Abstract
Four pigtailed macaques were inoculated with an infectious, apathogenic human immunodeficiency virus type 2 (HIV-2) molecular clone (HIV-2KR) and subsequently challenged with a highly pathogenic strain, HIV-2287, together with two naive control animals. After challenge, two animals inoculated with a high dose of the immunizing strain were protected from CD4 decline and immunodeficiency. To examine the role of genetic heterogeneity in protection, fragments of the env gene were amplified from peripheral blood mononuclear cell DNA and plasma RNA of challenged animals by PCR, examined by using a heteroduplex tracking assay (HTA), and sequenced. By HTA, variation was detected principally within the V1 and V2 regions of envelope. Extent of variation in viral DNA clones as assessed by HTA correlated with inoculum size, as did the degree of variation in sequences of clones derived from viral DNA. Conversely, a rapid reduction in the number of plasma viral RNA variants was noted by HTA at 8 weeks postinfection in protected animals; this reduction was not present in naive or unprotected macaques. Sequences derived from plasma viral RNA were found to be more closely related than corresponding viral DNA sequences, and protection correlated with a significant reduction in variation in plasma RNA sequences in animals given the identical inocula of HIV-2287. Nonsynonymous mutations were significantly less prevalent in the protected animals. An additional potential glycosylation site was predicted to be present in the V2 region in all but one clone, and amino acid signatures related to protection were identified in viral DNA and RNA clones within both the V1 and V2 regions. Examination of the role of viral variation in this HIV-2 live-virus vaccine model may provide valuable insights into immunopathogenesis.
Genetic variability is one of the major obstacles to the development of a broadly efficacious human immunodeficiency virus (HIV) vaccine (9, 45). Escape from host immunity mediated both variation by in the V3 region and in cytotoxic lymphocyte epitopes has been noted (39, 54, 55), and emergence of viral phenotypes characterized by different cellular tropism (10, 42, 44, 48), increased replicative capacity (10, 42), and induction of syncytia (SI phenotype) (41, 44) have been associated with rapid progression of disease. However, while genotypic variation may result from escape from host immunity or arise from intrinsic characteristics of viral replication, establishing a causal role for variation in immunopathogenesis has proved difficult, since relatively little information is available on the significance of heterogeneity in viral sequences amplified from plasma.
The env gene of HIV and related primate lentiviruses is a major site for viral variation (2, 7, 23), as might be expected in view of the role which the encoded surface envelope glycoprotein has been shown to play in determination of viral cell host range, replication rate, and induction of cytopathic effects. Residues present in the external envelope glycoprotein of HIV type 1 (HIV-1), HIV-2, and simian immunodeficiency virus (SIV) determine the character of both type-specific and conserved neutralizing epitopes. The V3 loop of HIV-1 is among the most variable regions of the envelope (28, 53–55), containing a potent type-specific neutralizing epitope (32, 53) as well as epitopes important for cellular cytotoxicity (43). However, while extensive variation in the V3 region of the HIV-1 envelope has been well documented, studies of recently infected individuals have indicated a restricted genetic repertoire (34), while others have found reduced variation in progressive disease (16, 47, 56). In HIV-1, the V1 and V2 domains contain type-specific conformational neutralizing epitopes (21) and are also extremely variable (51) compared to the C4 region, which contributes to viral binding to the CD4 molecule. In contrast, the V1, V2, and V4 regions of SIV seem to be the most heterogeneous (1–3, 6, 7, 38), whereas the V3 region is conserved. Relatively few studies have addressed HIV-2 env gene variability during the course of infection, although variability in V1, V2, V3 and V4 has been observed (5, 8, 50).
While HIV-2 is perhaps more closely related to SIV than to HIV-1 (8) and appears to be less pathogenic in humans (31), it is still a human virus, and it represents a useful model for understanding the relationship between genetic variation and pathogenicity, by virtue of the ability of some HIV-2 strains to both infect and produce disease in macaques and baboons (4, 20, 37, 40, 46), allowing a close and controlled examination of the kinetics of viral variation and replication. The availability of an HIV-2 strain (HIV-2287) which rapidly produces immunodeficiency (or progression) in naive Macaca nemestrina (22, 52), together with a live-virus model of immunization using an avirulent clone of HIV-2 (HIV-2KR) (27) which can induce protection from rapid CD4 depletion (nonprogression) after challenge with HIV-2287 (29), led us to examine the effects of both virus inoculum and host response on envelope polymorphisms in this HIV-2 macaque model. Viral variation was examined by the heteroduplex tracking assay (HTA) and sequencing of fragments amplified from peripheral blood mononuclear cells (PBMC) viral DNA as well as plasma viral RNA, using specimens from both naive and immunized animals.
Heteroduplex tracking revealed that the extent of initial viral DNA variation was related to inoculum size and suggested that variation in viral RNA species was less in protected animals than in naive control and unprotected animals. These findings were confirmed by sequencing of clones distinguishable by HTA, which revealed that viral DNA variation was significantly reduced in the protected animal that received a low inoculum of HIV-2287 and that significant reduction of the extent of variation in viral RNA sequences correlated with protection among animals given the same inoculum of HIV-2287.
MATERIALS AND METHODS
Animal immunization.
Four pigtailed macaques (M. nemestrina) were immunized by intravenous injection of either 104 (animals J90292 and F90350; designated 292 and 350, respectively) or 103 (animals F90404 and F90407; designated 404 and 407, respectively) syncytium-forming units (SFU) of HIV-2KR (27) derived from a Molt 4/clone 8 transfection culture (Table 1). All macaques were infected with HIV-2KR, as demonstrated by virus reisolation (three of four), DNA PCR (four of four), and gp36 peptide enzyme immunoassay (three of four). All experienced modest transient CD4 decline after HIV-2KR inoculation which resolved by 20 weeks postinoculation (wpi). HIV-2KR could not be isolated from any animal later than 26 wpi, and viral DNA was undetectable in all animals by 52 wpi of HIV-2KR. Fourteen months after HIV-2KR infection, these animals were challenged together with two naive controls (animals J92096 and F92147; designated 096 and 147, respectively) with HIV-2287 (29). The challenge virus was obtained by coculture of lymph node-derived lymphocytes from animal 287 with allogenic PBMCs. This animal had experienced rapid onset of immunodeficiency following inoculation with whole blood from another animal inoculated with HIV-2EHO (22). Challenge with HIV-2287 was performed via intravenous inoculation using either 105 50% tissue culture infectious doses (TCID50) (animals 292, 407, and 096) or 101 TCID50 (animals 350, 404, and 147). All macaques were infected with HIV-2287 after challenge, as demonstrated by virus reisolation performed 2 wpi. The kinetics of virus replication and immune responses have been described elsewhere (29).
TABLE 1.
Animals used in challenge experiment
| Animal | HIV-2KR inoculum (SFU) | HIV-2287 inoculum (TCID50) | CD4 decline after HIV-2287 inoculation | Immunodeficiency disease |
|---|---|---|---|---|
| 096 | None | 105 | Rapid | Rapid |
| 147 | None | 101 | Rapid | Rapid |
| 292 | 104 | 105 | None | None |
| 350 | 104 | 101 | None | None |
| 407 | 103 | 105 | Rapid | Delayed |
| 404 | 103 | 101 | Rapid | Delayed |
Plasma and PBMC separation; DNA extraction.
Blood was drawn from each animal at 2-week intervals after challenge, and PBMCs were isolated by 93% Ficoll-Hypaque gradient separation of blood diluted 1:1 with Hanks’ balanced salt solution, as previously described (27). Viral DNA was extracted by column separation (QIAamp kit; Qiagen) performed as specified by the manufacturer. Plasma was separated by either direct centrifugation of acid-citrate-dextrose-anticoagulated whole blood or by harvesting of diluted plasma (1:1) from Ficoll gradients. Both DNA and plasma were frozen at −80°C until examined.
PCR amplification of viral DNA sequences.
The DNA sequences encoding the V1-V2 and V3-V4-CD4 regions of HIV-2 gp130 were amplified by a first round of PCR using primers 243 (5′-ATG-TGT-GGA-GTC-TCT-TTG-AGA-CC-3′, from nucleotides [nt] 5328 to 5350 of the published HIV-2EHO sequence; GenBank locus HIV2EHOA, accession no. L14545) and 126 (5′-CAA-AGC-CAA-TTG-GTG-TTA-TC 3′, nt 6520 to 6539) to obtain a product of 1,212 bp (nt 5328 to 6539). Amplification was carried out starting from 500 ng of DNA in a final volume of 50 μl, in a mixture containing each primer at 1 μM, each deoxynucleoside triphosphate at 200 μM, 1.25 U of Taq DNA polymerase (Promega), 2.5 mM MgCl2, 50 mM KCl, and 10 mM Tris HCl (pH 8.3) in a Perkin-Elmer 480 thermocycler. PCR conditions were of 94°C for 45 s, 60°C for 30 s, and 72°C for 1 min 40 s. After column purification (QIAquick; Qiagen), 2 μl of the formerly amplified product was reamplified by using two sets of inner primers: V1-V2 (nt) (5352 to 5742) region primers 290 (5′-CAA-TAA-AAC-CAT-GTG-TTA-AAT-TAA-CC-3′, nt 5352-5377) plus 244 (5′-GCA-CAA-TAC-CTA-AAT-CTT-AAA-CTA-TCC-3′, nt 5716-5742), and V3-V4-CD4 (nt 5912-6539) region primers 240 (5′-GGT-AAA-GAC-AAT-AGG-ACT-ATC-ATA-AGC-3′, nt 5912 to 5938) plus 126, using the PCR conditions described above. The first and second sets of primers were designed to amplify a DNA fragment of 391 bp encompassing the V1-V2 region of gp120 and a 628-bp region including the V3, V4, and CD4-binding regions, respectively.
HTA.
Products from the second round of PCR were analyzed for heterogeneity by HTA (14, 15) using a radiolabeled single-stranded DNA (ssDNA) probe. To obtain the probe, PBMC-derived viral DNA env fragments obtained from two naive macaques 2 wpi with HIV-2287 were amplified by PCR, cloned into a pUC19 vector, and sequenced. The clone showing the highest overall homology with the consensus sequence (clone 147/2-14) was selected for the generation of the probe for the HTA. ssDNAs were prepared by PCR amplification of clone 147/2-14, using both 32P-labeled (probe) and biotinylated (capture) primers, followed by denaturation and removal of the biotinylated strand by incubation with M-280 streptavidin-bound magnetic beads (Dynabeads; Dynal) and processing in a magnetic particle concentrator (MPC-1; Dynal) (13a). The probe primer was end labeled with 32P by using 0.01 pM primer, 0.01 μCi of [γ-32P]dATP, 2 U of T4 polynucleotide kinase, and 1× polynucleotide kinase buffer (Boehringer Mannheim) in 10 μl. After incubation at 37°C for 30 min, the enzyme was inactivated at 95°C for 5 min and then cooled to 4°C in a thermocycler. Heteroduplexes were formed by denaturation and reannealing of 5 μl of the second-round PCR-amplified fragments in a 10-μl mixture containing annealing buffer (1 mM NaCl, 100 mM Tris HCl [pH 7.8], 20 mM EDTA) and 5,000 cpm of 32P-labeled ssDNA probe per sample. Denaturation was performed at 95°C for 2 min, followed by a 5-min incubation at 4°C in a thermocycler. ssDNA probe alone (5,000 cpm) and 5 μl of homoduplex control sample derived from amplification of probe plasmid 147/2-14 by using unlabeled primers were denatured and cooled in the same manner, and all samples were loaded onto a 5% neutral 1-mm-thick polyacrylamide gel (30%:0.8% acrylamide/bisacrylamide in 88 mM Tris-borate–2 mM EDTA [pH 8] [TBE buffer]) and run in TBE buffer on a Protean II vertical gel apparatus (Bio-Rad) for 2 h and 45 min at 550 V in a cold room.
Quantitative analysis of HTA results.
Bands were manually counted for numerical analysis. In addition, autoradiographs of gels prepared as described above were scanned (Silverscan III) under blue light to obtain 8-bit tagged image file format (TIFF) files. TIFF files were subjected to quantitative analysis, including determination of normalized Shannon entropy (SN) and mean mobility shift in a manner similar to that described by Delwart et al. (15). Briefly, ImageQuant software was used to determine the volume of cells within narrow (4-pixel-wide) grids (one column, 32 rows), drawn to extend from just below the position of the single-stranded probe to just below the position corresponding to migration of the homoduplex control in each lane, instead of averaging linear pixel values from density measurements along the analogous line. In addition, an affine transformation was applied to smooth the background values. In this manner, data from a complete gel could be obtained and exported to a spreadsheet for determinations as described elsewhere (15).
Screening of the clones for DNA sequencing.
Using the conditions described above for PCR amplification of viral DNA sequences), env fragments were amplified with primers 290 and 126. Products were analyzed on ethidium bromide-stained low-melting-point agarose gels, and 1,212-bp bands were excised, purified by lithium chloride precipitation, and cloned into plasmid pCRII by using a TA cloning kit (Invitrogen Corp., San Diego, Calif.) as specified by the manufacturer.
For each animal, time point, and source of material (DNA or RNA), 30 to 40 clones were obtained and screened by PCR using primers 290 and 126 for the presence of the correct-size insert. Orientation was determined by PCR using the forward primer [M13(−40) primer] with primer 126. The V1-V2 region was then amplified with inner primers 290 and 244 for screening by heteroduplex tracking using 32P-labeled V1-V2 region probe. Results from the HTA were used to identify clones representing the major viral variants in the viral DNA populations present in naive animal 096 at 8 and 18 wpi and in immunized animals 292 and 350 at 8 and 72 wpi. All clones within a sample (single animal, source, and time point) which exhibited distinct mobilities in the HTA assay were chosen for sequence analysis. For sequencing, clones were grown overnight and plasmid DNA was extracted by the alkaline lysis/polyethylene glycol precipitation procedure (Applied Biosystems, Inc., Perkin-Elmer). Template DNA (1 μg), 3.2 pmol of primer, and 9.5 μl of terminator premix (PRISM Ready Reaction DyeDeoxy Terminator Cycle Sequencing kit; Applied Biosystems) were combined in a final volume of 20 μl. Sequences were determined both in the forward and in the reverse direction, using primers M13(−40) and 244, respectively. Reactions were run in a Perkin-Elmer 480 thermal cycler (25 cycles; 95°C for 30 s, 45°C for 15 s, 60°C for 4 min), and products were stored at 4°C. Products were then spun on Centri-Sep columns (Princeton Separations, Inc.) to remove excess terminators according to the manufacturer’s specifications. Reaction mixtures were dried and resuspended in 4 μl of loading buffer (5:1 deionized formamide/dextran blue [30 mg/ml] in 50 mM EDTA [pH 8]), denatured at 90°C for 2 min, and cooled on ice. Each sample was loaded onto an Applied Biosystems 373A DNA sequencer and run in TBE buffer for 12 h.
RNA isolation.
Plasma RNA was isolated by the RNAzol method (Tel-Test, Inc., Friendswood, Tex.). Briefly, 400 μl of plasma was ultracentrifuged for 1 h at 17,000 rpm in a Contifuge 17RS centrifuge (Heraeus) at 4°C, and the pellet was lysed with 200 μl of RNAzol. RNA was extracted with 0.1 volume of chloroform (15 min on ice), and the aqueous phase was separated by spinning the suspension at 12,000 × g for 15 min at 4°C. RNA was then precipitated with 1 volume of isopropanol for 45 min at −20°C, spun again at 12,000 × g for 15 min at 4°C, and washed twice with 75% ethanol. The RNA pellet was then dried and resuspended in diethylpyrocarbonate-treated water.
RNA reverse transcription and PCR amplification of cDNA.
For reverse transcription of viral RNA, 1 μM reverse primer 126 was annealed (68°C, 8 min) to 2 μl of RNA template in a 14.2-μl reaction volume in a thermocycler; the product was rapidly cooled at 4°C and then added to a mixture containing reverse transcriptase buffer (25 mM Tris HCl [pH 8.3], 50 mM KCl, 2 mM MgCl2, 100 μg of bovine serum albumin/ml), 1 mM deoxynucleoside triphosphate, 10 mM dithiothreitol, and 200 U of Moloney murine leukemia virus reverse transcriptase (Gibco, BRL, Life Technologies) in a final volume of 20 μl. Reverse transcription was performed at 37°C for 45 min, followed by 2 min at 90°C and 2 min at 4°C. The reverse-transcribed product (10 μl) was added to a 40-μl mixture containing 50 pmol of forward primer 290, reverse transcriptase buffer, and 1.25 U of Taq polymerase. After amplification with outer primers 290 and 126 as described above, 1-μl volumes of products were amplified by using nested primers 290 and 244 to obtain the V1-V2 region fragments for analysis by HTA. All PCR conditions were the same as those used for the DNA amplification, except that the annealing temperature of the first round was 56°C.
RNA viremia in all animals, with the exception of time points after 20 wpi in animals 350 and 292, was such that 1,000 copies or more of plasma viral RNA (29) could be used for starting material for reverse transcription-PCRs, ensuring a statistically valid sample size.
Sequence analysis.
Sequences were initially manually aligned at the 5′ ends by identity with a short highly conserved nucleotide motif, and then a hierarchical multiple alignment algorithm was used (12). Nucleotide pairwise distances were determined by using both FASTA comparison (12) and the Kimura algorithm in the PHYLIP 3.5c DNADIST program (17). To compare distributions, mean pairwise distances were calculated, and both parametric (Satterthwaite approximation for t test for two samples with unequal means) and nonparametric (Wilcoxon rank-sum statistic) statistical tests were applied, using SAS System 6.11 software. The DNAML (18) program was used to obtain a phylogenetic tree, using aligned nucleic acid sequences. Predicted amino acid sequences were again aligned and manually adjusted by degapping. Amino acid sequence signature pattern analysis was performed with VESPA software (26).
Nucleotide sequence accession numbers.
The sequences determined in this work are available as GenBank accession no. AF064284 through AF064358, consecutively.
RESULTS
Clinical outcome of infection.
Infection of M. nemestrina with 104 (animals 292 and 350) and 103 (animals 404 and 407) SFU of the HIV-2KR molecular clone (27) resulted in transient low-level viremia and modest transient decline in CD4+ lymphocyte numbers (27). In contrast, inoculation with either high or low doses of HIV-2287, an isolate obtained after serial passage of HIV-2EHO in M. nemestrina, produced high levels of viremia, rapid decline of CD4+ lymphocyte numbers, and rapid development of immunodeficiency (29) in naive animals 096 and 147. The comparison of genomic variability in naive animals inoculated with HIV-2287 with that present in HIV-2KR-immunized macaques was limited by the rapid clinical deterioration of naive animals after inoculation (29). Samples from naive animals were available only for a limited time period after inoculation (18 weeks), after which animals were euthanized. A more complete comparison (2, 4, 6, 8, 52, 56 to 68, and up to 72 wpi) could be made between HIV-2KR-immunized animals which experienced CD4 decline (404 and 407) and those protected from CD4 decline (292 and 350) after HIV-2287 inoculation. Animals immunized by prior infection with HIV-2KR were found to survive longer without evidence of clinical disease, but only animals given the high HIV-2KR inoculum (292 and 350) were protected from CD4 decline (29) after challenge with either high- or low-dose HIV-2287 (Table 1). Reactivation of HIV-2KR following HIV-2287 challenge was not detectable, as judged by PCR using primers specific for either HIV-2KR or HIV-2287.
Distribution of genetic variation within the env gene.
To examine the distribution of genomic variation within the HIV-2 env gene following challenge, a large fragment of the HIV-2287 env gene (1,212 bp, nt 5328 to 6539), encompassing the V1 region through the CD4-binding region of the external envelope glycoprotein, was amplified. The number of copies in the starting material used for PCR could not be achieved in all animals at all time points: the low level of PBMC viral DNA copies observed in animals given the lower inoculum of HIV-2287 (147, 350, and 404) precluded the use of >20 copies of viral DNA as starting material in reactions at 8 wpi. In animal 350 and 404, <10 copies were present in 500 ng of genomic DNA; in animal 147, a predicted 13 copies were used at 8 wpi. In all other animals, more than 20 predicted copies of viral DNA were used at 8 wpi (as suggested by Delwart et al. [14, 15]), and for all other time points >100 copies of viral DNA were predicted to be present by quantitative PCR (29). For HTA, two subsections of this larger fragment were examined, including a fragment, V1, containing the V1 and V2 regions (nt 5352 to 5742) and a second fragment, CD4, containing the V3, V4, and CD4-binding regions (nt 5912 to 6539) of HIV-2287 gp130. Using a representative ssDNA probe obtained from a PBMC-derived viral DNA clone from animal 147 (see Materials and Methods), we compared the scope of variation in these two regions over time in naive and HIV-2KR-immunized animals (both protected and unprotected from CD4+ lymphocyte decline) after HIV-2287 inoculation.
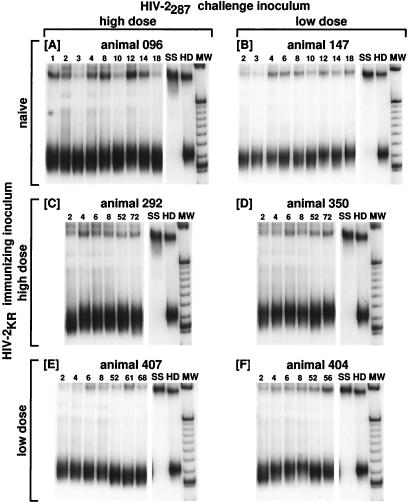
With the CD4 region probe, HTA of V3-V4-CD4 regions obtained from all HIV-2287-infected macaques revealed the presence of strong bands migrating near the homoduplex position at all time points, with few or no minor bands being detected (Fig. 1). This was not surprising in light of similar studies, where little variation was noted to occur in the V3 or CD4-binding region of the SIV envelope of infected macaques, though variation in V1, V2, and V4 was detected (1, 2, 7, 38). Although simple point mutations likely would have been detectable by this method, these fragments were not considered for further analysis. In contrast, many prevalent viral forms, as indicated by multiple bands, were present in HTA autoradiographs showing results of assays using the V1 probe (Fig. 2), while bands migrating near the homoduplex position were frequently not visible or very faint, indicating that viral forms closely related to probe were present only as a minor variant in these animals at these time points. Analysis of mean mobility shift confirms a profound difference in these regions (0.25 ± 0.004 for the CD4 region versus 0.64 ± 0.013 for the V1-V2 region, P < 0.0001 by Wilcoxon rank-sum test). The detection of bands migrating near the homoduplex position in animal 147 at all time points (Fig. 2B) is likely due to the fact that the HTA probe was derived from PBMC-derived viral DNA sequences from this animal at 2 wpi (see Materials and Methods).
FIG. 1.
HTA of PBMC-derived viral DNA sequences from the V3-V4-CD4 region of the HIV-2 envelope. Panels show the duplexes formed between the 32P-labeled ssDNA probe and the products of primers 240 and 126 amplifying the CD4 region of the viral DNA present in PBMCs obtained from animals at various times after inoculation. After hybridization, products were resolved on neutral polyacrylamide gels and autoradiographed (see Materials and Methods). (A) Animal 096, naive, challenged with high-dose HIV-2287; (B) animal 147, naive, challenged with low-dose HIV-2287; (C) animal 292, immunized with high-dose HIV-2KR and challenged with high-dose HIV-2287; (D) animal 350, immunized with high-dose HIV-2KR and challenged with low dose HIV-2287; (E) animal 407, immunized with low-dose HIV-2KR and challenged with high-dose HIV-2287; (F) animal 404, immunized with low-dose HIV-2KR and challenged with low-dose HIV-2287. Numbers above lanes correspond to the time point (week postinfection) to which the sample refers. SS, single-stranded 32P-labeled probe; HD, homoduplex band; MW, molecular weight marker. Collectively, the data show that when the CD4 probe is used, only one variant is detectable and it is conserved over time.
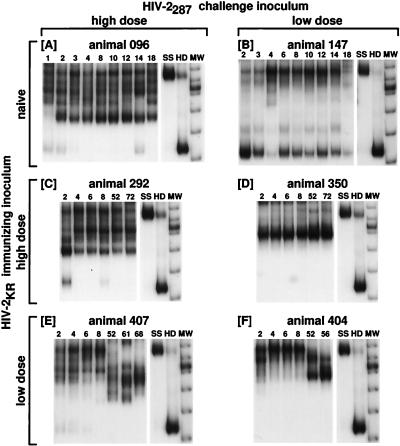
FIG. 2.
HTA of PBMC-derived viral DNA sequences from the V1-V2 region of the HIV-2 envelope. Panels show the duplexes formed between the 32P-labeled ssDNA probe and the products of primers 290 and 244 amplifying the V1 and V2 regions of viral DNA present in PBMCs obtained from animals at various times after inoculation (see Materials and Methods). (A) Animal 096, naive, challenged with high-dose HIV-2287; (B) animal 147, naive, challenged with low-dose HIV-2287; (C) animal 292, immunized with high-dose HIV-2KR and challenged with high-dose HIV-2287; (D) animal 350, immunized with high-dose HIV-2KR and challenged with low-dose HIV-2287; (E) animal 407, immunized with low-dose HIV-2KR and challenged with high-dose HIV-2287; (F) animal 404, immunized with low-dose HIV-2KR and challenged with low-dose HIV-2287. Numbers above lanes correspond to the time point (week postinfection) to which the sample refers. Note that in many animals, bands migrating near the homoduplex position are either not visible (A, animal 096 at 4 to 12 and 18 wpi; C, animal 292 at 4, 6, 52, and 72 wpi; D, animal 350 at 2 to 72 wpi; E, animal 407 at 8 to 68 wpi; F, animal 404 at 2 to 56 wpi) or are quite faint (A, animal 096 at 1, 2, 3, and 14 wpi; B, animal 147 at 4 and 18 wpi; C, animal 292 at 8 wpi; E, animal 407 at 2, 4, and 6 wpi). SS, single-stranded 32P-labeled probe; HD, homoduplex band; MW, molecular weight marker. Panels A, C, and E, from animals receiving the high HIV-2287 inoculum, display numerous bands; fewer bands are seen in panels B, D, and F, from animals receiving the low-dose HIV-2287 inoculum.
DNA heterogeneity in the V1-V2 region correlates with challenge dose.
When using the V1 probe, we found extensive variability in both naive and immunized animals (Fig. 2). Evolution of different major variants over time after inoculation was seen in several animals (compare variants in animal 407 [Fig. 2E] at 2 to 8 wpi versus 52 to 68 wpi and animal 404 [Fig. 2F] at 2 to 8 wpi versus 52 to 56 wpi), consistent with change due to or escape from exogenous pressure, such as immune selection, acting upon the V1 and V2 regions. However, we could establish no specific correlation between the number of variants and progression to immunodeficiency disease. Note that animal 292 (Fig. 2C), protected from disease and CD4 lymphocyte decline, exhibited a pattern of variants similar to that of animal 096 (Fig. 2A), which experienced rapid CD4 decline and progression of disease. Moreover, no dominant single genotype associated with protection or lack thereof could be identified.
A clear correlation appears to be present between variation in the V1-V2 region of viral DNA and the dose of HIV-2287 used for challenge (Table 2; Fig. 2). At 2, 4, and 8 wpi, macaques administered the low inoculum of HIV-2287 (naive animal 147 and HIV-2KR-experienced animals 350 and 404 [Table 2]) exhibited averages of 2.3, 2.0, and 1.8 distinct bands on HTA, respectively, whereas animals inoculated with the high dose of HIV-2287 (naive animal 096 and HIV-2KR-experienced animals 292 and 407) exhibited averages of 4.3, 3.8, and 3.7 distinct bands at the same early time points. At the last time points available (18 wpi for 147, 72 wpi for 350, 56 wpi for 404, 18 wpi for 096, 72 wpi for 292, and 68 wpi for 407), a similar difference in numbers of bands was seen, with low-dose animals averaging 2.3 distinct bands and high-dose animals averaging 3.7 distinct bands (overall, P = 0.0009 by Wilcoxon rank-sum test). Similarly, average SN values were significantly higher in animals given the high inoculum (0.78 ± 0.01 for high inoculum versus 0.67 ± 0.01 for low inoculum, P = 0.02 [Table 2]).
TABLE 2.
Numbers of viral DNA variants distinguishable by HTA and SN values of HTA lanes for DNA clones
| Animal | Inoculum
|
No. of viral DNA variants distinguishable by HTA
|
SN of HTA lanes
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HIV-2KR (SFU) | HIV-2287 (TCID50) | 2 wpi | 4 wpi | 8 wpi | Latea | 2 wpi | 4 wpi | 8 wpi | Late | |
| 096 | None | 105 | 5 | 4–5 | 4 | 4 | 0.83 | 0.84 | 0.84 | 0.83 |
| 147 | None | 101 | 3–4 | 3–4 | 2–3 | 2 | 0.69 | 0.70 | 0.68 | 0.69 |
| 292 | 104 | 105 | 5 | 4 | 4–5 | 4 | 0.80 | 0.80 | 0.79 | 0.70 |
| 350 | 104 | 101 | 2 | 1–2 | 2 | 2 | 0.71 | 0.72 | 0.69 | 0.65 |
| 407 | 103 | 105 | 3 | 3 | 2–3 | 3 | 0.72 | 0.68 | 0.82 | 0.70 |
| 404 | 103 | 101 | 1–2 | 1 | 1 | 3 | 0.64 | 0.64 | 0.70 | 0.64 |
Defined as 18 wpi for 147 and 096, 72 wpi for 350 and 292, 56 wpi for 404, and 68 wpi for 407.
Single V1-V2 variants emerge in plasma RNA from protected macaques.
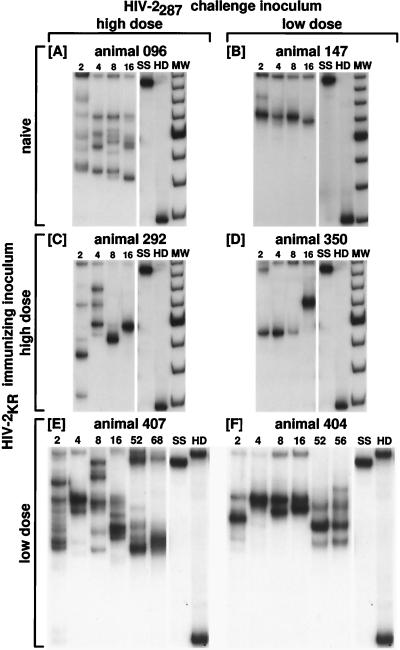
It is not clear from other studies whether examination of viral DNA sequences necessarily leads to an accurate assessment of the nature or course of viral variation during infection (15, 24, 33, 34, 36, 47, 56, 58). To investigate whether the number, type, or changes in quasispecies recovered from lymphocyte-derived viral DNA were a complete and accurate representation of viral heterogeneity, plasma RNA was isolated by the RNAzol method, reverse transcribed, and amplified by PCR, and resultant cDNA was analyzed by HTA (see Materials and Methods). Amplified products from weeks 2, 4, 8, and 16 were examined for every animal. Overall, heterogeneity in viral RNA sequences again correlated with viral inoculum (compare Fig. 3A, C, and E with Fig. 3B, D, and F). The average numbers of variants at 2, 4, 8, and 16 wpi were 1.7, 1.0, 1.3, and 1.3 in animals given low-dose HIV-2287 (Table 3, animals 147, 350, and 404) and 5.3, 3.3, 5.3, and 4.0 in animals given high-dose HIV-2287 (Table 3, animals 096, 292, and 407). Overall, this trend was significant (P = 0.0004 by the Wilcoxon rank-sum test). A significant difference was also seen in normalized entropy (Table 3) between animals given a high inoculum (SN = 0.68 ± 0.00) and animals given a low HIV-2287 inoculum (SN = 0.62 ± 0.01, P < 0.02). Among both naive (animal 147) and HIV-2KR experienced animals (animals 350 and 404) given a low inoculum of HIV-2287, early (weeks 2 to 8) heterogeneity was comparable in RNA and DNA samples. Viral RNA was also detectable by PCR at later time points in some animals, including 16 wpi for the naive controls 096 and 147 (Fig. 3A and B) and weeks 52 to 68 and 52 to 56 for unprotected immunized animals 407 and 404, respectively (Fig. 3E and F). Conversely, macaques immunized with the higher dose of HIV-2KR both exhibited rapid reduction of numbers of plasma RNA viral variants to a single HTA band (Fig. 3C and D) by week 16, a trend which was not exhibited by control animal 096 given the higher inoculum of HIV-2287 (Fig. 3A) or by animals 404 and 407, immunized with the lower dose of HIV-2KR, where multiple bands persist. This impression is also reflected by entropy values which decline from 0.68 to 0.56 from weeks 2 to 16 in animal 292, averaging 0.63 in protected and 0.66 in unprotected animals (Table 3, P = 0.05). Even when using up to 10 times the amount of plasma used for earlier time points, we were unable to amplify viral RNA from protected animals 292 and 350 after week 16 (20, 26, 35, 44, 52, and 72 wpi [data not shown]). Protection seems therefore to be associated with restriction of variation of viral RNA sequences in the V1-V2 region in immunized animals 292 and 350. These animals were also noted to clear circulating virus to levels below 80 to 200 copies/ml by 24 wpi, despite detection of persistent high levels of viral DNA (29).
FIG. 3.
HTA of plasma viral RNA-derived sequences from the V1-V2 region of the HIV-2 envelope. Panels show the duplexes formed between the 32P-labeled ssDNA probe and the products of primers 290 and 244 amplifying the V1 and V2 regions of viral RNA obtained from plasma at various times after inoculation (see Materials and Methods). (A) Animal 096, naive, challenged with high-dose HIV-2287; (B) animal 147, naive, challenged with low-dose HIV-2287; (C) animal 292, immunized with high-dose HIV-2KR and challenged with high-dose HIV-2287; (D) animal 350, immunized with high-dose HIV-2KR and challenged with low-dose HIV-2287; (E) animal 407, immunized with low-dose HIV-2KR and challenged with high-dose HIV-2287; (F) animal 404, immunized with low-dose HIV-2KR and challenged with low-dose HIV-2287. Numbers above lanes correspond to the time point (week postinfection) to which the sample refers. SS, single-stranded 32P-labeled probe; HD, homoduplex band; MW, molecular weight marker. Similar to viral DNA sequences of the V1-V2 region, panels A, C, and E, from animals receiving the high HIV-2287 inoculum, initially display numerous bands, whereas fewer bands are seen in panels B, D, and F, from animals receiving the low dose HIV-2287 inoculum. Note the decrease in number of bands seen in panel C by 8 to 16 wpi in the protected 292 animal receiving the high-dose HIV-2287 inoculum, in contrast to the persistence of multiple bands in animals 407 and 404, which were not protected from CD4 decline, and to the numerous bands still present in the naive unprotected animal at the same time point (A).
TABLE 3.
Numbers of plasma RNA viral variants distinguishable by HTA and SN values of HTA gel lanes for RNA clones
| Animal | Inoculum
|
No. of plasma RNA viral variants distinguishable by HTA
|
SN of HTA lanes
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HIV-2KR (SFU) | HIV-2287 (TCID50) | 2 wpi | 4 wpi | 8 wpi | 16 wpi | 2 wpi | 4 wpi | 8 wpi | 16 wpi | |
| 096 | None | 105 | 5 | 4 | 7 | 7 | 0.80 | 0.71 | 0.75 | 0.70 |
| 147 | None | 101 | 2 | 1 | 1 | 1 | 0.60 | 0.60 | 0.56 | 0.57 |
| 292 | 104 | 105 | 4 | 4 | 2 | 1 | 0.65 | 0.69 | 0.55 | 0.57 |
| 350 | 104 | 101 | 1 | 1 | 1 | 1 | 0.60 | 0.62 | 0.73 | 0.63 |
| 407 | 103 | 105 | 7 | 2 | 7 | 4 | 0.77 | 0.62 | 0.75 | 0.65 |
| 404 | 103 | 101 | 2 | 1 | 2 | 2 | 0.63 | 0.60 | 0.60 | 0.58 |
Selection of clones for sequence analysis.
To confirm the results revealed by HTA, to identify the nature of mutations responsible for generation of quasispecies within the V1-V2 region, including characterization of the single variants emerging in plasma RNA from protected versus unprotected animals, and to determine whether similarly migrating bands found at different time points represented identical variants (conserved species) or the coincidental evolution of the similarly divergent variants, cloning and sequencing were performed on selected clones. PCR-amplified V1-CD4 fragments from naive macaque 096 (high dose of HIV-2EHO) and protected macaques 292 and 350 (high- and low-dose HIV-2287, respectively) were cloned from viral DNA, including samples from both early (week 8) and late (week 18 or 72) time points. For plasma RNA, only animals 292 (weeks 8 and 16) and 096 (week 16) were considered.
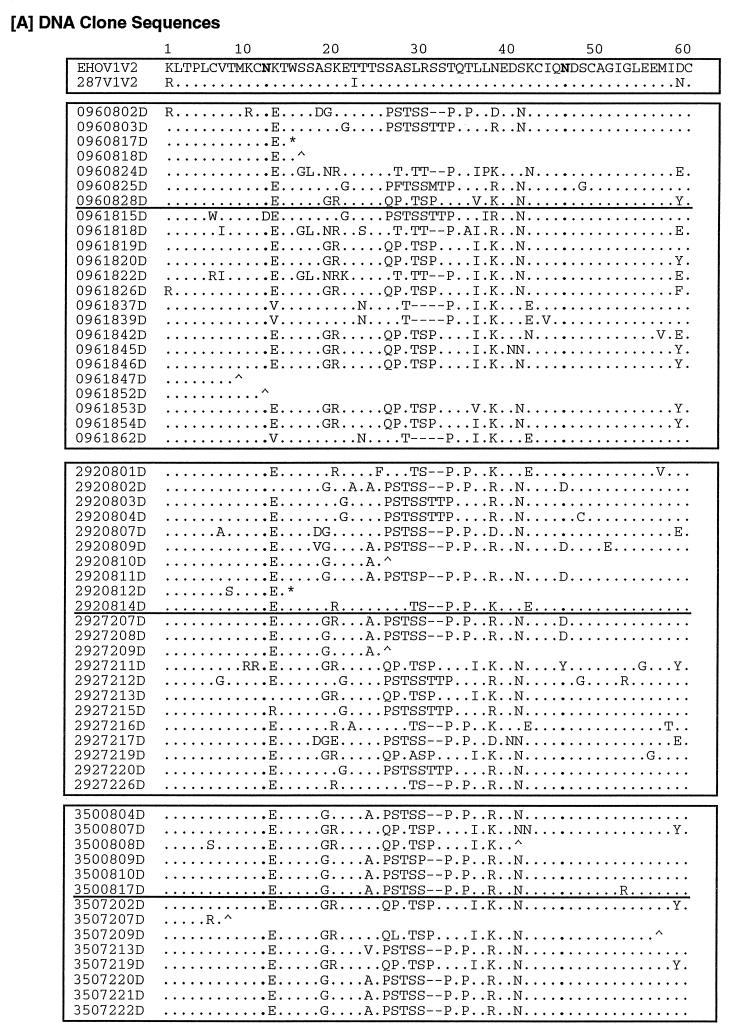
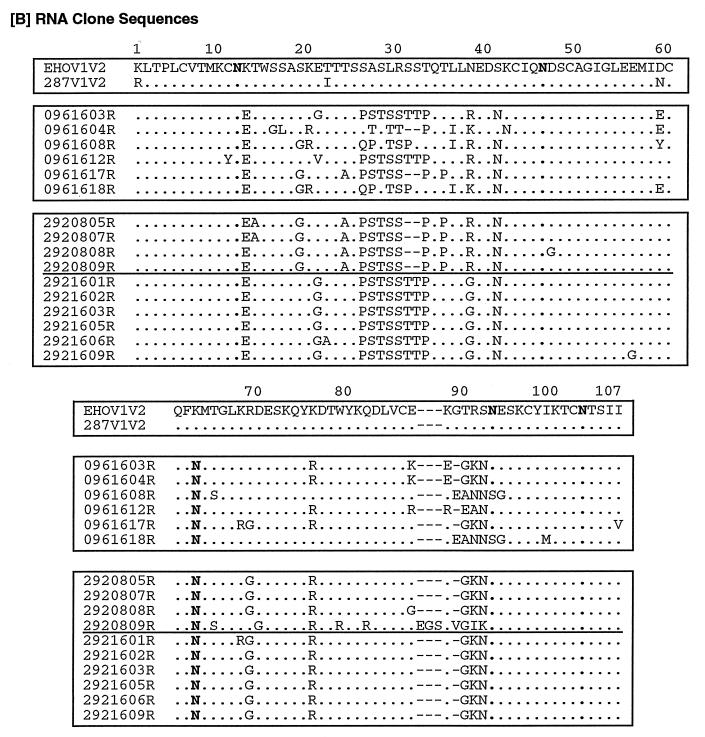
After amplification of the V1-V2 region by nested PCR, 30 to 40 clones from each animal at each time point were screened by HTA (see Materials and Methods). All clones which proved distinguishable by HTA, as well as a number of clones which appeared to be very similar, were sequenced. Figure 4a shows all distinguishable sequences, including those containing early or late frameshifts or stop codons, as well as some sequences found to be similar by HTA (e.g., 096/18/39-62 and clones 292/16/05-06-09). All of the sequences displayed a unique genotype in the V1-V2 region, the number and site of mutations being variable between isolates.
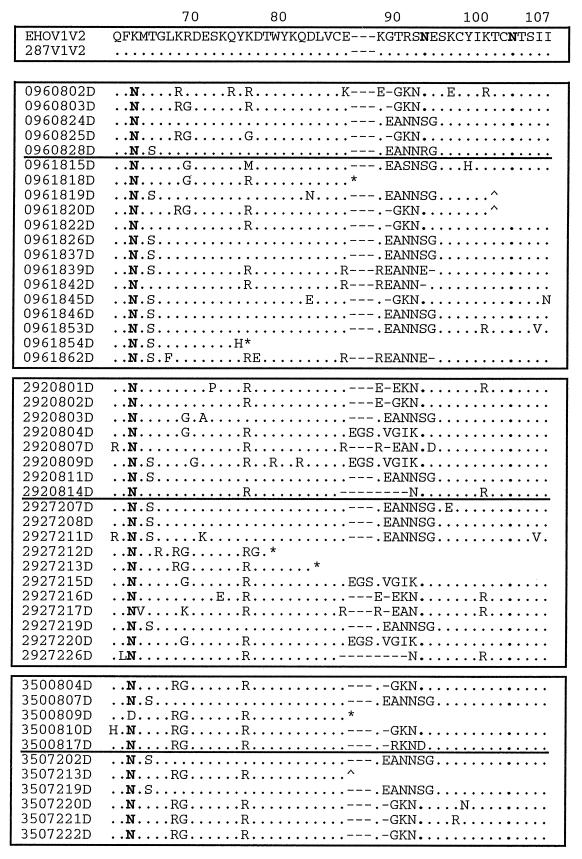
FIG. 4.
Predicted amino acid sequences of clones. Shown are the predicted amino acid sequences of DNA (A) and RNA (B) clones of the V1-V2 region of the HIV-2 envelope. The alignment numbering refers to the position in the HIV-2287 envelope fragment sequence. Each clone is designated by the animal number, followed by a two-digit number representing the week postinfection, a two-digit number indicating the clone, and “D” or “R”, designating the source as PBMC-derived viral DNA or plasma viral RNA. Included for comparison are sequences of the corresponding regions from HIV-2EHO (EHOV1V2) and an envelope clone of HIV-2287 (287V1V2). Multiple hierachical alignments were performed by using MULTALIN (12). •, identity; −, gap; ∧, frameshift; ∗, stop codon. Glycosylation sites (N) are in boldface.
Analysis of substitution frequencies.
To determine the significance of differences within sequence sets, the numbers of mutations resulting in amino acid changes and stop signals were compared in predicted sequences derived from DNA and RNA data from each animal, as well as between different animals. Overall, 6.7% of bases (1,718) were found to differ from that of the parental HIV-2EHO/HIV-2287 sequence, far in excess of the ∼0.1% which might be anticipated to occur during PCR amplification. This included 594 transversions, 1,124 transitions, 881 deletions, and 502 insertions, with 21,626 bases unchanged. Analysis of rates of base substitution in both DNA and RNA clones showed a slight excess in the number of mutations to A, a clear excess of changes to G, and no clear difference in the substitutions to C from that which would be predicted by random chance. A marked deficit in the number of mutations to T was also found, and a similar nonrandom (P < 0.001 by χ2 analysis) pattern was found in all subsets of sequences analyzed (including DNA only, RNA only, 8-wpi clones, 72-wpi clones, and subsets of clones from each individual animal).
Analysis of the effects of observed substitutions upon predicted amino acid sequences showed that most mutations were not silent (∼85% nonsynonymous [Fig. 4]). Using a set of 1,000 sequences constructed by using the observed frequencies of insertions, deletions, and substitutions, this finding differed significantly from that expected by random chance (15.6% versus 23.9% expected, P < < 0.001). The proportion of synonymous mutations in both DNA and RNA clones was significantly higher in the protected animal 292 (18.4%) than in the naive animal 096 given high-dose HIV-2287 (P < 0.01) but was still significantly less than the fraction expected by chance (P < 0.05). This observation held when we compared the total set of 096 sequences with DNA clones obtained from 292 at early times (292/08 [P < 0.05]), DNA clones at late times (292/72 [P < 0.05]), or RNA clones at late times (292/16 [P < 0.05]) or when we compared DNA clones obtained from 096 at late times either to the total set of 292 clones (P < 0.025) or to all subsets of 292 sequences (P < 0.05, 0.05, and 0.05, respectively). This result was primarily attributable to a reduced frequency of nonsynonymous mutations in the DNA of 292 at late time points and a very high incidence of nonsynonymous mutations in 096 at late time points.
Analysis revealed that frameshifts and stops were present only in DNA sequences; cysteines, tyrosines, and tryptophan residues were conserved; and introduction of glycosylation sites by mutation to asparagine was frequent. Mutations were in general conservative, especially with respect to basic (R) and hydrophilic (S, T) amino acids. In the sequenced sets of clones, 1,316 residues were substituted, 5,516 were unchanged, 113 were inserted and 284 were deleted, including 11 frameshifts and 67 stops in 59 DNA sequences but no frameshifts or stops in 16 clones obtained from RNA (P < 0.05, Fisher’s exact test). Cysteines were substituted only once for another residue (clone 292/08/04D, position 48) and highly conserved (625 residues). Three tyrosine residues (position 75, 80, and 98) and two tryptophan residues (position 15 and 79) were also highly conserved (for tyrosine, 3 of 185 positions substituted; for tryptophan, one insertion and seven substitutions of 142 positions [P < 0.001 for both]). In contrast, amino acid substitution introducing an asparagine was frequent (207 changes), producing a new predicted glycosylation site in 74 of 75 clones at position 67 relative to the HIV-2EHO/HIV-2287 sequence. A potential somatotropin type 2 motif of unknown significance was also noted to be conserved in 63 of 75 clones. The significance of the frequent mutation to asparagine (N) is uncertain, as it was common to both protected and unprotected animals.
Analysis of patterns in predicted amino acid sequences.
In an effort to determine if any specific motifs were associated with progression of disease versus protection, groups of peptide sequences were analyzed for signatures (Fig. 4). By both direct inspection of aligned sequences and the use of signature pattern analysis (26), we identified a number of motifs which appeared to differ in frequency between early and late times or between animals. The VESPA program identified residues in many motifs which were also noted by direct inspection (QPST, PSTSS, EAN, GR, and TILK [see below]).
In the V1 region, clusters of changed amino acids (PSTSS, residues 26 to 30) are represented in DNA clones at a higher frequency at week 8 than at week 18 or 72 (Fisher exact analysis, P = 0.019), the unprotected animal 096 showing at 18 wpi the lowest frequency (only 1 of 14 clones; P = 0.035). Conversely, other clusters (GR, residues 19 to 20; QPSTSP, residues 26 to 31; ILKEDN, residues 36 to 41) are present in DNA clones mainly at later times (P = 0.022, 0.038, and 0.003, respectively), showing the highest frequency in the unprotected animal (see clones 096/18/19, -20, -22, -26, -42, -45, -46, and -54).
Other motifs (PQPLLREDN, residues 33 to 41), although present with almost the same frequency at early and late times, are absent in all DNA clones of the unprotected animal both at week 8 and week 18, only occurring once in the RNA clone 096/16/17R (P = 0.005). Among the animals given the highest dose of challenge virus, a TTP motif (residues 31 to 33) tends to recur with higher frequency in the protected animal 292 (292/08/03 and -04 and 292/72/12, -15, and -20), whereas it is present in only two clones of the unprotected animal (096/08/03 to -15) (P = 0.079). Conversely, the motif RGDESKQYR (residues 68 to 76) is present at significantly higher frequency (P = 0.002) in the animal given the lowest dose of challenge virus (clones 350/08/04, -09, -10, and -17 and 350/72/13, -20, -21, and -22). The first three residues of this motif may represent an integrin binding (RGD) site. In the V2 region, clusters of amino acids (EAN, 89 to 91) appear in clones from all animals but are present with a higher frequency in clones obtained at late times than in clones obtained at earlier times (P = 0.065). A three-amino-acid insertion within EGSKVGIK (residues 88 to 95) is present at both early and late times and appears unique for the protected animal challenged with the high dose of HIV-2287 (292/08/04 to -09 and 292/72/15 to -20).
Clones derived from RNA show differences similar to those noted above for DNA clones. RNA clones 096/16/08 to -18 show amino acid motifs (residues 21 to 22) similar to those found in DNA clone 096/08/28 and clones 096/18/19, -20, -26, -42, -45, -46, -53, and -54 in the same region. In a similar fashion, RNA clones 096/16/3 to -12 and -17 bear a great similarity in the V1 region (amino acids 26 to 33) to DNA clones 096/08/02, -03, and -25 and to clone 096/18/15. In animal 292, the motif PSTSS (residues 26 to 30) already seen in DNA sequences is also observed in RNA sequences at both early (clones 5, 7, 8, and 9) and late (clones 1, -2, -5, -6, and -9) times. This motif is always preceded by an AS amino acid motif at early times, which is also a characteristic found in the DNA clones of protected animals as well as the motif PQPLLREDN (residues 33 to 41). This motif is always present in RNA clones at early times mainly in the protected animal and in DNA clones only in protected animals (P = 0.0052). The TTP motif (residues 31 to 33) is present as well in all RNA clones only at late times, and it is more frequent (P = 0.026) in the protected animal 292 than in the 096 clones from the unprotected animal (096/16/03 to -12). In the V2 region the cluster of amino acids EGSKVGIK (residues 88 to 95), which is unique among the DNA clones of the protected animal 292, is also present in an RNA clone from this animal (292/08/09). In RNA clones, the GKN motif (residues 90 to 92), also present in DNA clones but at a lower frequency, seems to completely substitute for the EANNSG motif (residues 89 to 94) in the same position (P = 0.0006, frequency in DNA versus RNA clones). Therefore, while their significance is unclear, amino acid signatures related to protection were identified within both the V1 and V2 regions in both DNA and RNA clones.
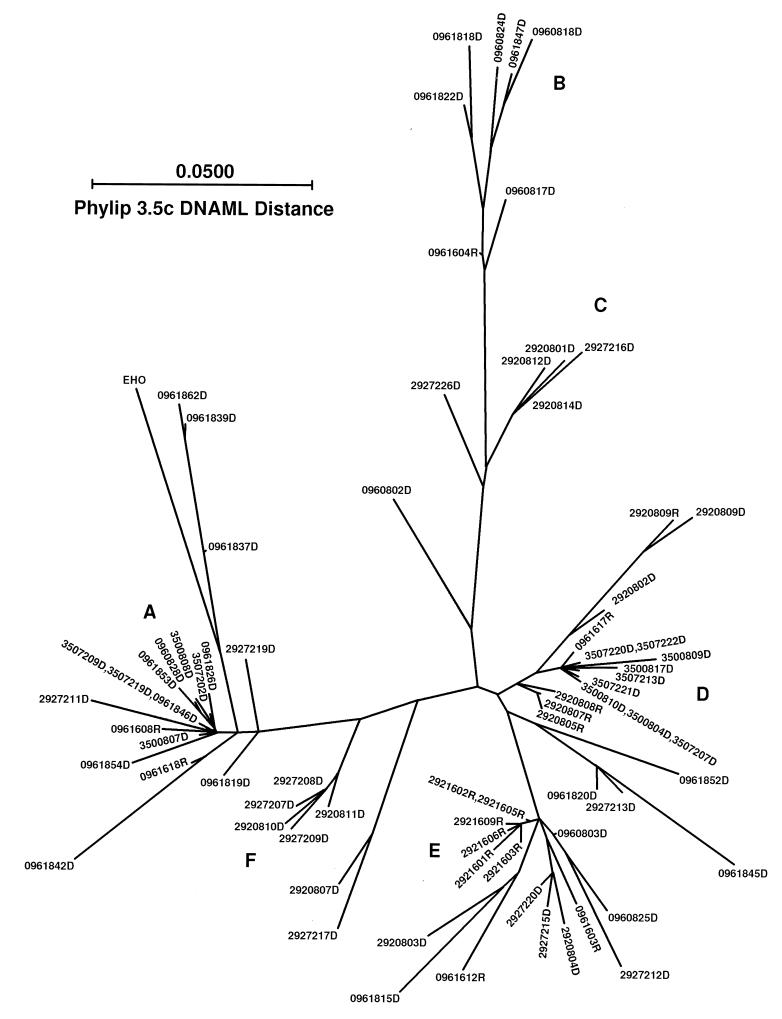
Phylogenetic analysis.
To examine the relationships among different clones, maximum-likelihood parsimony of nucleic acid sequences was also used (Fig. 5). Some of the sequences from the same animal with similar motifs noted above are seen to cluster together at specific terminal branches (Fig. 5, 350/08 and -72 at the extreme right [D] and left [A] branches, 292/08 and -72 in the mid-upper [C] and mid-lower [F] branches, and 096/08 and -18 in the upper branch [B]). However, 096/08 and -18 DNA clones and 292/08 and -72 DNA clones are distributed in branches throughout the tree, reflecting the higher heterogeneity of clones from animals receiving the high-dose challenge virus, as seen by HTA. Sequences from different animals are seen to be highly interrelated. Note that variants from animal 292 at 72 wpi can be seen clustering with groups of sequences derived from animal 096 at 8 and 18 wpi in the mid-lower portion of the tree (E), and sequences from animal 350 at 8 and 72 wpi cluster with sequences from animal 292 at 8 wpi in the right branch (D) and with 096/18 in the left branch (A), so that a specific relationship between clustering of clones derived from protected or unprotected animals was not evident.
FIG. 5.
Phylogenetic analysis of clones. Sequences of both PBMC-derived viral DNA and plasma virus RNA clones were aligned as in Fig. 4, and a phylogenetic tree was constructed by using the DNAML algorithm in the PHYLIP 3.5 package. Clones are designated as in Fig. 4. This is an unrooted tree drawn approximately to scale, based on interpolation of several pairs. Note that while there is clustering of clones obtained from a given animal at a given time point, some evidence of parallel evolution of sequences is present. Also, note the tight clustering of RNA sequences from protected animal 292 at 16 wpi.
Phylogenetic analysis of RNA sequences (Fig. 5) also showed that clones derived from animal 096 and 292 were sometimes closely related (lower right branches E and D). However, all RNA clones of animal 292 at 16 wpi are clustered together in lower middle branch E, and the 292 RNA clones at 8 wpi are all in right branch D, whereas RNA clones from animal 096 can be found in the terminal branches throughout the tree. The contrast in distribution between RNA clones from unprotected animal 096 and RNA clones from protected animal 292 reflects the restricted variation in RNA sequences from the protected animal already shown by HTA.
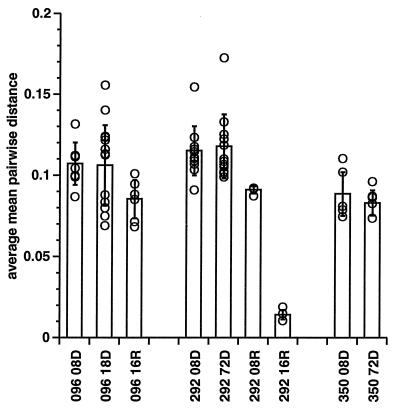
Total extent of genetic variation.
To examine the total extent of variation, distances between all 75 clones were derived by using both Hamming distances (identity) and a maximum-likelihood algorithm (17). The average mean pairwise distance (MPWD) for each clone and the distribution of distances between all members of each group were then compared (Fig. 6) with the average and distribution of clones from other groups, using both the Wilcoxon rank-sum statistic and the Satterthwaite approximation for t test for two samples with unequal means (SAS, nonparametric analysis of variance, and t-test analysis, respectively). When the DNA clones from different animals were compared at the same time point, the average MPWD (obtained by using Hamming distances of aligned sequences) of viral DNA sequences from unprotected animal 096 and protected animal 292 at 8 wpi given the higher inoculum of HIV-2287 did not show significant differences (MPWD ± standard deviation, 0.107 ± 0.013 versus 0.115 ± 0.015). Conversely, the MPWD of DNA sequences of clones from animal 350 (low HIV-2287 inoculum) were significantly smaller (0.089 ± 0.013) than those from clones from animal 096 or 292 at 8 wpi (P = 0.040 or 0.004, respectively, t test with unequal means), which is in agreement with the smaller number of bands seen on HTA in animals given the lower inoculum. Similarly, at 72 wpi the MPWD of DNA sequences of clones from animal 350 was also significantly smaller than that of clones from animal 292 (0.083 ± 0.008 versus 0.118 ± 0.020, P < 0.001). When comparing the DNA clones from the same animal at different time points, we noted no significant trend over time in MPWD in DNA clones (compare 096 DNA clones at 8 versus 18 wpi or 292 and 350 DNA clones at 8 versus 72 wpi). When MPWD of RNA and DNA clones were compared within the same animal at the same time points, the MPWD of RNA clones from animal 096 at 16 wpi, while smaller (0.085 ± 0.012 versus 0.106 ± 0.025), did not differ significantly (P = 0.075) from that of DNA clones obtained at 18 wpi. In contrast, the MWPD of RNA clones from animal 292 at 8 wpi was significantly smaller than that of DNA clones at the same time (P = 0.045, 0.091 ± 0.022 versus 0.115 ± 0.015), and at 16 wpi the MWPD of RNA clones from animal 292 was also significantly smaller than that of animal 096 (P < 0.001, 0.014 ± 0.003 versus 0.085 ± 0.012). The MWPD of RNA clones from animal 292 is also seen to decrease (P = 0.082) between 8 and 16 wpi, consistent with the loss of bands seen in the RNA HTA (Fig. 3) and the close clustering of 292/16 RNA clones seen in the phylogenetic tree (Fig. 5, branch E). The analysis of the total extent of variation, as determined by the MWPD of aligned sequences, confirms that distances in the DNA sequences between different animals at the same time points or within the same animal at different time points, even when statistically significant, do not show any association with protection. In contrast, variation in viral RNA species was reduced in the protected animal compared to the RNA and DNA species of the naive control at the same time point, confirming HTA results.
FIG. 6.
MPWD of groups of sequences. Shown are average MPWD (open bars), standard errors of the average distance (error bars), and individual MPWD (open circles) of sequences in various groups. Clones are designated as in Fig. 4, except that the clone number is not indicated. Distances were determined by the PHYLIP DNADIST program using the DNAML algorithm and by FASTA comparison using an identity matrix. MPWD are always lower for RNA sequences than for DNA sequences, and a significant difference is observed between the RNA clones from protected animal 292 16R and RNA clones from unprotected animal 096 16R.
DISCUSSION
Genetic variability has often been studied with the aim of finding a correlation with the viral phenotype and pathogenesis (10, 57). Sequential isolates or directly amplified clones within the same patients or animals have been examined to investigate the association of specific or unique species with progression to disease (10, 28, 36, 47, 49), as well as the role of the overall degree of variability in immunopathogenesis (10, 11, 13, 40, 41, 47, 57). While evidence from the studies noted above indicated a correlation between genotype, phenotype, and disease progression, recent studies have found a lack of correlation between sequence divergence and disease progression (51) and have found that acquisition of changes in the V3 loop indicative of development of an SI phenotype was not predictive (25, 56). The correlation between long-term nonprogressors and viral load or cytotoxic T-lymphocyte activity level was also found to be more significant than with specific viral phenotypes (11, 19). Similarly, some investigators examining overall variability have found early infection or nonprogression to be correlated with lower diversity (34–36, 47) and progressive disease to be associated with increasing quasispecies diversity (24, 33), even though convergence of specific groups or motifs (e.g., SI variants in the V3 loop) were observed (36, 47). In contrast, other investigators have noted higher diversity in nonprogressors (15, 56) or found diversity to correlate only with time after infection (30). Variants have also been described to differ significantly in various tissue compartments (7, 58).
In this study, two distinct regions inside the env gene were amplified by PCR: one encompassing the V1 and V2 regions (designated V1), and the other including the V3, V4, and CD4-binding regions (designated CD4). Viral polymorphism was first analyzed by HTA, followed by sequence analysis of V1-V2 clones (largely those which were distinguishable as unique by HTA screening). Comparison of the complexity of HTA patterns and quantitative analysis of HTA gel images obtained by using viral DNA did not reveal any correlation with protection from disease, or any trend with time, but rather indicated that complexity was related to inoculum size. In contrast, examination of plasma viral RNA by HTA revealed that a single variant was evident by 8 wpi in immunized animals which were protected from disease, whereas the naive control animal given a high-dose inoculum of HIV-2287, and both immunized animals which were not protected from CD4 decline, showed persistence of multiple variants.
To obtain additional and potentially complementary information concerning genetic diversity over time, we cloned and sequenced a subset of viral DNA- and plasma viral RNA-derived PCR products. This allowed comparison of HTA results with actual sequence data from the same amplification reactions from the same animals at the same time points. While HTA of PBMC-derived DNA clones did not reveal different patterns of variation between protected and unprotected animals, both HTA and sequence data established that viral DNA sequences were more restricted in animals given lower inocula. At 8 wpi, only low copy numbers were predicted to be present in PCR products for animals given 10 TCID50 of HIV-2287. The initial reduced diversity seen in animals given the lower HIV-2287 inoculum, therefore, is accompanied by reduced or delayed virus replication (29, 52) and may not represent an independent factor. The extent of heterogeneity in plasma RNA-derived sequences was less than that in viral DNA sequences for all animals and was further restricted in the protected animal given the high inoculum of HIV-2287 compared to the naive control at 16 wpi, when at least 1,000 copies of viral RNA was included in each reaction. Examination of the course of the extent of variation of viral DNA sequences over time was unrevealing. In contrast, variation in plasma viral RNA sequences was significantly and markedly restricted from 8 to 16 wpi in protected animal 292 (Fig. 6). This restriction of variability in RNA sequences over time in protected animal 292 would not seem to be consistent with those models of infection which predict that maintenance of diversity is associated with lack of progression, while narrowing of variation is (16, 33–36, 47). Rather, effective clearance of virus is associated with a significant restriction of the plasma virus repertoire (24). This is not reflected in viral DNA sequences, perhaps due to the accumulation of nonreplicating DNA variants in circulating PBMCs, emphasizing the importance of analyzing plasma viral RNA.
Overall, mutations to A and G predominated, and mutation to T was significantly underrepresented, but no evidence of hypermutation to A was seen in this sequence set. Not surprisingly, nonsynonymous mutations were seen more frequently than expected by chance in infected animals. However, if this is reflective of immune pressure, it is difficult to explain why protected animal 292, which completely cleared plasma viremia, exhibited the highest frequency of synonymous mutations. In general, mutations were conservative, especially with respect to basic (R) and hydrophilic (S, T) amino acids, and cysteine and tryptophan residues were highly conserved. Prior studies of variation in immunopathogenesis have not provided convincing evidence that any specific variant or motif is related to progression of disease or to protection from such progression. While the limited number of animals in the present study may limit conclusions, some of the differences in amino acid signatures noted are intriguing. A new potential glycosylation site was acquired by almost all sequences by 8 wpi. This is analogous to the acquisition of glycosylation sites in and around the SIV V1 region noted to occur during viral evolution in vivo (1). In addition, several motifs were seen more frequently within the amino acid sequences of protected animals. These included adjacent motifs PSTSS (residues 26 to 30), TTP motif (residues 31 to 33), and PQPLLREDN (residues 33 to 41) within the V1 region of envelope and the EGSKVGIK motif (residues 88 to 95) within the V2 region. While association with protection was significant (P < 0.05 by two-tailed Fisher exact test), analysis of individual amino acid substitutions did not reveal changes of anywhere near the magnitude (P < 10−14) seen in comparison of groups of pol sequences with residue changes induced by drug resistance (41).
The use of both HTA and sequencing directed by HTA screening of PCR clones would seem to provide a much more comprehensive and accurate picture of the scope and role of genomic variation in this model than either technique alone, while avoiding the extensive labor which would be involved in the exclusive use of sequencing, typically involving ∼30 clones per time point per animal. While the method used to select clones for sequencing did not result in analysis of a population of clones which were equally well represented in the starting material, it did allow the maximal extent of variation present within populations to be estimated without requiring the sequencing of hundreds of clones. Note also that neither HTA analysis nor sequencing indicated the emergence of a single predominant viral DNA clone, somewhat ameliorating objections to the selected sequence data. The use of sequence data validated HTA estimates of the total extent of variation. Similar analysis of an ongoing experiment with larger numbers of animals should provide more conclusive evidence on the nature and mechanism of viral variation in this live-virus vaccine model and its significance for protection.
ACKNOWLEDGMENTS
This project was made possible by National Collaborative Vaccine Development Group grant 5 U01 AI30238-05 and was also supported in part by a Simian Vaccine Evaluation Unit at the University of Washington Seattle Regional Primate Research Center subcontract (UCSD 95-6461). Sequencing and data analysis support for this project was also provided by the UCSD Center for AIDS Research Molecular Biology Core (grant 2 P30 AI36214-02). Support for visiting scientist Antonia Radaelli was provided through a grant from the Superior Institute of Health, AIDS Project, Rome, Italy.
We are grateful to E. L. Delwart for protocols and helpful discussion. We also thank Michael Wen, Richard Szubin, and Silvestre Ramos for technical assistance.
REFERENCES
- 1.Almond N, Jenkins, A. A, Heath A B, Kitchin P. Sequence variation in the env gene of simian immunodeficiency virus recovered from immunized macaques is predominantly in the V1 region. J Gen Virol. 1993;74:865–871. doi: 10.1099/0022-1317-74-5-865. [DOI] [PubMed] [Google Scholar]
- 2.Almond N, Jenkins A, Heath A B, Taffs L F, Kitchin P. The genetic evolution of the envelope gene of simian immunodeficiency virus in cynomolgus macaques infected with a complex virus pool. Virology. 1992;191:996–1002. doi: 10.1016/0042-6822(92)90280-3. [DOI] [PubMed] [Google Scholar]
- 3.Baier M, Dittmar M T, Cichutek K, Kurth R. Development in vivo of genetic variability of simian immunodeficiency virus. Proc Natl Acad Sci USA. 1991;88:8126–8130. doi: 10.1073/pnas.88.18.8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnett S W, Murthy K K, Herndier B G, Levy J A. An AIDS-like condition induced in baboons by HIV-2. Science. 1994;266:642–646. doi: 10.1126/science.7939718. [DOI] [PubMed] [Google Scholar]
- 5.Bayon-Auboyer M H, Boussin F D, Vogt G, Le Grand R, Vaslin B, Nicol-Jourdain I, Dormont D. Evolution of the human immunodeficiency virus type 2 envelope gene in preimmunized and persistently infected rhesus macaques. J Virol. 1994;68:3415–3420. doi: 10.1128/jvi.68.5.3415-3420.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burns D P, Desrosiers R C. Selection of genetic variants of simian immunodeficiency virus in persistently infected rhesus monkeys. J Virol. 1991;65:1843–1854. doi: 10.1128/jvi.65.4.1843-1854.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell B J, Hirsch V M. Extensive envelope heterogeneity of simian immunodeficiency virus in tissues from infected macaques. J Virol. 1994;68:3129–3137. doi: 10.1128/jvi.68.5.3129-3137.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Z, Tlefer P, Gettie A, Reed P, Zhang L, Ho D D, Marx P A. Genetic characterization of new West African simian immunodeficiency virus SIVsm: geographic clustering of household-derived SIV strains with human immunodeficiency virus type 2 subtypes and genetically diverse viruses from a single feral sooty mangabey troop. J Virol. 1996;70:3617–3627. doi: 10.1128/jvi.70.6.3617-3627.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng-Mayer C. HIV-1 variation: consequences for disease progression and vaccine strategies. Trends Microbiol. 1993;1:353–355. doi: 10.1016/0966-842x(93)90077-5. [DOI] [PubMed] [Google Scholar]
- 10.Connor R I, Ho D D. Human immunodeficiency virus type 1 variants with increased replicative capacity develop during the asymptomatic stage before disease progression. J Virol. 1994;68:4400–4408. doi: 10.1128/jvi.68.7.4400-4408.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Connor R I, Mohri H, Cao Y, Ho D D. Increased viral burden and cytopathicity correlate temporally with CD4+ T-lymphocyte decline and clinical progression in human immunodeficiency virus type 1-infected individuals. J Virol. 1993;67:1772–1777. doi: 10.1128/jvi.67.4.1772-1777.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corpet F. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 1988;16:10881–10890. doi: 10.1093/nar/16.22.10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Courgnaud V, Laure F, Fultz P N, Montagnier L, Brechot C, Sonigo P. Genetic differences accounting for evolution and pathogenicity of simian immunodeficiency virus from a sooty mangabey monkey after cross-species transmission to a pig-tailed macaque. J Virol. 1992;66:414–419. doi: 10.1128/jvi.66.1.414-419.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13a.Delwart, E. L. Personal communication.
- 14.Delwart E L, Shpaer E G, Louwagie J, McCutchan F E, Grez M, Rubsamen-Waigmann H, Mullins J I. Genetic relationships determined by a DNA heteroduplex mobility assay: analysis of HIV-1 env genes. Science. 1993;262:1257–1261. doi: 10.1126/science.8235655. [DOI] [PubMed] [Google Scholar]
- 15.Delwart E L, Sheppard H W, Walker B D, Goudsmit J, Mullins J I. Human immunodeficiency virus type 1 evolution in vivo tracked by DNA heteroduplex mobility assays. J Virol. 1994;68:6672–6683. doi: 10.1128/jvi.68.10.6672-6683.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delwart E L, Pan H, Sheppard H W, Wolpert D, Neumann A U, Korber B, Mullins J I. Slower evolution of human immunodeficiency virus type 1 quasispecies during progression to AIDS. J Virol. 1997;71:7498–7508. doi: 10.1128/jvi.71.10.7498-7508.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Felsenstein J. PHYLIP (phylogeny inference package) version 3.5c. Copyright J. Felsenstein and the Department of Genetics. Seattle: University of Washington; 1993. [Google Scholar]
- 18.Felsenstein J. The statistical approach to inferring evolutionary trees and what it tells us about parsimony and compatibility. In: Duncan T, Stuessy T F, editors. Cladistics: perspectives in the reconstruction of evolutionary history. New York, N.Y: Columbia University Press; 1984. pp. 169–191. [Google Scholar]
- 19.Ferbas J, Kaplan A H, Hausner M A, Hultin L E, Matud J L, Liu Z, Panicali D L, Nerng-Ho H, Detels R, Giorgi J V. Virus burden in long-term survivors of human immunodeficiency virus (HIV) infection is a determinant of anti-HIV CD8+ lymphocyte activity. J Infect Dis. 1995;172:329–339. doi: 10.1093/infdis/172.2.329. [DOI] [PubMed] [Google Scholar]
- 20.Franchini G, Markham P, Gard E, Fargnoli K, Keubaruwa S, Jagodzinski L, Robert-Guroff M, Lusso P, Ford G, Wong-Staal, F. F, Gallo R C. Persistent infection of rhesus macaques with a molecular clone of human immunodeficiency virus type 2: evidence of minimal genetic drift and low pathogenetic effects. J Virol. 1990;64:4462–4467. doi: 10.1128/jvi.64.9.4462-4467.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fung M S, Sun C R, Gordon W L, Liou R S, Chang T W, Sun W N, Daar E S, Ho D D. Identification and characterization of a neutralization site within the second variable region of human immunodeficiency virus type 1 gp120. J Virol. 1992;66:848–856. doi: 10.1128/jvi.66.2.848-856.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galabru J, Rey-Cuille M A, Hovanessian A G. Nucleotide sequence of the HIV-2 EHO genome, a divergent HIV-2 isolate. AIDS Res Hum Retroviruses. 1995;11:873–874. doi: 10.1089/aid.1995.11.873. [DOI] [PubMed] [Google Scholar]
- 23.Hahn B H, Gonda M A, Shaw G M, Popovic M, Hoxie J A, Gallo R C, Wong-Staal F. Genomic diversity of the acquired immune deficiency syndrome virus HTLV-III: different viruses exhibit greatest divergence in their envelope genes. Proc Natl Acad Sci USA. 1985;82:4813–4817. doi: 10.1073/pnas.82.14.4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hutto C, Zhou Y, He J, Geffin R, Hill M, Scott W, Wood C. Longitudinal studies of viral sequence, viral phenotype, and immunologic parameters of human immunodeficiency virus type 1 infection in perinatally infected twins with discordant disease courses. J Virol. 1996;70:3589–3598. doi: 10.1128/jvi.70.6.3589-3598.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kasper P, Kaiser R, Oldenburg J, Brackmann H H, Matz B, Schneweis K E. Parallel evolution in the V3 region of HIV type 1 after infection of hemophiliacs from a homogeneous source. AIDS Res Hum Retroviruses. 1994;10:1669–1678. doi: 10.1089/aid.1994.10.1669. [DOI] [PubMed] [Google Scholar]
- 26.Korber B, Myers G. Signature pattern analysis: a method for assessing viral sequence relatedness. AIDS Res Hum Retroviruses. 1992;8:1549–1560. doi: 10.1089/aid.1992.8.1549. [DOI] [PubMed] [Google Scholar]
- 27.Kraus G, Radaelli A, Talbott R, Leavitt M, Schmidt A, Badel P, Bartz C, Morton W, Wong-Staal, F. F, Looney D J. Characterization of a molecular clone of HIV type 2 infectious for Macaca nemestrina. AIDS Res Hum Retroviruses. 1998;14:65–77. doi: 10.1089/aid.1998.14.65. [DOI] [PubMed] [Google Scholar]
- 28.Kuiken C L, de Jong J J, Baan E, Keulen W, Tersmette M, Goudsmit J. Evolution of the V3 envelope domain in proviral sequences and isolates of human immunodeficiency virus type 1 during transition of the viral biological phenotype. J Virol. 1992;66:4622–4627. doi: 10.1128/jvi.66.7.4622-4627.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Looney D J, McClure J, Kent S, Radaelli A, Kraus G, Schmidt A, Steffy K, Greenberg P, Hu S-L, Morton W, Wong-Staal F. A minimally replicative HIV-2 live-virus vaccine protects M. nemestrina from disease after HIV-2287 challenge. Virology. 1998;242:150–160. doi: 10.1006/viro.1997.8992. [DOI] [PubMed] [Google Scholar]
- 30.Lukashov V V, Kuiken C L, Goudsmit J. Intrahost human immunodeficiency virus type 1 evolution is related to length of the immunocompetent period. J Virol. 1995;69:6911–6916. doi: 10.1128/jvi.69.11.6911-6916.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marlink R G, Ricard D, M’Boup S, Kanki P J, Romet-Lemonne J L, N’Doye I, Diop K, Simpson M A, Greco F, Chou M J, Degruttola V, Hsieh C-C, Boye C, Barin F, Denis F, McLane M F, Essex M. Clinical, hematologic, and immunologic cross-sectional evaluation of individuals exposed to human immunodeficiency virus type-2 (HIV-2) AIDS Res Hum Retroviruses. 1988;4:137–148. doi: 10.1089/aid.1988.4.137. [DOI] [PubMed] [Google Scholar]
- 32.Matthews T J, Langlois A J, Robey W G, Chang N T, Gallo R C, Fischinger P J, Bolognesi D P. Restricted neutralization of divergent human T-lymphotropic virus type III isolates by antibodies to the major envelope glycoprotein. Proc Natl Acad Sci USA. 1986;83:9709–9713. doi: 10.1073/pnas.83.24.9709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCutchan F E, Artenstein A W, Sanders-Buell E, Salminen M O, Carr J K, Mascola J R, Yu X F, Nelson K E, Khamboonruang C, Schmitt D, Kieny M P, McNeil J G, Burke D S. Diversity of the envelope glycoprotein among human immunodeficiency virus type 1 isolates of clade E from Asia and Africa. J Virol. 1996;70:3331–3338. doi: 10.1128/jvi.70.6.3331-3338.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McNearney T, Hornickova Z, Markham R, Birdwell A, Arens M, Saah A, Ratner L. Relationship of human immunodeficiency virus type 1 sequence heterogeneity to stage of disease. Proc Natl Acad Sci USA. 1992;89:10247–10251. doi: 10.1073/pnas.89.21.10247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nowak M A, Anderson R M, McLean A R, Wolfs T F, Goudsmit J, May R M. Antigenic diversity thresholds and the development of AIDS. Science. 1991;254:963–969. doi: 10.1126/science.1683006. [DOI] [PubMed] [Google Scholar]
- 36.Oka S, Ida S, Shioda T, Takebe Y, Kobayashi N, Shibuya, Y. Y, Ohyama K, Momota K, Kimura S, Shimada K. Genetic analysis of HIV-1 during rapid progression to AIDS in an apparently healthy man. AIDS Res Hum Retroviruses. 1994;10:271–277. doi: 10.1089/aid.1994.10.271. [DOI] [PubMed] [Google Scholar]
- 37.Otten R A, Brown B G, Simon M, Lupo L D, Parekh B S, Lairmore M D, Schable C A, Schochetman G, Rayfield M A. Differential replication and pathogenic effects of HIV-1 and HIV-2 in Macaca nemestrina. AIDS. 1994;8:297–306. doi: 10.1097/00002030-199403000-00002. [DOI] [PubMed] [Google Scholar]
- 38.Overbaugh J, Rudensey L M, Papenhausen M D, Benveniste R E, Morton W R. Variation in simian immunodeficiency virus env is confined to V1 and V4 during progression to simian AIDS. J Virol. 1991;65:7025–7031. doi: 10.1128/jvi.65.12.7025-7031.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Phillips R E, Rowland-Jones S, Nixon D F, Gotch F M, Edwards J P, Ogunlesi A O, Elvin J G, Rothbard J A, Bangham C R, Rizza C R, McMichael A J. Human immunodeficiency virus genetic variation that can escape cytotoxic T cell recognition. Nature. 1991;354:453–459. doi: 10.1038/354453a0. [DOI] [PubMed] [Google Scholar]
- 40.Putkonen P, Bottiger B, Warstedt K, Thorstensson R, Albert J, Biberfeld G. Experimental infection of cynomolgus monkeys (Macaca fascicularis) with HIV-2. J Acquired Immune Defic Syndr. 1989;2:366–373. [PubMed] [Google Scholar]
- 41.Richman D D, Bozzette S A. The impact of the syncytium-inducing phenotype of human immunodeficiency virus on disease progression. J Infect Dis. 1994;169:968–974. doi: 10.1093/infdis/169.5.968. [DOI] [PubMed] [Google Scholar]
- 42.Rudensey L M, Kimata J T, Benveniste R E, Overbaugh J. Progression to AIDS in macaques is associated with changes in the replication, tropism, and cytopathic properties of the simian immunodeficiency virus variant population. Virology. 1995;207:528–542. doi: 10.1006/viro.1995.1113. [DOI] [PubMed] [Google Scholar]
- 43.Safrit J T, Lee A Y, Andrews C A, Koup R A. A region of the third variable loop of HIV-1 gp120 is recognized by HLA-B7-restricted CTLs from two acute seroconversion patients. J Immunol. 1994;153:3822–3830. [PubMed] [Google Scholar]
- 44.Schuitemaker H, Koot M, Kootstra N A, Dercksen M W, de Goede R E, van Steenwijk R P, Lange J M, Schattenkerk J K, Miedema F, Tersmette M. Biological phenotype of human immunodeficiency virus type 1 clones at different stages of infection: progression of disease is associated with a shift from monocytotropic to T-cell-tropic virus population. J Virol. 1992;66:1354–1360. doi: 10.1128/jvi.66.3.1354-1360.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schultz A M, Koff W C. Prospects for an AIDS vaccine. Semin Immunol. 1990;2:351–359. [PubMed] [Google Scholar]
- 46.Stahl-Hennig C, Herchenroder O, Nick S, Evers M, Stille-Siegener M, Jentsch K D, Kirchhoff F, Tolle T, Gatesman T J, Luke W, Hunsmann G. Experimental infection of macaques with HIV-2ben, a novel HIV-2 isolate. AIDS. 1990;4:611–617. doi: 10.1097/00002030-199007000-00001. [DOI] [PubMed] [Google Scholar]
- 47.Strunnikova N, Ray S C, Livingston R A, Rubalcaba E, Viscidi R P. Convergent evolution within the V3 loop domain of human immunodeficiency virus type 1 in association with disease progression. J Virol. 1995;69:7548–7558. doi: 10.1128/jvi.69.12.7548-7558.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tersmette M, Lange J M, de Goede R E, de Wolf F, Eeftink-Schattenkerk J K, Schellekens P T, Coutinho R A, Huisman J G, Goudsmit J, Miedema F. Association between biological properties of human immunodeficiency virus variants and risk for AIDS and AIDS mortality. Lancet. 1989;1:983–985. doi: 10.1016/s0140-6736(89)92628-7. [DOI] [PubMed] [Google Scholar]
- 49.Tersmette M, Gruters R A, de Wolf F, de Goede R E, Lange J M, Schellekens P T, Goudsmit J, Huisman H G, Miedema F. Evidence for a role of virulent human immunodeficiency virus (HIV) variants in the pathogenesis of acquired immunodeficiency syndrome: studies on sequential HIV isolates. J Virol. 1989;63:2118–2125. doi: 10.1128/jvi.63.5.2118-2125.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tolle T, Petry H, Bachmann B, Hunsmann G, Luke W. Variability of the env gene in cynomolgus macaques persistently infected with human immunodeficiency virus type 2 strain ben. J Virol. 1994;68:2765–2771. doi: 10.1128/jvi.68.4.2765-2771.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang N, Zhu T, Ho D D. Sequence diversity of V1 and V2 domains of gp120 from human immunodeficiency virus type 1: lack of correlation with viral phenotype. J Virol. 1995;69:2708–2715. doi: 10.1128/jvi.69.4.2708-2715.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Watson A, Ranchalis J, Travis B, McClure J, Sutton W, Johnson P R, Hu S-L, Haigwood N. Plasma viremia in macaques infected with simian immunodeficiency virus: plasma viral load early in infection predicts survival. J Virol. 1997;71:284–290. doi: 10.1128/jvi.71.1.284-290.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weiss R A, Clapham P R, Weber J N, Dalgleish A G, Lasky L A, Berman P W. Variable and conserved neutralization antigens of human immunodeficiency virus. Nature. 1986;324:572–575. doi: 10.1038/324572a0. [DOI] [PubMed] [Google Scholar]
- 54.Wolfs T F, Zwart G, Bakker M, Valk M, Kuiken C L, Goudsmit J. Naturally occurring mutations within HIV-1 V3 genomic RNA lead to antigenic variation dependent on a single amino acid substitution. Virology. 1991;185:195–205. doi: 10.1016/0042-6822(91)90767-6. [DOI] [PubMed] [Google Scholar]
- 55.Wolfs T F, de Jong J J, Van den Berg H, Tijnagel J M, Krone W J, Goudsmit J. Evolution of sequences encoding the principal neutralization epitope of human immunodeficiency virus 1 is host dependent, rapid, and continuous. Proc Natl Acad Sci USA. 1990;87:9938–9942. doi: 10.1073/pnas.87.24.9938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wolinsky S M, Korber B T, Neumann A U, Daniels M, Kunstman K J, Whetsell A J, Furtado M R, Cao Y, Ho D D, Safrit J T, Koup R A. Adaptive evolution of human immunodeficiency virus-type 1 during the natural course of infection. Science. 1996;272:537–542. doi: 10.1126/science.272.5261.537. [DOI] [PubMed] [Google Scholar]
- 57.Zhu T, Mo H, Wang N, Nam D S, Cao Y, Koup R A, Ho D D. Genotypic and phenotypic characterization of HIV-1 patients with primary infection. Science. 1993;261:1179–1181. doi: 10.1126/science.8356453. [DOI] [PubMed] [Google Scholar]
- 58.Zhu T, Wang N, Carr A, Nam D S, Moor-Jankowski R, Cooper D A, Ho D D. Genetic characterization of human immunodeficiency virus type 1 in blood and genital secretions: evidence for viral compartmentalization and selection during sexual transmission. J Virol. 1996;70:3098–3107. doi: 10.1128/jvi.70.5.3098-3107.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]