Abstract
The transition of Epstein-Barr virus (EBV) from latency into the lytic cycle is associated with the expression of two immediate-early viral genes, BZLF1 and BRLF1. Overexpression of ZEBRA, the product of BZLF1, is sufficient to disrupt latency in B lymphocytes and epithelial cells by stimulating expression of lytic cycle genes, including BRLF1. The BRLF1 product Rta functions as a transcriptional activator in both B lymphocytes and epithelial cells. However, Rta has recently been reported to disrupt latency in an epithelial specific manner (S. Zalani, E. Holley-Guthrie, and S. Kenney, Proc. Natl. Acad. Sci. USA 93:9194–9199, 1996). Here we demonstrate that expression of Rta is also sufficient for disruption of latency in a permissive B-cell line. In HH514-16 cells, transfection of Rta leads to synthesis of ZEBRA, viral DNA replication, and late gene expression. However, Rta by itself is less potent than ZEBRA in the ability to activate most early and late lytic cycle genes. In light of previous work implicating ZEBRA in the activation of Rta, we suggest a cooperative model for EBV entry into the lytic cycle. Expression of either BZLF1 or BRLF1 triggers expression of the other immediate-early factor, and together these activators act individually or in synergy on downstream targets to activate the viral lytic cycle.
Latency, a state of limited viral gene expression observed for many viruses, is a prominent feature of infection by gammaherpesviruses. Epstein-Barr virus (EBV), a human gammaherpesvirus associated with both lymphoid and epithelial cell-derived human cancers, is latent in human B lymphocytes (for a review see reference 28). EBV can reactivate from B-lymphoid cells in vivo, an event that can be simulated in B-lymphoid cell culture by addition of a variety of inducing agents that include phorbol esters, n-butyrate, calcium ionophores, and cross-linking of surface immunoglobulin (3, 32, 34, 61). Induction of the switch between latency and lytic cycle gene expression by such stimuli is associated with expression of two immediate-early (IE) proteins that function as transcriptional activators, ZEBRA (Z EB replication activator), the product of the EBV BZLF1 gene, and Rta (R transactivator), the product of the BRLF1 gene. BZLF1 and BRLF1 are expressed simultaneously, usually within 2 h after application of an inducing stimulus (34, 37, 50, 52). ZEBRA and Rta then act on downstream promoters to activate a cascade of lytic gene expression, including in some permissive cell backgrounds, viral DNA replication, late gene expression, and the assembly of infectious virus (5, 8, 9, 11, 12, 22, 23, 27). ZEBRA also plays an essential role in lytic DNA replication (14, 46, 47).
The BRLF1 and BZLF1 genes are expressed from the virus in an overlapping transcription unit (33). The BRLF1 gene is expressed as 4.0- and 3.0-kb mRNAs driven from its upstream promoter, Rp; the two mRNAs are related through alternative splicing. The BZLF1 gene is contained as a bicistronic unit in these two messages. BZLF1 is also expressed as a monocistronic 1.0-kb mRNA that is controlled from a promoter immediately upstream the BZLF1 open reading frame (ORF), Zp (15, 16, 50). It is unclear, however, whether ZEBRA is efficiently translated from the bicistronic message in vivo (30).
A key unanswered question that is addressed in this report is whether ZEBRA or Rta is the principal controlling element of the latency to lytic cycle switch in B lymphocytes. An answer to this question is important to the basic understanding of the mechanisms of maintenance of latency and reactivation into the productive cycle. All current models assume that latency and reactivation are regulated through cellular control of Zp, the promoter that regulates BZLF1, and Rp, the promoter controlling BRLF1. Until now, considerable evidence has favored the dominant role of ZEBRA. Transfection of plasmids expressing ZEBRA into B-cell lines latently infected with EBV efficiently initiates the entire lytic cascade leading to production of virions (11, 19, 44, 53). As an early event, ZEBRA stimulates expression of Rta by acting on Rp, which contains binding sites for ZEBRA (29, 30, 50). If the lytic cascade is linear, with ZEBRA directing synthesis of Rta, entry into the lytic cycle should be accelerated by heterologous expression of BRLF1. Rta is a potent transcriptional activator that drives EBV gene expression by directly binding to responsive promoters that contain Rta response elements; Rta also activates other promoters that do not contain identifiable Rta response elements, presumably by an indirect mechanism (31, 41, 50, 58). In addition, Rta is known to synergize with ZEBRA in the activation of many viral genes (7, 8, 12, 23, 27, 42).
Available evidence about the capacity of Rta to activate EBV lytic gene expression in B cells is conflicting (4, 12, 57). Early reports indicated that transfection of Rta by itself could not disrupt latency (12). One study suggested that Rta might induce low-level lytic gene expression in lymphoblastoid cells but not in Burkitt’s lymphoma (BL)-derived cell lines (4). A recent study indicated that Rta disrupted latency in a cell-specific manner, exerting a positive effect in epithelial cells but not in lymphoid cells (57).
Several observations provoked us to reexamine the role of EBV Rta in control of lytic cycle gene expression in B cells. Since Rta is expressed in the same temporal class as ZEBRA, Rta should exert an essential regulatory function during the initiation of the lytic cycle. Homologues of EBV Rta are found in all members of the gammaherpesvirus family, while ZEBRA is often poorly conserved (36, 54). The homologue of EBV Rta, encoded in ORF 50 of human herpesvirus 8, a gammaherpesvirus associated with Kaposi’s sarcoma and primary effusion lymphoma, is capable of activating human herpesvirus 8 lytic gene expression in B cells derived from primary effusion lymphoma (51a). Our results indicate that EBV Rta can likewise activate lytic gene expression in B lymphocytes.
MATERIALS AND METHODS
Cell lines.
Cells were maintained in 5% CO2 at 37°C in RPMI 1640 supplemented with 8% fetal calf serum. HH514-16 is a clonal derivative of the P3J-HR-1 B-cell line derived from an EBV-positive BL that is permissive for viral replication (43); Raji is a human B-cell line derived from a BL containing an EBV strain that is defective for DNA replication and late gene expression (40). BJAB is a human B-cell line originating from an EBV-negative B-cell lymphoma (35); BJAB-B1 is the same cell line converted with the P3J-HR-1 EBV strain (18).
Antisera.
Anti-R is a polyclonal rabbit antiserum raised to a purified 320-amino-acid N-terminal fragment of R (R320) that was generated by using the pET expression system (Novagen). Anti-ZEBRA and anti-BLRF2 are polyclonal rabbit antisera raised to TrpE-BZLF1 and TrpE-BLRF2 fusion proteins (26, 48). R3 is a monoclonal antibody to BMRF1 (38), and SJ is a human serum with reactivity to EBNA1 and BFRF3 (48).
Chemical induction.
Cells were subcultured 3 days prior to drug treatment or transfection. Drug treatment consisted of the addition of either 10 ng of tetradecanoylphorbol-13-acetate (TPA) per ml or 3 mM sodium butyrate, or both, to induce the lytic cycle.
Expression vectors and reporter plasmids.
The ZEBRA expression vectors pBXG1-genomic Z (pBXG1/gZ) and CMV-genomic Z (CMV/gZ; CMV denotes cytomegalovirus) and their parent vectors pBXG1 and pHD1013 have been described previously (13, 17). CMV-RIE (CMV/Rta) contains the BRLF1 gene in the vector pHD1013 (23). The Rta expression vector RTS15 (pRTS/Rta), a kind gift of Diane Hayward, contains the BRLF1 gene linked to the simian virus 40 early promoter and is followed by a polyadenylation signal in plasmid pRTS2. RTS15-ΔHIII (pRTS) was generated by excising the EBV BRLF1 gene with HindIII and XbaI, filling in the sites, and religating the vector. The chloramphenicol acetyltransferase (CAT) construct ZpCAT contains the BamHI-NaeI fragment of the BZLF1 promoter cloned into pCAT basic (Promega); RpCAT was generated by cloning the PCR-amplified region spanning nucleotides (nt) 106123 to 107143 (1) of the BamHI R fragment of the EBV genome into the XbaI site in pCAT basic. RpΔTATACAT (equivalent to RpCAT with a 7-bp TATA box deletion) was constructed by a two-step PCR method. The first set of amplification reactions were carried out with primers 5′-GCTCTAGAAAAGGCCCTGTCGTCGGG (I) and 5′-GCCATTGGCATGGGCG (II) for the 5′ fragment and primers 5′-CCATGCCAATGGCTGACCAGTAATCCATGT (III) and 5′-GCTCTAGACCTGCGTCTGTTTGTG (IV) for the 3′ fragment. The second-step reaction contained the products of the first round as templates and primers I and IV. The reporter plasmid pGL2 basic+HMP has been described elsewhere (49). The bacterial R320 expression vector pET22-R320 was generated by cloning the BRLF1 ORF spanning residues 1 to 320 into the NdeI and SalI sites of pET22b (Novagen) in frame with the C-terminal His tag.
Transfections.
Transfections were carried out by electroporation (49). For Western analysis, 10 μg of plasmid DNA (5 μg of expression vector plus 5 μg of empty vector, either pBXG1 or pRTS) was used. Reporter assays were carried out with 10 μg of CAT construct plus 5 μg of expression vector pBXG1 or pRTS and 1 μg of pGL2 basic+HMP. For Southern analysis, the total amount of DNA per transfection was 20 μg (10 μg of each expression vector or one expression vector and pHD1013). For Northern analysis, cells were transfected with 5 μg of each expression vector.
Reporter assays.
CAT and luciferase assays were performed as described previously (49). CAT results represent the averages of at least two separate transfections and are standardized for transfection efficiency with luciferase.
Protein extracts and Western blots.
Cells were collected by centrifugation, washed once in phosphate-buffered saline, and resuspended in sodium dodecyl sulfate (SDS) sample buffer at 106 cells/10 μl. Prior to separation on SDS–12% polyacrylamide gels, samples (20 μl) were heated to 100°C for 5 min. Following electrophoresis, the proteins were transferred to nitrocellulose membranes by electroblotting and blocked in 5% nonfat dry milk overnight at 4°C. The blots were incubated with antiserum, diluted in 5% nonfat dry milk at 25°C for 2 h, then washed (three times for 10 min each) in TS (10 mM Tris [pH 7.5], 200 mM NaCl, 5% Tween 20), incubated with 125I-protein A for 1 h, and washed again. The membranes were exposed overnight with intensifying screens to Kodak XAR-5 film at −70°C.
RNA isolation and Northern blots.
Samples of 2.5 × 106 cells were harvested 30 h following transfection. Cellular RNA prepared with an RNeasy Mini Kit (Qiagen) was electrophoresed through a 1% agarose–6% formaldehyde gel in 20 mM MOPS (morpholinepropanesulfonic acid [pH 7.0]), the RNA transferred to a Nytran membrane, and probed with 32P-radiolabeled oligonucleotides. The BZLF1 oligonucleotide used spanned nt 93 to 128 in exon I of the BZLF1 gene. A radiolabeled 370-bp NcoI-PstI fragment of H1 RNA of RNase P was used as a probe to control for RNA loading (2).
DNA replication assay.
Three days following subculturing, triplicate aliquots of 107 cells were transfected and pooled into 24 ml of RPMI 1640–8% fetal calf serum. After a 3-day incubation period at 37°C, the cells were harvested and total DNA was extracted (49). After resuspension in TE (10 mM Tris [pH 8.0], 1 mM EDTA [pH 8.0]), 5 μg of each DNA sample was digested with BamHI for 5 h at 37°C. The fragments were separated on a 0.8% agarose–1× Tris-borate-EDTA gel and transferred to nitrocellulose in 20× SSC (3 M NaCl, 0.3 M sodium citrate). The blot was hybridized with a 32P-labeled 1.9-kb XhoI fragment covering the unique sequences adjacent to the terminal repeat region of the EBV genome (Xho 1.9 [45]) or with a 32-base oligonucleotide in this region (5′-CAGCTGTTTTCGTGGACTTTTATACAGTAAGG).
RESULTS
EBV Rta activates early lytic gene expression in Raji cells.
Zalani et al. reported that transfection of the BRLF1 gene under control of the CMV IE promoter into Raji cells, a BL B-lymphocyte cell line, failed to activate the EBV lytic cycle, as measured by the appearance of the EBV diffuse early antigen (EA-D), a lytic cycle product (57). In their experiments, however, Zalani et al. provided no data on the level of expression of the Rta protein. To monitor the expression of Rta while assessing its biologic activity, we used an antiserum raised in rabbits to the N-terminal 320 amino acids of Rta. Two constructs were used to express Rta in Raji cells. One, under control of the CMV promoter but lacking mRNA 3′-end processing signals (CMV/Rta), was identical to the construct used by Zalani et al. (57). The other, driven by the simian virus 40 promoter (pRTS/Rta), contained signals for mRNA 3′-end processing. Transfection of BZLF1 served as a positive control for activation of lytic cycle gene expression in B cells. As expected, transfection of BZLF1 activated EA-D and Rta (Fig. 1A, lane 3). High levels of Rta protein were expressed following transfection of pRTS/Rta but not of CMV/Rta (compare lanes 4 and 7). Transfection of plasmid pRTS/Rta also activated EA-D expression in Raji cells, while CMV/Rta did not. Since the same amount of EBNA1 was detected in all samples, the absence of Rta and EA-D signals in CMV/Rta-transfected cells was not due to a loading artifact. The level of EA-D expressed following transfection of pRTS/Rta was somewhat less than following transfection of BZLF1 (compare lanes 3 and 7). The failure of Zalani et al. to observe lytic cycle activation in lymphoid cells is likely to be due to a low level of expression of the EBV Rta protein from the CMV/Rta vector. In related experiments, we found that transfection of pRTS/Rta activated expression of mRNAs of several lytic cycle genes, including BaRF1 and BMRF1, in Raji cells (data not shown).
FIG. 1.
Activation of early and late viral genes following transfection of BRLF1 into B-cell lines. (A) Raji cells were untreated (lane 1) or were transfected with 5 μg of the indicated plasmids (lanes 2 to 7). Immunoblots prepared 72 h following transfection were probed sequentially with polyclonal rabbit antibody to Rta, with murine monoclonal antibody to EA-D, and with a human antibody to EBNA1. (B) HH514-16 cells were untreated (lane 1), chemically induced with TPA–n-butyrate (lane 2), or transfected with 10 μg of plasmid DNA (lanes 3 to 7). In lanes 4 and 6, the cells received 5 μg of activator and 5 μg of empty vector. In lanes 3 and 5, cells received only vector pBXG1 (lane 3) or pRTS (lane 5). In lane 7, both BZLF1 and BRLF1 were transfected. Immunoblots were probed sequentially with the indicated antisera. Anti-BZLF1 detects both the endogenous (top arrow) and transfected (bottom arrow) ZEBRA.
EBV Rta leads to the activation of late gene expression and lytic viral DNA replication in permissive B lymphocytes.
Due to a mutation in the EBV major DNA binding protein, Raji cells are not permissive for lytic viral DNA replication or production of virions. However, in cell lines such as HH514-16 (Fig. 1B), the latent EBV genome can be activated by inducing chemicals or transfection of BZLF1 to high-level viral lytic gene expression that proceeds to expression of late genes and production of progeny virus. Transfection of pRTS/Rta into HH514-16 cells led not only to the appearance of EA-D but also to the synthesis of low levels of ZEBRA from the endogenous virus (Fig. 1B, lane 6). Endogenous ZEBRA was readily distinguished from ZEBRA from the transfected expression vector due to a convenient polymorphism within BZLF1 (10, 25, 37). Moreover, transfection of pRTS/Rta also induced the expression of a 21-kDa virus structural protein encoded in BFRF3, a late viral gene (49). Transfection of HH514-16 cells with comparable amounts of vector induced neither early nor late genes. As in Raji cells (Fig. 1A), transfection of HH514-16 cells with BZLF1 was more potent than transfection of BRLF1 in activation of expression of EA-D; BFRF3 was also induced to a higher level by ZEBRA than by Rta (compare lanes 4 and 6). In B95-8 marmoset cells, another line that is completely permissive for EBV replication, transfection of BRLF1 also induced expression of EA-D and BFRF3 (not shown).
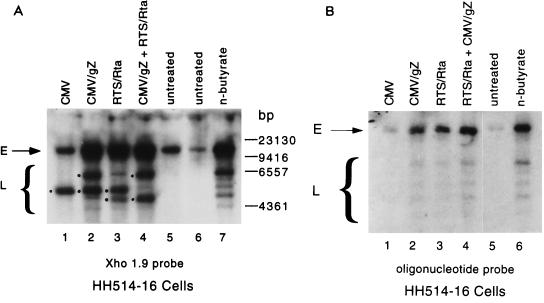
The appearance of a late viral polypeptide in HH514-16 cells following transfection of BRLF1 suggested that the EBV genome in these cells was induced into lytic replication. Lytic EBV replication is accompanied by amplification of the circular EBV episome and a complex DNA processing reaction that generates linear viral genomes with a heterogeneous collection of terminally repeated DNA fragments (45). Transfection of HH514-16 cells with BRLF1 or BZLF1 led to amplification of the genome and to the appearance of the ladder of terminal repeats (Fig. 2). The probe used in this assay also detected the transfected plasmids; however, an oligonucleotide probe that does not detect the transfected DNA also demonstrated plasmid amplification and terminal repeat processing following transfection of Rta (Fig. 2B, lane 3).
FIG. 2.
Activation of EBV DNA replication following transfection of BRLF1 into HH514-16 cells. Cells were transfected with 20 μg of total plasmid DNA containing vector alone (lane 1), vector plus genomic ZEBRA (lane 2), vector plus Rta (lane 3), or both activators (lane 4). Untreated cells (lane 5 and lane 6) or butyrate-treated cells (lane 7) were used as negative and positive controls. DNA digested with BamHI was analyzed by Southern blotting using either the Xho 1.9 probe (45) (A) or an oligonucleotide probe (B). Episomal EBV DNA (E) and input vector DNA (•) are indicated. L, linear DNA.
Rta strongly activates Rp linked to the CAT reporter in EBV-positive cells.
Since Rta activates ZEBRA expression and ultimately the entire lytic viral cascade in HH514-16 cells, it is likely to do so by acting on one or both promoters of the two IE genes, namely, Rp and Zp. This possibility was examined in the HH514-16 cell background in two ways: by the use of reporter-based transient transfection assays (Fig. 3) and by means of analysis of the viral transcripts originating from Rp and Zp (Fig. 4).
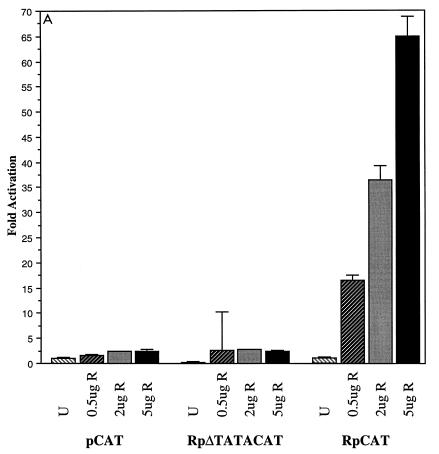
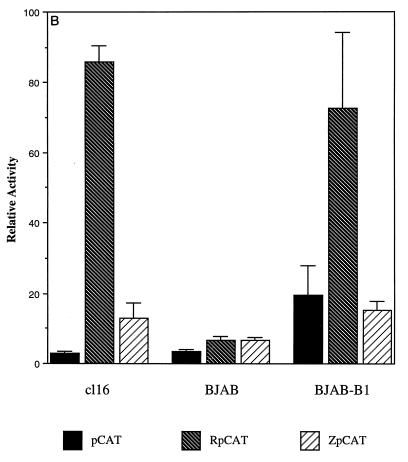
FIG. 3.
Comparison of transcriptional activation of CAT reporters containing Rp and Zp by Rta and ZEBRA. (A) Autostimulation of RpCAT by Rta. HH514-16 cells were transfected with 16 μg of total plasmid DNA with the indicated amount of activator, 10 μg of CAT reporter, and 1 μg of luciferase reporter. CAT and luciferase assays were performed 72 h following transfection. CAT activity was standardized for transfection efficiency by using the luciferase data. Fold activation is the ratio of CAT activity generated by a reporter in the presence of Rta (R) divided by the CAT activity in the presence of empty pRTS vector (U). The data for RpΔTATACAT are standardized together with those for RpCAT, where RpCAT transfected with empty vector equals 1. pCAT, reporter lacking any promoter or enhancer elements; RpΔTATACAT, 7-nt TATA box deletion in Rp. (B and C) Effects of different cell backgrounds on transcriptional activation of RpCAT and ZpCAT by Rta (B) and ZEBRA (C). For both panels B and C, fold activation for each cell line represents the CAT activity generated by the reporter pCAT, RpCAT, or ZpCAT in the presence of Rta or ZEBRA divided by the activity in the presence of empty vector. cl16, HH514-16.
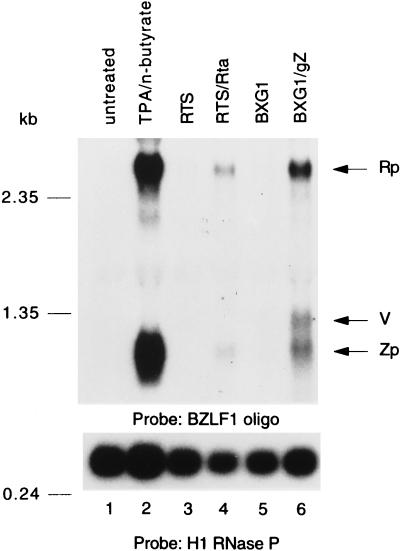
FIG. 4.
Activation of Rp- and Zp-driven transcripts from the latent EBV genome following transfection of Rta and ZEBRA. HH514-16 cells were untreated (lane 1), chemically induced (lane 2), or transfected with 5 μg of total plasmid DNA (5 μg of pRTS [lane 3] or pRTS/Rta [lane 4], 5 μg of pBXG1 [lane 5], or 4 μg of pBXG1 plus 1 μg of pBXG1/gZ [lane 6]). Total cell RNA prepared 28 h following transfection was analyzed by Northern blotting using a 33-nt oligonucleotide in BZLF1 as a probe. Transcripts initiating from Rp, Zp, and vector (V) are indicated.
In transient assays, Rta stimulated its own promoter powerfully, up to 65-fold (Fig. 3A). Stimulation of RpCAT was linearly related to the input dose of Rta expression plasmid and was dependent on the presence of an intact core promoter. Removal of the TATA element in plasmid RpΔTATACAT abolished any Rta-mediated activity.
The extent of Rta stimulation of RpCAT was markedly augmented in the presence of an endogenous EBV genome. In EBV-negative BJAB cells, Rta activated RpCAT weakly, about sixfold. To distinguish between an effect of cell background and the effect of the presence or absence of EBV, we performed the same assays with BJAB-B1 cells, which are BJAB cells that have been permanently converted to carriage of EBV (18). In BJAB-B1 cells, Rta stimulated RpCAT 70-fold, at least 10-fold more strongly than in the parental EBV-negative BJAB cells (Fig. 3B). Thus, RpCAT is strongly activated by Rta in an EBV-positive cell background.
Although ZpCAT was activated by Rta, this promoter was less responsive to Rta than was RpCAT in EBV-positive cells. In HH514-16 cells, Rta stimulated ZpCAT 12-fold; direct comparison of the responses of ZpCAT and RpCAT to Rta in HH514-16 cells revealed that RpCAT was about sevenfold more responsive than ZpCAT (Fig. 3B). In BJAB-B1 cells, Rta did not stimulate ZpCAT above the background level of stimulation of a pCAT basic vector that contained no promoter.
ZEBRA strongly activates the BRLF1 promoter in EBV-negative cells.
ZEBRA behaved very differently from Rta in the transient transfection assays (Fig. 3C). By contrast to Rta, ZEBRA was considerably more active in an EBV-negative than in an EBV-positive cell background. For example, ZEBRA stimulated RpCAT 80-fold in BJAB cells and only 4-fold in BJAB-B1 cells. ZEBRA stimulated ZpCAT 30-fold in EBV-negative BJAB cells but not at all in EBV-positive BJAB-B1 cells. The relatively weak activity of ZEBRA on RpCAT and ZpCAT reporters in an EBV-positive background was also seen in HH514-16 cells, where RpCAT was stimulated only 5-fold and ZpCAT was not stimulated at all. The differences observed in the activities of Rta and ZEBRA in the three different cell lines could not be accounted for by differences in levels of expression of the two proteins. For example, Rta was expressed at comparable levels in HH514-16 cells, where it was highly active, and in BJAB cells, where its activity was low. ZEBRA was expressed at lower levels in BJAB cells, where it was highly active, than in HH514-16 cells, where its activity was low. In BJAB-B1 cells, where ZEBRA showed little activity, its expression was comparable to that in BJAB cells. In summary, the transient transfection assays showed that Rta was a more potent transcriptional activator of RpCAT and ZpCAT in EBV-containing cell lines than was ZEBRA (data not shown).
Rta activates Rp and Zp from the virus.
A probe for BZLF1 detects the 4.0- and 3.0-kb mRNAs originating from Rp and the 1.0-kb mRNA originating from Zp. The 4.0-kb mRNA is characteristically less abundant. Figure 5 shows that chemical induction of the lytic cycle in HH514-16 cells by TPA and n-butyrate leads to the appearance of two abundant 3.0- and 1.0-kb mRNAs detected by a probe for BZLF1; this finding indicates that both Rp and Zp are active in HH514-16 cells. Transfection of ZEBRA also leads to the appearance of these two mRNAs as well as a third 1.3-kb mRNA that represents a transcript from the ZEBRA expression plasmid (29). Transfection of BRLF1 also leads to the appearance of the 3.0- and 1.0-kb mRNAs. Thus, overexpression of Rta following transfection of HH514-16 cells leads to activation of Rp and Zp, the two promoters that control the EBV lytic cascade.
FIG. 5.
Proposed model for disruption of latency in HH514-16 cells. The promoter regions of the BZLF1 and BRLF1 genes are illustrated. See Discussion for an explication of the model. H, repressive host factor (possibly histone); A, host cell activator; ZRE, ZEBRA response element; TRE, TPA response element, also known as ZII (16); Z, ZEBRA; R, Rta; X, a factor which mediates R activity on Rp and Zp.
DISCUSSION
Here we present three novel lines of evidence that emphasize the importance of the EBV Rta protein in events leading to activation of the viral lytic cascade in human B lymphocytes. First, transfection of Rta-expressing plasmids leads to the appearance of early and late viral lytic cycle polypeptides and, in permissive B cells, lytic viral DNA replication (Fig. 1 and 2). Second, in transient transfection assays, Rta powerfully stimulates its own promoter, Rp, in a manner that is dependent on the presence of an EBV genome (Fig. 3). Third, overexpression of Rta in permissive B cells leads to transcription from Rp and Zp, the two distinct promoters that control the IE genes BRLF1 and BZLF1 (Fig. 4).
Previous assumptions about the inability of Rta to drive lytic EBV gene expression in lymphoid cells may have been the result of the use of expression vectors that performed unequally in different cell types and yielded low or nondetectable levels of Rta protein in lymphocytes (Fig. 1A). When an appropriate expression system was used, transfection of Rta resulted in activation of both early and late genes. In HH514-16 cells, this property can be attributed in part to the ability of Rta to activate Zp and thus promote ZEBRA expression from the endogenous virus (Fig. 1B and 4). Once ZEBRA was induced in HH514-16 cells, lytic DNA replication and progression of the cycle to late gene expression were inevitable. In other cell lines, such as Raji, that are not fully permissive, transfection of BRLF1 also stimulated EA-D expression and several other lytic cycle genes (Fig. 1A and data not shown). Whether examined in fully permissive cells, such as HH514-16, or in Raji, Rta by itself was always less efficient at inducing lytic gene expression than was ZEBRA (Fig. 1B and data not shown). This finding suggests that maximal lytic EBV gene expression in B cells requires the joint and interactive contributions of Rta and ZEBRA.
In reporter-based transient assays Rta specifically and powerfully activated its own promoter, an autostimulatory activity that was maximal in EBV-positive cells (Fig. 3B). The EBV genome could contribute to Rp activation in one of several ways. Rp does not contain known binding sites for Rta (20); instead, it contains binding sites for cellular proteins including YY1, Sp1, and Zif268 (55, 56, 58). An EBV gene might induce or modify one or more cellular proteins enabling it to bind to Rp by an indirect mechanism. Alternatively, a latent or lytic EBV gene product that is induced by Rta may mediate the interaction between Rta and Rp. The identification of proteins that mediate Rta/Rp interactions remains a key unanswered problem.
By contrast to Rta, ZEBRA was relatively inefficient at activating Rp reporter constructs in EBV-positive cells and totally inactive at stimulating Zp reporters in this background (Fig. 3C). These findings raise the possibility that viral factors or virally induced cellular factors act at the posttranslational level to repress ZEBRA-mediated activity. ZEBRA is known to interact with a number of cellular factors that could provide this function (21, 24, 39, 51, 59, 60). In agreement with previous reports, ZEBRA was capable of stimulating these two promoters in an EBV-negative background (15). Nonetheless, transfection of ZEBRA into EBV-positive HH514-16 cells led to appearance of mRNAs initiated from Rp and Zp. These results suggest that in the context of latent EBV, ZEBRA may not autostimulate its own promoter directly but may do so indirectly either by activation of Rta or by contacting the Zp promoter through cellular factors.
In accordance with this idea, previous studies have shown that transfection of ZEBRA into Raji cells leads to the appearance of the 3.0-kb bicistronic mRNA driven from Rp but not the 1.0-kb monocistronic mRNA from Zp (29, 30). These previous studies using Raji cells suggested that ZEBRA’s capacity to stimulate Rp, rather than its ability to autostimulate Zp, was a pivotal event in disruption of latency. However, the pathway of activation of the endogenous virus following transfection of ZEBRA and Rta appears to differ among cell lines. The present experiments in HH514-16 cells show that Rta and ZEBRA each stimulate both Zp and Rp (Fig. 4). The experiments do not allow us to conclude whether each overexpressed protein stimulates both promoters, its own promoter, or the promoter of its partner IE gene. Nonetheless, the experiments clearly demonstrate that in permissive cells, both Zp and Rp become active as the result of transfection of either IE gene and suggest a mutual cross-stimulatory mechanism.
The experiments lead to the model proposed in Fig. 5, which should be valid for permissive cells such as HH514-16. During latency, both Rp and Zp are tightly repressed by cellular factors, by higher-order chromatin structure, and/or by the absence of specific activators. Following an inducing stimulus, some cellular factors or structures are displaced or modified by newly synthesized or modified cellular activators. On Zp, these may include AP-1 family members as well as other, unidentified proteins (6, 16, 29, 30). On Rp, Zif268, Sp1, or other proteins may be involved (56, 58). During an amplification or autostimulation event, Rta and ZEBRA activate each other’s promoter. Each of these proteins may also stimulate its own promoter; however, this capacity may be cell specific since in Raji cells Zp is not activated by ZEBRA (29, 30). In permissive cells, Rta activates both Rp and Zp in conjunction with an unidentified protein whose activity is enhanced in the presence of an EBV genome.
ACKNOWLEDGMENTS
This work was supported by grants CA12055, CA16038, and CA20036 from the NIH to G.M.
We thank T. Serio for helpful discussions and critical reading of the manuscript and S. D. Hayward for the gift of plasmid pRTS15.
REFERENCES
- 1.Baer R, Bankier A T, Biggin M D, Deininger P L, Farrell P J, Gibson T J, Hatful G, Hudson G S, Satchwell S C, Seguin C, Tuffnell P S, Barrell B G. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature. 1984;310:207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- 2.Bartkiewicz M, Gold H, Altman S. Identification and characterization of an RNA molecule that copurifies with RNase P activity from HeLa cells. Genes Dev. 1989;3:488–499. doi: 10.1101/gad.3.4.488. [DOI] [PubMed] [Google Scholar]
- 3.Bauer G, Hoefler P, zur Hausen H. Epstein-Barr virus induction by a serum factor. I. Induction and cooperation with additional inducers. Virology. 1982;121:184–194. doi: 10.1016/0042-6822(82)90128-3. [DOI] [PubMed] [Google Scholar]
- 4.Bogedain C, Alliger P, Schwarzmann F, Marschall M, Wolf H, Jilg W. Different activation of Epstein-Barr virus immediate-early and early genes in Burkitt lymphoma cells and lymphoblastoid cell lines. J Virol. 1994;68:1200–1203. doi: 10.1128/jvi.68.2.1200-1203.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buisson M, Manet E, Trescol-Biemont M C, Gruffat H, Durand B, Sergeant A. The Epstein-Barr virus (EBV) early protein EB2 is a posttranscriptional activator expressed under the control of EBV transcription factors EB1 and R. J Virol. 1989;63:5276–5284. doi: 10.1128/jvi.63.12.5276-5284.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cen H, McKnight L C. EBV-immortalized isogenic human B-cell clones exhibit differences in DNA-protein complex formation on the BZLF1 and BRLF1 promoter regions among latent, lytic and TPA-activated cell lines. Virus Res. 1994;31:89–107. doi: 10.1016/0168-1702(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 7.Chavrier P, Gruffat H, Chevallier-Greco A, Buisson M, Sergeant A. The Epstein-Barr virus (EBV) early promoter DR contains a cis-acting element responsive to the EBV transactivator EB1 and an enhancer with constitutive and inducible activities. J Virol. 1989;63:607–614. doi: 10.1128/jvi.63.2.607-614.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chevallier-Greco A, Gruffat H, Manet E, Calender A, Sergeant A. The Epstein-Barr virus (EBV) DR enhancer contains two functionally different domains: domain A is constitutive and cell specific, domain B is transactivated by the EBV early protein R. J Virol. 1989;63:615–623. doi: 10.1128/jvi.63.2.615-623.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chevallier-Greco A, Manet E, Chavrier P, Mosnier C, Daillie J, Sergeant A. Both Epstein-Barr virus (EBV)-encoded trans-acting factors, EB1 and EB2, are required to activate transcription from an EBV early promoter. EMBO J. 1986;5:3243–3249. doi: 10.1002/j.1460-2075.1986.tb04635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Countryman J, Jenson H, Seibl R, Wolf H, Miller G. Polymorphic proteins encoded within BZLF1 of defective and standard Epstein-Barr viruses disrupt latency. J Virol. 1987;61:3672–3679. doi: 10.1128/jvi.61.12.3672-3679.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Countryman J, Miller G. Activation of expression of latent Epstein-Barr herpesvirus after gene transfer with a small cloned subfragment of heterogeneous viral DNA. Proc Natl Acad Sci USA. 1985;82:4085–4089. doi: 10.1073/pnas.82.12.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cox M A, Leahy J, Hardwick J M. An enhancer within the divergent promoter of Epstein-Barr virus responds synergistically to the R and Z transactivators. J Virol. 1990;64:313–321. doi: 10.1128/jvi.64.1.313-321.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis M G, Huang E S. Transfer and expression of plasmids containing human cytomegalovirus immediate-early gene 1 promoter-enhancer sequences in eukaryotic and prokaryotic cells. Biotechnol Appl Biochem. 1988;10:6–12. [PubMed] [Google Scholar]
- 14.Fixman E D, Hayward G S, Hayward S D. Replication of Epstein-Barr Virus oriLyt: lack of a dedicated virally encoded origin-binding protein and dependence on Zta in cotransfection assays. J Virol. 1995;69:2998–3006. doi: 10.1128/jvi.69.5.2998-3006.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flemington E, Speck S H. Autoregulation of Epstein-Barr virus putative lytic switch gene BZLF1. J Virol. 1990;64:1227–1232. doi: 10.1128/jvi.64.3.1227-1232.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flemington E, Speck S H. Identification of phorbol ester response elements in the promoter of Epstein-Barr virus putative lytic switch gene BZLF1. J Virol. 1990;64:1217–1226. doi: 10.1128/jvi.64.3.1217-1226.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Francis A L, Gradoville L, Miller G. Alteration of a single serine in the basic domain of the Epstein-Barr virus ZEBRA protein separates its functions of transcriptional activation and disruption of latency. J Virol. 1997;71:3054–3061. doi: 10.1128/jvi.71.4.3054-3061.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fresen K-O, Merkt B, Bornkamm G W, zur Hausen H. Heterogeneity of Epstein-Barr virus originating from P3HR-1 cells. I. Studies on EBNA induction. Int J Cancer. 1977;19:317–323. doi: 10.1002/ijc.2910190306. [DOI] [PubMed] [Google Scholar]
- 19.Grogan E J, Jenson J, Countryman J, Heston L, Gradoville L, Miller G. Transfection of a rearranged viral DNA fragment WZhet, stably converts latent Epstein-Barr virus infection to productive infection in lymphoid cells. Proc Natl Acad Sci USA. 1987;84:1332–1336. doi: 10.1073/pnas.84.5.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gruffat H, Sergeant A. Characterization of the DNA-binding site repertoire for the Epstein-Barr virus transcription factor R. Nucleic Acids Res. 1994;22:1172–1178. doi: 10.1093/nar/22.7.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gutsch D E, Holley-Guthrie E A, Zhang Q, Stein B, Blanar M A, Baldwin A S, Kenney S C. The bZip transactivator of Epstein-Barr virus, BZLF1, functionally and physically interacts with the p65 subunit of NF-κ B. Mol Cell Biol. 1994;14:1939–1948. doi: 10.1128/mcb.14.3.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hardwick J M, Liebermann P M, Hayward S D. A new Epstein-Barr virus transactivator, R, induces expression of a cytoplasmic early antigen. J Virol. 1988;62:2274–2284. doi: 10.1128/jvi.62.7.2274-2284.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holley-Guthrie E A, Quinlivan E B, Mar E-C, Kenney S. The Epstein-Barr virus (EBV) BMRF1 promoter for early antigen (EA-D) is regulated by the EBV transactivators, BRLF1 and BZLF1, in a cell-specific manner. J Virol. 1990;64:3753–3759. doi: 10.1128/jvi.64.8.3753-3759.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hong Y, Holley-Guthrie E, Kenney S. The bZip dimerization domain of the Epstein-Barr virus BZLF1 (Z) protein mediates lymphoid-specific negative regulation. Virology. 1997;229:36–48. doi: 10.1006/viro.1996.8413. [DOI] [PubMed] [Google Scholar]
- 25.Jenson H B, Miller G. Polymorphisms of the region of the Epstein-Barr virus genome which disrupts latency. Virology. 1988;165:549–564. doi: 10.1016/0042-6822(88)90599-5. [DOI] [PubMed] [Google Scholar]
- 26.Katz D A, Baumann R P, Sun R, Kolman J L, Taylor N, Miller G. Viral proteins associated with the Epstein-Barr virus transactivator, ZEBRA. Proc Natl Acad Sci USA. 1992;89:378–382. doi: 10.1073/pnas.89.1.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kenney S, Holley-Guthrie E, Mar E-C, Smith M. The Epstein-Barr virus BMLF1 promoter contains an enhancer element that is responsive to the BZLF1 and BRLF1 transactivators. J Virol. 1989;63:3878–3883. doi: 10.1128/jvi.63.9.3878-3883.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kieff E. Epstein-Barr virus and its replication. In: Fields B N, editor. Fields virology. Vol. 2. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2343–2396. [Google Scholar]
- 29.Kolman J L, Taylor N, Gradoville L, Countryman J, Miller G. Comparing transcriptional activation and autostimulation by ZEBRA and ZEBRA/c-Fos chimeras. J Virol. 1996;70:1493–1504. doi: 10.1128/jvi.70.3.1493-1504.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Le Roux F, Sergeant A, Corbo L. Epstein-Barr virus (EBV) EB1/Zta protein provided in trans and competent for the activation of productive cycle genes does not activate the BZLF1 gene in the EBV genome. J Gen Virol. 1996;77:501–509. doi: 10.1099/0022-1317-77-3-501. [DOI] [PubMed] [Google Scholar]
- 31.Liu C, Sista N D, Pagano J S. Activation of the Epstein-Barr virus DNA polymerase promoter by the BRLF1 immediate-early protein is mediated through USF and E2F. J Virol. 1996;70:2545–2555. doi: 10.1128/jvi.70.4.2545-2555.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luka J, Kallin B, Klein G. Induction of the Epstein-Barr virus (EBV) cycle in latently infected cells by n-butyrate. Virology. 1979;94:228–231. doi: 10.1016/0042-6822(79)90455-0. [DOI] [PubMed] [Google Scholar]
- 33.Manet E, Gruffat H, Trescol-Biemont M C, Moreno N, Chambard P, Giot J F, Sergeant A. Epstein-Barr virus bicistronic mRNAs generated by facultative splicing code for two transcriptional trans-activators. EMBO J. 1989;8:1819–1826. doi: 10.1002/j.1460-2075.1989.tb03576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mellinghoff I, Daibata M, Humphreys R E, Mulder C, Takada K, Sairenji T. Early events in Epstein-Barr virus genome expression after activation: regulation by second messengers of B cell activation. Virology. 1991;185:922–928. doi: 10.1016/0042-6822(91)90574-u. [DOI] [PubMed] [Google Scholar]
- 35.Menezes J, Leifbold W, Klein G, Clements G. Establishment and characterization of an Epstein-Barr virus (EBV)-negative lymphoblastoid B cell line (BJA-B) from an exceptional, EBV-genome negative African Burkitt’s lymphoma. Biomedicine. 1975;22:276–284. [PubMed] [Google Scholar]
- 36.Nicholas J, Coles L S, Newman C, Honess R W. Regulation of the herpesvirus saimiri (HVS) delayed-early 110-kilodalton promoter by HVS immediate-early gene products and a homolog of the Epstein-Barr virus R trans activator. J Virol. 1991;65:2457–2466. doi: 10.1128/jvi.65.5.2457-2466.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Packham G, Brimmel M, Cook D, Sinclair A J, Farrell P J. Strain variation in Epstein-Barr virus immediate-early genes. Virology. 1993;192:541–550. doi: 10.1006/viro.1993.1070. [DOI] [PubMed] [Google Scholar]
- 38.Pearson G R, Vroman B, Chase B, Scully T, Hummel M, Kieff E. Identification of polypeptide components of the Epstein-Barr virus early antigen complex with monoclonal antibodies. J Virol. 1983;47:193–201. doi: 10.1128/jvi.47.1.193-201.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pfitzner E, Becker P, Rolke A, Schule R. Functional antagonism between the retinoic acid receptor and the viral transactivator BZLF1 is mediated by protein-protein interactions. Proc Natl Acad Sci USA. 1995;92:12265–12269. doi: 10.1073/pnas.92.26.12265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pulvertaft R J V. Cytology of Burkitt’s tumor (African lymphoma) Lancet. 1964;i:238–240. doi: 10.1016/s0140-6736(64)92345-1. [DOI] [PubMed] [Google Scholar]
- 41.Quinlivan E B, Holley-Guthrie E, Mar E-C, Smith M S, Kenney S. The Epstein-Barr virus BRLF1 immediate-early gene product transactivates the human immunodeficiency virus type 1 long terminal repeat by a mechanism which is enhancer independent. J Virol. 1990;64:1817–1820. doi: 10.1128/jvi.64.4.1817-1820.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quinlivan E B, Holley-Guthrie E A, Norris M, Gutsch D, Bachenheimer S L, Kenney S C. Direct BRLF1 binding is required for cooperative BZLF1/BRLF1 activation of the Epstein-Barr virus early promoter, BMRF1. Nucleic Acids Res. 1993;21:1999–2007. doi: 10.1093/nar/21.8.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rabson M, Heston L, Miller G. Identification of a rare Epstein-Barr virus variant that enhances early antigen expression in Raji cells. Proc Natl Acad Sci USA. 1983;80:2762–2766. doi: 10.1073/pnas.80.9.2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rooney C M, Rowe D T, Ragot T, Farrell P J. The spliced BZLF1 gene of Epstein-Barr virus (EBV) transactivates an early EBV promoter and induces the virus productive cycle. J Virol. 1989;63:3109–3116. doi: 10.1128/jvi.63.7.3109-3116.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sato H, Takimoto T, Tanaka S, Tanaka J, Raab-Traub N. Concatemeric replication of Epstein-Barr virus: structure of the termini in virus-producer and newly transformed cell lines. J Virol. 1990;64:5295–5300. doi: 10.1128/jvi.64.11.5295-5300.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schepers A, Pich D, Hammerschmidt W. A transcription factor with homology to the AP-1 family links RNA transcription and DNA replication in the lytic cycle of Epstein-Barr virus. EMBO J. 1993;12:3921–3929. doi: 10.1002/j.1460-2075.1993.tb06070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schepers A, Pich D, Mankertz J, Hammerschmidt W. cis-acting elements in the lytic origin of DNA replication of Epstein-Barr virus. J Virol. 1993;67:4237–4245. doi: 10.1128/jvi.67.7.4237-4245.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Serio T R, Angeloni A, Kolman J L, Gradoville L, Sun R, Katz D, Van Grunsven W, Middeldorp J, Miller G. Two 21-kilodalton components of the Epstein-Barr virus capsid antigen complex and their relationship to ZEBRA-associated protein p21 (ZAP21) J Virol. 1996;70:8047–8054. doi: 10.1128/jvi.70.11.8047-8054.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Serio T R, Kolman J L, Miller G. Late gene expression from the Epstein-Barr virus BcLF1 and BFRF3 promoters does not require DNA replication in cis. J Virol. 1997;71:8726–8734. doi: 10.1128/jvi.71.11.8726-8734.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sinclair A J, Brimmell M, Shanahan F, Farrell P J. Pathways of activation of the Epstein-Barr virus productive cycle. J Virol. 1991;65:2237–2244. doi: 10.1128/jvi.65.5.2237-2244.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sista N D, Pagano J S, Liao W, Kenney S. Retinoic acid is a negative regulator of the Epstein-Barr virus protein (BZLF1) that mediates disruption of latent infection. Proc Natl Acad Sci USA. 1993;90:3894–3898. doi: 10.1073/pnas.90.9.3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51a.Sun, R., S.-F. Lin, L. Gradoville, and G. Miller. A viral gene that activates lytic cycle expression of Kaposi’s sarcoma associated herpesvirus. Proc. Natl. Acad. Sci. USA, in press. [DOI] [PMC free article] [PubMed]
- 52.Takada K, Ono Y. Synchronous and sequential activation of latently infected Epstein-Barr virus genomes. J Virol. 1989;63:445–449. doi: 10.1128/jvi.63.1.445-449.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takada K, Shimuzu N, Sakuma S, Keating A. Transactivation of the latent Epstein-Barr virus (EBV) genome after transfection of the EBV BamHI Z DNA fragment. J Virol. 1986;57:1016–1022. doi: 10.1128/jvi.57.3.1016-1022.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Santen V L. Characterization of a bovine herpesvirus 4 immediate-early RNA encoding a homolog of the Epstein-Barr virus R transactivator. J Virol. 1993;67:773–784. doi: 10.1128/jvi.67.2.773-784.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zalani S, Coppage A, Holley-Guthrie E, Kenney S. The cellular YY1 transcription factor binds a cis-acting, negatively regulating element in the Epstein-Barr virus BRLF1 promoter. J Virol. 1997;71:3268–3274. doi: 10.1128/jvi.71.4.3268-3274.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zalani S, Holley-Guthrie E, Kenney S. The Zif268 cellular transcription factor activates expression of the Epstein-Barr virus immediate-early BRLF1 promoter. J Virol. 1995;69:3816–3823. doi: 10.1128/jvi.69.6.3816-3823.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zalani S, Holley-Guthrie E, Kenney S. Epstein-Barr viral latency is disrupted by the immediate-early BRLF1 protein through a cell-specific mechanism. Proc Natl Acad Sci USA. 1996;93:9194–9199. doi: 10.1073/pnas.93.17.9194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zalani S, Holley-Guthrie E A, Gutsch D E, Kenney S C. The Epstein-Barr virus immediate-early promoter BRLF1 can be activated by the cellular Sp1 transcription factor. J Virol. 1992;66:7282–7292. doi: 10.1128/jvi.66.12.7282-7292.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang Q, Gutsch D, Kenney S. Functional and physical interaction between p53 and BZLF1: implications for Epstein-Barr virus latency. Mol Cell Biol. 1994;14:1929–1938. doi: 10.1128/mcb.14.3.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang Q, Hong Y, Dorsky D, Holley-Guthrie E, Zalani S, Elshiekh N A, Kiehl A, Le T, Kenney S. Functional and physical interactions between the Epstein-Barr virus (EBV) proteins BZLF1 and BMRF1: effects on EBV transcription and lytic replication. J Virol. 1996;70:5131–5142. doi: 10.1128/jvi.70.8.5131-5142.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.zur Hausen H, O’Neil F, Freese U. Persisting oncogenic herpesviruses induced by the tumor promoter TPA. Nature. 1978;272:373–375. doi: 10.1038/272373a0. [DOI] [PubMed] [Google Scholar]