Abstract
Rabbit oral papillomavirus (ROPV) is a mucosatropic papillomavirus which naturally infects oral mucosal sites of domestic rabbits. In this study, we tested the hypothesis that rabbit genital mucosa is also susceptible to ROPV infection by using the athymic mouse xenograft system and adult immunocompetent rabbits. Subrenal xenografts of ROPV-infected rabbit vulvar and penile sheath tissues were strongly positive for ROPV infection by histologic, in situ hybridization, and Southern analyses. Direct inoculation of adult rabbit penises with infectious ROPV produced small raised lesions of approximately 1 by 1 by 1 mm that were ROPV positive by both in situ hybridization and Southern analyses and were also viral capsid antigen positive by immunohistological staining. Infection of rabbit genital tissues with ROPV may be a useful animal model for the study of genital tissue-targeting papillomaviruses.
Rabbit oral papillomavirus (ROPV) is a mucosatropic papillomavirus (PV) that causes tongue and oral cavity papillomas in domestic rabbits (8, 17, 20, 21, 25, 26). ROPV does not infect cutaneous sites, is antigenically unrelated to cottontail rabbit PV (CRPV), and induces papillomas which spontaneously regress (7, 17, 21). One additional contrast between ROPV- and CRPV-induced lesions of domestic rabbits is that papillomas induced by ROPV are virion rich (17) whereas those induced by CRPV are virion poor (23).
Currently, there are no laboratory animal model systems available for study of experimental infection and transmission of genital tissue-targeting PVs in intact hosts. Recent reports have described the detection and characterization of genital PVs in several primate species (3, 11, 15, 16, 19). However, isolation of infectious virions and experimental infection and transmission were not reported. It would thus be advantageous to develop a genital tissue-targeting mucosal PV animal model system in a small laboratory animal. A potential candidate for a genital tissue-targeting PV is ROPV, which is a spontaneous infection of domestic rabbits (17, 25, 26). ROPV, when combined with CRPV, may offer a unique animal model system for study of aspects of PV tissue specificity and host immunity and provide a close approximation to the human disease in which patients are susceptible to infection by mucosal and cutaneous tissue-targeting human PV (HPV) types.
Mucosatropic animal PV systems that are currently available for study include bovine PV type 4 infection of cattle and canine oral PV infection of dogs. Both of these mucosal tissue-targeting PVs are confined to oral mucosal tissues, the alimentary tract, and external lips of the mouth (2, 6, 18). The two models have been used to study some key events in PV infection of mucosal surfaces and host immune responses to PV infection (1, 4, 10, 24). A third PV mucosatropic model, involving an oral PV of hamsters, has been described recently (9). However, successful infection of genital mucosal tissues in these models has not been reported.
Our laboratory has recently produced high-titer stocks of infectious ROPV with the athymic mouse xenograft system (5). The seed stock of infectious ROPV was obtained from papillomas found on the undersurfaces of the tongues of several domestic New Zealand White rabbits. PCR amplification techniques indirectly detected ROPV DNA with primers designed from previously published partial ROPV sequence data (14). We have sequenced the entire genome of a cloned isolate of this virus, and the data confirm that the virus is ROPV (data not shown).
The objective of this study was to determine whether rabbit genital tissues were susceptible to ROPV infection. Infectivity studies were conducted in both immunodeficient (xenografted athymic mouse) and immunocompetent (adult male rabbit) environments. Previous attempts to directly infect rabbit genital tissues with ROPV were unsuccessful, leading to the conclusion that the virus infects only oral mucosal tissue (17, 25). However, some preliminary studies in our laboratory have suggested that ROPV may infect rabbit genital tissues when grafted under the renal capsules of athymic nude mice (5).
ROPV infection in xenografts of adult and newborn rabbit tissues.
Adult and newborn rabbits were initially used to analyze the tissue susceptibility of ROPV infection in an immunodeficient environment. Fragments of mucosal epithelial tissues from the tongue, penis, cervix, vagina, and larynx were incubated for 1 h in a 1:5 dilution of ROPV suspension at 37°C and surgically implanted beneath the renal capsules of athymic mice, as previously described (5, 13). For each source of mucosal tissue, between 8 and 16 xenografts (2 per mouse) were attempted. Grafts were harvested 60 days later for histological analysis, in situ hybridization, and Southern analysis. For Southern analysis, genomic DNA was digested with the SpeI restriction enzyme, which linearizes ROPV DNA, and electrophoresed on 2% agarose gels. DNA was detected with a 32P-labeled full-length ROPV genomic probe cloned into a modified pUC19 plasmid at the SpeI restriction site, according to the manufacturer’s instructions (New England Biolabs, Beverly, Mass.). In situ hybridization assays used a subgenomic fragment of ROPV (a 3.8-kb EcoRV fragment) that was cloned into plasmid pcDNA3 (Invitrogen, Carlsbad, Calif.). The 3.8-kb viral insert was purified from the plasmid, biotinylated (Amersham, Arlington Heights, Ill.), and used as an in situ probe for the detection of ROPV DNA. Control probes were HPV-11 and CRPV DNA, and control tissues were HPV-11-infected human tissues and CRPV-infected rabbit tissues. Xenografts from some of the mucosal sites were pooled for Southern analysis. The results of these experiments are summarized in Table 1.
TABLE 1.
Tissue susceptibilities of adult and newborn rabbit tissues to ROPV infection with the xenograft systema
| Donor rabbit | Tissue source | No. of viable xenograftsb | In situ hybridizationc | Southern analysis (pooled tissue)d |
|---|---|---|---|---|
| Adult | Tongue | 5 | 5/5 | 1/1 |
| Penis | 11 | 0/11 | 2/3 | |
| Cervix | 7 | 0/7 | 2/2 | |
| Vagina | 10 | 0/10 | 3/4 | |
| Larynx | 3 | 0/3 | 1/1 | |
| Penise | 4 | 1/4 | NTf | |
| Vaginae | 10 | 1/10 | NT | |
| Newborn | Tongue | 4g | 4/4 | 1/1g |
| Vulva | 6 | 5/6 | 6/6 | |
| Vagina | 4 | 0/4 | 3/4 | |
| Penile sheath | 4 | 3/4 | 4/4 |
Combined results of three experiments.
Number of viable xenografts at 60 days (subrenal grafts) or 30 days (subcutaneous grafts) after implanting of ROPV-infected mucosal tissue fragments. Some experiments represent pooled xenografts from the same tissue source.
Number of ROPV in situ hybridization-positive xenografts/number of xenografts examined.
Number of Southern analysis-positive pooled samples from xenografts/number of pooled samples examined.
Subcutaneous xenografts harvested after 30 days.
NT, not tested.
Pooled.
Histologically, xenografts derived from tongue tissue contained areas of acanthosis and vacuolation of squamous epithelial cells, with in situ hybridization-positive nuclei (5). In general, subrenal xenografts derived from adult rabbit tissues were considerably smaller than xenografts derived from newborn rabbit tissues. These observations are in agreement with our earlier studies, in which newborn tissues produced substantially larger xenografts than adult tissues when human tissues were infected with HPV-11 (12) or when rabbit tissues were infected with CRPV (unpublished observations). Subrenal xenografts derived from other adult mucosal tissues contained only a few areas of mild acanthosis and vacuolation of squamous epithelial cells and no in situ hybridization-positive nuclei (data not shown). Strong in situ hybridization signals, however, were obtained for 1 of 10 adult vaginal and 1 of 4 adult penile subcutaneous xenografts (Table 1), showing that adult genital tissues were susceptible to ROPV infection. Southern analysis of pooled xenografts from the adult mucosal tissues demonstrated strong signals for ROPV DNA from tongue and penile tissues and weak signals from the other mucosal tissues (Fig. 1; Table 1).
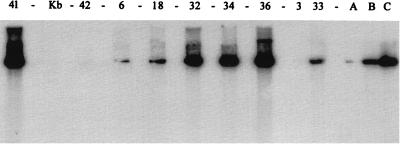
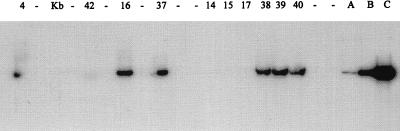
FIG. 1.
Southern blot analysis of ROPV DNA in adult penile or kit penile sheath xenografts in athymic mice. DNA was extracted from the xenografts, digested with SpeI, and loaded into wells of an agarose gel. After transfer of DNA to nylon membranes, the membranes were probed with a 32P-labeled ROPV DNA probe. Densitometric scanning of lanes was used to calculate the quantity of ROPV DNA with three standards (lanes A [100 pg], B [1,000 pg], and C [10,000 pg], insert ROPV DNA). Concentrations of ROPV DNA (picograms of ROPV DNA per microgram of total genomic DNA) for each sample were as follows: lane 41 (kit tongue xenograft), 12,500 pg; lane 42 (adult penis, uninfected), <10 pg; lanes 6, 18, and 3 (adult rabbit penile xenografts), 150, 600, and <10 pg, respectively; lanes 32, 34, 36, and 33 (kit rabbit penile xenografts), 12,500, 10,500, 12,500, and 40 pg, respectively. Lane Kb, markers.
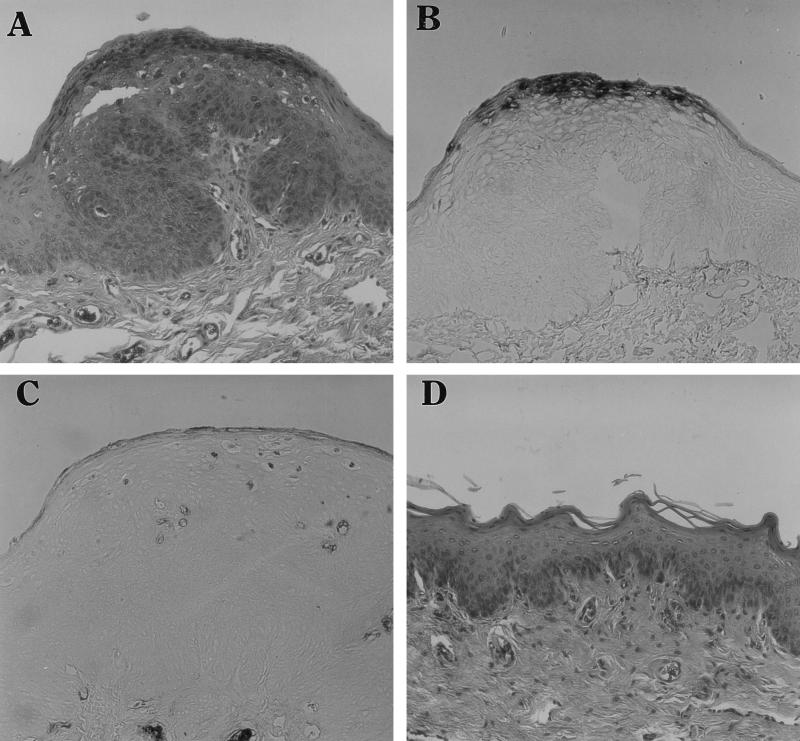
Newborn rabbit tissues were also tested for tissue susceptibility to ROPV infection. Four to eight xenografts (two per mouse) for each mucosal tissue type were attempted (Table 1). Xenografts from tongue (5), vulvar (Fig. 2A), and penile sheath (Fig. 2C) tissues contained marked hyperplasia and acanthosis of the squamous epithelial cells. Some vacuolated epithelial cells with clear cytoplasms, and epithelial cells with intranuclear inclusions, were present in the stratum spinosum. Numerous darkly stained in situ hybridization-positive nuclei were present in xenografts of the ROPV-infected newborn vulva (Fig. 2B) and penile sheath (Fig. 2D). Xenografts from vaginal tissues contained little or no histological changes (Fig. 2E) and no in situ hybridization-positive nuclei (data not shown). Southern analysis of individual newborn tissue xenografts yielded strong positive signals for tissues from the tongue, external penile sheath (Fig. 1), and vulva (Fig. 3) but only weak positive signals from vaginal tissues (data not shown).
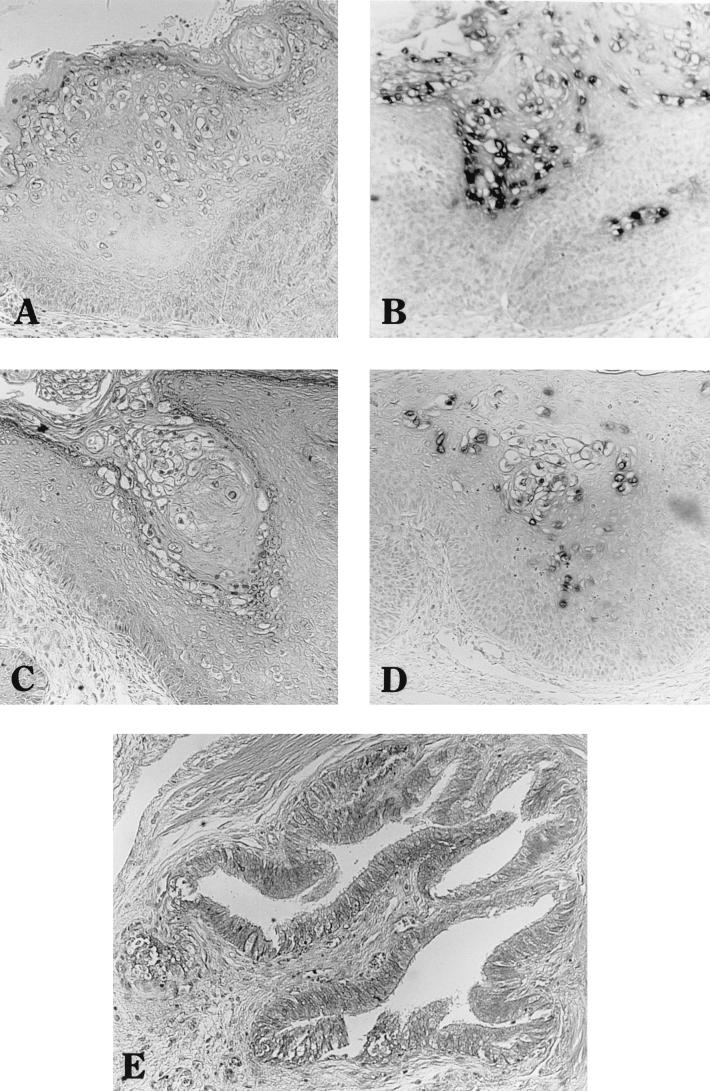
FIG. 2.
Histological and in situ hybridization analyses of ROPV-infected rabbit kit xenografts of vulva (A and B), penile sheath (C and D), and vagina (E). Hematoxylin and eosin staining of tissue sections is shown in panels A, C, and E; in situ hybridization with a subgenomic ROPV DNA probe of serial tissue sections is shown in panels B and D. Magnification, ×100.
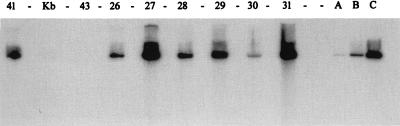
FIG. 3.
Southern blot analysis of ROPV DNA in kit vulvar xenografts in athymic mice. DNA was prepared as described in the legend to Fig. 1. After transfer of DNA to nylon membranes, the membranes were probed with a 32P-labeled ROPV DNA probe. Concentrations of ROPV DNA (picograms of ROPV DNA per microgram of total genomic DNA) were calculated as described in the legend to Fig. 1 and were as follows: lane 41 (kit tongue xenograft), 12,500 pg; lane 43 (adult vulva, uninfected), <10 pg; lanes 26 to 31 (kit vulva xenografts), 1,250, 34,000, 40, 670, 40, and 18,000 pg, respectively. Lanes A (100 pg), B (1,000 pg), and C (10,000 pg), insert ROPV DNA; lane Kb, markers.
Detection of genital ROPV infection in adult (immunocompetent) rabbits.
New Zealand White adult male rabbits were used to determine whether genital tissues in intact hosts supported ROPV infection. Seventeen rabbits (in a total of four experiments) were anesthetized, and the dorsal mucosal penile surfaces were superficially wounded by 10 to 20 needle punctures/penis with a 25-gauge needle held at a shallow angle to the mucosal surfaces. An undiluted virus suspension was placed onto the wounded areas, and the animals were allowed to recover from anesthesia. Animals were euthanized at 6 to 8 weeks postinfection, and penile tissues were harvested for histology, in situ hybridization, and Southern analysis. In one set of experiments, six male rabbits were infected with ROPV, of which three rabbits were treated with a regime of cyclosporine A (CsA) (22) to induce specific immune suppression. The dosing regime of CsA consisted of twice weekly subcutaneous injections of CsA (Sandoz Pharma Ltd., Basel, Switzerland) to achieve a dose of 20 mg/kg of body weight for 50 days, beginning on the day of ROPV infection.
After direct penile inoculation with ROPV, rabbits were examined at 28, 35, and 42 days postinfection for evidence of papilloma development. Small raised lesions measuring 1 by 1 by 1 mm were noted grossly by 28 to 35 days postinfection on 10 of 17 rabbits (see Fig. 5). These lesions quickly regressed by 50 to 60 days. Nine of the 17 infected rabbits were euthanized at 42 days (one at 56 days) postinfection, and penile tissues were harvested for histological analysis, in situ hybridization, and Southern analysis. The remaining eight rabbits were inspected weekly until the lesions had completely regressed, at 60 days postinfection. Of the nine infected rabbits from which tissues were harvested, several showed some histological evidence of viral infection (Fig. 4). These characteristics included acanthosis and hyperplasia of the squamous epithelium, strong in situ hybridization signals for ROPV DNA, and PV group-specific-antigen-positive nuclei, as revealed by immunohistological staining (Fig. 4). Penile DNA extracted from five of eight infected rabbits (including two rabbits with raised lesions) were strongly positive for ROPV DNA by Southern analysis (see Fig. 6).
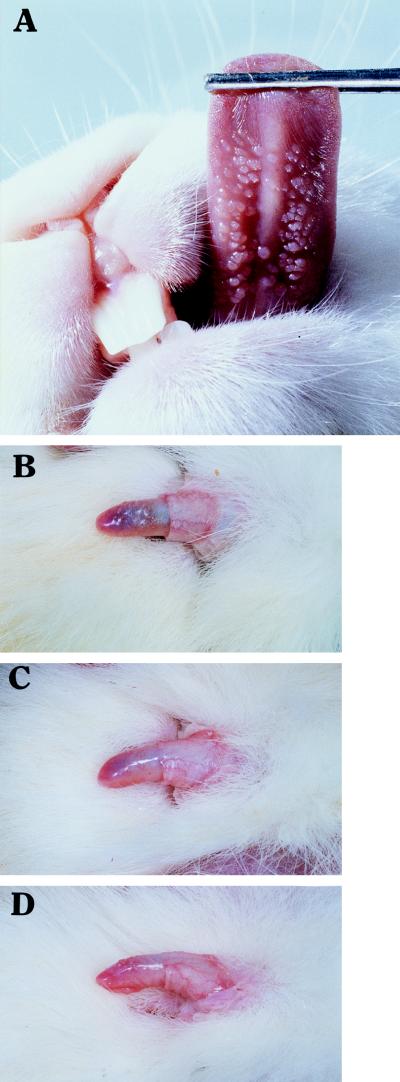
FIG. 5.
ROPV-challenged ventral lingual (magnification, ×1.66) (A) and dorsal penile (magnification, ×0.83) (B, C, and D) surfaces. Small discrete papillomas are easily observed on the underside of the tongue. Several small raised papillomas are seen on the dorsal surface of the penis. Rabbit G0850 (no CsA treatment) at day 30 (B) and day 56 (C) and rabbit G0856 (CsA treated) at day 56 (D) are shown. Note the regression of penile papillomas by day 56 for rabbit G0850. Confirmation that these lesions contained ROPV was conducted by in situ hybridization with ROPV DNA probes (Fig. 4).
FIG. 4.
Histologic, in situ hybridization, and capsid antigen immunostaining analyses at 56 days postinfection of adult rabbit penile epithelium of a CsA-treated rabbit infected with ROPV. (A, B, and C) Five-micrometer sections through penile papillomas. (D) Uninfected penile skin. Panels A and D show hematoxylin and eosin staining, panel B shows in situ hybridization with an ROPV DNA probe, and panel C shows capsid antigen immunohistological staining with the PV group-specific antigen antibody. Magnification, ×90.
FIG. 6.
Southern blot analysis of ROPV DNA in penile tissues of adult rabbits that were directly challenged with ROPV. Concentrations of ROPV DNA (picograms of ROPV DNA per microgram of total genomic DNA) were calculated as described in the legend to Fig. 1 and were as follows: lane 4 (adult tongue xenograft), 190 pg; lane 42 (adult penis, uninfected), <10 pg; lanes 16 to 40 (adult penile DNA from infected rabbits), 300, 250, <10, <10, <10, 20, 35, and 20 pg, respectively. Lanes A (100 pg), B (1,000 pg), and C (10,000 pg), insert ROPV DNA; lane Kb, markers.
Histological evidence of lymphocytic infiltration was also visible at 42 days postinfection in most of the penile sites (data not shown), suggesting an immune response possibly leading to regression. Consistent with these findings was the observation that these raised penile lesions regressed by 60 days (Fig. 5). While ROPV DNA was not detected in sites with lymphocytic infiltrates by in situ hybridization, Southern analysis revealed strong positive ROPV signals from penile DNA in five of eight inoculated rabbits. We also noted that ROPV lesions of the tongue that contained lymphocytic infiltrate were in situ hybridization negative, as observed for penile lesions with lymphocytic infiltrate (data not shown). These data suggest that the lymphocytes infiltrating regressing lesions may have an early effect upon the papillomas by reducing the levels of ROPV DNA detected by in situ hybridization. Also consistent with these findings was the observation of a strong in situ ROPV DNA signal and an absence of lymphocytic infiltrates in penile lesions of a rabbit treated with CsA (Fig. 5). We observed that ROPV-induced lesions of the tongues and penises of rabbits treated with CsA persisted longer than those of nonimmunosuppressed rabbits (Fig. 5).
Infectious ROPV is produced in ROPV-infected xenografted kit genital tissues.
Juvenile and young adult rabbits (three females and three males) were used to determine whether infectious ROPV virions had been successfully passaged through xenografted kit genital tissues. Small raised lesions measuring 2 by 2 by 1 mm were noted grossly on the ventral lingual surfaces of all six rabbits by 28 days postinfection (Fig. 5). Additionally, small raised lesions measuring 2 by 2 by 1 mm were noted on the dorsal penile surface of one male, and lesions measuring 1 by 1 by 1 mm were noted on a second rabbit (data not shown). A third rabbit showed no penile lesions. These experiments demonstrated that kit penile sheath tissues and kit vulvar tissues can support the full replication cycle of ROPV when the infected tissues are xenografted into athymic mice. This system may provide a method for developing a genital tissue-targeting variant of ROPV or a stock of ROPV with an increased titer of infectivity for genital tissues (our current stock of ROPV was prepared from tongue tissue xenografts).
Previous attempts to infect genital tissues with ROPV had been unsuccessful, leading investigators to conclude that the virus infects only oral tissues (17, 25). However, it is possible that the concentration of ROPV used for infection was insufficient for successful infection of genital mucosa. In these earlier studies, the challenged genital sites were grossly inspected only for signs of PV infection. In our studies, we used the athymic mouse xenograft system, which allowed us to infect fragments of oral and genital tissues with ROPV in an immunodeficient environment and thus produce high titers of infectious virions. We observed that genital mucosal tissues of intact hosts appeared to be less susceptible (by 1 to 2 orders of magnitude) to ROPV infection than the undersurfaces of the tongues (unpublished observations). In addition, the ROPV-induced oral and genital lesions in adult rabbits appeared to be very immunogenic and often quickly regressed. Earlier reports had suggested that the ventral lingual site was more susceptible to ROPV infection than other oral sites (17). Thus, the use of the athymic mouse xenograft system provided us with a method to produce high concentrations of infectious virus, which in turn increased the likelihood of successful infection of the less susceptible genital mucosal sites.
In summary, we have shown that (i) ROPV can infect rabbit genital mucosal tissues when transplanted subrenally into athymic mice, (ii) ROPV can directly infect genital tissues in adult immunocompetent rabbits, and (iii) infected genital tissues produce productive infections. These studies represent the first documented case of ROPV infection in a mucosal tissue other than in the oral cavity. ROPV infection of genital tissues may provide a new animal model for the study of a genital mucosal tissue-targeting PV. Such a laboratory animal model system will be particularly useful for study of immunity to PVs following direct infection of genital tissues of intact hosts, as well as of sexual transmission of PV infections from male to female and vice versa.
Acknowledgments
This work was supported by Public Health Service grants AI35138, AI37829, and RR07006 and the Jake Gittlen Memorial Golf Tournament.
We thank Martin Pickel for expert technical assistance.
REFERENCES
- 1.Bregman C L, Hirth R S, Sundberg J P, Christensen E F. Cutaneous neoplasms in dogs associated with canine oral papillomavirus vaccine. Vet Pathol. 1987;24:477–487. doi: 10.1177/030098588702400602. [DOI] [PubMed] [Google Scholar]
- 2.Campo M S, Moar M H, Jarrett W F, Laird H M. Presence and expression of bovine papillomavirus type 4 in tumours of the alimentary canal of cattle and its possible role: a new papillomavirus associated with alimentary cancer in cattle. Nature. 1980;286:180–182. doi: 10.1038/286180a0. [DOI] [PubMed] [Google Scholar]
- 3.Chan S Y, Ostrow R S, Faras A J, Bernard H U. Genital papillomaviruses (PVs) and epidermodysplasia verruciformis PVs occur in the same monkey species: implications for PV evolution. Virology. 1997;228:213–217. doi: 10.1006/viro.1996.8400. [DOI] [PubMed] [Google Scholar]
- 4.Chandrachud L M, Grindlay G J, McGarvie G M, O’Neil B W, Wagner E R, Jarrett W F H, Campo M S. Vaccination of cattle with the N-terminus of L2 is necessary and sufficient for preventing infection by bovine papillomavirus-4. Virology. 1995;211:204–208. doi: 10.1006/viro.1995.1392. [DOI] [PubMed] [Google Scholar]
- 5.Christensen N D, Cladel N M, Reed C A, Budgeon L R, Welsh P A, Patrick S D, Kreider J W. Laboratory production of infectious stocks of rabbit oral papillomavirus. J Gen Virol. 1996;77:1793–1798. doi: 10.1099/0022-1317-77-8-1793. [DOI] [PubMed] [Google Scholar]
- 6.Delius H, Van Ranst M A, Jenson A B, zur Hausen H, Sundberg J P. Canine oral papillomavirus genomic sequence: a unique 1.5-kb intervening sequence between the E2 and L2 open reading frames. Virology. 1994;204:447–452. doi: 10.1006/viro.1994.1552. [DOI] [PubMed] [Google Scholar]
- 7.DiGiacomo R F, Mare C J. Rabbit oral papillomavirus. In: Manning P J, Ringler D H, Newcomer C E, editors. The biology of the laboratory rabbit. 2nd ed. San Diego, Calif: Academic Press, Inc.; 1994. pp. 186–187. [Google Scholar]
- 8.Dominguez J A, Corella E L, Auro A. Oral papillomatosis in two laboratory rabbits in Mexico. Lab Anim Sci. 1981;31:71–73. [PubMed] [Google Scholar]
- 9.Iwasaki T, Maeda H, Kameyama Y, Moriyama M, Kanai S, Kurata T. Presence of a novel hamster oral papillomavirus in dysplastic lesions of hamster lingual mucosa induced by application of dimethylbenzanthracene and excisional wounding: molecular cloning and complete nucleotide sequence. J Gen Virol. 1997;78:1087–1093. doi: 10.1099/0022-1317-78-5-1087. [DOI] [PubMed] [Google Scholar]
- 10.Jarrett W F H, O’Neil B W, Gaukroger J M, Laird H M, Smith K T, Campo M S. Studies on vaccination against papillomaviruses: a comparison of purified virus, tumour extract and transformed cells in prophylactic vaccination. Vet Rec. 1990;126:449–452. [PubMed] [Google Scholar]
- 11.Kloster B E, Manias D A, Ostrow R S, Shaver M K, McPherson S W, Rangen S R S, Uno H, Faras A J. Molecular cloning and characterization of the DNA of two papillomaviruses from monkeys. Virology. 1988;166:30–40. doi: 10.1016/0042-6822(88)90143-2. [DOI] [PubMed] [Google Scholar]
- 12.Kreider J W, Howett M K, Stoler M H, Leure-Dupree A E, Lill N L, Zaino R J. Tissue-specific expression of human papillomavirus type 11. In: Steinberg B M, Brandsma J L, Taichman L B, editors. Cancer cells 5. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1987. pp. 215–222. [Google Scholar]
- 13.Kreider J W, Howett M K, Wolfe S A, Bartlett G L, Zaino R J, Sedlacek T V, Mortel R. Morphological transformation in vivo of human uterine cervix with papillomavirus from condylomata acuminata. Nature. 1985;317:639–641. doi: 10.1038/317639a0. [DOI] [PubMed] [Google Scholar]
- 14.O’Banion K M, Cialkowski M E, Reichmann M E, Sundberg J P. Cloning and molecular characterization of an oral papillomavirus of domestic rabbits. Virology. 1988;162:221–231. doi: 10.1016/0042-6822(88)90411-4. [DOI] [PubMed] [Google Scholar]
- 15.O’Banion M K, Sundberg J P, Shima A L, Reichmann M E. Venereal papilloma and papillomavirus in a colobus monkey (Colobus guereza) Intervirology. 1987;28:232–237. doi: 10.1159/000150020. [DOI] [PubMed] [Google Scholar]
- 16.Ostrow R S, McGlennen R C, Shaver M K, Kloster B E, Houser D, Faras A J. A rhesus monkey model for sexual transmission of papillomavirus isolated from a squamous cell carcinoma. Proc Natl Acad Sci USA. 1990;87:8170–8179. doi: 10.1073/pnas.87.20.8170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parsons R J, Kidd J G. Oral papillomatosis of rabbits: a virus disease. J Exp Med. 1942;77:233–250. doi: 10.1084/jem.77.3.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel K R, Smith K T, Campo M S. The nucleotide sequence and genome organization of bovine papillomavirus type-4. J Gen Virol. 1987;68:2117–2128. doi: 10.1099/0022-1317-68-8-2117. [DOI] [PubMed] [Google Scholar]
- 19.Rangan S R, Gutter A, Baskin G B, Anderson D. Virus associated papillomas in colobus monkeys (Colobus guereza) Lab Anim Sci. 1980;30:885–889. [PubMed] [Google Scholar]
- 20.Rdzok E J, Shipkowitz N L, Richter W R. Rabbit oral papillomatosis: ultrastructure of experimental infection. Cancer Res. 1966;26:160–165. [PubMed] [Google Scholar]
- 21.Richter W R, Shipkowitz N L, Rdzok E J. Oral papillomatosis of the rabbit: an electron microscope study. Lab Investig. 1964;13:430–438. [PubMed] [Google Scholar]
- 22.Shah A K, Sawchuk R J, Gratwohl A, Baldomero H, Speck B. Subcutaneous absorption of cyclosporine in rabbits. Transplant Proc. 1988;20:710–714. [PubMed] [Google Scholar]
- 23.Shope R E, Hurst E W. Infectious papillomatosis of rabbits, with a note on the histopathology. J Exp Med. 1933;58:607–624. doi: 10.1084/jem.58.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith K T, Campo M S. Amplification of specific DNA sequences in C127 mouse cells transformed by bovine papillomavirus type 4. Oncogene. 1989;4:409–413. [PubMed] [Google Scholar]
- 25.Sundberg J P, Junge R E, El Shazly M O. Oral papillomatosis in New Zealand White rabbits. Am J Vet Res. 1985;46:664–668. [PubMed] [Google Scholar]
- 26.Weisbroth S H, Scher S. Spontaneous oral papillomatosis in rabbits. J Am Vet Med Assoc. 1970;157:1940–1944. [PubMed] [Google Scholar]