Abstract
Prior to the adoption of widespread vaccination programs, mumps virus was the leading cause of virus-induced central nervous system (CNS) disease. Mumps virus-associated CNS complications in vaccinees continue to be reported; outside the United States, some of these complications have been attributed to vaccination with insufficiently attenuated neurovirulent vaccine strains. The development of potentially neurovirulent, live, attenuated mumps virus vaccines stems largely from the lack of an animal model that can reliably predict the neurovirulence of mumps virus vaccine candidates in humans. The lack of an effective safety test with which to measure mumps virus neurovirulence has also hindered analysis of the neuropathogenesis of mumps virus infection and the identification of molecular determinants of neurovirulence. In this report we show, for the first time, that mumps virus infection of the neonatal rat leads to developmental abnormalities in the cerebellum due to cerebellar granule cell migration defects. The incidence of the cerebellar abnormalities and other neuropathological and clinical outcomes of mumps virus infection of the neonatal rat brain demonstrated the ability of this model to distinguish neurovirulent (Kilham) from nonneurovirulent (Jeryl Lynn) mumps virus strains. Thus, this neonatal rat model may prove useful in evaluating the neurovirulence potential of new live, attenuated vaccine strains and may also be of value in elucidating the molecular basis of mumps virus neurovirulence.
Mumps virus, a member of the Paramyxoviridae family, contains a 15.3-kb enveloped, nonsegmented, negative-strand RNA virus within a lipid envelope. Humans are the only natural host of mumps virus infection, although nonhuman primates, rodents, and other species can be experimentally infected. Mumps virus is transmitted by droplet spread to the nasal mucosa or upper respiratory mucosal epithelium, resulting in viremia and dissemination to most organ systems, including the brain, with production of acute inflammatory disease (70). Cerebrospinal fluid pleocytosis has been detected in more than half of all mumps virus infections, a testament to the profound neurotropism of the virus (70). Prior to the widespread use of live, attenuated mumps virus vaccines, mumps virus was the leading cause of virus-induced central nervous system (CNS) disease (i.e., neurovirulence) (14, 30). The most frequent clinical CNS complication of wild-type mumps virus infection is aseptic meningitis (29, 46); other CNS complications include acute and chronic encephalitis (34, 47), hydrocephalus (52, 65), transverse myelitis (51, 69), and acute cerebellar ataxia (17, 28). Although the case fatality rate is only 1 in 10,000, nonfatal complications of mumps virus infection often lead to hospitalization and occasionally to permanent and severe neurological sequelae (25, 42, 50, 59).
Although vaccination programs have decreased the incidence of mumps virus infection worldwide (13), mumps virus outbreaks have not been completely eliminated, even in vaccinated populations. Mumps virus infections in vaccinees are due either to an infection with wild-type virus following primary vaccine failure (7, 24, 63) or to inoculation of a relatively neurovirulent mumps virus vaccine (e.g., Urabe AM9) (2, 16, 18, 45). Problems with excessive neurovirulence of live mumps virus vaccines primarily stem from the lack of an animal model capable of determining the strain’s neurovirulence potential for the human CNS. The lack of such an animal model has also hindered studies on the molecular basis for attenuation of mumps virus neurovirulence.
We previously demonstrated the sensitivity of the developing rat CNS to damage by perinatal viral infection with Borna disease virus (BDV) (4, 5, 12). The suggestion that mumps virus was particularly pathogenic for the rodent CNS was provided by a recent publication demonstrating enhanced susceptibility of neonatal hamsters to mumps virus-induced hydrocephalus (68). Here, we report an analysis of the developing rat CNS following intracranial inoculation with different strains of mumps virus, revealing that wild-type and vaccine strains of mumps virus could be discriminated according to disease induction in the neonatal rat. Also presented are associated strain-specific differences in in vitro replication in primate and rat neural and nonneural cell lines. Using these data, we propose a possible mechanism for the pathogenesis of mumps virus neurovirulence.
MATERIALS AND METHODS
Virus.
The hamster-neuroadapted wild-type Kilham (KH) strain (kindly provided by J. Wolinsky, University of Texas, Houston) and the attenuated Jeryl Lynn (JL) vaccine strain (MumpsVax; Merck Sharp & Dohme, West Point, Pa.) were used. Stocks of each strain were prepared from virus passaged twice on Vero cells in minimal essential medium (MEM; Gibco BRL, Gaithersburg, Md.) with 7% fetal bovine serum (FBS; Gibco BRL) at 37°C.
Plaque assay.
Viral titer was determined by plaque assay. Virus was serially diluted 10-fold, and 0.5 ml of each dilution was incubated for 1 h at 37°C on Vero cell monolayers in six-well plates. Viral inoculum was removed by aspiration; cell monolayers were rinsed with MEM, immediately covered with warm 0.5% Noble agar in 2× MEM (Quality Biologicals, Gaithersburg, Md.) supplemented with 7% FBS, and incubated at 37°C for 4 days. A second layer of agar containing 0.01% neutral red (Quality Biologicals) was subsequently added and incubated overnight, allowing for visualization of the plaques the following day. Virus was quantitated by counting the number of PFU and multiplying by the reciprocal of the dilution factor and volume plated.
Rats.
One-day-old Lewis rats (Harlan, Indianapolis, Ind.) were inoculated intracranially with 20 μl of either JL (n = 40) or KH (n = 60) containing 4 × 103 PFU. All inoculations were performed at the coronal suture approximately 1 to 2 mm left of midline. Duplicate litters of rats were inoculated with an equivalent volume of uninfected Vero cell supernatant (n = 20) to serve as controls. On days 3, 6, 9, 12, 19, and 26 postinoculation (p.i.), rats were weighed, and two control and six infected rats were deeply anesthetized and killed. Brains were removed aseptically and divided sagitally. Half of the brain, used for plaque assay for infectious virus titration, was homogenized into a 20% (wt/vol) suspension in MEM with 2% FBS by Dounce homogenization, followed by brief pulses of ultrasonic treatment, and clarified by centrifugation at 2,000 × g for 10 min. Rat body weights and viral titers were analyzed by two-way repeated-measures analysis of variance.
Histology and avidin-biotin immunohistochemistry.
The remainder of the brain was processed for histopathological and immunohistochemical analysis by fixation in 4% formalin overnight, paraffin embedding, and sagittal sectioning. Sectioned brain tissue was stained with hematoxylin and eosin and observed under light microscopy for the presence or absence of ventriculitis, hydrocephalus, meningitis, encephalitis, and developmental abnormalities. Avidin-biotin immunohistochemistry (Vector Laboratories, Burlingame, Calif.) was also performed by using a previously described method to identify inflammatory and glial cells in the cerebellum (11). Briefly, brain sections were incubated in blocking buffer (2% normal goat serum in phosphate-buffered saline) followed by incubation with either monoclonal mouse anti-major histocompatibility complex Ia (Chemicon, Temecula, Calif.) for detection of inflammatory cells or polyclonal rabbit anti-glial fibrillary acidic protein (Dako, Carpenteria, Calif.) for detection of glial cells. After being rinsed in phosphate-buffered saline, slides were incubated with secondary biotinylated anti-species immunoglobulin G antibodies and avidin-biotin-horseradish peroxidase complex (Vector Laboratories). A hydrogen peroxide-diaminobenzidine solution (Sigma, St. Louis, Mo.) was added, and the resultant brown precipitate was detected by light microscopy.
In vitro virus characterization.
Cultures of SK-N-SY5Y (human neuroblastoma; Joan Schwartz, National Institutes of Health, Bethesda, Md.), C6 (rat astrocytoma; American Type Culture Collection, Rockville, Md.), and Vero (monkey kidney; American Type Culture Collection) cells in six-well plates were infected with either JL or KH at a multiplicity of infection of 0.01 PFU/cell in MEM with 7% FBS. Supernatant was removed every 24 h over a 6- to 8-day period following infection, and the virus titer was determined by plaque assay. The onset and extent of cell fusion and lysis were evaluated qualitatively in the SK-N-SY5Y and C6 cell cultures and quantitatively in the Vero cell cultures.
For quantitative analysis, Vero cell monolayers were fixed in 100% ethanol at 20°C for 10 min, stained with KaryoMAX Giemsa stain (Gibco BRL) for 5 min, rinsed under tap water, and air dried. Stained Vero cell monolayers were examined by light microscopy, and the extent of cell-to-cell fusion and syncytium formation was determined by a modification of a previously described method (44). Briefly, four random 2.0- by 2.67-mm fields were examined by bright-field microscopy, and each field was scored as 0 (absence of cytopathic effects), 1+ (presence of discrete syncytium foci with 4 to 10 nuclei per polykaryocyte), 2+ (partially confluent syncytium foci containing 10 to 25 nuclei per polykaryocyte), or 3+ (confluent syncytia encompassing the entire field of view). The mean score was used to represent the overall fusion score. The time p.i. of the development of cell lysis (involving >50% of the monolayer) was also recorded. Statistical analysis was performed by one-way analysis of variance on data from four to six repetitions.
RESULTS
In vivo measurements of virulence. (i) Viral titers in rat brain.
One-day-old rats were inoculated with either the attenuated JL or the neurovirulent wild-type KH strain of mumps virus. Viral replication was confirmed by recovery of infectious virus from the brains of all mumps virus-inoculated rats between days 3 and 12 p.i. Maximum viral titers (PFU/milliliter) were recovered on day 3 p.i. from JL-infected rat brain (3.5 × 102 ± 62) and on day 6 p.i. from KH-infected rat brain (1.9 × 103 ± 152). Little to no virus was recovered after day 12 p.i. (a time coincident with the onset of neuroanatomical abnormalities) from any of the mumps virus-infected rats. Maximum viral titers in the KH-infected rats were significantly greater (P < 0.001) than those in the JL-infected rats. Virus was not recovered from any of the uninfected rats.
(ii) Rat weights.
Inhibition of normal weight gain in virus-infected neonatal rats compared to uninfected neonatal rats is a physiological indication of viral disease (57). Table 1 shows the mean weights of KH- and JL-infected rats and of uninfected rats at all time points tested. KH-infected rats weighed significantly less (P < 0.05) than uninfected rats from days 3 through 19 p.i. In contrast, no significant differences in weights between JL-infected and uninfected rats were measured at any time point. The mean percent inhibition of normal weight gain between days 3 and 19 p.i. in KH-infected rats was 16.6 ± 1.2, compared to 4.8 ± 0.7 in JL-infected rats (P < 0.001).
TABLE 1.
Mean weights of sham-, KH-, and JL-inoculated rats at various times p.i.
| Inoculum | Day p.i. | No. of rats | Mean wt (g) | SEM |
|---|---|---|---|---|
| Sham | 3 | 20 | 7.8 | 0.3 |
| KH | 3 | 60 | 6.8a | 0.2 |
| JL | 3 | 38 | 7.6 | 0.1 |
| Sham | 6 | 15 | 12.8 | 0.4 |
| KH | 6 | 54 | 10.7a | 0.3 |
| JL | 6 | 32 | 12.0 | 0.2 |
| Sham | 9 | 12 | 20.2 | 0.6 |
| KH | 9 | 48 | 16.1a | 0.3 |
| JL | 9 | 26 | 19.0 | 0.3 |
| Sham | 12 | 10 | 28.2 | 1.0 |
| KH | 12 | 42 | 23.8a | 0.4 |
| JL | 12 | 20 | 27.2 | 0.4 |
| Sham | 19 | 8 | 49.7 | 1.1 |
| KH | 19 | 35 | 40.9a | 0.6 |
| JL | 19 | 16 | 46.8 | 0.7 |
| Sham | 26 | 7 | 75.2 | 2.2 |
| KH | 26 | 30 | 71.5 | 1.4 |
| JL | 26 | 10 | 71.6 | 1.9 |
P < 0.05.
(iii) Neuropathology.
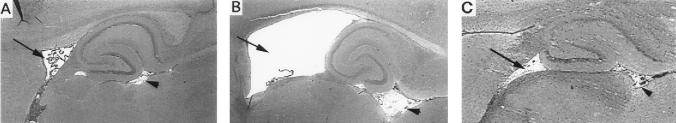
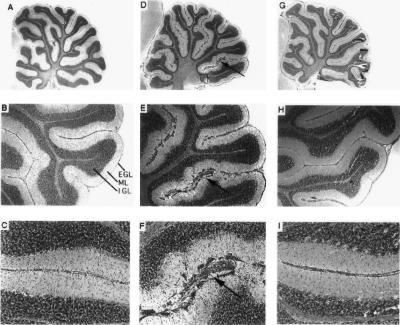
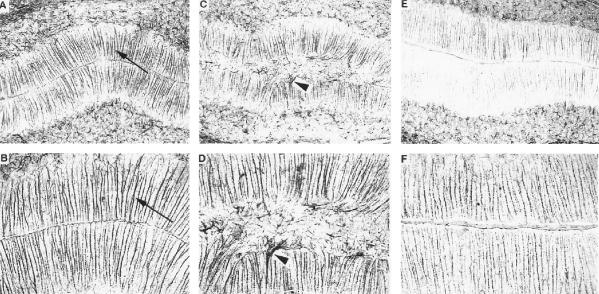
The increased virulence of KH relative to JL was also evident upon neuropathological evaluation of hematoxylin- and eosin-stained sagittal brain sections. The most striking neuroanatomical findings were (i) hydrocephalus of the lateral and third ventricles, first observed on day 12 p.i. (Fig. 1), with incidences of 72% (n = 18) in KH-infected rats and 12% (n = 17) in JL-infected rats, and (ii) abnormal cerebellar development, first observed on day 19 p.i. (Fig. 2), with incidences of 67% (n = 12) in KH-infected rats and 9% (n = 11) in JL-infected rats. The mumps virus-associated cerebellar abnormality was characterized by the anomalous retention of masses of granule cell neurons at the external germinal layer and scattered throughout the molecular layer. In these same rat brains, the cerebellar Bergmann glial fibers, normally arrayed in an orderly and parallel fashion, were disorganized and distorted (Fig. 3).
FIG. 1.
Sagittal sections through the brains of sham (A)-, KH (B)-, and JL (C)-inoculated rats on day 19 p.i. Note the hydrocephalus of the lateral (arrows) and third (arrowheads) ventricles in the KH-inoculated rat brain. Hematoxylin and eosin stained; magnification, ×40.
FIG. 2.
Sagittal sections through sham-, KH-, and JL-inoculated rat brains on day 19 p.i. Panels A (magnification, ×11), B (magnification, ×34), and C (magnification, ×85) show the cerebellum of a representative sham-inoculated rat. Note the smoothness and regularity of the external germinal layer (EGL), molecular layer (ML), and internal granule cell layer (IGL). Panels D to F (same magnifications as panels A to C, respectively) show the cerebellum of a representative KH-inoculated rat. Note the irregularity of the EGL, ML, and IGL and the anomalous masses of granule cell neurons (arrows). Panels G to I (same magnifications as panels A to C, respectively) show the cerebellum of a representative JL-inoculated rat. Note the similarity of the EGL, ML, and IGL to those of the sham-inoculated rats. All sections were stained with hematoxylin and eosin.
FIG. 3.
Bergmann glia, immunohistochemically stained with glial fibrillary acidic protein, in sagittal sections through the cerebella of sham-, KH-, and JL-inoculated rat brains on day 19 p.i. Panels A (magnification, ×100) and B (magnification, ×200) show the Bergmann glia (arrows) in the molecular layer of the cerebellum of a representative sham-inoculated rat. Note the smoothness of the molecular layer and the orderly parallel arrangement of the Bergmann glia. Panels C and D (same magnification as panels A and B, respectively) show the Bergmann glia (arrowheads) in the molecular layer of the cerebellum of a representative KH-inoculated rat. Note the irregularity of the molecular layer and the disorganization of the Bergmann glia. Panels E and F (same magnifications as panels A and B, respectively) show the Bergmann glia in the molecular layer of the cerebellum of a representative JL-inoculated rat. Note the similarity of the smoothness of the molecular layer and the orderly parallel arrangement of the Bergmann glia to those of the sham-inoculated rats.
There was little to no ventriculitis, meningitis, or encephalitis in any of the KH- or JL-infected rats at any time point. None of the sham-inoculated age-matched control rats exhibited either of these neuroanatomical findings.
In vitro measurements of virulence.
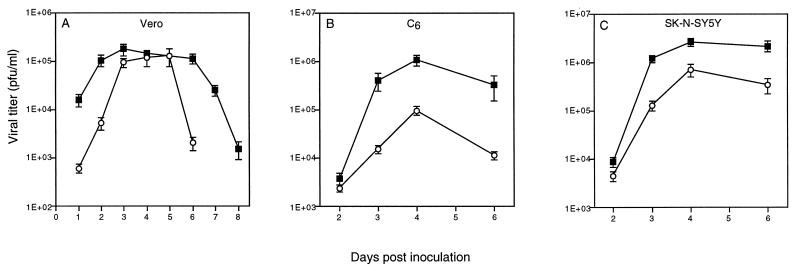
To determine the relative virulence of JL and KH in vitro, virus production and cytopathology were assessed in KH- and JL-infected neural (C6 and SK-N-SY5Y) and nonneural (Vero) cells. Peak viral titers in KH (1.8 × 105 ± 4.7 × 104)- and JL (1.3 × 105 ± 5.1 × 104)-infected Vero cells were not significantly different (P = 0.40) (Fig. 4A). In contrast, significant differences between peak viral titers were seen in KH (1.05 × 106 ± 7.9 × 104)- and JL (9.5 × 104 ± 3.6 × 104)-infected C6 cells (P < 0.001) (Fig. 4B) and in KH (2.6 × 106 ± 1.7 × 105)- and JL (6.9 × 105 ± 1.3 × 104)-infected SK-N-SY5Y cells (P < 0.001) (Fig. 4C). Results represent the averages of four determinations.
FIG. 4.
Amount of infectious virus recovered from supernatant of KH (solid squares)- and JL (open circles)-infected Vero (A), C6 astrocytoma (B), and SK-N-SY5Y neuroblastoma (C) cell lines over several days p.i.
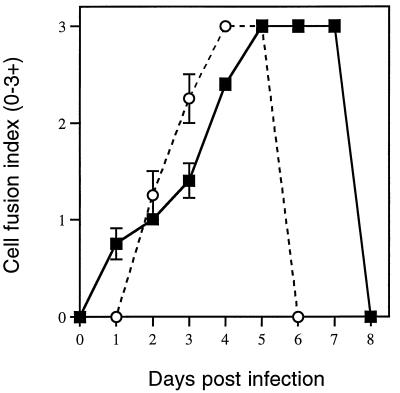
When the virus-specific cytopathic effects were evaluated following KH and JL infection of SK-N-SY5Y, C6, and Vero cells, in all three cell types, KH infection led to earlier evidence of infection (fusion) but slower progression to lysis than in JL-infected cells. Vero cells were chosen to quantitate the virus-specific cytopathic changes since, unlike the neural cells, Vero cells grow in uniform monolayers and replicate KH and JL to equivalent peak titers. A comparison of the time courses of cell fusion and cell lysis in Vero cell cultures infected with JL and KH is shown in Fig. 5. The early stages of cell fusion in KH-infected cultures were observed within 24 h p.i. and gradually increased to a point where the entire monolayer was fused (3+) by day 5 p.i. The fused monolayer remained intact until succumbing to cell lysis by day 8 p.i. In contrast, cell fusion in JL-infected cultures was not observed until day 2 p.i. and attained 3+ fusion by day 4 p.i., followed by rapid lysis the next day. Results represent the averages of data obtained from three to four infections.
FIG. 5.
Time course of cell fusion and cell lysis in Vero cell cultures infected with KH (solid squares) and JL (open circles). The day on which cell lysis was observed is represented by the last time point for each curve.
DISCUSSION
A major obstacle to the development of new live, attenuated mumps virus strains for use in vaccines is the assessment of the strains’ potential neurovirulence in humans. These concerns stem from historical and recent experience, as following use in countries outside the United States, new mumps vaccine strains were found to have unacceptable neurovirulence in humans. The risk of production of potentially neurovirulent new mumps virus vaccines may continue unless an animal model capable of reliably discriminating neurovirulent from nonneurovirulent human strains is developed. Neurovirulence testing of mumps virus vaccine strains is currently performed in monkeys (Macacus rhesus and Cercopithecus); however, in these animals clinical and pathological manifestations often do not correlate with the strain’s known neurovirulence in humans (36, 58). Similarly, the hamster, the most widely studied small-animal model of mumps virus pathogenesis, also lacks the ability to reliably discriminate neurovirulent from nonneurovirulent human strains (22, 32, 41, 71).
In the work described here, we developed a neonatal rat model of mumps virus infection that, based on clinical and pathological outcomes of infection, was capable of discriminating between two mumps virus strains of widely disparate neurovirulence, the neurovirulent KH strain and the nonneurovirulent JL vaccine strain. Our major findings in KH-infected rats were a reduction in weight gain and a high incidence of hydrocephalus and cerebellar abnormalities. In contrast, rats inoculated with the JL strain did not show statistically significant reductions in weight gain and exhibited only a small fraction of the incidence of neuroanatomical abnormalities. Validation of this rat model as a means of assessing the neurovirulence potential of new vaccine strains will require additional testing with a wide range of known human neurovirulent and nonneurovirulent mumps virus strains. Nevertheless, the potential applicability of the rat as an in vivo model for neurovirulence testing appears promising, since both monkeys (10, 58) and hamsters (22, 41, 71) have not been uniformly capable of distinguishing the attenuated JL strain from more virulent mumps virus strains.
This model also demonstrates the first evidence for mumps virus-associated developmental brain damage not linked to hydrocephalus, e.g., cerebellar neuron migration defects in mumps virus-infected rats. The delayed and inefficient cerebellar neuron migration was manifested by the prolonged presence of granule cells at the external germinal layer and deposits of ectopic granule cell neurons scattered throughout the molecular layer. Although the abnormally retained external germinal layer eventually dissipated, the retention of granule cells in the molecular layer persisted through at least 3 months p.i. (data not shown). In all cerebella displaying such abnormalities, Bergmann glial cells were morphologically abnormal, appearing tangled and distorted, unlike the uniform and parallel arrangement seen in uninfected or in unaffected KH- and JL-infected rats. Since Bergmann glial cells are responsible for guiding the migration of cerebellar granule cell neurons from the external germinal layer through the molecular layer to the internal granule cell layer (26, 27, 61), it is tempting to speculate that the morphologically abnormal Bergmann glia may be functionally abnormal as well and thus may have interrupted the normal migration of granule cells.
The granule cell migration abnormality reported here differs from the only other reported mumps virus-induced cerebellar abnormality, the Chiari type I malformation. The Chiari type I malformation is a secondary effect of the mumps virus-induced hydrocephalus and is manifested by elongation of the cerebellar vermis and flattening of the molecular layer and granule cell layers due to increased intracranial pressure (64). Unlike the Chiari type I malformation, the cerebellar neuronal migration abnormality reported here (Fig. 2) occurred in animals with and without hydrocephalus, suggesting that the development of ectopic granule cells is independent of the development of hydrocephalus.
Cerebellar developmental abnormalities have also been described for several other viral systems. Cerebellar neuronal migration abnormalities following BDV infection of neonatal rats are also likely to be due in part to disruption of normal migration of granule cell neurons. However, unlike the case for mumps virus-infected rats, granule cells from persistently BDV-infected rats that fail to migrate from the external germinal layer to the internal granule cell layer appear to die in situ and are not seen in the molecular layer (4). In neonatal infection of chicken embryos with influenza C virus, developmental defects are seen in conjunction with abnormal Purkinje cell arborization, attachment of granule cells, and migration (54). In other viral systems, direct lysis of cerebellar granule cell neurons (e.g., parvovirus [53]) or immune-mediated lysis of infected granule cells (e.g., lymphocytic choriomeningitis virus [19, 48] or reovirus type III [40, 56]) has also been associated with cerebellar developmental abnormalities.
To establish that the enhanced neurovirulence of KH was due to preferred replication in neural cells and not adaptation to the rodent, we examined in vitro virus replication and cytopathology kinetics of KH and JL in primate and rat neural and nonneural cell lines. In vitro, KH and JL replicated to similar titers in Vero cells, demonstrating that the two viral strains were equally replication competent in nonneural primate cells. In contrast, KH was able to replicate to significantly higher titers compared to JL in both rat astrocytoma (C6) and human neuroblastoma (SK-N-SY5Y) cell lines. These findings are consistent with the in vivo observation that neurovirulence may be associated with adaptation of the virus for replication in neural cells. The ability of JL to replicate well in nonneural primate cells and its apparent restriction of replication in neural cells from rodents and primates suggest that the relative nonneurovirulence of JL in the neonatal rat model is not due to a simple species restriction for this virus.
There is some indication that the increased in vivo neurovirulence of KH may be due to KH’s ability to persist and replicate in neurons with delayed lysis relative to JL. Delayed host cell lysis by KH may allow enhanced spread to and infection of neighboring neurons. Conversely, the rapidly lysed JL-infected neurons would likely result in a restricted ability to spread and replicate in the brain. This hypothesis is supported by in vitro data demonstrating KH’s tendency toward a relatively persistent infection compared to JL. Further data will be needed to confirm this hypothesis as a possible mechanism for the pathogenesis of mumps virus neurovirulence.
A related advantage of the development of the neonatal rat model of mumps virus pathogenesis is the elucidation of molecular determinants of mumps virus neurovirulence. To date, the majority of work in this area has focused on the analysis of the mumps virus hemagglutinin-neuraminidase surface protein, a glycoprotein responsible for viral attachment to cellular receptors and activation of the viral fusion protein. However, the association between alterations in the hemagglutinin-neuraminidase glycoprotein and changes in neurovirulence has been modest at best (1, 8, 9, 35, 38). Although alterations in similarly functioning proteins of many other RNA viruses (3, 20, 21, 31, 37, 55, 66, 67) have been linked to altered virulence, it is unlikely that any single gene is solely responsible for the neurovirulence potential of the virus. In fact, mutations in other coding and noncoding regions have also been linked to altered neurovirulence (6, 15, 23, 33, 39, 43, 49, 60, 62). The development of a clinically predictive model of mumps virus neurovirulence may allow for these and other changes at the molecular level to be evaluated in a biological disease model.
Additionally, by testing several other human CNS wild-type and vaccine strains, we may be able to validate the neonatal rat model as a means of preclinical neurovirulence safety testing and reduce or eliminate the use of monkeys for such purposes. With such a model and a better understanding of the molecular mechanisms of neurovirulence due to mumps virus, it may be possible to facilitate the production of safer, nonneurovirulent live, attenuated mumps virus vaccines.
ACKNOWLEDGMENTS
We thank Jerry Wolinsky for providing the Kilham virus strain and Ronald Lundquist and Kathleen Clouse for critical review of the manuscript.
REFERENCES
- 1.Afzal M A, Yates P J, Minor P D. Nucleotide sequence at position 1081 of the hemagglutinin-neuraminidase gene in the mumps Urabe vaccine strain. J Infect Dis. 1998;177:265–266. doi: 10.1086/517353. [DOI] [PubMed] [Google Scholar]
- 2.Balraj V, Miller E. Complications of mumps vaccines. Med Virol. 1995;5:219–227. [Google Scholar]
- 3.Bassel-Duby R, Spriggs D R, Tyler K L, Fields B N. Identification of attenuating mutations on the reovirus type 3 S1 double-stranded RNA segment with a rapid sequencing technique. J Virol. 1986;60:64–67. doi: 10.1128/jvi.60.1.64-67.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bautista J R, Rubin S A, Moran T H, Schwartz G J, Carbone K M. Developmental injury to the cerebellum following perinatal Borna disease virus infection. Dev Brain Res. 1995;90:45–53. doi: 10.1016/0165-3806(96)83485-7. [DOI] [PubMed] [Google Scholar]
- 5.Bautista J R, Schwartz G J, de la Torre J C, Moran T, Carbone K M. Early and persistent abnormalities in rats with neonatally acquired Borna disease virus infection. Brain Res Bull. 1994;34:31–40. doi: 10.1016/0361-9230(94)90183-x. [DOI] [PubMed] [Google Scholar]
- 6.Bradyopadhyay P K, Pritchard A, Jensen K, Lipton H L. A three-nucleotide insertion in the H stem-loop of the 5′ untranslated region of Theiler’s virus attenuates neurovirulence. J Virol. 1993;67:3691–3695. doi: 10.1128/jvi.67.6.3691-3695.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Briss P A, Fehrs L J, Parker R A, Wright P F, Sannella E C, Hutcheson R H, Schaffner W. Sustained transmission of mumps in a highly vaccinated population—assessment of primary vaccine failure and waning vaccine-induced immunity. J Infect Dis. 1994;169:77–82. doi: 10.1093/infdis/169.1.77. [DOI] [PubMed] [Google Scholar]
- 8.Brown E G, Dimock K, Wright K E. The Urabe AM9 mumps vaccine is a mixture of viruses differing at amino acid 335 of the hemagglutinin-neuraminidase gene with one form associated with disease. J Infect Dis. 1996;174:619–622. doi: 10.1093/infdis/174.3.619. [DOI] [PubMed] [Google Scholar]
- 9.Brown E G, Furesz J, Dimock K, Yarosh W, Contreras G. Nucleotide sequence analysis of Urabe mumps vaccine strain that caused meningitis in vaccine recipients. Vaccine. 1991;9:840–842. doi: 10.1016/0264-410x(91)90223-s. [DOI] [PubMed] [Google Scholar]
- 10.Buynak E B, Hilleman M R. Live attenuated mumps virus vaccine. 1. Vaccine development. Proc Soc Exp Biol Med. 1966;123:768–775. doi: 10.3181/00379727-123-31599. [DOI] [PubMed] [Google Scholar]
- 11.Carbone K M, Duchala C S, Griffin J W, Kincaid A L, Narayan O. Pathogenesis of Borna disease in rats: evidence that intra-axonal spread is the major route of dissemination and the determinant for disease incubation. J Virol. 1987;61:3431–3440. doi: 10.1128/jvi.61.11.3431-3440.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carbone K M, Park S W, Rubin S A, Waltrip R W, Vogelsang G B. Borna disease: association with a maturation defect in the cellular immune response. J Virol. 1991;65:6154–6164. doi: 10.1128/jvi.65.11.6154-6164.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control. Mumps: United States. Morbid Mortal Weekly Rep. 1987;36:151–155. [PubMed] [Google Scholar]
- 14.Cherry J D, Shields W D. Encephalitis and meningoencephalitis. In: Feigin R D, Cherry J D, editors. Textbook of pediatric infectious diseases. W. B. Philadelphia, Pa: Saunders; 1987. pp. 484–496. [Google Scholar]
- 15.Christodoulou C, Colbere-Garapin F, Macadem A, Taffs L F, Marsden S, Minor P D, Horaud F. Mapping of mutations associated with neurovirulence in monkeys infected with Sabin 1 poliovirus revertants selected at high temperature. J Virol. 1990;64:4922–4929. doi: 10.1128/jvi.64.10.4922-4929.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cizman M, Mozetic M, Radescek-Rakar R, Pleterski-Rigler D, Susec-Michieli M. Aseptic meningitis after vaccination against measles and mumps. Pediatr Infect Dis J. 1989;8:302–308. [PubMed] [Google Scholar]
- 17.Cohen H A, Ashkenazi A, Nussinovitch M, Amir J, Hart J, Frydman M. Mumps-associated acute cerebellar ataxia. Am J Dis Child. 1992;146:930–931. doi: 10.1001/archpedi.1992.02160200052025. [DOI] [PubMed] [Google Scholar]
- 18.Colville A, Pugh M. Mumps meningitis and measles, mumps and rubella vaccine. Lancet. 1992;340:786. doi: 10.1016/0140-6736(92)92322-7. [DOI] [PubMed] [Google Scholar]
- 19.Del Cerro M, Nathanson N, Monjan A A. Pathogenesis of cerebellar hypoplasia produced by lymphocytic choriomeningitis virus infection of neonatal rats. II. An ultrastructural study of the immune mediated pathology. Lab Investig. 1975;33:608–617. [PubMed] [Google Scholar]
- 20.Dietzchold B, Wunner W H, Wiktor T J, Lopes A D, Lafon M, Smith C L, Koprowski H. Characterization of an antigenic determinant of the glycoprotein that correlates with pathogenicity of rabies virus. Proc Natl Acad Sci USA. 1983;80:70–74. doi: 10.1073/pnas.80.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dropulic L K, Hardwick J M, Griffin D E. A single amino acid change in the E2 glycoprotein of Sindbis virus confers neurovirulence by altering an early step of virus replication. J Virol. 1997;71:6100–6105. doi: 10.1128/jvi.71.8.6100-6105.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ennis F A, Hopps H E, Douglas R D, Meyer H M., Jr Hydrocephalus in hamsters: induction by natural and attenuated mumps viruses. J Infect Dis. 1969;119:75–79. doi: 10.1093/infdis/119.1.75. [DOI] [PubMed] [Google Scholar]
- 23.Evans D M A, Dunn G, Minor P D, Schild G C, Cann A J, Stanway G, Almond J W, Currey K, Maizel J V., Jr Increased neurovirulence associated with a single nucleotide change in a noncoding region of the Sabin type 3 poliovaccine genome. Nature. 1985;314:548–550. doi: 10.1038/314548a0. [DOI] [PubMed] [Google Scholar]
- 24.German D, Ströhle A, Eggenberger K, Steiner C E, Matter L. An outbreak of mumps in a population partially vaccinated with the Rubini strain. Scand J Infect Dis. 1996;28:235–238. doi: 10.3109/00365549609027163. [DOI] [PubMed] [Google Scholar]
- 25.Hall R, Richards H. Hearing loss due to mumps. Arch Dis Child. 1987;62:189–191. doi: 10.1136/adc.62.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hatten M E. Riding the glial monorail: a common mechanism for glial-guided neuronal migration in different regions of the developing mammalian brain. Trends Neurosci. 1990;13:179–184. doi: 10.1016/0166-2236(90)90044-b. [DOI] [PubMed] [Google Scholar]
- 27.Hatten M E, Mason C A. Mechanisms of glial-guided neuronal migration in vitro and in vivo. Experientia. 1990;46:907–916. doi: 10.1007/BF01939383. [DOI] [PubMed] [Google Scholar]
- 28.Ichiba N, Miyake Y, Sato K, Oda M, Kimoto H. Mumps-induced opsoclonus-myoclonus and ataxia. Pediatr Neurol. 1988;4:224–227. doi: 10.1016/0887-8994(88)90035-5. [DOI] [PubMed] [Google Scholar]
- 29.Immunization Practices Advisory Committee. Mumps prevention. Morbid Mortal Weekly Rep. 1997;38:397–400. [Google Scholar]
- 30.Johnson R T. Viral infection of the nervous system. New York, N.Y: Raven Press; 1982. [Google Scholar]
- 31.Kawaoka Y, Naeve C W, Webster R G. Is virulence of H5N2 influenza viruses in chickens associated with the loss of carbohydrate from the hemagglutinin? Virology. 1984;139:303–316. doi: 10.1016/0042-6822(84)90376-3. [DOI] [PubMed] [Google Scholar]
- 32.Kilham L, Margolis G. Induction of congenital hydrocephalus in hamsters with attenuated and natural strains of mumps virus. J Infect Dis. 1975;132:462–466. doi: 10.1093/infdis/132.4.462. [DOI] [PubMed] [Google Scholar]
- 33.Kinney R M, Chang G-J, Tsuchiya K R, Sneider J M, Roehrig J T, Woodward T M, Trent D W. Attenuation of Venezuelan equine encephalitis virus strain TC-83 is encoded by the 5′-noncoding region and the E2 envelope glycoprotein. J Virol. 1993;67:1269–1277. doi: 10.1128/jvi.67.3.1269-1277.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koskiniemi M, Donner M, Pettay O. Clinical appearance and outcome in mumps encephalitis in children. Acta Pediatr Scand. 1983;72:603–609. doi: 10.1111/j.1651-2227.1983.tb09778.x. [DOI] [PubMed] [Google Scholar]
- 35.Kövamees J, Rydbeck R, Örvell C, Norrby E. Hemagglutinin-neuraminidase (HN) amino acid alterations in neutralization escape mutants of Kilham virus. Virus Res. 1990;17:119–130. doi: 10.1016/0168-1702(90)90073-k. [DOI] [PubMed] [Google Scholar]
- 36.Levenbuk I S, Nikolayeva M A, Chigirinsky A E, Ralf N M, Kozlov V G, Vardanyan N V, Sliopushkina V G, Kolomiets O L, Rukhamina M L, Grigoryeva L V. On the morphological evaluation of neurovirulence safety of attenuated mumps virus strains in monkeys. J Biol Stand. 1979;7:9–19. doi: 10.1016/s0092-1157(79)80033-5. [DOI] [PubMed] [Google Scholar]
- 37.Liebert U G, Flanagan S G, Baczko K, ter Meulen V, Rima B. Antigenic determinants of measles virus hemagglutinin associated with neurovirulence. J Virol. 1994;68:1486–1493. doi: 10.1128/jvi.68.3.1486-1493.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Love A, Rydbeck R, Kristensson K, Örvell C, Norrby E. Hemagglutinin-neuraminidase glycoprotein as a determinant of pathogenicity in mumps virus hamster encephalitis: analysis of mutants selected with monoclonal antibodies. J Virol. 1985;53:67–74. doi: 10.1128/jvi.53.1.67-74.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Macadem A, Pollard S, Ferguson G, Dunn G, Skuce R, Almond J W, Minor P D. The 5′ noncoding region of the type 2 poliovirus vaccine strain contains determinants of attenuation and temperature sensitivity. Virology. 1991;181:451–458. doi: 10.1016/0042-6822(91)90877-e. [DOI] [PubMed] [Google Scholar]
- 40.Margolis G, Kilham L, Gonatas N K. Reovirus type 3 encephalitis: observations of virus-cell interactions in neural tissues. Lab Investig. 1971;24:91–100. [PubMed] [Google Scholar]
- 41.McCarthy M, Jubelt B, Fay D B, Johnson R T. Comparative studies of five strains of mumps virus in vitro and in neonatal hamsters: evaluation of growth, cytopathogenicity, and neurovirulence. J Med Virol. 1980;5:1–15. doi: 10.1002/jmv.1890050102. [DOI] [PubMed] [Google Scholar]
- 42.McDonald R J, Moore D L, Quennec P. Clinical and epidemiologic features of mumps meningoencephalitis and possible vaccine-related disease. Pediatr Infect Dis J. 1989;8:751–755. doi: 10.1097/00006454-198911000-00004. [DOI] [PubMed] [Google Scholar]
- 43.McGoldrick A, Macadem A, Dunn G, Rowe A, Burlison J, Minor P D, Meredith J, Evans D J, Almond J W. Role of mutations G-480 and C-6203 in the attenuation phenotype of Sabin type 1 poliovirus. J Virol. 1995;69:7601–7605. doi: 10.1128/jvi.69.12.7601-7605.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Merz D C, Wolinsky J S. Conversion of nonfusing mumps virus infections to fusing infections by selective proteolysis of the HN glycoprotein. Virology. 1983;131:328–340. doi: 10.1016/0042-6822(83)90501-9. [DOI] [PubMed] [Google Scholar]
- 45.Miller E, Goldacre M, Pugh S, Colville A, Farrington P, Flower A, Nash J, MacFarlane L, Tettmar R. Risk of aseptic meningitis after measles, mumps and rubella vaccine in UK children. Lancet. 1993;341:979–982. doi: 10.1016/0140-6736(93)91069-x. [DOI] [PubMed] [Google Scholar]
- 46.Minor P D. Laboratory tests of mumps vaccines. Biologicals. 1997;25:35–40. doi: 10.1006/biol.1997.0058. [DOI] [PubMed] [Google Scholar]
- 47.Modlin J F, Orenstein W A, Brandling-Bennett A D. Current status of mumps in the United States. J Infect Dis. 1975;132:106–109. doi: 10.1093/infdis/132.1.106. [DOI] [PubMed] [Google Scholar]
- 48.Monjan A A, Cole G A, Nathanson N. Pathogenesis of cerebellar hypoplasia produced by lymphocytic choriomeningitis virus infection of neonatal rats: protective effects of immunosuppression with anti-lymphoid serum. Infect Immun. 1974;10:499–502. doi: 10.1128/iai.10.3.499-502.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ni H, Chang G-J, Xie H, Trent D W, Barrett A D T. Molecular basis of attenuation of neurovirulence of wild-type Japanese encephalitis virus strain SA14. J Gen Virol. 1995;76:409–413. doi: 10.1099/0022-1317-76-2-409. [DOI] [PubMed] [Google Scholar]
- 50.Nussinovitch M, Volovitz B, Varsano I. Complications of mumps requiring hospitalization in children. Eur J Pediatr. 1995;154:732–734. doi: 10.1007/BF02276717. [DOI] [PubMed] [Google Scholar]
- 51.Nussinovitch M, Brand N, Frydman M, Varsano I. Transverse myelitis following mumps in children. Acta Paediatr. 1992;81:183–184. doi: 10.1111/j.1651-2227.1992.tb12200.x. [DOI] [PubMed] [Google Scholar]
- 52.Ogata H, Oka K, Mitsudome A. Hydrocephalus due to acute aqueductal stenosis following mumps infection: report of a case and review of the literature. Brain Dev. 1992;14:417–419. doi: 10.1016/s0387-7604(12)80352-4. [DOI] [PubMed] [Google Scholar]
- 53.Oster-Granite M L, Herndon R M. The pathogenesis of parvovirus-induced cerebellar hypoplasia in the Syrian hamster, Mesocricetus auratus. Fluorescent antibody, foliation, cytoarchitectonic, Golgi and electron microscopic studies. J Comp Neurol. 1976;169:481–521. doi: 10.1002/cne.901690405. [DOI] [PubMed] [Google Scholar]
- 54.Parker M S, O’Callaghan R J, Smith D E, Spence H A. The effects of influenza C virus on the Purkinje cells of chick embryo cerebellum. Int J Dev Neurosci. 1994;12:461–470. doi: 10.1016/0736-5748(94)90030-2. [DOI] [PubMed] [Google Scholar]
- 55.Prehaud C, Coulon P, Lafay F, Thiers C, Flamand A. Antigenic site 2 of the rabies virus glycoprotein: structure and role in viral virulence. J Virol. 1988;62:1–7. doi: 10.1128/jvi.62.1.1-7.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Raine C S, Fields B N. Reovirus type 3 encephalitis—a virologic and ultrastructural study. J Neuropathol Exp Neurol. 1973;32:19–33. doi: 10.1097/00005072-197301000-00002. [DOI] [PubMed] [Google Scholar]
- 57.Richt J A, Vande-Woude S, Clements J E, Herzog S, Frese K, Stitz L, Narayan O. Molecular and immunopathological studies of Borna disease virus infection in rats. Behring Inst Mitt. 1991;89:163–176. [PubMed] [Google Scholar]
- 58.Rozina E E, Kaptsova T I, Sharova O K, Nikolayeva M A, Nesterova T P. Study of mumps virus invasiveness in monkeys. Acta Virol. 1984;28:107–113. [PubMed] [Google Scholar]
- 59.Russell R R, Donald J C. The neurological complications of mumps. Br Med J. 1958;2:27–35. doi: 10.1136/bmj.2.5087.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ryman K D, Xie H, Ledger T N, Campbell G A, Barrett A D T. Antigenic variants of yellow fever virus with an altered neurovirulence phenotype in mice. Virology. 1997;230:376–380. doi: 10.1006/viro.1997.8496. [DOI] [PubMed] [Google Scholar]
- 61.Shetty A K, Phillips D E. Effects of prenatal ethanol exposure on the development of Bergmann glia and astrocytes in the rat cerebellum: an immunohistochemical study. J Comp Neurol. 1992;321:19–32. doi: 10.1002/cne.903210103. [DOI] [PubMed] [Google Scholar]
- 62.Stein S B, Zhang L, Roos R P. Influence of Theiler’s murine encephalomyelitis virus 5′ untranslated region on translation and neurovirulence. J Virol. 1992;66:4508–4517. doi: 10.1128/jvi.66.7.4508-4517.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tabin R, Berclaz J, Dupuis G, Peter O. Résponse immune à divers vaccins anti-ourliens. Rev Med Suisse Rom. 1993;113:981–984. [PubMed] [Google Scholar]
- 64.Takano T, Uno M, Yamano T, Shimada M. Pathogenesis of cerebellar deformity in experimental Chiari type I malformation caused by mumps virus. Acta Neuropathol. 1994;87:168–173. doi: 10.1007/BF00296187. [DOI] [PubMed] [Google Scholar]
- 65.Timmons G D, Johnson K P. Aqueductal stenosis and hydrocephalus after mumps encephalitis. N Engl J Med. 1970;283:1505–1507. doi: 10.1056/NEJM197012312832707. [DOI] [PubMed] [Google Scholar]
- 66.Tucker P C, Lee S W, Bui N, Martinie D, Griffin D E. Amino acid changes in the Sindbis virus E2 glycoprotein that increase neurovirulence improve entry into neuroblastoma cells. J Virol. 1997;71:6106–6112. doi: 10.1128/jvi.71.8.6106-6112.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tucker P C, Strauss E G, Kuhn R J, Strauss J H, Griffin D E. Viral determinants of age-dependent virulence of Sindbis virus for mice. J Virol. 1993;67:4605–4610. doi: 10.1128/jvi.67.8.4605-4610.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Uno M, Takano T, Yamano T, Shimada M. Age dependent susceptibility in mumps-associated hydrocephalus: neuropathological features and brain barriers. Acta Neuropathol. 1997;94:207–215. doi: 10.1007/s004010050695. [DOI] [PubMed] [Google Scholar]
- 69.Venketasubramanian N. Transverse myelitis following mumps in an adult—a case report with MRI correlation. Acta Neurol Scand. 1997;96:328–330. doi: 10.1111/j.1600-0404.1997.tb00292.x. [DOI] [PubMed] [Google Scholar]
- 70.Wolinsky J S. Mumps virus. In: Fields B N, Knipe D M, Howley P M, Chanock R M, Melnick J L, Monath T P, Roizman B, Straus S E, editors. Fields virology. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 1243–1265. [Google Scholar]
- 71.Wolinsky J S, Stroop W G. Virulence and persistence of three prototype strains of mumps virus in newborn hamsters. Arch Virol. 1978;57:355–359. doi: 10.1007/BF01320075. [DOI] [PubMed] [Google Scholar]