Abstract
Background
Bacterial pathogens cause substantial diarrhea morbidity and mortality among children living in endemic settings, yet antimicrobial treatment is only recommended for dysentery or suspected cholera.
Methods
AntiBiotics for Children with severe Diarrhea was a 7-country, placebo-controlled, double-blind efficacy trial of azithromycin in children 2–23 months of age with watery diarrhea accompanied by dehydration or malnutrition. We tested fecal samples for enteric pathogens utilizing quantitative polymerase chain reaction to identify likely and possible bacterial etiologies and employed pathogen-specific cutoffs based on genomic target quantity in previous case-control diarrhea etiology studies to identify likely and possible bacterial etiologies.
Results
Among 6692 children, the leading likely etiologies were rotavirus (21.1%), enterotoxigenic Escherichia coli encoding heat-stable toxin (13.3%), Shigella (12.6%), and Cryptosporidium (9.6%). More than one-quarter (1894 [28.3%]) had a likely and 1153 (17.3%) a possible bacterial etiology. Day 3 diarrhea was less common in those randomized to azithromycin versus placebo among children with a likely bacterial etiology (risk difference [RD]likely, −11.6 [95% confidence interval {CI}, −15.6 to −7.6]) and possible bacterial etiology (RDpossible, −8.7 [95% CI, −13.0 to −4.4]) but not in other children (RDunlikely, −0.3% [95% CI, −2.9% to 2.3%]). A similar association was observed for 90-day hospitalization or death (RDlikely, −3.1 [95% CI, −5.3 to −1.0]; RDpossible, −2.3 [95% CI, −4.5 to −.01]; RDunlikely, −0.6 [95% CI, −1.9 to .6]). The magnitude of risk differences was similar among specific likely bacterial etiologies, including Shigella.
Conclusions
Acute watery diarrhea confirmed or presumed to be of bacterial etiology may benefit from azithromycin treatment.
Clinical Trials Registration
Keywords: bacterial diarrhea, azithromycin, molecular diagnostics, pediatric diarrhea, Shigella
Improved clinical outcomes among children with acute watery diarrhea of bacterial etiology in this reanalysis of the ABCD trial suggest that a short course of azithromycin may be warranted if bacterial etiologies could be identified at the point of care.
Diarrheal illness is the second leading cause of death in children aged <5 years [1]. Diarrhea deaths disproportionately occur in low- and middle-income countries [2]. The World Health Organization (WHO) recommends oral rehydration therapy, therapeutic zinc, and continued feeding for children with diarrhea, reserving antibiotics only for children with dysentery (presumptive shigellosis) or suspected Vibrio cholerae infection [3]. Despite these guidelines, children presenting to health facilities with moderate to severe diarrhea continue to die in the 3 months following diarrhea presentation, at an almost 9-fold higher rate than age- and community-matched children without diarrhea [4]. Children aged <2 years are also at high risk of linear growth faltering in the months following moderate to severe diarrhea [4, 5]. Importantly, linear growth faltering is on the pathway to stunting, which is associated with health and economic consequences that can extend into adulthood [6, 7].
The AntiBiotics for Children with severe Diarrhea (ABCD) study was a multicountry, randomized, double-blinded, placebo-controlled trial that tested whether a 3-day course of azithromycin reduced mortality or improved growth when administered to children presenting to facilities with acute watery diarrhea and at least 1 of the following: dehydration, severe stunting, or moderate wasting [8]. ABCD found no evidence of a mortality benefit but a small benefit in linear growth and risk of hospitalization (a secondary outcome) following a 3-day course of azithromycin [9].
Bacterial pathogens such as Shigella spp, enterotoxigenic Escherichia coli encoding heat-stable toxin (ST-ETEC), Campylobacter spp, and typical enteropathogenic E coli (tEPEC) are estimated to be responsible for less than half of watery diarrhea cases [10, 11] but are disproportionately associated with linear growth faltering [12, 13] and/or death [4, 14]. Since azithromycin is a broad-spectrum antibiotic with demonstrated efficacy against gram-negative bacteria such as Shigella spp [15, 16], Campylobacter spp [17, 18], and ETEC in adult travelers [19], we postulated that its potential benefit was diluted in ABCD by the presence of a significant proportion of children without a bacterial cause of their diarrhea. Utilizing molecular methods of pathogen detection and ascribing possible and likely etiologies based on pathogen quantity, which has been shown to associate with diarrhea in large case-control studies [10, 20], we sought to determine the effect of azithromycin on risk of death, hospitalization, diarrhea duration, and linear growth in children with and without bacterial etiologies.
METHODS
The ABCD methods and primary outcome results, which did not incorporate enteric pathogen testing, are described elsewhere [8, 9]. In summary, children in Bangladesh, India, Kenya, Malawi, Mali, Pakistan, and Tanzania aged 2–23 months presenting to health facilities with acute watery diarrhea were screened for eligibility between June 2017 and July 2019. Enrolled children had some or severe dehydration as defined by the WHO [21]; moderate wasting (11.5 cm ≤ mid-upper arm circumference [MUAC] <12.5 cm [if ≥6 months of age] or −2 < weight-for-length z-score [WLZ] < −3 [all ages]); and/or severe stunting (length-for-age z-score [LAZ] < −3). In addition, children had no indication for an antibiotic (ie, clinically defined dysentery, severe acute malnutrition, signs of other infections requiring antibiotics) and had not received antibiotics in the 14 days prior to presentation. Once enrolled, children were acutely managed, and a clinical history, physical examination, and caregiver interview were conducted. Whole stool or a flocked rectal swab if stool not passed (Pediatric FLOQswab, Copan Diagnostics, Murrieta, California, catalog number 5U002S) was collected prior to randomization and stored at −80°C. Children were randomized to a 3-day course of azithromycin (10 mg/kg/day) or placebo, and all doses were directly observed by the study team.
Follow-up visits were conducted at home or at a facility both 2 and 3 days after enrollment for directly observed therapy and a brief clinical assessment of adverse events and 90 days after enrollment to collect vital status, anthropometry (length, weight, MUAC), and history of hospitalization. An additional visit was conducted at 180 days postenrollment to assess vital status. Four months after trial initiation, 2 questions related to the diarrheal episode (presence of diarrhea and whether or not the diarrhea was worsening, staying the same, or improving) were added to the day 2/day 3 follow-up visit questionnaires.
Fecal samples from the first approximately 1000 children enrolled at each site were tested by quantitative polymerase chain reaction (qPCR) with a customized TaqMan array card (TAC) following the procedures described previously [22]. In brief, total nucleic acid was extracted from either stool or swab using the QIAamp Stool Fast DNA Mini kit (Qiagen, Valencia, California) [23]. Twenty microliters of the total nucleic acid from whole stool or 46 µK from rectal swab was mixed with AgPath One Step reverse-transcription PCR reagents (Thermo Fisher, Carlsbad, California) in a 100 µL reaction, then loaded into the TAC and run in a ViiA 7 or QuantStudio 7 Flex Real Time PCR system (Thermo Fisher). External controls, MS2 for RNA targets and phocine herpesvirus for DNA targets, were incorporated to monitor the extraction and amplification performance. One extraction blank was included per batch of extraction to rule out laboratory contamination. A cycle threshold (Ct) value of 35, determined previously as the analytical limit of detection [24], was applied. The data were validated only when the corresponding external control and extraction blank yielded valid results. Because we hypothesized that rectal swabs would be less sensitive than whole stool samples due to lower specimen quantity, we also obtained 433 paired rectal swab and whole stool samples from children enrolled at the Kenya site. Pathogen Ct values derived from rectal swabs were then adjusted by the mean Ct difference for each pathogen between the paired rectal swabs and whole stools (Supplementary Table 1 and Supplementary Figure 1). The ipaH gene, which is amplified both for Shigella and enteroinvasive Escherichia coli (EIEC), was assumed to be Shigella based on metagenomic sequencing and low rates of EIEC diarrhea found in similar settings/populations [25, 26].
We developed pathogen-specific cutoffs for assigning likely diarrhea etiology based on the quantity of pathogen DNA/RNA in the stool sample (ie, pathogen burden). Cutoffs were calculated by adapting statistical models previously developed in 2 large multisite studies of diarrhea in similar settings that included nondiarrheal controls, the 7-site Global Enteric Multicenter Study (GEMS) and the 8-site Malnutrition and the Consequences for Child Health and Development (MAL-ED) cohort study [10, 20]. Specifically, in a post hoc reanalysis of GEMS data, we performed qPCR testing of stool from 4077 children with moderate to severe watery diarrhea and 1:1 age-, sex-, village-, and season-matched controls to fit a multivariable conditional logistic regression model, where the outcome was case versus control status and the predictors were the quantity of nucleic acid for each enteric pathogen, with a random slope for each study site. For MAL-ED, we used qPCR testing of 6315 watery diarrheal stools and 28 444 monthly nondiarrheal stools to fit a generalized linear mixed model with an outcome of diarrheal stool versus nondiarrheal stool, and the predictors were sex, age in months, the quantity of nucleic acid for each pathogen, a random slope for each site, and a random effect for each individual child.
To calculate cutoffs (Supplementary Table 2), for each of GEMS and MAL-ED, we first estimated quantity-specific odds ratios (ORs) for Ct values from 35, the analytical limit of detection for the assay, to 15 by 0.001 increments by taking the median ORs for each Ct value from 10 000 random permutations from a normal distribution derived from the model coefficients and the variance-covariance, drawn equally from each of the site-specific models. We then calculated the episode-specific attributable fraction (AFe), where and ORi is the quantity-specific median OR. We then fit a loess regression to the relationship between Ct and AFe for all values of AFe between 0.2 and 0.8 inclusive and picked the highest Ct value with an AFe ≥0.5 (ie, majority attribution) as the cutoff (Supplementary Figures 2 and 3). Finally, we took the mean of the Ct values for each pathogen from GEMS and MAL-ED to arrive at the cutoffs for the present study. Only pathogens deemed attributable to diarrhea (AFe ≥0.5 in either GEMS or MAL-ED) were considered in the present analysis as no Ct cutoffs are available for pathogens not associated with diarrhea in case-control analyses.
Ct values less than or equal to the aforementioned established cutoffs were considered “likely” etiology. Ct values greater than the cutoffs but <35 were considered “possible” etiologies. “Unlikely” etiology was defined as a Ct value ≥35. To interrogate the effect of bacterial etiology on azithromycin efficacy, we compared outcomes between children in the 2 randomization arms within subgroups defined by likely, possible, and unlikely bacterial etiology. Bacteria considered as a likely or possible etiology included Campylobacter spp, tEPEC, ST-ETEC, Salmonella spp (typhoidal and nontyphoidal Salmonella), Shigella spp, and Vibrio cholerae. Children with a possible bacterial etiology in whom a virus or parasite was present at likely etiologic levels were reclassified in the unlikely bacterial etiology group. In additional exploratory analysis, we evaluated the effect of azithromycin in subsets of children with specific bacterial etiologies.
We evaluated the effect of azithromycin, in the above-defined subgroups, on the following outcomes: presence of diarrhea 3 days after enrollment, the combined outcome of death or hospitalization by day 90, and change in LAZ between enrollment and day 90. Day 3 diarrhea was not included in the prespecified outcomes of the main trial but was collected prospectively after the etiology substudy was conceived to be consistent with etiology-specific antibiotic trials that use diarrhea as a primary endpoint. Furthermore, death or hospitalization by day 90 was a secondary outcome in the main trial. The outcomes for this subgroup analysis were chosen based on the biologic plausibility that there might be a differential effect of azithromycin by bacterial etiology and had at least 80% power to detect an effect of azithromycin in the subgroup of children with a likely bacterial etiology, assuming approximately 25% would fall in this subgroup. We also reported on the outcome of death by day 90 and day 180; however, these outcomes were highly underpowered in the primary trial and were therefore anticipated to be underpowered in this subgroup analysis.
The effect of azithromycin on dichotomous outcomes was modeled using log-binomial (or Poisson if models did not converge [27]) mixed-effects models with an identity link (estimating risk difference) and ΔLAZ modeled with generalized linear mixed-effects models (estimating mean difference in difference). Each model included an indicator variable for bacterial etiology (likely, possible, unlikely) and randomization arm, as well as country (as an indicator variable) to account for country-stratified randomization, and interaction terms between randomization arm and each level of bacterial etiology. Evidence of effect modification by bacterial etiology was determined by likelihood ratio test. An α = .05 was used to determine statistical significance in this exploratory secondary analysis.
Ethical review committees from the WHO and each respective country institution approved the trial protocol, and informed consent was obtained from all caregivers of included participants. All analyses were done in Stata version 16.0 and R software.
RESULTS
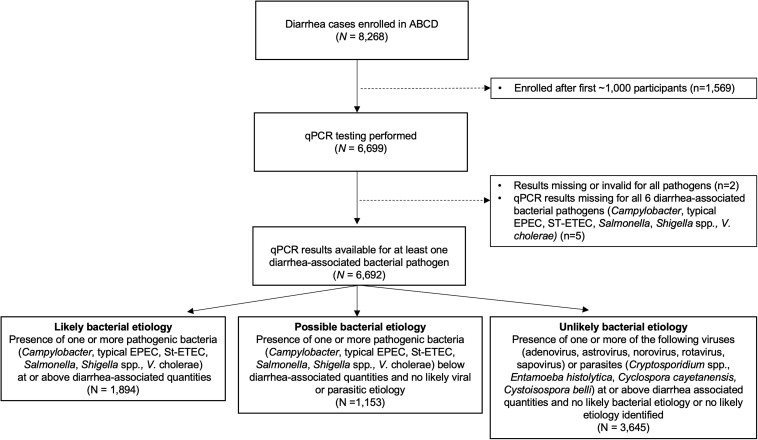
Of the 8268 children enrolled in the ABCD trial between July 2017 and July 2019, the first 6699 (81.0%) children's fecal samples were qPCR-tested, 6692 (99.9%) of which had valid qPCR results (Figure 1). Approximately one-third of fecal samples were rectal swabs (Bangladesh [17.2%], India [42.4%], Kenya [56.5%], Malawi [42.4%], Mali [61.2%], Pakistan [38.0%], Tanzania [14.8%]). The median age of children was 12 months (interquartile range [IQR], 7–17 months) and 53.9% were male (Table 1). Children experienced a median of 2 days of diarrhea (IQR, 1–3 days) before presenting to the health facility, 54.4% were dehydrated, 32.9% were stunted, and 46.4% had moderate wasting. Child characteristics were balanced between the 2 randomization arms.
Figure 1.
Participant flowchart describing children included in the subanalysis of diarrhea etiology among children enrolled in the AntiBiotics for Children with severe Diarrhea (ABCD) trial. Abbreviations: ABCD, AntiBiotics for Children with severe Diarrhea; EPEC, enteropathogenic Escherichia coli; qPCR, quantitative polymerase chain reaction; ST-ETEC, enterotoxigenic Escherichia coli encoding heat-stable toxin.
Table 1.
Participant Characteristics at Study Enrollment by Randomization Arm (N = 6692)
| Characteristic | Overall (N = 6692) | Azithromycin (n = 3347) | Placebo (n = 3345) | |||
|---|---|---|---|---|---|---|
| Enrollment site | ||||||
| Bangladesh (Jun 2017–Jan 2019) | 998 | (14.9%) | 500 | (14.9%) | 498 | (14.9%) |
| India (Sep 2017–May 2019) | 998 | (14.9%) | 501 | (15.0%) | 497 | (14.9%) |
| Kenya (Dec 2017–Jul 2019) | 1014 | (15.2%) | 502 | (15.0%) | 512 | (15.3%) |
| Malawi (Jan 2018–Jul 2019) | 691 | (10.3%) | 343 | (10.3%) | 348 | (10.4%) |
| Mali (Sep 2017–Jun 2019) | 1000 | (14.9%) | 498 | (14.9%) | 502 | (15.0%) |
| Pakistan (Sep 2017–Mar 2019) | 995 | (14.9%) | 498 | (14.9%) | 497 | (14.9%) |
| Tanzania (Sep 2017–Jul 2019) | 996 | (14.9%) | 505 | (15.1%) | 491 | (14.7%) |
| Child age | ||||||
| 2–5 mo | 967 | (14.5%) | 473 | (14.1%) | 494 | (14.8%) |
| 6–11 mo | 2799 | (41.8%) | 1408 | (42.1%) | 1391 | (41.6%) |
| 12–23 mo | 2926 | (43.7%) | 1466 | (43.8%) | 1460 | (43.6%) |
| Male sex | 3604 | (53.9%) | 1800 | (53.8%) | 1804 | (53.9%) |
| Duration of diarrhea prior to presentation, d, median (IQR) | 2 | (1–3) | 2 | (1–3) | 2 | (1–3) |
| No. of unusually loose or watery stools in last 24 h, median (IQR) | 6 | (5–9) | 6 | (5–9) | 6 | (5–9) |
| Dehydration status | ||||||
| None | 3053 | (45.6%) | 1545 | (46.2%) | 1508 | (45.1%) |
| Some | 3285 | (49.1%) | 1638 | (48.9%%) | 1647 | (49.2%) |
| Severe | 354 | (5.3%) | 164 | (4.9%) | 190 | (5.7%) |
| LAZ, median (IQR) | −1.4 | (−2.4 to −0.6) | −1.4 | (−2.3 to −0.6) | −1.4 | (−2.4 to −0.6) |
| Stuntinga | ||||||
| None | 4491 | (67.1%) | 2257 | (67.4%) | 2234 | (66.8%) |
| Moderate | 1209 | (18.1%) | 593 | (17.7%) | 616 | (18.4%) |
| Severe | 992 | (14.8%) | 497 | (14.9%) | 495 | (14.8%) |
| MUAC, cm, median (IQR) | 12.9 | (12.3–13.9) | 12.9 | (12.3–13.9) | 12.9 | (12.3–13.9) |
| WLZb, median (IQR) | −1.34 | (−2.1 to −0.4) | −1.4 | (−2.2 to −0.4) | −1.3 | (−2.1 to −0.4) |
| Wastingc | ||||||
| None | 3589 | (53.6%) | 1761 | (52.6%) | 1828 | (54.7%) |
| Moderate | 3103 | (46.4%) | 1586 | (47.4%) | 1517 | (45.4%) |
| No. of children <5 y of age in household, median (IQR) | 1 | (1–2) | 1 | (1–2) | 1 | (1–2) |
| Wealth quintile | ||||||
| Lowest | 864 | (12.9%) | 443 | (13.2%) | 421 | (12.6%) |
| Second | 1092 | (16.3%) | 546 | (16.3%) | 546 | (16.3%) |
| Middle | 1071 | (16.0%) | 552 | (16.5%) | 519 | (15.5%) |
| Fourth | 1779 | (26.6%) | 839 | (25.1%) | 940 | (28.1%) |
| Highest | 1886 | (28.2%) | 967 | (28.9%) | 919 | (27.5%) |
| Fecal sample type | ||||||
| Whole stool | 4094 | (61.2%) | 2025 | (60.5%) | 2069 | (61.9%) |
| Rectal swab | 2598 | (38.8%) | 1322 | (39.5%) | 1276 | (38.2%) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: IQR, interquartile range; LAZ, length-for-age z-score; MUAC, mid-upper arm circumference; WLZ, weight-for-length z-score.
aNo stunting defined as LAZ ≥ −2; moderate stunting defined as −3 ≤ LAZ < −2; severe stunting defined as LAZ < −3.
bThree participants missing WLZ at enrollment.
cNo wasting defined as MUAC ≥12.5 cm (if ≥6 months) or WLZ ≥ −2; moderate wasting defined as 11.5 cm ≤ MUAC <12.5 cm (if ≥6 mo) or −2 < WLZ < −3. Three participants missing WLZ at enrollment.
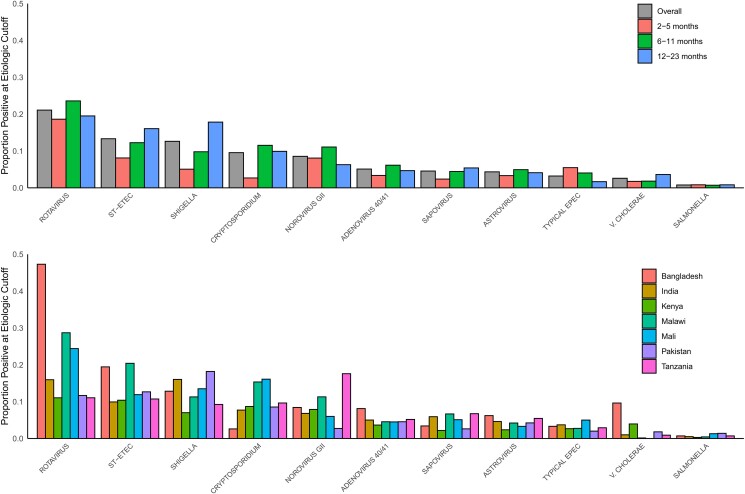
The 5 leading likely etiologies of diarrhea were rotavirus (21.1%), ST-ETEC (13.3%), Shigella (12.6%) Cryptosporidium (9.6%), and norovirus GII (8.6%) (Figure 2). Rotavirus was the leading likely etiology in each age category of 2–5 months, 6–11 months, and 12–23 months. The second and third leading likely etiologies were norovirus and ST-ETEC in 2- to 5-month-olds, Cryptosporidium and ST-ETEC in 6- to 11-month-olds, and Shigella and ST-ETEC among children aged 12–23 months. Site-specific attributable fractions are presented in Supplementary Table 3A and 3B and closely matched the distribution of pathogens determined using the Ct cutoffs (Supplementary Figure 4).
Figure 2.
Likely etiologies (determined by pathogen-specific diarrhea-attribution cycle threshold cutoffs) among AntiBiotics for Children with severe Diarrhea (ABCD) study participants by site and age. Showing etiologies with 10% prevalence or more. Abbreviations: EPEC, enteropathogenic Escherichia coli; ST-ETEC, enterotoxigenic Escherichia coli encoding heat-stable toxin.
More than one-quarter of children (1894 [28.3%]) had a likely bacterial cause of their diarrhea, and likely bacterial causes were balanced across the 2 arms (Supplementary Figure 5). The effect of azithromycin on the outcome of diarrhea on day 3 was modified by the presence of a bacterial etiology (interaction P < .001): Among children with a likely bacterial etiology, 11.8% in the azithromycin arm had diarrhea 3 days after randomization compared to 23.3% of children in the placebo group (risk difference [RD], −11.6 [95% confidence interval {CI}, −15.6 to −7.6]; Table 2). Among children with a possible bacterial etiology, 7.1% in the azithromycin arm had 3-day diarrhea versus 15.8% of placebo-treated children (RD, 8.7% [95% CI, −13.0% to −4.4%]). There was no difference between randomization arms on day 3 diarrhea among the subset of children without a bacterial etiology (RD, −0.3% [95% CI, −2.9% to 2.3%]). During the 90 days of follow-up, azithromycin was associated with fewer deaths/hospitalizations among children with a likely (RD, −3.1% [95% CI, −5.3% to −1.0%]) and possible (RD, −2.3% [95% CI, −4.5% to −.01%]) bacterial cause of diarrhea, which was not observed in children with an unlikely bacterial etiology (RD, −0.6 [95% CI, −1.9 to .6]), but testing for effect modification was not statistically significant (interaction P = .10). Magnitudes of association were similar when considering rectal swab samples only (Supplementary Table 4) and considering death and hospitalization as separate outcomes (Supplementary Table 5). Azithromycin treatment was associated with a modest improvement in ΔLAZ among children with an unlikely bacterial etiology (difference in difference, 0.06 [95% CI, .02–.10]), but the effect was smaller and imprecise in the children with a likely or presumed bacterial etiology and the effect modification could have been due to chance (interaction P = .6).
Table 2.
Effect of Azithromycin Within Subgroups Defined by Likely, Possible, and Unlikely Diarrhea Attribution to 1 or More of the Following Bacteria: Shigella spp, Enterotoxigenic Escherichia coli Encoding Heat-Stable Toxin, Typical Enteropathogenic E coli, Vibrio cholerae, Salmonella spp, and/or Campylobacter spp
| Outcome and Subgroup | Proportion With the Indicated Outcome/Mean Outcome | Evidence of Effect Modificationb | ||
|---|---|---|---|---|
| AZM | Placebo | Risk Difference or Difference in Differencea (95% CI) | ||
| Diarrhea on day 3c (n = 4835) | <.001 | |||
| Likely bacterial etiology (n = 1358) | 79/672 (11.8%) | 160/686 (23.3%) | −11.6% (−15.6% to −7.6%) | |
| Possible bacterial etiology (n = 815) | 28/392 (7.1%) | 67/423 (15.8%) | −8.7% (−13.0% to −4.4%) | |
| Unlikely bacterial etiology (n = 2662) | 173/1349 (12.8%) | 172/1313 (13.1%) | −0.31% (−2.9% to 2.3%) | |
| Death or hospitalization by day 90 (n = 6692) | .10 | |||
| Likely bacterial etiology (n = 1894) | 42/930 (4.5%) | 74/964 (7.7%) | −3.1% (−5.3% to −1.0%) | |
| Possible bacterial etiology (n = 1153) | 16/566 (2.8%) | 30/587 (5.1%) | −2.3% (−4.5% to −.01%) | |
| Unlikely bacterial etiology (n = 3645) | 63/1851 (3.4%) | 73/1794 (4.1%) | −0.62% (−1.90% to .61%) | |
| ΔLAZd, mean (SD) (n = 6406) | .58 | |||
| Likely bacterial etiology (n = 1813) | −0.18 (0.55) | −0.21 (0.56) | 0.03 (−.02 to .08) | |
| Possible bacterial etiology (n = 1106) | −0.13 (0.67) | −0.16 (0.61) | 0.03 (−.04 to .11) | |
| Unlikely bacterial etiology (n = 3487) | −0.13 (0.61) | −0.19 (0.59) | 0.06 (.02–.10) | |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: AZM, azithromycin; CI, confidence interval; ΔLAZ, difference in length-for-age z-score[LAZ] between enrollment and day-90; SD, standard deviation.
aEffect of azithromycin on outcome, adjusted for site (indicator) and baseline value (for ΔLAZ model). Risk difference per 100 child-years (for proportions) and difference of difference reported for mean values (ΔLAZ).
b P value from likelihood ratio test comparing nested models with and without additive interaction term between randomization arm and bacterial etiology group.
cQuestion added to questionnaire after recruitment began, therefore missing for some.
dChange between day 90 visit and enrollment visit.
Among the 1894 children with a likely bacterial etiology, we further explored the difference between randomization arms on all outcomes among those with diarrhea likely caused by ST-ETEC (n = 889), Shigella (n = 845), tEPEC (n = 215), V cholerae (n = 174), and Salmonella (n = 52). Only 6 children had Campylobacter quantities at likely etiologic levels, so effects were not explored. The proportion of children with diarrhea on day 3 was lower in children randomized to azithromycin compared to placebo in all subsets (other than Salmonella) and improved the change in LAZ for children with diarrhea attributed to Shigella and ST-ETEC (Table 3). Specifically, among children with Shigella, linear growth faltered less in those treated with azithromycin (mean ΔLAZ, −0.13 [standard deviation {SD}, 0.52]) than children treated with placebo (mean ΔLAZ, −0.21 [SD, 0.54]; difference in difference, 0.11 [95% CI, .04–.17]). Although there was not a statistically significant difference in terms of risk of death or hospitalization by day 90 between children randomized to azithromycin versus placebo in any of the bacteria groups, the magnitude of the effect of the point estimate ranged from 1 to 5 fewer deaths per 100 children in the azithromycin-treated group compared to the placebo group (Table 3 and Supplementary Table 6). Notably, 12.3% of the placebo-treated children with tEPEC were hospitalized or died during the 90-day follow-up, as did 7.6% of placebo-treated children with Shigella diarrhea and 7.2% with ST-ETEC diarrhea. In the unlikely bacterial etiology group, 4.1% of children died or were hospitalized during the 90-day follow-up period.
Table 3.
Effect of Azithromycin Among Subsets of Children With Specific Pathogenic Bacteria at Likely Etiologic Levels a
| Outcome | Diarrhea on Day 3b | Death or Hospitalization by Day 90 | ΔLAZc | ||||||
|---|---|---|---|---|---|---|---|---|---|
| AZM | Placebo | Risk Difference (95% CI)d | AZM | Placebo | Risk Difference (95% CI)d | AZM, Mean (SD) | Placebo, Mean (SD) | Difference in Difference (95% CI)e | |
| Likely ST-ETEC diarrhea (n = 889) | 33/300 (11.0%) | 67/316 (21.2%) | −11.2 (−16.8 to −5.6) | 22/431 (5.1%) | 33/458 (7.2%) | −1.5 (−4.6 to 1.5) | −0.17 (0.52) | −0.23 (0.54) | 0.08 (.01–.14) |
| Likely Shigella diarrhea (n = 845) | 36/301 (12.0%) | 82/316 (26.0%) | −14.0 (−20.0 to −8.0) | 19/411 (4.6%) | 33/434 (7.6%) | −2.9 (−6.0 to 0.27) | −0.13 (0.52) | −0.21 (0.54) | 0.11 (.04–.17) |
| Likely typical EPEC diarrhea (n = 215) | 10/80 (12.5%) | 24/82 (29.3%) | −15.6 (−28.3 to −3.0) | 6/109 (5.5%) | 13/106 (12.3%) | −4.9 (−11.7 to 1.9) | −0.27 (0.52) | −0.21 (0.54) | −0.01 (−.16 to .14) |
| Likely Vibrio cholerae diarrhea (n = 174) | 12/63 (19.1%) | 22/56 (39.3%) | −20.2 (−36.2 to −4.3) | 3/84 (3.6%) | 7/90 (7.8%) | −5.6 (−13.7 to 2.4) | −0.31 (0.5) | −0.13 (0.64) | −0.16 (−.34 to .03) |
| Likely Salmonella diarrhea (n = 52) | 2/17 (11.8%) | 4/22 (18.2%) | −6.4 (−29.8 to 16.9) | 0/25 (0%) | 1/27 (3.7%) | … | −0.09 (0.47) | −0.24 (0.81) | 0.07 (−.23 to .38) |
| Likely Campylobacter diarrhea (n = 6) | 0/2 (0%) | 1/2 (50%) | … | 0/3 (0%) | 0/3 (0%) | … | 0.003 (0.17) | −0.30 (0.31) | … |
Data are presented as no./No. (%) unless otherwise indicated.
Abbreviations: AZM, azithromycin; EPEC, enteropathogenic Escherichia coli; SD, standard deviation; ST-ETEC, enterotoxigenic Escherichia coli encoding heat-stable toxin.
a Shigella: cycle threshold (Ct) <28.7; ST-ETEC: Ct <25.4; typical EPEC: Ct <18.1; Vibrio cholerae: Ct <32.6; Salmonella: Ct <31.9; Campylobacter: Ct <16.3.
bQuestion added to questionnaire after recruitment began, therefore missing for some.
cChange between day 90 visit and enrollment visit.
dEffect of azithromycin on outcome, adjusted for site (indicator). Risk difference per 100 child-years.
eEffect of azithromycin on outcome, adjusted for site (indicator) and baseline length-for-age z-score. Difference of mean ΔLAZ.
DISCUSSION
In this subgroup analysis of a multicountry, placebo-controlled, randomized trial of azithromycin in conjunction with WHO standard of care to treat acute watery diarrhea, azithromycin was associated with shorter diarrhea duration and fewer deaths/hospitalizations in the subset of children with a likely bacterial etiology, with some residual benefit observed in children with bacteria detected at quantities below the pathogen-specific etiologic cutoffs. More than one-quarter of all enrolled children had a bacterial etiology identified, a proportion nearly identical (32%) to that observed among acute watery diarrhea cases in the GEMS study [10]. Antibiotic treatment efficacy studies of presumed or confirmed bacterial diarrhea have frequently used diarrhea presence after 2–6 days as a primary endpoint [15, 28–31] and here we show a benefit to azithromycin treatment of bacterial diarrhea in terms of the clinical outcome of diarrhea on day 3. Fewer than 9 children would be needed to treat to avert 1 case of day 3 diarrhea, and this effect was consistent across Shigella, ST-ETEC, tEPEC, and V cholerae, the most commonly identified bacterial etiologies.
While an effect of azithromycin on the secondary combined outcome of death or rehospitalization 90 days after diarrhea presentation was observed in the primary trial analysis [9], the absolute risk reduction was small (86 children would need to be treated to avert 1 death or hospitalization by day 90), a benefit likely diluted by enrollment of children with nonbacterial diarrhea. The present analysis supports this hypothesis in that the number needed to treat to prevent death or hospitalization by day 90 in children with likely bacterial diarrhea was >33. This effect size is comparable or greater than the effect size used to support the routine use of amoxicillin to treat nonsevere pneumonia, based on the outcome of treatment failure that also included death and/or hospitalization, albeit as the prespecified primary endpoint and not limited to bacterial pneumonia [32]. Clinical management guidelines in high-resource settings also indicate antibiotic treatment for some enteric bacteria, such as Shigella [33], and children with underlying malnutrition or other host vulnerabilities common in resource-limited settings may experience the greatest benefit of such treatment.
It is clear from this and other studies that dysentery captures only a small fraction of bacterial diarrhea [20, 34], and thus syndromic guidelines limiting antibiotics to children with dysentery leave many with bacterial diarrhea untreated. Indiscriminate use of antibiotics for diarrhea could promote antibiotic resistance; therefore, better diagnostic tools with high accuracy for bacterial diarrhea at the point-of-care are needed. Such tools could include rapid molecular or lateral flow tests, accurate biomarkers, or improved clinical scoring systems [35–38]. Rapid molecular testing for enteric bacteria was recently trialed in Botswana and, while underpowered, found a modest benefit of this test-and-treat strategy in preventing future diarrheal episodes [39]. A test-and-treat strategy could also limit antibiotic use; between 23% and 77% of children with watery diarrhea are treated with antibiotics empirically [12, 40, 41].
We observed a small residual clinical benefit of azithromycin treatment in diarrhea with bacteria identified but at a lower quantity than the prespecified cutoff for etiology attribution. The etiologic cutoffs derived from large observational studies were designed to favor specificity over sensitivity to confirm etiologies, and this finding suggests that some underattribution of bacterial etiology likely occurred. The cutoff used for Campylobacter was particularly stringent, due to subclinical carriage being particularly common in the GEMS and MAL-ED studies [10, 20] and a poor association between quantity detected and diarrhea, leading to possible underclassification of Campylobacter-attributed diarrhea. Post hoc analyses could possibly use treatment effect to further evaluate the test characteristics of these cutoffs and better identify the subset of diarrhea that benefits from antimicrobial therapy. It is also possible that the benefit of azithromycin treatment includes reduction in subclinical bacterial infections, an explanation consistent with the observed reduction in Shigella infections, and all-cause mortality reductions, among children randomized to mass azithromycin distribution in Niger [42, 43].
Interestingly, the modest effect of azithromycin on short-term linear growth was not observed in children with the combined groups of likely or possible bacterial etiology. Modest benefit was observed in some specific bacterial subgroups, such as Shigella and ST-ETEC. Shigella and ST-ETEC diarrhea have been consistently associated with short-term linear growth faltering [12, 13], and antibiotic treatment for Shigella was observed to have linear growth–promoting benefits in GEMS [12]. A modest, statistically significant benefit was observed in the subset of children without a bacterial etiology. This finding was surprising and needs to be assessed in other studies to exclude the possibility of a spurious result. If true, a potential mechanism could relate to a role for azithromycin on reducing inflammation in the context of environmental enteropathy [44] [45–47].
Strengths of this analysis include the large sample size of children with etiology-specific diarrhea (including Shigella, ST-ETEC, and tEPEC) as well as the merits of the original trial including it being double-blind, placebo-controlled, multicountry, implementing WHO standards of care, and with ascertainment of short- and longer-term outcomes representing a spectrum of diarrhea convalescence. Limitations include those related to any post hoc analysis; namely, that observed effects were due to other sources of bias, such as type 1 error that could occur by the inclusion of an outcome (day 3 diarrhea) that was not prespecified in the original trial and the use of a secondary outcome in the original trial (the combined outcome of death or hospitalization). We also had limited power for the primary outcome of mortality in subgroups, limiting our ability to formally test for effect modification by bacteria etiology in this primary endpoint. Assignment of likely and possible etiology is complicated with sensitive diagnostic methods, particularly in settings where asymptomatic carriage is high, but we attempted to overcome this by utilizing pathogen quantity cutoffs that erred on specificity. Other factors, such as age, underlying malnutrition, and previous enteric pathogen exposure also likely impact whether or not a given pathogen caused diarrhea, in addition to pathogen quantity. We did not have antimicrobial susceptibility data to confirm whether azithromycin was active against specific bacterial isolates. While azithromycin remains active against most Shigella, ETEC, Campylobacter, V cholerae, and Salmonella spp [48], it is possible that rates of azithromycin resistance in these gram-negative bacteria reduced the effect of azithromycin that we would have otherwise observed. The emergence of azithromycin resistance in enteric bacteria such as Shigella [49–51] makes it imperative that consideration of expanded use of azithromycin for bacterial watery diarrhea be weighed against the potential for increased resistance.
This reanalysis of the ABCD trial addresses evidence gaps in the use of antibiotic therapy, in conjunction with other WHO-indicated standards of care, for watery diarrhea of bacterial etiology among children in low- and middle-income settings. If confirmed, the use of azithromycin for acute watery diarrhea in young dehydrated or undernourished children living in settings of high bacterial diarrhea burden may be warranted if the subset of children with diarrhea who benefit from azithromycin can be identified.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Patricia B Pavlinac, Department of Global Health; Department of Epidemiology, University of Washington, Seattle, WA, USA.
James A Platts-Mills, Division of Infectious Diseases and International Health, Department of Medicine, University of Virginia, Charlottesville, Virginia, USA.
Jie Liu, School of Public Health, Qingdao University, Qingdao, China.
Hannah E Atlas, Department of Global Health.
Jean Gratz, Division of Infectious Diseases and International Health, Department of Medicine, University of Virginia, Charlottesville, Virginia, USA.
Darwin Operario, Division of Infectious Diseases and International Health, Department of Medicine, University of Virginia, Charlottesville, Virginia, USA.
Elizabeth T Rogawski McQuade, Department of Epidemiology, Rollins School of Public Health, Emory University, Atlanta, Georgia, USA.
Dilruba Ahmed, Laboratory Sciences and Services Division.
Tahmeed Ahmed, Nutrition and Clinical Services Division, International Centre for Diarrhoeal Disease Research, Dhaka, Bangladesh.
Tahmina Alam, Nutrition and Clinical Services Division, International Centre for Diarrhoeal Disease Research, Dhaka, Bangladesh.
Per Ashorn, Center for Child, Adolescent, and Maternal Health Research, Faculty of Medicine and Health Technology, Tampere University and Tampere University Hospital, Tampere, Finland.
Henry Badji, Centre pour le Développement des Vaccines, Bamako, Mali.
Rajiv Bahl, Department of Maternal, Newborn, Child, and Adolescent Health and Aging, World Health Organization, Geneva, Switzerland.
Naor Bar-Zeev, International Vaccine Access Center, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA.
Mohammod Jobayer Chisti, Nutrition and Clinical Services Division, International Centre for Diarrhoeal Disease Research, Dhaka, Bangladesh.
Jen Cornick, Clinical Research Programme, Malawi Liverpool Wellcome Trust, Blantyre, Malawi.
Aishwarya Chauhan, Center for Public Health Kinetics, New Delhi, India.
Ayesha De Costa, Department of Maternal, Newborn, Child, and Adolescent Health and Aging, World Health Organization, Geneva, Switzerland.
Saikat Deb, Center for Public Health Kinetics, New Delhi, India.
Usha Dhingra, Center for Public Health Kinetics, New Delhi, India.
Queen Dube, Department of Pediatrics, Queen Elizabeth Central Hospital, Blantyre, Malawi.
Christopher P Duggan, Division of Gastroenterology, Hepatology and Nutrition, Department of Nutrition, Boston Children's Hospital, Harvard T.H. Chan School of Public Health, Boston, Massachusetts, USA.
Bridget Freyne, Clinical Research Programme, Malawi Liverpool Wellcome Trust, Blantyre, Malawi; Institute of Infection, Veterinary and Ecological Sciences, University of Liverpool, Liverpool, United Kingdom; Department of Women and Children's Health, School of Medicine, University College Dublin, Dublin, Ireland.
Wilson Gumbi, Kenya Medical Research Institute–Wellcome Trust Research Programme, Kilifi, Kenya.
Aneeta Hotwani, Department of Pediatrics and Child Health, Aga Khan University, Karachi, Pakistan.
Mamun Kabir, Nutrition and Clinical Services Division, International Centre for Diarrhoeal Disease Research, Dhaka, Bangladesh.
Ohedul Islam, Nutrition and Clinical Services Division, International Centre for Diarrhoeal Disease Research, Dhaka, Bangladesh.
Furqan Kabir, Department of Pediatrics and Child Health, Aga Khan University, Karachi, Pakistan.
Irene Kasumba, Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore, Maryland, USA.
Upendo Kibwana, Department of Pediatrics and Child Health, Muhimbili University of Health and Allied Sciences, Dar es Salaam, Tanzania.
Karen L Kotloff, Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore, Maryland, USA; Department of Pediatrics, Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore, Maryland, USA.
Shaila S Khan, Nutrition and Clinical Services Division, International Centre for Diarrhoeal Disease Research, Dhaka, Bangladesh.
Victor Maiden, Clinical Research Programme, Malawi Liverpool Wellcome Trust, Blantyre, Malawi.
Karim Manji, Department of Pediatrics and Child Health, Muhimbili University of Health and Allied Sciences, Dar es Salaam, Tanzania.
Ashka Mehta, Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore, Maryland, USA.
Latif Ndeketa, Clinical Research Programme, Malawi Liverpool Wellcome Trust, Blantyre, Malawi.
Ira Praharaj, Department of Gastrointestinal Sciences, Christian Medical College, Vellore, India.
Farah Naz Qamar, Department of Pediatrics and Child Health, Aga Khan University, Karachi, Pakistan.
Sunil Sazawal, Center for Public Health Kinetics, New Delhi, India.
Jonathon Simon, Department of Maternal, Newborn, Child, and Adolescent Health and Aging, World Health Organization, Geneva, Switzerland.
Benson O Singa, Center for Clinical Research, Kenya Medical Research Institute, Nairobi, Kenya.
Sarah Somji, Department of Pediatrics and Child Health, Muhimbili University of Health and Allied Sciences, Dar es Salaam, Tanzania.
Samba O Sow, Centre pour le Développement des Vaccines, Bamako, Mali.
Milagritos D Tapia, Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore, Maryland, USA.
Caroline Tigoi, Kenya Medical Research Institute–Wellcome Trust Research Programme, Kilifi, Kenya.
Aliou Toure, Centre pour le Développement des Vaccines, Bamako, Mali.
Judd L Walson, Department of Global Health; Department of Epidemiology, University of Washington, Seattle, WA, USA; Infectious Diseases, Department of Pediatrics and Medicine, University of Washington, Seattle.
Mohammad Tahir Yousafzai, Kenya Medical Research Institute–Wellcome Trust Research Programme, Kilifi, Kenya.
Eric R Houpt, Division of Infectious Diseases and International Health, Department of Medicine, University of Virginia, Charlottesville, Virginia, USA.
for the AntiBiotics for Children with severe Diarrhea (ABCD) Study Group:
Muhammad Waliur Rahman, Irin Parvin, Md. Farhad Kabir, Pratibha Dhingra, Arup Dutta, Anil Kumar Sharma, Vijay Kumar Jaiswal, Churchil Nyabinda, Christine McGrath, Emily L Deichsel, Maurine Anyango, Kevin Mwangi Kariuki, Doreen Rwigi, Stephanie N Tornberg-Belanger, Fadima Cheick Haidara, Flanon Coulibaly, Jasnehta Permala-Booth, Dramane Malle, Nigel Cunliffe, Latif Ndeketa, Desiree Witte, Chifundo Ndamala, Shahida Qureshi, Sadia Shakoor, Rozina Thobani, Jan Mohammed, Rodrick Kisenge, Christopher R Sudfeld, Mohamed Bakari, Cecylia Msemwa, and Abraham Samma
Notes
Acknowledgments. We thank the children who participated in this trial and their families as well as the physicians, pharmacists, and research assistants who helped conduct the trial in each of the 7 countries. The data and safety monitoring board members provided critical guidance in the trial: Mathuram Santosham (chair), Yin Bun Cheung, Shinjini Bhatnagar, Elizabeth Molyneux, and Godwin Ndossi. We also thank additional members of the ABCD Study Group: Bangladesh: Muhammad Waliur Rahman, Irin Parvin, and Md. Farhad Kabir. India: Pratibha Dhingra, Arup Dutta, Anil Kumar Sharma, and Vijay Kumar Jaiswal. Kenya: Churchil Nyabinda, Christine McGrath, Emily L. Deichsel, Maurine Anyango, Kevin Mwangi Kariuki, Doreen Rwigi, and Stephanie N. Tornberg-Belanger. Mali: Fadima Cheick Haidara, Flanon Coulibaly, Jasnehta Permala-Booth, and Dramane Malle. Malawi: Nigel Cunliffe, Latif Ndeketa, Desiree Witte, and Chifundo Ndamala. Pakistan: Shahida Qureshi, Sadia Shakoor, Rozina Thobani, and Jan Mohammed. Tanzania: Rodrick Kisenge, Christopher R. Sudfeld, Mohamed Bakari, Cecylia Msemwa, and Abraham Samma.
Data availability. The de-identified participant and laboratory data that support the findings of this study are available from the corresponding author upon reasonable request and a signed data access agreement upon publication. Data requests should be sent to: deay@who.int.
Financial support. The trial and nested molecular diagnostics study was funded by the Bill & Melinda Gates Foundation (grant numbers OPP 1126331 and OPP 1179069). Funding to pay the Open Access publication charges for this article was provided by Division of Infectious Diseases and International Health, University of Virginia.
References
- 1. Global Burden of Disease Collaborative Network . Global Burden of Disease Study 2019 (GBD 2019) results. 2019. Available at: https://vizhub.healthdata.org/gbd-results/. Accessed 1 December 2021.
- 2. Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet 2014; 385:430–40. [DOI] [PubMed] [Google Scholar]
- 3. World Health Organization (WHO) . The treatment of diarrhoea. A manual for physicians and other senior health workers. Geneva, Switzerland: WHO, 2007. [Google Scholar]
- 4. Kotloff KL, Nataro JP, Blackwelder WC, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 2013; 382:209–22. [DOI] [PubMed] [Google Scholar]
- 5. Brander RL, Pavlinac PB, Walson JL, et al. Determinants of linear growth faltering among children with moderate-to-severe diarrhea in the Global Enteric Multicenter Study. BMC Med 2019; 17:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Scharf RJ, Rogawski ET, Murray-Kolb LE, et al. Early childhood growth and cognitive outcomes: findings from the MAL-ED study. Matern Child Nutr 2018; 14:e12584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nahar B, Hossain M, Mahfuz M, et al. Early childhood development and stunting: findings from the MAL-ED birth cohort study in Bangladesh. Matern Child Nutr 2020; 16:e12864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. ABCD Study Team . A double-blind placebo-controlled trial of azithromycin to reduce mortality and improve growth in high-risk young children with non-bloody diarrhoea in low resource settings: the Antibiotics for Children with Diarrhoea (ABCD) trial protocol. Trials 2020; 21:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Antibiotics for Children With Diarrhea (ABCD) Study Group; Ahmed T, Chisti MJ, Rahman MW, et al. Effect of 3 days of oral azithromycin on young children with acute diarrhea in low-resource settings: a randomized clinical trial. JAMA Netw Open 2021; 4:e2136726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu J, Platts-Mills JA, Juma J, et al. Use of quantitative molecular diagnostic methods to identify causes of diarrhoea in children: a reanalysis of the GEMS case-control study. Lancet 2016; 388:1291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Operario DJ, Platts-Mills JA, Nadan S, et al. Etiology of severe acute watery diarrhea in children in the Global Rotavirus Surveillance Network using quantitative polymerase chain reaction. J Infect Dis 2017; 216:220–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nasrin D, Blackwelder WC, Sommerfelt H, et al. Pathogens associated with linear growth faltering in children with diarrhea and impact of antibiotic treatment: the Global Enteric Multicenter Study. J Infect Dis 2021; 224:S848–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rogawski ET, Liu J, Platts-Mills JA, et al. Use of quantitative molecular diagnostic methods to investigate the effect of enteropathogen infections on linear growth in children in low-resource settings: longitudinal analysis of results from the MAL-ED cohort study. Lancet Glob Health 2018; 6:e1319–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pernica JM, Steenhoff AP, Welch H, et al. Correlation of clinical outcomes with Multiplex molecular testing of stool from children admitted to hospital with gastroenteritis in Botswana. J Pediatric Infect Dis Soc 2015; 5:312–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Basualdo W, Arbo A. Randomized comparison of azithromycin versus cefixime for treatment of shigellosis in children. Pediatr Infect Dis J 2003; 22:374–7. [PubMed] [Google Scholar]
- 16. Khan WA, Seas C, Dhar U, Salam MA, Bennish ML. Treatment of shigellosis: V. Comparison of azithromycin and ciprofloxacin. A double-blind, randomized, controlled trial. Ann Intern Med 1997; 126:697–703. [DOI] [PubMed] [Google Scholar]
- 17. Vukelic D, Trkulja V, Salkovic-Petrisic M. Single oral dose of azithromycin versus 5 days of oral erythromycin or no antibiotic in treatment of Campylobacter enterocolitis in children: a prospective randomized assessor-blind study. J Pediatr Gastroenterol Nutr 2010; 50:404–10. [DOI] [PubMed] [Google Scholar]
- 18. Hinterwirth A, Sie A, Coulibaly B, et al. Rapid reduction of Campylobacter species in the gut microbiome of preschool children after oral azithromycin: a randomized controlled trial. Am J Trop Med Hyg 2020; 103:1266–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sanders JW, Frenck RW, Putnam SD, et al. Azithromycin and loperamide are comparable to levofloxacin and loperamide for the treatment of traveler’s diarrhea in United States military personnel in Turkey. Clin Infect Dis 2007; 45:294–301. [DOI] [PubMed] [Google Scholar]
- 20. Platts-Mills JA, Liu J, Rogawski ET, et al. Use of quantitative molecular diagnostic methods to assess the aetiology, burden, and clinical characteristics of diarrhoea in children in low-resource settings: a reanalysis of the MAL-ED cohort study. Lancet Glob Health 2018; 6:e1309–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. World Health Organization (WHO) . Pocket book of hospital care for children: guidelines for the management of common illnesses with limited resources. Geneva, Switzerland: WHO, 2005. [PubMed] [Google Scholar]
- 22. Liu J, Gratz J, Amour C, et al. A laboratory-developed TaqMan array card for simultaneous detection of 19 enteropathogens. J Clin Microbiol 2013; 51:472–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu J, Gratz J, Amour C, et al. Optimization of quantitative PCR methods for enteropathogen detection. PLoS One 2016; 11:e0158199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu J, Kabir F, Manneh J, et al. Development and assessment of molecular diagnostic tests for 15 enteropathogens causing childhood diarrhoea: a multicentre study. Lancet Infect Dis 2014; 14:716–24. [DOI] [PubMed] [Google Scholar]
- 25. Liu J, Almeida M, Kabir F, et al. Direct detection of Shigella in stool specimens by use of a metagenomic approach. J Clin Microbiol 2018; 56:e01374-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Platts-Mills JA, Babji S, Bodhidatta L, et al. Pathogen-specific burdens of community diarrhoea in developing countries: a multisite birth cohort study (MAL-ED). Lancet Glob Health 2015; 3:e564–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004; 159:702–6. [DOI] [PubMed] [Google Scholar]
- 28. Helvaci M, Bektaslar D, Ozkaya B, Yaprak I, Umurtak B, Ertugrul A. Comparative efficacy of cefixime and ampicillin-sulbactam in shigellosis in children. Acta Paediatr Jpn 1998; 40:131–4. [DOI] [PubMed] [Google Scholar]
- 29. Prado D, Liu H, Velasquez T, Cleary TG. Comparative efficacy of pivmecillinam and cotrimoxazole in acute shigellosis in children. Scand J Infect Dis 1993; 25:713–9. [DOI] [PubMed] [Google Scholar]
- 30. Rodriguez RS, Chavez AZ, Galindo E. A randomized, controlled, single-blind study comparing furazolidone with trimethoprim-sulfamethoxazole in the empirical treatment of acute invasive diarrhea. Scand J Gastroenterol Suppl 1989; 169:47–53. [DOI] [PubMed] [Google Scholar]
- 31. Salam MA, Dhar U, Khan WA, Bennish ML. Randomised comparison of ciprofloxacin suspension and pivmecillinam for childhood shigellosis. Lancet 1998; 352:522–7. [DOI] [PubMed] [Google Scholar]
- 32. Jehan F, Nisar I, Kerai S, et al. Randomized trial of amoxicillin for pneumonia in Pakistan. N Engl J Med 2020; 383:24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. UpToDate. Shigella infection: Treatment and prevention in adults. Available at: https://www.uptodate.com/contents/shigella-infection-treatment-and-prevention-in-adults. Accessed 3 April 2023.
- 34. Tickell KD, Brander RL, Atlas HE, Pernica JM, Walson JL, Pavlinac PB. Identification and management of Shigella infection in children with diarrhoea: a systematic review and meta-analysis. Lancet Glob Health 2017; 5:e1235–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Brintz BJ, Haaland B, Howard J, et al. A modular approach to integrating multiple data sources into real-time clinical prediction for pediatric diarrhea. Elife 2021; 10:e63009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kim HJ. Efficacy of fecal calprotectin combined with stool hemoglobin in differentiating bacterial origin in acute gastroenteritis. Pediatr Emerg Care 2022; 38:e670–3. [DOI] [PubMed] [Google Scholar]
- 37. Raghavan R, Wang S, Dendukuri N, et al. Evaluation of LAMP for detection of Shigella from stool samples in children. Access Microbiol 2020; 2:acmi000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pernica JM, Steenhoff AP, Mokomane M, et al. Rapid enteric testing to permit targeted antimicrobial therapy, with and without Lactobacillus reuteri probiotics, for paediatric acute diarrhoeal disease in Botswana: a pilot, randomized, factorial, controlled trial. PLoS One 2017; 12:e0185177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pernica JM, Arscott-Mills T, Steenhoff AP, et al. Optimising the management of childhood acute diarrhoeal disease using a rapid test-and-treat strategy and/or Lactobacillus reuteri DSM 17938: a multicentre, randomised, controlled, factorial trial in Botswana. BMJ Glob Health 2022; 7:e007826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Auta A, Ogbonna BO, Adewuyi EO, Adeloye D, Strickland-Hodge B. Prevalence and factors associated with the use of antibiotics in non-bloody diarrhoea in children under 5 years of age in sub-Saharan Africa. Arch Dis Child 2019; 104:518–21. [DOI] [PubMed] [Google Scholar]
- 41. Seidman JC, John S, Mahfuz M, et al. Use of antibiotics in children younger than two years in eight countries: a prospective cohort study. Bull World Health Organ 2016; 95:49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Platts-Mills JA, Ayoub E, Zhang J, et al. Impact of biannual mass azithromycin treatment on enteropathogen carriage in children younger than 5 years in Niger. Clin Infect Dis 2022; 75:515–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Keenan JD, Bailey RL, West SK, et al. Azithromycin to reduce childhood mortality in sub-Saharan Africa. N Engl J Med 2018; 378:1583–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kosek MN. Causal pathways from enteropathogens to environmental enteropathy: findings from the MAL-ED birth cohort study. EBioMedicine 2017; 18:109–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Beigelman A, Isaacson-Schmid M, Sajol G, et al. Randomized trial to evaluate azithromycin’s effects on serum and upper airway IL-8 levels and recurrent wheezing in infants with respiratory syncytial virus bronchiolitis. J Allergy Clin Immunol 2015; 135:1171–8.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Segal LN, Clemente JC, Wu BG, et al. Randomised, double-blind, placebo-controlled trial with azithromycin selects for anti-inflammatory microbial metabolites in the emphysematous lung. Thorax 2017; 72:13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nair V, Loganathan P, Soraisham AS. Azithromycin and other macrolides for prevention of bronchopulmonary dysplasia: a systematic review and meta-analysis. Neonatology 2014; 106:337–47. [DOI] [PubMed] [Google Scholar]
- 48. World Health Organization (WHO) . Global antimicrobial resistance and use surveillance system (GLASS) report 2021. Geneva, Switzerland: WHO, 2021.
- 49. Houpt ER, Ferdous T, Ara R, et al. Clinical outcomes of drug-resistant shigellosis treated with azithromycin in Bangladesh. Clin Infect Dis 2021; 72:1793–8. [DOI] [PubMed] [Google Scholar]
- 50. Baker KS, Dallman TJ, Ashton PM, et al. Intercontinental dissemination of azithromycin-resistant shigellosis through sexual transmission: a cross-sectional study. Lancet Infect Dis 2015; 15:913–21. [DOI] [PubMed] [Google Scholar]
- 51. Nuzhat S, Das R, Das S, et al. Antimicrobial resistance in shigellosis: a surveillance study among urban and rural children over 20 years in Bangladesh. PLoS One 2022; 17:e0277574. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.