Abstract
Simple Summary
Zoonoses, particularly foodborne zoonoses, are a major problem worldwide, jeopardizing human and animal health and negatively impacting other aspects such as the economy, food systems, and human and animal wellness. This review focuses on the control of the foodborne pathogen Salmonella in pigs and pork, revising past and ongoing control programs, the control options in the food chain, and the impact of policies implemented worldwide. The objective is to take a closer look at the efforts made in the last 2–3 decades in Salmonella control in pigs, evidencing their strengths, pitfalls, and limitations and determining future directions for the efficient surveillance and control of this pathogen in the future.
Abstract
Pork is among the major sources of human salmonellosis in developed countries. Since the 1990s, different surveys and cross-sectional studies, both national and international (i.e., the baseline studies performed in the European Union), have revealed and confirmed the widespread non-typhoidal Salmonella serotypes in pigs. A number of countries have implemented control programs with different approaches and degrees of success. The efforts could be implemented either at farms, in post-harvest stages, or both. The current review revises the current state of the art in Salmonella in swine, the control programs ongoing or conducted in the past, and their strengths and failures, with particular attention to the weight of pre- and post-harvest control and the implications that both have for the success of interventions or mitigation after outbreaks. This review provides a novel perspective on Salmonella control in swine, a matter that still includes uncertainties and room for improvement as a question of public health and One Health.
Keywords: pig, zoonosis, public health, foodborne pathogen, mitigation
1. Introduction Scope and Aim of This Review
As a pathogen, Salmonella is a genus in which species and serovars have evolved to colonize different hosts and environments [1]. Among the serovars included in Salmonella enterica, subspecies enterica, a few have adapted to specific hosts, causing severe disease. For instance, host-specific serovars include Salmonella Typhi and S. Paratyphi in humans and the serovar S. Cholerasuis in pigs [2,3,4]. In contrast to these host-adapted serovars, the vast majority of Salmonella serovars have chosen a different evolutionary strategy, allowing them to colonize a wide range of hosts, including warm- and cold-blooded species. They are generically known as non-typhoidal serovars, a definition that refers to their tendency to restrict colonization to the intestine without causing systemic disease in the host, unlike the aforementioned serovars [3].
Over the last half-century, non-typhoidal serovars have successfully spread into food production animals, particularly in pig and poultry production. Industrialized production systems with high stock densities, coupled with subclinical outcomes of infection characterized by large pathogen shedding in feces with mild or no pathogenic signs, have facilitated the spread of Salmonella in poultry, turkey, pig, and calf productions [5,6,7,8,9].
Consequently, Salmonella is a major foodborne pathogen, causing approximately 150 million illnesses and 60,000 deaths globally each year [10]. Major sources of Salmonella infection include chicken, turkey meat, eggs, pork, and derived products [11,12]. Human salmonellosis, attributed to pork consumption, ranks among the most highly reported foodborne illnesses [12]. Since the 1990s, many countries have implemented control and surveillance programs aimed at reducing the risk of Salmonella transmission by pork into the food chain [13,14,15,16]. These programs target Salmonella control at different production stages, including feed mills, farms, and post-harvest control. Each strategy or approach has its strengths and shortcomings, leading to different perspectives among the main actors involved in pathogen control. This review aims to bring the readers closer to the control of Salmonella in pig production tackling aspects of the origin of control programs, their progression or even discontinuation, main aspects for pre and post-harvest control and legal aspects of Salmonella outbreaks in humans in different countries. This review provides a novel perspective on reviews focusing on Salmonella control in swine, an issue that still includes uncertainties and room for improvement in public health and One Health.
2. Identifying Relevant Literature
This study did not apply a systematic review or meta-analysis. Instead, the relevant literature on the different topics covered in the review was searched using different scientific databases (www.pubmed.com; accessed the last ie on 20 January 2024; www.sciencedirect.com; accessed the last ie on 20 January 2024) and the website search tool (www.google.es; accessed the last ie on 20 January 2024). Relevant scientific papers published in peer-reviewed English journals were identified using the following keyword combinations: (pig OR Salmonella OR farm OR slaughterhouse OR control OR program) AND (livestock OR swine OR farrow OR weaner OR finisher OR sow OR carcass) AND (acid OR vaccine OR antimic* OR HACCP, OR risk), among others. The search did not have any restriction on dates, so any document in the databases to date was searched to capture up-to-date data. To ensure a wide range of articles from different sources, additional searches were conducted using the reference lists of key articles.
3. Salmonella Control in Swine Production: Origins
Salmonella control in swine production was initiated in the 1990s and early 2000s by the establishment of national initiatives to reduce the burden of this pathogen in the food chain, encouraged in part by the results of programs in poultry production.
The pioneering program was the Danish Salmonella control program, established in 1993, with the development and implementation of a surveillance program for Danish pork and for slaughter pig herds in 1995 [13]. The program was a response to human cases of salmonellosis linked to pork, with a peak incidence in 1993 [17,18]. Other programs in Europe followed the Danish initiative. Chronologically, Ireland established a surveillance program for pig herds [14] in 1997, which remains in force today [19]. The German Salmonella Monitoring Program was established in 2002 through the German Quality Assurance System for the food chain, the so-called “QS-System” [20,21]. In the same year, the pig industry in the UK launched the Zoonosis Action Plan (ZAP) to categorize pig herds based on their Salmonella prevalence. The program was revised in 2008 under a new name, the “Zoonoses Control Program” [16], and was finally suspended in 2012 [22]. Finally, the Netherlands and Belgium established their respective programs in 2005. In the Netherlands, compulsory Salmonella monitoring in fattening pigs was initiated by the Product Boards for Livestock, Meat, and Eggs, whereas the Belgian program [23], named the National Salmonella Action Plan (SAP), originally targeted herds with more than 30 pigs and has since changed several times, currently operating on a voluntary basis [24]. For further information about control programs in European countries, specific reviews are accessible elsewhere [25].
Outside of European countries, few have undertaken actions to mitigate the pathogen in primary production. Despite the extensive literature on Salmonella epidemiology in Asian countries [26,27,28] and North and South American regions [29,30,31,32], only specific actions have been implemented in the United States [33]. In this country, the Food Safety and Inspection Service (FSIS) published a final rule on pathogen reduction (PR) and hazard analysis and critical control point (HACCP) systems in 1996. The final rule required meat and poultry establishments under federal inspection to take responsibility for preventing and reducing physical, chemical, and biological hazards throughout the food production process by implementing a system of science-based preventive controls, known as HACCP. Establishments must have an effective HACCP food safety system to comply with regulatory requirements, focusing on controlling hazards to prevent product adulteration. This approach relies on post-harvest decontamination for Salmonella control, with no surveillance in primary production. Undoubtedly, the approach taken to perform Salmonella control impacts the results and approaches to dealing with the pathogen, factors which will be introduced and discussed in subsequent sections.
4. Rationale of Salmonella Surveillance in Control Programs
Salmonella control can be approached from various perspectives or strategies, all valid but each with its weaknesses. Not previously mentioned, the most successful strategy, followed in Sweden, Norway, and Finland, is based on pre-harvest surveillance programs combined with an eradication strategy [34,35,36]. This strategy maintains Salmonella prevalence at 0% by identifying infected animals that are then condemned. However, this ideal strategy is only feasible when sporadic infections in production animals occur. In countries with a significant prevalence or larger production, characterized by higher movement and pig imports, this approach would fail, and the cost of eradication would be impractical. Therefore, in countries with a non-negligible herd and within-herd prevalence of Salmonella, more realistic options are needed to mitigate the risk of human infections.
All other European control programs rely on farm categorization based on serological Salmonella surveillance (Table 1). This on-farm categorization is based on the presence and quantification of antibodies against Salmonella-LPS, which demonstrate the on-farm contact between the pathogen and the animal [37]. Serology through Enzyme-Linked Immunosorbent Assays (ELISA) has been the technique of choice for several reasons. Firstly, it is less expensive than microbiological methods, can be easily automated, and Salmonella antibodies persist for long periods, overcoming the intermittent fecal shedding that may lead to false-negative results. Additionally, samples can be easily collected at the slaughterhouse (either sampling blood or meat juice samples). Thus, this technique has become the gold standard for Salmonella monitoring, with only the Danish control program conducting complementary bacteriological analyses [18].
Table 1.
Summary of surveillance programs in place since the 1990s in Salmonella control in pigs.
| Program (by Country) | Status | Farm Monitoring | Carcass Monitoring | Penalties | Demonstrated Impact | References |
|---|---|---|---|---|---|---|
| Denmark | Ongoing | Yes | Yes | Yes | Yes | [13,17,18] |
| Germany | Ongoing, voluntarily | Yes | No | Yes | No | [15,21] |
| Ireland | Ongoing, under animal health program | Yes | No | No | No | [14] |
| United Kingdom | Discontinued | Yes | No | No | No | [16,22] |
| Belgium | Ongoing, voluntarily | Yes | No | No | No | [24] |
| The Netherlands | Ongoing | Yes | No | No | No | [23] |
Despite herd surveillance and on-farm categorization based on Salmonella indirect burden estimation, not all programs act based on prevalence reduction or penalty systems considering their Salmonella serological results (Table 1). Indeed, only the Danish and German programs have established penalty systems that devalue carcass refunds from animals from infected farms [18,25]. Furthermore, some programs (such as in Denmark) also monitor carcass prevalence. Danish prevalence targets are based on carcass prevalence rather than herd prevalence [18]. Salmonella carcass prevalence provides complementary information to serological surveillance on the farm, reflecting the potential introduction of Salmonella into the food chain (cutting plants and retailers). Moreover, this relevant information can be used to assess the effectiveness of interventions taken to reduce prevalence, not only on the farm but also in post-harvest stages [38]. Carcass monitoring is also performed in EU countries, regardless if they have a control program or not, by the Competent Authorities and Food Business Operators, either by official requirements or internal audits. Although this carcass monitoring is not part of the control programs, the results may help to inform about the success of the interventions put in place. More information about carcass contamination and results from scientific studies can be found in specific reviews on the topic [38,39]. The national control programs currently underway do not establish penalties for either the supplier (farm) or processor (abattoir) associated with carcass prevalence, although purchasers or importers may establish regulations that include the absence of Salmonella in the products, thus establishing an indirect firewall against Salmonella-contaminated pork.
The aim of these programs is not just limited to detecting Salmonella carriers in the food chain but also to reducing the Salmonella burden over time. Have the current control programs and their policies achieved any reduction? This question will be discussed in the next section.
5. Outcomes of over Two Decades of Salmonella Surveillance
The ongoing programs mentioned above imply a significant economic investment and efforts by public bodies involved in official control, as well as industries, practitioners, and farmers participating in the surveillance. Indirectly, concern about Salmonella also generates costs in research about Salmonella etiology, epidemiology, and control. With this information in hand, the question to be asked is probably “is all this effort worthwhile?”.
A case study published by Alban and colleagues in 2012 [18] described the lessons learned in the Danish Salmonella surveillance and control program for pigs and pork. In 2001, its prevalence in pork produced by members of the Danish Agriculture & Food Council was 1.7%, according to that study. A decade later, in 2011 and onwards, carcass prevalence ranged between 1 and 1.4% [40]. According to the authors of the last reference, in 2015, 24 years after the beginning of the program, the Danish Salmonella program reduced the number of human cases associated with Danish pork by more than 95% [40]. Undoubtedly, these efforts show figures that highlight the benefits of the interventions. But what are the costs? According to Danish researchers, the initial expenses of the program were approximately EUR 13 million per year. Adjusting the program, costs were significantly reduced to approximately EUR 3 million per year. To be a pioneer implies risks and failures. Not everything in the Danish program is a successful story, and some interventions or decisions did not provide the desired results. Between 1996 and 2010, an eradication policy was pursued for a highly concerning strain from a human health perspective, the multi-resistant Salmonella Typhimurium DT104 [41]. The plan did not achieve the established targets, and the total expenses spent were enormous, reaching more than EUR 14 million [18]. The nature of the design made Salmonella TyphimuriumDT104 vanish from pig farms in the decade of the 2010s, replaced by other emerging strains [42,43].
The demonstrated success of the Danish control program, both in carcass prevalence and human-related cases, contrasts with the partial figures reported by other European control programs. The ZAP program in the UK, as mentioned above, was discontinued in 2015. The German control program aimed at reducing herds with high seroprevalence of Salmonella. According to data provided by Blaha and colleagues [44], although herds falling into the highest level category of Salmonella prevalence were able to reduce prevalence figures, thus lowering their intra-herd prevalence of Salmonella-antibody positive pigs and herds with mild or low prevalence used to increase in prevalence. As a result, the overall national frequency of Salmonella-antibody-positive pigs supplied to the slaughter plants did not show any sort of improvement [45]. Other programs such as the Irish or Belgian programs also did not observe any improvement in Salmonella figures [21]. Consequently, the Irish NSCP is under review by the Pig HealthCheck program of Animal Health Ireland [25].
Why have these programs failed? Probably, the question requires a complex analysis, but several reasons can be identified as major motives. Firstly, as mentioned above, different programs such as the Belgian, Irish, or Dutch do not include penalties for high-prevalence herds. Without that penalty, the motivation to reduce on-farm prevalence may not be as crucial. A recent study performed in Denmark observed differences among farmers’ perceptions of costs and actions toward Salmonella control, which might hamper the effectiveness of the penalty scheme as a regulatory instrument to influence farmers’ behavior [46].
Other potential challenges that may hamper the interventions taken could be related to the complex control of the pathogen [47,48,49,50], as well as human factors [51]. For instance, Blaha identified a “lean-back” attitude in farmers that succeeded in achieving a reduction in Salmonella prevalence, linking low prevalence serology with Salmonella-free herds, while the pathogen was still circulating there. That relaxation in the interventions taken to reduce Salmonella prevalence ended in an increase in prevalence finally [44]. A similar idea is reflected by Marier and colleagues in a study performed in the UK [52]. This study confirmed that farmers accepted their responsibility for controlling Salmonella in pork, even though their confidence in their ability to control Salmonella decreased over time, and believed that responsibility should be shared with the rest of the production chain, a fact that matches the perception of German studies [45].
The control of the pathogen seems to be complex according to the information gained so far in this article. However, complexity is not synonymous with impossibility. The next sections summarize the experience gained in Salmonella control both on farms and in post-farm stages.
6. Salmonella Control in Pig Production
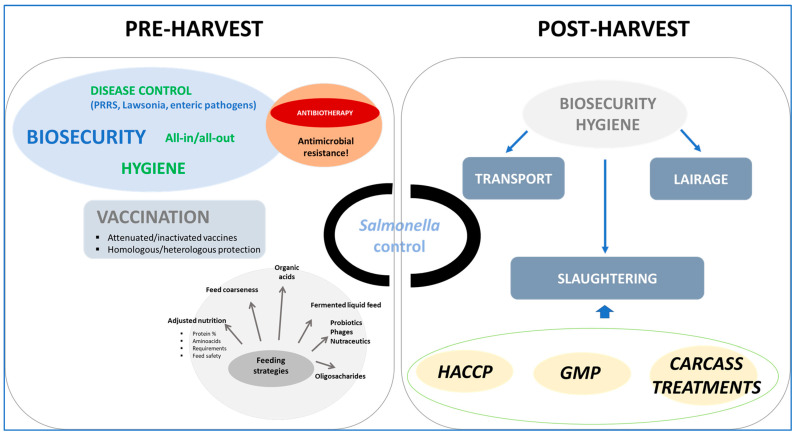
To assist surveillance programs in pig production, actions must be taken to mitigate Salmonella risk throughout the food chain. Salmonella mitigation strategies can be approached from different angles, meaning different production stages. Each intervention will have benefits but also weaknesses and risks of reinfection or recontamination. In this subsection, we aim to discuss the various alternatives available for Salmonella control (Figure 1). A thorough review of potential interventions at each production stage is not the objective of this review. Detailed and extensive reviews on this topic can be found elsewhere [39,44,47,48,51,52,53,54,55,56,57]. Nonetheless, several notes about the two main stages of pathogen control will be described in the subsequent paragraphs. This information will allow us to discuss the impact of on-farm and post-harvest control strategies for the control programs and the perception of the actors involved in Salmonella control.
Figure 1.
Summary of Salmonella control options in pre-harvest and post-harvest swine production.
6.1. The Control of Salmonella on the Farm
The study of control options to mitigate Salmonella prevalence and infection transmission on swine farms has been and is the subject of scientific research. Numerous published scientific peer-reviewed articles have evaluated in vivo control strategies or risk factors for Salmonella prevalence on swine farms, all aiming to extend the current knowledge in Salmonella control and identify tools and strategies to mitigate the risk of infection or the burden of this pathogen in live pigs. Despite the extensive research, variability in results is common, thus not providing absolute certainty about the formula to mitigate Salmonella on the farm.
In 2015, the FAO published a systematic review of Salmonella control in beef and pork, covering different steps in the food chain [58]. The FAO book report performed a thorough review of the options to control the pathogen on farms. Among all the interventions mentioned, experts highlighted several as the most efficient or useful. Biosecurity was highlighted as a good farm practice limiting the likelihood of pathogen introduction on the farm. Several studies have demonstrated that improved on-farm biosecurity limits the risk of on-farm Salmonella prevalence [59,60,61]. It seems logical to think that it is more plausible to introduce Salmonella by “four legs” than other potential sources such as feed, wild animals, or through water or semen. Nonetheless, all can be prevented with appropriate measures of external biosecurity on the farm, particularly restrictive policies for vehicles and visitors who come into contact with other herds. Measures of internal biosecurity, meaning limiting the transmission or circulation of the pathogen within the farm, seem to be less efficient than external biosecurity measures, probably due to lower or poorer internal biosecurity [32,62]. All-in/all-out husbandry, which includes emptying and cleaning barns and disinfection of facilities before introducing new animals, is the most useful tool to break infection cycles between animal batches [63]. Despite this activity being common among herds, risk factor studies have not demonstrated any clear benefit for Salmonella mitigation when this intervention is applied [59,64,65]. Different aspects like inefficient cleaning or commingling of animals from different batches may be behind the lack of benefits [66]. Thus, while introducing the pathogen on a farm can be prevented, once inside, breaking transmission is highly difficult.
The FAO document also mentions other interventions associated with feed or vaccination [58]. Feed serves as a vehicle for different strategies to mitigate Salmonella on the farm. Feed form [49,67,68], feed coarseness [69], feed additives [70,71,72], or probiotics administered through feed [73] have demonstrated their potential beneficial effect either by impacting the pathogen directly [74] or by improving intestinal health, thus limiting the opportunity for Salmonella to colonize the gut. Apart from the mentioned strategies, only vaccination is a strategy that can be performed on a large scale [58]. Again, different vaccines have been tested using different vaccination approaches [75,76,77,78]. Salmonella vaccines are not among the commonly used vaccines on farms, probably for different reasons; firstly, because they are not available in all countries, and secondly, because their efficacy is limited to the serotype infecting the animal [77]. While countries with serological surveillance may be reluctant to the use of vaccination due to interference with serological tests, they are extensively used in countries with clinical problems of salmonellosis like the USA (Fernando Leite, personal communication). Current policies to reduce antimicrobial use, together with the therapeutic use of ZnO in the EU [79], may be a reason to increase the use of vaccines in Salmonella control.
Apart from these interventions, other options are currently at a lower technology readiness level. For instance, phages, new nutraceuticals, or antimicrobial peptides are strategies with potential but with low field applicability right now [80,81,82]. There is not a magic formula for Salmonella control on the farm. Indeed, none of the options mentioned in this section exhibits enough robustness to successfully control the pathogen on its own. Controlling Salmonella on farms involves a combination of strategies, and their efficacy can vary based on farm-specific factors, management practices, and the Salmonella serotype involved. In a recent study evaluating factors associated with high and low seroprevalence on Irish farms, based on surveillance data from the National Control program, we demonstrated that low prevalence herds were related to farms in which aspects associated with biosecurity, feed coarseness, and herd health influenced the on-farm Salmonella prevalence [49].
In summary, a comprehensive and multifaceted approach, incorporating various control measures, is most effective in managing Salmonella on farms. The combination of biosecurity, sanitation, water and feed management, pest control, vaccination, monitoring, and employee training is key to reducing the prevalence and impact of Salmonella infections on pig farms.
6.2. Salmonella on Post Farm Stages, Epidemiology and Control
Post-harvest is the term that defines the production stages after the farm and which include transport of the animals to the slaughterhouse, their resting, slaughter and carcass fabrication and the latter stages of pork meat processing at the cutting plants and retailers. Again, the number of studies that have focused their aims on disclosing information about the dynamics of Salmonella in these stages and the potential mitigation strategies to be put in place is almost overwhelming. If readers want to extend their knowledge about Salmonella and post-harvest in pig production, we recommend a few review studies which go into depth into this topic [39,56,83,84,85].
There are a few studies that exemplify what happens in the Salmonella epidemiology after the farm. In 2001, Hurd and colleagues [86] performed a study in which 50 pigs were slaughtered on the farm and another 567 market-weight pigs were transported (mean distance, 169 km) to the slaughterhouse with 2 h to 3 h of holding in ante-mortem pens before slaughtering, as usual. Lymph nodes from both groups were collected and Salmonella prevalence was determined by microbiological detection of the pathogen. Interestingly, the prevalence at the slaughterhouse was five times higher than on the farm. In 2013, the European Food Safety Agency (EFSA) published the results of the analysis performed on carcasses from finisher pigs, as part of the cross-sectional study performed in member states to determine the basal prevalence of Salmonella in slaughter pigs [87]. From the results and conclusions obtained in the study, it is noteworthy to mention here that carcass prevalence was positively associated with on-farm prevalence, i.e., the higher the prevalence on the farm, the larger the number of contaminated carcasses detected. However, when farms with similar prevalence supplied different abattoirs, differences in carcass prevalence were observed among slaughterhouses. The third study to mention is a study in which the authors aimed at organizing the slaughtering by means of on-farm seroprevalence [88]. First, pigs from low prevalence farms were slaughtered followed by farms with high prevalence. Unexpectedly, when carcass prevalence was analyzed, a higher prevalence was obtained in carcasses from Salmonella-free and low Salmonella seroprevalence herds compared to high seroprevalence herds. Further typing of Salmonella isolates obtained from concomitant samples collected in the slaughterhouse environment revealed the link between carcasses from Salmonella-free herds and lairage environment, thus revealing new infections or contamination occurring in the slaughterhouse facilities.
We have chosen these three examples as they exemplify with accuracy the role of post-harvest in Salmonella epidemiology and control after the farm. The study of Hurd and colleagues, supported by results from subsequent studies [89,90,91,92], clearly highlights the potential hotspots in Salmonella new infections or re-infections after the farm. Indeed, the studies referenced and others [39] point out both transport and lairage as hotspots where new infections can occur and also where re-activation of infections can happen. The arrival of pigs from infected herds which spread Salmonella in these environments, sometimes in large concentrations, together with the rapid infection onset and fecal shedding in infected animals [93], explains the results observed by Hurd and colleagues in their study performed two decades ago. Is this information asserting that post-harvest is more important in Salmonella control than the on-farm prevalence?
The baseline Salmonella prevalence study performed in the EU and already mentioned [87,94] is the largest study performed so far, all over the world, involving a total of 19,159 slaughter pigs with validated results from 26 countries and monitored 5736 carcass swab samples in 146 slaughterhouses (from 13 Member States). The weighted prevalence of Salmonella contamination of carcasses was greater for slaughter pigs with Salmonella infection in lymph nodes compared to the pigs with un-infected lymph nodes. This result strongly evidences the link between on-farm status and carcass contamination risk. Nevertheless, when carcass contamination was compared in slaughterhouses with similar inputs of infected pig lymph nodes, differences were also observed, a fact which was repeated by other contemporary studies involving different slaughterhouses [89,92,95,96,97]. The result demonstrates that the impact of Salmonella inputs by infected animals in carcass contamination varies among establishments, indisputably associated with hygiene in the carcass fabrication.
And what about the third and last example? Well, on the one hand, it demonstrates that if we fail in Salmonella control in post-harvest, non-infected pigs become positive, but also that correct carcass processing, from a hygienic perspective, can mitigate external carcass contamination, even in highly infected batches.
The preceding section provided a brief overview of various control options that can be implemented on farms. While interventions on farms lack standardization, slaughterhouses have adhered to a standardized procedure for decades to uphold hygiene and ensure meat safety through the hazard analysis critical control points (HACCP) system. This system serves as a framework for monitoring the food system, aimed at reducing the risk of foodborne illness. Different points in the slaughter process or carcass fabrication can be incorporated into the HACCP plan of the abattoir. The control of endemic bacteria, including Salmonella can be performed through proper cleaning and disinfection with good manufacturing practices rules. According to Borch and colleagues [98], the following affiliation to CCPs made for specific steps during slaughter and dressing may serve as a guidance: (i) lairage (CP), (ii) killing (CP), (iii) scalding (CP), (iv) dehairing (CP), (v) singeing/flaming (CP), (vi) polishing (CP), (vii) circumoral incision and removal of the intestines (CCP), (viii) excision of the tongue, pharynx, and in particular the tonsils (CCP), (ix) splitting (CP), (x) post mortem inspection procedures (CCP) and (xi) deboning of the head (CCP). Most, if not all, of these CCPs have been highlighted by Salmonella post-harvest studies, but the most important CCPs associated with Salmonella contamination and spread, both between carcasses and within the slaughter environment, include carcass singeing, carcass scalding (considering water temperature and duration of scalding), carcass inspection (to detect the presence of fecal material), lacerations at evisceration [39,58] or carcass chilling [99,100]. Additionally, strategies such as hot water showers [18] and showers with antibacterial compounds (e.g., organic acids or disinfectants like peroxides) aid in mitigating carcass contamination at the conclusion of the fabrication process [48,101]. Individual plans in the slaughterhouse should identify and correct their CCPs and guarantee that strategies that help in Salmonella mitigation (singeing, potential hot washing or chilling) are run accordingly.
Apart from the strategies integrated into the HACCP plan, other measures contributing to Salmonella control involve enhancing hygiene in the slaughter process, encompassing both the environment and equipment. Moreover, mitigating the risk of cross-contamination during human carcass dressing activities is imperative, emphasizing strict hygiene practices such as tool sterilization (e.g., knives) and frequent glove changes [39,48]. Furthermore, interventions targeting the processing stage should be complemented by actions to limit the risk of new infections during transport and lairage, as discussed in previous reviews [39]. Enhancing cleaning protocols in both transport and lairage [64] and restricting transport duration or distance and minimizing lairage resting time are well-known practices effective in reducing Salmonella transmission risk.
7. Salmonella Control and Legal Aspects
The main objective of Salmonella control in livestock species is mitigating the risk for humans. The current legislation, actions taken or lack thereof have consequences in the aim just exposed. For the first time in this review, let us talk about Salmonella control in poultry within the EU. There are currently compulsory programs to control the bacteria in chickens, laying hens and breeders both in poultry and turkey meat production [102]. These control programs include microbiological samplings with Salmonella isolation, a fact that together with the zoonoses monitoring in humans [103] offers accurate information on the serotypes and strains circulating both in humans and animals and their associations. In the hypothetical case of human outbreaks, molecular typing methods allow us to trace back the case to the infection source [104,105]. The lack of bacteriological analyses in control programs, or even worse, the lack of control programs and involvement of some of the production stages in the actions taken to control Salmonella in swine, hampers clarifying and effectively cutting the outbreaks from swine origin. As an example, in 2015 and 2016, two outbreaks of the emerging monophasic variant of Salmonella Typhimurium occurred in the state of Washington in the United States [106,107]. Both outbreaks occurred by the consumption of roasted infected pork and involved over 40 human infections with hospitalizations [107]. According to the scientist involved in the investigations of the first outbreak in 2015 “Our investigation could not determine the relative importance of specific points in the pork production process that contributed to this outbreak” and “we were unable to assess practices or conduct environmental or animal testing at establishment A’s source farms because farms were reluctant to participate, and unclear jurisdictional authority of state agriculture agencies did not require farms to comply with our request”. Indeed, Salmonellosis is not listed as an animal reportable disease in the US despite its proven importance, which can involve large morbidity and relevant mortality rates in humans [108], a fact that limits potential investigations in live animals. Another recent outbreak in another country with no control program does not offer information about the source of infection either [109]. While in the references provided above [104,105], outbreaks could be traced back, the lack of official involvement of producers in official Salmonella surveillance and control in pigs [106,109] was an obstacle to disclosing the origin of both outbreaks. Furthermore, in the outbreak detected in the US, an investigation could prevent a second outbreak with pigs from the same origin occurring in 2016. The examples provided here clearly point out the importance of control programs and the involvement of all production stages in the mitigation of the first foodborne zoonosis worldwide [110]. When authorities excuse any of the actors from the Salmonella equation, for instance, primary production on the farm, despite their relevance in the equation, demonstrated by the information gathered here or elsewhere [47,48,51,56], commitment in the mitigation of the human risk fails, as pointed out by Kawami and colleagues [106].
8. Conclusions
Over the last 20 years, actions have been put in place to mitigate the main foodborne zoonosis in industrialized countries, which, together with antimicrobial resistance and slurry environmental pollution, are the major One Health problems to be tackled by the swine industry in the forthcoming years. The European Union led the initiative with consolidated compulsory programs in avian species which have ended up in half of the human Salmonellosis reported two decades ago. The success and progress in avian production contrast with the slow implementation of actions in the pig industry. A higher complexity of production systems, infection epidemiology, and lack of clear strategies to mitigate Salmonella have discouraged its control, with countries that have not implemented any control and others that have discontinued it. Unfortunately, outside the EU, there is not any leading country in Salmonella mitigation in pigs and pork, despite the frequent outbreaks observed and the evidenced risk for humans. Monitoring and surveillance programs offer valuable information to mitigate the pre-harvest and post-harvest Salmonella hazards. Countries with programs in place should, in our opinion, put in place actions to reduce prevalence both in animals and pork but avoid penalty systems as much as possible, which limit the profitability of the production and envisage a negative reaction to deal with Salmonella but also finding a strategy which does not waste the economic efforts of monitoring and control programs. In addition, the scientific knowledge and experience running control programs acquired through the last decades are useful to re-think and design new, where not in place, efficient and reliable programs to mitigate the pathogen, always considering economic constraints. As stated above, it is highly important to involve and encourage the participation of all the production stages and build awareness of the impact that health actions taken in primary production have on human and environmental health, named One Health.
Acknowledgments
We want to acknowledge the reviewers who participated in the review process. Their ideas im-proved the quality of the manuscript and the information provided here.
Author Contributions
H.A. and M.K. prepared the draft of the scope of the article. A.C., M.K. and H.A. contributed to the information search, discussions, writing and revision of the sections included in this article. H.A. conducted a final review of the information and A.C. prepared the tables and figures. M.K. also revised the English grammar of the article. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
Author Melvin Kramer is CEO in the company EHA consulting group. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Wray C. Mammalian salmonellosis. In: Beran G.W., Steele J.H., editors. Handbook of Zoonoses—Section A: Bacterial, Rickettsial, Chlamydial, and Mycotic. 2nd ed. CRC Press; Boca Raton, FL, USA: 1999. pp. 289–302. [Google Scholar]
- 2.Raffatellu M., Wilson R.P., Winter S.E., Bäumler A.J. Clinical pathogenesis of typhoid fever. J. Infect. Dev. Ctries. 2008;2:260–266. doi: 10.3855/jidc.219. [DOI] [PubMed] [Google Scholar]
- 3.Hiyoshi H., Tiffany C.R., Bronner D.N., Bäumler A.J. Typhoidal Salmonella serovars: Ecological opportunity and the evolution of a new pathovar. FEMS Microbiol. Rev. 2018;42:527–541. doi: 10.1093/femsre/fuy024. [DOI] [PubMed] [Google Scholar]
- 4.Pedersen K., Sørensen G., Löfström C., Leekitcharoenphon P., Nielsen B., Wingstrand A., Aarestrup F.M., Hendriksen R.S., Baggesen D.L. Reappearance of Salmonella serovar Choleraesuis var. Kunzendorf in Danish pig herds. Vet. Microbiol. 2015;176:282–291. doi: 10.1016/j.vetmic.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Wales A.D., Davies R.H. A critical review of Salmonella Typhimurium infection in laying hens. Avian Pathol. 2011;40:429–436. doi: 10.1080/03079457.2011.606799. [DOI] [PubMed] [Google Scholar]
- 6.Porter S., Strain S.A.J., Bagdonaite G., McDowell S.W., Bronckaers T., Sherrey M., Devine P., Pascual-Linaza A.V., Spence N., Porter R., et al. Trends in Salmonella serovars and antimicrobial resistance in pigs and poultry in Northern Ireland between 1997 and 2016. Vet. Rec. 2020;186:156. doi: 10.1136/vr.105640. [DOI] [PubMed] [Google Scholar]
- 7.Papadopoulou C., Davies R.H., Carrique-Mas J.J., Evans S.J. Salmonella serovars and their antimicrobial resistance in British turkey flocks in 1995 to 2006. Avian Pathol. 2009;38:349–357. doi: 10.1080/03079450903183678. [DOI] [PubMed] [Google Scholar]
- 8.Arguello H., Sørensen G., Carvajal A., Baggesen D.L., Rubio P., Pedersen K. Prevalence, serotypes and resistance patterns of Salmonella in Danish pig production. Res. Vet. Sci. 2013;95:334–342. doi: 10.1016/j.rvsc.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Mohler V.L., Izzo M.M., House J.K. Salmonella in calves. Vet. Clin. N. Am. Food Anim. Pract. 2009;25:37–54. doi: 10.1016/j.cvfa.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 10.Nemhauser J.B., editor. CDC Yellow Book 2024. Oxford University press; Oxford, UK: 2023. [Google Scholar]
- 11.Pires S.M., Vieira A.R., Hald T., Cole D. Source attribution of human salmonellosis: An overview of methods and estimates. Foodborne Pathog. Dis. 2014;1:667–676. doi: 10.1089/fpd.2014.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirk M.D., Pires S.M., Black R.E., Caipo M., Crump J.A., Devleesschauwer B., Döpfer D., Fazil A., Fischer-Walker C.L., Hald T., et al. World Health Organization Estimates of the Global and Regional Disease Burden of 22 Foodborne Bacterial, Protozoal, and Viral Diseases, 2010: A Data Synthesis. PLoS Med. 2015;12:e1001921. doi: 10.1371/journal.pmed.1001921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mousing J., Jensen P.T., Halgaard C., Bager F., Feld N., Nielsen B., Nielsen J.P., Bech-Nielsen S. Nation-wide Salmonella enterica surveillance and control in Danish slaughter swine herds. Prev. Vet. Med. 1997;29:247–261. doi: 10.1016/S0167-5877(96)01082-3. [DOI] [PubMed] [Google Scholar]
- 14.Quirke A.M., Leonard N., Kelly G., Egan J., Lynch P.B., Rowe T., Quinn P.J. Prevalence of Salmonella serotypes on pig carcasses from high- and low-risk herds slaughtered in three abattoirs. Berl. Munch. Tierarztl. Wochenschr. 2001;114:360–362. [PubMed] [Google Scholar]
- 15.Osterkorn K., Czerny C.P., Wittkowski G., Huber M. Sampling plan for the establishment of a serologic Salmonella surveillance for slaughter pigs with meat juice ELISA. Berl. Munch. Tierarztl. Wochenschr. 2001;114:30–34. [PubMed] [Google Scholar]
- 16.Snary E.L., Munday D.K., Arnold M.E., Cook A.J. Zoonoses action plan Salmonella monitoring programme: An investigation of the sampling protocol. J. Food Prot. 2010;73:488–494. doi: 10.4315/0362-028X-73.3.488. [DOI] [PubMed] [Google Scholar]
- 17.Baggesen D.L., Wegener H.C., Bager F., Stege H., Christensen J. Herd prevalence of Salmonella enterica infections in Danish slaughter pigs determined by microbiological testing. Prev. Vet. Med. 1996;26:201–213. doi: 10.1016/0167-5877(95)00563-3. [DOI] [Google Scholar]
- 18.Alban L., Baptista F.M., Møgelmose V., Sørensen L.L., Christensen H., Aabo S., Dahl J. Salmonella surveillance and control for finisher pigs and pork in Denmark—A case study. Food Res. Int. 2012;45:656–665. doi: 10.1016/j.foodres.2011.02.050. [DOI] [Google Scholar]
- 19.Deane A., Murphy D., Leonard F.C., Byrne W., Clegg T., Madigan G., Griffin M., Egan J., Prendergast D.M. Prevalence of Salmonella spp. in slaughter pigs and carcasses in Irish abattoirs and their antimicrobial resistance. Ir. Vet. J. 2022;75:4. doi: 10.1186/s13620-022-00211-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blaha T. The present state of the German QS Salmonella monitoring and reduction programme. Dtsch. Tierärztl. Wschr. 2004;111:324–326. [PubMed] [Google Scholar]
- 21.Merle R., Kösters S., May T., Portsch U., Blaha T., Kreienbrock L. Serological Salmonella monitoring in German pig herds: Results of the years 2003–2008. Prev. Vet. Med. 2011;99:229–233. doi: 10.1016/j.prevetmed.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 22.Anonymous. UK: New Direction for Zoonoses National Control Programme (ZNCP) 2012. [(accessed on 7 January 2024)]. Available online: https://www.pigprogress.net/Health-Diseases/Health/2012/6/UK-New-direction-for-Zoonoses-National-Control-Programme-ZNCP-PP008961W/
- 23.Van Der Wolf P.J. Monitoring for Salmonella in Swine in The Netherlands. [(accessed on 7 January 2024)]. Pig333. Available online: https://www.pig333.com/articles/monitoring-for-salmonella-in-swine-in-the-netherlands_12866/
- 24.Méroc E., Strubbe M., Vangroenweghe F., Czaplicki G., Vermeersch K., Hooyberghs J., Van der Stede Y. Evaluation of the Salmonella surveillance program in Belgian pig farms. Prev. Vet. Med. 2012;105:309–314. doi: 10.1016/j.prevetmed.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 25.Correia-Gomes C., Leonard F., Graham D. Description of control programmes for Salmonella in pigs in Europe. Progress to date? J. Food Saf. 2021;41:e12916. doi: 10.1111/jfs.12916. [DOI] [Google Scholar]
- 26.Kishima M., Uchida I., Namimatsu T., Osumi T., Takahashi S., Tanaka K., Aoki H., Matsuura K., Yamamoto K. Nationwide surveillance of Salmonella in the faeces of pigs in Japan. Zonoses Pub. Health. 2008;55:139–144. doi: 10.1111/j.1863-2378.2007.01105.x. [DOI] [PubMed] [Google Scholar]
- 27.Tian Y., Gu D., Wang F., Liu B., Li J., Kang X., Meng C., Jiao X., Pan Z. Prevalence and Characteristics of Salmonella spp. from a Pig Farm in Shanghai, China. Foodborne Pathog. Dis. 2021;18:477–488. doi: 10.1089/fpd.2021.0018. [DOI] [PubMed] [Google Scholar]
- 28.Ntakiyisumba E., Lee S., Won G. Identification of risk profiles for Salmonella prevalence in pig supply chains in South Korea using meta-analysis and a quantitative microbial risk assessment model. Food Res. Int. 2023;170:112999. doi: 10.1016/j.foodres.2023.112999. [DOI] [PubMed] [Google Scholar]
- 29.Haley C.A., Dargatz D.A., Bush E.J., Erdman M.M., Fedorka-Cray P.J. Salmonella prevalence and antimicrobial susceptibility from the National Animal Health Monitoring System Swine 2000 and 2006 studies. J. Food Prot. 2012;75:428–436. doi: 10.4315/0362-028X.JFP-11-363. [DOI] [PubMed] [Google Scholar]
- 30.Sanchez-Maldonado A.F., Aslam M., Service C., Narváez-Bravo C., Avery B.P., Johnson R., Jones T.H. Prevalence and antimicrobial resistance of Salmonella isolated from two pork processing plants in Alberta, Canada. Int. J. Food Microbiol. 2017;241:49–59. doi: 10.1016/j.ijfoodmicro.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Lopes G.V., Pissetti C., da Cruz Payão Pellegrini D., da Silva L.E., Cardoso M. Resistance phenotypes and genotypes of Salmonella enterica subsp. enterica isolates from feed, pigs, and carcasses in Brazil. J. Food. Prot. 2015;78:407–413. doi: 10.4315/0362-028X.JFP-14-274. [DOI] [PubMed] [Google Scholar]
- 32.Vico J.P., Lorenzutti A.M., Zogbi A.P., Aleu G., Sánchez I.C., Caffer M.I., Rosmini M.R., Mainar-Jaime R.C. Prevalence, associated risk factors, and antimicrobial resistance profiles of non-typhoidal Salmonella in large scale swine production in Cordoba, Argentina. Res. Vet. Sci. 2020;130:161–169. doi: 10.1016/j.rvsc.2020.03.003. [DOI] [PubMed] [Google Scholar]
- 33.Anon 2023. FSIS Guideline to Control Salmonella in Swine Slaughter and Pork Processing Establishments June 2023 USDA-FSIS. [(accessed on 8 January 2024)]; Available online: https://www.fsis.usda.gov/contact-us/askFSIS.
- 34.Carlsson U., Elvander M. Domestic and Wild Animals in Sweden. SVA National Veterinary Institute; Uppsala, Sweden: 2008. Surveillance and control programs; pp. 13–17. [Google Scholar]
- 35.Hofshagen M., Nygard K., Hauge K. The Surveillance and Control Programme for Salmonella in Live Animals, Eggs and Meat in Norway—Annual Report. National Veterinary Institute; Oslo, Norway: 2008. 10p [Google Scholar]
- 36.Huttunen A., Johansson T., Kostamo P., Kuronen P., Kuronen H., Laaksonen T. Salmonella Control and Occurence of Salmonella from 1995 to 2004. Finnish Food Safety Authority Evira; Helsinki, Finland: 2006. [(accessed on 8 January 2024)]. Available online: https://www.ruokavirasto.fi/en/about-us/publications/finnish-food-authority-publications/ [Google Scholar]
- 37.Nielsen B., Baggesen D., Bager F., Haugegaard J., Lind P. The serological response to Salmonella serovars typhimurium and infantis in experimentally infected pigs. The time course followed with an indirect anti-LPS ELISA and bacteriological examinations. Vet. Microbiol. 1995;47:205–218. doi: 10.1016/0378-1135(95)00113-1. [DOI] [PubMed] [Google Scholar]
- 38.O’Connor A.M., Wang B., Denagamage T., McKean J. Process mapping the prevalence of Salmonella contamination on pork carcass from slaughter to chilling: A systematic review approach. Foodborne Pathog. Dis. 2012;9:386–395. doi: 10.1089/fpd.2011.1040. [DOI] [PubMed] [Google Scholar]
- 39.Arguello H., Alvarez-Ordoñez A., Carvajal A., Rubio P., Prieto M. Role of slaughtering in Salmonella spreading and control in pork production. J. Food Prot. 2013;76:899–911. doi: 10.4315/0362-028X.JFP-12-404. [DOI] [PubMed] [Google Scholar]
- 40.Salmonella Control in the Pig and Pork Production in Denmark—Lessons Learnt. [(accessed on 20 December 2023)]. Available online: https://www.pig333.com/articles/salmonella-control-in-the-pig-and-pork-production-in-denmark-%E2%80%93-lessons_12329/#:~:text=Salmonella%20control%20in%20the%20pig%20and%20pork%20production%20in%20Denmark%20%E2%80%93%20lessons%20learnt&text=The%20success%20of%20the%20Danish,to%20control%20in%20primary%20production.
- 41.Møgelmose V., Bagger J., Nielsen B., Baggesen D.L. Reduction of multiresistant Salmonella TyphimuriumDT104 in Danish swineherds—New strategy; Proceedings of the 4th International Symposium on the Epidemiology and Control of Salmonella and Other Food Borne Pathogens in Pork; Leipzig, Germany. 2–5 September 2001; pp. 69–71. [Google Scholar]
- 42.Hopkins K.L., de Pinna E., Wain J. Prevalence of Salmonella enterica serovar 4,[5],12:i:- in England and Wales, 2010. Eurosurveillance. 2012;13:20275. doi: 10.2807/ese.17.37.20275-en. [DOI] [PubMed] [Google Scholar]
- 43.Argüello H., Sørensen G., Carvajal A., Baggesen D.L., Rubio P., Pedersen K. Characterization of the emerging Salmonella 4,[5],12:i:- in Danish animal production. Foodborne Pathog. Dis. 2014;11:366–372. doi: 10.1089/fpd.2013.1672. [DOI] [PubMed] [Google Scholar]
- 44.Blaha T. The German Salmonella Serological Monitoring Programme. Pig333. Com. [(accessed on 20 December 2023)]. Available online: https://www.pig333.com/articles/the-german-salmonella-serological-monitoring-programme_12395/
- 45.QS Anzahl Betriebe Mit Erhöhtem Salmonellenrisiko So Gering Wie Noch Nie. 2020. [(accessed on 8 January 2024)]. Available online: https://www.q-s.de/pressemeldungen/anzahl-betriebeerhoehtes-salmonellenrisiko-gering.html.
- 46.Olsen J.V., Christensen T., Jensen J.D. Pig Farmers’ Perceptions of Economic Incentives to Control Salmonella Prevalence at Herd Level. Front. Vet. Sci. 2021;8:647697. doi: 10.3389/fvets.2021.647697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boyen F., Haesebrouck F., Maes D., Van Immerseel F., Ducatelle R., Pasmans F. Non-typhoidal Salmonella infections in pigs: A closer look at epidemiology, pathogenesis and control. Vet. Microbiol. 2008;130:1–19. doi: 10.1016/j.vetmic.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 48.De Busser E.V., De Zutter L., Dewulf J., Houf K., Maes D. Salmonella control in live pigs and at slaughter. Vet. J. 2013;196:20–27. doi: 10.1016/j.tvjl.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 49.Argüello H., Manzanilla E.G., Lynch H., Walia K., Leonard F.C., Egan J., Duffy G., Gardiner G.E., Lawlor P.G. Surveillance Data Highlights Feed Form, Biosecurity, and Disease Control as Significant Factors Associated with Salmonella Infection on Farrow-to-Finish Pig Farms. Front. Microbiol. 2018;9:187. doi: 10.3389/fmicb.2018.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Galán-Relaño Á., Valero Díaz A., Huerta Lorenzo B., Gómez-Gascón L., Mena Rodríguez M.Á., Carrasco Jiménez E., Pérez Rodríguez F., Astorga Márquez R.J. Salmonella and Salmonellosis: An Update on Public Health Implications and Control Strategies. Animals. 2023;13:3666. doi: 10.3390/ani13233666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marier E., Piers Smith R., Ellis-Iversen J., Watson E., Armstrong D., Hogeveen H., Cook A.J. Changes in perceptions and motivators that influence the implementation of on-farm Salmonella control measures by pig farmers in England. Prev. Vet. Med. 2016;133:22–30. doi: 10.1016/j.prevetmed.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 52.Laanen M., Maes D., Hendriksen C., Gelaude P., De Vliegher S., Rosseel Y., Dewulf J. Pig, cattle and poultry farmers with a known interest in research have comparable perspectives on disease prevention and on-farm biosecurity. Prev. Vet. Med. 2014;115:1–9. doi: 10.1016/j.prevetmed.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 53.Berge A.C., Wierup M. Nutritional strategies to combat Salmonella in mono-gastric food animal production. Animal. 2012;6:557–564. doi: 10.1017/S1751731111002217. [DOI] [PubMed] [Google Scholar]
- 54.Wales A.D., Davies R.H. Salmonella Vaccination in Pigs: A Review. Zoonoses Public Health. 2017;64:1–13. doi: 10.1111/zph.12256. [DOI] [PubMed] [Google Scholar]
- 55.de la Cruz M.L., Conrado I., Nault A., Perez A., Dominguez L., Alvarez J. Vaccination as a control strategy against Salmonella infection in pigs: A systematic review and meta-analysis of the literature. Res. Vet. Sci. 2017;114:86–94. doi: 10.1016/j.rvsc.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 56.Rodrigues da Costa M., Pessoa J., Meemken D., Nesbakken T. A Systematic Review on the Effectiveness of Pre-Harvest Meat Safety Interventions in Pig Herds to Control Salmonella and Other Foodborne Pathogens. Microorganisms. 2021;9:1825. doi: 10.3390/microorganisms9091825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Viltrop A., Niine T., Tobias T., Sassu E.L., Bartolo I.D., Pavoni E., Alborali G.L., Burow E., Smith R.P. A Review of Slaughter Practices and Their Effectiveness to Control Microbial Salmonella spp., contamination of Pig Carcasses. J. Food Prot. 2023;86:100171. doi: 10.1016/j.jfp.2023.100171. [DOI] [PubMed] [Google Scholar]
- 58.FAO . Interventions for the Control of Non-Typhoidal Salmonella in Beef and Pork. FAO/WHO; Rome, Italy: 2016. [Google Scholar]
- 59.Beloeil P.A., Chauvin C., Proux K., Fablet C., Madec F., Alioum A. Risk factors for Salmonella seroconversion of fattening pigs in farrow-to-finish herds. Vet. Res. 2007;38:835–848. doi: 10.1051/vetres:2007034. [DOI] [PubMed] [Google Scholar]
- 60.Correia-Gomes C., Mendonça D., Vieira-Pinto M., Niza-Ribeiro J. Risk factors for Salmonella spp. in Portuguese breeding pigs using a multilevel analysis. Prev. Vet. Med. 2013;108:159–166. doi: 10.1016/j.prevetmed.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 61.Smith R.P., May H.E., Burow E., Meester M., Tobias T.J., Sassu E.L., Pavoni E., Di Bartolo I., Prigge C., Wasyl D., et al. Assessing pig farm biosecurity measures for the control of Salmonella on European farms. Epidemiol. Infect. 2023;151:e130. doi: 10.1017/S0950268823001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alarcón L.V., Allepuz A., Mateu E. Biosecurity in pig farms: A review. Porc. Health Manag. 2021;7:5. doi: 10.1186/s40813-020-00181-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dahl J., Wingstrand A., Nielsen B., Baggesen D.L. Eradication of Salmonella Typhimurium infection by the strategic movement of pigs. Vet. Rec. 1997;140:679–681. doi: 10.1136/vr.140.26.679. [DOI] [PubMed] [Google Scholar]
- 64.Beloeil P.A., Fravalo P., Fablet C., Jolly J.P., Eveno E., Hascoet Y., Chauvin C., Salvat G., Madec F. Risk factors for Salmonella enterica subsp. enterica shedding by market-age pigs in French farrow-to-finish herds. Prev. Vet. Med. 2004;63:103–120. doi: 10.1016/j.prevetmed.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 65.García-Feliz C., Carvajal A., Collazos J.A., Rubio P. Herd-level risk factors for faecal shedding of Salmonella enterica in Spanish fattening pigs. Prev. Vet. Med. 2009;91:130–136. doi: 10.1016/j.prevetmed.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 66.Walia K., Argüello H., Lynch H., Grant J., Leonard F.C., Lawlor P.G., Gardiner G.E., Duffy G. The efficacy of different cleaning and disinfection procedures to reduce Salmonella and Enterobacteriaceae in the lairage environment of a pig abattoir. Int. J. Food Microbiol. 2017;246:64–71. doi: 10.1016/j.ijfoodmicro.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 67.Farzan A., Friendship R.M., Dewey C.E., Warriner K., Poppe C., Klotins K. Prevalence of Salmonella spp. on Canadian pig farms using liquid or dry-feeding. Prev. Vet. Med. 2006;73:241–254. doi: 10.1016/j.prevetmed.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 68.Højberg O., Canibe N., Knudsen B., Jensen B.B. Potential rates of fermentation in digesta from the gastrointestinal tract of pigs: Effect of feeding fermented liquid feed. Appl. Environ. Microbiol. 2003;69:408–418. doi: 10.1128/AEM.69.1.408-418.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Van Immerseel F., Russell J.B., Flythe M.D., Gantois I., Timbermont L., Pasmans F., Haesebrouck F., Ducatelle R. The use of organic acids to combat Salmonella in poultry: A mechanistic explanation of the efficacy. Avian Pathol. 2006;35:182–188. doi: 10.1080/03079450600711045. [DOI] [PubMed] [Google Scholar]
- 70.Creus E., Pérez J.F., Peralta B., Baucells F., Mateu E. Effect of acidified feed on the prevalence of Salmonella in market-age pigs. Zoonoses Public Health. 2007;54:314–319. doi: 10.1111/j.1863-2378.2007.01069.x. [DOI] [PubMed] [Google Scholar]
- 71.Argüello H., Carvajal A., Costillas S., Rubio P. Effect of the addition of organic acids in drinking water or feed during part of the finishing period on the prevalence of Salmonella in finishing pigs. Foodborne Pathog. Dis. 2013;10:842–849. doi: 10.1089/fpd.2013.1497. [DOI] [PubMed] [Google Scholar]
- 72.Lynch H., Leonard F.C., Walia K., Lawlor P.G., Duffy G., Fanning S., Markey B.K., Brady C., Gardiner G.E., Argüello H. Investigation of in-feed organic acids as a low cost strategy to combat Salmonella in grower pigs. Prev. Vet. Med. 2017;139:50–57. doi: 10.1016/j.prevetmed.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 73.Barba-Vidal E., Castillejos L., Roll V.F.B., Cifuentes-Orjuela G., Moreno Muñoz J.A., Martín-Orúe S.M. The Probiotic Combination of Bifidobacterium longum subsp. infantis CECT 7210 and Bifidobacterium animalis subsp. lactis BPL6 Reduces Pathogen Loads and Improves Gut Health of Weaned Piglets Orally Challenged with Salmonella Typhimurium. Front. Microbiol. 2017;8:1570. doi: 10.3389/fmicb.2017.01570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Boyen F., Haesebrouck F., Vanparys A., Volf J., Mahu M., Van Immerseel F., Rychlik I., Dewulf J., Ducatelle R., Pasmans F. Coated fatty acids alter virulence properties of Salmonella Typhimurium and decrease intestinal colonization of pigs. Vet. Microbiol. 2008;132:319–327. doi: 10.1016/j.vetmic.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 75.Haesebrouck F., Pasmans F., Chiers K., Maes D., Ducatelle R., Decostere A. Efficacy of vaccines against bacterial diseases in swine: What can we expect? Vet. Microbiol. 2004;100:255–268. doi: 10.1016/j.vetmic.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 76.Haneda T., Okada N., Kikuchi Y., Takagi M., Kurotaki T., Miki T., Arai S., Danbara H. Evaluation of Salmonella enterica serovar Typhimurium and Choleraesuis slyA mutant strains for use in live attenuated oral vaccines. Comp. Immunol. Microbiol. Infect. Dis. 2011;34:399–409. doi: 10.1016/j.cimid.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 77.Arguello H., Carvajal A., Naharro G., Rubio P. Evaluation of protection conferred by a Salmonella Typhimurium inactivated vaccine in Salmonella-infected finishing pig farms. Comp. Immunol. Microbiol. Infect. Dis. 2013;36:489–498. doi: 10.1016/j.cimid.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 78.van der Wolf P., Meijerink M., Libbrecht E., Tacken G., Gijsen E., Lillie-Jaschniski K., Schüller V. Salmonella Typhimurium environmental reduction in a farrow-to-finish pig herd using a live attenuated Salmonella Typhimurium vaccine. Porc. Health Manag. 2021;7:43. doi: 10.1186/s40813-021-00222-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ortiz Sanjuán J.M., Manzanilla E.G., Cabrera-Rubio R., Crispie F., Cotter P.D., Garrido J.J., Argüello H. Using Shotgun Sequencing to Describe the Changes Induced by In-Feed Zinc Oxide and Apramycin in the Microbiomes of Pigs One Week Postweaning. Microbiol. Spectr. 2022;10:e0159722. doi: 10.1128/spectrum.01597-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jordá J., Lorenzo-Rebenaque L., Montoro-Dasi L., Marco-Fuertes A., Vega S., Marin C. Phage-Based Biosanitation Strategies for Minimizing Persistent Salmonella and Campylobacter Bacteria in Poultry. Animals. 2023;13:3826. doi: 10.3390/ani13243826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Walia K., Argüello H., Lynch H., Leonard F.C., Grant J., Yearsley D., Kelly S., Duffy G., Gardiner G.E., Lawlor P.G. Effect of strategic administration of an encapsulated blend of formic acid, citric acid, and essential oils on Salmonella carriage, seroprevalence, and growth of finishing pigs. Prev. Vet. Med. 2017;137:28–35. doi: 10.1016/j.prevetmed.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 82.Young I., Wilhelm B.J., Cahill S., Nakagawa R., Desmarchelier P., Rajić A. A Rapid Systematic Review and Meta-Analysis of the Efficacy of Slaughter and Processing Interventions to Control Non-Typhoidal Salmonella in Beef and Pork. J. Food Prot. 2016;79:2196–2210. doi: 10.4315/0362-028X.JFP-16-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ojha S., Kostrzynska M. Approaches for reducing Salmonella in pork production. J. Food Prot. 2007;70:2676–2694. doi: 10.4315/0362-028X-70.11.2676. [DOI] [PubMed] [Google Scholar]
- 84.Totton S.C., Glanville J.M., Dzikamunhenga R.S., Dickson J.S., O’Connor A.M. Systematic review of the magnitude of change in prevalence and quantity of Salmonella after administration of pathogen reduction treatments on pork carcasses. Anim. Health Res. Rev. 2016;17:39–59. doi: 10.1017/S1466252316000025. [DOI] [PubMed] [Google Scholar]
- 85.Blyth G.A.D., Connors L., Fodor C., Cobo E.R. The Network of Colonic Host Defense Peptides as an Innate Immune Defense against Enteropathogenic Bacteria. Front. Immunol. 2020;11:965. doi: 10.3389/fimmu.2020.00965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hurd H.S., McKean J.D., Griffith R.W., Wesley I.V., Rostagno M.H. Salmonella enterica infections in market swine with and without transport and holding. Appl. Environ. Microbiol. 2002;68:2376–2381. doi: 10.1128/AEM.68.5.2376-2381.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.EFSA Report of the Task Force on Zoonoses Data Collection on the Analysis of the baseline survey on the prevalence of Salmonella in slaughter pigs, Part, B. EFSA J. 2008;206:1–111. [Google Scholar]
- 88.Arguello H., Carvajal A., Alvarez-Ordoñez A., Jaramillo-Torres A.H., Rubio P. Effect of logistic slaughter on Salmonella contamination on pig carcasses. Food Res. Int. 2014;55:77–82. doi: 10.1016/j.foodres.2013.10.041. [DOI] [Google Scholar]
- 89.Swanenburg M., Urlings H.A., Snijders J.M., Keuzenkamp D.A., van Knapen F. Salmonella in slaughter pigs: Prevalence, serotypes and critical control points during slaughter in two slaughterhouses. Int. J. Food Microbiol. 2001;70:243–254. doi: 10.1016/S0168-1605(01)00545-1. [DOI] [PubMed] [Google Scholar]
- 90.O’Connor A.M., Gailey J., McKean J.D., Hurd H.S. Quantity and distribution of Salmonella recovered from three swine lairage pens. J. Food Prot. 2006;69:1717–1719. doi: 10.4315/0362-028X-69.7.1717. [DOI] [PubMed] [Google Scholar]
- 91.Boughton C., Egan J., Kelly G., Markey B., Leonard N. Quantitative examination of Salmonella spp. in the lairage environment of a pig abattoir. Foodborne Pathog. Dis. 2007;4:26–32. doi: 10.1089/fpd.2006.57. [DOI] [PubMed] [Google Scholar]
- 92.Arguello H., Carvajal A., Collazos J.A., Garcia-Feliz C., Rubio P. Prevalence and serovars of Salmonella enterica on pig carcasses, slaughtered pigs and the environment of four Spanish slaughterhouses. Food Res. Int. 2012;45:905–912. doi: 10.1016/j.foodres.2011.04.017. [DOI] [Google Scholar]
- 93.Fedorka-Cray P.J., Whipp S.C., Isaacson R.E., Nord N., Lager K. Transmission of Salmonella Typhimurium to swine. Vet. Micro. 1994;41:333–344. doi: 10.1016/0378-1135(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 94.EFSA Report of the Task Force on Zoonoses Data Collection on the analysis of the baseline survey on the prevalence of Salmonella in slaughter pigs, Part, A. EFSA J. 2008;135:1–111. [Google Scholar]
- 95.Hald T., Wingstrand A., Swanenburg M., von Altrock A., Thorberg B.M. The occurrence and epidemiology of Salmonella in European pig slaughterhouses. Epidemiol. Infect. 2003;131:1187–1203. doi: 10.1017/S0950268803001171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bonardi S., Bassi L., Brindani F., D’Incau M., Barco L., Carra E., Pongolini S. Prevalence, characterization and antimicrobial susceptibility of Salmonella enterica and Yersinia enterocolitica in pigs at slaughter in Italy. Int. J. Food Microbiol. 2013;163:248–257. doi: 10.1016/j.ijfoodmicro.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 97.Botteldoorn N., Herman L., Rijpens N., Heyndrickx M. Phenotypic and molecular typing of Salmonella strains reveals different contamination sources in two commercial pig slaughterhouses. Appl. Environ. Microbiol. 2004;70:5305–5314. doi: 10.1128/AEM.70.9.5305-5314.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Borch E., Nesbakken T., Christensen H. Hazard identification in swine slaughter with respect to foodborne bacteria. Int. J. Food Microbiol. 1996;30:9–25. doi: 10.1016/0168-1605(96)00988-9. [DOI] [PubMed] [Google Scholar]
- 99.Bolton D.J., Pearce R.A., Sheridan J.J., Blair I.S., McDowell D.A., Harrington D. Washing and chilling as critical control points in pork slaughter hazard analysis and critical control point (HACCP) systems. J. Appl. Microbiol. 2002;92:893–902. doi: 10.1046/j.1365-2672.2002.01599.x. [DOI] [PubMed] [Google Scholar]
- 100.Cê E.R., Giombelli A., Kich J.D., Moresco K.S., Miranda A., Pedrão M.R., Johann G., Badaró A.C.L., Hashimoto E.H., Machado-Lunkes A. Monitoring of Pig Slaughter Stages and Correlation in the Prevalence of Pathogens and Levels of Microorganisms That Indicate Microbiological Quality and Hygiene Using a Predictive Model. J. Food Prot. 2023;86:100034. doi: 10.1016/j.jfp.2022.100034. [DOI] [PubMed] [Google Scholar]
- 101.Mikołajczyk A. Evaluation of the effects of a mixture of organic acids and duration of storage on the survival of salmonella on turkey carcasses. J. Food Prot. 2015;78:585–589. doi: 10.4315/0362-028X.JFP-14-135. [DOI] [PubMed] [Google Scholar]
- 102.European Union . Directive 2003/99/EC of the European Parliament and of the Council of 17 November 2003 on the Monitoring of Zoonoses and Zoonotic Agents, Amending Council Decision 90/424/EEC and Repealing Council Directive 92/117/EEC. European Union; Brussels, Belgium: 2003. [Google Scholar]
- 103.EFSA. ECDC The European Union One Health 2022 Zoonoses Report. EFSA J. 2023;21:e8442. doi: 10.2903/j.efsa.2023.8442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kinross P., van Alphen L., Martinez Urtaza J., Struelens M., Takkinen J., Coulombier D., Makela P., Bertrand S., Mattheus W., Schmid D., et al. Multidisciplinary investigation of a multicountry outbreak of Salmonella Stanley infections associated with turkey meat in the European Union, August 2011 to January 2013. Eurosurveillance. 2014;19:20801. doi: 10.2807/1560-7917.ES2014.19.19.20801. [DOI] [PubMed] [Google Scholar]
- 105.Sarno E., Pezzutto D., Rossi M., Liebana E., Rizzi V. A Review of Significant European Foodborne Outbreaks in the Last Decade. J. Food Prot. 2021;84:2059–2070. doi: 10.4315/JFP-21-096. [DOI] [PubMed] [Google Scholar]
- 106.Kawakami V., Bottichio L., Lloyd J., Carleton H., Leeper M., Olson G., Li Z., Kissler B., Angelo K.M., Whitlock L., et al. Multidrug-Resistant Salmonella I 4,[5],12:i:- and Salmonella Infantis Infections Linked to Whole Roasted Pigs from a Single Slaughter and Processing Facility. J. Food Prot. 2019;82:1615–1624. doi: 10.4315/0362-028X.JFP-19-048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bearson B.L., Trachsel J.M., Holman D.B., Brunelle B.W., Sivasankaran S.K., Simmons M., Wasilenko J., Tillman G., Johnston J.J., Bearson S.M.D. Complete Genome Sequence of Multidrug-Resistant Salmonella enterica Serovar I 4,[5],12:i:- 2015 U.S. Pork Outbreak Isolate USDA15WA-1. Microbiol. Resour. Announc. 2019;8:e00791-19. doi: 10.1128/MRA.00791-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.USDA [(accessed on 19 January 2024)]; Available online: https://www.aphis.usda.gov/aphis/ourfocus/animalhealth/nvap/NVAP-Reference-Guide/Animal-Health-Emergency-Management/Notifiable-Diseases-and-Conditions.
- 109.Napoleoni M., Villa L., Barco L., Lucarelli C., Tiengo A., Baggio G., Dionisi A.M., Angellotti A., Ferretti E., Ruggeri S., et al. Monophasic Variant of Salmonella Typhimurium 4,[5],12:i:- (ACSSuGmTmpSxt Type) Outbreak in Central Italy Linked to the Consumption of a Roasted Pork Product (Porchetta) Microorganisms. 2023;15:2567. doi: 10.3390/microorganisms11102567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kuchenmüller T., Abela-Ridder B., Corrigan T., Tritscher A. World Health Organization initiative to estimate the global burden of foodborne diseases. Rev. Sci. Tech. 2013;32:459–467. doi: 10.20506/rst.32.2.2249. [DOI] [PubMed] [Google Scholar]