Abstract
Whole inactivated viral particles have been successfully used as vaccines for some viruses, but procedures historically used for inactivation can denature virion proteins. Results have been inconsistent, with enhancement of disease rather than protection seen in some notable instances following vaccination. We used the compound 2,2′-dithiodipyridine (aldrithiol-2; AT-2) to covalently modify the essential zinc fingers in the nucleocapsid (NC) protein of human immunodeficiency virus type 1 (HIV-1) or simian immunodeficiency virus (SIV) virions, thereby inactivating infectivity. The inactivated virus was not detectably infectious in vitro (up to 5 log units of inactivation). However, in contrast to virions inactivated by conventional methods such as heat or formalin treatment, viral and host cell-derived proteins on virion surfaces retained conformational and functional integrity. Thus, immunoprecipitation of AT-2-treated virions was comparable to precipitation of matched untreated virus, even when using antibodies to conformational determinants on gp120. AT-2 inactivated virions bound to CD4+ target cells and mediated virus-induced, CD4-dependent “fusion from without” comparably to native virions. However, viral entry assays demonstrated that the viral life cycle of AT-2-treated virions was arrested before initiation of reverse transcription. The major histocompatibility complex (MHC) class II molecules on the surface of AT-2-treated virions produced from MHC class II-expressing cells retained the ability to support class II-dependent, superantigen-triggered proliferative responses by resting T lymphocytes. These findings indicate that inactivation via this method results in elimination of infectivity with preservation of conformational and functional integrity of virion surface proteins, including both virally encoded determinants and proteins derived from the host cells in which the virus was produced. Such inactivated virions should provide a promising candidate vaccine antigen and a useful reagent for experimentally probing the postulated involvement of virion surface proteins in indirect mechanisms of HIV-1 pathogenesis.
The nucleocapsid (NC) proteins of all lentiviruses and oncornaviruses contain zinc finger motifs, which are among the most highly conserved elements in retroviral sequences (6, 27). Accumulating results from site-directed mutagenesis studies with several different retroviral systems implicate the NC protein in multiple distinct but essential aspects of the viral life cycle. Mutations that disrupt the capacity of the NC protein zinc fingers to coordinate zinc result in a phenotype characterized by profound impairment in packaging of viral genomic RNA into virions (11, 26, 50). More subtle mutations, in which the zinc-coordinating capacity of the NC protein is preserved but the sequence is altered, package viral genomic RNA at levels comparable to those in wild-type virus, but the resulting virions are incapable of productive infection, with the defect in infectivity mapping to critical preintegration steps of the viral life cycle (26a).
The key role of the NC protein at multiple essential steps in the viral life cycle and the highly conserved nature of the retroviral zinc finger motifs in the NC protein make it an attractive target for development of antiretroviral drugs (48, 50, 51). Indeed, a number of compounds have been identified that act via a variety of different mechanisms to covalently modify the NC zinc fingers, resulting in ejection of the coordinated zinc and loss of infectivity (38, 48, 51, 63, 64). Despite differences between detailed mechanisms of action for these compounds, the common mechanistic feature involves a preferential chemical attack on the zinc-coordinating cysteine sulfurs in the residues that make up the NC protein zinc fingers (38). According to this mechanism, it should be possible to identify compounds that can eject zinc from the zinc fingers yet should not affect proteins in which cysteine residues are already involved in disulfide linkages (e.g., viral envelope glycoproteins).
Such a mode of inactivation might provide certain advantages, since the conformational integrity of proteins on the virion surface would be preserved. This would be of interest from the dual perspectives of developing a potentially improved inactivated whole-particle vaccine immunogen and studying the functional and immunopathogenic properties of conformationally intact but noninfectious virons.
Virion surface proteins include both virally encoded proteins and proteins derived nonrandomly through budding from the host cells in which the virions were produced (2, 43). Viral proteins on the virion surface, such as the envelope glycoproteins, are essential for binding and entry into target cells and can also serve as a target for host immune responses (40, 55). Viral proteins have also been implicated in various immunopathogenic mechanisms, such as induction of anergy or apoptosis in human immunodeficiency virus type 1 (HIV-1) infection (1, 31, 58) as well as other viral infections (29). Host cell-derived proteins on the HIV-1 virion surface include major histocompatibility complex (MHC) class II molecules, notably HLA-DR (2, 43). These proteins have been shown to be capable of inducing protective immunity against in vivo challenge with simian immunodeficiency virus (SIV) propagated in human cells (3, 12). As cell surface molecules physiologically involved in immunoregulatory cell-cell recognition events (21, 22, 25), MHC class II proteins may also have the potential to mediate immunopathogenic effects when displayed on the surface of virions.
In this study, we examined HIV-1 virions whose infectivity was abrogated by using the prototypical NC zinc finger targeting compound, 2,2′-dithiodipyridine (aldrithiol-2 [AT-2]). Our analysis focused on assays intended to assess the conformational and functional integrity of virion surface proteins, comparing AT-2-inactivated virus to native virus and to virions inactivated by classical means such as heat treatment or formaldehyde fixation. Our findings indicate that the surface proteins of AT-2-treated virus are conformationally and functionally intact, but the virions are not infectious, with the block to infectivity occurring after virion binding and membrane fusion but before reverse transcription.
MATERIALS AND METHODS
Viruses.
HIV-1MN/H9 clone 4 and HIV-1LAI/H9 were propagated in H9 cells, as described previously (44). SIVmne was obtained from supernatants of the cloned E11S cell line, derived from a culture of HuT-78 cells infected with SIVmne (5, 24). Where indicated, concentrated virus preparations were produced by sucrose gradient banding in a continuous-flow centrifuge (7). Primary HIV-1 isolates 92US727 and 91US054 were obtained from short-term cocultures of peripheral blood mononuclear cells (PBMC) from HIV-1-infected subjects with phytohemagglutinin (PHA)-activated (PHA-M [GIBCO, Grand Island, N.Y.]; 1:100 for 72 h), IL-2-supported (20 U/ml) PBMC from HIV-1-seronegative volunteer donors. All virus stocks were stored at −70°C or in vapor-phase liquid nitrogen until use. Microvesicles, used as a control reagent, were isolated from supernatants of uninfected H9 cell cultures in a manner identical to that used for virus preparation from infected cells (7).
Virus titer determinations.
Virus titers were determined, essentially as described previously (39), with H9 cells, AA2 cells (10, 62), or PHA-stimulated human PBMC blasts (72 h), as indicated, with or without the addition of Polybrene (Sigma, St. Louis, Mo.). Briefly, 2 × 106 indicator cells in 1-ml volumes were inoculated with serial 10-fold dilutions of each native or inactivated (see below) virus stock and incubated overnight (14 to 16 h). After being washed, inoculated cells were seeded at 105 cells in 250 μl in 96-well culture plates, using 16 replicates per dilution or treatment. Cells were cultured in RPMI 1640 with 10% heat-inactivated fetal bovine serum and 2 mM l-glutamine (complete medium); 100 μl of medium was replaced twice weekly. Cultures of PBMC blasts were also supplemented with 20 IU of interleukin-2 per ml. On day 10 postinoculation, supernatants were harvested and tested for p24CA content as an index of productive infection by using a capture enzyme-linked immunosorbent assay (AIDS Vaccine Program, National Cancer Institute—Frederick Cancer Research and Development Center [NCI-FCRDC], Frederick, Md.). For SIV titer determinations, serial dilutions of virus in quadruplicate were inoculated onto AA2 clone 5 cells and cultures were monitored for 21 days, with twice-weekly changes of medium. Culture supernatants were monitored for p28CA. Wells containing >100 pg of p24 per ml or 300 pg of p28CA per ml were scored as positive, and the 50% tissue culture infective dose (TCID50) was calculated by the method of Reed and Muench (47).
Virus inactivation procedures.
For all procedures, frozen virus stocks were quickly thawed at 37°C in a water bath. Heat inactivation was carried out at 56°C in a water bath for 2 h with frequent mixing. Virus was then kept on ice until used (within 2 h). Formaldehyde inactivation was performed as described previously (46). Briefly, virus preparations were treated with a 1:80 solution of buffered formalin (1:2,000 formaldehyde; Sigma, St. Louis, Mo.) in phosphate-buffered saline (PBS) for 24 h at 37°C. Formalin was then neutralized with 2% sodium bisulfite in PBS. For inactivation with AT-2, a stock solution of AT-2 (100 mM in dimethyl sulfoxide [Aldrich, Milwaukee, Wis.]) was prepared and added directly to virus to produce the desired AT-2 concentration. Virus preparations were treated for 1 h at 37°C and then kept on ice until used (within 2 h). At the conclusion of the inactivating procedures, treatment agents were removed by ultrafiltration with a centrifugal filtration device with a 500-kDa cutoff (Centriprep 500; Amicon, Beverly, Mass.). Filtrations were done at 4°C and resulted in at least a 1:375 reduction in the concentration of the chemical inactivating agents. Control virus preparations were sham treated and processed in parallel with inactivated samples.
Western blot analysis.
HIV-1MN was treated with AT-2 or heat as described above. Samples were centrifuged for 1 h at 17,000 × g (4°C) to pellet the virus. Samples for electrophoresis were run separately on sodium dodecyl sulfate-polyacrylamide (4 to 20% gradient) gels (NOVEX, San Diego, Calif.) under reducing and nonreducing conditions. Proteins were transferred onto polyvinylidere difluoride membranes, stained with 0.5% (wt/vol) Ponceau S stain, and detected by immunoblot analysis with monospecific polyvalent goat antiserum prepared against purified viral NC and enhanced chemilumenscence reagents (Amersham, Arlington, Ill.).
Cell lines.
The H9, A3.01, and Sup-T1 cell lines were obtained from the National Institute of Allergy and Infectious Diseases AIDS Research and Reference Reagent Program (Rockville, Md.). The AA2 cell line (62) was provided by R. Benveniste (NCI-FCRDC). All cell lines were mycoplasma negative (PCR mycoplasma detection kit; American Type Culture Collection, Rockville, Md.) and were cultured in complete medium (RPMI 1640, 10% heat-inactivated fetal bovine serum, 2 mM l-glutamine, 100 U of penicillin G per ml, 100 μg of streptomycin sulfate per ml).
Antibody reagents.
The generation and characterization of polyclonal goat antibodies against human MHC class I and class II and a polyclonal goat antibody raised against microvesicles prepared from cultures of H9 cells have been described previously (2, 7). The murine monoclonal antibody 48d (also listed as 4.8D) (59) and the neutralizing monoclonal antibody IgG1b12 (32, 60), both directed against conformational determinants on gp120, were obtained from the AIDS Research and Reference Reagent Program (Rockville, Md.).
Primary cells.
PBMC were isolated by density centrifugation (Ficoll-Hypaque; Sigma) from leukopacks obtained from healthy, HIV-1 seronegative donors at either the National Institutes of Health, Bethesda, Md. or NCI-FCRDC.
Whole-particle immunoprecipitation assay.
A whole-particle immunoprecipitation assay was performed as described previously (2). Briefly, comparable input amounts of native or inactivated virus preparations were incubated with empirically optimized concentrations of each antibody preparation (or PBS) for 1 h at 37°C, then overnight at 4°C, with rocking. Formalin-fixed Staphylococcus aureus Cowan strain (GIBCO) was then added, and after incubation at 20°C for 30 min, virions with bound antibody were immunoprecipitated by centrifugation (2,000 × g for 30 min). To calculate the percent clearance of viral particles, the residual virus content of the supernatant after immunoprecipitation was determined by p24 capture immunoassay and compared to the p24 content of the same virus preparation after identical precipitation with PBS instead of antibody. Clearance by a particular antibody in this assay is indicative of the presence of intact antigen immunoreactive with that antibody on the surface of the virions (2). For purposes of comparison, data were normalized by considering the maximal amount of clearance achieved by each antibody for precipitation of native virus to be 100% relative clearance. The relative percent clearance of inactivated virus preparations by each antibody was determined by comparing the extent of clearance to this 100% value. Maximal absolute clearance values for native virus ranged from 90% (anti-H9 antibody) to 35% (48d antibody).
Virus binding assay.
An immunofluorescence flow cytometric whole-particle virion binding assay was performed by using modifications to a previously reported assay format (56, 65). The A3.01 cell line expresses CD4 and CXCR4 but does not express HLA-DR. Virus propagated in HLA-DR expressing cells incorporates host cell-derived HLA-DR into the viral envelope (7). Thus, acquisition of HLA-DR reactivity by A3.01 cells following incubation with HLA-DR-containing virions can be used as a criterion of virion binding.
Briefly, A3.01 cells (2 × 105 per condition) were preincubated at 4°C for 30 min with either staining buffer (calcium- and magnesium-free PBS with 1% [wt/vol] bovine serum albumin) or unlabeled anti-Leu 3a (5 μg/ml [Becton Dickinson Immunocytometry Systems, San Jose, Calif.]) and then washed once. The cells were then incubated with 100 μl of staining buffer or native or inactivated virus preparation at 37°C for 30 min and washed twice. Immunofluorescent staining was performed (4°C for 30 min) with fluorochrome-conjugated monoclonal antibodies to HLA-DR (phycoerythrin conjugated) and CD4 (anti-Leu 3a, fluorescein isothiocyanate conjugated) and OKT4 (fluorescein isothiocyanate conjugated) with nonspecific antibody binding measured by using isotype-matched monoclonal antibodies of irrelevant specificity, conjugated to the appropriate fluorochromes (all antibodies from Becton-Dickinson Immunocytometry Systems except for OKT4 [Ortho Diagnostics, Raritan, N.J.]). Following antibody staining, the cells were washed three times and fixed in 1% paraformaldehyde for 30 min at 4°C before being analyzed on a FACScan flow cytometer with Cell Quest software (Becton Dickinson Immunocytometry Systems).
Fusion from without.
To test the ability of AT-2-treated virus to mediate CD4-dependent, HIV-1 envelope glycoprotein-mediated “fusion from without” (15, 28), we incubated Sup T1 cells (105 cells/well/50 μl in 96-well flat-bottom plates), which are highly susceptible to HIV-1-induced cell fusion, at 37°C with matched concentrated preparations of HIV-1 or AT-2-inactivated HIV-1 (HIV-1MN/H9 clone 4; 50 μl/well). The presence of characteristic syncytia was evaluated by inverted phase-contrast microscopy 1 to 3 h following virus addition. Syncytia present at this time are due to fusion from without (that is, due to the input virus inoculum), since this is insufficient time for infection to result in cell surface expression of envelope glycoproteins and resulting syncytia (fusion from within). The CD4 dependence of fusion was determined by preincubating target cells with anti-Leu 3a (25 μg/ml) for 30 min before the addition of virus.
Viral entry assay.
To determine the stage of the viral life cycle at which infectivity was arrested for inactivated virus preparations, we performed a viral entry assay in which reverse-transcribed viral DNA species were quantified by a real-time PCR assay. Briefly, PHA-activated, interleukin-2-supported PBMC from HIV-1-seronegative donors were inoculated with native or inactivated virus (1,600 TCID50, 9,000 pg of HIV-1 p24 per 2.5 × 106 cells). After being washed, the inoculated cells were cultured in complete medium and aliquots were harvested at 24 and 48 h postinoculation. A parallel control culture was treated with zidovudine (AZT; 20 μM) and ddI (20 μM), with pretreatment of the target cells for 2 h before inoculation; compounds were present for the duration of the cultures. After being harvested, the washed, dry cell pellets were cryopreserved at −70°C until used for processing and analysis.
The pellets were lysed and total DNA was extracted with commercial reagents (PureGene kit; Gentra Systems, Minneapolis, Minn.) as recommended by the manufacture. HIV-1 strong-stop DNA, indicative of initiation of reverse transcription, and HIV-1 gag DNA, indicative of completion of first-strand DNA synthesis, were quantitated by a real-time PCR assay on an ABI Prism 7700 sequence detection system. The underlying principles and operation of this instrument are reviewed in detail elsewhere (26c, 36, 57a). For the present assays (57b), the following reagent sets were used: strong stop, forward primer, 5′-GGT CTC TCT GGT TAG ACC A-3′ (455 to 473); reverse primer, 5′-CAC ACT GAC TAA AAG GGT CTG-3′ (593 to 573); probe, 5′-(R)TAG TGT GTG CCC GTC TGT TGT GTG ACT(Q)-3′ (554 to 580); Gag, forward primer, 5′-GiC ATC AiG CAG CCA TGC AAA T-3′ (1366 to 1387); reverse primer, 5′-CAT iCT ATT TGT TCi TGA AGG GTA CTA G-3′ (1507 to 1480, where i indicates an inosine residue inserted to avoid bias in amplification based on sequence mismatch at positions where mismatches have been documented among sequenced HIV-1 isolates [45a]); probe, 5′-(R)TCA ATG AGG AAG CTG CAG AAT GGG AT(Q)-3′ (1402 to 1427) (based on the reference sequence for HIV-1 isolate HXB2 [GenBank accession no. K03455]), where R indicates the reporter fluorochrome (6-carboxyfluorescein) and Q indicates the quencher dye 6-carboxytetramethylrhodamine conjugated though a linker arm nucleotide (LAN [36]). (Fluorescent probes for HIV-1 gag and strong-stop DNA were obtained from DNA Sciences, Inc., San Diego, Calif.) In addition, each specimen was analyzed for the copy number for a unique sequence from the coding region for porphobilinogen deaminase (PBGD) (14a, 26b) by using a fluorescent probe purchased from the Applied Biosystems Division of Perkin-Elmer (Foster City, Calif.). Since this sequence is present at two copies per diploid cell and there are no pseudogene sequences, quantitative analysis of this sequence in a given specimen provides an internal control, allowing normalization of HIV sequences relative to the number of diploid genome equivalents of DNA present in the specimen. The average interassay coefficient of variation for the real-time PCR assays for HIV-1 gag and strong-stop and PBGD DNA was <15%, with a nominal threshold sensitivity of 3 DNA copy equivalents per reaction.
MHC class II-dependent, superantigen-triggered proliferation of resting T lymphocytes.
Superantigens trigger polyclonal proliferation of T lymphocytes through a mechanism that is dependent on the presentation of the superantigen to the T-cell receptor complex by MHC class II molecules (13, 35). We have shown previously that the MHC class II molecules incorporated into HIV-1 virions produced from MHC class II-expressing cells are capable of providing the necessary MHC class II proteins to support this effect (53). We therefore performed a comparative evaluation of the ability of native virions and matched virion preparations inactivated by different means to support superantigen-triggered proliferation. PBMC were isolated from leukopacks by density centrifugation. Monocytes were removed by two rounds of plastic adherence (37°C for 1 h and overnight) and recovered separately. Resting T lymphocytes were prepared by passage of nonadherent cells through T-cell enrichment columns (R & D Systems, Minneapolis, Minn.) as recommended by the manufacturer. To ensure that cells were not activated at the initiation of functional assays, T lymphocytes recovered from the T-cell preparation columns were cultured in complete medium with no activating agents for 48 h before use.
To measure superantigen-triggered proliferative responses, resting T cells were seeded in 96-well culture plates at 5 × 104 cells per well (triplicate wells for each condition), incubated alone or with various additions to the cultures for 3 days, and pulsed with [3H]thymidine (1 μCi/well; specific activity, 6.8 Ci/mmol [NEN Life Sciences, Boston, Mass.]) during the final 8 h of culture. The cells were harvested, and [3H]thymidine incorporation was measured as an index of proliferation by liquid scintillation counting (LKB Microbeta; LKB, Rockville, Md.). Culture conditions included T cells alone, T cells plus autologous adherent cells, T cells plus superantigen (staphylococcal enterotoxin A, 100 ng/ml [Sigma]), T cells plus autologous adherent cells plus superantigen, T cells plus native or inactivated virus, and T cells plus native or inactivated virus plus superantigen.
RESULTS
The effectiveness of inactivation of HIV-1 infectivity by treatment with AT-2 was evaluated, and the conformational and functional preservation of virion surface structures after inactivation was assessed. AT-2 treatment eliminated the infectivity of HIV-1, as measured by the inability of treated virus to replicate in highly permissive AA-2 cells, H9 cells, or PHA-treated human lymphoblasts in vitro. As shown in Table 1, the heat, formaldehyde, and AT-2 treatments completely inactivated the viral stocks tested, providing 3 to >4 log units of inactivation. In additional experiments, AT-2 treatment demonstrated in excess of 5 log units of inactivation, with treatment concentrations greater than 100 μM completely inactivating all virus stocks tested in the studies summarized in Table 1, including multiple different HIV-1 viral isolates, as well as viruses produced from both T-cell lines and primary PBMC. Since AT-2 targets a structure that is conserved across all lentiviruses, we were also able to evaluate AT-2 inactivation of SIV infectivity. Following AT-2 treatment, residual SIV infectivity was undetectable, even after purification and 1,000-fold concentration of the inactivated virus (Table 1). In separate dose-effect experiments with virus stocks of different infectivities, complete inactivation of HIV-1 was achieved at AT-2 doses greater than 300 μM, with a 50% reduction at about 30 μM AT-2 (Table 2).
TABLE 1.
Inactivation of HIV-1 and SIV by AT-2, heat, or formalin treatment
| Virus stock | Concn | Amt of p24/p28GAG (pg/ml) | Target cellsa | Infectious titer (TCID50)b after inactivation by:
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| No inactivation | AT-2
|
Formalin | Heat | ||||||
| 1,000 μM | 250 μM | 100 μM | |||||||
| HIV-13B | 1X | 1.7 × 105 | AA2 | 2.1 × 104 | 0 | —c | 15 | 0 | 0 |
| HIV-13B | 1X | 1.7 × 105 | H9 | 6.6 × 103 | 0 | — | |||
| HIV-1MN | 1X | 8.9 × 105 | H9 | 4.3 × 103 | 0 | — | 0 | 0 | 0 |
| HIV-1MN | 1X | 8.9 × 105 | H9 | 4.3 × 103 | 0 | — | 63 | 0 | 0 |
| HIV-1MN | 1X | 8.9 × 105 | AA2 | 5.1 × 104 | 0 | — | 0 | — | — |
| HIV-1MN | 1X | 8.9 × 105 | AA2 | 2.1 × 105 | 0 | — | 0 | — | — |
| HIV-191US054 | 1X | 3.3 × 104 | PHAB | 5.6 × 103 | — | 0 | 271 | — | — |
| HIV-192US657 | 1X | 2.2 × 104 | PHAB | 4.2 × 103 | — | 0 | 0 | — | — |
| SIVMNE, P3612 | 1X | — | — | 3.2 × 105 | — | — | — | — | — |
| SIVMNE, P3612 | 1,000X | 9.9 × 107 | AA2 | 3.2 × 108 | — | — | — | — | — |
| SIVMNE, P3611 | 1,000X | 3.1 × 107 | AA2 | — | 0 | — | — | — | — |
Titers were obtained with H9 cells, AA2 cells, or PHA blasts (PHAB), as indicated and described in Materials and Methods.
TCID50 indicates the calculated reciprocal dilution of viral stock for detectable p24CA production (>100 pg/ml) in 50% of 16 replicate cultures after 10 days. Representative results are shown for various HIV-1 stocks, including long-term laboratory-maintained isolates HIV3B and HIVMN propagated in H9 cells, and primary isolates 91US054 (302054) and 92US657 (301657), propagated in PBMC, as well as SIVMNE, from SIVMNE/HuT-78 clone E11S cells. With these virus stocks, treatment with concentrations of AT-2 in excess of 100 μM gave complete inactivation. In more than 10 independent experiments, treatment with 1,000 μM AT-2 eliminated all detectable infectivity. Also shown are titers for two consecutive large-scale preparations (30 liters) of SIVMNE, clone E11S, produced for biochemical studies (7a). SIV was treated with AT-2 prior to concentration.
—, not tested.
TABLE 2.
Effect of AT-2 treatment concentration on HIV-1MNa
| AT-2 concn (μM) | TCID50b |
|---|---|
| 0 | 4,500 |
| 10 | 3,200 |
| 30 | 2,200 |
| 100 | 770 |
| 300 | 0 |
| 1,000 | 0 |
Native HIV-1 virion preparations (HIV-1MN, 892,987 pg of p24 per ml) and preparations treated with different concentrations of AT-2 were subjected to titer determination on AA2 cells as described in Materials and Methods.
Residual infectivity after treatment is shown, demonstrating complete inactivation with treatment concentrations of >300 μM, with a 50% reduction at approximately 30 μM.
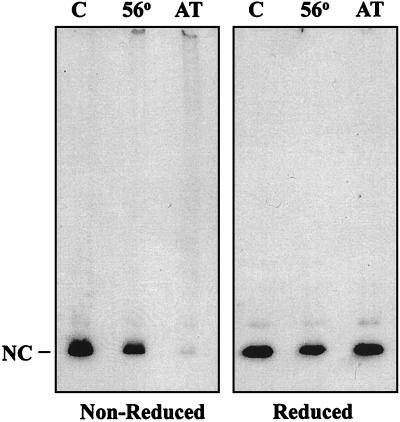
The mechanism of HIV-1 inactivation by AT-2 is proposed to be via covalent modification of the zinc fingers in the NC (p7) protein of HIV-1, with ejection of the coordinated Zn2+, resulting in disruption of multiple aspects of viral replication (38). The Western blot analysis in Fig. 1 shows that the p7NC protein in AT-2-treated HIV-1 is modified so that it does not migrate to the expected position on gel electrophoresis, with other studies demonstrating AT-2-mediated cross-linking of p7 into high-molecular-weight aggregates (data not shown). Complete chemical reduction of the virus extract with 3% 2-mercaptoethanol in sample buffer resulted in the reappearance of the p7NC band at the correct molecular weight (Fig. 1). Heating HIV-1 at 56°C for 2 h did not affect the migration of p7NC, although the treated virus was not detectably infectious.
FIG. 1.
Western blot analysis of AT-2-treated HIV-1. HIV-1 preparations treated with AT-2 (lanes AT) or heat (56°C for 2 h) (lanes 56) and untreated controls (lanes C) were lysed and electrophoresed in 4 to 20% polyacrylamide gradient gels under nonreducing (left) or strongly reducing (right) conditions. The proteins were blotted, and developed with antiserum to p7NC. The left panel shows that p7NC does not migrate to its normal position in AT-2-treated virions. Upon complete chemical reduction of AT-2-treated virus (right panel), the p7NC band reappears.
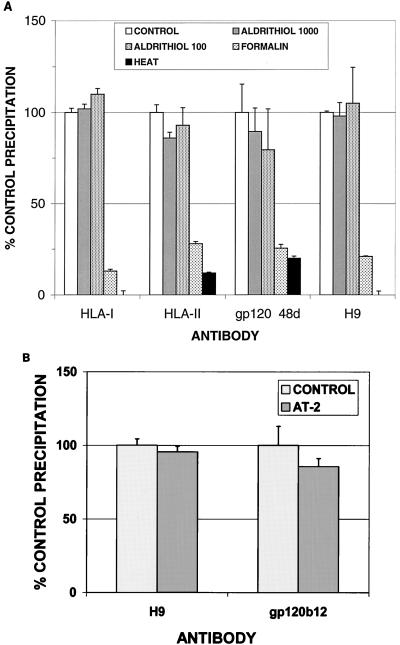
To assess the integrity of viral and cellular proteins on the surface of noninfectious virions inactivated by AT-2 or other means, whole-particle immunoprecipitation was performed. The antibodies used were directed against a variety of determinants on the virion surface, including both linear and conformational determinants, and both viral and host cell-derived proteins. As shown in Fig. 2A, immunoprecipitation of AT-2-inactivated virions was comparable to precipitation of native virions with all antibodies tested, including comparable immunoprecipitation with the monoclonal antibody 48d, which recognizes a conformational determinant on gp120 (59). Figure 2B demonstrates comparable precipitation of native and AT-2-inactivated virions by using IgG1b12, a potently neutralizing monoclonal antibody that reacts with a conformational determinant (32, 60). In marked contrast, immunoprecipitation of both formaldehyde-inactivated virions and heat-inactivated virions was greatly decreased compared to native virus, including minimal immunoprecipitation by the 48d monoclonal antibody, demonstrating the virtually complete denaturation or loss of conformational antigenic determinants on virions following these modes of inactivation (Fig. 2A).
FIG. 2.
(A) Whole-particle immunoprecipitation of HIV-1. HIV-1MN, either untreated or inactivated by different methods as described in the text, was precipitated with antisera to cellular proteins (HLA class I or class II) or viral proteins (gp120, 48d, which recognizes a conformationally sensitive epitope [59]) or H9, an antiserum raised against microvesicle preparations derived from H9 cells (7). All inactivated virus preparations used for these experiments were not detectably infectious. Results shown are the mean ± 1 standard error of the mean for triplicate determinations for each condition in two separate experiments. (B) Whole-particle immunoprecipitation of untreated or AT-2-inactivated HIV-1MN by anti-H9 antiserum or monoclonal antibody IgG1b12, a potently neutralizing antibody to a conformational determinant on gp120 (32, 60). Results shown are the mean ±1 standard error of the mean for triplicate determinations in four separate experiments.
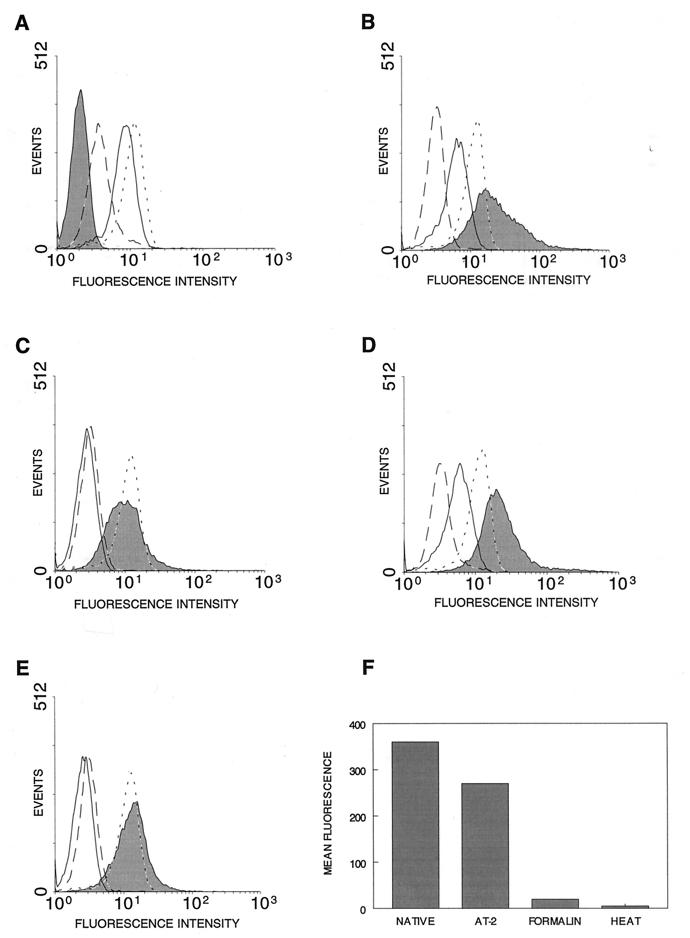
The results of the immunoprecipitation studies described above suggested that conformational determinants on AT-2-inactivated virus were preserved, including determinants on the envelope glycoprotein gp120. To further investigate whether the envelope glycoprotein was functionally intact on AT-2-inactivated virus, we performed a flow cytometry-based virion binding assay. Figure 3A shows that the A3.01 cell line expresses CD4, demonstrated by reactivity with both anti-Leu 3a and OKT4 monoclonal antibodies, but does not express HLA-DR. After incubation of target cells with native virions produced from HLA-DR-positive H9 cells, the cells became positive for HLA-DR staining, with a concomitant decrease in anti-Leu 3a staining but no change in OKT4 staining, consistent with binding of virions to the target cells (Fig. 3B). The HLA-DR staining reflects the MHC class II determinants on the surface of virions bound to the target cells. Staining for the Leu 3a determinant is decreased, since this epitope is involved in interactions with HIV-1 gp120 (4) and since binding of virions blocks antibody access. Staining with OKT4 is not decreased, since this epitope on CD4 is sufficiently removed from the gp120 interaction site. The acquired HLA-DR staining is not attributable to target cell binding of HLA-DR-containing microvesicles present in the virus preparation (7), since incubation of target cells with high concentrations of purified microvesicles derived from uninfected cultures of the same cells used to produce the virus did not result in acquisition of HLA-DR reactivity (data not shown). Preincubation of the target cells with unlabeled anti-Leu 3a before the addition of virions inhibited the acquisition of HLA-DR staining (Fig. 3C). Not all binding was blockable by anti-Leu 3a pretreatment, perhaps reflecting CD4-independent binding (65). However, all infectivity was blockable by anti-Leu 3a (data not shown). Similar studies with AT-2-inactivated virus demonstrated CD4-dependent binding comparable to that of native virions (Fig. 3D and E). In contrast, binding of virions inactivated by either heat or formaldehyde treatment was markedly decreased relative to that of native virions (Fig. 3F).
FIG. 3.
Virion binding demonstrated by flow cytometry. For panels A to E, the solid line indicates Leu 3a staining, the dotted line indicates OKT4 staining, the dashed line indicates isotype-matched irrelevant-specificity control staining, and the shaded area indicates HLA-DR staining. Ten thousand cells were evaluated in each analysis. (A) In the absence of added HIV-1 virions, A3.01 cells are CD4+ (anti-Leu 3a and OKT4 staining) and HLA-DR−. (B) Incubation of A3.01 cells with native HIV-1 produced from HLA-DR-expressing cells results in acquisition of HLA-DR staining and a decrease in anti-Leu 3a but not OKT4 staining, reflecting binding of virions. (C) Preincubation of A3.01 cells with unlabeled anti-Leu 3a prior to incubation with HIV-1 virions and subsequent staining blocks anti-Leu 3a staining and substantially reduces virion binding, reflected in decreased acquisition of HLA-DR staining. (D) Acquisition of HLA-DR reactivity and loss of anti-Leu 3a staining following incubation with AT-2-treated HIV-1 virions were comparable to those in native virions (compare to panel B). (E) Binding of AT-2-inactivated HIV-1 virions is authentic, as reflected by the ability of target cell preincubation with unlabeled anti-Leu 3a to inhibit acquisition of HLA-DR staining (compare to panel C). (F) Binding of AT-2-inactivated HIV-1 virions, as indicated by acquisition of HLA-DR staining, is comparable to that of native virions, while virions inactivated by formalin or heat treatment show virtually no anti-Leu 3a-inhibitable binding. The plot shows mean channel fluorescence for HLA-DR staining following incubation of target cells with comparable amounts (p24CA content) of each virus preparation. Results are representative of four independent assays.
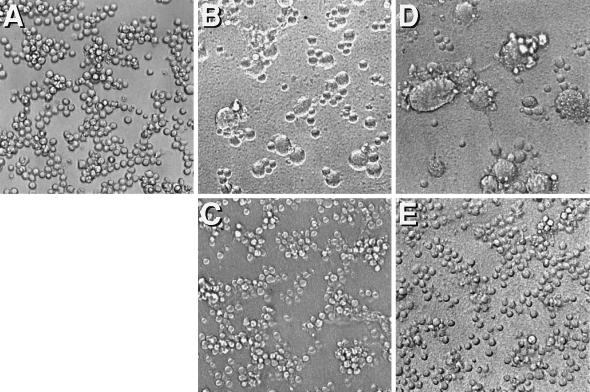
To evaluate whether bound AT-2-inactivated virus was capable of undergoing post-CD4 binding-induced conformational changes and resulting membrane fusion events, we tested the ability of such virions to mediate fusion from without (15). As shown in Fig. 4, inactivated virions rendered noninfectious by AT-2 treatment were nevertheless able to mediate CD4-dependent fusion from without comparably to matched native virion preparations.
FIG. 4.
HIV-1 induced fusion from without of Sup T1 cells. (A) Sup T1 cells, highly susceptible to CD4-dependent, HIV-1 envelope glycoprotein-mediated cell fusion. (B) Following a 3-h incubation with concentrated native HIV-1, characteristic syncytia are seen, reflecting virion-mediated fusion from without. (C) Fusion mediated by native virions is inhibited by prior incubation of cells with anti-Leu 3a. (D) AT-2-inactivated virions mediate fusion from without comparable to native virus. (E) Fusion mediated by AT-2-inactivated virions is inhibited by anti-Leu 3a. Magnification, ×200.
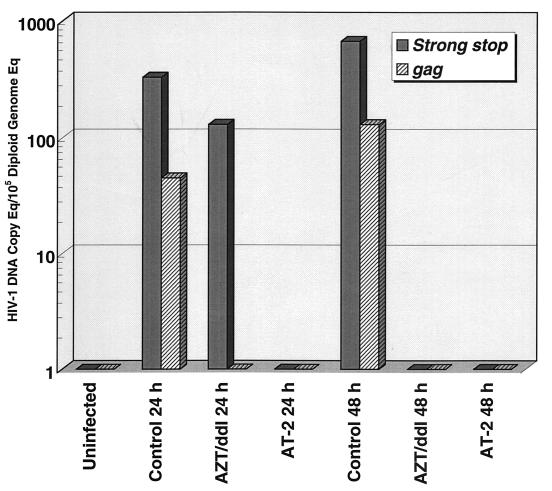
Having confirmed that AT-2-inactivated virus was capable of binding to target cells and undergoing postbinding conformational changes and membrane fusion events comparably to native virions, we next used a viral entry assay to test whether viral entry (57), uncoating, and reverse transcription occurred normally. The viral entry assay used is based on quantitative real-time PCR analysis for HIV reverse transcription intermediates, with normalization of the results based on a single-copy genomic sequence. Quantitation of HIV-1 gag DNA copy equivalents per 100,000 diploid genome equivalents showed a time-dependent accumulation of defined reverse transcription intermediates in cultures inoculated with untreated virus (Fig. 5). Treatment of target cells with the reverse transcriptase inhibitors AZT and ddI completely blocked accumulation of gag DNA. AT-2 treatment of virions that eliminated infectivity also prevented the synthesis of gag DNA. Quantitation of AZT- and ddI-inhibitable synthesis of HIV-1 strong-stop DNA sequences showed that initiation of reverse transcription was also blocked for AT-2-treated virions compared to native virions, after accounting for the noninhibitable strong-stop DNA that may reflect intravirion endogenous reverse transcription (61, 66). As was observed for cells treated with AZT and ddI inoculated with native virions, no new strong-stop DNA was made in untreated cells inoculated with AT-2-treated virions (Fig. 5), indicating that although such treated virions bind to target cells comparably to native virions and can mediate the fusion of viral envelope and host cell membranes, further progression of the viral life cycle is blocked before the initiation of reverse transcription.
FIG. 5.
Viral DNA in cells exposed to native and AT-2-inactivated HIV-1. Human 3-day PHA-induced lymphoblasts were exposed to HIV-1, and cell pellets were collected at 24 and 48 h. Total DNA from the cell pellets was prepared and tested by real-time PCR for reverse-transcribed HIV-1 gag and strong-stop DNA. Results were normalized based on copies of DNA coding for PBGD, as described in the text. Note the time-dependent accumulation of gag and strong-stop DNA in untreated controls and the absence of gag DNA in AZT- and ddI-treated cultures. AT-2 treatment of HIV-1 prevents the production of both gag and strong-stop DNA. Results shown are averages of duplicate determinations of the DNA copy number for each condition in one of three experiments with consistent results. In a separate experiment in which low levels of gag DNA were produced from virions treated with a suboptimal concentration of AT-2, no productive infection was observed (data not shown), suggesting that AT-2-treated virions may also be deficient in the ability to complete other post-reverse transcription, pre-integration steps of the viral life cycle in which p7NC has been implicated (26a).
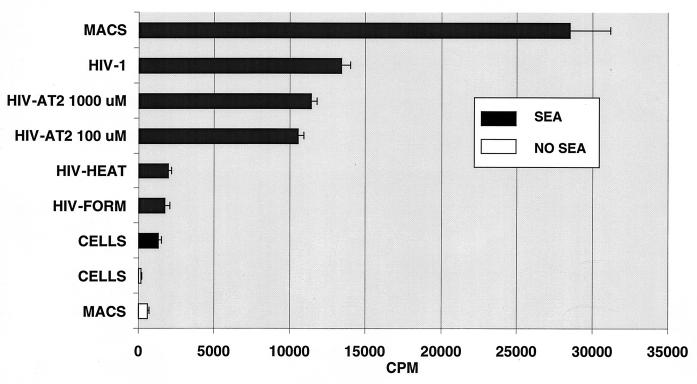
MHC class II-dependent, superantigen-triggered proliferation of resting T lymphocytes was measured as a reflection of the functional integrity of MHC class II determinants on inactivated virions. Neither cultures of resting T cells alone, resting T cells plus macrophages, or resting T cells plus superantigen showed meaningful proliferative responses (Fig. 6). HIV alone did not induce T-cell proliferation under these conditions, either in native form (53) or after AT-2 treatment (data not shown). As expected, when resting T cells were cultured with superantigen in the presence of autologous monocytes/macrophages to provide a source of MHC class II, a vigorous proliferative response was observed. The MHC class II present on native virions was sufficient to support superantigen-triggered proliferation by resting T cells, in the absence of other sources of class II. AT-2-inactivated virions supported superantigen-triggered proliferation comparably to native virions, while virions inactivated by either heat or formaldehyde treatment were not capable of supporting the superantigen triggered proliferative response (Fig. 6).
FIG. 6.
Superantigen-induced T-cell proliferation. Resting human peripheral blood lymphoid cells alone, in the presence of autologous macrophages alone, or in the presence of SEA alone do not proliferate, as measured by [3H]-thymidine incorporation during the last 8 h of a 3-day incubation (bottom three bars). SEA exposure in the presence of MHC class II present on macrophages induces strong proliferation (top bar). HIV-1 produced from MHC class II-expressing cells was able to support the superantigen-triggered proliferative response when present as the only source of class II in the culture, and AT-2 inactivated virus was as active as untreated HIV-1. Formalin or heat treatment of HIV-1 rendered virions incapable of supporting superantigen-induced proliferative responses. Representative results from one of three experiments are shown; each bar represents the mean of triplicate cultures ±1 standard error of the mean.
DISCUSSION
Whole inactivated virions have been used successfully as vaccines for numerous viruses (16, 17, 41, 42, 46), but traditional means of viral inactivation can denature virion surface proteins. Results have been inconsistent, with examples of both effective protection and disease exacerbation associated with inactivated virus vaccines (9, 14, 33). Formalin treatment has been shown to be poor in preserving the antigenicity of SIV (18), consistent with our observations in this study. The unique mechanism of retroviral inactivation mediated by compounds targeting the NC protein zinc fingers suggested that this approach might be a means of inactivating infectivity while preserving the conformational integrity of virion surface proteins. We tested this hypothesis by inactivating HIV-1 stocks with the prototype zinc finger-modifying compound AT-2 and performing comparative testing of native virus and AT-2-inactivated virus, along with matched virus preparations inactivated with heat or formaldehyde treatment. These inactivated virions were characterized by using a battery of assays to probe the conformational and functional integrity of both virally encoded and host cell-derived virion surface proteins.
Treatment with more than 100 μM AT-2 completely inactivated the detectable infectivity of all HIV-1 stocks tested (more than 5 log units), including multiple different primary isolates propagated in primary PBMC and isolates adapted for growth in T-cell lines (Table 1). Inactivation of SIV infectivity has also been observed (Table 1) (7a). However, in marked contrast to virus inactivated by heat or formaledehyde treatment (18, 54), AT-2 treatment preserved the conformational and functional integrity of surface proteins on inactivated HIV-1 virions. Thus, AT-2-inactivated virions were immunoprecipitated comparably to native virions, even using monoclonal antibodies to conformational determinants on gp120 (Fig. 1 and 2). The conformational and functional integrity of host cell-derived proteins on the surface of AT-2-inactivated virions was also preserved, as reflected by the ability to support MHC class II-dependent, superantigen-triggered proliferation by resting T lymphocytes (Fig. 6). AT-2-inactivated virions showed CD4-dependent binding to target cells comparable to native virions (Fig. 3). Treated virions were capable of mediating membrane fusion (Fig. 4), although infectivity was blocked before initiation of reverse transcription (Fig. 5). In combination with biochemical data suggesting cross-linking of p7NC in treated virions (37), these results are consistent with a blockade based on cross-linking of the NC protein in treated virions, preventing full uncoating and initiation of reverse transcription following fusion of the virion envelope with the target cell plasma membrane (57).
Maintenance of conformational and functional integrity by AT-2-inactivated virions suggests that such virions may be a useful vaccine antigen. Oligomeric forms of HIV-1 envelope glycoprotein have been shown to be more effective in inducing antibodies to conformational determinants important for broad neutralizing activity than have monomeric forms of envelope glycoprotein (8, 19, 20, 52). Noninfectious but conformationally intact whole virions may represent a further improvement in this regard. Such virions may be particularly useful as the boost component of “prime-boost” vaccination regimens involving live vectors such as attenuated poxviruses (45) or in vaccination schemes involving DNA immunization (34). The use of inactivated virions produced from mutant virus strains engineered for improved immunogenicity, especially for induction of neutralizing antibodies, may provide even greater utility (49).
Another potential application of these observations is the inactivation of HIV-1 in patient blood samples used in hospital or research laboratories, reducing the infectious hazards associated with the manipulation of such samples. The effects of AT-2 on serum chemistry analyses and cellular metabolism are under investigation. Since this mode of inactivation can be expected to be effective against all retroviruses that contain zinc finger motifs in their nucleocapsid proteins, it may also be relevant to enhancing the safety of biological products such as monoclonal antibodies produced from cell lines that may harbor endogenous retroviruses.
The virions produced by AT-2 inactivation, by virtue of the conformational and functional integrity of their surface proteins, may also provide a useful reagent for studies to evaluate the potential role of virions and virion proteins in the immunopathogenesis of HIV-1 infection, independent of cytopathic or other consequences of productive viral infection, including proposed “bystander” mechanisms of CD4 cell depletion (23, 30). The postulated involvement of the HIV-1 envelope glycoprotein in inducing anergy or priming cells for subsequent apoptotic cell death may perhaps be best explored by using conformationally authentic but noninfectious virions. Similarly, the possible contributions of host cell-derived molecules on the virion surface can also be investigated.
Analysis of retroviral NC proteins by site-directed mutagenesis is providing insight into the critical role of this protein in several different stages of the viral life cycle (26). The essential functions mediated by the NC protein make it an attractive target for antiretroviral drug development as well, with the identification of compounds capable of inactivating the virus via p7NC interactions providing good leads for drug development (51, 63). The mechanism of action of inactivation by these compounds also results in virions that may be useful as a candidate vaccine and may also facilitate basic studies of retroviral pathogenesis in yet another applied aspect of investigation of this fascinating, yet still incompletely understood, viral protein.
ACKNOWLEDGMENTS
We thank P. Grove for assistance with the preparation of the manuscript and B. Kane for electrophoresis.
This project has been funded with Federal funds from the National Cancer Institute, National Institutes of Health, under contract NO1-CO-56000.
REFERENCES
- 1.Ameisen J C, Capron A. Cell dysfunction and depletion in AIDS: the programmed cell death hypothesis. Immunol Today. 1991;12:102–105. doi: 10.1016/0167-5699(91)90092-8. [DOI] [PubMed] [Google Scholar]
- 2.Arthur L O, Bess J W, Jr, Sowder R C, Benveniste R E, Mann D L, Chermann J C, Henderson L E. Cellular proteins bound to immunodeficiency viruses: implications for pathogenesis and vaccines. Science. 1992;258:1935–1938. doi: 10.1126/science.1470916. [DOI] [PubMed] [Google Scholar]
- 3.Arthur L O, Bess J W, Jr, Urban R G, Strominger J L, Morton W R, Mann D L, Henderson L E, Benveniste R E. Macaques immunized with HLA-DR are protected from challenge with simian immunodeficiency virus. J Virol. 1995;69:3117–3124. doi: 10.1128/jvi.69.5.3117-3124.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Attanasio R, Dilley D, Buck D, Maino V C, Lohman K L, Kanda P, Kennedy R C. Structural characterization of a cross-reactive idiotype shared by monoclonal antibodies specific for the human CD4 molecule. J Biol Chem. 1991;266:14611–14619. [PubMed] [Google Scholar]
- 5.Benveniste R E, Hill R W, Eron L J, Csaikl U M, Knott W B, Henderson L E, Sowder R C, Nagashima K, Gonda M A. Characterization of clones of HIV-1 infected HuT 78 cells defective in gag gene processing and of SIV clones producing large amounts of envelope glycoprotein. J Med Primatol. 1990;19:351–366. [PubMed] [Google Scholar]
- 6.Berg J M, Shi Y. The galvanization of biology: a growing appreciation for the roles of zinc. Science. 1996;271:1081–1085. doi: 10.1126/science.271.5252.1081. [DOI] [PubMed] [Google Scholar]
- 7.Bess J W, Jr, Gorelick R J, Bosche W J, Henderson L E, Arthur L O. Microvesicles are a source of contaminating cellular proteins found in purified HIV-1 preparations. Virology. 1997;230:134–144. doi: 10.1006/viro.1997.8499. [DOI] [PubMed] [Google Scholar]
- 7a.Bess, J. W., Jr., et al. Unpublished data.
- 8.Broder C C, Earl P L, Long D, Abedon S T, Moss B, Doms R W. Antigenic implications of human immunodeficiency virus type 1 envelope quaternary structure: oligomer-specific and -sensitive monoclonal antibodies. Proc Natl Acad Sci USA. 1994;91:11699–11703. doi: 10.1073/pnas.91.24.11699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown F. Review of accidents caused by incomplete inactivation of viruses. Dev Biol Stand. 1993;81:103–107. [PubMed] [Google Scholar]
- 10.Chaffee S, Leeds J M, Matthews T J, Weinhold K J, Skinner M, Bolognesi D P, Hershfield M S. Phenotypic variation in the response to the human immunodeficiency virus among derivatives of the CEM T and WIL-2 B cell lines. J Exp Med. 1988;168:605–621. doi: 10.1084/jem.168.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan B, Musier-Forsyth K. The nucleocapsid protein specifically anneals tRNALys-3 onto a noncomplementary primer binding site within the HIV-1 RNA genome in vitro. Proc Natl Acad Sci USA. 1997;94:13530–13535. doi: 10.1073/pnas.94.25.13530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan W L, Rodgers A, Grief C, Almond N, Ellis S, Flanagan B, Silvera P, Bootman J, Stott J, Kent K, Bomford R. Immunization with class I human histocompatibility leukocyte antigen can protect macaques against challenge infection with SIVmac-32H. AIDS. 1995;9:223–228. [PubMed] [Google Scholar]
- 13.Chatila T, Geha R S. Superantigens. Curr Opin Immunol. 1992;4:74–78. doi: 10.1016/0952-7915(92)90129-3. [DOI] [PubMed] [Google Scholar]
- 14.Chin J, Magoffin R L, Shearer L A, Schieble J H, Lennette E H. Field evaluation of a respiratory syncytial virus vaccine and a trivalent parainfluenza virus vaccine in a pediatric population. Am J Epidemiol. 1969;89:449–463. doi: 10.1093/oxfordjournals.aje.a120957. [DOI] [PubMed] [Google Scholar]
- 14a.Chretien S, Dubart A, Beaupain D, Raich N, Grandchamp B, Rosa J, Goossens M, Romeo P H. Alternative transcription and splicing of the human porphobilinogen deaminase gene result either in tissue-specific or in housekeeping expression. Proc Natl Acad Sci USA. 1988;85:6–10. doi: 10.1073/pnas.85.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clavel F, Charneau P. Fusion from without directed by human immunodeficiency virus particles. J Virol. 1994;68:1179–1185. doi: 10.1128/jvi.68.2.1179-1185.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clemens, R., A. Safary, A. Hepburn, C. Roche, W. J. Stanbury, and F. E. Andre. 1995. Clinical experience with an inactivated hepatitis A vaccine. J. Infect. Dis. 171(Suppl. 1):S44–S49. [DOI] [PubMed]
- 17.Cranage M P, Baskerville A, Ashworth L A, Dennis M, Cook N, Sharpe S, Farrar G, Rose J, Kitchin P A, Greenaway P J. Intrarectal challenge of macaques vaccinated with formalin-inactivated simian immunodeficiency virus. Lancet. 1992;339:273–274. doi: 10.1016/0140-6736(92)91335-6. [DOI] [PubMed] [Google Scholar]
- 18.Cranage M P, McBride B W, Rud E W. The simian immunodeficiency virus transmembrane protein is poorly immunogenic in inactivated virus vaccine. Vaccine. 1995;13:895–900. doi: 10.1016/0264-410x(95)00008-o. [DOI] [PubMed] [Google Scholar]
- 19.Earl P L, Broder C C, Doms R W, Moss B. Epitope map of human immunodeficiency virus type 1 gp41 derived from 47 monoclonal antibodies produced by immunization with oligomeric envelope protein. J Virol. 1997;71:2674–2684. doi: 10.1128/jvi.71.4.2674-2684.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Earl P L, Broder C C, Long D, Lee S A, Peterson J, Chakrabarti S, Doms R W, Moss B. Native oligomeric human immunodeficiency virus type 1 envelope glycoprotein elicits diverse monoclonal antibody reactivities. J Virol. 1994;68:3015–3026. doi: 10.1128/jvi.68.5.3015-3026.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Engleman E G, Benike C J, Grumet F C, Evans R L. Activation of human T lymphocyte subsets: helper and suppressor/cytotoxic T cells recognize and respond to distinct histocompatibility antigens. J Immunol. 1981;127:2124–2129. [PubMed] [Google Scholar]
- 22.Engleman E G, Benike C J, Metzler C, Gatenby P A, Evans R L. Blocking of human T lymphocyte functions by anti-Leu-2 and anti-Leu-3 antibodies: differential inhibition of proliferation and suppression. J Immunol. 1983;130:2623–2628. [PubMed] [Google Scholar]
- 23.Finkel T H, Tudor-Williams G, Banda N K, Cotton M F, Curiel T, Monks C, Baba T W, Ruprecht R M, Kupfer A. Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV- and SIV-infected lymph nodes. Nat Med. 1995;1:129–134. doi: 10.1038/nm0295-129. [DOI] [PubMed] [Google Scholar]
- 24.Gale M J, Jr, Ledbetter J A, Schieven G L, Jonker M, Morton W R, Benveniste R E, Clark E A. CD4 and CD8 T cells from SIV-infected macaques have defective signaling responses after perturbation of either CD3 or CD2 receptors. Int Immunol. 1990;2:849–858. doi: 10.1093/intimm/2.9.849. [DOI] [PubMed] [Google Scholar]
- 25.Germain R N. Antigen processing and presentation. In: Paul W E, editor. Fundamental immunology. 3rd ed. New York, N.Y: Raven Press; 1993. pp. 629–676. [Google Scholar]
- 26.Gorelick R J, Chabot D J, Ott D E, Gagliardi T D, Rein A, Henderson L E, Arthur L O. Genetic analysis of the zinc finger in the Moloney murine leukemia virus nucleocapsid domain: replacement of zinc-coordinating residues with other zinc-coordinating residues yields noninfectious particles containing genomic RNA. J Virol. 1996;70:2593–2597. doi: 10.1128/jvi.70.4.2593-2597.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26a.Gorelick, R. J., et al. Unpublished data.
- 26b.Grandchamp B, De Verneuil H, Beaumont C, Chretien S, Walter O, Nordmann Y. Tissue-specific expression of porphobilinogen deaminase. Two isoenzymes from a single gene. Eur J Biochem. 1987;162:105–110. doi: 10.1111/j.1432-1033.1987.tb10548.x. [DOI] [PubMed] [Google Scholar]
- 26c.Heid C A, Stevens J, Livak K J, Williams P M. Real time quantitative PCR. Genome Res. 1996;6:986–994. doi: 10.1101/gr.6.10.986. [DOI] [PubMed] [Google Scholar]
- 27.Henderson L E, Copeland T D, Sowder R C, Smythers G W, Oroszlan S. Primary structure of the low molecular weight nucleic acid-binding proteins of murine leukemia viruses. J Biol Chem. 1981;256:8400–8406. [PubMed] [Google Scholar]
- 28.Hernandez L D, Hoffman L R, Wolfsberg T G, White J M. Virus-cell and cell-cell fusion. Annu Rev Cell Dev Biol. 1996;12:627–661. doi: 10.1146/annurev.cellbio.12.1.627. [DOI] [PubMed] [Google Scholar]
- 29.Hilleman M R. Overview: practical insights from comparative immunology and pathogenesis of AIDS, hepatitis B, and measles for developing an HIV vaccine. Vaccine. 1995;13:1733–1740. doi: 10.1016/0264-410x(95)00114-g. [DOI] [PubMed] [Google Scholar]
- 30.Kameoka M, Suzuki S, Kimura T, Fujinaga K, Auwanit W, Luftig R B, Ikuta K. Exposure of resting peripheral blood T cells to HIV-1 particles generates CD25+ killer cells in a small subset, leading to induction of apoptosis in bystander cells. Int Immunol. 1997;9:1453–1462. doi: 10.1093/intimm/9.10.1453. [DOI] [PubMed] [Google Scholar]
- 31.Kaneko H, Hishikawa T, Sekigawa I, Hashimoto H, Okumura K, Kaneko Y. Role of tumour necrosis factor-alpha (TNF-alpha) in the induction of HIV-1 gp120-mediated CD4+ T cell anergy. Clin Exp Immunol. 1997;109:41–46. doi: 10.1046/j.1365-2249.1997.4231325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kessler J A, Jr, McKenna P M, Emini E A, Chan C P, Patel M D, Gupta S K, Mark III G E, Barbas III C F, Burton D R, Conley A J. Recombinant human monoclonal antibody IgG1b12 neutralizes diverse human immunodeficiency virus type 1 primary isolates. AIDS Res Hum Retroviruses. 1997;13:575–582. doi: 10.1089/aid.1997.13.575. [DOI] [PubMed] [Google Scholar]
- 33.Kim H W, Canchola J G, Brandt C D, Pyles G, Chanock R M, Jensen K, Parrott R H. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89:422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- 34.Kim J J, Weiner D B. DNA gene vaccination for HIV. Springer Semin Immunopathol. 1997;19:175–194. doi: 10.1007/BF00870267. [DOI] [PubMed] [Google Scholar]
- 35.Kotzin B L, Leung D Y, Kappler J, Marrack P. Superantigens and their potential role in human disease. Adv Immunol. 1993;54:99–166. doi: 10.1016/s0065-2776(08)60534-9. [DOI] [PubMed] [Google Scholar]
- 36.Livak K J, Flood S J, Marmaro J, Giusti W, Deetz K. Oligonucleotides with fluorescent dyes at opposite ends provide a quenched probe system useful for detecting PCR product and nucleic acid hybridization. PCR Methods Applic. 1995;4:357–362. doi: 10.1101/gr.4.6.357. [DOI] [PubMed] [Google Scholar]
- 37.Loo J A, Holler T P, Sanchez J, Gogliotti R, Maloney L, Reily M D. Biophysical characterization of zinc ejection from HIV nucleocapsid protein by anti-HIV 2,2′-dithiobis[benzamides] and benzisothiazolones. J Med Chem. 1996;39:4313–4320. doi: 10.1021/jm960253w. [DOI] [PubMed] [Google Scholar]
- 38.McDonnell N B, De Guzman R N, Rice W G, Turpin J A, Summers M F. Zinc ejection as a new rationale for the use of cystamine and related disulfide-containing antiviral agents in the treatment of AIDS. J Med Chem. 1997;40:1969–1976. doi: 10.1021/jm970147+. [DOI] [PubMed] [Google Scholar]
- 39.McDougal J S, Cort S P, Kennedy M S, Cabridilla C D, Feorino P M, Francis D P, Hicks D, Kalyanaraman V S, Martin L S. Immunoassay for the detection and quantitation of infectious human retrovirus, lymphadenopathy-associated virus (LAV) J Immunol Methods. 1985;76:171–183. doi: 10.1016/0022-1759(85)90489-2. [DOI] [PubMed] [Google Scholar]
- 40.Moore J P, Trkola A, Dragic T. Co-receptors for HIV-1 entry. Curr Opin Immunol. 1997;9:551–562. doi: 10.1016/s0952-7915(97)80110-0. [DOI] [PubMed] [Google Scholar]
- 41.Murdin A D, Barreto L, Plotkin S. Inactivated poliovirus vaccine: past and present experience. Vaccine. 1996;14:735–746. doi: 10.1016/0264-410x(95)00211-i. [DOI] [PubMed] [Google Scholar]
- 42.Murphey-Corb M, Martin L N, Davison-Fairburn B, Montelaro R C, Miller M, West M, Ohkawa S, Baskin G B, Zhang J Y, Putney S D, Allison A C, Eppstein D A. A formalin-inactivated whole SIV vaccine confers protection in macaques. Science. 1989;246:1293–1297. doi: 10.1126/science.2555923. [DOI] [PubMed] [Google Scholar]
- 43.Ott D E. Cellular proteins in HIV virions. Rev Med Virol. 1997;7:167–180. doi: 10.1002/(sici)1099-1654(199709)7:3<167::aid-rmv199>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 44.Ott D E, Nigida S M, Jr, Henderson L E, Arthur L O. The majority of cells are superinfected in a cloned cell line that produces high levels of human immunodeficiency virus type 1 strain MN. J Virol. 1995;69:2443–2450. doi: 10.1128/jvi.69.4.2443-2450.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paoletti E. Applications of pox virus vectors to vaccination: an update. Proc Natl Acad Sci USA. 1996;93:11349–11353. doi: 10.1073/pnas.93.21.11349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45a.Piatak M, Jr, Luk K C, Williams B, Lifson J D. Quantitative competitive polymerase chain reaction for accurate quantitation of HIV DNA and RNA species. Biotechniques. 1993;14:70–81. [PubMed] [Google Scholar]
- 46.Potash L. Methods in human virus vaccine preparation. Methods Virol. 1968;4:371–464. [Google Scholar]
- 47.Reed L J, Muench H. A simple method of estimating fifty per cent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 48.Rein A, Ott D E, Mirro J, Arthur L O, Rice W, Henderson L E. Inactivation of murine leukemia virus by compounds that react with the zinc finger in the viral nucleocapsid protein. J Virol. 1996;70:4966–4972. doi: 10.1128/jvi.70.8.4966-4972.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reitter J N, Means R E, Desrosiers R C. A role for carbohydrates in immune evasion in AIDS. Nat Med. 1998;4:679–684. doi: 10.1038/nm0698-679. [DOI] [PubMed] [Google Scholar]
- 50.Rice W G, Schaeffer C A, Harten B, Villinger F, South T L, Summers M F, Henderson L E, Bess J W, Jr, Arthur L O, McDougal J S, et al. Inhibition of HIV-1 infectivity by zinc-ejecting aromatic C-nitroso compounds. Nature. 1993;361:473–475. doi: 10.1038/361473a0. [DOI] [PubMed] [Google Scholar]
- 51.Rice W G, Supko J G, Malspeis L, Buckheit R W, Jr, Clanton D, Bu M, Graham L, Schaeffer C A, Turpin J A, Domagala J, Gogliotti R, Bader J P, Halliday S M, Coren L, Sowder R C, Jr, Arthur L O, Henderson L E. Inhibitors of HIV nucleocapsid protein zinc fingers as candidates for the treatment of AIDS. Science. 1995;270:1194–1197. doi: 10.1126/science.270.5239.1194. [DOI] [PubMed] [Google Scholar]
- 52.Richardson T M, Jr, Stryjewski B L, Broder C C, Hoxie J A, Mascola J R, Earl P L, Doms R W. Humoral response to oligomeric human immunodeficiency virus type 1 envelope protein. J Virol. 1996;70:753–762. doi: 10.1128/jvi.70.2.753-762.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rossio J L, Bess J, Jr, Henderson L E, Cresswell P, Arthur L O. HLA class II on HIV particles is functional in superantigen presentation to human T cells: implications for HIV pathogenesis. AIDS Res Hum Retroviruses. 1995;11:1433–1439. doi: 10.1089/aid.1995.11.1433. [DOI] [PubMed] [Google Scholar]
- 54.Sattentau Q J. Conservation of HIV-1 gp120 neutralizing epitopes after formalin inactivation. AIDS. 1995;9:1383–1385. doi: 10.1097/00002030-199512000-00017. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 55.Sattentau Q J, Moore J P. The role of CD4 in HIV binding and entry. Philos Trans R Soc London B Ser. 1993;342:59–66. doi: 10.1098/rstb.1993.0136. [DOI] [PubMed] [Google Scholar]
- 56.Schols D, Pauwels R, Desmyter J, De Clercq E. Presence of class II histocompatibility DR proteins on the envelope of human immunodeficiency virus demonstrated by FACS analysis. Virology. 1992;189:374–376. doi: 10.1016/0042-6822(92)90719-6. [DOI] [PubMed] [Google Scholar]
- 57.Stein B S, Gowda S D, Lifson J D, Penhallow R C, Bensch K G, Engleman E G. pH-independent HIV entry into Cd4-positive T cells via virus envelope fusion to the plasma membrane. Cell. 1987;49:659–668. doi: 10.1016/0092-8674(87)90542-3. [DOI] [PubMed] [Google Scholar]
- 57a.Suryanarayana K, Wiltrout T A, Vasquez G M, Hirsch V M, Lifson J D. Plasma SIV RNA viral load determination by real-time quantification of product generation in reverse transcriptase-polymerase chain reaction. AIDS Res Hum Retroviruses. 1998;14:183–189. doi: 10.1089/aid.1998.14.183. [DOI] [PubMed] [Google Scholar]
- 57b.Suryanarayara, K., et al. Unpublished data.
- 58.Tamma S M, Chirmule N, McCloskey T W, Oyaizu N, Kalyanaraman V S, Pahwa S. Signals transduced through the CD4 molecule interfere with TCR/CD3-mediated ras activation leading to T cell anergy/apoptosis. Clin Immunol Immunopathol. 1997;85:195–201. doi: 10.1006/clin.1997.4424. [DOI] [PubMed] [Google Scholar]
- 59.Thali M, Moore J P, Furman C, Charles M, Ho D D, Robinson J, Sodroski J. Characterization of conserved human immunodeficiency virus type 1 gp120 neutralization epitopes exposed upon gp120-CD4 binding. J Virol. 1993;67:3978–3988. doi: 10.1128/jvi.67.7.3978-3988.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Trkola A, Pomales A B, Yuan H, Korber B, Maddon P J, Allaway G P, Katinger H, Barbas III C F, Burton D R, Ho D D. Cross-clade neutralization of primary isolates of human immunodeficiency virus type 1 by human monoclonal antibodies and tetrameric CD4-IgG. J Virol. 1995;69:6609–6617. doi: 10.1128/jvi.69.11.6609-6617.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Trono D. Partial reverse transcripts in virions from human immunodeficiency and murine leukemia viruses. J Virol. 1992;66:4893–4900. doi: 10.1128/jvi.66.8.4893-4900.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsai C C, Follis K E, Yarnall M, Deaver L E, Benveniste R E, Sager P R. In vitro screening for antiretroviral agents against simian immunodeficiency virus (SIV) Antiviral Res. 1990;14:87–98. doi: 10.1016/0166-3542(90)90046-a. [DOI] [PubMed] [Google Scholar]
- 63.Tummino P J, Harvey P J, McQuade T, Domagala J, Gogliotti R, Sanchez J, Song Y, Hupe D. The human immunodeficiency virus type 1 (HIV-1) nucleocapsid protein zinc ejection activity of disulfide benzamides and benzisothiazolones: correlation with anti-HIV and virucidal activities. Antimicrob Agents Chemother. 1997;41:394–400. doi: 10.1128/aac.41.2.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tummino P J, Scholten J D, Harvey P J, Holler T P, Maloney L, Gogliotti R, Domagala J, Hupe D. The in vitro ejection of zinc from human immunodeficiency virus (HIV) type 1 nucleocapsid protein by disulfide benzamides with cellular anti-HIV activity. Proc Natl Acad Sci USA. 1996;93:969–973. doi: 10.1073/pnas.93.3.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ugolini S, Mondor I, Parren P W, Burton D R, Tilley S A, Klasse P J, Sattentau Q J. Inhibition of virus attachment to CD4+ target cells is a major mechanism of T cell line-adapted HIV-1 neutralization. J Exp Med. 1997;186:1287–1298. doi: 10.1084/jem.186.8.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang H, Zhang Y, Spicer T, Henrard D, Poiesz B J. Nascent human immunodeficiency virus type 1 reverse transcription occurs within an enveloped particle. J Virol. 1995;69:3675–3682. doi: 10.1128/jvi.69.6.3675-3682.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]