Abstract
Nutri-Score is a front-of-package (FOP) labeling designed to assist consumers in selecting healthier options at the point of purchase and ultimately enhance their health. This study aims to evaluate the association between the Nutri-Score system and incident abdominal obesity (AO) in community-dwelling older adults. A prospective cohort of 628 individuals aged ≥ 60 were recruited in Spain between 2008–2010 and were reexamined between 2015–2017. Dietary intake was evaluated utilizing a validated computerized dietary history. Food was categorized based on the Nutri-Score system into five levels from A (green, representing the best quality) to E (red, representing the poorest quality). A five-color Nutri-Score dietary index (5-CNS DI) in g/day/kg was calculated for each participant. AO was determined by a waist circumference (WC) of ≥102 cm for men and ≥88 cm for women. Logistic regression models were adjusted for the main potential confounders. During a mean six-year follow-up, 184 incident cases of AO occurred. The odds ratio (OR) and 95% confidence interval (CI) for AO, when comparing the highest and lowest quartiles of the 5-CNS DI, were 2.45 (1.17–5.14), with a p-value for trend of 0.035. In sensitivity analyses, the OR was 2.59 (1.22–5.52, p-trend: 0.032) after adjustment for WC at baseline, and 1.75 (0.74–4.18, p-trend: 0.316) after adjustment for ultra-processed food consumption. In conclusion, less favorable food-consumption ratings in the Nutri-Score are associated with incident AO in the elderly. These findings support the use of this FOP system to potentially improve metabolic health.
Keywords: abdominal obesity, Seniors-ENRICA 1 study, five-color Nutri-Score, metabolic risk, cohort study
1. Introduction
Front-of-pack (FOP) nutrition labelling such as the Nutri-Score system aims to help consumers in selecting healthier food options at the point of purchase, with the goal being to enhance their overall health [1]. These are policy instruments that, when combined with educational initiatives, can promote healthy eating habits and mitigate the risk of diet-related chronic diseases [2]. FOP nutrition labels employ nutrient-profiling frameworks to evaluate the nutritional value of food products, displaying their health attributes in an accessible visual format [2]. Their purpose is to provide consumers with nutritional information that is both easy to comprehend and read, while also motivating the food manufacturing industry to enhance the nutritional quality of their products [3].
A recent systematic review of the relevant literature suggests that FOP nutrition labelling leads to significant improvements in food choices [4]. Specifically, the Nutri-Score system enhanced consumers’ capability to accurately assess food based on its nutritional value, encouraged their decision-making in selecting healthier options, and augmented their proficiency in selecting suitable portion sizes [5].
Furthermore, in several population-based cohorts, a less favorable Nutri-Score rating food consumption has been prospectively associated with a higher risk of chronic diseases [5]. This association has been shown for cardiovascular disease [6], metabolic syndrome [7], decline in renal function [8], incident frailty [9], cancer [10,11], as well as all-cause mortality [12].
Abdominal obesity (AO) is a well-known risk factor for cardiometabolic disorders. In older adults specifically, AO is a risk factor for cardiometabolic disease independent of general obesity [13]. In this population, AO is also associated with increased risk of disability [14] and sarcopenic obesity [15].
Hence, this study’s objective was to conduct a prospective evaluation of the association between food intake categorized according to the Nutri-Score system, and the onset of abdominal obesity (AO) in older adults residing in the community, while thoroughly adjusting for an extensive range of potential confounding factors.
2. Materials and Methods
2.1. Research Methodology and Participant Selection
Data were obtained from the Seniors-ENRICA 1 cohort, the methodology of which has been previously documented [16]. This cohort was established in Spain between 2008–2010 with 3521 community-based individuals aged ≥ 60 years. Data were gathered by qualified staff in three consecutive stages. At baseline, a telephone interview was conducted to obtain information on sociodemographic factors, lifestyle, and morbidity. Subsequently, two home visits were conducted to gather urine and blood specimens, assess anthropometric variables and blood pressure, and collect dietary information. Participants were followed-up with until 31 December 2015, achieving an average follow-up duration of six years, at which point data collection was updated including a physical examination.
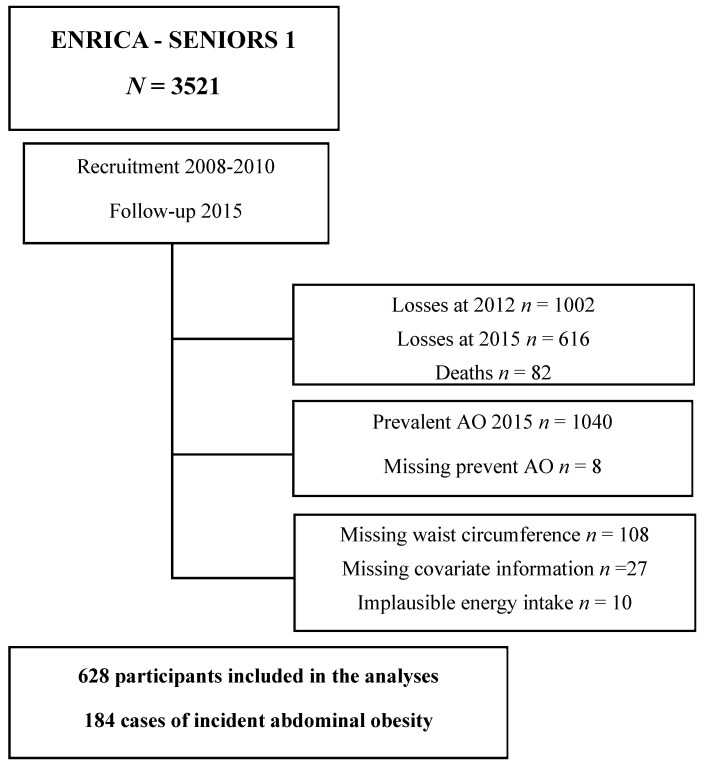
Within the cohort of 3521 participants, 1618 were excluded due to attrition during the follow-up periods in 2012 and 2015, with 1002 and 616 participants lost in each year, respectively. During follow-up, 82 deaths occurred. Of the remaining 1821 participants, 1040 were excluded because they had prevalent AO at baseline, and 8 because of missing data on waist circumference at baseline. We also excluded 10 participants with implausible energy intake (<600 or >4200 Kcal/day for men, or <400 or >3500 Kcal/day for women), and 27 who lacked data on other covariables (weight, body mass index (BMI), hypertension, diabetes, food consumption, and hours of TV viewing). Thus, the final analyses were carried out on 628 participants, among which 184 developed incident abdominal obesity (AO) during the follow-up period (Figure 1).
Figure 1.
Participant flowchart.
The Clinical Research Ethics Committee of the University Hospital of La Paz in Madrid, Spain approved the baseline and follow-up studies. All participants provided written informed consent.
2.2. Research Variables
2.2.1. Dietary Intake Evaluation and Calculation of the Nutri-Score
Dietary intake over the past year was evaluated through a validated computerized dietary history tool (DH-ENRICA) that was administered by skilled interviewers. This questionnaire gathers uniform data on 880 food items and 184 recipes for commonly consumed dishes in Spain. A collection of 129 photographs were used to estimate food portion sizes [17]. Consumption was considered habitual if a food was consumed at least once every two weeks. Intakes of energy and nutrients were determined utilizing standard Spanish food composition databases [18,19].
The Nutri-Score is a FOP labelling system that classifies products according to their nutritional value [20,21]. In our study, this labelling system was applied to each food product suitable for packaging and consumed by the study participants using the updated algorithm of 2022 for food and 2023 for beverages [22,23]. A score was assigned based on the nutrient content of the packaged food. Fresh produce (predominantly fruits and vegetables) and unprocessed meat and fish were omitted from the calculation of the Nutri-Score. For each packaged food item consumed, positive points were assigned ranging from 0 to 10 based on its content of four specific elements: total energy (kj), sugars (g), saturated fats (g), and sodium (mg). Conversely, foods were allocated negative points, varying from 0 to −5, depending on their amounts of five beneficial components: proportion of fruits, vegetables, legumes, nuts, and healthy oils, as well as dietary fiber (g), and protein (g). Consequently, a continuous Nutri-Score from −15 to +40 was determined for each consumed food item, where a higher score indicates a lower nutritional quality. This continuous score was segmented into five categories, resulting in a five-color Nutri-Score (5-CNS) assigned to each food item. Subsequently, foods were allocated into one of five classifications, represented by letters (A, B, C, D, and E) and associated colors, ranging from dark green for letter A, denoting superior nutritional quality, through to light green (B), yellow (C), orange (D), and dark orange (E) for the lowest nutritional quality [21].
For each participant, we calculated the following four dietary indexes (DI) based on their food consumption and the Nutri-Score previously assigned to each food:
The five-color Nutri-Score dietary index (5-CNS DI, in grams per day per kilogram): This index was determined by totaling the consumed quantities of each packaged food and beverage (grams per day), each multiplied by its respective 5-CNS rating (where A is scored as 1 and E as 5), and then dividing this total by the individual’s body weight in kilograms. In the present analysis this DI was considered the main exposure.
The continuous Nutri-Score dietary index (in grams per day per kilogram): This index was computed by summing the consumption amounts of all packaged foods and beverages (grams per day), each amount multiplied by its respective continuous Nutri-Score value (which varies from −15 to +40). This sum was divided by the individual’s body weight in kilograms.
The five-color Nutri-Score dietary index (5-CNS DI as a percentage of energy intake): This index was computed using the 5-CNS DI, where food consumption is represented as a percentage of total energy intake (percent energy per kilogram).
The continuous Nutri-Score dietary index (as a percentage of energy intake): This index was derived by calculating the continuous Nutri-Score DI, where food consumption is quantified as a percentage of total energy intake (percent energy per kilogram).
2.2.2. Abdominal Obesity
Waist circumference measurements were taken at baseline (2008–2010) and, upon conclusion of the follow-up phase (in 2015), through in-home physical assessments conducted by qualified staff. Incident AO was calculated according to the World Health Organization recommended cut-off points as waist circumference ≥ 102 cm in men and ≥88 cm in women in 2015 [24].
2.2.3. Other Variables
At the beginning of the study, sociodemographic, lifestyle, and morbidity data were collected. Weight and height measurements were conducted at participants’ homes under standardized conditions, and BMI was calculated. Self-reported data were collected regarding sex, age, educational level, smoking habits, former drinker status, time spent watching TV, and leisure time physical activity [16]. Hypertension was defined as blood pressure ≥ 140 and/or 90 mmHg, or use of antihypertensive medication. Participants in the study also provided information on physician-diagnosed conditions, including chronic respiratory disease, coronary heart disease, stroke, osteoarthritis/arthritis, cancer, depression requiring treatment, and diabetes. Participants disclosed all medications they were consuming, which were verified by a nurse against the actual medicine packages kept at the participants’ homes. Finally, the Mediterranean Diet Adherence Score (MEDAS), a 14-item validated tool designed to measure the adherence to the Mediterranean diet which methods are reported elsewhere, was assessed [25]. Ultra-processed food (UPF) was categorized based on the NOVA classification system [26].
2.3. Statistical Analysis
Based on the four derived Nutri-Score DIs, participants were classified into sex-specific quartiles. To assess the risk of abdominal obesity within these sex-specific quartiles for each Nutri-Score DI, logistic regression analysis was conducted and odds ratios (OR) along with their respective 95% confidence intervals (CI) were determined. The p-value for linear trends was calculated by using the quartiles of each DI as a continuous variable. Three logistic regression models were constructed, incorporating adjustments for potential confounding variables. Model 1 was adjusted for sex, age, educational level (primary or less, secondary, and university), and total energy intake (kcal/d). Model 2 was additionally adjusted for smoking (current, former, and never smoker), physical activity at leisure time (Mets/h/week), time watching TV (h/week), total sleep time (minutes/day), total ethanol consumption (g/day), BMI (continuous), chronic obstructive pulmonary disease/asthma, coronary heart disease, hypercholesterolemia, hypertension, diabetes, cancer (yes/no), arthrosis (yes/no), arthritis (yes/no), and number of medications (0, 1–3, >3). Model 3 was further adjusted for the MEDAS without wine (0–13 points). Sensitivity analyses were performed by adjusting for waist circumference (cm) at baseline and for UPF consumption (classified as NOVA-4).
To assess the dose–response relationship, restricted cubic-splines with three knots (10th, 50th, and 90th percentile) were fitted, showing the ORs for the association of AO throughout the continuous Nutri-Score DI in g/day/kg as well as their 95% CI. Data analysis was conducted utilizing STATA/SE version 14.1, developed by StataCorp in College Station, TX, USA. Statistical significance was established at a two-sided p-value of less than 0.05.
3. Results
Of the 628 participants included in the analyses (44.9% women; mean (SD) 6.02 ± 5.76 years), 184 developed AO after a mean six-year follow-up. The mean (SD) for the 5-CNS DI in g/day/kg at baseline was 33.5 (12.7). In the lowest quartile (best nutritional quality) of the 5-CNS DI, the consumption of foods labelled A was 146 (108) grams per day and 39 (35) grams per day for foods labelled E. Within the highest quartile (poorer nutritional quality), the amounts were 161 (91) and 164 (152) grams per day, respectively. Participants in the highest quartile of the 5-CNS DI per day per kilogram exhibited higher energy consumption and reduced intake of fresh foods, presented with a lower BMI, and showed a lower likelihood of hypertension and medication use. However, they were more inclined to have been former drinkers and demonstrated increased consumption of UPF compared to those in the lowest quartile (Table 1).
Table 1.
Baseline characteristics of cohort participants according to sex-specific quartiles of five-color Nutri-Score dietary index (5-CNS DI) in g/day/kg (n = 628).
| Five-Color Nutri-Score (5-CNS DI) in g/Day/kg | ||||||
|---|---|---|---|---|---|---|
| Q1 (Best Diet Quality) |
Q2 | Q3 | Q4 (Worse Diet Quality) |
p for Linear Trend | ||
| n | 158 | 162 | 162 | 146 | ||
| 5-CNS DI in g/day/kg, mean ± SD | 20.2 ± 4.10 | 28.8 ± 24.43 | 35.7 ± 2.39 | 50.6 ± 12.2 | <0.001 | |
| Continuous Nutri-Score DI in g/day/kg, mean ± SD | 20.3 ± 20.09 | 31.9 ± 21.0 | 46.1 ± 24.1 | 76.3 ± 42.0 | <0.001 | |
| 5-CNS DI based on the % of energy, mean ± SD | 1.76 ± 0.44 | 2.08 ± 0.33 | 2.24 ± 0.38 | 2.45 ± 0.40 | <0.001 | |
| ontinuous Nutri-Score DI based on the % of energy, mean ± SD | 3.72 ± 2.48 | 4.55± 2.03 | 5.36 ± 2.29 | 6.10 ± 2.57 | <0.001 | |
| Packaged foods (g/d), mean ± SD | ||||||
| Label A | 146 ± 108 | 155 ± 92.8 | 167 ± 95.7 | 161 ± 91.1 | 0.092 | |
| Label B | 223 ± 108 | 289 ± 123 | 315 ± 146 | 362 ± 202 | <0.001 | |
| Label C | 144 ± 83.2 | 230 ± 98.9 | 290 ± 120 | 375 ± 207 | <0.001 | |
| Label D | 45.7 ± 44.4 | 59.4 ± 53.5 | 75.2 ± 61.8 | 116 ± 106 | <0.001 | |
| Label E | 38.9 ± 35.4 | 59.8 ± 43.5 | 89.4 ± 65.0 | 164 ± 152 | <0.001 | |
| Energy (Kcal), mean ± SD | 1663 ± 410 | 1945 ± 450 | 2141 ± 458 | 2373 ± 470 | <0.001 | |
| Sex (women), % | 44.3 | 45.1 | 44.4 | 45.9 | 0.823 | |
| Age, mean ± SD | 67.3 ± 5.80 | 66.6 ± 5.37 | 66.9 ± 5.31 | 67.3 ± 6.56 | 0.886 | |
| Educational level (%) | 0.714 † | |||||
| Primary or less | 36.7 | 41.4 | 41.4 | 41.1 | ||
| Secondary | 29.8 | 29.0 | 28.4 | 34.3 | ||
| University | 33.5 | 29.6 | 30.3 | 24.7 | ||
| Smoking, % | 0.700 † | |||||
| Current smoker | 14.6 | 14.8 | 11.7 | 16.4 | ||
| Former smoker | 33.5 | 26.5 | 28.4 | 28.8 | ||
| Never smoker | 51.9 | 58.6 | 59.9 | 54.8 | ||
| Former drinker status, % | 6.33 | 7.41 | 4.32 | 13.01 | 0.094 | |
| Physical activity at leisure time (Mets/h/week), mean ± SD | 24.7 ± 15.7 | 25.8 ± 17.4 | 22.9 ± 14.4 | 24.3 ± 16.7 | 0.453 | |
| Time watching TV (h/week), mean ± SD | 15.4± 9.46 | 15.3 ± 8.93 | 14.6 ± 10.6 | 15.7 ± 8.47 | 0.956 | |
| Total sleep time (minutes/day), mean ± SD | 428 ± 85.8 | 439 ± 81.4 | 434 ± 81.0 | 435 ± 77.1 | 0.578 | |
| Body Mass Index (kg/m2), mean ± SD | 26.2 ± 2.44 | 25.7 ± 2.43 | 25.6± 2.49 | 24.9 ± 2.82 | <0.001 | |
| Waist circumference (cm), mean ± SD | 89.4 ± 8.69 | 88.3 ± 9.12 | 88.9 ± 9.0 | 86.7 ± 9.85 | 0.026 | |
| Ethanol consumption (g/day), mean ± SD | 10.6 ± 16.4 | 10.7 ± 17.9 | 12.9 ± 18.2 | 9.12 ± 15.0 | 0.781 | |
| Coronary heart disease, % | 0.00 | 2.47 | 0.00 | 0.68 | 0.885 | |
| Chronic respiratory disease, % | 3.16 | 6.17 | 4.94 | 5.48 | 0.465 | |
| Hypertension, % | 60.1 | 61.7 | 61.1 | 53.4 | 0.432 † | |
| Diabetes, % | 10.8 | 10.49 | 6.17 | 5.48 | 4.779 † | |
| Cancer, % | 1.90 | 1.23 | 3.09 | 1.37 | 0.928 | |
| Osteoarthritis, % | 34.8 | 35.8 | 35.2 | 37.0 | 0.739 | |
| Arthritis, % | 5.70 | 7.41 | 8.64 | 9.59 | 0.183 | |
| Number of medications, % | 0.751 † | |||||
| 0 | 33.5 | 37.0 | 38.3 | 41.1 | ||
| 1–3 | 55.7 | 49.4 | 51.9 | 47.3 | ||
| >3 | 10.8 | 13.6 | 9.88 | 11.6 | ||
| MEDAS Score (excluding wine), mean ± SD | 7.77 ± 1.65 | 7.48 ± 1.68 | 7.17 ± 1.57 | 6.61 ± 1.72 | <0.001 | |
| Ultra-processed food (g/day) | 116 ± 80 | 180 ± 98 | 245 ± 119 | 400 ± 223 | <0.001 | |
SD: standard deviation; 5-CNS: five-color Nutri-Score; DI: dietary index; † chi-squared test. Categories of the five-color Nutri-Score (5-CNS DI) in g/day/kg: Q1 (9.1523.95); Q2: (24.00–31.12); Q3 (38.98–31.23); Q4 (39.13–96.43) in men and Q1 (11.80–26.70); Q2: (26.84–33.56); Q3 (33.57–41.13); Q4 (41.21–120.49) in women.
In the fully adjusted model, OR (95% CI) for AO across the increasing quartiles of the 5-CNS Dietary Index (DI) per gram per day per kilogram were as follows: 1 (reference), 1.29 (0.72–2.32), 1.25 (0.66–2.36), and 2.45 (1.17–5.14); with a p-value for trend at 0.035. The risk of AO increased by 24% (0–53%) with a 10-unit increase in this DI. When comparing the highest to the lowest quartiles of the continuous Nutri-Score DI in g/day/kg, the OR (95% CI) for AO was 2.52 (1.22–5.20), p-trend = 0.023, with an 8% (0–17%) increased risk of AO per 10-unit increase in this DI (Table 2).
Table 2.
Risk of abdominal obesity over a six-year follow-up according to the Nutri-Score dietary indexes in g/day/kg (n = 628).
| Five-Color Nutri-Score (5-CNS DI) in g/Day/kg | ||||||
|---|---|---|---|---|---|---|
| Sex-Specific Quartiles of the 5-CNS DI in g/day/kg | ||||||
| Q1 (Best Diet Quality) |
Q2 | Q3 | Q4 (Worse Diet Quality) |
p-Trend | Per 10 Unit-Increment | |
| Interquartile range (g/day/kg) | 17.78–23.46 | 27.04–30.46 | 34.13–37.62 | 42.94–54.78 | ||
| Cases/n | 47/158 | 44/162 | 39/162 | 41/146 | ||
| Model 1, OR (95% CI) | 1 (Ref.) | 0.94 (0.56–1.56) | 0.83 (0.48–1.44) | 1.08 (0.59–1.99) | 0.923 | 0.98 (0.82–1.17) |
| Model 2, OR (95% CI) | 1 (Ref.) | 1.34 (0.75–2.39) | 1.32 (0.70–2.46) | 2.71 (1.33–5.50) | 0.012 | 1.28 (1.04–1.57) |
| Model 3, OR (95% CI) | 1 (Ref.) | 1.29 (0.72–2.32) | 1.25 (0.66–2.36) | 2.45 (1.17–5.14) | 0.035 | 1.24 (1.00–1.53) |
| Continuous Nutri-Score DI in g/day/kg | ||||||
| Sex-Specific Quartiles of the Continuous Nutri-Score DI in g/day/kg | ||||||
|
Q1
(Best Diet Quality) |
Q2 | Q3 |
Q4
(Worse Diet Quality) |
p -Trend | Per 10-Unit Increment | |
| Interquartile range (g/day/kg) | 1.16–14.32 | 26.52–35.75 | 45.12–54.07 | 69.01–100.82 | ||
| Cases/n | 38/161 | 45/160 | 41/159 | 47/148 | ||
| Model 1, OR (95% CI) | 1 (Ref.) | 1.43 (0.85–2.40) | 1.36 (0.79–2.33) | 2.14 (1.19–3.87) | 0.022 | 1.05 (0.99–1.11) |
| Model 2, OR (95% CI) | 1 (Ref.) | 1.62 (0.90–2.89) | 1.59 (0.87–2.92) | 2.73 (1.41–5.31) | 0.006 | 1.09 (1.02–1.17) |
| Model 3, OR (95% CI) | 1 (Ref.) | 1.58 (0.87–2.84) | 1.52 (0.81–2.85) | 2.52 (1.22–5.20) | 0.023 | 1.08 (1.00–1.17) |
5-CNS: five-color Nutri-Score, DI: dietary index, OR: odds ratio, CI: confidence intervals. Model 1 was adjusted for sex, age (continuous), total energy intake (kcal/d), and educational level (primary or less, secondary, and university); Model 2 was adjusted for smoking (current, former, and never smoker), former drinker status (yes/no), physical activity at leisure time (METS/h/week), time watching TV (h/week), total sleep time (minutes/day), total ethanol consumption (grams/day), body mass index (continuous), chronic obstructive pulmonary disease/asthma (yes/no), coronary heart disease (yes/no), hypercholesterolemia (yes/no), hypertension (yes/no), diabetes (yes/no), cancer (yes/no), arthrosis (yes/no), arthritis (yes/no), and number of medications (0, 1–3, >3). Model 3 was further adjusted by MEDAS index score excluding wine (0–13 points).
Comparable results were observed for the two DIs based on the percentage of energy; the respective values stood at 2.03 (0.98–4.23), p-trend = 0.024, for the 5-CNS DI, and 2.24 (1.07–4.68), p-trend = 0.031, for the continuous Nutri-Score DI (Table 3).
Table 3.
Risk of abdominal obesity over a six-year follow-up according to Nutri-Score dietary indexes based on percentage of energy (n = 628).
| Five Color Nutri-Score DI (5-CNS DI) Based on the Percentage of Energy | ||||||
|---|---|---|---|---|---|---|
| Sex-Specific Quartiles of the 5-CNS DI Based on the % of Energy | ||||||
| Q1 (Best Diet Quality) |
Q2 | Q3 | Q4 (Worse Diet Quality) |
p-Trend | Per 1-Unit Increment | |
| Interquartile range (% of energy) | 1.44–1.74 | 1.93–2.06 | 2.20–2.32 | 2.51–2.87 | ||
| Cases/n | 35/157 | 41/158 | 51/157 | 44/156 | ||
| Model 1, OR (95% CI) | 1 (Ref.) | 1.33 (0.78–2.26) | 1.86 (1.10–3.12) | 1.60 (0.92–2.78) | 0.045 | 1.48 (0.98–2.24) |
| Model 2, OR (95% CI) | 1 (Ref.) | 1.41 (0.78–2.56) | 2.29 (1.26–4.14) | 2.19 (1.16–4.14) | 0.005 | 1.88 (1.15–3.07) |
| Model 3, OR (95% CI) | 1 (Ref.) | 1.37 (0.74–2.53) | 2.19 (1.17–4.11) | 2.03 (0.98–4.23) | 0.024 | 1.76 (0.98–3.15) |
| Continuous Nutri-Score DI Based on the % of Energy | ||||||
| Sex-Specific Quartiles of the Continuous Nutri-Score DI Based on the % of Energy | ||||||
|
Q1
(Best Diet Quality) |
Q2 | Q3 |
Q4
(Worse Diet Quality) |
p -Trend | Per 1-Unit Increment | |
| Interquartile range (% of energy) | 1.58–2.74 | 3.66–4.367 | 5.08–5.84 | 6.95–8.95 | ||
| Cases/n | 31/160 | 45/157 | 46/158 | 49/153 | ||
| Model 1, OR (95% CI) | 1 (Ref.) | 1.77 (1.0–3.0) | 1.90 (1.11–3.25) | 2.27 (1.32–3.93) | 0.004 | 1.08 (1.00–1.16) |
| Model 2, OR (95% CI) | 1 (Ref.) | 1.74 (0.96–3.17) | 2.15 (1.18–3.92) | 2.34 (1.25–4.35) | 0.007 | 1.08 (0.99–1.17) |
| Model 3, OR (95% CI) | 1 (Ref.) | 1.72 (0.92–3.19) | 2.09 (1.10–4.00) | 2.24 (1.07–4.68) | 0.031 | 1.05 (0.95–1.17) |
5-CNS: five-color Nutri-Score, DI: dietary index, OR: odds ratio, CI: confidence intervals. Model 1 was adjusted for sex, age (continuous), total energy intake (kcal/d), and educational level (primary or less, secondary, and university); Model 2 was adjusted for smoking (current, former, and never smoker), former drinker status (yes/no), physical activity at leisure time (METS/h/week), time watching TV (h/week), total sleep time (minutes/day), total ethanol consumption (grams/day), body mass index (continuous), chronic obstructive pulmonary disease/asthma (yes/no), coronary heart disease (yes/no), hypercholesterolemia (yes/no), hypertension (yes/no), diabetes (yes/no), cancer (yes/no), arthrosis (yes/no), arthritis (yes/no), and number of medications (0, 1–3, >3). Model 3 was further adjusted by MEDAS index score excluding wine (0–13 points).
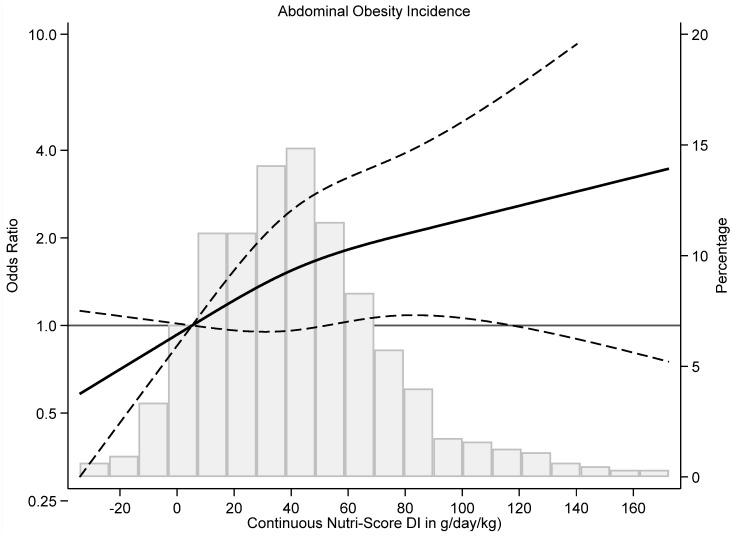
When the dose–response was assessed using the continuous Nutri-Score DI, a positive linear relationship was observed which indicated that the higher the index, the higher the incidence of AO (Figure 2).
Figure 2.
Restricted-cubic splines of association of continuous Nutri-Score DI in g/day/kg with risk of abdominal obesity in Seniors-ENRICA 1 study (from 2008–2010 and 2015). Analyses were adjusted as in Model 3 in Table 2 [adjustment for sex, age (continuous), total energy intake (kcal/d), and educational level (primary or less, secondary, and university), smoking (current, former, and never smoker former drinker status (yes/no), physical activity at leisure time (METS/h/week), time watching TV (h/week), total sleep time (minutes/day), total ethanol consumption (grams/day), body mass index (continuous), chronic obstructive pulmonary disease/asthma (yes/no), coronary heart disease (yes/no), hypercholesterolemia (yes/no), hypertension (yes/no), diabetes (yes/no), cancer (yes/no), arthrosis (yes/no), arthritis (yes/no), number of medications (0, 1–3, >3). MEDAS index score excluding wine (0–13 points)].
In the sensitivity analyses, the OR (95% CI) for AO after adjusting for waist circumference at baseline when comparing extreme quartiles of the 5-CNS DI was 2.59 (1.22–5.52); p-trend = 0.032. In addition, the correlation between the 5-CNS DI and UPF consumption was 0.68. The OR (95% CI) after adjustment for UPF consumption at baseline was: 1.75 (0.74–4.18); p-trend = 0.316 (Table S1). Comparable results were obtained for the continuous Nutri-Score DI in g/day/kg and in the percentage of energy (Table S2).
4. Discussion
In this cohort of community-dwelling older adults from Spain, individuals following a diet with a less favorable Nutri-Score exhibited approximately a 24% higher risk of abdominal obesity (AO) for every 10-unit increase in the 5-CNS DI (g/day/kg). This association persisted after adjustment for BMI as well as adherence to the Mediterranean diet. The findings indicate that a diet of lower nutritional quality, evaluated using the Nutri-Score system, is significantly associated to a higher incidence of abdominal obesity in the elderly population.
Our findings align with current research on the topic. The influence of food’s nutritional quality (levels of saturated fat, salt, calories, sugar, etc.) is widely recognized [27]. Several epidemiological and interventional studies have shown the association between the quality of the food consumed and various diseases. Regarding the individual components of the Nutri-Score, the higher incidence and prevalence of obesity was associated with higher consumption of energy [28], sugar [29], saturated fat [30], and sodium [31], but a protective association was found with higher consumption of fruits and vegetables [32], fiber [33], and protein [34]. Indeed, FOP such as the Nutri-Score intend to be a synthesis of these evidences and, as such, several prospective cohorts (i.e., SU. VI.MAX, the NutriNet-Santé, the EPIC, and the Seniors-ENRICA 1 cohort), have shown that a less favorable Nutri-Score is associated with numerous diseases [6,7,8,9,10,11] and all-cause mortality [12].
In addition to the association derived from each nutrient component of the Nutri-Score (part of the algorithm for its calculation) which has been widely studied, another possible underlying mechanism of this association may be related to the degree of processing of the foods consumed. Studies have shown that about 84% of foods labelled D or E in the Nutri-Score system are highly processed or UPF [35]. A recent systematic review of prospective studies showed a link between the consumption of UPF and a higher incidence of obesity, along with an elevated risk of cardiometabolic conditions [36]. In a study from Sandoval-Insausti et al. [37], an association between UPF consumption and incident AO was identified. These findings have been reproduced in other worldwide cohorts such as the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil) cohort [38], the Ribeirão Preto cohort [39], the NutriNet-Santé cohort [40], the University of Navarra Follow-Up (SUN) cohort study [41], the China Health and Nutrition Survey (CHNS) [42], the EPIC [43], and the National Diet and Nutrition Survey Rolling Program (NDNS) in the United Kingdom [44]. All showed a positive association between UPF consumption and the development of AO.
Biological mechanisms have been proposed to link the degree of food processing with AO beyond its nutritional quality including the content of additives added to these products. Additives have been associated with changes in the gut microbiota’s composition promoting inflammatory diseases [45], with potentially important implications for body weight and adiposity [46]. Additionally, UPFs are generally packaged in plastics, and numerous plasticizers (such as bisphenol A) have been shown to be associated with obesity, possibly acting as endocrine disruptors [47].
In our analyses, 48.3% of the association between 5-CNS DI and AO was explained by UPF consumption, and the remainder could be explained by the Nutri-Score components themselves (energy, sugar, saturated fat, sodium, fruits and vegetables, fiber, and protein content). Therefore, the extent of food processing and the nutritional quality, as denoted by the Nutri-Score, are interconnected, representing complementary aspects (since UPF tends to have a higher score in the 5-CNS DI and at the same time tends to have a lower nutritional quality) [27]. Considering these two dimensions together, our results make it possible to improve dietary advice by promoting the consumption of products with a better Nutri-Score, which generally tend to be not only those with a better nutritional quality, but also those with the lowest level of processing.
Public health strategies are essential to guide consumer choices and achieve a substantial population impact on preventing cardiovascular disease through diet [6]. Among FOP, the graphical design of Nutri-Score facilitates nutritional literacy, thus favoring consumer choice [1,48,49,50]. Nevertheless, there is also evidence to suggest that the Nutri-Score system may require more public education compared to other intuitive symbols (such as warning labels) [51]. For example, it is often thought that Nutri-Score ‘punishes’ some healthy products such as olive oil, previously categorized as C. Nevertheless, Nutri-Score is intended to make it simpler to compare foods within the same category. If we compare different types of oil, olive oil gets the best score. This concept requires people to understand how to interpret the Nutri-Score properly.
Another aspect that encourages the use of FOPs is the pressure on companies to adapt and improve the nutritional quality of their products. However, there is limited evidence on the impact of FOP nutrition labelling on food reformulation [52]. The impact of such policies on reformulation is likely to be greater if they are mandatory, coordinated with other regulations, and thoroughly monitored and evaluated [52]. In the European Union, the use of FOPs is recommended but not mandatory. This may be the reason why the impact of FOPs on reformulation may be limited (achieving only minor changes). In addition, policies targeting reformulation and wider food system policies will be needed to significantly improve diets.
The relationship between dietary patterns guided by the Nutri-Score system and abdominal obesity holds significant social relevance. First, the Nutri-Score is practical and easy-to-understand, making it accessible and useful for individuals with lower levels of education and older adults. The Nutri-Score system also enhances consumer empowerment by boosting their capacity to make well-informed decisions. The prevalence of AO among older adults in Spain is high and very difficult to reverse. It is estimated that 61.6% of older adults in Spain already have AO, with this prevalence being even higher among women (69.7%) [53]. AO is also associated with an increased risk of disability [14], and an increase in fat mass may be associated with sarcopenic obesity [15]. Several epidemiological studies suggest that this syndrome is associated with accelerated functional decline, increased risk of multiple diseases and, ultimately, increased mortality [15]. Therefore, any effort to reduce the incidence of AO may be beneficial.
The study also presents certain limitations. First, the number of cases of incident AO was relatively low due to the high prevalence of AO at baseline. However, the association attained was statistically significance. Second, repeated dietary measurements were not considered, and diet may have changed since baseline. Third, because diet was self-reported, recall bias cannot completely be ruled out. Fourth, despite comprehensive adjustments in the statistical analyses for numerous potential confounding factors, the possibility of some residual confounding cannot be entirely excluded. Our study also has several strengths. First, prospective design makes it more likely that the time order of the association will be examined. Second, dietary intake was evaluated using a validated dietary history that was gathered by trained personnel. Third, the findings remained robust following adjustments for numerous potential confounders.
5. Conclusions
A diet characterized by a less favorable Nutri-Score rating is prospectively associated with an increased risk of developing AO. Our findings endorse the adoption of the Nutri-Score front-of-pack (FOP) labeling system as an effective public health measure to improve dietary nutritional quality.
Abbreviations
| 5-CNS DI | Five-Color Nutri-Score Dietary Index |
| BMI | Body mass index |
| CHNS | China Health and Nutrition Survey study and the National Diet |
| CI | Confidence intervals |
| DH-ENRICA | validated computer-based dietary history from the Study on Nutrition and Cardiovascular risk factors in Spain |
| DI | Dietary Index |
| ELSA-Brasil cohort | The Brazilian Longitudinal Study of Adult Health, cohort |
| ENRICA | Study on Nutrition and Cardiovascular risk factors in Spain |
| EPIC | European Prospective Investigation into Cancer and Nutrition cohort |
| FOP | Front-of-packages |
| NDNS | Nutrition Survey Rolling Programme |
| MEDAS | Mediterranean Diet Adherence Score |
| OR | Odds Ratio |
| SD | Standard Deviation |
| Seniors-ENRICA Cohort | Seniors Study on Nutrition and Cardiovascular risk factors in Spain cohort |
| SU.VI.MAX | The Supplementation en Vitamines et Mineraux Antioxydants cohort |
| SUN | University of Navarra Follow-Up cohort study |
| UPF | Ultra-processed food |
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu16071020/s1, Table S1. Sensitivity analysis for the association between the Nutri-Score Dietary Indexes in g/day/kg and the risk of abdominal obesity (N = 628). Table S2. Sensitivity analysis for the association between the Nutri-Score Dietary Indexes based on the percentage of energy and the risk of abdominal obesity (N = 628).
Author Contributions
Conceptualization, P.G.-C.; methodology, J.R.-G. and P.G.-C.; software, J.R.-G. and D.M.M.; validation, P.G.-C.; formal analysis, J.R.-G. and D.M.M.; investigation, J.R.-G.; resources, J.R.B., F.R.-A., and P.G.-C.; data curation, J.R.-G. and P.G.-C.; writing—original draft preparation, J.R.-G.; writing—review and editing, D.M.M., C.D.-V., H.S.-I., M.R.-A., J.R.B., F.R.-A. and P.G.-C.; visualization, J.R.-G. and D.M.M.; supervision, P.G.-C.; project administration, P.G.-C.; funding acquisition, P.G.-C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The baseline and follow-up studies were approved by the Clinical Research Ethics Committee of the University Hospital of La Paz (Comité Ético de Investigación Clínica del Hospital Universitario La Paz), Madrid, Spain on 2 June 2015, with the approval code HULP:PI-1793.
Informed Consent Statement
All participants provided written informed consent.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
FIS grants 17/1709, 20/144 and 22/01111 (Instituto de Salud Carlos III, State Secretary of R+D+I, and FEDER/FSE), and the FACINGLCOVID-CM project-Funding REACT EU Program (Comunidad de Madrid and the European Regional Development Fund. ERDF. European Union).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Temple N.J. Front-of-Package Food Labels: A Narrative Review. Appetite. 2020;144:104485. doi: 10.1016/j.appet.2019.104485. [DOI] [PubMed] [Google Scholar]
- 2.Cecchini M., Warin L. Impact of Food Labelling Systems on Food Choices and Eating Behaviours: A Systematic Review and Meta-Analysis of Randomized Studies. Obes. Rev. 2016;17:201–210. doi: 10.1111/obr.12364. [DOI] [PubMed] [Google Scholar]
- 3.Kanter R., Vanderlee L., Vandevijvere S. Front-of-Package Nutrition Labelling Policy: Global Progress and Future Directions. Public Health Nutr. 2018;21:1399–1408. doi: 10.1017/S1368980018000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shangguan S., Afshin A., Shulkin M., Ma W., Marsden D., Smith J., Saheb-Kashaf M., Shi P., Micha R., Imamura F., et al. A Meta-Analysis of Food Labeling Effects on Consumer Diet Behaviors and Industry Practices. Am. J. Prev. Med. 2019;56:300–314. doi: 10.1016/j.amepre.2018.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andreeva V.A., Egnell M., Touvier M., Galan P., Julia C., Hercberg S. International Evidence for the Effectiveness of the Front-of-Package Nutrition Label Called Nutri-Score. Cent. Eur. J. Public Health. 2021;29:76–79. doi: 10.21101/cejph.a6239. [DOI] [PubMed] [Google Scholar]
- 6.Adriouch S., Julia C., Kesse-Guyot E., Méjean C., Ducrot P., Péneau S., Donnenfeld M., Deschasaux M., Menai M., Hercberg S., et al. Prospective Association between a Dietary Quality Index Based on a Nutrient Profiling System and Cardiovascular Disease Risk. Eur. J. Prev. Cardiol. 2016;23:1669–1676. doi: 10.1177/2047487316640659. [DOI] [PubMed] [Google Scholar]
- 7.Julia C., Fézeu L.K., Ducrot P., Méjean C., Péneau S., Touvier M., Hercberg S., Kesse-Guyot E. The Nutrient Profile of Foods Consumed Using the British Food Standards Agency Nutrient Profiling System Is Associated with Metabolic Syndrome in the SU.VI.MAX Cohort. J. Nutr. 2015;145:2355–2361. doi: 10.3945/jn.115.213629. [DOI] [PubMed] [Google Scholar]
- 8.Montero-Salazar H., Guallar-Castillón P., Banegas J.R., Åkesson A., Rey-García J., Rodríguez-Artalejo F., Donat-Vargas C. Food Consumption Based on the Nutrient Profile System Underlying the Nutri-Score and Renal Function in Older Adults. Clin. Nutr. 2022;41:1541–1548. doi: 10.1016/j.clnu.2022.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Rey-García J., Donat-Vargas C., Sandoval-Insausti H., Banegas J.R., Dominguez L.J., Rodríguez-Artalejo F., Guallar-Castillón P. Less Favourable Food Consumption Ratings in the Five-Color Nutri-Score Are Associated with Incident Frailty in Older Adults. Age Ageing. 2023;52:afad142. doi: 10.1093/ageing/afad142. [DOI] [PubMed] [Google Scholar]
- 10.Donnenfeld M., Julia C., Kesse-Guyot E., Méjean C., Ducrot P., Péneau S., Deschasaux M., Latino-Martel P., Fezeu L., Hercberg S., et al. Prospective Association between Cancer Risk and an Individual Dietary Index Based on the British Food Standards Agency Nutrient Profiling System. Br. J. Nutr. 2015;114:1702–1710. doi: 10.1017/S0007114515003384. [DOI] [PubMed] [Google Scholar]
- 11.Deschasaux M., Huybrechts I., Murphy N., Julia C., Hercberg S., Srour B., Kesse-Guyot E., Latino-Martel P., Biessy C., Casagrande C., et al. Nutritional Quality of Food as Represented by the FSAm-NPS Nutrient Profiling System Underlying the Nutri-Score Label and Cancer Risk in Europe: Results from the EPIC Prospective Cohort Study. PLoS Med. 2018;15:e1002651. doi: 10.1371/journal.pmed.1002651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donat-Vargas C., Sandoval-Insausti H., Rey-Garciá J., Ramón Banegas J., Rodríguez-Artalejo F., Guallar-Castillón P. Five-Color Nutri-Score Labeling and Mortality Risk in a Nationwide, Population-Based Cohort in Spain: The Study on Nutrition and Cardiovascular Risk in Spain (ENRICA) Am. J. Clin. Nutr. 2021;113:1301–1311. doi: 10.1093/ajcn/nqaa389. [DOI] [PubMed] [Google Scholar]
- 13.Hirani V. Generalised and Abdominal Adiposity Are Important Risk Factors for Chronic Disease in Older People: Results from a Nationally Representative Survey. J. Nutr. Health Aging. 2011;15:469–478. doi: 10.1007/s12603-011-0051-3. [DOI] [PubMed] [Google Scholar]
- 14.Guallar-Castillón P., Sagardui-Villamor J., Banegas J.R., Graciani A., Fornés N.S., López García E., Rodríguez-Artalejo F. Waist Circumference as a Predictor of Disability among Older Adults. Obesity. 2007;15:233–244. doi: 10.1038/oby.2007.532. [DOI] [PubMed] [Google Scholar]
- 15.Stenholm S., Harris T.B., Rantanen T., Visser M., Kritchevsky S.B., Ferrucci L. Sarcopenic Obesity: Definition, Cause and Consequences. Curr. Opin. Clin. Nutr. Metab. Care. 2008;11:693–700. doi: 10.1097/MCO.0b013e328312c37d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodríguez-Artalejo F., Graciani A., Guallar-Castillón P., León-Muñoz L.M., Zuluaga M.C., López-García E., Gutiérrez-Fisac J.L., Taboada J.M., Aguilera M.T., Regidor E., et al. Justificación y Métodos Del Estudio Sobre Nutrición y Riesgo Cardiovascular En España (ENRICA) Rev. Esp. Cardiol. 2011;64:876–882. doi: 10.1016/j.recesp.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 17.Guallar-Castillón P., Sagardui-Villamor J., Balboa-Castillo T., Sala-Vila A., Astolfi M.J.A., Pelous M.D.S., León-Muñoz L.M., Graciani A., Laclaustra M., Benito C., et al. Validity and Reproducibility of a Spanish Dietary History. PLoS ONE. 2014;9:e86074. doi: 10.1371/journal.pone.0086074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moreiras O., Carvajal A., Cabrera L., Cuadrado C. Tablas de Composición de Alimentos. 11th ed. Ediciones Pirámide; Madrid, Spain: 2007. [Google Scholar]
- 19.Ferrán A., Zamora R., Cervera P. In: Tablas de Composición de Alimentos Del CESNID. Centre d’Ensenyament Superior de Nitricióí Dietètica (CESNID), editor. Edicions Universitat de Barcelona; Barcelona, Spain: 2004. [Google Scholar]
- 20.Julia C., Kesse-Guyot E., Touvier M., Méjean C., Fezeu L., Hercberg S. Application of the British Food Standards Agency Nutrient Profiling System in a French Food Composition Database. Br. J. Nutr. 2014;112:1699–1705. doi: 10.1017/S0007114514002761. [DOI] [PubMed] [Google Scholar]
- 21.Chantal J., Serge H. Development of a New Front-of-Pack Nutrition Label in France: The Five-Colour Nutri-Score. Public Health Panor. 2017;3:537–820. [Google Scholar]
- 22.Scientific Committee of the Nutri-Score Update Report from the Scientific Committee of the Nutri-Score 2022. 2022. [(accessed on 30 March 2024)]. Available online: www.aesan.gob.es/AECOSAN/docs/documentos/Nutri_Score/2022_main_algorithm_report_update_FINAL.pdf.
- 23.Scientific Committee of the Nutri-Score 2023 Update of the Nutri-Score Algorithm for Beverages 2023, 1–104. [(accessed on 30 March 2024)]. Available online: www.aesan.gob.es/AECOSAN/docs/documentos/Nutri_Score/report_beverages_2023.pdf.
- 24.World Health Organization . Waist Circumference and Waist-Hip Ratio: Report of a WHO Expert Consultation, Geneva, 8–11 December 2008. World Health Organization; Geneva, Switzerland: 2011. [Google Scholar]
- 25.Martínez-González M.A., García-Arellano A., Toledo E., Salas-Salvadó J., Buil-Cosiales P., Corella D., Covas M.I., Schröder H., Arós F., Gómez-Gracia E., et al. A 14-Item Mediterranean Diet Assessment Tool and Obesity Indexes among High-Risk Subjects: The PREDIMED Trial. PLoS ONE. 2012;7:e43134. doi: 10.1371/journal.pone.0043134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monteiro C.A., Levy R.B., Claro R.M., de Castro I.R.R., Cannon G. A New Classification of Foods Based on the Extent and Purpose of Their Processing. Cad. Saude Publica. 2010;26:2039–2049. doi: 10.1590/S0102-311X2010001100005. [DOI] [PubMed] [Google Scholar]
- 27.Touvier M., Srour B., Hercberg S., Galan P., Kesse-Guyot E., Julia C. Health Impact of Foods: Time to Switch to a 3D-Vision. Front. Nutr. 2022;9:1958–1972. doi: 10.3389/fnut.2022.966310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Romieu I., Dossus L., Barquera S., Blottière H.M., Franks P.W., Gunter M., Hwalla N., Hursting S.D., Leitzmann M., Margetts B., et al. Energy Balance and Obesity: What Are the Main Drivers? Cancer Causes Control. 2017;28:247–258. doi: 10.1007/s10552-017-0869-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faruque S., Tong J., Lacmanovic V., Agbonghae C., Minaya D.M., Czaja K. The Dose Makes the Poison: Sugar and Obesity in the United States—A Review. Pol. J. Food Nutr. Sci. 2019;69:219–233. doi: 10.31883/pjfns/110735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phillips C.M., Kesse-Guyot E., McManus R., Hercberg S., Lairon D., Planells R., Roche H.M. High Dietary Saturated Fat Intake Accentuates Obesity Risk Associated with the Fat Mass and Obesity-Associated Gene in Adults. J. Nutr. 2012;142:824–831. doi: 10.3945/jn.111.153460. [DOI] [PubMed] [Google Scholar]
- 31.Ma Y., He F.J., MacGregor G.A. High Salt Intake: Independent Risk Factor for Obesity? Hypertension. 2015;66:843–849. doi: 10.1161/HYPERTENSIONAHA.115.05948. [DOI] [PubMed] [Google Scholar]
- 32.Nour M., Lutze S.A., Grech A., Allman-Farinelli M. The Relationship between Vegetable Intake and Weight Outcomes: A Systematic Review of Cohort Studies. Nutrients. 2018;10:1626. doi: 10.3390/nu10111626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dayib M., Larson J., Slavin J. Dietary Fibers Reduce Obesity-Related Disorders: Mechanisms of Action. Curr. Opin. Clin. Nutr. Metab. Care. 2020;23:445–450. doi: 10.1097/MCO.0000000000000696. [DOI] [PubMed] [Google Scholar]
- 34.Fappi A., Mittendorfer B. Dietary Protein Intake and Obesity-Associated Cardiometabolic Function. Curr. Opin. Clin. Nutr. Metab. Care. 2020;23:380–386. doi: 10.1097/MCO.0000000000000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Romero Ferreiro C., Lora Pablos D., Gómez de la Cámara A. Two Dimensions of Nutritional Value: Nutri-Score and NOVA. Nutrients. 2021;13:2783. doi: 10.3390/nu13082783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen X., Zhang Z., Yang H., Qiu P., Wang H., Wang F., Zhao Q., Fang J., Nie J. Consumption of Ultra-Processed Foods and Health Outcomes: A Systematic Review of Epidemiological Studies. Nutr. J. 2020;19:86. doi: 10.1186/s12937-020-00604-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sandoval-Insausti H., Jiménez-Onsurbe M., Donat-Vargas C., Rey-García J., Banegas J.R., Rodríguez-Artalejo F., Guallar-Castillón P. Ultra-Processed Food Consumption Is Associated with Abdominal Obesity: A Prospective Cohort Study in Older Adults. Nutrients. 2020;12:2368. doi: 10.3390/nu12082368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Canhada S.L., Luft V.C., Giatti L., Duncan B.B., Chor D., de Jesus M da Fonseca M., Matos S.M.A., Molina M.D.C.B., Barreto S.M., Levy R.B., et al. Ultra-Processed Foods, Incident Overweight and Obesity, and Longitudinal Changes in Weight and Waist Circumference: The Brazilian Longitudinal Study of Adult Health (ELSA-Brasil) Public Health Nutr. 2020;23:1076–1086. doi: 10.1017/S1368980019002854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.da Silva Magalhães E.I., de Oliveira B.R., Rudakoff L.C.S., de Carvalho V.A., de Almeida Fonseca Viola P.C., Arruda S.P.M., de Carvalho C.A., da Silva Coelho C.C.N., Bragança M.L.B.M., Bettiol H., et al. Sex-Dependent Effects of the Intake of NOVA Classified Ultra-Processed Foods on Syndrome Metabolic Components in Brazilian Adults. Nutrients. 2022;14:3126. doi: 10.3390/nu14153126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beslay M., Srour B., Méjean C., Allès B., Fiolet T., Debras C., Chazelas E., Deschasaux M., Wendeu-Foyet M.G., Hercberg S., et al. Ultra-Processed Food Intake in Association with BMI Change and Risk of Overweight and Obesity: A Prospective Analysis of the French NutriNet-Santé Cohort. PLoS Med. 2020;17:e1003256. doi: 10.1371/journal.pmed.1003256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Deus Mendonça R., Marcal Pimenta A., Gea A., de la Fuente-Arrillaga C., Martinez-Gonzalez M.A., Lopes A.C.S., Bes-Rastrollo M. Ultraprocessed Food Consumption and Risk of Overweight and Obesity: The University of Navarra Follow-Up (SUN) Cohort Study. Am. J. Clin. Nutr. 2017;105:1012. doi: 10.3945/ajcn.116.135004. [DOI] [PubMed] [Google Scholar]
- 42.Li M., Shi Z. Ultra-Processed Food Consumption Associated with Overweight/Obesity among Chinese Adults—Results from China Health and Nutrition Survey 1997–2011. Nutrients. 2021;13:2796. doi: 10.3390/nu13082796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cordova R., Kliemann N., Huybrechts I., Rauber F., Vamos E.P., Levy R.B., Wagner K.-H., Viallon V., Casagrande C., Nicolas G., et al. Consumption of Ultra-Processed Foods Associated with Weight Gain and Obesity in Adults: A Multi-National Cohort Study. Clin. Nutr. 2021;40:5079–5088. doi: 10.1016/j.clnu.2021.08.009. [DOI] [PubMed] [Google Scholar]
- 44.Rauber F., Campagnolo P.D.B., Hoffman D.J., Vitolo M.R. Nutrition, Metabolism & Cardiovascular Diseases Consumption of Ultra-Processed Food Products and Its Effects on Children’ s Lipid pro Fi Les: A Longitudinal Study. Nutr. Metab. Cardiovasc. Dis. 2019;25:116–122. doi: 10.1016/j.numecd.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 45.Zinöcker M.K., Lindseth I.A. The Western Diet-Microbiome-Host Interaction and Its Role in Metabolic Disease. Nutrients. 2018;10:365. doi: 10.3390/nu10030365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rauber F., Steele E.M., da Costa Louzada M.L., Millett C., Monteiro C.A., Levy R.B. Ultra-Processed Food Consumption and Indicators of Obesity in the United Kingdom Population (2008–2016) PLoS ONE. 2020;15:e0232676. doi: 10.1371/journal.pone.0232676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heindel J.J., Newbold R., Schug T.T. Endocrine Disruptors and Obesity. Nat. Rev. Endocrinol. 2015;11:653–661. doi: 10.1038/nrendo.2015.163. [DOI] [PubMed] [Google Scholar]
- 48.Gómez-Donoso C., Martínez-González M.Á., Perez-Cornago A., Sayón-Orea C., Martínez J.A., Bes-Rastrollo M. Association between the Nutrient Profile System Underpinning the Nutri-Score Front-of-Pack Nutrition Label and Mortality in the SUN Project: A Prospective Cohort Study. Clin. Nutr. 2021;40:1085–1094. doi: 10.1016/j.clnu.2020.07.008. [DOI] [PubMed] [Google Scholar]
- 49.Egnell M., Kesse-Guyot E., Galan P., Touvier M., Rayner M., Jewell J., Breda J., Hercberg S., Julia C. Impact of Front-of-Pack Nutrition Labels on Portion Size Selection: An Experimental Study in a French Cohort. Nutrients. 2018;10:1268. doi: 10.3390/nu10091268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Galan P., Babio N., Salas-Salvadó J. Nutri-Score: Front-of-Pack Nutrition Label Useful for Public Heath in Spain Which Is Supported by as Strong Scientific Background. Nutr. Hosp. 2019;36:1213–1222. doi: 10.20960/nh.02848. [DOI] [PubMed] [Google Scholar]
- 51.Acton R.B., Jones A.C., Kirkpatrick S.I., Roberto C.A., Hammond D. Taxes and Front-of-Package Labels Improve the Healthiness of Beverage and Snack Purchases: A Randomized Experimental Marketplace. Int. J. Behav. Nutr. Phys. Act. 2019;16:46. doi: 10.1186/s12966-019-0799-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vandevijvere S., Vanderlee L. Effect of Formulation, Labelling, and Taxation Policies on the Nutritional Quality of the Food Supply. Curr. Nutr. Rep. 2019;8:240–249. doi: 10.1007/s13668-019-00289-x. [DOI] [PubMed] [Google Scholar]
- 53.Gutiérrez-Fisac J.L., Guallar-Castillón P., León-Muñoz L.M., Graciani A., Banegas J.R., Rodríguez-Artalejo F. Prevalence of General and Abdominal Obesity in the Adult Population of Spain, 2008-2010: The ENRICA Study. Obes. Rev. 2012;13:388–392. doi: 10.1111/j.1467-789X.2011.00964.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is contained within the article.