Abstract
We assessed human immunodeficiency virus (HIV) load in plasma and semen during primary HIV infection using serial samples of semen and plasma during the first 24 weeks after diagnosis in untreated participants and those who started antiretroviral therapy (ART) immediately at diagnosis. In the absence of treatment, semen viral load was >1000 copies/mL in almost all specimens (83%) collected 2–10 weeks after the estimated date of HIV acquisition and remained >1000 copies/mL in 35% of untreated participants at the last observed time point. Thus, in the absence of ART, semen viral load remained at a level consistent with transmissibility throughout primary infection.
Keywords: HIV, viral load, antiretroviral therapy (ART), plasma, seminal plasma, blood, semen
Viral load dynamics was assessed among those randomized to deferred versus immediate ART during primary HIV-1 infection. Among treated participants, VL in plasma and semen reduced to <1000 copies/mL; in the absence of ART, semen VL remained >1000 copies/mL throughout primary infection.
Treatment as prevention (TasP) strategies initiate antiretroviral therapy (ART) soon after a human immunodeficiency virus (HIV) diagnosis and can reduce forward transmission by suppressing viral replication [1–3]. However, most cluster randomized trials of TasP in heterosexual populations in Africa did not demonstrate expected reductions in HIV incidence [4–7], suggesting that TasP strategies are nuanced.
Plasma viral load (VL) is a predictor of forward transmission [8]; large epidemiologic studies have documented few transmissions from individuals with suppressed plasma VL. Plasma VL generally correlates well with the biologically relevant measure of VL in semen or rectal secretions, but this is not always the case [9].
To investigate the potential consequences of even short delays in treatment during primary HIV infection when VL is high, we analyzed serial samples of semen and plasma during the first 24 weeks after diagnosis in untreated participants and those who started ART immediately at diagnosis. We also assessed whether plasma VL reliably predicts semen VL during this period.
METHODS
Study Population
The present study includes 66 participants from the Sabes study, conducted from 2013 to 2017 in Lima, Peru [10]. Sabes enrolled 2109 HIV-negative men who have sex with men and transgender women who were tested monthly for HIV antibodies and RNA. Participants diagnosed with acute (HIV seronegative and HIV RNA positive) or recent (HIV seropositive with HIV RNA negative test within 3 months) HIV infection were randomized to initiate ART immediately (immediate arm) or 24 weeks later (deferred arm). This analysis includes 29 immediate arm and 37 deferred arm participants. If deferred arm participants met local treatment criteria prior to 24 weeks, they were offered ART and censored at ART initiation. Immediate and deferred arm participants are referred to as treated and untreated for the purpose of this study.
Self-Administered Questionnaires
Participants reported demographics (eg, age, self-identified gender), substance use including the Alcohol Use Disorders Identification Test (AUDIT; https://auditscreen.org/about/scoring-audit/), and sexual behaviors using a computer-assisted self-interview at study visits.
Time Since HIV Infection
We used estimated date of detectable infection (EDDI) and Fiebig stage [11] at the time of randomization to assess time since HIV infection. EDDI was calculated using a published online calculator that incorporates all HIV testing data from each participant [12].
Laboratory Data
CD4 cell count and CD4/CD8 ratio were assessed at weeks 0, 4, 12, and 24; HIV-1 RNA in plasma and semen were measured at week 0 and at 1, 2, 4, 8, 12, and 24 weeks after randomization (Abbott m2000 real-time HIV-1 assay; Abbott Molecular). The lower limits of quantification (LLOQ) were approximately 40 copies/mL for plasma and 160 to 640 copies/mL for semen, which reflects the differing dilution factors. In the event of missing samples, the sample from the closest available time point was analyzed.
Participants were tested for sexually transmitted infections (STI) at weeks 0, 12, and 24 and as indicated clinically, and treated according to local guidelines. Urine samples and rectal swabs were tested for chlamydia and gonorrhea (APTIMA Combo 2 Assay; Hologic Gen-Probe, Inc).
Specimen Collection
Semen was collected by masturbation after ≥3 days of sexual abstinence. Seminal plasma was prepared by centrifugation of 1 mL of semen at 800g for 10 minutes and stored at −80°C. HIV-negative semen was used to obtain the necessary sample volume for processing, thus diluting the semen 4–16 times. Blood was drawn at semen collection time points, and plasma was stored at −80°C.
Viral Suppression
We defined viral suppression in both plasma and semen as 2 consecutive measurements of <1000 copies/mL. While our limit of detection in semen was 160–640 copies/mL, we used the approximation of 1000 copies/mL as the likely threshold for transmissibility. In prior studies, we found this level to be the minimum needed to support HIV infection in vitro; in a study of 232 semen samples, only 1 of 24 HIV coculture-positive samples had seminal plasma VL < 2.8 log copies/mL [9, 13].
Statistical Analysis
Differences between treatment arms were evaluated using χ2 or Fischer exact analysis for categorical variables and 2-sample t tests on means for continuous variables. We used the Kruskal-Wallis test for differences in semen VL at baseline comparing Fiebig stages 1–4 to Fiebig stages 5–6, and to test whether there was a difference in baseline VL by randomization arm.
Spaghetti plots tracked VL decay in plasma and semen by treatment arm; the value for participants achieving the LLOQ in semen (160–640 copies/mL) or plasma (40 copies/mL) was plotted at one-half the LLOQ. Kaplan-Meier curves plotted by compartment (semen and plasma) and treatment arm show the survival functions to the first of at least 2 consecutive visits with VL <1000 copies/mL. Cox proportional hazard models were used to compare treatment arms for semen and plasma VL separately. We compared VL in plasma and semen to assess how well plasma VL predicts semen VL during the high viremia seen in primary infection.
We calculated the area under the curve (AUC) using Riemann sums for log10 VLs for each individual, using time to viral suppression (ie, 2 subsequent measures below the LLOQ) or until the end of follow-up. We quantified the longitudinal association between cumulative AUC and treatment arm using linear mixed effects models. We used a density-dependent decay model to compare treatment arms for both semen and plasma (Supplementary Material).
Ethics
This study was approved by institutional review boards at the Fred Hutchinson Cancer Center, Asociación Civil Impacta Salud y Educación, and the Asociación Vía Libre.
RESULTS
Sixty-six individuals with acute or recent HIV infection were included in this analysis. At enrollment, participants reported an average of 9 partners in the last 3 months (range, 1–60). Three-quarters reported unprotected receptive anal intercourse (mean, 4.8 partners; range, 1–55) and two-thirds reported unprotected insertive anal intercourse (mean, 8 partners; range, 1–40). Twenty-nine participants began ART (Atripla, Efavirenz/Emtricitabine/Tenofovir [n = 28] or Stribild, Elvitegravir/Cobicistat/Emtricitabine/Tenofovir [n = 1]) immediately, while 37 participants were randomized to the deferred arm. Gender, age, AUDIT score, Fiebig stage category, CD4/CD8 ratio, or CD4 counts were comparable between the immediate and deferred arms (Supplementary Table 1). The mean time between EDDI and randomization at the enrollment visit was 36.8 days (SD 17.3) in the immediate arm and 43.2 days (SD 18.2) in the deferred arm.
Plasma Viral Load
Plasma VL at baseline was slightly higher among immediate versus deferred arm participants (P = .035). The likelihood of achieving viral suppression in plasma was significantly higher in the immediate arm (Supplementary Figure 1; hazard ratio [HR], 28.61; P < .0001). In contrast to the rapid suppression of VL in the immediate arm, only 1 participant in the deferred arm achieved viral suppression by week 12 (Figure 1A and 1B). The differences in timing and extent of suppression in plasma were reflected in AUC analysis (Supplementary Figure 2A). There was persistently higher VL in blood over time as seen by a larger cumulative AUC over the 24-week period in the deferred compared to the immediate arm (P < .001).
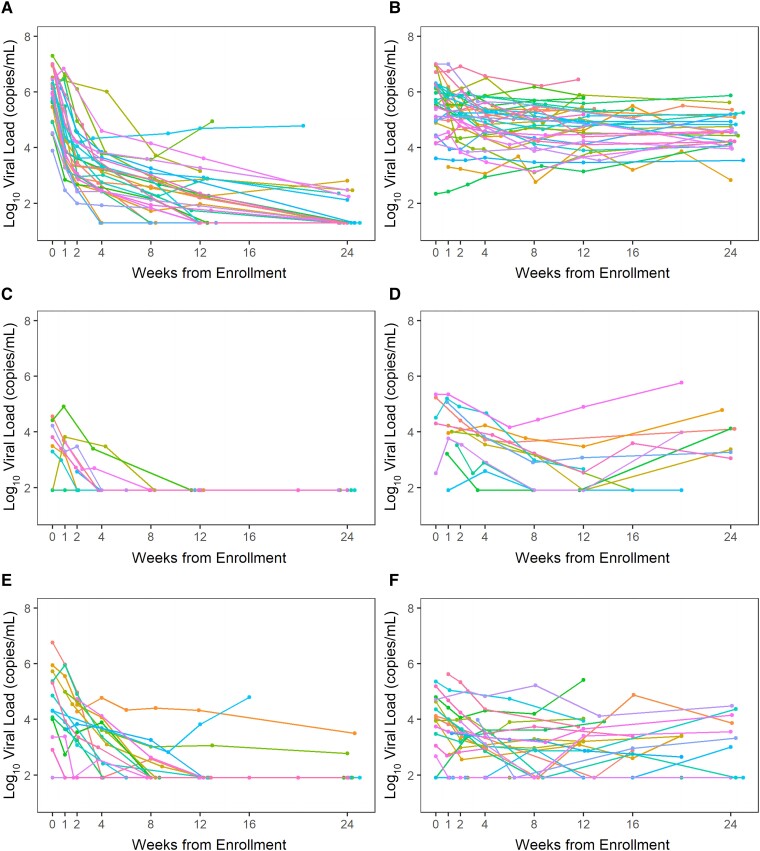
Figure 1.
Viral load in plasma and semen during primary infection in treated (immediate arm) and untreated (deferred arm) study participants: (A) plasma, immediate arm; (B) plasma, deferred arm; (C) semen, immediate arm, Fiebig stage 1–4; (D) semen, deferred arm, Fiebig stage 1–4; (E) semen, immediate arm, Fiebig stage 5–6; and (F) semen, deferred arm, Fiebig stage 5–6. Fiebig stage correlated well with time from EDDI to the randomization visit; 95% (21 of 22) participants in Fiebig stages 1–4 attended the randomization visit within 5 weeks of EDDI and 91% (40 of 44) participants in Fiebig stages 5–6 had their randomization visit ≥5 weeks after EDDI. The median VLs in semen among participants who were in Fiebig stages 1–4 versus Fiebig 5–6 at the randomization visit were similar (4.22 log10 copies/mL [IQR, 3.49–4.51] vs 4.05 log10 copies/mL [IQR, 3.04–4.80]; P = .99). The initial rise in semen VL, which peaked at approximately 5 weeks and subsequent decayed, (as shown in Figure 2) may be responsible for the similarity in the median and IQR for semen VL for Fiebig stages 1–4 versus Fiebig stages 5–6. Different colors indicate values for individual participants. Abbreviations: EDDI, estimated date of detectable infection; IQR, interquartile range; VL, viral load.
Semen Viral Load
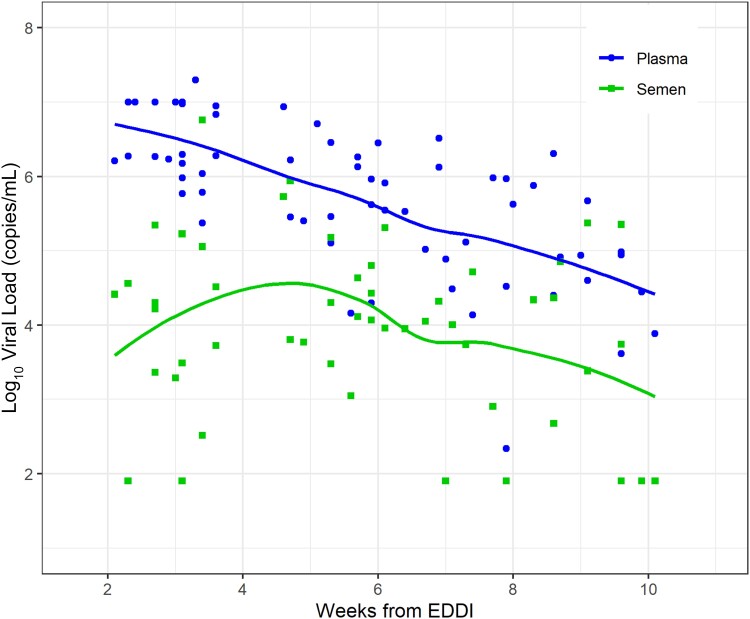
At the baseline visit, we detected high-level viremia in semen, consistent with early seeding of this compartment after HIV acquisition. Fifty-five participants (83%) had semen VL > 1000 copies/mL, which did not differ by randomization arm (P = .7). We observed a slight increase in VL in semen in the initial weeks from EDDI (weeks 2–5), and then a decline that paralleled VL in plasma beginning 5 weeks from EDDI (Figure 2).
Figure 2.
Viral load in semen and plasma in the absence of treatment (ie, at diagnosis) by weeks from estimated date of detectible infection (EDDI) for all study participants. The Loess method was used to generate smoothed curves for semen and plasma viral load (VL). Dots show individual VL values and the lines show the Loess smoothed fit. At enrollment and randomization, time from EDDI ranged from 2 to 14 weeks. Semen VL increased slightly in the initial weeks after EDDI (weeks 2–5), and then declined paralleling plasma VL, beginning 5 weeks from EDDI.
Nearly all immediate arm participants achieved semen viral suppression by week 12 (Figure 1C and 1E). One of 10 participants who began treatment during Fiebig stages 1–4 failed to achieve viral suppression; 3 of 19 who began treatment during Fiebig stages 5–6 did not achieve viral suppression in semen by week 12 and remained unsuppressed at week 24 (Figure 1E). Among deferred arm participants, semen VL remained >1000 copies/mL in 35% at the last observed time point (Figure 1D and 1F).
As observed in plasma, the likelihood of achieving viral suppression in semen was significantly higher in the immediate versus the deferred arm (HR, 2.33; P = .007; Supplementary Figure 1). We observed persistent VL in semen in untreated participants as shown by a larger AUC in the deferred compared to the immediate arm (P = .005; Supplementary Figure 2B).
After 12 weeks of ART in the immediate treatment arm, 3 participants were diagnosed with urethral STIs and 4 were diagnosed with rectal STIs. All participants diagnosed with a urethral STI (3 of 3) and 75% (3 of 4) of participants diagnosed with a rectal STI maintained viral suppression in semen. In contrast, among untreated participants 5 of 8 diagnosed with a urethral STI and 8 of 15 diagnosed with a rectal STI had an increase in semen VL above 1000 copies/mL.
Plasma VL as a Proxy for Semen VL
Defining suppression as VL below LLOQ (≤40 copies/mL in plasma or ≤160 copies/mL in semen) as is used for “undetectable equals untransmittable” (U = U), we identified 18 participants who were never suppressed in either plasma or semen, and 20 who achieved suppression in both compartments. Only 1 participant who achieved suppression in plasma was never suppressed in semen. This participant achieved viral suppression in plasma by week 4 and remained suppressed until week 24. In contrast, their semen VL ranged from 18 800 to 59 200 copies/mL during weeks 4–12 and reached 3200 copies/mL at week 24. In contrast, 27 participants who never suppressed VL in plasma achieved VL suppression in semen.
DISCUSSION
Our results suggest that seeding of the genital tract occurs during the initial weeks after HIV infection, resulting in high semen VL during early primary infection. When ART was initiated immediately, VL in both plasma and semen reduced to < 1000 copies/mL in line with previous reports [14–17]; this level likely will not support onward transmission. Our results may provide a possible explanation for findings in the PopART trial in Zambia and South Africa, which found that up to 30% of infections are attributable to forward transmission within a year of HIV acquisition [18]. These inferences are consistent with our findings that HIV is detectable in semen shortly after HIV acquisition and remains at transmissible levels throughout primary infection in the absence of ART.
While semen VL is likely the best predictor of transmissibility for sexually transmitted HIV, it is usually not available and thus plasma is often used as a proxy. We found that plasma VL is a good, if conservative, predictor of semen VL during primary HIV infection, as it is in chronic infection.
We observed that suppression of semen VL was maintained in ART-treated participants diagnosed with STIs; in contrast, our results suggest an impact of STIs on semen VL in untreated participants, consistent with other reports [19].
In 4 TasP community-randomized trials in heterosexual populations in Africa [4–7], reductions of HIV incidence attributable to universal ART access were less than expected and nonsignificant in most cases. Low levels of linkage to care were cited as a contributing cause and the trials that showed the greatest impact [5, 7] were those with the most significant HIV testing and linkage activities [20]. Rapid linkage after HIV acquisition is important because throughout the 24-week postdiagnosis study period, VL remained elevated in semen in approximately 50% of untreated participants. At enrollment, participants reported an average of 9 partners (range, 1–60) in the last 3 months, with a large majority reporting anal intercourse without a condom. Our expanded TasP strategy emphasized frequent HIV testing by serology and HIV RNA detection and facilitated timely linkage to care using peer navigators, as rapid detection, counseling, and treatment of HIV infection is needed to reduce the risk of transmission to multiple partners.
Strengths of this study are the randomized prospective study design, frequent follow-up, and monthly sampling, providing linked laboratory, epidemiologic, and behavioral data over time. Limitations include a relatively small sample size and use of self-reported data, although any bias is unlikely to be differential by randomization group.
Detection of HIV during early infection and rapid initiation of ART may be essential to reducing HIV incidence in key populations such as transgender women and men who have sex with men. This study underscores the critical need for timely HIV detection through continued scale-up of testing and linkage in the era of universal ART access. Future research should seek to investigate the population-level impact and cost-effectiveness of strategies that seek to identify HIV infection shortly after acquisition and rapidly initiate ART.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Supplementary Material
Notes
Author contributions. T. G., A. D., and R. W. C. contributed substantially to the conception or design of the work, and the acquisition, analysis, and interpretation of data. A. K. U., S. Holte, and S. D. contributed substantially to the conception or design of the work, and analysis and interpretation of data. Y. W. performed analysis and interpretation of data. J. R. L. and J. R. contributed substantially to the conception or design of the work and the acquisition of data. R. A., G. D., and C. G. acquired data. S. Harb and S. P. acquired and analyzed data. All authors drafted the manuscript or revising it critically for important intellectual content, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Acknowledgments. We thank the Sabes Study Team and participants. We also thank Gilead Sciences (Foster City, CA) and Merck Sharp & Dohme Corp. (Kenilworth, NJ) for donating the study drugs.
Financial support. This work was supported by the National Institute on Drug Abuse, US National Institutes of Health (grant number DA032106 to A. D.). T. G. received funding from the International AIDS Society-National Institute on Drug Abuse-French National Agency for Research on AIDS and Viral Hepatitis HIV and Drug Use Research Fellowship Program 2014–2016.
Contributor Information
Trupti Gilada, Unison Medicare and Research Center, Mumbai, India.
Angela K Ulrich, Center for Infectious Disease Research and Policy, University of Minnesota, Minneapolis, Minnesota, USA; Division of Environmental Health Science, University of Minnesota, Minneapolis, Minnesota, USA.
Yixin Wang, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA.
Javier R Lama, Asociación Civil Impacta Salud y Educación, Lima, Peru.
Ricardo Alfaro, Centro de Investigaciones Tecnológicas Biomédicas y Medioambientales, Lima, Peru.
Socorro Harb, School of Medicine, University of Washington, Seattle, Washington, USA.
Glenda Daza, School of Medicine, University of Washington, Seattle, Washington, USA.
Sarah Holte, Public Health Sciences Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA; Department of Global Health, University of Washington, Seattle, Washington, USA; Department of Biostatistics, University of Washington, Seattle, Washington, USA.
Siavash Pasalar, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA.
Jessica Rios, Pharmaceutical Product Development, Inc, Lima, Peru.
Carmela Ganoza, HIV Vaccine Trials Network, Lima, Peru.
Sayan Dasgupta, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA.
Robert W Coombs, School of Medicine, University of Washington, Seattle, Washington, USA.
Ann Duerr, Vaccine and Infectious Disease Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA; Public Health Sciences Division, Fred Hutchinson Cancer Center, Seattle, Washington, USA; Department of Global Health, University of Washington, Seattle, Washington, USA.
References
- 1. Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet 2009; 373:48–57. [DOI] [PubMed] [Google Scholar]
- 3. Leyre L, Kroon E, Vandergeeten C, et al. Abundant HIV-infected cells in blood and tissues are rapidly cleared upon ART initiation during acute HIV infection. Sci Transl Med 2020; 12:eaav3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Havlir DV, Balzer LB, Charlebois ED, et al. HIV testing and treatment with the use of a community health approach in rural Africa. N Engl J Med 2019; 381:219–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hayes RJ, Donnell D, Floyd S, et al. Effect of universal testing and treatment on HIV incidence—HPTN 071 (PopART). N Engl J Med 2019; 381:207–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Iwuji CC, Orne-Gliemann J, Larmarange J, et al. Universal test and treat and the HIV epidemic in rural South Africa: a phase 4, open-label, community cluster randomised trial. Lancet HIV 2018; 5:e116–25. [DOI] [PubMed] [Google Scholar]
- 7. Makhema J, Wirth KE, Pretorius Holme M, et al. Universal testing, expanded treatment, and incidence of HIV infection in Botswana. N Engl J Med 2019; 381:230–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. N Engl J Med 2000; 342:921–9. [DOI] [PubMed] [Google Scholar]
- 9. Stekler J, Sycks BJ, Holte S, et al. HIV dynamics in seminal plasma during primary HIV infection. AIDS Res Hum Retrov 2008; 24:1269–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lama JR, Brezak A, Dobbins JG, et al. Design strategy of the sabes study: diagnosis and treatment of early HIV infection among men who have sex with men and transgender women in Lima, Peru, 2013–2017. Am J Epidemiol 2018; 187:1577–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fiebig EW, Wright DJ, Rawal BD, et al. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS 2003; 17:1871–9. [DOI] [PubMed] [Google Scholar]
- 12. Pilcher CD, Porco TC, Facente SN, et al. A generalizable method for estimating duration of HIV infections using clinical testing history and HIV test results. AIDS 2019; 33:1231–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Coombs RW, Speck CE, Hughes JP, et al. Association between culturable human immunodeficiency virus type 1 (HIV-1) in semen and HIV-1 RNA levels in semen and blood: evidence for compartmentalization of HIV-1 between semen and blood. J Infect Dis 1998; 177:320–30. [DOI] [PubMed] [Google Scholar]
- 14. Gutierrez-Valencia A, Benmarzouk-Hidalgo OJ, Rivas-Jeremías I, et al. Viral kinetics in semen with different antiretroviral families in treatment-naive human immunodeficiency virus-infected patients: a randomized trial. Clin Infect Dis 2017; 65:551–6. [DOI] [PubMed] [Google Scholar]
- 15. Chéret A, Durier C, Mélard A, et al. Impact of early cART on HIV blood and semen compartments at the time of primary infection. PLoS One 2017; 12:e0180191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ghosn J, Assoumou L, Lascoux-Combe C, et al. HIV-1 RNA kinetics in blood plasma and in seminal plasma of men starting a dolutegravir-based triple-combination regimen at the time of primary HIV-1 infection. J Infect Dis 2022; 225:116–20. [DOI] [PubMed] [Google Scholar]
- 17. Mariaggi AA, Bauer R, Charre C, et al. HIV-1-RNA and total HIV-1-DNA loads in the genital compartment in men receiving dolutegravir- versus darunavir-based combined ART (cART) regimens during primary HIV infection. J Antimicrob Chemother 2022; 77:735–9. [DOI] [PubMed] [Google Scholar]
- 18. Fraser C. Viral networks and their implications for prevention policy. HIV Research for Prevention Conference (HIVR4P), PL03.1, February 2021.
- 19. Cohen MS, Council OD, Chen JS. Sexually transmitted infections and HIV in the era of antiretroviral treatment and prevention: the biologic basis for epidemiologic synergy. J Int AIDS Soc 2019; 22:e25355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Johnson L. The past is prologue: can lessons from the TasP trials point the way forward? HIV Research for Prevention Conference (HIVR4P), PL04.01, February 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.