Abstract
Simple Summary
The initial treatment for patients with advanced-stage follicular lymphoma is usually a combo of immunochemotherapy called R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) or R-bendamustine (R-B, for short). After six cycles of R-CHOP, continuing with rituximab for two years (maintenance therapy) has demonstrated a reduction in relapses. However, determining if this approach works well after R-B has yet to be confirmed. Here, we collected data from 476 FL patients from 17 GELTAMO centers and evaluated the efficacy of both regimens followed by rituximab maintenance therapy in untreated follicular lymphoma patients. We found a better response with R-B and relapses were more frequent with R-CHOP. During the initial treatment, low blood counts were more frequent with R-CHOP but, during maintenance therapy, they were more frequent with R-B and so were infectious complications. After six years, 79% and 67% of R-B- and R-CHOP-treated patients, respectively, did not have evidence of the disease but the number of deaths was the same in both groups. In conclusion, R-B followed by rituximab maintenance therapy in patients with previously untreated follicular lymphoma showed better responses and fewer relapses, without any extra side effects in an elderly population. During maintenance, patients had more issues when using R-B but deaths were the same in both groups.
Abstract
Rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) and R-bendamustine (R-B) are the most common frontline treatment strategies for advanced-stage follicular lymphoma (FL). After R-CHOP induction therapy, using rituximab for maintenance therapy notably improves outcomes; however, whether this can be achieved by using the same approach after R-B therapy is still being determined. This retrospective analysis compared 476 FL patients from 17 GELTAMO centers who received R-based regimens followed by rituximab maintenance therapy for untreated advanced-stage FL. The complete response rate at the end of induction was higher with R-B and relapses were more frequent with R-CHOP. During induction, cytopenias were significantly more frequent with R-CHOP and so was the use of colony-stimulating factors. During maintenance therapy, R-B showed more neutropenia and infectious toxicity. After a median follow-up of 81 months (95% CI: 77–86), the 6-year rates of progression-free survival (PFS) were 79% (95% CI: 72–86) for R-bendamustine vs. 67% (95% CI: 61–73) for R-CHOP (p = 0.046), and 6-year overall survival (OS) values were 91% (95% CI: 86–96) for R-B vs. 91% (95% CI: 87–94) for R-CHOP (p = 0.49). In conclusion, R-B followed by rituximab maintenance therapy in patients with previously untreated FL resulted in significantly longer PFS than R-CHOP, with older patients also benefiting from this treatment without further toxicity. Adverse events during maintenance were more frequent with R-B without impacting mortality.
Keywords: follicular lymphoma, rituximab, R-bendamustine, R-CHOP, maintenance
1. Introduction
Follicular lymphoma (FL) is the most common type of indolent lymphoma [1,2,3], representing nearly 30% of all non-Hodgkin lymphomas (NHLs). It is characterized by an indolent course with an estimated overall survival (OS) of over 10 years [4]. However, FL remains an incurable hematological malignancy with a characteristic course of multiple relapses and with heterogeneous clinical behavior, since about 20% of patients experience rapid progression after initial treatment [5] or histological transformation to aggressive lymphoma [6] (2% of patients per year), which confers a poor prognosis.
The stage of the disease, tumor burden, and symptoms strongly determine the therapy decision, since the commonly used prognostic indices, such as the FLIPI [7,8], do not help in this choice.
Chemoimmunotherapy is the most common treatment strategy for advanced disease and high tumor burden if GELF criteria are met [9,10]. The most commonly used combinations are rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) and R-bendamustine (RB); or R-cyclophosphamide, vincristine, and prednisone (CVP) for elderly or comorbid patients [11,12,13]. R-CHOP and RB have been compared in two non-inferiority phase III clinical trials [12,14]. Patients treated with RB presented longer progression-free survival (PFS), but overall survival (OS) was similar to that of patients treated with R-CHOP. Other anti-CD20 monoclonal antibodies, like obinutuzumab, have been evaluated successfully in combinations with bendamustine, CHOP, or CVP and are an option, although they are not available in Spain [15].
After R-CHOP induction therapy, using rituximab as maintenance therapy notably improved outcomes in patients with FL, showing a 20% difference in 10-year PFS compared with patients who had not received it, without significant differences in OS [15,16,17]. Although R-B has demonstrated excellent efficacy in this setting, the role of rituximab as maintenance therapy after this combination must be clarified, since some studies have indicated that maintenance therapy after bendamustine could increase the risk of toxicity, especially infectious toxicity [18]. Therefore, we aimed to compare both R-based regimens followed by rituximab maintenance therapy for untreated advanced-stage follicular FL and assess the outcomes in terms of efficacy and toxicity.
2. Material and Methods
2.1. Patients
This is a retrospective, multicenter, observational study conducted following the Declaration of Helsinki and approved by the institutional ethics committee of Hospital Universitario Son Espases. We retrospectively assessed all patients with 1–3a high tumor burden FL from 17 GELTAMO centers, treated with either R-Bendamustine or R-CHOP as first-line therapy for whom two years of rituximab maintenance treatment was planned, between January 2013 and January 2022. The decision on the chosen treatment was made following the local protocols of each center. All included patients received full doses of treatment. To mitigate selection bias, patients who did not undergo the intended therapy (R-chemo followed by R maintenance) were excluded from the analysis if the deviation was due to arbitrary decisions unrelated to toxicity, disease progression, or death. This approach mirrors the criteria used in clinical trials, where patients who do not adhere to the trial protocol are not included in the efficacy analysis. The outcome and toxicity were evaluated. In no case were patients excluded based on their response to or toxicity developed from frontline therapy, ensuring an unbiased comparison. Refractoriness was defined as no response or the increase in the lesion sizes or new lesions appearing at the end of induction or the 6 months after. Early progression (POD24) was defined as an increase in the lesion sizes or appearance of new lesions in the 24 months since treatment began, and relapse was defined as an increase in lesion size or appearance of new lesions at any time, different from the previous definitions. Only high tumor burden patients that met criteria for treatment [10] were included in the efficacy analysis. Response assessment was conducted with PET-CT at the end of induction, and follow-up was performed according to local guidelines. Transformations were confirmed histologically. No centralized assessment was performed.
2.2. Statistical Analysis
Variables following binomial distributions (i.e., response rate) were expressed as frequencies and percentages. Comparisons between qualitative variables were performed using the Fisher Exact Test or Chi-square. Comparisons between quantitative and qualitative variables were performed through nonparametric tests (U of Mann–Whitney or Kruskal–Wallis).
Time to event variables (OS and PFS) were measured from the date of frontline therapy onset and were estimated according to the Kaplan–Meier method. Comparisons between the variables of interest were performed by the log-rank test. Multivariate analysis with the variables that appeared to be significant in the univariate analysis as well as potential confounders was carried out according to the Cox proportional hazard regression model, using forward stepwise regression procedures. All p-values reported were 2-sided and statistical significance was defined at p < 0·05.
3. Results
From 476 FL patients who initially fulfilled the criteria to be included in this study, 71 were excluded because they had not received rituximab maintenance despite not having had toxicity/death or progression. Then, 405 patients were analyzed, 245 treated with R-CHOP and 160 with R-bendamustine. Table 1 shows global patient characteristics and split by treatment type.
Table 1.
Global patient characteristics and by treatment.
| Global Group (N = 405) |
R-CHOP (N = 245) |
R-BENDA (N = 160) |
p | |
|---|---|---|---|---|
| Median months from biopsy to initial treatment median (range) | 0.90 (0–144) | 0.73 (0–144) | 1.30 (0–66) | <0.001 |
| Median age at first line (range) | 59 (21–100) | 57 (21–83) | 62 (30–100) | 0.003 |
| Sex Male Female Missing |
201 (50%) 202 (50%) 2 |
125 (51%) 119 (49%) |
76 (48%) 83 (52%) |
0.54 |
| Age (years) ≤60 >60 Missing |
208 (52%) 188 (47%) 9 |
138 (56%) 107 (44%) |
70 (46%) 81 (54%) |
0.062 |
| Ann Arbor stage I-II bulky III-IV Missing |
31 (8%) 361 (92%) 13 |
20 (8%) 219 (92%) |
11 (7%) 142 (93%) |
0.71 |
| B symptoms present No Yes Missing |
242 (64%) 136 (35%) 27 |
154 (65%) 81 (34%) |
88 (61%) 55 (38%) |
0.44 |
| ECOG performance status 0–1 2–4 Missing |
307 (93%) 23 (7%) 75 |
186 (92%) 16 (8%) |
121 (94%) 7 (5%) |
0.51 |
| Bone marrow involvement No Yes Missing |
198 (50%) 195 (50%) 12 |
128 (53%) 115 (47%) |
70 (47%) 80 (53%) |
0.25 |
| FLIPI score 0–1 2 3–5 Missing |
66 (17) 145 (38) 171 (45) 23 |
39 (17%) 78 (33%) 118 (50%) |
27 (18%) 67 (46%) 53 (36%) |
0.020 |
| Histological grade 1 2 3a Missing |
145 (39) 151 (41) 75 (20) 34 |
82 (36%) 85 (37%) 60 (26%) |
63 (44%) 66 (46%) 15 (10%) |
<0.001 |
| Induction regimen R-CHOP R-Bendamustine |
245 (60) 160 (40) |
--- | --- | --- |
| Rituximab maintenance: Yes No |
392 (97%) 13 (3%) |
237 (97%) 8 (3%) |
155 (97%) 5 (3%) |
1 |
The R-CHOP-treated group was composed of younger patients, with a shorter time from biopsy to start of treatment; grade 3a was more frequently represented, as well as higher-risk patients. The median age in the R-bendamustine group was higher. Both groups received the maintenance therapy with similar proportions (97% in each cohort). However, eight patients in the R-CHOP cohort and five in the R-Bendamustine cohort (comprising 3% of both cohorts) could not initiate maintenance therapy. The primary reason for this was early progression (82%), while 18% were related to toxicity from the frontline regimen.
The outcome of the overall patient population and according to the treatment received is shown in Table 2. The complete response (CR) rate at the end of induction was higher with R-bendamustine and relapses were more frequent with R-CHOP. The transformation rate was similar in both groups, as was early progression (POD24) and death.
Table 2.
Overall patient population outcome and according to the treatment received.
|
Global Group (N = 405) |
R-CHOP (N = 245) |
R-BENDA (N = 160) |
p | |
|---|---|---|---|---|
| Median follow-up (95%CI) | 81 (77–86) | 96 (88–103) | 68 (60–75) | |
Response:
|
316 (78%) 77 (19%) 6 (1.5%) 6 (1.5%) |
180 (73%) 56 (23%) 3 (1%) 6 (2%) |
136 (85%) 21 (13%) 3 (2%) 0 (0%) |
0.014 |
Relapse/progression:
|
77 (19%) 30 (7%) 298 (74%) |
62 (25%) 20 (8%) 164 (67%) |
16 (10%) 10 (6%) 134 (84%) |
<0.001 |
Transformation:
|
19 (5%) 364 (95%) |
14 (6%) 216 (94%) |
5 (3%) 148 (97%) |
0.24 |
| POD24: | 39 (10%) | 25 (10%) | 14 (9%) | 0.73 |
| Death: | 50 (12%) | 34 (14%) | 16 (10%) | 0.28 |
Causes of death:
|
19 (5%) 13 (3%) 7 (2%) 11 (3%) |
15 (6%) 6 (2%) 5 (2%) 8 (3%) |
4 (2%) 7 (4%) 2 (1%) 3 (2%) |
0.28 |
Regarding toxicity, the characteristics are listed in Table 3 and Table 4. During induction (Table 3), prophylaxis against pneumocystis was more frequently used in the R-CHOP group and anti-herpes in the R-bendamustine group. There was no difference in the appearance of infections between both groups. Global and severe cytopenias were significantly more frequent with R-CHOP, as well as the use of colony-stimulating factors. Treatment discontinuation was more frequent with R-bendamustine (Table 4). During maintenance therapy (Table 4), anti-herpes prophylaxis was more frequent in the R-bendamustine group and secondary prophylaxis with colony-stimulating factors. Discontinuation due to toxicity was more frequent in the R-bendamustine group and due to disease progression in the R-CHOP group. Severe neutropenia, as well as infections, were also more frequent in the R-bendamustine group. We did not find differences in the incidence of secondary neoplasms. Supplementary Table S1 shows the distribution of toxicity by age with the different chemo regimens.
Table 3.
Toxicity during induction and supportive agents.
|
Global Group (N = 405) |
R-CHOP (N = 245) |
R-BENDA (N = 160) |
p | |
|---|---|---|---|---|
| Pneumocystis carinii prophylaxis (induction): | 184 (48%) | 125 (55%) | 59 (39%) | 0.003 |
| Herpes prophylaxis (induction): | 124 (32%) | 64 (28%) | 60 (39%) | 0.02 |
G-CSF during induction:
|
158 (42%) 114 (30%) 107 (28%) |
69 (30%) 97 (42%) 65 (28%) |
89 (60%) 17 (11%) 42 (28%) |
<0.001 |
| Median number of cycles (range) | 6 (2–8) | 6 (3–8) | 6 (2–8) | <0.001 |
| 1st line discontinuation: | 13 (3%) | 4 (2%) | 9 (6%) | 0.04 |
Neutropenia:
|
167 (43%) 66 (17%) 157 (40%) |
82 (35%) 51 (22%) 102 (43%) |
85 (55%) 15 (10%) 55 (35%) |
<0.001 |
Anemia:
|
255 (65%) 126 (32%) 14 (3%) |
125 (52%) 104 (43%) 12 (5%) |
130 (84%) 22 (14%) 2 (1%) |
<0.001 |
Thrombocytopenia:
|
327 (82%) 68 (16%) 2 (1%) |
196 (81%) 45 (19%) 1 (0.4%) |
131 (84%) 23 (15%) 1 (1%) |
0.46 |
Liver toxicity:
|
377 (95%) 13 (3%) 1 (1%) |
226 (94%) 11 (5%) 4 (2%) |
151 (98%) 2 (1%) 1 (1%) |
0.14 |
Renal toxicity:
|
388 (98%) 5 (1%) 3 (1%) |
238 (99%) 2 (1%) 1 (0.5%) |
150 (97%) 3 (2%) 2 (1%) |
0.39 |
| Infections: | 93 (24%) | 61 (25%) | 32 (21%) | 0.33 |
Infections during induction:
|
94 (24%) 299 (76%) 55 (14%) 39 (10%) |
61 (25%) 179 (75%) 35 (15%) 26 (11%) |
33 (22%) 120 (78%) 20 (13%) 13 (8%) |
0.4 0.65 |
Infections during maintenance:
|
64 (19%) 277 (81%) 49 (14%) 15 (4%) |
27 (13%) 177 (87%) 20 (10%) 7 (3%)) |
37 (27%) 100 (73%) 29 (21%) 8 (6%) |
<0.001 0.006 |
| Dermatologic toxicity: | 35 (9%) | 20 (9%) | 15 (10%) | 0.72 |
| Hospitalization: | 70 (18%) | 48 (20%) | 22 (14%) | 0.18 |
Table 4.
Toxicity during maintenance therapy and supportive agents.
|
Global Group (N = 405) |
R-CHOP (N = 245) |
R-BENDA (N = 160) |
p | |
|---|---|---|---|---|
| Pneumocystis carinii prophylaxis (maintenance): | 165 (45%) | 109 (49%) | 57 (40%) | 0.13 |
| Herpes prophylaxis (maintenance): | 113 (31%) | 55 (25%) | 58 (39%) | 0.004 |
G-CSF during maintenance:
|
312 (87%) 9 (2%) 39 (11%) |
200 (91%) 6 (3%) 13 (6%) |
112 (79%) 3 (2%) 26 (18%) |
<0.001 |
| Rituximab maintenance: | 392 (97%) | 237 (97%) | 155 (97%) | 1 |
| Maintenance discontinuation: | 75 (19%) | 29 (12%) | 46 (30%) | <0.001 |
Causes of discontinuation:
|
17 (22%) 31 (41%) 28 (27%) |
7 (24%) 4 (14%) 18 (62%) |
10 (21%) 27 (57%) 10 (21%) |
<0.001 |
Neutropenia:
|
286 (77%) 42 (11%) 44 (12%) |
190 (84%) 20 (9%) 15 (7%) |
96 (65%) 22 (15%) 29 (20%) |
<0.001 |
Anemia:
|
324 (88%) 42 (11%) 3 (1%) |
196 (88%) 26 (12%) 1 (0.4%) |
128 (88%) 16 (11%) 2 (1%) |
0.62 |
Thrombocytopenia:
|
331 (89%) 31 (8%) 9 (2%) |
199 (88%) 21 (9%) 6 (3%) |
132 (91%) 10 (7%) 3 (2%) |
0.66 |
| Infections: | 68 (18%) | 27 (12%) | 41 (28%) | <0.001 |
| Severe infections | ||||
| Hospitalization: | 25 (7%) | 13 (6%) | 12 (8%) | 0.4 |
Second malignancies:
|
29 (7%) 257 (63%) 119 (29%) |
16 (6%) 156 (64%) 73 (30%) |
13 (8%) 101 (63%) 46 (29%) |
0.82 |
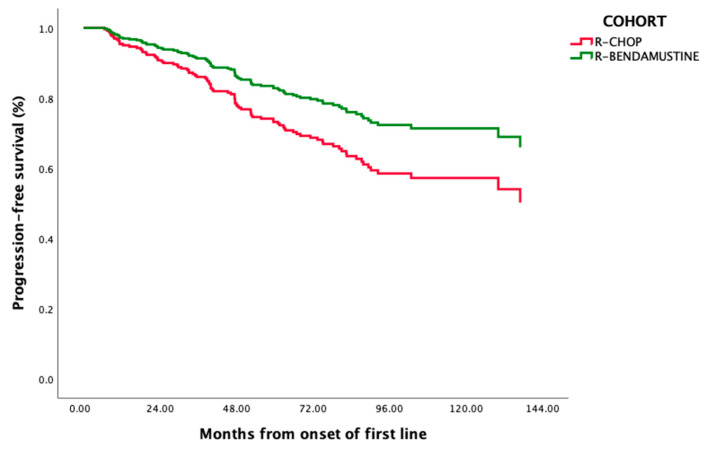
After a median follow-up of 81 months (95%CI: 77–86) (68 (60–75) for R-bendamustine and 96 (88–103) for R-CHOP), 6-year PFS (95%CI) was 71% (66–76) and 6-year OS (95%CI) was 91% (88–94) (Supplementary Figure S1). The six-year rate of PFS was 79% (95%CI: 72–86) for R-bendamustine vs. 67% (95%CI: 61–73) for R-CHOP (p = 0.046) and 6-year OS was 91% (95%CI: 86–96) for R-bendamustine vs. 91% (95%CI: 87–94) for R-CHOP (p = 0.49) (Figure 1).
Figure 1.
Kaplan–Meier estimation of PFS comparing R-CHOP and R-bendamutine.
Supplementary Table S2 shows the impact of different variables on 6-year PFS and OS. Patient age, lymphoma stage, ECOG-PS, FLIPI, and treatment regimen impacted PFS. Gender, ECOG-PS, and FLIPI impacted OS. Supplementary Table S3 shows the same analysis but only for grade 3A FL patients. Table 5 shows the multivariate analysis in which we included all significant variables in the univariate analysis as well as potential confounders (those variables not equally distributed between both cohorts: time to treatment and histological grade). We identified FLIPI 3–5 (HR 6.58 (1.13–2.62); p = 0.01) and induction regimen R-CHOP (HR 1.65 (1.01–2.71) p = 0.045) as independently associated with worse PFS and age > 60 years (HR 6.52 (2.7–15.74); p < 0.001), ECOG PS 2–4 (HR 4.39 (1.97–9.79); p < 0.001), and male gender (HR 1.51 (1.07–2.13); p = 0.018) with lower OS. Supplementary Table S3 shows the impact of different variables on 6-year PFS and OS for grade 3a FL patients.
Table 5.
Multivariate analyses.
| PFS | HR (95%CI) | p |
|---|---|---|
| R-CHOP | 1.65 (1.01–2.71) | 0.045 |
| FLIPI 3–5 | 6.58 (1.13–2.62) | 0.01 |
| OS | ||
| Age > 60 | 6.52 (2.7–15.74) | <0.001 |
| ECOG > 1 | 4.39 (1.97–9.79) | <0.001 |
| Male gender | 1.51 (1.07–2.13) | 0.018 |
4. Discussion
This analysis of the first-line treatment in low-grade FL patients showed that PFS was longer for patients treated with R-bendamustine than those treated with R-CHOP, with OS being almost identical among the two groups. This study analyzed the impact of rituximab maintenance, identifying a higher discontinuation rate due to toxicity for patients treated with R-bendamustine and a higher discontinuation rate due to progression in the R-CHOP group. However, it is important to note that the risk factor characteristics are not equally distributed between the groups due to the retrospective nature of the study.
The greater efficacy of R-bendamustine in terms of PFS has been previously identified, both in clinical trials and in real-world settings. Although Brigth [12] and StiL [14,19] studies were designed with a noninferiority endpoint, their results showed superior efficacy of bendamustine against CHOP. Notably, in these studies, maintenance was not used. This could in part explain the better results for our analysis, where the PFS achieved at six years for bendamustine was 79%, compared with the median of 69 months in the StiL study and 55% at five years in the Bright study. It should also be noted that these studies included other indolent lymphomas, even though most patients were follicular lymphoma patients [12,14]. Chemotherapy was not randomized in the Gallium study, as this comparison was not the study’s objective. However, the estimated PFS at three years was 73% for R-chemotherapy, similar to our results [15]. A recent meta-analysis also showed superiority of bendamustine over CHOP in terms of PFS, with or without maintenance rituximab, without differences in OS [20].
A question that is of interest to address is whether, given these results, all patients need maintenance after induction. The advantage in PFS in favor of RB vs. RCHOP could indicate that patients who achieve CR with RB, especially those older or with comorbidities that increase the risk of infectious complications, may not receive maintenance. As Hill et al. suggest in their retrospective article [21], it is likely that the benefit of maintenance after R-B is especially limited to those patients who do not achieve a profound response with induction. However, we cannot answer this question clearly with our data because the number of patients who achieve PR at the end of induction with R-B is small. Indeed, our study demonstrates that the PFS at six years in patients who performed maintenance was more than 10% superior in the R-bendamustine group compared to that of R-CHOP, without an increase in toxicity, which could be in favor of maintenance use. The PETReA trial is ongoing; a randomized clinical trial looking for the impact of avoiding maintenance for patients with CR after induction therapy will undoubtedly address this question [22].
In the real-world context, data also suggest bendamsutine’s superiority, although there are contradictory data here. Mondello et al. published an analysis focused on patients with grade 3a FL, demonstrating a higher PFS in favor of bendamustine and no differences in OS; none received maintenance [23]. However, a German study showed strikingly superior OS for R-CHOP compared with R-bendamustine, with no difference in PFS, attributing these results to grade 3a LF heterogeneity. Importantly, in this analysis, only 34% of patients in the R-CHOP arm and 75% in the R-bendamustine arm received maintenance [24]. Other similar retrospective analysis recently published from Italy, also focused on 3a FL, did not show these differences, finding similar results for both R-CHOP and R-bendamustine in terms of PFS and OS [25]. In our study, the sub-analysis in the population with grade 3a FL showed no differences for PFS or OS between the regimens used. However, this population in our study is small.
Regarding toxicity, the nature of the adverse events identified during this study was consistent with the known safety profiles of the treatments evaluated. Interestingly, severe cytopenias were more frequent with R-CHOP during the induction period but severe neutropenia and infections were more frequent with R-bendamustine during rituximab maintenance. However, the frequency of fatal adverse events was similar between the two groups and the distribution of second neoplasms was also similar. Although initial clinical trials attributed less toxicity to R-bendamustine than to R-CHOP [12,14], other studies have reported more severe infection frequency with bendamustine than CHOP because it was associated with marked and prolonged reductions in T-cell counts [18]. Certainly, our analysis shows a higher rate of severe infections during maintenance in the bendamustine group but without any impact on mortality from this cause.
As previously reported, our analysis identified age as a risk factor related to OS [26,27]. Interestingly, in our study, the median age of patients receiving bendamustine was higher than R-CHOP. But, when evaluating the toxicity of patients older than 60, there was no higher risk of severe infection frequency than that of the younger group, nor hospitalization or second malignancies. Severe neutropenia was more common in the elderly during induction but with the same frequency between R-bendamustine and R-CHOP. Likewise, we found a higher frequency of anemia and thrombocytopenia in the elderly but of a mild nature. These data suggest that bendamustine is also a valid option in elderly patients.
Bendamustine has recently been discredited. This fact is due to the impact it could have on T lymphocytes regarding the necessity of a future CAR-T cell therapy [28,29]. However, considering this scheme’s prolonged progression-free survival in the first line of treatment, its use as initial therapy could be encouraged, meaning that it will be far away in time from a hypothetical lymphoapheresis.
The limitations of this study are mainly related to the retrospective design of our analysis. On the other hand, we also consider the limitation of not having a control group without maintenance to assess this impact directly. However, in this sense, the criterion of only including patients with maintenance makes the cohort more homogeneous to assess efficacy. On the other hand, the 3a FL population is underrepresented in our analysis, which does not allow us to conclude for this population. Finally, although the median follow-up of the bendamustine group was beyond three years, it was significantly shorter compared with R-CHOP. Another significant limitation previously mentioned is that groups are not entirely comparable since, being a retrospective analysis, risk factor characteristics are not equally distributed between arms. Finally, we want to highlight a limitation of this study regarding toxicity analysis since cardiotoxicity, associated with using anthracyclines and cyclophosphamide [30,31], was not evaluated.
5. Conclusions
In conclusion, the results of this multicenter study show that the use of R-bendamustine followed by rituximab maintenance in patients with previously untreated follicular lymphoma resulted in significantly longer PFS compared to patients treated with R-CHOP. The frequency of high-grade adverse events was higher with this regimen during maintenance regarding neutropenia and infectious toxicity, conferring more significant therapy discontinuation in this group, without impact in mortality. Older patients also benefit from this regimen without further toxicity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers16071285/s1, Figure S1: OS comparing R-CHOP vs R-Bendamustine; Table S1: Toxicity distribution by age with different chemo regimens; Table S2: Impact of different variables on six-year OS and PFS (univariate analysis). Table S3: Impact of different variables on six-year OS and PFS in grade 3A FL patients.
Author Contributions
M.B.-O., A.G., A.S. and J.-M.S. were responsible for conception and design; A.C., A.J.-U., J.L., P.V., R.d.O., A.D.l.F., C.A., B.P.-M., R.C., J.P., P.M., J.P.d.O., B.N., M.I., S.G.d.V., D.G.B., P.F.-C., R.d.C. and J.D.-G. collected and assembled data; M.B.-O. and A.G. were responsible for data analysis and interpretation; M.B.-O., A.G., A.S. and J.-M.S. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study protocol was approved by the Ethical Committee of Son Espases Universitary Hospital (Approval number: IB 4145/20, approval date: 19 June 2020).
Informed Consent Statement
All patients signed the informed consent document.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Bastos-Oreiro M., Muntañola A., Panizo C., Gonzalez-Barca E., de Villambrosia S.G., Córdoba R., López J.L.B., González-Sierra P., Terol M.J., Gutierre A., et al. RELINF: Prospective epidemiological registry of lymphoid neoplasms in Spain. A project from the GELTAMO group. Ann. Hematol. 2020;99:799–808. doi: 10.1007/s00277-020-03918-6. [DOI] [PubMed] [Google Scholar]
- 2.Swerdlow S.H., Campo E., Pileri S.A., Harris N.L., Stein H., Siebert R., Advani R., Ghielmini M., Salles G.A., Zelenetz A.D., et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375–2390. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alaggio R., Amador C., Anagnostopoulos I., Attygalle A.D., de Oliveira Araujo I.B., Berti E., Bhagat G., Borges A.M., Boyer D., Calaminici M., et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia. 2022;36:1720–1748. doi: 10.1038/s41375-022-01620-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sarkozy C., Maurer M.J., Link B.K., Ghesquieres H., Nicolas E., Thompson C.A., Traverse-Glehen A., Feldman A.L., Allmer C., Slager S.L., et al. Cause of Death in Follicular Lymphoma in the First Decade of the Rituximab Era: A Pooled Analysis of French and US Cohorts. J. Clin. Oncol. 2019;37:144–152. doi: 10.1200/JCO.18.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casulo C., Byrtek M., Dawson K.L., Ghesquieres H., Nicolas E., Thompson C.A., Traverse-Glehen A., Feldman A.L., Allmer C., Slager S.L., et al. Early Relapse of Follicular Lymphoma After Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone Defines Patients at High Risk for Death: An Analysis From the National LymphoCare Study. J. Clin. Oncol. 2015;33:2516–2522. doi: 10.1200/JCO.2014.59.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Montoto S., Davies A.J., Matthews J., Calaminici M., Norton A.J., Amess J., Vinnicombe S., Waters R., Rohatiner A.Z.S., Lister T.A. Risk and clinical implications of transformation of follicular lymphoma to diffuse large B-cell lymphoma. J. Clin. Oncol. 2007;25:2426–2433. doi: 10.1200/JCO.2006.09.3260. [DOI] [PubMed] [Google Scholar]
- 7.Solal-Céligny P., Roy P., Colombat P., White J., Armitage J.O., Arranz-Saez R., Au W.Y., Bellei M., Brice P., Caballero D., et al. Follicular Lymphoma International Prognostic Index. Blood. 2004;104:1258–1265. doi: 10.1182/blood-2003-12-4434. [DOI] [PubMed] [Google Scholar]
- 8.Pastore A., Jurinovic V., Kridel R., White J., Armitage J.O., Arranz-Saez R., Au W.Y., Bellei M., Brice P., Caballero D., et al. Integration of gene mutations in risk prognostication for patients receiving first-line immunochemotherapy for follicular lymphoma: A retrospective analysis of a prospective clinical trial and validation in a population-based registry. Lancet Oncol. 2015;16:1111–1122. doi: 10.1016/S1470-2045(15)00169-2. [DOI] [PubMed] [Google Scholar]
- 9.Dreyling M., Ghielmini M., Rule S., Salles G., Vitolo U., Ladetto M., ESMO Guidelines Committee Newly diagnosed and relapsed follicular lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016;27:v83–v90. doi: 10.1093/annonc/mdw400. [DOI] [PubMed] [Google Scholar]
- 10.Brice P., Bastion Y., Lepage E., Brousse N., Haïoun C., Moreau P., Straetmans N., Tilly H., Tabah I., Solal-Céligny P. Comparison in low-tumor-burden follicular lymphomas between an initial no-treatment policy, prednimustine, or interferon alfa: A randomized study from the Groupe d’Etude des Lymphomes Folliculaires. Groupe d’Etude des Lymphomes de l’Adulte. J. Clin. Oncol. 1997;15:1110–1117. doi: 10.1200/JCO.1997.15.3.1110. [DOI] [PubMed] [Google Scholar]
- 11.Hiddemann W., Kneba M., Dreyling M., Schmitz N., Lengfelder E., Schmits R., Reiser M., Metzner B., Harder H., Hegewisch-Becker S., et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: Results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2005;106:3725–3732. doi: 10.1182/blood-2005-01-0016. [DOI] [PubMed] [Google Scholar]
- 12.Flinn I.W., van der Jagt R., Kahl B., Wood P., Hawkins T., MacDonald D., Simpson D., Kolibaba K., Issa S., Chang J., et al. First-Line Treatment of Patients With Indolent Non-Hodgkin Lymphoma or Mantle-Cell Lymphoma With Bendamustine Plus Rituximab Versus R-CHOP or R-CVP: Results of the BRIGHT 5-Year Follow-Up Study. J. Clin. Oncol. 2019;37:984–991. doi: 10.1200/JCO.18.00605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marcus R., Imrie K., Belch A., Cunningham D., Flores E., Catalano J., Solal-Celigny P., Offner F., Walewski J., Raposo J., et al. CVP chemotherapy plus rituximab compared with CVP as first-line treatment for advanced follicular lymphoma. Blood. 2005;105:1417–1423. doi: 10.1182/blood-2004-08-3175. [DOI] [PubMed] [Google Scholar]
- 14.Rummel M.J., Niederle N., Maschmeyer G., Banat G.A., von Grünhagen U., Losem C., Kofahl-Krause D., Heil G., Welslau M., Balser C., et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: An open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet. 2013;381:1203–1210. doi: 10.1016/S0140-6736(12)61763-2. [DOI] [PubMed] [Google Scholar]
- 15.Marcus R., Davies A., Ando K., Klapper W., Opat S., Owen C., Phillips E., Sangha R., Schlag R., Seymou J.F., et al. Obinutuzumab for the First-Line Treatment of Follicular Lymphoma. N. Engl. J. Med. 2017;377:1331–1344. doi: 10.1056/NEJMoa1614598. [DOI] [PubMed] [Google Scholar]
- 16.Salles G., Seymour J.F., Offner F., López-Guillermo A., Belada D., Xerri L., Feugier P., Bouabdallah R., Catalano J.V., Brice P., et al. Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): A phase 3, randomised controlled trial. Lancet. 2011;377:42–51. doi: 10.1016/S0140-6736(10)62175-7. [DOI] [PubMed] [Google Scholar]
- 17.Bachy E., Seymour J.F., Feugier P., Offner F., López-Guillermo A., Belada D., Xerri L., Catalano J.V., Brice P., Lemonnier F., et al. Sustained Progression-Free Survival Benefit of Rituximab Maintenance in Patients With Follicular Lymphoma: Long-Term Results of the PRIMA Study. J. Clin. Oncol. 2019;37:2815–2824. doi: 10.1200/JCO.19.01073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hiddemann W., Barbui A.M., Canales M.A., Cannell P.K., Collins G.P., Dürig J., Forstpointner R., Herold M., Hertzberg M., Klanova M., et al. Immunochemotherapy With Obinutuzumab or Rituximab for Previously Untreated Follicular Lymphoma in the GALLIUM Study: Influence of Chemotherapy on Efficacy and Safety. J. Clin. Oncol. 2018;36:2395–2404. doi: 10.1200/JCO.2017.76.8960. [DOI] [PubMed] [Google Scholar]
- 19.Rummel M.J., Maschmeyer G., Ganser A., Heider A., von Gruenhagen U., Losem C., Heil G., Welslau M., Balser C., Kaiser U., et al. Bendamustine plus rituximab (B-R) versus CHOP plus rituximab (CHOP-R) as first-line treatment in patients with indolent lymphomas: Nine-year updated results from the StiL NHL1 study. J. Clin. Oncol. 2017;35((Suppl. 15)):7501. doi: 10.1200/JCO.2017.35.15_suppl.7501. [DOI] [Google Scholar]
- 20.Wang Y., Zhou S., Qi X., Yang F., Maurer M.J., Habermann T.M., Witzig T.E., Wang M.L., Nowakowski G.S. Efficacy of front-line immunochemotherapy for follicular lymphoma: A network meta-analysis of randomized controlled trials. Blood Cancer J. 2022;12:1. doi: 10.1038/s41408-021-00598-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hill B.T., Nastoupil L., Winter A.M., Becnel M.R., Cerhan J.R., Habermann T.M., Link B.K., Maurer M.J., Fakhri B., Reddy P., et al. Maintenance rituximab or observation after frontline treatment with bendamustine-rituximab for follicular lymphoma. Br. J. Haematol. 2019;184:524–535. doi: 10.1111/bjh.15720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pettitt A.R., Barrington S., Kalakonda N., Khan U.T., Jackson R., Carruthers S., Oates M., Lin K., Ardeshna K., Eyre T., et al. Ncri Petrea Trial: A Phase 3 Evaluation of Pet-Guided, Response-Adapted Therapy in Patients with Previously Untreated, Advanced-Stage, High-Tumour-Burden Follicular Lymphoma. Hematol. Oncol. 2019;37((Suppl. 2)):67–68. doi: 10.1002/hon.35_2629. [DOI] [Google Scholar]
- 23.Mondello P., Steiner N., Willenbacher W., Cerchione C., Nappi D., Mauro E., Ferrero S., Cuzzocrea S., Mian M. Bendamustine plus Rituximab Versus R-CHOP as First-Line Treatment for Patients with Follicular Lymphoma Grade 3A: Evidence from a Multicenter, Retrospective Study. Oncologist. 2018;23:454–460. doi: 10.1634/theoncologist.2017-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pouyiourou M., Meyer A., Stroux A., Viardot A., La Rosée P., Maschmeyer G., Kämpfe D., Kahl C., Vucinic V., Monecke A., et al. First-line treatment with R-CHOP or rituximab-bendamustine in patients with follicular lymphoma grade 3A-results of a retrospective analysis. Ann. Hematol. 2020;99:2821–2829. doi: 10.1007/s00277-020-04171-7. [DOI] [PubMed] [Google Scholar]
- 25.Margiotta-Casaluci G., Bigliardi S., Cocito F., Meli E., Petrucci L., Nicolosi M., Annibali O., Boccomini C., Bozzoli V., Castellino A., et al. Comparison of first-line treatment with bendamustine plus rituximab versus R-CHOP for patients with follicular lymphoma grade 3A: Results of a retrospective study from the Fondazione Italiana Linfomi. Front. Oncol. 2023;13:1120967. doi: 10.3389/fonc.2023.1120967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma S. Risk Factors of Follicular Lymphoma. Expert Opin. Med. Diagn. 2012;6:323–333. doi: 10.1517/17530059.2012.686996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alig S., Jurinovic V., Pastore A., Haebe S., Schmidt C., Zoellner A.K., Dreyling M., Unterhalt M., Hoster E., Hiddemann W., et al. Impact of age on clinical risk scores in follicular lymphoma. Blood Adv. 2019;3:1033–1038. doi: 10.1182/bloodadvances.2019032136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iacoboni G., Martin Lopez A.A., Jalowiec K.A., Rejeski K., Kwon M., Jalowiec K.A., Amat P., Reguera-Ortega J.L., Gallur L., Blumenberg V., et al. Recent Bendamustine Treatment before Apheresis Has a Negative Impact on Outcomes in Patients with Large B-Cell Lymphoma Receiving Chimeric Antigen Receptor T-Cell Therapy. Blood. 2022;140((Suppl. 1)):1592–1594. doi: 10.1182/blood-2022-169783. [DOI] [PubMed] [Google Scholar]
- 29.Wang M., Munoz J., Goy A., Wang M., Munoz J., Goy A., Locke F.L., Jacobson C.A., Hill B.T., Timmerman J.M., et al. Three-Year Follow-Up of KTE-X19 in Patients With Relapsed/Refractory Mantle Cell Lymphoma, Including High-Risk Subgroups, in the ZUMA-2 Study. J. Clin. Oncol. 2022;41:555. doi: 10.1200/JCO.21.02370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leszek P., Klotzka A., Bartuś S., Leszek P., Klotzka A., Bartuś S., Burchardt P., Czarnecka A.M., Długosz-Danecka M., Gierlotka M., et al. A practical approach to the 2022 ESC cardio-oncology guidelines: Comments by a team of experts-cardiologists and oncologists. Pol. Heart J. 2023;81:1047–1063. doi: 10.33963/v.kp.96840. [DOI] [PubMed] [Google Scholar]
- 31.Lyon A.R., López-Fernández T., Couch L.S., Asteggiano R., Aznar M.C., Bergler-Klein J., Boriani G., Cardinale D., Cordoba R., Cosyns B. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS) Eur. Heart J. 2022;43:4229–4361. doi: 10.1093/eurheartj/ehac244. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.