Abstract
Simple Summary
Cervical cancer occurring during pregnancy represents a rare and challenging situation and requires multidisciplinary care. Both diagnosis and treatment should take into consideration maternal and fetal health, while not compromising the chances for cure. Currently, guidelines for the management of cervical cancer occurring during pregnancy are provided by international consensus meetings with expert panels. However, the current guidelines do not discuss the various approaches that can be found within the literature, such as alternative staging modalities or innovative surgical approaches. Our systematic review, performed with the “Cancer Associé à La Grossesse” (CALG) network, aims to fill the gap on current issues, including both neoadjuvant chemotherapy and surgical strategies to preserve fertility.
Abstract
Cancer during pregnancy is defined as a tumor diagnosed in a pregnant woman or up to 1-year post-partum. While being a rare disease, cervical cancer is probably one of the most challenging medical conditions, with the dual stake of treating the cancer without compromising its chances for cure, while preserving the pregnancy and the health of the fetus and child. To date, guidelines for gynecological cancers are provided through international consensus meetings with expert panels, giving insights on both diagnosis, treatment, and obstetrical care. However, these expert guidelines do not discuss the various approaches than can be found within the literature, such as alternative staging modalities or innovative surgical approaches. Also, the obstetrical care of women diagnosed with cervical cancer during pregnancy requires specific considerations that are not provided within our current standard of care. This systematic review aims to fill the gap on current issues with regards to the management of cervical cancer during pregnancy and provide future directions within this evolving landscape.
Keywords: cervical cancer, pregnancy, chemotherapy, radiation therapy, fetal outcome
1. Introduction
Cancer during pregnancy is defined as a tumor diagnosed in a pregnant woman or up to 1-year post-partum. Among them, cervical cancer is one of the most frequently diagnosed, alongside breast and ovarian cancers [1,2,3], and occurs in approximately 1.6–11.1 cases per 100,000 pregnancies, with 3% of cervical cancers being diagnosed during pregnancy [4,5,6,7]. Its incidence is on the rise, with a crude increase of 2.9% suggested over a 30-year period in the Danish Cancer Registry [8].
Cervical cancer during pregnancy is probably one of the most challenging medical conditions, with the dual stake of treating the cancer without compromising its chances for cure, while preserving the pregnancy and the health of the fetus and child. Over the years, the treatment strategy has gradually changed from radical treatment with termination of pregnancy to more conservative approaches, allowing even fertility-sparing strategies [9,10,11,12]. To date, guidelines for gynecological cancers have been provided through international consensus meetings with expert panels [10,11,12], giving insights on both diagnosis, treatment, and obstetrical care. Since the publication of the 3rd edition of these guidelines in 2019, several articles have reported advances in both diagnosis and treatment that may improve the management of these patients.
This systematic review aims to fill the gap on current issues with regards to the management of cervical cancer during pregnancy and provide future directions within this evolving landscape.
2. Materials and Methods
2.1. Eligibility Criteria
All studies reporting data on the management of cervical cancer during pregnancy were included in this systematic review. Studies were deemed eligible if they reported either oncological, obstetrical, or pediatric outcomes.
2.2. Information Sources and Search Strategy
A systematic review was performed on PubMed in January 2022 and updated in August 2023. We used the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines for reporting [13]. The keyword “pregnancy” (MeSH term) and “cervix cancer” (MeSH term) were used. The systematic review followed the PRISMA recommendations. The protocol has not been registered.
2.3. Selection Process
A consensus on article selection was found between two reviewers (J.L.G. and M.K.). For every study, the following data were retrieved: publication year, number of patients, study design, tumor histology, stage, term of pregnancy, treatment, delivery modality, and maternal and fetal outcomes. Pediatric outcomes were also retrieved, if available.
2.4. Synthesis Method
All studies meeting the inclusion criteria were selected for narrative synthesis. The results have been reported narratively and summarized in tables when appropriate.
3. Results
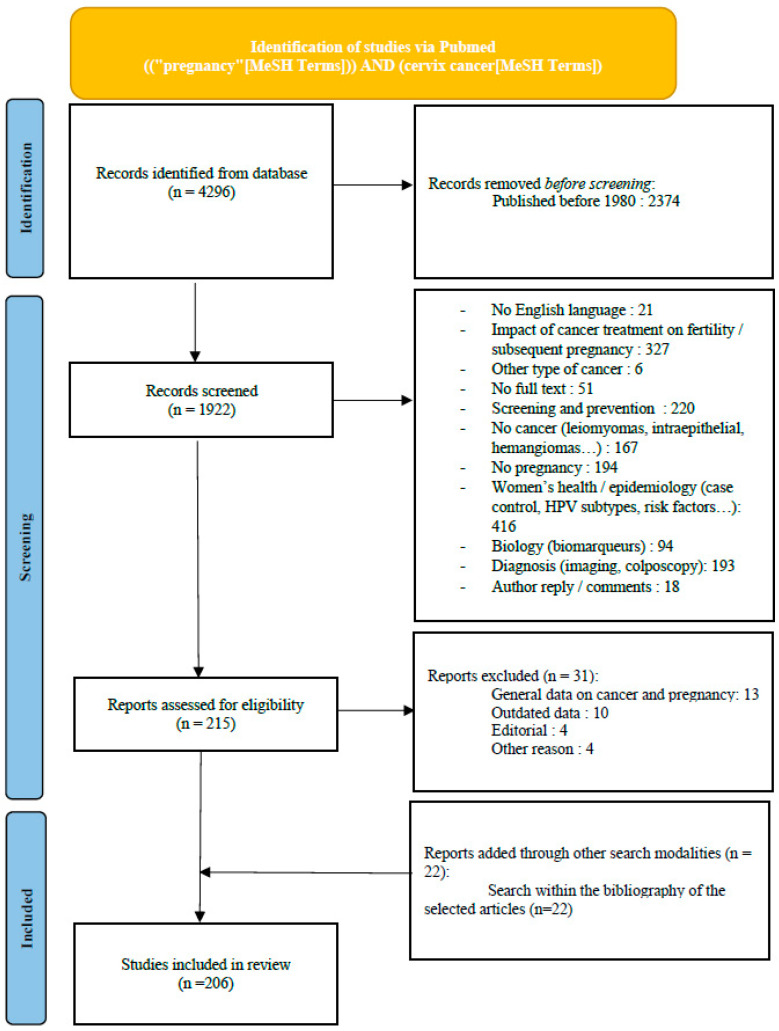
A flow chart of the literature screening is shown in Figure 1. The search allowed retrieval of a total of 4296 articles. The research was narrowed to articles published after 1980 in order to exclude outdated data. A total of 1922 articles were screened. After reading both titles and abstracts, 1707 articles were excluded (diagnosis technique, impact of cancer treatment on fertility, prevention…). Two hundred and fifteen articles were assessed for eligibility. Thirty-two articles were further excluded. We added 22 articles through searches within the bibliographies of other articles. Finally, 206 articles were included in this review.
Figure 1.
Flow chart.
A total of 67 cases and 30 reviews were collected and reported in Supplementary Tables S1 and S2, respectively.
Cervical Cancer during Pregnancy: Opportunity for Screening or Risk-Factor?
Approximately 30% of women diagnosed with cervical cancer are in their childbearing years, with a maximum of incidence reached around the age of 40 [14]. Taken together with the tendency to postpone childbirth in developed countries, these facts might explain the rise in incidence in cervical cancer diagnosed during pregnancy in the past decades [8].
Cervical cancer is one of the most frequently diagnosed cancers during pregnancy, alongside breast and ovarian cancers [1,2,3,8]. The incidence of cervical cancer is lower in pregnant women than in non-pregnant women. However, the incidence of cervical cancer during pregnancy is on the rise, which may partly be explained by the current tendency to delay childbearing. Data obtained from large cancer networks indicate that pregnant patients are more likely to be diagnosed with early-stage cervical cancer, with only 26% of them diagnosed with stage II-IV cancer, compared to 52% in non-pregnant women [15]. Yet, data from national registries remain quite rare and this finding may not be applicable to other countries. Whether pregnancy is a risk factor for development of cervical cancer or not remains an unanswered question. Persistent human papilloma virus (HPV) is firmly established as the main carcinological mechanism associated with the development of both cervical squamous cell carcinoma (SCC) [16] and adenocarcinoma [17]. Estradiol, for which blood levels are elevated during pregnancy, has been suggested to be associated with development of HPV-related tumors, through both inactivation of retinoblastoma protein (pRb) and downregulation of phosphatase and tensin homolog (PTEN) [18]. Additionally, estrogens have long been suggested to influence the development of cervical adenocarcinomas [19,20]. Also, immunohistochemical analyses of cervical cancers diagnosed during pregnancy revealed that almost all tumors express estrogen receptors [21]. As high-level evidence is lacking, a hormone-dependent carcinogenesis cannot be entirely excluded. Last but not least, the shift in maternal immunity, with both inhibition of the Th1 pathway and enhancement of the Treg pathway, has been shown to enhance immune tolerance, which could ultimately lead to cervical cancer progression [22]. Specific investigations on both tumor biology and tumor micro-environment are warranted before making any conclusions on this subject.
4. Diagnosis
As for non-pregnant women, cervical cytology with HPV testing, colposcopy, and biopsy can be safely performed during pregnancy. Cervical curettage, which can be performed as a complementary procedure at the time of colposcopy, should not be performed on pregnant women, as it is associated with both an increase in abortion and premature delivery rate [23].
Magnetic resonance imaging (MRI) is one of the reference imaging modalities for cervical cancer and allows assessment of both local (parametrium, vagina, uterus) and regional extension [24,25]. Due to its non-ionizing properties, it is the preferred imaging modality during pregnancy and has proven non-deleterious effects on the fetus regardless of the term of the pregnancy [26]. Current guidelines advocate against the use of Gadolinium [12], both due to its low added value in parametrial evaluation and due to its risks for the fetus [27]. However, it may provide a benefit in detecting small invasive cervical lesions. The 11th European Symposium on Urogenital Radiology recently stated in favor of the administration of Gadolinium to pregnant women, if deemed necessary [28].
The challenges associated with pelvic MRI in pregnancy are multiple. First, T2 sequences can be artifacted due to fetal movement, thereby limiting their interpretation. Second, cervical distension occurring during pregnancy can complicate the measurement of the tumor size. Only one trial assessed the clinical impact of pelvic MRI in the particular case of cervical cancer occurring during pregnancy. It showed its value in both diagnosis and follow-up under neoadjuvant chemotherapy (NAC). A good correlation between MRI findings and pathology specimens was also demonstrated [29]. Fetal movements had no consequence on MRI interpretation and could be minimized by using ultrafast MR sequences.
The pitfalls of MRI usually include its low specificity with regards to lymph node evaluation [30]. This evaluation can be even more challenging during pregnancy, because of the ectopic decidual reaction that can occur in the pelvic and para-aortic lymph nodes [31,32,33]. In this setting, dual positron emission tomography (PET)/MR imaging showed both a high sensitivity and a high specificity for local and nodal extension [34], even if the small size of the cohort precludes any conclusions on both sensitivity or specificity. If the fetal radiation dose after administration of 18F-FDG remains a concern, Zanotti-Fregonara et al. reported it to range from 5.2 mSv in early pregnancy to 1.4 mSv in late pregnancy [35], which remains well below the thresholds for deterministic effects (Table 1).
Table 1.
Fetal risk caused by ionizing radiation, according to the dose and term of pregnancy.
| 0–100 mGy | 100–500 mGy | >500 mGy | |
|---|---|---|---|
| 0–2 WG | Death of the embryo | ||
| 2–8 WG | No demonstrated effects | Risk of malformation | |
| 8–24 WG | No demonstrated effects | Risk of reducing intellectual abilities | High risk of mental retardation |
| 24–39 WG | No demonstrated effects | Decrease of all determinist side effects |
Abbreviations: mGy = miliGray, WG = week of gestation.
To date, guidelines advocate performing pelvic +/− para-aortic lymph node dissections as a staging modality both for tumors smaller or larger than 2 cm diagnosed before 22 to 25 weeks of pregnancy. PET/MRI may be of future value for patients that cannot undergo the lymph node dissection procedure (i.e., in their third trimester of pregnancy). Additional studies are necessary to determine both the benefit and safety of this procedure in pregnant patients.
4.1. Cancer Treatment during Pregnancy
4.1.1. General Considerations
In situations where pregnancy preservation is not aimed for, termination of pregnancy should be performed without delay and cancer management should be similar to that for non-pregnant women. Both termination of pregnancy and diagnosis of cancer represent situations at high-risk of emotional distress, so the patient should be counseled towards specific psychological care.
Specific attention should be given to symptoms caused by the tumor. In the presence of major bleeding, cancer treatment should be initiated without delay, regardless of the term of the pregnancy.
4.1.2. Surgery
Conization and Trachelectomy
Conization with clear margins is an option in international guidelines as the treatment for T1a1–T1a2 squamous carcinoma, regardless of LVSI status and T1b1 LVSI negative tumors with negative nodes [36]. However, during pregnancy, conization is tricky since it may cause bleeding (5–15%), spontaneous abortion, preterm premature rupture of membranes, preterm labor, and infection. Data are conflicting on the subject, with authors reporting up to 5–15% bleeding rates and spontaneous abortion rates of 25% and some other authors reporting no significant intraoperative or post-operative complications and deliveries at term, with/without cerclage placement [37,38,39,40,41,42,43,44,45,46,47,48,49]. Goldberg suggested a cone cerclage prior to conization to prevent major hemorrhage [46]. Cold-knife conizations have been evaluated in large but ancient series [39,40]. Modern literature reports prefer loop-excision or more marginally laser conization [38,41,44,50,51]. Loop excision may be the preferred technique but with higher rates of non-in sano resections. The ideal timing during pregnancy also remains to be determined: some authors advocate for the first trimester given the cervix is not as hyperemic at this stage, but some other authors advocate waiting until the second trimester to avoid the association of the procedure with a spontaneous, and likely unrelated, miscarriage [44,50,52,53].
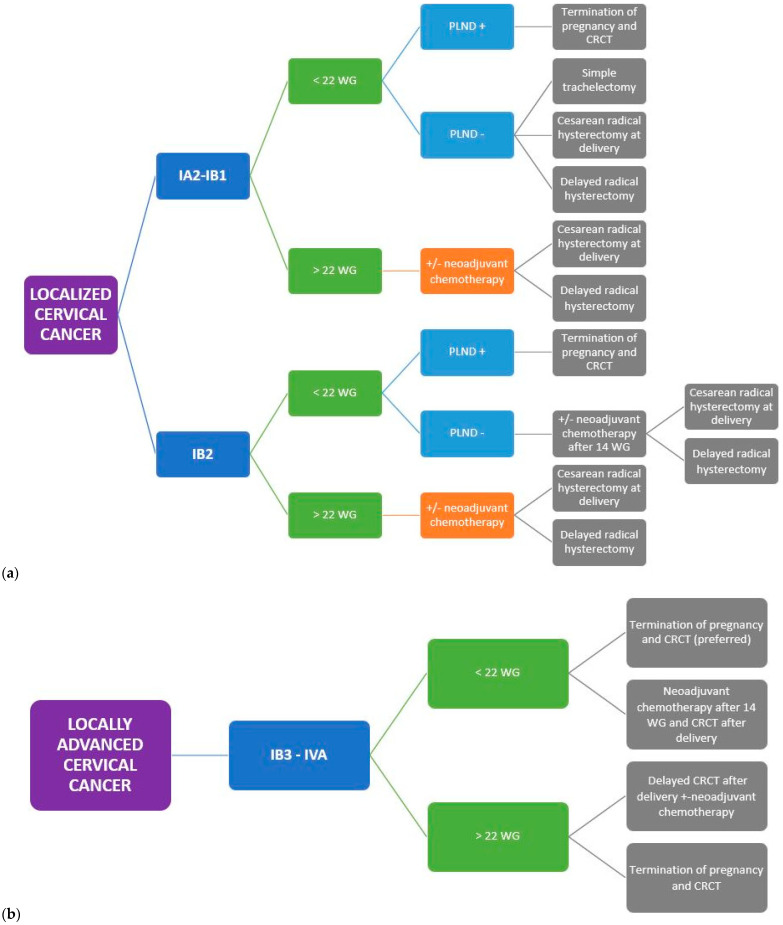
Current guidelines advocate that simple trachelectomy can be proposed for pregnant women diagnosed with FIGO IA2-IB tumors < 2 cm with negative nodes [11,36] (Figure 2). The data from the SHAPE trial presented at the 2023 ASCO Annual Meeting may comfort the surgeon in de-escalating surgery towards a simple trachelectomy when the selection criteria are met (IA2-IB1 squamous cell/adenocarcinoma/adenosquamous carcinoma < 2 cm with less than 50% stromal invasion and negative nodes) [54].
Figure 2.
(a) Summary of the management of localized cervical cancer occurring during pregnancy. (b) Summary of the management of locally advanced cervical cancer occurring during pregnancy Abbreviations: WG: weeks of gestation, PLND: pelvic lymph node dissection, CRCT: concomitant radio-chemotherapy.
One of the greatest motivations to perform radical trachelectomy (RT) during pregnancy is to enable both continuation of the pregnancy and cancer treatment without delay. From an oncological point of view, a systematic review totaling more than 600 cases confirmed an overall recurrence rate < 5% and a death rate < 3%, demonstrating the safety of this approach for small invasive cervical tumors [55]. RT in pregnancy has been investigated by many teams, as first-line therapy for patients diagnosed with tumors <2 cm (≤IB1 according to FIGO 2018) [33,56,57,58,59,60,61,62,63,64,65,66] or, in rarer situations, for patients diagnosed with tumors >2 cm (≥IB2 according to FIGO 2018) [67,68,69,70,71,72,73,74,75]. RT can be performed either through abdominal [58,59,60,63,66,67,69,72,74,76] or vaginal approach (Dargent’s method) [56,57,61,71,73,75,77,78], each technique being associated with its own advantages and drawbacks. Vaginal approach allows for less manipulation of the pregnant uterus and thus may carry a lower risk of spontaneous abortion. Abdominal approach allows for a wider resection of the parametrium (including contiguous parametrial nodes), up to the level of type III hysterectomy [79]. Some teams believe that large parametrial resection may not be mandatory in this setting [57], but a higher recurrence rate and cancer-related death were demonstrated with vaginal approach [80]. Whatever the approach, trachelectomy remains a heavy surgery associated with long operating times ranging from 3.5 [59] to 7.5 h [74] and blood loss of up to 2.5 L [72]. Fetal risks for RT performed during pregnancy include spontaneous abortion, premature rupture of the membranes, preterm delivery, chorioamniotitis, and fetal death. Many teams attempted to adapt their procedure when performed during pregnancy. Iwami et al. proposed to perform a daily vaginal disinfection with povidone iodine, bed rest, and administration of ritodrine (β2 adrenoreceptor agonist) and ulinastatin (urinary trypsin inhibitor) vaginal suppository until delivery, and report improved pregnancy courses with this strategy [57]. The administration of vaginal progesterone did not reduce the rate of preterm birth in a case–control study led by Sato et al. [81]. Abdominal radical trachelectomy with ligation of both uterine arteries has been suggested to be a risk for premature birth, intrauterine growth retardation (IUGR), and death, due to increased fetal hypoxia [67]. Some teams advocates in favor of either bilateral [66,72] or unilateral [59,74,76] preservation. Recent data, with procedures adapted to pregnancy, seem to support the safety for mother and child of both vaginal and abdominal approaches, but the small number of patients hampers these conclusions. Thus, radical trachelectomy performed through either vaginal or abdominal approach is still not recommended during pregnancy due to poor fetal outcomes, with a high risk of early abortions and neural tube defects when performed during the first trimester of pregnancy [11].
Cesarean-Radical Hysterectomy (CRH) vs. Delayed Radical Hysterectomy (RH)
CRH was the preferred modality of surgical therapy across studies [56,59,68,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114]. Its main indications were residual disease after radical trachelectomy, IB1 tumors in patients who do not wish to undergo fertility-preservation surgery, or for IB2-3 tumors after neoadjuvant chemotherapy (Figure 2). Allowing performance of both delivery and cancer treatment in the same operating time avoids a further delay, which could be deleterious in terms of cancer prognosis. Excellent oncological outcomes were demonstrated across trials, with only 14 recurrences out of 189 patients [86,106,107,110,115,116]. No survival difference was found between CRH compared to RH performed among non-pregnant women [99,110]. CRH is not necessary in situations when an in sano resection with either conization or trachelectomy has already been performed.
Compared to delayed RH, CRH is at risk of significantly higher blood loss (2033 cc vs. 425 cc) [99]. In a national survey, women undergoing CRH had a higher rate of perioperative complications compared to those undergoing RH (45.1% vs. 32.1%), consisting mostly in hemorrhage, bowel obstruction, and pyelonephritis. Mortality rates were similar between the two groups (0% vs. 0.2%) [117]. To date, ESMO guidelines suggest to proceed with radical surgery concomitantly to cesarean delivery in qualified centers [118].
Ovarian Transposition
Ovarian transposition has been performed at the time of the hysterectomy by a few teams in order to preserve hormonal production when adjuvant radiation therapy was deemed likely [84,87,94,98,119,120,121]. Ovarian metastasis from cervical cancer remains rare [122]. In a nationwide multicenter study examining consecutive cases of surgically treated women with clinical stage IB-IIB cervical cancer, onset of ovarian metastasis was associated with adenocarcinoma histology, lympho-vascular space invasion, uterine corpus tumor invasion, and nodal metastases. In the absence of these five risk factors, ovarian metastasis remained anecdotal (0.14%) [123]. An accurate selection of patients is necessary in this setting, and to date no specific guidelines have been made on this topic.
Pelvic Lymph Node Dissection (PNLD) vs. Sentinel Lymph Node Dissection (SLND)
Pathologic assessment of pelvic +/− para-aortic lymph nodes is mandatory before starting any treatment, as its results will guide therapeutic decisions for both the oncological approach and pregnancy management. The current guidelines on cervical cancer during pregnancy advocate its realization for patients diagnosed with IB1 or IB2 tumors before 22 weeks of gestation [12] (Figure 2). Usually, PLND is not performed after this term because the dimension of the gravid uterus may preclude the ability to access the pelvic lymph nodes. When performed by experienced surgeons, this technique does present few maternal or fetal morbidity or mortality risks. Only one case of spontaneous abortion occurred at 12 weeks of pregnancy, yet lymphadenectomy was performed in association with radical trachelectomy [71].
Few data are available with regards to SLND for pregnant cervical cancer patients, but this technique represents an attractive alternative to PLND, as it could enable a further decrease in intervention time and blood loss. Sentinel node mapping using radioactive tracers is contraindicated for cervical cancer—although it is not for vulvar cancer. Indocyanine green may be promising in this setting, as it has already been shown not to cross the placenta after intravenous injection [124]. Papadia et al. reported SNLD performed with indocyanine green on two patients and recorded no adverse events [125]. The scarcity of data on this subject precludes any conclusion on the conduction of sentinel lymph node dissection during pregnancy, and further trials are warranted before its introduction in routine care.
4.1.3. Chemotherapy
Neoadjuvant chemotherapy (NAC) is the preferred option for patients diagnosed with FIGO 2018 IA2-IB1 cervical tumors after 22 weeks of gestation and for patients diagnosed with IB2-IB3 cervical tumors regardless of the term [12] (Figure 2). NAC allows for a double compromise: treating the cancer while postponing local treatment until fetal maturity.
Chemotherapy administered during pregnancy is associated with specific risks with regards to the pregnancy. Chemotherapy is contra-indicated during the first trimester of pregnancy, being associated with a 10 to 20% rate of malformations and high-risk of miscarriage [126]. After 14 weeks of gestation, its administration is feasible and associated with a low rate of fetal adverse effects. Based on the results of several prospective trials, Platinum-based chemotherapy with either Paclitaxel or Docetaxel represents the most prescribed regimen in the neo-adjuvant setting [127]. This regimen has been widely used across trials [62,69,82,83,84,85,87,88,90,92,103,104,106,108,109,111,112,121,128,129,130,131,132,133,134,135,136,137,138,139,140] and was usually associated with a favorable tolerance profile, with no report of grade 3–4 adverse events. General guidelines advocate in favor of the use of Carboplatin instead of Cisplatin in pregnant women to avoid ototoxicity in children exposed in utero. Taxanes during pregnancy are deemed safe, with their main adverse events consisting mostly of non-severe anaphylactic reactions and occurrence of preterm contractions [106,141].
Caution should also be exerted with regard to drugs usually prescribed during chemotherapy. Premedication with corticosteroids and Ondansetron appears to be safe regardless of the term of the pregnancy, but insufficient data are available with regards to the use of Aprepitant. Growth factors are contraindicated, thereby emphasizing the crucial need for hematological monitoring and dose adjustment. Maternal anemia is a multifactorial issue (due to dilution, tumor bleeding, and the hematological toxicity of chemotherapy), thus requiring early detection and adequate supplementation.
Trials investigating the role of NAC for cervical cancer revealed promising results, with response rates ranging from 66% to 85% and considerable rates of pathological complete responses [142]. The EORTC 5594 trial demonstrated similar survival outcomes with NAC followed by surgery compared to chemoradiotherapy (CRT), with 5-year overall survival (OS) of 72% and 76% respectively. However, a significant proportion of patients could not undergo the planned surgery (24%), due to either toxicity, progression, or an insufficient response to NAC [143]. NAC followed by surgery also demonstrated inferior disease-free survival compared to CRT in randomized trials, with 18.6% experiencing local first recurrence in the NAC group vs. 13.5% in the CRT group [144]. Additionally, a report of rapid progression on NAC in a pregnant patient has been published in a patient with locally advanced disease [145].
Caution needs to be exerted when NAC is advocated as first-line therapy for pregnant cervical cancer patients, given the poor prognosis in the event of tumor progression. Patients diagnosed with bulky tumors may be more appropriately counselled towards CRT associated with termination of pregnancy, especially if the diagnosis is made during the first trimester (Figure 2). If the patient wishes to continue the pregnancy, both clinical and MRI assessment must be arranged at each cycle to eliminate tumor progression.
4.1.4. Radiation Therapy
Pelvic radiation therapy is contraindicated during pregnancy as it is known to induce fetal death and spontaneous abortion after 2–3 weeks of treatment [146]. CRT has been performed for locally advanced tumors after premature delivery in patients diagnosed during their third trimester of pregnancy [119,147]. Although current guidelines recommend termination of pregnancy for locally advanced tumors, some patients choose to proceed with the pregnancy. In this situation, some authors reported an approach using NAC until fetal maturity, followed by premature delivery and CRT [128,131,133,135,138] (Figure 2).
One team reported chemoradiation therapy (CRT) with fetuses in utero for advanced-stage cervical carcinoma diagnosed during the first trimester of pregnancy [145]. The patients were diagnosed with large tumors (>8 cm), which made uterine evacuation impossible through vaginal approach. After 3 weeks of CRT, tumor regression allowed vaginal evacuation, avoiding the risk of tumor spread with hysterotomy. CRT with the fetus in utero seems feasible without major short-term adverse events, either urinary or digestive. While this approach is not exempted from ethical dilemmas and psychological distress, it must be restricted to complex situations where the cancer spread requires immediate treatment.
4.1.5. Delaying Definitive Treatment
Delaying definitive treatment to improve fetal outcomes, although beneficial to the fetus, may carry an additional risk of tumor progression. However, the clinical data are reassuring and do not report either significant disease progression or loss of chance during pregnancy. Morice et al. retrieved published data on delayed treatment and described the outcomes for 76 patients diagnosed mostly with localized tumors (96% rate of FIGO 2009 IB tumors). Even with a median delay of 16 weeks, excellent oncological outcomes were demonstrated, with a 95% survival rate at a mean follow-up of 37.5 months [148]. Amongst them, Lee et al. reported outcomes after delayed treatment for 12 patients. Two patients, diagnosed with FIGO IB2 and IB3 (FIGO 2018) cervical cancer, died of the disease, with a mean delays of treatment of 6 and 4 weeks, respectively [110]. Favero et al. reported the outcomes of 14 node-negative patients after an average treatment delay of 17 weeks. Nine of them underwent CRH, while five underwent RT approximately 6 weeks after delivery. All patients remained disease-free at a mean follow-up of 38 months [114]. Ishioka et al. reported planned delay of treatment for patients diagnosed with IB1-IIB cervical cancer. No apparent tumor growth was reported, although surgery had to be preempted for one patient due to pain (parametrial invasion), emphasizing the importance of patient selection in this setting [101].
According to the most recent guidelines, a delay in definitive treatment may be considered feasible for patients diagnosed with IA2-IB3 tumors diagnosed after 22 W, subject to close monitoring [12], with the aim to obtain fetal maturity (>37 weeks of gestation, WG) (Figure 2). The ESMO guidelines also support delayed treatment and careful monitoring of the patient at all early stages [118]. When progressive disease is observed, either termination of pregnancy or NACT should be advocated.
4.1.6. Rare Histological Types
Few conclusions can be drawn from case reports relating to cervical cancer of rare histology diagnosed during pregnancy. In decreasing frequency order, we retrieved cases about treating neuroendocrine carcinomas [97,113,120,121,136,137,138,139,140,149,150,151,152,153,154], clear cell carcinomas [134,135,155], and sarcoma [98].
Most patients diagnosed with neuroendocrine tumors at the first or early second trimester of pregnancy underwent termination of pregnancy [121,136,151,154] to prevent any delay in cancer management. Three out of fifteen patients were diagnosed with stage FIGO 2018 IVB disease and died within months after the diagnosis [137,150,152]. Three additional patients diagnosed with localized disease died from either lung or liver metastases in less than a year [113,137,140], demonstrating the particularly poor prognosis of this disease.
Specific guidelines were published for the management of small cell cervical cancer in 2011. A multimodal approach is recommended for early stages, including surgical resection, pelvic radiotherapy, and chemotherapy [156]. Of the cases collected in Supplementary Table S1, most patients were diagnosed with locally advanced disease due to either vaginal or parametrial invasion and underwent NAC followed by radiation therapy [138,149,153,154]. All patients remained disease-free after a follow-up ranging from 14 months to 5 years. NAC consisted mostly of platinum-based regimens (with Etoposide/Paclitaxel) or poly-chemotherapy with Cyclophosphamide and Adriamycin. For metastatic disease, platinum-based chemotherapy was usually advocated. Even if initial response rates are high (50–70% in the non-pregnant population), recurrence and progression are frequent and the outcomes remained poor [156].
4.2. Obstetrical Management
Planned Delivery
Current guidelines advocate conducting the pregnancy to its full-term (>37 WG). A 3-week interval is usually advocated between the last chemotherapy and delivery to avoid hematopoietic suppression. A full blood count is mandatory in order to evaluate the degree of anemia, thrombopenia, and neutropenia—all the more if delivery and cancer treatment are planned at the same time.
Among the 27 patients undergoing vaginal delivery [68,86,94,99,107,113,115,140,150,157,158,159,160,161,162,163,164,165], 9 patients experienced recurrence at the episiotomy site [86,115,157,158,162,163,164,165]. In other situations, cervical cancer was revealed by metastasis at the episiotomy site several months after delivery, having remained occult during the pregnancy [157,162,164,166,167,168,169,170]. The metastatic lesion was described as either a swelling, a nodular or ulcerative lesion, or even an abscess. The etiology of these recurrences appears to be related to local seeding at the time of delivery, rather than metastasis through a vascular or lymphatic route. Vaginal delivery was the most significant predictor of recurrence in a large cohort of 83 pregnant women, with an odds ratio (OR) of 6.9. On the other hand, only one report described a recurrence where Cesarean section had been performed [171]. Cesarean delivery should be the preferred method in pregnant women diagnosed with invasive cervical cancer [12].
In a large series of women in California with pregnancy-associated cervical cancer, higher rates of cesarean section (OR 3.7; 95% CI 2.6, 5.2), hospitalization > 5 days (maternal: OR 14.1; 95% CI 9.2, 21.5 neonatal: OR 5.2; 95% CI 3.6, 7.5), low birth weight (OR 5.5; 95% CI 3.7, 8.1), very low birth weight (OR 6.9; 95% CI 3.7, 12.8), prematurity (OR 4.7; 95% CI 3.2, 6.7), and fetal deaths (OR 5.5; 95% CI 2.0, 14.8) were reported compared to non-cancer pregnant controls [172].
4.3. Pediatric Management
4.3.1. In Utero Surveillance
At the time of cancer diagnosis, it is necessary to assess the exact term of the pregnancy and to exclude fetal anomalies through ultrasonography and specific screening, according to guidelines. During cancer treatment, specific attention should be given to fetal anemia after chemotherapy administration, as its complications range from fetal hydrops to fetal death. Currently, peak systolic velocimetry of the middle cerebral artery is considered as the best method and brings the advantage of being reproducible throughout the pregnancy [173].
4.3.2. Pediatric Management
Most of the concerns for the neonates are related to premature delivery. Morbidity attributable to prematurity includes respiratory distress syndromes, ranging from transient oxygen need to invasive ventilation [58,75,82,89,109,129,135,137,139] and including hypoglycemia [135,139] and necrotizing enterocolitis [75]. No death due to premature delivery was reported in the setting of cervical cancer associated with pregnancy. During the past decades, neonatal intensive care has been improved significantly and both infant morbidity and mortality have continued to decline, with survival rates above 90% for children born after their 27th week of gestation in a recent national survey [174]. Still, the ESMO recommends delivery at term (>37 weeks) in order to decrease morbidity, which can already be significant due to cancer treatment [127]. Specialized care (neonatology—intensive care) is advised after delivery and needs to be tailored to each clinical course.
Although extremely rare, transmission of cervical cancer from mother to child has been reported in the literature [152,175]. Two routes of mother-to-child cancer metastases can be identified. Hematological dissemination can occur through placental metastases and has been described for aggressive cancers such as melanoma and small cell cervical carcinomas [152]. Recently, two cases of lung metastases from cervical cancer have been reported, raising concerns about the possibility of metastasis after inhalation of cancer cells at the time of delivery [175]. In these specific situations, a follow-up with a pediatrician from a reference center should be initiated, along with regular clinical examination. Atypical and persistent symptoms in a child with this type of history require the utmost vigilance and targeted examinations.
Precautions should be taken with regards to breastfeeding in patients who have received chemotherapy during pregnancy. Cisplatin has been detected in breast milk at concentrations up to 10% of those in the maternal blood [109]. Breastfeeding is not contraindicated after cancer treatment, provided that there is a minimum delay of 3 weeks between the last chemotherapy and its beginning [12].
Due to their low molecular weight (less than 600 kDa), most cytotoxic agents cross the placenta [176]. Cisplatin concentrations in the umbilical cord and amniotic fluid were shown to be up to 31–65% and 13–42% of the amount in the maternal blood, respectively [112,177]. The paclitaxel concentration in the fetal umbilical cord has been shown to rise up to 15% of the maternal blood concentration 3 h after paclitaxel administration. Even if very few toxicities have been reported for both fetuses and children exposed to chemotherapeutic agents during pregnancy, most studies are hampered by their short follow-up and do not report on the long-term potential complications. After in utero chemotherapy exposure, both neonates and infants need specific examination performed by a trained neonatologist or pediatrician. Hematological, liver, and renal parameters should be checked. In case of Cisplatin exposure, special attention must be paid with respect to hearing function and cardiac malformations [178]. Induced malignancies in the child, such as rhabdomyosarcoma or acute myeloid leukemia, have been reported in the literature after in utero exposure to platinum and paclitaxel [85,139]. While these events remain anecdotal, their severity requires them to be kept in mind.
4.4. Prognosis
Several factors may be associated with an impaired prognosis for cancer in pregnant women. First, diagnostic difficulties may arise due to the limitations of certain procedures during pregnancy (PET/CT for instance) that may ultimately result in under-staging. However, surgical staging via PNLD, the gold-standard method for lymph node evaluation, can be performed until the end of the second trimester of pregnancy. Second, the contraindication to perform local procedures (radiotherapy, radical hysterectomy) may worsen the prognosis if the alternative approach (either delay or NAC) is inappropriate. Nonetheless, the oncological outcomes of cervical cancer diagnosed during pregnancy seem relatively similar with those of cervical cancer diagnosed in the global population. Large epidemiological studies showed similar maternal and neonatal morbidity for patients diagnosed during pregnancy than for non-pregnant patients [15,179,180,181,182,183]. Only one trial showed a 1.24 age-adjusted hazards ratio for cancer-related deaths, compared with non-pregnant patients (confidence interval between 0.84 and 1.81) [184]. The limitations of these national surveys include their retrospective nature and their long inclusion period. Standardization of the management of patients with cervical cancer during pregnancy through the development of international guidelines will likely improve the management of these patients.
5. Discussion
The management of cervical cancer or pre-cancerous lesions during pregnancy is complex and can jeopardize both the pregnancy and maternal prognosis. Additionally, it seems that the very presence of HPV lesions favors obstetrical complications, even if the precise physiopathology is not well known [185,186]. It is therefore essential to act on primary prevention. The real-life efficacies of HPV vaccination programs have been demonstrated in several national surveys, with significant reductions in the risk of invasive cervical cancer [187,188]. While health institutions in France recently opted in favor of an enlargement of the population targeted for HPV vaccination, some authors even propose a vaccination program during the postpartum period [189]. The second prevention lever against HPV lesions is represented by their early detection by cervical smear. Since 2019, HPV testing has replaced the classic cytology from 30 to 65 years in screening in France [190]. While the classic obstacles to this screening are the fear of the gynecological examination and the absence of gynecological follow-up, urine HPV self-testing could replace the classic smear test for those populations that escape screening and has already been validated in pregnant women [191].
Advances in cervical cancer in general may also benefit patients diagnosed during pregnancy. Recent data on the safety of MRI at all terms (without Gadolinium injection) and laparoscopic lymph-node staging (up to a certain term and in trained teams) have made it possible to improve staging and to more easily offer active surveillance.
Locally advanced cervical cancer during pregnancy is one of the most challenging situations, as it theoretically requires CRT, which is incompatible with preservation of the pregnancy. For women who wish to proceed with the pregnancy, NAC represents the only therapeutic option, but there is a risk of tumor progression and altered maternal prognosis. One of the options could be to potentiate the tumor response with an additional chemotherapeutic agent to maintain the pregnancy more serenely. One of the leads could be the use of Bevacizumab, which has demonstrated its value in PFS and OS in metastatic cervical cancer [192]. However, IV antiangiogenic drugs have not been the subject of any publications during pregnancy and could potentially be very toxic for both mother and fetus (vascular, renal, pre-eclampsia). Long-standing experience with thalidomide suggests that great caution should be exercised, and antiangiogenic drugs are therefore contraindicated during pregnancy. PDL1 inhibitors have recently shown efficacy in PFS and OS in first-line metastatic cervical cancer [193]. They are also of growing interest as adjuvant therapy after CRT in locally advanced cancers [194,195,196,197]. In these challenging situations, one could imagine proposing immunotherapy as a complement to NAC to increase the response rate in pregnant women. However, as treatment with anti-PD-1 and anti-PD-L1 monoclonal antibodies enhance the functional activity of T cells towards tumor cells, they simultaneously abrogate the established fetomaternal immunotolerance [198]. Preclinical data show that anti-PD1 and PDL-1 cross the placental barrier, especially in the 2nd and 3rd trimesters. Additionally, adverse effects on both the fetus and pregnancy have been described in animals and in some case reports [198]. Thus, preliminary data on checkpoint inhibitors and pregnancy are scarce at best and even worrisome, discouraging their further implementation in clinical trials. As Tisotumab–Vedotin recently showed a durable antitumor response in recurrent or metastatic cervical cancer in a phase II trial [199] and was recently granted accelerated approval by the FDA, further safety data on pregnant women will likely be provided in the coming years. Cervical cancer during pregnancy requires management in expert centers to optimize maternal oncological treatment while ensuring fetal development. Based on our experience, we aimed to summarize our “red flags” for both mother and child in order to fill the gap with the current guidelines (Table 2).
Table 2.
“Red flags” for diagnosis and follow-up of cervical cancer diagnosed during pregnancy.
|
At the time of diagnosis: General care Evaluate maternal psychological distress—refer for psychological support Tumor staging
|
|
Treatment during pregnancy: General care Evaluate maternal psychological distress—refer for psychological support Chemotherapy
|
|
Before delivery:
General care
|
|
After delivery: General care Evaluate maternal psychological distress—refer for psychological support Tumor staging
Long-term follow-up required with a trained pediatrician, particularly if neoadjuvant chemotherapy was performed during pregnancy |
Abbreviations: MRI = Magnetic Resonance Imaging, PET/CT = Positron Emission Tomography/Computed Tomography.
A multidisciplinary team involving a trained gynecologist, oncologist, radiation oncologist, neonatologist, pediatrician, and psychologist is required at all stages of the treatment. In France, the “Cancer Associé à La Grossesse” (CALG) network provides specific counseling and a dedicated tumor board for the management of women diagnosed with cancer during pregnancy.
Our systematic review carries some limitations. Mostly, it is based on either case reports or small series, with no prospective data existing on this topic. Also, the search modality intended to be quite complete, but the use of the keyword “cervix”, with no additional search, might result in the loss of some information—despite a thorough search within the bibliography of the selected articles.
6. Conclusions
Cervical cancers occurring during pregnancy require a multidisciplinary team and management in expert centers. Therapeutic advances in the past decades have made it possible to improve both fetus and infant prognosis through the reduction of the termination of pregnancy rate and improvement of neonatology techniques. The occurrence of cervical cancer during pregnancy does not appear to adversely affect maternal prognosis, whether because of increased aggressiveness or inadequate treatment. More investigations are necessary with regards to imaging modalities such as PET-MRI and surgical techniques such a RT or SLND, since they represent promising alternatives. Long-term follow-up of infants, especially those exposed to cytotoxic agents in utero, is necessary.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers16071341/s1, Table S1: case reports of cervical cancer diagnosed during pregnancy. Stage has been updated according to the FIGO 2018 classification; Table S2: series of cervical cancer diagnosed during pregnancy. Stage has been updated according to the FIGO 2018 classification.
Author Contributions
J.L.G.: conceptualization, data curation, formal analysis, methodology, project administration, validation, writing. M.K.: conceptualization, project administration, validation, writing. E.L., L.S. and F.L.: project administration, validation, writing (review and editing). All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Creasman W.T. Cancer and Pregnancy. Ann. N. Y. Acad. Sci. 2001;943:281–286. doi: 10.1111/j.1749-6632.2001.tb03809.x. [DOI] [PubMed] [Google Scholar]
- 2.Kobayashi Y., Tabata T., Omori M., Kondo E., Hirata T., Yoshida K., Sekine M., Itakura A., Enomoto T., Ikeda T. A Japanese Survey of Malignant Disease in Pregnancy. Int. J. Clin. Oncol. 2019;24:328–333. doi: 10.1007/s10147-018-1352-x. [DOI] [PubMed] [Google Scholar]
- 3.Sekine M., Kobayashi Y., Tabata T., Sudo T., Nishimura R., Matsuo K., Grubbs B.H., Enomoto T., Ikeda T. Malignancy during Pregnancy in Japan: An Exceptional Opportunity for Early Diagnosis. BMC Pregnancy Childbirth. 2018;18:50. doi: 10.1186/s12884-018-1678-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nguyen C., Montz F.J., Bristow R.E. Management of Stage I Cervical Cancer in Pregnancy. Obstet. Gynecol. Surv. 2000;55:633–643. doi: 10.1097/00006254-200010000-00022. [DOI] [PubMed] [Google Scholar]
- 5.Pentheroudakis G., Pavlidis N. Cancer and Pregnancy: Poena Magna, Not Anymore. Eur. J. Cancer. 2006;42:126–140. doi: 10.1016/j.ejca.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 6.Kärrberg C., Rådberg T., Holmberg E., Norström A. Support for Down-Staging of Pregnancy-Associated Cervical Cancer. Acta Obstet. Gynecol. Scand. 2015;94:654–659. doi: 10.1111/aogs.12645. [DOI] [PubMed] [Google Scholar]
- 7.Norström A., Jansson I., Andersson H. Carcinoma of the Uterine Cervix in Pregnancy: A Study of the Incidence and Treatment in the Western Region of Sweden 1973 to 1992. Acta Obstet. Gynecol. Scand. 1997;76:583–589. doi: 10.3109/00016349709024589. [DOI] [PubMed] [Google Scholar]
- 8.Eibye S., Kjær S.K., Mellemkjær L. Incidence of Pregnancy-Associated Cancer in Denmark, 1977–2006. Obstet. Gynecol. 2013;122:608–617. doi: 10.1097/AOG.0b013e3182a057a2. [DOI] [PubMed] [Google Scholar]
- 9.Morice P., Narducci F., Mathevet P., Marret H., Daraï E., Querleu D. Recommandations de la Société française d’urologie gynécologique, de la Société française de chirurgie pelvienne et du Collège national des gynécologues et obstétriciens français sur la prise en charge des cancers invasifs du col utérin pendant la grossesse. Gynécologie Obstétrique Fertil. 2009;37:959–963. doi: 10.1016/j.gyobfe.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Amant F., Halaska M.J., Fumagalli M., Steffensen K.D., Lok C., Van Calsteren K., Han S.N., Mir O., Fruscio R., Uzan C., et al. Gynecologic Cancers in Pregnancy: Guidelines of an International Consensus Meeting. Int. J. Gynecol. Cancer. 2009;19((Suppl. S1)):S1YS12. doi: 10.1111/IGC.0b013e3181a1d0ec. [DOI] [PubMed] [Google Scholar]
- 11.Amant F., Halaska M.J., Fumagalli M., Dahl Steffensen K., Lok C., Van Calsteren K., Han S.N., Mir O., Fruscio R., Uzan C., et al. Gynecologic Cancers in Pregnancy: Guidelines of a Second International Consensus Meeting. Int. J. Gynecol. Cancer. 2014;24:394–403. doi: 10.1097/IGC.0000000000000062. [DOI] [PubMed] [Google Scholar]
- 12.Amant F., Berveiller P., Boere I.A., Cardonick E., Fruscio R., Fumagalli M., Halaska M.J., Hasenburg A., Johansson A.L.V., Lambertini M., et al. Gynecologic Cancers in Pregnancy: Guidelines Based on a Third International Consensus Meeting. Ann. Oncol. 2019;30:1601–1612. doi: 10.1093/annonc/mdz228. [DOI] [PubMed] [Google Scholar]
- 13.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arbyn M., Weiderpass E., Bruni L., de Sanjosé S., Saraiya M., Ferlay J., Bray F. Estimates of Incidence and Mortality of Cervical Cancer in 2018: A Worldwide Analysis. Lancet Glob. Health. 2020;8:e191–e203. doi: 10.1016/S2214-109X(19)30482-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halaska M.J., Uzan C., Han S.N., Fruscio R., Dahl Steffensen K., Van Calster B., Stankusova H., Marchette M.D., Mephon A., Rouzier R., et al. Characteristics of Patients with Cervical Cancer during Pregnancy: A Multicenter Matched Cohort Study. An Initiative from the International Network on Cancer, Infertility and Pregnancy. Int. J. Gynecol. Cancer. 2019;29:676–682. doi: 10.1136/ijgc-2018-000103. [DOI] [PubMed] [Google Scholar]
- 16.Castellsagué X. Natural History and Epidemiology of HPV Infection and Cervical Cancer. Gynecol. Oncol. 2008;110((Suppl. S2)):S4–S7. doi: 10.1016/j.ygyno.2008.07.045. [DOI] [PubMed] [Google Scholar]
- 17.Bosch F.X., Lorincz A., Muñoz N., Meijer C.J., Shah K.V. The Causal Relation between Human Papillomavirus and Cervical Cancer. J. Clin. Pathol. 2002;55:244–265. doi: 10.1136/jcp.55.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le Guevelou J., Lebars S., Kammerer E., Gabory L., Vergez S., Janot F., Baujat B., Righini C., Jegoux F., Dufour X., et al. Head and Neck Cancer during Pregnancy. Head Neck. 2019;41:3719–3732. doi: 10.1002/hed.25877. [DOI] [PubMed] [Google Scholar]
- 19.Lacey J.V., Brinton L.A., Barnes W.A., Gravitt P.E., Greenberg M.D., Hadjimichael O.C., McGowan L., Mortel R., Schwartz P.E., Kurman R.J., et al. Use of Hormone Replacement Therapy and Adenocarcinomas and Squamous Cell Carcinomas of the Uterine Cervix. Gynecol. Oncol. 2000;77:149–154. doi: 10.1006/gyno.2000.5731. [DOI] [PubMed] [Google Scholar]
- 20.Richardson A., Watson L., Persic M., Phillips A. Safety of Hormone Replacement Therapy in Women with a History of Cervical Adenocarcinoma. Post Reprod. Health. 2021;27:167–173. doi: 10.1177/20533691211028518. [DOI] [PubMed] [Google Scholar]
- 21.Robertson D.I., Paslawski D., Duggan M.A., Stuart G.C.E., Nation J.G. Estrogen and Progesterone Receptor, Human Papillomavirus, and DNA Ploidy Analysis in Invasive Carcinoma of the Cervix in Pregnancy. Am. J. Clin. Pathol. 1993;100:18–21. doi: 10.1093/ajcp/100.1.18. [DOI] [PubMed] [Google Scholar]
- 22.Lin W., Niu Z., Zhang H., Kong Y., Wang Z., Yang X., Yuan F. Imbalance of Th1/Th2 and Th17/Treg during the Development of Uterine Cervical Cancer. Int. J. Clin. Exp. Pathol. 2019;12:3604–3612. [PMC free article] [PubMed] [Google Scholar]
- 23.Pretorius R.G., Belinson J.L. Colposcopy. Minerva Ginecol. 2012;64:173–180. [PubMed] [Google Scholar]
- 24.Merz J., Bossart M., Bamberg F., Eisenblaetter M. Revised FIGO Staging for Cervical Cancer—A New Role for MRI. Rofo. 2020;192:937–944. doi: 10.1055/a-1198-5729. [DOI] [PubMed] [Google Scholar]
- 25.Hricak H., Gatsonis C., Chi D.S., Amendola M.A., Brandt K., Schwartz L.H., Koelliker S., Siegelman E.S., Brown J.J., McGhee R.B., et al. Role of Imaging in Pretreatment Evaluation of Early Invasive Cervical Cancer: Results of the Intergroup Study American College of Radiology Imaging Network 6651–Gynecologic Oncology Group 183. J. Clin. Oncol. 2005;23:9329–9337. doi: 10.1200/JCO.2005.02.0354. [DOI] [PubMed] [Google Scholar]
- 26.Kanal E., Barkovich A.J., Bell C., Borgstede J.P., Bradley W.G., Froelich J.W., Gilk T., Gimbel J.R., Gosbee J., Kuhni-Kaminski E., et al. ACR Guidance Document for Safe MR Practices: 2007. Am. J. Roentgenol. 2007;188:1447–1474. doi: 10.2214/AJR.06.1616. [DOI] [PubMed] [Google Scholar]
- 27.Ray J.G., Vermeulen M.J., Bharatha A., Montanera W.J., Park A.L. Association Between MRI Exposure During Pregnancy and Fetal and Childhood Outcomes. JAMA. 2016;316:952–961. doi: 10.1001/jama.2016.12126. [DOI] [PubMed] [Google Scholar]
- 28.Webb J.A., Thomsen H.S., Morcos S.K. The Use of Iodinated and Gadolinium Contrast Media during Pregnancy and Lactation. Eur. Radiol. 2005;15:1234–1240. doi: 10.1007/s00330-004-2583-y. [DOI] [PubMed] [Google Scholar]
- 29.Balleyguier C., Fournet C., Ben Hassen W., Zareski E., Morice P., Haie-Meder C., Uzan C., Gouy S., Duvillard P., Lhommé C. Management of Cervical Cancer Detected during Pregnancy: Role of Magnetic Resonance Imaging. Clin. Imaging. 2013;37:70–76. doi: 10.1016/j.clinimag.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 30.Liu B., Gao S., Li S. A Comprehensive Comparison of CT, MRI, Positron Emission Tomography or Positron Emission Tomography/CT, and Diffusion Weighted Imaging-MRI for Detecting the Lymph Nodes Metastases in Patients with Cervical Cancer: A Meta-Analysis Based on 67 Studies. Gynecol. Obstet. Investig. 2017;82:209–222. doi: 10.1159/000456006. [DOI] [PubMed] [Google Scholar]
- 31.Wu D.C., Hirschowitz S., Natarajan S. Ectopic Decidua of Pelvic Lymph Nodes: A Potential Diagnostic Pitfall. Arch. Pathol. Lab. Med. 2005;129:e117–e120. doi: 10.5858/2005-129-e117-EDOPLN. [DOI] [PubMed] [Google Scholar]
- 32.Ashraf M., Boyd C.B., Beresford W.A. Ectopic Decidual Cell Reaction in Para-Aortic and Pelvic Lymph Nodes in the Presence of Cervical Squamous Cell Carcinoma during Pregnancy. J. Surg. Oncol. 1984;26:6–8. doi: 10.1002/jso.2930260103. [DOI] [PubMed] [Google Scholar]
- 33.Dharan M. Hyaline Globules in Ectopic Decidua in a Pregnant Woman with Cervical Squamous Cell Carcinoma. Diagn. Cytopathol. 2009;37:696–698. doi: 10.1002/dc.21113. [DOI] [PubMed] [Google Scholar]
- 34.Ishiguro T., Nishikawa N., Ishii S., Yoshihara K., Haino K., Yamaguchi M., Adachi S., Watanabe T., Soeda S., Enomoto T. PET/MR Imaging for the Evaluation of Cervical Cancer during Pregnancy. BMC Pregnancy Childbirth. 2021;21:288. doi: 10.1186/s12884-021-03766-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zanotti-Fregonara P., Stabin M.G. New Fetal Radiation Doses for (18) F-FDG Based on Human Data. J. Nucl. Med. 2017;58:1865–1866. doi: 10.2967/jnumed.117.195404. [DOI] [PubMed] [Google Scholar]
- 36.Cibula D., Raspollini M.R., Planchamp F., Centeno C., Chargari C., Felix A., Fischerová D., Jahnn-Kuch D., Joly F., Kohler C., et al. ESGO/ESTRO/ESP Guidelines for the Management of Patients with Cervical Cancer—Update 2023. Int. J. Gynecol. Cancer. 2023;33:649–666. doi: 10.1136/ijgc-2023-004429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sánchez-Cuerda C., Cuadra M., Gámir S., Lobo P., Elices M., Cabrera Y. Cervical Adenocarcinoma in Situ during Pregnancy and Subsequent Fertility-Sparing Therapy Challenge. Int. J. Gynecol. Obstet. 2022;158:21–26. doi: 10.1002/ijgo.13948. [DOI] [PubMed] [Google Scholar]
- 38.Robinson W.R., Webb S., Tirpack J., Degefu S., O’Quinn A.G. Management of Cervical Intraepithelial Neoplasia during Pregnancy with LOOP Excision. Gynecol. Oncol. 1997;64:153–155. doi: 10.1006/gyno.1996.4546. [DOI] [PubMed] [Google Scholar]
- 39.Averette H.E., Nasser N., Yankow S.L., Little W.A. Cervical Conization in Pregnancy. Analysis of 180 Operations. Am. J. Obstet. Gynecol. 1970;106:543–549. doi: 10.1016/0002-9378(70)90039-6. [DOI] [PubMed] [Google Scholar]
- 40.Hannigan E.V., Whitehouse H.H., Atkinson W.D., Becker S.N. Cone Biopsy during Pregnancy. Obstet. Gynecol. 1982;60:450–455. [PubMed] [Google Scholar]
- 41.Mitsuhashi A., Sekiya S. Loop electrosurgical excision procedure (LEEP) during first trimester of pregnancy. Int. J. Gynecol. Obstet. 2000;71:237–239. doi: 10.1016/S0020-7292(99)00173-3. [DOI] [PubMed] [Google Scholar]
- 42.Dunn T.S., Ginsburg V., Wolf D. Loop-Cone Cerclage in Pregnancy: A 5-Year Review. Gynecol. Oncol. 2003;90:577–580. doi: 10.1016/S0090-8258(03)00395-0. [DOI] [PubMed] [Google Scholar]
- 43.Lacour R.A., Garner E.I.O., Molpus K.L., Ashfaq R., Schorge J.O. Management of Cervical Adenocarcinoma in Situ during Pregnancy. Am. J. Obstet. Gynecol. 2005;192:1449–1451. doi: 10.1016/j.ajog.2004.12.079. [DOI] [PubMed] [Google Scholar]
- 44.Hunter M.I., Monk B.J., Tewari K.S. Cervical Neoplasia in Pregnancy. Part 1: Screening and Management of Preinvasive Disease. Am. J. Obstet. Gynecol. 2008;199:3–9. doi: 10.1016/j.ajog.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 45.Griffin D., Manuck T.A., Hoffman M.S. Adenocarcinoma in Situ of the Cervix in Pregnancy. Gynecol. Oncol. 2005;97:662–664. doi: 10.1016/j.ygyno.2004.12.060. [DOI] [PubMed] [Google Scholar]
- 46.Goldberg G.L., Altaras M.M., Block B. Cone Cerclage in Pregnancy. Obstet. Gynecol. 1991;77:315–317. doi: 10.1097/00006250-199102000-00033. [DOI] [PubMed] [Google Scholar]
- 47.Demeter A., Sziller I., Csapó Z., Szánthó A., Papp Z. Outcome of Pregnancies after Cold-Knife Conization of the Uterine Cervix during Pregnancy. Eur. J. Gynaecol. Oncol. 2002;23:207–210. [PubMed] [Google Scholar]
- 48.Herod J., Decruze S., Patel R. A Report of Two Cases of the Management of Cervical Cancer in Pregnancy by Cone Biopsy and Laparoscopic Pelvic Node Dissection. BJOG Int. J. Obstet. Gynaecol. 2010;117:1558–1561. doi: 10.1111/j.1471-0528.2010.02723.x. [DOI] [PubMed] [Google Scholar]
- 49.Ben-Arie A., Levy R., Lavie O., Edwards C., Kaplan A. Conservative Treatment of Stage IA2 Squamous Cell Carcinoma of the Cervix During Pregnancy. Obstet. Gynecol. 2004;104:1129–1131. doi: 10.1097/01.AOG.0000143261.45225.ec. [DOI] [PubMed] [Google Scholar]
- 50.Seki N., Kodama J., Kusumoto T., Nakamura K., Hongo A., Hiramatsu Y. Complications and Obstetric Outcomes after Laser Conization during Pregnancy. Eur. J. Gynaecol. Oncol. 2010;31:399–401. [PubMed] [Google Scholar]
- 51.Fambrini M., Penna C., Fallani M.G., Pieralli A., Mattei A., Scarselli G., Taddei G.L., Marchionni M. Feasibility and outcome of laser CO2 conization performed within the 18th week of gestation. Int. J. Gynecol. Cancer. 2007;17:127–131. doi: 10.1111/j.1525-1438.2007.00802.x. [DOI] [PubMed] [Google Scholar]
- 52.Siegler E., Amit A., Lavie O., Auslender R., Mackuli L., Weissman A. Cervical Intraepithelial Neoplasia 2, 3 in Pregnancy: Time to Consider Loop Cone Excision in the First Trimester of Pregnancy? J. Low. Genit. Tract Dis. 2014;18:162–168. doi: 10.1097/LGT.0b013e318299c0af. [DOI] [PubMed] [Google Scholar]
- 53.Robova H., Rob L., Pluta M., Kacirek J., Halaska M., Strnad P., Schlegerova D. Squamous Intraepithelial Lesion-Microinvasive Carcinoma of the Cervix during Pregnancy. Eur. J. Gynaecol. Oncol. 2005;26:611–614. [PubMed] [Google Scholar]
- 54.Plante M., Kwon J.S., Ferguson S., Samouëlian V., Ferron G., Maulard A., de Kroon C., Van Driel W., Tidy J., Marth C., et al. An International Randomized Phase III Trial Comparing Radical Hysterectomy and Pelvic Node Dissection (RH) vs Simple Hysterectomy and Pelvic Node Dissection (SH) in Patients with Low-Risk Early-Stage Cervical Cancer (LRESCC): A Gynecologic Cancer Intergroup Study Led by the Canadian Cancer Trials Group (CCTG CX.5-SHAPE) J. Clin. Oncol. 2023;41:LBA5511. doi: 10.1200/JCO.2023.41.17_suppl.LBA5511. [DOI] [Google Scholar]
- 55.Jolley J.A., Battista L., Wing D.A. Management of Pregnancy after Radical Trachelectomy: Case Reports and Systematic Review of the Literature. Am. J. Perinatol. 2007;24:531–539. doi: 10.1055/s-2007-986680. [DOI] [PubMed] [Google Scholar]
- 56.Saso S., Sawyer R., O’Neill N.M., Tzafetas M., Sayasneh A., Hassan Hamed A., Elliott F., Thum M.-Y., Ghaem-Maghami S., Lee M.-J., et al. Trachelectomy during Pregnancy: What Has Experience Taught Us?: Early Stage Cervical Cancer and Pregnancy. J. Obstet. Gynaecol. Res. 2015;41:640–645. doi: 10.1111/jog.12594. [DOI] [PubMed] [Google Scholar]
- 57.Iwami N., Ishioka S., Endo T., Baba T., Nagasawa K., Takahashi M., Sugio A., Takada S., Mariya T., Mizunuma M., et al. First Case of Vaginal Radical Trachelectomy in a Pregnant Japanese Woman. Int. J. Clin. Oncol. 2011;16:737–740. doi: 10.1007/s10147-011-0209-3. [DOI] [PubMed] [Google Scholar]
- 58.Kyrgiou M., Horwell D., Farthing A. Laparoscopic Radical Abdominal Trachelectomy for the Management of Stage IB1 Cervical Cancer at 14 Weeks’ Gestation: Case Report and Review of the Literature. BJOG Int. J. Obstet. Gynaecol. 2015;122:1138–1143. doi: 10.1111/1471-0528.13392. [DOI] [PubMed] [Google Scholar]
- 59.Abu-Rustum N.R., Tal M.N., DeLair D., Shih K., Sonoda Y. Radical Abdominal Trachelectomy for Stage IB1 Cervical Cancer at 15-Week Gestation. Gynecol. Oncol. 2010;116:151–152. doi: 10.1016/j.ygyno.2009.10.042. [DOI] [PubMed] [Google Scholar]
- 60.Mandic A., Novakovic P., Nincic D., Zivaljevic M., Rajovic J. Radical Abdominal Trachelectomy in the 19th Gestation Week in Patients with Early Invasive Cervical Carcinoma: Case Study and Overview of Literature. Am. J. Obstet. Gynecol. 2009;201:e6–e8. doi: 10.1016/j.ajog.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 61.Ferriaoli D., Buenerd A., Marchiolè P., Constantini S., Venturini P.L., Mathevet P. Early Invasive Cervical Cancer During Pregnancy: Different Therapeutic Options to Preserve Fertility. Int. J. Gynecol. Cancer. 2012;22:842–849. doi: 10.1097/IGC.0b013e31824ff142. [DOI] [PubMed] [Google Scholar]
- 62.Puchar A., Boudy A.S., Selleret L., Arfi A., Owen C., Bendifallah S., Darai E. Invasive and in Situ Cervical Cancer Associated with Pregnancy: Analysis from the French Cancer Network (CALG: Cancer Associé à La Grossesse) Clin. Transl. Oncol. 2020;22:2002–2008. doi: 10.1007/s12094-020-02343-5. [DOI] [PubMed] [Google Scholar]
- 63.Rodolakis A., Thomakos N., Sotiropoulou M., Kypriotis K., Valsamidis D., Bourgioti C., Moulopoulou L.E., Vlachos G., Loutradis D. Abdominal Radical Trachelectomy for Early-Stage Cervical Cancer During Pregnancy: A Provocative Surgical Approach. Overview of the Literature and a Single-Institute Experience. Int. J. Gynecol. Cancer. 2018;28:1743–1750. doi: 10.1097/IGC.0000000000001357. [DOI] [PubMed] [Google Scholar]
- 64.Umemoto M., Ishioka S., Mizugaki Y., Fujibe Y., Mariya T., Kawamata A., Mizuuchi M., Morishita M., Baba T., Saito T. Obstetrical Prognosis of Patients Who Underwent Vaginal Radical Trachelectomy during Pregnancy. J. Obstet. Gynaecol. Res. 2019;45:1167–1172. doi: 10.1111/jog.13964. [DOI] [PubMed] [Google Scholar]
- 65.Yoshihara K., Ishiguro T., Chihara M., Shima E., Adachi S., Isobe M., Haino K., Yamaguchi M., Sekine M., Kashima K., et al. The Safety and Effectiveness of Abdominal Radical Trachelectomy for Early-Stage Cervical Cancer During Pregnancy. Int. J. Gynecol. Cancer. 2018;28:782–787. doi: 10.1097/IGC.0000000000001218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ungár L., Smith J.R., Pálfalvi L., Del Priore G. Abdominal Radical Trachelectomy During Pregnancy to Preserve Pregnancy and Fertility. Obstet. Gynecol. 2006;108:811–814. doi: 10.1097/01.AOG.0000216015.15415.5f. [DOI] [PubMed] [Google Scholar]
- 67.Karateke A., Cam C., Celik C., Baykal B., Tug N., Ozbasli E., Tosun O.A. Radical Trachelectomy in Late Pregnancy: Is It an Option? Eur. J. Obstet. Gynecol. Reprod. Biol. 2010;152:112–113. doi: 10.1016/j.ejogrb.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 68.Salas P.I., González-Benitez C., De Santiago J., Zapardiel I. Polypoid Adenocarcinoma of the Cervix during Pregnancy Managed with Conservative Treatment. Int. J. Gynecol. Obstet. 2015;130:202–203. doi: 10.1016/j.ijgo.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 69.Yi X., Ding J., Zhang Y., Liu X., Cheng H., Li X., Zhou X., Hua K. Laparoscopic Radical Trachelectomy Followed by Chemotherapy in a Pregnant Patient with Invasive Cervical Cancer. Int. J. Gynecol. Obstet. 2015;131:101–102. doi: 10.1016/j.ijgo.2015.04.035. [DOI] [PubMed] [Google Scholar]
- 70.Salvo G., Frumovitz M., Pareja R., Lee J., Ramirez P.T. Simple Trachelectomy with Pelvic Lymphadenectomy as a Viable Treatment Option in Pregnant Patients with Stage IB1 (≥2 cm) Cervical Cancer: Bridging the Gap to Fetal Viability. Gynecol. Oncol. 2018;150:50–55. doi: 10.1016/j.ygyno.2018.05.021. [DOI] [PubMed] [Google Scholar]
- 71.Alouini S., Rida K., Mathevet P. Cervical Cancer Complicating Pregnancy: Implications of Laparoscopic Lymphadenectomy. Gynecol. Oncol. 2008;108:472–477. doi: 10.1016/j.ygyno.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 72.Aoki Y., Inamine M., Ohishi S., Nagai Y., Masamoto H. Radical Abdominal Trachelectomy for IB1 Cervical Cancer at 17 Weeks of Gestation: A Case Report and Literature Review. Case Rep. Obstet. Gynecol. 2014;2014:926502. doi: 10.1155/2014/926502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bravo E., Parry S., Alonso C., Rojas S. Radical Vaginal Trachelectomy and Laparoscopic Pelvic Lymphadenectomy in IB1 Cervical Cancer during Pregnancy. Gynecol. Oncol. Case Rep. 2012;2:78–79. doi: 10.1016/j.gynor.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Enomoto T., Yoshino K., Fujita M., Miyoshi Y., Ueda Y., Koyama S., Kimura T., Tomimatsu T., Kimura T. A Successful Case of Abdominal Radical Trachelectomy for Cervical Cancer during Pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011;158:365–366. doi: 10.1016/j.ejogrb.2011.04.048. [DOI] [PubMed] [Google Scholar]
- 75.Kolomainen D.F., Bradley R.J., Larsen-Disney P., Shepherd J.H. Radical Vaginal Trachelectomy at 16weeks’ Gestation: A Case Report. Gynecol. Oncol. Case Rep. 2013;5:28–30. doi: 10.1016/j.gynor.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Căpîlna M.E., Szabo B., Becsi J., Ioanid N., Moldovan B. Radical Trachelectomy Performed During Pregnancy: A Review of the Literature. Int. J. Gynecol. Cancer. 2016;26:758–762. doi: 10.1097/IGC.0000000000000655. [DOI] [PubMed] [Google Scholar]
- 77.Van De Nieuwenhof H.P., Van Ham M.A.P.C., Lotgering F.K., Massuger L.F.A.G. First Case of Vaginal Radical Trachelectomy in a Pregnant Patient. Int. J. Gynecol. Cancer. 2008;18:1381–1385. doi: 10.1111/j.1525-1438.2008.01193.x. [DOI] [PubMed] [Google Scholar]
- 78.Sioutas A., Schedvins K., Larson B., Gemzell-Danielsson K. Three Cases of Vaginal Radical Trachelectomy during Pregnancy. Gynecol. Oncol. 2011;121:420–421. doi: 10.1016/j.ygyno.2010.12.357. [DOI] [PubMed] [Google Scholar]
- 79.Einstein M.H., Park K.J., Sonoda Y., Carter J., Chi D.S., Barakat R.R., Abu-Rustum N.R. Radical Vaginal versus Abdominal Trachelectomy for Stage IB1 Cervical Cancer: A Comparison of Surgical and Pathologic Outcomes. Gynecol. Oncol. 2009;112:73–77. doi: 10.1016/j.ygyno.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cao D.Y., Yang J.X., Wu X.H., Chen Y.L., Li L., Liu K.J., Cui M.H., Xie X., Wu Y.M., Kong B.H., et al. Comparisons of Vaginal and Abdominal Radical Trachelectomy for Early-Stage Cervical Cancer: Preliminary Results of a Multi-Center Research in China. Br. J. Cancer. 2013;109:2778–2782. doi: 10.1038/bjc.2013.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sato Y., Hidaka N., Sakai A., Kido S., Fujita Y., Okugawa K., Yahata H., Kato K. Evaluation of the Efficacy of Vaginal Progesterone in Preventing Preterm Birth after Abdominal Trachelectomy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021;259:119–124. doi: 10.1016/j.ejogrb.2021.02.009. [DOI] [PubMed] [Google Scholar]
- 82.Bader A., Petru E., Winter R. Long-Term Follow-up after Neoadjuvant Chemotherapy for High-Risk Cervical Cancer during Pregnancy. Gynecol. Oncol. 2007;105:269–272. doi: 10.1016/j.ygyno.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 83.Caluwaerts S., Van Calsteren K., Mertens L., Lagae L., Moerman P., Hanssens M., Wuyts K., Vergote I., Amant F. Neoadjuvant Chemotherapy Followed by Radical Hysterectomy for Invasive Cervical Cancer Diagnosed during Pregnancy: Report of a Case and Review of the Literature. Int. J. Gynecol. Cancer. 2006;16:905–908. doi: 10.1111/j.1525-1438.2006.00223.x. [DOI] [PubMed] [Google Scholar]
- 84.Peculis L.D., Ius Y., Campion M., Friedlander M., Hacker N. Stage IB2 Adenosquamous Cervical Cancer Diagnosed at 19-Weeks’ Gestation. Aust. N. Z. J. Obstet. Gynaecol. 2015;55:94–97. doi: 10.1111/ajo.12281. [DOI] [PubMed] [Google Scholar]
- 85.De Vincenzo R., Tortorella L., Ricci C., Cavaliere A.F., Zannoni G.F., Cefalo M.G., Scambia G., Fagotti A. Locally Advanced Cervical Cancer Complicating Pregnancy: A Case of Competing Risks from the Catholic University of the Sacred Heart in Rome. Gynecol. Oncol. 2018;150:398–405. doi: 10.1016/j.ygyno.2018.06.028. [DOI] [PubMed] [Google Scholar]
- 86.Heron D.E., Axtel A., Edwards R.P. Villoglandular Adenocarcinoma of the Cervix Recurrent in an Episiotomy Scar: A Case Report in a 32-Year-Old Female. Int. J. Gynecol. Cancer. 2005;15:366–371. doi: 10.1136/ijgc-00009577-200503000-00030. [DOI] [PubMed] [Google Scholar]
- 87.Karam A., Feldman N., Holschneider C.H. Neoadjuvant Cisplatin and Radical Cesarean Hysterectomy for Cervical Cancer in Pregnancy. Nat. Clin. Pract. Oncol. 2007;4:375–380. doi: 10.1038/ncponc0821. [DOI] [PubMed] [Google Scholar]
- 88.Palaia I., Pernice M., Graziano M., Bellati F., Panici P.B. Neoadjuvant Chemotherapy plus Radical Surgery in Locally Advanced Cervical Cancer during Pregnancy: A Case Report. Am. J. Obstet. Gynecol. 2007;197:e5–e6. doi: 10.1016/j.ajog.2007.07.034. [DOI] [PubMed] [Google Scholar]
- 89.Schorge J.O., Russo A.L., Greene M.F., Woythaler M.A., Oliva E. Case 21-2017: A 28-Year-Old Pregnant Woman with Endocervical Carcinoma. N. Engl. J. Med. 2017;377:174–182. doi: 10.1056/NEJMcpc1703511. [DOI] [PubMed] [Google Scholar]
- 90.Guo Y., Zhang D., Li Y., Wang Y. A Case of Successful Maintained Pregnancy after Neoadjuvant Chemotherapy plus Radical Surgery for Stage IB3 Cervical Cancer Diagnosed at 13 Weeks. BMC Pregnancy Childbirth. 2020;20:202. doi: 10.1186/s12884-020-02895-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hurteau J. Villoglandular Adenocarcinoma of the Cervix: A Case Report. Obstet. Gynecol. 1995;85:906–908. doi: 10.1016/0029-7844(94)00418-D. [DOI] [PubMed] [Google Scholar]
- 92.Islam S., Mukhopadhyay L., Howells R. Neo-Adjuvant Chemotherapy and Radical Surgery for Stage 1B Cervical Cancer in Pregnancy. J. Obstet. Gynaecol. 2012;32:191–192. doi: 10.3109/01443615.2011.645094. [DOI] [PubMed] [Google Scholar]
- 93.Lavie O., Segev Y., Peer G., Gutterman E., Sagie S., Auslnader R. Conservative Management for Villoglandular Papillary Adenocarcinoma of the Cervix Diagnosed during Pregnancy Followed by a Successful Term Delivery: A Case Report and a Review of the Literature. Eur. J. Surg. Oncol. EJSO. 2008;34:606–608. doi: 10.1016/j.ejso.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 94.Lurie S., Dgani R., Gorbacz S., Borenstein R. Invasive Papillary Serous Adenocarcinoma of the Endocervix in Pregnancy; a Case Report. Eur. J. Obstet. Gynecol. Reprod. Biol. 1991;40:79–81. doi: 10.1016/0028-2243(91)90049-Q. [DOI] [PubMed] [Google Scholar]
- 95.Marnitz S., Schmittel A., Bolbrinker J., Schmidt F.-P., Fons G., Kalache K., Schneider A., Köhler C. The Therapeutic Management of a Twin Pregnancy Complicated by the Presence of Cervical Cancer, Following Laparoscopic Staging and Chemotherapy, with an Emphasis on Cisplatin Concentrations in the Fetomaternal Compartments Amnion Fluid, Umbilical Cord, and Maternal Serum. Fertil. Steril. 2009;92:1748.e1–1748.e4. doi: 10.1016/j.fertnstert.2009.07.1000. [DOI] [PubMed] [Google Scholar]
- 96.Powell J.L., Bock K.A., Gentry J.K., White W.C., Ronnett B.M. Metastatic Endocervical Adenocarcinoma Presenting as a Virilizing Ovarian Mass During Pregnancy. Obstet. Gynecol. 2002;100:1129–1133. doi: 10.1016/s0029-7844(02)02205-6. [DOI] [PubMed] [Google Scholar]
- 97.Ohwada M., Suzuki M., Hironaka M., Irie T., Sato I. Neuroendocrine Small Cell Carcinoma of the Uterine Cervix Showing Polypoid Growth and Complicated by Pregnancy. Gynecol. Oncol. 2001;81:117–119. doi: 10.1006/gyno.2000.6117. [DOI] [PubMed] [Google Scholar]
- 98.Meseci E., Onculoglu C., Ince U., Teomete M., Eser S.K., Demirkıran F. Embryonal Rhabdomyosarcoma of the Uterine Cervix in a Pregnant Woman. Taiwan. J. Obstet. Gynecol. 2014;53:423–425. doi: 10.1016/j.tjog.2013.10.042. [DOI] [PubMed] [Google Scholar]
- 99.Bigelow C.A., Horowitz N.S., Goodman A., Growdon W.B., Del Carmen M., Kaimal A.J. Management and Outcome of Cervical Cancer Diagnosed in Pregnancy. Am. J. Obstet. Gynecol. 2017;216:276.e1–276.e6. doi: 10.1016/j.ajog.2016.10.034. [DOI] [PubMed] [Google Scholar]
- 100.Mazghi A.E., Bouhafa T., Loukili K., Kacemi H.E., Lalya I., Hassouni K. Prise en charge du cancer du col utérin durant la grossesse: À propos de 05 cas. Pan Afr. Med. J. 2014;19:245. doi: 10.11604/pamj.2014.19.245.5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ishioka S., Ezaka Y., Endo T., Nagasawa K., Shimizu A., Sato A., Inoue M., Saito T. Outcomes of Planned Delivery Delay in Pregnant Patients with Invasive Gynecologic Cancer. Int. J. Clin. Oncol. 2009;14:321–325. doi: 10.1007/s10147-008-0861-4. [DOI] [PubMed] [Google Scholar]
- 102.Ribeiro F., Correia L., Paula T., Santana I., Vieira Pinto L., Borrego J., Jorge A.F. Cervical Cancer in Pregnancy: 3 Cases, 3 Different Approaches. J. Low. Genit. Tract Dis. 2013;17:66–70. doi: 10.1097/LGT.0b013e31824d6fb8. [DOI] [PubMed] [Google Scholar]
- 103.Ricci C., Scambia G., De Vincenzo R. Locally Advanced Cervical Cancer in Pregnancy: Overcoming the Challenge. A Case Series and Review of the Literature. Int. J. Gynecol. Cancer. 2016;26:1490–1496. doi: 10.1097/IGC.0000000000000795. [DOI] [PubMed] [Google Scholar]
- 104.Vercellino G.F., Koehler C., Erdemoglu E., Mangler M., Lanowska M., Malak A.-H., Schneider A., Chiantera V. Laparoscopic Pelvic Lymphadenectomy in 32 Pregnant Patients with Cervical Cancer: Rationale, Description of the Technique, and Outcome. Int. J. Gynecol. Cancer. 2014;24:364–371. doi: 10.1097/IGC.0000000000000064. [DOI] [PubMed] [Google Scholar]
- 105.Baltzer J., Regenbrecht M.E., Kopcke W., Zander J. Carcinoma of the Cervix and Pregnancy. Int. J. Gynaecol. Obstet. 1990;31:317–323. doi: 10.1016/0020-7292(90)90908-4. [DOI] [PubMed] [Google Scholar]
- 106.Fruscio R., Villa A., Chiari S., Vergani P., Ceppi L., Dell’Orto F., Dell’Anna T., Chiappa V., Bonazzi C.M., Milani R., et al. Delivery Delay with Neoadjuvant Chemotherapy for Cervical Cancer Patients during Pregnancy: A Series of Nine Cases and Literature Review. Gynecol. Oncol. 2012;126:192–197. doi: 10.1016/j.ygyno.2012.04.027. [DOI] [PubMed] [Google Scholar]
- 107.Germann N., Haie-Meder C., Morice P., Lhomme C., Duvillard P., Hacene K., Gerbaulet A. Management and Clinical Outcomes of Pregnant Patients with Invasive Cervical Cancer. Ann. Oncol. 2005;16:397–402. doi: 10.1093/annonc/mdi084. [DOI] [PubMed] [Google Scholar]
- 108.Huang H., Quan Y., Qi X., Liu P. Neoadjuvant Chemotherapy with Paclitaxel plus Cisplatin before Radical Surgery for Locally Advanced Cervical Cancer during Pregnancy: A Case Series and Literature Review. Medicine. 2021;100:e26845. doi: 10.1097/MD.0000000000026845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lanowska M., Köhler C., Oppelt P., Schmittel A., Gottschalk E., Hasenbein K., Schneider A., Marnitz S. Addressing Concerns about Cisplatin Application during Pregnancy. J. Perinat. Med. 2011;39:279–285. doi: 10.1515/jpm.2011.015. [DOI] [PubMed] [Google Scholar]
- 110.Lee J.-M., Lee K.-B., Kim Y.-T., Ryu H.-S., Kim Y.-T., Cho C.-H., Namkoong S.-E., Lee K.-H., Choi H.-S., Kim K.-T. Cervical Cancer Associated with Pregnancy: Results of a Multicenter Retrospective Korean Study (KGOG-1006) Am. J. Obstet. Gynecol. 2008;198:92.e1–92.e6. doi: 10.1016/j.ajog.2007.06.077. [DOI] [PubMed] [Google Scholar]
- 111.Li J., Wang L., Zhang B., Peng Y., Lin Z. Neoadjuvant Chemotherapy with Paclitaxel plus Platinum for Invasive Cervical Cancer in Pregnancy: Two Case Report and Literature Review. Arch. Gynecol. Obstet. 2011;284:779–783. doi: 10.1007/s00404-011-1943-5. [DOI] [PubMed] [Google Scholar]
- 112.Marnitz S., Köhler C., Oppelt P., Schmittel A., Favero G., Hasenbein K., Schneider A., Markman M. Cisplatin Application in Pregnancy: First in Vivo Analysis of 7 Patients. Oncology. 2010;79:72–77. doi: 10.1159/000320156. [DOI] [PubMed] [Google Scholar]
- 113.Xia T., Gao Y., Wu B., Yang Y. Clinical Analysis of Twenty Cases of Cervical Cancer Associated with Pregnancy. J. Cancer Res. Clin. Oncol. 2015;141:1633–1637. doi: 10.1007/s00432-014-1886-x. [DOI] [PubMed] [Google Scholar]
- 114.Favero G., Lanowska M., Schneider A., Marnitz S., Köhler C. Laparoscopic Pelvic Lymphadenectomy in a Patient with Cervical Cancer Stage Ib1 Complicated by a Twin Pregnancy. J. Minim. Invasive Gynecol. 2010;17:118–120. doi: 10.1016/j.jmig.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 115.Hoopmann M., Valter M., Kurbacher C., Possover M., Mallmann P.K. Chemopersistent Early Recurrence in the Perineal Tear Scar of an Intrapartally Diagnosed Cervical Cancer. Gynecol. Oncol. 2003;91:272–274. doi: 10.1016/S0090-8258(03)00475-X. [DOI] [PubMed] [Google Scholar]
- 116.Dede M., Deveci G., Deveci M.S., Yenen M.C., Goktolga U., Dilek S., Gunhan O. Villoglandular Papillary Adenocarcinoma of the Uterine Cervix in a Pregnant Woman: A Case Report and Review of Literature. Tohoku J. Exp. Med. 2004;202:305–310. doi: 10.1620/tjem.202.305. [DOI] [PubMed] [Google Scholar]
- 117.Matsuo K., Mandelbaum R.S., Matsuzaki S., Licon E., Roman L.D., Klar M., Grubbs B.H. Cesarean Radical Hysterectomy for Cervical Cancer in the United States: A National Study of Surgical Outcomes. Am. J. Obstet. Gynecol. 2020;222:507–511.e2. doi: 10.1016/j.ajog.2020.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Peccatori F.A., Azim H.A., Jr., Orecchia R., Hoekstra H.J., Pavlidis N., Kesic V., Pentheroudakis G., ESMO Guidelines Working Group Cancer, Pregnancy and Fertility: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-up. Ann. Oncol. 2013;24((Suppl. S6)):Vi160–Vi170. doi: 10.1093/Annonc/Mdt199. [DOI] [PubMed] [Google Scholar]
- 119.Fox C.R., Burdeaux S., Downing K.T. A 33-Year-Old Woman in the Third Trimester of Pregnancy Diagnosed with Advanced-Staged Squamous Cell Cervical Carcinoma by Magnetic Resonance Imaging and Biopsy. Am. J. Case Rep. 2021;22:e933639. doi: 10.12659/AJCR.933639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Perrin L., Bell J., Ward B. Small Cell Carcinoma of the Cervix of Neuroendocrine Origin Causing Obstructed Labour. Aust. N. Z. J. Obstet. Gynaecol. 1996;36:85–87. doi: 10.1111/j.1479-828X.1996.tb02932.x. [DOI] [PubMed] [Google Scholar]
- 121.Tsao A.S., Roth L.M., Sandler A., Hurteau J.A. Cervical Primitive Neuroectodermal Tumor. Gynecol. Oncol. 2001;83:138–142. doi: 10.1006/gyno.2001.6339. [DOI] [PubMed] [Google Scholar]
- 122.Bizzarri N., Pedone Anchora L., Kucukmetin A., Ratnavelu N., Korompelis P., Fedele C., Bruno M., Di Fiore G.L.M., Fagotti A., Fanfani F., et al. Risk of Ovarian Recurrence after Ovarian Conservation in Early-Stage Cervical Cancer Treated with Radical Surgery: A Propensity Match Analysis. Eur. J. Surg. Oncol. 2021;47:2158–2165. doi: 10.1016/j.ejso.2021.04.009. [DOI] [PubMed] [Google Scholar]
- 123.Matsuo K., Shimada M., Yamaguchi S., Kanao H., Nakanishi T., Saito T., Kamiura S., Iwata T., Mikami M., Sugiyama T. Identifying a Candidate Population for Ovarian Conservation in Young Women with Clinical Stage IB–IIB Cervical Cancer. Int. J. Cancer. 2018;142:1022–1032. doi: 10.1002/ijc.31084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rudolf H., Goretzlehner G., Brugmann E., Töwe J., Rudolf K. Assessment of Liver Function Using Indocyanine Green (Ujoviridin) during Normal Pregnancy, during Labor and in Puerperium. Zentralbl. Gynakol. 1977;99:1548–1553. [PubMed] [Google Scholar]
- 125.Papadia A., Mohr S., Imboden S., Lanz S., Bolla D., Mueller M.D. Laparoscopic Indocyanine Green Sentinel Lymph Node Mapping in Pregnant Cervical Cancer Patients. J. Minim. Invasive Gynecol. 2016;23:270–273. doi: 10.1016/j.jmig.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 126.Weisz B., Meirow D., Schiff E., Lishner M. Impact and Treatment of Cancer during Pregnancy. Expert Rev. Anticancer Ther. 2004;4:889–902. doi: 10.1586/14737140.4.5.889. [DOI] [PubMed] [Google Scholar]
- 127.Marth C., Landoni F., Mahner S., McCormack M., Gonzalez-Martin A., Colombo N., ESMO Guidelines Committee Cervical Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-up. Ann. Oncol. 2017;28((Suppl. S4)):Iv72–Iv83. doi: 10.1093/Annonc/Mdx220. Erratum in: Ann. Oncol. 2018, 29 (Suppl. S4), Iv262. [DOI] [PubMed] [Google Scholar]
- 128.Dawood R., Instone M., Kehoe S. Neo-Adjuvant Chemotherapy for Cervical Cancer in Pregnancy: A Case Report and Literature Review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013;171:205–208. doi: 10.1016/j.ejogrb.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 129.Kayahashi K., Mizumoto Y., Myojo S., Mitani Y., Tajima A., Fujiwara H. A Successful Case of Neoadjuvant Chemotherapy and Radical Hysterectomy during Pregnancy for Advanced Uterine Cervical Cancer Accompanied by Neonatal Erythroderma: Radical Hysterectomy after NAC in Pregnancy. J. Obstet. Gynaecol. Res. 2018;44:2003–2007. doi: 10.1111/jog.13746. [DOI] [PubMed] [Google Scholar]
- 130.Marana H.R.C., Moreira de Andrade J., do Carmo da Silva Mathes Â., Duarte G., Pereira da Cunha S., Bighetti S. Chemotherapy in the Treatment of Locally Advanced Cervical Cancer and Pregnancy. Gynecol. Oncol. 2001;80:272–274. doi: 10.1006/gyno.2000.6055. [DOI] [PubMed] [Google Scholar]
- 131.Levy L., Meuwly J.-Y., Sarivalasis A., Achtari C., Mathevet P., Herrera F.G. Survival of the Fetus: Cervical Cancer and Pregnancy, a Challenging Combination. Lancet. 2020;396:725. doi: 10.1016/S0140-6736(20)31794-3. [DOI] [PubMed] [Google Scholar]
- 132.De Oliveira A.F., de Souza L.R.M.F., Paschoini M.C., Murta E.F.C., Nomelini R.S. Chemotherapy for Cervical Cancer in Pregnancy. J. Obstet. Gynaecol. 2019;39:425–426. doi: 10.1080/01443615.2018.1474187. [DOI] [PubMed] [Google Scholar]
- 133.Seamon L.G., Downey G.O., Harrison C.R., Doss B., Carlson J.W. Neoadjuvant Chemotherapy Followed by Post-Partum Chemoradiotherapy and Chemoconsolidation for Stage IIIB Glassy Cell Cervical Carcinoma during Pregnancy. Gynecol. Oncol. 2009;114:540–541. doi: 10.1016/j.ygyno.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 134.Ayhan A., Dursun P., Karakaya B.K., Ozen O., Tarhan C. Neoadjuvant Chemotherapy Followed by Cesarean Radical Hysterectomy in a Triplet Pregnancy Complicated by Clear Cell Carcinoma of the Cervix: A Case Presentation and Literature Review. Int. J. Gynecol. Cancer. 2012;22:1198–1202. doi: 10.1097/IGC.0b013e31825e0d5a. [DOI] [PubMed] [Google Scholar]
- 135.Boyd A., Cowie V., Gourley C. The Use of Cisplatin to Treat Advanced-Stage Cervical Cancer During Pregnancy Allows Fetal Development and Prevents Cancer Progression: Report of a Case and Review of the Literature. Int. J. Gynecol. Cancer. 2009;19:273–276. doi: 10.1111/IGC.0b013e31819bcff8. [DOI] [PubMed] [Google Scholar]
- 136.Komiyama S., Nishio E., Torii Y., Kawamura K., Oe S., Kato R., Hasagawa K., Abe M., Kuroda M., Udagawa Y. A Case of Primary Uterine Cervical Neuroendocrine Tumor with Meningeal Carcinomatosis Confirmed by Diagnostic Imaging and Autopsy. Int. J. Clin. Oncol. 2011;16:581–586. doi: 10.1007/s10147-010-0155-5. [DOI] [PubMed] [Google Scholar]
- 137.Carillon M.-A., Emmanuelli V., Castelain B., Taieb S., Collinet P., Vinatier D., Lesoin A., Chevalier-Evain V., Leblanc E., Narducci F. Cancer du col utérin invasif et grossesse: Cinq cas observés à Lille de 2002 à 2009. Évaluation des pratiques en référence aux recommandations françaises. J. Gynécologie Obstétrique Biol. Reprod. 2011;40:514–521. doi: 10.1016/j.jgyn.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 138.Smyth E.C., Korpanty G., McCaffrey J.A., Mulligan N., Carney D.N. Small-Cell Carcinoma of the Cervix at 23 Weeks Gestation. J. Clin. Oncol. 2010;28:E295–E297. doi: 10.1200/JCO.2009.25.5604. [DOI] [PubMed] [Google Scholar]
- 139.Surbone A., Achtari C. Embryonal Rhabdomyosarcoma in a Child Exposed to Chemotherapy in Utero: A Mere Coincidence? Eur. J. Obstet. Gynecol. Reprod. Biol. 2016;207:235. doi: 10.1016/j.ejogrb.2016.09.030. [DOI] [PubMed] [Google Scholar]
- 140.Zhang X., Gao Y., Yang Y. Treatment and Prognosis of Cervical Cancer Associated with Pregnancy: Analysis of 20 Cases from a Chinese Tumor Institution. J. Zhejiang Univ.-Sci. B. 2015;16:388–394. doi: 10.1631/jzus.B1400251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zagouri F., Korakiti A.-M., Zakopoulou R., Kyriazoglou A., Zografos E., Haidopoulos D., Apostolidou K., Papatheodoridi M.A., Dimopoulos M.A. Taxanes during Pregnancy in Cervical Cancer: A Systematic Review and Pooled Analysis. Cancer Treat. Rev. 2019;79:101885. doi: 10.1016/j.ctrv.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 142.Lapresa M., Parma G., Portuesi R., Colombo N. Neoadjuvant Chemotherapy in Cervical Cancer: An Update. Expert Rev. Anticancer. Ther. 2015;15:1171–1181. doi: 10.1586/14737140.2015.1079777. [DOI] [PubMed] [Google Scholar]
- 143.Kenter G., Greggi S., Vergote I., Katsaros D., Kobierski J., Massuger L., van Doorn H.C., Landoni F., Van Der Velden J., Reed N.S., et al. Results from Neoadjuvant Chemotherapy Followed by Surgery Compared to Chemoradiation for Stage Ib2-IIb Cervical Cancer, EORTC 55994. J. Clin. Oncol. 2019;37:5503. doi: 10.1200/JCO.2019.37.15_suppl.5503. [DOI] [PubMed] [Google Scholar]
- 144.Gupta S., Maheshwari A., Parab P., Mahantshetty U., Hawaldar R., Sastri Chopra S., Kerkar R., Engineer R., Tongaonkar H., Ghosh J., et al. Neoadjuvant Chemotherapy Followed by Radical Surgery Versus Concomitant Chemotherapy and Radiotherapy in Patients with Stage IB2, IIA, or IIB Squamous Cervical Cancer: A Randomized Controlled Trial. J. Clin. Oncol. 2018;36:1548–1555. doi: 10.1200/JCO.2017.75.9985. [DOI] [PubMed] [Google Scholar]
- 145.Benhaim Y., Haie-Meder C., Lhommé C., Pautier P., Duvillard P., Castaigne D., Morice P. Chemoradiation Therapy in Pregnant Patients Treated for Advanced-Stage Cervical Carcinoma during the First Trimester of Pregnancy: Report of Two Cases. Int. J. Gynecol. Cancer. 2007;17:270–274. doi: 10.1111/j.1525-1438.2007.00783.x. [DOI] [PubMed] [Google Scholar]
- 146.Sood A. Cervical Cancer Diagnosed Shortly after Pregnancy: Prognostic Variables and Delivery Routes. Obstet. Gynecol. 2000;95:832–838. doi: 10.1097/00006250-200006000-00010. [DOI] [PubMed] [Google Scholar]
- 147.Jadoul P., Querleu D., Squifflet J.-L., Donnez J. Recurrence during Pregnancy of a Conservatively Treated Early-Stage Cervical Squamous Cell Carcinoma. Fertil. Steril. 2011;95:e13–e290. doi: 10.1016/j.fertnstert.2010.06.056. [DOI] [PubMed] [Google Scholar]
- 148.Morice P., Uzan C., Gouy S., Verschraegen C., Haie-Meder C. Gynaecological Cancers in Pregnancy. Lancet. 2012;379:558–569. doi: 10.1016/S0140-6736(11)60829-5. [DOI] [PubMed] [Google Scholar]
- 149.Balderston K.D., Tewari K., Gregory W.T., Berman M.L., Kucera P.R. Neuroendocrine Small Cell Uterine Cervix Cancer in Pregnancy: Long-Term Survival Following Combined Therapy. Gynecol. Oncol. 1998;71:128–132. doi: 10.1006/gyno.1998.5104. [DOI] [PubMed] [Google Scholar]
- 150.Canto M.J., Pons N., Mendez I., Reus A., Ojeda F. Metastatic Small-Cell Carcinoma of the Cervix during Pregnancy. J. Obstet. Gynaecol. 2014;34:204–205. doi: 10.3109/01443615.2013.845550. [DOI] [PubMed] [Google Scholar]
- 151.Feng X., Zhang L., Tan Y., Feng A., Luo F., Xu M., Ye H., Zhu H., Zhou P., Li H. Primitive Neuroectodermal Tumor of the Cervix Diagnosed during Pregnancy: A Rare Case Report with Discussion. BMC Pregnancy Childbirth. 2021;21:382. doi: 10.1186/s12884-021-03859-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Herskovic E., Ryan M., Weinstein J., Wadhwani N.R. Maternal to Fetal Transmission of Cervical Carcinoma. Pediatr. Radiol. 2014;44:1035–1038. doi: 10.1007/s00247-013-2858-z. [DOI] [PubMed] [Google Scholar]
- 153.Leung T.W., Lo S.H., Wong S.F., Yuen M.K., Wong F.C.S., Tung S.Y., Sze W.K., SK O. Small Cell Carcinoma of the Cervix Complicated by Pregnancy. Clin. Oncol. 1999;11:123–125. doi: 10.1053/clon.1999.9026. [DOI] [PubMed] [Google Scholar]
- 154.Li W.W.H., Yau T.N., Leung C.W.L., Pong W.M., Chan M.Y.M. Large-Cell Neuroendocrine Carcinoma of the Uterine Cervix Complicating Pregnancy. Hong Kong Med. J. 2009;15:69–72. [PubMed] [Google Scholar]
- 155.Terada T. Clear Cell Adenocarcinoma of the Uterine Cervix in a Young Pregnant Woman: A Case Report with Immunohistochemical Study. Med. Oncol. 2011;28:290–293. doi: 10.1007/s12032-009-9410-x. [DOI] [PubMed] [Google Scholar]
- 156.Gardner G.J., Reidy-Lagunes D., Gehrig P.A. Neuroendocrine Tumors of the Gynecologic Tract: A Society of Gynecologic Oncology (SGO) Clinical Document. Gynecol. Oncol. 2011;122:190–198. doi: 10.1016/j.ygyno.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 157.Goldman N. Late Recurrence of Squamous Cell Cervical Cancer in an Episiotomy Site after Vaginal Delivery. Obstet. Gynecol. 2003;101:1127–1129. doi: 10.1016/S0029-7844(02)02337-2. [DOI] [PubMed] [Google Scholar]
- 158.Baloglu A., Uysal D., Aslan N., Yigit S. Advanced Stage of Cervical Carcinoma Undiagnosed during Antenatal Period in Term Pregnancy and Concomitant Metastasis on Episiotomy Scar during Delivery: A Case Report and Review of the Literature. Int. J. Gynecol. Cancer. 2007;17:1155–1159. doi: 10.1111/j.1525-1438.2007.00926.x. [DOI] [PubMed] [Google Scholar]
- 159.Can N.T.T., Robertson P., Zaloudek C.J., Gill R.M. Cervical Squamous Cell Carcinoma Metastatic to Placenta. Int. J. Gynecol. Pathol. 2013;32:516–519. doi: 10.1097/PGP.0b013e3182763178. [DOI] [PubMed] [Google Scholar]
- 160.Van Calsteren K., Hanssens M., Moerman P., Orye G., Bielen D., Vergote I., Amant F. Successful Conservative Treatment of Endocervical Adenocarcinoma Stage Ib1 Diagnosed Early in Pregnancy. Acta Obstet. Gynecol. Scand. 2008;87:250–253. doi: 10.1080/00016340701870794. [DOI] [PubMed] [Google Scholar]
- 161.van Vliet W., van Loon A.J., ten Hoor K.A., Boonstra H. Cervical Carcinoma during Pregnancy: Outcome of Planned Delay in Treatment. Eur. J. Obstet. Gynecol. Reprod. Biol. 1998;79:153–157. doi: 10.1016/S0301-2115(98)00022-0. [DOI] [PubMed] [Google Scholar]
- 162.Copeland L.J., Saul P.B., Sneige N. Cervical Adenocarcinoma: Tumor Implantation in the Episiotomy Sites of Two Patients. Gynecol. Oncol. 1987;28:230–235. doi: 10.1016/0090-8258(87)90219-8. [DOI] [PubMed] [Google Scholar]
- 163.Cliby W.A., Dodson M.K., Podratz K.C. Cervical Cancer Complicated by Pregnancy: Episiotomy Site Recurrences Following Vaginal Delivery. Obstet. Gynecol. 1994;84:179–182. [PubMed] [Google Scholar]
- 164.Khalil A.M., Khatib R.A., Mufarrij A.A., Tawil A.N., Issa P.Y. Squamous Cell Carcinoma of the Cervix Implanting in the Episiotomy Site. Gynecol. Oncol. 1993;51:408–410. doi: 10.1006/gyno.1993.1313. [DOI] [PubMed] [Google Scholar]
- 165.Gordon A.N., Jensen R., Jones H.W., 3rd Squamous Carcinoma of the Cervix Complicating Pregnancy: Recurrence in Episiotomy after Vaginal Delivery. Obstet. Gynecol. 1989;73:850–852. [PubMed] [Google Scholar]
- 166.Burgess S.P., Waymont B. Implantation of a Cervical Carcinoma in an Episiotomy Site. Case Report. Br. J. Obstet. Gynaecol. 1987;94:598–599. doi: 10.1111/j.1471-0528.1987.tb03159.x. [DOI] [PubMed] [Google Scholar]
- 167.Van Dam P.A., Irvine L., Lowe D.G., Fisher C., Barton D.P., Shepherd J.H. Carcinoma in Episiotomy Scars. Gynecol. Oncol. 1992;44:96–100. doi: 10.1016/0090-8258(92)90019-F. [DOI] [PubMed] [Google Scholar]
- 168.Van Den Broek N.R., Lopes A.D., Ansink A., Monaghan J.M. ‘“Microinvasive”’ Adenocarcinoma of the Cervix Implanting in an Episiotomy Scar. Gynecol. Oncol. 1995;59:297–299. doi: 10.1006/gyno.1995.0025. [DOI] [PubMed] [Google Scholar]
- 169.Amanie J., Pearcey R.G., Honore L., Sloboda R. Metastatic Adenocarcinoma of the Cervix in a Delivery-Induced Traumatic Lower Vaginal Tear. Gynecol. Oncol. 2005;96:857–859. doi: 10.1016/j.ygyno.2004.11.040. [DOI] [PubMed] [Google Scholar]
- 170.Greenlee R.M., Chervenak F.A., Tovell H.M. Incisional Recurrence of a Cervical Carcinoma. Report of a Case. JAMA. 1981;246:69–70. doi: 10.1001/jama.1981.03320010049032. [DOI] [PubMed] [Google Scholar]
- 171.Sivanesaratnam V., Jayalakshmi P., Loo C. Surgical Management of Early Invasive Cancer of the Cervix Associated with Pregnancy. Gynecol. Oncol. 1993;48:68–75. doi: 10.1006/gyno.1993.1011. [DOI] [PubMed] [Google Scholar]
- 172.Dalrymple J.L., Gilbert W.M., Leiserowitz G.S., Cress R., Xing G., Danielsen B., Smith L.H. Pregnancy-Associated Cervical Cancer: Obstetric Outcomes. J. Matern. Fetal Neonatal Med. 2005;17:269–276. doi: 10.1080/14767050500123962. [DOI] [PubMed] [Google Scholar]
- 173.Halaska M.J., Komar M., Vlk R., Tomek V., Skultety J., Robova H., Rob L. A Pilot Study on Peak Systolic Velocity Monitoring of Fetal Anemia after Administration of Chemotherapy during Pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014;174:76–79. doi: 10.1016/j.ejogrb.2013.12.021. [DOI] [PubMed] [Google Scholar]
- 174.Ancel P.Y., Goffinet F., EPIPAGE-2 Writing Group. Kuhn P., Langer B., Matis J., Hernandorena X., Chabanier P., Joly-Pedespan L., Lecomte B., et al. Survival and Morbidity of Preterm Children Born at 22 through 34 Weeks’ Gestation in France in 2011: Results of the EPIPAGE-2 Cohort Study. JAMA Pediatr. 2015;169:230.e8. doi: 10.1001/jamapediatrics.2014.3351. [DOI] [PubMed] [Google Scholar]
- 175.Arakawa A., Ichikawa H., Kubo T., Motoi N., Kumamoto T., Nakajima M., Yonemori K., Noguchi E., Sunami K., Shiraishi K., et al. Vaginal Transmission of Cancer from Mothers with Cervical Cancer to Infants. N. Engl. J. Med. 2021;384:42–50. doi: 10.1056/NEJMoa2030391. [DOI] [PubMed] [Google Scholar]
- 176.Berretta M., Di Francia R., Lleshi A., De Paoli P., Li Volti G., Bearz A., Del Pup L., Tirelli U., Michieli M. Antiblastic Treatment, for Solid Tumors, during Pregnancy: A Crucial Decision. Int. J. Immunopathol. Pharmacol. 2012;25:1–19. doi: 10.1177/03946320120250S201. [DOI] [PubMed] [Google Scholar]
- 177.Kohler C., Oppelt P., Favero G., Morgenstern B., Runnebaum I., Tsunoda A., Schmittel A., Schneider A., Mueller M., Marnitz S. How Much Platinum Passes the Placental Barrier? Analysis of Platinum Applications in 21 Patients with Cervical Cancer during Pregnancy. Am. J. Obstet. Gynecol. 2015;213:206.e1–206.e5. doi: 10.1016/j.ajog.2015.02.022. [DOI] [PubMed] [Google Scholar]
- 178.Geijteman E.C.T., Wensveen C.W.M., Duvekot J.J., van Zuylen L. A Child with Severe Hearing Loss Associated with Maternal Cisplatin Treatment during Pregnancy. Obstet. Gynecol. 2014;124:454–456. doi: 10.1097/AOG.0000000000000389. [DOI] [PubMed] [Google Scholar]
- 179.Al-Halal H., Kezouh A., Abenhaim H.A. Incidence and Obstetrical Outcomes of Cervical Intraepithelial Neoplasia and Cervical Cancer in Pregnancy: A Population-Based Study on 8.8 Million Births. Arch. Gynecol. Obstet. 2013;287:245–250. doi: 10.1007/s00404-012-2475-3. [DOI] [PubMed] [Google Scholar]
- 180.Allen D.G., Planner R.S., Tang P.T.M., Scurry J.P., Weerasir T. Invasive Cervical Cancer in Pregnancy. Aust. N. Z. J. Obstet. Gynaecol. 1995;35:408–412. doi: 10.1111/j.1479-828X.1995.tb02154.x. [DOI] [PubMed] [Google Scholar]
- 181.Manuel-Limson G.A., Ladines-Llave C.A., Sotto L.S.J., Manalo A.M. Cancer of the Cervix in Pregnancy: A 31-Year Experience at the Philippine General Hospital. J. Obstet. Gynaecol. Res. 1997;23:503–509. doi: 10.1111/j.1447-0756.1997.tb00879.x. [DOI] [PubMed] [Google Scholar]
- 182.Pettersson B.F., Andersson S., Hellman K., Hellström A.-C. Invasive Carcinoma of the Uterine Cervix Associated with Pregnancy: 90 Years of Experience. Cancer. 2010;116:2343–2349. doi: 10.1002/cncr.24971. [DOI] [PubMed] [Google Scholar]
- 183.Vandervange N., Weverling G., Ketting B., Ankum W., Samlal R., Lammes F. The Prognosis of Cervical Cancer Associated with Pregnancy: A Matched Cohort Study. Obstet. Gynecol. 1995;85:1022–1026. doi: 10.1016/0029-7844(95)00059-Z. [DOI] [PubMed] [Google Scholar]
- 184.Eibye S., Krüger Kjær S., Nielsen T.S.S., Mellemkjær L. Mortality Among Women with Cervical Cancer During or Shortly After a Pregnancy in Denmark 1968 to 2006. Int. J. Gynecol. Cancer. 2016;26:951–958. doi: 10.1097/IGC.0000000000000708. [DOI] [PubMed] [Google Scholar]
- 185.Condrat C.E., Filip L., Gherghe M., Cretoiu D., Suciu N. Maternal HPV Infection: Effects on Pregnancy Outcome. Viruses. 2021;13:2455. doi: 10.3390/v13122455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hooda R., Baghla N., Malik N., Kaushik S. To Evaluate the Role of Placental Human Papilloma Virus (HPV) Infection as a Risk Factor for Spontaneous Preterm Birth: A Prospective Case Control Study. J. Perinat. Med. 2022;50:427–432. doi: 10.1515/jpm-2021-0317. [DOI] [PubMed] [Google Scholar]
- 187.Lei J., Ploner A., Elfström K.M., Wang J., Roth A., Fang F., Sundström K., Dillner J., Sparén P. HPV Vaccination and the Risk of Invasive Cervical Cancer. N. Engl. J. Med. 2020;383:1340–1348. doi: 10.1056/NEJMoa1917338. [DOI] [PubMed] [Google Scholar]
- 188.Falcaro M., Castañon A., Ndlela B., Checchi M., Soldan K., Lopez-Bernal J., Elliss-Brookes L., Sasieni P. The Effects of the National HPV Vaccination Programme in England, UK, on Cervical Cancer and Grade 3 Cervical Intraepithelial Neoplasia Incidence: A Register-Based Observational Study. Lancet. 2021;398:2084–2092. doi: 10.1016/S0140-6736(21)02178-4. [DOI] [PubMed] [Google Scholar]
- 189.Avni-Singer L., Oliveira C.R., Torres A., Shapiro E.D., Niccolai L.M., Sheth S.S. Evaluation of an Inpatient Postpartum Human Papillomavirus Immunization Program. Obstet. Gynecol. 2020;136:1006–1015. doi: 10.1097/AOG.0000000000004097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Évaluation de la Recherche des Papillomavirus Humains (HPV) en Dépistage Primaire des Lésions Précancéreuses et Cancéreuses du col de l’utérus et de la Place du Double Immuno-Marquage p16/Ki67. [(accessed on 2 May 2022)]. Available online: https://www.has-sante.fr/jcms/c_2806160/fr/evaluation-de-la-recherche-des-papillomavirus-humains-hpv-en-depistage-primaire-des-lesions-precancereuses-et-cancereuses-du-col-de-l-uterus-et-de-la-place-du-double-immuno-marquage-p16/ki67.
- 191.Franciscatto L.G., Silva C.M.D., Barcellos R.B., Angeli S., Silva M.S.N., Almeida S.E.M., Rossetti M.L.R. Comparison of Urine and Self-Collected Vaginal Samples for Detecting Human Papillomavirus DNA in Pregnant Women. Int. J. Gynaecol. Obstet. Off. Organ. Int. Fed. Gynaecol. Obstet. 2014;125:69–72. doi: 10.1016/j.ijgo.2013.09.031. [DOI] [PubMed] [Google Scholar]
- 192.Tewari K.S., Sill M.W., Penson R.T., Huang H., Ramondetta L.M., Landrum L.M., Oaknin A., Reid T.J., Leitao M.M., Michael H.E., et al. Final Overall Survival of the Phase III Randomised Trial of Chemotherapy with and without Bevacizumab for Advanced Cervical Cancer: An NRG Oncology/Gynecologic Oncology Group Study. Lancet Lond. Engl. 2017;390:1654–1663. doi: 10.1016/S0140-6736(17)31607-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Colombo N., Dubot C., Lorusso D., Caceres M.V., Hasegawa K., Shapira-Frommer R., Tewari K.S., Salman P., Hoyos Usta E., Yañez E., et al. Pembrolizumab for Persistent, Recurrent, or Metastatic Cervical Cancer. N. Engl. J. Med. 2021;385:1856–1867. doi: 10.1056/NEJMoa2112435. [DOI] [PubMed] [Google Scholar]
- 194.Duska L.R., Scalici J.M., Temkin S.M., Schwarz J.K., Crane E.K., Moxley K.M., Hamilton C.A., Wethington S.L., Petroni G.R., Varhegyi N.E., et al. Results of an Early Safety Analysis of a Study of the Combination of Pembrolizumab and Pelvic Chemoradiation in Locally Advanced Cervical Cancer. Cancer. 2020;126:4948–4956. doi: 10.1002/cncr.33136. [DOI] [PubMed] [Google Scholar]
- 195.Roussy G., Campus C., Paris G. Randomized Phase II Trial Assessing the Inhibitor of Programmed Cell Death Ligand 1 (PD-L1) Immune Checkpoint Atezolizumab in Locally Advanced Cervical Cancer. [(accessed on 1 November 2022)]; Available online: https://clinicaltrials.gov/study/NCT03612791?cond=Randomized%20Phase%20II%20Trial%20Assessing%20the%20Inhibitor%20of%20Programmed%20Cell%20Death%20Ligand%20&rank=1.
- 196.Mayadev J.S., Enserro D., Lin Y.G., Da Silva D.M., Lankes H.A., Aghajanian C., Ghamande S., Moore K.N., Kennedy V.A., Fracasso P.M., et al. Sequential Ipilimumab After Chemoradiotherapy in Curative-Intent Treatment of Patients with Node-Positive Cervical Cancer. JAMA Oncol. 2020;6:92–99. doi: 10.1001/jamaoncol.2019.3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Merck Sharp & Dohme LLC A Randomized, Phase 3, Double-Blind Study of Chemoradiotherapy with or without Pembrolizumab for the Treatment of High-Risk, Locally Advanced Cervical Cancer (KEYNOTE-A18/ENGOT-Cx11/GOG-3047) [(accessed on 1 November 2022)]; Available online: https://clinicaltrials.gov/study/NCT04221945?cond=A%20Randomized,%20Phase%203,%20Double-Blind%20Study%20of%20Chemoradiotherapy%20with%20or%20without%20Pembrolizumab%20for%20the%20Treatment%20of%20&rank=1.
- 198.Borgers J.S.W., Heimovaara J.H., Cardonick E., Dierickx D., Lambertini M., Haanen J.B.A.G., Amant F. Immunotherapy for Cancer Treatment during Pregnancy. Lancet Oncol. 2021;22:e550–e561. doi: 10.1016/S1470-2045(21)00525-8. [DOI] [PubMed] [Google Scholar]
- 199.Coleman R.L., Lorusso D., Gennigens C., González-Martín A., Randall L., Cibula D., Lund B., Woelber L., Pignata S., Forget F., et al. Efficacy and Safety of Tisotumab Vedotin in Previously Treated Recurrent or Metastatic Cervical Cancer (innovaTV 204/GOG-3023/ENGOT-Cx6): A Multicentre, Open-Label, Single-Arm, Phase 2 Study. Lancet Oncol. 2021;22:609–619. doi: 10.1016/S1470-2045(21)00056-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are contained within the article and Supplementary Materials.