Abstract
We report the isolation and propagation of human papillomavirus type 16, the main agent of cervical cancer, using human foreskin fragments implanted in severe combined immunodeficiency mice. The infection produced viral particles, and with each passage of the virus it caused lesions identical to intraepithelial neoplasia, the precursor to carcinoma.
Human papillomaviruses (HPVs) are small DNA viruses that infect stratified squamous epithelia. Over 100 different HPV genotypes have been identified or characterized, and an association between the genotypes and the tissues they infect has been established (3). For example, HPVs infecting the anogenital region form a group distinct from the HPVs infecting the nongenital epithelia. Anogenital HPVs cause lesions ranging from anogenital warts (condylomata acuminata) to squamous cell cancer of the cervix, including intraepithelial neoplasias (3). Each anogenital HPV carries a different oncogenic risk. For example, HPV types 6 and 11 (HPV-6 and -11) are the agents of condyloma acuminatum and low-grade intraepithelial neoplasias, while HPV-16 and -18 cause intraepithelial neoplasias and cancers.
The inability to passage HPVs in vitro has been a major obstacle to working with these viruses. In 1985, Kreider et al. used human cervix fragments infected with an extract of pooled human condylomas and grafted them under the renal capsule of athymic (nude) mice to isolate the first HPV and reproduce some of the histologic features of HPV infection (27). This isolate, HPV-11Hershey, was subsequently passaged in other human tissues, primarily human foreskins, using the same animal model (17, 24–26). We subsequently showed that this viral strain could be more efficiently propagated and provide a more versatile model by using the severe combined immunodeficiency (SCID) mouse (9).
HPV-11Hershey was until recently the sole HPV reported to have been propagated and passaged experimentally. Kreider et al. described the isolation of HPV-1, the agent of plantar warts, in the athymic mouse xenograft model using human fetal foot skin (28), but passage of the isolate was not described. In a chamber grafted on the panniculus carnosus of SCID mice, Sterling et al. obtained the differentiation of a uterine cervical cell line (W12) stably infected with HPV-16 that produced histologic features of intraepithelial neoplasia, as well as viral antigen and particles; however, the virus was not passaged through uninfected cells or tissues (41). More recently, Brandsma et al. were able to introduce with a gene gun naked full genomic HPV-16 DNA in human foreskin fragments that were subsequently grafted onto the flank of SCID mice (12). Lesions that had histologic features of HPV infection and contained HPV antigen and DNA from type 16 developed, but the production of virions and the passage of the infection to uninfected tissue were not demonstrated. Other investigators have successfully grafted human lesions of recurrent respiratory papillomatosis or epidermodysplasia verruciformis and observed the maintenance of the full differentiation of the grafts, but they did not isolate and propagate the HPVs contained in these tumors (29, 39). In 1997, Christensen et al. isolated simultaneously HPV-40 and HPV-LVX82/MM7 from an anal condyloma coinfected with these two viruses (14). These two relatively uncommon viruses, which are found in anogenital condylomas and intraepithelial neoplasias, were subsequently copassaged.
Production of HPV particles and associated cytopathic effects in vitro have been demonstrated in differentiated epithelia developed on a raft system, either by using naturally infected cell lines or by transfection of HPV DNA into keratinocytes (19, 32, 33). However, these promising models have not yet permitted the propagation of free viral particles in vitro. In the present study, we report the isolation and passage of HPV-16, and the concomitant production of intraepithelial neoplasia, using the human xenograft SCID mouse model.
First isolation of HPV.
Fragments of single biopsies of clinical condyloma acuminatum from 11 patients were collected and snap-frozen in liquid nitrogen. (Protocols were approved by the University of Rochester Committee on Animal Research, Highland Hospital [Rochester, N.Y.] Human Investigation Committee, and the Investigational Review Board of the University of Rochester.) Each was separately ground in phosphate-buffered saline with sterile sand, using mortar and pestle, as previously described (9, 10). The material was clarified by centrifugation at 1,000 × g for 10 min, and the supernatant was stored at −80°C. Individual aliquots of these 11 sample suspensions were pooled and pelleted by centrifugation at 100,000 × g for 1 h at 4°C. The single pellet was resuspended in 760 μl of phosphate-buffered saline (identified as original inoculum) before being aliquoted and stored at −80°C. A neonatal human foreskin from routine circumcision was prepared as previously reported (9, 10) and cut into fragments of 1 by 1 mm. These were incubated in 250 μl of the original inoculum for 1 h at 37°C. One graft was implanted under the skin of the external ear, and another was implanted under the renal capsule on both sides of three 5- to 8-week-old female SCID (C.B-17/Icr Tac-scidfDF) mice (Taconic Farms). The mice were sacrificed 12 weeks later. None of the grafts implanted at the ear site grew. Of the six renal grafts, only one enlarged slightly; the cubic root of its three perpendicular dimensions, or geometric mean diameter (GMD), was 1.82 mm, compared to approximately 1 mm for the original implant.
The grafts were split; one part was fixed in buffered formalin, and the other part was snap-frozen in liquid nitrogen. Histologic (hematoxylin-eosin stain) criteria of HPV infection were preestablished and were the presence of any two of the following elements: acanthosis, koilocytosis, and parakeratosis (8–10, 22). All of the grafts had a normal histology except the enlarged graft, which was positive for HPV. It had the cytoarchitecture of an epidermal cyst, with evidence of acanthosis and parakeratosis. Koilocytes were essentially absent, but a basaloid hyperproliferation with several unusual mitotic figures in the upper third of the stratum acanthosum was noted. All of these features were consistent with intraepithelial neoplasia (Fig. 1). This graft was also strongly positive for the presence of HPV capsid antigen (data not shown), as determined by a previously described streptavidin-peroxidase method (10, 43). The primary antibodies were from pre- and postimmune sera from a rabbit immunized with a β-galactose fusion protein encoded by a 480-bp fragment derived from the L1 open reading frame of HPV-6b and bearing the common papillomavirus antigen (42). In the whole study, positive controls were included and the corresponding uninfected foreskins were used as negative controls. Viral DNA was extracted from the frozen sample and submitted to PCR amplification using the degenerate L1 primers MY09 and MY11 (30). An approximately 450-bp fragment consistent with the presence of HPV was amplified.
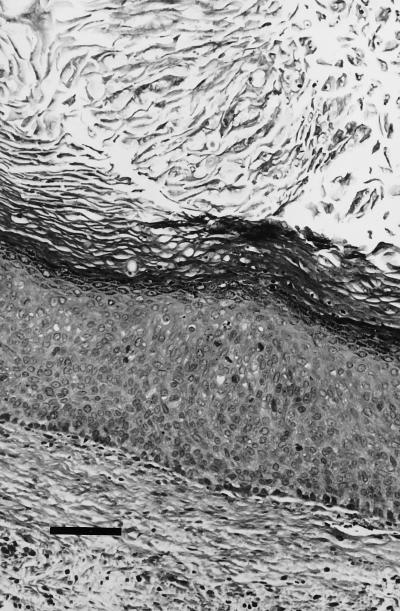
FIG. 1.
First isolation; histology (hematoxylin-eosin stain) of the single renal graft positive for the presence of HPV after the first passage. In addition to the acanthosis and parakeratosis, there is a mild basaloid proliferation and several mitotic figures are present in the stratum spinosum (bar, 600 μm).
Second passage and typing of the HPV isolate.
The HPV-positive graft was processed as for the preparation of the original inoculum by low- and high-speed centrifugation; 60 μl of the resuspended lysate was allocated for HPV DNA PCR, and 450 μl was used for further passage. A neonatal human foreskin prepared as before was incubated in 225 μl of the lysate for 1 h at 37°C. Single infected grafts were implanted under the renal capsule on both sides of six 5- to 8-week-old female SCID mice. The experiment was repeated, using a different foreskin on six additional SCID mice. These mice were sacrificed 19 weeks later. Of the 24 implants placed under the renal capsule of the 12 mice, 20 were still visible at euthanasia 19 weeks later, and all had the appearance of solid cysts. Their mean (standard deviation [SD]) GMD was 1.98 (0.87) mm. Fifteen grafts were ultimately available for histology, and five had evidence of HPV infection, including acanthosis and parakeratosis, as well as basaloid proliferation, dyskeratosis, and mitoses high in the stratum spinosum. None had koilocytosis. One of the five grafts was positive for capsid antigen by immunocytochemistry (data not shown). Transmission electron microscopy of a paraffin-embedded section (16) of that sample revealed the presence of intranuclear clusters of 30-nm particles in cells of the stratum granulosum (data not shown). The inoculum prepared for the subsequent passage from 11 of the collected grafts contained approximately 55-nm viral particles (Fig. 2) whose morphology was consistent with papillomavirus, as shown by electron microscopy after negative staining with 2% phosphotungstic acid.
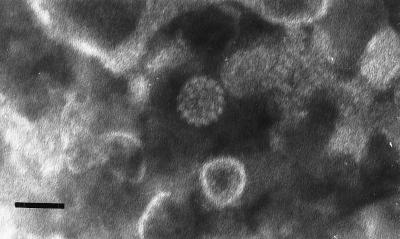
FIG. 2.
First isolation. The electron micrograph of a negatively stained preparation of the inoculum prepared from grafts collected from the second-passage experiment shows a 55-nm viral particle of morphology similar to that of papillomavirus capsids among cellular debris (bar, 55 nm).
The DNA in the same preparation was amplified by PCR with the MY09 and MY11 primers, and the amplicon was cloned into the pAMP cloning system (Gibco-BRL, Gaithersburg, Md.). It was also amplified by PCR using HPV-16 L1-specific primers, by methods previously described (37). The resulting amplification product was then cloned into a baculovirus transfer vector, pVL-1392, for expression in insect cells. Both the MY09-MY11 and full L1 amplicons were independently sequenced in both directions, using standard methods on a model 373 series Dye-Deoxy DNA sequencer (Applied Biosystem, Inc. Foster City, Calif.). The MY09-MY11 amplicon sequence was identical to that of the reference strain (34, 38) except at seven nucleotide positions (A6693C, G6719A, A6801T, C6852T, C6863T, C6968T, and G6992A). Five of the base changes were silent, one caused a conservative change from threonine to serine (A6801T), and one yielded a nonconservative substitution from threonine to proline (A6693C). DNA sequence of the full L1 clone from that preparation contained the same changes in the overlapping region, as well as 11 additional nucleotide substitutions outside the region amplified by MY09 and MY11 (G5696A, C5826T, T5909C, C6163A, A6178C, C6240G, T6245C, G6252A, A6432G, C6557T, and G7058T). These mutations resulted in seven amino acid changes: histidine to tyrosine (C5862T), threonine to asparagine (C6163A), asparagine to threonine (A6178C), histidine to aspartic acid (C6240G), glycine to serine (G6252A), threonine to alanine (A6432G), and leucine to phenylalanine (G7058T). All of these nucleotide substitutions fall in positions where variation has also been observed in HPV-16 variants, except for G6252A (35). In keeping with a naming practice established with the strain HPV-11Hershey isolated in the athymic mouse model (17), we refer to this isolate as HPV-16Rochester-1k.
Third passage of HPV-16Rochester-1k.
Eleven grafts recovered from the second passage were pooled. As before, they were ground and subjected to low- and high-speed centrifugation to prepare an infectious inoculum. A neonatal human foreskin was prepared and cut into squares of 1 by 1 mm for implantation under the renal capsule and 3 by 3 mm for subcutaneous grafting. Each group of fragments was incubated separately for 1 h at 37°C in 125 μl of the inoculum. The renal grafts were grafted in the usual manner, one per kidney, in six 6-week-old male SCID mice. Individual subcutaneous grafts were implanted under each flank in replacement of two stacked 1-cm-diameter round glass coverslips that had been placed 2 weeks earlier to elicit the formation of a vascular bed (41). The experiment was replicated a total of four times with different foreskin donors. The mice were sacrificed 27 weeks postgrafting. From 35 surviving mice, we retrieved 30 renal and 28 subcutaneous grafts with mean (SD) GMDs of 2.65 (0.77) and 2.75 (0.83) mm, respectively. The renal implants (GMD of ≈1 mm), which were twice as small as the subcutaneous implants (GMD of ≈2 mm), eventually reached the same size. Only four of the grafts were examined by histology. All demonstrated the features of HPV infection and of moderate to severe intraepithelial neoplasia already observed after the first passage (Fig. 3). The DNA from a viral inoculum prepared with available portions of 48 of the collected grafts was amplified by PCR with the MY09 and MY11 primers. The amplicon was probed with an extensive series of type-specific oligonucleotide probes corresponding to the anogenital HPV types 6, 11, 16, 18, 26, 31, 33, 35, 39, 40, 42, 45, 51, 52, 53, 54, 55, 56, 57, 58, 59, 66, 68, and 73 and the novel types MM4, LVX-82/MM7, and MM8 (2, 21, 31). It contained only HPV-16.
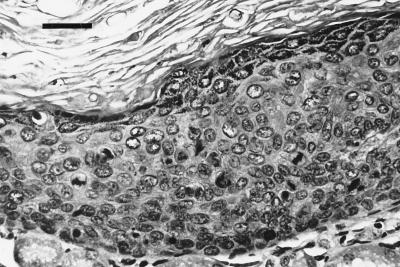
FIG. 3.
First isolation. Histology (hematoxylin-eosin stain) of one of the renal grafts from the third passage experiment shows prominent signs of intraepithelial neoplasia with basaloid proliferation, nuclear pleomorphism, dyskeratosis, and multiple aberrant mitoses throughout the stratum spinosum (bar, 100 μm).
Second isolation and propagation of HPV-16.
Using the original inoculum prepared from clinical lesions, we attempted to reisolate the virus. A human neonatal foreskin was prepared as before and cut into 3- by 3-mm squares that were incubated for 1 h at 37°C in 50 μl of the original viral inoculum. Each infected foreskin fragment was implanted subcutaneously in three 6- to 7-week-old male SCID mice to replace a cephalad and a caudad stack of two 1-cm-diameter round glass coverslips that had been placed under the left flank 2 weeks earlier. Four weeks later, the skin overlaying the caudad grafts was incised and the grafts were left exposed externally. The mice were sacrificed 24 weeks after grafting. The grafts were collected and split in two parts, one for formalin fixation and the other for freezing. The experiment was duplicated with another foreskin.
One of the six grafted mice died. In the five surviving mice, 9 of 10 implanted grafts were recovered. Their mean (SD) GMD was 2.50 (0.71) mm. Three of the five grafts that had been externalized remained so, forming small papillomas (Fig. 4). The internal grafts formed solid cysts. Eight of the grafts were interpretable by histology and immunocytochemistry. Five, including one of the external grafts, had histologic evidence for the presence of HPV (acanthosis and parakeratosis) (data not shown); one of them was also positive by immunocytochemistry, and three others had features of intraepithelial neoplasia (basaloid proliferation, dyskeratosis, and dyskaryosis).
FIG. 4.
Second isolation. A mouse from the first-passage experiment demonstrates an externalized, cutaneous graft. Magnified view of the lesion (insert) reveals a papillomatous appearance.
Four of the five grafts were used to prepare, in the manner already described, an inoculum for a second passage. Three sets of glass coverslips were implanted on each side under the flank skin of 12 6-week-old male SCID mice. Twenty days later, the coverslip stacks were removed and each was replaced with 3- by 3-mm square foreskin fragments that had been incubated in 100 μl of the viral inoculum. The foreskin fragments implanted on the left side of the animals came from a different donor than the fragments implanted on the right side. Twelve weeks after grafting, the cephalad grafts on each mouse were externalized through an incision to allow the graft to become cutaneous. The mice were sacrificed 16 weeks after grafting. Two of the 12 mice died before the end of the experiment. In the 10 surviving mice, 56 of the 60 implants were present at euthanasia; none were external. Their mean (SD) GMD was 2.05 (0.50) mm. Thirteen of the 53 available for histology were not interpretable because of incomplete or altered histologic architecture. Of the 40 interpretable grafts, 34 were positive for the presence of HPV (acanthosis and parakeratosis), and 21 of those exhibited the features of intraepithelial neoplasia, grades 1 to 2 (Fig. 5). Only 1 of the 40 samples was positive for HPV capsid by immunocytochemistry. From the viral inoculum prepared from 42 of the retrieved grafts, PCR with the MY09 and MY11 primers amplified a DNA fragment that was cloned into pAMP and whose sequence was found to be identical to that of the reference HPV-16 strain (34, 38). This strain is referred as HPV-16Rochester-1sc.
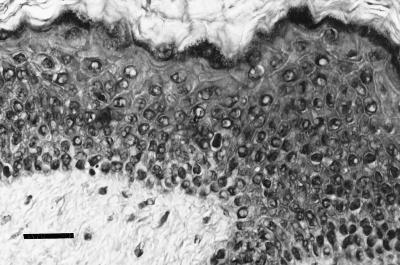
FIG. 5.
Second isolation. Histology (hematoxylin-eosin) of a subcutaneous graft from the second-passage experiment exhibits features of acanthosis, parakeratosis, and dyskeratosis (bar, 100 μm).
We were able to repeatedly propagate HPV-16 in human foreskin fragments grafted in the SCID mouse and to obtain intraepithelial neoplasia in the implants. We obtained two distinct lineages for the propagation of HPV-16, one through renal implants (HPV-16Rochester-1k) and one through subcutaneous implants (HPV-16Rochester-1sc). By extrusion, we were also able to convert some of the subcutaneous grafts into cutaneous lesions. These lesions had the appearance of small papillomas without any clinical attributes of malignancy, which is consistent with the HPV-16-associated papules found on penile skin (1).
The histopathology of the lesions that we observed varied from benign to premalignant. Because some of the lesions were cystic, some of the features of HPV infection such as papillomatosis and hyperkeratosis could not be assessed. None of the samples we reviewed had frank koilocytosis. Most HPV-16-associated lesions of the penis reveal intraepithelial neoplasia on histology, and koilocytosis is an inconsistent component of anogenital intraepithelial neoplasia (1, 22). However, Brandsma et al. reported that koilocytosis was a prominent element in the histology of lesions induced by the transfection of full-genomic HPV-16 DNA in human foreskins subsequently grafted onto SCID mice (12). The histology of the majority of our lesions containing HPV also revealed mild to severe intraepithelial neoplasia, as seen clinically in penile lesions associated with HPV-16 (1). Keratinocytes transfected with HPV-16 DNA and implanted subcutaneously in the athymic mouse can differentiate and exhibit histologic features similar but not strictly identical to those of intraepithelial neoplasia, and they do not produce viral particles (18, 44). W12 cells, which are derived from a low-grade cervical neoplasia lesion, have retained episomal HPV-16 DNA (40, 41). When grafted on the flank panniculus carnosus of SCID mice, the cells differentiate, display some of the histologic features of the original lesion, and produce viral particles. This and other data established a link between HPV-16 and intraepithelial neoplasia. However, our study demonstrates for the first time that an intraepithelial neoplasia lesion whose histology is identical to the natural lesion can be created by infecting normal skin with an inoculum containing HPV-16 viral particles, the presumed natural agent of infection. It thus fulfills one of the classic Koch’s postulates of being able to reproduce the disease after inoculation of the healthy host (20). Of note, in the human xenograft athymic mouse model, neither HPV-40 nor HPV-LVX82/MM7 reproduced the intraepithelial neoplasia lesions with which they can be associated in the natural host (14). However, in contrast to HPV-16, neither of these two viruses appears to be associated with anogenital cancer (11, 31). In our study, depending somewhat on the experiment, variation existed in the grades, from mild to severe, of the intraepithelial neoplasias and in the nature of the histologic features, such as differentiation, nuclear abnormalities, and mitotic activity. This variation may possibly be a reflection of the titer of the inoculum and the susceptibility of the foreskin donor. No squamous carcinoma was noted, which is not surprising considering that it takes about a decade for high-grade lesions to evolve into cervical cancer (36).
The presence of HPV in the lesions was confirmed by the detection of viral capsid antigen in some of the samples. These samples were few, an observation in agreement with the finding that only 5 to 8% of clinical bowenoid papillomatosis lesions (a variant of intraepithelial neoplasia of the external genitalia) exhibit this antigen (13, 23). The demonstration of approximately 55-nm viral particles resembling HPV in the inoculum prepared from the lesions of a second passage of the isolate excludes any carryover from the original inoculum. Furthermore, since it is reasonable to assume that these capsids were of HPV-16, it is remarkable that the xenograft SCID mouse model allowed the production of extractable virions, something that has not been reported previously for HPV-16 or intraepithelial neoplasias.
HPV-16 was identified by PCR and DNA sequencing of the MY09-MY11 amplicon. The sequence of HPV-16Rochester-1k differed from the prototype sequence at seven nucleotide positions, only one resulting in a significant amino acid change. This DNA sequence was confirmed in a separate clone of the full L1 open reading frame. At least two reasons may account for why the HPV-16Rochester-1k sequence differs from that of HPV-16Rochester-1sc. The DNA sequences were each derived from single clones that may represent different variants in the original inoculum. Alternatively, nucleotide errors may have been introduced in the HPV-16Rochester-1k amplicon by the Taq polymerase during PCR. This is less likely given the concordance of two independent DNA sequences of the HPV-16Rochester-1k amplicon. Conversely, the sequencing error might have been in the HPV-16Rochester-1sc. Further passage and sequencing of the isolate should clarify this issue. The inoculum prepared from the third passage in the first set of experiments contained only HPV-16 when tested, after PCR amplification, with type-specific oligonucleotide probes corresponding to the vast majority of anogenital HPV types. The isolation of HPV-16 from clinical condylomata acuminata was surprising since this genotype is rarely, if ever, found in penile condylomata acuminata (1). However, when we looked back at the histologic diagnosis of the lesions biopsied, we found that 2 of the 11 patients had mild to moderate penile intraepithelial neoplasia, while the remainder had condyloma acuminatum. This finding is significant because in a previous study we had found HPV-16 in 17 (36%) of 47 penile intraepithelial neoplasias (15). It is therefore conceivable that different HPV-16 strains from different patients were present in the original inoculum. Since this isolation of HPV-16, we have attempted five other isolations and propagations of HPV types, using the human xenograft SCID mouse model. All were successful and yielded three HPV-6 and two new HPV-11 strains (4, 5, 7). The percentages of grafts positive for HPV by histology after a first passage were, respectively, 79, 43, and 21% for our three HPV-6 isolates and 62 and 50% for our two HPV-11 isolates, compared with 17% for HPV-16Rochester-1k and 63% for HPV-16Rochester-1sc. These variable rates do not permit conclusions regarding the growth advantage of a particular genotype over another, and we cannot explain why other HPV types did not grow along with HPV-16. As with HPV-11Hershey, we noted that HPV-16-infected renal grafts grew larger than subcutaneous grafts (6). The ease of isolating new genital HPV strains in the human xenograft SCID mouse model contrasts with several personal failed attempts and the sporadic success of others using the athymic mouse (14, 27). It supports our view of the advantages of the SCID mouse over the athymic mouse (9). Another potential factor in our success was the addition of a high-speed centrifugation step in the preparation of the viral inoculum (27), since a high viral titer may be essential for infectivity (14, 24).
Our observations are further confirmation that HPV-16 directly causes intraepithelial neoplasia. The availability of this HPV-16 human xenograft model offers novel possibilities. One can now produce infectious particles of an oncogenic HPV and study the full replicative cycle of the virus. It provides a model that seems to faithfully reproduce the natural lesion and thus permits the investigation of an authentic pathogenesis of HPV-induced premalignant lesions. Finally, it allows for the evaluation of antiviral strategies.
Acknowledgments
This work was supported by contract NIH-NO1-AI-35159 from the National Institutes of Health.
We are indebted to Mark H. Stoler for interpreting the pathology of the patient biopsies. We thank Debbie Pilc, University of Rochester Xenograft Facility, for excellent assistance, and we thank Elizabeth Woodward and her staff for providing access to foreskins.
REFERENCES
- 1.Barrasso R, De Brux J, Croissant O, Orth G. High prevalence of papillomavirus-associated penile intraepithelial neoplasia in sexual partners of women with cervical intraepithelial neoplasia. N Engl J Med. 1987;317:916–923. doi: 10.1056/NEJM198710083171502. [DOI] [PubMed] [Google Scholar]
- 2.Bauer H M, Greer C E, Manos M M. Detection of genital human papillomavirus using PCR. In: Herrington C S, McGee J O, editors. Diagnostic molecular pathology: a practical approach. Oxford, England: Oxford University Press; 1992. pp. 131–152. [Google Scholar]
- 3.Bonnez W. Papillomavirus. In: Richman D D, Whitley R J, Hayden F G, editors. Clinical virology. 1st ed. New York, N.Y: Churchill Livingstone; 1997. pp. 569–611. [Google Scholar]
- 4.Bonnez, W. Unpublished data.
- 5.Bonnez W, Borkhuis C, DaRin C, de Mesy Jensen K L, Beutner K, Van Nest G, Greer C. Abstracts of the 15th International Papillomavirus Workshop, Gold Coast, Queensland, Australia. 1996. Growth and propagation of human papillomavirus (HPV) type 6 in the human skin xenograft-severe combined immunodeficiency (SCID) mouse model; p. 152. [Google Scholar]
- 6.Bonnez W, DaRin C, Borkhuis C, Rose R. Abstracts of the 13th International Papillomavirus Conference, Amsterdam, The Netherlands. 1994. Effect of graft location (renal capsule versus subcutis) and animal gender on growth of HPV-11-infected human xenografts in the severe combined immunodeficiency (SCID) mouse; p. P8. [Google Scholar]
- 7.Bonnez W, DaRin C, Reichman R C, de Mesy Jensen K. Abstracts of the 16th International Papillomavirus Conference, Siena, Italy. 1997. Growth and propagation of two new HPV-11 strains in the human xenograft severe combined immunodeficiency (SCID) mouse model; p. 482. [Google Scholar]
- 8.Bonnez W, Rose R C, Borkhuis C, Da Rin C, Reichman R C. Evaluation of the temperature sensitivity of human papillomavirus (HPV) type 11 using the human xenograft severe combined immunodeficiency (SCID) mouse model. J Clin Microbiol. 1994;32:1575–1577. doi: 10.1128/jcm.32.6.1575-1577.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonnez W, Rose R C, Da Rin C, Borkhuis C, de Mesy Jensen K L, Reichman R C. Propagation of human papillomavirus type 11 in human xenografts using the severe combined immunodeficiency (SCID) mouse and comparison to the nude mouse model. Virology. 1993;197:455–458. doi: 10.1006/viro.1993.1611. [DOI] [PubMed] [Google Scholar]
- 10.Bonnez W, Rose R C, Reichman R C. Antibody-mediated neutralization of human papillomavirus type 11 (HPV-11) infection in the nude mouse. Detection of HPV-11 mRNAs. J Infect Dis. 1992;165:376–380. doi: 10.1093/infdis/165.2.376. [DOI] [PubMed] [Google Scholar]
- 11.Bosch F X, Manos M M, Muñoz N, Sherman M, Jansen A M, Peto J, Schiffman M H, Moreno V, Kurman R, Shah K V International Biological Study on Cervical Cancer (IBSCC) Study Group. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. J Natl Cancer Inst. 1995;87:796–802. doi: 10.1093/jnci/87.11.796. [DOI] [PubMed] [Google Scholar]
- 12.Brandsma J L, Brownstein D G, Xiao W, Longley B J. Papilloma formation in human foreskin xenografts after inoculation of human papillomavirus type 16 DNA. J Virol. 1995;69:2716–2721. doi: 10.1128/jvi.69.4.2716-2721.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braun L, Farmer E R, Shah K V. Immunoperoxidase localization of papillomavirus antigen in cutaneous warts and bowenoid papulosis. J Med Virol. 1983;12:187–193. doi: 10.1002/jmv.1890120304. [DOI] [PubMed] [Google Scholar]
- 14.Christensen N D, Koltun W A, Cladel N M, Budgeon L R, Reed C A, Kreider J W, Welsh P A, Patrick S D, Yang H. Coinfection of human foreskin fragments with multiple human papillomavirus types (HPV-11, -40, and -LVX82/MM7) produces regionally separate HPV infections within the same athymic mouse xenograft. J Virol. 1997;71:7337–7344. doi: 10.1128/jvi.71.10.7337-7344.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Demeter L M, Stoler M H, Bonnez W, Pappas P, Corey L, Strussenberg J, Reichman R C. Penile intraepithelial neoplasia: clinical presentation and an analysis of the physical state of human papillomavirus DNA. J Infect Dis. 1993;168:38–46. doi: 10.1093/infdis/168.1.38. [DOI] [PubMed] [Google Scholar]
- 16.di Sant’Agnese P A, de Mesy-Jensen K L. Diagnostic electron microscopy on reembedded (“popped off”) areas of large Spurr epoxy sections. Ultrastruct Pathol. 1984;6:247–253. doi: 10.3109/01913128409018580. [DOI] [PubMed] [Google Scholar]
- 17.Dollard S C, Chow L T, Kreider J W, Broker T R, Lill N L, Howett M K. Characterization of an HPV type 11 isolate propagated in human foreskin implants in nude mice. Virology. 1989;171:294–297. doi: 10.1016/0042-6822(89)90542-4. [DOI] [PubMed] [Google Scholar]
- 18.Dürst M, Bosch F X, Glitz D, Schneider A, zur Hausen H. Inverse relationship between human papillomavirus (HPV) type 16 early gene expression and cell differentiation in nude mouse epithelial cysts and tumors induced by HPV-positive human cell lines. J Virol. 1991;65:796–804. doi: 10.1128/jvi.65.2.796-804.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frattini M G, Lim H B, Doorbar J, Laimins L A. Induction of human papillomavirus type 18 late gene expression and genomic amplification in organotypic cultures from transfected DNA templates. J Virol. 1997;71:7068–7072. doi: 10.1128/jvi.71.9.7068-7072.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fredericks D N, Relman D A. Sequence-based identification of microbial pathogens: a reconsideration of Koch’s postulates. Clin Microbiol Rev. 1996;9:18–33. doi: 10.1128/cmr.9.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hildesheim A, Schiffman M H, Gravitt P E, Glass A G, Greer C E, Zhang T, Scott D R, Rush B B, Lawler P, Sherman M E, Kurman R J, Manos M M. Persistence of type-specific human papillomavirus infection among cytologically normal women. J Infect Dis. 1994;169:235–240. doi: 10.1093/infdis/169.2.235. [DOI] [PubMed] [Google Scholar]
- 22.Jacques S M, Lawrence W D. Dermatopathology of anogenital skin. In: Murphy G F, editor. Dermatopathology—a practical guide to common disorders. W. B. Philadelphia, Pa: Saunders Company; 1995. pp. 407–420. [Google Scholar]
- 23.Jenson A B, Kurman R J, Lancaster W D. Detection of papillomavirus common antigens in lesions of skin and mucosa. Clin Dermatol. 1985;3:56–63. doi: 10.1016/0738-081x(85)90049-5. [DOI] [PubMed] [Google Scholar]
- 24.Kreider J, Howett M K, Stoler M H, Zaino R J, Welsh P. Susceptibility of various human tissues to transformation in vivo with human papillomavirus type 11. Int J Cancer. 1987;39:459–465. doi: 10.1002/ijc.2910390409. [DOI] [PubMed] [Google Scholar]
- 25.Kreider J W, Howett M K, Leure-Dupree A E, Zaino R J, Weber J A. Laboratory production in vivo of infectious human papillomavirus type 11. J Virol. 1987;61:590–593. doi: 10.1128/jvi.61.2.590-593.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kreider J W, Howett M K, Lill N L, Bartlett G L, Zaino R J, Sedlacek T V, Mortel R. In vivo transformation of human skin with human papillomavirus type 11 from condylomata acuminata. J Virol. 1986;59:369–376. doi: 10.1128/jvi.59.2.369-376.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kreider J W, Howett M K, Wolfe S A, Bartlett G L, Zaino R J, Sedlacek T V, Mortel R. Morphological transformation in vivo of human uterine cervix with papillomavirus from condylomata acuminata. Nature. 1985;317:639–641. doi: 10.1038/317639a0. [DOI] [PubMed] [Google Scholar]
- 28.Kreider J W, Patrick S D, Cladel N M, Welsh P A. Experimental infection with human papillomavirus type 1 of human hand and foot skin. Virology. 1990;177:415–417. doi: 10.1016/0042-6822(90)90503-j. [DOI] [PubMed] [Google Scholar]
- 29.Majewski S, Breitburd F, Skopinska M, Croissant O, Jablonska S, Orth G. A mouse model for studying epidermodysplasia-verruciformis-associated carcinogenesis. Int J Cancer. 1994;56:727–730. doi: 10.1002/ijc.2910560519. [DOI] [PubMed] [Google Scholar]
- 30.Manos M M, Ting Y, Lewis A J, Broker T R, Wolinsky S M. Use of polymerase chain reaction amplification for the detection of genital human papillomaviruses. Cancer Cells. 1989;7:209–214. [Google Scholar]
- 31.Manos M M, Waldman J, Zhang T Y, Greer C G, Eichinger M H, Schiffman M H, Wheeler C M. Epidemiology and partial nucleotide sequence of four novel genital human papillomaviruses. J Infect Dis. 1994;170:1096–1099. doi: 10.1093/infdis/170.5.1096. [DOI] [PubMed] [Google Scholar]
- 32.Meyers C, Frattini M G, Hudson J B, Laimins L A. Biosynthesis of human papillomavirus from a continuous cell line upon epithelial differentiation. Science. 1992;257:971–973. doi: 10.1126/science.1323879. [DOI] [PubMed] [Google Scholar]
- 33.Meyers C, Mayer T J, Ozbun M A. Synthesis of infectious human papillomavirus type 18 in differentiating epithelium transfected with viral DNA. J Virol. 1997;71:7381–7386. doi: 10.1128/jvi.71.10.7381-7386.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Myers G, Bernard H-U, Delius H, Baker C, Icenogle J, Halpern A, Wheeler C. Human papillomaviruses 1995—a compilation and analysis of nucleic acid and amino acid sequences. Los Alamos, N.Mex: Los Alamos National Laboratory; 1995. [Google Scholar]
- 35.Myers G, Halpern A, Baker C, McBride A, Wheeler C, Doorbar J. Human papillomaviruses 1996—a compilation and analysis of nucleic acid and amino acid sequences. Los Alamos, N.Mex: Los Alamos National Laboratory; 1996. [Google Scholar]
- 36.Noda K. Cervical intraepithelial neoplasia and microinvasive carcinoma of the cervix. Curr Top Pathol. 1992;85:57–80. doi: 10.1007/978-3-642-75941-3_3. [DOI] [PubMed] [Google Scholar]
- 37.Rose R C, Bonnez W, Da Rin C, McCance D J, Reichman R C. Serologic differentiation of human papillomavirus (HPV) types 11, 16, and 18, using recombinant virus-like particles (VLPs) J Gen Virol. 1994;75:2445–2449. doi: 10.1099/0022-1317-75-9-2445. [DOI] [PubMed] [Google Scholar]
- 38.Seedorf K, Kraemmer G, Dürst M, Suhai S, Roewekamp W G. Human papillomavirus type 16 DNA sequence. Virology. 1985;145:181–185. doi: 10.1016/0042-6822(85)90214-4. [DOI] [PubMed] [Google Scholar]
- 39.Sexton C J, Williams A T, Topley P, Shaw R J, Lovegrove C, Leigh I, Stables J N. Development and characterization of a novel xenograft model permissive for human papillomavirus DNA amplification and late gene expression. J Gen Virol. 1995;76:3107–3112. doi: 10.1099/0022-1317-76-12-3107. [DOI] [PubMed] [Google Scholar]
- 40.Stanley M A, Browne H M, Appleby M, Minson A C. Properties of a non tumorigenic human cervical keratinocyte cell line. Int J Cancer. 1989;43:672–676. doi: 10.1002/ijc.2910430422. [DOI] [PubMed] [Google Scholar]
- 41.Sterling J, Stanley M, Gatward G, Minson T. Production of human papillomavirus type 16 virions in a keratinocyte cell line. J Virol. 1990;64:6305–6307. doi: 10.1128/jvi.64.12.6305-6307.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strike D G, Bonnez W, Rose R C, Reichman R C. Expression in Escherichia coli of seven DNA segments comprising the complete L1 and L2 open reading frames of human papillomavirus type 6b and the location of the “common antigen.”. J Gen Virol. 1989;70:543–555. doi: 10.1099/0022-1317-70-3-543. [DOI] [PubMed] [Google Scholar]
- 43.Wilbur D C, Reichman R C, Stoler M H. Detection of infection by human papillomavirus in genital condylomata. A comparison study using immunocytochemistry and in situ nucleic acid hybridization. Am J Clin Pathol. 1988;89:505–510. doi: 10.1093/ajcp/89.4.505. [DOI] [PubMed] [Google Scholar]
- 44.Woodworth C D, Waggoner S, Barnes W, Stoler M H, DiPaolo J A. Human cervical and foreskin epithelial cells immortalized by human papillomavirus DNAs exhibit dysplastic differentiation in vivo. Cancer Res. 1990;50:3709–3715. [PubMed] [Google Scholar]