Abstract
Background.
Pancreas transplant volumes are limited because of poor utilization of “extended criteria grafts.” Prolonged cold ischemia is a risk factor associated with poor allograft survival. We aimed to establish the feasibility of transplantation using grafts subjected to prolonged cold ischemia and determine whether these grafts could be optimized using normothermic ex vivo perfusion (NEVP) in a porcine model.
Methods.
The study population consisted of 35 to 40 kg male Yorkshire pigs in an allotransplantation model with a 3-d survival plan for recipients. Control grafts were subjected to cold storage (CS) in a University of Wisconsin solution for 21 to 24 h (n = 6), whereas the test group received an additional 3 h NEVP after CS of 21 h (n = 5).
Results.
The 3-d survival was 60% in the NEVP arm versus 0% in the control arm (P = 0.008; log rank). Graft parenchyma was 60% to 70% preserved in the NEVP arm at necropsy on gross appearance. In addition, the islet function was well preserved, and both the pancreas (including the islets) and the duodenal morphology were maintained histologically. The intravenous glucose tolerance test on the day of euthanasia was in the normoglycemic range for 80% of cases in the NEVP arm.
Conclusions.
Optimization of pancreas grafts exposed to extended CS with NEVP seems promising at rescuing and reanimating these grafts for transplantation, resulting in significantly improved survival in a porcine pancreas transplant model.
Pancreas transplantation has shown promising results in extending and improving the quality of life of patients with diabetes mellitus. However, complications related to ischemia–reperfusion injury (IRI), such as graft pancreatitis and vascular thrombosis, can negatively impact outcomes.1 Despite significant advances, a recent database has shown a utilization rate of only 9% for pancreas grafts.2 The causes for this could be multifactorial, mostly attributed to organ-related factors, including cold ischemia time (CIT), graft steatosis, donor age, and mode of death (cardiac versus brain death), rather than the availability of organs.3 Factors such as organ recovery, packaging, transportation from the retrieval center to the recipient hospital (across cities or provinces), back-table preparation, and recipient characteristics might all contribute to increasing the cold preservation time of the graft.4,5 Studies have shown prolonged CS of the pancreas graft (>12 h) to be associated with graft failure, thereby leading to the inclusion of this factor in the calculation of the pancreas donor risk index.6
The standard CIT for pancreas grafts has been 5 to 7 h at most centers worldwide, including ours.7 However, there has been a shift toward the utilization of extended criteria grafts (ECD), including the grafts subjected to prolonged CS (>12 h) to address the problem of organ shortage.8 In the past 2 decades, ex vivo machine perfusion has emerged as a promising tool for assessing graft viability and mitigating the preservation injury before implantation, especially in lung, kidney, and liver transplantations.9 In the realm of pancreas transplantation, the applicability of ex vivo machine perfusion has mostly been limited to discarded human grafts and porcine models in experimental trials.10,11 Despite the limited evidence in the literature, normothermic ex vivo perfusion (NEVP) has shown promising results in alleviating preservation injury in pancreas transplantation, mostly in donation after brain death models.12,13 One of the earliest evidence on the role of NEVP in pancreas grafts damaged by prolonged CS was established by Barlow et al10 (Cambridge group) in 2015 in discarded human grafts. Based on this, we aimed to establish the feasibility of pancreas transplantation using grafts subjected to prolonged CS (≥21 h) in standard preservation solution and optimize the same using NEVP before implantation of the graft in porcine survival models, with a hypothesis that NEVP could reanimate grafts damaged by prolonged CIT. To our knowledge, this is the first attempt at using NEVP for pancreas grafts damaged by prolonged CIT in an animal allotransplantation survival model in the world.
MATERIALS AND METHODS
Animals and Study Protocol
The study was approved by the Animal Care Committee of the University Health Network Research Institute, Ontario, Canada (Protocol ID: AUP 6245.1). Fifteen-week-old male Yorkshire pigs were used for the transplant model (35–40 kg). All animals received humane care and all procedures were performed in accordance with the “Principles of Laboratory Animal Care” and the “Guide for the Care of Laboratory Animals” published by the National Society for Medical Research and by the National Institutes of Health, respectively. The study was reported in compliance with the ARRIVE guidelines.14
The study protocol is outlined in Figure 1. Briefly, the pancreatoduodenal grafts were procured from the donor pig (minimal warm ischemia/ heart beating donation model) and were stored for ≥21 h in the UW solution at 4 °C in an insulated organ container (day 1). The recipient pig (survival animal) was pancreatectomized (Iatrogenic Diabetes) the following day (day 2) and the retrieved grafts were implanted after 21 to 24 h of either cold storage (CS) alone (in the control arm) or subjected CS plus to 3 h of NEVP followed by implantation (in the test arm). The recipient animals in both groups were followed for up to 3 d (72 h) after transplantation. An intravenous glucose tolerance test (IVGTT) was performed on day 3 on the animals that survived. The animals were subsequently euthanized using established humane protocols. Both the groups (control and test) were performed alternately in sequence by the same surgeon (S.R.) and first assistant (C.P.) throughout the study period.
FIGURE 1.
Summary of the study protocol: heart beating extended CS model. (3-d survival model). CS, cold storage; NEVP, normothermic ex vivo perfusion.
Surgery and Anesthesia Protocol (Donor Operation)
The anesthetic and surgical procedures in the donor operation were performed according to the step-by-step protocol described in detail previously by our group.15 A policy of minimal handling of the native pancreas was followed to ensure a minimally inflamed graft at the end of the operation. After systemic administration of heparin (500 IU/kg bodyweight) through the central venous catheter, blood was drained and collected (usually 800–1000 mL) in bags containing Citrate Phosphate Dextrose Saline Adenine Glucose-Mannitol (CPD/SAG-M), used later as a component in the perfusate used in NEVP. This was followed by cross-clamping of the suprahepatic abdominal aorta and injection of potassium chloride (10 mL) to induce cardiac death (minimum warm ischemia). Immediately after this, cold flushing with 1 L Ringer lactate (primed with 10 000 IU heparin), followed by 1 L of UW solution (primed with 10 000 IU heparin) and venting out of the blood through a lateral opening in the portal vein and infrahepatic inferior vena cava were performed. This was followed by dissection of the graft en masse with the spleen, surrounding fasciae and retroperitoneal tissues, and intact aorta, taking care to preserve the celiac trunk and the superior mesenteric branches. The graft was subsequently flushed with 500 mL of UW solution and stored in an iced organ box at 4 °C for ≥21 h (24 h in the first 2 cases and 21 h in the remaining cases).
Protocol for NEVP
The basic setup of the NEVP circuit is a modification of the neonatal cardiopulmonary bypass and S3 heart-lung machine (see Figure S1, SDC, http://links.lww.com/TXD/A640). The principle of the machine used at our center has been described in detail by Mazilescu et al13 and more recently by Parmentier et al15 from our center. The settings and components used in the current study are detailed in Table 1. Oxygen/carbon dioxide was provided continuously throughout the perfusion at a concentration different from what was described previously by our group (91%/9% versus 95%/5%; 2 L/min described previously). This was based on the hypothesis of augmenting the vascular flow of the graft (vasoconstriction due to prolonged CS) utilizing the anti-inflammatory and vasodilatory effects of higher carbon dioxide concentration and some promising preliminary results from our center.16 Similarly, for dialysate, we used a higher concentration of salt (1.5 versus 1 g/L as previously described) based on lower edema and more physiological perfusion milieu achieved by us in a series of our previous perfusion experiments.15 At the back-table, all arterial branches were tied off, and the spleen was removed. Next, the aorta was cannulated using a 1/4” × 3/8” cannula (in the NEVP group). The portal vein was left open into the organ chamber without cannulation. The distal duodenal end was also cannulated using a Malecot catheter to allow for enteric output measurements during perfusion (in the NEVP group). The graft was flushed with 300 mL of 5% human serum albumin before placing it on the pump and with 500 mL of UW solution after off-pump before implantation (the NEVP group). Graft edema during NEVP (and after graft reperfusion postimplantation) was assessed hourly on a semiquantitative scale from 0 to 3 (0: no edema, 1: mild edema, 2: moderate edema, 3: severe edema) and recorded.
TABLE 1.
Components and settings of the normothermic ex vivo perfusion used in the study (n = 5)
| Perfusate (1×) | Dialysate (per 1 L) | Pressure, flow, and temperature settings |
|---|---|---|
| RL: 200 mL | NaHCO3 (8.4%): 27 mL | Arterial pressure: 20–25 mm Hg in first 30 min, followed by 15–17 mm Hg (rest of 3 h) |
| Steen’s solution: 150 mL | KHCO3 (8.4%): 3 mL | Arterial flow: 90–120 mL/h |
| Leucocyte-depleted red cell concentrate: 125 mL | Commercial hemodialysis concentrate: 22 mL | O2/CO2 conc: 91%/9%; 2 L/min |
| DRO water: 27 mL | NaCl: 1500 mg | Temperature of organ chamber: 37 °C |
| NaHCO3 (8.4%): 8 mL | Sodium pyruvate: 27.5 mg | Temperature of the perfusion reservoir: 36–37 °C |
| Calcium gluconate (10%): 1.8 mL | All of the above are to be mixed in DRO water (solvent) to prepare a final solution of 3 L | |
| Heparin (10 000 IU/10 mL): 1 mL | ||
| Solu-Medrol: 250 mg | ||
| Aprotinin (protease inhibitor): 30 mL (15 mg) | ||
| Epoprostenol infusion: 8 mL/h (0.5 mg dissolved in 250 mL RL) | ||
| Aprotinin infusion: 10 mL/h (5 mg/h) |
DRO, double reverse osmosis; KHCO3, potassium bicarbonate; NaCl, sodium chloride; NaHCO3, sodium bicarbonate; O2/CO2, oxygen/carbon dioxide; RL, Ringer’s lactate.
Surgery and Anesthesia Protocol (Recipient Operation)
The anesthesia and surgery protocol in recipient operation followed the same steps as described by our group previously.17 Broad-spectrum antibiotics (metronidazole 500 mg and cefazolin 1 gm) and proton pump inhibitor (pantoprazole 20 mg IV) were administered at induction. The surgery comprised of 2 parts: pancreatectomy and graft implantation. Surgery started with fascial dissection along the groove between the pancreatic head (duodenal lobe), duodenojejunal flexure, and the transverse colon. The duodenal lobe was mobilized from the underlying main portal vein trunk, and the pancreatic tail (splenic lobe) mobilized from the underlying splenic vein and splenoportal junction. The pancreatic duct was identified, ligated, and divided, and the pancreatectomy was completed by removing the native pancreas en masse. After removing the graft from ice (Control group) or the NEVP machine and flushing with the UW solution (Test group), the recipient inferior vena cava was partially occluded using the side biting Satinsky clamp and venous anastomosis performed with the graft portal vein in an end-side continuous manner. This was followed by arterial anastomosis between the graft aorta (proximal end) and the recipient aorta in an end-side continuous manner using the Parachute technique. After securing an adequate hemostasis with Tranexamic acid and stabilizing hemodynamics by adjusting the inotropic infusion, bowel anastomosis was performed in a side-to-side continuous manner between the graft duodenum and recipient jejunal loop. Abdominal closure was performed in 2 layers (rectus sheath followed by skin) in a continuous manner. The central venous catheter was secured in the neck by creating a subcutaneous tunnel as described elsewhere by our group.17 The animal was transferred to the housing facility, extubated, and monitored for the next 72 h (3 d). Postoperatively, the animals received cefazolin 1 g IV twice per day and methylprednisolone 500 mg IV per day till sacrifice, besides the appropriate analgesics and intravenous fluids. Oral cyclosporine 300 mg was attempted to be given twice per day but was not tolerated by the animals in most cases.
Sample Collection
For the test arm, blood gas analyses of the perfusate were performed hourly during NEVP. A part of each blood sample retrieved from the donor and recipient at different time points of the experiment was centrifuged, and the supernatant was stored at –80 °C for later analysis. For amylase, lipase, lactate dehydrogenase (LDH), and creatinine phosphokinase (CPK) measurements, samples were sent to the Toronto General Hospital Core Laboratory for analysis with the Abbott Architect Chemistry Analyzer using the manufacturer’s reagents. Coagulation profile (prothrombin time, international normalized ratio, and D-dimer) was performed for all recipients at 3 h after reperfusion and daily till the day of euthanasia. An IVGTT was performed 72 h after transplantation (or at euthanasia, whichever is earlier) by administrating 50 mL of dextrose 50% (Baxter Corporation, Mississauga, ON, Canada) to the recipient animals. Glucose levels were monitored for 2 h and samples were taken at multiple time points (0–120 min). For measurement of C-peptide, ELISA kits (R&D Systems, Toronto, ON, Canada, and Mercodia, Winston Salem, NC) were used according to manufacturer’s instructions.
Histology
Biopsies were taken from all grafts in both groups before implantation (sham: after 21 h CS for the control group and after 3 h of NEVP for the NEVP group) and 1 h after reperfusion. At sacrifice, 4 biopsies were taken from the pancreatoduodenal graft, which included 1 from each region in the pancreas and 1 from the duodenum; the graft was split into 4 regions as described by Taylor et al.18 All samples were placed in 10% neutral buffered formalin and transferred to 70% alcohol after 36 to 48 h. Following paraffin embedding and sectioning (3 μm), hematoxylin and eosin (H&E)-stained sections were used to score fat and parenchyma necrosis as well as islet cell integrity on a semiquantitative scale from 0 to 3 (0: no changes, 1: mild changes, 2: moderate changes, 3: severe changes) by a pathologist blinded to the experimental groups. Additionally, duct inflammation and the integrity of islet cells were assessed (per 40 high-power fields). Terminal deoxynucleotidyl transferase deoxynucleotide triphosphate nick end labeling and Caspase staining were also performed additionally and graded (<30%, 30%–60%, and >60%). Also, sections of the tissues were stored separately in RNA later solution at 4 °C and snap frozen in liquid nitrogen and stored at –80 °C.
Statistical Analysis
The continuous variables were expressed as mean ± SD (range) and categorical variables were expressed as percentages. A mixed effect analysis using 2-way ANOVA was performed for the continuous variables across different time points. Kaplan-Meier survival curves were plotted for both groups and compared using the log-rank test. A P value of <0.05 was set as statistically significant. All analyses were done using GraphPad Prism (version 9.5.1) software.
RESULTS
Animal and Graft Characteristics
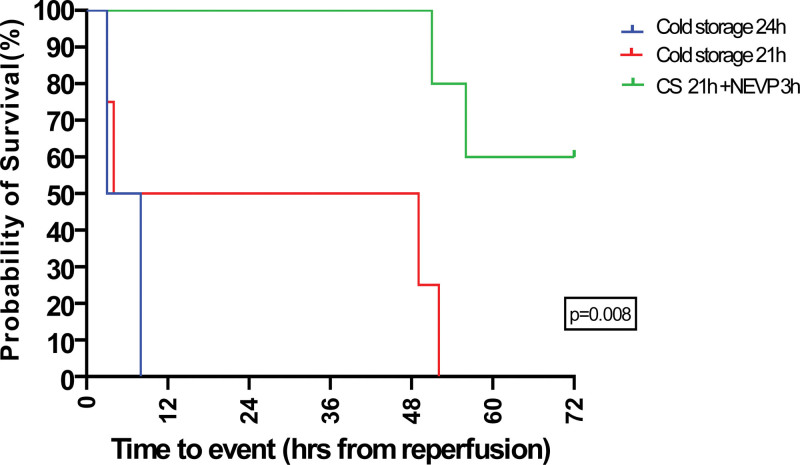
There was a total of 11 experiments in the entire cohort. There were 6 in the control arm (n = 2: 24 h CS alone and n = 4: 21 h CS alone) and 5 in the NEVP arm (21 h CS followed by 3 h of NEVP). The first 2 experiments (24 h CS alone) were performed on a pilot trial basis and the CS duration was decreased to 21 h subsequently for the next 4 in the control arm. Figure 2 shows the comparison of 3-d survival among the 2 groups. The 3-d survival was 60% (n = 3) in the NEVP group versus 0% in the control group (P = 0.008). None of the animals survived beyond 3 h of reperfusion in the 24 h CS control group (n = 2); both presenting a disseminated intravascular coagulopathy like clinical picture, diffuse bleeding, and hemorrhagic shock. D-dimer levels (as a marker for disseminated intravascular coagulopathy) were assessed for the test and control groups, illustrated in Figure S2 (SDC, http://links.lww.com/TXD/A640. The mean graft weight at retrieval was 180.6 ± 17.47 g, which increased to 276.8 ± 49.91 g after 3 h of NEVP, with an average increase of 57.56% ± 26.75%. Figure 3 shows the comparison of graft appearance after 60 min of reperfusion in the NEVP and control groups. Patchy subcapsular hemorrhages and grade 2 and 3 edema were seen in all grafts in the NEVP group, compared with grade 1 and 2 edema and a more congested dusky appearance of the duodenum in the control group.
FIGURE 2.
Comparison of survival of the animals among the controls (n = 2 + 4): CS 24 h (n = 2) and CS 21 h (n = 4) and test: CS 21 h + NEVP 3 h (n = 5); target survival: 72 h; log-rank test (P < 0.05 significant). CS, cold storage; NEVP, normothermic ex vivo perfusion.
FIGURE 3.
Graft appearance 60 min after implantation/in vivo reperfusion in recipient animal compared between control and test/NEVP groups: white arrowhead pointing at the duodenum after reperfusion in the control group compared with black arrowhead pointing at the duodenum after reperfusion in the NEVP group. NEVP, normothermic ex vivo perfusion.
NEVP Parameters (Hemodynamic and Biochemical)
The trend of arterial flow and the intravascular resistance ([arterial pressure × 100/arterial pressure] per 100 g of tissue weight) >3 h of NEVP for the test group was studied (Figure S3, SDC, http://links.lww.com/TXD/A640). The mean arterial pressure at baseline was 17 ± 2.1 mm Hg (mean arterial flow of 118 ± 9.5 mL/min) and was 15.4 ± 1.2 mm Hg (mean arterial flow of 122 ± 8.4 mL/min) at the end of 3 h. The trend of partial pressure of oxygen (arterial and venous) and mean lactate levels in the perfusate were assessed >3 h of NEVP and revealed an excellent oxygen extraction ratio (Figure S3, SDC, http://links.lww.com/TXD/A640).
Posttransplantation Graft Function Assessment
There was no significant difference in mean blood lactate and glucose levels at any time point compared between the 2 groups (P = 0.32 and 0.16, respectively; Figure 4A). The mean amylase levels were 10 912 ± 4172.8 U/L on postoperative day (POD) 1 in the NEVP group compared with 10 678 ± 7182.2 U/L in the control group, which decreased to 4757.3 ± 3022.8 U/L on POD 3 for the former (P = 0.56; Figure 4B). The serum CPK and LDH levels were also compared between the 2 groups (Figure S4, SDC, http://links.lww.com/TXD/A640). The endocrine function of the graft was assessed by the intravenous glucose challenge test (IVGTT) and C-peptide levels for the NEVP group (Figures 5). The glucose levels peaked to a mean of 15.16 ± 5.36 mmol/L at 10 min and dropped to 5.76 ± 6.3 mmol/L (normoglycemic) at 120 min in the NEVP group (Figure 5A). None of the controls survived till 72 h for an accurate comparison of the IVGTT trend with the NEVP group. However, in the 2 cases that survived till 48 to 50 h, an IVGTT performed before the euthanasia revealed a severely hypoglycemic trend. The corresponding mean C-peptide levels at different time points of IVGTT and on POD 1 for the NEVP group are shown in Figure 5B.
FIGURE 4.
Comparison of the trend of serum lactate and glucose levels (A) and serum amylase and lipase levels (B) across different time points in the perioperative period, between control (n = 2 + 4) and NEVP/test (n = 5) groups; amylase test vs control: P = 0.56; lipase test vs control: P = 0.65. CS, cold storage; NEVP, normothermic ex vivo perfusion; POD, postoperative day; Prep, postreperfusion.
FIGURE 5.
Endocrine function of the graft. A, Intravenous glucose challenge test/IVGTT at different time points >120 min. Note that the normoglycemic range was maintained at 80% (n = 4) of the test group (CS 21 h + NEVP 3 h). B, Mean C-peptide levels in the test group (CS 21 h + NEVP 3 h) at different select corresponding time points of IVGTT and postoperative d 1. BL, baseline; CS, cold storage; GTT, glucose tolerance test; IVGTT, intravenous glucose tolerance test; NEVP, normothermic ex vivo perfusion; POD, postoperative day.
Graft Morphology at Necropsy
A comparison of the gross morphology of the graft at necropsy is shown in Figure S5 (SDC, http://links.lww.com/TXD/A640. The duodenum was preserved with good vascular integrity in the NEVP group, while it showed complete transmural gangrene with impending perforations in the control group. There was preservation of 60% to 70% of the pancreatic parenchyma in 4 cases of the NEVP group and in only 1 case of the control group. Table 2 shows a comparison of the histological grading of the parenchyma, duct, and islets between 2 cases in both groups H&E staining of the sections revealed a marked transmural necrosis with ischemic changes in the duodenum in both the controls, with relatively well-preserved architecture of parenchyma and duodenum in the NEVP groups, with no evidence of clots or microthrombi in the parenchyma (Figure 6).
TABLE 2.
Histological assessment of the tissues among the biological replicates in 2 closely comparable cases (selected, based on the comparability of perioperative physiological parameters and trend of biochemical markers between the cases in both groups), in control (n = 2) and NEVP (n = 2) groups by H&E and TUNEL staining (grading: 0: none, 1: mild, 2: moderate, 3: severe; for ducts: 0: absent and 1: present)
| H&E | TUNEL | ||||||
|---|---|---|---|---|---|---|---|
| Group | Fat necrosis | Parenchyma | Hemorrhage | Duct integrity | Autolysis | Islets (× 4×) | Staining percent |
| Test 1 (Prep 60 min) | 0 | 1 | 0 | 1 | 1 | 1 | NA |
| Control 1 (Prep 60 min) | 1 | 2–3 | 1 | 1 | 1 | 2 | NA |
| Test 2 (Prep 60 min) | 1–2 | 3 | 1 | 0 | 1 | 1 | NA |
| Control 2 (Prep 60 min) | 1 | 1 | 0 | 1 | 1 | 3 | NA |
| Test 1 (Corpus nec) | 1 | 1 | 0 | 0 | 0 | 2 | 30%–60% |
| Control 1 (Corpus nec) | 1–2 | 3 | 2–3 | 0 | 2 | 0 | >60% |
| Test 2 (Corpus nec) | 1 | 1–2 | 1 | 1 | 0 | 2 | 30%–60% |
| Control 2 (Corpus nec) | 1–2 | 2–3 | 2 | 1 | 1 | 1 | 30%–60% |
| Test 1 (Duod nec) | NA | 1 | 1 | NA | 1 | NA | NA |
| Control 1 (Duod nec) | NA | 3 | 3 | NA | 3 | NA | NA |
| Test 2 (Duod nec) | NA | 0–1 | 1 | NA | 0 | NA | NA |
| Control 2 (Duod nec) | NA | 3 | 3 | NA | 3 | NA | NA |
Duod, duodenum; H&E, hematoxylin and eosin; Islet number, per high-power field; NA, not applicable; NEVP, normothermic ex vivo perfusion; Prep, postreperfusion; nec, necropsy; TUNEL, terminal deoxynucleotidyl transferase deoxynucleotide triphosphate nick end labeling.
FIGURE 6.
Histological comparison of the pancreas parenchyma (corpus) in H&E (Control group in the bottom row and NEVP group on the top row). A, Marked parenchymal inflammatory infiltration with autolysis in the control group vs relatively preserved architecture in the NEVP group; duodenal section in NEVP and control groups on H&E. B, Marked distortion of the glands and crypts in the control group vs well-preserved crypt and glandular architecture in the NEVP group; and TUNEL staining of pancreas parenchyma. C, Yellow areas denote TUNEL staining, with nearly 30%–60% in both NEVP and control groups. H&E, hematoxylin and eosin; NEVP, normothermic ex vivo perfusion; TUNEL, terminal deoxynucleotidyl transferase deoxynucleotide triphosphate nick end labeling.
DISCUSSION
This study demonstrates the feasibility of pancreas transplantation of grafts subjected to prolonged CIT (defined as >21 h) in porcine 3-d survival models using NEVP. Duodenal gangrene with sepsis was identified as the most common cause of mortality, followed by endocrine failure of the graft, leading to severe hypoglycemia and failure to thrive in the control arm. However, adding 3 h of NEVP after CS augmented these grafts, resulting in duodenal vascular integrity and better endocrine function, with superior overall survival.
Prolonged CIT has been identified as a known risk factor for graft failure after pancreas transplantation. Humar et al19 demonstrated a significant correlation of duodenal leak rates with prolonged CIT, with a 5.8% leak rate associated with a CIT of 15 to 20 h, increasing to 14.1% between 20 and 25 h and 16.7% with a CIT of >25 h. A subsequent analysis by Rudolph et al20 reported a clear trend of lower graft survival with ≥12 h of CIT in pancreas transplantation. Therefore, these grafts fall under the category of “extended criteria” or “marginal donor grafts” in pancreas transplantation, limiting their utilization. Despite the known detrimental effects of cold ischemia beyond 20 h, we chose to start with a 21 to 24 h timeline with the idea of pushing the limits for the feasibility of NEVP in reviving such presumably damaged grafts for transplantation, which could be motivated to expand the limits of NEVP in “extended criteria grafts.”
Besides slowing down cellular metabolism, cold ischemia exerts its detrimental effects by inhibiting ATP generation, leading to the accumulation of toxic metabolites, which on reperfusion, lead to the generation of reactive oxygen species, causing downstream inflammatory cascade in the recipient and systemic inflammatory response syndrome.21 A recent study by Prudhomme et al22 established the feasibility of pancreas allotransplantation in a porcine survival model using grafts (Heart beating donors) subjected to a CIT of 2 and 6 h. The authors compared the outcome between the grafts subjected to CS alone and the ones receiving hypothermic oxygenated machine perfusion for the same duration. They reported noninferiority of the latter compared with CS in terms of survival and endocrine and exocrine graft function. Another experimental model by Ogbemudia et al23 in 2021 compared the outcome of hypothermic oxygenated machine perfusion versus CS for 6 h by subjecting the grafts to 1 h of normothermic regional perfusion for reperfusion after ischemia. They reported better perfusion characteristics, decreased macroscopic edema, and normal macroscopic appearance compared with CS during reperfusion, suggesting a role of ex situ machine perfusion in alleviating tissue injuries associated with cold ischemia. Conversely, our study group has been centered on using normothermic perfusion (NEVP) in the porcine pancreas allotransplantation models. A recent study by Mazilescu et al13 from our center in 2022 established the feasibility of NEVP in pancreatectomized porcine diabetic model. The authors demonstrated excellent graft function, with preserved islet integrity and physiologic and endocrine function after pancreas transplantation using grafts subjected to 3 h of NEVP.
In the present study, the addition of 3 h of NEVP was found to “salvage” the pancreas graft damaged by prolonged cold ischemia injury. Subjecting to a physiologic temperature (36–37 °C) “conditioned” these grafts to reperfusion injury. Prolonged CIT causes vasoconstriction of the capillaries and vascular arcades. Perfusing these grafts at a lower arterial pressure (15–20 mm Hg on NEVP) before implantation could aid in reestablishing the graft microcirculation, especially around the pancreatoduodenal groove. This correlated with the macroscopic appearance of the graft duodenum, which was congested and dusky 60 min after graft implantation in the CS alone group (evolving into transmural gangrene by the day of euthanasia) and hyperemic and well perfused in the NEVP arm.
Besides the risks of bowel leaks and graft failure in solid organ transplantation, cold ischemia is also detrimental to islet function, as evident by the blunted insulin secretory response on the glucose challenge test.24 A noteworthy finding of the present study was the difference in endocrine function between the 2 groups. IVGTT revealed a normoglycemic response (physiologic range) for 80% of the animals in the test groups, whereas it was severely hypoglycemic in the control group. However, histologic evaluation of the graft parenchyma (H&E) revealed preserved islet numbers in both test and control groups. This further substantiated the hypothesis that cold ischemia probably stuns the islet function without grossly affecting the numbers in most cases. This functional “stunning” of the islets was salvaged by NEVP, thereby restoring the endocrine function of these grafts damaged by prolonged CIT.
In the experimental models of pancreas transplantation, not much has been studied about the feasibility and outcome of using grafts subjected to prolonged CIT using the standard preservative solution(s). One of the earliest reports of preserving the integrity of such grafts came from Japan in 1988 by Kuroda et al,25 who introduced the “2-layer method” of preserving the graft pancreas in an interface between Euro-Collins solution (later replaced by UW) and perfluorocarbons, with a sustained supply of oxygen to the graft during the period of cold ischemia.26 Although it showed some promising results in islet isolation from such grafts, the results could not be extrapolated in solid organ transplantation in any of the experimental models so far.
Our study had a few limitations. The technique is challenging to replicate, considering the sensitivity of the already damaged graft related to handling, temperature fluctuations, and pressure changes while on the machine (NEVP). The markers of tissue injury (amylase, lipase, LDH, and CPK) were found to be elevated in the serum at 3 h after reperfusion of the implanted graft. However, the levels mostly stabilized during the next 3 d of recovery of the animal, suggesting a “resolving acute pancreatitis” like picture. This could be attributed to the sensitivity of the organ to the factors mentioned previously, therefore limiting the reliability and clinical replicability of this model currently. The inability to provide adequate immunosuppression in these animals postoperatively (intolerability to oral cyclosporine) could also be an attributable factor to pancreatitis-like picture after transplantation, constituting a limitation of the study protocol. Although we assessed the integrity of the graft parenchyma, pancreatic duct, and acini immediately postreperfusion (after 60 min) and also at necropsy (72 h postreperfusion) on an objective scale (Table 2), there was no significant difference noted in terms of tissue damage at the corresponding time points to appreciate the effect of a presumed acute rejection (due to lack of immunosuppression) over an IRI. Identifying these subtle variations at different time points postreperfusion would possibly be more appreciated at the molecular level, something that we are currently working on. Also, we are currently underway on assessing the feasibility of perfusing the pancreas grafts on an NEVP machine for a longer period (10–12 h), with an idea to further optimize the graft functional outcome and hope to come up with definitive evidence of the advantage of the same in future, besides exploring the possibility of gene therapy using viral vectors in NEVP (future direction). Besides this, more objective evidence of tissue oxygenation, ATP production, and tissue injury markers need to be analyzed ahead, which form a part of our future direction in subsequent phases of this novel experimental model.27,28 Identifying biomolecular targets of alleviating IRI in such grafts could pave the path for more physiological ex perfusion in future and is something that we also aim to analyze in the near future. At this point, not much is known, and not much has been substantiated with evidence regarding the most appropriate way to assess organ viability during NEVP. The focus of NEVP is to optimize the graft for an adequate function after implantation and minimize reperfusion injury. Currently, there is no standardized method to assess graft viability on NEVP, given the fact that all pancreases on NEVP secrete C peptide and therefore, are functionally active. However, minimizing injury to the graft during the entire process of graft retrieval to implantation and also, while on NEVP, with an idea to minimize graft pancreatitis is the only way to ensure a good transplantation outcome using these vulnerable grafts.
Finally, the study also attempted to examine the extremes of cold ischemia in grafts, which might not be clinically relevant for many transplant programs. This time was chosen to try demonstrating the maximal difference between the 2 groups and for logistical reasons. However, we feel this could be extrapolated to grafts with less CIT but is beyond what would be considered clinically acceptable. Even after a short CIT, there appears to be some benefit with NEVP compared with grafts left in CS (article in preparation).
To conclude, the present study is a pilot attempt at salvaging and reanimating pancreatic grafts damaged by prolonged cold ischemia using NEVP in an allotransplantation survival model. The current study is also the first of its kind in the world and North America to have shown a demonstrable benefit of reviving the pancreatic grafts damaged by prolonged cold ischemic injury using NEVP, thereby deeming them suitable to be used for solid organ transplantation. This is with the hope of extending the clinical applicability of ex vivo perfusion for pancreas transplantation, where the threshold for rejection of grafts remains much higher compared with other solid organ transplantation, thereby augmenting utilization of the “extended criteria” grafts in the future, significantly increasing the pancreatic donor pool.
Supplementary Material
Footnotes
S.R. participated in acquisition of data, research design, analysis of data, drafting the article, critical analysis and revision, and final approval of the article. C.P., M.K., S.G., and M.S. participated in research design, drafting the article, critical revision, final approval of the article. E.N., F.C.N., C.H., and T.P.C. participated in drafting the article, critical revision, and final approval of the article. S.N.K. participated in analytic tools, drafting the article, critical revision, final approval of the article. T.W.R. is a chief supervisor of the article and participated in primary conceptualization, drafting the article, critical revision, and final approval of the article.
The authors declare no conflicts of interest.
S.R. and T.W.R. were funded by Ajmera Transplant Centre Fellowship award (March 2022–2023), Ajmera Transplant Centre, Toronto General Hospital, Toronto, ON, Canada. T.W.R. was funded by the Government of Canada’s New Frontiers in Research Fund.
The detailed animal study-related data may be available on request to the corresponding author (T.W.R.).
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantationdirect.com).
Contributor Information
Samrat Ray, Email: samrat.ray@uhn.ca.
Catherine Parmentier, Email: catherine.parmentier@uhn.ca.
Masataka Kawamura, Email: kawamukawamu@gmail.com.
Sujani Ganesh, Email: sujani.ganesh@uhn.ca.
Emmanuel Nogueira, Email: emmanuelnog@gmail.com.
Francisco Calderon Novoa, Email: Francisco.CalderonNovoa@uhn.ca.
Christian Hobeika, Email: Christian.Hobeika@uhn.ca.
Markus Selzner, Email: markus.selzner@uhn.ca.
REFERENCES
- 1.Jenssen T, Hartmann A, Birkeland KI. Long-term diabetes complications after pancreas transplantation. Curr Opin Organ Transplant. 2017;22:382–388. [DOI] [PubMed] [Google Scholar]
- 2.Canadian Institute for Health Information. Annual Statistics on Organ Replacement in Canada: Dialysis, Transplantation and Donation, 2009 to 2018. CIHI; 2019. [Google Scholar]
- 3.Cornateanu SM, O’Neill S, Dholakia S, et al. Pancreas utilization rates in the UK - an 11-year analysis. Transpl Int. 2021;34:1306–1318. [DOI] [PubMed] [Google Scholar]
- 4.Mei S, Huang Z, Dong Y, et al. Pancreas preservation time as a predictor of prolonged hospital stay after pancreas transplantation. J Int Med Res. 2021;49:300060520987059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finger EB, Radosevich DM, Bland BJ, et al. Comparison of recipient outcomes following transplant from local versus imported pancreas donors. Am J Transplant. 2012;12:447–457. [DOI] [PubMed] [Google Scholar]
- 6.Axelrod DA, Sung RS, Meyer KH, et al. Systematic evaluation of pancreas allograft quality, outcomes and geographic variation in utilization. Am J Transplant. 2010;10:837–845. [DOI] [PubMed] [Google Scholar]
- 7.Spetzler VN, Goldaracena N, Marquez MA, et al. Duodenal leaks after pancreas transplantation with enteric drainage—characteristics and risk factors. Transpl Int. 2015;28:720–728. [DOI] [PubMed] [Google Scholar]
- 8.Gruessner RW, Gruessner AC. The current state of pancreas transplantation. Nat Rev Endocrinol. 2013;9:555–562. [DOI] [PubMed] [Google Scholar]
- 9.Kaths JM, Echeverri J, Chun YM, et al. Continuous normothermic ex vivo kidney perfusion improves graft function in donation after circulatory death pig kidney transplantation. Transplantation. 2017;101:754–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barlow AD, Hamed MO, Mallon DH, et al. Use of ex vivo normothermic perfusion for quality assessment of discarded human donor pancreases. Am J Transplant. 2015;15:2475–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamaoui K, Gowers S, Sandhu B, et al. Development of pancreatic machine perfusion: translational steps from porcine to human models. J Surg Res. 2018;223:263–274. [DOI] [PubMed] [Google Scholar]
- 12.Kuan KG, Wee MN, Chung WY, et al. A study of normothermic hemoperfusion of the porcine pancreas and kidney. Artif Organs. 2017;41:490–495. [DOI] [PubMed] [Google Scholar]
- 13.Mazilescu LI, Parmentier C, Kalimuthu SN, et al. Normothermic ex situ pancreas perfusion for the preservation of porcine pancreas grafts. Am J Transplant. 2022;22:1339–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Percie du Sert N, Ahluwalia A, Alam S, et al. Reporting animal research: explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 2020;18:e3000411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parmentier C, Ray S, Mazilescu L, et al. Normothermic ex vivo pancreas perfusion for the preservation of pancreas allografts before transplantation. J Vis Exp. 2022;27:185. [DOI] [PubMed] [Google Scholar]
- 16.Ray S, Parmentier C, Kawamura M, et al. 234.3: establishing the most physiological perfusion milieu for normothermic ex vivo pancreas perfusion in porcine models: how far have we reached? Transplantation. 2022;106:S94–S95. [Google Scholar]
- 17.Ray S, Parmentier C, Mazilescu L, et al. Surgical tips and tricks for performing porcine pancreas transplantation. J Vis Exp. 2022;20:185. [DOI] [PubMed] [Google Scholar]
- 18.Taylor M, Baicu S. Hypothermic perfusion of pancreas: emphasis on preservation prior to islet isolation. In: Uygun K, Lee CY, eds. Organ Preservation and Reengineering. Artech House Publisher; 2011:85–104. [Google Scholar]
- 19.Humar A, Ramcharan T, Kandaswamy R, et al. Technical failures after pancreas transplants: why grafts fail and the risk factors—a multivariate analysis. Transplantation. 2004;78:1188–1192. [DOI] [PubMed] [Google Scholar]
- 20.Rudolph EN, Dunn TB, Sutherland DER, et al. Optimizing outcomes in pancreas transplantation: impact of organ preservation time. Clin Transplant. 2017;31:e13035. [DOI] [PubMed] [Google Scholar]
- 21.Wu MY, Yiang GT, Liao WT, et al. Current mechanistic concepts in ischemia and reperfusion injury. Cell Physiol Biochem. 2018;46:1650–1667. [DOI] [PubMed] [Google Scholar]
- 22.Prudhomme T, Kervella D, Ogbemudia AE, et al. Successful pancreas allotransplantations after hypothermic machine perfusion in a novel diabetic porcine model: a controlled study. Transpl Int. 2021;34:353–364. [DOI] [PubMed] [Google Scholar]
- 23.Ogbemudia AE, Hakim G, Dengu F, et al. Development of ex situ normothermic reperfusion as an innovative method to assess pancreases after preservation. Transpl Int. 2021;34:1630–1642. [DOI] [PubMed] [Google Scholar]
- 24.Ryan EA, Lakey JR, Rajotte RV, et al. Clinical outcomes and insulin secretion after islet transplantation with the Edmonton protocol. Diabetes. 2001;50:710–719. [DOI] [PubMed] [Google Scholar]
- 25.Kuroda Y, Kawamura T, Suzuki Y, et al. A new, simple method for cold storage of the pancreas using perfluorochemical. Transplantation. 1988;46:457–460. [DOI] [PubMed] [Google Scholar]
- 26.Agrawal A, Gurusamy K, Powis S, et al. A meta-analysis of the impact of the two-layer method of preservation on human pancreatic islet transplantation. Cell Transplant. 2008;17:1315–1322. [DOI] [PubMed] [Google Scholar]
- 27.Martin JL, Costa ASH, Gruszczyk AV, et al. Succinate accumulation drives ischaemia-reperfusion injury during organ transplantation. Nat Metab. 2019;1:966–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prudhomme T, Mulvey JF, Young LAJ, et al. Ischemia-reperfusion injuries assessment during pancreas preservation. Int J Mol Sci. 2021;22:5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.