Abstract
Measles virus (MV) infection of the human central nervous system (CNS) typically involves widespread infection of neurons. However, little is known about how they become infected, how defective virus arises and accumulates, or how virus spreads among the cells of the CNS. In vitro studies of viral interactions with human neuronal cells may contribute to the resolution of such issues. In mixed cultures containing differentiated human neuronal (hNT2) cells and neuroepithelial cells, immunofluorescence studies show that the neurons, unlike both their NT2 progenitors and the neuroepithelial cells, are not initially susceptible to MV infection. This is possibly due to their lack of expression of CD46, a known cell surface receptor for MV. Later in the course of infection, however, both MV proteins and genomic RNA become detectable in their processes, where they contact infected, fully permissive neuroepithelial cells. Such a mechanism of virus transfer may be involved in the initiation and spread of persistent MV infection in diseases such as subacute sclerosing panencephalitis. Furthermore, mutated defective virus may readily accumulate and spread without the need, at any stage, for viral maturation and budding.
Measles virus (MV) is the etiological agent of the rare neurological diseases subacute sclerosing panencephalitis (SSPE) and measles inclusion body encephalitis (4, 20). In neuropathological studies of these diseases, the cells most often found to be MV infected are neurons and oligodendrocytes (1, 7). Molecular studies of central nervous system (CNS) tissue have demonstrated attenuation of viral gene expression at the level of both transcription and translation of viral mRNAs, affecting mainly the envelope genes coding for the matrix (M), fusion (F), and hemagglutinin (H) proteins (3, 17). Studies on SSPE tissues in this laboratory suggest that defective MV spreads in a cephalocaudal direction, probably by passage from neuron to neuron (1). However, there is little understanding of how neuronal infection is initiated, how defective virus may arise in neurons, and how the virus spreads from cell to cell.
Down-regulation of MV gene expression can occur in vitro in human glial cells, through the operation of a cell type-dependent regulation of viral mRNA transcription and a differentiation-dependent regulation of translation (18). Furthermore, the suppression of MV growth (involving inhibition of both synthesis of viral RNAs and phosphorylation of viral proteins) by papaverine treatment, which increases endogenous cyclic AMP, has been shown to be most prominent in neuroblastoma cell lines rather than epidermoid, glial, or oligodendroglioma cells (21). However, it is not known how relevant such studies are to the situation in the MV-infected human CNS. Therefore, to analyze aspects of MV infection and spread more fully, we have studied these phenomena in vitro in cultures containing differentiated human neuronal cells.
Cells with a human CNS neuronal phenotype are available for in vitro studies of virus-cell interaction.
The NT2 cell line, derived from a human teratocarcinoma, exhibits properties characteristic of a committed neuronal precursor at an early stage of development (2, 15). NT2 cells can be induced by retinoic acid to differentiate in vitro into a mixture of postmitotic CNS neurons expressing all well-defined neuronal markers and elaborate axonal and dendritic processes (hNT neurons) and large, flat, neuroepithelium-like cells (14). In this study, we examined the effect of this neuronal cell differentiation on MV replication and spread. NT2 cells (Stratagene) were grown in Dulbecco’s modified Eagle medium (Gibco) containing 10% fetal bovine serum, 4 mM glutamine, 0.1% penicillin, and 0.1% streptomycin. Differentiation with retinoic acid (Sigma) was carried out as described previously (15). Cells were characterized by using the following antibodies: anti-CAM5.2, a monoclonal antibody (MAb) which recognizes keratins 8 and 18 (neat; Becton Dickinson); a 1:50 antivimentin MAb from Dako; a 1:50 polyclonal antibody (PAb) from ICN; a 1:50 antineurofilament MAb from Dako; a 1:50 antisynaptophysin PAb from Dako; a 1:50 anti-NSE MAb from Dako; and 1:100 anti-GFAP MAb from Dako. A MAb to CD46 (1:100), a putative MV receptor on human cells, was obtained from J. Schneider-Schaulies, Würzburg, Germany. Prior to immunocytochemical staining (except for CD46), coverslips were washed twice in Hanks balanced salt solution (Gibco), fixed in ice-cold acetone for 10 min, and air dried. For CD46 immunocytochemistry analysis, coverslips remained unfixed during incubation with the primary antibody and then were fixed in acetone before application of the secondary antibody. All incubations with primary and secondary antibodies were for 30 min at 37°C with two washes in 10 mM phosphate-buffered saline (PBS) between steps. Secondary antibodies were obtained from Dako (fluorescein, 1:50) or Sigma (Cy3, 1:100). In some preparations, a propidium iodide (Sigma) counterstain was used to visualize nuclei. After further washes in PBS, coverslips were mounted in Citifluor (Amersham). Negative controls for all immunofluorescence involved omission of the primary antibody from the procedure. All fluorescence was examined and photographed on a Zeiss Axioplan microscope fitted with an MC 100 camera system. Selected preparations were further examined by confocal microscopy using a krypton-argon laser on a Leica TCS NT laser scanning confocal microscope. Dual composite images, throughout the entire thickness of the cell layers, were generated and examined for the presence or absence of virus in cells and cell processes.
NT2 cells, before retinoic acid differentiation, were identified by their extensive cytoplasmic content of CAM5.2, cytokeratins, actin, vimentin, and CD46 (Fig. 1a and k). After retinoic acid-induced differentiation, immunocytochemical characterization revealed two distinct cellular populations, 75% with the above-described immunocytochemical phenotype 2, 15; Fig. 1b and l) and about 25% with a neuronal phenotype characterized by NFP and synaptophysin positivity (Fig. 1c) but with no detectable CD46 expression (Fig. 1l). To analyze the fine structure of the contacts between adjacent NT2 cells and between hNT2 and neuroepithelial cells in the mixed differentiated cultures, cells growing in 25-cm2 flasks were fixed in 2.5% glutaraldehyde and embedded in situ as previously described (10). Undifferentiated NT2 cells (Fig. 2A) were compact, with regular-shaped nuclei and no lengthy cell processes. The plasma membranes of contiguous NT2 cells often lay very close together but did not form defined junctions. In mixed, uninfected cultures of differentiated hNT2 cells and neuropeithelial cells, the neuronal cells had pleomorphic nuclei and multiple long cell processes. These were observed to terminate either as growth cones or, where they touched the surfaces of neuroepithelial cells, as clublike structures (Fig. 2E). At such contact points, the contacting cells were modified but did not form defined junctions.
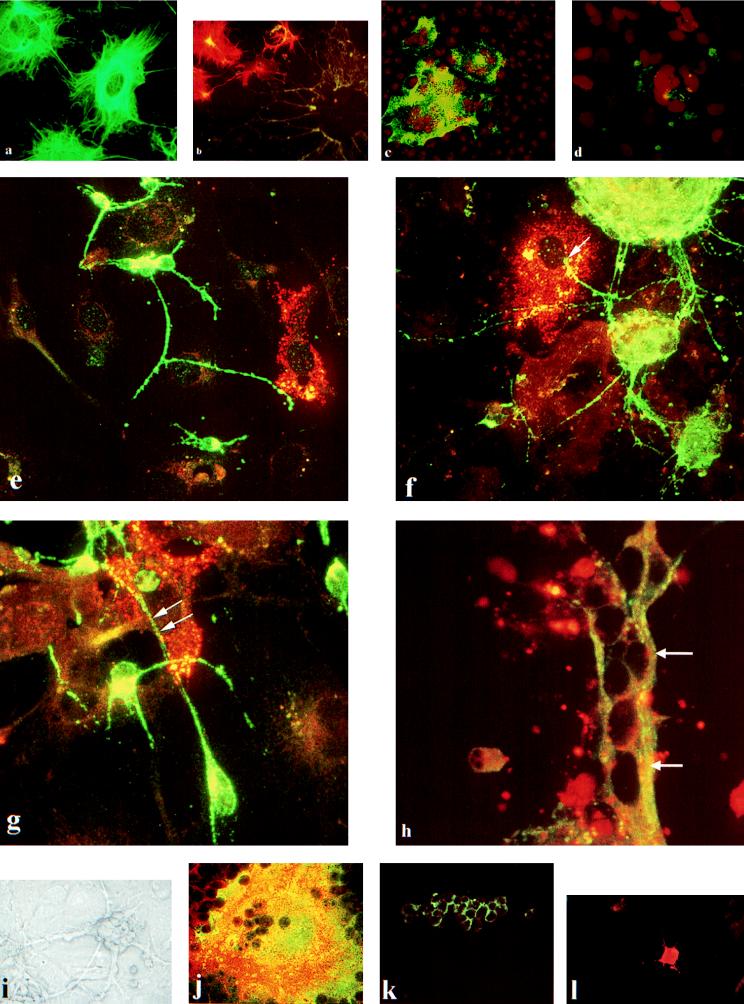
FIG. 1.
(a) Cytokeratin expression in undifferentiated NT2 cells. (b) Dual labeling for cytokeratin-positive neuroepithelial cells (Cy3, orange-red) and synaptophysin-positive hNT neurons (FITC, green) in mixed, differentiated cultures. (c) Strong expression of MV nucleocapsid protein in infected, undifferentiated NT2 cells. (d) Weak expression of MV fusion protein in infected, undifferentiated NT2 cells. Both c and d are 72 h postinfection and are propidium iodide counterstained. (e to h) Composite confocal images along the optical axis showing synaptophysin-positive hNT cells (FITC) and MV (Cy3) in mixed, differentiated cultures. (e) At 24 h postinfection, MV positivity is confined to neuroepithelial cells. At 48 h (f) and 72 h (g) postinfection, there is strong MV expression in neuroepithelial cells. However, viral protein is also present in the synaptophysin-positive processes where they are in contact with virus-positive neuroepithelial cells. (h) At 96 h postinfection, MV nucleocapsid RNA (arrows) is evident in a synaptophysin-positive process. (i) Phase contrast photomicrograph of relatively pure hNT2 cells. (j) Dual confocal image of vimentin (Cy3) present in MV-infected syncytium of neuroepithelial cells (FITC). CD46 is expressed in undifferentiated NT2 cells (k) and in neuroepithelial cells in mixed, differentiated cultures (l). This photographic plate was made from 35-mm slides which were scanned into ADOBE photoshop by using a Kodak RFS film scanner. The images were then sized and made into composites. Resolution was 300 pixels/in.
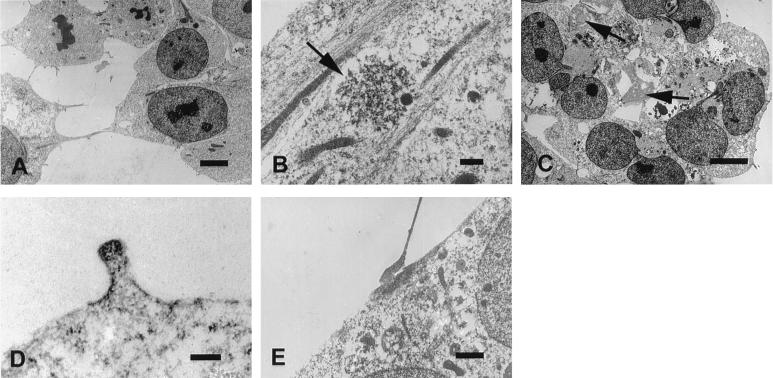
FIG. 2.
Ultrastructure of cell cultures. (A) Uninfected NT2 cells (bar, 5 μm). (B) Viral inclusion (arrow) in cytoplasm of infected NT2 cells (bar, 1 μm). (C) Virus inclusions (arrows) in syncytium in infected NT2 culture (bar, 0.5 μm). (D) Rare, aberrant budding structure in infected NT2 culture, showing virus-modified plasma membrane (bar, 200 nm). (E) Cell contact in a mixed culture of uninfected, retinoic acid-induced, differentiated hNT2 and NE cells (bar, 1 μm). The photographic plate was made from 35-mm slides which were scanned into ADOBE photoshop by using a Kodak RFS film scanner. The images were then sized and made into composites. Resolution was 300 pixels/in.
Confluent monolayers of NT2 cells growing on glass coverslips were infected with MV before and after differentiation.
A stock pool of the CAM strain of MV (obtained from U. Liebert, Institute for Virology, Würzburg, Germany) was used at a multiplicity of infection of 1. Dishes of coverslips were also mock infected with tissue culture medium to serve as negative controls. Coverslips were removed and fixed in acetone for MV immunocytochemical analysis after 8, 16, 24, 48, 72, 96, 120, and 168 h. MV proteins and genomic RNA were detected by immunofluorescence and in situ hybridization on cultures of undifferentiated NT2 cells and on mixed cultures of hNT2 neurons and neuroepithelial cells). An antinucleocapsid MAb to MV (1:2,000) was obtained from Harlan, Seralab. All other MAbs to MV proteins were obtained from C. Orvell, Stockholm, Sweden, and used at the dilutions described previously (6). For determination of numbers of virus-positive cells, eight microscope fields at a magnification of ×40 were assessed in propidium iodide-counterstained preparations, and the results were expressed as percentages. Dual fluorescence analysis was carried out with mixed cultures of differentiated NT2 cells which had been infected with MV for cell-specific markers and virus. Briefly, coverslips were incubated in rabbit PAbs to synaptophysin or vimentin and then in fluorescein-conjugated swine anti-rabbit immunoglobulin G. Following washes in PBS, sections were incubated in MAbs against the viral proteins and then in Cy.3-conjugated sheep anti-mouse immunoglobulin G. Appropriate negative controls for virus-infected cells produced no fluorescence signals, and mock-infected cultures of NT2 or hNT2 cells were consistently immunofluorescence negative for viral proteins.
MV readily infects undifferentiated NT2 cells with localized formation of syncytia but without production of infectious virus.
Indirect immunofluorescence analysis of undifferentiated NT2 cells infected with the CAM strain of MV demonstrated a small number of infected cells 24 h postinfection. With increasing time postinfection, the number of infected cells did not increase significantly. However, these isolated infected cells gave rise to focal syncytia by 48 h postinfection which increased in size with increasing time. As indicated in Table 1, strong expression of nucleocapsid and H proteins was observed in infected cells at all time points (Fig. 1c). With the two different MAbs used against both the F and M proteins, only weak expression of F (Fig. 1d) or M protein was observed. However, these MAbs produced strong reactions in Vero cells (Table 1). Extensive ultrastructural analysis of MV-infected NT2 cells revealed that in isolated infected cells, viral nucleocapsid was seen dispersed or in small clumps scattered throughout the cytoplasm (Fig. 2B). Virus-infected syncytia (Fig. 2C) contained dense viral factories and massed intermediate filaments. In contrast to infection of Vero cells, viral nucleocapsids in syncytia and other virus-infected cells were not found aligned beneath the plasma membrane. Furthermore, a virally modified plasma membrane was seen once only in an aberrant, budlike outgrowth (Fig. 2D). However, no evidence of the normal stages of MV budding was found and mature virions were not seen. At selected time points, the medium from the infected cells was retained for analysis of virus infectivity. Aliquots of medium taken from infected cells were incubated for 48 and 96 h on Vero or NT2 cells growing on coverslips. Cells were then fixed for viral immunocytochemical analysis as described above. When medium collected from virus-infected NT2 cells at various time points postinfection was inoculated onto fresh NT2 or Vero cells, no virus infection could be demonstrated after 48, 72, or 120 h by indirect immunofluorescence. The absence of detectable infectious virus in the medium from such infected cultures is probably due to the relatively low level and focal production of viral M and F proteins and the consequent failure of maturation and budding. This suggests that replication of the virus in this cell line is inhibited, an explanation which is supported by the lack of viral budding observed by electron microscopy. It is possible that factors intrinsic to or induced in these cells attenuate primary viral gene expression, as has been proposed for glial and neuroblastoma cells in culture (9, 16, 18, 19). Such factors may produce high rates of mutation in the viral M and F genes, leading to establishment of the persistent infection in this cell line which has been observed in a parallel study (5). The clonal origin of NT2 cells (15) suggests that these controlling factors should be present in 100% of the cells, similar to the expression of the MV receptor CD46. Other factors which may be involved in the establishment of infection and viral persistence in this cell line are the stage of the cell cycle and the clonality of the cell line, which may have changed, with different subclones being more or less susceptible to viral infection.
TABLE 1.
Characteristics of cultured cells and their response to MV infection
| Cell type | Marker expressiona
|
Response to MV (CAM strain) infection
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Actin | Vim. | Cyk. | Syn. | NFP | GFAP | CD46 | Virion infectability | Infectability by cell contact | MV protein expressionb
|
Syncytium formation | Infectious virus production | ||||
| N | H | M | F | ||||||||||||
| NT2c | E | E | E | NX | NX | NX | E | Yes | Yes | +++ | +++ | + | + | Yes | No |
| NEd | E | E | E | NX | NX | NX | E | Yes | Yes | +++ | +++ | +++ | +++ | Yes | Yes |
| hNTe | E | NX | NX | E | E | NX | NX | No | Yes | (−) | (−) | (−) | (−) | No | No |
| Vero | E | E | E | NX | NX | NX | E | Yes | Yes | +++ | +++ | +++ | +++ | Yes | Yes |
Vim., vimentin; Cyk., cytokeratins; Syn., synaptophysin; E, expressed; NX, not expressed.
N, nucleocapsid; (−), trace expression in neuronal processes, only at point of contact with syncytium; +, weak expression; +++, strong expression.
Undifferentiated neuroepithelial cells.
Differentiated neuroepithelial cells.
Differentiated neuronal cells.
Neurons and neuroepithelial cells resulting from retinoic acid-induced differentiation of NT2 cells differ from their precursors and from each other in their response to MV infection.
The identity of infected cells in the mixed cultures was confirmed by dual labeling for vimentin and viral nucleocapsid protein, which demonstrated that it was the cells with a neuroepithelial phenotype (i.e., the nonneuronal cells) which were virus infected (Fig. 1j). Dual immunofluorescence analysis for synaptophysin and viral proteins on coverslips from infected cultures of hNT2 cells and neuroepithelial cells produced the time course of infection summarized in Table 2. At 24 h postinfection, dual labeling revealed the presence of isolated virus-infected cells with neuroepithelial characteristics. However, none of the synaptophysin-positive hNT2 cells contained any viral antigens (Fig. 1e). Virus infection for 24, 48, or 72 h had no effect on CD46 expression at the surfaces of any of the three cell types (NT2, hNT2, or neuroepithelial).
TABLE 2.
Expression of MV proteins determined by immunocytochemical analysis in mixed cultures of NE and hNT2 cells after retinoic acid-induced differentiationa
| Time (h) postinfection | Nucleocapsid protein
|
M protein
|
F protein
|
H protein
|
||||
|---|---|---|---|---|---|---|---|---|
| NE | hNT2 | NE | hNT2 | NE | hNT2 | NE | hNT2 | |
| 24 | + | − | + | − | + | − | + | − |
| 48 | +++ | + | +++ | + | ++ | − | +++ | + |
| 72 | ++++ | + | ++++ | + | +++ | + | ++++ | + |
| 96 | ++++ | + | ++++ | + | +++ | + | ++++ | + |
| 120 | +++++ | + | +++++ | + | ++++ | + | +++++ | + |
Percentages of cells positive: −, 0; +, 1 to 15; ++, 16 to 30; +++, 31 to 50; ++++, 51 to 75; +++++, 76 to 100.
Human neurons (hNT2) in mixed culture, refractory to early direct infection with virions, become infected later, at points where their long cell processes contact virus-infected neuroepithelial syncytia.
At 48 h postinfection, many virus-positive neuroepithelial cells were found, both as isolated cells and as syncytia. However, unlike the situation with the undifferentiated NT2 cells, all four viral proteins (viral nucleocapsid, H, M, and F proteins) were readily expressed in infected neuroepithelial cells and infectious virus was produced from these cultures—i.e., viral antigen was detected on NT2 or Vero cells which were inoculated with medium from infected mixed cultures of differentiated hNT2 and neuroepithelial cells. This suggests that one of the effects of retinoic acid-induced differentiation on NT2 cells is to produce a cell type which expresses the same cell characterization markers but has a modified virus-cell interaction so that steps such as the synthesis of viral RNAs are no longer inhibited. While the majority of synaptophysin-positive hNT2 cells contained no virus, a few neuronal processes which were in contact with viral syncytia contained detectable amounts of the nucleocapsid, H, and M proteins. This trend towards increasing numbers of virus-infected neuroepithelial cells and formation and growth of syncytia continued through all of the time points studied. With increasing time postinfection, however, no more than a few synaptophysin-positive hNT2 cells were observed to contain virus, and this was always in the processes which were in close contact with virus-infected neuroepithelial syncytia (Fig. 1f and g). In some examples of the infection of synaptophysin-positive neuronal processes, the virus appeared to have been anterogradely transported down the process, away from the point of contact with the virus-positive neuroepithelial syncytium. To determine if MV RNA was present in neuronal processes, digoxigenin-labeled single-stranded RNA probes to the N gene of MV were prepared as previously described (11). For in situ hybridization, coverslips were pretreated with protease at 0.05 mg/ml in PBS for 10 min at room temperature. Hybridization and immunodetection of the digoxigenin-labeled hybrids were carried out as described previously, by using streptavidin-fluorescein isothiocyanate (FITC) as an end point reporter molecule. On selected coverslips, following in situ hybridization, synaptophysin was detected by immunofluorescence as described above. In situ hybridization for detection of viral genomic RNA at 24, 48, and 72 h postinfection demonstrated viral RNA in isolated neuroepithelial cells or syncytia at all time points. Dual labeling (viral genomic RNA demonstrated by Cy.3, synaptophysin demonstrated by FITC) showed that a few neuronal processes in contact with syncytia did contain viral genomic RNA (Fig. 1h). In situ hybridization for viral genomic RNA was consistently negative on mock-infected cultures of NT2 or hNT2 cells. An ultrastructural search of mixed infected cultures by electron microscopy did not result in detection of cellular contacts containing viral nucleocapsids.
The nonsusceptibility of cultured human neurons (hNT2) to MV virion infection may be due to their lack of expression of CD46, a known cell surface receptor for MV (13) which is differentially expressed on neuronal precursors (NT2), differentiated neurons (hNT2), and neuroepithelial cells.
However, this study demonstrates that hNT2 neurons can apparently become infected via their long cell processes where they have contact with adjacent infected cells. This suggests; therefore, that for human neurons to become infected in this in vitro situation, the virus must pass to them directly from the adjacent infected neuroepithelial cells by a direct intracellular route, most likely involving cell fusion at the points of contact between the two cell types. Such a process may be due to the presence of virus-encoded proteins in the neuroepithelial cell plasma membrane. Whether MV receptors other than CD46 are present at these contact points remains to be determined. The fate of any virus which enters the process of a differentiated neuron is of interest. It is possible that transcription and translation of the viral RNA are attenuated, allowing viral persistence but only low levels of viral protein production. In this context, it is significant that viral RNA has been detected in neurons in parts of the brains of patients with SSPE where immunocytochemical techniques failed to detect viral antigens (1, 8). Furthermore, suppressed viral replication has been demonstrated in neuronal cell lines when cellular conditions were experimentally modified by increasing endogenous cyclic AMP with papaverine treatment (12, 21).
In view of the number of elongated cell processes and specialized intercellular contacts in the CNS involving both neurons and glia, such a mechanism of cell-to-cell virus transfer may be involved in the spread of MV infection in diseases such as SSPE, in which production of infectious virus particles is known to be low or absent (3). Relatively few hNT2 neurons become infected in mixed cultures, and the process is dependent on the presence of a sufficient proportion of neuroepithelial cells. In experiments in which highly purified cultures of differentiated hNT2 cells (with very few neuroepithelial cells) growing in flasks (Fig. 1i) or on coverslips were exposed to virus, no viral infection was observed at any time point postinfection. Such an apparently inefficient mechanism of neuronal infection may also help to explain why SSPE occurs months or years after acute measles. Relatively few neurons may initially become infected, and the virus may persist in these cells undetected by immune control. However, the nature of the factors which control virus spread and disease onset after these prolonged periods of time remains unknown.
Acknowledgments
We thank Roy Creighton for expert assistance with the preparation of the composite photographic plates.
REFERENCES
- 1.Allen I V, McQuaid S, McMahon J, Kirk J, McConnell R. The significance of measles virus antigen and genome distribution in the CNS in SSPE for mechanisms of viral spread and demyelination. J Neuropathol Exp Neurol. 1996;55:471–480. doi: 10.1097/00005072-199604000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Andrews P W, Damjanov I, Simon D, Banting G, Carlin C, Dracopoli N C, Fogh J. Pluripotent embryonal carcinoma clones derived from the human teratocarcinoma cell line Tera-2: differentiation in vivo and in vitro. Lab Invest. 1984;50:147–162. [PubMed] [Google Scholar]
- 3.Billeter M A, Cattaneo R. Mutations and A/1 hypermutations in measles virus persistent infections. In: Kinsbury D, editor. The paramyxoviruses. New York, N.Y: Plenum; 1991. pp. 323–345. [Google Scholar]
- 4.Connolly J H, Allen I V, Hurwitz L J, Millar J H D. Subacute sclerosing panencephalitis: clinical, pathological, epidemiological, and virological findings in three patients. Q J Med. 1968;37:625–644. [PubMed] [Google Scholar]
- 5.Cosby, S. L., and S. Galbraith. Unpublished data.
- 6.Cosby S L, McQuaid S, Duffy N, Lyons C, Rima B K, Allan G M, McCullough S J, Kennedy S, Smyth J A, McNeilly F, Craig C, Orvell C. Characterization of a seal morbillivirus. Nature. 1988;336:115–116. [Google Scholar]
- 7.Esiri M M, Oppenheimer D R, Brownell B, Haire M. Distribution of measles antigen and immunoglobulin-containing cells in the CNS in subacute sclerosing panencephalitis (SSPE) and atypical measles encephalitis. J Neurol Sci. 1981;53:29–43. doi: 10.1016/0022-510x(82)90078-8. [DOI] [PubMed] [Google Scholar]
- 8.Haase A T, Swoveland P, Stowring L, Ventura P, Johnson K P, Norrby E, Gibbs C J., Jr Measles virus genome in infections of the central nervous system. J Infect Dis. 1981;144:154–160. doi: 10.1093/infdis/144.2.154. [DOI] [PubMed] [Google Scholar]
- 9.Kraus E, Schneider-Schaulies S, Miyasaka M, Tamatani T, Sedgewick J. Augmentation of major histocompatibility complex class I and ICAM expression on glial cells following measles virus infection: evidence for the role of type-1 interferon. Eur J Immunol. 1992;22:175–182. doi: 10.1002/eji.1830220126. [DOI] [PubMed] [Google Scholar]
- 10.McCormick D, Wallace I, Kirk J, Dinsmore S, Allen I. The establishment and characterization of a cell line and mouse xenografts from a human malignant melanoma. Br J Exp Pathol. 1983;64:103–115. [PMC free article] [PubMed] [Google Scholar]
- 11.McQuaid S, McMahon J, Allen G M. A comparative study of digoxigenin and biotin labeled DNA and RNA probes for in situ hybridization. Biotech Histochem. 1995;70:147–154. doi: 10.3109/10520299509108331. [DOI] [PubMed] [Google Scholar]
- 12.Miller C A, Carrigan D R. Reversible repression and activation of measles virus infection in neural cells. Proc Natl Acad Sci USA. 1982;79:1629–1633. doi: 10.1073/pnas.79.5.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naniche D, Wild T F, Babourdin-Combe C, Gerlier D. A monoclonal antibody recognizes a human cell surface glycoprotein involved in measles virus binding. J Gen Virol. 1992;73:2617–2624. doi: 10.1099/0022-1317-73-10-2617. [DOI] [PubMed] [Google Scholar]
- 14.Pleasure S J, Page C, Lee V M-Y. Pure, postmitotic, polarized human neurons derived from NTera 2 cells provide a system for expressing exogenous proteins in terminally differentiated neurons. J Neurosci. 1992;12:1802–1815. doi: 10.1523/JNEUROSCI.12-05-01802.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pleasure S J, Lee V M-Y. NTera cells: a human cell line which displays characteristics expected of a human committed neuronal progenitor cell. J Neurosci Res. 1993;35:585–602. doi: 10.1002/jnr.490350603. [DOI] [PubMed] [Google Scholar]
- 16.Rataul S M, Hirano A, Wong T C. Irreversible modification of measles virus RNA in vitro by nuclear RNA-unwinding activity in human neuroblastoma cells. J Virol. 1992;66:1769–1773. doi: 10.1128/jvi.66.3.1769-1773.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schneider-Schaulies S, ter Meulen V. Molecular aspects of measles virus induced central nervous system diseases. In: Roos R P, editor. Molecular neurovirology. Clifton, N.J: Humana; 1992. pp. 419–449. [Google Scholar]
- 18.Schneider-Schaulies S, Schneider-Schaulies J, Bayer M, Loffler S, ter Meulen V. Spontaneous and differentiation-dependent regulation of measles virus gene expression in human glial cells. J Virol. 1993;67:3375–3383. doi: 10.1128/jvi.67.6.3375-3383.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schneider-Schaulies S, Schneider-Schaulies J, ter Meulen V. Differential induction of cytokines by primary or persistent measles virus infections in human glial cells. Virology. 1993;195:219–228. doi: 10.1006/viro.1993.1363. [DOI] [PubMed] [Google Scholar]
- 20.Ter Meulen V, Stephenson J R, Kreth H W. Subacute sclerosing panencephalitis. Comp Virol. 1983;18:105–159. [Google Scholar]
- 21.Yoshikawa Y, Yamanouchi K. Effects of papaverine treatment on replication of measles virus in human neural and nonneural cells. J Virol. 1984;50:489–496. doi: 10.1128/jvi.50.2.489-496.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]