Abstract
The use of conventional chemotherapy in conjunction with targeted and immunotherapy drugs has emerged as an option to limit the severity of side effects in patients diagnosed with head and neck cancer (HNC), particularly oropharyngeal cancer (OPC). OPC prevalence has increased exponentially in the past 30 years due to the prevalence of human papillomavirus (HPV) infection. This study reports a comprehensive review of clinical trials registered in public databases and reported in the literature (PubMed/Medline, Scopus, and ISI web of science databases). Of the 55 clinical trials identified, the majority (83.3%) were conducted after 2015, of which 77.7% were performed in the United States alone. Eight drugs have been approved by the FDA for HNC, including both generic and commercial forms: bleomycin sulfate, cetuximab (Erbitux), docetaxel (Taxotere), hydroxyurea (Hydrea), pembrolizumab (Keytruda), loqtorzi (Toripalimab-tpzi), methotrexate sodium (Trexall), and nivolumab (Opdivo). The most common drugs to treat HPV-associated OPC under these clinical trials and implemented as well for HPV-negative HNC include cisplatin, nivolumab, cetuximab, paclitaxel, pembrolizumab, 5-fluorouracil, and docetaxel. Few studies have highlighted the necessity for new drugs specifically tailored to patients with HPV-associated OPC, where molecular mechanisms and clinical prognosis are distinct from HPV-negative tumors. In this context, we identified most mutated genes found in HPV-associated OPC that can represent potential targets for drug development. These include TP53, PIK3CA, PTEN, NOTCH1, RB1, FAT1, FBXW7, HRAS, KRAS, and CDKN2A.
Keywords: head and neck cancer, oropharyngeal cancer, clinical trial, chemotherapy, immunotherapy, drug discovery
1. Introduction
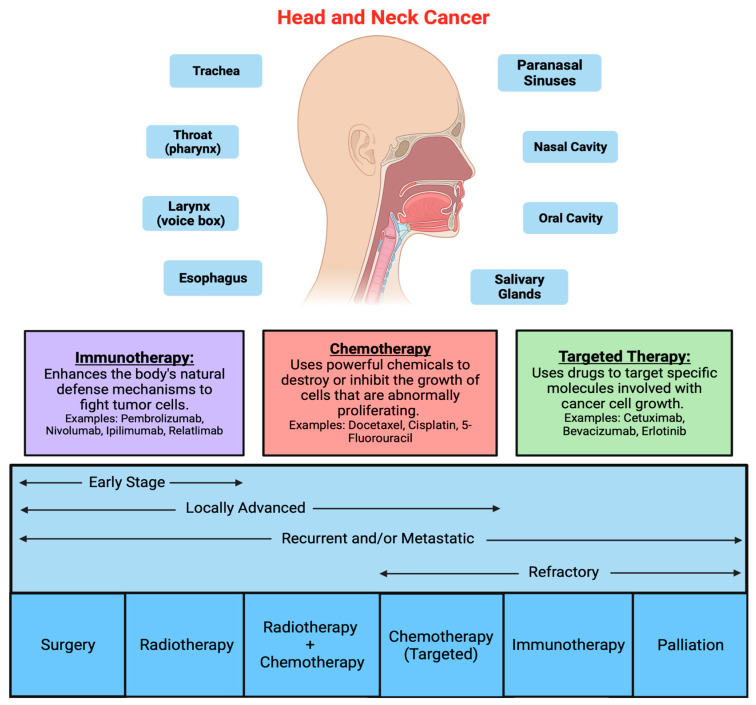
Head and neck mucosal cancer (HNC) involves a heterogeneous group of malignant tumors that can affect different sites of the oral cavity, pharynx, and larynx [1,2,3]. The prevalence of these cancers has increased during the last 30 years [1,2,3]. The main risk factors associated with HNC are alcohol and tobacco consumption, followed by human papillomavirus (HPV) infection [1,2,4], which significantly impacts the patient’s prognosis [5,6,7]. Treatment plans are based on the clinical and pathological stage of the cancer and consist of surgery, radiation therapy, chemotherapy, immunotherapy, or a combination of these treatments’ modalities [8,9] (Figure 1). Surgery is the primary treatment for most resectable oral cancers as well as many larynx cancers. Most tumors in the head and neck region are diagnosed at advanced stages, where chemotherapy combined with radiotherapy are standard treatment. However, this treatment approach is associated with toxicity and severe side effects [10,11,12]. At present, patients with HNC have one of the lowest survival rates among cancer patients despite recent advances in therapeutic discovery [2,13].
Figure 1.
Head and neck cancer (HNC) involves a heterogeneous group of malignant tumors that can affect different sites of the oral cavity, pharynx, and larynx and upper respiratory tract. Treatment plans are based on the clinical and pathological stage of the cancer and consist of surgery, radiation therapy, chemotherapy (red box), immunotherapy (purple box), target therapy (green box), or a combination of these treatments’ modalities.
New therapeutic approaches have been developed in an attempt to improve efficacy while mitigating the undesirable side effects of chemotherapy and chemoradiation and improving the quality of life of patients [2,10]. Several targeted chemo- and immune-therapeutics have been integrated into de-intensified strategies to improve or maintain response rates while minimizing treatment-related morbidities. Due to its unique etiology and superior prognosis, patients with HPV-positive oropharyngeal cancer (OPC), the most common HPV-associated HNC, may benefit from de-escalated strategies. Currently, eight semi-synthetic or synthetic agents have been approved for use against all HNC subtypes, including bleomycin sulfate, cetuximab (Erbitux), docetaxel (Taxotere), hydroxyurea (Hydrea), pembrolizumab (Keytruda), loqtorzi (Toripalimab-tpzi), methotrexate sodium (Trexall), and nivolumab (Opdivo) [14]. Furthermore, based on a systematic data collection from the literature, our team has recently identified commonly mutated genes in HPV-positive OPC, which may provide avenues for novel therapeutic target selection and drug development [15]. This comprehensive review explored the status of approved therapies (chemotherapy, immunotherapy, and target therapy), therapies that are currently under investigation, and potential investigational drugs and treatment strategies that can be further studied in the context of head and neck cancer research.
2. Materials and Methods
This study did not require ethical approval or informed consent, as the analyses were carried out based on data from previously published clinical trials and the published literature.
2.1. Literature Search
This review was carried out through searches in the PubMed/Medline (1946 to 2023), Scopus, and International Statistical Institute (ISI) web of science databases. Briefly, the search included keywords and mesh terms such as “head and neck cancer”, “oropharyngeal cancer”, “chemotherapy”, “drugs”, “treatment”, “chemoradiotherapy”, and “pharmacotherapy”.
2.2. Clinical Trials Selection: Inclusion and Exclusion Criteria
The data from the clinical trials were extracted from the World Health Organization (WHO), International Clinical Trials Registry Platform (ICTRP) (http://apps.who.int/trialsearch/ accessed on 1 February 2024), Current Controlled trials (www.controlledtrials.com/ accessed on 1 February 2024), and Clinical Trials (www.clinicaltrials.gov/ accessed on 1 February 2024).
Filters were applied to select the interventional clinical trials considered in “recruiting”, “not recruiting”, “active, not recruiting”, and “applying by invitation”. The search was performed until 21 November 2023. Clinical trials whose primary objective was treatment, which evaluated the use of drugs combined or not with radiotherapy and immunotherapy, were included. Clinical trials focused only on prevention, supportive care, basic science, behavior, diagnosis, nutritional/supplemental treatment, and radiotherapy alone were excluded. Data extraction included the drugs used for the chemotherapy (CT), the NCT number (number of the register), clinical trial status, HPV status, clinical intervention, clinical phase, population, the date that the study started and was completed, and the country.
2.3. Genes Involved in HPV-Associated OPC
Based on our recent publication compiling data from 38 studies retrieved from four databases (Medline, PubMed, Web of Science, and Scopus), we identified the most cited genes in relation to HPV-associated OPC. These studies spanned 8311 patients across 12 countries. The mutated genes identified most often in these 38 studies included TP53 (n = 22), PIK3CA (n = 20), PTEN (n =16), NOTCH1 (n = 14), RB1 (n = 13), FAT1 (n = 13), FBXW7 (n = 12), HRAS (n = 10), KRAS (n = 10), and CDKN2A (n = 10) [15]. TP53 was the most cited mutated gene among the studies reviewed. These genes were then used to identify potential targets in OPC-related HNC and to assess the feasibility of ongoing clinical trial strategies.
3. Results
3.1. Current Chemotherapy Strategies for the Treatment of HNC
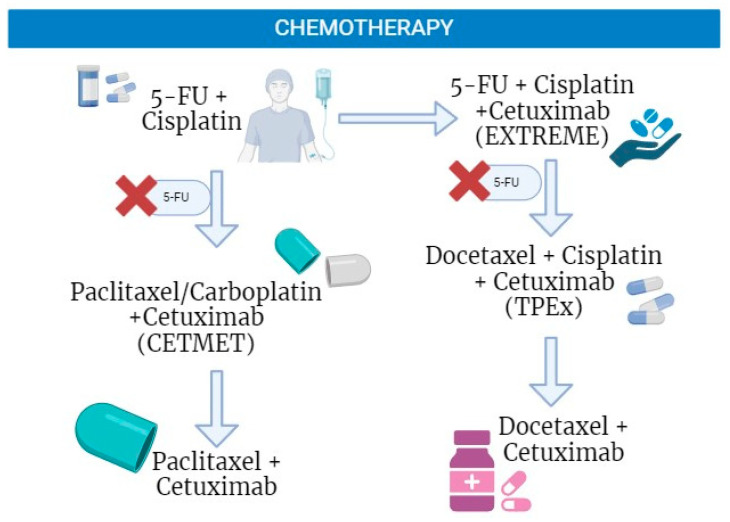
According to the American Society of Clinical Oncology (ASCO) and the European Society for Medical Oncology (ESMO) clinical guidelines for HNC treatment [9], the most used chemotherapy drugs for HNC are cisplatin, 5-FU, cetuximab, docetaxel, and paclitaxel (Figure 2). Treatment combinations are proposed based on the status of the disease progression and contra-indications.
Figure 2.
Current chemotherapy regimens used in the treatment of head and neck cancer. Many clinical trials have studied the benefits of combination therapy for the treatment of head and neck cancer. These studies have concluded that combinations of cetuximab, taxanes, and cisplatin are beneficial treatment alternatives to the EXTREME regimen. This figure was generated using biorender.com.
Cisplatin is an established chemotherapy used in the treatment of solid cancers, including HNC [16]. The compound crosslinks DNA, impeding DNA repair and inducing apoptosis [17]. Similarly, 5-fluorouracil (5-FU) inhibits DNA synthesis and is commonly combined with platinum chemotherapies, though it is less frequently used in antineoplastic therapies [18,19,20]. For refractory tumors, the epidermal growth factor receptor (EGFR) inhibitor cetuximab, and taxanes like docetaxel and paclitaxel, are currently accepted chemotherapies [21,22,23]. Combinations of these drugs have been found to improve patient survival outcomes compared to the use of a single treatment modality [19,23]. The combination of platinum chemotherapy, 5-FU, and cetuximab, known as the EXTREME regimen, aimed to improve overall survival (OS) [24]. The inclusion of cetuximab has shown slightly increased OS (median OS of 10.1 months) compared to platinum–fluorouracil alone (median OS of 7.4 months), at the expense of more toxicity which limited its widespread adoption. Currently, the Keynote 48 regimen is recommended as the standard first-line treatment for patients with recurrent or metastatic HNC [11,23,24]. However, for HPV-positive HNC, recent studies have cautioned the use of adjuvant cetuximab due to worse survival outcomes in comparison to standard platinum chemotherapy [25]. Inferior cetuximab responses in these patients further support the need for specific treatment recommendations based on HPV status.
Taxanes, such as paclitaxel and docetaxel, are semi-synthetic drugs that block the progression of the cell cycle [26,27,28]. Paclitaxel is an option for patients not eligible for platinum therapy [29]. The inclusion of taxanes in chemotherapy may reduce adverse side effects and the number of treatment cycles needed [24]. Studies that used taxanes have shown OS equal to or greater than 10.2 months [30], 14.7 months [23], and 21.3 months [31], and a progression-free survival (PFS) of 6.5 months [30], 5.2 months [23], and 5.8 months [31] compared to the EXTREME regimen which demonstrated an OS of 10.1 months and PFS of 5.6 months [32]. An exception to this increase in OS was observed in a study performed by Klinghammer et al. [33] that obtained a median OS of 8.9 months using docetaxel.
The inclusion of taxanes in chemotherapy provided alternatives to treat HNC patients [23,30]. The replacement of 5-FU with paclitaxel (100 mg/m2) was proposed in a phase II trial as first-line treatment in patients with HNC [34,35]. 5-FU causes adverse side effects, including oral mucositis and acute skin reactions, in addition to longer hospitalization for continuous intravenous infusion [34,35]. The switch to paclitaxel resulted in an overall response rate (ORR) of 40%, a median progression-free survival (PFS) of 5.2 months, and a median OS of 14.7 months [23]. Another phase II trial examined the CETMET regimen, consisting of cetuximab and paclitaxel/carboplatin, as a therapy for HNC. With a median PFS of 6.5 months, and a median OS of 10.2 months, this regimen had similar efficacy and less toxicity than standard treatment with cetuximab and 5-FU/cisplatin or carboplatin [36]. These findings coincide with a retrospective study evaluating a combination of cetuximab, paclitaxel, and carboplatin, which reported good tolerability and survival outcomes similar to the EXTREME regimen [8].
Similarly, docetaxel is another alternative in the EXTREME regimen [36]. Though one phase II trial found high toxicity rates, lower median OS, and no improvements in PFS with docetaxel in this regimen [33], a retrospective evaluation of biweekly treatment with docetaxel (50 mg/m2) and cetuximab (500 mg/m2) was shown to be safe and effective, with a median OS of 8.3 months and PFS of 4.0 months [31]. In platinum-resistant patients, a phase I/II trial revealed promising anti-tumor activity with docetaxel (75 mg/m2) and pembrolizumab (200 mg) followed by pembrolizumab maintenance therapy, with an ORR of 22.7%, median PFS of 5.8 months, and a median OS of 21.3 months [36]. These results are further supported by trials assessing regimens of docetaxel, cisplatin, and cetuximab, which reported high efficacies and patient survival, and favorable tolerability [22,24,37]. Therefore, treatment with cetuximab and docetaxel or paclitaxel is an alternative treatment strategy with satisfactory PFS and OS for cisplatin-resistant patients [29,31].
3.2. Immunotherapy for HNC Patients
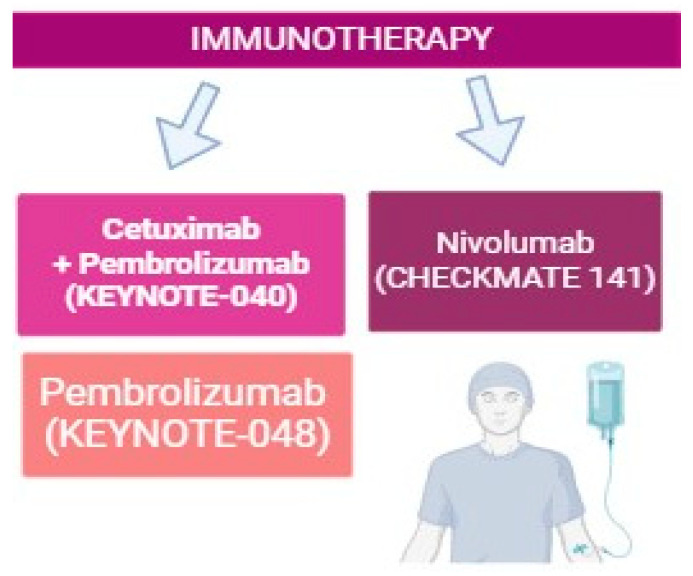
Recently, immunotherapy has emerged at the forefront of anticancer therapy, with two PD-1 inhibitors, pembrolizumab and nivolumab, being approved for use in HNC (Figure 3) [38,39]. The CheckMate 141 trial compared treatment with nivolumab (3 mg per kilogram of body weight) to standard, single-agent, systemic therapy (methotrexate, docetaxel, or cetuximab) [40] Nivolumab treatment resulted in a longer OS and fewer toxicities compared to standard therapy [40]. Rischin et al. [10] demonstrated that the use of pembrolizumab as a monotherapy or in combination with chemotherapy, in addition to prolonging survival, maintains the quality of life of patients, and can be used as a first-line treatment for HNC [10].
Figure 3.
Current immunotherapy regimens used in the treatment of head and neck cancer. Three landmark clinical studies, the Keynote-040, Keynote-048, and Checkmate 141 trials, were crucial in the approval of PD-1/PD-L1 immunotherapies in HNC. This figure was generated using biorender.com.
The phase III clinical trial KEYNOTE-40 assessed the efficacy of standard treatment with methotrexate, docetaxel, or cetuximab with pembrolizumab in the second line or beyond setting [41]. Tumor PD-L1 expression predicted better outcomes for pembrolizumab, with a favorable safety profile and median OS of 8.4 months [41]. Similarly, the KEYNOTE-048 trial compared pembrolizumab in combination with chemotherapy (platinum and 5-FU) to cetuximab with chemotherapy as a first-line treatment [42]. The study reported an improved OS with immunotherapy, demonstrating that pembrolizumab in combination with chemotherapy is effective as a first-line treatment across all subgroups and pembrolizumab alone for patients whose tumors express PDL-1 in more than 1% of tumor cells [42]. During a 4-year follow-up period, both the first-line pembrolizumab and pembrolizumab associated with chemotherapy demonstrated a survival rate improvement in comparison to cetuximab with chemotherapy, with a subset of patients achieving a sustainable remission which was not previously possible with standard chemotherapy [42].
In all large phase III clinical studies, immunotherapy has been shown to be effective regardless of HPV status. Particularly, immunotherapies have emerged as potential de-escalated treatment options for HPV-positive HNC, with HPV-positive patients having some superior outcomes under these therapies. A study by Ferris et al. [40] reported a higher median OS following nivolumab treatment in patients with HPV-positive HNC, compared to those with HPV-negative tumors [40]. This corroborates the need for treatment individualization and de-escalation for HPV-associated HNC.
3.3. Current Status of Clinical Studies on HNC Treatments
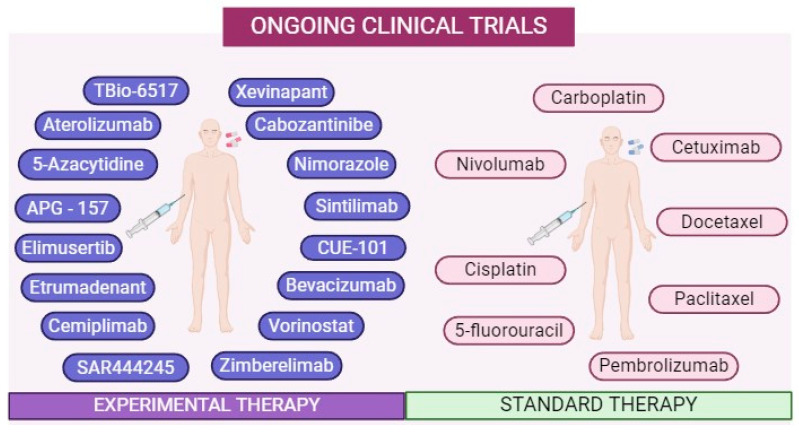
Seventy-three clinical trials were found in HNC, 55 of which were related to OPC (Figure 4 and Table 1). Of these 55 clinical trials, 22 also included cancer in the oral cavity. The most common drugs used to treat HPV-related OPC are the same drugs used to treat HPV-negative HNC, with platinum and taxane-based systemic chemotherapies remaining the most common (Figure 4, Table 1). Indeed, platinum chemotherapy remains the most used intervention in the current clinical trials (n = 42), with cisplatin being the most common (n = 28), followed by carboplatin (n = 14) (Table 1). This is followed by taxane-based chemotherapies (n = 15), with paclitaxel being used more often in clinical trials (n = 12) than docetaxel (n = 3) (Table 1). Contrarily, other systemic chemotherapies like 5-fluorouracil (n = 4), gemcitabine hydrochloride (n = 1), hydroxyurea (n = 1), mitomycin C (n = 1), and 5-azacytidine (n = 1), are less widely used in the current clinical trials (Table 1). Similarly, targeted chemotherapies and novel immunomodulatory agents that remain experimental therapies in HNC, such as carbozantinib (n = 1), CUE-101 (n = 1), and TBio-6517 (n = 1), among others, are much less common among the current trials (Table 1, Figure 4). However, in HPV-positive HNC, immunotherapies are often incorporated into clinical trials (n = 15), with the PD-1 inhibitors nivolumab (n = 8) and pembrolizumab (n = 6) being the predominant immunotherapies studied in HNC (Table 1). Though they are slightly less common, targeted chemotherapies like cetuximab (n = 9), xevinapant (n = 1), vorinostat (n = 1), and bevacizumab (n = 1) are also implemented in some clinical studies (n = 12), with cetuximab being the most used (Table 1), likely due to its approval in this cancer type. In an attempt to reduce the side effects associated with radiation, many clinical trials involve a reduction in radiotherapy doses as a primary intervention (n = 5) or based on patient risk levels (n = 8), in combination with chemotherapy with or without immunotherapy (Table 1 and Figure 4).
Figure 4.
Summary of the drugs in ongoing clinical trials registered in public platforms (https://clinicaltrials.gov). The panel on the right represents the most common drugs used to treat both HPV-positive and HPV-negative oropharyngeal cancer. The panel on the left represents the novel therapeutics currently in phase I, phase II, and phase III clinical trials. This image was created using biorender.com.
Table 1.
Ongoing clinical trials using chemotherapies, targeted therapies, and immunotherapies to treat OPC.
| NCT Number | Study Status | HPV Status | Interventions | Phase | Sample Size | Start and Completion Date | Country |
|---|---|---|---|---|---|---|---|
| NCT05724602 | NR | + | Debio 1143, PLCB | 2 | 230 | 09/2023–10/2029 | NP |
| NCT05721755 | NYR | + | CBDCA, CDDP, 5-FU, PTX, MK-3475 | 3 | 290 | 04/2023–03/2030 | NP |
| NCT05608369 | NYR | − | CDDP, SAHA | 2 | 64 | 05/2023–02/2024 | US |
| NCT05541016 | NYR | + | CDDP, TXT | 2 | 320 | 02/2023–08/2029 | US |
| NCT05535023 | NYR | + | SAR444245, REGN2810 | 2 | 26 | 02/2023–10/2024 | US |
| NCT05419089 | R | + | RDR, CDDP | 2 | 199 | 07/2022–06/2027 | US |
| NCT05317000 | NYR | + | 5-AC, NIVO | 1 | 50 | 02/2023–02/2026 | NP |
| NCT05312710 | R | NM | APG-157 | 2 | 24 | 04/2022–04/2023 | US |
| NCT05268614 | R | + | RT or RDR, CDDP or CBDCA and PTX | 2 | 250 | 05/2022–06/2032 | US |
| NCT05136196 | R | + | Cabozantinib S- malate, NIVO | 2 | 150 | 10/2022–10/2025 | US |
| NCT05108870 | R | + | CBDCA, PTX | 1, 2 | 98 | 08/2022–01/2026 | US |
| NCT05063552 | R | + | MPDL328OA, rhuMab-VEGF, CBDCA, C225, CDDP, TXT | 2, 3 | 430 | 12/2021–12/2027 | US |
| NCT04900623 | R | + | RT or RDR, CDDP or CBDCA and PTX | 2 | 75 | 07/2021–06/2032 | US |
| NCT04892875 | NYR | +/− | AB122, AB 928, CDDP | 1 | 24 | 02/2023–04/2025 | US |
| NCT04862650 | R | + | CBDCA, REGN2810, PTX | 2 | 42 | 11/2021–12/2024 | US |
| NCT04852328 | R | + | CUE-101 | 2 | 30 | 12/2021–10/2025 | US |
| NCT04718415 | R | NM | IBI308, PTX, CBDCA | 2 | 25 | 01/2021–05/2026 | CN |
| NCT04576091 | R | + | BAY-1895344, MK-3475 | 1 | 37 | 02/2021–04/2026 | US |
| NCT04572100 | R | + | PTX, CBDCA RT or RDR | 1 | 36 | 10/2020–03/2023 | US |
| NCT04502407 | R | + | RT, CDDP | 2 | 36 | 02/2021–09/2025 | US |
| NCT04444869 | R | + | CDDP | 2 | 28 | 09/2020–06/2025 | US |
| NCT04301011 | ANR | + | MK-3475 | 1,2 | 27 | 06/2020–12/ 2023 | US, CA, KR |
| NCT04180215 | R | + | HB-201, HB-202 | 1, 2 | 200 | 12/2019–06/2025 | US |
| NCT04124198 | R | NM | CDDP, Nimorazole | - | 138 | 03/2019–01/2029 | DK |
| NCT04106362 | R | + | C225, CDDP | 2 | 70 | 01/2020–07/2024 | US |
| NCT03829722 | ANR | + | NIVO, CBDCA, PTX | 2 | 26 | 09/2019–09/2024 | US |
| NCT03822897 | ANR | + | RT ± CDDP | 2 | 103 | 02/2019–12/2024 | CA |
| NCT03799445 | R | + | MDX-CTLA-4, NIVO | 2 | 180 | 07/2019–12/2023 | US |
| NCT03715946 | ANR | + | NIVO, RDR | 2 | 42 | 11/2018–11/2023 | US |
| NCT03646461 | ANR | E | PCI-32765, C225, NIVO | 2 | 5 | 10/2018–05/2024 | US |
| NCT03621696 | ANR | + | CDDP, RT | 2 | 63 | 10/2018–03/2026 | US |
| NCT03410615 | ANR | + | CDDP, MEDI4736, CP-675 | 2 | 129 | 01/2018–07/2026 | BE, CA |
| NCT03383094 | R | + | MK-3475, CDDP | 2 | 114 | 03/2018–06/2024 | US |
| NCT03370276 | ANR | + | NIVO, C225 | 1, 2 | 95 | 12/2017–11/2023 | US |
| NCT03323463 | R | + | RDR, CDDP, CBDCA, 5-FU | 2 | 300 | 10/2017–10/2024 | US |
| NCT03258554 | ANR | + | C225, MEDI4736 | 2, 3 | 493 | 12/2017–12/2025 | US |
| NCT03215719 | R | + | RT or RDR, CDDP | 2 | 54 | 07/2017–12/2025 | US |
| NCT03174275 | ANR | - | MEDI4736, CBDCA, nab-PTX, CDDP |
2 | 39 | 12/2017–12/ 2026 | US |
| NCT03107182 | ANR | + | nab-PTX, CBDCA, NIVO, CDDP, HU, 5-FU |
2 | 76 | 06/2017–07/2023 | US |
| NCT03088059 | R | - | BIBW 2992 MA2, PD-332991, IPH2201, MEDI4736, CJNJ-6765200, INCAGN01876 |
2 | 340 | 11/2017–12/2025 | BE |
| NCT03082534 | ANR | NM | MK-3475, C225 | 2 | 78 | 03/2017–05/2024 | US |
| NCT03077243 | ANR | + | RT or RDR, CDDP | 2 | 215 | 12/2016–02/2026 | US |
| NCT02918955 | R | NM | RT or RDR, CDDP | 3 | 65 | 10/2016–03/2030 | CH |
| NCT02586207 | ANR | NM | CDDP, MK-3475 | 1 | 59 | 11/2015–09/2023 | US |
| NCT02573493 | ANR | + | nab-PTX, CDDP, C225 | 2 | 96 | 04/2016–12/2029 | US |
| NCT02369458 | ANR | + | MTC, HSP-130 | 2 | 48 | 04/2015–06/2023 | US |
| NCT02281955 | ANR | + | RT or RDR, CDDP | 2 | 115 | 08/2014–11/2024 | US |
| NCT02254278 | ANR | + | CDDP, RDR | 2 | 316 | 10/2014–05/2024 | US |
| NCT02229656 | ANR | − | RT, AZD2281 | 1 | 12 | 09/2014–01/2024 | NL |
| NCT01855451 | ANR | + | C225, RT, CDDP | 3 | 189 | 06/2013–08/2023 | AU |
| NCT01706939 | ANR | + | RDR, CBDCA | 3 | 23 | 09/2012–05/2035 | US |
| NCT00956007 | ANR | NM | C225, RT | 3 | 703 | 11/2009–08/2029 | US |
| NCT00544414 | ANR | E | CDDP, TXT, 5-FU, dFdCyd, leucovorin | 2 | 30 | 06/2000–12/2023 | NP |
| NCT00494182 | ANR | NM | CBDCA, PTX, Sorafenib | 2 | 48 | 04/2007–05/2023 | US |
| NCT03719690 | ANR | NM | Tipifarnib | 2 | 284 | 11/2018–05/2023 | US |
Abbreviations: NM: Not mentioned; NR: Not recruiting; ANR: Active, not recruiting; R: Recruiting; NYR: Not yet recruiting; NP: Not provided; E: Evaluate; US: United States; CN: China; AU: Australia; NL: Netherlands; BE: Belgium; CA: Canada; DK: Denmark; CH: Switzerland; KR: Korea. RT: Standard Radiotherapy; RDR: Reduced Dose Radiation; PTX: paclitaxel; 5-FU: 5-Fluorouracil; CDDP: Cisplatin; C225: Cetuximab; TXT: Docetaxel; Debio 1143: Xevinapant; MK-3475: Pembrolizumab; PLCB: Placebo; CBDCA: Carboplatin; SAHA: Vorinostat; dFdCyd: Gemcitabine hydrochloride; HU: Hydroxyurea; NIVO: Nivolumab; MTC: Mitomycin-C; MDX-CTLA-4: Ipilimumab; 5-AC: 5-azacytidine; rhuMab-VEGF: Bevacizumab.
The United States (n = 42; 77.7%) was the country with the highest number of ongoing clinical trials, followed by Canada (n = 3; 5.5%). These clinical trials began between the years 2000 and 2023 and will be completed between 2023 and 2032. The majority of the clinical trials (83.3%) commenced after 2015. Most studies (n = 44; 81.48%) considered HPV status as an inclusion criterion, while ten studies (18.52%) had no mention of HPV. Regarding the HPV status of participants, the p16 protein was the biological marker used to identify HPV positivity [43].
3.4. Novel Targets for Drug Treatments in HNC
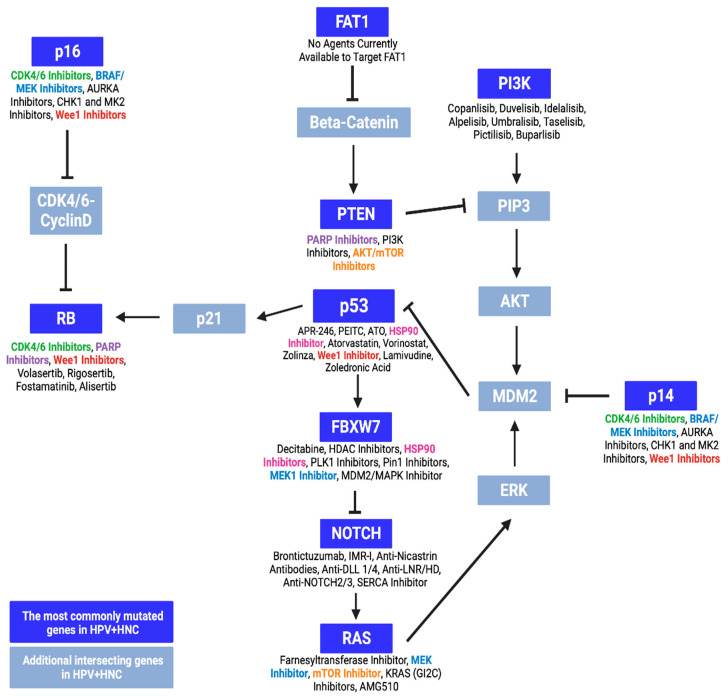
Despite differences in patient outcomes based on HPV infections, the drugs approved and recommended for use in HNC remain the same regardless of HPV status. This leads to HPV-positive patients with superior prognoses undergoing unnecessarily intense treatments which can greatly reduce their quality of life. For this reason, strategies to de-intensify treatments in HPV-positive patients have been explored, including the use of targeted chemotherapy, immunotherapy, and neoadjuvant approach [44]. Due to the distinct genetic landscape and etiology of HPV-positive HNC, it is increasingly recognized as a unique HNC subtype, with its genetic and mutational profile offering new targets for de-escalated drug therapies. Recently, our group identified, based on public data repository, the genes that are commonly mutated in HPV-positive HNC, including TP53, PIK3CA, PTEN, NOTCH1, RB1, FAT1, FBXW7, HRAS, KRAS, and CDKN2A [15]. These genes were involved in proliferative and apoptotic mechanisms, supporting cancer cell growth when dysregulated [15]. As such, drugs that target these genes and their signaling pathways could provide alternative de-intensified treatment strategies for HPV-positive HNC (Figure 5).
Figure 5.
Proteins expressed by the most commonly mutated genes in HPV-associated head and neck cancer (HNC) and their intersecting pathways. These mutated genes include TP53, PIK3CA, PTEN, NOTCH1, RB1, FAT1, FBXW7, HRAS, KRAS, and CDKN2A. The genes and proteins in blue are intersecting genes in the HPV-associated HNC pathway. The drugs currently available, or in development, to target these mutations are listed below with their respective genes/proteins. Some common drugs include HSP90 inhibitors, BRAF/MEK inhibitors, CDK4/6 inhibitors, PARP inhibitors, mTOR inhibitors, and Wee1 inhibitors. Different colored font was added to indicate that certain treatments can target multiple genes and pathways.
Such drugs include cyclin-dependent kinase (CDK) 4 and 6 inhibitors, which may be effective in HNCs with mutated RB1 and CDK2NA, that encode the tumor suppressors RB and p14/p16, respectively (Figure 5). The improper functioning of these regulators will lead to uncontrolled proliferation and cancer [45]. Though three CDK4/6 inhibitors are currently approved in the treatment of breast cancer, none have been approved for HNC despite early clinical and preclinical studies demonstrating their potential application [46]. Particularly, the CDK4/6 inhibitor palbociclib has shown promise when combined with cetuximab. When used as an adjuvant to radiotherapy in HNC, this combined therapy has shown promising efficacy and tolerability in a phase I study [47]. In patients with HPV-negative HNC, the addition of palbociclib to cetuximab induced moderate therapeutic responses, comparable to cetuximab with a placebo [48]. Further analyses have also shown improved survival with palbociclib in HNC patients with CDKN2A and PI3KCA mutations [49]. However, this inhibitor has not shown any clinical benefit in HPV-positive HNC, with palbociclib showing superior anti-tumor activity against HPV-negative HNC cells compared to HPV-positive cells in vitro [50]. The drug has also been found to induce significant adverse effects despite its efficacy in CDKN2A-mutated HNC [51]. Another phase 2 trial (NCT02101034) investigated palbociclib and cetuximab in cetuximab-resistant HPV-related OPC, but only one out of 24 patients achieved an objective response, suggesting that further investigation of this combination is not justified.
Other checkpoint inhibitors with potential applications in HNC are Wee1 inhibitors, which prevent cell cycle arrest [52]. Cancer cells with mutated tumor suppressors involved in the G1/S checkpoint, such as p53 [52] and RB [53], will rely on G2/M arrest for DNA repair, resulting in mitotic catastrophe and synthetic lethality when this mechanism is inhibited [52,54]. Though Wee1 inhibitors have shown promising anti-tumor effects in vitro and in vivo, few studies explore their clinical efficacy in HNC [52]. Nevertheless, in patients with HNC, the Wee1 inhibitor adavosertib has shown favorable safety, tumor response, and survival as an adjuvant to platinum chemoradiotherapy [55] as well as high tolerability and overall responses when combined with cisplatin and docetaxel [56].
Poly-adenosine diphosphate-ribose polymerase (PARP) inhibitors have also emerged as potential treatment alternatives in HNC, particularly among patients with mutations in RB1 and PTEN. These genes encode tumor suppressors that play a role in cell cycle checkpoints and DNA repair [54,57]. As PARP is also involved in the repair of DNA damage, its inhibition in RB1- or PTEN-deficient cancer cells can lead to genomic instability and cell death [58,59,60]. Despite promising preclinical results [61], PARP inhibitors have not been widely accepted in clinical settings for the treatment of HNC. However, they have resulted in favorable outcomes as adjuvants in preliminary clinical studies [61]. When combined with radiotherapy or chemoradiotherapy for HNC, the PARP inhibitor olaparib has been found to maintain high survival outcomes with good safety and tolerability [62,63]. Similarly, the combination of olaparib with carboplatin and the PD-1 inhibitor pembrolizumab was found to induce an overall response rate of 67% when administered as a primary treatment for recurrent or metastatic HNC. As such, PARP inhibitors may provide alternative treatments for HNC, though further investigations are needed for their implementation.
BRAF and MEK inhibitors are commonly used in combination treatments for other cancers with high rates of RAS and CDKN2A mutations [61,64,65]. With the success of these inhibitors in other cancers, their application in HNC could provide new treatment avenues. Though the effects of these drugs in HNC remains unknown, two studies have shown that the MEK inhibitor trametinib may be beneficial against HNC in vitro. The inhibitor has been shown to improve the efficacy of PD-1/PD-L1 immunotherapies in HNC cells [66] and to induce partial cell death in HNC cell lines [67]. Further investigations are required to understand the implications of BRAF and MEK as potential targets in HNC.
Similarly, the farnesyltransferase inhibitor tipifarnib may be a possible alternative treatment for HNC patients with RAS mutations, particularly HRAS mutations (Figure 5). Though larger studies are underway (NCT03719690), the drug has shown promising responses and survival rates in HNC patients with HRAS mutations [68].
HSP90 inhibitors may also be possible treatment options in HNC, particularly in tumors with mutated TP53 genes [69]. As certain cancers rely on the HSP90-mediated stabilization of mutated p53, these inhibitors have been shown to promote cancer cell death and improve survival in vivo [70]. HSP90 inhibitors have also been shown to reduce the expression of pro-proliferative agents, including mutated p53, and promote the production of p21, which halts the cell cycle in vitro [71]. Despite these findings, research on HSP90 inhibitors in HNC is limited, with only one study reporting their benefit in enhancing platinum chemoradiotherapy in HNC cell lines [72].
Finally, AKT and mTOR inhibitors may also be beneficial in HNC treatments, particularly for patients with mutations in PIK3CA, PTEN, and RAS genes. Indeed, the inhibition of downstream effectors of the anti-apoptotic PI3K/AKT pathway has shown success in other cancer types with these mutations [73,74] and has shown promise for HNC treatment in preclinical studies [75]. Few clinical trials have assessed the efficacy of AKT inhibitors in HNC, and mostly reported unfavorable tumor responses [75,76,77]. However, a recent meta-analysis has highlighted the potential benefits of mTOR inhibitors as adjuvants in combination therapies for HNC, though further research is required as these drugs did not induce significant tumor responses when administered alone [78].
4. Discussion
Regardless of the patient’s HPV status, cisplatin, nivolumab, cetuximab, paclitaxel, pembrolizumab, 5-fluorouracil, and docetaxel are the drugs currently used to treat HNC. Due to the high toxicity of most available treatment options, the search for a therapeutic drug presenting high specificity, efficacy, tolerability, and reduced side effects is under investigation and the subject of ongoing clinical trials. Several factors are involved for the selection of the ideal therapy, including the balance between the impact on the patient’s quality of life and the real clinical benefits regarding the survival rates [79].
HPV-associated HNC has clinical and molecular behaviors that differ from HPV-negative tumors [22,37]. Indeed, despite both HNC subtypes having similar differential gene expression, superior prognostic outcomes are seen in HPV-positive HNC [6]. For this reason, novel de-escalated strategies should be clinically trialed in HPV-positive patients to reduce adverse effects. These include advances in adjuvant and neoadjuvant chemotherapies and immunotherapies, a reduction in treatment intensity, or the incorporation of new drugs. Taxanes have shown promise in the treatment of HNC, inducing favorable outcomes when integrated into combination therapies, and providing alternatives for platinum-refractory tumors [21,22,23]. Similarly, the EGFR inhibitor cetuximab has been successfully applied in HNC treatments [32], though strategies involving the drug have been under debate for HPV-positive patients [25]. Conversely, PD-1/PD-L1 inhibitors like nivolumab and pembrolizumab have shown great efficacy against HNC, particularly in HPV-positive patients. Although these drugs have been approved for use in HNC, their success may translate into de-intensified strategies for HPV-positive patients. With a small number of clinical trials in favor of individualizing treatment for HPV-associated HNC, further investigations are needed to broaden treatment options in these patients and to reach a conclusion. Clinical trials focusing on experimental drugs are critical in bridging current treatment gaps and improving patient prognosis and quality of life.
Currently, clinical data that support the use of alternative drugs for the treatment of the 10 most mutated genes (TP53, PIK3CA, PTEN, NOTCH1, RB1, FAT1, FBXW7, HRAS, KRAS, and CDKN2A [15]) in HPV-positive HNC are limited. Despite showing potential as novel HNC targets, few registered ongoing clinical trials are investigating drugs that may target these genes (Table 1). Potential treatment alternatives include BRAF/MEK inhibitors, which have been useful in the treatment of various cancers like CDKN2A-negative melanoma, and CDK4/6 inhibitors, which have been approved to treat breast cancer. Apart from FAT1, the 10 commonly mutated genes mentioned above are druggable, warranting the exploration of potential targeted therapies. However, the successes of other novel therapeutic strategies in HNC cannot be denied. Xevinapant, a pro-apoptotic agent, has shown promising improvements in reducing mortality risks and sustaining locoregional control, though the latter was non-significant at 3-year follow-up [80,81]. Similarly, alternative chemotherapy dosing regimens, like metronomic chemotherapy, have been found to improve progression-free and overall survival [82,83]. Nevertheless, these strategies are not yet validated in the clinical context of HNC, as research on this cancer type is limited, especially for HPV-associated OPC. Thus, further research is required to elucidate the implications of new gene targets and therapeutic regimens in HNC.
5. Conclusions
Despite ongoing efforts to enhance the prognosis of patients with HNC through diverse therapeutic approaches, the complexity and heterogeneity of the disease have delayed the desired revolutionary advancements in treatment. Numerous medications have undergone investigation in clinical trials, either as a single treatment or in combination with other drugs. The effectiveness of chemotherapy, chemoradiotherapy, targeted therapy, and immunotherapy in treating HNC varies based on factors such as disease stage, comorbidities, age, and prior treatments. Immunotherapy has gained prominence due to the pivotal role of the immune system in HNC carcinogenesis. While targeted therapeutics capitalize on molecular insights into cancer biology, their response is limited by the intricate interplay of multiple cell-signaling pathways. To establish their efficacy across diverse HNC cohorts and stages, additional clinical trials that involve innovative approaches and/or targets are necessary.
Author Contributions
Conception and design: S.D.d.S.; development of methodology: I.d.A.F.M., M.A. (Megan Araujo), J.B., M.A. (Mai Atique) and F.F.; acquisition of data: I.d.A.F.M. and M.A. (Megan Araujo); writing, review, and/or revision of the manuscript: I.d.A.F.M., M.A. (Megan Araujo), J.B., M.A. (Mai Atique), F.F., P.R.F.B., K.E., M.H., M.A.-J., M.M., A.M. and S.D.d.S.; administrative, technical, or material support: P.R.F.B., M.H., M.A.-J. and S.D.d.S.; study supervision: S.D.d.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study did not require ethical approval.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research was funded by Emerging Leaders in the Americas Program (IM) through Global Affairs Canada International Scholarships Program (2022–2023). This work was supported by FRQ-S/RSBO (Soutien du RiSBOd aux projets structurants majeurs and major infrastructure), NCOHR (New Frontier Seed Grant), CIHR (2022–2027). This research was made possible thanks to the support of the Dr. Arthur Rosenberg Memorial Fellowship Graduate Scholarship Fund. The authors acknowledge all the valuable support from the Head and Neck Foundation (Jewish General Hospital—Faculty of Medicine—McGill University) and the Marvin Carsley Research Fund.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Guidi A., Codecà C., Ferrari D. Chemotherapy and immunotherapy for recurrent and metastatic head and neck cancer: A systematic review. Med. Oncol. 2018;35:37. doi: 10.1007/s12032-018-1096-5. [DOI] [PubMed] [Google Scholar]
- 2.Liang J., Yang B., Zhou X., Han Q., Zou J., Cheng L. Stimuli-responsive drug delivery systems for head and neck cancer therapy. Drug Deliv. 2021;28:272–284. doi: 10.1080/10717544.2021.1876182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kiyota N., Tahara M., Mizusawa J., Kodaira T., Fujii H., Yamazaki T., Mitani H., Iwae S., Fujimoto Y., Onozawa Y., et al. Head and Neck Cancer Study Group of the Japan Clinical Oncology Group (JCOG-HNCSG). Weekly Cisplatin Plus Radiation for Postoperative Head and Neck Cancer (JCOG1008): A Multicenter, Noninferiority, Phase II/III Randomized Controlled Trial. J. Clin. Oncol. 2022;40:1980–1990. doi: 10.1200/JCO.21.01293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morand G.B., Cardona I., Cruz SB S.C., Mlynarek A.M., Hier M.P., Alaoui-Jamali M.A., da Silva S.D. Therapeutic Vaccines for HPV-Associated Oropharyngeal and Cervical Cancer: The Next De-Intensification Strategy? Int. J. Mol. Sci. 2022;23:8395. doi: 10.3390/ijms23158395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morand G.B., Diaconescu A., Ibrahim I., Lamarche G., Ruas J.S., Dalfen J., Hier M.P., Alaoui-Jamali M.A., Maschietto M., da Silva S.D. Molecular prognostic indicators in HPV-positive oropharyngeal cancer: An updated review. Clin. Exp. Metastasis. 2022;39:407–416. doi: 10.1007/s10585-022-10148-9. [DOI] [PubMed] [Google Scholar]
- 6.Yom S.S., Torres-Saavedra P., Caudell J.J., Waldron J.N., Gillison M.L., Xia P., Truong M.T., Kong C., Jordan R., Subramaniam R.M., et al. Reduced-Dose Radiation Therapy for HPV-Associated Oropharyngeal Carcinoma (NRG Oncology HN002) J. Clin. Oncol. 2021;39:956–965. doi: 10.1200/JCO.20.03128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Concha-Benavente F., Srivastava R.M., Trivedi S., Lei Y., Chandran U., Seethala R.R., Ferris R.L. Identification of the Cell-Intrinsic and -Extrinsic Pathways Downstream of EGFR and IFNγ That Induce PD-L1 Expression in Head and Neck Cancer. Cancer Res. 2016;76:1031–1043. doi: 10.1158/0008-5472.CAN-15-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forman R., Deshpande H., Burtness B., Bhatia A.K. Efficacy and toxicity of weekly paclitaxel, carboplatin, and cetuximab as induction chemotherapy or in cases of metastases or relapse for head and neck cancer with a focus on elderly or frail patients. Head. Neck. 2022;44:1777–1786. doi: 10.1002/hed.27077. [DOI] [PubMed] [Google Scholar]
- 9.Mesia R., Iglesias L., Lambea J., Martínez-Trufero J., Soria A., Taberna M., Trigo J., Chaves M., García-Castaño A., Cruz J. SEOM clinical guidelines for the treatment of head and neck cancer (2020) Clin. Transl. Oncol. 2021;23:913–921. doi: 10.1007/s12094-020-02533-1. Erratum in Clin. Transl. Oncol. 2021, 23, 1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rischin D., Harrington K.J., Greil R., Soulières D., Tahara M., de Castro G., Jr., Psyrri A., Braña I., Neupane P., Bratland Å., et al. Pembrolizumab alone or with chemotherapy for recurrent or metastatic head and neck squamous cell carcinoma: Health-related quality-of-life results from KEYNOTE-048. Oral Oncol. 2022;128:105815. doi: 10.1016/j.oraloncology.2022.105815. [DOI] [PubMed] [Google Scholar]
- 11.Guo Y., Luo Y., Zhang Q., Huang X., Li Z., Shen L., Feng J., Sun Y., Yang K., Ge M., et al. First-line treatment with chemotherapy plus cetuximab in Chinese patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck: Efficacy and safety results of the randomised, phase III CHANGE-2 trial. Eur. J. Cancer. 2021;156:35–45. doi: 10.1016/j.ejca.2021.06.039. [DOI] [PubMed] [Google Scholar]
- 12.Lacas B., Carmel A., Landais C., Wong S.J., Licitra L., Tobias J.S., Burtness B., Ghi M.G., Cohen E.E.W., Grau C., et al. MACH-NC Collaborative Group. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 107 randomized trials and 19,805 patients, on behalf of MACH-NC Group. Radiother. Oncol. 2021;156:281–293. doi: 10.1016/j.radonc.2021.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y.J., Chang J.T., Liao C.T., Wang H.M., Yen T.C., Chiu C.C., Lu Y.C., Li H.F., Cheng A.J. Head and neck cancer in the betel quid chewing area: Recent advances in molecular carcinogenesis. Cancer Sci. 2008;99:1507–1514. doi: 10.1111/j.1349-7006.2008.00863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. [(accessed on 2 February 2024)]; Available online: https://www.cancer.gov/about-cancer/treatment/drugs/head-neck.
- 15.Atique M., Muniz I., Farshadi F., Hier M., Mlynarek A., Macarella M., Maschietto M., Nicolau B., Alaoui-Jamali M.A., da Silva S.D. Genetic Mutations Associated with Inflammatory Response Caused by HPV Integration in Oropharyngeal Squamous Cell Carcinoma. Biomedicines. 2024;12:24. doi: 10.3390/biomedicines12010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pendleton K.P., Grandis J.R. Cisplatin-Based Chemotherapy Options for Recurrent and/or Metastatic Squamous Cell Cancer of the Head and Neck. Clin. Med. Insights Ther. 2013;5:CMT-S10409. doi: 10.4137/CMT.S10409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mittal A., Sharma A. Current status of systemic therapy in head and neck cancer. J. Chemother. 2022;34:9–24. doi: 10.1080/1120009X.2021.1955201. [DOI] [PubMed] [Google Scholar]
- 18.Havelka A.M., Berndtsson M., Olofsson M.H., Shoshan M.C., Linder S. Mechanisms of action of DNA-damaging anticancer drugs in treatment of carcinomas: Is acute apoptosis an “off-target” effect? Mini Rev. Med. Chem. 2007;7:1035–1039. doi: 10.2174/138955707782110196. [DOI] [PubMed] [Google Scholar]
- 19.Longley D.B., Harkin D.P., Johnston P.G. 5-fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer. 2003;3:330–338. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- 20.Novaes V.C.N., Ervolino E., Fernandes G.L., Cunha C.P., Theodoro L.H., Garcia V.G., de Almeida J.M. Influence of the treatment with the antineoplastic agents 5-fluorouracil and cisplatin on the severity of experimental periodontitis in rats. Support Care Cancer. 2022;30:1967–1980. doi: 10.1007/s00520-021-06586-y. [DOI] [PubMed] [Google Scholar]
- 21.Haddad R., Tishler R.B., Norris C.M., Mahadevan A., Busse P., Wirth L., Goguen L.A., Sullivan C.A., Costello R., Case M.A., et al. Docetaxel, cisplatin, 5-fluorouracil (TPF)-based induction chemotherapy for head and neck cancer and the case for sequential, combined-modality treatment. Oncologist. 2003;8:35–44. doi: 10.1634/theoncologist.8-1-35. [DOI] [PubMed] [Google Scholar]
- 22.Posner M.R., Hershock D.M., Blajman C.R., Mickiewicz E., Winquist E., Gorbounova V., Tjulandin S., Shin D.M., Cullen K., Ervin T.J., et al. TAX 324 Study Group. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N. Engl. J. Med. 2007;357:1705–1715. doi: 10.1056/NEJMoa070956. [DOI] [PubMed] [Google Scholar]
- 23.Tahara M., Kiyota N., Yokota T., Hasegawa Y., Muro K., Takahashi S., Onoe T., Homma A., Taguchi J., Suzuki M., et al. Phase II trial of combination treatment with paclitaxel, carboplatin and cetuximab (PCE) as first-line treatment in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck (CSPOR-HN02) Ann. Oncol. 2018;29:1004–1009. doi: 10.1093/annonc/mdy040. [DOI] [PubMed] [Google Scholar]
- 24.Guigay J., Aupérin A., Fayette J., Saada-Bouzid E., Lafond C., Taberna M., Geoffrois L., Martin L., Capitain O., Cupissol D., et al. TTCC, and UniCancer Head and Neck groups. Cetuximab, docetaxel, and cisplatin versus platinum, fluorouracil, and cetuximab as first-line treatment in patients with recurrent or metastatic head and neck squamous-cell carcinoma (GORTEC 2014-01 TPExtreme): A multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2021;22:463–475. doi: 10.1016/S1470-2045(20)30755-5. [DOI] [PubMed] [Google Scholar]
- 25.Rieckmann T., Kriegs M. The failure of cetuximab-based de-intensified regimes for HPV-positive OPSCC: A radiobiologists perspective. Clin. Transl. Radiat. Oncol. 2019;17:47–50. doi: 10.1016/j.ctro.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nawara H.M., Afify S.M., Hassan G., Zahra M.H., Seno A., Seno M. Paclitaxel-Based Chemotherapy Targeting Cancer Stem Cells from Mono- to Combination Therapy. Biomedicines. 2021;9:500. doi: 10.3390/biomedicines9050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newman D.J., Cragg G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020;83:770–803. doi: 10.1021/acs.jnatprod.9b01285. [DOI] [PubMed] [Google Scholar]
- 28.Cardona-Mendoza A., Olivares-Niño G., Díaz-Báez D., Lafaurie G.I., Perdomo S.J. Chemopreventive and Anti-tumor Potential of Natural Products in Oral Cancer. Nutr. Cancer. 2022;74:779–795. doi: 10.1080/01635581.2021.1931698. [DOI] [PubMed] [Google Scholar]
- 29.Bernad I.P., Trufero J.M., Urquizu L.C., Pazo Cid R.A., de Miguel A.C., Agustin M.J., Lanzuela M., Antón A. Activity of weekly paclitaxel-cetuximab chemotherapy in unselected patients with recurrent/metastatic head and neck squamous cell carcinoma: Prognostic factors. Clin. Transl. Oncol. 2017;19:769–776. doi: 10.1007/s12094-016-1604-z. [DOI] [PubMed] [Google Scholar]
- 30.Tsakonas G., Specht L., Kristensen C.A., Moreno M.H.C., Cange H.H., Soderstrom K., Friesland S. Randomized Phase II Study with Cetuximab in Combination with 5-FU and Cisplatin or Carboplatin vs. Cetuximab in Combination with Paclitaxel and Carboplatin for Treatment of Patients with Relapsed or Metastatic Squamous Cell Carcinoma of the Head and Neck (CETMET Trial) Cancers. 2020;12:3110. doi: 10.3390/cancers12113110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fuereder T., Minichsdorfer C., Mittlboeck M., Wagner C., Heller G., Putz E.M., Oberndorfer F., Müllauer L., Aretin M.B., Czerny C., et al. Pembrolizumab plus docetaxel for the treatment of recurrent/metastatic head and neck cancer: A prospective phase I/II study. Oral. Oncol. 2022;124:105634. doi: 10.1016/j.oraloncology.2021.105634. [DOI] [PubMed] [Google Scholar]
- 32.Rivera F., García-Castaño A., Vega N., Vega-Villegas M.E., Gutiérrez-Sanz L. Cetuximab in metastatic or recurrent head and neck cancer: The EXTREME trial. Expert Rev. Anticancer Ther. 2009;9:1421–1428. doi: 10.1586/era.09.113. [DOI] [PubMed] [Google Scholar]
- 33.Klinghammer K., Gauler T., Dietz A., Grünwald V., Stöhlmacher J., Knipping S., Schroeder M., Guntinas-Lichius O., Frickhofen N., Lindeman H.W., et al. Cetuximab, fluorouracil and cisplatin with or without docetaxel for patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck (CeFCiD): A n open-label phase II randomised trial (AIO/IAG-KHT trial 1108) Eur. J. Cancer. 2019;122:53–60. doi: 10.1016/j.ejca.2019.08.018. [DOI] [PubMed] [Google Scholar]
- 34.Rades D., Seidl D., Janssen S., Bajrovic A., Hakim S.G., Wollenberg B., Schild S.E. Do we need 5-FU in addition to cisplatin for chemoradiation of locally advanced head-and-neck cancer? Oral Oncol. 2016;57:40–45. doi: 10.1016/j.oraloncology.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 35.Tribius S., Kronemann S., Kilic Y., Schroeder U., Hakim S., Schild S.E., Rades D. Radiochemotherapy including cisplatin alone versus cisplatin + 5-fluorouracil for locally advanced unresectable stage IV squamous cell carcinoma of the head and neck. Strahlenther Onkol. 2009;185:675–681. doi: 10.1007/s00066-009-1992-x. [DOI] [PubMed] [Google Scholar]
- 36.Posch D., Fuchs H., Kornek G., Grah A., Pammer J., Aretin M.B., Fuereder T. Docetaxel plus cetuximab biweekly is an active regimen for the first-line treatment of patients with recurrent/metastatic head and neck cancer. Sci. Rep. 2016;6:32946. doi: 10.1038/srep32946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Szturz P., Vinches M., Remenár É., van Herpen C.M.L., Abdeddaim C., Stewart J.S., Fortpied C., Vermorken J.B. Prognostic factor analysis and long-term results of the TAX 323 (EORTC 24971) study in unresectable head and neck cancer patients. Eur. J. Cancer. 2021;156:109–118. doi: 10.1016/j.ejca.2021.07.034. [DOI] [PubMed] [Google Scholar]
- 38.Yeh J., Guddati A.K. Cost-effectiveness analysis of nivolumab compared to pembrolizumab in the treatment of recurrent or metastatic squamous cell carcinoma of the head and neck. Am. J. Cancer Res. 2020;10:1821–1826. [PMC free article] [PubMed] [Google Scholar]
- 39.Cohen E.E.W., Bell R.B., Bifulco C.B., Burtness B., Gillison M.L., Harrington K.J., Le Q.T., Lee N.Y., Leidner R., Lewis R.L., et al. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of squamous cell carcinoma of the head and neck (HNSCC) J. Immunother Cancer. 2019;7:184. doi: 10.1186/s40425-019-0662-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferris R.L., Blumenschein G., Jr., Fayette J., Guigay J., Colevas A.D., Licitra L., Harrington K., Kasper S., Vokes E.E., Even C., et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016;375:1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cohen E.E.W., Soulières D., Le Tourneau C., Dinis J., Licitra L., Ahn M.J., Soria A., Machiels J.P., Mach N., Mehra R., et al. KEYNOTE-040 investigators. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): A randomised, open-label, phase 3 study. Lancet. 2019;393:156–167. doi: 10.1016/S0140-6736(18)31999-8. Erratum in Lancet 2019, 393, 132. [DOI] [PubMed] [Google Scholar]
- 42.Burtness B., Harrington K.J., Greil R., Soulières D., Tahara M., de Castro G., Jr., Psyrri A., Basté N., Neupane P., Bratland Å., et al. KEYNOTE-048 Investigators. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet. 2019;394:1915–1928. doi: 10.1016/S0140-6736(19)32591-7. Erratum in Lancet 2021, 397, 2252. [DOI] [PubMed] [Google Scholar]
- 43.Kumar B., Cordell K.G., Lee J.S., Worden F.P., Prince M.E., Tran H.H., Wolf G.T., Urba S.G., Chepeha D.B., Teknos T.N., et al. EGFR, p16, HPV Titer, Bcl-xL and p53, sex, and smoking as indicators of response to therapy and survival in oropharyngeal cancer. J. Clin. Oncol. 2008;26:3128–3137. doi: 10.1200/JCO.2007.12.7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sadeghi N., Khalife S., Mascarella M.A., Ramanakumar A.V., Richardson K., Joshi A.S., Bouganim N., Taheri R., Fuson A., Siegel R. Pathologic response to neoadjuvant chemotherapy in HPV-associated oropharynx cancer. Head Neck. 2020;42:417–425. doi: 10.1002/hed.26022. [DOI] [PubMed] [Google Scholar]
- 45.Riess C., Irmscher N., Salewski I., Strüder D., Classen C.F., Große-Thie C., Junghanss C., Maletzki C. Cyclin-dependent kinase inhibitors in head and neck cancer and glioblastoma-backbone or add-on in immune-oncology? Cancer Metastasis Rev. 2021;40:153–171. doi: 10.1007/s10555-020-09940-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shah M., Nunes M.R., Stearns V. CDK4/6 Inhibitors: Game Changers in the Management of Hormone Receptor–Positive Advanced Breast Cancer? Oncology. 2018;32:216–222. [PMC free article] [PubMed] [Google Scholar]
- 47.Ngamphaiboon N., Pattaranutaporn P., Lukerak S., Siripoon T., Jinawath A., Arsa L., Shantavasinkul P.C., Taonam N., Trachu N., Jinawath N., et al. A Phase I Study of the CDK4/6 Inhibitor, Palbociclib in Combination with Cetuximab and Radiotherapy (IMRT) for Locally Advanced Head and Neck Squamous Cell Carcinoma. Clin. Cancer Res. 2023;30:294–303. doi: 10.1158/1078-0432.CCR-23-2303. [DOI] [PubMed] [Google Scholar]
- 48.Adkins D.R., Lin J.C., Sacco A., Ley J., Oppelt P., Vanchenko V., Komashko N., Yen C.J., Wise-Draper T., Lopez-Picazo Gonzalez J., et al. Palbociclib and cetuximab compared with placebo and cetuximab in platinum-resistant, cetuximab-naïve, human papillomavirus-unrelated recurrent or metastatic head and neck squamous cell carcinoma: A double-blind, randomized, phase 2 trial. Oral Oncol. 2021;115:105192. doi: 10.1016/j.oraloncology.2021.105192. [DOI] [PubMed] [Google Scholar]
- 49.Adkins D., Ley J., Cohen J., Oppelt P. The Potential for Selective Cyclin-Dependent Kinase 4/6 Inhibition in the Therapy for Head and Neck Squamous Cell Carcinoma. Cancer J. 2022;28:377–380. doi: 10.1097/PPO.0000000000000617. [DOI] [PubMed] [Google Scholar]
- 50.Gadsden N.J., Fulcher C.D., Li D., Shrivastava N., Thomas C., Segall J.E., Prystowsky M.B., Schlecht N.F., Gavathiotis E., Ow T.J. Palbociclib Renders Human Papilloma Virus-Negative Head and Neck Squamous Cell Carcinoma Vulnerable to the Senolytic Agent Navitoclax. Mol. Cancer Res. 2021;19:862–873. doi: 10.1158/1541-7786.MCR-20-0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pisick E.P., Rothe M., Mangat P.K., Garrett-Mayer L., Worden F.P., Bauman J.R., Schilsky R.L. Palbociclib (P) in patients (pts) with head and neck cancer (HNC) with CDKN2A loss or mutation: Results from the Targeted Agent and Profiling Utilization Registry (TAPUR) study. JCO. 2021;39:6043. doi: 10.1200/JCO.2021.39.15_suppl.6043. [DOI] [Google Scholar]
- 52.Khan S., Swiecicki P., Doroshow D. Mitotic Checkpoints and the Role of WEE1 Inhibition in Head and Neck Squamous Cell Carcinoma. Cancer J. 2022;28:381–386. doi: 10.1097/PPO.0000000000000613. [DOI] [PubMed] [Google Scholar]
- 53.Al Baghdadi T., Halabi S., Garrett-Mayer E., Mangat P.K., Ahn E.R., Sahai V., Alvarez R.H., Kim E.S., Yost K.J., Rygiel A.L., et al. Palbociclib in Patients with Pancreatic and Biliary Cancer with CDKN2A Alterations: Results from the Targeted Agent and Profiling Utilization Registry Study. JCO Precis Oncol. 2019;3:1–8. doi: 10.1200/PO.19.00124. [DOI] [PubMed] [Google Scholar]
- 54.Linn P., Kohno S., Sheng J., Kulathunga N., Yu H., Zhang Z., Voon D., Watanabe Y., Takahashi C. Targeting RB1 Loss in Cancers. Cancers. 2021;13:3737. doi: 10.3390/cancers13153737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chera B.S., Sheth S.H., Patel S.A., Goldin D., Douglas K.E., Green R.L., Shen C.J., Gupta G.P., Moore D.T., Grilley Olson J.E., et al. Phase 1 trial of adavosertib (AZD1775) in combination with concurrent radiation and cisplatin for intermediate-risk and high-risk head and neck squamous cell carcinoma. Cancer. 2021;127:4447–4454. doi: 10.1002/cncr.33789. [DOI] [PubMed] [Google Scholar]
- 56.Méndez E., Rodriguez C.P., Kao M.C., Raju S., Diab A., Harbison R.A., Konnick E.Q., Mugundu G.M., Santana-Davila R., Martins R., et al. A Phase I Clinical Trial of AZD1775 in Combination with Neoadjuvant Weekly Docetaxel and Cisplatin before Definitive Therapy in Head and Neck Squamous Cell Carcinoma. Clin. Cancer Res. 2018;24:2740–2748. doi: 10.1158/1078-0432.CCR-17-3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mendes-Pereira A.M., Martin S.A., Brough R., McCarthy A., Taylor J.R., Kim J.S., Waldman T., Lord C.J., Ashworth A. Synthetic lethal targeting of PTEN mutant cells with PARP inhibitors. EMBO Mol. Med. 2009;1:315–322. doi: 10.1002/emmm.200900041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zamalloa L.G., Pruitt M.M., Hermance N.M., Gali H., Flynn R.L., Manning A.L. RB loss sensitizes cells to replication-associated DNA damage after PARP inhibition by trapping. Life Sci. Alliance. 2023;6:e202302067. doi: 10.26508/lsa.202302067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zoumpoulidou G., Alvarez-Mendoza C., Mancusi C., Ahmed R.M., Denman M., Steele C.D., Mittnacht S. Therapeutic vulnerability to PARP1,2 inhibition in RB1-mutant osteosarcoma. Nat. Commun. 2021;12:7064. doi: 10.1038/s41467-021-27291-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Glorieux M., Dok R., Nuyts S. Novel DNA targeted therapies for head and neck cancers: Clinical potential and biomarkers. Oncotarget. 2017;8:81662–81678. doi: 10.18632/oncotarget.20953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moutafi M., Economopoulou P., Rimm D., Psyrri A. PARP inhibitors in head and neck cancer: Molecular mechanisms, preclinical and clinical data. Oral Oncol. 2021;117:105292. doi: 10.1016/j.oraloncology.2021.105292. [DOI] [PubMed] [Google Scholar]
- 62.Navran A., Al-Mamgani A., Elzinga H., Kessels R., Vens C., Tesselaar M., van den Brekel M., de Haan R., van Triest B., Verheij M. Phase I feasibility study of Olaparib in combination with loco-regional radiotherapy in head and neck squamous cell carcinoma. Clin. Transl. Radiat Oncol. 2023;44:100698. doi: 10.1016/j.ctro.2023.100698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Karam S.D., Reddy K., Blatchford P.J., Waxweiler T., DeLouize A.M., Oweida A., Somerset H., Marshall C., Young C., Davies K.D., et al. Final Report of a Phase I Trial of Olaparib with Cetuximab and Radiation for Heavy Smoker Patients with Locally Advanced Head and Neck Cancer. Clin. Cancer Res. 2018;24:4949–4959. doi: 10.1158/1078-0432.CCR-18-0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kreuger I.Z.M., Slieker R.C., van Groningen T., van Doorn R. Therapeutic Strategies for Targeting CDKN2A Loss in Melanoma. J. Invest. Dermatol. 2023;143:18–25.e1. doi: 10.1016/j.jid.2022.07.016. [DOI] [PubMed] [Google Scholar]
- 65.Spagnolo F., Dalmasso B., Tanda E., Potrony M., Puig S., van Doorn R., Kapiteijn E., Queirolo P., Helgadottir H., Ghiorzo P. Efficacy of BRAF and MEK Inhibition in Patients with BRAF-Mutant Advanced Melanoma and Germline CDKN2A Pathogenic Variants. Cancers. 2021;13:2440. doi: 10.3390/cancers13102440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kang S.H., Keam B., Ahn Y.O., Park H.R., Kim M., Kim T.M., Kim D.W., Heo D.S. Inhibition of MEK with trametinib enhances the efficacy of anti-PD-L1 inhibitor by regulating anti-tumor immunity in head and neck squamous cell carcinoma. Oncoimmunology. 2018;8:e1515057. doi: 10.1080/2162402X.2018.1515057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gurbi B., Brauswetter D., Pénzes K., Varga A., Krenács T., Dános K., Birtalan E., Tamás L., Csala M. MEK Is a Potential Indirect Target in Subtypes of Head and Neck Cancers. Int. J. Mol. Sci. 2023;24:2782. doi: 10.3390/ijms24032782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ho A.L., Brana I., Haddad R., Bauman J., Bible K., Oosting S., Wong D.J., Ahn M.J., Boni V., Even C., et al. Tipifarnib in Head and Neck Squamous Cell Carcinoma with HRAS Mutations. J. Clin. Oncol. 2021;39:1856–1864. doi: 10.1200/JCO.20.02903. Erratum in J. Clin. Oncol. 2021, 39, 3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li M., Sun D., Song N., Chen X., Zhang X., Zheng W., Yu Y., Han C. Mutant p53 in head and neck squamous cell carcinoma: Molecular mechanism of gainoffunction and targeting therapy (Review) Oncol. Rep. 2023;50:162. doi: 10.3892/or.2023.8599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alexandrova E.M., Yallowitz A.R., Li D., Xu S., Schulz R., Proia D.A., Moll U.M. Improving survival by exploiting tumour dependence on stabilized mutant p53 for treatment. Nature. 2015;523:352–356. doi: 10.1038/nature14430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lin K., Rockliffe N., Johnson G.G., Sherrington P.D., Pettitt A.R. Hsp90 inhibition has opposing effects on wild-type and mutant p53 and induces p21 expression and cytotoxicity irrespective of p53/ATM status in chronic lymphocytic leukaemia cells. Oncogene. 2008;27:2445–2455. doi: 10.1038/sj.onc.1210893. [DOI] [PubMed] [Google Scholar]
- 72.McLaughlin M., Barker H.E., Khan A.A., Pedersen M., Dillon M., Mansfield D.C., Patel R., Kyula J.N., Bhide S.A., Newbold K.L., et al. HSP90 inhibition sensitizes head and neck cancer to platin-based chemoradiotherapy by modulation of the DNA damage response resulting in chromosomal fragmentation. BMC Cancer. 2017;17:86. doi: 10.1186/s12885-017-3084-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Janku F., Wheler J.J., Westin S.N., Moulder S.L., Naing A., Tsimberidou A.M., Fu S., Falchook G.S., Hong D.S., Garrido-Laguna I., et al. PI3K/AKT/mTOR inhibitors in patients with breast and gynecologic malignancies harboring PIK3CA mutations. J. Clin. Oncol. 2012;30:777–782. doi: 10.1200/JCO.2011.36.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Janku F., Tsimberidou A.M., Garrido-Laguna I., Wang X., Luthra R., Hong D.S., Naing A., Falchook G.S., Moroney J.W., Piha-Paul S.A., et al. PIK3CA mutations in patients with advanced cancers treated with PI3K/AKT/mTOR axis inhibitors. Mol. Cancer Ther. 2011;10:558–565. doi: 10.1158/1535-7163.MCT-10-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Marquard F.E., Jücker M. PI3K/AKT/mTOR signaling as a molecular target in head and neck cancer. Biochem. Pharmacol. 2020;172:113729. doi: 10.1016/j.bcp.2019.113729. [DOI] [PubMed] [Google Scholar]
- 76.Ma B.B., Goh B.C., Lim W.T., Hui E.P., Tan E.H., Lopes G., Lo K.W., Li L., Loong H., Foster N.R., et al. Multicenter phase II study of the AKT inhibitor MK-2206 in recurrent or metastatic nasopharyngeal carcinoma from patients in the mayo phase II consortium and the cancer therapeutics research group (MC1079) Investig. New Drugs. 2015;33:985–991. doi: 10.1007/s10637-015-0264-0. [DOI] [PubMed] [Google Scholar]
- 77.Argiris A., Cohen E., Karrison T., Esparaz B., Mauer A., Ansari R., Wong S., Lu Y., Pins M., Dancey J., et al. A phase II trial of perifosine, an oral alkylphospholipid, in recurrent or metastatic head and neck cancer. Cancer Biol. Ther. 2006;5:766–770. doi: 10.4161/cbt.5.7.2874. [DOI] [PubMed] [Google Scholar]
- 78.Patel J., Nguyen S.A., Ogretmen B., Gutkind J.S., Nathan C.A., Day T. mTOR inhibitor use in head and neck squamous cell carcinoma: A meta-analysis on survival, tumor response, and toxicity. Laryngoscope Investig. Otolaryngol. 2020;5:243–255. doi: 10.1002/lio2.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Golusinski P., Corry J., Poorten V.V., Simo R., Sjögren E., Mäkitie A., Kowalski L.P., Langendijk J., Braakhuis B.J.M., Takes R.P., et al. De-escalation studies in HPV-positive oropharyngeal cancer: How should we proceed? Oral Oncol. 2021;123:105620. doi: 10.1016/j.oraloncology.2021.105620. [DOI] [PubMed] [Google Scholar]
- 80.Tao Y., Sun X.S., Pointreau Y., Le Tourneau C., Sire C., Kaminsky M.C., Coutte A., Alfonsi M., Calderon B., Boisselier P., et al. Extended follow-up of a phase 2 trial of xevinapant plus chemoradiotherapy in high-risk locally advanced squamous cell carcinoma of the head and neck: A randomised clinical trial. Eur. J. Cancer. 2023;183:24–37. doi: 10.1016/j.ejca.2022.12.015. [DOI] [PubMed] [Google Scholar]
- 81.Sun X.S., Tao Y., Le Tourneau C., Pointreau Y., Sire C., Kaminsky M.C., Coutte A., Alfonsi M., Boisselier P., Martin L., et al. Debio 1143 and high-dose cisplatin chemoradiotherapy in high-risk locoregionally advanced squamous cell carcinoma of the head and neck: A double-blind, multicentre, randomised, phase 2 study. Lancet Oncol. 2020;21:1173–1187. doi: 10.1016/S1470-2045(20)30327-2. [DOI] [PubMed] [Google Scholar]
- 82.Patil V.M., Noronha V., Joshi A., Muddu V.K., Dhumal S., Bhosale B., Arya S., Juvekar S., Banavali S., D’Cruz A., et al. A prospective randomized phase II study comparing metronomic chemotherapy with chemotherapy (single agent cisplatin), in patients with metastatic, relapsed or inoperable squamous cell carcinoma of head and neck. Oral Oncol. 2015;51:279–286. doi: 10.1016/j.oraloncology.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 83.Patil V., Noronha V., Dhumal S.B., Joshi A., Menon N., Bhattacharjee A., Kulkarni S., Ankathi S.K., Mahajan A., Sable N., et al. Low-cost oral metronomic chemotherapy versus intravenous cisplatin in patients with recurrent, metastatic, inoperable head and neck carcinoma: An open-label, parallel-group, non-inferiority, randomised, phase 3 trial. Lancet Glob. Health. 2020;8:e1213–e1222. doi: 10.1016/S2214-109X(20)30275-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.