Abstract
Background: The comparison of retinal perfusion in the eyes of patients with systemic sclerosis (SSc) and in healthy controls using optical coherence tomography angiography (OCTA). The correlation between nailfold capillaroscopy results and OCTA findings among SSc. Methods: The study enrolled 31 patients with systemic sclerosis and 41 healthy controls. OCTA was performed in both groups to assess the retinal vasculature in the superficial (SCP) and deep (DCP) capillary plexuses and the foveal avascular zone (FAZ) area. Nailfold capillaroscopy (NC) was performed in SSc patients and compared to the FAZ area and the superficial and the deep vessel density. Results: In the SSc group, the parafoveal vessel density in DCP was significantly higher in relation to the mean value (p < 0.0001) and in each quadrant of the macula (p < 0.0001) compared to healthy subjects (p < 0.0001). The patients with early scleroderma patterns in capillaroscopy had a larger superficial and deep FAZ (p = 0.0104, p = 0.0076, respectively) than those with active and late patterns. There was a statistically significant difference in the FAZ when comparing early to active (p < 0.0001) and early to late scleroderma patterns (p < 0.0001). A statistically significant difference was found in the type of interstitial lung disease and the deep FAZ area (p = 0.0484). SSc patients with nonspecific interstitial pneumonia (NSIP) had a larger FAZ than those with usual interstitial pneumonia (UIP) (p = 0.0484). Moreover, NSIP cases had a higher parafoveal mean superficial vessel density than those with UIP (p = 0.0471). Conclusions: Our investigation showed that the peripheral microvascular system correlates with ocular microcirculatory impairment. The results indicate the important role of OCTA in the diagnosis, monitoring, and prognosis of microvascular changes in SSc.
Keywords: systemic sclerosis, scleroderma, retinal perfusion, nailfold capillaroscopy, optical coherence tomography angiography, OCTA
1. Introduction
Systemic sclerosis (SSc) is a rare and chronic autoimmune disease of the connective tissue [1]. It affects about 2.5 million people worldwide, with a female predominance [2]. The disease is divided into two main subtypes: limited cutaneous SSc (lcSSc) and diffuse cutaneous SSc (dcSSc) [3]. SSc manifests through an abnormal immunological response, vasculopathy, and fibrosis, leading to organ dysfunction [1]. The complex interplay of these pathological processes considerably affects vital organs such as the lungs, heart, kidneys, muscles, skin, and eyes [4].
These cumulative effects lead to a quality-of-life deterioration and increased mortality. Microvasculopathy is an important and initial pathogenic link responsible for early SSc manifestations like Raynaud’s phenomenon and changes in nailfold capillaries [5].
Endothelial damage is one of the mechanisms leading to disorders of vascular hemostasis, platelet activation, and thrombosis, causing coagulopathy and microangiopathy.
Moreover, it has been proven that structural changes appear in the microvasculatory system before inflammation and fibrosis and play a crucial role in the diagnostic process and treatment response in those with scleroderma [5,6].
Nailfold capillaroscopy (NC) is a non-invasive tool included in the diagnostic criteria for SSc in the 2013 American Collage of Rheumatology (ACR) and the European League Against Rheumatism (EULAR) guidelines. It is used in everyday practice to evaluate the number, size, and shape of the blood vessels in the nailfold microcirculation [6,7,8].
Currently, changes in NC are categorized using Cutolo et al.’s classification into three patterns: early, active, and late [9]. However, vascular pathologies can occur in other organs, including the eye, especially the retinal microvasculature system.
Optical coherence tomography angiography (OCTA) is a non-invasive imaging method that provides highly detailed, three-dimensional images of the entire microvasculature of the retina and choroid, enabling retinal perfusion assessment. It identifies the retinal vessels by detecting and measuring the movement of the blood cells in them without dye injection [10,11]. A wide variety of ocular manifestations has been reported in SSc patients, including the anterior and posterior segments of the eye [12,13,14,15,16]. However, there are inconsistent data regarding retinal perfusion measured using OCTA in SSc patients compared to capillaroscopy results.
The aim of our study was to compare the microvasculature changes in patients with SSc to those in healthy volunteers using OCTA and to evaluate the correlation between the peripheral microvascular changes seen in NC and the characteristics of scleroderma and the OCTA results obtained in the study group.
2. Materials and Methods
This cross-sectional study was conducted between March 2022 and May 2023 at the Department of Ophthalmology of the Military Institute of Aviation Medicine in Warsaw, Poland. The study was approved by the Ethics Committee of the Military Institute of Aviation Medicine and followed the tenets of the Declaration of Helsinki. The participants signed a written consent form after the detailed explanation of the nature of the study.
The study group consisted of patients with scleroderma from the Department and Polyclinic of Systemic Connective Tissue Diseases of the National Institute of Geriatrics, Rheumatology and Rehabilitation in Warsaw, Poland. Healthy controls were recruited during routine visits to the Ophthalmology Department of the Military Institute of Aviation Medicine.
The exclusion criteria encompassed refractive defects exceeding −3 D and +3 D; the presence of eye diseases such glaucoma, retinal and choroidal pathologies, and uveitis; and low-quality OCTA images.
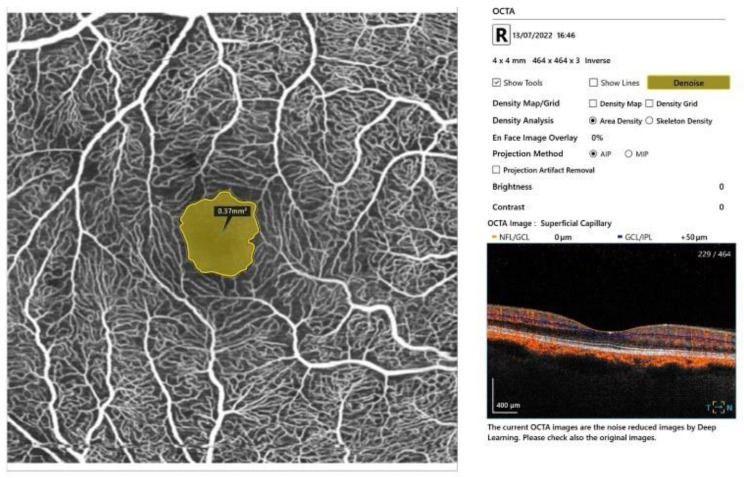
Every participant underwent a complete ophthalmic examination, including a refraction test, best-corrected visual acuity (BCVA), tonometry, segment slit-lamp examination of the anterior segment, and dilated fundus evaluation using a 90-diopter (D) lens. BCVA was measured monocularly using LogMAR charts (Lighthouse International, New York, NY, USA) at a distance of 5 m. OCT and OCTA were performed using SS-OCT (Xephilio® OCT-A1, Canon, Tokyo, Japan). The OCTA scans covered a region of 4 mm × 4 mm. The scan automatically inserted fovea-centered circles at the macula, detecting the superficial capillary plexus (SCP) and the deep capillary plexus (DCP). The SCP network was segmented from 3 μm beneath the internal limiting membrane to 15 μm below the inner plexiform layer (IPL). The DCP was segmented from 15 to 70 μm below the IPL. The foveal vessel density was defined as the area of the small circle, with a diameter of 1 mm. The parafoveal vessel density was defined as the area of the outer circle. It was automatically divided into four quadrants: superior (S), inferior (I), nasal (N), and temporal (T). The vessel density was automatically calculated as a percentage, according to the area occupied by blood vessels, by the software. The average vessel density of the SCP and DCP in the four quadrants was calculated. The foveal avascular zone (FAZ) in the deep and the superficial plexus was measured using the automated procedure provided with the software. Two scans for each eye were captured, and the best one in terms of quality was considered for the analysis (Figure 1).
Figure 1.
Representative OCTA report of patient with SSc. An image of the macular superficial capillary plexus with the FAZ area measured.
Moreover, all SSc patients underwent a clinical evaluation, laboratory examination, and nailfold capillaroscopy. The data collected were the type of SSc, i.e., limited or diffuse cutaneous scleroderma; interstitial lung disease defined as nonspecific interstitial pneumonia (NSIP) or usual interstitial pneumonia (UIP); hypertension; elevated proBNP; and the treatment method—mycophenolate mofetil (MMf) or methotrexat (MTX)—as well as sildenafil or amlodipine administration.
In this study, nailfold capillaroscopy was conducted using the Dino-Lite capillaroscope. The device was set up according to the manufacturer’s guidelines, and participants were positioned to expose the nailfold area for examination. The same expert systematically captured the images, ensuring consistency in lighting, focus, and positioning.
The objective was to classify scleroderma patterns according to the Cutolo classification and assess various parameters, including the vessel number, hemorrhages, branched-shaped vessels, giant capillaries, and avascular areas. This classification was based on the presence of specific capillary abnormalities associated with different disease stages.
The number of vessels detected in each capillaroscopy image was categorized as follows: normal—over 7, reduced—between 6 and 4, and very reduced—3 or fewer capillaries. This classification provided insights into the microvascular changes associated with scleroderma progression.
Hemorrhages, branched-shaped vessels, giant capillaries, and avascular areas were meticulously documented for each participant. These parameters can contribute to a comprehensive understanding of the microvascular abnormalities characteristic of scleroderma [17].
Regular quality control checks were performed on the Dino-Lite capillaroscope, and calibration images were captured to ensure accurate color representation and resolutions.
The study’s endpoint was to evaluate and describe the scleroderma patterns based on the Cutolo classification and the categorized vessel numbers. Additionally, a detailed description of other capillaroscopy parameters, including hemorrhages, branched-shaped vessels, giant capillaries, and avascular areas, was provided.
Another goal was to evaluate the correlation between the nailfold capillaroscopy results and the OCTA parameters in patients with systemic sclerosis.
The final aim of the study was to compare the OCTA parameters in the study group to those of healthy controls.
Statistical Analysis
Categorical traits were depicted through integers and percentages. Numerical variables were described by the weighted arithmetic mean, median, standard deviation, and minimum-to-maximum values.
For contingency tables, a chi-squared test of independency or Fisher’s exact test (for small cell numbers) was applied. The normality of distribution was assessed by using the Shapiro–Wilk W test. The homogeneity of variances was assessed by Levene’s test. An analysis of variance (for normally distributed variables) or generalized linear models (for non-normally distributed ones) were applied to assess differences in numerical variables between the study groups. All models were controlled for age and gender.
Due to the fact that the multifactor analyses encompassed measurements for two eyes per patient, standard error correction consisting of intra-subject correlations was applied.
In order to illustrate and describe the degree of separability in selected angiological measurements by the prevalence of scleroderma, ROC curves were plotted and corresponding cut-off points were proposed, adding sensitivity and specificity values. In this context, the Youden index was applied.
A level of p < 0.05 was deemed statistically significant. All statistical procedures were performed by using STATGRAPHICS Centurion, version 19.4 (Statgraphics Technologies, Inc., The Plains, VA, USA).
3. Results
A total of 61 eyes of 31 patients with systemic sclerosis were included in this study and compared to the OCTA data of 81 eyes of 41 healthy controls. There was no significant difference between the scleroderma patients and controls in terms of the age and gender distribution.
In our study group, 71.4% of patients had diffuse cutaneous systemic sclerosis and 28.6% had limited cutaneous SSc. The nailfold capillaroscopy findings in this group revealed 5 patients with an early pattern, 12 patients with an active pattern, and 9 patients with a late pattern. Among the SSc patients, only one had pulmonary arterial hypertension (PAH), eight subjects had finger ulcers, and eight suffered from hypertension. A total of 13 participants were treated with sildenafil and 14 with amlodipide. Interstitial lung disease was observed in 12 patients (67% NSIP and 33% UIP). Elevated proBNP was detected in 17.8% of the study group (Table 1 and Table 2).
Table 1.
Baseline characteristics of the study participants (n = 72).
| Trait |
n M (SD) * |
% Me (Q1–Q3) ** |
|---|---|---|
| Gender: | ||
| - Female | 49 | 68.1 |
| - Male | 23 | 31.9 |
| Age (year) | 41.3 (11.2) | 40.0 (32.0–53.0) |
| Scleroderma | 28 | 38.9 |
* For discrete variables: n—number; %—percentage. ** For numerical traits: M—mean; SD—standard deviation; Me—median; Q—quartile.
Table 2.
Baseline characteristics of the scleroderma group (n = 31).
| Trait |
n M (SD) |
% Me (Q1–Q3) |
|---|---|---|
| Gender: | ||
| - Female | 22 | 71.0 |
| - Male | 9 | 29.0 |
| Scleroderma: | ||
| - Localized | 8 | 28.6 |
| - Systemic | 20 | 71.4 |
| Capillaroscopy | 27 | 96.4 |
| Scleroderma pattern: | ||
| - None | 2 | 7.1 |
| - Early | 5 | 17.9 |
| - Active | 12 | 42.9 |
| - Late | 9 | 32.1 |
| PM/SCL | 1 | 3.6 |
| PC TH fibrillarin | 3 | 10.7 |
| SCI70 | 17 | 54.8 |
| Centromere | 8 | 25.8 |
| Number of vessels: | ||
| - Normal | 2 | 7.1 |
| - Reduced | 20 | 71.5 |
| - Very reduced | 6 | 21.4 |
| Avascular area | 16 | 57.1 |
| Giant capillaries | 14 | 50.0 |
| Hemorrhages | 11 | 39.3 |
| Branched vessels | 10 | 35.7 |
| MES | 2.2 (1.1) | 2.5 (1.1–3.1) |
| Interstitial lung disease | 12 | 42.9 |
| PAH-1 | 2 | 7.1 |
| NSIP | 7 | 22.6 |
| UIP | 4 | 14.3 |
| MMF | 13 | 41.9 |
| MTX | 7 | 25.0 |
| mRSS | 8.1 (7.9) | 2.0 (2.0–12.0) |
| Finger ulcers | 8 | 28.6 |
| RVSP > 35 mmHg | 3 | 1.1 |
| Elevated proBNP | 5 | 18.5 |
| Arterial hypertension | 8 | 25.8 |
| Administration of sildenafil | 13 | 41.9 |
| Administration of amlodipine | 12 | 38.7 |
Missing data were pair-wise deleted. For discrete variables: n—number; %—percentage. For numerical traits: M—mean; SD—standard deviation; Me—median; Q—quartile.
There was no statistically significant difference in the superficial vessel density (SVD) or the FAZ measurements between the study group and the controls. However, the parafoveal deep vessel density (DVD) was significantly higher in SSc patients than in control subjects, considering both its mean value (p < 0.0001) and every quadrant: superior, inferior, temporal, and nasal (respectively, p < 0.0001) (Table 3).
Table 3.
Descriptive statistics for the selected numerical measures of the study participants’ eyes.
| Investigated Trait | Cases (n = 61 Eyes) |
Controls (n = 81 Eyes) |
p-Value ** | ||
|---|---|---|---|---|---|
| M (SD) * | Me (Q1–Q3) * | M (SD) * | Me (Q1–Q3) * | ||
| Age (years) | 46.5 (9.7) | 48 (40–54) | 44.1 (9.6) | 41 (35–55) | =0.1520 |
| FAZ superficial | 0.260 (0.104) | 0.26 (0.17–0.35) | 0.246 (0.095) | 0.25 (0.18–0.30) | =0.5361 |
| FAZ deep | 0.248 (0.176) | 0.18 (0.12–0.37) | 0.202 (0.147) | 0.16 (0.12–0.24) | =0.1527 |
| Foveal SVD | 23.3 (5.5) | 23.0 (12.3–38.6) | 24.0 (3.9) | 23.5 (22.0–26.5) | =0.3767 |
| Parafoveal SVD superior | 41.1 (2.9) | 41.1 (19.5–26.6) | 41.7 (1.6) | 41.8 (41.0–42.7) | =0.0655 |
| Parafoveal SVD inferior | 41.9 (2.0) | 41.8 (40.2–42.5) | 42.0 (1.1) | 41.9 (41.1–42.7) | =0.5463 |
| Parafoveal SVD temporal | 39.1 (3.5) | 39.2 (37.6–41.1) | 39.7 (1.8) | 39.5 (38.5–40.5) | =0.0705 |
| Parafoveal SVD nasal | 40.1 (3.1) | 40.3 (38.6–41.5) | 40.6 (1.7) | 40.5 (39.6–41.8) | =0.1247 |
| Parafoveal mean SVD | 40.6 (2.5) | 40.3 (39.7–41.6) | 41.0 (1.3) | 40.8 (40.2–41.8) | =0.0713 |
| Foveal DVD | 27.4 (7.6) | 29.6 (23.2–32.5) | 28.0 (9.0) | 32.4 (21.4–35.0) | =0.8505 |
| Parafoveal DVD superior | 41.8 (2.7) | 42.1 (41.0–43.6) | 38.9 (5.0) | 41.3 (32.8–42.1) | <0 . 0001 |
| Parafoveal DVD inferior | 42.0 (3.1) | 42.3 (41.4–43.8) | 38.8 (5.3) | 41.3 (32.3–42.1) | <0.0001 |
| Parafoveal DVD temporal | 42.1 (2.6) | 42.6 (40.5–44.3) | 38.2 (5.3) | 40.8 (32.5–41.9) | <0.0001 |
| Parafoveal DVD nasal | 42.0 (3.7) | 42.6 (41.2–44.1) | 39.2 (5.7) | 41.7 (31.8–42.9) | <0.0001 |
| Mean parafoveal DVD | 42.0 (2.5) | 42.1 (41.1–43.8) | 38.8 (5.3) | 41.4 (32.1–42.0) | <0.0001 |
* M—mean; Me—median; SD—standard deviation; minimum-to-maximum values. ** A multifactor model, controlled for age and gender, was implemented. Bold values indicate statistical significance.
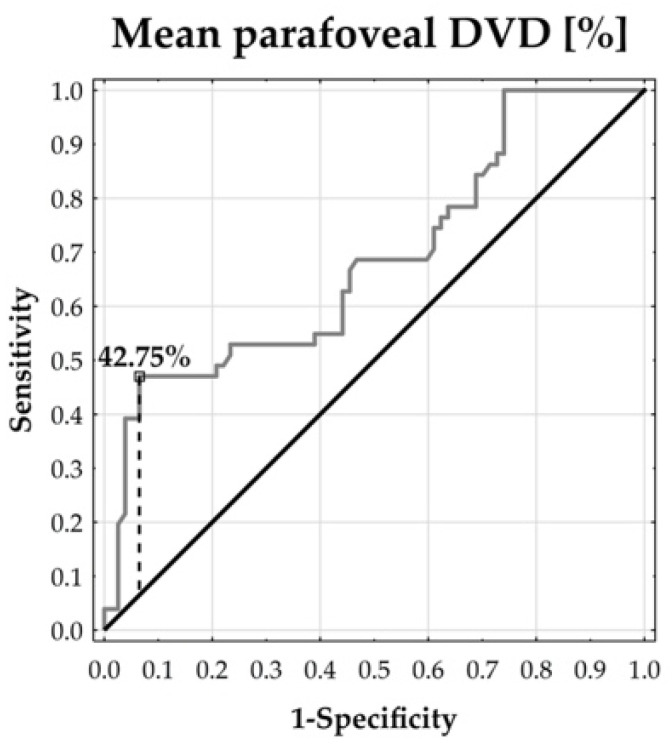
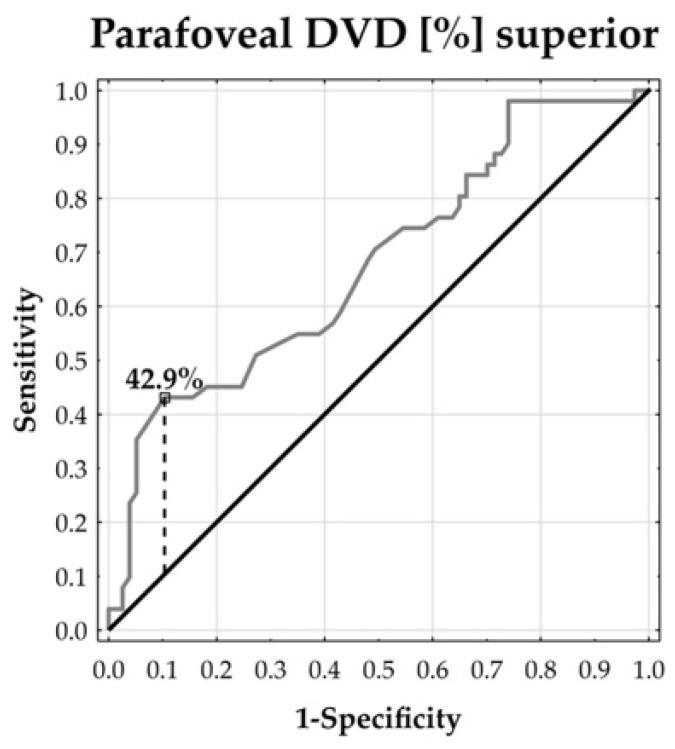
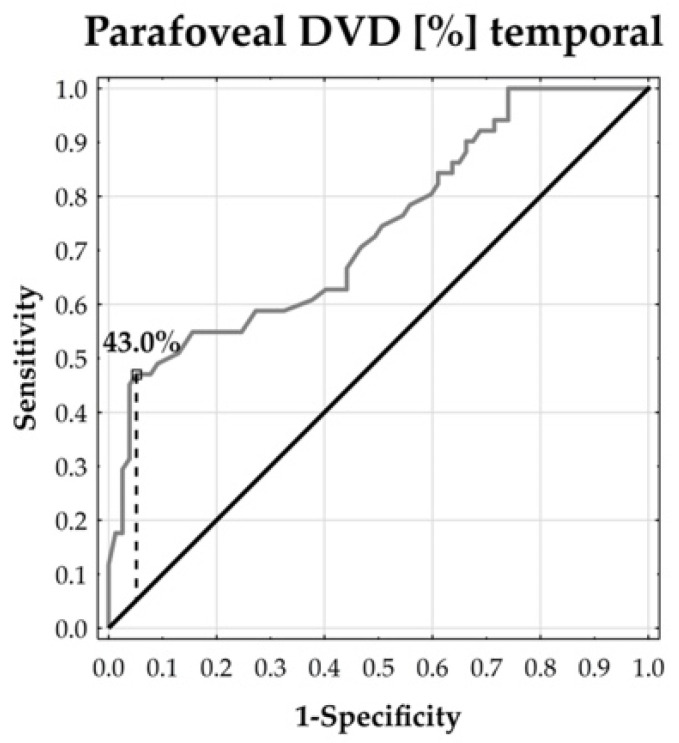
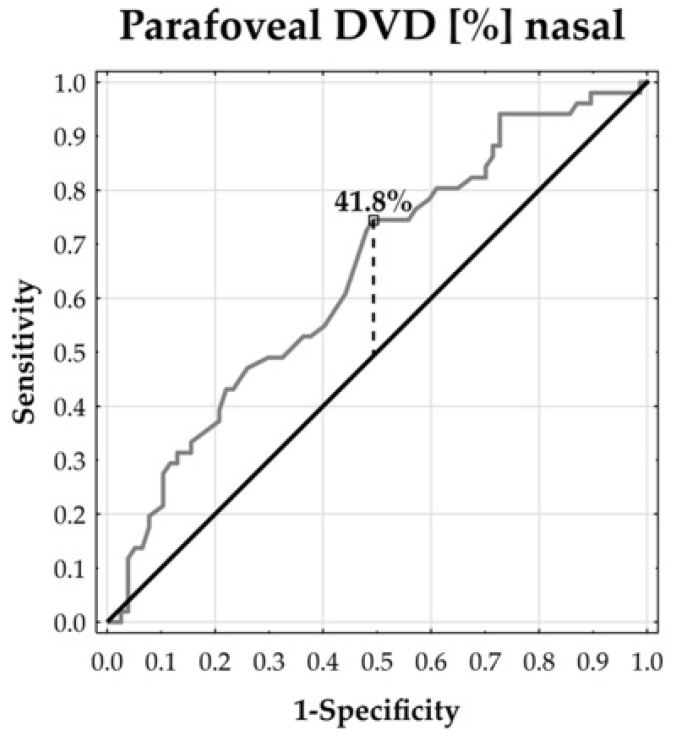
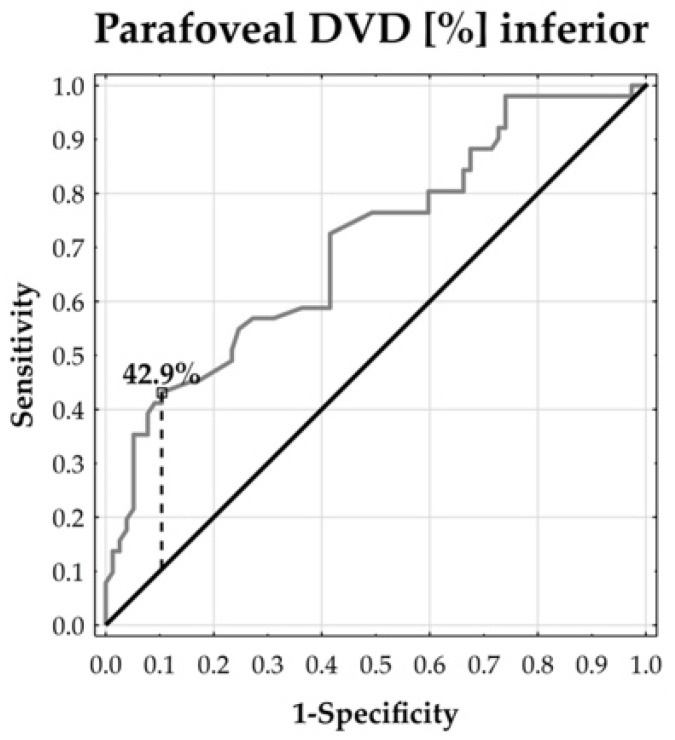
The ROC curves demonstrated that the parafoveal DVD could distinguish between the patients and healthy subjects. The temporal and inferior parafoveal DVDs revealed the largest separability, reflected by the corresponding highest area under the curve (AUC: 73.57% and 70.47%, respectively, both at p < 0.0001). In addition, the temporal parafoveal DVD’s sensitivity was 47.06% and its specificity reached 94.81%. The inferior parafoveal DVD’s sensitivity was 43.14% and its specificity was 86.61%. This means that the temporal and inferior DVDs tended to enable the accurate specification of true negative cases, whereas the identification of true positive cases remained moderate (Figure 2, Figure 3, Figure 4, Figure 5 and Figure 6).
Figure 2.
ROC curve for mean parafoveal DVD. AUC = 68.49% (p = 0.0002).
Figure 3.
ROC curve for superior DVD. AUC = 67.71% (p = 0.0003).
Figure 4.
ROC curve for temporal DVD. AUC = 73.57% (p < 0.0001).
Figure 5.
ROC curve for nasal DVD. AUC = 64.46% (p = 0.0034).
Figure 6.
ROC curve for inferior DVD. AUC = 70.47% (p < 0.0001).
We compared the OCTA parameters, i.e., the superficial FAZ, deep FAZ, parafoveal mean SVD, and parafoveal mean DVD, to the capillaroscopy findings and scleroderma parameters (Table 4, Table 5, Table 6 and Table 7).
Table 4.
Descriptive statistics for the superficial FAZ in the study participants by selected clinical conditions (n = 28).
| Investigated Trait | Statistical Parameter | p-Value | |
|---|---|---|---|
| M (SD) | Me (Q1–Q3) | ||
| Localized scleroderma | 0.271 (0.084) | 0.270 (0.240–0.345) | =0.6331 |
| Systemic scleroderma | 0.255 (0.115) | 0.250 (0.150–0.330) | |
| Scleroderma pattern: | =0.0104 | ||
| ● Early | 0.399 (0.073) | 0.390 (0.350–0.445) | |
| ● Active | 0.239 (0.088) | 0.250 (0.150–0.300) | |
| ● Late * | 0.222 (0.089) | 0.230 (0.140–0.280) | |
| SCI70 | 0.256 (0.121) | 0.245 (0.145–0.350) | =0.6184 |
| Centromere | 0.271 (0.084) | 0.270 (0.240–0.345) | |
| Number of vessels: | =0.4979 | ||
| ● Normal | 0.322 (0.080) | 0.315 (0.255–0.390) | |
| ● Reduced | 0.260 (0.112) | 0.260 (0.160–0.345) | |
| ● Very reduced | 0.237 (0.080) | 0.240 (0.220–0.280) | |
| Avascular areas: | =0.7091 | ||
| ● Yes | 0.255 (0.100) | 0.275 (0.155–0.340) | |
| ● No | 0.267 (0.111) | 0.250 (0.180–0.340) | |
| Giant capillaries: | =0.1753 | ||
| ● Yes | 0.239 (0.086) | 0.250 (0.150–0.300) | |
| ● No | 0.282 (0.117) | 0.270 (0.180–0.380) | |
| Hemorrhages: | =0.0198 | ||
| ● Yes | 0.216 (0.087) | 0.225 (0.135–0.280) | |
| ● No | 0.285 (0.106) | 0.270 (0.230–0.350) | |
| Branched vessels: | =0.7631 | ||
| ● Yes | 0.268 (0.100) | 0.270 (0.180–0.350) | |
| ● No | 0.257 (0.107) | 0.250 (0.175–0.320) | |
| Interstitial lung disease: | =0.9067 | ||
| ● Yes | 0.258 (0.105) | 0.270 (0.150–0.350) | |
| ● No | 0.262 (0.106) | 0.250 (0.180–0.340) | |
| NSIP | 0.286 (0.102) | 0.270 (0.230–0.380) | =0.1248 |
| UIP | 0.185 (0.101) | 0.155 (0.120–0.250) | |
| MMF | 0.286 (0.118) | 0.270 (0.210–0.380) | =0.4313 |
| MTX | 0.238 (0.095) | 0.250 (0.140–0.300) | |
| Finger ulcers: | =0.4087 | ||
| ● Yes | 0.241 (0.105) | 0.250 (0.140–0.330) | |
| ● No | 0.269 (0.106) | 0.255 (0.180–0.350) | |
| Arterial hypertension: | =0.5162 | ||
| ● Yes | 0.242 (0.092) | 0.245 (0.140–0.340) | |
| ● No | 0.267 (0.111) | 0.270 (0.180–0.350) | |
| Administration of sildenafil: | =0.0178 | ||
| ● Yes | 0.218 (0.098) | 0.215 (0.140–0.300) | |
| ● No | 0.292 (0.103) | 0.280 (0.240–0.350) | |
| Administration of amlodipine: | =0.0626 | ||
| ● Yes | 0.292 (0.121) | 0.280 (0.180–0.380) | |
| ● No | 0.231 (0.083) | 0.240 (0.170–0.300) | |
M—mean; SD—standard deviation; Me—median; Q—quartile. * Scleroderma pattern, post-hoc multiple comparisons: early vs. active p < 0.0001; early vs. late p < 0.0001; active vs. late NS. Bold values indicate statistical significance.
Table 5.
Descriptive statistics for the deep FAZ (%) in the study participants by selected clinical conditions (n = 28).
| Investigated Trait | Statistical Parameter | p-Value | |
|---|---|---|---|
| M (SD) | Me (Q1–Q3) | ||
| Localized scleroderma | 0.237 (0.171) | 0.190 (0.125–0.240) | =0.9996 |
| Systemic scleroderma | 0.237 (0.152) | 0.180 (0.110–0.370) | |
| Scleroderma pattern: | =0.0076 | ||
| ● Early | 0.456 (0.142) | 0.425 (0.345–0.555) | |
| ● Active | 0.197 (0.119) | 0.160 (0.110–0.260) | |
| ● Late * | 0.173 (0.132) | 0.110 (0.100–0.330) | |
| SCI70 | 0.243 (0.163) | 0.180 (0.100–0.390) | =0.9997 |
| Centromere | 0.237 (0.171) | 0.190 (0.125–0.240) | |
| Number of vessels: | =0.1350 | ||
| ● Normal | 0.435 (0.252) | 0.410 (0.220–0.650) | |
| ● Reduced | 0.227 (0.140) | 0.185 (0.110–0.335) | |
| ● Very reduced | 0.182 (0.114) | 0.120 (0.110–0.290) | |
| Avascular areas: | =0.2698 | ||
| ● Yes | 0.214 (0.138) | 0.120 (0.105–0.350) | |
| ● No | 0.263 (0.177) | 0.190 (0.150–0.260) | |
| Giant capillaries: | =0.2396 | ||
| ● Yes | 0.210 (0.120) | 0.180 (0.120–0.290) | |
| ● No | 0.262 (0.185) | 0.210 (0.110–0.390) | |
| Hemorrhages: | =0.0152 | ||
| ● Yes | 0.174 (0.079) | 0.160 (0.115–0.240) | |
| ● No | 0.271 (0.179) | 0.220 (0.110–0.390) | |
| Branched vessels: | =0.4219 | ||
| ● Yes | 0.267 (0.135) | 0.300 (0.150–0.390) | |
| ● No | 0.225 (0.166) | 0.180 (0.110–0.275) | |
| Interstitial lung disease: | =0.3505 | ||
| ● Yes | 0.270 (0.150) | 0.315 (0.100–0.390) | |
| ● No | 0.222 (0.160) | 0.170 (0.110–0.260) | |
| NSIP | 0.324 (0.142) | 0.370 (0.330–0.390) | =0.0484 |
| UIP | 0.140 (0.112) | 0.110 (0.070–0.210) | |
| MMF | 0.247 (0.147) | 0.190 (0.120–0.390) | =0.3844 |
| MTX | 0.193 (0.109) | 0.190 (0.110–0.220) | |
| Finger ulcers: | =0.3582 | ||
| ● Yes | 0.207 (0.129) | 0.135 (0.110–0.330) | |
| ● No | 0.249 (0.171) | 0.190 (0.110–0.370) | |
| Arterial hypertension: | =0.2474 | ||
| ● Yes | 0.195 (0.119) | 0.150 (0.120–0.260) | |
| ● No | 0.251 (0.171) | 0.190 (0.110–0.370) | |
| Administration of sildenafil: | =0.0222 | ||
| ● Yes | 0.181 (0.125) | 0.115 (0.100–0.300) | |
| ● No | 0.279 (0.173) | 0.210 (0.150–0.420) | |
| Administration of amlodipine: | =0.0780 | ||
| ● Yes | 0.278 (0.177) | 0.210 (0.120–0.390) | |
| ● No | 0.200 (0.137) | 0.140 (0.100–0.300) | |
M—mean; SD—standard deviation; Me—median; Q—quartile. * Scleroderma pattern, post-hoc multiple comparisons: early vs. active p < 0.0001; early vs. late p < 0.0001; active vs. late NS. Bold values indicate statistical significance.
Table 6.
Descriptive statistics for the parafoveal mean SVD (%) in the study participants by selected clinical conditions (n = 28).
| Investigated Trait | Statistical Parameter | p-Value | |
|---|---|---|---|
| M (SD) | Me (Q1–Q3) | ||
| Localized scleroderma | 40.68 (2.25) | 40.01 (39.36–42.47) | =0.6807 |
| Systemic scleroderma | 40.93 (1.95) | 40.35 (39.95–41.62) | |
| Scleroderma pattern: | =0.6174 | ||
| ● Early | 41.57 (1.79) | 40.97 (40.29–42.88) | |
| ● Active | 40.86 (2.24) | 40.22 (39.70–43.25) | |
| ● Late | 40.37 (2.12) | 40.22 (38.77–41.63) | |
| SCI70 | 41.10 (2.07) | 40.46 (40.04–42.63) | =0.6908 |
| Centromere | 40.68 (2.25) | 40.01 (39.36–42.48) | |
| Number of vessels: | =0.1146 | ||
| ● Normal | 42.83 (1.75) | 40.94 (41.34–44.33) | |
| ● Reduced | 40.65 (1.97) | 40.29 (39.36–41.63) | |
| ● Very reduced | 40.64 (2.12) | 40.22 (39.75–40.35) | |
| Avascular areas: | =0.3032 | ||
| ● Yes | 40.56 (1.97) | 40.26 (39.72–41.60) | |
| ● No | 41.17 (2.12) | 40.97 (39.75–43.25) | |
| Giant capillaries: | =0.7616 | ||
| ● Yes | 40.93 (2.24) | 40.59 (39.70–43.25) | |
| ● No | 40.75 (1.88) | 40.35 (39.75–41.63) | |
| Hemorrhages: | =0.1405 | ||
| ● Yes | 40.26 (1.81) | 40.17(39.55–41.31) | |
| ● No | 41.16 (2.12) | 40.35 (39.85–43.63) | |
| Branched vessels: | =0.5786 | ||
| ● Yes | 41.10 (1.40) | 40.57 (40.30–41.63) | |
| ● No | 40.74 (2.26) | 40.22 (39.36–42.48) | |
| Interstitial lung disease: | =0.2209 | ||
| ● Yes | 41.38 (1.88) | 41.07 (40.22–43.63) | |
| ● No | 40.60 (2.09) | 40.22 (39.40–41.63) | |
| NSIP | 42.21 (1.69) | 41.62 (40.57–43.80) | =0.0471 |
| UIP | 39.81 (1.40) | 39.54 (38.69–40.94) | |
| MMF | 40.76 (1.51) | 40.35 (39.95–41.58) | =0.8716 |
| MTX | 41.10 (2.43) | 40.97 (39.70–43.25) | |
| Finger ulcers: | =0.3372 | ||
| ● Yes | 40.43 (2.00) | 40.32 (39.70–41.05) | |
| ● No | 41.05 (2.10) | 40.35 (39.75–43.25) | |
| Arterial hypertension: | =0.7358 | ||
| ● Yes | 41.02 (2.07) | 40.32 (39.70–41.63) | |
| ● No | 40.76 (2.12) | 40.22 (39.75–41.63) | |
| Administration of sildenafil: | =0.3159 | ||
| ● Yes | 40.44 (1.79) | 40.32 (39.75–41.05) | |
| ● No | 41.09 (2.27) | 40.22 (39.70–43.38) | |
| Administration of amlodipine: | =0.9567 | ||
| ● Yes | 40.80 (1.77) | 40.30 (39.80–41.63) | |
| ● No | 40.84 (2.39) | 40.29 (39.22–43.63) | |
M—mean; SD—standard deviation; Me—median; Q—quartile. Bold values indicate statistical significance.
Table 7.
Descriptive statistics for the parafoveal mean DVD (%) in the study participants by selected clinical conditions (n = 28).
| Investigated Trait | Statistical Parameter | p-Value | |
|---|---|---|---|
| M (SD) | Me (Q1–Q3) | ||
| Localized scleroderma | 42.55 (1.44) | 42.34 (41.46–43.93) | =0.6199 |
| Systemic scleroderma | 42.21 (2.47) | 42.12 (41.15–43.90) | |
| Scleroderma pattern: | =0.4437 | ||
| ● Early | 41.57 (4.02) | 43.54 (39.21–44.06) | |
| ● Active | 42.51 (1.50) | 42.04 (41.47–43.80) | |
| ● Late | 42.08 (1.70) | 41.22 (41.07–44.38) | |
| SCI70 | 42.01 (2.64) | 42.04 (40.94–44.09) | =0.6192 |
| Centromere | 42.55 (1.44) | 42.34 (41.46–43.93) | |
| Number of vessels: | =0.3402 | ||
| Normal | 43.84 (0.52) | 43.84 (43.40–44.29) | |
| Reduced | 42.24 (2.35) | 42.44 (41.36–43.85) | |
| Very reduced | 41.99 (1.60) | 41.47 (41.22–41.63) | |
| Avascular areas: | =0.7687 | ||
| ● Yes | 42.42 (1.49) | 41.76 (41.22–43.75) | |
| ● No | 42.23 (2.76) | 43.05 (41.45–44.25) | |
| Giant capillaries: | =0.3396 | ||
| ● Yes | 42.65 (1.51) | 42.44 (41.47–43.90) | |
| ● No | 42.03 (2.62) | 42.00 (41.15–44.23) | |
| Hemorrhages: | =0.8356 | ||
| ● Yes | 42.42 (1.23) | 42.10 (41.49–43.55) | |
| ● No | 42.28 (2.54) | 43.05 (41.22–44.35) | |
| Branched vessels: | =0.1093 | ||
| ● Yes | 43.14 (1.32) | 43.38 (42.12–43.90) | |
| ● No | 42.00 (2.34) | 41.69 (41.19–43.93) | |
| Interstitial lung disease: | =0.2237 | ||
| ● Yes | 42.92 (1.73) | 43.54 (41.07–44.38) | |
| ● No | 42.07 (2.29) | 42.00 (41.27–43.63) | |
| NSIP | 43.58 (1.50) | 43.80 (43.37–44.45) | =0.1244 |
| UIP | 41.46 (1.66) | 40.82 (40.44–42.49) | |
| MMF | 41.88 (2.79) | 42.13 (41.22–43.70) | =0.5305 |
| MTX | 42.70 (1.63) | 43.35 (41.47–44.25) | |
| Finger ulcers: | =0.8485 | ||
| ● Yes | 42.42 (1.54) | 42.10 (41.07–43.70) | |
| ● No | 42.28 (2.43) | 42.47 (41.27–44.25) | |
| Arterial hypertension: | =0.9677 | ||
| ● Yes | 42.31 (1.78) | 41.61 (41.45–44.45) | |
| ● No | 42.28 (2.32) | 41.12 (41.22–43.90) | |
| Administration of sildenafil: | =0.8966 | ||
| ● Yes | 42.23 (1.45) | 41.94 (41.07–43.48) | |
| ● No | 42.32 (2.62) | 42.95 (41.47–44.35) | |
| Administration of amlodipine: | =0.3429 | ||
| ● Yes | 41.95 (2.64) | 41.72 (41.47–44.23) | |
| ● No | 42.59 (1.64) | 42.44 (41.15–43.90) | |
M—mean; SD—standard deviation; Me—median; Q—quartile.
Patients with an early scleroderma pattern in capillaroscopy showed a larger superficial FAZ than those with active and late patterns (p = 0.0104).
There was also a statistically significant difference when comparing the FAZ in the early to active (p < 0.0001) and early to late scleroderma patterns (p < 0.0001). However, there was no statistically significant difference in the comparison between the active and late phases.
Moreover, the superficial FAZ was larger among patients without hemorrhages (p = 0.0198). Similar results were observed in the deep FAZ according to the scleroderma pattern in capillaroscopy (p = 0.0076) and in the presence of hemorrhages (p = 0.0152). A statistically significant difference was found regarding interstitial lung diseases, i.e., NSIP and UIP, in reference to the deep FAZ area (p = 0.0484). SSc patients with NSIP had a larger FAZ than UIP patients (p = 0.0484). Moreover, NSIP subjects had a higher parafoveal mean superficial vessel density than UIP patients (p = 0.471). Furthermore, patients undergoing sildenafil treatment demonstrated a decreased FAZ in both the superficial and deep capillary plexuses (p = 0.0178, p = 0.0222, respectively) (Table 4 and Table 5).
4. Discussion
Vasculopathy is the main and initial step in the pathogenesis of SSc and is not only observed in the skin but also in the internal organs [3]. The vascular injury, followed by impaired neovascularization and vascular remodeling, leads to capillary dilation, stenosis of the arterioles, and the loss of small capillaries [18]. The retinal circulatory system, as one of the most active tissues in the body, is susceptible to injury. Microvasculatory changes in SSc mostly affect the arterioles and small capillaries; therefore, the retinal microvasculature seems to be ideal for disease observation and early change detection.
Some previous studies have revealed retinal findings in SSc patients. Ushiyama et al. demonstrated a higher incidence of retinal pathologies compared to healthy controls (p = 0.011), consisting of hard exudates, vascular tortuosity, microhemorrhages, and macular degeneration [19]. In addition, Gomes et al. observed retinal microvascular abnormalities including arteriolar narrowing, vascular tortuosity, and arteriovenous nicking [14]
In our study, we used OCTA to examine the retinal microvasculature in patients with SSc and in a healthy group.
The FAZ is the central vessel-free area of the macula, surrounded by a network of capillaries. Studies suggest that systemic diseases correlate with the FAZ size. Kok et al. revealed a decreased FAZ area in Ssc patients [20]. In contrast, no statistically significant differences were observed in comparison to healthy subjects regarding the FAZ size in other studies conducted by Hekimsoy et al. [21], Mihalovic et al. [22], and Cutolo et al. [23]. Their findings were similar to ours.
Interestingly, we discovered an increased parafoveal deep vessel density in SSc patients compared to controls. Scleroderma microangiopathy includes reduced normal vessels, avascular areas, and capillary neovascularization, leading to the formation of enlarged and bushy capillaries, as observed in NC [24]. Carnevli et al. [25] revealed abnormal capillaries in the OCTA images of SSc patients, such as megacapillaries, as found in NC. These pathologic giant capillaries and branched vessels might be detected by OCTA and increase the vessel density. However, in most studies, the authors have demonstrated decreased percentages in VD. Hekimsoy et al. [21] revealed a lower superficial vessel density and deep vessel density in the fovea in patients compared to the control group. According to the DVD, similar results were observed by Carnevli et al. [25]. Rothe et al. demonstrated a reduced macular VD in the superficial capillary plexus [26]. Kok et al. demonstrated a reduced capillary density in both the deep and superficial plexuses among SSc patients with retinopathy [20].
The presence of characteristic hallmarks observed in peripheral capillaroscopy correlate with internal organ involvement, e.g., the lungs and kidneys. Furthermore, the changes observed in NC are considered to reflect the stages of microangiopathy [27].
Ushiyama et al. compared the nailfold capillary findings of SSc patients with the retinal findings of SSc patients without them, and the results showed no significant correlation between the two groups [19]. In contrast, our study showed a significant correlation between the nailfold capillaroscopy and OCTA findings among SSc patients. Firstly, the superficial and the deep FAZ areas were correlated with the scleroderma patterns. Our study demonstrated enlarged superficial and deep areas in the early phase of the disease and decreased areas in the late phase. An early pattern is defined as a few giant capillaries, the presence of hemorrhages without the loss of capillaries, and normal capillary perfusion [28]. As the disease progresses, we observe extensive avascular areas, branched capillaries, and the disorganization of the normal capillary architecture [28]. An enlarged FAZ in the early stages of the disease could suggest the dominance of the ischemic and atrophic stages, as well as in the retinal microcirculatory system. Furthermore, we observed a decreased FAZ area in the late pattern due to the presence of abnormal, branched vessels detected by OCTA. To our knowledge, this is the first study that shows FAZ area changes in relation to the stages of NC. Consequently, hemorrhages, characteristic of the early phase of SSc, correlated positively with an increased superficial FAZ.
However, we did not observe a statistically significant correlation between avascular areas, giant capillaries, and branched vessels as detected via the NC and OCTA parameters.
Mihailovic et al., in their study, revealed a significant correlation between the nailfold capillary density and the VD of the choriocapillaris [22]. However, we did not find a correlation between the vessel density and the scleroderma pattern or number of vessels in NC.
Other studies have analyzed OCTA parameters with pulmonary tests in SSc patients. Cerasuolo et al. detected a correlation between chorioretinal perfusion and the diffusion capacity of the lungs for carbon monoxide (DLCO) in scleroderma patients [18].
However, Cutolo et al. did not find a significant difference in the OCTA parameters between SSc patients with interstitial lung disease (ILD) and those without it [23]. Our results showed a significant correlation between the type of lung disease, the FAZ size, and the parafoveal superficial vessel density. SSc patients with nonspecific interstitial pneumonia (NSIP) had a larger FAZ than those with usual interstitial pneumonia (UIP) (p = 0.0484). Moreover, NSIP cases had a higher parafoveal mean superficial vessel density than those with UIP (p = 0.0471).
Moreover, we studied the effects of vasodilator drugs used to manage the consequences of scleroderma vasculopathy, such as digital ulcers, Raynaud’s syndrome, and pulmonary hypertension. We observed a decreased FAZ in both the superficial and deep capillary plexuses in those treated with sildenafil when compared to the SSc group without vasodilator therapy. However, we did not find a significant difference in the OCTA parameters in patients treated with amlodipine. These results indicate that vasodilator therapy can influence the OCTA parameters, reducing the suspected ischemic effect and FAZ enlargement. Polak et al. [29], in their study, investigated the effects of sildenafil on the retinal blood flow in 12 healthy male patients. Their results showed increased retinal blood flow compared to the placebo group (p = 0.029) and no effect on vasodilatation in the retinal arteries or veins.
In our analysis, we did not find significant differences in the OCTA parameters according to hypertension or the immunosuppressive treatment method.
Our study demonstrates the correlation between the peripheral microvascular system and ocular microcirculatory impairment. The strengths of the study included the size of the rheumatological dataset. We also acknowledge the limitations of the research, which include the limited number of participants and the cross-sectional nature of the study.
Nonetheless, our study is the first to reveal a significant correlation between the stage of the disease according to the NC findings and the OCTA parameters.
5. Conclusions
Our investigation showed that the peripheral microvascular system is correlated with ocular microcirculatory impairment. OCTA is a useful tool for the assessment of microvascular impairment in scleroderma patients. Based on our study, we suggest regular ophthalmic examination in SSc patients for the early detection and management of ocular manifestations.
However, further studies are needed on larger numbers of patients to corroborate our results.
Author Contributions
Conceptualization, K.P. and J.G.; methodology, K.P., M.R. and K.R.-P.; software, K.P., M.R., K.R.-P. and J.G.; validation, K.P., J.G. and M.R.; formal analysis, K.P.; investigation, K.P., M.R. and K.R.-P.; resources, M.O. and R.R.; data curation, K.P., M.R. and K.R.-P.; writing—original draft preparation, K.P. and M.R.; writing—review and editing, K.P. and J.G. visualization, K.P. and M.R.; supervision, J.G.; project administration, K.P., K.R.-P., M.R. and J.G. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was approved by the Ethics Committee of the Military Institute of Aviation Medicine and followed the tenets of the Declaration of Helsinki. The ethical approval code was 1/2022; the approval date was 9 February 2022.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Gomes B.d.A., Santhiago M.R., Magalhães P., Kara-Junior N., Azevedo M.N., Moraes H.V., Jr. Ocular findings in patients with systemic sclerosis. Clinics. 2011;66:379–385. doi: 10.1590/S1807-59322011000300003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lear T.B., Lockwood K.C., Larsen M., Tuncer F., Kennerdell J.R., Morse C., Valenzi E., Tabib T., Jurczak M.J., Kass D.J., et al. Kelch-like protein 42 is a profibrotic ubiquitin E3 ligase involved in systemic sclerosis. J. Biol. Chem. 2020;295:4171–4180. doi: 10.1074/jbc.AC119.012066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nikpour M., Stevens W.M., Herrick A.L., Proudman S.M. Epidemiology of systemic sclerosis. Best Pract. Res. Clin. Rheumatol. 2010;24:857–869. doi: 10.1016/j.berh.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Krasowska D., Rudnicka L., Dańczak-Pazdrowska A., Chodorowska G., Woźniacka A., Lis-Święty A., Czuwara J., Maj J., Majewski S., Sysa-Jędrzejowska A., et al. Systemic sclerosis—Diagnostic and therapeutic recommendations of the Polish Dermatological Society. Part 1: Diagnosis and monitoring. Dermatol. Rev./Przegl. Dermatol. 2017;104:483–498. doi: 10.5114/dr.2017.71214. [DOI] [Google Scholar]
- 5.Saygin D., Highland K.B., Tonelli A.R. Microvascular involvement in systemic sclerosis and systemic lupus erythematosus. Microcirculation. 2019;26:e12440. doi: 10.1111/micc.12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pawlik K., Bohdziewicz A., Maciejewska M., Prado J., Czuwara J., Olszewska M., Rudnicka L. Evaluation of cutaneous microcirculation in systemic sclerosis. An update. Dermatol. Rev. 2023;110:499–517. doi: 10.5114/dr.2023.131385. [DOI] [Google Scholar]
- 7.Lambova S.N., Müller-Ladner U. Nailfold capillaroscopy in systemic sclerosis—State of the art: The evolving knowledge about capillaroscopic abnormalities in systemic sclerosis. J. Scleroderma Relat. Disord. 2019;4:200–211. doi: 10.1177/2397198319833486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith V., Herrick A.L., Ingegnoli F., Damjanov N., De Angelis R., Denton C.P., Distler O., Espejo K., Foeldvari I., Frech T., et al. Standardisation of nailfold capillaroscopy for the assessment of patients with Raynaud’s phenomenon and systemic sclerosis. Autoimmun. Rev. 2020;19:102458. doi: 10.1016/j.autrev.2020.102458. [DOI] [PubMed] [Google Scholar]
- 9.Cutolo M., Sulli A., Pizzorni C., Accardo S. Nailfold videocapillaroscopy assessment of microvascular damage in systemic sclerosis. J. Rheumatol. 2000;27:155–160. [PubMed] [Google Scholar]
- 10.Jia Y., Tan O., Tokayer J., Potsaid B., Wang Y., Liu J.J., Kraus M.F., Subhash H., Fujimoto J.G., Hornegger J., et al. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt. Express. 2012;20:4710–4725. doi: 10.1364/OE.20.004710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paczwa K., Gołębiewska J. Optical Coherence Tomography and Optical Coherence Tomography Angiography in Ophthalmology. Pol. J. Aviat. Med. Bioeng. Psychol. 2020;26:45–54. doi: 10.13174/pjambp.17.05.2023.05. [DOI] [Google Scholar]
- 12.de A. F., Gomes B., Santhiago M.R., de Azevedo M.N.L., Moraes H.V., Jr. Evaluation of dry eye signs and symptoms in patients with systemic sclerosis. Graefe’s Arch. Clin. Exp. Ophthalmol. 2012;250:1051–1056. doi: 10.1007/s00417-012-1938-3. [DOI] [PubMed] [Google Scholar]
- 13.Sahin Atik S., Koc F., Akin Sari S., Sefi Yurdakul N., Ozmen M., Akar S. Anterior segment parameters and eyelids in systemic sclerosis. Int. Ophthalmol. 2016;36:577–583. doi: 10.1007/s10792-015-0165-4. [DOI] [PubMed] [Google Scholar]
- 14.Kozikowska M., Luboń W., Kucharz E., Mrukwa-Kominek E. Ocular manifestations in patients with systemic sclerosis. Reumatologia. 2020;58:401–406. doi: 10.5114/reum.2020.102004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tailor R., Gupta A., Herrick A., Kwartz J. Ocular manifestations of scleroderma. Surv. Ophthalmol. 2009;54:292–304. doi: 10.1016/j.survophthal.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 16.Szucs G., Szekanecz Z., Aszalos Z., Gesztelyi R., Zsuga J., Szodoray P., Kemeny-Beke A. A Wide Spectrum of Ocular Manifestations Signify Patients with Systemic Sclerosis. Ocul. Immunol. Inflamm. 2021;29:81–89. doi: 10.1080/09273948.2019.1657467. [DOI] [PubMed] [Google Scholar]
- 17.Cutolo M., Pizzorni C., Secchi M.E., Sulli A. Capillaroscopy. Best Pract. Res. Clin. Rheumatol. 2008;22:1093–1108. doi: 10.1016/j.berh.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Asano Y. Systemic sclerosis. J. Dermatol. 2018;45:128–138. doi: 10.1111/1346-8138.14153. [DOI] [PubMed] [Google Scholar]
- 19.Ushiyama O., Ushiyama K., Yamada T., Koarada S., Tada Y., Suzuki N., Ohta A., Nagasawa K. Retinal findings in systemic sclerosis: A comparison with nailfold capillaroscopic patterns. Ann. Rheum. Dis. 2003;62:204–207. doi: 10.1136/ard.62.3.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kök M., Ayan A., Fatih Küçük M., Erol M.K., Yaprak L. Evaluation of the direct effects on retinal and choroidal microvascularity of systemic scleroderma. Microvasc. Res. 2021;136:104166. doi: 10.1016/j.mvr.2021.104166. [DOI] [PubMed] [Google Scholar]
- 21.Kılınç Hekimsoy H., Şekeroğlu M.A., Koçer A.M., Akdoğan A. Analysis of retinal and choroidal microvasculature in systemic sclerosis: An optical coherence tomography angiography study. Eye. 2020;34:763–770. doi: 10.1038/s41433-019-0591-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mihailovic N., Lahme L., Braasch S., Rosenberger F., Eter N., Ehrchen J., Alnawaiseh M. Altered ocular microvasculature in patients with systemic sclerosis and very early disease of systemic sclerosis using optical coherence tomography angiography. Sci. Rep. 2022;12:10990. doi: 10.1038/s41598-022-14377-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cutolo C.A., Cere A., Toma P., Cannavacciuolo T., Toma C., Balito S., Gerli V., Smith V., Sulli A., Paolino S., et al. Peripheral and ocular microvascular alterations in systemic sclerosis: Observations from capillaroscopic assessments, perfusion peripheral analysis, and optical coherence tomography angiography. Rheumatol. Int. 2024;44:107–118. doi: 10.1007/s00296-023-05495-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manetti M., Guiducci S., Ibba-Manneschi L., Matucci-Cerinic M. Mechanisms in the loss of capillaries in systemic sclerosis: Angiogenesis versus vasculogenesis. J. Cell. Mol. Med. 2010;14:1241–1254. doi: 10.1111/j.1582-4934.2010.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carnevali A., Giannaccare G., Gatti V., Battaglia C., Randazzo G., Yu A.C., Pellegrini M., Ferragina F., Toro M.D., Bruno C., et al. Retinal microcirculation abnormalities in patients with systemic sclerosis: An explorative optical coherence tomography angiography study. Rheumatology. 2021;60:5827–5832. doi: 10.1093/rheumatology/keab258. [DOI] [PubMed] [Google Scholar]
- 26.Rothe M., Rommel F., Klapa S., Humrich J.Y., Nieberding R., Lange T., Sochurek J.A.M., Plöttner P., Grisanti S., Riemekasten G., et al. Evaluation of retinal microvascular perfusion in systemic sclerosis: A case–control study. Ann. Rheum. Dis. 2019;78:857–858. doi: 10.1136/annrheumdis-2018-214541. [DOI] [PubMed] [Google Scholar]
- 27.Wielgosz E. The usefulness of capillaroscopy for assessment of microcirculation disturbances during treatment. Forum Reumatol. 2019;5:65–69. doi: 10.5603/FR.2019.0009. [DOI] [Google Scholar]
- 28.Lemmers J.M.J., Velauthapillai A., van Herwaarden N., Vonk M.C. Change of the microvascularization in systemic sclerosis, a matter of air. Best Pract. Res. Clin. Rheumatol. 2021;35:101683. doi: 10.1016/j.berh.2021.101683. [DOI] [PubMed] [Google Scholar]
- 29.Polak K., Wimpissinger B., Berisha F., Georgopoulos M., Schmetterer L. Effects of Sildenafil on Retinal Blood Flow and Flicker-Induced Retinal Vasodilatation in Healthy Subjects. Investig. Ophthalmol. Vis. Sci. 2003;44:4872–4876. doi: 10.1167/iovs.03-0177. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.