necroptosis [136]. Non-small cell lung cancer cell killing was enhanced by ablative hypofractionated radiation therapy through preferential stimulation of necroptosis [137]. In keeping with these results, response of human colon cancer cell lines to supra-normal temperatures was associated with autophagy, apoptosis, and necroptosis [138]. In ovarian cancer, ceramide nanoliposomes were found to be necroptosis-inducing and MLKL-dependent chemotherapeutic reagents [139]. In breast cancer cells, apoptosis resistance was overcome by carbon nanodots for on-demand chemophotothermal therapy combination to elicit necroptosis [140]. Immunogenic necroptosis played a role in the anti-tumor photodynamic action of BAM-SiPc, a silicon(IV) phthalocyanine-based photosensitizer [141].
8. Concluding remarks
Accumulating evidence suggests that certain cancer cells can undergo necroptosis under some physical or chemical stimuli, and such triggering necroptosis could be a promising alternative strategy for killing cancer cells that fail to die by apoptosis. Moreover, important biological issues remain as follows: (i) Most studies on cancer and necroptosis were using cell-based assays in vitro, and thus more in vivo studies need to be performed to check the efficacy of necroptosis on killing cancer cells. (ii) Dissecting the functional role of necroptosis and underlying mechanism in tumor development and cancer therapy will be important for taking advantage of necroptosis-based anti-cancer therapies. (iii) More physiological and pathological systems need to be defined to study necroptosis. So far, in the necroptosis field, most of the studies were relatively artificial as caspase inhibition, TNFα treatment, or gene knockouts are needed to induce necroptosis. (iv) Cancer immunotherapy has been proved successful. It is fascinating to further investigate if induction of necroptosis can increase the efficacy of cancer immunotherapies. It will be crucial to know the specific roles regarding which DAMPs and other signaling molecules are released during necroptosis on the immune response and cancer immune surveillance and tumor promotion. In summary, RIPK1/RIPK3/MLKL-dependent necroptosis as a new form of cell death would be further explored to develop new anti-cancer therapies to overcome the resistance to proapoptotic chemotherapeutic agents.
Acknowledgements
We apologize for not citing all relevant reports owing to space limitations. This work was supported in part by funding from NIH (R35CA253027 to W.W.).
Data Availability
No data was used for the research described in the article.
References
- [1].Hanahan D, Weinberg RA, Hallmarks of cancer: the next generation, Cell 144 (5) (2011) 646–674. [DOI] [PubMed] [Google Scholar]
- [2].Cookson BT, Brennan MA, Pro-inflammatory programmed cell death, Trends Microbiol 9 (3) (2001) 113–114. [DOI] [PubMed] [Google Scholar]
- [3].Tsvetkov P, Coy S, Petrova B, Dreishpoon M, Verma A, Abdusamad M, Rossen J, Joesch-Cohen L, Humeidi R, Spangler RD, Eaton JK, Frenkel E, Kocak M, Corsello SM, Lutsenko S, Kanarek N, Santagata S, Golub TR, Copper induces cell death by targeting lipoylated TCA cycle proteins, Science 375 (6586) (2022) 1254–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B 3rd; Stockwell BR, Ferroptosis: an iron-dependent form of nonapoptotic cell death, Cell 149 (5) (2012) 1060–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chen X, Kang R, Kroemer G, Tang D, Broadening horizons: the role of ferroptosis in cancer, Nat. Rev. Clin. Oncol 18 (5) (2021) 280–296. [DOI] [PubMed] [Google Scholar]
- [6].Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J, Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury, Nat. Chem. Biol 1 (2) (2005) 112–119. [DOI] [PubMed] [Google Scholar]
- [7].Tait SW, Green DR, Caspase-independent cell death: leaving the set without the final cut, Oncogene 27 (50) (2008) 6452–6461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Christofferson DE, Yuan J, Necroptosis as an alternative form of programmed cell death, Curr. Opin. Cell Biol 22 (2) (2010) 263–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Welz PS, Wullaert A, Vlantis K, Kondylis V, Fernandez-Majada V, Ermolaeva M, Kirsch P, Sterner-Kock A, van Loo G, Pasparakis M, FADD prevents RIP3-mediated epithelial cell necrosis and chronic intestinal inflammation, Nature 477 (7364) (2011) 330–334. [DOI] [PubMed] [Google Scholar]
- [10].Gunther C, Martini E, Wittkopf N, Amann K, Weigmann B, Neumann H, Waldner MJ, Hedrick SM, Tenzer S, Neurath MF, Becker C, Caspase-8 regulates TNF-alpha-induced epithelial necroptosis and terminal ileitis, Nature 477 (7364) (2011) 335–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dannappel M, Vlantis K, Kumari S, Polykratis A, Kim C, Wachsmuth L, Eftychi C, Lin J, Corona T, Hermance N, Zelic M, Kirsch P, Basic M, Bleich A, Kelliher M, Pasparakis M, RIPK1 maintains epithelial homeostasis by inhibiting apoptosis and necroptosis, Nature 513 (7516) (2014) 90–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bonnet MC, Preukschat D, Welz PS, van Loo G, Ermolaeva MA, Bloch W, Haase I, Pasparakis M, The adaptor protein FADD protects epidermal keratinocytes from necroptosis in vivo and prevents skin inflammation, Immunity 35 (4) (2011) 572–582. [DOI] [PubMed] [Google Scholar]
- [13].Gautheron J, Vucur M, Reisinger F, Cardenas DV, Roderburg C, Koppe C, Kreggenwinkel K, Schneider AT, Bartneck M, Neumann UP, Canbay A, Reeves HL, Luedde M, Tacke F, Trautwein C, Heikenwalder M, Luedde T, A positive feedback loop between RIP3 and JNK controls non-alcoholic steatohepatitis, EMBO Mol. Med 6 (8) (2014) 1062–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Roychowdhury S, McMullen MR, Pisano SG, Liu X, Nagy LE, Absence of receptor interacting protein kinase 3 prevents ethanol-induced liver injury, Hepatology 57 (5) (2013) 1773–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ofengeim D, Ito Y, Najafov A, Zhang Y, Shan B, DeWitt JP, Ye J, Zhang X, Chang A, Vakifahmetoglu-Norberg H, Geng J, Py B, Zhou W, Amin P, Berlink Lima J, Qi C, Yu Q, Trapp B, Yuan J, Activation of necroptosis in multiple sclerosis, Cell Rep 10 (11) (2015) 1836–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ito Y, Ofengeim D, Najafov A, Das S, Saberi S, Li Y, Hitomi J, Zhu H, Chen H, Mayo L, Geng J, Amin P, DeWitt JP, Mookhtiar AK, Florez M, Ouchida AT, Fan JB, Pasparakis M, Kelliher MA, Ravits J, Yuan J, RIPK1 mediates axonal degeneration by promoting inflammation and necroptosis in ALS, Science 353 (6299) (2016) 603–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Degterev A, Boyce M, Yuan J, A decade of caspases, Oncogene 22 (53) (2003) 8543–8567. [DOI] [PubMed] [Google Scholar]
- [18].Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S, Bodmer JL, Schneider P, Seed B, Tschopp J, Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule, Nat. Immunol 1 (6) (2000) 489–495. [DOI] [PubMed] [Google Scholar]
- [19].Degterev A, Hitomi J, Germscheid M, Ch’en IL, Korkina O, Teng X, Abbott D, Cuny GD, Yuan C, Wagner G, Hedrick SM, Gerber SA, Lugovskoy A, Yuan J, Identification of RIP1 kinase as a specific cellular target of necrostatins, Nat. Chem. Biol 4 (5) (2008) 313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Dynek JN, Goncharov T, Dueber EC, Fedorova AV, Izrael-Tomasevic A, Phu L, Helgason E, Fairbrother WJ, Deshayes K, Kirkpatrick DS, Vucic D, c-IAP1 and UbcH5 promote K11-linked polyubiquitination of RIP1 in TNF signalling, EMBO J 29 (24) (2010) 4198–4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bertrand MJ, Milutinovic S, Dickson KM, Ho WC, Boudreault A, Durkin J, Gillard JW, Jaquith JB, Morris SJ, Barker PA, cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination, Mol. Cell 30 (6) (2008) 689–700. [DOI] [PubMed] [Google Scholar]
- [22].Varfolomeev E, Goncharov T, Fedorova AV, Dynek JN, Zobel K, Deshayes K, Fairbrother WJ, Vucic D, c-IAP1 and c-IAP2 are critical mediators of tumor necrosis factor alpha (TNFalpha)-induced NF-kappaB activation, J. Biol. Chem 283 (36) (2008) 24295–24299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ikeda F, Deribe YL, Skanland SS, Stieglitz B, Grabbe C, Franz-Wachtel M, van Wijk SJ, Goswami P, Nagy V, Terzic J, Tokunaga F, Androulidaki A, Nakagawa T, Pasparakis M, Iwai K, Sundberg JP, Schaefer L, Rittinger K, Macek B, Dikic I, SHARPIN forms a linear ubiquitin ligase complex regulating NF-kappaB activity and apoptosis, Nature 471 (7340) (2011) 637–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gerlach B, Cordier SM, Schmukle AC, Emmerich CH, Rieser E, Haas TL, Webb AI, Rickard JA, Anderton H, Wong WW, Nachbur U, Gangoda L, Warnken U, Purcell AW, Silke J, Walczak H, Linear ubiquitination prevents inflammation and regulates immune signalling, Nature 471 (7340) (2011) 591–596. [DOI] [PubMed] [Google Scholar]
- [25].Haas TL, Emmerich CH, Gerlach B, Schmukle AC, Cordier SM, Rieser E, Feltham R, Vince J, Warnken U, Wenger T, Koschny R, Komander D, Silke J, Walczak H, Recruitment of the linear ubiquitin chain assembly complex stabilizes the TNF-R1 signaling complex and is required for TNF-mediated gene induction, Mol. Cell 36 (5) (2009) 831–844. [DOI] [PubMed] [Google Scholar]
- [26].Moquin DM, McQuade T, Chan FK, CYLD deubiquitinates RIP1 in the TNFalpha-induced necrosome to facilitate kinase activation and programmed necrosis, PLoS One 8 (10) (2013), e76841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wang L, Du F, Wang X, TNF-alpha induces two distinct caspase-8 activation pathways, Cell 133 (4) (2008) 693–703. [DOI] [PubMed] [Google Scholar]
- [28].Wright A, Reiley WW, Chang M, Jin W, Lee AJ, Zhang M, Sun SC, Regulation of early wave of germ cell apoptosis and spermatogenesis by deubiquitinating enzyme CYLD, Dev. Cell 13 (5) (2007) 705–716. [DOI] [PubMed] [Google Scholar]
- [29].Wertz IE, O’Rourke KM, Zhou H, Eby M, Aravind L, Seshagiri S, Wu P, Wiesmann C, Baker R, Boone DL, Ma A, Koonin EV, Dixit VM, Deubiquitination and ubiquitin ligase domains of A20 downregulate NF-kappaB signalling, Nature 430 (7000) (2004) 694–699. [DOI] [PubMed] [Google Scholar]
- [30].Varfolomeev E, Blankenship JW, Wayson SM, Fedorova AV, Kayagaki N, Garg P, Zobel K, Dynek JN, Elliott LO, Wallweber HJ, Flygare JA, Fairbrother WJ, Deshayes K, Dixit VM, Vucic D, IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis, Cell 131 (4) (2007) 669–681. [DOI] [PubMed] [Google Scholar]
- [31].Vince JE, Wong WW, Khan N, Feltham R, Chau D, Ahmed AU, Benetatos CA, Chunduru SK, Condon SM, McKinlay M, Brink R, Leverkus M, Tergaonkar V, Schneider P, Callus BA, Koentgen F, Vaux DL, Silke J, IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis, Cell 131 (4) (2007) 682–693. [DOI] [PubMed] [Google Scholar]
- [32].Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, Chan FK, Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation, Cell 137 (6) (2009) 1112–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Newton K, Dugger DL, Wickliffe KE, Kapoor N, de Almagro MC, Vucic D, Komuves L, Ferrando RE, French DM, Webster J, Roose-Girma M, Warming S, Dixit VM, Activity of protein kinase RIPK3 determines whether cells die by necroptosis or apoptosis, Science 343 (6177) (2014) 1357–1360. [DOI] [PubMed] [Google Scholar]
- [34].Wang H, Sun L, Su L, Rizo J, Liu L, Wang LF, Wang FS, Wang X, Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3, Mol. Cell 54 (1) (2014) 133–146. [DOI] [PubMed] [Google Scholar]
- [35].Cai Z, Jitkaew S, Zhao J, Chiang HC, Choksi S, Liu J, Ward Y, Wu LG, Liu ZG, Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis, Nat. Cell Biol 16 (1) (2014) 55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Mandal P, Berger SB, Pillay S, Moriwaki K, Huang C, Guo H, Lich JD, Finger J, Kasparcova V, Votta B, Ouellette M, King BW, Wisnoski D, Lakdawala AS, DeMartino MP, Casillas LN, Haile PA, Sehon CA, Marquis RW, Upton J, Daley-Bauer LP, Roback L, Ramia N, Dovey CM, Carette JE, Chan FK, Bertin J, Gough PJ, Mocarski ES, Kaiser WJ, RIP3 induces apoptosis independent of pronecrotic kinase activity, Mol. Cell 56 (4) (2014) 481–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Sun L, Wang H, Wang Z, He S, Chen S, Liao D, Wang L, Yan J, Liu W, Lei X, Wang X, Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase, Cell 148 (1–2) (2012) 213–227. [DOI] [PubMed] [Google Scholar]
- [38].Fulda S, Promises and challenges of smac mimetics as cancer therapeutics, Clin. Cancer Res 21 (22) (2015) 5030–5036. [DOI] [PubMed] [Google Scholar]
- [39].Vanlangenakker N, Vanden Berghe T Vandenabeele, P, Many stimuli pull the necrotic trigger, an overview, Cell Death Differ 19 (1) (2012) 75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Pasparakis M, Vandenabeele P, Necroptosis and its role in inflammation, Nature 517 (7534) (2015) 311–320. [DOI] [PubMed] [Google Scholar]
- [41].He S, Liang Y, Shao F, Wang X, Toll-like receptors activate programmed necrosis in macrophages through a receptor-interacting kinase-3-mediated pathway, Proc. Natl. Acad. Sci. USA 108 (50) (2011) 20054–20059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kaiser WJ, Sridharan H, Huang C, Mandal P, Upton JW, Gough PJ, Sehon CA, Marquis RW, Bertin J, Mocarski ES, Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL, J. Biol. Chem 288 (43) (2013) 31268–31279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Upton JW, Kaiser WJ, Mocarski ES, DAI/ZBP1/DLM-1 complexes with RIP3 to mediate virus-induced programmed necrosis that is targeted by murine cytomegalovirus vIRA, Cell Host Microbe 11 (3) (2012) 290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Lin J, Kumari S, Kim C, Van TM, Wachsmuth L, Polykratis A, Pasparakis M, RIPK1 counteracts ZBP1-mediated necroptosis to inhibit inflammation, Nature 540 (7631) (2016) 124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Jiao H, Wachsmuth L, Kumari S, Schwarzer R, Lin J, Eren RO, Fisher A, Lane R, Young GR, Kassiotis G, Kaiser WJ, Pasparakis M, Z-nucleic-acid sensing triggers ZBP1-dependent necroptosis and inflammation, Nature 580 (7803) (2020) 391–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Lee S, Karki R, Wang Y, Nguyen LN, Kalathur RC, Kanneganti TD, AIM2 forms a complex with pyrin and ZBP1 to drive PANoptosis and host defence, Nature 597 (7876) (2021) 415–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Zhang T, Yin C, Boyd DF, Quarato G, Ingram JP, Shubina M, Ragan KB, Ishizuka T, Crawford JC, Tummers B, Rodriguez DA, Xue J, Peri S, Kaiser WJ, Lopez CB, Xu Y, Upton JW, Thomas PG, Green DR, Balachandran S, Influenza Virus Z-RNAs induce ZBP1-mediated necroptosis, Cell 180 (6) (2020) 1115–1129, e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Robinson N, McComb S, Mulligan R, Dudani R, Krishnan L, Sad S, Type I interferon induces necroptosis in macrophages during infection with Salmonella enterica serovar Typhimurium, Nat. Immunol 13 (10) (2012) 954–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].McComb S, Cessford E, Alturki NA, Joseph J, Shutinoski B, Startek JB, Gamero AM, Mossman KL, Sad S, Type-I interferon signaling through ISGF3 complex is required for sustained Rip3 activation and necroptosis in macrophages, Proc. Natl. Acad. Sci. USA 111 (31) (2014) E3206–E3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Hitomi J, Christofferson DE, Ng A, Yao J, Degterev A, Xavier RJ, Yuan J, Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway, Cell 135 (7) (2008) 1311–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Elliott PR, Leske D, Wagstaff J, Schlicher L, Berridge G, Maslen S, Timmermann F, Ma B, Fischer R, Freund SMV, Komander D, Gyrd-Hansen M, Regulation of CYLD activity and specificity by phosphorylation and ubiquitin-binding CAP-Gly domains, Cell Rep 37 (1) (2021), 109777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Wu W, Zhu H, Fu Y, Shen W, Xu J, Miao K, Hong M, Xu W, Liu P, Li J, Clinical significance of down-regulated cylindromatosis gene in chronic lymphocytic leukemia, Leuk. Lymphoma 55 (3) (2014) 588–594. [DOI] [PubMed] [Google Scholar]
- [53].Liu P, Xu B, Shen W, Zhu H, Wu W, Fu Y, Chen H, Dong H, Zhu Y, Miao K, Xu W, Li J, Dysregulation of TNFalpha-induced necroptotic signaling in chronic lymphocytic leukemia: suppression of CYLD gene by LEF1, Leukemia 26 (6) (2012) 1293–1300. [DOI] [PubMed] [Google Scholar]
- [54].Massoumi R, Kuphal S, Hellerbrand C, Haas B, Wild P, Spruss T, Pfeifer A, Fassler R, Bosserhoff AK, Down-regulation of CYLD expression by Snail promotes tumor progression in malignant melanoma, J. Exp. Med 206 (1) (2009) 221–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Hellerbrand C, Bumes E, Bataille F, Dietmaier W, Massoumi R, Bosserhoff AK, Reduced expression of CYLD in human colon and hepatocellular carcinomas, Carcinogenesis 28 (1) (2007) 21–27. [DOI] [PubMed] [Google Scholar]
- [56].Feng X, Song Q, Yu A, Tang H, Peng Z, Wang X, Receptor-interacting protein kinase 3 is a predictor of survival and plays a tumor suppressive role in colorectal cancer, Neoplasma 62 (4) (2015) 592–601. [DOI] [PubMed] [Google Scholar]
- [57].Nugues AL, El Bouazzati H Hetuin D Berthon C Loyens A Bertrand E Jouy N Idziorek T Quesnel B, RIP3 is downregulated in human myeloid leukemia cells and modulates apoptosis and caspase-mediated p65/RelA cleavage, Cell Death Dis 5 (2014), e1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Koo GB, Morgan MJ, Lee DG, Kim WJ, Yoon JH, Koo JS, Kim SI, Kim SJ, Son MK, Hong SS, Levy JM, Pollyea DA, Jordan CT, Yan P, Frankhouser D, Nicolet D, Maharry K, Marcucci G, Choi KS, Cho H, Thorburn A, Kim YS, Methylation-dependent loss of RIP3 expression in cancer represses programmed necrosis in response to chemotherapeutics, Cell Res 25 (6) (2015) 707–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].He GW, Gunther C, Thonn V, Yu YQ, Martini E, Buchen B, Neurath MF, Sturzl M, Becker C, Regression of apoptosis-resistant colorectal tumors by induction of necroptosis in mice, J. Exp. Med 214 (6) (2017) 1655–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Wu M, Wei W, Xiao X, Guo J, Xie X, Li L, Kong Y, Lv N, Jia W, Zhang Y, Expression of SIRT1 is associated with lymph node metastasis and poor prognosis in both operable triple-negative and non-triple-negative breast cancer, Med Oncol (2012). [DOI] [PubMed] [Google Scholar]
- [61].Colbert LE, Fisher SB, Hardy CW, Hall WA, Saka B, Shelton JW, Petrova AV, Warren MD, Pantazides BG, Gandhi K, Kowalski J, Kooby DA, El-Rayes BF, Staley CA, Adsay NV, Curran WJ Jr, Landry JC, Maithel SK, Yu DS, Pronecrotic mixed lineage kinase domain-like protein expression is a prognostic biomarker in patients with early-stage resected pancreatic adenocarcinoma, Cancer 119 (17) (2013) 3148–3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Forbes SA, Bhamra G, Bamford S, Dawson E, Kok C, Clements J, Menzies A, Teague JW, Futreal PA, Stratton MR, The catalogue of somatic mutations in cancer (COSMIC), Curr. Protoc. Hum. Genet 10 (2008) 11. Chapter 10, Unit. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Murphy JM, Czabotar PE, Hildebrand JM, Lucet IS, Zhang JG, Alvarez-Diaz S, Lewis R, Lalaoui N, Metcalf D, Webb AI, Young SN, Varghese LN, Tannahill GM, Hatchell EC, Majewski IJ, Okamoto T, Dobson RC, Hilton DJ, Babon JJ, Nicola NA, Strasser A, Silke J, Alexander WS, The pseudokinase MLKL mediates necroptosis via a molecular switch mechanism, Immunity 39 (3) (2013) 443–453. [DOI] [PubMed] [Google Scholar]
- [64].Hockendorf U, Yabal M, Herold T, Munkhbaatar E, Rott S, Jilg S, Kauschinger J, Magnani G, Reisinger F, Heuser M, Kreipe H, Sotlar K, Engleitner T, Rad R, Weichert W, Peschel C, Ruland J, Heikenwalder M, Spiekermann K, Slotta-Huspenina J, Gross O, Jost PJ, RIPK3 restricts myeloid leukemogenesis by promoting cell death and differentiation of leukemia initiating cells, Cancer Cell 30 (1) (2016) 75–91. [DOI] [PubMed] [Google Scholar]
- [65].Geserick P, Wang J, Schilling R, Horn S, Harris PA, Bertin J, Gough PJ, Feoktistova M, Leverkus M, Absence of RIPK3 predicts necroptosis resistance in malignant melanoma, Cell Death Dis 6 (2015), e1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Najafov A, Zervantonakis IK, Mookhtiar AK, Greninger P, March RJ, Egan RK, Luu HS, Stover DG, Matulonis UA, Benes CH, Yuan J, BRAF and AXL oncogenes drive RIPK3 expression loss in cancer, PLoS Biol 16 (8) (2018), e2005756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Li X, Guo J, Ding AP, Qi WW, Zhang PH, Lv J, Qiu WS, Sun ZQ, Association of mixed lineage kinase domain-like protein expression with prognosis in patients with colon cancer, Technol. Cancer Res Treat 16 (4) (2017) 428–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].McCormick KD, Ghosh A, Trivedi S, Wang L, Coyne CB, Ferris RL, Sarkar SN, Innate immune signaling through differential RIPK1 expression promote tumor progression in head and neck squamous cell carcinoma, Carcinogenesis 37 (5) (2016) 522–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Ertao Z, Jianhui C, Kang W, Zhijun Y, Hui W, Chuangqi C, Changjiang Q, Sile C, Yulong H, Shirong C, Prognostic value of mixed lineage kinase domain-like protein expression in the survival of patients with gastric caner, Tumour Biol 37 (10) (2016) 13679–13685. [DOI] [PubMed] [Google Scholar]
- [70].He L, Peng K, Liu Y, Xiong J, Zhu FF, Low expression of mixed lineage kinase domain-like protein is associated with poor prognosis in ovarian cancer patients, Onco Targets Ther 6 (2013) 1539–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Jiao D, Cai Z, Choksi S, Ma D, Choe M, Kwon HJ, Baik JY, Rowan BG, Liu C, Liu ZG, Necroptosis of tumor cells leads to tumor necrosis and promotes tumor metastasis, Cell Res 28 (8) (2018) 868–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Seehawer M, Heinzmann F, D’Artista L, Harbig J, Roux PF, Hoenicke L, Dang H, Klotz S, Robinson L, Dore G, Rozenblum N, Kang TW, Chawla R, Buch T, Vucur M, Roth M, Zuber J, Luedde T, Sipos B, Longerich T, Heikenwalder M, Wang XW, Bischof O, Zender L, Necroptosis microenvironment directs lineage commitment in liver cancer, Nature 562 (7725) (2018) 69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Workenhe ST, Nguyen A, Bakhshinyan D, Wei J, Hare DN, MacNeill KL, Wan Y, Oberst A, Bramson JL, Nasir JA, Vito A, El-Sayes N, Singh SK, McArthur AG, Mossman KL, De novo necroptosis creates an inflammatory environment mediating tumor susceptibility to immune checkpoint inhibitors, Commun. Biol 3 (1) (2020) 645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Takemura R, Takaki H, Okada S, Shime H, Akazawa T, Oshiumi H, Matsumoto M, Teshima T, Seya T, PolyI:C-induced, TLR3/RIP3-dependent necroptosis backs up immune effector-mediated tumor elimination in vivo, Cancer Immunol. Res 3 (8) (2015) 902–914. [DOI] [PubMed] [Google Scholar]
- [75].Qi JL, He JR, Jin SM, Yang X, Bai HM, Liu CB, Ma YB, Aeruginosa P Mediated Necroptosis in Mouse Tumor Cells Induces Long-Lasting Systemic Antitumor Immunity, Front Oncol 10 (2020), 610651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Sharapova TN, Romanova EA, Sashchenko LP, Yashin DV, FasL on the surface of Tag7 (PGRP-S)-activated lymphocytes induces necroptosis in HLA-negative tumor cells with the involvement of lysosomes and mitochondria, Biochimie 152 (2018) 174–180. [DOI] [PubMed] [Google Scholar]
- [77].Kesarwani P, Chakraborty P, Gudi R, Chatterjee S, Scurti G, Toth K, Simms P, Husain M, Armeson K, Husain S, Garrett-Mayer E, Vasu C, Nishimura MI, Mehrotra S, Blocking TCR restimulation induced necroptosis in adoptively transferred T cells improves tumor control, Oncotarget 7 (43) (2016) 69371–69383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Takaki H, Shime H, Matsumoto M, Seya T, Tumor cell death by pattern-sensing of exogenous RNA: Tumor cell TLR3 directly induces necroptosis by poly(I:C) in vivo, independent of immune effector-mediated tumor shrinkage, Oncoimmunology 6 (10) (2017), e1078968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Yamauchi T, Fujishima F, Hashimoto M, Tsunokake J, Akaishi R, Gokon Y, Ueki S, Ozawa Y, Fukutomi T, Okamoto H, Sato C, Taniyama Y, Nakamura T, Nakaya N, Kamei T, Sasano H, Necroptosis in esophageal squamous cell carcinoma: an independent prognostic factor and its correlation with tumor-infiltrating lymphocytes, Cancers 13 (17) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Lan H, Liu Y, Liu J, Wang X, Guan Z, Du J, Jin K, Tumor-associated macrophages promote oxaliplatin resistance via METTL3-mediated m(6)A of TRAF5 and necroptosis in colorectal cancer, Mol. Pharm 18 (3) (2021) 1026–1037. [DOI] [PubMed] [Google Scholar]
- [81].Fu W, Li H, Fu H, Zhao S, Shi W, Sun M, Li Y, The SIRT3 and SIRT6 promote prostate cancer progression by inhibiting necroptosis-mediated innate immune response, J. Immunol. Res 2020 (2020) 8820355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Li L, Yu S, Zang C, Low necroptosis process predicts poor treatment outcome of human papillomavirus positive cervical cancers by decreasing tumor-associated macrophages M1 polarization, Gynecol. Obstet. Invest 83 (3) (2018) 259–267. [DOI] [PubMed] [Google Scholar]
- [83].Lomphithak T, Akara-Amornthum P, Murakami K, Hashimoto M, Usubuchi H, Iwabuchi E, Unno M, Cai Z, Sasano H, Jitkaew S, Tumor necroptosis is correlated with a favorable immune cell signature and programmed death-ligand 1 expression in cholangiocarcinoma, Sci. Rep 11 (1) (2021) 11743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Feng W, Shi W, Liu S, Liu H, Liu Y, Ge P, Zhang H, Fe(III)-shikonin supramolecular nanomedicine for combined therapy of tumor via ferroptosis and necroptosis, Adv. Health Mater 11 (2) (2022), e2101926. [DOI] [PubMed] [Google Scholar]
- [85].Chen C, Xiao W, Huang L, Yu G, Ni J, Yang L, Wan R, Hu G, Shikonin induces apoptosis and necroptosis in pancreatic cancer via regulating the expression of RIP1/RIP3 and synergizes the activity of gemcitabine, Am. J. Transl. Res 9 (12) (2017) 5507–5517. [PMC free article] [PubMed] [Google Scholar]
- [86].Markowitsch SD, Juetter KM, Schupp P, Hauschulte K, Vakhrusheva O, Slade KS, Thomas A, Tsaur I, Cinatl J Jr., Michaelis M, Efferth T, Haferkamp A, Juengel E, Shikonin reduces growth of docetaxel-resistant prostate cancer cells mainly through necroptosis, Cancers 13 (4) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Wang Y, Hao F, Nan Y, Qu L, Na W, Jia C, Chen X, PKM2 inhibitor shikonin overcomes the cisplatin resistance in bladder cancer by inducing necroptosis, Int J. Biol. Sci 14 (13) (2018) 1883–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Shahsavari Z, Karami-Tehrani F, Salami S, Targeting cell necroptosis and apoptosis induced by shikonin via receptor interacting protein kinases in estrogen receptor positive breast cancer cell line, MCF-7, Anticancer Agents Med. Chem 18 (2) (2018) 245–254. [DOI] [PubMed] [Google Scholar]
- [89].Saito Y, Moriya S, Kazama H, Hirasawa K, Miyahara K, Kokuba H, Hino H, Kikuchi H, Takano N, Hiramoto M, Tsukahara K, Miyazawa K, Amino acid starvation culture condition sensitizes EGFR-expressing cancer cell lines to gefitinib-mediated cytotoxicity by inducing atypical necroptosis, Int J. Oncol 52 (4) (2018) 1165–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Ge D, Tao HR, Fang L, Kong XQ, Han LN, Li N, Xu YX, Li LY, Yu M, Zhang H, 11-methoxytabersonine induces necroptosis with autophagy through AMPK/mTOR and JNK pathways in human lung cancer cells, Chem. Pharm. Bull 68 (3) (2020) 244–250. [DOI] [PubMed] [Google Scholar]
- [91].Wang Q, Wang P, Zhang L, Tessema M, Bai L, Xu X, Li Q, Zheng X, Saxton B, Chen W, Willink R, Li Z, Zhang L, Belinsky SA, Wang X, Zhou B, Lin Y, Epigenetic regulation of RIP3 suppresses necroptosis and increases resistance to chemotherapy in nonsmall cell lung cancer, Transl. Oncol 13 (2) (2020) 372–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Sun W, Yu J, Gao H, Wu X, Wang S, Hou Y, Lu JJ, Chen X, Inhibition of lung cancer by 2-Methoxy-6-Acetyl-7-methyljuglone through induction of necroptosis by targeting receptor-interacting protein 1, Antioxid. Redox Signal 31 (2) (2019) 93–108. [DOI] [PubMed] [Google Scholar]
- [93].Wu M, Jiang Z, Duan H, Sun L, Zhang S, Chen M, Wang Y, Gao Q, Song Y, Zhu X, Zhang L, Deoxypodophyllotoxin triggers necroptosis in human non-small cell lung cancer NCI-H460 cells, Biomed. Pharm 67 (8) (2013) 701–706. [DOI] [PubMed] [Google Scholar]
- [94].Jing L, Song F, Liu Z, Li J, Wu B, Fu Z, Jiang J, Chen Z, MLKL-PITPalpha signaling-mediated necroptosis contributes to cisplatin-triggered cell death in lung cancer A549 cells, Cancer Lett 414 (2018) 136–146. [DOI] [PubMed] [Google Scholar]
- [95].Khing TM, Po WW, Sohn UD, Fluoxetine enhances anti-tumor activity of paclitaxel in gastric adenocarcinoma cells by triggering apoptosis and necroptosis, Anticancer Res 39 (11) (2019) 6155–6163. [DOI] [PubMed] [Google Scholar]
- [96].Han Q, Ma Y, Wang H, Dai Y, Chen C, Liu Y, Jing L, Sun X, Resibufogenin suppresses colorectal cancer growth and metastasis through RIP3-mediated necroptosis, J. Transl. Med 16 (1) (2018) 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Xie X, Zhao Y, Ma CY, Xu XM, Zhang YQ, Wang CG, Jin J, Shen X, Gao JL, Li N, Sun ZJ, Dong DL, Dimethyl fumarate induces necroptosis in colon cancer cells through GSH depletion/ROS increase/MAPKs activation pathway, Br. J. Pharm 172 (15) (2015) 3929–3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Sakle NS, More SA, Mokale SN, Chemomodulatory effects of Alysicarpus vaginalis extract via mitochondria-dependent apoptosis and necroptosis in breast cancer, Nutr. Cancer 72 (7) (2020) 1243–1253. [DOI] [PubMed] [Google Scholar]
- [99].Mann J, Yang N, Montpetit R, Kirschenman R, Lemieux H, Goping IS, BAD sensitizes breast cancer cells to docetaxel with increased mitotic arrest and necroptosis, Sci. Rep 10 (1) (2020) 355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Zheng XL, Yang JJ, Wang YY, Li Q, Song YP, Su M, Li JK, Zhang L, Li ZP, Zhou B, Lin Y, RIP1 promotes proliferation through G2/M checkpoint progression and mediates cisplatin-induced apoptosis and necroptosis in human ovarian cancer cells, Acta Pharm. Sin 41 (9) (2020) 1223–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Lu Z, Wu C, Zhu M, Song W, Wang H, Wang J, Guo J, Li N, Liu J, Li Y, Xu H, Ophiopogonin D’ induces RIPK1dependent necroptosis in androgendependent LNCaP prostate cancer cells, Int. J. Oncol 56 (2) (2020) 439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Philipp S, Sosna J, Plenge J, Kalthoff H, Adam D, Homoharringtonine, a clinically approved anti-leukemia drug, sensitizes tumor cells for TRAIL-induced necroptosis, Cell Commun. Signal 13 (2015) 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Wang Y, Zhao M, He S, Luo Y, Zhao Y, Cheng J, Gong Y, Xie J, Wang Y, Hu B, Tian L, Liu X, Li C, Huang Q, Necroptosis regulates tumor repopulation after radiotherapy via RIP1/RIP3/MLKL/JNK/IL8 pathway, J. Exp. Clin. Cancer Res 38 (1) (2019) 461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Barati M, Javidi MA, Darvishi B, Shariatpanahi SP, Mesbah Moosavi ZS, Ghadirian R, Khani T, Sanati H, Simaee H, Shokrollahi Barough M, Farahmand L, Madjid Ansari A, Necroptosis triggered by ROS accumulation and Ca(2+) overload, partly explains the inflammatory responses and anti-cancer effects associated with 1Hz, 100 mT ELF-MF in vivo, Free Radic. Biol. Med 169 (2021) 84–98. [DOI] [PubMed] [Google Scholar]
- [105].Chen WY, Chen YL, Lin HW, Chang CF, Huang BS, Sun WZ, Cheng WF, Stereotactic body radiation combined with oncolytic vaccinia virus induces potent anti-tumor effect by triggering tumor cell necroptosis and DAMPs, Cancer Lett 523 (2021) 149–161. [DOI] [PubMed] [Google Scholar]
- [106].Chen W, Wang X, Zhao B, Zhang R, Xie Z, He Y, Chen A, Xie X, Yao K, Zhong M, Yuan M, CuS-MnS2 nano-flowers for magnetic resonance imaging guided photothermal/photodynamic therapy of ovarian cancer through necroptosis, Nanoscale 11 (27) (2019) 12983–12989. [DOI] [PubMed] [Google Scholar]
- [107].Wang HH, Wu ZQ, Qian D, Zaorsky NG, Qiu MH, Cheng JJ, Jiang C, Wang J, Zeng XL, Liu CL, Tian LJ, Ying GG, Meng MB, Hao XS, Yuan ZY, Ablative hypofractionated radiation therapy enhances non-small cell lung cancer cell killing via preferential stimulation of necroptosis in vitro and in vivo, Int J. Radiat. Oncol. Biol. Phys 101 (1) (2018) 49–62. [DOI] [PubMed] [Google Scholar]
- [108].Mouratidis PX, Rivens I, Ter Haar G, A study of thermal dose-induced autophagy, apoptosis and necroptosis in colon cancer cells, Int J. Hyperth 31 (5) (2015) 476–488. [DOI] [PubMed] [Google Scholar]
- [109].Zhang X, Kitatani K, Toyoshima M, Ishibashi M, Usui T, Minato J, Egiz M, Shigeta S, Fox T, Deering T, Kester M, Yaegashi N, Ceramide nanoliposomes as a MLKL-dependent, necroptosis-inducing, chemotherapeutic reagent in ovarian cancer, Mol. Cancer Ther 17 (1) (2018) 50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Nicosia A, Cavallaro G, Costa S, Utzeri MA, Cuttitta A, Giammona G, Mauro N, Carbon nanodots for on demand chemophotothermal therapy combination to elicit necroptosis: overcoming apoptosis resistance in breast cancer cell lines, Cancers 12 (11) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Zhang Y, Cheung YK, Ng DKP, Fong WP, Immunogenic necroptosis in the anti-tumor photodynamic action of BAM-SiPc, a silicon(IV) phthalocyanine-based photosensitizer, Cancer Immunol. Immunother 70 (2) (2021) 485–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Akimoto M, Maruyama R, Kawabata Y, Tajima Y, Takenaga K, Antidiabetic adiponectin receptor agonist AdipoRon suppresses tumour growth of pancreatic cancer by inducing RIPK1/ERK-dependent necroptosis, Cell Death Dis 9 (8) (2018) 804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Alvarez-Diaz S, Preaudet A, Samson AL, Nguyen PM, Fung KY, Garnham AL, Alexander WS, Strasser A, Ernst M, Putoczki TL, Murphy JM, Necroptosis is dispensable for the development of inflammation-associated or sporadic colon cancer in mice, Cell Death Differ 28 (5) (2021) 1466–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Bevan MJ, Cross-priming, Nat Immunol 7 (4) (2006) 363–365. [DOI] [PubMed] [Google Scholar]
- [115].Cekay MJ, Roesler S, Frank T, Knuth AK, Eckhardt I, Fulda S, Smac mimetics and type II interferon synergistically induce necroptosis in various cancer cell lines, Cancer Lett 1 (410) (2017) 228–237. [DOI] [PubMed] [Google Scholar]
- [116].Chiu HW, Su YC, Hong JR, Betanodavirus B2 protein triggers apoptosis and necroptosis in lung cancer cells that suppresses autophagy, Oncotarget 8 (55) (2017) 94129–94141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Jin G, Lan Y, Han F, Sun Y, Liu Z, Zhang M, Liu X, Zhang X, Hu J, Liu H, Wang B, Smac mimetic‑induced caspase‑independent necroptosis requires RIP1 in breast cancer, Mol Med Rep 13 (1) (2016) 359–366. [DOI] [PubMed] [Google Scholar]
- [118].Jin G, Liu Y, Xu P, Jin G, Induction of Necroptosis in Human Breast Cancer Drug-Resistant Cells by SMAC Analog LCL161 After Caspase Inhibition Requires RIP3, Pharmazie 74 (6) (2019) 363–368, 1. [DOI] [PubMed] [Google Scholar]
- [119].Kaczmarek A, Vandenabeele P, Krysko DV, Necroptosis: the release of damage-associated molecular patterns and its physiological relevance, Immunity 38 (2) (2013) 209–223. [DOI] [PubMed] [Google Scholar]
- [120].Kaku Y, Tsuchiya A, Kanno T, Nishizaki T, HUHS1015 induces necroptosis and caspase-independent apoptosis of MKN28 human gastric cancer cells in association with AMID accumulation in the nucleus, Anticancer Agents Med Chem 15 (2) (2015) 242–247. [DOI] [PubMed] [Google Scholar]
- [121].Kang YJ, Bang BR, Han KH, Hong L, Shim EJ, Ma J, Lerner RA, Otsuka M, Regulation of NKT cell-mediated immune responses to tumours and liver inflammation by mitochondrial PGAM5-Drp1 signalling, Nat Commun 6 (2015) 8371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Lee YJ, Nam HS, Cho MK, Lee SH, Arctigenin induces necroptosis through mitochondrial dysfunction with CCN1 upregulation in prostate cancer cells under lactic acidosis, Mol Cell Biochem 467 (1–2) (2020) 45–56. [DOI] [PubMed] [Google Scholar]
- [123].Le DT, Jung S, Quynh NTN, Sandag Z, Lee BS, Kim S, Lee H, Lee H, Lee MS, Inhibitory role of AMP‑activated protein kinase in necroptosis of HCT116 colon cancer cells with p53 null mutation under nutrient starvation, Int J Oncol 54 (2) (2019) 702–712. [DOI] [PubMed] [Google Scholar]
- [124].Lim JH, Oh S, Kim L, Suh YJ, Ha YJ, Kim JS, Kim HJ, Park MH, Kim YS, Cho Y, Kwak SM, Lee HL, Kim YS, Ryu JS, Low-level expression of necroptosis factors indicates a poor prognosis of the squamous cell carcinoma subtype of non-small-cell lung cancer, Transl Lung Cancer Res 10 (3) (2021) 1221–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Liu ZY, Wu B, Guo YS, Zhou YH, Fu ZG, Xu BQ, Li JH, Jing L, Jiang JL, Tang J, Chen ZN, Necrostatin-1 reduces intestinal inflammation and colitis-associated tumorigenesis in mice, Am J Cancer Res 5 (10) (2015) 3174–3185. [PMC free article] [PubMed] [Google Scholar]
- [126].Liu X, Zhang N, Wang D, Zhu D, Yuan Q, Zhang X, Qian L, Niu H, Lu Y, Ren G, Tian K, Yuan H, Downregulation of reticulocalbin-1 differentially facilitates apoptosis and necroptosis in human prostate cancer cells, Cancer Sci 109 (4) (2018) 1147–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Mao Q, Zhuang Q, Shen J, Chen Z, Xue D, Ding T, He X, MiRNA-124 regulates the sensitivity of renal cancer cells to cisplatin-induced necroptosis by targeting the CAPN4-CNOT3 axis, Transl Androl Urol 10 (9) (2021) 3669–3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Qiu X, Klausen C, Cheng JC, Leung PC, CD40 ligand induces RIP1-dependent, necroptosis-like cell death in low-grade serous but not serous borderline ovarian tumor cells, Cell Death Dis 6 (8) (2015), e1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Sakanashi F, Shintani M, Tsuneyoshi M, Ohsaki H, Kamoshida S Apoptosis, necroptosis and autophagy in colorectal cancer: Associations with tumor aggressiveness and p53 status, Pathol Res Pract 215 (7) (2019), 152425. Jul. [DOI] [PubMed] [Google Scholar]
- [130].Scaffidi P, Misteli T, Bianchi ME, Release of chromatin protein HMGB1 by necrotic cells triggers inflammation, Nature 418 (6894) (2002) 191–195. [DOI] [PubMed] [Google Scholar]
- [131].Seneviratne D, Ma J, Tan X, Kwon YK, Muhammad E, Melhem M, DeFrances MC, Zarnegar R, Genomic instability causes HGF gene activation in colon cancer cells, promoting their resistance to necroptosis, Gastroenterology 148 (1) (2015) 181–191, e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Sonkusre P, Cameotra SS, Biogenic selenium nanoparticles induce ROS-mediated necroptosis in PC-3 cancer cells through TNF activation, J Nanobiotechnology 15 (1) (2017) 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Strilic B, Yang L, Albarran-Juarez J, Wachsmuth L, Han K, Muller UC, Pasparakis M, Offermanns S, Tumour-cell-induced endothelial cell necroptosis via death receptor 6 promotes metastasis, Nature 536 (7615) (2016) 215–218. [DOI] [PubMed] [Google Scholar]
- [134].Tang X, Li Y, Liu L, Guo R, Zhang P, Zhang Y, Zhang Y, Zhao J, Su J, Sun L, Liu Y, Sirtuin 3 (sirt3)induces apoptosis and necroptosis by regulating mutant p53 expression in small‑cell lung cancer, Oncol Rep 43 (2) (2020) 591–600. Feb. [DOI] [PubMed] [Google Scholar]
- [135].Wang HY, Zhang B, Cobalt chloride induces necroptosis in human colon cancer HT-29 cells, Asian Pac J Cancer Prev 16 (6) (2015) 2569–2574. [DOI] [PubMed] [Google Scholar]
- [136].Wu Y, Zhou BP, Inflammation: a driving force speeds cancer metastasis, Cell Cycle 8 (20) (2009) 3267–3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Xie Y, Zhao Y, Shi L, Li W, Chen K, Li M, Chen X, Zhang H, Li T, Matsuzawa-Ishimoto Y, Yao X, Shao D, Ke Z, Li J, Chen Y, Zhang X, Cui J, Cui S, Leng Q, Cadwell K, Li X, Wei H, Zhang H, Li H, Xiao H, Gut epithelial TSC1/mTOR controls RIPK3-dependent necroptosis in intestinal inflammation and cancer, J Clin Invest 130 (4) (2020) 2111–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Yan S, Li Q, Zhang D, Wang X, Xu Y, Zhang C, Guo D, Bao Y, Necroptosis pathway blockage attenuates PFKFB3 inhibitor-induced cell viability loss and genome instability in colorectal cancer cells, Am J Cancer Res 11 (5) (2021) 2062–2080. [PMC free article] [PubMed] [Google Scholar]
- [139].Yashin DV, Romanova EA, Ivanova OK, Sashchenko LP, The Tag7-Hsp70 cytotoxic complex induces tumor cell necroptosis via permeabilisation of lysosomes and mitochondria, Biochimie 123 (2016) 2–6. [DOI] [PubMed] [Google Scholar]
- [140].Yatim N, Jusforgues-Saklani H, Orozco S, Schulz O, Barreira da Silva R; Reis e Sousa C; Green DR; Oberst A; Albert ML, RIPK1 and NF-kappaB signaling in dying cells determines cross-priming of CD8(+) T cells, Science 350 (6258) (2015) 328–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Ye YC, Wang HJ, Yu L, Tashiro S, Onodera S, Ikejima T, RIP1-mediated mitochondrial dysfunction and ROS production contributed to tumor necrosis factor alpha-induced L929 cell necroptosis and autophagy, Int Immunopharmacol 14 (4) (2012) 674–682. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.


