Abstract
Background
People with suspected malaria may harbor Plasmodium falciparum undetected by rapid diagnostic test (RDT). The impact of these subpatent infections on the risk of developing clinical malaria is not fully understood.
Methods
We analyzed subpatent P. falciparum infections using a longitudinal cohort in a high-transmission site in Kenya. Weighted Kaplan-Meier models estimated the risk difference (RD) for clinical malaria during the 60 days following a symptomatic subpatent infection. Stratum-specific estimates by age and transmission season assessed modification.
Results
Over 54 months, we observed 1128 symptomatic RDT-negative suspected malaria episodes, of which 400 (35.5%) harbored subpatent P. falciparum. Overall, the 60-day risk of developing clinical malaria was low following all episodes (8.6% [95% confidence interval, 6.7%–10.4%]). In the low-transmission season, the risk of clinical malaria was slightly higher in those with subpatent infection, whereas the opposite was true in the high-transmission season (low-transmission season RD, 2.3% [95% confidence interval, .4%–4.2%]; high-transmission season RD, −4.8% [−9.5% to −.05%]).
Conclusions
The risk of developing clinical malaria among people with undetected subpatent infections is low. A slightly elevated risk in the low-transmission season may merit alternate management, but RDTs identify clinically relevant infections in the high-transmission season.
Keywords: diagnosis, malaria, Plasmodium falciparum, rapid diagnostic test, survival analysis
The difference in risk of clinical malaria following a subpatent infection compared with an uninfected episode varies by transmission season. Rapid diagnostic tests identify most clinically relevant infections; however, elevated risk in the low-transmission season suggests consideration of alternate strategies.
The World Health Organization recommends parasitologic confirmation before treatment for malaria to enhance rational use of antimalarials. Testing options include smear microscopy and rapid diagnostic tests (RDTs), which can have comparable lower limits of detection. RDTs for Plasmodium falciparum, which typically detect histidine-rich protein (HRP) 2, have largely replaced microscopy as the standard diagnostic tool for malaria [1]; in 2021, >400 million RDTs were sold globally [2]. While conventional RDTs can detect quantities above 100–200 parasites per microliter of blood, many P. falciparum infections are below the limit of detection of conventional RDTs and missed in routine testing [3, 4]. More sensitive diagnostics, such as high-sensitivity RDTs and molecular detection methods, can detect lower parasite densities [5], but these methods are not in wide clinical use. Some subpatent P. falciparum infections, defined as those that are present by molecular test detection but absent by clinical diagnostic tests, could progress to clinical malaria.
It is unclear whether detecting lower-parasite-density infections would enhance the management of suspected malaria. The natural history of low-density infections in different endemic settings is incompletely understood [6–8]. Clear evidence exists that some infections in symptomatic people remain undetected by conventional RDTs [6, 8], but few studies have directly investigated the natural course and clinical consequences in symptomatic patients of these undetected P. falciparum infections. One study of febrile Tanzanian children <5 years old in a malaria-hypoendemic setting reported no difference in negative clinical outcomes between uninfected children and those with untreated subpatent P. falciparum infections [9]. Otherwise, there is a paucity of longitudinal studies of the natural history of low-density infections and the clinical consequences of untreated low-density infections in additional age groups and transmission settings. Estimating the risk of future clinical sequalae for those whose infections are not detected by RDTs can inform treatment decision-making in high-transmission settings for people with suspected malaria and for the choice of diagnostics to evaluate suspected malaria.
We investigated the clinical consequences of untreated subpatent P. falciparum malaria infection among symptomatic patients with negative RDT results. Using data from a 54-month longitudinal cohort in a high-transmission setting in Western Kenya, we compared the risk of subsequent clinical malaria—defined as symptomatic, RDT-positive infection—between people with subpatent P. falciparum infections and those uninfected. We hypothesized that, because RDTs are believed to adequately detect parasites at densities that routinely cause clinical symptoms [7], the 60-day risk of symptomatic RDT-positive malaria would be similar between subpatently infected and uninfected people.
METHODS
Ethical Statement
The study protocol was approved by the ethical review committees of Duke University (no. Pro0008200) and Moi University (no. 2017/36); that of the University of North Carolina at Chapel Hill deemed this analysis exempt. We obtained written informed consent from all participants or their parent for those <18 years old, who also provided assent if they were >8 years old.
Study Site and Participants
We analyzed data collected from a cohort of people aged 1–85 years living in 75 households in Webuye, Western Kenya. The cohort was first enrolled in 2017 with 38 households in 3 villages selected by radial sampling in an area of high malaria transmission [10, 11]. In 2020, we expanded to 75 households across 5 villages. Throughout the study, when participants experienced symptoms of suspected malaria, they contacted study, staff who administered an RDT (Carestart Malaria HRP2 Pf; Accessbio), using capillary blood. RDT-positive participants were treated with artemether-lumefantrine. Participants also provided dried blood spots (DBSs) at the time of RDT testing. In addition, as described elsewhere [12], households were visited weekly for morning collections by vacuum aspiration of mosquitos, which were morphologically graded for genus and sex. Demographic and behavioral questionnaires were administered monthly.
Sample Processing Procedures
Sample processing has been described elsewhere [11]. Briefly, each DBS was tested for P. falciparum using a real-time quantitative polymerase chain reaction (PCR) assay, which consistently detects parasite densities as low as 0.1/μL whole blood [13]. For the first 14 months of the study, P. falciparum–positive samples were genotyped using amplicon deep sequencing to identify haplotypes [12].
Exposure, Outcome, and Covariate Assessment
After excluding symptomatic RDT-positive episodes which resulted in treatment, we divided the analytic population of symptomatic RDT-negative episodes into exposed (subpatent) and unexposed (uninfected) episodes, based on positivity for P. falciparum by real time PCR. We excluded 40 episodes (6% of the data) with either inconclusive RDTs (n = 2) or missing DBSs (n = 38). For all analyses, the outcome was clinical malaria, defined as symptomatic, RDT-positive P. falciparum infection observed within 60 days after the index RDT-negative episode. We included age, sex, transmission season, and bed net use as covariates. We categorized age (<5, 5–15, or >15 years) using standard categories [14, 15]. Transmission season was categorized as low or high transmission according to the number of female Anopheles mosquitos collected across the study site in the 14 days before RDT testing (low transmission, ≤75 mosquitos; high transmission, >75 mosquitos).
From May to July 2020, mosquito collection was interrupted owing to the COVID-19 pandemic, and these months were categorized as the high-transmission season based on historical patterns. Bed net use was assessed during a monthly behavioral survey, and regular use was defined as reporting sleeping under a bed net >5 nights in the week preceding the survey administered just before RDT testing. If bed net use data were missing, we used information from the previous month's behavioral questionnaire.
Analysis Population
Our primary analysis population consisted of all symptomatic, RDT-negative episodes experienced by cohort participants who had not received antimalarials for their current illness. We also defined 2 secondary subpopulations. The febrile population comprised episodes during which the participant's measured temperature exceeded 37.4°C or the participant reported a recent history of fever, or both. The low-density population comprised all uninfected episodes and subpatent episodes in which the parasite density in the infection was ≤100/μL, in order to account for potential RDT technical failures.
Statistical Analysis
We assessed bivariate associations between covariates and exposure status using Pearson χ2 tests for categorical variables or Wilcoxon rank sum tests for continuous variables. For each, P values were adjusted using the Bonferroni correction to account for repeated measures across participants of up to 22 episodes, the maximum number observed in any participant.
To compare the incidence of subsequent clinical malaria between symptomatic episodes with and without subpatent P. falciparum, we used stratified Kaplan-Meier survival curves for clinical malaria after exposed and unexposed episodes. We used stabilized inverse probability (IP) weights to account for confounding by age, sex, bed net use, and transmission season. We also used stabilized IP weights to account for informative censoring by age and transmission season. For both sets of IP weights, directed acyclic graph analyses determined the minimally sufficient adjustment set of covariates. We estimated both sets of weights using logistic regression, multiplied them together, and applied them to the Kaplan-Meier function [16]. We calculated weighted risk differences (RDs) between groups using IP-weighted Kaplan-Meier curves and used bootstrapped standard error estimation to estimate Wald-type 95% confidence intervals (CIs). We repeated these statistical methods in our secondary febrile and low-density subpopulations and in all subanalyses.
The unit of analysis was an episode; thus, participants could contribute >1 RDT-negative episode to the analysis, and Kaplan-Meier functions included an individual-level clustering term. Participants were followed up until they developed clinical malaria or were censored at 60 days, whichever came first. If a participant had another RDT-negative episode within 60 days of an index episode, the follow-up from that index episode was censored, and they reentered the analysis as a new RDT-negative index episode. For those in whom clinical malaria developed within 60 days, we calculated summary statistics for the number of days in between an index episode and clinical malaria outcome event. We repeated these analyses within subgroups defined by transmission season (high or low) and age group (<5, 5–15, or >15 years) to assess modification. We also conducted sensitivity analyses to test the effects of different censoring criteria (see Supplementary Materials). Using parasite genotype data from the first 14 months of the study, we compared the unique pfcsp haplotypes detected in subpatent index infections and future RDT-positive infections.
RESULTS
Index Episode Characteristics
Within our main cohort of 757 participants, we observed ≥1 symptomatic, RDT-negative episode in 347 participants (Table 1). This subgroup experienced 1128 RDT-negative episodes, of which 59.9% were in female participants, 56.0% were among participants >15 years old, 69.9% occurred during low-transmission season, and 80.3% occurred in people reporting regular bed net use. The most common symptoms prompting RDT testing were reported fever (61.0%) and aches (40.7%).
Table 1.
Characteristics of Symptomatic Rapid Diagnostic Test–Negative Index Episodes
| Episode Characteristics |
Plasmodium falciparum Real-Time PCR Result, No. (%) of Episodesa |
P Valueb | ||
|---|---|---|---|---|
| Overall (N = 1128) | Positive (n = 400) | Negative (n = 728) | ||
| Village | ||||
| Kinesamo | 353 (31.3) | 123 (30.8) | 230 (31.6) | >.99 |
| Lurare | 77 (6.8) | 20 (5.0) | 57 (7.8) | |
| Maruti | 263 (23.3) | 98 (24.5) | 165 (22.7) | |
| Nangili | 81 (7.2) | 21 (5.2) | 60 (8.2) | |
| Sitabicha | 354 (31.4) | 138 (34.5) | 216 (29.7) | |
| Sex | ||||
| Female | 676 (59.9) | 246 (61.5) | 430 (59.1) | >.99 |
| Male | 452 (40.1) | 154 (38.5) | 298 (40.9) | |
| Age, y | ||||
| <5 | 176 (15.6) | 59 (14.8) | 117 (16.1) | >.99 |
| 5–15 | 320 (28.4) | 112 (28.0) | 208 (28.6) | |
| >15 | 632 (56.0) | 229 (57.2) | 403 (55.4) | |
| Transmission seasonc | ||||
| Low | 788 (69.9) | 253 (63.2) | 535 (73.5) | .01 |
| High | 340 (30.1) | 147 (36.8) | 193 (26.5) | |
| Regular bed net used | ||||
| No | 222 (19.7) | 81 (20.2) | 141 (19.4) | >.99 |
| Yes | 906 (80.3) | 319 (79.8) | 587 (80.6) | |
| Duration of illness, median (IQR), d | 2.0 (1.0–3.0) | 2.0 (1.0–3.0) | 2.0 (1.0–3.0) | >.99 |
| Symptomse | ||||
| Fever | 688 (61.0) | 228 (57.0) | 460 (63.2) | >.99 |
| Aches | 459 (40.7) | 158 (39.5) | 301 (41.3) | >.99 |
| Chills | 201 (17.8) | 68 (17.0) | 133 (18.3) | >.99 |
| Cough | 181 (16.0) | 59 (14.8) | 122 (16.8) | >.99 |
| Congestion | 131 (11.6) | 44 (11.0) | 87 (12.0) | >.99 |
| Vomiting | 63 (5.6) | 27 (6.8) | 36 (4.9) | >.99 |
| Diarrhea | 42 (3.7) | 18 (4.5) | 24 (3.3) | >.99 |
| Otherf | 444 (39.4) | 148 (37.0) | 296 (40.7) | >.99 |
Abbreviations: IQR, interquartile range; PCR, polymerase chain reaction.
aData represent no. (%) of participants unless otherwise specified.
bComputed using Pearson χ2 tests for categorical variables or Wilcoxon rank sum tests for continuous values, with Bonferroni correction for repeated measures.
cClassified by the number of female Anopheles mosquitoes collected in the 2 weeks following the index episode.
dRegular bed net use was defined as sleeping under a net >5 nights in the previous week.
eSome participants experienced >1 symptom per episode.
fOther symptoms include headache, stomachache, nausea, loss of appetite, fatigue, back pain, weakness, joint pain, and chest pain.
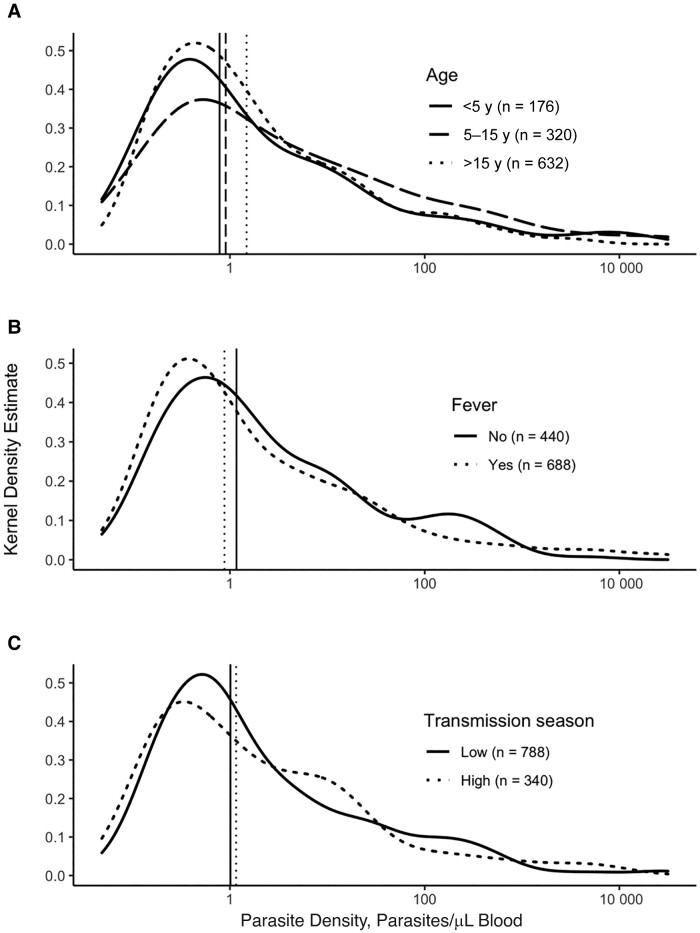
Across these 1128 symptomatic, RDT-negative episodes, 400 (35.5%) were positive for P. falciparum by real-time PCR (Table 1). PCR-positive episodes were significantly more likely to occur in the high-transmission season (36.8%) compared to uninfected episodes (26.5%) (P < .01) and were not associated with village, sex, age, or other covariates. Among these 400 subpatent infections, the median parasite density (interquartile range) was 1.02/μL (0.34–8.24/μL) of blood, consistent with the negative result by RDT (Figure 1).
Figure 1.
Distribution of parasite densities in rapid diagnostic test–negative subpatent infections. Kernel density curves show the distribution of parasite densities across age groups (A), febrile status (B), and transmission seasons (C). Vertical lines represent the median parasite density in each group. Febrile episodes were defined as episodes during which the participant's measured temperature exceeded 37.4°C, the participant reported a recent history of fever, or both. The transmission season was categorized as low or high transmission according to the number of female Anopheles mosquitos collected across the study site in the 14 days before evaluation (low transmission, ≤75 mosquitos; high transmission, >75 mosquitos).
Clinical Malaria Events
Clinical malaria occurred following 7.7% of RDT-negative episodes (n = 87). The median time to event (interquartile range) was 25 (15–41) days, the median parasite density for outcome events was 864/μL (45.1–6840.2/μL), and the most common symptoms prompting repeated RDT testing were reported fever (82.8%) and aches (46.0%). With Kaplan-Meier survival analysis, the overall 60-day risk of clinical malaria after a symptomatic, RDT-negative evaluation was 8.5% (95% CI, 6.7%–10.4%).
Associations of Subpatent Infections With Clinical Malaria
We recorded 34 clinical malaria events after the 400 subpatent episodes and 53 events after the 728 uninfected episodes. In survival analyses, the risk of clinical malaria over 60 days was similar between infected (9.0%) and uninfected (8.7%) groups (RD, 0.3% [95% CI, −1.9% to 2.6%]), suggesting that the presence of parasites in symptomatic people who test negative by RDT does not increase the risk of clinical malaria.
We next estimated the risk of clinical malaria in the febrile and low-density subpopulations. Among 688 febrile, RDT-negative episodes, 228 (33.1%%) were subpatent and 460 (66.9%) were uninfected. We observed 38 clinical malaria episodes, 14 following subpatent and 24 following uninfected episodes. The overall 60-day risk of malaria after febrile RDT-negative episodes was 7.6% (95% CI, 5.4%–9.7%). Similar to the primary analysis, the risk of subsequent clinical malaria following febrile RDT-negative episodes was similar between the subpatent (7.7%) and uninfected (9.3%) groups (RD, −1.6% [95% CI, −3.9% to .8%]). We also did not observe a significant RD in the low-parasite-density subpopulation (n = 1089) (RD, −0.9% [95% CI, −2.8% to 1.0%]). In an additional analysis stratified by age, RDs for clinical malaria between infected and uninfected episodes were minimal for children aged <5 years (RD, −2.3% [95% CI, −8.4% to 3.1%]), school-aged children (1.8% [−2.8% to 6.3%]), and adults (0.6% [−1.6% to 2.8%]).
Clinical Malaria Risk by Transmission Season
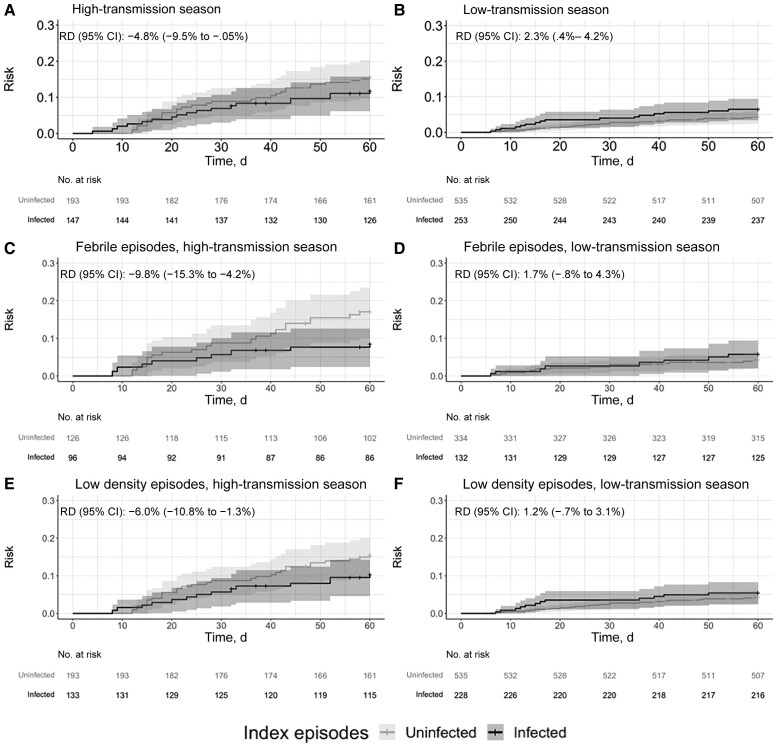
Surprisingly, we observed contrasting RDs between transmission seasons. During the low-transmission season, the risk of malaria was significantly higher after infected than after uninfected episodes (7.1% vs 4.7%; RD, 2.3% [CI, .4%–4.2%]). In contrast, during the high-transmission season, the risk of malaria was significantly lower after an infected than after an uninfected episode (13.0% vs 17.8%; RD, −4.8% [95% CI, −9.53% to −.05%]) (Figure 2).
Figure 2.
Risk of clinical malaria following a symptomatic, index rapid diagnostic test (RDT)–negative episode stratified by transmission season among the total population (A and B) and the febrile (C and D) and low-density (E and F) subpopulations. Cumulative incidence functions from inverse probability–weighted Kaplan-Meier estimation indicating time to clinical malaria after symptomatic RDT-negative episodes. Crosses indicate censoring at either the date of the next RDT-negative episode or the end of the follow-up period (60 days). Shaded areas represent 95% confidence intervals (CIs). Sixty-day risk differences (RDs) were calculated using the weighted Kaplan-Meier curves.
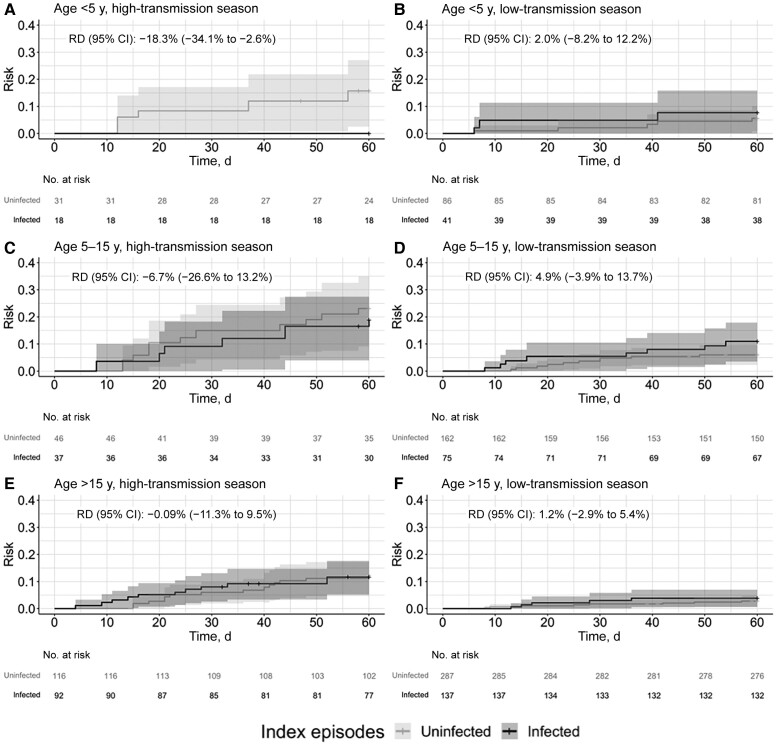
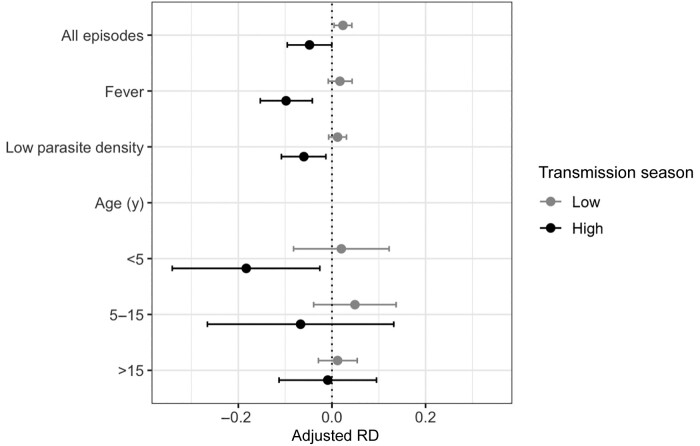
We conducted additional analyses stratified by transmission season across our predefined subgroups. Although power was limited in these stratified subpopulations, we observed that the risk of clinical malaria was consistently lower following a subpatent episode during the high-transmission season and slightly higher during the low-transmission season across febrile and low-parasite-density subpopulations (Figure 2) and age groups (Figure 3). RDs stratified by transmission season are summarized in Table 2 and Figure 4.
Figure 3.
Risk of clinical malaria following a symptomatic rapid diagnostic test (RDT)–negative episode stratified transmission season in people <5 y (A and B), 5–15 y (C and D), and >15 y (E and F). Cumulative incidence functions from inverse probability–weighted Kaplan-Meier estimation indicating time to clinical malaria following symptomatic RDT-negative episodes. Crosses indicate censoring on either the date of the next RDT-negative episode or at the end of the follow-up period (60 days). Shaded areas represent the 95% confidence intervals (CIs). Sixty-day risk differences (RDs) were calculated using the weighted Kaplan-Meier curves.
Table 2.
Risk of Clinical Malaria After Subpatent Episodes Stratified by Transmission Season
| Participant Population | High-Transmission Season | Low-Transmission Season | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Subpatent Episodes | Uninfected Episodes | Adjusted RDa (95% CI) | Subpatent Episodes | Uninfected Episodes | Adjusted RDa (95% CI) | |||||
| No. | Risk, % | No. | Risk, % | No. | Risk, % | No. | Risk, % | |||
| All episodes | 147 | 13.0 | 193 | 17.8 | −4.8 (−9.5 to −.05) | 253 | 7.1 | 535 | 4.7 | 2.3 (.4 to 4.2) |
| Fever | 96 | 9.3 | 126 | 19.0 | −9.8 (−15.3 to −4.2) | 132 | 6.4 | 334 | 4.7 | 1.7 (−.8 to 4.3) |
| Low parasite density | 228 | 11.5 | 535 | 17.6 | −6.0 (−10.8 to −1.3) | 133 | 5.9 | 193 | 4.7 | 1.2 (−.7 to 3.1) |
| Participant age, y | ||||||||||
| <5 | 18 | 0 | 31 | 18.3 | −18.3 (−34.1 to −2.6) | 41 | 8.3 | 86 | 6.3 | 2.0 (−8.2 to 12.2) |
| 5–15 | 37 | 19.5 | 46 | 26.2 | −6.7 (−26.6 to 13.2) | 75 | 11.5 | 162 | 6.6 | 4.9 (−3.9 to 13.7) |
| >15 | 92 | 13.1 | 116 | 14.0 | −.09 (−11.3 to 9.5) | 137 | 4.3 | 287 | 3.0 | 1.2 (−2.9 to 5.4) |
Abbreviations: CI, confidence interval; RD: risk difference.
aRDs were adjusted using inverse probability weights for confounding and informative censoring.
Figure 4.
Risk of clinical malaria following a subpatent, rapid diagnostic test (RDT)–negative infection stratified by transmission season, displayed as inverse probability (IP)–weighted risk differences (RDs) and 95% confidence intervals (CIs) of clinical malaria in people with or without subpatent infection. Subgroup analyses were conducted among febrile RDT-negative episodes and low-parasite-density infections, while stratified analyses were conducted for different age groups. All analyses were stratified by transmission season. Dots indicate RDs; lines, 95% CIs. In the primary and subgroup analyses, IP weights for confounding included age, sex, and bed net use, while IP weights for informative censoring included age. In the analyses stratified by age group and transmission season, IP weights for confounding included sex and bed net use.
The results of the sensitivity analyses can be found in the Supplementary Table. We observed the same pattern of lower risk of clinical malaria after a subpatent infection compared with an uninfected episode during the high-transmission season, and we noted a slightly increased risk during the low-transmission season. Broadly, the RDs were similar to those in the main analyses, although most were not statistically significant. Collectively, these analyses suggest that the risk of clinical malaria after a subpatent infection is highly influenced by parasite exposure during the high- and low-transmission seasons.
Parasite Genotypes in Index and Outcome Infections
Parasite genotypes were available for 83 subpatent infections, after which we observed 7 clinical malaria outcomes. In 5 of the 7 subpatent index infections, the subsequent episode of malaria shared ≥1 parasite haplotype with the initial infection, suggesting that some malaria events following subpatent infections were genetically related to the index infection.
DISCUSSION
We used a 54-month longitudinal cohort to investigate the association between symptomatic subpatent P. falciparum infections and subsequent clinical malaria. We observed that, following episodes of suspected malaria during which people tested negative for P. falciparum with an RDT, the risk of subsequent clinical malaria was low among those with a subpatent P. falciparum infection. In addition, in an exploratory analysis of modification by season, the comparative risk with uninfected people was modified by transmission season; subpatent infections were associated with a slightly increased risk of subsequent clinical malaria during the low-transmission season and a reduced risk during the high-transmission season. Taken together, these findings suggest that though the slightly elevated risk in the low-transmission season may merit alternate management, RDTs identify the majority of clinically relevant infections.
We observed that transmission season influenced the risk of malaria following a subpatent infection. Compared with those who were uninfected, those with subpatent infections had a slightly elevated risk of clinical malaria during the low-transmission season and a reduced risk during the high-transmission season, a pattern that was also observed in the febrile and low-density populations and in children <5 years old. To our knowledge, our study is the first to analyze clinical outcomes for subpatent infections in a longitudinal cohort across multiple transmission seasons in a high-transmission setting. One explanation of our findings could be that subpatent symptomatic infections do indeed confer some mildly increased risk of malaria during low-transmission season, when exposure to incident infections is limited owing to the paucity of vectors. This could be counteracted during high-transmission seasons by some protective benefit that prevents or forestalls malaria, consistent with our group’s prior observation that the presence of persistent parasites limits the symptomaticity of newly acquired, superinfecting parasites [17] and with evidence that blood-stage infections enhance adaptive immune responses [18]. Because the high-transmission season is characterized by the exposure to many infectious bites with diverse parasites, undetected and untreated subpatent infections may attenuate the clinical impact of newly acquired parasites or enhance immunity among people with parasitemia [16, 19].
The clinical significance of the increased risk during the low-transmission season is unclear. Given the low risk of malaria among people with subpatent infections during the low-transmission season (7.1%), the 2-percentage-point increase in risk might be minimal. Alternate strategies during the low-transmission season, including more sensitive clinical diagnostics and presumptive prescribing of antimalarials for later use, could be useful for detecting and treating subpatent infections that may progress to clinical malaria.
Clinical malaria following a subpatent infection associated with symptoms was rare, and our investigation into haplotype sharing between index and subsequent infections suggests that some of these rare events occurred after index subpatent infections that were “preclinical.” Of the 83 subpatent index infections with available haplotype data, only 7 were followed by clinical malaria episodes, of which 5 shared ≥1 haplotype between index and secondary infections. Although we cannot make decisive conclusions from this limited analysis, the short time to clinical malaria and the presence of ≥1 shared haplotype between index and secondary infections suggest that some of these subpatent infections may represent a preclinical phase. Such infections, not yet above the density threshold for RDTs at the time of testing, could have progressed to be detectable shortly thereafter.
This study has several strengths. The availability of a comparator group consisting of symptomatic P. falciparum–negative episodes allowed us to form a sample representative of people with untreated suspected malaria, which made our results more generalizable to our target population. In addition, our study design used identical mechanisms for the ascertainment of exposures and outcomes, namely, self-reported symptoms. As a result, only participants in the overall cohort who sought care in this way were able to enter the analysis, enhancing the ability to rigorously capture outcome events. Finally, using IP weighted Kaplan-Meier survival curves takes advantage of our longitudinal study design and unequal follow-up time between participants. This approach is more interpretable and does not have the methodologic issues of using a Cox proportional hazards model [20]. IP weighting standardizes the population such that one survival curve represents the entire sample if all episodes were subpatent infections and the other represents the entire sample if all were uninfected episodes [21], allowing us to interpret our findings as the average effect in the population [22], which is more interpretable than the conditional estimates produced by other methods.
These analyses are subject to limitations. Parasitic genetic data were unavailable for the majority of the study period, which precluded a comprehensive investigation of haplotype sharing between index and subsequent infections. However, we still observed evidence of identical parasite haplotypes in index and outcome infections, demonstrating an ability to observe some expected “prepatent” infections. In addition, some exposures may have been misclassified owing to parasites which do not express the HRP2 antigen that is detected by RDTs [23]. We did not assess HRP2 deletions among parasites in this study, though these deletions have proved to be rare in western Kenya [24–26] and the multiplicity of parasite clones we have observed in this cohort would also “mask” the effect of individual parasites lacking HRP2 within complex infections. Finally, our definitions of transmission season were empiric and based on contemporary mosquito collections, and they may therefore deviate from traditional perceptions of a community's transmission season, which are subject to monthly and yearly variability.
In our longitudinal study of follow-up after symptomatic RDT-negative episodes, clinical malaria was less likely after a subpatent P. falciparum infection than after an uninfected, symptomatic episode during the high-transmission season. Although clinical malaria was slightly more likely following a subpatent infection compared with an uninfected episode in the low-transmission season, this difference was minimal. The absence of a clinically significant increased risk after undetected, untreated infections supports the notion that current malaria RDTs adequately identify the large majority of clinically relevant P. falciparum infections. In areas without substantial HRP2-deleted parasites, negative results with conventional RDTs should prompt evaluation for alternate causes of symptoms.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Erica E Zeno, Department of Epidemiology, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, USA; Division of Infectious Diseases, School of Medicine, Duke University, Durham, North Carolina, USA.
Andrew A Obala, School of Medicine, College of Health Sciences, Moi University, Eldoret, Kenya.
Brian Pence, Department of Epidemiology, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, USA.
Elizabeth Freedman, Division of Infectious Diseases, School of Medicine, Duke University, Durham, North Carolina, USA.
Judith N Mangeni, School of Public Health, College of Health Sciences, Moi University, Eldoret, Kenya.
Jessica T Lin, Division of Infectious Diseases, School of Medicine, University of North Carolina at Chapel Hill, USA.
Lucy Abel, Academic Model Providing Access to Healthcare, Moi Teaching and Referral Hospital, Eldoret, Kenya.
Jessie K Edwards, Department of Epidemiology, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, USA.
Emily W Gower, Department of Epidemiology, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, USA.
Steve M Taylor, Division of Infectious Diseases, School of Medicine, Duke University, Durham, North Carolina, USA; Duke Global Health Institute, Duke University, Durham, North Carolina, USA.
Notes
Acknowledgments. We thank J. Kipkoech Kirui, I. Khaoya, L. Marango, E. Mukeli, E. Nalianya, J. Namae, L. Nukewa, E. Wamalwa, and A. Wekesa (each of Moi University) for their operational expertise in the field; J. Grassia, B. Freedman, and J. Decurzio (each of Duke University) for their assistance with laboratory processing; K. Sumner (of Duke University) for sequence data processing; W. Prudhomme-O’Meara (of Duke University) for her feedback during the early phases of this project; and Z. Lapp and C. Markwalter (each of Duke University) for their helpful analytic advice. Ultimately, we are indebted to the Webuye cohort members for their participation in the study.
Author contributions. E. E. Z. and S. M. T. conceptualized the study. E. E. Z., B. P., J. T. L., J. K. E., E. W. G., and S. M. T. developed the methodology. E. E. Z. performed formal analysis and visualized the data. S. M. T. acquired funding. E. F. performed the investigation. A. A. O., L. A., J. N. M., and S. M. T. administered and supervised the project. E. E. Z. and S. M. T. wrote the original manuscript draft. E. E. Z., B. P., J. T. L., J. N. M., J. K. E., E. W. G., and S. M. T. reviewed and edited the manuscript.
Data availability. Data are available at https://github.com/duke-malaria-collaboratory/Sub-patent-risk.
Financial support. This work was supported by National Institute of Allergy and Infectious Diseases (grants R21AI126024 to W. PrudhommeO'Meara and R01AI146849 to W. PrudhommeO'Meara and S. M. T.) and an Infectious Disease Epidemiology Pre-Doctoral Training Fellowship from the University of North Carolina at Chapel Hill (grant T32AI070114 to E. E. Z.).
References
- 1. Aidoo M, Incardona S. Ten years of universal testing: how the rapid diagnostic test became a game changer for malaria case management and improved disease reporting. Am J Trop Med Hyg 2021; 106:29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization. World malaria report 2022. 2022. https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2022. Accessed 20 March 2023.
- 3. Slater HC, Ross A, Ouedraogo AL, et al. Assessing the impact of next-generation rapid diagnostic tests on Plasmodium falciparum malaria elimination strategies. Nature 2015; 528:S94–101. [DOI] [PubMed] [Google Scholar]
- 4. Wu L, van den Hoogen LL, Slater H, et al. Comparison of diagnostics for the detection of asymptomatic Plasmodium falciparum infections to inform control and elimination strategies. Nature 2015; 528:S86–93. [DOI] [PubMed] [Google Scholar]
- 5. Slater HC, Ding XC, Knudson S, et al. Performance and utility of more highly sensitive malaria rapid diagnostic tests. BMC Infect Dis 2022; 22:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stresman GH, Stevenson JC, Ngwu N, et al. High levels of asymptomatic and subpatent Plasmodium falciparum parasite carriage at health facilities in an area of heterogeneous malaria transmission intensity in the Kenyan highlands. Am J Trop Med Hyg 2014; 91:1101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. World Health Organization . Meeting report of the WHO Evidence Review Group on low-density malaria infections. 2017. https://www.who.int/docs/default-source/malaria/mpac-documentation/mpac-oct2017-erg-malaria-low-density-infections-session2-confidential-version.pdf?sfvrsn=ef11225f_2. Accessed 26 September 2023.
- 8. Kobayashi T, Kanyangarara M, Laban NM, et al. Characteristics of subpatent malaria in a pre-elimination setting in southern Zambia. Am J Trop Med Hyg 2019; 100:280–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hartley MA, Hofmann N, Keitel K, et al. Clinical relevance of low-density Plasmodium falciparum parasitemia in untreated febrile children: a cohort study. PLoS Med 2020; 17:e1003318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. O’Meara WP, Simmons R, Bullins P, et al. Mosquito exposure and malaria morbidity: a microlevel analysis of household mosquito populations and malaria in a population-based longitudinal cohort in western Kenya. J Infect Dis 2020; 221:1176–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sumner KM, Mangeni JN, Obala AA, et al. Impact of asymptomatic Plasmodium falciparum infection on the risk of subsequent symptomatic malaria in a longitudinal cohort in Kenya. Elife 2021; 10:e68812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sumner KM, Freedman E, Abel L, et al. Genotyping cognate Plasmodium falciparum in humans and mosquitoes to estimate onward transmission of asymptomatic infections. Nat Commun 2021; 12:909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Taylor SM, Sumner KM, Freedman B, Mangeni JN, Obala AA, Prudhomme O’Meara W. Direct estimation of sensitivity of Plasmodium falciparum rapid diagnostic test for active case detection in a high-transmission community setting. Am J Trop Med Hyg 2019; 101:1416–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bousema T, Drakeley C. Epidemiology and infectivity of Plasmodium falciparum and Plasmodium vivax gametocytes in relation to malaria control and elimination. Clin Microbiol Rev 2011; 24:377–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bousema T, Drakeley C. Determinants of malaria transmission at the population level. Cold Spring Harb Perspect Med 2017; 7:a025510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sondo P, Derra K, Lefevre T, et al. Genetically diverse Plasmodium falciparum infections, within-host competition and symptomatic malaria in humans. Sci Rep 2019; 9:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sumner KM, Freedman E, Mangeni JN, et al. Exposure to diverse Plasmodium falciparum genotypes shapes the risk of symptomatic malaria in incident and persistent infections: a longitudinal molecular epidemiologic study in Kenya. Clin Infect Dis 2021; 73:1176–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Odera DO, Tuju J, Mwai K, et al. Anti-merozoite antibodies induce natural killer cell effector function and are associated with immunity against malaria. Sci Transl Med 2023; 15:eabn5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McKenzie FE, Killeen GF, Beier JC, Bossert WH. Seasonality, parasite diversity, and local extinctions in Plasmodium falciparum malaria. Ecology 2001; 82:2673–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hernan MA. The hazards of hazard ratios. Epidemiology 2010; 21:13–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cole SR, Hernan MA. Adjusted survival curves with inverse probability weights. Comput Methods Programs Biomed 2004; 75:45–9. [DOI] [PubMed] [Google Scholar]
- 22. Brookhart MA, Wyss R, Layton JB, Sturmer T. Propensity score methods for confounding control in nonexperimental research. Circ Cardiovasc Qual Outcomes 2013; 6:604–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Verma AK, Bharti PK, Das A. HRP-2 deletion: a hole in the ship of malaria elimination. Lancet Infect Dis 2018; 18:826–7. [DOI] [PubMed] [Google Scholar]
- 24. Vera-Arias CA, Holzschuh A, Oduma CO, et al. High-throughput Plasmodium falciparum hrp2 and hrp3 gene deletion typing by digital PCR to monitor malaria rapid diagnostic test efficacy. Elife 2022; 11:e72083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nderu D, Kimani F, Thiong’o K, et al. Plasmodium falciparum histidine-rich protein (PfHRP2 and 3) diversity in Western and Coastal Kenya. Sci Rep 2019; 9:1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rogier E, McCaffery JN, Nace D, et al. Plasmodium falciparum pfhrp2 and pfhrp3 gene deletions from persons with symptomatic malaria infection in Ethiopia, Kenya, Madagascar, and Rwanda. Emerg Infect Dis 2022; 28:608–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.