Abstract
Mouse AIDS (MAIDS) induced in C57BL/6 mice by infection with a replication-defective retrovirus (Du5H) combines extensive lymphoproliferation and profound immunodeficiency. Although B cells are the main target of viral infection, recent research has focused on CD4+ T cells, the activation of which is a key event in MAIDS induction and progression. A preliminary observation of increased expression of B7 molecules on B cells in MAIDS prompted us to address the possible involvement of the CD28/B7 costimulatory pathway in MAIDS. Mice infected with the MAIDS-inducing viral preparation were treated with murine fusion protein CTLA4Ig (3 × 50 μg/week given intraperitoneally), a competitive inhibitor of physiological CD28-B7 interactions. In CTLA4Ig-treated animals, the onset of the disease was delayed, lymphoproliferation progressed at a much slower rate than in untreated mice, and the loss of in vitro responsiveness to mitogens was reduced. Relative expression of Du5H did not differ between treated and untreated animals. These results suggest that the CD28/B7 costimulatory pathway contributes to MAIDS development.
Mouse AIDS (MAIDS) is induced by murine leukemia viruses present in a virus mixture recovered from a radiation-induced lymphoma of C57BL/6 mice (28). The pathogenic agent is a replication-defective retrovirus, designated BM5def or Du5H, with a single open reading frame that encodes a mutant Pr60gag protein (3). The syndrome is characterized by rapid and persistent proliferation of B and CD4+ T cells, hypergammaglobulinemia, phenotypic abnormalities of lymphocyte subsets, and increasingly severe defects in both cell-mediated and humoral immunity.
MAIDS pathogenesis clearly implies crucial interactions between B- and T-lymphocyte subsets: although B cells are the main target of the pathogenic retrovirus (17), development of the disease is strictly dependent on the presence of functional CD4+ T cells (41); chronic T-cell activation and induction of anergy are considered to be major histocompatibility complex class II antigen (Ag) dependent with virus-infected B cells acting as viral Ag-presenting cells (APC) (9).
The activation of CD4+ T lymphocytes requires two signals from the APC (1). Ligation of the T-cell-associated receptor (TCR) complex by Ag in association with the class II major histocompatibility complex determines the specificity of the response, while ligation of various accessory molecules on the T-cell surface acts as the second nonspecific costimulatory signal (27). One of the most potent costimulatory activating signals relies upon the interaction of surface TCR CD28 with its counterreceptors B7.1 (CD80) and B7.2 (CD86) on APC (5, 6, 11, 20, 25). Lymphocyte activation and regulation of immune responses partly proceed through a modulation of the level of expression of these counterreceptors. Activated T cells also express CTLA4 as a second receptor that binds avidly to both B7.1 and B7.2 (24); its contribution to costimulation is less well defined than that of CD28 (23). Increased surface expression of B7.1 and B7.2 has been detected on APC in response to various stimuli, including mitogens and cytokines (14).
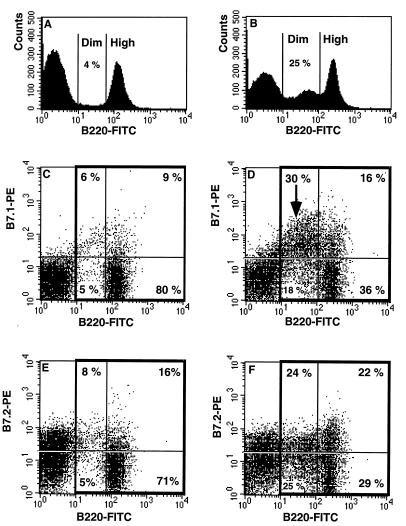
First, we examined whether B-cell expansion and activation in MAIDS are associated with an increased level of expression of B7 molecules. Two-color flow cytometry was performed to demonstrate B7.1 and B7.2 on B220+ spleen cells. Briefly, 106 cells were preincubated with 1 μg of an anti-FcγRII antibody (CD32) (Fc block; Pharmingen, San Diego, Calif.) prior to labeling with fluorescein isothiocyanate-labeled anti-B220 (RA3-682) and a biotin-conjugated anti-CD80 (B7.1) (16-10A1; monoclonal hamster immunoglobulin G [IgG]) antibodies or an anti-CD86 (B7.2) (GL1; monoclonal rat IgG2a kappa) antibody, all purchased from Pharmingen, and counterstaining with streptavidin-phycoerythrin. Individual suspensions from four controls and five mice with MAIDS (infected for 10 weeks) were analyzed. Enhanced expression of B7.1 and B7.2 was demonstrated on B cells from mice with MAIDS by comparison with uninfected controls (Fig. 1). The B7.1 molecule was detected on 15% of B220+ cells in controls, and this fraction rose to 46% in infected mice (Fig. 1C and D; P < 0.001). B7.2 had a higher basal level of expression than B7.1 and was detected on 24% of B220+ control splenocytes. In infected mice with MAIDS, there was a significant B7.2 upregulation that was detected on 46% of B220+ cells (Fig. 1E and F; P < 0.001).
FIG. 1.
MAIDS is associated with overexpression of B7 costimulatory molecules on B cells. The staining profile of SP cells from uninfected C57BL/6 mice (A, C, and E) and mice with MAIDS at 10 weeks postinfection (B, D, and F) are compared. These are representative results obtained from four or five mice analyzed separately in each group. MAIDS is associated with an expansion of B cells expressing B220 at low densities (B220dim cells), accounting for 25% of the lymphocyte population (B) versus 4% in controls (A). B220+ cells, contained within the bold boxes, were evaluated for B7.1 (C and D) and B7.2 (E and F) expression. B7.1 was expressed on 46% ± 1.22% of the B220+ cells in mice with MAIDS (D) versus 15% ± 0.75% of those in controls (C). In mice with MAIDS, overexpression of B7.1 was due mainly to expansion of the B7.1+ B220dim population (D, arrow). In controls (E), 24% ± 2.8% of the B220+ cells were B7.2+; in infected mice (F), this fraction rose to 46% ± 2.7%, equally distributed between B220dim and B220hi cells. FITC, fluorescein isothiocyanate.
Thereafter, we investigated the effect of a systemic blockade of CD28/B7 interactions by CTLA4Ig on MAIDS development. CTLA4Ig (prepared by Bristol-Myers Squibb Pharmaceutical Research Institute, Seattle, Wash.) is a fusion protein made up of the extracellular domain of CTLA4 and the Fc portion of a murine IgG that binds avidly to both B7.1 and B7.2 and acts as a soluble inhibitor of CD28-CTLA4/B7 interactions (26).
C57BL/6 mice were inoculated intraperitoneally (i.p.) at 4, 5, and 6 weeks of age with 0.5 ml of a viral preparation containing the Du5H virus pseudotyped with the nonpathogenic G6T2 helper RadLV, obtained as a culture supernatant of Du5H-transfected SIM.R fibroblasts chronically infected with G6T2 (16). The viral preparation was determined by XC plaque assay (34) to contain 10 × 103 PFU of ecotropic virus/ml. Treatment with CTLA4Ig (three i.p. injections of 50 μg weekly) was initiated 1 week before viral inoculation and continued throughout the observation period. Control infected and untreated mice received i.p. injections of phosphate-buffered saline instead of CTLA4Ig. Uninfected mice treated with CTLA4Ig were also examined.
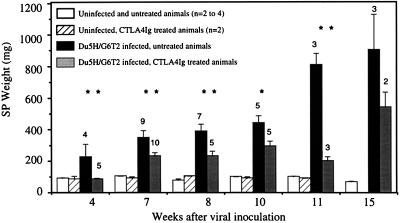
MAIDS is characterized by an early lymphoproliferative process which can be first approximated by recording spleen (SP) and lymph node (LN) weights. At different time points after viral inoculation (zero time was the first injection), we compared populations of infected mice having received CTLA4Ig or phosphate-buffered saline and corresponding uninfected controls (Fig. 2). Infected mice rapidly developed significant splenomegaly, with an SP weight averaging 2.5 times that of sham-inoculated controls at week 4. By contrast, none of the five infected mice undergoing CTLA4Ig treatment showed any increase in SP weight at that time. Nevertheless, at later time points, CTLA4Ig treatment did not prevent the occurrence of splenomegaly in infected animals, although its amplitude remained much lower than that of untreated counterparts. The inhibitory effect of CTLA4Ig treatment on MAIDS-associated lymphoproliferation was still very significant at week 11; at that time, the SP weights of infected, CTLA4Ig-treated mice were more than 50% lower than those of their untreated counterparts. The inhibitory effect of CTLA4Ig treatment on lymphadenopathy development was in the same range as that observed for splenomegaly (data not shown).
FIG. 2.
Systemic administration of CTLA4Ig inhibits MAIDS-associated lymphoproliferation. Each column represents the mean SP weight ± the standard error of the mean for each experimental group. The number of infected mice studied at each time point is indicated at the top of the corresponding column. Administration of CTLA4Ig to uninfected controls did not induce any variation in SP weight. In infected animals, CTLA4Ig administration delayed the onset of the lymphoproliferative process and significantly reduced its amplitude. Statistical significance of the differences between untreated and treated, infected groups was tested by a two-tailed Student t test or the Mann-Whitney test. ∗, P < 0.05; ∗∗, P < 0.025.
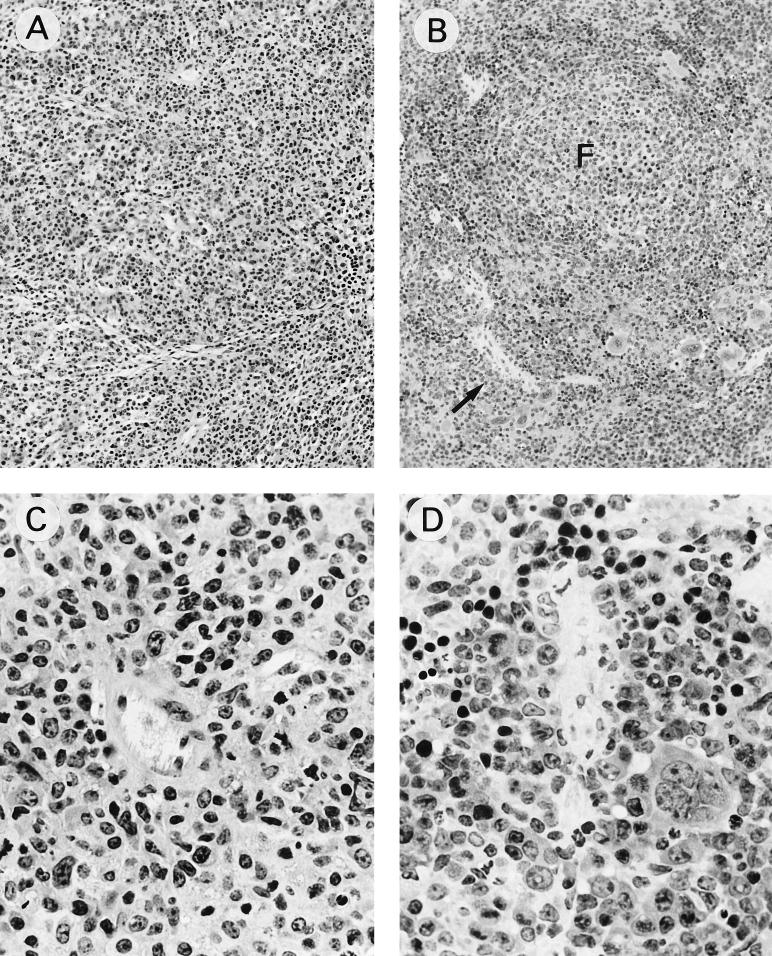
Histopathological modifications observed in the SP after Du5H/G6T2 infection were similar to those previously described after LP-BM5 infection (12); they comprised follicular activation and enlargement, together with a widening of periarteriolar lymphoid sheaths, due to the accumulation of blastic lymphoid cells, leading to progressive obliteration of the normal SP architecture. Paralleling the delayed occurrence of macroscopical splenomegaly or lymphadenopathy, the histological modifications in SPs from CTLA4Ig-treated, infected animals were delayed and encompassed a pattern of pathological changes similar to that observed in the untreated group (Fig. 3).
FIG. 3.
Histologic comparisons of SPs from untreated (A and C) or CTLA4Ig-treated (B and D) mice infected for 15 weeks. Tissue samples were fixed in 4% paraformaldehyde, embedded in glycolmethacrylate (JB Polyscience), and semithin sectioned before staining with hematoxylin and eosin. (A) SP from an untreated infected mouse showing obliteration of the white pulp-red pulp demarcation (magnification, ×100) due to diffuse infiltration by large, blastic cells displaying immunoblastic or plasmocytoid differentiation. (C) Several mitoses were observed (magnification, ×400). (B) SP from a CTLA4Ig-treated, infected animal showing white pulp expansion consisting of follicular enlargement (F) and widening of the adjacent periarteriolar lymphoid sheaths (arrow) (magnification, ×100). (D) Follicular and periarteriolar cells that are blastic, similar to those in untreated mice, and display high mitotic activity. Extension of the lymphoid infiltration around central arterioles is much less prominent than in panel C (magnification, ×400).
To measure the effect of CTLA4Ig treatment on MAIDS-associated immunodeficiency, proliferative responses of lymphocytes to different mitogens were monitored. Aliquots containing 2 × 105 LN or SP lymphocytes from individual healthy and experimental mice were cultured in triplicate in 96-well microtest plates for 72 h with concanavalin A (ConA; Boehringer, Mannheim, Germany) at 5 μg/ml or with lipopolysaccharide (LPS; Difco, Detroit, Mich.) at 10 μg/ml. During the last 4 h of culture, cells were incubated with 0.4 μCi of [3H]thymidine (6.7 Ci/mmol) (Dupont, NEN Products, Boston, Mass.) and collected with a cell harvester (Skatron, Sterling, Va.) onto glass fiber filters. The incorporated precursor was counted in a scintillation analyzer (Tri-Carb; Packard, Meriden, Conn.). Results were expressed as comparative proliferation indexes, calculated as the ratio of the specific proliferation (counts per minute of mitogen-stimulated culture minus counts per minute of unstimulated culture) of cells from infected mice to the specific proliferation of cells from uninfected controls.
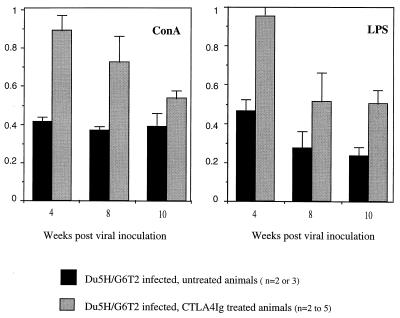
Figure 4 compares the proliferative responses of LN lymphocytes from treated and untreated, infected mice to ConA and LPS. Uninfected, CTLA4Ig-treated controls showed mitogen-induced expansion in the same range as that of untreated controls when absolute quantification (in counts per minute) was used. Significant anergy to both the B-cell (LPS) and T-cell (ConA) mitogens induced in infected mice was already noticed at week 4, with a response index of 40%, and changed little later on. In contrast, treatment with CTLA4Ig prevented the early anergy, which was manifest only from week 8, with response indexes intermediate between those of the control and infected groups. Studies performed on splenocytes led to similar observations. The beneficial effect of CTLA4Ig was maintained until late stages of the disease (week 10 postinfection).
FIG. 4.
Chronic administration of CTLA4Ig partially preserves immune function in Du5H/G6T2-infected mice. Proliferative responses of LN lymphocytes from treated and untreated infected mice to ConA and LPS in vitro are compared. Data are expressed as comparative proliferation indexes. Columns represent means of the data obtained from two to five mice in each group + the standard error of the mean. In infected animals, the onset of immune anergy is early, affecting both B- and T-cell responses. The short-term effect of CTLA4Ig administration is preservation of immune responses in vitro early after infection (week 4). At later time points, CTLA4Ig does not prevent the immune anergy but the level of proliferative responses is higher in treated than in untreated, infected animals.
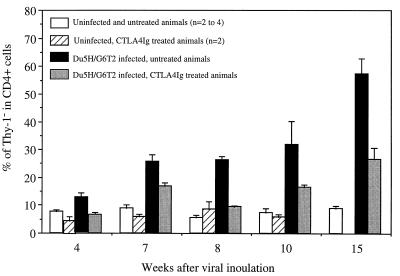
MAIDS-associated phenotypical abnormalities comprise the expansion of a subset of CD4+ T cells lacking Thy-1 expression (15, 32). Fluorescence-activated cell sorter (FACS) analysis of SP and LN cells stained with antibodies to CD4 and Thy-1.2 was done to assess and compare the phenotypical shifts of lymphocytes in the different experimental groups. In CTLA4Ig-treated, infected animals, the relative percentages of the main lymphoid subsets did not differ significantly from those observed in infected, untreated mice (data not shown). The percentage of CD4+ cells lacking Thy-1 was less than 10% in SPs and LNs of control animals, and this percentage was not modified by CTLA4Ig administration. After Du5H/G6T2 inoculation, this level increased rapidly to 25% in LNs at week 7, reaching up to 50% 15 weeks after infection. In infected mice undergoing CTLA4Ig treatment, expansion of the CD4+ Thy-1− subset took place at a much lower rate, accounting for only 27% of CD4+ cells at week 15 (Fig. 5).
FIG. 5.
Expansion of the CD4+ Thy-1− population is delayed by CTLA4Ig treatment. LN single-cell suspensions were stained with a phycoerythrin-conjugated rat anti-mouse CD4 monoclonal antibody (GK 1.5) and a fluorescein isothiocyanate-conjugated rat anti-mouse Thy-1.2 monoclonal antibody (30-H12) (Pharmingen) as previously described (32). The fraction of CD4+ cells that were Thy-1− was determined by FACS analysis. The columns represent the mean calculated fractions plus the standard error of the mean.
B cells are the primary target of Du5H infection (17), and their expansion accounts for a large part of the lymphoproliferation in MAIDS. Proliferating, infected B cells were previously described as blastlike cells with a low surface density of B220 Ag (17). As assessed by FACS analysis, CTLA4Ig treatment did not prevent the blastic shift of the B-cell subset, nor did it affect the proportion of B220dim B cells in the entire B-cell population (data not shown). We therefore examined whether CTLA4Ig treatment modified the viral load, estimated as defective gag mRNA expression.
Transcripts for Du5H Gag protein in SP cells were detected by the reverse transcriptase (RT)-PCR technique. RNA samples were prepared from SPs of individual animals by the RNAzol B method (Biotecx, Houston, Tex.), and 2-μg individual samples were reacted with RT. Defective Gag- or hypoxanthine phosphoribosyltransferase (HPRT)-specific cDNA sequences were amplified by a 30-cycle PCR which was verified to be below saturating conditions. The primers used for the Gag sequence had the sequences 5′-CCTCTTCCTTTATCGACACT-3′ and 5′-ATTAGGGGGGGAATAGCTCG-3′, corresponding to nucleotides 1282 to 1301 and 1499 to 1518, respectively, of the published sequence (38). The primers used for HPRT had the sequences 5′-GTTGGATACAGGCCAGACTTTGTTG-3′ and 5′-GATTCAACTTGCGCTCATCTTAGGC-3′ (39). The DNA of pDu5H was used as a positive control for the defective Gag sequence. Quantitation of defective Gag included normalization to the amplification of the HPRT message.
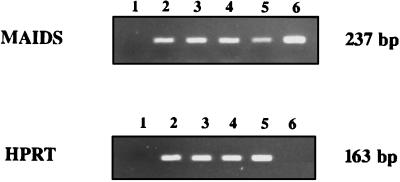
Interestingly, defective Gag mRNA relative expression was not found to be modified by CTLA4Ig treatment when semiquantitatively assessed with respect to HPRT mRNA expression (Fig. 6), and the effects of CTLA4Ig could not be ascribed to a reduction of the viral load.
FIG. 6.
CTLA4Ig treatment does not reduce defective Gag mRNA expression. cDNA obtained from reaction of RNA samples of individual mice with RT were amplified by either defective-Gag- or HPRT-specific primers for 30 cycles. The bands for the two products appeared on a 2% agarose gel at the expected migration points, corresponding to 237 and 163 bp, respectively. Lanes: 2 and 3, samples from untreated, infected mice (SP weights, 450 and 477 mg); 4 and 5, samples from CTLA4Ig-treated, infected mice (SP weights, 165 and 199 mg); 1, negative control for PCR (water); 6, specificity control for PCR using a J1 plasmid containing the defective Du5H sequence. No amplification was obtained after PCR of RNA samples (data not shown). These results are representative of two to five mice per group.
The mechanisms leading to CD4+ T-cell activation in MAIDS remain controversial. Simard et al. proposed that a soluble factor might be responsible for the abnormal activation process (37), whereas experiments done by Morse and his group suggested the involvement of a “classical” TCR-mediated activation pathway (9). In this study, CTLA4Ig treatment inhibited CD4+ T-cell expansion, limited the expansion of the Thy-1− fraction, and partially preserved the ability to respond to mitogens in vitro. This observation underlies a novel argument in favor of the concept that both T-cell activation and induction of anergy occur as a result of abnormal signaling through the TCR. Our findings are not inconsistent with the hypothesis of “superantigenic” TCR triggering (18, 29), inasmuch as the role of CD28 costimulation has been demonstrated in different superantigenic models of T-cell stimulation in the mouse (2, 22, 33).
Blockade of CD28 cosignals to CD4+ T cells may, in turn, affect MAIDS progression in several ways. In certain in vivo models, CD28 costimulation promotes the production of Th-2 type cytokines by naive T cells (35). MAIDS has been reported to be associated with a Th0-to-Th2 switch (7); interference with the Th2 differentiation by CTLA4Ig might, interestingly, account for part of the observed effect. In an in vivo model using CD28-deficient mice, resistance to toxic shock syndrome is associated with nearly complete impairment of gamma interferon and tumor necrosis factor alpha secretion (36). This observation is interesting in view of the role of both cytokines in the pathogenesis of MAIDS (8, 30, 31, 40). The expression of CD28/B7 and that of CD40/CD40L are interdependent (13); in vitro CD28 costimulation can, under some circumstances, regulate T-helper cell function for B-cell activation via a CD40/CD40L-dependent pathway (21). Interestingly, MAIDS development is markedly inhibited in mice treated with a monoclonal antibody to CD40L (10). Whether some of the effects mediated by CTLA4Ig are due to interference with CD40/CD40L signalling or whether the two pathways have complementary additive or multiplicative effects remains to be determined. In addition to signals delivered to the T cell, interaction between CD28 and B7 counterreceptors might deliver signals to B7-bearing APC and CTLA4Ig might inhibit these putative signals.
CTLA4Ig has proven its efficacy as a potent immunosuppressor agent in diverse in vitro or in vivo models of immune responses via inhibition or modulation of the activation of Ag-specific, reactive, CD4+ T cells (19, 26). The results reported here are the first demonstration of the efficacy of CTLA4Ig in the blockade of a clinically malignant lymphoproliferative process. Our findings gain relevance with regard to the recent demonstration of an interaction between a defective Gag protein and the proto-oncogene c-Abl, supporting the theory that Du5H acts as an oncogene (4).
Acknowledgments
We acknowledge M. C. Petit and G. Zeimers for technical assistance and P. Jolicoeur for providing the virus.
This work was supported, in part, by the Fund for Scientific Medical Research and the Centre Anticancéreux près l’Université de Liège. L.D. is a Research Assistant of the Fonds National de la Recherche Scientifique. S.C. is supported by grants from the Fonds pour la Formation à la Recherche dans l’Industrie et l’Agriculture. S.D. and M.-A.D. are Research Fellows of the FIRST program of the Walloon Region (programme de Formation et d’Impulsion à la Recherche Scientifique et Technologique). M.M. is a Research Associate of the Fonds National de la Recherche Scientifique.
REFERENCES
- 1.Bretscher P. The two-signal model of lymphocyte activation twenty-one years later. Immunol Today. 1992;13:74–76. doi: 10.1016/0167-5699(92)90138-W. [DOI] [PubMed] [Google Scholar]
- 2.Champagne E, Scarpellino L, Lane P, Acha-Orbea H. CD28/CTLA4-B7 interaction is dispensable for T cell stimulation by mouse mammary tumor virus superantigen but not for B cell differentiation and virus dissemination. Eur J Immunol. 1996;26:1595–1602. doi: 10.1002/eji.1830260728. [DOI] [PubMed] [Google Scholar]
- 3.Chattopadhyay S K, Morse III H C, Makino M, Ruscetti S K, Hartley J W. Defective virus is associated with induction of murine retrovirus-induced immunodeficiency syndrome. Proc Natl Acad Sci USA. 1989;86:3862–3866. doi: 10.1073/pnas.86.10.3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dupraz P, Rebai N, Klein S J, Beaulieu N, Jolicoeur P. The murine AIDS virus Gag precursor protein binds to the SH3 domain of c-Abl. J Virol. 1997;71:2615–2620. doi: 10.1128/jvi.71.4.2615-2620.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freeman G J, Borriello F, Hodes R J, Reiser H, Gribben J G, Ng J W, Kim J, Goldberg J M, Hathcock K, Laszlo G, Lombard L A, Wang S, Gray G S, Nadler L M, Sharpe A H. Murine B7-2, an alternative CTLA4 counter-receptor that costimulates T cell proliferation and interleukin 2 production. J Exp Med. 1993;178:2185–2192. doi: 10.1084/jem.178.6.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galvin F, Freeman G J, Razi-Wolf Z, Hall W J, Benacerraf B, Nadler L, Reiser H. Murine B7 antigen provides a sufficient costimulatory signal for antigen-specific and MHC-restricted T cell activation. J Immunol. 1992;149:3802. [PubMed] [Google Scholar]
- 7.Gazzinelli R T, Makino M, Chattopadhyay S K, Snapper C M, Sher A, Hügin A W, Morse H C., III CD4+ subset regulation in viral infection. Preferential activation of Th2 cells during progression of retrovirus-induced immunodeficiency in mice. J Immunol. 1992;148:182–188. [PubMed] [Google Scholar]
- 8.Giese N A, Gazzinelli R T, Actor J K, Morawetz R A, Sarzotti M, Morse H C., III Retrovirus-elicited interleukin-12 and tumour necrosis factor-α as inducers of interferon-γ-mediated pathology in mouse AIDS. Immunology. 1996;87:467–474. doi: 10.1046/j.1365-2567.1996.492569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giese N A, Giese T, Morse H C., III Murine AIDS is an antigen-driven disease: requirements for major histocompatibility complex class II expression and CD4+ T cells. J Virol. 1994;68:5819–5824. doi: 10.1128/jvi.68.9.5819-5824.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Green K A, Crassi K M, Laman J D, Schoneveld A, Strawbridge R R, Foy T M, Noelle R J, Green W R. Antibody to the ligand for CD40 (gp39) inhibits murine AIDS-associated splenomegaly, hypergammaglobulinemia, and immunodeficiency in disease-susceptible C57BL/6 mice. J Virol. 1996;70:2569–2575. doi: 10.1128/jvi.70.4.2569-2575.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harding F A, McArthur J G, Gross J A, Raulet D H, Allison J P. CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature. 1992;356:607–609. doi: 10.1038/356607a0. [DOI] [PubMed] [Google Scholar]
- 12.Hartley J W, Fredrickson T N, Yetter R A, Makino M, Morse H C., III Retrovirus-induced murine acquired immunodeficiency syndrome: natural history of infection and differing susceptibility of inbred mouse strains. J Virol. 1989;63:1223–1231. doi: 10.1128/jvi.63.3.1223-1231.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hathcock K S, Hodes R J. Role of the CD28-B7 costimulatory pathways in T cell-dependent B cell responses. Adv Immunol. 1996;62:131–166. doi: 10.1016/s0065-2776(08)60429-0. [DOI] [PubMed] [Google Scholar]
- 14.Hathcock K S, Laszlo G, Pucillo C, Linsley P S, Hodes R J. Comparative analysis of B7-1 and B7-2 costimulatory ligands: expression and function. J Exp Med. 1994;180:631–640. doi: 10.1084/jem.180.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holmes K L, Morse III H C, Makino M, Hardy R R, Hayakawa K. A unique subset of normal murine CD4+ T cells lacking Thy-1 is expanded in a murine retrovirus-induced immunodeficiency syndrome, MAIDS. Eur J Immunol. 1990;20:2783–2787. doi: 10.1002/eji.1830201237. [DOI] [PubMed] [Google Scholar]
- 16.Huang M, Simard C, Jolicoeur P. Immunodeficiency and clonal growth of target cells induced by helper-free defective retrovirus. Science. 1989;246:1614–1617. doi: 10.1126/science.2480643. [DOI] [PubMed] [Google Scholar]
- 17.Huang M, Simard C, Kay D G, Jolicoeur P. The majority of cells infected with the defective murine AIDS virus belong to the B-cell lineage. J Virol. 1991;65:6562–6571. doi: 10.1128/jvi.65.12.6562-6571.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hügin A W, Vacchio M S, Morse H C., III A virus-encoded “superantigen” in a retrovirus-induced immunodeficiency syndrome of mice. Science. 1991;252:424–427. doi: 10.1126/science.1850169. [DOI] [PubMed] [Google Scholar]
- 19.Judge T A, Tang A, Spain L M, Gratiot-Deans J, Sayegh M H, Turka L A. The in vivo mechanism of action of CTLA4Ig. J Immunol. 1996;156:2294–2299. [PMC free article] [PubMed] [Google Scholar]
- 20.June C H, Bluestone J A, Nadler L M, Thompson C B. The B7 and CD28 receptor families. Immunol Today. 1994;15:321–331. doi: 10.1016/0167-5699(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 21.Klaus S J, Pinchuk L M, Ochs H D, Law C L, Fanslow W C, Armitage R J, Clark E A. Costimulation through CD28 enhances T cell-dependent B cell activation via CD40-CD40L interaction. J Immunol. 1994;152:5643–5652. [PubMed] [Google Scholar]
- 22.Lane P, Haller C, McConnell F. Evidence that induction of tolerance in vivo involves active signaling via a B7 ligand-dependent mechanism: CTLA4-Ig protects Vβ8+ T cells from tolerance induction by the superantigen staphylococcal enterotoxin B. Eur J Immunol. 1996;26:858–862. doi: 10.1002/eji.1830260420. [DOI] [PubMed] [Google Scholar]
- 23.Linsley P S. Distinct roles for CD28 and cytotoxic T lymphocyte-associated molecule-4 receptors during T cell activation? J Exp Med. 1995;182:289–292. doi: 10.1084/jem.182.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Linsley P S, Brady W, Grosmaire L, Ledbetter J A, Damle N. CTLA-4 is a second receptor for the B cell activation antigen B7. J Exp Med. 1991;174:561–570. doi: 10.1084/jem.174.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Linsley P S, Ledbetter J A. The role of the CD28 receptor during T cell responses to antigens. Annu Rev Immunol. 1993;11:191–212. doi: 10.1146/annurev.iy.11.040193.001203. [DOI] [PubMed] [Google Scholar]
- 26.Linsley P S, Ledbetter J A. Immunoligands: CTLA4Ig and the CD28 T-cell costimulatory pathway. In: Austen K F, Burakoff S J, Rosen F S, Strom T B, editors. Therapeutic immunology. Cambridge, Mass: Blackwell Science; 1996. pp. 406–418. [Google Scholar]
- 27.Liu Y, Linsley P S. Costimulation of T-cell growth. Curr Opin Immunol. 1992;4:265–270. doi: 10.1016/0952-7915(92)90075-p. [DOI] [PubMed] [Google Scholar]
- 28.Mistry P B, Duplan J F. Propriétés biologiques d’un virus isolé d’une radioleucémie C57Bl. Bull Cancer. 1973;60:287–300. [Google Scholar]
- 29.Morse H C, III, Chattopadhyay S K, Makino M, Fredrickson T N, Hügin A W, Hartley J W. Retrovirus-induced immunodeficiency in the mouse: MAIDS as a model for AIDS. AIDS. 1992;6:607–621. doi: 10.1097/00002030-199207000-00001. [DOI] [PubMed] [Google Scholar]
- 30.Morse H C, III, Giese N, Morawetz R, Tang Y, Gazzinelli R, Kim W K, Chattopadhyay S, Hartley J W. Cells and cytokines in the pathogenesis of MAIDS, a retrovirus-induced immunodeficiency syndrome of mice. Springer Semin Immunopathol. 1995;17:231–245. doi: 10.1007/BF00196167. [DOI] [PubMed] [Google Scholar]
- 31.Morse, H. C., III, R. A. Morawetz, N. A. Giese, S. K. Chattopadhyay, Y. Tang, T. N. Fredrickson, and J. W. Hartley. 1997. Immunologic havoc induced by the 60 kD Gag protein of the MAIDS defective virus. Leukemia 11(Suppl. 3):167–169. [PubMed]
- 32.Moutschen M P, Colombi S, Deprez M, Van Wijk F, Hotermans C, Martin M T, Greimers R, Boniver J. Population dynamics of CD4+ T cells lacking Thy-1 in murine retrovirus-induced immunodeficiency syndrome (MAIDS) Scand J Immunol. 1994;39:216–224. doi: 10.1111/j.1365-3083.1994.tb03363.x. [DOI] [PubMed] [Google Scholar]
- 33.Muraille E, De Smedt T, Thielemans K, Urbain J, Moser M, Leo O. Activation of murine T cells by bacterial superantigens requires B7-mediated costimulation. Cell Immunol. 1995;162:315–320. doi: 10.1006/cimm.1995.1084. [DOI] [PubMed] [Google Scholar]
- 34.Rowe W P, Pugh W E, Hartley J W. Plaque assay techniques for murine leukemia viruses. Virology. 1970;42:1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- 35.Rulifson I C, Sperling A I, Fields P E, Fitch F W, Bluestone J A. CD28 costimulation promotes the production of Th2 cytokines. J Immunol. 1997;158:658–665. [PubMed] [Google Scholar]
- 36.Saha B, Harlan D M, Lee K P, June C H, Abe R. Protection against lethal toxic shock by targeted disruption of the CD28 gene. J Exp Med. 1996;183:2675–2680. doi: 10.1084/jem.183.6.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simard C, Huang M, Jolicoeur P. Murine AIDS is initiated in the lymph nodes draining the site of inoculation, and the infected B cells influence T cells located at distance, in noninfected organs. J Virol. 1994;68:1903–1912. doi: 10.1128/jvi.68.3.1903-1912.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suzuki K, Makino M, Okada Y, Kinoshita J, Yui R, Kanazawa H, Asakura H, Fujiwara M, Mizuochi T, Komuro K. Exocrinopathy resembling Sjögren’s syndrome induced by a murine retrovirus. Lab Investig. 1993;69:430–435. [PubMed] [Google Scholar]
- 39.Svetic A, Finkelman F D, Jian Y C, Dieffenbach C W, Scott D E, Mccarthy K F, Steinberg A D, Gause W C. Cytokine gene expression after in vivo primary immunization with goat antibody to mouse IgD antibody. J Immunol. 1991;147:2391–2397. [PubMed] [Google Scholar]
- 40.Uehara S, Hitoshi Y, Numata F, Makino M, Howard M, Mizuochi T, Takatsu K. An Ifn-γ dependent pathway plays a critical role in the pathogenesis of murine immunodeficiency syndrome induced by LP-BM5 murine leukemia viruses. Int Immunol. 1994;12:1937–1947. doi: 10.1093/intimm/6.12.1937. [DOI] [PubMed] [Google Scholar]
- 41.Yetter R A, Buller R M, Lee J S, Elkins K L, Mosier D E, Fredrickson T N, Morse H C., III CD4+ T cells are required for development of a murine retrovirus-induced immunodeficiency syndrome (MAIDS) J Exp Med. 1988;168:623–635. doi: 10.1084/jem.168.2.623. [DOI] [PMC free article] [PubMed] [Google Scholar]