Abstract
Mixed infection of cells with both Moloney murine leukemia virus (MoMLV) and related or heterologous viruses produces progeny pseudotype virions bearing the MoMLV genome encapsulated by the envelope of the other virus. In this study, pseudotype formation between MoMLV and the prototype parainfluenza virus Sendai virus (SV) was investigated. We report for the first time that SV infection of MoMLV producer cells results in the formation of MoMLV(SV) pseudotypes, which display a largely extended host range compared to that of MoMLV particles. This could be associated with SV hemagglutinin-neuraminidase (SV-HN) glycoprotein incorporation into MoMLV envelopes. In contrast, solitary incorporation of the other SV glycoprotein, SV fusion protein (SV-F), resulted in a distinct and narrow extension of the MoMLV host range to asialoglycoprotein receptor (ASGP-R)-positive cells (e.g., cultured human hepatoma cells). Since stably ASGP-R cDNA-transfected MDCK cells, but not parental ASGP-R-negative MDCK cells, were found to be transduced by MoMLV(SV-F) pseudotypes and transduction of ASGP-R-expressing cells was found to be inhibited by ASGP-R antiserum, a direct proof for the ASGP-R-restricted tropism of MoMLV(SV-F) pseudotypes was provided. Cultivation of ASGP-R-positive HepG2 hepatoma cells on Transwell-COL membranes led to a significant enhancement of MoMLV(SV-F) titers in subsequent flowthrough transduction experiments, thereby suggesting the importance of ASGP-R accessibility at the basolateral domain for MoMLV(SV-F) pseudotype transduction. The availability of such ASGP-R-restricted MoMLV(SV-F)-pseudotyped vectors opens up new perspectives for future liver-restricted therapeutic gene transfer applications.
Formation of viral pseudotypes is a well-known natural phenomenon frequently occurring during a dual infection by different enveloped viruses (38). Based on this principle, recombinant technology allows a deliberate and systematic modification of the natural tropism of a large variety of viruses and virus-based vector systems (2, 9, 21, 26, 35). Several investigators have described retroviral pseudotypes based on Moloney murine leukemia virus (MoMLV) vectors whose host cell range has been altered by substitution of envelope proteins from related and heterologous viruses (4, 9, 17, 23, 28). However, employment of the pseudotype technology for the targeting of MoMLV pseudotype virions to the hepatocyte-specific asialoglycoprotein receptor (ASGP-R) has not been described so far.
The ASGP-R functions as an uptake system for desialylated glycoproteins (32), and its ligand specificity is defined by the recognition of terminal galactose residues in defined biantennary, triantennary, and tetra-antennary oligosaccharide structures (13). Interestingly, Sendai virus (SV), a paramyxovirus, has been found to interact with the ASGP-R (19). Usually, the host cell tropism of wild-type SV is determined by its two surface glycoproteins: SV hemagglutinin-neuraminidase (SV-HN) binds to sialic acid-containing ganglioside receptors (SA-R) ubiquitously expressed on the surface of virtually all eucaryotic cells (20), followed by SV fusion glycoprotein (SV-F)-mediated fusion of the viral envelope with the cell membrane (16). Infection studies after enzymatic destruction of conventional SV SA-R revealed that only ASGP-R-expressing cells are still infectable by SV (3). Interestingly, SV infection via ASGP-R was found to be nearly as efficient as infection via conventional SA-R. It was concluded that SV-F—beyond its well-characterized membrane fusion property—also functions as a ligand for the hepatocyte-specific ASGP-R (3, 19), since it exhibits suitable carbohydrate structures for the interaction with the ASGP-R. A potential pseudotype formation between MoMLV and SV has not been investigated yet. However, formation of stable pseudotypes between vesicular stomatitis virus (VSV) and SV (15) or the closely related SV5 (6) was demonstrated a long time ago. Since pseudotype formation between MoMLV and VSV (35) as well as between VSV and SV has been documented, it was tempting to speculate that pseudotype formation between MoMLV and SV could also take place.
In this study, formation of MoMLV(SV) pseudotypes was investigated both by SV infection and expression of recombinant SV-F in ecotropic MoMLV packaging cells. Our results demonstrate that MoMLV-based retroviral vectors can be pseudotyped with both SV envelope glycoproteins, SV-HN and SV-F, and that MoMLV(SV-F) pseudotypes are targeted specifically to ASGP-R-expressing cells.
Generation of MoMLV(SV) pseudotypes.
The interaction between SV glycoproteins SV-HN and SV-F and the MoMLV envelope was studied by introducing the neor gene as a selectable marker with the postulated pseudotype populations into cells capable or incapable of supporting MoMLV transduction. Since hamster cells are not susceptible to infection with MoMLV-based retroviruses because of the absence of retrovirus-specific cell surface receptors (12), but are susceptible to infection with SV (25), potential MoMLV(SV) pseudotypes were assayed on BHK-21 hamster cells. For generation of MoMLV(SV) pseudotypes, ecotropic PE501 packaging cells (24) (5 × 105 cells per 60-mm-diameter petri dish) were transiently transfected with the retroviral vector pLXSN (22) containing the neor gene under the control of the simian virus 40 (SV40) enhancer and promoter (Fig. 1) and additionally infected with SV 24 h following transfection by incubation with SV (diluted in serum-free Dulbecco’s modified Eagle’s medium [DMEM] at a multiplicity of infection of 10) for 1 h. The SV-containing medium was then replaced by DMEM supplemented with 1% Nutridoma SR, which was employed as serum replacement, since serum-free conditions were required for subsequent SV-F0 precursor activation by acetylated trypsin. Twelve hours later, the virus medium potentially containing pseudotype particles as well as wild-type SV was harvested, and conversion of F0 precursor protein molecules into the fusion-active F1-F2 form was performed by treatment with acetylated trypsin (1 μg/ml) for 30 min at 37°C. Since this activation was performed only once prior to transduction, a continuous spreading out of the SV wild type in the target cell monolayers could not take place (30). Therefore, destruction of the target cell monolayers was avoided, enabling the detection of putative MoMLV(SV) pseudotype particles. The activated virus suspension was used for Polybrene-enhanced (5 μg/ml) transduction of both BHK-21 cells and mouse NIH 3T3 fibroblasts, a cell line susceptible to MoMLV infection. Supernatants obtained with serum-free DMEM containing 1% Nutridoma SR medium yielded 3.8 × 103 CFU/ml (Table 1) on NIH 3T3 cells. Interestingly, BHK-21 cells lacking the ecotropic receptor were also found to be able to survive G418 selection at a titer of 2.0 × 103 CFU/ml (Table 1). In contrast, BHK-21 control cultures either infected with SV (Table 1) or transduced with ecotropic retrovirus (pLXSN) did not (Table 1). In a further control experiment, it was not possible to transduce BHK-21 cells with a mixture of recombinant ecotropic retrovirus and SV (data not shown), which demonstrates that SV by itself is not able to facilitate the entry of ecotropic MoMLV into recipient cells lacking the ecotropic receptor. Taken together, these results indicate that MoMLV(SV) pseudotype particles have been generated following SV infection of ecotropic retrovirus-producing packaging cells, which exhibit an extended host range compared to MoMLV virions. This extended host cell tropism of MoMLV(SV) pseudotypes is determined by the receptor binding properties of the incorporated SV-HN glycoprotein, which binds to ubiquitously expressed sialic acid-containing receptors (20). Therefore, recombinant MoMLV(SV-[HN+F]) pseudotypes are supposed to exhibit a largely extended host cell tropism [covering virtually all eucaryotic cell types and tissues and thereby resembling MoMLV(VSV-G) pseudotype properties (4, 9)]. In contrast, a hepatocyte-restricted pattern is expected for MoMLV(SV-F) pseudotypes as a consequence of SV-F’s function as a specific ligand for the hepatocyte-specific ASGP-R (3, 19). Since MoMLV pseudotype vectors restricted to a solid human organ have not been described so far, we next focused on the generation of recombinant MoMLV(SV-F) pseudotypes.
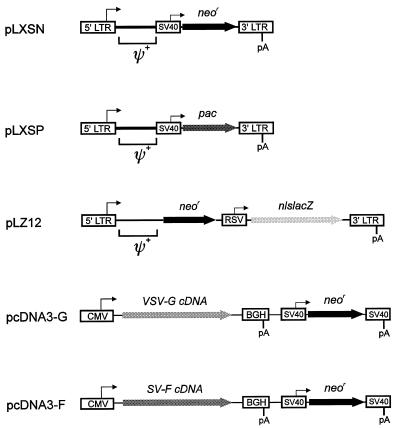
FIG. 1.
Retroviral vectors and virus surface protein expression vectors. Retroviral vector pLXSN (22) contains the neomycin phosphotransferase reporter gene (neor) under the control of the SV40 enhancer and early promoter. In order to generate retroviral vector pLXSP, which transduces the puromycin resistance gene (pac [i.e., puromycin acetyltransferase]), pLXSN was digested with NcoI, followed by ligation of the resulting 5,146-bp pLXSN fragment to the 848-bp fragment of NcoI-digested plasmid pBSpacΔP (8) containing the 3′-end of the SV40 early promoter together with the entire pac gene. Retroviral double-reporter vector pLZ12 (29) contains the neor gene under the control of the MoMLV long terminal repeat (LTR) promoter and enhancer and the β-galactosidase reporter gene attached to a nuclear location signal (nlslacZ) under the control of a truncated Rous sarcoma virus (RSV) LTR. VSV-G expression vector pcDNA3-G was constructed by inserting the 1.6-kb VSV-G cDNA XhoI-XhoI fragment of plasmid pSVGL1 (27) into the XhoI site of mammalian expression vector pcDNA3 (Invitrogen, Leek, The Netherlands). SV-F expression vector pcDNA3-F was constructed by ligation of the 1.7-kb SV-F cDNA EcoRI-XhoI fragment of pUC29-F (14) with EcoRI-XhoI-digested plasmid pcDNA3. Transient and stable transfections of all plasmids were carried out with 3 μg of DNA and 20 μl of Lipofectamine (Life Technologies, Eggenstein, Germany) per 5 × 105 packaging cells according the manufacturer’s instructions. 5′ LTR, Moloney murine sarcoma virus LTR; 3′ LTR-pA, MoMLV LTR; RSV, truncated RSV LTR; ψ+, extended packaging signal; CMV, human cytomegalovirus immediate-early promoter; SV40, SV40 early promoter and enhancer; SV-pA, SV40 polyadenylation site; BGH-pA, bovine growth hormone polyadenylation site. The figure is not drawn to scale.
TABLE 1.
Pseudotyping of MoMLV particles with envelope proteins SV-HN and SV-Fa
| Retroviral vector | SV infection | Viral titer (103 CFU/ml) on cells
|
|
|---|---|---|---|
| NIH 3T3 | BHK-21 | ||
| pLXSN | − | 3.8 | 0.0 |
| pLXSN | + | 2.4 | 2.0 |
| pUC18 | + | 0.0 | 0.0 |
Ecotropic packaging cells PE501 were transfected with retroviral vector pLXSN or mock plasmid pUC18. Twenty-four hours later, cells were infected with SV (+) or transfection medium was replaced with fresh medium (−). Thirty-six hours after transfection, supernatants were used to transduce NIH 3T3 and BHK-21 cells, followed by G418 selection with 600 μg of G418 per ml for both cell lines.
Generation of recombinant MoMLV(SV-F) pseudotypes.
Retroviral vector pLXSN and surface glycoprotein expression vectors pcDNA3-F (SV-F [Fig. 1]) or pcDNA3-G (VSV-G, positive control [Fig. 1]) were transiently cotransfected into ecotropic packaging cell line PE501. Subsequently, the produced supernatant was employed for transduction of ASGP-R-negative BHK-21 and NIH 3T3 cells as well as ASGP-R-positive HepG2 human hepatoma cells. As expected, viral supernatant generated by pLXSN–pcDNA3-G cotransfection of PE501 packaging cells (positive control) was found to transduce both tested target cell lines, NIH 3T3 and BHK-21 (Table 2), serving as an internal standard for the feasibility of this completely transient approach of generating functional MoMLV pseudotypes.
TABLE 2.
Pseudotyping of MoMLV particles with recombinant SV-F or VSV-Ga
| Surface protein expression vector | Viral titer (102 CFU/ml) on cells
|
||
|---|---|---|---|
| NIH 3T3 | BHK-21 | HepG2 | |
| pcDNA3 | 19.0 | 0.0 | 0.0 |
| pcDNA3-G | 6.0 | 1.8 | NDb |
| pcDNA3-F | 2.4 | 0.0 | 1.0 |
Ecotropic packaging cells PE501 were cotransfected with retroviral vector pLXSN and a surface protein expression vector (pcDNA3-F or pcDNA3-G) or parental plasmid pcDNA3. Forty-eight hours after transfection, activated supernatants were used to transduce NIH 3T3, BHK-21 and HepG2 cells, followed by G418 selection (with 600 μg of G418 per ml for NIH 3T3 and BHK-21 cells and 1,000 μg of G418 per ml for HepG2 cells).
ND, not determined.
Transduction with supernatant generated by pLXSN–pcDNA3-F cotransfection resulted in a titer of 1.0 × 102 CFU/ml on ASGP-R-positive HepG2 cells, whereas ASGP-R-negative BHK-21 target cells were found not to be transduced at all (Table 2). Supernatant generated by pLXSN–pcDNA3 cotransfection of PE501 packaging cells (negative control) was only able to transduce NIH 3T3 target cells, but failed to transduce the cell lines BHK-21 and HepG2 (Table 2). These results demonstrate that recombinant MoMLV(SV-F) pseudotypes have been generated, which, as a consequence of the specific SV-F–ASGP-R interaction, are able to transduce only ASGP-R-positive cells (e.g., ASGP-R-positive human hepatoma cell line HepG2). The decreased efficiency of NIH 3T3 target cell transduction with MoMLV(SV-F) pseudotypes (2.4 × 102 versus 3.8 × 103 CFU/ml when ecotropic MoMLV particles are used [Table 2]) might reflect a direct inhibition of REV env receptor binding and/or target cell fusion function by SV-F. Alternatively, incorporation of SV-F into the MoMLV(SV-F) envelope might diminish the absolute number of incorporated REV env molecules, thereby reducing virion affinity to the ecotropic receptor.
Generally, for efficient pseudotyping of MoMLV virions, packaging cell lines exhibiting a stable expression of the heterologous glycoprotein are required. However, the efficient expression of viral glycoproteins can be toxic to mammalian cells, as demonstrated for VSV-G (4). Concerning SV-F, efficient accumulation on the surface of packaging cells potentially could lead to syncytium formation, which is a prominent feature of the cytopathic effect produced by parainfluenza viruses in cell culture (30). To directly address potential side effects of stable SV-F expression on MoMLV packaging cells, we therefore first aimed at the generation of stable monotransfected pseudotype packaging cells (additionally expressing only the SV-F cDNA).
Generation of stable SV-F expressing pseudotype packaging cell line FE21.
Cell line PE501 was stably transfected with SV-F expression vector pcDNA3-F. Following G418 selection (with 600 μg of G418 per ml), resistant clones were picked and expanded. Subsequently, individual clones were analyzed for their transduction capability following transient transfection with retroviral vector pLXSN. Of all 59 clones tested, the supernatant of clone FE21 demonstrated the highest virus titers on HepG2 recipient cells (data not shown). Therefore, FE21 pseudotype packaging cells were characterized in detail for SV-F mRNA transcription (reverse transcription-PCR [RT-PCR]) and SV-F protein expression (Western blotting).
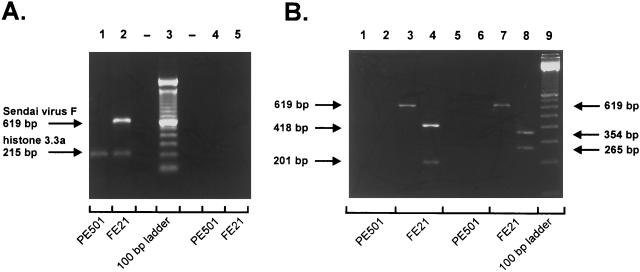
For detection of SV-F mRNA by RT-PCR, total cellular RNA was extracted by the method of Chomczynski and Sacchi (5). First-strand cDNA was synthesized by Super Script II reverse transcriptase (Life Technologies, Eggenstein, Germany) from 2 μg of total RNA using 25 ng of oligo(dT)12–18 primer per μl and 200 U of Super Script II per reaction. Primer sequences for SV-F (forward primer, 5′TTGTGTTGAGTCCAGATTGACC3′; reverse primer, 5′ATATGTGTCCCTCGGTATACGG3′) and murine histone 3.3a (forward primer, 5′CCACTGAACTTCTGATCCGC3′; reverse primer, 5′GCGTGCTAGCTGGATATCTT3′) were designed by retrieving published sequence information for the SV-F gene (SV strain Fushimi; sequence identification [ID], PAMSNDFP) and murine histone 3.3a gene (sequence ID, MMZ85979) from the EMBL databases (European Molecular Biology Laboratories, Heidelberg, Germany). Samples of the RT reaction mixture were employed for PCR with 0.2 μM SV-F- or histone 3.3a-specific primers and 2 U of Taq DNA polymerase (Qiagen, Hilden, Germany). RT-PCR of FE21 total RNA employing SV-F-specific primers generated an expected SV-F-specific fragment of 619 bp (Fig. 2A, lane 2). Subsequent digests of this SV-F-specific PCR product by HaeIII and MunI gave rise to the calculated SV-F fragment patterns (HaeIII, 418 and 201 bp; MunI, 354 and 265 bp; Fig. 2B, lanes 4 and 8). In contrast, RT-PCR of PE501 total RNA with SV-F-specific primers produced no PCR product at all (Fig. 2B, lanes 1 and 2), whereas a control amplification of either PE501 or FE21 total RNA with histone 3.3a-specific primers yielded equal amounts of a 215-bp histone 3.3a PCR product (Fig. 2A, lanes 1 and 2). These results demonstrate that FE21 pseudotype packaging cells specifically transcribe the stably transfected SV-F cDNA, although the transcription level is low: 44 amplification cycles were required to generate easily detectable amounts of SV-F PCR product, whereas 26 amplification cycles were sufficient to generate comparable amounts of the histone 3.3a PCR product (Fig. 2A).
FIG. 2.
(A) RT-PCR detection of SV-F cDNA transcription in cell line FE21 stably transfected with SV-F expression plasmid pcDNA3-F. Five microliters of SV-F amplification product and 2 μl of histone 3.3a amplification product were loaded together per lane and separated in a 1.5% agarose gel. Lanes: 1, RT and amplification of PE501 total RNA; 2, RT and amplification of FE21 total RNA; 3, 100-bp DNA ladder; 4, amplification of PE501 total RNA without preceding RT; 5, amplification of FE21 total RNA without preceding RT. Amplifications without RT were carried out to confirm the absence of contaminating DNA in total RNA preparations. (B) Restriction analysis of amplified SV-F product (expected fragment sizes: HaeIII digest of SV-F PCR product, 418 and 201 bp; MunI digest of SV-F PCR product, 354 and 265 bp). Fifteen microliters of SV-F amplification products (total amount, 100 μl) was subjected to restriction digests with HaeIII or MunI, and the whole samples were separated on a 3.5% agarose gel. For undigested controls, 7.5 μl of amplification products was loaded per lane. Lanes: 1, RT and amplification of PE501 total RNA; 2, HaeIII digest of PE501 amplification product; 3, RT and amplification of FE21 total RNA; 4, HaeIII digest of FE21 amplification product; 5, same as lane 1; 6, MunI digest of PE501 amplification product; 7, same as lane 3; 8, MunI digest of FE21 amplification product; 9, 100-bp DNA ladder.
Next, FE21 pseudotype packaging cells were investigated for SV-F glycoprotein expression. Western blotting analysis employing both an SV-F-specific monoclonal antibody (MAb) and an SV-F-specific polyclonal antiserum resulted only in the detection of a very faint band 63 kDa in size, which specifically represents the SV-F precursor protein (F0) not cleaved by protease with trypsin specificity (data not shown). Similar results were obtained with all other stably SV-F-expressing pseudotype packaging cell lines generated in parallel. This finding suggests that ecotropic PE501 packaging cells only tolerate expression of very small amounts of SV-F, thus allowing the outgrowth of low-SV-F-expressing G418-resistant PE501 clones only.
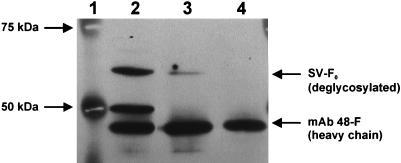
To increase the sensitivity of SV-F glycoprotein detection, the following modifications were introduced. First, FE21 cell lysate aliquots containing 2 mg of total protein were immunoprecipitated. For this purpose, protein G-Sepharose beads (Pharmacia Biotech, Freiburg im Breisgau, Germany) were incubated with hybridoma supernatants (diluted 1:5 in phosphate-buffered saline) containing SV-F MAb 48-F (34) for 1 h at 4°C and subsequently added to cell lysates. The mixtures were further incubated for 1 h at 4°C, and then the tertiary protein G-antigen-antibody complexes were collected by centrifugation and washed three times with a mixture of 20 mM HEPES (pH 7.4), 150 mM NaCl, 10% glycerol, and 1% Nonidet P-40. Second, protein G-Sepharose-bound SV-F glycoprotein was deglycosylated, since SV-F is known to be expressed in different glycosylation patterns (reference 37 and our unpublished observation of SV-F glycosylation patterns following PE501 infection by SV); thereby, all glycosylation forms of SV-F are summed up in a single band of 60 kDa. Samples were deglycosylated with 2,500 New England Biolabs units of PNGase F per sample according the manufacturer’s instructions (New England Biolabs, Schwalbach, Germany). Deglycosylated samples were separated on a discontinuous sodium dodecyl sulfate-polyacrylamide gel (stacking gel, 5% acrylamide-bisacrylamide [29:1]; resolving gel, 10% acrylamide-bisacrylamide [29:1]). Protein transfer on polyvinylidene difluoride membrane and enhanced chemiluminescence (ECL)-Western blotting were performed according to the manufacturer’s instructions (Amersham Buchler, Braunschweig, Germany) with a 1:20 dilution of primary SV-F MAb 48-F and a 1:4,000 dilution of a secondary horseradish peroxidase-labeled antimouse antibody. In SV-infected PE501 cells (positive control [Fig. 3, lane 2]) the modifications used led to the detection of an intense band of 60 kDa representative for deglycosylated SV-F protein. Lysates of FE21 pseudotype packaging cells exhibited a band of the same size of much less intensity (Fig. 3, lane 3), which corresponds to the low-SV-F-expressing status of stable PE501 clones generated by G418 selection. Uninfected, untransfected PE501 cells (negative control [Fig. 3, lane 3]) were not found to express SV-F. Since SV-F MAb 48-F was used for both immunoprecipitation and for Western blotting, MAb 48-F was detected in all samples (Fig. 3, lanes 2 to 4). Unexpectedly, immunoprecipitated, deglycosylated lysates of SV-infected PE501 cells gave rise to an additional band of approximately 50 kDa (Fig. 3, lane 2). While this band corresponds to the deglycosylated F1 fragment size of cleaved SV-F0 precursor proteins, a clear-cut explanation for SV-F1 generation could not be provided, since samples had not been treated with trypsin.
FIG. 3.
Detection of SV-F expression after stable transfection of PE501 cells with SV-F expression vector pcDNA3-F by immunoprecipitation and Western blotting. Lanes: 1, protein molecular mass marker; 2, PE501 cells infected by SV (positive control); 3, clone FE21 stably transfected with pcDNA3-F; 4, PE501 cells, neither infected nor transfected (negative control).
Taken together, RT-PCR and Western blotting confirmed that FE21 packaging cells indeed express the SV-F glycoprotein, albeit in very small amounts. However, when the standard cytomegalovirus immediate-early promoter-based mammalian expression vector (pcDNA3) which was used for SV-F expression throughout this study (pcDNA3-F) was employed for the expression of an nlslacZ reporter cassette (pcDNA3-nlslacZ) in control transfection experiments, high levels of β-galactosidase reporter gene expression were observed (data not shown). Therefore, a potential cytotoxic effect of high-level, constitutive SV-F expression cannot be ruled out. This concern is underlined by the observation of a total loss of expression of measles virus fusion protein (MV-F, which is related to SV-F) after only three passages when expressed by a recombinant VSV/MV-F virus (31), suggesting a strong selective pressure for the elimination of MV-F protein expression. On the other hand, the inclusion efficiency of heterologous membrane proteins, which is known to be heavily dependent on high-level expression of these proteins at the cell surface budding site, was found to be a major determinant concerning MoMLV pseudotype transduction efficiencies (33). It can be concluded from this finding that an inducible SV-F expression system might be required for the generation of stable, high-level SV-F-expressing, high-titer MoMLV(SV-F) producer cell lines.
As a consequence of low SV-F expression as well as low virus titers, it was not possible to detect SV-F directly as an integral component of MoMLV(SV-F) pseudotypes employing concentrated supernatants of FE21 cells transiently transfected with retroviral vector pLXSN (data not shown). Nevertheless, supernatants of pLXSN-transfected FE21 cells were able to transduce ASGP-R-positive HepG2 cells (Table 3), indicating the incorporation of SV-F in the retroviral envelope at a functional level.
TABLE 3.
Generation of MoMLV(SV-F) pseudotypes by stable SV-F-expressing packaging cellsa
| Retroviral vector | Transduction method | Detection method | Viral titer (102 CFU/ml or FFU/ml) on cellsb
|
||
|---|---|---|---|---|---|
| NIH 3T3 (A−/E+) | BHK-21 (A−/E−) | HepG2 (A+/E−) | |||
| pLXSN | Static | G418 selection | 5.0 | 0.0 | 4.1 |
| pLZ12 | Static | β-Galactosidase assay | 3.3 | 0.0 | 2.0 |
| pLZ12 | Flowthrough | β-Galactosidase assay | 12.8 | 0.0 | 17.5 |
Ecotropic packaging FE21 cells stably expressing SV-F protein were transfected with retroviral vector. Forty-eight hours after transfection, activated supernatants were used to transduce NIH 3T3, BHK-21, and HepG2 cells, followed by G418 selection or direct histochemical staining for β-galactosidase expression.
A+, ASGP-R positive; A−, ASGP-R negative; E+, ecotropic receptor positive; E−, ecotropic receptor negative.
To further investigate the transduction specificity of retroviral particles generated with cell line FE21, packaging cells were transfected with retroviral vector pLXSN or double-reporter retroviral vector pLZ12 (11) (Fig. 1), which transduces not only the neor gene but also an nlslacZ gene (Fig. 1), thereby enabling a rapid histochemical detection of transgene expression within 48 h after transduction (30). Incubation of produced supernatants with ASGP-R-expressing human hepatoma HepG2 recipient cells generated both G418-resistant clones and β-galactosidase-positive cells (Table 3), thereby demonstrating the formation of recombinant MoMLV(SV-F) pseudotypes. Similar results were obtained for a second ASGP-R-positive human hepatoma cell line, HuH-7 (data not shown). Incubation with ASGP-R-negative recipient cells of nonmouse, nonrat origin (BHK-21 cells) did not exhibit any transduction events (Table 3), again indicating an ASGP-R-restricted tropism of MoMLV(SV-F) pseudotypes. MoMLV(SV-F) pseudotype titers obtained with stable SV-F-expressing pseudotype packaging cell line FE21 and retroviral vector pLXSN were found to be only slightly increased compared to those obtained by cotransfection of PE501 cells with retroviral vector pLXSN and SV-F expression plasmid pcDNA3-F (4.1 × 102 CFU/ml versus 1.0 × 102 CFU/ml on ASGP-R-positive HepG2 cells). Therefore, we investigated whether low accessibility of ASGP-R molecules was at least in part responsible for the low titers obtained by the standard static transduction procedure.
Improved access to the ASGP-R on the basolateral cell domain enhances MoMLV(SV-F) pseudotype transduction efficiency.
HepG2 cells plated out on petri dishes are known to express the ASGP-R in a polarized manner, where most of the receptor molecules are located on the basolateral domain (13, 32). This feature is similar to the in vivo situation of normal hepatocytes which express the ASGP-R almost exclusively on the sinusoidal (i.e., basolateral) plasma membrane (13). Therefore, the ASGP-R molecules are supposed not to be presented to pseudotype particles moving in Brownian motion on top of the HepG2 cell monolayer. In order to improve the accessibility of the ASGP-R for MoMLV(SV-F) pseudotype transduction, target cells were plated out and cultivated on collagen-coated Transwell-COL cell culture membrane inserts, which display an estimated porosity of 50%, thereby potentially improving access to basolaterally located ASGP-R molecules. For flowthrough transduction (7), medium was completely removed from the Transwell-COL insert as well as from the outer chamber. Subsequently, 3 ml of virus-containing medium produced after transient transfection of cell line FE21 with retroviral vector pLZ12 was applied to the Transwell-COL insert, thereby inducing a flowthrough by gravity. Every 45 min, medium was removed from the outer chamber and transferred again to the Transwell-COL insert. After 3 h, the virus medium was completely removed from both chambers and replaced by regular growth medium. This procedure resulted in a MoMLV(SV-F) titer of 17.5 × 102 focus-forming units (FFU)/ml on HepG2 cells (Table 3), which represents an almost ninefold increase in titer in comparison to static (i.e., conventional) MoMLV(SV-F) pseudotype transduction (2.0 × 102 FFU/ml [Table 3]). Furthermore, BHK-21 cells (negative control) were again found not to be transduced (Table 3). Since flowthrough transduction of NIH 3T3 cells (positive control) only resulted in a fourfold increase in titer (3.3 × 102 versus 12.8 × 102 FFU/ml [Table 3]), the stronger enhancement of MoMLV(SV-F) titers on HepG2 cells could be due to improved accessibility of ASGP-R receptor molecules under flowthrough conditions and not only to enhanced transduction rates obtained by the flowthrough procedure itself (7). To our knowledge, the MoMLV flowthrough transduction procedure has been applied for the first time to a cell surface receptor specifically sorted to the basolateral domain, and our results provide evidence that an efficient SV-F–ASGP-R ligand-receptor interaction is of major importance for the efficiency of the ASGP-R-restricted MoMLV(SV-F) pseudotype transduction.
Genetic proof for the ASGP-R restriction of MoMLV(SV-F) pseudotypes.
To further specify the tropism of MoMLV(SV-F) pseudotypes restricted to ASGP-R-positive target cells, a pair of cell lines known to differ only with respect to ASGP-R expression was required. For this purpose, cell line MDCK (Madin-Darby canine kidney cells, which cannot be transduced by ecotropic retrovirus) and a derivative cell line stably transfected with the ASGP-R cDNA—thereby constituting new cell line M12 (10)—were used in further transduction experiments. Since M12 cells constitutively express both the histidinol (hisD) and the neomycin resistance (neor) genes as a result of the stable transfection procedure, retroviral vector pLXSP, which transduces the puromycin resistance gene (pac), was employed for the transient transfection of stable SV-F-expressing cell line FE21. Subsequently, the produced pseudotype supernatants were incubated on cell lines MDCK, M12, and NIH 3T3 (positive control), followed by continuous selection with puromycin (2 μg/ml for MDCK and M12 cells, 5 μg/ml for NIH 3T3 cells). Parental cell line MDCK did not exhibit any transduction event at all, whereas cell line M12 constitutively expressing the ASGP-R predominantly at the basolateral domain (10) exhibited 7.2 × 102 CFU/ml. Transduction of NIH 3T3 cells (i.e., transduction via the ecotropic receptor) resulted in a titer of 5.5 × 102 CFU/ml, indicating a similar efficiency for ecotropic receptor-mediated and ASGP-R-mediated transductions. These results provide direct genetic proof for the ASGP-R-restricted tropism of MoMLV(SV-F) pseudotypes.
MoMLV(SV-F)-mediated transduction of ASGP-R-positive cells is blocked by ASGP-R antiserum.
To investigate whether MoMLV(SV-F) transduction via the ASGP-R can be blocked by a receptor-specific antibody, M12 cells were incubated with polyclonal ASGP-R antiserum prior to transduction and during incubation of cells with pseudotype virus. To prevent internalization and recirculation of ASGP-R molecules, all steps had to be carried out at 4°C (13). Antiserum was diluted in serum-free DMEM, and cells were incubated with diluted antibody for 60 min prior to transduction. Subsequently, the antiserum solution was removed from the cells and replaced by antiserum-supplemented virus supernatants, and transduction was carried out for 60 min at 4°C. Following transduction, the virus-antiserum mixture was removed, and cells were washed two times with complete growth medium and incubated for 24 h at 37°C. In the absence of ASGP-R-specific antiserum, MoMLV(SV-F) transduction at 4°C yielded a titer of 5.4 × 102 CFU/ml on M12 cells, whereas in the presence of antiserum (at a concentration of 2 mg of protein/ml), transduction resulted in a titer of 1.2 × 102 CFU/ml, which represents a decrease of almost 80%. Since increasing antiserum concentrations did not increase inhibition of transduction, the remaining transduction rate may represent the proportion of recycled ASGP-R molecules [newly accessible for MoMLV(SV-F) pseudotypes] following uptake and internalization of ASGP-R–antibody complexes (despite the fact that this process should be minimized at 4°C). Interestingly, transduction of NIH 3T3 cells was completely blocked at 4°C, independent of the presence or absence of antiserum, whereas transduction at 37°C was not affected in the presence of the ASGP-R antiserum.
Trypsin-mediated activation of SV-F0 is dispensable for MoMLV(SV-F) pseudotype transduction.
Recently, we have demonstrated that ASGP-R-mediated infections by SV require SV-F0 precursor activation (by trypsin cleavage in vitro or by proteases with trypsin-like specificity in vivo [3]). Following cleavage of SV-F0 into subunits SV-F1 and SV-F2 during particle maturation, SV-F1 first binds to the ASGP-R and second exerts fusion of cellular and viral membranes. We have now investigated whether the ASGP-R-mediated MoMLV(SV-F) pseudotype transduction requires the presence of fusion-active SV-F1 subunits or whether the copresence of ecotropic retroviral env proteins [coincorporated into the MoMLV(SV-F) pseudotype envelope] is sufficient for the fusion process. For this purpose, trypsin-treated and untreated preparations of MoMLV(SV-F) pseudotypes were generated after transient transfection of FE21 cells with the nlslacZ gene-containing vector pLZ12 (for HepG2 transduction) or with pac gene-containing vector pLXSP (for M12 transduction) and compared for their transduction capabilities. Interestingly, untreated pseudotype preparations were found to transduce as efficiently as trypsin-activated preparations. (i) Titers for HepG2 recipient cells were found to be 17.5 × 102 FFU/ml in flowthrough transductions and 2.0 × 102 FFU/ml in static transductions for both trypsin-treated and untreated preparations. (ii) Titers for M12 recipient cells in static transductions were 7.2 × 102 CFU/ml for either approach. These results demonstrate that for ASGP-R-mediated MoMLV(SV-F) pseudotype transduction, the presence of fusion-active SV-F subunits is not strictly required, and the presence of the retroviral env proteins might be advantageous beyond a potential stabilization of the pseudotyped envelope. The observation that ASGP-R-mediated MoMLV(SV-F) pseudotype transduction does not require fusion-active (i.e., trypsin cleaved) SV-F proteins suggests that binding is mediated by SV-F0 protein, whereas the retroviral env protein promotes fusion of viral and cellular membranes. As a consequence, generation of pseudotype particles under serum-free conditions and subsequent SV-F0 activation might be dispensable as long as retroviral env proteins are coexpressed in MoMLV(SV-F) pseudotype packaging cells.
In this context, a crucial question currently under investigation is whether MoMLV(SV-F) pseudotypes require the additional presence of MoMLV env proteins for efficient transduction of ASGP-R-positive target cells. It is of interest that reconstituted SV envelopes containing exclusively fusion-active SV-F glycoproteins on their surface (denominated as F-virosomes) were previously found to bind to and subsequently fuse with ASGP-R-positive HepG2 cells. Following binding and fusion, direct release of F-virosomal contents into the cytosol was detected (1). Furthermore, we have demonstrated that SV infection of ASGP-R-positive but SA-R-depleted target cells does take place only after the SV-F–ASGP-R ligand-receptor interaction and cleavage of SV-F0 precursor proteins into fusion-active subunits SV-F1 and SV-F2 (3, 18). These data suggest that SV-F alone should be sufficient to mediate both ASGP-R binding and fusion with cellular plasma membranes in MoMLV(SV-F) pseudotypes lacking MoMLV env proteins.
The hepatocyte-restricted expression of the ASGP-R makes it an attractive receptor for liver-specific targeting of chemotherapeutic agents (1) and therapeutic genes (36). Our data provide evidence that the ASGP-R can be exploited for a retrovirus-mediated liver gene transfer by usage of MoMLV(SV-F)-pseudotypes. Therefore, the advantages of retroviral gene transfer (e.g., sustained gene expression) could be combined with liver restriction of therapeutic gene transfer, thus potentially facilitating a future in vivo vector delivery. Further attempts will be made to enhance SV-F expression in retrovirus packaging cells, which should lead to increased SV-F incorporation into pseudotype particles, thereby improving achievable MoMLV(SV-F) pseudotype titers.
In conclusion, our results demonstrate that (i) SV envelope proteins HN and F can be incorporated into ecotropic MoMLV particles, thereby leading to an extended host cell tropism of the generated pseudotype particles; (ii) recombinant MoMLV(SV-F) pseudotypes can be generated and are able to transduce ASGP-R-positive target cells specifically (as demonstrated by transduction of ASGP-R-positive cell lines HepG2 and M12 and inhibition of ASGP-R-dependent transduction with ASGP-R antiserum); and (iii) improved access to ASGP-R molecules enhances the efficiency of SV-F- and ASGP-R-mediated transductions (as evidenced by flowthrough transduction).
Acknowledgments
Retroviral vector pLXSN and retroviral packaging cell line PE501 were kindly provided by A.D. Miller (Fred Hutchinson Cancer Research Center, Seattle, Wash.). Retroviral vector pLZ12 was kindly provided by P. van Hoegen and P. Förg (German Cancer Research Center, Heidelberg, Germany). Plasmid pSVGL1 was kindly provided by J. K. Rose (Yale University School of Medicine, New Haven, Conn.). Cell lines MDCK and M12 were kindly provided by M. Spiess (Biocenter, University of Basel, Basel, Switzerland). Antiserum to the human ASGP-R (goat) was kindly provided by G. Ashwell (National Institutes of Health, Bethesda, Md.). We are grateful to S. Lambrecht for excellent technical assistance. We thank S. Wesselborg and E. Rossmann for help in performing immunoprecipitations and C. D. Gross, W. A. Wybranietz, and F. T. C. Graepler for carefully reading and discussing the manuscript.
This work was supported by grants from the Deutsche Forschungsgemeinschaft (La 649/11-1); the Bundesministerium für Bildung, Wissenschaft, Forschung, Technologie (Programm “Gesundheitsforschung 2000,” 01 KV 9532); and the fortüne-program of the Medical Faculty of the Eberhard-Karls-University, Tübingen (F.1281011).
REFERENCES
- 1.Bagai S, Sarkar D P. Fusion-mediated microinjection of lysozyme into HepG2 cells through hemagglutinin neuraminidase-depleted Sendai virus envelopes. J Biol Chem. 1994;269:1966–1972. [PubMed] [Google Scholar]
- 2.Bennett R P, Nelle T D, Wills J W. Functional chimeras of the Rous sarcoma virus and human immunodeficiency virus Gag proteins. J Virol. 1993;67:6487–6498. doi: 10.1128/jvi.67.11.6487-6498.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bitzer M, Lauer U, Baumann C, Spiegel M, Gregor M, Neubert W J. Sendai virus efficiently infects cells via the asialoglycoprotein receptor and requires the presence of cleaved F0 precursor proteins for this alternative route of cell entry. J Virol. 1997;71:5481–5486. doi: 10.1128/jvi.71.7.5481-5486.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burns J, Friedmann T, Driever W, Burrascano M, Yee J-K. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc Natl Acad Sci USA. 1993;90:8033–8037. doi: 10.1073/pnas.90.17.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 6.Choppin P, Compans R W. Phenotypic mixing of envelope proteins of the parainfluenza virus SV5 and vesicular stomatitis virus. J Virol. 1970;5:609–616. doi: 10.1128/jvi.5.5.609-616.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chuck A S, Palsson B O. Consistent and high rates of gene transfer can be obtained using flow-through transduction over a wide range of retroviral titers. Hum Gene Ther. 1996;7:743–750. doi: 10.1089/hum.1996.7.6-743. [DOI] [PubMed] [Google Scholar]
- 8.De la Luna S, Soria I, Pulido D, Ortin J, Jimenez A. Efficient transformation of mammalian cells with constructs containing a puromycin-resistance marker. Gene. 1988;62:121–126. doi: 10.1016/0378-1119(88)90585-9. [DOI] [PubMed] [Google Scholar]
- 9.Emi N, Friedmann T, Yee J-K. Pseudotype formation of murine leukemia virus with the G protein of vesicular stomatitis virus. J Virol. 1991;65:1202–1207. doi: 10.1128/jvi.65.3.1202-1207.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuhrer C, Geffen I, Huggel K, Spiess M. The two subunits of the asialoglycoprotein receptor contain different sorting information. J Biol Chem. 1994;269:3277–3282. [PubMed] [Google Scholar]
- 11.Galileo D S, Gray G E, Owens G C, Majors J, Sanes J R. Neurons and glia arise from a common progenitor in chicken optic tectum: demonstration with two retroviruses and cell-type-specific antibodies. Proc Natl Acad Sci USA. 1990;87:458–462. doi: 10.1073/pnas.87.1.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gazdar A F, Ole H, Lalley P, Moss W W, Minna J D, Francke U. Identification of mouse chromosomes required for murine leukemia virus replication. Cell. 1977;11:949–956. doi: 10.1016/0092-8674(77)90306-3. [DOI] [PubMed] [Google Scholar]
- 13.Geffen I, Spiess M. Asialoglycoprotein receptor. Int Rev Cytol. 1992;137B:181–219. doi: 10.1016/s0074-7696(08)62605-4. [DOI] [PubMed] [Google Scholar]
- 14.Gerner F M. Stable synthesis of recombinant Sendai virus surface glycoproteins F and HN in eucaryotic cells. Diploma thesis. Germany: Ludwig-Maximilians-University Munich; 1995. [Google Scholar]
- 15.Kimura Y. Phenotypic mixing of vesicular stomatitis virus with HVJ (Sendai virus) Jpn J Microbiol. 1973;17:373–381. doi: 10.1111/j.1348-0421.1973.tb00788.x. [DOI] [PubMed] [Google Scholar]
- 16.Lamb R A. Paramyxovirus fusion: a hypothesis for changes. Virology. 1993;197:1–11. doi: 10.1006/viro.1993.1561. [DOI] [PubMed] [Google Scholar]
- 17.Landau N R, Littman D R. Packaging system for rapid production of murine leukemia virus vectors with variable tropism. J Virol. 1992;66:5110–5113. doi: 10.1128/jvi.66.8.5110-5113.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leyrer S, Bitzer M, Lauer U, Kramer J, Neubert W J, Sedlmeier R. Sendai virus-like particles devoid of HN protein infect cells via the human asialoglycoprotein receptor. J Gen Virol. 1998;79:683–687. doi: 10.1099/0022-1317-79-4-683. [DOI] [PubMed] [Google Scholar]
- 19.Markwell M A K, Portner A, Schwartz L. An alternative route of infection for viruses: entry by means of the asialoglycoprotein receptor of a Sendai virus mutant lacking its attachment protein. Proc Natl Acad Sci USA. 1985;82:978–982. doi: 10.1073/pnas.82.4.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Markwell M A K, Svennerholm L, Paulson J C. Specific gangliosides function as host cell receptors for Sendai virus. Proc Natl Acad Sci USA. 1981;78:5406–5410. doi: 10.1073/pnas.78.9.5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mebatsion T, Conzelmann K-K. Specific infection of CD4+ target cells by recombinant rabies virus pseudotypes carrying the HIV-1 envelope spike protein. Proc Natl Acad Sci USA. 1996;93:7310–7314. doi: 10.1073/pnas.93.21.11366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller A D, Rosman G J. Improved retroviral vectors for gene transfer and expression. BioTechniques. 1988;7:980–990. [PMC free article] [PubMed] [Google Scholar]
- 23.Miller A D, Garcia J V, von Suhr N, Lynch C M, Wilson C, Eiden M V. Construction and properties of retrovirus packaging cells based on gibbon ape leukemia virus. J Virol. 1991;65:2220–2224. doi: 10.1128/jvi.65.5.2220-2224.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller A D, Miller D G, Garcia J V, Lynch C M. Use of retroviral vectors for gene transfer and expression. Methods Enzymol. 1993;217:581–599. doi: 10.1016/0076-6879(93)17090-r. [DOI] [PubMed] [Google Scholar]
- 25.Mottet G, Curran J, Roux L. Intracellular stability of nonreplicating paramyxovirus nucleocapsids. Virology. 1990;176:1–7. doi: 10.1016/0042-6822(90)90223-e. [DOI] [PubMed] [Google Scholar]
- 26.Naldini L, Blömer U, Gallay P, Ory D, Mulligan R, Gage F H, Verma I M, Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 27.Rose J K, Bergmann J E. Expression from cloned cDNA of cell surface secreted forms of the glycoprotein of vesicular stomatitis virus in eucaryotic cells. Cell. 1982;30:753–762. doi: 10.1016/0092-8674(82)90280-x. [DOI] [PubMed] [Google Scholar]
- 28.Salmons B, Saller R M, Baumann J G, Günzburg W H. Construction of retroviral vectors for targeted delivery and expression of therapeutic genes. Leukemia. 1995;9:S53–S60. [PubMed] [Google Scholar]
- 29.Sanes J R, Rubenstein J L, Nicolas J F. Use of a recombinant retrovirus to study post-implantation cell lineage in mouse embryos. EMBO J. 1986;5:3133–3142. doi: 10.1002/j.1460-2075.1986.tb04620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scheid A, Choppin P W. Identification of biological activities of paramyxovirus glycoproteins. Activation of cell fusion, hemolysis, and infectivity by proteolytic cleavage of an inactive precursor protein of Sendai virus. Virology. 1974;57:475–490. doi: 10.1016/0042-6822(74)90187-1. [DOI] [PubMed] [Google Scholar]
- 31.Schnell M J, Buonocore L, Kretzschmar E, Johnson E, Rose J K. Foreign glycoproteins expressed from recombinant vesicular stomatitis viruses are incorporated efficiently into virus particles. Proc Natl Acad Sci USA. 1996;93:11359–11365. doi: 10.1073/pnas.93.21.11359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stockert R J. The asialoglycoprotein receptor: relationships between structure, function, and expression. Physiol Rev. 1995;75:591–609. doi: 10.1152/physrev.1995.75.3.591. [DOI] [PubMed] [Google Scholar]
- 33.Suomalainen M, Garoff H. Incorporation of homologous and heterologous proteins into the envelope of Moloney murine leukemia virus. J Virol. 1994;68:4879–4889. doi: 10.1128/jvi.68.8.4879-4889.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Willenbrink W, Neubert W J. Long-term replication of Sendai virus defective interfering particle nucleocapsids in stable helper cell lines. J Virol. 1994;68:8413–8417. doi: 10.1128/jvi.68.12.8413-8417.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Witte O N, Baltimore D. Mechanism of formation of pseudotypes between vesicular stomatitis virus and murine leukemia virus. Cell. 1977;11:505–511. doi: 10.1016/0092-8674(77)90068-x. [DOI] [PubMed] [Google Scholar]
- 36.Wu G Y, Wu C H. Targeted delivery and expression of foreign genes in hepatocytes. In: Wu G Y, Wu C H, editors. Liver diseases: targeted diagnosis and therapy using specific receptors and ligands. New York, N.Y: Marcel Dekker; 1991. pp. 127–149. [PubMed] [Google Scholar]
- 37.Yoshima H, Nakanishi M, Okada Y, Kobata A. Carbohydrate structures of HVJ (Sendai virus) glycoproteins. J Biol Chem. 1981;256:5355–5361. [PubMed] [Google Scholar]
- 38.Zavada J. The pseudotypic paradox. J Gen Virol. 1982;63:15–24. doi: 10.1099/0022-1317-63-1-15. [DOI] [PubMed] [Google Scholar]