INTRODUCTION:
Adult T-cell leukemia/lymphoma (ATL) is a rare, aggressive subtype of peripheral T-cell lymphoma developing after many years of chronic, asymptomatic infection with the retrovirus human T-cell lymphotropic virus type 1 (HTLV-1). HTLV-1 is endemic to certain geographic areas of the world, and primary infection generally occurs in infancy through mother-to-child transmission via breastfeeding. In less than 5% of infected individuals, a decades-long pathogenic process culminates in the development of ATL. Aggressive subtypes of ATL are life-threatening and challenging to treat, with median overall survival typically less than one year in the absence of allogeneic hematopoietic cell transplantation (alloHCT). Owing to the rarity of this illness, prospective large-scale clinical trials have been challenging to perform, and treatment recommendations are largely founded upon limited evidence. Herein, we review the current therapeutic options for ATL, providing a broad literature overview of the foremost clinical trials and reports of this disease. We emphasize our own treatment paradigm, which is broadly based upon disease subtype, patient fitness, and intent to perform alloHCT. Finally, we highlight recent advances in understanding ATL disease biology and important ongoing clinical trials that we foresee as informative and potentially practice-changing.
Keywords: human T-cell lymphotropic virus, adult T-cell leukemia/lymphoma, peripheral T-cell lymphoma, mogamulizumab
HUMAN T-CELL LYMPHOTROPIC VIRUS TYPE 1 (HTLV-1)
Human T-cell lymphotropic virus type 1 (HTLV-1) is a retrovirus with a worldwide geographic distribution. Current estimates suggest that 5–10 million individuals worldwide may be infected, with areas of high viral prevalence in southwest regions of Japan, the Caribbean, sub-Saharan Africa, South America, and sporadic areas in the southwestern Pacific Ocean.1 The predominant mode of transmission is felt to be mother-to-child through breastfeeding, with sexual intercourse and blood contact as additional likely modes of spread.2 Once contracted, HTLV-1 infects CD4+ T-cells through viral integration into host genomes as a single proviral copy.2,3 While most infected individuals remain asymptomatic carriers throughout their lifespan, a minority (approximately 10%) develop HTVL-1-associated diseases.2 The most well-recognized of these diseases are adult T-cell leukemia/lymphoma (ATL) and HTLV-1-associated myelopathy/tropic spastic paraparesis, though multiple other sequelae have been described with lower frequency.4
ADULT T-CELL LEUKEMIA/LYMPHOMA
In less than 5% of HTLV-1-infected individuals, an incompletely understood pathogenic process results in the HTLV-1-associated peripheral T-cell lymphoma (PTCL) subtype ATL (Figure 1). The risk of developing ATL is highest in individuals with high proviral loads (>4% of infected mononuclear cells), whose lifetime risk may exceed 20%.5,6 Other risk factors for ATL include advanced age and a family history of ATL.5 The time between infection and disease onset is long, with a median age at presentation in Japan of 60 to 70 years; the median age in other regions is one-two decades lower for unclear reasons.7–9 This prolonged disease onset implies a multistep oncogenic process, and, indeed, clones of pre-malignant HTLV-1-infected cells can be detected in peripheral blood samples up to ten years prior to the development of ATL.10 Small studies using targeted mutational sequencing over time support a multi-hit model in which HTLV-1-infected cells with oncogenic driver mutations persist stably for many years, followed by the accumulation of additive or synergistic mutations which ultimately result in overt neoplasia.10 In particular, mutations in genes encoding for members of the T-cell receptor (TCR), STAT3, and NOTCH pathways have recently been shown to result in subclonal transcriptomic aberrations that can result in accelerated proliferative potential and disease onset.11
Figure 1. Schematic Representation.
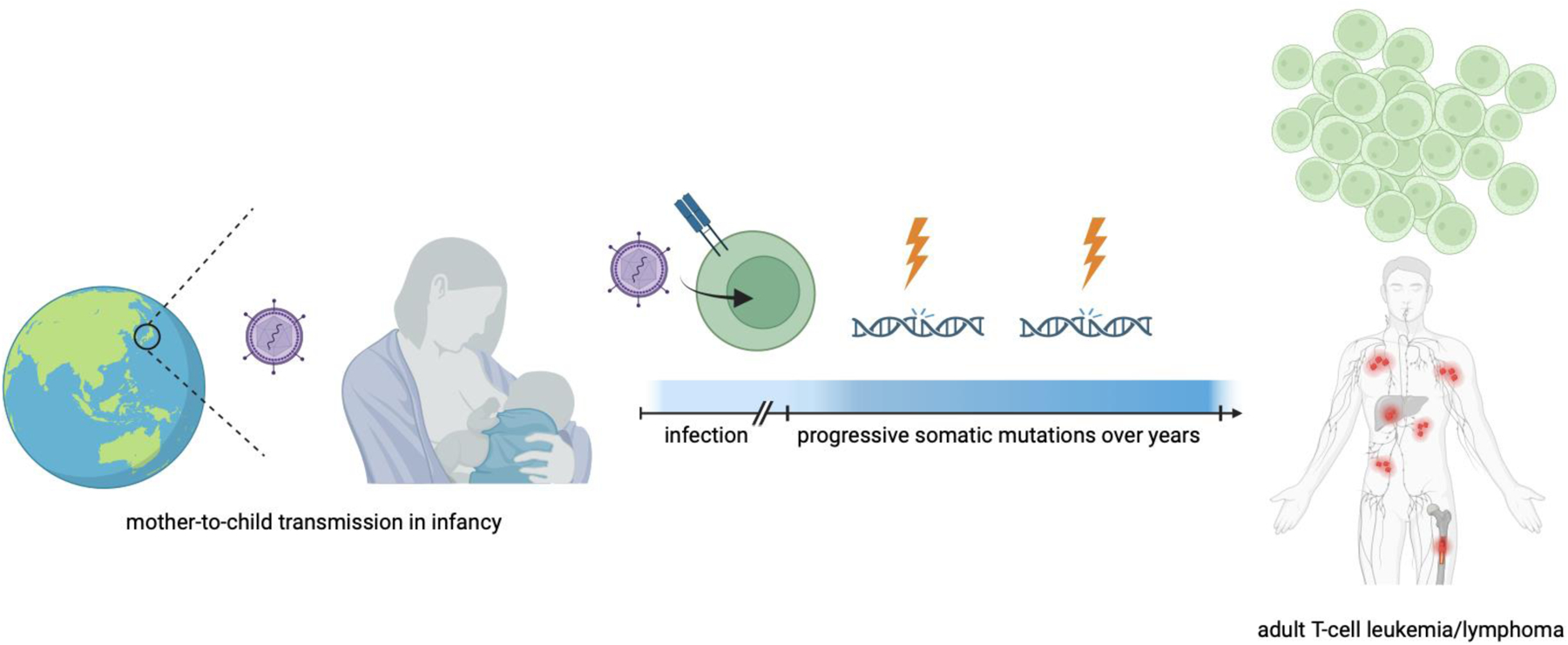
Human T-cell lymphotropic virus type 1 (HTLV1) exists with high prevalence in various geographical regions, most notably Japan, the Caribbean islands, sub-Saharan Africa, South America, and regions of Oceania. The virus is predominantly spread mother-to-child through breastfeeding and infects CD4+ T cells. In less than 5% of infected individuals, a multihit pathogenic process occurs over decades as multiple somatic mutations occur, eventually resulting in an HTLV-1 associated peripheral T-cell lymphoma known as adult T-cell leukemia/lymphoma (ATL). ATL has varied manifestations and subtypes (see text). Created in BioRender.com.
ATL has varied clinical presentations that historically have been divided into four subtypes based on clinical behavior, laboratory features, and pattern of organ involvement. This classification, referred to as the Shimoyama classification, remains unchanged since its original description in the early 1990s.12 The most common subtype is the ‘acute’ subtype, characterized by a frequently rapid progressive clinical course with leukemic disease and various tumor lesions. The ‘lymphoma’ subtype, accounting for approximately 20% of cases, is defined by histologically proven lymphadenopathy, absence of lymphocytosis, and variable extranodal organ involvement and laboratory abnormalities. The acute and lymphoma subtypes are considered aggressive, whereas the remaining two subtypes—’chronic’ and ‘smoldering’—have historically been considered indolent although may accelerate to display more aggressive clinical features (see additional comments on chronic unfavorable disease in section, “Indolent Disease: Chronic and Smoldering Subtypes”). The diagnosis of ATL is established based on histopathologic and immunophenotypic review of tumor lesions, peripheral blood flow cytometry and smear review, and HTLV-1 serology. Staging is performed preferentially with 18F-FDG positron emission tomography/computed tomography (PET/CT) and bone marrow aspirate and biopsy.
The prognosis of ATL is generally poor: in the original report of the Shimoyama classification describing 818 cases from between 1983 and 1987, the median overall survival (OS) for all patients was just 5.4 months.12 By subtype, estimated four-year OS rates for acute, lymphoma, chronic, and smoldering disease were 5%, 6%, 27%, and 63%, respectively. In an updated national query of 1,665 patients in Japan diagnosed between 2000 and 2009, survival only marginally improved, with estimated four-year OS rates for acute, lymphoma, chronic, and smoldering disease of 11%, 16%, 36%, and 52%, respectively.13 These survival improvements may be due to increased use of and improved supportive care during/after allogeneic hematopoietic stem cell transplantation (alloHCT). Still, for both acute and lymphoma subtypes, median OS remained less than one year.13 Moreover, as described below, the term ‘indolent’ that is often used to group the chronic and smoldering subtypes together is misleading when applied to all patients. When patients with indolent ATL subtypes are able to be observed, median OS is still less than five years.14 Certain patients with higher-risk indolent disease subtypes have poor survival outcomes equivalent to those with aggressive subtypes.15 A recent multinational effort of over 800 patients from Japan, Latin America, the Caribbean, and the United States reported a three-year overall survival for all patients at just 21%.16
CURRENT THERAPEUTIC APPROACHES: UPFRONT THERAPY
The treatment of ATL is primarily dictated by disease subtype/behavior, patient fitness/comorbidities, goals of care, and alloHCT donor availability. Below we review the current therapeutic approaches for each subtype, with an emphasis on recent advances and ongoing clinical trials (Tables 1 and 2). Of note, when analyzing reports of novel treatment regimens, it is important to consider both response- and survival-based efficacy endpoints and to examine rates of consolidation with alloHCT depending on the clinical context for use to fully contextualize results achieved.
Table 1.
Recent Therapeutic Updates in Adult T-cell Leukemia/Lymphoma (ATL).
| Trial | Design | Population | Response Rates | Survival | Notes |
|---|---|---|---|---|---|
| mogamulizumab 75 | open-label randomized trial of mogamulizumab vs. inv.’s choice | R/R aggressive ATL with ≥ 1 prior systemic therapy |
mogamulizumab: ORR: 11% best response: 34% inv.’s choice: ORR: 0% best response: 0% |
median PFS: 0.93 (moga) vs. 0.88 m median OS*: 4.9 (moga) vs. 6.9 m |
mogamulizumab is not approved in US for use in R/R ATL but is NCCN listed |
| CHOP + mogamulizumab 37 | single-arm efficacy evaluation of CHOP x 3 followed by mogamulizumab x 8 | untreated, transplant-ineligible Japanese patients with ATL | ORR: 87.5% |
12-month PFS: 26.6% 12-month OS: 52.6% |
mogamulizumab + more intensive chemotherapy is approved in Japan (not US) based on prior studies38 |
| valemetostat 97 | open-label, single-arm, phase II | R/R aggressive ATL after prior moga or ≥ 1 systemic therapy if moga contraindicated |
ORR: 48.0% CR: 20.0% |
median PFS: 7.4 m median OS: 16.4 m |
valemetostat is approved in Japan for R/R ATL |
Crossover allowed.
CHOP, cyclophosphamide, doxorubicin, vincristine, prednisone; inv.’s choice, investigator’s choice; moga, mogamulizumab; NCCN, National Comprehensive Cancer Network; ORR, objective response rate; OS, overall survival; PFS, progression-free survival, US, United States.
Table 2.
Ongoing Trials in Adult T-cell Leukemia/Lymphoma (ATL).
| Trial | Regimen | Population | Design | Notes |
|---|---|---|---|---|
| NCT04301076 | lenalidomide + EPOCH | untreated acute, lymphoma, or poor-risk chronic subtype ATL | phase I | NCI sponsored, estimated completion 2023 |
| NCT03264131 | BV-CHEP | untreated acute, lymphoma, or poor-risk chronic subtype ATL | pilot/phase I | interim results,33 showing 75% ORR in 8 patients |
| NCT04502446 | CTX130 | R/R T-cell lymphoma, including ATL | phase I | anti-CD70 allogeneic CRISPR-Cas9-engineered T cells, interim results91 |
| recombinant human IL-15 (rhIL-15) combinations (NCT02689453, NCT04185220) | rhIL-15 + mogamulizumab or alemtuzumab | R/R T-cell lymphoma, including ATL | phase I | NCI sponsored |
| NCT04848046 | NK cell therapy + mogamulizumab | R/R ATL or CTCL | phase I | IL-21 expanded, off-the-shelf NK cell therapy |
| NCT02737046 | belinostat + ZDV +/− IFN | aggressive ATL with persistent circulating disease after prior chemotherapy or ZDV/IFN | phase II | interim results of 6 patients reported, potential to achieve complete molecular CR92 |
| NCT04703192 (VALENTINE-PTCL01) | valemetostat | R/R T-cell lymphoma, including ATL | phase II | enrollment complete, registration study |
| JCOG1111C | IFN/AZT versus watchful waiting | indolent ATL (symptomatic smoldering or chronic w/o unfavorable features) | open-label, randomized phase III | led by Japanese Clinical Oncology Group (JCOG) |
BV, brentuximab vedotin; CR, complete response; CTCL, cutaneous T-cell lymphoma; EPOCH, etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin; IFN, interferon; NCI, National Cancer Institute; NK, natural killer; ORR, objective response rate; R/R, relapsed/refractory; ZDV, zidovudine.
Aggressive Disease: Lymphoma or Acute Subtypes
The mainstay of curative-intent therapy in fit patients with lymphoma or acute subtype associated with significant tumor bulk is combination chemotherapy.17–22 No regimen is clearly superior, and use differs globally based on historic practice patterns and drug availability. In Japan, the vincristine, cyclophosphamide, doxorubicin, and prednisone (VCAP), doxorubicin, ranimustine, and prednisone (AMP), and vindesine, etoposide, carboplatin, and prednisone (VCEP) protocol is widely adopted.19 Support for this regimen comes from the randomized Japan Clinical Oncology Group Study JCOG9801, in which 118 patients with aggressive ATL (including unfavorable chronic subtype15) were assigned to receive either VCAP/AMP/VCEP or biweekly CHOP, with both arms receiving intrathecal prophylaxis against central nervous system (CNS) disease. While the study failed to meet the prespecified hazard ratio of 0.6 for OS in the VCAP/AMP/VCEP arm, the OS rate at three years was numerically improved in the VCAP/AMP/VCEP arm (24% versus 13%; HR 0.75, 95% CI, 0.50–1.13). Key secondary endpoints showed improved one-year progression-free survival (PFS) in the VCAP/AMP/VCEP group (28% vs. 16%; HR 0.78, 95% CI 0.52–1.14), as well as increased complete response (CR) rate (40% vs. 21%, p=0.02). Despite these improvements, VCAP/AMP/VECP is generally not curative and is a challenging regimen for patients, with less than one third of patients able to complete planned treatment, including three treatment-related deaths in JCOG9801. Of note, a modified version of VCAP/AMP/VECP omitting ranimustine and substituting vincristine for vindesine has been used in the United States (US) in a single report.23
As vindesine and ranimustine are not available outside of Japan, intensified anthracycline-based regimens, such as EPOCH/CHOEP (etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin) and hyperCVAD (cyclophosphamide, vincristine, doxorubicin, and dexamethasone plus cytarabine/methotrexate) are commonly used initial regimens elsewhere.20,22,24 The evidence for these regimens is sparse and generally extrapolated from trials of such regimens in nodal-based PTCLs which variably included patients with ATL.25–27 In a multi-institutional retrospective report of 89 cases from North America, CHOP-/CHOEP-like regimens resulted in overall response rates exceeding 60%, but responses were generally not durable, with median OS of only 19.4 and 37.3 weeks for acute and lymphoma subtypes, respectively.28 While the regimen of brentuximab vedotin plus CHP (BV-CHP) is approved by the United States Food and Drug Administration (FDA) for adults with previously untreated CD30-expressing PTCL, the phase 3 ECHELON-2 trial that led to this approval included only seven patients with ATL.29 As this trial demonstrated PFS and OS benefits over CHOP in the entire intent-to-treat population, BV-CHP can be considered in patients with CD30-expressing ATL. An interim analysis of BV-CHEP in eight patients with aggressive ATL showed an overall response rate of 75%, enabling three patients to proceed to alloHCT.30 This regimen has been used elsewhere in the upfront treatment of T-cell lymphomas,31 and we await final results of this trial specifically in ATL (NCT03264131).
The addition of antiviral therapy (usually zidovudine) and interferon-alpha (IFN-α) to chemotherapy has been shown in retrospective evaluations to improve response rates over chemotherapy alone, resulting in overall response rates of 81% (39% CR) versus 49% (36% CR) in a historic cohort of 73 patients with aggressive subtypes from the United Kingdom (UK).32 EPOCH followed by up to one year of zidovudine, lamivudine, and IFN-α was evaluated in a small prospective study led by the AIDS Malignancy Consortium in 19 patients, with 11 patients achieving an overall response (two CR).22 In both this study and the UK cohort, however, relapse rates were high and survival was short, with median OS of just 9 and 13 months, respectively.22,32 An additional regimen of EPOCH with bortezomib and raltegravir, also led by the AIDS Malignancy Consortium, was prospectively evaluated in a trial of 18 patients, with 12 patients achieving an overall response (three CR).21 Again, responses were short-lived and survival was short, with median PFS and OS of just 5.8 and 6.2 months, respectively.21 Thus, while the addition of antivirals may improve response rates, there remains no consensus and no clear survival advantages.33
The addition of the anti-CCR4 monoclonal antibody mogamulizumab (discussed in detail below, see Current Therapeutic Approaches: Relapsed/Refractory Disease) to upfront combination therapy has been attempted in Japan.34,35 In a randomized phase II trial of 54 patients with aggressive ATL, mogamulizumab plus VCAMP/AMP/VECP resulted in an ORR of 86% (52% CR) versus 75% (33% CR) with VCAMP/AMP/VECP alone.35 This study met the prespecified target of a 15% difference in CR rate between the investigational and control arms. Still, relapses were frequent, with median PFS of 8.5 months with mogamulizumab plus VCAMP/AMP/VECP versus 6.3 months in the chemotherapy only arm. Long-term follow-up showed 3-year median OS rate of 45% in the investigational arm versus 50% in the control arm.36 Based on these results, mogamulizumab is approved in Japan for use with VACMP/AMP/VECP. Of note, mogamulizumab plus VCAMP/AMP/VECP resulted in greater hematologic toxicity and several serious adverse events including interstitial lung disease, numerous infectious complications that were not observed with chemotherapy alone (including cytomegalovirus viremia and pneumonia and unspecified viral encephalitis), and two treatment-related deaths.36 Similarly, in an untreated population of 24 transplant-ineligible patients, sequential CHOP followed by mogamulizumab resulted in ORR of 88% and one-year PFS and OS rates of 27% and 53%, respectively.34 Finally, a retrospective analysis of 77 patients with newly diagnosed ATL treated with chemotherapy alone or chemotherapy plus mogamulizumab showed no significant survival advantage in those treated with upfront mogamulizumab; no concerning safety signal was noted between arms.37
While mogamulizumab is an important agent in treating relapsed and refractory (R/R) ATL as discussed below, optimal strategies for its incorporation into upfront regimens have not been defined. It is important to note that mogamulizumab has a consistent compartmental therapeutic effect, with greater effect on disease in the blood than in skin, nodes, or viscera, which may factor into treatment decisions.34,35,37 In addition, it is critical to recognize that receipt of mogamulizumab prior to alloHCT has been shown to increase the risk of steroid-refractory graft-versus-host-disease (GVHD), non-relapse mortality, and overall mortality, likely due to a deleterious effect on non-malignant T cells such as regulatory T cells (Treg).38–41 Some experts avoid mogamulizumab prior to alloHCT.33 Others recommend a minimum “washout” period of at least 50 days to mitigate against this complication—this cutoff is derived from a Japanese series that evaluated the impact of pre-alloHCT mogamulizumab receipt and found that an interval of less than 50 days between last mogamulizumab administration and alloHCT was associated with inferior OS on multivariable analyses (HR 2.02, 95% CI 1.37–2.98).42
Patients with acute, bulky disease are treated similarly to those with lymphoma subtype disease.33 Antiviral/interferon therapy alone for patients with acute, bulky/aggressive disease is typically avoided given that patients with pure lymphoma subtype have survival disadvantages when treated with antiviral/interferon therapy alone.43 However, for those with purely acute/leukemic disease, antiviral/interferon therapy is a valid therapeutic option, taking into consideration the pace of disease, intent of therapy, and patient fitness.43 In an international meta-analysis of antiviral/interferon therapy in various ATL subtypes, patients with acute subtype who were treated with antiviral/interferon alone and achieved a CR (30% of all acute subtype patients) had a prolonged overall survival at five years of 82% (versus 12% in those achieving less than CR).43
Despite substantial advances in defining the molecular underpinnings of ATL, upfront therapy selection based on tumor mutational profiling is not yet routine. Recent important efforts have identified TP53 mutations and CD28 gene-related activating mutations as each being associated with unfavorable prognosis, regardless of disease subtype or therapeutic modality used.44–47 For example, in a recently reported series of 177 Japanese patients evaluating outcomes by TP53-mutation status, outcomes were worse among those with TP53 mutations regardless of therapeutic modality.44 Altogether, 38% of patients’ disease harbored either a TP53 single nucleotide variant, insertion-deletions, or copy number variation; in the entire cohort, median OS in those with and without TP53-mutated ATL was 1.0 and 6.7 years, respectively (p<0.01). Moreover, even among those who received alloHCT, median OS was less in those with TP53-mutated ATL (n=16) compared to those without (n=27, 0.4 years versus not reached, p<0.01). Further defining the significance of these alterations and incorporating such findings into routine clinical practice and treatment paradigms will represent important advances in treating ATL.
CNS evaluation and prophylaxis is strongly recommended by multiple consensus guidelines in patients with aggressive subtypes, as the risk of CNS involvement exceeds 10%.48–50 There have been no prospective evaluations of specific prophylaxis regimens or mode/timing of delivery, and practices generally mirror those for treating aggressive B-cell lymphomas. For patients with circulating leukemic disease, we generally debulk this disease when safe (asymptomatic and no evidence of CNS involvement on imaging) and feasible to minimize the theoretical risk of CNS seeding during traumatic lumbar puncture.
Finally, an ongoing trial sponsored by the US National Cancer Institute (NCT04301076) is testing lenalidomide plus EPOCH in patients with treatment-naïve ATL. This study is based on demonstrable clinical activity for lenalidomide in treating patients with R/R ATL (see below). Providers should be mindful of this important multi-institutional effort and consider referring potentially eligible patients.
Aggressive Disease: Allogeneic Transplant
Despite high response rates to upfront combination chemotherapy, responses are generally not durable and nearly all patients with aggressive subtypes will relapse without consolidative therapy. The only potentially curative approach in treating aggressive ATL remains alloHCT, and consideration of alloHCT in first or subsequent remission is recommended for all eligible patients with aggressive subtypes of disease.48–50 While no randomized data exist, select prospective reports and multiple retrospective series show the curative potential of alloHCT.51–60 Most data are derived from Japanese series; an early report from three Japanese transplant registries of 386 patients who underwent alloHCT between 1996 and 2005 demonstrated rates of three-year OS, disease-associated mortality, and treatment-related mortality of 33%, 21%, and 37%, respectively.54 A more modern prospective series of 90 patients with aggressive ATL who underwent alloHCT between 2015 and 2018 showed two-year PFS and OS rates of 31% and 44%, respectively.59 Two-year relapse and non-relapse mortality rates were 46% and 23%, respectively.59
Non-Japanese series from the European Society for Blood and Marrow Transplantation as well a single-center experience from our institution have validated the feasibility and efficacy of alloHCT in non-Japanese patients with aggressive ATL.52,58 A meta-analysis of 18 studies comprising 1,767 patients with ATL who underwent alloHCT reported pooled OS, PFS, relapse, and non-relapse mortality rates of 40%, 37%, 36%, and 29%, respectively.60 This work validates the potential for prolonged remission with alloHCT in treating ATL, although relapses and transplant-related mortality remain high. Nuances regarding donor type and conditioning regimens are outside the scope of this review and are discussed elsewhere.33,50 In those who are transplant-ineligible, a maintenance approach with antiviral therapy or oral chemotherapy has been attempted.22,61
Indolent Disease: Chronic and Smoldering Subtypes
Neither chronic nor smoldering ATL are truly indolent diseases. In a series of 90 Japanese patients with newly diagnosed indolent ATL subtypes between 1974 and 2003, most of whom were observed at diagnosis in a “watch and wait” approach, the median survival was 4.1 years with an estimated 10-year survival rate of only 25%.62 There was notably no observable plateau in survival curves. Moreover, multiple reports have described an ‘unfavorable’ chronic subtype defined as having a low serum albumin, high lactate dehydrogenase (LDH), or high blood urea nitrogen concentration that has a similar prognosis to aggressive ATL.15 Therefore, while no randomized data refutes a surveillance strategy, our view and others33 generally favors attempts at treatment in indolent ATL if available and tolerable unless a patient is asymptomatic. A prospective trial of antiviral therapy versus active monitoring in indolent ATL is ongoing in Japan (JCOG1111C).
For indolent disease, antiviral/interferon therapy is the preferred first-line treatment strategy.63–66 In the aforementioned meta-analysis of 254 patients from the UK, median and five-year OS rates for chronic and smoldering ATL were not reached and 76%, respectively.43 Most of the patients with indolent disease (74%) were treated with upfront antiviral/interferon therapy only. For patients with indolent subtype ATL who received upfront antiviral/interferon only, overall survival was excellent at 100% beyond five years, recognizing the potential that identifying such patients may reflect a selection bias for those felt suitable for such therapy. Emerging data suggest that zidovudine and interferon function through the induction of p53 signaling and apoptosis,67 and that inactivating TP53 mutations may confer resistance.44,68 Dosing and maintenance strategies are largely empiric; guidelines from the NCCN recommend ZDV 1000 mg daily with IFN-α 5 million units daily (which can be substituted with peginterferon if available).48 Higher doses of both with ZDV at 1.5 grams daily and interferon up to 10 million units can be used if tolerated with careful dose uptitration.33 In responding patients, no clear “maintenance” treatment strategy is currently defined. In our practice, we typically continue therapy indefinitely if tolerated, and doses can be reduced to mitigate side effects.23 Given the potential for durable responses with antiviral/interferon therapy alone, responding patients with indolent subtypes do not require upfront alloHCT.
Finally, there are a group of patients with disease seemingly confined to the skin, sometimes referred to as a ‘primary cutaneous variant’.69 While skin-directed therapies can be applied to these patients, this entity is incompletely understood, with some evidence suggesting poor outcomes for certain patients, especially those with nodulo-tumoral and erythrodermic manifestations.14,69,70
CURRENT THERAPUETIC APPROACHES: RELAPSED/REFRACTORY DISEASE
Patients with relapsed/refractory (R/R) ATL have been underrepresented in prospective trials of novel agents for R/R PTCLs. For example, in the phase II trials that led to regulatory approval for belinostat, pralatrexate, and romidepsin for PTCL, the proportion of patients with ATL was <5% or patients with ATL were excluded.71–73 Below is a discussion of agents that have undergone dedicated evaluation and emerging therapies under investigation.74
In Japan, mogamulizumab is approved in the treatment of R/R ATL.74–77 In the original phase II trial of mogamulizumab, an ORR of 50% was observed (31% CR).75 ORR by subtype for acute, lymphoma, and unfavorable chronic were 43%, 33%, and 83%, respectively. Prolonged survival analysis of this study showed a median PFS and OS of 5.2 and 14.4 months, respectively. Survival differed by subtype, with three-year OS rate for acute, lymphoma, and unfavorable chronic subtype of 7%, 17%, and 67%, respectively. Patients who developed an on-treatment rash, a common side effect of mogamulizumab, survived longer, implying that this complication reflects a treatment effect given the mechanism of mogamulizumab and the hypothesized pathogenesis of rash due to this agent.78,79 In a post-marketing surveillance study of 597 patients from Japan who received mogamulizumab for R/R ATL, the overall best response rate was 58% (by compartment: blood, 82%; skin, 56%; lymph nodes, 46%)76 and median OS was 5.5 months.
In a non-Japanese, multi-national randomized phase II trial, 71 patients with R/R ATL (acute, lymphoma, or chronic) were randomized to receive mogamulizumab or investigator’s choice (most commonly, gemcitabine/oxaliplatin).80 By independent review, the best response rate in the mogamulizumab arm was 28% versus 8% in the investigator’s choice arm. The confirmed ORR, defined as maintaining a response through a second assessment eight weeks later, was 11% in the mogamulizumab arm versus 0% in the investigator’s choice arm. Median PFS in the mogamulizumab and investigator’s choice arms was 0.93 versus 0.88 months, respectively. Mogamulizumab is not FDA-approved for use in the United States but can be obtained off-label and is compendium-listed by the NCCN.48 Importantly, loss of CCR4 expression via mutation or deletion, as well as somatic CCR7 gene alterations, have emerged as mechanisms of resistance to mogamulizumab; conversely, gain-of-function CCR4 mutations are associated with improved survival in patients with ATL treated with mogamulizumab.81–84 When possible, tumor genomic profiling that includes CCR4 and CCR7 can be performed to cautiously inform clinical decision making when equipoise exists. Mogamulizumab has notably been used in those who recur after alloHCT in a small series of eight patients with some efficacy (three CRs, two patients with continued CR beyond two years) and no emergence or progression of GVHD.85 Further safety data are needed in this setting.
Another agent with demonstrable activity in R/R ATL is the oral immunomodulatory drug lenalidomide. A multicenter phase II trial of Japanese patients with R/R ATL showed ORR of 42% and median time to progression of 3.8 months.86 A similar trial conducted in the United States saw no responses in four patients and closed due to limited accrual.87 Lenalidomide has been used in recurrent disease after alloHCT, although new onset and worsening GVHD is observed.85 Small cases series and prospective trials have been conducted with single-agent pralatrexate,88 romidepsin,89 bortezomib,90 arsenic trioxide,91,92 and alemtuzumab93 with limited successes. A multicenter phase II trial of tucidinostat, an oral histone deacetylase inhibitor, has recently been reported.94 Among 23 patients with R/R aggressive ATL who had previously received chemotherapy and mogamulizumab, tucidinostat resulted on ORR of 30%, with median PFS and OS of 1.7 and 7.9 months, respectively.
Other investigational therapies worth noting include an allogeneic CD70-directed T-cell therapy95 and the combination of the histone deacetylase inhibitor belinostat with antiviral therapy.96 The latter combination was evaluated in a phase II trial in patients with aggressive ATL who had persistent circulating disease after prior chemotherapy or antiviral therapy.96 Among six patients, clinical activity was observed in five patients, with three patients achieving a complete response and two patients maintaining partial remission. Recent pre-clinical work suggests a potential role for certain small molecule inhibitors, including those inhibiting the JAK/STAT, PI3K, NF-kB, and CDK6 networks.97–99 In our practice, we prioritize clinical trial enrollment for patients with R/R disease whenever possible. In the absence of a clinical trial, we select any of the above agents or other agents used in the treatment of R/R T-cell lymphomas based on prior regimens, goals of care, anticipated toxicities, GVHD status if post-alloHCT, and patient fitness and comorbidities.
A promising agent in development is valemetostat, an epigenetic therapy that functions as a dual inhibitor of enhancer of zeste homolog 1 (EZH1) and EZH2. EZH2 is widely overexpressed in T-cell lymphomas.100,101 In a dedicated phase II study of valemetostat in Japan for patients with R/R ATL, a high ORR of 48% was observed in 25 patients, 24 of whom had been previously treated with mogamulizumab.102 Median PFS and OS were 7.4 and 16.4 months, respectively. Noting small numbers, responses varied by subtype with ORR of 63%, 17%, and 33% for acute, lymphoma, and chronic unfavorable subtypes, respectively. Of note, this study excluded patients with relapsed disease after alloHCT. Based on these results, valemetostat was approved in Japan in September 2022 for the treatment of R/R ATL. In a separate phase I trial of valemetostat in all R/R non-Hodgkin lymphomas across Japan and the United States, an ORR of 56% was seen in PTCL.103 The global VALENTINE-PTCL01 phase II trial (NCT04703192) of valemetostat in PTCL, including ATL, has completed accrual and results are awaited.
CONCLUSION
ATL is a complication of chronic HTLV-1 infection for which therapeutic progress has been challenging. Nationwide surveys from Japan show only marginal survival improvements over nearly 30 years, and the only reliably curative approach for those with aggressive disease remains intensive combination chemotherapy followed by alloHCT, a strategy that relatively few patients achieve. Key questions in the current treatment of ATL are which induction regimen for patients with aggressive disease is superior, how to incorporate novel active agents, such as mogamulizumab, lenalidomide, and potentially valemetostat, into upfront regimens, and how to optimize allotransplantation strategies. Treatment is currently dictated by disease subtype, but as tremendous work by global investigators has begun to detail the genomic landscape of this disease,104,105 our hope is that truly targeted, rational therapies will allow for tailored treatment strategies. The rarity of this entity remains a major hurdle to dedicated study, and concerted, international efforts are needed to move forward.
Footnotes
DISCLOSURES: We have no disclosures.
REFERENCES AND RECOMMENDED READING:
• Of importance
- 1.Gessain A, Cassar O. Epidemiological Aspects and World Distribution of HTLV-1 Infection. Front Microbiol. 2012;(3):388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gonçalves DU, Proietti FA, Ribas JGR, et al. Epidemiology, Treatment, and Prevention of Human T-Cell Leukemia Virus Type 1-Associated Diseases. Clin Microbiol Rev. 2010;23(3):577–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cook LB, Rowan AG, Melamed A, Taylor GP, Bangham CRM. HTLV-1–infected T cells contain a single integrated provirus in natural infection. Blood. 2012;120(17):3488–3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schierhout G, McGregor S, Gessain A, et al. Association between HTLV-1 infection and adverse health outcomes: a systematic review and meta-analysis of epidemiological studies. Lancet Infect Dis. 2020;20(1):133–143. [DOI] [PubMed] [Google Scholar]
- 5.Iwanaga M, Watanabe T, Utsunomiya A, et al. Human T-cell leukemia virus type I (HTLV-1) proviral load and disease progression in asymptomatic HTLV-1 carriers: a nationwide prospective study in Japan. Blood. 2010;116(8):1211–1219. [DOI] [PubMed] [Google Scholar]
- 6.Demontis MA, Hilburn S, Taylor GP. Human T cell lymphotropic virus type 1 viral load variability and long-term trends in asymptomatic carriers and in patients with human T cell lymphotropic virus type 1-related diseases. AIDS Res Hum Retroviruses. 2013;29(2):359–364. [DOI] [PubMed] [Google Scholar]
- 7.Suzumiya J, Ohshima K, Tamura K, et al. The International Prognostic Index predicts outcome in aggressive adult T-cell leukemia/lymphoma: analysis of 126 patients from the International Peripheral T-Cell Lymphoma Project. Ann Oncol. 2009;20(4):715–721. [DOI] [PubMed] [Google Scholar]
- 8.Iwanaga M Epidemiology of HTLV-1 Infection and ATL in Japan: An Update. Front Microbiol. 2020;11:1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shah UA, Shah N, Qiao B, et al. Epidemiology and survival trend of adult T-cell leukemia/lymphoma in the United States. Cancer. 2020;126(3):567–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rowan AG, Dillon R, Witkover A, et al. Evolution of retrovirus-infected premalignant T-cell clones prior to adult T-cell leukemia/lymphoma diagnosis. Blood. 2020;135(23):2023–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamagishi M, Kubokawa M, Kuze Y, et al. Chronological genome and single-cell transcriptome integration characterizes the evolutionary process of adult T cell leukemia-lymphoma. Nat Commun. 2021;12(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shimoyama M Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma. A report from the Lymphoma Study Group (1984–87). Br J Haematol. 1991;79(3):428–437. [DOI] [PubMed] [Google Scholar]
- 13.Katsuya H, Ishitsuka K, Utsunomiya A, et al. Treatment and survival among 1594 patients with ATL. Blood. 2015;126(24):2570–2577. [DOI] [PubMed] [Google Scholar]
- 14.Bittencourt AL, Vieira MDG, Brites CR, Farre L, Barbosa HS. Adult T-cell leukemia/lymphoma in Bahia, Brazil: analysis of prognostic factors in a group of 70 patients. Am J Clin Pathol. 2007;128(5):875–882. [DOI] [PubMed] [Google Scholar]
- 15.Tsukasaki K, Hermine O, Bazarbachi A, et al. Definition, Prognostic Factors, Treatment, and Response Criteria of Adult T-Cell Leukemia-Lymphoma: A Proposal From an International Consensus Meeting. J Clin Oncol. 2009;27(3):459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valcarcel B, Sano H, Katsuya H, et al. Clinical Features, Treatment Patterns, and Outcomes Among 837 Patients with Adult T-Cell Leukemia-Lymphoma in the Real-World Setting: A Comparison of Endemic Regions. Blood. 2022;140(Supplement 1):1072–1073. [Google Scholar]
- 17.Tsukasaki K, Tobinai K, Shimoyama M, et al. Deoxycoformycin-containing combination chemotherapy for adult T-cell leukemia-lymphoma: Japan Clinical Oncology Group Study (JCOG9109). Int J Hematol. 2003;77(2):164–170. [DOI] [PubMed] [Google Scholar]
- 18.Yamada Y, Tomonaga M, Fukuda H, et al. A new G-CSF-supported combination chemotherapy, LSG15, for adult T-cell leukaemia-lymphoma: Japan Clinical Oncology Group Study 9303. Br J Haematol. 2001;113(2):375–382. [DOI] [PubMed] [Google Scholar]
- 19.Tsukasaki K, Utsunomiya A, Fukuda H, et al. VCAP-AMP-VECP compared with biweekly CHOP for adult T-cell leukemia-lymphoma: Japan Clinical Oncology Group study JCOG9801. J Clin Oncol. 2007;25(34):5458–5464. [DOI] [PubMed] [Google Scholar]
- 20.Alduaij A, Butera JN, Treaba D, Castillo J. Complete remission in two cases of adult T-Cell Leukemia/Lymphoma treated with Hyper-CVAD: A case report and review of the literature. Clin Lymphoma Myeloma Leuk. 2010;10(6):480–483. [DOI] [PubMed] [Google Scholar]
- 21.Ratner L, Rauch D, Abel H, et al. Dose-adjusted EPOCH chemotherapy with bortezomib and raltegravir for human T-cell leukemia virus-associated adult T-cell leukemia lymphoma. Blood Cancer J. 2016;6(3):e408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ratner L, Harrington W, Feng X, et al. Human T cell leukemia virus reactivation with progression of adult T-cell leukemia-lymphoma. PLoS One. 2009;4(2):e4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malpica L, Pimentel A, Reis IM, et al. Epidemiology, clinical features, and outcome of HTLV-1–related ATLL in an area of prevalence in the United States. Blood Adv. 2018;2(6):607–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toriyama E, Imaizumi Y, Taniguchi H, et al. EPOCH regimen as salvage therapy for adult T-cell leukemia-lymphoma. Int J Hematol. 2018;108(2):167–175. [DOI] [PubMed] [Google Scholar]
- 25.Maeda Y, Nishimori H, Yoshida I, et al. Dose-adjusted EPOCH chemotherapy for untreated peripheral T-cell lymphomas: a multicenter phase II trial of West-JHOG PTCL0707. Haematologica. 2017;102(12):2097–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmitz N, Trümper L, Ziepert M, et al. Treatment and prognosis of mature T-cell and NK-cell lymphoma: an analysis of patients with T-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood. 2010;116(18):3418–25. [DOI] [PubMed] [Google Scholar]
- 27.Escalón MP, Liu NS, Yang Y, et al. Prognostic factors and treatment of patients with T-cell non-Hodgkin lymphoma: the M. D. Anderson Cancer Center experience. Cancer. 2005;103(10):2091–8. [DOI] [PubMed] [Google Scholar]
- 28.Phillips AA, Shapira I, Willim RD, et al. A critical analysis of prognostic factors in North American patients with human T-cell lymphotropic virus type-1-associated adult T-cell leukemia/lymphoma: a multicenter clinicopathologic experience and new prognostic score. Cancer. 2010;116(14):3438–3446. [DOI] [PubMed] [Google Scholar]
- 29.Horwitz S, O’Connor OA, Pro B, et al. Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): a global, double-blind, randomised, phase 3 trial. Lancet. 2019;393(10168):229–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dittus C, Weinstock MJ, Barnes JA, et al. Interim Results of a Multicenter Pilot Study Evaluating Brentuximab Vedotin with Cyclophosphamide, Doxorubicin, Etoposide, and Prednisone (BV-CHEP) for the Treatment of Aggressive Adult T-Cell Leukemia/Lymphoma. Blood. 2021;138(Supplement 1):1395–1395. [Google Scholar]
- 31.Herrera AF, Zain J, Savage KJ, et al. Brentuximab Vedotin Plus Cyclophosphamide, Doxorubicin, Etoposide, and Prednisone (CHEP-BV) Followed By BV Consolidation in Patients with CD30-Expressing Peripheral T-Cell Lymphomas. Blood. 2021;138(Supplement 1):133–133. [Google Scholar]
- 32.Hodson A, Crichton S, Montoto S, et al. Use of zidovudine and interferon alfa with chemotherapy improves survival in both acute and lymphoma subtypes of adult T-cell leukemia/lymphoma. J Clin Oncol. 2011;29(35):4696–4701. [DOI] [PubMed] [Google Scholar]
- 33.Cook LB, Phillips AA. How I treat adult T-cell leukemia/lymphoma. Blood. 2021;137(4):459–470. [DOI] [PubMed] [Google Scholar]
- 34.Tanimoto K, Kato K, Aoki T, et al. CHOP Plus Sequential Mogamulizumab As First-Line Therapy for Untreated Adult T-Cell Leukemia-Lymphoma. Blood. 2021;138(Supplement 1):1393–1393. [Google Scholar]
- 35.Ishida T, Jo T, Takemoto S, et al. Dose-intensified chemotherapy alone or in combination with mogamulizumab in newly diagnosed aggressive adult T-cell leukaemia-lymphoma: A randomized phase II study. Br J Haematol. 2015;169(5):672–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ishida T, Jo T, Takemoto S, et al. Follow-up of a randomised phase II study of chemotherapy alone or in combination with mogamulizumab in newly diagnosed aggressive adult T-cell leukaemia-lymphoma: impact on allogeneic haematopoietic stem cell transplantation. Br J Haematol. 2019;184(3):479–483. [DOI] [PubMed] [Google Scholar]
- 37.Jo T, Matsuzaka K, Shioya H, et al. Mogamulizumab Plus EPOCH Therapy for Patients With Newly Diagnosed Aggressive Adult T-cell Leukemia/lymphoma. Anticancer Res. 2020;40(9):5237–5243. [DOI] [PubMed] [Google Scholar]
- 38.Sugio T, Kato K, Aoki T, et al. Mogamulizumab Treatment Prior to Allogeneic Hematopoietic Stem Cell Transplantation Induces Severe Acute Graft-versus-Host Disease. Biology of Blood and Marrow Transplantation. 2016;22(9):1608–1614. [DOI] [PubMed] [Google Scholar]
- 39.Fuji S, Inoue Y, Utsunomiya A, et al. Pretransplantation anti-CCR4 antibody mogamulizumab against adult T-cell leukemia/lymphoma is associated with significantly increased risks of severe and corticosteroid-refractory graft-versus-host disease, nonrelapse mortality, and overall mortality. J Clin Oncol. 2016;34(28):3426–3433. [DOI] [PubMed] [Google Scholar]
- 40.Inoue Y, Fuji S, Tanosaki R, Fukuda T. Pretransplant mogamulizumab against ATLL might increase the risk of acute GVHD and non-relapse mortality. Bone Marrow Transplant. 2016;51(5):725–727. [DOI] [PubMed] [Google Scholar]
- 41.Haji S, Kiyasu J, Choi I, et al. Administration of an anti-CC chemokine receptor 4 monoclonal antibody, mogamulizumab, before allogeneic bone marrow transplantation for adult T-cell leukemia/lymphoma. Bone Marrow Transplant. 2016;51(3):432–434. [DOI] [PubMed] [Google Scholar]
- 42.Fuji S, Inoue Y, Utsunomiya A, et al. Pretransplantation Anti-CCR4 Antibody Mogamulizumab Against Adult T-Cell Leukemia/Lymphoma Is Associated With Significantly Increased Risks of Severe and Corticosteroid-Refractory Graft-Versus-Host Disease, Nonrelapse Mortality, and Overall Mortality. J Clin Oncol. 2016;34(28):3426–3433. [DOI] [PubMed] [Google Scholar]
- 43.Bazarbachi A, Plumelle Y, Ramos JC, et al. Meta-analysis on the use of zidovudine and interferon-alfa in adult T-cell leukemia/lymphoma showing improved survival in the leukemic subtypes. J Clin Oncol. 2010;28(27):4177–4183. [DOI] [PubMed] [Google Scholar]
- 44.Sakamoto Y, Ishida T, Masaki A, et al. Clinical significance of TP53 mutations in adult T-cell leukemia/lymphoma. Br J Haematol. 2021;195(4):571–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sakamoto Y, Ishida T, Masaki A, et al. Clinicopathological significance of CD28 overexpression in adult T-cell leukemia/lymphoma. Cancer Sci. 2022;113(1):349–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sakamoto Y, Ishida T, Masaki A, et al. Clinical significance of CD28 gene-related activating alterations in adult T-cell leukaemia/lymphoma. Br J Haematol. 2021;192(2):281–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nakahata S, Morishita K. The role of CD28 in adult T-cell leukaemia/lymphoma. Br J Haematol. 2021;192(2):235–236. [DOI] [PubMed] [Google Scholar]
- 48.Horwitz SM, Ansell S, Ai WZ, et al. T-Cell Lymphomas, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2022;20(3):285–308. [DOI] [PubMed] [Google Scholar]
- 49.Fox CP, Ahearne MJ, Pettengell R, et al. Guidelines for the management of mature T- and natural killer-cell lymphomas (excluding cutaneous T-cell lymphoma): a British Society for Haematology Guideline. Br J Haematol. 2022;196(3):507–522. [DOI] [PubMed] [Google Scholar]
- 50.Cook LB, Fuji S, Hermine O, et al. Revised adult T-cell leukemia-lymphoma international consensus meeting report. J Clin Oncol. 2019;37(8):677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fuji S, Fujiwara H, Nakano N, et al. Early application of related SCT might improve clinical outcome in adult T-cell leukemia/lymphoma. Bone Marrow Transplantation 2016 51:2. 2015;51(2):205–211. [DOI] [PubMed] [Google Scholar]
- 52.Bazarbachi A, Cwynarski K, Boumendil A, et al. Outcome of patients with HTLV-1-associated adult T-cell leukemia/lymphoma after SCT: a retrospective study by the EBMT LWP. Bone Marrow Transplant. 2014;49(10):1266–1268. [DOI] [PubMed] [Google Scholar]
- 53.Fuji S, Yamaguchi T, Inoue Y, et al. Development of a modified prognostic index for patients with aggressive adult T-cell leukemia-lymphoma aged 70 years or younger: possible risk-adapted management strategies including allogeneic transplantation. Haematologica. 2017;102(7):1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hishizawa M, Kanda J, Utsunomiya A, et al. Transplantation of allogeneic hematopoietic stem cells for adult T-cell leukemia: a nationwide retrospective study. Blood. 2010;116(8):1369–1376. [DOI] [PubMed] [Google Scholar]
- 55.Fukushima T, Miyazaki Y, Honda S, et al. Allogeneic hematopoietic stem cell transplantation provides sustained long-term survival for patients with adult T-cell leukemia/lymphoma. Leukemia. 2005;19(5):829–834. [DOI] [PubMed] [Google Scholar]
- 56.Kami M, Hamaki T, Miyakoshi S, et al. Allogeneic haematopoietic stem cell transplantation for the treatment of adult T-cell leukaemia/lymphoma. Br J Haematol. 2003;120(2):304–309. [DOI] [PubMed] [Google Scholar]
- 57.Utsunomiya A, Miyazaki Y, Takatsuka Y, et al. Improved outcome of adult T cell leukemia/lymphoma with allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplantation 2001;27(1):15–20. [DOI] [PubMed] [Google Scholar]
- 58.Epstein-Peterson ZD, Ganesan N, Barker JN, et al. Outcomes of adult T-Cell leukemia/lymphoma with allogeneic stem cell transplantation: single-institution experience. Leuk Lymphoma. 2021;62(9):2177–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ito A, Nakano N, Tanaka T, et al. Improved survival of patients with aggressive ATL by increased use of allo-HCT: a prospective observational study. Blood Adv. 2021;5(20):4156–4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Iqbal M, Reljic T, Klocksieben F, et al. Efficacy of Allogeneic Hematopoietic Cell Transplantation in Human T Cell Lymphotropic Virus Type 1-Associated Adult T Cell Leukemia/Lymphoma: Results of a Systematic Review/Meta-Analysis. Biol Blood Marrow Transplant. 2019;25(8):1695–1700. [DOI] [PubMed] [Google Scholar]; • Meta-Analysis of allogenetic transplant in ATL.
- 61.Matsushita K, Matsumoto T, Ohtsubo H, et al. Long-term maintenance combination chemotherapy with OPEC/MPEC (vincristine or methotrexate, prednisolone, etoposide and cyclophosphamide) or with daily oral etoposide and prednisolone can improve survival and quality of life in adult T-cell leukemia/lymphoma. Leuk Lymphoma. 1999;36(1–2):67–75. [DOI] [PubMed] [Google Scholar]
- 62.Takasaki Y, Iwanaga M, Imaizumi Y, et al. Long-term study of indolent adult T-cell leukemia-lymphoma. Blood. 2010;115(22):4337–4343. [DOI] [PubMed] [Google Scholar]
- 63.Gill PS, Harrington W, Kaplan MH, et al. Treatment of adult T-cell leukemia-lymphoma with a combination of interferon alfa and zidovudine. N Engl J Med. 1995;332(26):1744–1748. [DOI] [PubMed] [Google Scholar]
- 64.Hermine O, Bouscary D, Gessain A, et al. Brief report: treatment of adult T-cell leukemia-lymphoma with zidovudine and interferon alfa. N Engl J Med. 1995;332(26):1749–1751. [DOI] [PubMed] [Google Scholar]
- 65.White JD, Wharfe G, Stewart DM, et al. The Combination of Zidovudine and Interferon AIpha-2B in the Treatment of Adult T-Cell Leukemia/Lymphoma. Leuk Lymphoma. 2001;40(42067):287–294. [DOI] [PubMed] [Google Scholar]
- 66.Hermine O, Allard I, Lévy V, et al. A prospective phase II clinical trial with the use of zidovudine and interferon-alpha in the acute and lymphoma forms of adult T-cell leukemia/lymphoma. Hematol J. 2002;3(6):276–282. [DOI] [PubMed] [Google Scholar]
- 67.Kinpara S, Kijiyama M, Takamori A, et al. Interferon-α (IFN-α) suppresses HTLV-1 gene expression and cell cycling, while IFN-α combined with zidovudine induces p53 signaling and apoptosis in HTLV-1-infected cells. Retrovirology. 2013;10(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Datta A, Bellon M, Sinha-Datta U, et al. Persistent inhibition of telomerase reprograms adult T-cell leukemia to p53-dependent senescence. Blood. 2006;108(3):1021–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tsukasaki K, Imaizumi Y, Tokura Y, et al. Meeting report on the possible proposal of an extranodal primary cutaneous variant in the lymphoma type of adult T-cell leukemia-lymphoma. J Dermatol. 2014;41(1):26–28. [DOI] [PubMed] [Google Scholar]
- 70.Sawada Y, Hino R, Hama K, et al. Type of skin eruption is an independent prognostic indicator for adult T-cell leukemia/lymphoma. Blood. 2011;117(15):3961–3967. [DOI] [PubMed] [Google Scholar]
- 71.O’Connor OA, Pro B, Pinter-Brown L, et al. Pralatrexate in patients with relapsed or refractory peripheral T-cell lymphoma: results from the pivotal PROPEL study. J Clin Oncol. 2011;29(9):1182–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.O’Connor OA, Horwitz S, Masszi T, et al. Belinostat in Patients With Relapsed or Refractory Peripheral T-Cell Lymphoma: Results of the Pivotal Phase II BELIEF (CLN-19) Study. J Clin Oncol. 2015;33(23):2492–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Coiffier B, Pro B, Prince HM, et al. Results From a Pivotal, Open-Label, Phase II Study of Romidepsin in Relapsed or Refractory Peripheral T-Cell Lymphoma After Prior Systemic Therapy. J Clin Oncol. 2012;30(6):631–636. [DOI] [PubMed] [Google Scholar]
- 74.Yamamoto K, Utsunomiya A, Tobinai K, et al. Phase I study of KW-0761, a defucosylated humanized anti-CCR4 antibody, in relapsed patients with adult T-cell leukemia-lymphoma and peripheral T-cell lymphoma. J Clin Oncol. 2010;28(9):1591–1598. [DOI] [PubMed] [Google Scholar]
- 75.Ishida T, Joh T, Uike N, et al. Defucosylated anti-CCR4 monoclonal antibody (KW-0761) for relapsed adult T-cell leukemia-lymphoma: a multicenter phase II study. J Clin Oncol. 2012;30(8):837–842. [DOI] [PubMed] [Google Scholar]
- 76.Ishitsuka K, Yurimoto S, Tsuji Y, et al. Safety and effectiveness of mogamulizumab in relapsed or refractory adult T-cell leukemia-lymphoma. Eur J Haematol. 2019;102(5):407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ishida T, Utsunomiya A, Jo T, et al. Mogamulizumab for relapsed adult T-cell leukemia-lymphoma: Updated follow-up analysis of phase I and II studies. Cancer Sci. 2017;108(10):2022–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hirotsu KE, Neal TM, Khodadoust MS, et al. Clinical Characterization of Mogamulizumab-Associated Rash During Treatment of Mycosis Fungoides or Sézary Syndrome. JAMA Dermatol. 2021;157(6):700–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.de Masson A, Darbord D, Dobos G, et al. Macrophage-derived CXCL9 and CXCL11, T-cell skin homing, and disease control in mogamulizumab-treated CTCL patients. Blood. 2022;139(12):1820–1832. [DOI] [PubMed] [Google Scholar]
- 80.Phillips AA, Fields PA, Hermine O, et al. Mogamulizumab versus investigator’s choice of chemotherapy regimen in relapsed/refractory adult T-cell leukemia/lymphoma. Haematologica. 2019;104(5):993–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Randomized trial of mogamulizumab versus investigator’s choice in R/R ATL outside of Asia.
- 81.Sakamoto Y, Ishida T, Masaki A, et al. CCR4 mutations associated with superior outcome of adult T-cell leukemia/lymphoma under mogamulizumab treatment. Blood. 2018;132(7):758–761. [DOI] [PubMed] [Google Scholar]
- 82.Sakamoto Y, Ishida T, Masaki A, et al. CCR7 alterations associated with inferior outcome of adult T-cell leukemia/lymphoma under mogamulizumab treatment. Hematol Oncol. 2022;40(5):876–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tanaka N, Mori S, Kiyotani K, et al. Genomic determinants impacting the clinical outcome of mogamulizumab treatment for adult T-cell leukemia/lymphoma. Haematologica. 2022;107(10):2418–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Beygi S, Duran GE, Fernandez-Pol S, et al. Resistance to mogamulizumab is associated with loss of CCR4 in cutaneous T-cell lymphoma. Blood. 2022;139(26):3732–3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sakamoto H, Itonaga H, Sawayama Y, et al. Treatment with mogamulizumab or lenalidomide for relapsed adult T-cell leukemia/lymphoma after allogeneic hematopoietic stem cell transplantation: The Nagasaki transplant group experience. Hematol Oncol. 2020;38(2):162–170. [DOI] [PubMed] [Google Scholar]
- 86.Ishida T, Fujiwara H, Nosaka K, et al. Multicenter Phase II Study of Lenalidomide in Relapsed or Recurrent Adult T-Cell Leukemia/Lymphoma: ATLL-002. J Clin Oncol. 2016;34(34):4086–4093. [DOI] [PubMed] [Google Scholar]
- 87.Phillips AA, Giddings J, Lee SM, Horwitz SM. Lenalidomide in patients with Relapsed or Refractory HTLV-1 Related Adult T cell Leukemia/Lymphoma (ATLL). Int J Blood Res Disord. 2015;2:3. [Google Scholar]
- 88.Lunning MA, Gonsky J, Ruan J, et al. Pralatrexate in Relapsed/Refractory HTLV-1 Associated Adult T-Cell Lymphoma/Leukemia: A New York City Multi-Institutional Experience. Blood. 2012;120(21):2735. [Google Scholar]
- 89.Mukhi N, Verma V, Ahmed A, et al. Romidepsin in Relapsed/Refractory HTLV-1 Associated Adult T-Cell Lymphoma/Leukemia: A Case Series. Blood. 2015;126(23):5113. [Google Scholar]
- 90.Ishitsuka K, Utsunomiya A, Katsuya H, et al. A phase II study of bortezomib in patients with relapsed or refractory aggressive adult T-cell leukemia/lymphoma. Cancer Sci. 2015;106(9):1219–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ishitsuka K, Suzumiya J, Aoki M, et al. Therapeutic potential of arsenic trioxide with or without interferon-α for relapsed/refractory adult T-cell leukemia/lymphoma. Haematologica. 2007;92(5):719–720. [DOI] [PubMed] [Google Scholar]
- 92.Hermine O, Dombret H, Poupon J, et al. Phase II trial of arsenic trioxide and alpha interferon in patients with relapsed/refractory adult T-cell leukemia/lymphoma. Hematol J. 2004;5(2):130–134. [DOI] [PubMed] [Google Scholar]
- 93.Sharma K, Janik JE, O’Mahony D, et al. Phase II Study of Alemtuzumab (CAMPATH-1) in Patients with HTLV-1-Associated Adult T-cell Leukemia/lymphoma. Clin Cancer Res. 2017;23(1):35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Utsunomiya A, Izutsu K, Jo T, et al. Oral histone deacetylase inhibitor tucidinostat (HBI‐8000) in patients with relapsed or refractory adult T‐cell leukemia/lymphoma: Phase IIb results. Cancer Sci. 2022;113(8):2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Iyer SP, Sica RA, Ho PJ, et al. S262: The Cobalt-LYM Study of CTX130: A Phase I Dose Escalation Study of CD70-Targeted Allogeneic CRISPR-CAS9-Engineered CAR T Cells in Patients with Relapsed/Refractory (R/R) T-cell Malignancies. Hemasphere. 2022;6:163–164. [DOI] [PubMed] [Google Scholar]
- 96.Pongas G, Toomey NL, Reis IM, et al. Safety and Efficacy of Belinostat Trial with Zidovudine Plus Interferon for HTLV-1 Related Adult T-Cell Leukemia-Lymphoma: Interim Results. Blood. 2022;140(Supplement 1):9448–9449. [Google Scholar]
- 97.Daenthanasanmak A, Bamford RN, Yoshioka M, et al. Triple combination of BET plus PI3K and NF-κB inhibitors exhibit synergistic activity in adult T-cell leukemia/lymphoma. Blood Adv. 2022;6(7):2346–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ishio T, Kumar S, Shimono J, et al. Genome-wide CRISPR screen identifies CDK6 as a therapeutic target in adult T-cell leukemia/lymphoma. Blood. 2022;139(10):1541–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Daenthanasanmak A, Lin Y, Zhang M, et al. Enhanced efficacy of JAK1 inhibitor with mTORC1/C2 targeting in smoldering/chronic adult T cell leukemia. Transl Oncol. 2021;14(1):100913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shi M, Shahsafaei A, Liu C, Yu H, Dorfman DM. Enhancer of zeste homolog 2 is widely expressed in T-cell neoplasms, is associated with high proliferation rate and correlates with MYC and pSTAT3 expression in a subset of cases. Leuk Lymphoma. 2015;56(7):2087–91. [DOI] [PubMed] [Google Scholar]
- 101.Simon C, Chagraoui J, Krosl J, et al. A key role for EZH2 and associated genes in mouse and human adult T-cell acute leukemia. Genes Dev. 2012;26(7):651–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Izutsu K, Makita S, Nosaka K, et al. An Open-Label, Single-Arm, Phase 2 Trial of Valemetostat in Relapsed or Refractory Adult T-Cell Leukemia/Lymphoma. Blood. 2022;Sep 23(blood.2022016862.). [Google Scholar]; • Phase 2 trial of valemetostat in R/R ATL, leading to approval in Japan.
- 103.Ishitsuka K, Izutsu K, Maruyama D, et al. First in-human study of the EZH1 and EZH2 dual inhibitor valemetostat (DS-3201B) in patients with relapsed or refractory non-Hodgkin lymphoma. Hematol Oncol. 2021;39(S2). [Google Scholar]
- 104.Kataoka K, Nagata Y, Kitanaka A, et al. Integrated molecular analysis of adult T cell leukemia/lymphoma. Nat Genet. 2015;47(11):1304–1315. [DOI] [PubMed] [Google Scholar]
- 105.Kogure Y, Kameda T, Koya J, et al. Whole-genome landscape of adult T-cell leukemia/lymphoma. Blood. 2022;139(7):967–982. [DOI] [PMC free article] [PubMed] [Google Scholar]


