Abstract
In the aging process, physiological decline occurs, posing a substantial threat to the physical and mental well-being of the elderly and contributing to the onset of age-related diseases. While traditional perspectives considered the maintenance of life as influenced by a myriad of factors, including environmental, genetic, epigenetic, and lifestyle elements such as exercise and diet, the pivotal role of symbiotic microorganisms had been understated. Presently, it is acknowledged that the intestinal microbiota plays a profound role in overall health by signaling to both the central and peripheral nervous systems, as well as other distant organs. Disruption in this bidirectional communication between bacteria and the host results in dysbiosis, fostering the development of various diseases, including neurological disorders, cardiovascular diseases, and cancer. This review aims to delve into the intricate biological mechanisms underpinning dysbiosis associated with aging and the clinical ramifications of such dysregulation. Furthermore, we aspire to explore bioactive compounds endowed with functional properties capable of modulating and restoring balance in this aging-related dysbiotic process through epigenetics alterations.
Keywords: aging, dysbiosis, intestinal microbiota, polyphenols, epigenetics
1. Introduction
Aging is characterized as a gradual and continuous process occurring throughout an organism’s lifespan, resulting in physiological alterations. It encompasses a complex interplay of social, cultural, biological, and psychological factors. The global demographic of the elderly is shaped by health conditions, extending beyond mere disease absence to encompass indices of independence and functionality, providing a comprehensive perspective on well-being [1]. The aging process is influenced by a complex interplay of genetic and environmental factors. Over time, these factors contribute to the gradual functional deterioration of cells, tissues, and organs, leading to a decline in physiological integrity. This can potentially increase vulnerability to various pathologies, making aging a significant factor in overall mortality risk [2,3]. The aging process involves characteristics based on three premises: the first involves its manifestation associated with age, the second is characterized by the acceleration of aging, which can be accentuated experimentally, and the third involves the opportunity to decelerate or reverse aging through therapeutic interventions. In this sense, López-Otín and colleagues proposed an expansion related to aging markers, suggesting twelve characteristics of aging that include genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, deficient macroautophagy, dysregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, altered intercellular communication, chronic inflammation, and dysbiosis [4].
In the aging process, a progressive decline in physiological function poses a significant threat to the physical and mental well-being of the elderly population, contributing significantly to the onset of age-related diseases. Preventing such maladies, enhancing life quality, and preserving elderly health have become focal points in various research endeavors. Historically, the preservation of life was conventionally attributed to a complex interplay of environmental, genetic, epigenetic factors, and lifestyle components, including physical activity and dietary habits. However, the substantive role played by symbiotic microorganisms in this context was previously underappreciated [5,6].
The balance between commensal and pathogenic bacterial populations is crucial for the integrity of the intestinal microbiome, playing a vital role in maintaining host health and homeostasis. Microbial residents in the human intestine actively contribute to the production and synthesis of essential compounds, such as vitamins, amino acids, short-chain fatty acids, and various metabolites through intricate enzymatic activities and metabolic pathways. A significant portion of these metabolites form the necessary foundation for the activities of epigenetic enzymes. These biochemical processes are integral to functions like food digestion, xenobiotic metabolism, and the composition of bioactive molecules. Disruption of this delicate balance, termed microbial dysbiosis, is characterized by a diminished diversity of bacterial species within the intestinal microflora and a decline in beneficial bacteria. Such dysbiosis can compromise intestinal permeability, leading to an altered microbial landscape that may contribute to increased permeability. Consequently, this disruption adversely affects nutrient absorption, food metabolization, and immune system modulation. The resulting perturbations in the intestinal microflora have the potential to precipitate complications and influence the development of various pathological conditions [7,8].
This comprehensive review aims to explore the intricate mechanisms influencing the composition of the intestinal microbiota throughout the aging process. Furthermore, it will investigate the potential of polyphenols as a strategic tool to foster the preservation of a robust and balanced intestinal microbiota.
2. Microbiota Dynamics in the Aging Process
The intestinal microbiota comprises microorganisms inhabiting the human intestine and undergoes changes with age. From birth, the intestinal microbiota aids in the maturation of the immune system and contributes to homeostasis. Studies in both humans and animal models suggest microbial alterations in the aging intestine, even under healthy conditions [9,10,11]. The population of bacterial phyla, comprising over 90% of the intestinal microbiota, is principally composed of Bacteroidetes and Firmicutes. The remaining phyla, present in lesser abundance, comprise diverse species, some of which may contribute metabolites and essential functions to facilitate healthy aging [12].
The microbiota composition varies within individuals across different segments of the gastrointestinal tract, particularly between the cecum and the rectum. Additionally, there is a noticeable gradient in diversity and bacterial load from the oropharynx to the jejunum, with a prevalence of Proteobacteria and Firmicutes. Conversely, an increase in bacterial load is observed from the ileum to the colon, characterized by a predominance of Firmicutes, Bacteroidetes, and Proteobacteria. Reports suggest that the microbiota may exhibit greater interindividual variability in bacterial composition, and variations between segments within an individual can lead to differences in the metabolism of dietary molecules and medications. These variations contribute to an increased susceptibility to various diseases [13,14,15,16,17].
Throughout life, changes in the diversity of the intestinal microbiota occur. Naturally, in early life, the intestinal microbiota is well established, but subtle variations persist until around the age of 40. A certain stability is achieved during this period, but it can be lost over the aging process due to the aging of prokaryotic and eukaryotic symbionts, resulting in a dysbiosis state [18,19,20,21].
After the age of 70, noticeable alterations in the composition of the intestinal microbiota occur as a consequence of the cumulative impact of various stressors, the physiological aging processes within the gastrointestinal system, and shifts in lifestyle and dietary habits. These changes typically manifest as a reduction in microbial biodiversity, accompanied by an increase in opportunistic Gram-negative bacteria and a decrease in species purportedly associated with health-promoting functions [9,22,23].
Alongside changes in microbiota diversity, aging also results in a reduction in the microbiota’s metabolic capacity, lower levels of short-chain fatty acids, and potential connections with age-related conditions. These may include disruptions in intestinal transit, weight loss, reduced appetite, cognitive decline, frailty, vitamin D deficiency, hypertension, diabetes, arthritis, sarcopenia, and more [24,25,26]. Moreover, during the aging process, there is a decline in the function of the intestinal barrier modulated by intestinal microbiota, leading to a decrease in tight junction proteins and mucins production. Research has indicated that the presence of A. muciniphila is diminished in individuals with age-associated disorders compared to their healthy counterparts of the same age, underscoring the potential benefits of supplementing with A. muciniphila to enhance the thickness of colonic mucus. Age-related intestinal disorders have been observed to reduce the thickness of colonic mucus [27,28]. In addition, a probiotic combination featuring strains of Lactobacillus and Enterococcus isolated from healthy infants, when administered to elderly mice with dysbiosis, facilitated a reduction in intestinal permeability. This effect was achieved through an increase in bile salt hydrolase activity, inducing a taurine-dependent expression of tight junction proteins (Zo-1 and occludin). These findings suggest that microbiota-based therapies hold promise for potentially reversing the age-related deterioration in intestinal barrier function [29,30,31].
Intestinal commensal bacteria exert a substantial influence on individual nutrition, metabolism, and the production of microbiota-derived metabolites. Notably, short-chain fatty acids are promptly absorbed into the plasma through the individual’s intestinal epithelium. Emerging studies propose that interventions based on microbiota-derived metabolites may positively impact healthy aging, as these metabolites demonstrate the potential to influence human longevity [32,33,34,35,36]. Noteworthy findings indicate that the suppression of folate metabolism entails anti-aging effects in individuals. Intriguingly, this effect appears to be indirectly mediated by bacterial influence, underscoring the pivotal role of the microbiota and microbial metabolites in human health. This underscores the importance of exploring strategies aimed at extending longevity, particularly given that elderly individuals often exhibit elevated serum levels of inflammatory cytokines (IL-6) and c-reactive protein (CRP) [37,38,39,40,41,42].
Human studies reveal a decline in the population of microorganisms within the aging intestinal microbiota, characterized by reduced Firmicutes and Bifidobacteria. Concurrently, there is an elevation in Bacteroidetes and pro-inflammatory Enterobacteriaceae. Functionally, the microbiota of elderly individuals diverges from that of their younger counterparts; notably, young individuals exhibit a higher production of short-chain fatty acids compared to the elderly [43,44,45,46], which in turns helps the immune system.
The aging process is intricately connected to changes in the immune system, known as immunosenescence, and inflammation. Immunosenescence involves a decline in immune functions, affecting the ability to combat infections and respond to new antigens. This condition also contributes to increased autoimmunity and inefficient wound healing. Simultaneously, inflammation often results from immunosenescence, marked by reduced serum levels of anti-inflammatory cytokines like IL-10, along with a decrease in pro-inflammatory cytokines such as IL-1β, IL-6, IL-8, and TNF-α. These immunological and inflammatory alterations play crucial roles in shaping the aging immune system and its responses [47].
The aging process induces alterations in the intestinal microbiota, disrupting innate immunity balance. This is linked to an increased proliferation and redistribution of myeloid cells, showcasing a significant elevation in peripheral-origin antigen-presenting cells (APCs) in the brain. The accumulation of myeloid cells associated with aging may positively correlate with changes in the intestinal microbiota, including a reduction in Akkermansia and behavioral deficits [48]. As aging advances, the functions of chemotaxis, phagocytosis, and the production of reactive oxygen species (ROS) may suffer impairment. Nonetheless, the proportions of circulating neutrophils do not appear to be affected. Animal models demonstrate improved survival in endotoxin-induced septic shock. The microbiota influences neutrophil aging through Toll-like receptor (TLR) and MyD88 signaling. In animal models using germ-free (GF) mice or those treated with antibiotics, microbiota depletion reduced the number of circulating aged neutrophils, mediated by decreased CXCR4 and increased CD68 [49].
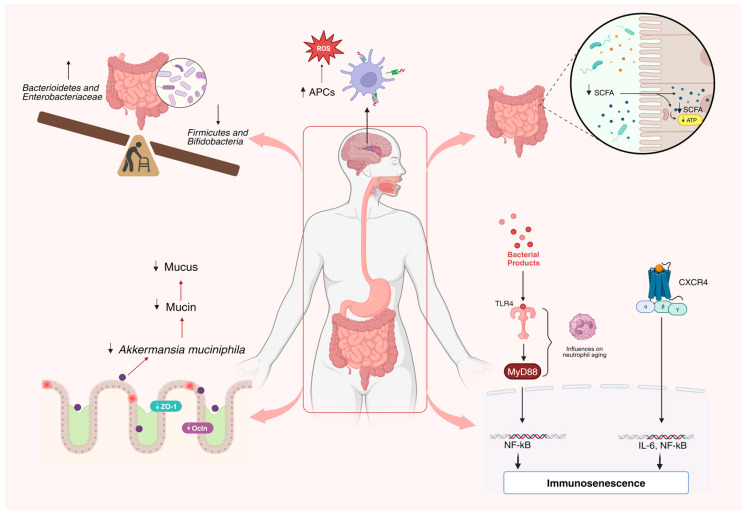
It is crucial to emphasize the coordinated regulation of the commensal microbiota, the preservation of the epithelial barrier, and the controlled secretion and re-absorption of enzymes and nutrients. The dysregulation of these homeostatic mechanisms results in an imbalance, promoting intestinal dysbiosis and chronic inflammation. Consequently, this heightens the risk of diseases and negatively impacts epithelial regeneration, potentially leading to the development of dysplasias and cancers [50,51]. Recognizing the intricate interactions involved in intestinal regeneration, encompassing epithelial functions, the commensal microbiota, and the immune system, is crucial. Exploring strategies for intervening and preventing gastrointestinal diseases is imperative, especially considering that aging is a significant risk factor for various age-related conditions, including chronic and inflammatory diseases, not to mention those affecting the gastrointestinal tract. The elderly population becomes more susceptible to inflammatory and infectious diseases [50] (Figure 1).
Figure 1.
Impact of aging on the composition of the intestinal microbiota. Significant changes in the intestinal microbiota composition arise from various stress factors linked to aging, resulting in a dysbiotic state. This imbalance entails reduced microbial diversity, fostering an uptick in opportunistic Gram-negative bacteria while decreasing species associated with health benefits. Furthermore, these shifts disrupt innate immunity, notably increasing antigen-presenting cells in the brain. Myeloid cell buildup reduces Akkermansia muciniphila, diminishing colonic mucus and heightening IL6-mediated inflammation. Additionally, alterations in tight junction expression (Zo-1 and occludin) contribute to changes in intestinal permeability [9,12,22,23,29,30,31,47,48].
3. Impact of the Gut Microbiota on Age-Associated Diseases
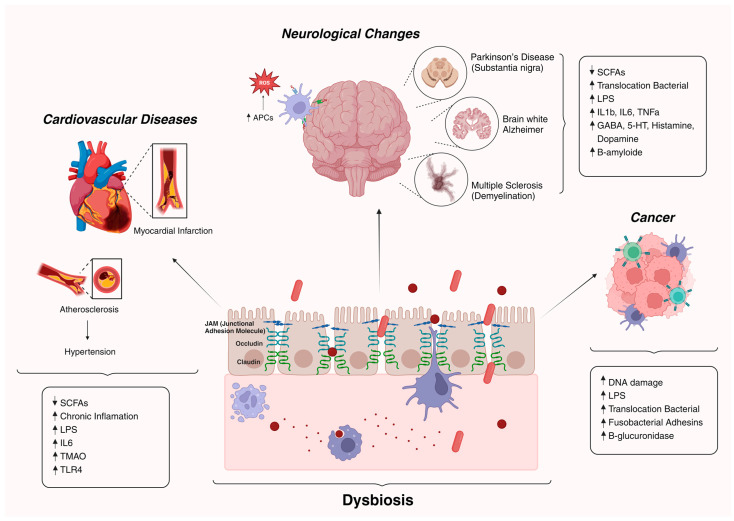
The intestinal microbiota significantly influences overall health through complex bidirectional signaling with the central and peripheral nervous systems and other distant organs. Disruption of this intricate communication between bacteria and the host leads to dysbiosis, thereby fostering the development of various diseases, including neurological disorders, cardiovascular conditions, and cancer, among others [4] (Figure 2).
Figure 2.
Diseases associated with age promoted by dysbiotic condition. Changes in the intestinal microbiota during the aging process significantly impact health, heightening vulnerability to age-associated ailments such as neurodegenerative and cardiovascular afflictions, alongside conditions conducive to cancer development [4].
3.1. Neurological Changes and Dysbiosis
Communication involving the brain–gut axis is associated with metabolic, endocrine, neural, and immune pathways, which can act independently or cooperatively [34]. Detrimental alterations in the gut microbiota are not intrinsically linked to the aging process. Some evidence suggests that when individuals are healthy, age may not significantly impact the composition of the gut microbiota, except during phases of rapid neurodevelopment (such as late adolescence and emerging adulthood). Across both young and older adulthood, there is considerable variability in the microbiota among individuals, yet there is relative stability within an individual [52]. The role of the intestinal microbiota in modulating central physiological and pathological processes is based on the ability of bacteria to produce bioactive products that provide insight into the mechanisms involved [34].
Increased intestinal permeability may result from a decrease in short-chain fatty acids (SCFAs), inducing cell apoptosis and disrupting the expression of tight junction proteins. This disruption facilitates the translocation of bacterial products, including LPS, microbial toxins, and even whole bacterial cells into circulating tissues. Consequently, there is heightened transport of bacterial metabolites to the brain, leading to increased neuroinflammation and further promoting the dysbiotic state. Beyond the intestinal barrier, dysbiosis is associated with the breakdown of the blood–brain barrier. This can exacerbate the reduction in intestinal integrity, triggering systemic inflammation correlated with various metabolic, neuroinflammatory, neuropsychiatric, and neurodevelopmental or neurodegenerative conditions. Elevated levels of circulating pro-inflammatory cytokines such as IL-1β, IL-6, TNF-α, and CRP (C-reactive protein) characterize these associations [53,54,55,56,57,58]. Furthermore, several studies indicate that peripheral inflammation can compromise the integrity of the blood–brain barrier, enabling the spread of inflammation to the central nervous system. Additionally, there were adverse effects on tight junction proteins like Occludin and Claudin-5, coupled with an upregulation of caspase-3 levels in the cortex and hippocampus. This implies that the apoptotic cascade triggered by the inflammatory process and oxidative stress could contribute to neuronal death in specific brain regions [59].
The link between brain interaction and bacterial products influencing behavior is intriguing. Various genera and species of bacteria can produce metabolites involved in mood-related functions and behavioral and cognitive functions, acting as neurotransmitters or precursors of neurotransmitters such as gamma-aminobutyric acid (GABA), serotonin (5-HT), histamine, and dopamine [60,61]. For instance, Bifidobacterium infantis increases tryptophan levels in blood plasma, affecting central serotonin transmission. GABA can be produced by Lactobacillus and Bifidobacterium; norepinephrine by Escherichia, Bacillus, and Saccharomyces species; serotonin by Streptococcus, Candida, Escherichia, and Enterococcus species; dopamine by various bacteria; and acetylcholine by Lactobacillus [62].
Metabolic functions involving the individual and intestinal microbiota encompass the production of essential metabolites like short-chain fatty acids. These include acetate, propionate, and butyrate, which operate through G protein-coupled receptors or histone deacetylases. These metabolites play a crucial role in brain–gut communication and may represent potential therapeutic targets for neurodevelopmental and neurodegenerative disorders, as well as in the progression of neurological changes [63,64].
The gradual accumulation of abnormal proteins in the central nervous system may characterize the development of neurological disorders, including Alzheimer’s disease (AD), Parkinson’s disease (PD), and Multiple Sclerosis (MS) [65]. AD is a progressive neurodegenerative syndrome linked to the buildup of misfolded amyloid-β (Aβ) protein fibrils and oligomers. Additionally, there are neurofibrillary tangles composed of hyperphosphorylated tau protein found in the cerebral cortex and other brain regions [66,67].
An elevated Firmicutes/Bacteroidetes ratio characterizes intestinal dysbiosis, potentially leading to the accumulation of amyloid precursor proteins from the early stages of Alzheimer’s disease (AD) [68]. A study found increased specific bacterial populations, including Actinomycetes and Bacteroides, in the intestines of Alzheimer’s disease (AD) patients compared to the control group [69]. This rise in pathogenic bacteria, along with elevated E. coli/Shigella, is linked to heightened pro-inflammatory cytokines (interleukin IL-1 and CXCL2) expression and NLRP3 inflammasome activation [70]. Clinical research revealed lower levels of anti-inflammatory Bacteroides fragilis and Eubacterium rectale in the intestinal tract of elderly individuals with cognitive impairment, while pro-inflammatory bacteria like E. coli and Shigella were more prevalent [71,72]. Strains such as Bacillus subtilis, E. coli, Salmonella, Mycobacterium, Mycobacterium tuberculosis, and Staphylococcus aureus produced functional extracellular amyloid fibers [66]. Certain microorganisms released LPS, amyloid, and other immunogenic mixtures into the surrounding environment, promoting Aβ accumulation and inflammation, activating the signaling pathway in AD pathogenesis. Bacterial amyloid protein is associated with molecular and cellular adaptation, adhesion stimulation, aggregation, biofilm formation, tissue invasion, bacterial colonization, and pathogen infectivity [73]. Conversely, treatment with Clostridium butyricum improved cognitive function in rats, reducing neuronal apoptosis, increasing brain-derived neurotrophic factors, and decreasing pro-inflammatory cytokines [74].
PD is a progressive neurodegenerative condition characterized by the accumulation of α-synuclein (α-syn) in dopaminergic nerve cells within the substantia nigra region of the brain. This process leads to the formation of Lewy bodies, which are round lamellated eosinophilic cytoplasmic inclusions. Despite ongoing research, the precise mechanisms underlying PD pathogenesis remain unclear and are likely multifactorial in nature, giving rise to various theories about its origin [75].
Aging significantly increases the risk of developing and progressing PD, impacting multiple cellular pathways and contributing to neurodegeneration [76]. The molecular perturbations tolerated by young neurons may have more severe consequences in aged neurons [77]. Investigations suggest a correlation between gastrointestinal abnormalities in individuals with PD and intestinal dysbiosis, along with α-synuclein deposits in the Enteric Nervous System (ENS) [78,79,80,81]. Research exploring the gut microbiome in PD observed a significant reduction in Prevotellaceae species, rather than relative Enterobacteriaceae counts, in fecal samples from individuals with PD [82]. Intestinal dysbiosis associated with PD, leading to increased Akkermansia and decreased SCFA-producing bacteria, may cause intestinal permeability and inflammation. This, in turn, exposes the intestinal neural plexus to toxins like lipopolysaccharides (LPS) and pesticides, potentially triggering the abnormal aggregation of α-synuclein fibrils and the formation of Lewy bodies [82]. Patients with PD display a distinct microbial composition compared to healthy individuals or those with other neurological disorders [83,84,85].
The intestinal microbiota in PD patients shows a reduced presence of bacteria producing SCFAs, particularly butyrate, such as Faecalibacterium prausnitzii and Lachnospiraceae, known for their anti-inflammatory properties [86,87,88]. Additionally, a murine model study suggested that certain bacterial species, like Proteus mirabilis, may contribute to the development of motor deficiencies and potentially play a role in PD [89]. The gut microbiota also influences the metabolism of medications used to alleviate PD symptoms. For instance, the primary PD drug, levodopa (L-dopa), undergoes decarboxylation to dopamine by tyrosine decarboxylase from Enterococcus faecalis and is converted to metatyramine by dopamine dehydroxylase from Eggerthella lenta A2 [90,91]. Moreover, L-dopa accumulates on the surface of Helicobacter pylori in vitro, possibly affecting the L-dopa concentration in PD patients and leading to motor symptom fluctuations [92,93].
MS is a chronic immune-mediated neurological disease affecting the central nervous system (CNS), causing damage to axons and demyelination, and impacting around 2.3 million people worldwide. The pathophysiology of MS is intricate and not fully understood [94,95]. Its incidence is higher in women [96,97,98]. The primary pathogenic feature involves the development of demyelinated inflammatory plaques in the CNS, affecting gray or white matter in the spinal cord and brain. This triggers a neuroinflammatory response, leading to the demyelination of specialized cells, including oligodendrocytes, and subsequent neurodegeneration. The abnormal permeability of the blood–brain barrier (BBB) allows immune system cells to infiltrate CNS neuronal cells, initiating demyelination. Myelin antigen-specific T cells (CD8+ and CD4+ T cells) cross the BBB, contributing to events that result in the formation of demyelinating lesions [99,100,101]. The gut microbiota influences and modulates the balance between pro- and anti-inflammatory T cells in gut-associated lymphoid tissue [102,103]. Studies have characterized the gut microbiota profile of patients with various forms of MS [104,105,106].
It has been reported that there is a reduction in bacteria from the Actinobacteria phylum, such as Collinsella [107,108], Slackia [108], and Adlercreutzia [107], in MS patients. Conversely, other groups reported an increase in Bifidobacterium [109,110] and Eggerthella [109] in MS patients. Regarding the Bacteroidetes phylum, research indicated a decrease in species like Prevotella [107,108,109,111,112], Parabacteroides [107,111], Butyricimonas [108], and Bacteroides [109] in MS patients. Bacteria from the Firmicutes phylum, including Blautia and Dorea (from the Lachnospiraceae family) [107], Streptococcus [109,112], and Ruminococcus [113], were found in higher abundance in MS patients. Conversely, species like Lactobacillus, Coprobacillus, Erysipelotrichaceae, and Veillonellaceae [107], as well as Lachnospiraceae, Ruminococcaceae [110], and Clostridia [109,113], were reduced in these patients. Concerning the Proteobacteria phylum, an increase in the abundance of Pseudomonas, Mycoplana, Haemophilus [107], Bilophila [110], and Acinetobacter [110] was observed in MS patients, while the presence of Sutterella was decreased [109]. Interestingly, one study reported an increase in Sutterella in MS patients undergoing disease-modifying therapies [108]. Additionally, studies have noted an increase in the abundance of Akkermansia species, belonging to the Verrucomicrobia phylum, in MS patients [108,111,114].
Based on these findings, it can be posited that the intestinal microbiota holds promise for research in the realm of neurological disorders, particularly neurodegenerative diseases. Moreover, the intricate interactions between gut microorganisms and medications currently employed in the treatment of neurodegenerative conditions require further investigation. A more in-depth exploration of the pathological mechanisms associated with dysbiosis can significantly enhance advancements in early diagnosis, amplify the therapeutic effects of existing medications, and foster the development of new therapeutic targets and drugs for neurodegeneration treatment.
3.2. Cardiovascular Diseases and Dysbiosis
Cardiovascular diseases (CVDs) stand out as the primary cause of global mortality, including conditions such as coronary artery disease, cerebrovascular disease, and peripheral arterial disease. The established risk factors for CVDs encompass atherosclerosis, hypertension, diabetes, dyslipidemia, obesity, and mental health disorders. Emerging research suggests a crucial role for the intestinal microbiota in maintaining cardiovascular health, with dysbiosis potentially contributing to the initiation and progression of cardiovascular diseases [115]. For instance, investigations reveal a clear positive correlation of Atopobium with various anthropometric variables, such as waist circumference, weight, and body mass index, as well as with fat and protein intake reported in a 24-h dietary recall study [116]. Moreover, metagenomic studies have demonstrated a positive correlation between Clostridium and the formation of metabolites like trimethylamine N-oxide (TMAO), along with a positive correlation between Clostridium histolyticum and Clostridium perfringens and waist circumference, weight, body mass index, and fat mass. This suggests that both the aforementioned Clostridium and Atopobium species can be considered markers of inflammation and CVD risk [116,117].
The intestinal microbiota actively participates in the metabolism of choline, phosphatidylcholine, and carnitine, key compounds implicated in the production of metabolites such as TMAO. Scientific evidence indicates that TMAO not only influences cholesterol and bile acid regulation but also correlates with early-stage atherosclerosis and an increased long-term risk of cardiovascular disease-related mortality [118]. Additionally, studies indicate that metabolites from the intestinal microbiota can contribute not only to inflammation but also to the development of high blood pressure [119]. Furthermore, the overproduction of metabolites such as ammonia and ammonium hydroxide, derived from the fermentation of proteins by the intestinal microbiota, disrupts the tight junctions between intestinal epithelial cells. This results in further increases in microbial translocation and systemic inflammation [120,121].
Some studies have explored the link between gut microbiota and markers of low-grade chronic inflammation in humans [122,123,124,125,126,127]. A systematic review of human studies revealed that the abundances of Faecalibacterium, Bifidobacterium, Ruminococcus, and Prevotella are inversely associated with various markers of low-grade inflammation, including high-sensitivity C-reactive protein and interleukin (IL)-6. Understanding the connections between the intestinal microbiota and markers of low-grade inflammation in humans holds promise for developing therapeutic strategies to prevent and treat atherosclerotic cardiovascular disease, considering the intestinal microbiota’s interaction with the innate and adaptive immune systems [57]. Similarly, another investigation found a contrasting association between Prevotella and inflammatory markers. An increased abundance of specific Prevotella species was linked to low-grade inflammation in systemic diseases like rheumatoid arthritis [127]. The study also noted that individuals with obesity exhibited a lower abundance of Prevotella species in the intestine. Furthermore, Prevotella abundance showed an inverse association with lipopolysaccharide (LPS) and high-sensitivity C-reactive protein [127].
LPS, a known inflammatory mediator found in Gram-negative bacteria, is primarily distributed in the intestine and oral cavity. Studies have demonstrated that LPS can induce vascular oxidative stress by activating Toll-like receptor 4 (TLR4), resulting in endothelial dysfunction and vascular inflammation. Notably, individuals with cardiovascular diseases exhibit elevated fecal levels of LPS compared to their healthy counterparts. It is worth mentioning that the activity of LPS can be discerned based on the distinct lipid A structures of LPS originating from different bacterial sources [128].
An increased presence of opportunistic pathogens originating from the host, such as Escherichia coli, Clostridium ramosum, Bacteroides caccae, and Eggerthella lenta, along with a decrease in bacteria that synthesize short-chain fatty acids (SCFAs) like Roseburia, Faecalibacterium, and Eubacterium rectale, has been associated with a higher risk of developing cardiovascular diseases. This highlights that both the types and abundance of microorganisms in the intestinal microbiota serve as risk factors influencing the development of cardiovascular diseases [129,130].
In patients at high risk of stroke, there is a reduction in butyrate-producing bacteria, such as those from the Lachnospiraceae and Ruminococcaceae families. This reduction leads to decreased fecal butyrate levels and simultaneous increases in intestinal pathogens, particularly those from the Enterobacteriaceae and Veillonellaceae families [131]. Moreover, there is an observed rise in the prevalence of opportunistic pathogens like Megasphaera, Enterobacter, and Clostridium difficile [132,133,134]. In a post-stroke clinical study, a decrease in the abundance of Roseburia, Bacteroides, and Faecalibacterium prausnitzii was noted, accompanied by an increase in Enterobacteriaceae, Bifidobacteriaceae, and Clostridium difficile [135].
Finally, atheroma plaques from patients with coronary artery disease (CAD) have been found to contain pathogenic species such as Staphylococcus, Proteus vulgaris, Klebsiella pneumoniae, and Streptococcus species [131]. In these patients, there is an increase in the abundance of Lactobacillus, Streptococcus, Escherichia, Shigella, and Enterococcus species, accompanied by a reduction in Faecalibacterium, Subdoligranulum, Roseburia, Eubacterium rectale, and Bacteroides fragilis species. The latter group is known to regulate T cell functions in the intestinal mucosa, providing anti-inflammatory effects and protecting the intestinal barrier [131,136].
In general, extensive research has been conducted on the involvement of the intestinal microbiota in the development of cardiovascular diseases (CVDs). Understanding these interactions is crucial for enhancing the effectiveness of current CVD therapies, ensuring better suitability and response to treatment, and fostering the development of new prophylactic approaches aimed at cardiovascular health through the manipulation of the intestinal microbiota.
3.3. Cancer and Dysbiosis
The intestinal microbiota significantly influences the development and progression of various cancers by modulating metabolic and genetic processes. Microorganisms residing in tissues can increase cancer risk, shape immune responses, and enhance the effectiveness of anticancer treatments [137,138,139,140,141,142,143,144].
The mechanisms through which tissue-resident microbiota may promote cancer development are not fully explored. However, they are associated with inducing inflammatory responses that create a favorable environment for tumors and breaking down tissue barriers, facilitating the translocation of metabolites from the intestinal microbiota. Due to systemic effects, intestinal microbes are implicated in inducing cancer in various organs, including the skin and brain. The dynamic interaction between the resident microbiota and the host involves specific tissue factors, pH alterations, antimicrobial presence, mucin, and bile acids, among others. The microbiota’s impact is mediated by microbial diversity, nutrient availability, competition between species, and adaptive mechanisms [145,146].
It has been reported that gut microbiota’s metabolism can influence cancer progression. The microbiota may exert carcinogenic effects directly or indirectly through its products, involving three key mechanisms in carcinogenesis. Firstly, it can induce DNA damage, alter gene expression, and stimulate proliferation. Secondly, it may promote protumorigenic microbial niches, including biofilm formation. Lastly, imbalances in the immune response can contribute to its carcinogenic impact [147]. Certain bacteria, such as Escherichia coli, Bacteroides fragilis enterotoxigenica, and Fusobacterium nucleatum, have demonstrated virulence factors linked to colorectal cancer development. Fusobacterial adhesins like Fap2, RadD, and FadA, expressed by Fusobacterium nucleatum, contribute to bacterial aggregation, adhesion to dysplastic tissues, and biofilm formation [148]. Colicin B2, produced by E. coli, induces DNA damage [149,150], while CagA, a cytotoxin from Helicobacter pylori, known for inducing inflammatory pathways in gastric cancer, may also play a role in colorectal cancer tumorigenesis [151]. Additionally, recent research has unveiled insights into the metabolites of the intestinal microbiota. Studies indicate that gut microbial β-glucuronidase (βG) found in specific strains is implicated in promoting azoxymethane-induced gut microbial dysbiosis and intestinal tumorigenesis [152,153].
The intestinal microbiota can impact tumor development beyond the gastrointestinal tract [154]. Evidence indicates that intestinal bacteria can travel to adjacent tissues, influencing tumor progression. For instance, a study using fluorescence-labeled commensal bacteria in a murine model demonstrated bacterial translocation from the intestine’s luminal compartment to the pancreas [155]. It is proposed that bacteria, like Gammaproteobacteria, translocate from the intestine to pancreatic tumors, metabolizing the active form of the drug gemcitabine and diminishing its effectiveness. The suggested route for this translocation is likely through the pancreatic duct, which communicates with the duodenum [156].
Moreover, it is theorized that molecules such as metabolites, antigens from intestinal bacteria, and pathogen-associated molecular patterns can be transported to the liver through the hepatic portal vein. Given that about 70% of the liver’s blood supply comes from the intestinal circulation, the liver becomes a crucial site for interactions between signals from the intestinal microbiota and the immune system. Studies indicate that molecules from intestinal bacteria, including secondary bile acids (BAs), lipopolysaccharide (LPS), and lipoteichoic acid (LTA), can either drive carcinogenesis or suppress antitumor immunity in the liver [30,157,158,159].
These findings highlight the profound impact of the intestinal microbiota on the human immune system and its recognition as a crucial factor in modulating antitumor immunity and therapeutic outcomes. Monitoring the microbiological composition of this organ could aid in identifying individuals at a higher risk of developing tumors, and modulating the microbiota in these individuals may have implications for cancer prevention. Recent studies revealing the presence of intestinal bacterial species in previously considered sterile tissues underscore the need for the further exploration of microbial colonization sources and the composition and function of the microbiota in these locations.
4. Polyphenolic Compounds and Their Significance in Shaping the Intestinal Microbiota
The preservation of a healthy intestinal microbiota, where the predominance of beneficial bacteria surpasses that of pathogenic ones, engenders various health advantages and supports immune functions. Nevertheless, several endogenous and exogenous factors, including nutritional status, age, genetic conditions, and immune status, can perturb the intestinal equilibrium, leading to the onset of gastrointestinal disorders such as irritable bowel syndrome, antibiotic-associated diarrhea, and inflammatory bowel disease [160,161]. Much research evidence suggests that polyphenols, secondary metabolites derived from the metabolism of vegetables and fruits, possess health-promoting properties and contribute to the prevention of chronic diseases such as coronary heart disease, type 2 diabetes, neurodegenerative diseases, and certain types of cancer [162,163]. Evidence indicates that the prolonged daily intake of polyphenols can modulate the intestinal microbiota, fostering a beneficial microbial ecosystem that significantly enhances human health. It has been established that polyphenol consumption induces alterations in various aspects of the intestinal microbiota composition, including changes in the abundance of Bifidobacterium, Proteobacteria, and Helicobacter [164]. Moreover, experimental studies conducted in both pre-clinical and clinical phases suggest that polyphenolic compounds play a pivotal role in augmenting beneficial intestinal bacteria, such as Akkermansia muciniphila, while concurrently reducing the prevalence of pathogenic microbial species such as Flexispira, Prevotella, Adlercreutzia, Dessulfovibrio, Bifidobacterium, Lactobacillus, Deferribacteres, and Actinobacteria [165,166].
Polyphenols exert their antimicrobial effects through diverse mechanisms, which can vary depending on the structural differences in the cell membranes of Gram-positive and Gram-negative bacteria. The concentration-dependent binding of polyphenols to bacterial cell membranes alters membrane functionality, impeding bacterial growth. This interaction, particularly with catechins, induces the production of H2O2, affecting the permeability of microbial cell membranes. Notably, this phenomenon is observed across various bacterial species, including Bordetella bronchiseptica, E. coli, Klebsiella pneumoniae, Serratia marcescens, Pseudomonas aeruginosa, Salmonella choleraesuis, Bacillus subtilis, and Staphylococcus aureus. Moreover, polyphenols, such as epicatechin gallate, have demonstrated the ability to sensitize bacteria to the effects of antibiotics, a promising aspect for addressing antibiotic-resistant strains, as evidenced in methicillin-resistant S. aureus treated with beta-lactam antibiotics [167].
In vitro investigations have underscored curcumin’s potential as a compound for rectifying disrupted intestinal permeability. Employing CaCo2 cell studies, curcumin showcased inhibitory effects on the disruption of the intestinal epithelial barrier, neutralized IL-1β secretion induced by LPS, and prevented occludin junction protein disruption [168,169]. Noteworthy actions of curcumin included the inhibition of IL-1β-induced MAPK p38 activation, the augmentation of phosphorylation in occludin junction proteins, and their preservation of their normal arrangement [169]. In vivo assessments in rat models further affirmed curcumin’s efficacy, improving tight junction structure, suppressing serum TNF-α and LPS concentrations, and positively modulating occludin expression in the intestinal mucosa [170]. Another study corroborated these findings, demonstrating that curcumin significantly enhanced intestinal barrier function by restoring alkaline phosphatase activity and the expression of tight junction proteins ZO-1 and claudin-1 [171].
Ohno et al., in an in vivo study utilizing animal models, demonstrated the remarkable anti-inflammatory properties of a newly developed nanoparticle curcumin, a polyphenol derivative, in mice with DSS-induced colitis. The intervention led to a significant reduction in pro-inflammatory mediators, restrained Treg expansion, and elevated fecal butyrate levels. Notably, curcumin effectively suppressed NF-κB activation and modulated the expression of pro-inflammatory mediators within the colonic epithelial cells of treated mice [172]. Additionally, alternative anti-inflammatory mechanisms of curcumin were proposed, including the attenuation of LPS-induced inflammation and inhibition of the TLR4/MyD88/NF-κB signaling pathways [173,174]. The compound exhibited a noteworthy capability to inhibit NF-κB nuclear translocation and downregulate pro-inflammatory genes implicated in cancer pathogenesis [175].
In an in vitro model, flavan-3-ol monomers such as (+)catechin and (-)epicatechin have demonstrated the capacity to enhance the bacterial population in the colon, particularly influencing the growth of Clostridium coccoides, Eubacterium rectale, and E. coli compared to Lactobacillus spp. and Bifidobacterium spp., which remained unaltered [176]. Moreover, in an animal model of colon cancer, the extract from red wine, rich in proanthocyanidins, was shown to modulate the predominance of bacterial species, favoring Bacteroides, Bifidobacterium, and Lactobacillus spp. over Bacteroidetes, Propionibacterium, and Clostridium spp. [177]. In a separate study utilizing a rodent model of dextran sulfate sodium (DSS)-induced colitis, grape resveratrol was found to stimulate the fecal cell count of Lactobacillus and Bifidobacterium spp. [178].
A study conducted on healthy adults treated with a grape seed extract rich in proanthocyanidins for two weeks revealed a significant increase in Bifidobacteria [179]. In vivo investigations have suggested that monomeric flavan-3-ols and abundant sources of flavan-3-ols, such as grape seed extract, green tea, currants, and chocolate, possess the ability to modulate the intestinal microbiota, inducing alterations in beneficial bacteria like Lactobacillus spp. However, both in vitro and in vivo studies have demonstrated the inhibition of other groups, such as Clostridium spp. [176,180,181,182].
5. Polyphenolic Compounds, Metabolization, and Their Significance in Modulating Intestinal Microbiota during Aging
The metabolization of polyphenols, a critical process for unlocking their bioactive potential, begins as these compounds enter the intricate pathways of the human digestive system. The efficiency of polyphenol absorption is influenced by various physicochemical factors, including the size of the molecule, the presence of functional groups, its spherical shape, lipophilicity, and solubility [183]. Once absorbed, the phenolic compounds are the subject of intense metabolization, which leads to metabolites with more bioavailability than the phenolic compounds [184].
Polyphenols, once ingested, are processed through a series of metabolic reactions within the gastrointestinal system [185,186]. These compounds are initially metabolized in the intestine (predominantly phase II reactions) and the liver (both phase I and II reactions) [187,188]. The liver’s phase I metabolism is primarily facilitated by the cytochrome P450 enzymes. In the intestine, enzymes like lactase-phlorizin hydrolase and cytosolic β-glucosidase transform these compounds into forms that can be readily absorbed [186]. These forms are further modified through glucuronidation, sulfation, and methylation, depending on the species inhabiting the intestine, enhancing their solubility and thus enabling their elimination through bile and urine [188]. Furthermore, more than 90% of dietary polyphenols remain unabsorbed in the small intestine, along with hydrophilic conjugates formed in the liver, which are transported to the colon, where they encounter diverse bacteria capable of extensive metabolism [186,189]. These bacteria have enzymes capable of breaking down various polyphenol structures, including the separation of sugar molecules from aglycones and the breakdown of complex rings within the polyphenol structures. The gut microflora also processes conjugates by detaching sugars and sulfates, converting them back into their aglycone form [188]. These microbes play a crucial role in converting polyphenols into low-molecular-weight phenolic metabolites, which exhibit improved bioavailability and health effects. By acting in a “prebiotic-like” manner, polyphenols can modify the composition of gut microbiota, favoring the growth of beneficial strains like Bifidobacterium and Lactobacilli while reducing the population of pathogens such as Clostridium perfringens and Clostridium histolyticum [190]. Notably, the metabolism of polyphenols by gut microbiota varies among individuals due to differences in microbiota composition, leading to diverse health outcomes even with similar daily polyphenol intake [191]. It is equally important that these mechanisms are crucial as they limit any potential toxic effects of polyphenols and also regulate their bioavailability within the body [192].
The onset and advancement of age-related diseases are closely linked to lifestyle and diet. There is a rising interest in polyphenol-rich foods due to their potential to safeguard neurons from damage, alleviate neuroinflammation, and enhance cognitive functions, learning, and memory [193].
An aging model study based on in silico prediction and in vitro transport studies across endothelial cells of the blood–brain barrier, at circulating concentrations, evaluating the potential of known bioavailable phenolic sulfates, arising from the colonic metabolism of berries, provided evidence of differential transport, probably related to the chemical structure, observed that the metabolites of polyphenolic compounds can exert neuroprotective effects when reaching the brain, crossing the blood–brain barrier, observing that preconditioning with phenolic sulfates favored an improvement in cellular responses to oxidative, excitotoxic, and inflammatory injuries, attributing that this attenuation of neuroinflammation was achieved through the modulation of the NF-κB pathway [194].
Bondonno and colleagues in a randomized clinical trial model with healthy individuals observed that the use of apples rich in flavonoids plays a role in the vasodilatory response, attributed to the abundance of quercetin and (-)-epicatechin in apples, improving endothelial function, reducing blood pressure, and providing benefits to cardiovascular health [195]. Some data related to the benefits of polyphenols in modulating the microbiota are still limited; however, these data allow us to raise hypotheses that these abundant flavonoids in apples may offer benefits to cardiovascular health in elderly individuals, since this age group represents an increased risk for changes in the circulatory system [196].
Saccon et al. conducted a comprehensive investigation into the impact of a senolytic drug combination, namely Dasatinib and Quercetin (D+Q), recognized for its efficacy in diminishing senescent cell burden in aged mice. Employing an aged Balb/c female mouse model, the study aimed to discern the treatment’s effects on senescence markers, inflammatory processes, and the microbial milieu within the intestinal tract. The findings unveiled significant alterations in the expression of senescence-associated genes, such as p16 and p21, alongside inflammatory genes, including IL-1β, IL-6, TNF-α, Mcp1, and Cxcl1, discerned in both the large and small intestines. Moreover, a discernable shift in the intestinal microbiota was noted, characterized by an augmentation of Firmicutes coupled with a diminution of Verrucomicrobia across all samples. Intriguingly, an elevated Gram-positive to Gram-negative taxa ratio was identified specifically in the ileum but not in the descending segments of the intestine, namely the cecum and colon. The nuanced microbiome signatures observed in conjunction with the amelioration of senescence and inflammation post-senolytic treatment highlight intricate associations in the intestinal ecosystems of aged mice [197].
It is known that curcumin possesses anti-inflammatory, antitumoral, and other beneficial properties [198,199,200,201]. In the context of aging, studies in aged rat models have revealed that curcumin can directly modulate the intestinal microbiota, primarily due to its low bioavailability and absorption. The consumption of curcumin has been demonstrated to induce changes in the bacterial proportions of the intestinal microbiota, favoring an increase in the abundance of Bifidobacteria and Lactobacillus while reducing the levels of Prevotellaceae, Choriobacteria, Enterobacteriaceae, and Enterococcus [202,203,204].
In a study scrutinizing aging-induced alterations in mice, the salutary effects of chlorogenic acid (CGA) and epicatechin-3-gallate (EGCG), principal polyphenolic constituents of coffee and green tea, respectively, were evaluated. Noteworthily, both compounds exhibited discernible intestinal fortification, either in concert or in isolation. The amalgamated administration of CGA+EGCG, harnessing its prowess in antioxidant and anti-inflammatory spheres, coupled with its microbiota-modulating capacities, efficaciously ameliorated cognitive deficits and fortified the intestinal barrier’s functional integrity in comparison to their solitary counterparts. This holistic approach not only thwarted the accrual of D-galactose-induced reactive oxygen species but also augmented the overall oxidative prowess. The supplementation with CGA+EGCG effectively curbed the expression of pro-inflammatory cytokines—namely, TNFα, IFNγ, IL1β, and IL6—culminating in the mitigation of intestinal inflammation. Furthermore, it orchestrated a harmonious modulation of the D-galactose-disrupted intestinal microbiome, orchestrating a restitution of Firmicutes/Bacteroidetes equilibrium in the aged murine cohort. This intervention intricately orchestrated a reduction in the abundance of Lactobacilliaceae, Erysipelotrichaceae, and Derribacteriacea, concomitant with an elevation in the proportions of Lachnospiraceae, Muribaculaceae, and Rikenellaceae [205].
Diets enriched with polyphenolic compounds have exhibited pronounced efficacy in modulating intestinal permeability and the composition of the gut microbiota in the elderly population. In a clinical study involving elderly subjects subjected to a polyphenol-rich dietary regimen featuring theobromine and methylxanthine derivatives from cocoa and/or green tea, a significant positive correlation was observed between polyphenol-rich diets and the abundance of butyrate-producing bacteria, notably from the Clostridiales order and genera Roseburia, Butyricicoccus, and Faecalibacterium. Conversely, an inverse relationship with zonulin levels was noted. Furthermore, a comprehensive multi-omics analysis unveiled direct correlations between specific polyphenol metabolites, namely hydroxyphenylpropionic acid sulfate, 2-methylpyrogallol sulfate, and catechol sulfate, and the abundance of Butyricicoccus. Intriguingly, hydroxyphenylpropionic acid sulfate and 2-methylpyrogallol sulfate exhibited a negative correlation with Methanobrevibacter. This intricate interplay was underscored by participant age, baseline zonulin concentrations, and shifts in Porphyromonadaceae abundance, emerging as pivotal determinants steering the impact of polyphenol-rich diets on zonulin dynamics. These findings postulate that understanding the intricate relationships among polyphenol consumption, intestinal permeability, and the intricate landscape of the gut microbiota in the elderly holds promise for tailoring precision dietary interventions in this demographic [206].
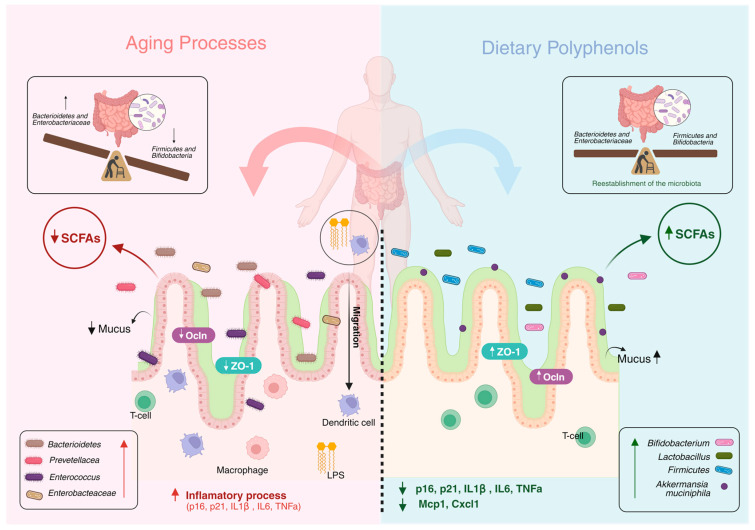
The mentioned studies suggest that polyphenolic compounds confer health benefits mediated through the modulation of the gut microbiota, preventing and/or ameliorating inflammatory conditions (Figure 3). Additionally, they are associated with preventing neuroinflammation, inflammatory diseases, cardiovascular diseases, and other age-related ailments (Table 1).
Figure 3.
Benefits of polyphenolic compounds in modulating intestinal microbiota. Dysbiosis associated with aging promotes changes in the intestinal microbiota, leading to changes in the proportions of the Firmicutes/Bacterioidetes phyla, observed by the increase in the proportions of Pretovellaceae, Enterobacteaceae, and Enterococcus, and changes in the intestinal barrier mediated by the reduction in colonic mucus, favoring the development of the inflammatory process. However, the use of polyphenolic compounds confers health benefits mediated by the modulation of the intestinal microbiota, favoring an increase in the proportions of Fircumutes/Bacterioidetes, Bifidobacterium, and Lactobacillus, in addition to preventing inflammatory conditions, observed by the inhibition of inflammatory mediator genes such as p16, p21, IL1β, IL6, TNFα, Mcp1, and Cxcl1. Polyphenolic compounds favor the increase in the production of SCFA, such as butyrate, and the increase in colonic mucus, which plays an important role in protecting the intestinal barrier [194,195,197,202,203,204,205,206].
Table 1.
Effects of polyphenols on intestinal microbiota during aging.
| Compound | Study Design | N | Age | Dose | Intervention Time | Effect | Reference |
|---|---|---|---|---|---|---|---|
| Grape extracts | In vitro gastrointestinal digestion with Lactobacillus and Bifidobacterium strains | 2 mg/mL | 48 h | Prebiotic effect ↑ Bifodobacteria |
[207] | ||
| Resveratrol + H2O2 | Human intestinal cell line Caco-2 | H2O2 (500 μM) plus Resveratrol (80 μM) | 24 h | ↓ ROS Improved cell viability |
[208] | ||
| Quercetin + Dasatinib | Female Balb/c aged mice | 20 | 18 months | Dasatinib (5 mg/kg) plus Quercetin (50 mg/kg) | 3 consecutive days every 2 weeks over a 10-week period administered by oral gavage | ↓ p16, p21, IL1β, IL6, TNFα, Mcp1, Cxcl1 ↑ Firmicutes ↓ Verrucomicrobia |
[190] |
| Tea polyphenols | Female Sprague Dawley | 60 | 3–4 months | 300 mg/kg | 12 weeks | Improved intestinal permeability Improved intestinal dysbiosis ↓ TLR4, IRAK, p-IκBα, NF-κB, p65 in the hippocampus ↑ Bacteroidetes/Firmicutes ratio ↑ Bacteroides, Lactobacillus, and Bifidobacterium |
[209] |
| Herbal saponins | Male C57BL/6 mice | 50 | 8 weeks old | 500 mg/kg | 15 days | [210] | |
| Resveratrol | Male Fischer F344 rats | 12 | Adults | 1 mg/kg/day | 20 days | ↑ Bifidobacterium ↑ Lactobacillus ↓ Cox-2 and PGE2 Protection of colonic structures |
[178] |
| Black raspberries (BRBs) | Male F-344 rats | 32 | 4–5 weeks old | 5% whole BRB powder, 0.2% BRB anthocyanins, or 2.25% of the residue fraction | 6 weeks | ↑ Anaerostipes, Ruminococcus, Coprobacillus, and ↓ Acetivibrio, Anaerotruncus spp. by 5% whole BRB ↑ A. muciniphila by the whole BRB and the residue |
[211] |
| Polyphenol-rich diet | Clinical trial | 51 | ≥60 years old | 3 daily portions (1391 mg/day of dietary polyphenols) | 8 weeks | Improved intestinal permeability ↑ Butyrate ↑ Roseburia ↑ Butyricicoccus ↑ Faecalibacterium ↑ Butyricicoccus ↓ Methanobrevibacter |
[199] |
| Green tea liquid | Randomized clinical trial | 12 | 27–46 years old | 400 mL/day | 14 days | ↑ α-Diversity; changed microbial composition; ↑ Relative abundance of (family) Lachnospiraceae and Bifidobactericeae; ↑ Bifidobacterium and SCFA-producing genus Roseburia, Feacalibacterium, Eubacterium, Blautia, Coprococcus, and Dorea; ↑ Predicted microbial genes for LPS biosynthesis, glutathione metabolism, and glycosaminoglycan degradation |
[212] |
| De-alcoholized red wine | Randomized, crossover, controlled study | 10 | 48 ± 2 years old | 733.02 ± 23.61 mg gallic acid equivalent/day | 20 days | ↑ Fusobacteria, Bacteroidetes, Enterococcus, Blautia coccoides–Eubacterium rectale, Bifidobacterium, and Eggerthella lenta. ↓ Cholesterol and C-reactive protein |
[213] |
6. The Role of Microbiota and Epigenetics during Aging
Recent studies have highlighted that epigenetic changes play a crucial role in how diet and the makeup of gut bacteria impact the body, both in normal and abnormal conditions [214,215,216,217,218,219,220]. This concept, known as the ‘microbiota–nutrient metabolism–host epigenetics axis’, explores how these factors intersect. Epigenetics involves changes passed down through cell division, influencing gene activity through DNA methylation and histone modifications [221,222]. A notable aspect is ‘epigenetic drift’, a phenomenon where DNA methylation patterns change with age. Generally, there is a decrease in methylation in areas with heterochromatin repeats and an increase, or hypermethylation, in promoter CpG regions [223,224]. Histone modifications, including acetylation and methylation, are crucial for cellular aging [225]. Histone methylation levels, regulated by methyltransferases and demethylases, can activate or repress transcription [226]. Gene expression is also influenced by noncovalent epigenetic mechanisms like miRNAs and lncRNAs [227]. These collectively control access to DNA or RNA transcripts, impacting the cell’s transcriptional activities. Epigenetic regulation, shaped by the microbiota, plays a key role in host physiology. This influence occurs through microbial synthesis, regulation of enzymes, and activation of cellular processes affecting epigenetic pathways [218,219,220].
Enzymes like DNA/histone methyltransferases and acetyltransferases modify epigenetics, relying on specific substrates. While the body produces many of these substrates, the microbiota is increasingly recognized as a source. The microbiota generates various compounds influencing epigenetic enzymes [220,228]. Notably, gut microbes produce folate and B vitamins, crucial for DNA or histone methylation. Beneficial microbes like Bifidobacterium and Lactobacillus synthesize folate through one-carbon metabolism, producing S-adenosylmethionine (SAM), a key substrate for DNA and histone methylation [229]. Changes in bacterial composition impact SAM availability, influencing host DNA or histone methylation. Short-chain fatty acids (SCFAs), exclusively produced by gut microbes, result from the fermentation of indigestible carbohydrates. SCFAs inhibit histone deacetylases (HDACs), modifying chromatin and enhancing specific gene expression.
In germ-free mice, both gut and systemic levels of short-chain fatty acids (SCFAs) are notably low [230,231,232]. However, supplementing these mice with SCFAs replicates many transcriptional and epigenetic effects induced by the microbiota [233]. SCFAs not only inhibit HDACs but also influence the tricarboxylic acid (TCA) cycle, impacting enzymes like ten-eleven translocation (TET) methylcytosine dioxygenases, involved in DNA methylation [217]. The microbiota significantly influences DNA methylation patterns in various mammalian cells and tissues [234]. Comparing intestinal epithelial cells (IECs) with germ-free (GF) and conventional (CNV) mice reveals that microbiota exposure leads to DNA hypomethylation and the increased expression of anti-bacterial and anti-inflammatory genes [235]. This decrease in methylation is associated with the heightened activity of DNA demethylase enzymes like Tet3 and Dnmt1 in CNV IECs [236,237]. Deleting Tet3 in IECs increases overall DNA methylation, including in areas sensitive to microbiota changes. In colitis cases induced by dextran sulfate sodium (DSS), CNV IECs show more pronounced hypomethylation compared to GF mice [235].
Human studies have identified correlations between DNA methylation in colon biopsies, microbiota composition, inflammation levels, and diseases like ulcerative colitis (UC) and Crohn’s disease [238]. In UC, linked to colorectal cancer, disease progression correlates with microbial imbalance [239,240]. Specifically, elevated Fusobacterium in UC patients is associated with increased DNA methylation in colorectal-cancer-related genes [241]. Colorectal-cancer-associated microbiota correlates with DNA methylation patterns and induces hyperplasia and DNA methylation in GF mice [240], highlighting the intricate interplay between microbiota and DNA methylation [242].
Microbiota also influence host chromatin through post-transcriptional histone modifications. These modifications, covalently attached to histone tails, crucially alter chromatin structure and regulate gene expression [243]. Gut microbiota impacts the global acetylation and methylation of histones H3 and H4, affecting various tissues (colon, liver, adipose tissue) with diet-dependent variations [243]. Introducing short-chain fatty acids (SCFAs) to germ-free mice partly reinstates histone modifications and gene expression changes influenced by the microbiota [217]. In the intestinal lining, variations in H3K4me3, a histone methylation type linked to active gene transcription, were observed in GF mice compared to conventional mice, resembling patterns found in inflammatory bowel disease (IBD) patients [244]. Gallic acid from the microbiota regulates this modification at Wnt-responsive gene promoters in a colorectal cancer mouse model [245].
Histone deacetylase (HDAC) enzymes, particularly HDAC3, highly expressed in the intestinal epithelium, play a critical role in maintaining intestinal balance dependent on the microbiota [246]. Deleting HDAC3 in intestinal epithelial cells (HDAC3ΔIEC) increases H3K9 acetylation, gene expression, impacts Paneth cell balance, and exacerbates dextran sulfate sodium (DSS)-induced inflammation, observed in conventional mice but not in germ-free mice. This highlights HDAC3′s crucial role in normal host–commensal microbe interactions [247].
Research has unveiled the intricate relationship between the host’s epigenome and gut microbiota, a dynamic interplay influenced by dietary choices. The introduction of specific nutrients prompts temporary modifications in the gut microbiome’s composition, thereby intricately impacting the host’s health [248]. Various dietary components, such as fiber, glucosinolates, fats, and polyphenols—processed by gut bacteria—exert profound effects on the host’s epigenetic landscape, shaping outcomes in health and disease [162].
Polyphenols have emerged as focal points for their potential antimicrobial properties and prebiotic attributes, fostering microbial enzyme activities [249]. These activities give rise to compounds capable of eliciting meaningful epigenetic changes in the host [250]. Notably, compounds like curcumin, epigallocatechin gallate (EGCG), and resveratrol derived from plants yield the power to modify the gut microbiota, thereby influencing the host’s epigenetic profile [251]. Curcumin, for instance, stands out for enhancing colon cell function by inhibiting histone deacetylases (HDACs) [252,253]. In a groundbreaking study utilizing an AOM/IL10 knockout model, curcumin demonstrated the ability to prevent the development of colonic tumors, coinciding with an observed increase in microbial diversity [254]. Furthermore, EGCG counteracts metabolic challenges induced by high-fat diets, upregulating DNA methyltransferase 1 (DNMT1) and leading to the reduced methylation of CpG sites in the colon. It also suppresses HDAC activity and diminishes CpG site hypermethylation in colon cancer cells [255]. Resveratrol, through supplementation, induces significant shifts in the composition of gut microbiota in individuals with metabolic syndrome [256]. Additionally, resveratrol-treated mice exhibit heightened Sirtuin 1 expression and a concomitant decrease in pro-inflammatory cytokines, hinting at anti-inflammatory effects [256].
In summary, the intricate interplay between polyphenolic compounds and the gut microbiota stands as a promising advancement, addressing dysbiosis and its associated epigenetic nuances. This area holds potential for pioneering nutritional interventions aimed at combatting the intricate biological effects of aging.
7. Conclusions
The aging process triggers biological shifts that influence the dynamics of the intestinal microbiota, potentially reshaping the proportions of essential microbial phyla like Bacteroidetes, Firmicutes, Proteobacteria, and Actinobacteria. The intestinal microbiota serves as a linchpin, orchestrating various physiological and metabolic functions dependent on the delicate equilibrium of bacteria. Factors including lifestyle choices, diet, genetics, epigenetics, and environmental influences contribute to shifts in the intestinal microbiota, laying the groundwork for age-related maladies. Epigenetic modifications, driven by diverse dietary regimes, play a pivotal role in the intricate communication between the gut microbiota and the host’s cellular framework. This emphasizes the growing need to explore effective avenues for modulating the intestinal microbiota to promote health. Enter polyphenol-rich compounds, emerging as stalwart allies in treating and preventing various diseases. Recognized for their anti-inflammatory, antioxidant, and immunomodulatory virtues, polyphenols are increasingly acknowledged for their potential interweaving with the complex dance of the intestinal microbiota in the trajectory of diverse diseases. Moreover, there is a growing focus on how the microbiota can initiate epigenetic adjustments, with polyphenolic compounds potentially holding the key to counteracting these age-related epigenetic changes. While the data in this domain are still evolving, these insights underscore the urgency of delving deeper into the effects of polyphenolic compounds in concert with intestinal microbiota modulation throughout the aging process. This expedition seeks to leverage the functional attributes of bioactive compounds to adeptly modulate and realign processes linked to aging.
Author Contributions
All authors made a substantial contribution to all aspects of the preparation of this manuscript. All authors have read and agreed to the published version of this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
Q.C.P. (88887.823956/2023-00), I.M.F (88887.910407/2023-00), and TWdS (88887.837642/2023-00) hold a CAPES research M.L.R. holds a CNPq research contract (305402/2019-6).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.de Mendonça J.M.B., de Abigalil A.P.C., Pereira P.A.P., Yuste A., de Souza Ribeiro J.H. O sentido do envelhecer para o idoso dependente. Cien. Saude Colet. 2021;26:57–65. doi: 10.1590/1413-81232020261.32382020. [DOI] [PubMed] [Google Scholar]
- 2.Gladyshev T.V., Gladyshev V.N. A Disease or not a Disease? Aging as a Pathology. Trends Mol. Med. 2016;22:995. doi: 10.1016/j.molmed.2016.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soria-Valles C., López-Otín C. iPSCs: On the Road to Reprogramming Aging. Trends Mol. Med. 2016;22:713–724. doi: 10.1016/j.molmed.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 4.López-Otín C., Blasco M.A., Partridge L., Serrano M., Kroemer G. Hallmarks of aging: An expanding universe. Cell. 2023;186:243–278. doi: 10.1016/j.cell.2022.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Dato S., Rose G., Crocco P., Monti D., Garagnani P., Franceschi C., Passarino G. The genetics of human longevity: An intricacy of genes, environment, culture and microbiome. Mech. Ageing Dev. 2017;165:147–155. doi: 10.1016/j.mad.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 6.Smith P., Willemsen D., Popkes M., Metge F., Gandiwa E., Reichard M., Valenzano D.R. Regulation of life span by the gut microbiota in the short-lived african turquoise killifish. eLife. 2017;6:e27014. doi: 10.7554/eLife.27014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belizário J.E., Faintuch J., Garay-Malpartida M. New frontiers for treatment of metabolic diseases. Mediat. Inflamm. 2018;2018:2037838. doi: 10.1155/2018/2037838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Srivastava S., Singh A., Sandeep K., Yadav D. Epigenetic Regulation of Gut Microbial Dysbiosis. Indian J. Microbiol. 2021;61:125–129. doi: 10.1007/s12088-021-00920-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeJong E.N., Surette M.G., Bowdish D.M.E. The Gut Microbiota and Unhealthy Aging: Disentangling Cause from Consequence. Cell Host Microbe. 2020;28:180–189. doi: 10.1016/j.chom.2020.07.013. [DOI] [PubMed] [Google Scholar]
- 10.Langille M.G.I., Meehan C.J., Koenig J.E., Dhanani A.S., Rose R.A., Howlett S.E., Beiko R.G. Microbial shifts in the aging mouse gut. Microbiome. 2014;2:50. doi: 10.1186/s40168-014-0050-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vemuri R., Gundamaraju R., Shastri M.D., Shukla S.D., Kalpurath K., Ball M., Tristram S., Shankar E.M., Ahuja K., Eri R. Gut Microbial Changes, Interactions, and Their Implications on Human Lifecycle: An Ageing Perspective. Biomed Res. Int. 2018;2018:4178607. doi: 10.1155/2018/4178607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sommer F., Bäckhed F. The gut microbiota--masters of host development and physiology. Nat. Rev. Microbiol. 2013;11:227–238. doi: 10.1038/nrmicro2974. [DOI] [PubMed] [Google Scholar]
- 13.Fei N., Bernabé B.P., Lie L., Baghdan D., Bedu-Addo K., Plange-Rhule J., Forrester T.E., Lambert E.V., Bovet P., Gottel N., et al. The human microbiota is associated with cardiometabolic risk across the epidemiologic transition. PLoS ONE. 2019;14:e0215262. doi: 10.1371/journal.pone.0215262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiao L., Kourkoumpetis T., Hutchinson D., Ajami N.J., Hoffman K., White D.L., Graham D.Y., Hair C., Shah R., Kanwal F., et al. Spatial Characteristics of Colonic Mucosa-Associated Gut Microbiota in Humans. Microb. Ecol. 2022;83:811–821. doi: 10.1007/s00248-021-01789-6. [DOI] [PubMed] [Google Scholar]
- 15.Kerimi A., Kraut N.U., da Encarnacao J.A., Williamson G. The gut microbiome drives inter- and intra-individual differences in metabolism of bioactive small molecules. Sci. Rep. 2020;10:19590. doi: 10.1038/s41598-020-76558-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vuik F.E.R., Dicksved J., Lam S.Y., Fuhler G.M., van der Laan L.J.W., van de Winkel A., Konstantinov S.R., Spaander M.C.W., Peppelenbosch M.P., Engstrand L., et al. Composition of the mucosa-associated microbiota along the entire gastrointestinal tract of human individuals. United Eur. Gastroenterol. J. 2019;7:897. doi: 10.1177/2050640619852255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yeoh Y.K., Chen Z., Hui M., Wong M.C.S., Ho W.C.S., Chin M.L., Ng S.C., Chan F.K.L., Chan P.K.S. Impact of inter- and intra-individual variation, sample storage and sampling fraction on human stool microbial community profiles. PeerJ. 2019;2019:e6172. doi: 10.7717/peerj.6172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benjamin H. Biologic versus chronologic age. J. Gerontol. 1947;2:217–227. doi: 10.1093/geronj/2.3.217. [DOI] [PubMed] [Google Scholar]
- 19.de la Cuesta-Zuluaga J., Kelley S.T., Chen Y., Escobar J.S., Mueller N.T., Ley R.E., McDonald D., Huang S., Swafford A.D., Knight R., et al. Age- and Sex-Dependent Patterns of Gut Microbial Diversity in Human Adults. mSystems. 2019;4:e00261-19. doi: 10.1128/mSystems.00261-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simon J.C., Marchesi J.R., Mougel C., Selosse M.A. Host-microbiota interactions: From holobiont theory to analysis. Microbiome. 2019;7:5. doi: 10.1186/s40168-019-0619-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yatsunenko T., Rey F.E., Manary M.J., Trehan I., Dominguez-Bello M.G., Contreras M., Magris M., Hidalgo G., Baldassano R.N., Anokhin A.P., et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clements S.J., Carding S.R. Diet, the intestinal microbiota, and immune health in aging. Crit. Rev. Food Sci. Nutr. 2018;58:651–661. doi: 10.1080/10408398.2016.1211086. [DOI] [PubMed] [Google Scholar]
- 23.Koh A., De Vadder F., Kovatcheva-Datchary P., Bä Ckhed F. Leading Edge Review From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell. 2016;165:1332–1345. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 24.Ríos-Covián D., Ruas-Madiedo P., Margolles A., Gueimonde M., De los Reyes-Gavilán C.G., Salazar N. Intestinal Short Chain Fatty Acids and their Link with Diet and Human Health. Front. Microbiol. 2016;7:185. doi: 10.3389/fmicb.2016.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ley R.E., Turnbaugh P.J., Klein S., Gordon J.I. Microbial ecology: Human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 26.Ursell L.K., Haiser H.J., Van Treuren W., Garg N., Reddivari L., Vanamala J., Dorrestein P.C., Turnbaugh P.J., Knight R. The Intestinal Metabolome—An Intersection Between Microbiota and Host. Gastroenterology. 2014;146:1470. doi: 10.1053/j.gastro.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parrish A.R. The impact of aging on epithelial barriers. Tissue Barriers. 2017;5:e1343172. doi: 10.1080/21688370.2017.1343172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qi Y.F., Goel R., Kim S., Richards E.M., Carter C.S., Pepine C.J., Raizada M.K., Buford T.W. Intestinal Permeability Biomarker Zonulin is Elevated in Healthy Aging. J. Am. Med. Dir. Assoc. 2017;18:810.e1. doi: 10.1016/j.jamda.2017.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmadi S., Wang S., Nagpal R., Wang B., Jain S., Razazan A., Mishra S.P., Zhu X., Wang Z., Kavanagh K., et al. A human-origin probiotic cocktail ameliorates aging-related leaky gut and inflammation via modulating the microbiota/taurine/tight junction axis. JCI Insight. 2020;5:e132055. doi: 10.1172/jci.insight.132055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh H., Torralba M.G., Moncera K.J., DiLello L., Petrini J., Nelson K.E., Pieper R. Gastro-intestinal and oral microbiome signatures associated with healthy aging. GeroScience. 2019;41:907. doi: 10.1007/s11357-019-00098-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Der Lugt B., Van Beek A.A., Aalvink S., Meijer B., Sovran B., Vermeij W.P., Brandt R.M.C., De Vos W.M., Savelkoul H.F.J., Steegenga W.T., et al. Akkermansia muciniphila ameliorates the age-related decline in colonic mucus thickness and attenuates immune activation in accelerated aging Ercc1−/Δ7 mice. Immun. Ageing. 2019;16:6. doi: 10.1186/s12979-019-0145-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maslowski K.M., Vieira A.T., Ng A., Kranich J., Sierro F., Yu D., Schilter H.C., Rolph M.S., MacKay F., Artis D., et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Myers J.S. Factors associated with changing cognitive function in older adults: Implications for nursing rehabilitation. Rehabil. Nurs. 2008;33:117–123. doi: 10.1002/j.2048-7940.2008.tb00215.x. [DOI] [PubMed] [Google Scholar]
- 34.Nicholson J.K., Holmes E., Kinross J., Burcelin R., Gibson G., Jia W., Pettersson S. Host-gut microbiota metabolic interactions. Science. 2012;336:1262–1267. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- 35.Wikoff W.R., Anfora A.T., Liu J., Schultz P.G., Lesley S.A., Peters E.C., Siuzdak G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc. Natl. Acad. Sci. USA. 2009;106:3698. doi: 10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamada M., Arai H., Yoshimura K., Kajiwara Y., Sonoda T., Nishiguchi S., Aoyama T. Nutritional Supplementation during Resistance Training Improved Skeletal Muscle Mass in Community-Dwelling Frail Older Adults. J. Frailty Aging. 2012;1:64–70. doi: 10.14283/jfa.2012.12. [DOI] [PubMed] [Google Scholar]
- 37.Cabreiro F., Au C., Leung K.Y., Vergara-Irigaray N., Cochemé H.M., Noori T., Weinkove D., Schuster E., Greene N.D.E., Gems D. Metformin Retards Aging in C. elegans by Altering Microbial Folate and Methionine Metabolism. Cell. 2013;153:228. doi: 10.1016/j.cell.2013.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Espinoza S., Walston J.D. Frailty in older adults: Insights and interventions. Cleve. Clin. J. Med. 2005;72:1105–1112. doi: 10.3949/ccjm.72.12.1105. [DOI] [PubMed] [Google Scholar]
- 39.Kenyon C.J. The genetics of ageing. Nature. 2010;464:504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- 40.Martin-Montalvo A., Mercken E.M., Mitchell S.J., Palacios H.H., Mote P.L., Scheibye-Knudsen M., Gomes A.P., Ward T.M., Minor R.K., Blouin M.J., et al. Metformin improves healthspan and lifespan in mice. Nat. Commun. 2013;4:2192. doi: 10.1038/ncomms3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Romero R., Erez O., Hüttemann M., Maymon E., Panaitescu B., Conde-Agudelo A., Pacora P., Yoon B.H., Grossman L.I. Metformin, the aspirin of the 21st century: Its role in gestational diabetes, prevention of preeclampsia and cancer, and the promotion of longevity. Am. J. Obstet. Gynecol. 2017;217:282. doi: 10.1016/j.ajog.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Virk B., Correia G., Dixon D.P., Feyst I., Jia J., Oberleitner N., Briggs Z., Hodge E., Edwards R., Ward J., et al. Excessive folate synthesis limits lifespan in the C. elegans: E. coli aging model. BMC Biol. 2012;10:67. doi: 10.1186/1741-7007-10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bosco N., Noti M. The aging gut microbiome and its impact on host immunity. Genes Immun. 2021;22:289. doi: 10.1038/s41435-021-00126-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hansson G.C. Role of mucus layers in gut infection and inflammation. Curr. Opin. Microbiol. 2012;15:57. doi: 10.1016/j.mib.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jeffery I.B., Lynch D.B., O’Toole P.W. Composition and temporal stability of the gut microbiota in older persons. ISME J. 2016;10:170. doi: 10.1038/ismej.2015.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu L., Zeng T., Zinellu A., Rubino S., Kelvin D.J., Carru C. A Cross-Sectional Study of Compositional and Functional Profiles of Gut Microbiota in Sardinian Centenarians. mSystems. 2019;4:e00325-19. doi: 10.1128/mSystems.00325-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Conway J., Certo M., Lord J.M., Mauro C., Duggal N.A. Understanding the role of host metabolites in the induction of immune senescence: Future strategies for keeping the ageing population healthy. Br. J. Pharmacol. 2022;179:1808–1824. doi: 10.1111/bph.15671. [DOI] [PubMed] [Google Scholar]
- 48.Honarpisheh P., Reynolds C.R., Conesa M.P.B., Manchon J.F.M., Putluri N., Bhattacharjee M.B., Urayama A., McCullough L.D., Ganesh B.P. Dysregulated Gut Homeostasis Observed Prior to the Accumulation of the Brain Amyloid-β in Tg2576 Mice. Int. J. Mol. Sci. 2020;21:1711. doi: 10.3390/ijms21051711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roy-O’Reilly M.A., Ahnstedt H., Spychala M.S., Munshi Y., Aronowski J., Sansing L.H., McCullough L.D. Aging exacerbates neutrophil pathogenicity in ischemic stroke. Aging. 2020;12:436. doi: 10.18632/aging.102632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Clemente J.C., Ursell L.K., Parfrey L.W., Knight R. The Impact of the Gut Microbiota on Human Health: An Integrative View. Cell. 2012;148:1258–1270. doi: 10.1016/j.cell.2012.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gonda T.A., Tu S., Wang T.C. Chronic inflammation, the tumor microenvironment and carcinogenesis. Cell Cycle. 2009;8:2005–2013. doi: 10.4161/cc.8.13.8985. [DOI] [PubMed] [Google Scholar]
- 52.Madison A.A., Kiecolt-Glaser J.K. The Gut Microbiota and Nervous System: Age-Defined and Age-Defying. Semin. Cell Dev. Biol. 2021;116:98. doi: 10.1016/j.semcdb.2020.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dumitrescu L., Marta D., Dănău A., Lefter A., Tulbă D., Cozma L., Manole E., Gherghiceanu M., Ceafalan L.C., Popescu B.O. Serum and Fecal Markers of Intestinal Inflammation and Intestinal Barrier Permeability Are Elevated in Parkinson’s Disease. Front. Neurosci. 2021;15:689723. doi: 10.3389/fnins.2021.689723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Felger J.C. Role of Inflammation in Depression and Treatment Implications. Handb. Exp. Pharmacol. 2019;250:255–286. doi: 10.1007/164_2018_166. [DOI] [PubMed] [Google Scholar]
- 55.Haran J.P., Bhattarai S.K., Foley S.E., Dutta P., Ward D.V., Bucci V., McCormick B.A. Alzheimer’s Disease Microbiome Is Associated with Dysregulation of the Anti-Inflammatory P-Glycoprotein Pathway. MBio. 2019;10:e00632-19. doi: 10.1128/mBio.00632-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shi N., Li N., Duan X., Niu H. Interaction between the gut microbiome and mucosal immune system. Mil. Med. Res. 2017;4:14. doi: 10.1186/s40779-017-0122-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van den Munckhof I.C.L., Kurilshikov A., ter Horst R., Riksen N.P., Joosten L.A.B., Zhernakova A., Fu J., Keating S.T., Netea M.G., de Graaf J., et al. Role of gut microbiota in chronic low-grade inflammation as potential driver for atherosclerotic cardiovascular disease: A systematic review of human studies. Obes. Rev. 2018;19:1719–1734. doi: 10.1111/obr.12750. [DOI] [PubMed] [Google Scholar]
- 58.Wasser C.I., Mercieca E.C., Kong G., Hannan A.J., McKeown S.J., Glikmann-Johnston Y., Stout J.C. Gut dysbiosis in Huntington’s disease: Associations among gut microbiota, cognitive performance and clinical outcomes. Brain Commun. 2020;2:110. doi: 10.1093/braincomms/fcaa110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cordaro M., Scuto M., Siracusa R., D’amico R., Filippo Peritore A., Gugliandolo E., Fusco R., Crupi R., Impellizzeri D., Pozzebon M., et al. Effect of N-palmitoylethanolamine-oxazoline on comorbid neuropsychiatric disturbance associated with inflammatory bowel disease. FASEB J. 2020;34:4085–4106. doi: 10.1096/fj.201901584RR. [DOI] [PubMed] [Google Scholar]
- 60.Clarke G., Stilling R.M., Kennedy P.J., Stanton C., Cryan J.F., Dinan T.G. Minireview: Gut Microbiota: The Neglected Endocrine Organ. Mol. Endocrinol. 2014;28:1221. doi: 10.1210/me.2014-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dinan T.G., Cryan J.F. Gut instincts: Microbiota as a key regulator of brain development, ageing and neurodegeneration. J. Physiol. 2017;595:489. doi: 10.1113/JP273106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lyte M. Microbial endocrinology and the microbiota-gut-brain axis. Adv. Exp. Med. Biol. 2014;817:3–24. doi: 10.1007/978-1-4939-0897-4_1. [DOI] [PubMed] [Google Scholar]
- 63.Samuel B.S., Shaito A., Motoike T., Rey F.E., Backhed F., Manchester J.K., Hammer R.E., Williams S.C., Crowley J., Yanagisawa M., et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc. Natl. Acad. Sci. USA. 2008;105:16767. doi: 10.1073/pnas.0808567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Soliman M.L., Rosenberger T.A. Acetate supplementation increases brain histone acetylation and inhibits histone deacetylase activity and expression. Mol. Cell. Biochem. 2011;352:173–180. doi: 10.1007/s11010-011-0751-3. [DOI] [PubMed] [Google Scholar]
- 65.Jucker M., Walker L.C. Propagation and spread of pathogenic protein assemblies in neurodegenerative diseases. Nat. Neurosci. 2018;21:1341. doi: 10.1038/s41593-018-0238-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pistollato F., Cano S.S., Elio I., Vergara M.M., Giampieri F., Battino M. Role of gut microbiota and nutrients in amyloid formation and pathogenesis of Alzheimer disease. Nutr. Rev. 2016;74:624–634. doi: 10.1093/nutrit/nuw023. [DOI] [PubMed] [Google Scholar]
- 67.Friedland R.P. Mechanisms of molecular mimicry involving the microbiota in neurodegeneration. J. Alzheimer’s Dis. 2015;45:349–362. doi: 10.3233/JAD-142841. [DOI] [PubMed] [Google Scholar]
- 68.Brandscheid C., Schuck F., Reinhardt S., Schäfer K.H., Pietrzik C.U., Grimm M., Hartmann T., Schwiertz A., Endres K. Altered Gut Microbiome Composition and Tryptic Activity of the 5xFAD Alzheimer’s Mouse Model. J. Alzheimer’s Dis. 2017;56:775–788. doi: 10.3233/JAD-160926. [DOI] [PubMed] [Google Scholar]
- 69.Zhuang Z.Q., Shen L.L., Li W.W., Fu X., Zeng F., Gui L., Lü Y., Cai M., Zhu C., Tan Y.L., et al. Gut Microbiota is Altered in Patients with Alzheimer’s Disease. J. Alzheimer’s Dis. 2018;63:1337–1346. doi: 10.3233/JAD-180176. [DOI] [PubMed] [Google Scholar]
- 70.Cattaneo A., Cattane N., Galluzzi S., Provasi S., Lopizzo N., Festari C., Ferrari C., Guerra U.P., Paghera B., Muscio C., et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol. Aging. 2017;49:60–68. doi: 10.1016/j.neurobiolaging.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 71.Garcez M.L., Jacobs K.R., Guillemin G.J. Microbiota Alterations in Alzheimer’s Disease: Involvement of the Kynurenine Pathway and Inflammation. Neurotox. Res. 2019;36:424–436. doi: 10.1007/s12640-019-00057-3. [DOI] [PubMed] [Google Scholar]
- 72.Schlegel P., Novotny M., Klimova B., Valis M. “Muscle-Gut-Brain Axis”: Can Physical Activity Help Patients with Alzheimer’s Disease Due to Microbiome Modulation? J. Alzheimer’s Dis. 2019;71:861–878. doi: 10.3233/JAD-190460. [DOI] [PubMed] [Google Scholar]
- 73.Zou B., Li J., Ma R.X., Cheng X.Y., Ma R.Y., Zhou T.Y., Wu Z.Q., Yao Y., Li J. Gut Microbiota is an Impact Factor based on the Brain-Gut Axis to Alzheimer’s Disease: A Systematic Review. Aging Dis. 2023;14:694. doi: 10.14336/AD.2022.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Krüger J.F., Hillesheim E., Pereira A.C.S.N., Camargo C.Q., Rabito E.I. Probiotics for dementia: A systematic review and meta-analysis of randomized controlled trials. Nutr. Rev. 2021;79:160–170. doi: 10.1093/nutrit/nuaa037. [DOI] [PubMed] [Google Scholar]
- 75.Vendrik K.E.W., Ooijevaar R.E., de Jong P.R.C., Laman J.D., van Oosten B.W., van Hilten J.J., Ducarmon Q.R., Keller J.J., Kuijper E.J., Contarino M.F. Fecal Microbiota Transplantation in Neurological Disorders. Front. Cell. Infect. Microbiol. 2020;10:98. doi: 10.3389/fcimb.2020.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sorboni S.G., Moghaddam H.S., Jafarzadeh-Esfehani R., Soleimanpour S. A Comprehensive Review on the Role of the Gut Microbiome in Human Neurological Disorders. Clin. Microbiol. Rev. 2022;35:e0033820. doi: 10.1128/CMR.00338-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Poewe W., Seppi K., Tanner C.M., Halliday G.M., Brundin P., Volkmann J., Schrag A.E., Lang A.E. Parkinson disease. Nat. Rev. Dis. Prim. 2017;3:17013. doi: 10.1038/nrdp.2017.13. [DOI] [PubMed] [Google Scholar]
- 78.Miraglia F., Colla E. Microbiome, Parkinson’s Disease and Molecular Mimicry. Cells. 2019;8:222. doi: 10.3390/cells8030222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dogra N., Mani R.J., Katare D.P. The Gut-Brain Axis: Two Ways Signaling in Parkinson’s Disease. Cell. Mol. Neurobiol. 2022;42:315–332. doi: 10.1007/s10571-021-01066-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nielsen S.D., Pearson N.M., Seidler K. The link between the gut microbiota and Parkinson’s Disease: A systematic mechanism review with focus on α-synuclein transport. Brain Res. 2021;1769:147609. doi: 10.1016/j.brainres.2021.147609. [DOI] [PubMed] [Google Scholar]
- 81.Pant A., Bisht K.S., Aggarwal S., Maiti T.K. Human gut microbiota and Parkinson’s disease. Prog. Mol. Biol. Transl. Sci. 2022;192:281–307. doi: 10.1016/BS.PMBTS.2022.08.004. [DOI] [PubMed] [Google Scholar]
- 82.Scheperjans F., Aho V., Pereira P.A.B., Koskinen K., Paulin L., Pekkonen E., Haapaniemi E., Kaakkola S., Eerola-Rautio J., Pohja M., et al. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov. Disord. 2015;30:350–358. doi: 10.1002/mds.26069. [DOI] [PubMed] [Google Scholar]
- 83.Hirayama M., Ohno K. Parkinson’s Disease and Gut Microbiota. Ann. Nutr. Metab. 2021;77((Suppl. S2)):28–35. doi: 10.1159/000518147. [DOI] [PubMed] [Google Scholar]
- 84.Hasegawa S., Goto S., Tsuji H., Okuno T., Asahara T., Nomoto K., Shibata A., Fujisawa Y., Minato T., Okamoto A., et al. Intestinal Dysbiosis and Lowered Serum Lipopolysaccharide-Binding Protein in Parkinson’s Disease. PLoS ONE. 2015;10:e0142164. doi: 10.1371/journal.pone.0142164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ullah H., Arbab S., Tian Y., Liu C.Q., Chen Y., Qijie L., Khan M.I.U., Hassan I.U., Li K. The gut microbiota–brain axis in neurological disorder. Front. Neurosci. 2023;17:1225875. doi: 10.3389/fnins.2023.1225875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Unger M.M., Spiegel J., Dillmann K.U., Grundmann D., Philippeit H., Bürmann J., Faßbender K., Schwiertz A., Schäfer K.H. Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat. Disord. 2016;32:66–72. doi: 10.1016/j.parkreldis.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 87.Barichella M., Severgnini M., Cilia R., Cassani E., Bolliri C., Caronni S., Ferri V., Cancello R., Ceccarani C., Faierman S., et al. Unraveling gut microbiota in Parkinson’s disease and atypical parkinsonism. Mov. Disord. 2019;34:396–405. doi: 10.1002/mds.27581. [DOI] [PubMed] [Google Scholar]
- 88.Petrov V.A., Saltykova I.V., Zhukova I.A., Alifirova V.M., Zhukova N.G., Dorofeeva Y.B., Tyakht A.V., Kovarsky B.A., Alekseev D.G., Kostryukova E.S., et al. Analysis of Gut Microbiota in Patients with Parkinson’s Disease. Bull. Exp. Biol. Med. 2017;162:734–737. doi: 10.1007/s10517-017-3700-7. [DOI] [PubMed] [Google Scholar]
- 89.Choi J.G., Kim N., Ju I.G., Eo H., Lim S.M., Jang S.E., Kim D.H., Oh M.S. Oral administration of Proteus mirabilis damages dopaminergic neurons and motor functions in mice. Sci. Rep. 2018;8:1275. doi: 10.1038/s41598-018-19646-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.van Kessel S.P., Frye A.K., El-Gendy A.O., Castejon M., Keshavarzian A., van Dijk G., El Aidy S. Gut bacterial tyrosine decarboxylases restrict levels of levodopa in the treatment of Parkinson’s disease. Nat. Commun. 2019;10:310. doi: 10.1038/S41467-019-08294-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rekdal V.M., Bess E.N., Bisanz J.E., Turnbaugh P.J., Balskus E.P. Discovery and inhibition of an interspecies gut bacterial pathway for Levodopa metabolism. Science. 2019;364:1055. doi: 10.1126/SCIENCE.AAU6323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pierantozzi M., Pietroiusti A., Brusa L., Galati S., Stefani A., Lunardi G., Fedele E., Sancesario G., Bernardi G., Bergamaschi A., et al. Helicobacter pylori eradication and l-dopa absorption in patients with PD and motor fluctuations. Neurology. 2006;66:1824–1829. doi: 10.1212/01.wnl.0000221672.01272.ba. [DOI] [PubMed] [Google Scholar]
- 93.Niehues M., Hensel A. In-vitro interaction of L-dopa with bacterial adhesins of Helicobacter pylori: An explanation for clinicial differences in bioavailability? J. Pharm. Pharmacol. 2009;61:1303–1307. doi: 10.1211/jpp/61.10.0005. [DOI] [PubMed] [Google Scholar]
- 94.Doshi A., Chataway J. Multiple sclerosis, a treatable disease. Clin. Med. 2017;17:530–536. doi: 10.7861/clinmedicine.17-6-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Olsson T., Barcellos L.F., Alfredsson L. Interactions between genetic, lifestyle and environmental risk factors for multiple sclerosis. Nat. Rev. Neurol. 2017;13:26–36. doi: 10.1038/nrneurol.2016.187. [DOI] [PubMed] [Google Scholar]
- 96.Schwendimann R.N., Alekseeva N. Gender issues in multiple sclerosis. Int. Rev. Neurobiol. 2007;79:377–392. doi: 10.1016/S0074-7742(07)79017-7. [DOI] [PubMed] [Google Scholar]
- 97.Harbo H.F., Gold R., Tintora M. Sex and gender issues in multiple sclerosis. Ther. Adv. Neurol. Disord. 2013;6:237–248. doi: 10.1177/1756285613488434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Airas L. Hormonal and gender-related immune changes in multiple sclerosis. Acta Neurol. Scand. 2015;132:62–70. doi: 10.1111/ane.12433. [DOI] [PubMed] [Google Scholar]
- 99.Frohman E.M., Racke M.K., Raine C.S. Multiple sclerosis--the plaque and its pathogenesis. N. Engl. J. Med. 2006;354:942–955. doi: 10.1056/NEJMra052130. [DOI] [PubMed] [Google Scholar]
- 100.DePaula-Silva A.B. The Contribution of Microglia and Brain-Infiltrating Macrophages to the Pathogenesis of Neuroinflammatory and Neurodegenerative Diseases during TMEV Infection of the Central Nervous System. Viruses. 2024;16:119. doi: 10.3390/v16010119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee C.Y., Chan K.H. Personalized Use of Disease-Modifying Therapies in Multiple Sclerosis. Pharmaceutics. 2024;16:120. doi: 10.3390/pharmaceutics16010120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yissachar N., Zhou Y., Ung L., Lai N.Y., Mohan J.F., Ehrlicher A., Weitz D.A., Kasper D.L., Chiu I.M., Mathis D., et al. An Intestinal Organ Culture System Uncovers a Role for the Nervous System in Microbe-Immune Crosstalk. Cell. 2017;168:1135–1148.e12. doi: 10.1016/j.cell.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rooks M.G., Garrett W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016;16:341–352. doi: 10.1038/nri.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chu F., Shi M., Lang Y., Shen D., Jin T., Zhu J., Cui L. Gut Microbiota in Multiple Sclerosis and Experimental Autoimmune Encephalomyelitis: Current Applications and Future Perspectives. Mediators Inflamm. 2018;2018:8168717. doi: 10.1155/2018/8168717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shahi S.K., Freedman S.N., Mangalam A.K. Gut microbiome in multiple sclerosis: The players involved and the roles they play. Gut Microbes. 2017;8:607–615. doi: 10.1080/19490976.2017.1349041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Freedman S.N., Shahi S.K., Mangalam A.K. The “Gut Feeling”: Breaking Down the Role of Gut Microbiome in Multiple Sclerosis. Neurotherapeutics. 2018;15:109. doi: 10.1007/s13311-017-0588-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen J., Chia N., Kalari K.R., Yao J.Z., Novotna M., Soldan M.M.P., Luckey D.H., Marietta E.V., Jeraldo P.R., Chen X., et al. Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci. Rep. 2016;6:28484. doi: 10.1038/srep28484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jangi S., Gandhi R., Cox L.M., Li N., Von Glehn F., Yan R., Patel B., Mazzola M.A., Liu S., Glanz B.L., et al. Alterations of the human gut microbiome in multiple sclerosis. Nat. Commun. 2016;7:12015. doi: 10.1038/ncomms12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Miyake S., Kim S., Suda W., Oshima K., Nakamura M., Matsuoka T., Chihara N., Tomita A., Sato W., Kim S.W., et al. Dysbiosis in the Gut Microbiota of Patients with Multiple Sclerosis, with a Striking Depletion of Species Belonging to Clostridia XIVa and IV Clusters. PLoS ONE. 2015;10:e0137429. doi: 10.1371/journal.pone.0137429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tremlett H., Fadrosh D.W., Faruqi A.A., Zhu F., Hart J., Roalstad S., Graves J., Lynch S., Waubant E., Aaen G., et al. Gut microbiota in early pediatric multiple sclerosis: A case-control study. Eur. J. Neurol. 2016;23:1308–1321. doi: 10.1111/ene.13026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cekanaviciute E., Yoo B.B., Runia T.F., Debelius J.W., Singh S., Nelson C.A., Kanner R., Bencosme Y., Lee Y.K., Hauser S.L., et al. Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc. Natl. Acad. Sci. USA. 2017;114:10713–10718. doi: 10.1073/pnas.1711235114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cosorich I., Dalla-Costa G., Sorini C., Ferrarese R., Messina M.J., Dolpady J., Radice E., Mariani A., Testoni P.A., Canducci F., et al. High frequency of intestinal TH17 cells correlates with microbiota alterations and disease activity in multiple sclerosis. Sci. Adv. 2017;3:e1700492. doi: 10.1126/sciadv.1700492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cantarel B.L., Waubant E., Chehoud C., Kuczynski J., Desantis T.Z., Warrington J., Venkatesan A., Fraser C.M., Mowry E.M. Gut microbiota in multiple sclerosis: Possible influence of immunomodulators. J. Investig. Med. 2015;63:729–734. doi: 10.1097/JIM.0000000000000192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Berer K., Gerdes L.A., Cekanaviciute E., Jia X., Xiao L., Xia Z., Liu C., Klotz L., Stauffer U., Baranzini S.E., et al. Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proc. Natl. Acad. Sci. USA. 2017;114:10719–10724. doi: 10.1073/pnas.1711233114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sanchez-Rodriguez E., Egea-Zorrilla A., Plaza-Díaz J., Aragón-Vela J., Muñoz-Quezada S., Tercedor-Sánchez L., Abadia-Molina F. The Gut Microbiota and Its Implication in the Development of Atherosclerosis and Related Cardiovascular Diseases. Nutrients. 2020;12:605. doi: 10.3390/nu12030605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Klinder A., Shen Q., Heppel S., Lovegrove J.A., Rowland I., Tuohy K.M. Impact of increasing fruit and vegetables and flavonoid intake on the human gut microbiota. Food Funct. 2016;7:1788–1796. doi: 10.1039/C5FO01096A. [DOI] [PubMed] [Google Scholar]
- 117.Koeth R.A., Lam-Galvez B.R., Kirsop J., Wang Z., Levison B.S., Gu X., Copeland M.F., Bartlett D., Cody D.B., Dai H.J., et al. l-Carnitine in omnivorous diets induces an atherogenic gut microbial pathway in humans. J. Clin. Investig. 2019;129:373–387. doi: 10.1172/JCI94601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Roncal C., Martínez-Aguilar E., Orbe J., Ravassa S., Fernandez-Montero A., Saenz-Pipaon G., Ugarte A., Estella-Hermoso de Mendoza A., Rodriguez J.A., Fernández-Alonso S., et al. Trimethylamine-N-Oxide (TMAO) Predicts Cardiovascular Mortality in Peripheral Artery Disease. Sci. Rep. 2019;9:15580. doi: 10.1038/s41598-019-52082-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gomez-Arango L.F., Barrett H.L., McIntyre H.D., Callaway L.K., Morrison M., Dekker Nitert M. Increased Systolic and Diastolic Blood Pressure Is Associated With Altered Gut Microbiota Composition and Butyrate Production in Early Pregnancy. Hypertension. 2016;68:974–981. doi: 10.1161/HYPERTENSIONAHA.116.07910. [DOI] [PubMed] [Google Scholar]
- 120.Tang W.H.W., Kitai T., Hazen S.L. Gut Microbiota in Cardiovascular Health and Disease. Circ. Res. 2017;120:1183–1196. doi: 10.1161/CIRCRESAHA.117.309715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Witkowski M., Weeks T.L., Hazen S.L. Gut Microbiota and Cardiovascular Disease. Circ. Res. 2020;127:553–570. doi: 10.1161/CIRCRESAHA.120.316242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pearson T.A., Mensah G.A., Alexander R.W., Anderson J.L., Cannon R.O., Criqui M., Fadl Y.Y., Fortmann S.P., Hong Y., Myers G.L., et al. Markers of inflammation and cardiovascular disease: Application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.CIR.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 123.Gil-Cruz C., Perez-Shibayama C., de Martin A., Ronchi F., van der Borght K., Niederer R., Onder L., Lütge M., Novkovic M., Nindl V., et al. Microbiota-derived peptide mimics drive lethal inflammatory cardiomyopathy. Science. 2019;366:881–886. doi: 10.1126/science.aav3487. [DOI] [PubMed] [Google Scholar]
- 124.Schiffrin E.J., Parlesak A., Bode C., Bode J.C., van’t Hof M.A., Grathwohl D., Guigoz Y. Probiotic yogurt in the elderly with intestinal bacterial overgrowth: Endotoxaemia and innate immune functions. Br. J. Nutr. 2009;101:961–966. doi: 10.1017/S0007114508055591. [DOI] [PubMed] [Google Scholar]
- 125.Dong H., Rowland I., Thomas L.V., Yaqoob P. Immunomodulatory effects of a probiotic drink containing Lactobacillus casei Shirota in healthy older volunteers. Eur. J. Nutr. 2013;52:1853–1863. doi: 10.1007/s00394-012-0487-1. [DOI] [PubMed] [Google Scholar]
- 126.Valentini L., Pinto A., Bourdel-Marchasson I., Ostan R., Brigidi P., Turroni S., Hrelia S., Hrelia P., Bereswill S., Fischer A., et al. Impact of personalized diet and probiotic supplementation on inflammation, nutritional parameters and intestinal microbiota—The “RISTOMED project”: Randomized controlled trial in healthy older people. Clin. Nutr. 2015;34:593–602. doi: 10.1016/j.clnu.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 127.Larsen J.M. The immune response to Prevotella bacteria in chronic inflammatory disease. Immunology. 2017;151:363–374. doi: 10.1111/imm.12760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yoshida N., Yamashita T., Kishino S., Watanabe H., Sasaki K., Sasaki D., Tabata T., Sugiyama Y., Kitamura N., Saito Y., et al. A possible beneficial effect of Bacteroides on faecal lipopolysaccharide activity and cardiovascular diseases. Sci. Rep. 2020;10:13009. doi: 10.1038/s41598-020-69983-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Franzosa E.A., Hsu T., Sirota-Madi A., Shafquat A., Abu-Ali G., Morgan X.C., Huttenhower C. Sequencing and beyond: Integrating molecular “omics” for microbial community profiling. Nat. Rev. Microbiol. 2015;13:360–372. doi: 10.1038/nrmicro3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yan Q., Gu Y., Li X., Yang W., Jia L., Chen C., Han X., Huang Y., Zhao L., Li P., et al. Alterations of the gut microbiome in hypertension. Front. Cell. Infect. Microbiol. 2017;7:381. doi: 10.3389/fcimb.2017.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ahmad A.F., Dwivedi G., O’Gara F., Caparros-Martin J., Ward N.C. The gut microbiome and cardiovascular disease: Current knowledge and clinical potential. Am. J. Physiol. Heart Circ. Physiol. 2019;317:H923–H938. doi: 10.1152/ajpheart.00376.2019. [DOI] [PubMed] [Google Scholar]
- 132.Li N., Weng X., Sun C., Wu X., Lu M., Si Y., Ye X., Wang T., Yu X., Zhao X., et al. Change of intestinal microbiota in cerebral ischemic stroke patients. BMC Microbiol. 2019;19:191. doi: 10.1186/s12866-019-1552-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Xia G.H., You C., Gao X.X., Zeng X.L., Zhu J.J., Xu K.Y., Tan C.H., Xu R.T., Wu Q.H., Zhou H.W., et al. Stroke dysbiosis index (SDI) in gut microbiome are associated with brain injury and prognosis of stroke. Front. Neurol. 2019;10:397. doi: 10.3389/fneur.2019.00397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yin J., Liao S.X., He Y., Wang S., Xia G.H., Liu F.T., Zhu J.J., You C., Chen Q., Zhou L., et al. Dysbiosis of Gut Microbiota With Reduced Trimethylamine-N-Oxide Level in Patients With Large-Artery Atherosclerotic Stroke or Transient Ischemic Attack. J. Am. Hear. Assoc. Cardiovasc. Cerebrovasc. Dis. 2015;4:e002699. doi: 10.1161/JAHA.115.002699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Karlsson F.H., Fåk F., Nookaew I., Tremaroli V., Fagerberg B., Petranovic D., Bäckhed F., Nielsen J. Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat. Commun. 2012;3:1245. doi: 10.1038/ncomms2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tuomisto S., Huhtala H., Martiskainen M., Goebeler S., Lehtimäki T., Karhunen P.J. Age-dependent association of gut bacteria with coronary atherosclerosis: Tampere Sudden Death Study. PLoS ONE. 2019;14:e0221345. doi: 10.1371/journal.pone.0221345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Arthur J.C., Perez-Chanona E., Mühlbauer M., Tomkovich S., Uronis J.M., Fan T.J., Campbell B.J., Abujamel T., Dogan B., Rogers A.B., et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science. 2012;338:120. doi: 10.1126/science.1224820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Baruch E.N., Youngster I., Ben-Betzalel G., Ortenberg R., Lahat A., Katz L., Adler K., Dick-Necula D., Raskin S., Bloch N., et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science. 2021;371:602–609. doi: 10.1126/science.abb5920. [DOI] [PubMed] [Google Scholar]
- 139.Cheng W.Y., Wu C.Y., Yu J. The role of gut microbiota in cancer treatment: Friend or foe? Gut. 2020;69:1867. doi: 10.1136/gutjnl-2020-321153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Davar D., Dzutsev A.K., McCulloch J.A., Rodrigues R.R., Chauvin J.M., Morrison R.M., Deblasio R.N., Menna C., Ding Q., Pagliano O., et al. Fecal microbiota transplant overcomes resistance to anti–PD-1 therapy in melanoma patients. Science. 2021;371:595. doi: 10.1126/science.abf3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sears C.L., Pardoll D.M. Perspective: Alpha-Bugs, Their Microbial Partners, and the Link to Colon Cancer. J. Infect. Dis. 2011;203:306. doi: 10.1093/jinfdis/jiq061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tsoi H., Chu E.S.H., Zhang X., Sheng J., Nakatsu G., Ng S.C., Chan A.W.H., Chan F.K.L., Sung J.J.Y., Yu J. Peptostreptococcus anaerobius Induces Intracellular Cholesterol Biosynthesis in Colon Cells to Induce Proliferation and Causes Dysplasia in Mice. Gastroenterology. 2017;152:1419–1433.e5. doi: 10.1053/j.gastro.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 143.Wu S., Rhee K.J., Albesiano E., Rabizadeh S., Wu X., Yen H.R., Huso D.L., Brancati F.L., Wick E., McAllister F., et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat. Med. 2009;15:1016. doi: 10.1038/nm.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zeng J., Zhang G., Chen C., Li K., Wen Y., Zhao J., Wu P. Alterations in Urobiome in Patients With Bladder Cancer and Implications for Clinical Outcome: A Single-Institution Study. Front. Cell. Infect. Microbiol. 2020;10:555508. doi: 10.3389/fcimb.2020.555508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dey P., Chaudhuri S.R. Cancer-Associated Microbiota: From Mechanisms of Disease Causation to Microbiota-Centric Anti-Cancer Approaches. Biology. 2022;11:757. doi: 10.3390/biology11050757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Guo Y., Kitamoto S., Kamada N. Microbial adaptation to the healthy and inflamed gut environments. Gut Microbes. 2020;12:1857505. doi: 10.1080/19490976.2020.1857505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Li Z., Ke X., Zuo D., Wang Z., Fang F., Li B. New Insights into the Relationship between Gut Microbiota and Radiotherapy for Cancer. Nutrients. 2023;15:48. doi: 10.3390/nu15010048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Clay S.L., Fonseca-Pereira D., Garrett W.S. Colorectal cancer: The facts in the case of the microbiota. J. Clin. Investig. 2022;132:e155101. doi: 10.1172/JCI155101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Dalmasso G., Cougnoux A., Delmas J., Darfeuille-Michaud A., Bonnet R. The bacterial genotoxin colibactin promotes colon tumor growth by modifying the tumor microenvironment. Gut Microbes. 2014;5:675–680. doi: 10.4161/19490976.2014.969989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Buc E., Dubois D., Sauvanet P., Raisch J., Delmas J., Darfeuille-Michaud A., Pezet D., Bonnet R. High prevalence of mucosa-associated E. coli producing cyclomodulin and genotoxin in colon cancer. PLoS ONE. 2013;8:e56964. doi: 10.1371/journal.pone.0056964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Shmuely H., Passaro D., Figer A., Niv Y., Pitlik S., Samra Z., Koren R., Yahav J. Relationship between Helicobacter pylori CagA status and colorectal cancer. Am. J. Gastroenterol. 2001;96:3406–3410. doi: 10.1111/j.1572-0241.2001.05342.x. [DOI] [PubMed] [Google Scholar]
- 152.Cheng K.W., Tseng C.H., Chen I.J., Huang B.C., Liu H.J., Ho K.W., Lin W.W., Chuang C.H., Huang M.Y., Leu Y.L., et al. Inhibition of gut microbial β-glucuronidase effectively prevents carcinogen-induced microbial dysbiosis and intestinal tumorigenesis. Pharmacol. Res. 2022;177:106115. doi: 10.1016/j.phrs.2022.106115. [DOI] [PubMed] [Google Scholar]
- 153.Pollet R.M., D’Agostino E.H., Walton W.G., Xu Y., Little M.S., Biernat K.A., Pellock S.J., Patterson L.M., Creekmore B.C., Isenberg H.N., et al. An Atlas of β-Glucuronidases in the Human Intestinal Microbiome. Structure. 2017;25:967–977.e5. doi: 10.1016/j.str.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Matson V., Chervin C.S., Gajewski T.F. Cancer and the Microbiome—Influence of the Commensal Microbiota on Cancer, Immune Responses, and Immunotherapy. Gastroenterology. 2021;160:600. doi: 10.1053/j.gastro.2020.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pushalkar S., Hundeyin M., Daley D., Zambirinis C.P., Kurz E., Mishra A., Mohan N., Aykut B., Usyk M., Torres L.E., et al. The Pancreatic Cancer Microbiome Promotes Oncogenesis by Induction of Innate and Adaptive Immune Suppression. Cancer Discov. 2018;8:403–416. doi: 10.1158/2159-8290.CD-17-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Geller L.T., Barzily-Rokni M., Danino T., Jonas O.H., Shental N., Nejman D., Gavert N., Zwang Y., Cooper Z.A., Shee K., et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science. 2017;357:1156–1160. doi: 10.1126/science.aah5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Dapito D.H., Mencin A., Gwak G.Y., Pradere J.P., Jang M.K., Mederacke I., Caviglia J.M., Khiabanian H., Adeyemi A., Bataller R., et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell. 2012;21:504–516. doi: 10.1016/j.ccr.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ma C., Han M., Heinrich B., Fu Q., Zhang Q., Sandhu M., Agdashian D., Terabe M., Berzofsky J.A., Fako V., et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science. 2018;360:eaan5931. doi: 10.1126/science.aan5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Loo T.M., Kamachi F., Watanabe Y., Yoshimoto S., Kanda H., Arai Y., Nakajima-Takagi Y., Iwama A., Koga T., Sugimoto Y., et al. Gut Microbiota Promotes Obesity-Associated Liver Cancer through PGE2-Mediated Suppression of Antitumor Immunity. Cancer Discov. 2017;7:522–538. doi: 10.1158/2159-8290.CD-16-0932. [DOI] [PubMed] [Google Scholar]
- 160.Bouhnik Y., Vahedi K., Achour L., Attar A., Salfati J., Pochart P., Marteau P., Flourié B., Bornet F., Rambaud J.C. Short-chain fructo-oligosaccharide administration dose-dependently increases fecal bifidobacteria in healthy humans. J. Nutr. 1999;129:113–116. doi: 10.1093/jn/129.1.113. [DOI] [PubMed] [Google Scholar]
- 161.Verdu E.F., Collins S.M. Irritable bowel syndrome. Best Pract. Res. Clin. Gastroenterol. 2004;18:315–321. doi: 10.1016/j.bpg.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 162.Cardona F., Andrés-Lacueva C., Tulipani S., Tinahones F.J., Queipo-Ortuño M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013;24:1415–1422. doi: 10.1016/j.jnutbio.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 163.Hervert-Hernández D., Goñi I. Dietary Polyphenols and Human Gut Microbiota: A Review. Food Rev. Int. 2011;27:154–169. doi: 10.1080/87559129.2010.535233. [DOI] [Google Scholar]
- 164.Kuhn P., Kalariya H.M., Poulev A., Ribnicky D.M., Jaja-Chimedza A., Roopchand D.E. Grape polyphenols reduce gut-localized reactive oxygen species associated with the development of metabolic syndrome in mice. PLoS ONE. 2018;13:e0198716. doi: 10.1371/journal.pone.0198716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Jiao X., Wang Y., Lin Y., Lang Y., Li E., Zhang X., Zhang Q., Feng Y., Meng X., Li B. Blueberry polyphenols extract as a potential prebiotic with anti-obesity effects on C57BL/6 J mice by modulating the gut microbiota. J. Nutr. Biochem. 2019;64:88–100. doi: 10.1016/j.jnutbio.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 166.Qiao Y., Sun J., Xia S., Tang X., Shi Y., Le G. Effects of resveratrol on gut microbiota and fat storage in a mouse model with high-fat-induced obesity. Food Funct. 2014;5:1241–1249. doi: 10.1039/c3fo60630a. [DOI] [PubMed] [Google Scholar]
- 167.Stapleton P.D., Shah S., Ehlert K., Hara Y., Taylor P.W. The β-lactam-resistance modifier (–)-epicatechin gallate alters the architecture of the cell wall of Staphylococcus aureus. Microbiology. 2007;153:2093. doi: 10.1099/mic.0.2007/007807-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Faralli A., Shekarforoush E., Ajalloueian F., Mendes A.C., Chronakis I.S. In vitro permeability enhancement of curcumin across Caco-2 cells monolayers using electrospun xanthan-chitosan nanofibers. Carbohydr. Polym. 2019;206:38–47. doi: 10.1016/j.carbpol.2018.10.073. [DOI] [PubMed] [Google Scholar]
- 169.Wang J., Ghosh S.S., Ghosh S. Curcumin improves intestinal barrier function: Modulation of intracellular signaling, and organization of tight junctions. Am. J. Physiol. Cell Physiol. 2017;312:C438–C445. doi: 10.1152/ajpcell.00235.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hou H.T., Qiu Y.M., Zhao H.W., Li D.H., Liu Y.T., Wang Y.Z., Su S.H. Effect of curcumin on intestinal mucosal mechanical barrier in rats with non-alcoholic fatty liver disease. Zhonghua Gan Zang Bing Za Zhi. 2017;25:134–138. doi: 10.3760/CMA.J.ISSN.1007-3418.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 171.Ghosh S.S., Bie J., Wang J., Ghosh S. Oral Supplementation with Non-Absorbable Antibiotics or Curcumin Attenuates Western Diet-Induced Atherosclerosis and Glucose Intolerance in LDLR−/− Mice—Role of Intestinal Permeability and Macrophage Activation. PLoS ONE. 2014;9:e108577. doi: 10.1371/journal.pone.0108577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ohno M., Nishida A., Sugitani Y., Nishino K., Inatomi O., Sugimoto M., Kawahara M., Andoh A. Nanoparticle curcumin ameliorates experimental colitis via modulation of gut microbiota and induction of regulatory T cells. PLoS ONE. 2017;12:e0185999. doi: 10.1371/journal.pone.0185999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Fu Y., Gao R., Cao Y., Guo M., Wei Z., Zhou E., Li Y., Yao M., Yang Z., Zhang N. Curcumin attenuates inflammatory responses by suppressing TLR4-mediated NF-κB signaling pathway in lipopolysaccharide-induced mastitis in mice. Int. Immunopharmacol. 2014;20:54–58. doi: 10.1016/j.intimp.2014.01.024. [DOI] [PubMed] [Google Scholar]
- 174.Zhu H.T., Bian C., Yuan J.C., Chu W.H., Xiang X., Chen F., Wang C.S., Feng H., Lin J.K. Curcumin attenuates acute inflammatory injury by inhibiting the TLR4/MyD88/NF-κB signaling pathway in experimental traumatic brain injury. J. Neuroinflammation. 2014;11:59. doi: 10.1186/1742-2094-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Jobin C., Bradham C.A., Russo M.P., Juma B., Narula A.S., Brenner D.A., Sartor R.B. Curcumin Blocks Cytokine-Mediated NF-κB Activation and Proinflammatory Gene Expression by Inhibiting Inhibitory Factor I-κB Kinase Activity. J. Immunol. 1999;163:3474–3483. doi: 10.4049/jimmunol.163.6.3474. [DOI] [PubMed] [Google Scholar]
- 176.Tzounis X., Vulevic J., Kuhnle G.G.C., George T., Leonczak J., Gibson G.R., Kwik-Uribe C., Spencer J.P.E. Flavanol monomer-induced changes to the human faecal microflora. Br. J. Nutr. 2008;99:782–792. doi: 10.1017/S0007114507853384. [DOI] [PubMed] [Google Scholar]
- 177.Tombola F., Campello S., De Luca L., Ruggiero P., Del Giudice G., Papini E., Zoratti M. Plant polyphenols inhibit VacA, a toxin secreted by the gastric pathogen Helicobacter pylori. FEBS Lett. 2003;543:184–189. doi: 10.1016/S0014-5793(03)00443-5. [DOI] [PubMed] [Google Scholar]
- 178.Larrosa M., Yañéz-Gascón M.J., Selma M.V., González-Sarrías A., Toti S., Cerón J.J., Tomás-Barberán F., Dolara P., Espín J.C. Effect of a low dose of dietary resveratrol on colon microbiota, inflammation and tissue damage in a DSS-induced colitis rat model. J. Agric. Food Chem. 2009;57:2211–2220. doi: 10.1021/jf803638d. [DOI] [PubMed] [Google Scholar]
- 179.Yamakoshi J., Tokutake S., Kikuchi M., Kubota Y., Konishi H., Mitsuoka T. Effect of Proanthocyanidin-Rich Extract from Grape Seeds on Human Fecal Flora and Fecal Odor. Microb. Ecol. Health Dis. 2001;13:25–31. [Google Scholar]
- 180.Molan A.L., Liu Z., Kruger M. The ability of blackcurrant extracts to positively modulate key markers of gastrointestinal function in rats. World J. Microbiol. Biotechnol. 2010;26:1735–1743. doi: 10.1007/s11274-010-0352-4. [DOI] [Google Scholar]
- 181.Tzounis X., Rodriguez-Mateos A., Vulevic J., Gibson G.R., Kwik-Uribe C., Spencer J.P.E. Prebiotic evaluation of cocoa-derived flavanols in healthy humans by using a randomized, controlled, double-blind, crossover intervention study. Am. J. Clin. Nutr. 2011;93:62–72. doi: 10.3945/ajcn.110.000075. [DOI] [PubMed] [Google Scholar]
- 182.Viveros A., Chamorro S., Pizarro M., Arija I., Centeno C., Brenes A. Effects of dietary polyphenol-rich grape products on intestinal microflora and gut morphology in broiler chicks. Poult. Sci. 2011;90:566–578. doi: 10.3382/ps.2010-00889. [DOI] [PubMed] [Google Scholar]
- 183.Tringali C. Special Issue: From Natural Polyphenols to Synthetic Bioactive Analogues. Molecules. 2020;25:2772. doi: 10.3390/molecules25122772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Marín L., Miguélez E.M., Villar C.J., Lombó F. Bioavailability of dietary polyphenols and gut microbiota metabolism: Antimicrobial properties. Biomed Res. Int. 2015;2015:905215. doi: 10.1155/2015/905215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Aragonès G., Danesi F., Del Rio D., Mena P. The importance of studying cell metabolism when testing the bioactivity of phenolic compounds. Trends Food Sci. Technol. 2017;69:230–242. doi: 10.1016/j.tifs.2017.02.001. [DOI] [Google Scholar]
- 186.Kawabata K., Yoshioka Y., Terao J. Role of Intestinal Microbiota in the Bioavailability and Physiological Functions of Dietary Polyphenols. Molecules. 2019;24:370. doi: 10.3390/molecules24020370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lewandowska H., Kalinowska M., Lewandowski W., Stepkowski T.M., Brzóska K. The role of natural polyphenols in cell signaling and cytoprotection against cancer development. J. Nutr. Biochem. 2016;32:1–19. doi: 10.1016/j.jnutbio.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 188.Liu Z., Hu M. Natural polyphenol disposition via coupled metabolic pathways. Expert Opin. Drug Metab. Toxicol. 2007;3:389–406. doi: 10.1517/17425255.3.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Manach C., Williamson G., Morand C., Scalbert A., Rémésy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005;81:230S–242S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- 190.Dueñas M., Muñoz-González I., Cueva C., Jiménez-Girón A., Sánchez-Patán F., Santos-Buelga C., Moreno-Arribas M.V., Bartolomé B. A survey of modulation of gut microbiota by dietary polyphenols. Biomed Res. Int. 2015;2015:850902. doi: 10.1155/2015/850902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Filosa S., Di Meo F., Crispi S. Polyphenols-gut microbiota interplay and brain neuromodulation. Neural Regen. Res. 2018;13:2055–2059. doi: 10.4103/1673-5374.241429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Hoyles L., Swann J. The Handbook of Metabolic Phenotyping. Elsevier; Amsterdam, The Netherlands: 2019. Influence of the Human Gut Microbiome on the Metabolic Phenotype; pp. 535–560. Chapter 18. [DOI] [Google Scholar]
- 193.Pandey K.B., Rizvi S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009;2:270. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Figueira I., Garcia G., Pimpão R.C., Terrasso A.P., Costa I., Almeida A.F., Tavares L., Pais T.F., Pinto P., Ventura M.R., et al. Polyphenols journey through blood-brain barrier towards neuronal protection. Sci. Rep. 2017;7:11456. doi: 10.1038/s41598-017-11512-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Bondonno C.P., Yang X., Croft K.D., Considine M.J., Ward N.C., Rich L., Puddey I.B., Swinny E., Mubarak A., Hodgson J.M. Flavonoid-rich apples and nitrate-rich spinach augment nitric oxide status and improve endothelial function in healthy men and women: A randomized controlled trial. Free Radic. Biol. Med. 2012;52:95–102. doi: 10.1016/j.freeradbiomed.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 196.Bondonno N.P., Bondonno C.P., Blekkenhorst L.C., Considine M.J., Maghzal G., Stocker R., Woodman R.J., Ward N.C., Hodgson J.M., Croft K.D. Flavonoid-Rich Apple Improves Endothelial Function in Individuals at Risk for Cardiovascular Disease: A Randomized Controlled Clinical Trial. Mol. Nutr. Food Res. 2018;62:1700674. doi: 10.1002/mnfr.201700674. [DOI] [PubMed] [Google Scholar]
- 197.Saccon T.D., Nagpal R., Yadav H., Cavalcante M.B., Nunes A.D.D.C., Schneider A., Gesing A., Hughes B., Yousefzadeh M., Tchkonia T., et al. Senolytic Combination of Dasatinib and Quercetin Alleviates Intestinal Senescence and Inflammation and Modulates the Gut Microbiome in Aged Mice. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2021;76:1895. doi: 10.1093/gerona/glab002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Akbik D., Ghadiri M., Chrzanowski W., Rohanizadeh R. Curcumin as a wound healing agent. Life Sci. 2014;116:1–7. doi: 10.1016/j.lfs.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 199.Menon V.P., Sudheer A.R. Antioxidant and anti-inflammatory properties of curcumin. Adv. Exp. Med. Biol. 2007;595:105–125. doi: 10.1007/978-0-387-46401-5_3. [DOI] [PubMed] [Google Scholar]
- 200.Pricci M., Girardi B., Giorgio F., Losurdo G., Ierardi E., Di Leo A. Curcumin and Colorectal Cancer: From Basic to Clinical Evidences. Int. J. Mol. Sci. 2020;21:2364. doi: 10.3390/ijms21072364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Shahriari M., Kesharwani P., Johnston T.P., Sahebkar A. Anticancer potential of curcumin-cyclodextrin complexes and their pharmacokinetic properties. Int. J. Pharm. 2023;631:122474. doi: 10.1016/j.ijpharm.2022.122474. [DOI] [PubMed] [Google Scholar]
- 202.Bielak-Zmijewska A., Grabowska W., Ciolko A., Bojko A., Mosieniak G., Bijoch Ł., Sikora E. The Role of Curcumin in the Modulation of Ageing. Int. J. Mol. Sci. 2019;20:1239. doi: 10.3390/ijms20051239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Di Meo F., Margarucci S., Galderisi U., Crispi S., Peluso G. Curcumin, Gut Microbiota, and Neuroprotection. Nutrients. 2019;11:2426. doi: 10.3390/nu11102426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Skyvalidas D., Mavropoulos A., Tsiogkas S., Dardiotis E., Liaskos C., Mamuris Z., Roussaki-Schulze A., Sakkas L.I., Zafiriou E., Bogdanos D.P. Curcumin mediates attenuation of pro-inflammatory interferon γ and interleukin 17 cytokine responses in psoriatic disease, strengthening its role as a dietary immunosuppressant. Nutr. Res. 2020;75:95–108. doi: 10.1016/j.nutres.2020.01.005. [DOI] [PubMed] [Google Scholar]
- 205.Wei R., Su Z., Mackenzie G.G. Chlorogenic acid combined with epigallocatechin-3-gallate mitigates D-galactose-induced gut aging in mice. Food Funct. 2023;14:2684–2697. doi: 10.1039/D2FO03306B. [DOI] [PubMed] [Google Scholar]
- 206.Peron G., Gargari G., Meroño T., Miñarro A., Lozano E.V., Escuder P.C., González-Domínguez R., Hidalgo-Liberona N., Del Bo C., Bernardi S., et al. Crosstalk among intestinal barrier, gut microbiota and serum metabolome after a polyphenol-rich diet in older subjects with “leaky gut”: The MaPLE trial. Clin. Nutr. 2021;40:5288–5297. doi: 10.1016/j.clnu.2021.08.027. [DOI] [PubMed] [Google Scholar]
- 207.Rodríguez-Costa S., Cardelle-Cobas A., Roca-Saavedra P., Porto-Arias J.J., Miranda J.M., Cepeda A. In vitro evaluation of the prebiotic effect of red and white grape polyphenolic extracts. J. Physiol. Biochem. 2018;74:101–110. doi: 10.1007/s13105-017-0573-1. [DOI] [PubMed] [Google Scholar]
- 208.Zhang Z.D., Tao Q., Bai L.X., Qin Z., Liu X.W., Li S.H., Yang Y.J., Ge W.B., Li J.Y. The Transport and Uptake of Resveratrol Mediated via Glucose Transporter 1 and Its Antioxidant Effect in Caco-2 Cells. Molecules. 2023;28:4569. doi: 10.3390/molecules28124569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Song C., Zhang Y., Cheng L., Shi M., Li X., Zhang L., Zhao H. Tea polyphenols ameliorates memory decline in aging model rats by inhibiting brain TLR4/NF-κB inflammatory signaling pathway caused by intestinal flora dysbiosis. Exp. Gerontol. 2021;153:111476. doi: 10.1016/j.exger.2021.111476. [DOI] [PubMed] [Google Scholar]
- 210.Chen L., Tai W.C.S., Hsiao W.L.W. Dietary saponins from four popular herbal tea exert prebiotic-like effects on gut microbiota in C57BL/6 mice. J. Funct. Foods. 2015;17:892–902. doi: 10.1016/j.jff.2015.06.050. [DOI] [Google Scholar]
- 211.Pan P., Lam V., Salzman N., Huang Y.W., Yu J., Zhang J., Wang L.S. Black raspberries and their anthocyanin and fiber fractions alter the composition and diversity of gut microbiota in F-344 rats. Nutr. Cancer. 2017;69:943. doi: 10.1080/01635581.2017.1340491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Yuan X., Long Y., Ji Z., Gao J., Fu T., Yan M., Zhang L., Su H., Zhang W., Wen X., et al. Green Tea Liquid Consumption Alters the Human Intestinal and Oral Microbiome. Mol. Nutr. Food Res. 2018;62:e1800178. doi: 10.1002/mnfr.201800178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Queipo-Ortuño M.I., Boto-Ordóñez M., Murri M., Gomez-Zumaquero J.M., Clemente-Postigo M., Estruch R., Cardona Diaz F., Andrés-Lacueva C., Tinahones F.J. Influence of red wine polyphenols and ethanol on the gut microbiota ecology and biochemical biomarkers. Am. J. Clin. Nutr. 2012;95:1323–1334. doi: 10.3945/ajcn.111.027847. [DOI] [PubMed] [Google Scholar]
- 214.Takahashi K. Influence of bacteria on epigenetic gene control. Cell. Mol. Life Sci. 2014;71:1045–1054. doi: 10.1007/s00018-013-1487-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Alenghat T., Artis D. Epigenomic regulation of host-microbiota interactions. Trends Immunol. 2014;35:518–525. doi: 10.1016/j.it.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Hullar M.A.J., Fu B.C. Diet, the gut microbiome, and epigenetics. Cancer J. 2014;20:170–175. doi: 10.1097/PPO.0000000000000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Krautkramer K.A., Kreznar J.H., Romano K.A., Vivas E.I., Barrett-Wilt G.A., Rabaglia M.E., Keller M.P., Attie A.D., Rey F.E., Denu J.M. Diet-Microbiota Interactions Mediate Global Epigenetic Programming in Multiple Host Tissues. Mol. Cell. 2016;64:982–992. doi: 10.1016/j.molcel.2016.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Qin Y., Wade P.A. Crosstalk between the microbiome and epigenome: Messages from bugs. J. Biochem. 2018;163:105–112. doi: 10.1093/jb/mvx080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Gerhauser C. Impact of dietary gut microbial metabolites on the epigenome. Philos. Trans. R. Soc. B Biol. Sci. 2018;373:20170359. doi: 10.1098/rstb.2017.0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Miro-Blanch J., Yanes O. Epigenetic Regulation at the Interplay Between Gut Microbiota and Host Metabolism. Front. Genet. 2019;10:638. doi: 10.3389/fgene.2019.00638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Allis C.D., Jenuwein T. The molecular hallmarks of epigenetic control. Nat. Rev. Genet. 2016;17:487–500. doi: 10.1038/nrg.2016.59. [DOI] [PubMed] [Google Scholar]
- 222.Rozek L.S., Dolinoy D.C., Sartor M.A., Omenn G.S. Epigenetics: Relevance and Implications for Public Health. Annu. Rev. Public Health. 2014;35:105. doi: 10.1146/annurev-publhealth-032013-182513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Mendelsohn A.R., Larrick J.W. Epigenetic Drift Is a Determinant of Mammalian Lifespan. Rejuvenation Res. 2017;20:430–436. doi: 10.1089/rej.2017.2024. [DOI] [PubMed] [Google Scholar]
- 224.Sen P., Shah P.P., Nativio R., Berger S.L. Epigenetic Mechanisms of Longevity and Aging. Cell. 2016;166:822–839. doi: 10.1016/j.cell.2016.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Paluvai H., Di Giorgio E., Brancolini C. The Histone Code of Senescence. Cells. 2020;9:466. doi: 10.3390/cells9020466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Yi S.J., Kim K. New Insights into the Role of Histone Changes in Aging. Int. J. Mol. Sci. 2020;21:8241. doi: 10.3390/ijms21218241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Cech T.R., Steitz J.A. The noncoding RNA revolution-trashing old rules to forge new ones. Cell. 2014;157:77–94. doi: 10.1016/j.cell.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 228.Shock T., Badang L., Ferguson B., Martinez-Guryn K. The interplay between diet, gut microbes, and host epigenetics in health and disease. J. Nutr. Biochem. 2021;95:108631. doi: 10.1016/j.jnutbio.2021.108631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Rossi M., Amaretti A., Raimondi S. Folate production by probiotic bacteria. Nutrients. 2011;3:118–134. doi: 10.3390/nu3010118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Chen H., Wang Z., Cai H., Zhou C. Progress in the microbial production of S-adenosyl-L-methionine. World J. Microbiol. Biotechnol. 2016;32:153. doi: 10.1007/s11274-016-2102-8. [DOI] [PubMed] [Google Scholar]
- 231.Furusawa Y., Obata Y., Fukuda S., Endo T.A., Nakato G., Takahashi D., Nakanishi Y., Uetake C., Kato K., Kato T., et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 232.Arpaia N., Campbell C., Fan X., Dikiy S., Van Der Veeken J., Deroos P., Liu H., Cross J.R., Pfeffer K., Coffer P.J., et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Chang P.V., Hao L., Offermanns S., Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc. Natl. Acad. Sci. USA. 2014;111:2247–2252. doi: 10.1073/pnas.1322269111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Etchegaray J.P., Mostoslavsky R. Interplay between Metabolism and Epigenetics: A Nuclear Adaptation to Environmental Changes. Mol. Cell. 2016;62:695–711. doi: 10.1016/j.molcel.2016.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Ansari I., Raddatz G., Gutekunst J., Ridnik M., Cohen D., Abu-Remaileh M., Tuganbaev T., Shapiro H., Pikarsky E., Elinav E., et al. The microbiota programs DNA methylation to control intestinal homeostasis and inflammation. Nat. Microbiol. 2020;5:610–619. doi: 10.1038/s41564-019-0659-3. [DOI] [PubMed] [Google Scholar]
- 236.Poupeau A., Garde C., Sulek K., Citirikkaya K., Treebak J.T., Arumugam M., Simar D., Olofsson L.E., Bäckhed F., Barrès R. Genes controlling the activation of natural killer lymphocytes are epigenetically remodeled in intestinal cells from germ-free mice. FASEB J. 2019;33:2719–2731. doi: 10.1096/fj.201800787R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Ryan F.J., Ahern A.M., Fitzgerald R.S., Laserna-Mendieta E.J., Power E.M., Clooney A.G., O’Donoghue K.W., McMurdie P.J., Iwai S., Crits-Christoph A., et al. Colonic microbiota is associated with inflammation and host epigenomic alterations in inflammatory bowel disease. Nat. Commun. 2020;11:1512. doi: 10.1038/s41467-020-15342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Eaden J.A., Abrams K.R., Mayberry J.F. The risk of colorectal cancer in ulcerative colitis: A meta-analysis. Gut. 2001;48:526–535. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Linson E.A., Hanauer S.B. Epidemiology of Colorectal Cancer in Inflammatory Bowel Disease—the Evolving Landscape. Curr. Gastroenterol. Rep. 2021;23:16. doi: 10.1007/s11894-021-00816-3. [DOI] [PubMed] [Google Scholar]
- 240.Tahara T., Hirata I., Nakano N., Tahara S., Horiguchi N., Kawamura T., Okubo M., Ishizuka T., Yamada H., Yoshida D., et al. Potential link between Fusobacterium enrichment and DNA methylation accumulation in the inflammatory colonic mucosa in ulcerative colitis. Oncotarget. 2017;8:61917. doi: 10.18632/oncotarget.18716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Sobhani I., Bergsten E., Couffin S., Amiot A., Nebbad B., Barau C., de’Angelis N., Rabot S., Canoui-Poitrine F., Mestivier D., et al. Colorectal cancer-associated microbiota contributes to oncogenic epigenetic signatures. Proc. Natl. Acad. Sci. USA. 2019;116:24285–24295. doi: 10.1073/pnas.1912129116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Ramos-Molina B., Sánchez-Alcoholado L., Cabrera-Mulero A., Lopez-Dominguez R., Carmona-Saez P., Garcia-Fuentes E., Moreno-Indias I., Tinahones F.J. Gut Microbiota Composition Is Associated With the Global DNA Methylation Pattern in Obesity. Front. Genet. 2019;10:613. doi: 10.3389/fgene.2019.00613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Zhao Y., Garcia B.A. Comprehensive Catalog of Currently Documented Histone Modifications. Cold Spring Harb. Perspect. Biol. 2015;7:a025064. doi: 10.1101/cshperspect.a025064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Kelly D., Kotliar M., Woo V., Jagannathan S., Whitt J., Moncivaiz J., Aronow B.J., Dubinsky M.C., Hyams J.S., Markowitz J.F., et al. Microbiota-sensitive epigenetic signature predicts inflammation in Crohn’s disease. JCI Insight. 2018;3:122104. doi: 10.1172/jci.insight.122104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Kadosh E., Snir-Alkalay I., Venkatachalam A., May S., Lasry A., Elyada E., Zinger A., Shaham M., Vaalani G., Mernberger M., et al. The gut microbiome switches mutant p53 from tumour-suppressive to oncogenic. Nature. 2020;586:133–138. doi: 10.1038/s41586-020-2541-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Alenghat T., Osborne L.C., Saenz S.A., Kobuley D., Ziegler C.G.K., Mullican S.E., Choi I., Grunberg S., Sinha R., Wynosky-Dolfi M., et al. Histone deacetylase 3 coordinates commensal-bacteria-dependent intestinal homeostasis. Nature. 2013;504:153–157. doi: 10.1038/nature12687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Whitt J., Woo V., Lee P., Moncivaiz J., Haberman Y., Denson L., Tso P., Alenghat T. Disruption of Epithelial HDAC3 in Intestine Prevents Diet-Induced Obesity in Mice. Gastroenterology. 2018;155:501–513. doi: 10.1053/j.gastro.2018.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.David L.A., Maurice C.F., Carmody R.N., Gootenberg D.B., Button J.E., Wolfe B.E., Ling A.V., Devlin A.S., Varma Y., Fischbach M.A., et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Duda-Chodak A. The Inhibitory Effect of Polyphenols on Human Gut Microbiota—PubMed. [(accessed on 23 January 2024)]; Available online: https://pubmed.ncbi.nlm.nih.gov/23211303/
- 250.Evans L.W., Athukorala M., Martinez-Guryn K., Ferguson B.S. The Role of Histone Acetylation and the Microbiome in Phytochemical Efficacy for Cardiovascular Diseases. Int. J. Mol. Sci. 2020;21:4006. doi: 10.3390/ijms21114006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Dent P., Booth L., Roberts J.L., Poklepovic A., Hancock J.F. (Curcumin+sildenafil) enhances the efficacy of 5FU and anti-PD1 therapies in vivo. J. Cell. Physiol. 2020;235:6862–6874. doi: 10.1002/jcp.29580. [DOI] [PubMed] [Google Scholar]
- 252.Mcfadden R.M.T., Larmonier C.B., Shehab K.W., Midura-Kiela M., Ramalingam R., Harrison C.A., Besselsen D.G., Chase J.H., Caporaso J.G., Jobin C., et al. The Role of Curcumin in Modulating Colonic Microbiota During Colitis and Colon Cancer Prevention. Inflamm. Bowel Dis. 2015;21:2483–2494. doi: 10.1097/MIB.0000000000000522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Remely M., Ferk F., Sterneder S., Setayesh T., Roth S., Kepcija T., Noorizadeh R., Rebhan I., Greunz M., Beckmann J., et al. EGCG Prevents High Fat Diet-Induced Changes in Gut Microbiota, Decreases of DNA Strand Breaks, and Changes in Expression and DNA Methylation of Dnmt1 and MLH1 in C57BL/6J Male Mice. Oxid. Med. Cell. Longev. 2017;2017:3079148. doi: 10.1155/2017/3079148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Saldanha S.N., Kala R., Tollefsbol T.O. Molecular mechanisms for inhibition of colon cancer cells by combined epigenetic-modulating epigallocatechin gallate and sodium butyrate. Exp. Cell Res. 2014;324:40–53. doi: 10.1016/j.yexcr.2014.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Walker J.M., Eckardt P., Aleman J.O., da Rosa J.C., Liang Y., Iizumi T., Etheve S., Blaser M.J., Breslow J.L., Holt P.R. The effects of trans-resveratrol on insulin resistance, inflammation, and microbiota in men with the metabolic syndrome: A pilot randomized, placebo-controlled clinical trial. J. Clin. Transl. Res. 2019;4:122. doi: 10.18053/jctres.04.201802.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Zhang L., Hui X.U.E., Zhao G., Qiao C., Xiaomei S.U.N., Pang C., Zhang D. Curcumin and resveratrol suppress dextran sulfate sodium-induced colitis in mice. Mol. Med. Rep. 2019;19:3053. doi: 10.3892/mmr.2019.9974. [DOI] [PMC free article] [PubMed] [Google Scholar]