Abstract
A trans-encapsidation assay was established to study the specificity of picornavirus RNA encapsidation. A poliovirus replicon with the luciferase gene replacing the capsid protein-coding region was coexpressed in transfected HeLa cells with capsid proteins from homologous or heterologous virus. Successful trans-encapsidation resulted in assembly and production of virions whose replication, upon subsequent infection of HeLa cells, was accompanied by expression of luciferase activity. The amount of luciferase activity was proportional to the amount of trans-encapsidated virus produced from the cotransfection. When poliovirus capsid proteins were supplied in trans, >2 × 106 infectious particles/ml were produced. When coxsackievirus B3, human rhinovirus 14, mengovirus, or hepatitis A virus (HAV) capsid proteins were supplied in trans, all but HAV showed some encapsidation of the replicon. The overall encapsidation efficiency of the replicon RNA by heterologous capsid proteins was significantly lower than when poliovirus capsid was used. trans-encapsidated particles could be completely neutralized with specific antisera against each of the donor virus capsids. The results indicate that encapsidation is regulated by specific viral nucleic acid and protein sequences.
Picornaviridae is a family of icosahedral, plus-sense, single-stranded RNA viruses that includes important human and animal pathogens such as poliovirus, human rhinovirus (HRV), hepatitis A virus (HAV), and foot-and-mouth disease virus. Although the overall genetic organization is quite conserved among the different genera of picornaviruses, sequence conservation at both the amino acid and nucleic acid levels between the different genera of viruses varies to a great extent (25). Enteroviruses are more closely related to rhinoviruses than to the cardioviruses and aphthoviruses. HAV is by far the most distantly related virus among the picornaviruses. Many aspects of virus attachment, entry, translation, and replication biochemistry have been elucidated over the last several decades, but surprisingly little is known about the mechanisms of viral RNA encapsidation into virions.
It is generally believed that specific signals present in RNA sequence or structure determine the interaction with viral capsid proteins that result in encapsidation. Recent work with retroviruses and coronaviruses showed that specific viral RNA segments in genomic RNA are responsible for the specificity of encapsidation. For the coronavirus mouse hepatitis virus, a 69-nucleotide (nt) sequence in the genome RNA was necessary and sufficient for an RNA to be encapsidated into viral particles (12, 35). Although the encapsidation signals are somewhat different for each retrovirus, generally the primary signals are located near the 5′ end and are multipartite elements (18, 21). For picornaviruses, studies using defective interfering particles (17, 20) and chimeric viruses (16, 19, 26, 28) have shown that the regions required for internal initiation of translation in the 5′ untranslated region, as well as the entire P1 or capsid region, do not contain specific signals for encapsidation of poliovirus RNA. The absence of any requirement for P1 coding sequences for RNA encapsidation has allowed replacement of this region with foreign genes and has led to the development of a replicon which can serve as an expression vector for gene delivery (3, 27, 36).
Efficient packaging of defective interfering particles by viral capsid proteins supplied by nondefective homologous viruses during coinfection demonstrated that encapsidation could occur in trans. Earlier work showed that poliovirus and other enterovirus capsid proteins could trans-encapsidate each other’s RNAs when cells were coinfected with the two different viruses (15, 33). It has also been shown that poliovirus capsid protein expressed from a recombinant vaccinia virus could provide capsid protein in trans (4).
In this study, we have utilized a recombinant replicon to measure trans-encapsidation of poliovirus RNA by other picornavirus capsid proteins. The results show that although intergenus recognition of RNA by capsid proteins does occur, it occurs with varying efficiencies. These data support the existence of specific encapsidation signals that determine RNA-protein interactions required for efficient virion assembly.
MATERIALS AND METHODS
Plasmid constructs.
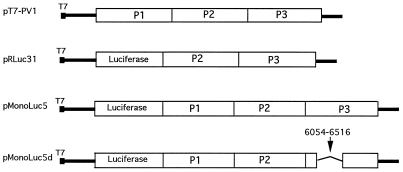
All of the plasmid constructs used were under T7 promoter control. Plasmid pT7-PV1 is an infectious cDNA clone of poliovirus type 1 which has been described before (13). Plasmid pRLuc31 (2) and pMonoLuc5 were generously provided by R. Andino, University of California, San Francisco. The pRLuc31 plasmid has a luciferase reporter gene replacing nearly the entire P1 capsid region of poliovirus type 1 cDNA. The RNA transcripts from this construct are replication competent and produce a luciferase signal upon transfection. Plasmid pMonoLuc5 contains a complete poliovirus cDNA with the luciferase gene inserted in frame just upstream of the poliovirus polyprotein-coding sequence. The construct was designed so that an engineered 3C proteinase cleavage site was created between luciferase and VP0 to generate a native VP0 N terminus upon cleavage by 3C. This construct produces all of the poliovirus capsid proteins and is replication competent, but the RNA is not packaged into infectious viral particles due to its increased length. Plasmid pMonoLuc5 is a modified form of pMonoLuc5 constructed by deletion of the HindIII fragment between nt 6054 and 6516 of the poliovirus genome. The deletion produces a truncated viral 3D protein of only 23 amino acids before a frameshift generating 4 additional amino acids and a TGA stop codon. All of the poliovirus cDNA constructs are shown schematically in Fig. 1. Plasmid pT7-HRV14 contains the complete HRV14 cDNA, and T7 transcripts from this construct are infectious upon transfection (31). Construct pCBV3-0 is an infectious human coxsackievirus B3 cDNA clone (9). Plasmid pMC16 contains mengovirus cDNA, and its transcripts are also infectious upon transfection (11). Plasmid pT7/HAV1 is an infectious HAV cDNA clone which has been described previously (14).
FIG. 1.
Schematic representation of poliovirus cDNA constructs used in this study. pT7-PV1 encodes the complete poliovirus genome, pRLuc31 encodes a poliovirus replicon in which the luciferase gene is substituted for the viral capsid coding sequence, pMonoLuc5d contains the complete poliovirus cDNA with the luciferase gene inserted upstream of the capsid coding sequences, and pMonoLuc5d contains a large out-of-frame deletion from nt 6054 to 6516 in the P3 coding region.
Vaccinia virus and transfection.
Recombinant vaccinia virus v-TF7-3, expressing T7 RNA polymerase, was provided by B. Moss, National Institutes of Health. A one-step infection/plasmid transfection procedure has been described before (29). Briefly, 0.5 ml of Dulbecco minimal essential medium (DMEM) containing 10 μl of Lipofectin (Bethesda Research Laboratories), v-TF7-3 (5 PFU/cell), and various plasmids described above (2 μg) were added to HeLa cell monolayers at 50 to 75% confluence in six-well plates. Unless specifically indicated, cells were incubated in a CO2 incubator at 37°C for 6 h. At the end of the transfection, cells were washed twice with DMEM and 1 ml of DMEM containing 10% bovine calf serum was added to each well. Cells were further incubated at 37°C for an additional 36 h.
trans-encapsidation and luciferase assay.
trans-encapsidation was assayed by cotransfection of HeLa cells with pRLuc31 plus a second plasmid that provided capsid proteins by using the transfection procedure described above and outlined in Fig. 2. Following transfection, the supernatant was collected and extracted with an equal volume of chloroform, followed by RNase (15 μg/ml) and DNase (20 U/ml) treatment for 20 min at 37°C. The treated supernatants were used to infect fresh cultures of HeLa cells in 12-well plates, and the presence of trans-encapsidated virus was scored by assay of luciferase activity after 8 h at 37°C (Fig. 2). For luciferase assay, the medium was removed and cells were lysed directly on the plates by the addition of 150 μl of lysis buffer (Promega). Ten microliters of the cell lysate was used to assay luciferase activity in a Monolight 2010 luminometer (Analytical Laboratory) by using a luciferase assay kit (Promega). Luciferase activity was expressed as relative luciferase units (RLU). All experiments were carried out in duplicate.
FIG. 2.
trans-encapsidation assay. HeLa cell monolayers were cotransfected with pRLuc31 and capsid donor plasmid in the presence of recombinant vaccinia virus v-TF7-3, expressing T7 RNA polymerase. Progeny viruses recovered from the transfection are labelled passage 1 (Pass 1) virus; progeny viruses recovered from subsequent infection of HeLa cells with passage 1 virus are labelled passage 2 (Pass 2) virus.
Antisera.
High-titer human antipoliovirus serum was from one of the authors (E.E.). Because it is difficult to obtain a negative human antipoliovirus serum, normal chimpanzee serum was used as a negative control. Guinea pig anti-HRV14 was purchased from the American Type Culture Collection, Manassas, Va. Normal guinea pig serum was provided by A. Tenner, University of California, Irvine; horse anti-coxsackievirus B3 was provided by S. Tracy, University of Nebraska; and mouse antimengovirus was a convalescent serum from a mengovirus-infected BALB/c mouse and was kindly provided by S. Nguyen, Zymo Research, Inc.
Virus titer.
Plaque assays were performed as described previously (30). For luciferase-containing particles, titers were determined by infecting confluent cells on cover slips in a six-well plate with dilutions of transfected cell supernatant. After 6 h, cells were fixed with acetone-methanol (1:1) for 10 min at −20°C. The coverslips were prepared for immunofluorescence analysis with rabbit anti-2C serum (30). The percentage of fluorescent cells was measured by microscopy in 10 random fields.
RESULTS
trans-encapsidation of a poliovirus replicon by poliovirus capsid proteins.
An assay was designed to measure trans-encapsidation of a poliovirus replicon RNA (transcript of pRLuc31 [Fig. 1]) containing luciferase coding sequences in lieu of the capsid protein genes. HeLa cell monolayers were cotransfected with the replicon construct and a second plasmid to provide a source of capsid proteins. Recombinant vaccinia virus v-TF7-3 provided a source of T7 RNA polymerase which transcribed both plasmids. If expression of capsid proteins by the second plasmid resulted in encapsidation of the replicon RNA (Fig. 2), then virus particles containing the luciferase gene would be produced. These particles (passage 1 virus) could be detected during a subsequent round of infection in HeLa cells, since replication would be accompanied by production of luciferase activity and passage 2 virus. The trans-encapsidation assay is outlined in Fig. 2.
Figure 3A shows that pRLuc 31 RNA was trans-encapsidated by poliovirus capsid protein provided in trans by pT7-PV1, the plasmid containing infectious poliovirus cDNA, or by pMonoLuc5, a plasmid containing the complete poliovirus cDNA with the luciferase gene inserted upstream of the poliovirus coding sequences (Fig. 1). The latter construct produces RNA which is replication competent but cannot be encapsidated because the inserted luciferase gene lengthens the RNA by over 23%, which renders this RNA too long for encapsidation (1). The strong luciferase signal observed 8 h after inoculation of HeLa cells with nuclease-treated transfected cell supernatant indicated efficient production of trans-encapsidated virus particles during the initial double transfection. Although transfection with pMonoLuc5 or pRLuc31 alone produced significant amounts of luciferase activity in the transfected cell lysates (data not shown), they did not produce infectious progeny viruses, as indicated by the absence of luciferase activity produced during passage 1 virus infection. Plaque assay showed that cells transfected with pT7-PV1 alone or together with pRLuc31 produced a high titer of poliovirus (108 to 109 PFU/ml [see below]), whereas no virus was detected after transfection with pMonoLuc5.
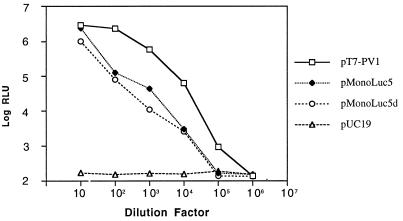
FIG. 3.
trans-encapsidation of poliovirus replicon pRLuc31 RNA with different sources of poliovirus capsid proteins. (A) Luciferase activity from trans-encapsidated poliovirus replicon with different capsid donors. Plasmid pRLuc31 was cotransfected with the three indicated poliovirus capsid protein donors or control plasmid pUC19. Progeny viruses (passage 1 [Pass 1]) were used to infect HeLa cells, and luciferase production was measured at 8 h postinfection. (B) Luciferase activity from second passage (Pass 2) virus. Virus recovered from the experiment shown in panel A was used to infect fresh HeLa cell cultures, and luciferase activity was monitored after 8 h.
Cotransfection with pT7-PV1 and pRLuc31 produced both native and trans-encapsidated viruses. This mixture of passage 1 viruses was able to infect HeLa cells and produce second-passage progeny particles which still contained both native virus and encapsidated pRLuc31 replicon, which was again detectable by production of luciferase activity during a subsequent passage (Fig. 3B). Continued passage of this mixture of viruses resulted in a progressive decrease in luciferase activity with each passage (not shown). Initial cotransfection with pRLuc31 and pMonoLuc5, however, produced only pRLuc31 RNA-containing viral particles; thus, these particles could infect cells for only one passage (Fig. 3B).
Both pT7-PV1 and pMonoLuc5 donated capsid proteins expressed from RNAs that replicated following the cotransfection event. To determine if poliovirus capsid proteins expressed in transfected cells directly from T7 RNA polymerase transcripts of plasmid constructs without viral RNA replication could mediate trans-encapsidation of the poliovirus replicon, a deletion was introduced into pMonoLuc5 so that only a truncated 3D sequence was produced and the donor RNA was no longer able to replicate (pMonoLuc5d [Fig. 1]). This construct was used as a capsid protein donor in a trans-encapsidation assay. Figure 4 shows that capsid proteins expressed from nonreplicating transcripts of pMonoLuc5d were used to encapsidate the poliovirus replicon RNA with an efficiency similar to that from replicating capsid donor RNA.
FIG. 4.
trans-encapsidation of the poliovirus replicon by the capsid protein from a nonreplicating construct, pMonoLuc5d. trans-encapsidation was carried out as described in the text, and luciferase activity (RLU) was monitored after 8 h of infection with passage 1 virus.
In contrast, construction of pRLuc31 containing a site-specific lethal mutation in the 2C gene, in which a Pro residue at position 131 was replaced with an Asn codon (30), allowed us to demonstrate that replication of the replicon was required for its trans-encapsidation. The pRLuc31/2Cpro transcripts produced by T7 RNA polymerase in the transfected cell produced no infectious particles in the presence of pT7-PV1 or pMonoLuc5 as a source of capsid proteins (data not shown). Thus, the encapsidation process appears to be coupled to RNA replication and not the result of assembly between viral RNAs and capsid proteins present in the same cell.
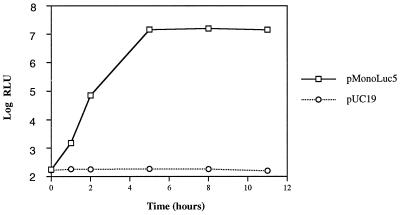
To assess the relative efficiency of this transfection-based encapsidation system, trans-encapsidated viral titers were estimated from a tissue culture infectious dose assayed by luciferase activity induced by serial dilutions of passage 1 virus. The kinetics of replication of trans-encapsidated virus were first determined by using luciferase activity as a marker for virus replication (Fig. 5). The accumulation rate of luciferase activity was similar to published poliovirus replication kinetics. Luciferase activity peaked at about 5 h postinfection and then gradually dropped with time after 10 h. The three capsid protein donor plasmids shown in Fig. 1 were tested for their abilities to produce capsid proteins used for trans-encapsidation of the poliovirus-luciferase replicon. Each was used in a cotransfection assay, and serial dilutions of passage 1 progeny virus were incubated with HeLa cell monolayers. According to the kinetic analysis shown in Fig. 5, cultures were harvested for luciferase assay after 8 h. Figure 6 shows that there is a range of linear correspondence between the amount of trans-encapsidated virus and the induced luciferase activity observed at 8 h postinfection. Plasmid pT7-PV1 was approximately 1 log unit more efficient than either pMonoLuc5 or pMonoLuc5d at producing trans-encapsidated virus particles. The reason for this difference is not clear. The trans-encapsidated virus from the above experiment was also used to determine infectivity by immunofluorescence with antibody against poliovirus 2C protein. Since the encapsidated virus can only initiate one cycle of infection, the infected cell number should reflect the infectious titer of the virus. The infectivity of the virus encapsidated by pMonoLuc5 from Fig. 6 was 2.1 × 106/ml based on immunofluorescence.
FIG. 5.
Kinetics of postinfection luciferase expression after infection with trans-encapsidated virus. trans-encapsidation of the poliovirus replicon pRLuc31 was carried out as described in the text, and passage 1 virus trans-encapsidated by capsid protein expressed from pMonoLuc5 was used to infect HeLa cells. Samples were harvested at the indicated times for luciferase assay, with results expressed as RLU.
FIG. 6.
Estimated infectivity of trans-encapsidated viruses. Supernatants from transfected cells containing passage 1 viruses were used to make 10-fold serial dilutions in DMEM, and 0.1 ml was used to infect HeLa cell monolayers in 24-well plates. Luciferase activity was measured 8 h postinfection.
trans-encapsidation of the poliovirus replicon by other picornavirus capsid proteins.
The ability to quantitatively assess trans-encapsidation efficiency allowed us to investigate the trans-encapsidation of the poliovirus replicon by other picornavirus coat proteins. The picornavirus cDNAs used were: coxsackievirus B3 cDNA (pCVB3-0), HRV14 cDNA (pT7-HRV14), mengovirus cDNA (pMC16) and cell culture-adapted human HAV HM175 cDNA (pT7/HAV1). The viral cDNA constructs were cotransfected with the poliovirus replicon pRLuc31 described above, and putative passage 1 progeny viruses were assayed for their abilities to induce luciferase activity upon subsequent infection. Figure 7 shows that all picornavirus capsid proteins except those from HAV could trans-encapsidate the poliovirus replicon RNA.
FIG. 7.
trans-encapsidation of a poliovirus replicon by different donor picornavirus coat proteins. Plasmid cDNA constructs encoding different picornavirus capsid proteins were used to cotransfect HeLa cells, and trans-encapsidation was monitored by luciferase activity after infection of HeLa cells with progeny virus.
trans-encapsidation of the poliovirus RNA occurred most efficiently by the homologous poliovirus capsid proteins. Other picornavirus capsid proteins were significantly less efficient at encapsidating poliovirus replicon RNA; another enterovirus (coxsackievirus B3) and HRV14 capsid proteins packaged approximately 1 to 2 log units less virus, and a cardiovirus (mengovirus) capsid was even less efficient. No trans-encapsidation was detected with HAV proteins. Table 1 shows an estimate of the amount of trans-encapsidated virus produced by the different viral capsid donors in three separate experiments. In addition, the table shows the amount of each infectious picornavirus recovered following the initial cotransfection event. All of the viral cDNAs generated relatively high-titer virus within approximately a log unit of each other, suggesting that there was sufficient capsid protein produced in the transfected cells for trans-encapsidation to occur. In contrast to the other picornavirus cDNAs, the HAV cDNA showed no detectable trans-encapsidation. In addition, there was no infectious HAV recovered from the transfection, even after prolonged incubation of over 72 h. Varying the vaccinia virus concentration from 0.1 to 10 PFU/cell had no effect on production of infectious HAV.
TABLE 1.
Relative efficiency of trans-encapsidation of poliovirus replicon by different viral capsids
| Capsid used for trans-encapsidationa | Virus recovered from transfection (PFU/ml [expt 1b]) | Relative efficiency of trans-encapsidation (%)c
|
||
|---|---|---|---|---|
| Expt 1 | Expt 2 | Expt 3 | ||
| Poliovirus | 4 × 108 | 100 | 100 | 100 |
| Coxsackievirus B3 | 2 × 108 | 6.1 | 3.7 | n.d. |
| HRV14d | 1 × 107 | 0.23 | n.d. | 1.2 |
| Mengovirus | 7 × 107 | 0.06 | n.d. | 0.6 |
| HAV | Negative | Negative | n.d. | n.d. |
Viral capsids were produced following transfection with the corresponding viral cDNA constructs described in Materials and Methods.
Experiment 1 is the experiment represented in Fig. 7.
Relative efficiency was calculated by arbitrarily setting poliovirus trans-encapsidation at 100%, based on luciferase activity after infection. Values represent the percentage of luciferase activity resulting from infection of trans-encapsidated replicon. n.d., not done.
Plaque assay mixture for HRV14 was incubated at 34°C.
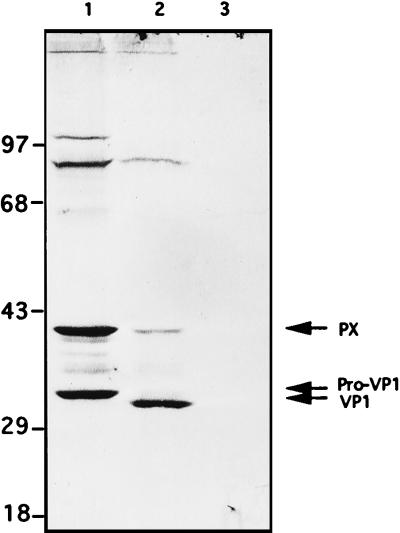
The reasons that there was no detectable trans-encapsidation of poliovirus replicon RNA by HAV capsid proteins are not fully understood. Western blot analysis of transfected HeLa cells showed that although viral capsid protein was produced and the amount of VP1-related capsid protein PX (VP1-2A) was comparable to that found in lysates from FRhK4 cells productively infected with HAV (16), there was almost no detectable VP1 of correct size produced (Fig. 8). Other experiments showed that the formation of mature VP1 in HAV-infected FRhK4 cells is a relatively slow process (not shown). Processing of VP1 precursors may require an undefined multistep proteolytic process to form mature VP1. The major products containing HAV VP1 sequences in the transfected HeLa cells are PX and a minor intermediate-sized protein (denoted pro-VP1 [Fig. 8]) which may be an intermediate processing product from PX to mature VP1.
FIG. 8.
HAV VP1 immunoblot analysis following transfection with HAV cDNA. Lane 1, transfection of HeLa cells by pT7-HAV1 in the presence of recombinant vaccinia virus expressing T7 RNA polymerase; lane 2, FRhK4 cells infected for 3 weeks with HAV; lane 3, HeLa cells transfected with pUC19 plasmid as a negative control. Eighty micrograms of total protein was loaded in each gel lane. Numbers on the left are molecular masses (in kilodaltons) of marker proteins.
Confirmation of the specificity of the intergenus trans-encapsidated viruses was obtained by neutralization of the luciferase induction ability of the passage 1 virus by specific antisera. Supernatants from transfected cells were incubated with either corresponding specific antisera or control sera prior to infection of HeLa cells. Cultures were harvested after 8 h for luciferase assay. Figure 9 shows that luciferase activity was abrogated after incubation with serum directed only against the specific capsid protein donor; neither antipoliovirus serum nor normal host serum caused significant reduction in induction of luciferase activity, the marker for virus replication, during subsequent infection.
FIG. 9.
Serum neutralization of trans-encapsidated viruses. Viruses resulting from transfection and trans-encapsidation with different picornavirus capsid donors were incubated with corresponding specific antisera or control sera diluted 1:200 in DMEM at 37°C for 20 min before inoculation of HeLa cell monolayers. Each trans-encapsidated virus was also tested against poliovirus-specific antiserum. Since different antisera were used for each trans-encapsidated virus, values are comparable only within a set of an individual virus, not between different virus sets. PV, poliovirus; CBV3, coxsackievirus B3; G. pig, guinea pig; MV, mengovirus; NC, negative control with uninfected HeLa cell lysate.
DISCUSSION
We have established a simple transfection-based trans-encapsidation system to analyze picornavirus encapsidation. By analysis of luciferase signals following infection of cells with transfected cell supernatants, the ability of different picornavirus capsid proteins to encapsidate a poliovirus replicon containing the luciferase gene can be easily determined. The assay was shown to be quantitative, with linear correspondence between luciferase activity and the amount of trans-encapsidated virus produced over a broad range. Capsid proteins from different genera of picornaviruses showed varying efficiencies of trans-encapsidation. Another enterovirus, coxsackievirus B3, was the most efficient in trans-encapsidation of the poliovirus replicon. The rhinovirus and cardiovirus capsid proteins could also encapsidate the poliovirus replicon, albeit much less efficiently than homologous poliovirus capsids. Human HAV capsid protein provided in trans showed no detectable encapsidation of the poliovirus replicon.
Although HAV does not replicate in the HeLa cell cultures used in these tests, the full-length RNA was translated to generate capsid proteins (Fig. 8). It appears, however, that processing of VP1-containing precursors may be incomplete or incorrect in the HeLa cell cultures. It is not known if this is the reason for the failure of HeLa cells to produce infectious virus. Viral RNA replication in these cells has not been examined.
Early studies by Holland and Cords showed that coinfection of HeLa cells with poliovirus and coxsackievirus B1 produced “hybrid” viruses (15). More recently, it was shown that poliovirus capsid protein supplied in trans by recombinant vaccinia virus would encapsidate a poliovirus replicon expressing heterologous proteins (27). Our data show that poliovirus capsid provided by translation of RNA amplified after direct transfection could also efficiently trans-encapsidate a poliovirus replicon. These results suggest that compartmentalization of the poliovirus replication complex does not restrict capsid proteins expressed from different cellular compartments to be used for viral particle assembly. Different picornaviral capsid proteins provided in trans showed greatly varying abilities to encapsidate the poliovirus replicon. Production of high-titer native viruses from the different infectious constructs (other than HAV) during the transfection step demonstrated that there was sufficient capsid protein produced inside the cells. Therefore, the reduced ability of other picornavirus capsids to encapsidate a poliovirus replicon is due to incompatibilities between poliovirus encapsidation signals and the coat proteins of the other picornaviruses. This suggests that encapsidation is determined by some specific recognition between poliovirus RNA or nucleocapsid complex and the coat proteins. This recognition could be mediated by either RNA-protein or protein-protein interactions. Several proteins in addition to Vpg have been demonstrated to form complexes with viral RNAs (2, 8, 23), and some viral nonstructural proteins have been proposed to be encapsidated into virus particles (24). These proteins may play a role in the RNA encapsidation process.
With a nonreplicating viral RNA as the capsid donor for the replicon RNA, the cotransfection procedure described here generated over 106 trans-encapsidated infectious units/ml. The high sensitivity of the luciferase assay used to monitor the production of particles will allow us to analyze encapsidation with RNAs and/or capsid proteins that have been subjected to specific mutagenesis. In this way, the specific signals involved in RNA-protein recognition for encapsidation may be defined.
All viruses need to assemble their nucleic acids with capsid proteins to form progeny viruses. Studies from other viruses showed that the encapsidation process is a highly specific and regulated event. Numerous strategies have evolved for packaging of viral genetic material. In the cases of some bacteriophages (7, 22) and parvoviruses (10), viral DNAs containing specific sequences are threaded into empty particles. For tobacco mosaic virus, encapsidation is initiated by binding of an aggregate of capsid protein to a defined RNA origin (32). A similar pathway is likely used by turnip crinkle virus (34). For picornaviruses, no specific sequence elements essential for viral encapsidation have been identified. The observed coupling between RNA replication and encapsidation (5, 6) strongly suggests that picornavirus packaging may be mechanistically linked to the RNA replication process.
After this manuscript was submitted, Porter et al. reported a similar study from which it was concluded that other enterovirus capsid proteins were unable to trans-encapsidate a poliovirus replicon (27a). In their experiments, capsids were donated by coinfection with heterologous viruses (bovine enterovirus, coxsackievirus A21, coxsackievirus B3, and enterovirus 70) as opposed to transfection with viral cDNAs, which were utilized in our study. Evidently, the differences in the kinetics and possible compartmentalization of capsid protein production during these two types of replication cycles accounted for the discrepant results.
ACKNOWLEDGMENTS
We are grateful for S. Tracy, B. Semler, and A. Palmenberg for providing plasmids and antisera.
This work was supported by grants AI 26350 and AI 17386 from the National Institutes of Health.
REFERENCES
- 1.Alexander L, Lu H H, Wimmer E. Polioviruses containing picornavirus type 1 and/or type 2 internal ribosomal entry site elements: genetic hybrids and the expression of a foreign gene. Proc Natl Acad Sci USA. 1994;91:1406–1410. doi: 10.1073/pnas.91.4.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andino R, Rieckhof G E, Achacoso P L, Baltimore D. Poliovirus RNA synthesis utilizes an RNP complex formed around the 5′-end of viral RNA. EMBO J. 1993;12:3587–3598. doi: 10.1002/j.1460-2075.1993.tb06032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andino R, Silvera D, Suggett S D, Achacoso P L, Miller C J, Baltimore D, Feinberg M B. Engineering poliovirus as a vaccine vector for the expression of diverse antigens. Science. 1994;265:1448–1451. doi: 10.1126/science.8073288. [DOI] [PubMed] [Google Scholar]
- 4.Ansardi D C, Porter D C, Morrow C D. Complementation of a poliovirus defective genome by a recombinant vaccinia virus which provides poliovirus P1 capsid precursor in trans. J Virol. 1993;67:3684–3690. doi: 10.1128/jvi.67.6.3684-3690.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baltimore D. The replication of picornaviruses. In: Levy H B, editor. The biochemistry of viruses. New York, N.Y: Marcel Dekker; 1969. pp. 101–176. [Google Scholar]
- 6.Barton D J, Black E P, Flanegan J B. Complete replication of poliovirus in vitro: preinitiation RNA replication complexes require soluble cellular factors for the synthesis of Vpg-linked RNA. J Virol. 1995;69:5516–5527. doi: 10.1128/jvi.69.9.5516-5527.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Black L W. DNA packaging in dsDNA bacteriophages. Annu Rev Microbiol. 1989;43:267–292. doi: 10.1146/annurev.mi.43.100189.001411. [DOI] [PubMed] [Google Scholar]
- 8.Blyn L B, Swiderek K M, Richards O, Stahl D C, Semler B L, Ehrenfeld E. Poly(rC) binding protein 2 binds to stem-loop IV of the poliovirus RNA 5′ noncoding region: identification by automated liquid chromatography-tandem mass spectrometry. Proc Natl Acad Sci USA. 1996;93:11115–11120. doi: 10.1073/pnas.93.20.11115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chapman N M, Tu Z, Tracy S, Gauntt C J. An infectious cDNA copy of the genome of a non-cardiovirulent coxsackievirus B3 strain: its complete sequence analysis and comparison to the genomes of cardiovirulent Coxsackieviruses. Arch Virol. 1994;135:115–130. doi: 10.1007/BF01309769. [DOI] [PubMed] [Google Scholar]
- 10.Cotmore S F, Tattersall P. A genome-linked copy of the NS-1 polypeptide is located outside of infectious parvovirus particles. J Virol. 1989;63:3902–3911. doi: 10.1128/jvi.63.9.3902-3911.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duke G M, Palmenberg A C. Cloning and synthesis of infectious cardiovirus RNAs containing short, discrete poly(C) tracts. J Virol. 1989;63:1822–1826. doi: 10.1128/jvi.63.4.1822-1826.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fosmire J A, Hwang K, Makino S. Identification and characterization of a coronavirus packaging signal. J Virol. 1992;66:3522–3530. doi: 10.1128/jvi.66.6.3522-3530.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haller A A, Semler B L. Linker scanning mutagenesis of the internal ribosome entry site of poliovirus RNA. J Virol. 1992;66:5075–5086. doi: 10.1128/jvi.66.8.5075-5086.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harmon S A, Richards O C, Summers D F, Ehrenfeld E. The 5′-terminal nucleotides of hepatitis A virus RNA, but not poliovirus RNA, are required for infectivity. J Virol. 1991;65:2757–2760. doi: 10.1128/jvi.65.5.2757-2760.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holland J J, Cords C E. Maturation of poliovirus RNA with capsid protein coded by heterologous enteroviruses. Proc Natl Acad Sci USA. 1964;51:1082–1085. doi: 10.1073/pnas.51.6.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jia X-Y, Tesar M, Summers D F, Ehrenfeld E. Replication of hepatitis A virus with chimeric 5′ nontranslated regions. J Virol. 1996;70:2861–2868. doi: 10.1128/jvi.70.5.2861-2868.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuge S, Saito I, Nomoto A. Primary structure of poliovirus defective-interfering particle genomes and possible generation mechanisms of the particles. J Mol Biol. 1986;192:473–487. doi: 10.1016/0022-2836(86)90270-6. [DOI] [PubMed] [Google Scholar]
- 18.Laughrea M, Jette L, Mak J, Kleiman L, Liang C, Wainberg M A. Mutations in the kissing-loop hairpin of human immunodeficiency virus type 1 reduce viral infectivity as well as genomic RNA packaging and dimerization. J Virol. 1997;71:3397–3406. doi: 10.1128/jvi.71.5.3397-3406.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu H H, Wimmer E. Poliovirus chimeras replicating under the translation control of genetic elements of hepatitis C virus reveal unusual properties of the internal ribosomal entry site of hepatitis C virus. Proc Natl Acad Sci USA. 1996;93:1412–1417. doi: 10.1073/pnas.93.4.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lundquist R E, Sullivan M, Maizel J. Characterization of a new isolate of poliovirus defective interfering particles. Cell. 1979;18:759–769. doi: 10.1016/0092-8674(79)90129-6. [DOI] [PubMed] [Google Scholar]
- 21.McBride M S, Panganiban A T. The human immunodeficiency virus type 1 encapsidation site is a multipartite RNA element composed of functional hairpin structures. J Virol. 1996;70:2963–2973. doi: 10.1128/jvi.70.5.2963-2973.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKenna R, Xia D, Willingmann P, Ilag L L, Krishnaswamy S, Rossmann M G, Olson N H, Baker T S, Incardona N L. Atomic structure of single-stranded DNA bacteriophage phi X174 and its functional implication. Nature. 1992;355:137–143. doi: 10.1038/355137a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meerovitch K, Pelletier J, Sonenberg N. A cellular protein that binds to the 5′-noncoding region of poliovirus RNA: implications for internal translation initiation. Genes Dev. 1989;3:1026–1034. doi: 10.1101/gad.3.7.1026. [DOI] [PubMed] [Google Scholar]
- 24.Newman J F, Piatti P G, Gorman B M, Burrage T G, Ryan M D, Flint M, Brown F. Foot-and-mouth disease virus particles contain replicase protein 3D. Proc Natl Acad Sci USA. 1994;91:733–737. doi: 10.1073/pnas.91.2.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palmenberg A C. Sequence alignments of picornaviral capsid proteins. In: Semler B L, Ehrenfeld E, editors. Molecular aspects of picornavirus infection and detection. Washington, D.C: American Society for Microbiology; 1989. pp. 211–241. [Google Scholar]
- 26.Percy N, Barclay W S, Sullivan M, Almond J W. A poliovirus replicon containing the chloramphenicol acetyltransferase gene can be used to study the replication and encapsidation of poliovirus RNA. J Virol. 1992;66:5040–5046. doi: 10.1128/jvi.66.8.5040-5046.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Porter D C, Ansardi D C, Morrow C D. Encapsidation of poliovirus replicons encoding the complete human immunodeficiency virus type 1 gag gene by using a complementation system which provides the P1 capsid protein in trans. J Virol. 1995;69:1548–1555. doi: 10.1128/jvi.69.3.1548-1555.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27a.Porter D C, Ansardi D C, Wang J, McPherson S, Moldoveanu Z, Morrow C D. Demonstration of the specificity of poliovirus encapsidation using a novel replicon which encodes enzymatically active firefly luciferase. Virology. 1998;243:1–11. doi: 10.1006/viro.1998.9046. [DOI] [PubMed] [Google Scholar]
- 28.Richardson A J, Percy N, Rohll J B, Barclay W S, Li Q, Moon D H, Almond J W. Abstracts of the IX meeting of the European Study Group on the Molecular Biology of Picornaviruses”. Gmunden, Austria. 1996. Studies on picornavirus encapsidation. [Google Scholar]
- 29.Tesar M, Pak I, Jia X Y, Richards O C, Summers D F, Ehrenfeld E. Expression of hepatitis A virus precursor protein P3 in vivo and in vitro: polyprotein processing of the 3CD cleavage site. Virology. 1994;198:524–533. doi: 10.1006/viro.1994.1063. [DOI] [PubMed] [Google Scholar]
- 30.Teterina N L, Zhou W D, Cho M W, Ehrenfeld E. Inefficient complementation activity of poliovirus 2C and 3D proteins for rescue of lethal mutations. J Virol. 1995;69:4245–4254. doi: 10.1128/jvi.69.7.4245-4254.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Todd S, Semler B L. Structure-infectivity analysis of the human rhinovirus genomic RNA 3′ non-coding region. Nucleic Acids Res. 1996;24:2133–2142. doi: 10.1093/nar/24.11.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turner D R, Joyce L E, Butler P J G. The tobacco mosaic virus assembly origin RNA: functional characteristics defined by directed mutagenesis. J Mol Biol. 1988;203:531–547. doi: 10.1016/0022-2836(88)90190-8. [DOI] [PubMed] [Google Scholar]
- 33.Wecker E, Lederhilger G. Genomic masking produced by double-infection of HeLa cells with heterotypic polioviruses. Proc Natl Acad Sci USA. 1964;52:705–709. doi: 10.1073/pnas.52.3.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei N, Heaton L A, Morris T J, Harrison S C. Structure and assembly of turnip crinkle virus. VI. Identification of coat protein binding sites on the RNA. J Mol Biol. 1990;214:85–95. doi: 10.1016/0022-2836(90)90148-F. [DOI] [PubMed] [Google Scholar]
- 35.Woo K, Joo M, Narayanan K, Kim K H, Makino S. Murine coronavirus packaging signal confers packaging to nonviral RNA. J Virol. 1997;71:824–827. doi: 10.1128/jvi.71.1.824-827.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yim T J, Tang S, Andino R. Poliovirus recombinants expressing hepatitis B virus antigens elicited a humoral immune response in susceptible mice. Virology. 1996;218:61–70. doi: 10.1006/viro.1996.0166. [DOI] [PubMed] [Google Scholar]