Abstract
(1) Background: Cardiogenic shock (CS) is associated with high morbidity and mortality. Frailty and cardiovascular diseases are intertwined, commonly sharing risk factors and exhibiting bidirectional relationships. The relationship of frailty and non-acute myocardial infarction with cardiogenic shock (non-AMI-CS) is poorly described. (2) Methods: We retrospectively analyzed the National Inpatient Sample from 2016 to 2020 and identified all hospitalizations for non-AMI-CS. We classified them into frail and non-frail groups according to the hospital frailty risk score cut-off of 5 and compared in-hospital outcomes. (3) Results: A total of 503,780 hospitalizations for non-AMI-CS were identified. Most hospitalizations involved frail adults (80.0%). Those with frailty had higher odds of in-hospital mortality (adjusted odds ratio [aOR] 2.11, 95% confidence interval [CI] 2.03–2.20, p < 0.001), do-not-resuscitate status, and discharge to a skilled nursing facility compared with those without frailty. They also had higher odds of in-hospital adverse events, such as acute kidney injury, delirium, and longer length of stay. Importantly, non-AMI-CS hospitalizations in the frail group had lower use of mechanical circulatory support but not rates of cardiac transplantation. (4) Conclusions: Frailty is highly prevalent among non-AMI-CS hospitalizations. Those accompanied by frailty are often associated with increased rates of morbidity and mortality compared to those without frailty.
Keywords: cardiogenic shock, frailty, non-acute myocardial infarction
1. Introduction
Frailty is characterized by increased vulnerability to internal and external stressors and has been demonstrated to be associated with functional limitations and susceptibility to adverse events [1]. The presence of frailty places individuals with cardiovascular diseases at an elevated risk of experiencing complications, longer hospital stays, and major adverse cardiovascular events [2]. As the aging population continues to grow, the anticipated rise in frailty is concerning, posing an increasingly significant global health burden [3,4,5].
Individuals who present with non-acute myocardial infarction (AMI) cardiogenic shock (CS) often suffer from common cardiovascular conditions such as malignant arrhythmias, valvular heart disease, cardiomyopathies, and myocarditis. These underlying chronic comorbidities can independently contribute to reduced physical function, sarcopenia, disability, inflammation, and end-organ dysfunction [6,7]. The association between frailty and CS deserves attention due to the rising prevalence of non-AMI-CS-related hospitalizations, accounting for up to 70% of all CS hospitalizations in certain areas [8,9,10]. Despite this, there has been little investigation of the association between frailty and non-AMI-CS [11,12].
This study aims to investigate the relationship between frailty and outcomes among hospitalizations for non-AMI-CS cases. Our objectives include determining the prevalence of frailty among non-AMI-CS hospitalizations and analyzing its correlations with in-hospital outcomes, with the aim of underscoring the need for systematic frailty assessment in hospitalizations for acute cardiovascular illness.
2. Materials and Methods
2.1. Data Source
We performed a retrospective cohort study using the National Inpatient Sample (NIS), the largest all-payer inpatient healthcare database in the United States, designed to produce national estimates of inpatient utilization, access, costs, outcomes, and quality [13]. Developed for the Healthcare Cost and Utilization Project (HCUP) and sponsored by the Agency for Healthcare Research and Quality, the NIS collects data from more than 7 million admissions annually and approximates 35 million hospitalizations across 49 participating states when weights are applied. The NIS covers more than 97% of the total population, allowing the study of specific conditions and procedures on a national level. The NIS protects patient confidentiality by excluding state and hospital identifiers, thereby guaranteeing anonymity, and because of this strictly deidentified nature of the database, our study was exempt from the purview of our institutional review board. The NIS is openly available and can be accessed through the public website of the HCUP [13].
2.2. Study Population and Covariates
We identified all admissions with CS coded in either primary or secondary diagnoses from the years 2016 to 2020 [14]. Afterward, we excluded patients aged less than 18 years and entries that were missing values for demographics, hospital characteristics, primary payer, median income, day of admission, in-hospital mortality, or length of hospital stay (LOS). We excluded all patients with any diagnosis of AMI. From the remaining dataset, we collected data on demographics (sex, age, race), hospital characteristics (region, bed size, urban location), primary payer, median income, and day of admission (weekday, weekend), which are given in the database. Among all admissions, we detected the presence of multiple comorbidities; listed in Table 1. We also calculated the hospital frailty risk score, a validated measure of clinical frailty, for each admission by bestowing prespecified scores to 109 individual International Classification of Diseases, Tenth Revision, and Clinical Modification codes (Table S1) [15]. A hospital frailty risk score of at least 5 defined frailty, consistent with the definition used by the previous literature [16,17,18]. The hospital frailty risk score has been tested and validated among several studies, demonstrating a correlation between mortality, functional impairment, and quality-of-life outcomes [15,19]. All the comorbidities and procedural data we used were established based on the International Classification of Diseases, Tenth Revision, Clinical Modification and International Classification of Diseases, Tenth Revision, Procedural Coding System codes. These codes are listed in Table S2.
2.3. Study Outcomes
We set our primary outcome as in-hospital mortality. Secondary outcomes consisted of do-not-resuscitate status (DNR), palliative care consult, disposition to a skilled nursing facility, use of mechanical circulatory support (MCS), heart transplant, intracranial hemorrhage, gastrointestinal hemorrhage, acute kidney injury, delirium, LOS, and total hospital cost. Using an intra-aortic balloon pump, percutaneous left ventricular assist device, durable left ventricular assist device, or extracorporeal membranous oxygenation defines MCS. We calculated total hospital cost by multiplying the given total hospital charge with the cost-to-charge ratios available in cost-to-charge files on an ancillary website of HCUP [20].
2.4. Statistical Analysis
We applied hospital-level discharge weights to all entries when performing all statistical analyses to produce national estimates. We used the chi-square and Kruskal–Wallis H tests to compare categorical and continuous covariates in the baseline characteristics, respectively. To select covariates included in statistical adjustment, we first examined all baseline characteristics in a correlation matrix to confirm that no two covariates had a Pearson correlation coefficient above 0.80 (Table S3). Second, we confirmed that all covariates had a variance inflation factor below 3 and a tolerance value above 0.1. Third, we double-checked the absence of multicollinearity in an eigensystem analysis of covariance. After resolving multicollinearity, we inserted all covariates in a multivariable logistic regression model comparing non-AMI-CS admissions with and without frailty and identified significant covariates by stepwise selection. Age, sex, smoking, diabetes mellitus, hyperlipidemia, obesity, heart failure, chronic ischemic heart disease, valvular heart disease, previous percutaneous coronary intervention, previous coronary artery bypass grafting, previous stroke, previous pacemaker, chronic obstructive pulmonary disease, pulmonary hypertension, end-stage renal disease, deficiency anemia, malnutrition, major depression, and weekend admission were used to adjust all statistical models.
We used univariable and multivariable logistic regression to produce crude odds ratios and adjust odds ratios (aOR), respectively, to compare binary outcomes. We used linear regression when comparing secondary outcomes, such as LOS and total hospital cost. We performed a subgroup analysis stratified to younger (age 18–64) and older (age ≥ 65) adults. All statistical tests were two-sided, and p-values < 0.05 were considered significant. Data curation and all statistical analyses were performed using SAS, version 9.4 (SAS Institute, Cary, NC, USA). Figure production was assisted by R version 4.0.5 (R Foundation for Statistical Computing, Vienna, Austria).
3. Results
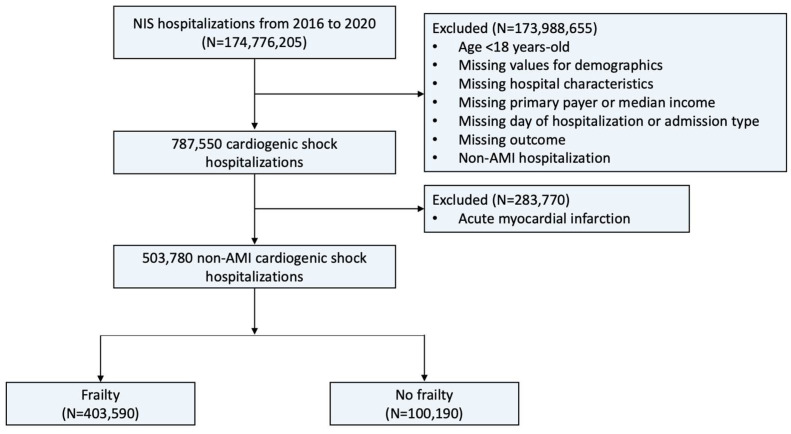
A total of 503,780 admissions for non-AMI-CS were identified (Figure 1). Most (80.1%) occurred in the frail, while a minority (19.9%) occurred in the non-frail. The frail group had a higher median age compared with the non-frail group (68 vs. 65, p < 0.001) (Table 1). The percentage of hospitalizations for patients classified as Black was greater in the frail group (19.8% vs. 17.7%, p < 0.001). The frail group had a higher prevalence of diabetes mellitus, heart failure, chronic ischemic heart disease, atrial fibrillation, previous stroke, chronic kidney disease, malnutrition, and dementia but a lower prevalence of hypertension.
Figure 1.
Flowchart of this study. Description: The flowchart illustrates the patient selection process used in this study. Abbreviations: AMI, acute myocardial infarction; NIS, National Inpatient Sample.
Table 1.
Baseline characteristics of non-AMI-CS admissions with and without frailty.
| Frailty (+) | Frailty (−) | p-Value | |
|---|---|---|---|
| Number of admissions | 403,590 | 100,190 | |
| Male sex (%) | 61.1 | 63.3 | <0.001 |
| Age, mean (Q1–Q3), years | 68 (57–77) | 65 (54–73) | <0.001 |
| Race (%) | <0.001 | ||
| White | 64.9 | 67.1 | |
| Black | 19.8 | 17.7 | |
| Hispanic | 8.7 | 8.6 | |
| Asian | 3.0 | 3.0 | |
| AI/AN | 0.6 | 0.7 | |
| Other | 3.0 | 3.0 | |
| Comorbidities (%) | |||
| Smoking | 33.3 | 33.6 | 0.442 |
| Hypertension | 11.8 | 20.2 | <0.001 |
| Diabetes mellitus | 38.6 | 34.5 | <0.001 |
| Hyperlipidemia | 39.2 | 45.2 | <0.001 |
| Obesity | 18.2 | 18.1 | 0.761 |
| Heart failure | 71.7 | 63.2 | <0.001 |
| Chronic ischemic heart disease | 17.5 | 15.6 | <0.001 |
| Atrial fibrillation | 45.1 | 38.5 | <0.001 |
| Valvular heart disease | 13.0 | 15.4 | <0.001 |
| Peripheral artery disease | 6.9 | 6.1 | <0.001 |
| Previous PCI | 0.9 | 1.3 | <0.001 |
| Previous CABG | 7.9 | 8.9 | <0.001 |
| Previous stroke | 10.0 | 7.3 | <0.001 |
| Previous pacemaker | 3.5 | 3.8 | 0.087 |
| COPD | 24.9 | 20.1 | <0.001 |
| Pulmonary hypertension | 20.8 | 19.6 | <0.001 |
| Chronic kidney disease | 47.7 | 22.5 | <0.001 |
| End-stage renal disease | 9.8 | 4.0 | <0.001 |
| Liver cirrhosis | 8.0 | 5.9 | <0.001 |
| History of malignancy | 7.3 | 8.1 | <0.001 |
| Deficiency anemia | 7.4 | 5.9 | <0.001 |
| Malnutrition | 17.3 | 8.8 | <0.001 |
| Dementia | 6.6 | 0.9 | <0.001 |
| Major depression | 0.9 | 0.8 | 0.173 |
| HFRS, median (Q1-Q3) | 8.8 (7.0–11.3) | 3.4 (2.2–4.2) | <0.001 |
| Hospital characteristics (%) | |||
| Hospital region | <0.001 | ||
| Northwest | 16.5 | 19.8 | |
| Midwest | 22.8 | 19.5 | |
| South | 40.1 | 41.5 | |
| West | 20.7 | 19.2 | |
| Hospital bed size | <0.001 | ||
| Small | 12.9 | 11.9 | |
| Medium | 23.6 | 22.2 | |
| Large | 63.5 | 65.9 | |
| Urban location | <0.001 | ||
| Rural | 3.5 | 3.7 | |
| Urban non-teaching | 14.5 | 13.4 | |
| Urban teaching | 82.0 | 82.9 | |
| Primary payer (%) | <0.001 | ||
| Medicare | 63.5 | 53.6 | |
| Medicaid | 13.1 | 14.1 | |
| Private insurance | 17.9 | 25.5 | |
| Self-pay | 2.9 | 3.5 | |
| No charge | 0.2 | 0.3 | |
| Others | 2.6 | 3.1 | |
| Median income (%) | 0.002 | ||
| Quartile 1 | 31.5 | 30.2 | |
| Quartile 2 | 26.1 | 26.9 | |
| Quartile 3 | 23.2 | 23.4 | |
| Quartile 4 | 19.1 | 19.5 | |
| Day of admission (%) | <0.001 | ||
| Weekday | 77.2 | 81.9 | |
| Weekend | 22.8 | 18.1 | |
Abbreviations: AI/AN, American Indian/Alaska Native; AMI, acute myocardial infarction; CABG, coronary artery bypass graft; COPD, chronic obstructive pulmonary disease; CS, cardiogenic shock; HFRS, hospital frailty risk score; PCI, percutaneous coronary intervention; Q, quartile.
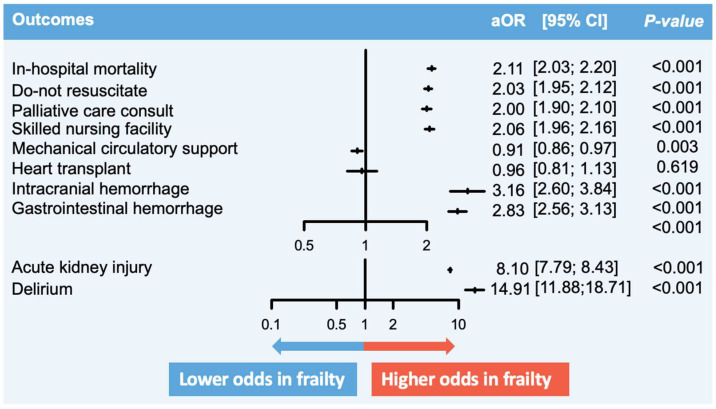
In admissions for non-AMI-CS, in-hospital death occurred in 35.1% of the frail group compared to 20.4% in the non-frail group (Table 2). Frailty was associated with significantly higher odds of in-hospital mortality (aOR 2.11, 95% confidence interval [CI] 2.03–2.20, p < 0.001). The presence of frailty was also associated with higher odds of receiving a palliative care consultation (aOR 2.00, 95% CI 1.90–2.10, p < 0.001) and having a DNR order placed (aOR 2.03, 95% CI 1.95–2.12, p < 0.001). Frailty had higher odds of disposition to a skilled nursing facility (aOR 2.06, 95% CI 1.96–2.16, p < 0.001). It had lower odds of being managed with MCS (aOR 0.91, 95% CI 0.86–0.97, p = 0.003). No significant difference in the odds of heart transplant was observed (aOR 0.96, 95% CI 0.81–1.13, p = 0.619). Frailty was associated with higher in-hospital morbidities, including intracerebral hemorrhage, gastrointestinal hemorrhage, acute kidney injury, and delirium. The LOS and total hospital cost were significantly higher in the frail. No significant difference was seen between univariable and multivariable models (Figure 2).
Table 2.
Comparison of outcomes in non-AMI-CS with and without frailty.
| Outcome | Frailty (+) | Frailty (−) | Crude Odds Ratio | p-Value | Adjusted Odds Ratio a | p-Value |
|---|---|---|---|---|---|---|
| In-hospital mortality (%) | 35.1 | 20.4 | 2.11 (2.03–2.20) | <0.001 | 2.11 (2.03–2.20) | <0.001 |
| Do not resuscitate (%) | 30.2 | 16.2 | 2.24 (2.14–2.33) | <0.001 | 2.03 (1.95–2.12) | <0.001 |
| Palliative care consultation (%) | 23.3 | 12.1 | 2.21 (2.10–2.31) | <0.001 | 2.00 (1.90–2.10) | <0.001 |
| Skilled nursing facility (%) | 26.2 | 12.9 | 2.39 (2.28–2.51) | <0.001 | 2.06 (1.96–2.16) | <0.001 |
| MCS (%) | 11.2 | 13.4 | 0.82 (0.77–0.87) | <0.001 | 0.91 (0.86–0.97) | 0.003 |
| Heart transplant (%) | 1.3 | 1.5 | 0.88 (0.75–1.04) | 0.125 | 0.96 (0.81–1.13) | 0.619 |
| Intracranial hemorrhage (%) | 1.7 | 0.6 | 2.98 (2.46–3.61) | <0.001 | 3.16 (2.60–3.84) | <0.001 |
| Gastrointestinal hemorrhage (%) | 6.8 | 2.3 | 3.05 (2.76–3.37) | <0.001 | 2.83 (2.56–3.13) | <0.001 |
| Acute kidney injury (%) | 73.1 | 29.2 | 6.59 (6.35–6.84) | <0.001 | 8.10 (7.79–8.43) | <0.001 |
| Delirium (%) | 5.8 | 0.4 | 15.92 (12.69–19.97) | <0.001 | 14.91 (11.88–18.71) | <0.001 |
| Length of stay (days ± SD) | 13.1 ± 15.6 | 8.4 ± 10.1 | 4.77 (4.55–5.00) b | <0.001 | 4.12 (3.90–4.33) c | <0.001 |
| Total hospital cost (USD ± SD) | 60,777 ± 88,028 | 42,792 ± 59,142 | 17,985 (16,699–19,270) b | <0.001 | 17,277 (16,031–18,522) c | <0.001 |
a Adjusted for age, sex, smoking, diabetes mellitus, hyperlipidemia, obesity, heart failure, chronic ischemic heart disease, valvular heart disease, previous PCI, previous CABG, previous stroke, previous pacemaker, chronic obstructive pulmonary disease, pulmonary hypertension, end-stage renal disease, deficiency anemia, malnutrition, major depression, and weekend admission. b Crude mean difference with 95% confidence interval. c Adjusted mean difference with 95% confidence interval. Abbreviations: AMI, acute myocardial infarction; CABG, coronary artery bypass graft; CS, cardiogenic shock; MCS, mechanical circulatory support; SD, standard deviation.
Figure 2.
Comparison of in-hospital outcomes between non-AMI-CS in frailty versus no frailty. Description: The figure summarizes the key findings of this study. The vertical lines represent the aOR, while the perpendicular horizontal lines represent the 95% CI. aOR > 1 signifies that the odds of the particular outcome are higher in AMI-CS hospitalizations with frailty, and vice versa. Abbreviations: AMI, acute myocardial infarction; aOR, adjusted odds ratio; CI, confidence interval; CS, cardiogenic shock.
Stratification to age cut-off of 65 years showed that 216,375 (42.9%) non-AMI-CS occurred in younger adults, while 287,405 (57.1%) occurred in older adults. Although the prevalence of frailty was high regardless of age group, it was lower in younger adults compared with older adults (76.9% vs. 82.5%, p < 0.001). The results were largely similar in younger adults, except for the odds of MCS, which were not different between the frail and non-frail (aOR 1.05, 95% CI 0.97–1.12, p < 0.001) (Table S3). Similar results were seen in the older population, in which frailty was associated with higher odds of in-hospital mortality, DNR, palliative care consult, skilled nursing facility, heart transplant, intracerebral hemorrhage, gastrointestinal hemorrhage, acute kidney injury, and delirium, but lower odds of MCS.
4. Discussion
In this national retrospective analysis, we aimed to explore the association between frailty and non-AMI-CS hospitalizations. Our findings highlight an 80% prevalence of frailty, coupled with in-hospital morbidity and mortality. The frail subset also demonstrated a clear propensity for multi-morbidity (≥2 chronic illnesses). Importantly, frailty was linked to a reduced likelihood of cardiac interventions, notably MCS, but with no impact on cardiac transplantation rates.
Frailty’s impact on prognostic outcomes has been extensively explored in acute cardiovascular diseases such as AMI and decompensated heart failure. However, our understanding of frailty in the non-AMI-CS population remains limited [12]. This topic is important due to the high rates of frailty in individuals with cardiovascular diseases. The rates vary widely, ranging from 12.6% to 70%. These variations are likely attributable to differences in diagnostic tools, the absence of frailty assessment, and variations in acute cardiovascular conditions and baseline characteristics. Notably, this current study revealed a staggering 80% prevalence of frailty in non-AMI-CS [21,22]. This elevated prevalence and the associated heightened mortality risk emphasizes the imperative for standardized frailty assessments, facilitating tailored interventions and care discussions in clinical practice and patient-centered decision making [1,23].
It comes as no surprise that frailty was associated with a higher incidence of in-hospital complications and increased mortality, similar to prior studies [2,21,22,24]. This propensity is likely related to the presence of multi-morbidity, including diabetes, hypertension, heart failure, coronary artery disease, peripheral artery disease, and valvular dysfunction [25]. These comorbid conditions can potentially compromise physical activity and cognitive function. Consequently, such patients are rendered more vulnerable to stressors, with their capacity to rebound from these stressors potentially impaired [26,27]. In addition, frailty is intricately linked to hemodynamic alterations, subclinical vascular modifications, and autonomic dysfunction, further complicating the intricate hemodynamic balance and potentially altering compensatory mechanisms during acute decompensation as in CS [28,29]. Moreover, frail individuals often have diminished independence, often present late, and are more likely to be managed conservatively [30,31,32]. Lastly, there are higher in-hospital complications, such as delirium, which could prolong and lead to long-term sequelae (physical and psychological) [33,34]. Therefore, frail hospitalizations are often complicated with higher in-hospital mortality and morbidity, raising the question of whether frailty hospitalizations should be approached differently.
This study highlights that frailty is not exclusive to octogenarians and can manifest in a younger subset of patients. The mean age for those hospitalized with frailty was 66.1 years, necessitating age differentiation from frailty [35,36]. Furthermore, in line with existing evidence, frail adults are less likely to receive invasive interventions and evidence-based strategies, including MCS and intensive medical therapy [37,38]. For instance, in a post hoc analysis from the GUIDE-IT trial, those with a high frailty burden and heart failure were less likely to achieve goal-directed medical therapy [39]. Despite ongoing uncertainty surrounding the benefits of intensive strategies in this population, compounded by potential risks and complications, identifying a specific subset of the frail who may benefit from more intensive approaches remains an unmet challenge. Developing a targeted approach for using MCS in this patient population may result in improved clinical outcomes and minimize potential complications. Alternatively, identifying patients who are highly unlikely to benefit from MCS under any circumstance could lead providers to have earlier discussions about the goals of care with patients [40,41]. Lastly, this study found no differences in cardiac transplant rates between frail and non-frail groups. This is likely because frail individuals are prioritized in the national emergency priority system due to their critical condition. Observational data showed shorter waiting times for frail patients (0.6 years vs. 0.2 years) [42].
Several limitations should be acknowledged in this retrospective study. This observational, administrative data analysis relies on diagnosis codes and the associated coding errors related to institutional practice or individual bias. Reliance on administrative data lacks clinical information related to the severity and duration of preceding frailty and the CS state. Moreover, although AMI was excluded, the etiology of non-AMI-CS was unknown due to the limitations of the database. Additionally, the severity of comorbidities such as diabetes and hypertension are unknown, which could also impact frailty differently. While hospital frailty risk scores have been validated and have demonstrated a fair to moderate overlap with the Fried and Rockwood scales, it is important to note that comorbidities can influence the score rather than frailty alone. Each entry provided in the NIS consists of hospitalizations and not patients, so our findings should be interpreted at the hospitalization level and after considering that the same patient can be included more than once, given the absence of patient-level identifiers. Lastly, the specifics of shared decision making regarding invasive or conservative therapy are unknown, posing a potential risk of selection bias.
5. Conclusions
In this nationally representative sample of hospitalizations for non-AMI-CS, frailty emerged as a noteworthy factor associated with elevated mortality rates and in-hospital complications. Hospitalizations characterized by frailty were less likely to undergo invasive interventions. Further studies are needed to investigate the potential benefits of interventions targeting frailty in this patient population.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm13072078/s1, Table S1: ICD-10-CM codes used to calculate the Hospital Frailty Risk Score; Table S2: List of the ICD-10-CM codes used, Table S3: Subgroup analysis according to younger (age < 65 years) and older (≥65 years) patients.
Author Contributions
D.Y.P.: conceptualization, project administration, supervision, data curation, formal analysis, investigation, validation, methodology, resources, software, visualization, writing original draft, review, and editing. Y.J.: conceptualization, project administration, supervision, investigation, validation, methodology, resources, visualization, writing original draft, review, and editing. Y.A.: conceptualization, validation, writing—review and editing. T.C.: validation, writing—review and editing. H.B.B.: conceptualization, validation, writing—review and editing. N.S.: conceptualization, validation, writing—review and editing. G.B.: conceptualization, validation, writing—review and editing. C.D.: conceptualization, validation, writing—review and editing. S.V.R.: conceptualization, validation, writing—review and editing. A.A.D.: conceptualization, validation, writing—review and editing. M.G.N.: conceptualization, project administration, supervision, investigation, validation, methodology, resources, review, and editing. M.D.S.: conceptualization, project administration, supervision, investigation, validation, methodology, resources, review, and editing. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The NIS protects patient confidentiality by excluding State and hospital identifiers, thereby guaranteeing anonymity, and because this strictly deidentified nature of the database, our study was exempt from the purview of our institutional review board.
Informed Consent Statement
Not applicable.
Data Availability Statement
The NIS is openly available and can be accessed through the public website of the HCUP.
Conflicts of Interest
Nanna MG: Dr. Nanna reports current research support from the American College of Cardiology Foundation supported by the George F. and Ann Harris Bellows Foundation, the Patient-Centered Outcomes Research Institute (PCORI), the Yale Claude D. Pepper Older Americans Independence Center (P30AG021342), and the National Institute on Aging/National Institutes of Health from R03AG074067 (GEMSSTAR award). Dr. Nanna also reports being a consultant for Heartflow and Merck. Coles T: Dr. Coles has research funding from Pfizer and Merck. Bosworth HB: Hayden Bosworth reports research funding through his institution from BeBetter Therapeutics, Boehringer Ingelheim, Esperion, Improved Patient Outcomes, Merck, NHLBI, Novo Nordisk, Otsuka, Sanofi, Veterans Administration, Elton John Foundation, Hilton Foundation, Pfizer. He also provides consulting services for Abbott, Esperion, Imatar, Novartis, Sanofi, Vidya, Walmart, and Webmed. He was also on the board of directors of Preventric Diagnostics. Ahmad Y: Dr. Ahmad is a Consultant for Cardiovascular Systems Inc. and Shockwave and serves on the Medical Advisory Board of Boston Scientific. Damluji AA: Dr. Damluji receives research funding from (1) the Pepper Scholars Program of the Johns Hopkins University Claude D. Pepper Older Americans Independence Center funded by the National Institute on Aging P30-AG021334; (2) mentored patient-oriented research career development award from the National Heart, Lung, and Blood Institute K23-HL153771; (3) The NIH National Institute of Aging R01-AG078153; (4) the Patient-Centered Outcomes Research Institute (PCORI). The rest of the authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Walsh B., Fogg C., Harris S., Roderick P., de Lusignan S., England T., Clegg A., Brailsford S., Fraser S.D. Frailty transitions and prevalence in an ageing population: Longitudinal analysis of primary care data from an open cohort of adults aged 50 and over in England, 2006–2017. Age Ageing. 2023;52:afad058. doi: 10.1093/ageing/afad058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ijaz N., Buta B., Xue Q.-L., Mohess D.T., Bushan A., Tran H., Batchelor W., deFilippi C.R., Walston J.D., Bandeen-Roche K., et al. Interventions for Frailty Among Older Adults With Cardiovascular Disease. J. Am. Coll. Cardiol. 2022;79:482–503. doi: 10.1016/j.jacc.2021.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quinlan N., Marcantonio E.R., Inouye S.K., Gill T.M., Kamholz B., Rudolph J.L. Vulnerability: The crossroads of frailty and delirium. J. Am. Geriatr. Soc. 2011;59((Suppl. S2)):S262–S268. doi: 10.1111/j.1532-5415.2011.03674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Damluji A.A., Chung S.-E., Xue Q.-L., Hasan R.K., Moscucci M., Forman D.E., Bandeen-Roche K., Batchelor W., Walston J.D., Resar J.R., et al. Frailty and cardiovascular outcomes in the National Health and Aging Trends Study. Eur. Heart J. 2021;42:3856–3865. doi: 10.1093/eurheartj/ehab468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Damluji A.A., Cohen M.G. The Influence of Frailty on Cardiovascular Disease: The Time for a “Frailty Academic Research Consortium” Is Now! Circ. Cardiovasc. Interv. 2022;15:e011669. doi: 10.1161/CIRCINTERVENTIONS.121.011669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vahdatpour C., Collins D., Goldberg S. Cardiogenic Shock. J. Am. Heart Assoc. 2019;8:e011991. doi: 10.1161/JAHA.119.011991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Damluji A.A., Alfaraidhy M., AlHajri N., Rohant N.N., Kumar M., Al Malouf C., Bahrainy S., Ji Kwak M., Batchelor W.B., Forman D.E., et al. Sarcopenia and Cardiovascular Diseases. Circulation. 2023;147:1534–1553. doi: 10.1161/CIRCULATIONAHA.123.064071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Papolos A.I., Kenigsberg B.B., Berg D.D., Alviar C.L., Bohula E., Burke J.A., Carnicelli A.P., Chaudhry S.-P., Drakos S., Gerber D.A., et al. Management and Outcomes of Cardiogenic Shock in Cardiac ICUs With Versus Without Shock Teams. J. Am. Coll. Cardiol. 2021;78:1309–1317. doi: 10.1016/j.jacc.2021.07.044. [DOI] [PubMed] [Google Scholar]
- 9.Berg D.D., Bohula E.A., Morrow D.A. Epidemiology and causes of cardiogenic shock. Curr. Opin. Crit. Care. 2021;27:401–408. doi: 10.1097/MCC.0000000000000845. [DOI] [PubMed] [Google Scholar]
- 10.Berg D.D., Bohula E.A., Diepen S.v., Katz J.N., Alviar C.L., Baird-Zars V.M., Barnett C.F., Barsness G.W., Burke J.A., Cremer P.C., et al. Epidemiology of Shock in Contemporary Cardiac Intensive Care Units. Circ. Cardiovasc. Qual. Outcomes. 2019;12:e005618. doi: 10.1161/CIRCOUTCOMES.119.005618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thiele H., Ohman E.M., Desch S., Eitel I., de Waha S. Management of cardiogenic shock. Eur. Heart J. 2015;36:1223–1230. doi: 10.1093/eurheartj/ehv051. [DOI] [PubMed] [Google Scholar]
- 12.Walker D.M., Gale C.P., Lip G., Martin-Sanchez F.J., McIntyre H.F., Mueller C., Price S., Sanchis J., Vidan M.T., Wilkinson C., et al. Editor’s Choice—Frailty and the management of patients with acute cardiovascular disease: A position paper from the Acute Cardiovascular Care Association. Eur. Heart J. Acute Cardiovasc. Care. 2018;7:176–193. doi: 10.1177/2048872618758931. [DOI] [PubMed] [Google Scholar]
- 13.HCUP Databases. Healthcare Cost and Utilization Project (HCUP) [(accessed on 28 November 2022)];2022 Available online: https://www.hcup-us.ahrq.gov/nisoverview.jsp.
- 14.Damluji A.A., Bandeen-Roche K., Berkower C., Boyd C.M., Al-Damluji M.S., Cohen M.G., Forman D.E., Chaudhary R., Gerstenblith G., Walston J.D., et al. Percutaneous Coronary Intervention in Older Patients With ST-Segment Elevation Myocardial Infarction and Cardiogenic Shock. J. Am. Coll. Cardiol. 2019;73:1890–1900. doi: 10.1016/j.jacc.2019.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilbert T., Neuburger J., Kraindler J., Keeble E., Smith P., Ariti C., Arora S., Street A., Parker S., Roberts H.C., et al. Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: An observational study. Lancet. 2018;391:1775–1782. doi: 10.1016/S0140-6736(18)30668-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McAlister F.A., Savu A., Ezekowitz J.A., Armstrong P.W., Kaul P. The hospital frailty risk score in patients with heart failure is strongly associated with outcomes but less so with pharmacotherapy. J. Intern. Med. 2020;287:322–332. doi: 10.1111/joim.13002. [DOI] [PubMed] [Google Scholar]
- 17.Orlandi M., Dover D.C., Sandhu R.K., Hawkins N.M., Kaul P., McAlister F.A. The Introduction of Direct Oral Anticoagulants Has Not Resolved Treatment Gaps for Frail Patients With Nonvalvular Atrial Fibrillation. Can. J. Cardiol. 2022;38:77–84. doi: 10.1016/j.cjca.2021.09.021. [DOI] [PubMed] [Google Scholar]
- 18.Rottler M., Ocskay K., Sipos Z., Görbe A., Virág M., Hegyi P., Molnár T., Erőss B., Leiner T., Molnár Z. Clinical Frailty Scale (CFS) indicated frailty is associated with increased in-hospital and 30-day mortality in COVID-19 patients: A systematic review and meta-analysis. Ann. Intensive Care. 2022;12:17. doi: 10.1186/s13613-021-00977-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eckart A., Hauser S.I., Haubitz S., Struja T., Kutz A., Koch D., Neeser O., Meier M.A., Mueller B., Schuetz P. Validation of the hospital frailty risk score in a tertiary care hospital in Switzerland: Results of a prospective, observational study. BMJ Open. 2019;9:e026923. doi: 10.1136/bmjopen-2018-026923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cost-to-Charge Ratio for Inpatient Files. Healthcare Cost and Utilization Project (HCUP) [(accessed on 28 November 2022)];2022 Available online: https://www.hcup-us.ahrq.gov/db/ccr/ip-ccr/ip-ccr.jsp.
- 21.Ekerstad N., Javadzadeh D., Alexander K.P., Bergström O., Eurenius L., Fredrikson M., Gudnadottir G., Held C., Ängerud K.H., Jahjah R., et al. Clinical Frailty Scale classes are independently associated with 6-month mortality for patients after acute myocardial infarction. Eur. Heart J. Acute Cardiovasc. Care. 2022;11:89–98. doi: 10.1093/ehjacc/zuab114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Volle K., Delmas C., Ferrières J., Toulza O., Blanco S., Lairez O., Lhermusier T., Biendel C., Galinier M., Carrié D., et al. Prevalence and Prognosis Impact of Frailty Among Older Adults in Cardiac Intensive Care Units. CJC Open. 2021;3:1010–1018. doi: 10.1016/j.cjco.2021.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jain R., Duval S., Adabag S. How Accurate Is the Eyeball Test? Circ. Cardiovasc. Qual. Outcomes. 2014;7:151–156. doi: 10.1161/CIRCOUTCOMES.113.000329. [DOI] [PubMed] [Google Scholar]
- 24.Rockwood K., Howlett S.E., MacKnight C., Beattie B.L., Bergman H., Hébert R., Hogan D.B., Wolfson C., McDowell I. Prevalence, attributes, and outcomes of fitness and frailty in community-dwelling older adults: Report from the Canadian study of health and aging. J. Gerontol. A Biol. Sci. Med. Sci. 2004;59:1310–1317. doi: 10.1093/gerona/59.12.1310. [DOI] [PubMed] [Google Scholar]
- 25.Hongisto M., Lassus J., Tarvasmäki T., Sionis A., Sans-Rosello J., Tolppanen H., Kataja A., Jäntti T., Sabell T., Lindholm M.G., et al. Mortality risk prediction in elderly patients with cardiogenic shock: Results from the CardShock study. ESC Heart Fail. 2021;8:1398–1407. doi: 10.1002/ehf2.13224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vetrano D.L., Palmer K., Marengoni A., Marzetti E., Lattanzio F., Roller-Wirnsberger R., Lopez Samaniego L., Rodríguez-Mañas L., Bernabei R., Onder G. Frailty and Multimorbidity: A Systematic Review and Meta-analysis. J. Gerontol. Ser. A. 2018;74:659–666. doi: 10.1093/gerona/gly110. [DOI] [PubMed] [Google Scholar]
- 27.Afilalo J., Lauck S., Kim D.H., Lefèvre T., Piazza N., Lachapelle K., Martucci G., Lamy A., Labinaz M., Peterson M.D., et al. Frailty in Older Adults Undergoing Aortic Valve Replacement. J. Am. Coll. Cardiol. 2017;70:689–700. doi: 10.1016/j.jacc.2017.06.024. [DOI] [PubMed] [Google Scholar]
- 28.Lujic S., Randall D.A., Simpson J.M., Falster M.O., Jorm L.R. Interaction effects of multimorbidity and frailty on adverse health outcomes in elderly hospitalised patients. Sci. Rep. 2022;12:14139. doi: 10.1038/s41598-022-18346-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Docherty N.G., Delles C., D’Haese P., Layton A.T., Martínez-Salgado C., Vervaet B.A., López-Hernández F.J. Haemodynamic frailty—A risk factor for acute kidney injury in the elderly. Ageing Res. Rev. 2021;70:101408. doi: 10.1016/j.arr.2021.101408. [DOI] [PubMed] [Google Scholar]
- 30.Debain A., Loosveldt F.A., Knoop V., Costenoble A., Lieten S., Petrovic M., Bautmans I. Frail older adults are more likely to have autonomic dysfunction: A systematic review and meta-analysis. Ageing Res. Rev. 2023;87:101925. doi: 10.1016/j.arr.2023.101925. [DOI] [PubMed] [Google Scholar]
- 31.Sanchis J., Bueno H., Miñana G., Guerrero C., Martí D., Martínez-Sellés M., Domínguez-Pérez L., Díez-Villanueva P., Barrabés J.A., Marín F., et al. Effect of Routine Invasive vs Conservative Strategy in Older Adults With Frailty and Non-ST-Segment Elevation Acute Myocardial Infarction: A Randomized Clinical Trial. JAMA Intern. Med. 2023;183:407–415. doi: 10.1001/jamainternmed.2023.0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dodson J.A., Hochman J.S., Roe M.T., Chen A.Y., Chaudhry S.I., Katz S., Zhong H., Radford M.J., Udell J.A., Bagai A., et al. The Association of Frailty With In-Hospital Bleeding Among Older Adults With Acute Myocardial Infarction. JACC Cardiovasc. Interv. 2018;11:2287–2296. doi: 10.1016/j.jcin.2018.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boyer S., Trimouillas J., Cardinaud N., Gayot C., Laubarie-Mouret C., Dumoitier N., Rudelle K., Druet-Cabanac M., Laroche M.-L., Tchalla A. Frailty and functional dependence in older population: Lessons from the FREEDOM Limousin—Nouvelle Aquitaine Cohort Study. BMC Geriatr. 2022;22:128. doi: 10.1186/s12877-022-02834-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Noriega F.J., Vidán M.T., Sánchez E., Díaz A., Serra-Rexach J.A., Fernández-Avilés F., Bueno H. Incidence and impact of delirium on clinical and functional outcomes in older patients hospitalized for acute cardiac diseases. Am. Heart J. 2015;170:938–944. doi: 10.1016/j.ahj.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 35.Pauley E., Lishmanov A., Schumann S., Gala G.J., van Diepen S., Katz J.N. Delirium is a robust predictor of morbidity and mortality among critically ill patients treated in the cardiac intensive care unit. Am. Heart J. 2015;170:79–86.e1. doi: 10.1016/j.ahj.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 36.Loecker C., Schmaderer M., Zimmerman L. Frailty in Young and Middle-Aged Adults: An Integrative Review. J. Frailty Aging. 2021;10:327–333. doi: 10.14283/jfa.2021.14. [DOI] [PubMed] [Google Scholar]
- 37.Hanlon P., Blane D.N., Macdonald S., Mair F.S., O’Donnell C.A. Our response to rising frailty in younger people must address prevention burden. Lancet Healthy Longev. 2021;2:e245. doi: 10.1016/S2666-7568(21)00052-0. [DOI] [PubMed] [Google Scholar]
- 38.Matsuzawa Y., Konishi M., Akiyama E., Suzuki H., Nakayama N., Kiyokuni M., Sumita S., Ebina T., Kosuge M., Hibi K., et al. Association between gait speed as a measure of frailty and risk of cardiovascular events after myocardial infarction. J. Am. Coll. Cardiol. 2013;61:1964–1972. doi: 10.1016/j.jacc.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 39.Damluji A.A., Forman D.E., Wang T.Y., Chikwe J., Kunadian V., Rich M.W., Young B.A., Page R.L., DeVon H.A., Alexander K.P. Management of Acute Coronary Syndrome in the Older Adult Population: A Scientific Statement From the American Heart Association. Circulation. 2023;147:e32–e62. doi: 10.1161/CIR.0000000000001112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khan M.S., Segar M.W., Usman M.S., Singh S., Greene S.J., Fonarow G.C., Anker S.D., Felker G.M., Januzzi J.L., Butler J., et al. Frailty, Guideline-Directed Medical Therapy, and Outcomes in HFrEF: From the GUIDE-IT Trial. JACC Heart Fail. 2022;10:266–275. doi: 10.1016/j.jchf.2021.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murali-Krishnan R., Iqbal J., Rowe R., Hatem E., Parviz Y., Richardson J., Sultan A., Gunn J. Impact of frailty on outcomes after percutaneous coronary intervention: A prospective cohort study. Open Heart. 2015;2:e000294. doi: 10.1136/openhrt-2015-000294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muthiah K., Wilhelm K., Robson D., Raju H., Aili S.R., Jha S.R., Pierce R., Fritis-Lamora R., Montgomery E., Gorrie N., et al. Impact of frailty on mortality and morbidity in bridge to transplant recipients of contemporary durable mechanical circulatory support. J. Heart Lung Transplant. 2022;41:829–839. doi: 10.1016/j.healun.2022.02.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The NIS is openly available and can be accessed through the public website of the HCUP.