Abstract
Recent usage of Virtual Reality (VR) technology in surgical training has emerged because of its cost-effectiveness, time savings, and cognition-based feedback generation. However, the quantitative evaluation of its effectiveness in training is still not studied thoroughly. This paper demonstrates the effectiveness of a VR-based surgical training simulator in laparoscopic surgery and investigates how stochastic modeling represented as Continuous-time Markov-chain (CTMC) can be used to explicit the training status of the surgeon. By comparing the training in real environments and in VR-based training simulators, the authors also explore the validity of the VR simulator in laparoscopic surgery. The study further aids in establishing learning models of surgeons, supporting continuous evaluation of training processes for the derivation of real-time feedback by CTMC-based modeling.
Keywords: Continuous-time Markov Chain (CTMC), Virtual Reality, Surgical Training, Proficiency Evaluation, Learning Curves, Laparoscopic Surgery
I. Introduction
Surgeons require practicing skills ranging from simple wound closure to highly complex diagnostic and therapeutic procedures. Thus, surgical training has been on the verge of a seismic shift in how one can give the level of surgical training expected of a modern surgeon. Surgical training has traditionally been an opportunity-based learning strategy centered on an operating room apprenticeship. This “see one, do one, teach one” approach to surgical training was commonly typified by this Halstedian method (Higgins et al., [31]). As a result of this apprenticeship model, surgical training was extended to gather enough surgical experience to reach a subjective degree of operative experience, which is time-consuming and costly (Franzese and Stringer [15]).
Surgical education has changed to overcome these limitations by adopting new technology such as virtual reality (VR). The adoption of VR has seen a surge of interest for training surgical skills both inside and outside of the operating room (Seymour [68]). Incorporating VR with the physics-level of simulations for surgical operations allows the transfer of techniques learned in a skills lab to the operating room. Moreover, artificial tissue movements and the movements of surgical tools generated in computer simulation would be accessible to trainees. In laparoscopic surgery, these technical skills often lead to a prolonged learning curve. Thus, one can investigate the advantages of using VR-based training models in laparoscopic surgery, which can provide an objective assessment of technical ability, while retaining realism, and measuring self-confidence in a controlled laboratory setting. The VR-based training system also enables generating a large set of training data, stimulating research to apply advanced statistical methods and machine learning techniques for learning model investigation as Rogers et al. studied [62].
Although VR-based training has the potential to contribute significant advantages in surgical training for new skills and procedures, quantitative evaluation of skills acquired within the simulated environment is still limited. Thus, this study aimed to investigate the learning curve of training in a real environment versus in a VR environment, identifying any competitive advantage of VR-based training. The second goal was to demonstrate the use of continuous assessments of surgical skills during training for identifying surgical skill deficiencies to provide targeted and individualized feedback. To answer our research questions, we have studied four different learning process methods: Hidden-Markov Models (HMM), learning curves, Generalized Estimating Equation (GEE), and Cumulative Sum (CUSUM). After investigating other modeling methods, the authors found the competitive advantage of using the Continuous-Time Markov Chain (CTMC) over others. Therefore, the authors proposed a new approach to model the learning process using Continuous Time Markov Chains (CTMC) by capturing learning variability, sudden accuracy drops, and simultaneous consideration of time and accuracy together. The proposed model was validated by training data for a simulated laparoscopic surgery skill.
The remainder of this paper is as follows. Section II shows the literature review results of how VR has been used in general surgery and laparoscopic surgery while summarizing the learning curve models in surgical training. Section III utilizes the traditional learning model via learning curves to compare laparoscopic surgery practices in real versus VR-based environments. Section IV proposes a new learning modeling approach using CTMC, examining its merits compared to the traditional one. Section V concludes with observations and findings from the proposed approach.
II. Literature Review
The traditional surgical training methods have supported generations of surgeons, but it is not enough due to cost effectiveness, time management, and the procedure’s safety. This forced surgeons to examine the possibility of incorporating an advanced technology like VR to tackle these challenges [28, 34, 44, 59, 65, 81]. It was also shown that patients had excellent outcomes when the surgical procedure was simulated through VR first the operation was started [33, 35, 69, 77]. Moreover, VR-based training provided efficient guidance for trainees [21, 31, 47] and revealed the group using VR training showed higher accuracy scores [17, 20, 37, 55, 68], less operating time [4, 17, 50, 80, 85], and a better understanding of procedural knowledge [22, 40, 48, 78]. Researchers also revealed that surgical platforms consisting of interactive user interface and guidance reduce complexity in getting used to VR as a training tool [8, 12, 14, 42, 82]. Table I summarizes how those benefits of VR-based training are utilized in each type of surgery.
TABLE I.
Recent Development of VR in Surgical Training
| Type of Surgery | Methodology | References |
|---|---|---|
| General Surgery | Comparison: Experimental vs Control groups |
Aggarwal et al. (2006)
Gurusamy et al. (2008) |
| Theoretical studies | Haluck et al. (2000) | |
| Machine Learning | Kim et al. (2017) | |
| Osteotomy | Comparison: Experimental vs Control groups | Pulijala et al. (2017) |
| Theoretical studies | Hsieh et al. (2002), Sayadi et al. (2019) | |
| Descriptive Statistics | Wilson et al. (2020) | |
| Heart | Theoretical studies | Wang and Wu (2021), Friedl et al. (2002), Falah et al. (2002) |
| Comparison: Augmented vs Virtual Reality | Silva et al. (2018) | |
| Brain | Descriptive Statistics | Bracq et al. (2017) |
| Comparison: Experimental vs Control groups | Phaneuf et al. (2014), Fried et al. (2010) | |
| Cataract | Comparison: Experimental vs Control groups | Beauchamp et al. (2020), Thomsen et al. (2017), Thomsen et al. (2017) |
| Theoretical studies | Lama et al. (2013) | |
| Tendon repair | Comparison: Experimental vs Control groups | Mok et al. (2021) |
| Neuro | Machine Learning | Schwartz et al. (2019) |
| Theoretical studies | Fiani et al. (2020), Alaraj et al. (2011) | |
| Spine | Comparison: Experimental vs Control groups | Luca et al. (2020), Xin et al. (2019) |
| Theoretical studies | Pfandler et al. (2017) | |
| Arthroscopy | Theoretical studies | Muller et al. (1995) |
| Descriptive Statistics | Gomoll et al. (2007), Gomoll et al. (2008), Jacobsen et al. (2015) |
The authors’ literature review is three-fold. Firstly, the authors investigated the advanced role of VR in laparoscopic surgery. Secondly, learning curve approaches in a surgery application are majorly summarized. Lastly, the advanced statistical modeling, such as Hidden Markov Chain (HMM), Generalized Estimating Equation (GEE), and Cumulative Sum (CUSUM) approaches, are also summarized.
2.1. VR-based training in laparoscopic surgery
Reducing operating time and improving the accuracy are two major advantages of using VR-based simulators in laparoscopic surgery. For example, Grantcharov et al. [24–25] showed that VR-based training stimulated learning faster and improved movement scores while reducing errors, while Munz et al. [52] demonstrated that completion time was more rapid by reducing the necessary movements. Aggarwal et al. [1] showed VR shortens the learning curve as a time- and cost-effective training model. Portelli et al. [60] also concluded that VR improves efficiency in the trainee’s surgical practice and improves quality with reduced error rates and improved tissue handling. Gurusamy et al. [26–27] revealed that VR decreased time, errors, and increased accuracy, whereas Larsen et al. [43] verified that using a VR simulator aided trainees’ proficiency as their operation time was halved.
It was also studied that VR-based training could be more accurate than video-based training. Alaker et al. [3] exhibited that a VR-based simulator was more effective than video-based training, while Yiannakopoulou et al. [86] indicated VR could provide alternative means of video-based practicing while improving performance in surgery. Phe et al. [58] and Botden et al. [7] illustrated that a VR simulator could offer better realism and haptic feedback based on trainees’ skill levels. Hart et al. [30] also showed a VR as an essential part of clinical training, supporting trainees to practice surgical tools. Instead, to maximize the efficiency of VR-based training, Aggarwal et al. [2] pointed out that junior trainees were recommended to acquire pre-requisite skill levels before entering an operating room. Table II summarizes and classifies all the work based on the types of design of experiments and methodologies.
TABLE II.
VR-based Surgical Training in Laparoscopic Surgery
| Design of Experiments | Methodology | References |
|---|---|---|
| VR vs Physical simulator | Descriptive Statistics | Taba et al. (2021) |
| Comparison: Experimental vs Control groups | Gurusamy et al. (2009), Papanikolaou et al. (2019) | |
| VR vs real | Descriptive Statistics | Larsen et al. (2009), Munz et al. (2007) |
| Machine Learning Comparison: Experimental vs Control groups |
Alaker et al. (2016)
Grantcharov et al. (2004) |
|
| VR vs trad mentoring | Meta-analysis | Gurusamy et al. (2008) |
| Meta-analysis & Descriptive Statistics | Portelli et al. (2020) | |
| Theoretical Studies | Yiannakopoulou et al. (2015), Harta and Karthigasua (2007) | |
| Descriptive Statistics | Aggarwal et al. (2007) | |
| VR | Descriptive Statistics | Aggarwal et al. (2006) |
| Jain et al. (2020) | ||
| Experimental vs Control groups | Phé et al. (2017) | |
| Machine Learning | Botden et al. (2007) |
2.2. Traditional learning curve modeling in surgical training
Developing learning curves requires a proper selection of independent (predictor) and dependent (response) variables to derive general causality. This subsection summarizes relevant work for developing the learning curve and its related parameters in surgical training. Feldman et al. [13] suggested two parameters called learning plateau (intercept) and learning late (slop) while assuming that the improvement of surgical proficiency over time follows an S-curve such as y = a – b/x. These smooth improvement in skill acquisition and performance over trial was also investigated by Bosse et al. [6], provided with high and low-frequency intermittent feedback. Khan et al. [39] instead considered procedural variables, including experiences and supervision levels, to develop a learning curve using logistic regression. Subramonian and Muir [73] investigated responses by measuring surgeons’ skills and techniques whereas Suguita et al. [74] evaluated their average operating time for learning curve development.
Time factors have been heavily considered for the learning curve analysis. Brunckhorst et al. [9] investigated the effect of the time factors on learning curves in VR-based training, whereas Howells et al. [32] examined the time factor affecting the learning curve by showing that even with a time delay (e.g., six months later after the trainees were exposed to the surgical procedure for the first time) in training, repeating it again can improve their proficiency. Uribe et al. [79] also proved that novices initially displayed a steeper learning curve, while Leijte et al. [45] observed a performance delay in minimal invasive surgery compared to robot-assisted surgery.
The other consideration for developing learning curves is classifying trainees into several groups based on their expertise [54]. Papachristofi et al. [53] suggested that learning curves were different based on the trainees’ prior knowledge, whereas Hardon et al. [29] investigated the expertise based on force and motion factors during surgery. Grantcharov et al. [24–25] further investigated that trainers’ proficiency differences could not be captured by changing the function parameters but by requiring different learning kernels. A comprehensive review of learning curve modeling in surgical training was performed by Chan et al. [10]. The challenge of using learning curves is the trainee-specific nature, which requires carefully selecting the curve’s kernel structure for generalization. Moreover, if more than two response variables (e.g., completion time and accuracy) are of interest, then it requires more than two learning curves per trainee. The authors explain the universality of the proposed CTMC-based model in Section 3.
2.3. Other Modeling Methods in Surgical Training
Hidden Markov Models (HMM) are widely used to model the training process in surgery when high-fidelity data is provided. Especially if surgical instrument trajectory data is given, HMM was shown to supports to detect hidden states beyond the movement [46]. The HMM approach was widely used to decompose all the surgical tasks by Rosen et al. [63], which helps to develop objective performance metrics [49]. From the analysis of high-fidelity data, HMM also supports classifying surgeons based on their surgical proficiency [64], and it helps to provide a continuous evaluation by temporal and motion-based analysis [23]. As HMM requires high-resolution data (motion and tracking data) to recognize hidden learning phases, it could be challenging to provide an intuitive and practical learning model to derive the best time to support additional feedback.
Generalized Estimating Equation (GEE) modeling is appropriate when one collects repeated training data per participant by Aggarwal et al. [2], especially the modeler is interested in investigating the covariance structure among predictors. Since GEE identifies the correlation structures in repeated trials, it also supports to demonstrate the interaction effects on proficiency achievement in laparoscopic-guided surgery [38, 87]. GEE can also be used to generate an objective surgical proficiency measure, such as a visual analog scale (VAS), as studied by Zhang et al. [87]. The recent advance in using GEE in surgical training has been studied and comprehensively summarized in the review papers by Chang et al. and Jin et al. [11, 36].
Cumulative-sum (CUSUM) can also be used to demonstrate learning processes. CUSUM was originally designed to detect the small process shift in quality control, but it is also used to magnitude the improvement over the trials [19]. The benefit of using CUSUM in modeling learning processes is to detect learning improvement when the reading in charts exceeds control limits [5, 16]. Sood et al. [72] demonstrated that the learning curve could be estimated using a CUSUM chart, aiding in determining the length of training time, whereas Smith et al. [70] claimed the CUSUM chart itself could support trainees’ learning processes. All the literature review results of different modeling approaches are summarized in Table III.
TABLE III.
Learning Models for Surgical Training
| Methodology | References |
|---|---|
| Generalized Estimating Equation (GEE) | Aggarwal et al. (2007) |
| Chang et al., (2020) | |
| Jin et al. (2021) | |
| Kauffman (2020) | |
| Portelli et al. (2020) | |
| Zhang et al. (2022) | |
| Learning Curves | Chan et al. (2021) |
| Feldman et al. (2009) | |
| Hardon et al. (2021) | |
| Leijte et al. (2020) | |
| Wong et al. (2022) | |
| Hidden Markov Models (HMM) | Megali et al. (2006) |
| Leong et al. (2006), Gorantla and Esfahani (2019), Rosen et al. (2002), Saravanan and Menold (2022). | |
| Cumulative-Sum (CUSUM) | Fraser et al. (2005) |
| Fu et al. (2020) | |
| Perivoliotis et al (2022) | |
| Sultana et al. (2019) |
As shown in Fraser et al. [16], it also requires separate CUSUM analyses when looking at different criterion levels (e.g., junior, intermediate, and senior in their case). Also, trainees can show a “back and forth” pattern in learning when advancing to the next stage because the trainee requires time to get familiar with the achievement (Feldman et al. [13]). As our learning process is not linear, multiple threshold values are required and it is even possible that the threshold value (δ) itself could be a function of time (δ(t)). To address the variability shown right after the state transition in the learning process, the authors proposed a new approach based on CTMC. Because our proposed CTMC model has two levels (high-level states and sub-states), it can help to keep track of the continuous evaluation of learning processes. The details of our model are explained in Section 3.
III. Traditional Learning Curve-Based Modeling
3.1. Description of Surgical Process for Training
Table IV explains the experimental configuration. The surgical skills training task performed was the intracorporal suturing with knot tying task of the Fundamentals of Laparoscopic Surgery (FLS) curriculum. The task was performed either in a standard FLS box trainer or using the Virtual Basic Laparoscopic Skill Trainer - Suturing Simulator (VBLaST-SS(c)) following the same task procedures [71]. For the task, a Penrose drain is placed on a Velcro strip inside the trainer. The subject uses two needle drivers to feed a needle and suture through two marked targets on the Penrose drain and complete three knots intracorporeally to close a slit in the drain. The task ends after three knots have been completed and the suture has been trimmed. Task completion is limited to 10 min (600 sec). The overall performance score is based on the completion time, error in needle placement, knot security, and slit closure, following the equation published in Korndorffer et al. [41]. The proficiency time was set at 112 sec with deviation from the marked targets of less than 1 mm [66, 71]. Fifteen trainees who were pre-medical or 1st to 3rd year medical students participated the training and those participants completed the task for multiple repetitions over 15 days within a three-week period. The study was approved by the University at Buffalo Institutional Review Board under protocols STUDY00000750 and STUDY00004789 and all participants provided written informed consent. Two figures in Fig. 1 illustrate the practice in physical box (in a real environment) and VR-based training.
TABLE IV.
Experimental Configuration
| Configuration | Experimental Settings |
|---|---|
| Number of Trainees | In a Real Environment: 7 |
| In a VR Environment: 8 | |
| Training Environments | Real vs Virtual-Reality (VR) |
| Measurements | Accuracy vs Completion Time |
| Analysis | Learning Curve Fitting vs CTMC |
Fig. 1.
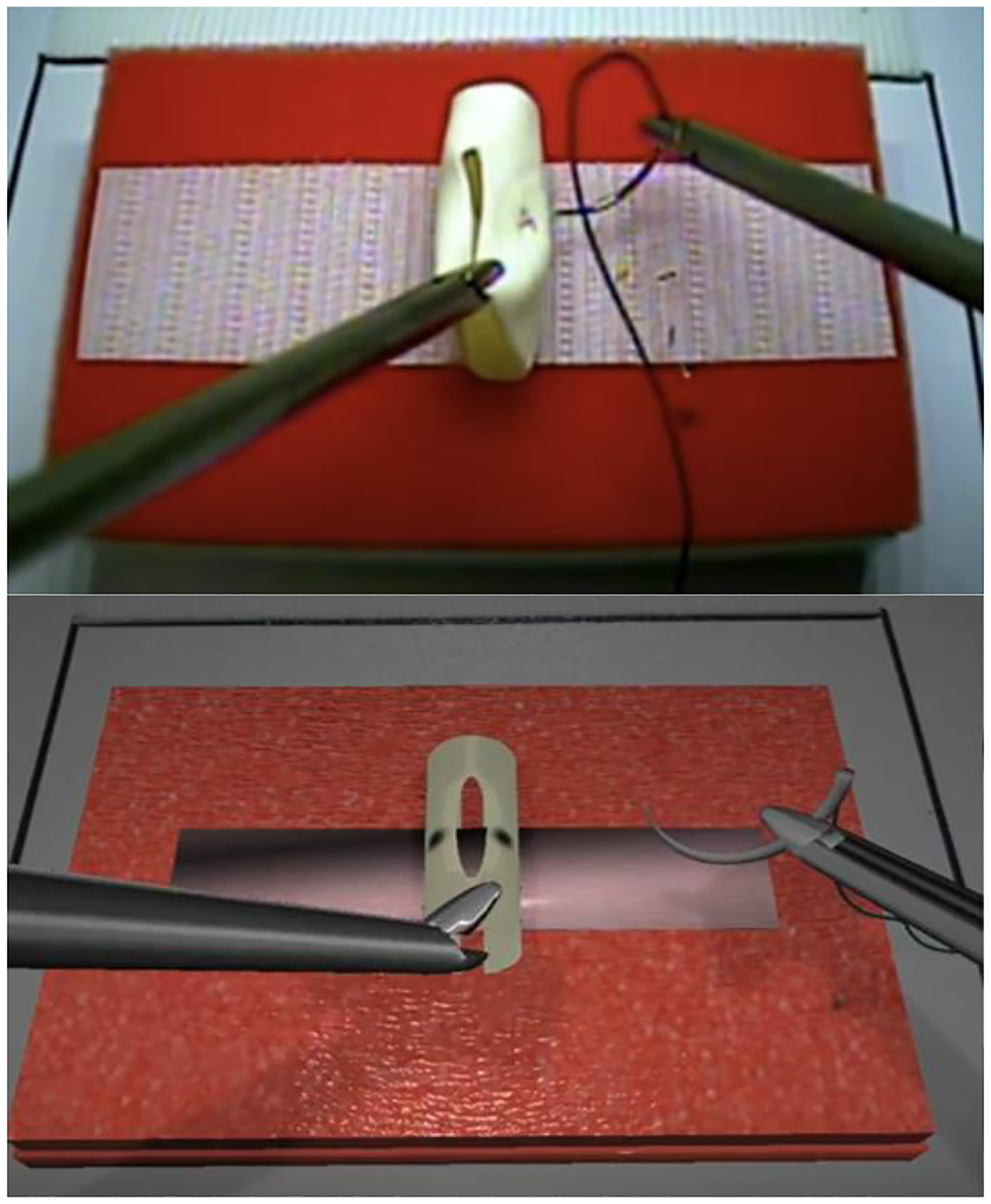
Practice in physical box trainer (top) versus VR-based training (bottom)
3.2. Learning Process using Learning Curve Fitting
Generally, surgical proficiency is expected to mature as the number of practice trials accumulates. Thus, researchers have developed the relationship between practice trials and surgical ability to demonstrate surgeons’ learning processes. Once researchers have training data with respect to surgical precision and/or time data over the number of trials, one can fit the data to a certain function using different kernels such as polynomial, Gaussian, and sigmoid functions to oblige in classifying trainees into their proficiency levels. The authors have performed polynomial fitting by taking data sets of training accuracy and time in two different surgical training settings: training in a real environment versus in a VR environment. Fig. 2 illustrates four different trainees’ scores over trials, and the red line demonstrates the best-fitted line using the maximum likelihood approach. The degree of 3 polynomial functions () were used to derive the fitted lines.
Fig. 2.
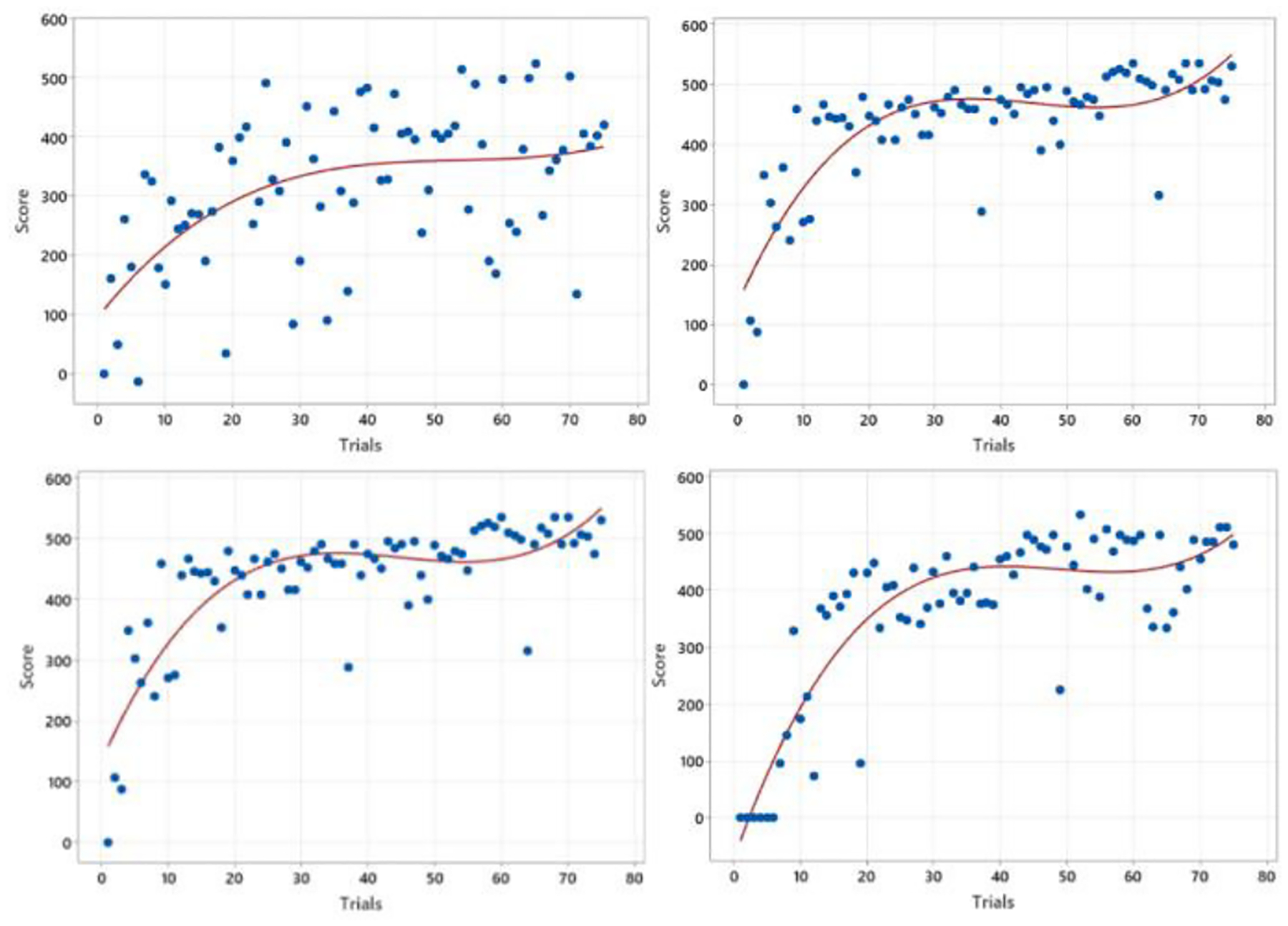
Four learning curve fitting results of accuracy scores vs training trials in real surgery practicing.
Fig. 2 displays that all four trainees’ accuracy scores have grown over the trials, revealing their learning processes. Moreover, within the first few trials, the slopes of the learning curves are more elevated than in the later trials, demonstrating a steep learning curve representing an initial learning barrier at an earlier phase. However, Fig 2 also illustrates some limitations of the learning curve approaches in modeling surgical training processes. Firstly, the fitted line still carries a considerable variability, weakening the expressiveness of the line as a representative of learning processes. For example, the top left figure exhibits an evident variability, indicating that most observations are far from the best fitting line. The other three figures also depict frequent outliers, conveying a sudden significant performance drop even at a later learning phase. Those variabilities, which the fitted line cannot capture, require an advanced analysis. Thus, further discussions will be followed at the end of this section by investigating R-squared values. Another limitation observed in Fig. 2. is the significant differences among the fitted lines of individual results, indicating the possibilities of discrepancies in function types. In specific, not just differences in the parameter values of the similar kernel (function), each result requires different kernels, preventing generalizing the learning processes across individuals. For instance, it is sufficient to use the degree of three polynomial function to find the best fit in the bottom left figure; however, a Gaussian kernel would work well to describe the learning pattern in the bottom right figure. These observations (and limitations) demonstrate the challenges of learning process generalization regardless of the trainees, supporting the need for proposing a new approach for mimicking learning processes.
Fig. 3 exhibits the completion time of one training procedure over trials. As shown in the four different figures in Fig. 3, the results by the four trainees describe that the completion time has reduced as they practiced more, which is consistent with the expectation. However, the learning curve approach has not clearly demonstrated the variability problems of training time either. Even between a few successive trials, their completion times are wildly distinct, indicating a limitation of the expressive power of the single fitted line. Compared to the performance score graph (Fig. 2), all four figures in Fig. 3 suggest that those functions are based on the same kernels; however, another limitation is observed - saturation. For example, when comparing the top-right and bottom-right figures, it is evident that the bottom figure shows a trainee’s maturity in terms of time, while the other trainee from the top figure may still need extra training due to his/her time variability. However, the fitted line cannot convey such information, leaving the time variability unconsidered.
Fig. 3.
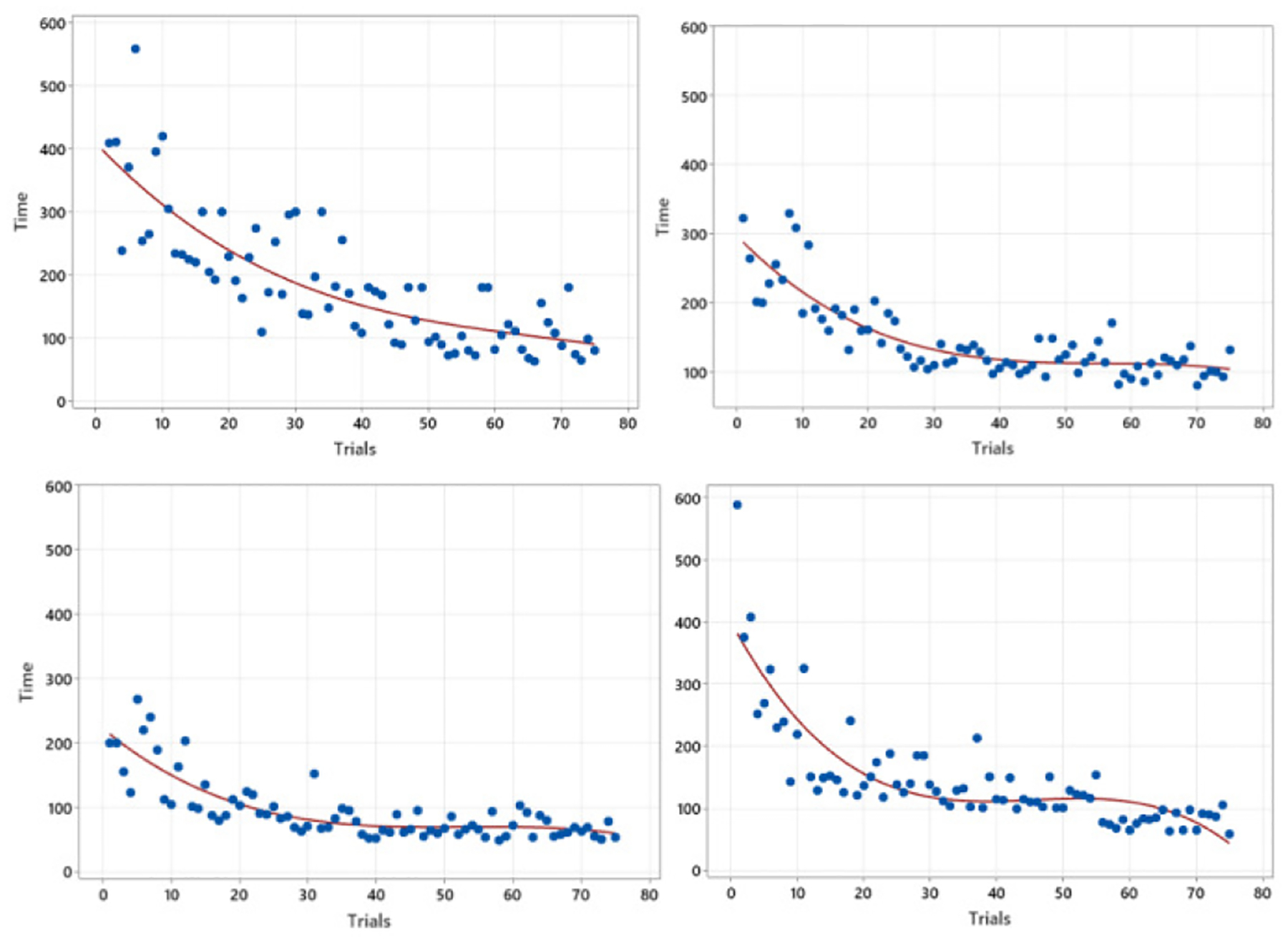
Completion time changes over practice trials in real surgical training.
As investigated in Fig. 2 and 3, two proficiency measures, including accuracy and time, were considered separately in the learning curve approaches. Thus, it is required to capture both overall performance score and time together to represent the learning processes, leading the authors to propose a new modeling approach using the CTMC. Another advantage of using CTMC is its capability for generalization. As in Fig. 2, it is possible that each trainee requires different learning kernels for modeling his/her learning process, prohibiting the generalized guidelines for trainees. However, the CTMC modeling approach allows generalizing all the trainees’ learning processes, which will be investigated at the end of this section. Finally, the learning curve approach makes it unattainable to compare two different training processes in real versus VR environments. The following Fig. 4 shows the learning curve derived in a VR-training environment to investigate the limitation of using a learning curve in comparison between real and VR-based training.
Fig. 4.
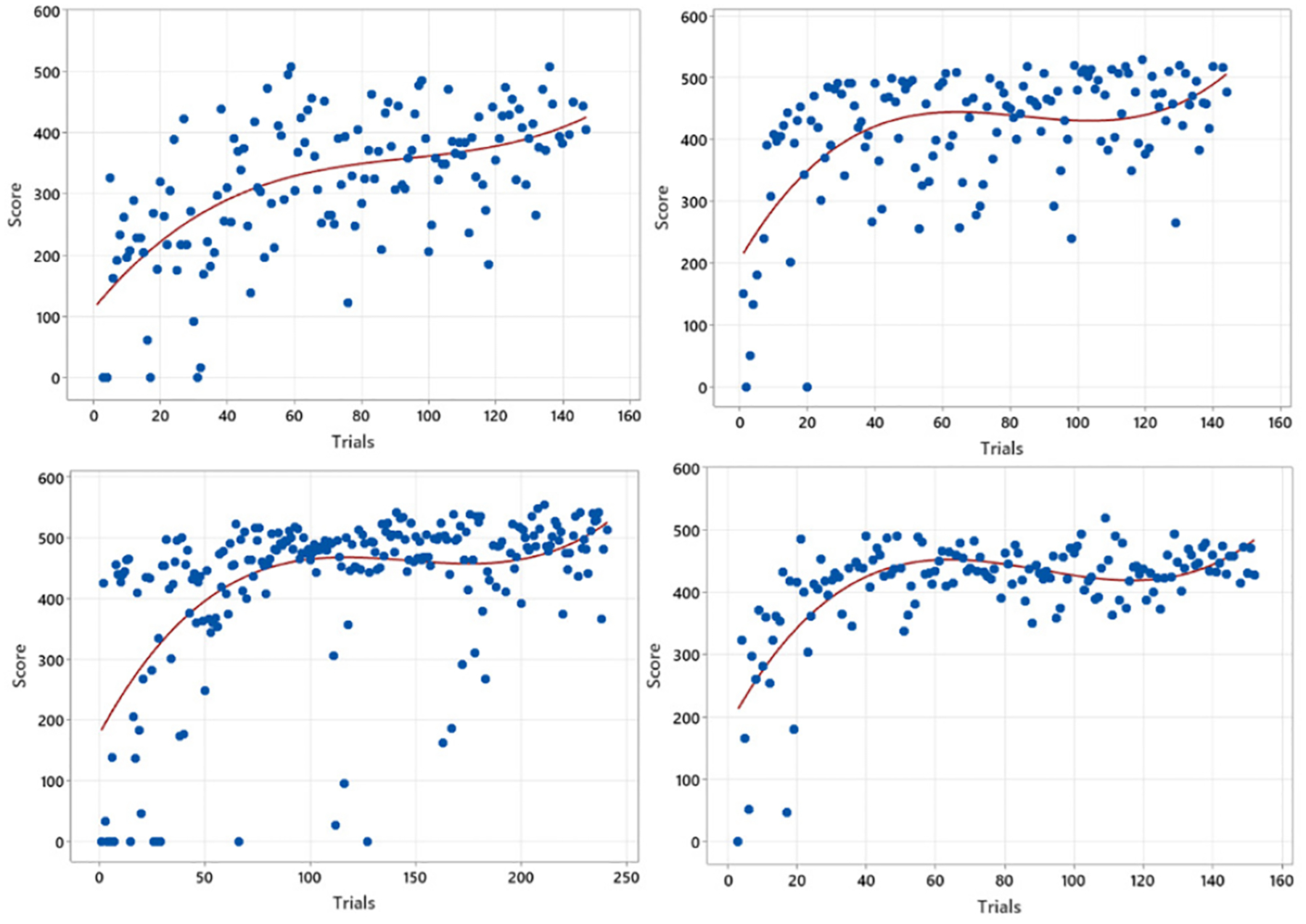
Four Learning Curve Fitting results of scores vs trials in VR-based surgery practicing.
Fig. 4 illustrates four selected trainees’ accuracy scores over practice trials in a VR environment. Trainees’ learning processes within VR show similar patterns as in a real environment, as studied in Fig. 2, presenting that trainees’ performance scores have enhanced as the number of trials accumulates. The steeper slope in an earlier learning phase also denotes an initial learning barrier in the processes. However, the figures also demonstrate the difficulty of modeling the learning process in a VR environment using a fitted line. Firstly, more observations do not lie the fitted line close enough, indicating a higher variability than a real-environment practice. Secondly, as shown in the top-left and bottom-left figures, sudden accuracy drops are marked even after numerous practice trials, weakening the fitted line’s expressiveness in the learning process in a VR environment. Finally, all the fitted lines may require different kernels as described in a real environment configuration. Fig. 4, similar to Fig. 2, suggests that the modeling of learning processes should tackle the sudden accuracy drops, which the fitted line couldn’t capture enough. The authors will demonstrate the capability of CTMC-based modeling in explaining those variabilities.
Fig. 5 shows the trainee’s completion time of one training procedure over the number of trials. As investigated in Fig. 3, one can claim that the completion time drops with practice, indicating the similarity of learning processes in real and VR training environments. However, the variability at a later training phase also diminishes faster compared to the real environment, implying that a VR-based practice can support the trainees to become familiar with the surgical procedure in a shorter amount of time. Therefore, Fig. 5 suggests the potential advantage of VR-based surgical training as a complementary learning system, supporting lower initial learning barriers as well as improving trainees’ confidence before they perform surgery in a real environment.
Fig. 5.
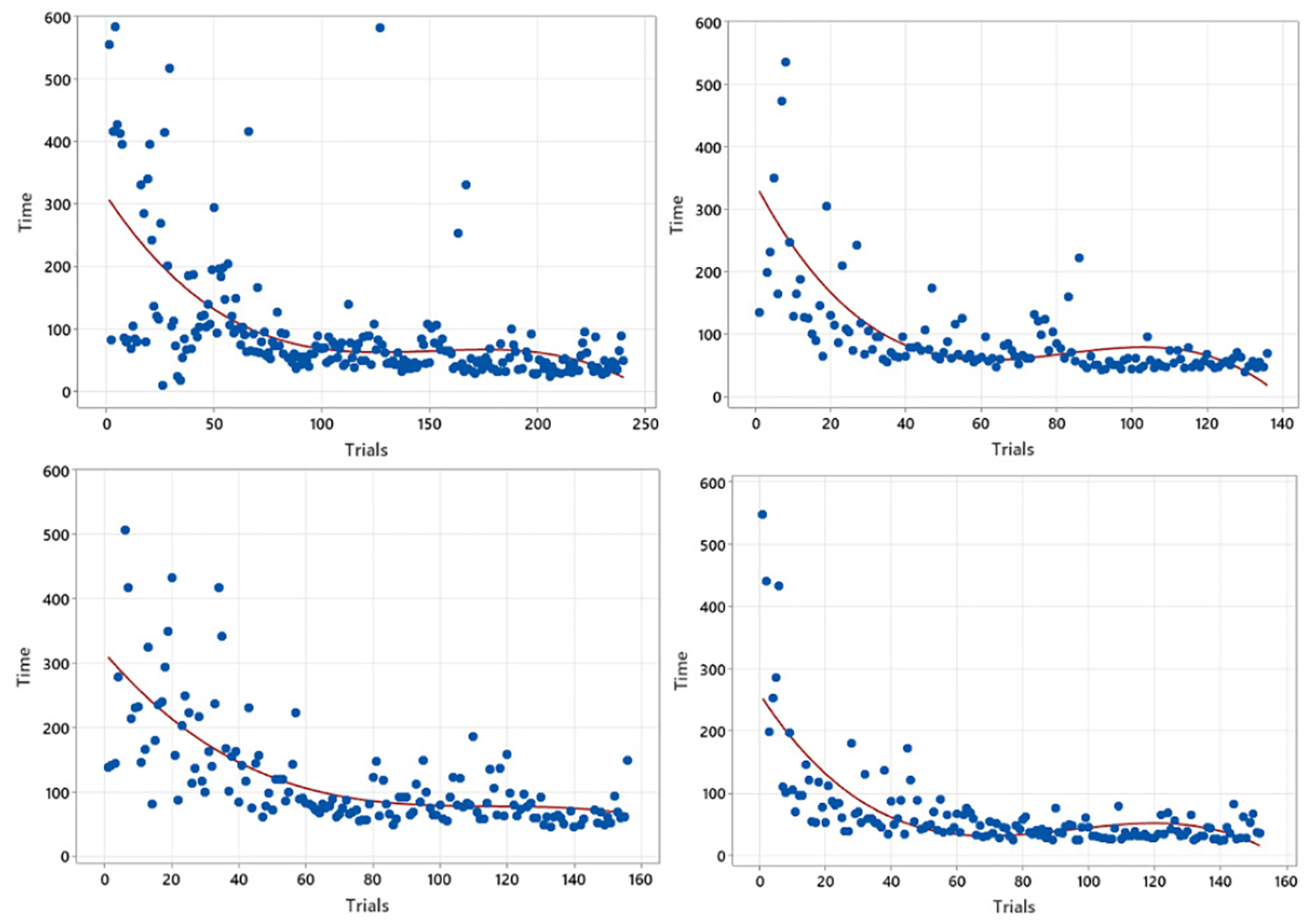
Four Learning Curve Fitting results of times vs trials in VR-based surgery practicing.
To conclude the discussions of learning curve modeling approaches, the authors studied two representative statistics of the R-square values of the fitted results. Table V summarizes the average and the standard deviation of the R-square values of all the trainee’s fitted results in a real and a VR practice environment. As shown in the table, it is evident that the R-square values of the fitted lines are higher in a real practice environment than in a VR environment in terms of both accuracy and time. The result indicates that advanced approaches are required to incorporate the variability, which the best-fitted line cannot capture. Secondly, since R-squared values are lower in training in a VR experiment, one should consider a different approach when especially analyzing the learning processes using a VR setting. Finally, a smaller standard deviation of R-square values in VR training represents that it could be easier to generalize the learning processes in a VR, requiring a new modeling technique to demonstrate all phases over trials. Therefore, the authors introduce a new modeling approach to the learning processes using the Continuous-time Markov chain (CTMC) to enrich the model’s expressiveness, capturing more variability, especially in VR-based training.
TABLE V.
Mean and Standard Deviation of R2 Values
| Measures | Mean | Standard Deviation |
|---|---|---|
| Accuracy Scores in Real | 0.5514 | 0.2323 |
| Accuracy Scores in VR | 0.4847 | 0.1611 |
| Completion Time in Real | 0.6789 | 0.2956 |
| Completion Time in VR | 0.5215 | 0.1033 |
IV. CTMC-Based Learning Process Modeling
This section introduces the CTMC-based modeling of learning processes in surgical training by addressing the following four limitations of learning curve approaches: demonstrating learning variability, incorporating a sudden performance drop, revealing differences between learning in a real and VR environment, and considering both accuracy and time together. Within the CTMC modeling, it is required to define state sets (S), transition probabilities (T), and rates (average time staying at each state). Firstly, the authors identified four different high-level learning states based on trainees’ performance scores, named Stages 1, 2, 3, and 4. Stage 1 corresponds to the performance score range between 0 and 199, Stage 2 between 200 and 369, Stage 3 between 370 and 477, and Stage 4 over 477 [16, 41]. The Stage 4 threshold is based on the target proficiency score described above [17]. The cutoff for the top-level score 477 in CTMC was based on the proficiency requirement described in the FLS training instructions [16, 66]. According to the instructions, the ultimate proficiency is achieved with a completion time less than 112 seconds, and an accuracy within 1 mm deviation (error score of 10), for a total score of 478 [66].
As the training trial repeats, it is expected that the trainee’s stage will advance, assuming that there is no backward movement such as moving down from Stages 4 to 3, 3 to 2, or 2 to 1. Instead, the authors included low-level learning states (named “sub-states” in each stage) to represent the learning variability and a sudden accuracy drop. The following tables show both high- and low-level states within the proposed CTMC-based learning model.
As discussed, one can admit that the trainees’ skills (in terms of accuracy and time) will enhance as they practice repeatedly. Thus, the authors introduced the low-level state “Mature” in Stages 1, 2, and 3, representing the situation where the trainee hit the next level’s score for the first time, which works as an absorbing state. Since it is an absorbing state, the chain always advances to a higher stage. Once one’s learning process reaches the “Mature” state in Stages 1, 2, and 3, the chain moves to the next stage and never returns to the previous stage. At the same time, “Immature” and “In-Progress” sub-states in each stage illustrate that trainees require enough trials to progress to the next stage while demonstrating accuracy drops and fluctuation of the surgical proficiency in learning processes. For example, if one’s progress stays in an “Immature” sub-state in Stage 2, a trainee has advanced to Stage 2 by scoring the accuracy value between 200 and 369 once, but he/she scored below 200 in his latest training trial. The second column in Table VI shows all the accuracy score ranges of higher-level states (Stages), and the last column describes the corresponding accuracy score in each lower-level state.
TABLE VI.
States in Two-Stage Markov Chain Model
| High-level States | Accuracy Score Range | Sub-States | Accuracy Score Range |
|---|---|---|---|
| Stage 1 | [0, 199] | Immature | [0, 199] |
| Mature | over 199 | ||
| Stage 2 | [200, 369] | Immature | [0, 199] |
| In-Progress | [200, 369] | ||
| Mature | over 369 | ||
| Stage 3 | [370, 477] | Immature | [0, 369] |
| In-Progress | [370, 476] | ||
| Mature | over 477 | ||
| Stage 4 | over 477 | Immature | [0, 369] |
| In-Progress | [370, 476] | ||
| Mature | over 477 |
Fig. 6 shows a proposed CTMC describing training processes in a real environment by incorporating all seven participants’ practice results. Four high-level states along with nine sub-states are determined, as shown below. The transition matrix (T) demonstrating the transition probability from one state to the other is also developed by considering the likelihood of accuracy changes in two subsequent trials. Thus, the matrix dimension is nine by nine, considering the number of sub-states. Since all the “Mature” states in Stages 1, 2, and 3 are absorbing states, the transition probabilities from to , from to and from to are all 1, meaning the trainee advanced to the next stage. Notations in Equation (1) represent both high- and low-level states.
Fig. 6.
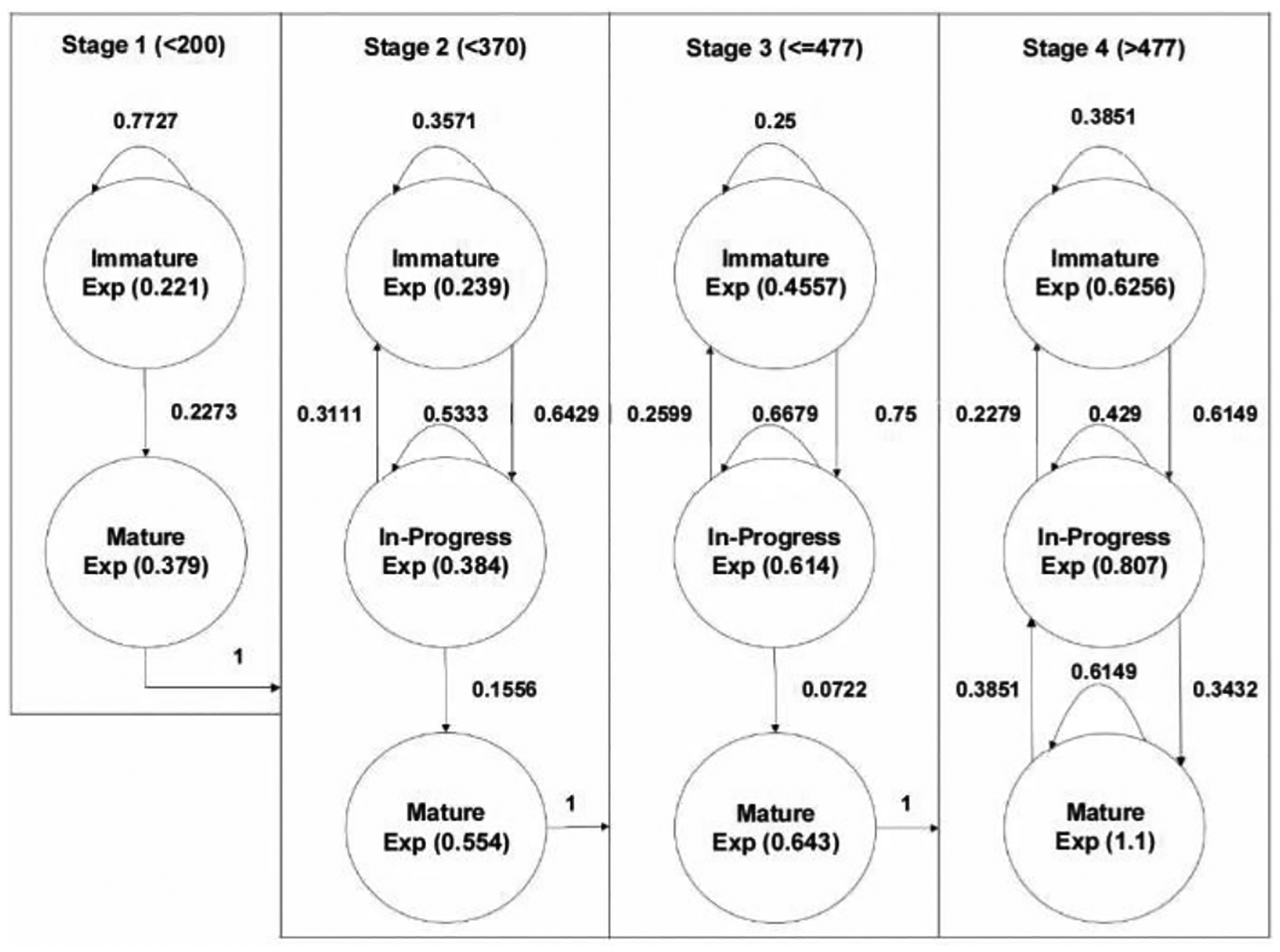
CTMC modeling results of practicing using real environment
| (1) |
The corresponding transition matrix (T) is as derived below. The authors observed trainees’ subsequent attempts and calculated the occurrence of the next trials’ accuracy score to derive the transition probability.
Once the learning process was modeled with the CTMC, the general Markov Chain analytics were applied to characterize the learning process. For example, it can be analytically derived based on the average number of trials to advance to the next stage (Stages 1 to 2, 2 to 3, and 3 to 4), as all the mature states are absorbing states. The transit matrices (Q) in each stage (named Q1, Q2, and Q3, respectively) were also developed under the consideration of transition probabilities only between transient states, as in Equation (2).
| (2) |
Then, the average number of trials (N) required to advance to the next stage can be obtained by reading the first element of the vector derived by the following formula: , where I, Q, and 1 represent an identity matrix, a transient matrix, and vector of 1, respectively. On average, 4.35, 11.09, and 20.57 trials were required to advance to the next stage from 1 to 2, from 2 to 3, and from 3 to 4, respectively. This observation is consistent with the traditional learning theory that more additional efforts are required to advance to the next stage after the trainee passes the earlier learning phase (i.e., it is more challenging to become an expert). Moreover, one can estimate each state’s rate values by considering the completion time in each trial, as shown in Table VII. The authors have empirically proved that the rate values follow an exponential distribution, satisfying the assumption of the Markov Chain model. This will be investigated at the end of this section.
TABLE VII.
Rate Values in a Real Environment
| High-level States | Sub-States | Rate (× 10−2) | Average Completion Time (sec) |
|---|---|---|---|
| Stage 1 | Immature | 0.221 | 451.74 |
| Mature | 0.379 | 263.80 | |
| Stage 2 | Immature | 0.239 | 418.41 |
| In-Progress | 0.384 | 260.42 | |
| Mature | 0.554 | 180.51 | |
| Stage 3 | Immature | 0.456 | 219.30 |
| In-Progress | 0.614 | 162.87 | |
| Mature | 0.643 | 155.52 | |
| Stage 4 | Immature | 0.626 | 159.74 |
| In-Progress | 0.807 | 123.92 | |
| Mature | 1.1 | 90.91 |
The trainee took in each training state. Rate values were estimated by taking the reciprocal values of the average practice time, implying how fast a trainee completes a trial in each learning state. As shown in the table, it is evident that a trainee spends less time as she advances to a higher level, indicating her progress in learning. Moreover, even if she is at a higher level, it is also possible that she could produce a sudden proficiency drop, resulting in an increased completion time in the same stage. The table also shows that regardless of Stages where the trainee is in, the completion time drops (the rate values increase) as she moves from “Immature” to “In-Progress” and from “In-Progress” to “Mature, demonstrating the trainee’s performance improves in each Stage.
Now, the same analogy can be applied to the VR-based training data. The authors have developed the CTMC model along with a transition matrix to mimic the learning process in a VR training environment. The transition matrix (T) was also derived in the same way as before.
Fig. 7 shows CTMC modeling results in a VR training environment by incorporating all eight participants’ practice results. Corresponding transient matrices are as follows: One can use the same formula, to find the expected number of trials to advance the next stages by checking its first element, as discussed before. On average, 3.1, 8.83, and 29.68 trials are required to advance to Stages 2, 3, and 4, respectively.
Fig. 7.
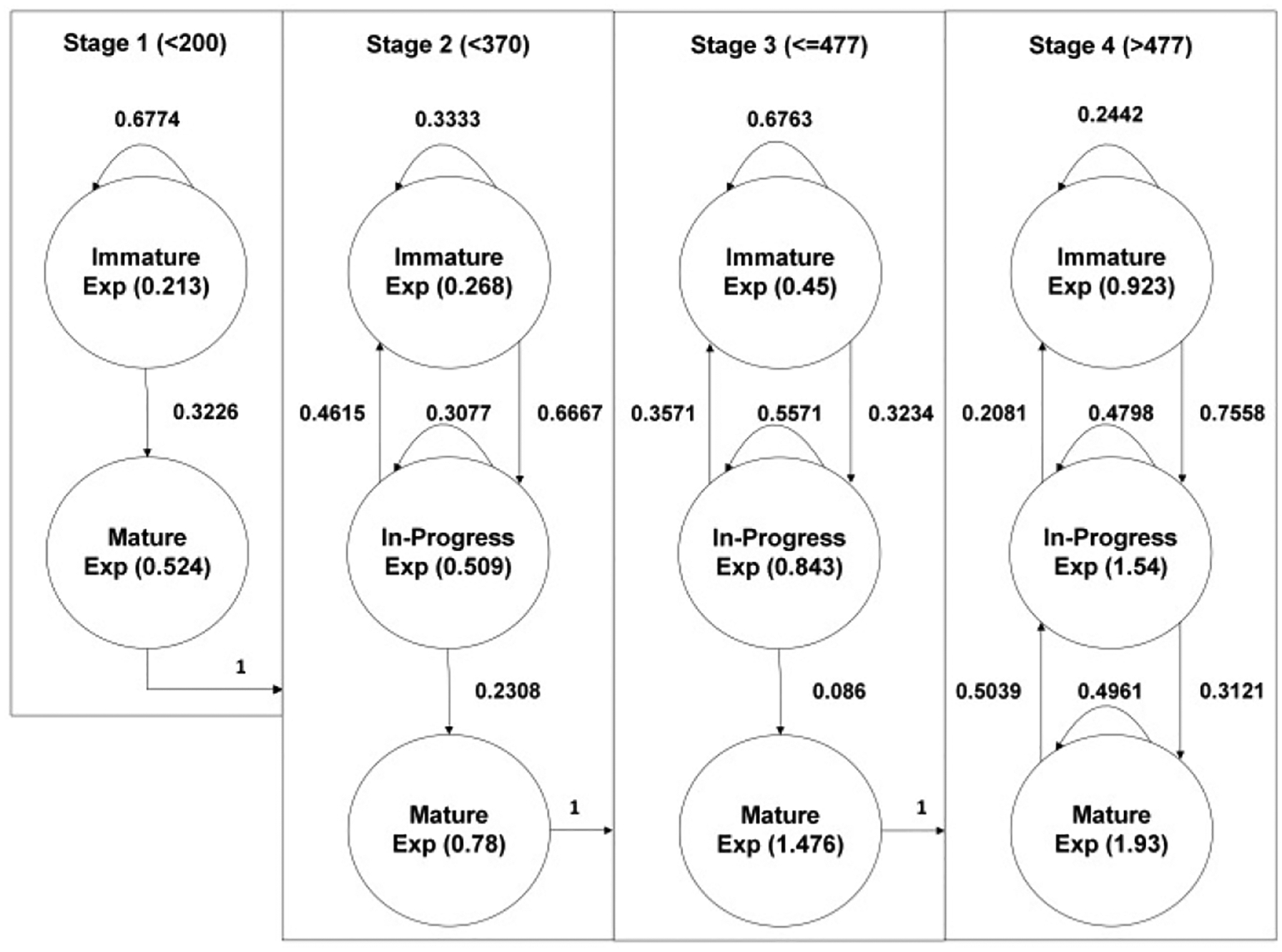
CTMC modeling results of practicing in a VR environment
Table VIII summarizes the average number of trials to advance to the next stage in real- and VR-based training environments. Compared to the learning process in a real environment, it requires fewer trials to reach Stages 2 and 3, as shown in Table VIII, representing that the VR-based training aided in bending the initial learning barriers. However, after the practitioners reach Stage 3, it requires more trials of experiments to reach Stage 4, meaning that a trainee has to practice more in a VR environment to score more than 477. This result indicates that even though the VR-based training model helps you reduce the initial barrier in surgical training, it becomes less effective for trainees at a later learning phase (who have already overcome the initial learning barrier) to become fully trained. It also implies that a higher level of resolution and complexity of the training model is required to support the trainees with advanced skills.
TABLE VIII.
Average Number of Trials for Advancing
| From | To | Average # of Trials in Real Env. | Average # of Trials in VR Env. |
|---|---|---|---|
| Stage 1 | Stage 2 | 4.35 | 3.1 |
| Stage 2 | Stage 3 | 8.83 | 11.09 |
| Stage 3 | Stage 4 | 20.57 | 29.68 |
Table IX shows the average completion time and the rate values at each state in VR-based training. Compared to training in a real environment, the “Immature” state in Stage 1 is more prolonged, indicating that trainees take more time to become familiar with the VR setting. After the trainee advances to Stage 2, the rate value increases as the sub-state moves forward, indicating sequential improvement in learning in each stage. Overall, compared to Table VIII, one can observe that VR experiments result in less completion per trial, indicating that VR could assist better for trainees to mature faster compared to training in a real environment.
TABLE IX.
Rate Values in a VR Environment
| High-level States | Sub-States | Rate (× 10−2) | Average Completion Time (sec) |
|---|---|---|---|
| Stage 1 | Immature | 0.213 | 469.48 |
| Mature | 0.524 | 190.84 | |
| Stage 2 | Immature | 0.268 | 373.13 |
| In-Progress | 0.509 | 196.46 | |
| Mature | 0.78 | 128.21 | |
| Stage 3 | Immature | 0.45 | 222.22 |
| In-Progress | 0.843 | 118.62 | |
| Mature | 1.476 | 67.75 | |
| Stage 4 | Immature | 0.923 | 108.34 |
| In-Progress | 1.54 | 64.94 | |
| Mature | 1.93 | 51.81 |
The authors checked whether the proposed model satisfies the major assumption of CTMC that all the rate values in the proposed CTMC model should follow Exponential distribution. The distribution fitting was carried out using rate values in all states through the goodness-of-fit (GOF). The authors selected six representative continuous distributions, including Normal, Lognormal, Exponential, Weibull, Gamma, and Exponential distributions, and fitted the completion time data into each distribution. Then, both Akaike Information Criterion (AIC) and Bayesian Information Criterion (BIC) values were derived to find the best distribution representing the rate values. Tables IX and X present both AIC and BIC values of each distribution’s fitting results in real and VR environments, respectively.
TABLE X.
AIC and BIC Values in a Real Environment
| Exp | Uniform | Normal | Gamma | Weibull | Log. N. | |
|---|---|---|---|---|---|---|
| AIC | 388.20 | 367.41 | 362.70 | 362.70 | 361.46 | 360.7 |
| BIC | 389.50 | 370.00 | 365.29 | 365.29 | 364.05 | 363.3 |
| Exp | Normal | Uniform | Gamma | Weibull | Log. N. | |
| AIC | 154.27 | 151.30 | 150.28 | 149.75 | 149.53 | 149.53 |
| BIC | 154.67 | 152.10 | 151.07 | 150.54 | 150.32 | 150.32 |
| Exp | Gamma | Log. N. | Normal | Weibull | Uniform | |
| AIC | 290.92 | 257.91 | 256.12 | 254.30 | 253.55 | 250.62 |
| BIC | 292.05 | 260.18 | 258.39 | 256.57 | 255.82 | 252.89 |
| Uniform | Exp | Normal | Gamma | Log.N. | Weibull | |
| AIC | 1052.63 | 1026.57 | 1010.59 | 995.5 | 988.15 | 991.58 |
| BIC | 1057.39 | 1028.95 | 1015.35 | 1000.26 | 992.91 | 996.34 |
| Uniform | Exp | Normal | Weibull | Log.N. | Gamma | |
| AIC | 707.3 | 637.35 | 639.42 | 607.84 | 576.9 | 553.22 |
| BIC | 711.31 | 639.36 | 643.43 | 611.85 | 580.92 | 557.24 |
| Exp | Gamma | Log.N. | Normal | Weibull | Uniform | |
| AIC | 85.45 | 64.92 | 64.26 | 63.09 | 61.15 | 60.89 |
| BIC | 85.53 | 65.08 | 64.42 | 63.25 | 61.31 | 61.04 |
| Exp | Normal | Uniform | Weibull | Log.N. | Gamma | |
| AIC | 1790.99 | 1645.08 | 1605.23 | 1564.87 | 1541.3 | 1513.78 |
| BIC | 1796.88 | 1650.98 | 1608.18 | 1570.77 | 1547.19 | 1519.68 |
| Exp | Uniform | Normal | Weibull | Log.N. | Gamma | |
| AIC | 5382.53 | 5295.1 | 4879.54 | 4843.24 | 4771.06 | 4753.92 |
| BIC | 5386.79 | 5303.61 | 4888.05 | 4851.75 | 4779.57 | 4762.43 |
| Exp | Uniform | Weibull | Normal | Gamma | Log.N. | |
| AIC | 3127.56 | 2689.05 | 2583.29 | 2576.16 | 2566.32 | 2562.29 |
| BIC | 3131.32 | 2696.56 | 2590.81 | 2583.67 | 2573.83 | 2569.8 |
Both Tables X and XI are displayed in decreasing order of AIC & BIC values from left to right. Table X observes that the highest AIC & BIC values are produced when fitting to Exponential distribution in most of the cases. Only two states show that the Uniform distribution fits better; however, uniform distribution works far worse in all the other states, indicating that exponential would be the best distribution to represent the randomness of rate values in each state. On the other hand, in a VR environment, the exponential distribution beats all the other distributions, as shown in Table XI. Both AIC and BIC values are highest in the case of exponential distributions in all the states. Therefore, one can conclude that the exponential distribution is the best distribution to represent the variability of the rate values (average completion time) in states within the proposed CTMC model. It is confirmed that the proposed model satisfies the basic assumption of CTMC.
TABLE XI.
AIC and BIC Values in a VR Environment
| Exp | Gamma | Log.N. | Normal | Weibull | Uniform | |
|---|---|---|---|---|---|---|
| AIC | 329.2 | 297.22 | 295.47 | 293.26 | 291.81 | 273.86 |
| BIC | 330.34 | 299.49 | 297.74 | 295.53 | 294.08 | 276.13 |
| Exp | Weibull | Gamma | Normal | Log.N. | Uniform | |
| AIC | 157.27 | 130.72 | 130.47 | 130.42 | 130.37 | 126.17 |
| BIC | 157.67 | 131.52 | 131.26 | 131.22 | 131.16 | 126.96 |
| Exp | Uniform | Gamma | Weibull | Log.N. | Normal | |
| AIC | 500.81 | 430.32 | 418.46 | 416.96 | 416.7 | 415.52 |
| BIC | 502.45 | 433.6 | 421.73 | 420.23 | 419.97 | 418.8 |
| Exp | Gamma | Log.N. | Normal | Weibull | Uniform | |
| AIC | 138.28 | 104.6 | 104.4 | 104.11 | 103.42 | 97.15 |
| BIC | 138.68 | 105.39 | 105.2 | 104.90 | 104.21 | 97.95 |
| Exp | Gamma | Log.N. | Normal | Weibull | Uniform | |
| AIC | 295.99 | 266.65 | 264.98 | 263.58 | 262.83 | 256.68 |
| BIC | 297.12 | 268.92 | 267.25 | 265.85 | 265.1 | 258.95 |
| Exp | Weibull | Normal | Log.N. | Gamma | Uniform | |
| AIC | 586.87 | 476.28 | 473.72 | 471.58 | 471.13 | 463.6 |
| BIC | 588.74 | 480.02 | 477.47 | 475.32 | 474.87 | 467.34 |
| Exp | Uniform | Normal | Weibull | Log.N. | Gamma | |
| AIC | 500.08 | 452.38 | 450.97 | 450.11 | 444.87 | 444.15 |
| BIC | 501.79 | 455.81 | 454.4 | 453.54 | 448.3 | 447.58 |
| Exp | Uniform | Weibull | Gamma | Normal | Log.N. | |
| AIC | 1154.23 | 986.64 | 954.24 | 952.55 | 950.86 | 950.16 |
| BIC | 1156.83 | 991.83 | 959.43 | 957.74 | 956.05 | 955.35 |
| Exp | Log.N. | Gamma | Normal | Wi. | Uniform | |
| AIC | 1257.02 | 990.35 | 995.23 | 984.02 | 978.11 | 969.38 |
| BIC | 1259.76 | 995.83 | 1000.70 | 989.49 | 983.58 | 974.85 |
V. Conclusion
This work proposes a new modeling framework to depict the learning procedures in surgical training. The proposed CTMC-based model of the learning processes captures learning variability induced by trainees and a sudden performance drop at the later learning phase, which supports to find the best time to provide additional feedback in the learning process. Secondly, our proposed model helps to identify a “stagnation” phase by relying on the advanced analytics of CTMC. One can compare the expected number of trials for a trainee to advance to the next stage analytically. Thus, if a trainee performs more than the average number of trials and remains in the same stage, we can provide any additional feedback to facilitate their training process. Thirdly, through CTMC, we can identify differences in the surgical learning processes in a real versus VR environment, which could be used to identify additional aids to support trainees at a later learning phase by investigating the rate and transition probabilities. Finally, CTMC can represent three different measures, including accuracy scores, trials, and completion time, together in one graph, which is simple and intuitive compared to the other learning methods we described. The competitive advantages of the proposed CTMC-model demonstrate the validity of the VR-based training model.
Acknowledgment
Research reported in this publication was supported by the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health under Award Number R44EB019802 and by the Medical Technology Enterprise Consortium (MTEC) award #W81XWH2090019 (2020-628).
Biographies

Seunghan Lee is an assistant professor in the Industrial and Systems Engineering Department at Mississippi State University.
His research encompasses modeling and analysis of data-driven systems under uncertainty using simulation and stochastics. Major application areas include disaster relief and mitigation efforts, smart city and homeland security, and healthcare operations.
Dr. Lee received the Institute of Industrial and Systems Engineering (IISE) Annual Meetings Best Paper Awards in the Service & Work Systems Track and the Security Engineering Track in 2016 and 2018, respectively. He is also a member of IISE and the Institute of Operation Research and Management Sciences (INFORMS).

Amar Sadanand Shetty received the bachelor’s degree in Mechanical Engineering from University of Mumbai, Mumbai, India, in 2018 and currently pursuing Master of Science in Industrial and Systems Engineering (Operations Research specialization) from University at Buffalo, The State University of New York.
Mr. Shetty’s research interest includes process optimization using mathematical modelling, statistical data analysis, system analysis using stochastics process, mechanical process like thermodynamic system. The research applies to areas like Supply Chain Planning, Warehousing, Temperature-controlled system. In 2015, Mr. Shetty was awarded with Academic Award for Engineering Drawing Exam by University of Mumbai. He is a member of IISE.

Lora A. Cavuoto received her B.S. degree in Biomedical Engineering and M.S. in Occupational Ergonomics and Safety from the University of Miami, Coral Gables, FL, USA, in 2006 and 2008, respectively. She received M.S. and Ph.D. degrees in Industrial and Systems Engineering from Virginia Tech, Blacksburg, VA, USA, in 2009 and 2012, respectively.
She is currently an Associate Professor of Industrial and Systems Engineering at the University at Buffalo, Buffalo, NY, USA. She is the author of more than 60 journal papers. Her research interests include surgical training, motor skills development, occupational ergonomics, fatigue, and wearable sensor applications in industry. She serves as a scientific editor for Applied Ergonomics and an associate editor for Human Factors and Ergonomics in Manufacturing & Service Industries.
Contributor Information
Seunghan Lee, Industrial and Systems Engineering Department at Mississippi State University..
Amar Sadanand Shetty, University at Buffalo, The State University of New York..
Lora Cavuoto, Industrial and Systems Engineering at the University at Buffalo, Buffalo, NY, USA.
References
- [1].Aggarwal R, Grantcharov TP, Eriksen JR, Blirup D, Kristiansen VB, Funch-Jensen P, & Darzi A (2006). An evidence-based virtual reality training program for novice laparoscopic surgeons. Annals of surgery, 244(2), 310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Aggarwal R, Ward J, Balasundaram I, Sains P, Athanasiou T, & Darzi A (2007). Proving the effectiveness of virtual reality simulation for training in laparoscopic surgery. Annals of surgery, 246(5), 771–779. [DOI] [PubMed] [Google Scholar]
- [3].Alaker M, Wynn GR, & Arulampalam T (2016). Virtual reality training in laparoscopic surgery: a systematic review & meta-analysis. International Journal of Surgery, 29, 85–94. [DOI] [PubMed] [Google Scholar]
- [4].Alaraj A, Lemole MG, Finkle JH, Yudkowsky R, Wallace A, Luciano C, … & Charbel FT (2011). Virtual reality training in neurosurgery: review of current status and future applications. Surgical neurology international, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Biau DJ, Williams SM, Schlup MM, Nizard RS, & Porcher R (2008). Quantitative and individualized assessment of the learning curve using LC-CUSUM. Journal of British Surgery, 95(7), 925–929. [DOI] [PubMed] [Google Scholar]
- [6].Bosse HM, Mohr J, Buss B, Krautter M, Weyrich P, Herzog W, … & Nikendei C (2015). The benefit of repetitive skills training and frequency of expert feedback in the early acquisition of procedural skills. BMC medical education, 15(1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Botden SM, Buzink SN, Schijven MP, & Jakimowicz JJ (2007). Augmented versus virtual reality laparoscopic simulation: What is the difference? World journal of surgery, 31(4), 764–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bracq MS, Michinov E, Arnaldi B, Caillaud B, Gibaud B, Gouranton V, & Jannin P (2019). Learning procedural skills with a virtual reality simulator: An acceptability study. Nurse education today, 79, 153–160. [DOI] [PubMed] [Google Scholar]
- [9].Brunckhorst O, Ahmed K, Nehikhare O, Marra G, Challacombe B, & Popert R (2015). Evaluation of the learning curve for holmium laser enucleation of the prostate using multiple outcome measures. Urology, 86(4), 824–829. [DOI] [PubMed] [Google Scholar]
- [10].Chan KS, Wang ZK, Syn N, & Goh BK (2021). Learning curve of laparoscopic and robotic pancreas resections: a systematic review. Surgery, 170(1), 194–206. [DOI] [PubMed] [Google Scholar]
- [11].Chang EJ, Mandelbaum RS, Nusbaum DJ, Violette CJ, Matsushima K, Klar M, … & Matsuo K (2020). Vesicoureteral injury during benign hysterectomy: minimally invasive laparoscopic surgery versus laparotomy. Journal of minimally invasive gynecology, 27(6), 1354–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Falah J, Khan S, Alfalah T, Alfalah SF, Chan W, Harrison DK, & Charissis V (2014, August). Virtual Reality medical training system for anatomy education. In 2014 Science and information conference (pp. 752–758). IEEE. [Google Scholar]
- [13].Feldman LS, Cao J, Andalib A, Fraser S, Fried GM. A method to characterize the learning curve for performance of a fundamental laparoscopic simulator task: defining “learning plateau” and “learning rate.” Surgery. 2009;146(2):381–386. [DOI] [PubMed] [Google Scholar]
- [14].Fiani B, De Stefano F, Kondilis A, Covarrubias C, Reier L, & Sarhadi K (2020). Virtual Reality in Neurosurgery:“Can You See It?”– A Review of the Current Applications and Future Potential. World neurosurgery, 141, 291–298. [DOI] [PubMed] [Google Scholar]
- [15].Franzese CB, & Stringer SP (2007). The evolution of surgical training: perspectives on educational models from the past to the future. Otolaryngologic Clinics of North America, 40(6), 1227–1235. [DOI] [PubMed] [Google Scholar]
- [16].Fraser SA, Feldman LS, Stanbridge D, Fried GM. Characterizing the learning curve for a basic laparoscopic drill. Surg Endosc. 2005;19(12):1572–1578. [DOI] [PubMed] [Google Scholar]
- [17].Fried GM, Feldman LS, Vassiliou MC, Fraser SA, Stanbridge D, Ghitulescu G, and Andrew CG, Proving the value of simulation in laparoscopic surgery, Annals of Surgery, vol. 240, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Fried MP, Sadoughi B, Gibber MJ, Jacobs JB, Lebowitz RA, Ross DA, … & Schaefer SD (2010). From virtual reality to the operating room: the endoscopic sinus surgery simulator experiment. Otolaryngology—Head and Neck Surgery, 142(2), 202–207. [DOI] [PubMed] [Google Scholar]
- [19].Fu Y, Cavuoto L, Qi D, Panneerselvam K, Arikatla VS, Enquobahrie A, … & Schwaitzberg SD (2020). Characterizing the learning curve of a virtual intracorporeal suturing simulator VBLaST-SS©. Surgical endoscopy, 34(7), 3135–3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gélinas-Phaneuf N, Choudhury N, Al-Habib AR, Cabral A, Nadeau E, Mora V, … & Del Maestro RF (2014). Assessing performance in brain tumor resection using a novel virtual reality simulator. International journal of computer assisted radiology and surgery, 9(1), 1–9. [DOI] [PubMed] [Google Scholar]
- [21].Gomoll AH, O’toole RV, Czarnecki J, & Warner JJ (2007). Surgical experience correlates with performance on a virtual reality simulator for shoulder arthroscopy. The American journal of sports medicine, 35(6), 883–888. [DOI] [PubMed] [Google Scholar]
- [22].Gomoll AH, Pappas G, Forsythe B, & Warner JJ (2008). Individual skill progression on a virtual reality simulator for shoulder arthroscopy: a 3-year follow-up study. The American journal of sports medicine, 36(6), 1139–1142. [DOI] [PubMed] [Google Scholar]
- [23].Gorantla KR, & Esfahani ET (2019, July). Surgical skill assessment using motor control features and hidden Markov model. In 2019 41st annual international conference of the IEEE engineering in medicine and biology society (EMBC) (pp. 5842–5845). IEEE. [DOI] [PubMed] [Google Scholar]
- [24].Grantcharov TP, Bardram L, Funch-Jensen P, & Rosenberg J (2003). Learning curves and impact of previous operative experience on performance on a virtual reality simulator to test laparoscopic surgical skills. The American journal of surgery, 185(2), 146–149. [DOI] [PubMed] [Google Scholar]
- [25].Grantcharov TP, Kristiansen VB, Bendix J, Bardram L, Rosenberg J, & Funch-Jensen P (2004). Randomized clinical trial of virtual reality simulation for laparoscopic skills training. Journal of British Surgery, 91(2), 146–150. [DOI] [PubMed] [Google Scholar]
- [26].Gurusamy KS, Aggarwal R, Palanivelu L, & Davidson BR (2009). Virtual reality training for surgical trainees in laparoscopic surgery. Cochrane database of systematic reviews, (1). [DOI] [PubMed] [Google Scholar]
- [27].Gurusamy K, Aggarwal R, Palanivelu L, & Davidson BR (2008). Systematic review of randomized controlled trials on the effectiveness of virtual reality training for laparoscopic surgery. Journal of British Surgery, 95(9), 1088–1097. [DOI] [PubMed] [Google Scholar]
- [28].Haluck RS, & Krummel TM (2000). Computers and virtual reality for surgical education in the 21st century. Archives of surgery, 135(7), 786–792. [DOI] [PubMed] [Google Scholar]
- [29].Hardon SF, van Gastel LA, Horeman T, & Daams F (2021). Assessment of technical skills based on learning curve analyses in laparoscopic surgery training. Surgery, 170(3), 831–840. [DOI] [PubMed] [Google Scholar]
- [30].Hart R, & Karthigasu K (2007). The benefits of virtual reality simulator training for laparoscopic surgery. Current Opinion in Obstetrics and Gynecology, 19(4), 297–302. [DOI] [PubMed] [Google Scholar]
- [31].Higgins M, Madan CR, & Patel R (2021). Deliberate practice in simulation-based surgical skills training: a scoping review. Journal of Surgical Education, 78(4), 1328–1339. [DOI] [PubMed] [Google Scholar]
- [32].Howells NR, Auplish S, Hand GC, Gill HS, Carr AJ, & Rees JL (2009). Retention of arthroscopic shoulder skills learned with use of a simulator: Demonstration of a learning curve and loss of performance level after a time delay. JBJS, 91(5), 1207–1213. [DOI] [PubMed] [Google Scholar]
- [33].Hsieh MS, Tsai MD, & Chang WC (2002). Virtual reality simulator for osteotomy and fusion involving the musculoskeletal system. Computerized medical imaging and graphics, 26(2), 91–101. [DOI] [PubMed] [Google Scholar]
- [34].Jain S, Lee S, Barber SR, Chang EH, & Son YJ (2020). Virtual reality-based hybrid simulation for functional endoscopic sinus surgery. IISE Transactions on Healthcare Systems Engineering, 10(2), 127–141. [Google Scholar]
- [35].Jacobsen ME, Andersen MJ, Hansen CO, & Konge L (2015). Testing basic competency in knee arthroscopy using a virtual reality simulator: exploring validity and reliability. JBJS, 97(9), 775–781. [DOI] [PubMed] [Google Scholar]
- [36].Jin C, Dai L, & Wang T (2021). The application of virtual reality in the training of laparoscopic surgery: a systematic review and meta-analysis. International Journal of Surgery, 87, 105859. [DOI] [PubMed] [Google Scholar]
- [37].Johnson SJ, Guediri SM, Kilkenny C, & Clough PJ (2011). Development and validation of a virtual reality simulator: human factors input to interventional radiology training. Human Factors, 53(6), 612–625. [DOI] [PubMed] [Google Scholar]
- [38].Kauffman JD, Nguyen ATH, Litz CN, Farach SM, DeRosa JC, Gonzalez R, … & Chandler NM (2020). Laparoscopic-guided versus transincisional rectus sheath block for pediatric single-incision laparoscopic cholecystectomy: A randomized controlled trial. Journal of pediatric surgery, 55(8), 1436–1443. [DOI] [PubMed] [Google Scholar]
- [39].Khan N, Abboudi H, Khan MS, Dasgupta P, & Ahmed K (2014). Measuring the surgical ‘learning curve’: methods, variables and competency. Bju Int, 113(3), 504–508. [DOI] [PubMed] [Google Scholar]
- [40].Kim Y, Kim H, & Kim YO (2017). Virtual reality and augmented reality in plastic surgery: a review. Archives of plastic surgery, 44(3), 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Korndorffer JR Jr, Dunne JB, Sierra R, Stefanidis D, Touchard CL, & Scott DJ (2005). Simulator training for laparoscopic suturing using performance goals translates to the operating room. Journal of the American College of Surgeons, 201(1), 23–29. [DOI] [PubMed] [Google Scholar]
- [42].Lam CK, Sundaraj K, & Sulaiman MN (2013). Virtual reality simulator for phacoemulsification cataract surgery education and training. Procedia Computer Science, 18, 742–748. [Google Scholar]
- [43].Larsen CR, Soerensen JL, Grantcharov TP, Dalsgaard T, Schouenborg L, Ottosen C, … & Ottesen BS (2009). Effect of virtual reality training on laparoscopic surgery: randomised controlled trial. Bmj, 338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Lee GI, & Lee MR (2018). Can a virtual reality surgical simulation training provide a self-driven and mentor-free skills learning? Investigation of the practical influence of the performance metrics from the virtual reality robotic surgery simulator on the skill learning and associated cognitive workloads. Surgical endoscopy, 32(1), 62–72. [DOI] [PubMed] [Google Scholar]
- [45].Leijte E, de Blaauw I, Van Workum F, Rosman C, & Botden S (2020). Robot assisted versus laparoscopic suturing learning curve in a simulated setting. Surgical endoscopy, 34(8), 3679–3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Leong JJ, Nicolaou M, Atallah L, Mylonas GP, Darzi AW, & Yang GZ (2006, October). HMM assessment of quality of movement trajectory in laparoscopic surgery. In International Conference on Medical Image Computing and Computer-Assisted Intervention (pp. 752–759). Springer, Berlin, Heidelberg. [DOI] [PubMed] [Google Scholar]
- [47].Lopez-Beauchamp C, Singh GA, Shin SY, & Magone MT (2020). Surgical simulator training reduces operative times in resident surgeons learning phacoemulsification cataract surgery. American journal of ophthalmology case reports, 17, 100576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Luca A, Giorgino R, Gesualdo L, Peretti GM, Belkhou A, Banfi G, & Grasso G (2020). Innovative Educational Pathways in Spine Surgery: Advanced Virtual Reality–Based Training. World Neurosurgery, 140, 674–680. [DOI] [PubMed] [Google Scholar]
- [49].Megali G, Sinigaglia S, Tonet O, & Dario P (2006). Modelling and evaluation of surgical performance using hidden Markov models. IEEE Transactions on Biomedical Engineering, 53(10), 1911–1919. [DOI] [PubMed] [Google Scholar]
- [50].Mok TN, Chen J, Pan J, Ming WK, He Q, Sin TH, … & Zha Z (2021). Use of a Virtual Reality Simulator for Tendon Repair Training: Randomized Controlled Trial. JMIR serious games, 9(3), e27544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Müller WK, Ziegler R, Bauer A, & Soldner EH (1995). Virtual reality in surgical arthroscopic training. Journal of Image Guided Surgery, 1(5), 288–294. [DOI] [PubMed] [Google Scholar]
- [52].Munz Y, Almoudaris AM, Moorthy K, Dosis A, Liddle AD, & Darzi AW (2007). Curriculum-based solo virtual reality training for laparoscopic intracorporeal knot tying: objective assessment of the transfer of skill from virtual reality to reality. The American journal of surgery, 193(6), 774–783. [DOI] [PubMed] [Google Scholar]
- [53].Papachristofi O, Jenkins D, & Sharples LD (2016). Assessment of learning curves in complex surgical interventions: a consecutive case-series study. Trials, 17(1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Papanikolaou IG, Haidopoulos D, Paschopoulos M, Chatzipapas I, Loutradis D, & Vlahos NF (2019). Changing the way we train surgeons in the 21th century: a narrative comparative review focused on box trainers and virtual reality simulators. European Journal of Obstetrics & Gynecology and Reproductive Biology, 235, 13–18. [DOI] [PubMed] [Google Scholar]
- [55].Park J, MacRae H, Musselman LJ, Rossos P, Hamstra SJ, Wolman S, & Reznick RK (2007). Randomized controlled trial of virtual reality simulator training: transfer to live patients. The American journal of surgery, 194(2), 205–211. [DOI] [PubMed] [Google Scholar]
- [56].Perivoliotis K, Baloyiannis I, Mamaloudis I, Volakakis G, Valaroutsos A, & Tzovaras G (2022). Change point analysis validation of the learning curve in laparoscopic colorectal surgery: Experience from a non-structured training setting. World Journal of Gastrointestinal Endoscopy, 14(6), 387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Pfandler M, Lazarovici M, Stefan P, Wucherer P, & Weigl M (2017). Virtual reality-based simulators for spine surgery: a systematic review. The Spine Journal, 17(9), 1352–1363. [DOI] [PubMed] [Google Scholar]
- [58].Phé V, Cattarino S, Parra J, Bitker MO, Ambrogi V, Vaessen C, & Rouprêt M (2017). Outcomes of a virtual-reality simulator-training programme on basic surgical skills in robot-assisted laparoscopic surgery. The International Journal of Medical Robotics and Computer Assisted Surgery, 13(2), e1740. [DOI] [PubMed] [Google Scholar]
- [59].Pulijala Y, Ma M, Pears M, Peebles D, & Ayoub A (2018). Effectiveness of immersive virtual reality in surgical training—a randomized control trial. Journal of Oral and Maxillofacial Surgery, 76(5), 1065–1072. [DOI] [PubMed] [Google Scholar]
- [60].Portelli M, Bianco SF, Bezzina T, & Abela JE (2020). Virtual reality training compared with apprenticeship training in laparoscopic surgery: a meta-analysis. The Annals of The Royal College of Surgeons of England, 102(9), 672–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Reinhard Friedl MD (2002). Virtual reality and 3D visualizations in heart surgery education. In The Heart surgery forum (Vol. 2001, p. 03054). [PubMed] [Google Scholar]
- [62].Rogers MP, DeSantis AJ, Janjua H, Barry TM, & Kuo PC (2021). The future surgical training paradigm: Virtual reality and machine learning in surgical education. Surgery, 169(5), 1250–1252. [DOI] [PubMed] [Google Scholar]
- [63].Rosen J, Solazzo M, Hannaford B, & Sinanan M (2002). Task decomposition of laparoscopic surgery for objective evaluation of surgical residents’ learning curve using hidden Markov model. Computer Aided Surgery, 7(1), 49–61. [DOI] [PubMed] [Google Scholar]
- [64].Saravanan P, & Menold J (2022). Deriving Effective Decision-Making Strategies of Prosthetists: Using Hidden Markov Modeling and Qualitative Analysis to Compare Experts and Novices. Human Factors, 64(1), 188–206. [DOI] [PubMed] [Google Scholar]
- [65].Sayadi LR, Naides A, Eng M, Fijany A, Chopan M, Sayadi JJ, … & Widgerow AD (2019). The new frontier: a review of augmented reality and virtual reality in plastic surgery. Aesthetic surgery journal, 39(9), 1007–1016. [DOI] [PubMed] [Google Scholar]
- [66].Scott DJ, & Ritter E (2018). Fundamentals of Laparoscopic Surgery technical skills proficiency-based training curriculum. https://www.flsprogram.org/wp-content/uploads/2014/02/Proficiency-Based-Curriculum-updated-May-2019-v24-.pdf [DOI] [PubMed]
- [67].Selvander M, & Åsman P (2012). Virtual reality cataract surgery training: learning curves and concurrent validity. Acta ophthalmologica, 90(5), 412–417. [DOI] [PubMed] [Google Scholar]
- [68].Seymour NE (2008). VR to OR: a review of the evidence that virtual reality simulation improves operating room performance. World journal of surgery, 32(2), 182–188. [DOI] [PubMed] [Google Scholar]
- [69].Silva JN, Southworth M, Raptis C, & Silva J (2018). Emerging applications of virtual reality in cardiovascular medicine. JACC: Basic to Translational Science, 3(3), 420–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Smith SE, Tallentire VR, Spiller J, Wood SM, & Cameron HS (2012). The educational value of using cumulative sum charts. Anaesthesia, 67(7), 734–740. [DOI] [PubMed] [Google Scholar]
- [71].Society of American Gastrointestinal and Endoscopic Surgeons (SAGES). FLS Manual Skills Written Instructions and Performance Guidelines (2014). https://www.flsprogram.org/wp-content/uploads/2014/03/Revised-Manual-Skills-Guidelines-February-2014.pdf. [Google Scholar]
- [72].Sood A, Ghani KR, Ahlawat R, Modi P, Abaza R, Jeong W, … & Bhandari M (2014). Application of the statistical process control method for prospective patient safety monitoring during the learning phase: robotic kidney transplantation with regional hypothermia (IDEAL phase 2a–b). European urology, 66(2), 371–378. [DOI] [PubMed] [Google Scholar]
- [73].Subramonian K, & Muir G (2004). The ‘learning curve’in surgery: what is it, how do we measure it and can we influence it?. BJU international, 93(9), 1173–1174. [DOI] [PubMed] [Google Scholar]
- [74].Suguita FY, Essu FF, Oliveira LT, Iuamoto LR, Kato JM, Torsani MB, … & Andraus W (2017). Learning curve takes 65 repetitions of totally extraperitoneal laparoscopy on inguinal hernias for reduction of operating time and complications. Surgical endoscopy, 31(10), 3939–3945. [DOI] [PubMed] [Google Scholar]
- [75].Sultana A, Nightingale P, Marudanayagam R, & Sutcliffe RP (2019). Evaluating the learning curve for laparoscopic liver resection: a comparative study between standard and learning curve CUSUM. HPB, 21(11), 1505–1512 [DOI] [PubMed] [Google Scholar]
- [76].Taba JV, Cortez VS, Moraes WA, Iuamoto LR, Hsing WT, Suzuki MO, … & Andraus W (2021). The development of laparoscopic skills using virtual reality simulations: A systematic review. Plos one, 16(6), e0252609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Thomsen ASS, Bach-Holm D, Kjærbo H, Højgaard-Olsen K, Subhi Y, Saleh GM, … & Konge L (2017). Operating room performance improves after proficiency-based virtual reality cataract surgery training. Ophthalmology, 124(4), 524–531. [DOI] [PubMed] [Google Scholar]
- [78].Thomsen ASS, Smith P, Subhi Y, Cour ML, Tang L, Saleh GM, & Konge L (2017). High correlation between performance on a virtual-reality simulator and real-life cataract surgery. Acta ophthalmologica, 95(3), 307–311. [DOI] [PubMed] [Google Scholar]
- [79].Uribe SJI, Ralph WM Jr, Glaser AY, & Fried MP (2004). Learning curves, acquisition, and retention of skills trained with the endoscopic sinus surgery simulator. American journal of rhinology, 18(2), 87–92. [PubMed] [Google Scholar]
- [80].Wang H, & Wu J (2021). A virtual reality based surgical skills training simulator for catheter ablation with real-time and robust interaction. Virtual Reality & Intelligent Hardware, 3(4), 302–314. [Google Scholar]
- [81].Wilson G, Zargaran A, Kokotkin I, Bhaskar J, Zargaran D, & Trompeter A (2020). Virtual Reality and Physical Models in Undergraduate Orthopaedic Education: A Modified Randomised Crossover Trial. Orthopedic Research and Reviews, 12, 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Winkler-Schwartz A, Bissonnette V, Mirchi N, Ponnudurai N, Yilmaz R, Ledwos N, … & Del Maestro RF (2019). Artificial intelligence in medical education: best practices using machine learning to assess surgical expertise in virtual reality simulation. Journal of surgical education, 76(6), 1681–1690. [DOI] [PubMed] [Google Scholar]
- [83].Winkler-Schwartz A, Yilmaz R, Mirchi N, Bissonnette V, Ledwos N, Siyar S, … & Del Maestro R (2019). Machine learning identification of surgical and operative factors associated with surgical expertise in virtual reality simulation. JAMA network open, 2(8), e198363–e198363. [DOI] [PubMed] [Google Scholar]
- [84].Wong SW, & Crowe P (2022). Factors affecting the learning curve in robotic colorectal surgery. Journal of Robotic Surgery, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Xin B, Chen G, Wang Y, Bai G, Gao X, Chu J, … & Liu T (2019). The efficacy of immersive virtual reality surgical simulator training for pedicle screw placement: a randomized double-blind controlled trial. World neurosurgery, 124, e324–e330. [DOI] [PubMed] [Google Scholar]
- [86].Yiannakopoulou E, Nikiteas N, Perrea D, & Tsigris C (2015). Virtual reality simulators and training in laparoscopic surgery. International Journal of Surgery, 13, 60–64. [DOI] [PubMed] [Google Scholar]
- [87].Zhang L, Huo H, Li H, Luo M, Wang F, Zhou Y, … & Zhang Y (2022). Transumbilical Single-incision Laparoscopic Surgery for Harvesting Rib and Costal Cartilage. Plastic and Reconstructive Surgery Global Open, 10(3). [DOI] [PMC free article] [PubMed] [Google Scholar]


