Abstract
We have previously shown that the presence of the CD4 cytoplasmic tail is critical for human immunodeficiency virus (HIV)-induced apoptosis (J. Corbeil, M. Tremblay, and D. D. Richman, J. Exp. Med. 183:39–48, 1996). We have pursued our investigation of the role of the CD4 transduction pathway in HIV-induced apoptosis. To do this, wild-type and mutant forms of the CD4 cytoplasmic tail were stably expressed in the lymphoblastoid T-cell line A2.01. Apoptosis was prevented when CD4 truncated at residue 402 was expressed; however, cells expressing mutated receptors that do not associate with p56lck (mutated at the dicysteine motif and truncated at residue 418) but which conserved proximal domains of the cytoplasmic tail underwent apoptosis like wild-type CD4. The differences between wild-type and mutated receptors in the induction of apoptosis were not related to levels of p56lck or NF-κB activation. Initial signaling through the CD4 receptor played a major role in the sensitization of HIV-infected T cells to undergo apoptosis. Incubation of HIV-infected cells with monoclonal antibody (MAb) 13B8-2, which binds to CD4 in a region critical for dimerization of the receptor, prevented apoptosis without inhibiting HIV replication. Moreover, the apoptotic process was not related to Fas-Fas ligand interaction; however, an antagonistic anti-Fas MAb (ZB-4) enhanced apoptosis in HIV-infected cells without inducing apoptosis in uninfected cells. These observations demonstrate that CD4 signaling mediates HIV-induced apoptosis by a mechanism independent of Fas-Fas ligand interaction, does not require p56lck signaling, and may involve a critical region for CD4 dimerization.
Human immunodeficiency virus (HIV) infection in vivo is characterized by high levels of continuous viral replication (19, 39, 40, 72). Similarly, CD4 cells in HIV-infected patients are undergoing a dynamic process with increased levels of destruction and replacement to maintain steady state. Virus replication may be directly involved in the destruction of HIV-infected CD4+ T cells. In addition, there is a general state of immune system activation in HIV-infected patients that contributes to enhanced apoptosis of both infected and bystander cells in vivo (27, 55).
Apoptosis is a cellular suicide process regulated by both internal and external factors (56, 68). The various stimuli triggering apoptosis are assumed to converge to a common executioner pathway that involves the release of cytochrome c from the mitochondria into the cytoplasm and activation of caspase family proteases (2, 38, 64). The cellular changes associated with apoptosis include exposure of phosphatidylserine on the plasma membrane externally and nuclear damage typified by chromatin condensation and oligonucleosomal DNA fragmentation.
Apoptosis has been shown to mediate HIV cytopathology in vitro (35, 49, 67) and may contribute to CD4+-T-cell depletion in vivo. The HIV env gene products gp120 and gp41 have been reported to induce apoptosis by engagement of the CD4 receptor (50), while cross-linking of bound gp120 and T-cell receptor has been shown to prime for activation-induced apoptosis (5). Other viral genes like tat may accelerate Fas-mediated apoptosis in association with gp120 (74). vpr has also been demonstrated to be capable of inducing or suppressing apoptosis (3, 59, 62). Signaling through Fas may contribute significantly to T-cell depletion in HIV-1-infected patients (6, 24, 25, 45); however, the role of Fas in inducing apoptosis in HIV-infected cells remains to be fully characterized. The potency of the Fas-Fas ligand interaction to induce apoptosis appears to differ between T-cell lines and primary T cells (32, 57).
We previously demonstrated that productive HIV-1 infection triggered apoptosis in lymphoblastoid T-cell lines and that the cytoplasmic tail of CD4 was required for apoptosis (20, 21). We showed that HIV-induced apoptosis was prevented in cells expressing a truncated CD4 mutant that lacks the whole CD4 cytoplasmic tail (truncation at residue 402). In this study, we examined the role of the CD4 signaling and Fas signaling pathways in HIV-induced apoptosis in A2.01 lymphoblastoid T cells expressing wild-type or mutated CD4 receptors. We also addressed the role of NF-κB in the control of HIV-induced apoptosis in cells expressing wild-type or mutated CD4 receptors, since NF-κB activation modulates apoptosis (4, 7, 34, 52). The cytoplasmic tail of the CD4 receptor is functionally important for CD4-mediated signal transduction during T-cell activation (31, 71). This function is dependent on the association of protein tyrosine kinase p56lck with CD4 (69, 70). Therefore, we determined whether the association of CD4 with p56lck was required for HIV-induced apoptosis. HIV-infected cells expressing CD4 constructs that did not associate with p56lck but conserved all or part of the cytoplasmic tail (substitution in the dicysteine motif and truncation at residue 418, respectively) underwent apoptosis. Moreover, p56lck signaling was not rescued in cells expressing CD4 mutants that do not associate with p56lck, demonstrating that p56lck signaling is dispensable for HIV-induced apoptosis. Initial signaling through the CD4 receptor was found to be critical for HIV-induced apoptosis. Prolonged presence of the CD4 receptor on the surface of HIV-infected cells did not appear to enhance the level of apoptosis. The anti-CD4 antibody 13B8-2 that interacts with a critical region for CD4 dimerization was able to prevent the apoptosis of productively HIV-infected cells while not inhibiting virus replication, corroborating the essential role of CD4 signaling in HIV-induced apoptosis. HIV-induced apoptosis was not mediated by Fas-Fas ligand interaction, since the Fas-Fc decoy was unable to prevent apoptosis in HIV-infected cells.
MATERIALS AND METHODS
Antibodies.
Murine monoclonal antibody (MAb) OKT-4 (anti-human CD4) was used as ascites (hybridoma from the American Type Culture Collection). The fluorescein isothiocyanate (FITC)-labeled OKT4 MAb and the 13B8-2 MAb were purchased from Johnson & Johnson (Raritan, N.J.) and Immunotech Inc. (Westbrook, Maine), respectively. FITC-conjugated goat anti-mouse immunoglobulin G (IgG) was obtained from Biosource International (Camarillo, Calif.). The rabbit antiserum against lck and the peroxidase-coupled polyclonal goat anti-mouse Ig were purchased from Pharmingen (San Diego, Calif.). 4G10 MAb (antiphosphotyrosine) was obtained from Upstate Biotechnology (Lake Placid, N.Y.). Peroxidase-labeled goat anti-rabbit IgG was obtained from The Binding Site Inc. (San Diego, Calif.). The goat anti-mouse IgG(Fab′)2 was obtained from Biosource International. The agonistic CD95 IgM MAb (clone CH-11) was obtained from Upstate Biotechnology. The soluble Fas receptor decoy (human Fas-Fc Ig fusion protein) was a generous gift from C. Ware (La Jolla Institute for Allergy and Immunology, La Jolla, Calif.). The anti-Fas IgG1 antibody clone ZB4 was from Immunotech Inc. The biotin-conjugated mouse anti-human Fas ligand MAb (clone NOK-1) was obtained from Pharmingen.
Generation of stably transfected CD4 cell lines.
Plasmids encoding wild-type or mutant CD4 receptors that interfere with Nef-associated motifs (LL-to-AA substitution in the dileucine motif at residues 413 and 414 and M-to-T substitution at residue 407) and that partially lack the CD4 cytoplasmic tail (truncation at residue 418) were obtained from D. Trono (University of Geneva, Geneva, Switzerland). Other CD4 mutants that disrupt the association with p56lck (CC-to-AA substitution in the dicysteine motif at residues 420 and 422) or lack the CD4 cytoplasmic tail (truncation at residue 402) were described previously (21). The CD4 sequences were subsequently cloned as XhoI-BamHI fragments in the retroviral expression vector pLPONL (76) and were transfected in the packaging cell line 293GP (Viagene, La Jolla, Calif.) by calcium phosphate coprecipitation. 293GP cells were selected for neomycin resistance with G418 (500 μg/ml), and the resistant population was used to generate pseudotyped virus. 293GP clones were transfected with 20 μg of an expression plasmid for vesicular stomatitis virus protein (pHCMV-G) by calcium phosphate coprecipitation. The pseudotyped virions were collected 72 h after transfection. Culture supernatants were filtered and used to infect the lymphoblastoid T-cell line A2.01, obtained from Rafick-Pierre Sekaly (Institut de Recherches Cliniques de Montréal, Montréal, Canada), in the presence of Polybrene (8 μg/ml). Resistant A2.01 cell populations were selected for each of the constructs, and cells expressing CD4 on their surface were sorted under sterile conditions by fluorescence-activated cell sorting (with the MAb OKT-4). For each cell line, we used flow cytometry to select bulk populations expressing comparable levels of CD4 at the cell surface, by using Quantum Simply Cellular microbeads (Flow Cytometry Standards Corp., San Juan, P.R.) and the MAb OKT-4 conjugated to FITC. Wild-type and mutated CD4 cell lines were grown in RPMI 1640 medium (Gibco-BRL, Gaithersburg, Md.) supplemented with 10% (vol/vol) fetal bovine serum, 2 mM glutamine, penicillin, and streptomycin. The stable transfected cells were maintained under G418 (500 μg/ml) selection.
Virus preparation, in vitro HIV-1 infection, and p24 ELISA.
High-titer stocks of HIV-1LAI (2.5 × 106 50% tissue culture infective doses [TCID]50/ml) were prepared and subjected to titer determination by end-point dilution (44). High titer stocks were prepared by inoculating CEM cells at a multiplicity of infection (moi) of 0.001 TCID50/ml and growing the cells for 10 days. A 10-ml volume of culture was added to 400 ml of uninfected CEM cells (5 × 105 cells/ml) and grown for 5 to 7 days until abundant syncytia were present. The cells were pelleted (300 × g for 10 min) and resuspended in 1/100 of the initial volume for 8 h. The supernatant was clarified by centrifugation (800 × g for 10 min).
The infection protocol was identical for each of the cell lines. Cells were passaged the day before infection in the absence of G418 selection. Briefly, 5 × 106 to 10 × 106 cells were infected with HIV-1LAI at a moi of 0.1. After a 2-h inoculation period at 37°C in 5% CO2 in air, the cells were washed three times in phosphate-buffered saline (PBS) to remove inoculum virus and resuspended at a density of 5 × 105 cells/ml in RPMI 1640 medium.
CD4+ T cells were isolated from peripheral blood mononuclear cells as described by Spina et al. (61). Purified CD4+ T cells were cultured in fresh medium (RPMI 1640 medium supplemented with 10% [vol/vol] fetal bovine serum, 2 mM glutamine, penicillin, and streptomycin) containing recombinant interleukin-2 (rIL-2) (final concentration, 40 U/ml; DuPont-New England Nuclear Research Products, Boston, Mass.) and stimulated with phytohemagglutinin (3 μg/ml; Sigma). After 72 h of stimulation, CD4+ T cells were infected with HIV-1LAI at a MOI of 0.1. After a 2-h inoculation period at 37°C in 5% CO2 in air, the cells were washed three times in PBS to remove inoculum virus and resuspended at a density of 106 cells/ml in fresh medium containing rIL-2 (final concentration, 40 U/ml).
HIV-1 p24 antigen was measured by enzyme-linked immunosorbent assay (ELISA) as described by the manufacturer (Coulter Corp., Miami, Fla.).
Assays for apoptosis. (i) DNA fragmentation and cell cycle analysis.
Cells (2 × 106) were washed in PBS, resuspended in 30% ethanol in PBS, and kept at 4°C until analyzed. The cells were stained with propidium iodide for DNA content analysis as previously described (23, 66).
(ii) Fluorochrome-labeled annexin-V binding.
Phosphatidylserine on the outer lamella of apoptotic cell membranes was detected with FITC-conjugated annexin-V (R&D System, Minneapolis, Minn.). Differentiation from necrotic cells was performed with 7-AAD as specified by the manufacturer.
(iii) Light microscopy.
Cells displaying characteristic condensation and fragmentation of nuclear chromatin and permeable to trypan blue were counted as apoptotic, as previously described (42).
Quantification of surface CD4 receptor expression by flow cytometry.
Uninfected and HIV-infected cells (106) were washed in PBS and resuspended in 200 μl of PBS with 2% fetal bovine serum. The fluorescein-conjugated anti-CD4 MAb OKT-4 was added (20 μl) to the cells, which were then kept at 4°C for 30 min for staining. The cells were then washed in PBS, resuspended in 500 μl of 1% paraformaldehyde in PBS, and kept in the dark at 4°C until analyzed by fluorescence-activated cell sorting. CD4 cell surface expression was quantified with Quantum Simply Cellular microbeads and analyzed by flow cytometry (Elite; Coulter).
Immunoprecipitation of the CD4 receptor.
Cells expressing wild-type or mutated CD4 (107 cells) were washed with ice-cold PBS and lysed on ice with 500 μl of lysis buffer (1% Nonidet P-40 [NP-40], 20 mM HEPES [pH 7.9], 150 mM NaCl, 20 mM NaF, 1 mM sodium pyrophosphate, 1 mM EDTA) in the presence of protease inhibitors (10 μg of leupeptin per ml, 10 μg of aprotinin per ml, and 1 mM phenylmethylsulfonyl fluoride) and the tyrosine phosphatase inhibitor sodium orthovanadate at 1 mM. The lysates were cleared by centrifugation, and their protein concentration was determined by a Bradford assay. For immunoprecipitations, 1-mg protein aliquots of lysates were precleared with protein G-Sepharose (Pharmacia, Uppsala, Sweden) and incubated for 2 h at 4°C with mouse IgG1 OKT-4 MAb coupled to protein A-Sepharose beads. The beads were washed three times with the following buffers. Buffer 1 (50 mM Tris HCl [pH 7.5], 150 mM NaCl, 0.1% NP-40), buffer 2 (50 mM Tris HCl [pH 7.5], 500 mM NaCl, 0.1% NP-40), and buffer 3 (10 mM Tris HCl [pH 7.5], 500 mM NaCl). They were denatured by the addition of 1× nonreducing sodium dodecyl sulfate (SDS) sample buffer. Denatured samples were resolved by SDS-polyacrylamide gel electrophoresis (PAGE) and transferred to a nitrocellulose membrane. The membranes were blocked in SuperBlock blocking buffer in Tris-buffered saline (TBS; Pierce, Rockford, Ill.) for 45 min at room temperature, and CD4-associated p56lck was detected by immunoblotting with rabbit antiserum against lck as the primary antibody followed by peroxidase-labeled anti-rabbit IgG. The blot was developed by using the enhanced chemiluminescence (ECL) detection system (Amersham Inc., Cleveland, Ohio) as specified by the manufacturer.
Cross-linking, antiphosphotyrosine immunoblots, and kinase assays of p56lck.
Antibody-mediated cross-linking was performed as previously described (75). A total of 5 × 106 cells were centrifuged, washed once in ice-cold PBS, and incubated with 250 μl of the appropriate MAb at saturating concentration for 30 min. The cells were washed twice in ice-cold PBS, resuspended in 100 μl of medium without fetal bovine serum, and transferred to an Eppendorf tube. After 3 min at 37°C, 2 μg of goat anti-mouse IgG(Fab′)2 was added to the cells and the mixture was incubated for 2 min. The cells were washed in ice-cold PBS and lysed on ice with 200 μl of lysis buffer as described above. The lysates were cleared by centrifugation, and their protein concentration was determined by the Bradford assay. For antiphosphotyrosine, the lysates were denatured by the addition of 2× nonreducing SDS sample buffer. Denatured samples were resolved by SDS-PAGE and transferred to a nitrocellulose membrane. Proteins phosphorylated on tyrosine residues were detected by immunoblotting with MAb 4G10 followed by peroxidase-coupled polyclonal goat anti-mouse Ig. The blot was developed by the enhanced chemiluminescence method. For the kinase assay, the lysates were incubated for 2 h at 4°C with rabbit anti-lck coupled to protein A-Sepharose beads (Pharmacia). The beads were washed once with PBS–0.5% NP-40, once with 0.5 M LiCl–50 mM Tris HCl (pH 7.5), and once with distilled H2O. The beads were then incubated at room temperature for 10 min in 30 μl of kinase buffer (50 mM Tris HCl [pH 7.4], 10 mm MnCl2) containing 10 μCi of [γ-32P]ATP before being washed in distilled H2O and analyzed by SDS-PAGE and autoradiography.
Nuclear extract preparation and EMSAs.
Nuclear extracts were prepared as previously described (54). The pellet (5 × 106 to 1 × 107 cells) was washed once in ice-cold PBS, resuspended with 5 volumes of buffer A (10 mM HEPES [pH 7.9], 10 mM Tris [pH 7.8], 60 mM KCl, 1 mM EDTA, 1 mM dithiothreitol [DTT], 1 mM phenylmethylsulfonyl fluoride), and incubated on ice. Cells were lysed by the addition of 2.5 μl of 5% NP-40, and lysis was microscopically monitored by trypan blue staining. Nuclei were pelleted at 1,200 × g for 1 min, washed once in 5 volumes of buffer A, and resuspended in 200 μl of buffer B (250 mM Tris [pH 7.8], 60 mM KCl, 1 mM DTT, 1 mM phenylmethylsulfonyl fluoride. After the nuclei had been transferred to Eppendorf tubes, three freeze-thaw cycles were performed with dry ice and a 37°C water bath. Nuclei were removed by centrifugation at 14,000 × g for 15 min. The protein concentration of the supernatant was determined by the micro-Bradford assay, and the supernatants were stored at −80°C until used. The binding reactions were performed in a volume of 20 μl containing 2 μl of binding buffer (100 mM Tris [pH 7.5], 500 mM NaCl, 10 mM DTT, 10 mm EDTA, 50% glycerol), 1 μl of 10 mM DTT, 2 μl of poly(dI-dC) (0.25 mg/ml), 10 μg of protein extract (for NF-κB and Oct-1 electrophoretic mobility shift assay [EMSA]), and 2 μl of radioactively labeled DNA probe (10,000 cpm/μl). After incubation at room temperature for 15 min, the reaction products were separated on a 6% polyacrylamide gel. In competition experiments, unlabeled probes were added in excess to the reaction mixture before the addition of 32P-labelled probes.
RNA isolation and RT-PCR amplification.
Total RNA was extracted from 2 × 106 cells with the RNeasy total RNA kit (Qiagen Inc., Chatsworth, Calif.). RNA samples (5 μg) were then mixed with 300 ng of oligonucleotide (dT015 primer [Promega]), heated at 65°C for 5 min, and cooled on ice. First-strand cDNA synthesis was carried out at 37°C for 1 h in a total volume of 50 μl containing 50 mM Tris HCl (pH 8.3), 75 mM KCl, 3 mM MgCl2, 10 mM DTT, 4 mM deoxynucleoside triphosphates, 10 U of RNase inhibitor (Promega), and 50 U of Moloney murine leukemia virus reverse transcriptase (RT) (Gibco-BRL). Each cDNA mixture (5 μl) was subsequently amplified in a total volume of 100 μl containing 20 mM Tris-HCl (pH 8.7) at 20°C, 16 mM (NH4)2SO4, 2.5 mM MgCl2, 200 mM deoxynucleoside triphosphates, 700 ng of each primer, and 2.5 U of Taq DNA polymerase (Perkin-Elmer). Primer sequences for the Fas ligand 5′-agctcttccacctgcagaag-3′ (sense) and 5′-cctcaaaattgaccagagagag-3′ (antisense) were designed to amplify a 504-bp product. Primer sequences for β-actin 5′-gacgaggcccagagcaagagagg-3′ (sense) and 5′-gatccacatctgctggaaggtggac-3′ (antisense) were designed to amplify a 350-bp product of the β-actin transcript and were used as a control for RNA extraction. Amplification was carried out in a thermal cycler (Perkin-Elmer) for 2 min at 94°C and for 35 cycles (Fas ligand) or 30 cycles (β-actin) as follows: for Fas ligand, 1 min at 94°C, 1 min at 56°C, and 1.5 min at 72°C with a final elongation for 8.5 min at 72°C; for β-actin, 15 s at 94°C and 1 min at 68°C with a final elongation for 10 min at 72°C.
RESULTS
The CD4 cytoplasmic tail, independent of its association with p56lck, is a determinant for HIV-induced apoptosis during acute HIV infection.
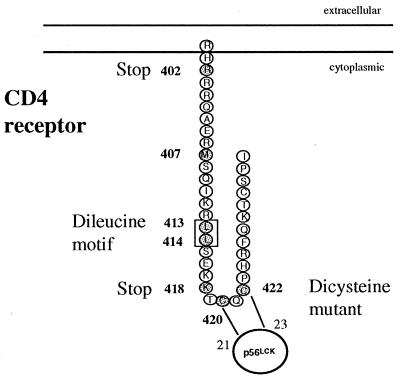
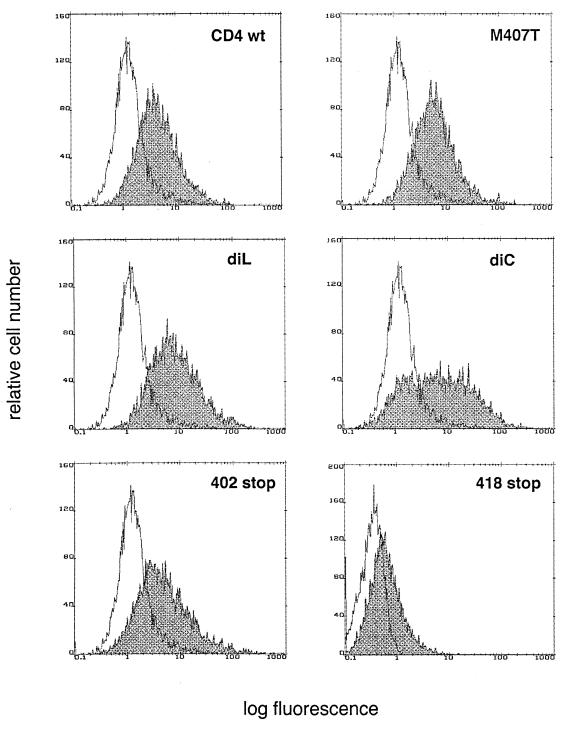
To investigate the role of the CD4 transduction pathway in HIV-induced apoptosis, CD4 wild-type and mutated receptors (Fig. 1) were stably expressed in the lymphoblastoid T-cell line A2.01, which does not express CD4. The mutations included the disruption of the association of CD4 and p56lck (AA substitutions in the dicysteine motif at residues 420 and 422) or interference with Nef-associated motifs (AA substitutions in the dileucine motif at residues 413 and 414 or substitution at residue 407) (1, 41, 60) and partial or total truncations of the CD4 cytoplasmic tail (at residues 418 and 402, respectively). These variants were expressed at the surface of A2.01 cells at comparable levels with a mean density of 40,000 to 50,000 CD4 receptors per cell, which approximates the number of receptors found on primary CD4+ T cells (Fig. 2).
FIG. 1.
Schematic representation of CD4 and the mutants investigated in this study. The CC-to-AA substitution in the dicysteine motif 420 and 422 disrupts CD4-p56lck association, the LL-to-AA substitution in the dileucine motif 413 and 414 and the M-to-T mutation at residue 407 interfere with Nef-associated motifs, and the truncations at amino acid residues 418 and 402 respectively (418 Stop and 402 Stop) partially or totally delete the CD4 cytoplasmic tail. These constructs were stably expressed in the lymphoblastoid T-cell line A2.01.
FIG. 2.
Cell surface expression of CD4 wild-type (wt) or mutants stably expressed in the lymphoblastoid T-cell line A2.01. Surface levels of CD4 were evaluated by flow cytometry after staining with FITC-labelled OKT-4 anti-CD4 antibody. The abscissa gives the relative fluorescence intensity on a logarithmic scale. The mutant truncated at residue 418 was generated later, and the flow cytometry profile corresponds to an independent analysis. diL, dileucine; diC, dicysteine.
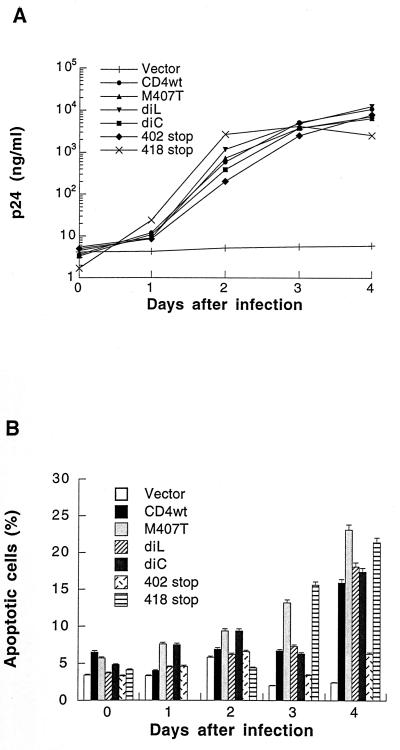
Apoptosis was evaluated in these cells after infection with HIV-1LAI at a MOI of 0.1 TCID50/ml. The level of viral production, determined by ELISA for HIV p24 antigen, was comparable for each of the constructs (Fig. 3A). Apoptosis was assessed by several methods: flow cytometry (both cell cycle analysis and fluorochrome labeled annexin-V binding), fluorescent DNA dye (Hoechst dye uptake), and light microscopy (nuclear condensation and fragmentation). These different techniques provided very similar percentages of apoptosis. Apoptosis was measured after HIV infection of the cell lines expressing different CD4 constructs, using flow cytometry with FITC-conjugated annexin-V (Fig. 3B).
FIG. 3.
Apoptosis in CD4 cells expressing wild-type or mutant CD4 infected with HIVLAI. (A) Virus production assessed by p24 antigen ELISA in CD4 wild-type (wt) and mutants infected with HIVLAI (MOI, 0.1). (B) Apoptosis assessed by flow cytometry (fluorochrome-labelled annexin-V binding). The results presented are representative of five independent experiments. The mutant truncated at residue 418 was generated later, and the results presented are representative of three independent experiments. diL, dileucine; diC, dicysteine.
Cells expressing the mutation at the dileucine motif and the mutation at residue 407 underwent apoptosis like those expressing the wild-type CD4. Higher levels of apoptosis were consistently observed in HIV-infected cells expressing the mutation at residue 407. Cell death was also observed after infection of cells expressing the mutation at the dicysteine motif, which does not associate with p56lck. This demonstrated the ability of HIV to induce apoptosis when CD4-p56lck association was disrupted. This was further confirmed with the CD4 construct truncated in the CD4 cytoplasmic tail at residue 418. This mutant could be expressed only with a lower mean intensity of fluorescence compared to the CD4 mutant truncated at residue 402 (Fig. 2); however, cells expressing this truncated mutant receptor displayed comparable levels of apoptosis to those expressing the wild-type CD4 (21.4% on day 4 after infection) (Fig. 3B). Despite similar levels of viral production, apoptosis was abrogated in infected cells expressing the mutant truncated at residue 402. These results demonstrate that CD4-p56lck association is dispensable for HIV-induced apoptosis and that the first 18 amino acids of the CD4 cytoplasmic tail are sufficient to mediate the process. Cell cycle analysis and DNA profiles confirmed that cells expressing wild-type CD4 and constructs with mutations at residue 407, the dileucine motif, and the dicysteine motif, all underwent apoptosis while those expressing a truncation at residue 402 displayed little apoptosis during HIV infection. DNA profiles consistently showed a diminution of the proportion of cells in the G1 phase of the cell cycle, a concomittant increase in the fraction of cells in G2/M, and the appearance of cells with a sub-G1 DNA content, representing apoptotic cells. The DNA profile of the cells expressing the mutant truncated at residue 402 showed a constant and specific accumulation of aneuploid cells upon HIV infection but no apoptotic cells (sub-G1 DNA) during the 4 days of observation (data not shown).
HIV-induced apoptosis is independent of NF-κB transactivation.
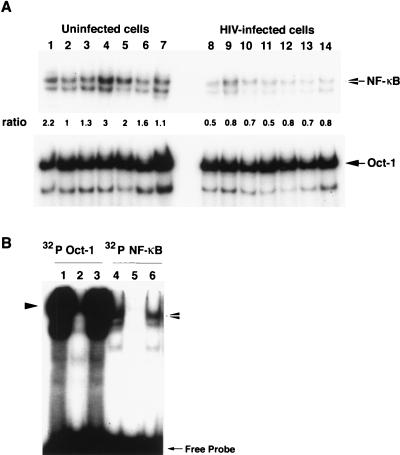
NF-κB may either protect from or promote apoptosis (4, 7, 34, 52). We examined whether cells expressing distinct forms of the CD4 receptor, with their associated differential susceptibility to undergo apoptosis during HIV infection, differed in NF-κB activation. NF-κB activity in uninfected and HIV-infected A2.01 cells expressing wild-type and truncated CD4 were analyzed by EMSA. To determine whether the apoptotic phenotype in HIV-infected cells was related to different degrees of NF-κB activation, NF-κB DNA binding activity was analyzed before infection and 3 days after infection (when cells were undergoing apoptosis). Equal amounts of protein from nuclear extracts of cells expressing wild-type or mutated CD4 were analyzed with a 32P-labeled DNA probe encompassing a consensus binding site for NF-κB (Fig. 4A, upper panel). Basal levels of NF-κB activity were detectable in wild-type and mutated CD4 cells. Three days after infection, NF-κB binding activity was diminished in infected cells compared to uninfected cells, regardless of whether they expressed wild-type or mutated CD4. NF-κB activity was quantified by phosphorimaging, and ratios were obtained by normalizing to the level of endogenous Oct-1 activity. The ratios confirmed the general decrease in NF-κB activity in cells expressing wild-type or mutated CD4 independently of their apoptotic phenotype. The DNA-binding activity of the ubiquitous factor Oct-1, used as input control, was similar in cells expressing wild-type and mutant CD4 (Fig. 4A, lower panel). The specificity of the transcription factor binding was confirmed by a competition assay performed with a molar excess of unlabeled NF-κB oligonucleotide probe (Fig. 4B). The propensity of cells expressing wild-type and mutated CD4 to undergo apoptosis did not correlate with any distinctive modification in NF-κB activation. The apoptotic outcome was neither predetermined by initial level of NF-κB activity nor related to a specific level of NF-κB activity during HIV-induced apoptosis. These results demonstrate that NF-κB activity is not involved in the induction of cell death during HIV infection of the lymphoblastoid T-cell line A2.01.
FIG. 4.
DNA-binding activity of NF-κB during HIV-induced apoptosis in cells expressing wild-type or mutant CD4. (A) Effect of HIV-infection on NF-κB activation in wild-type and mutant CD4. Equal amounts of nuclear extracts (10 μg) were subjected to EMSA and analyzed with 32P-labeled DNA probes encompassing a consensus binding site for NF-κB and the ubiquitous factor Oct-1. Nuclear extracts of uninfected cells (lanes 1 to 7) or cells infected with HIVLAI for 3 days (lanes 8 to 14) expressing CD4 wild type (lanes 1 and 8), mutants 407 M to T (lane 2 and 9), dileucine (lane 3 and 10), dicysteine (lane 4 and 11), 402 truncated (lanes 5 and 12), and 418 truncated (lanes 6 and 13) and the vector control (lane 7 and 14) were analyzed. The levels of NF-κB transactivation in wild-type and truncated CD4 mutants were quantified by phosphorimaging, and ratios were obtained by normalizing to the level of Oct-1 activity. (B) Competition experiments to establish the specificity of Oct-1 (lanes 1 to 3) and NF-κB (lanes 4 to 6) oligonucleotides. Nuclear extracts (5 μg) from cells expressing CD4 wild-type were subjected to EMSA. Lanes: 1, positive control with 32P-labelled Oct-1 oligonucleotide; 2, 32P-labelled Oct-1 oligonucleotide incubated with an excess of unlabeled Oct-1 oligonucleotide (competitor); 3, 32P-labelled Oct-1 oligonucleotide incubated with an excess of unlabeled NF-κB oligonucleotide (non competitor); 4, positive control with 32P labelled NF-κB oligonucleotide; 5, 32P-labelled NF-κB oligonucleotide incubated with an excess of unlabeled NF-κB oligonucleotide (competitor); 6, 32P-labelled NF-κB oligonucleotide incubated with an excess of unlabeled Oct-1 oligonucleotide (noncompetitor).
The CD4 cytoplasmic tail is critical for HIV-induced apoptosis independent of p56lck signaling.
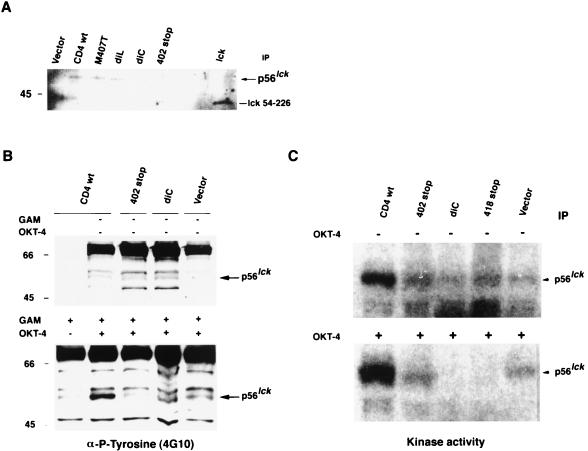
The reduced levels of HIV-induced apoptosis in cells expressing a truncated CD4 molecule indicated that HIV signaling through the CD4 receptor may activate a signal transduction pathway leading to apoptosis. To further characterize the signal mediated through the CD4 cytoplasmic tail and determine whether p56lck signaling may be involved despite the disruption of p56lck-CD4 association (18), we examined the patterns of protein tyrosine phosphorylation and tyrosine kinase activity of p56lck upon CD4 stimulation in cells expressing wild-type CD4 and variants of CD4 mutated to not associate with p56lck. The association of CD4 with p56lck was comparable in A2.01 cells expressing wild-type CD4 and in those expressing CD4 mutated at the dileucine motif and residue 407 (Fig. 5A). As expected, p56lck did not coimmunoprecipitate with CD4 in cells expressing the dicysteine or truncated mutants, in contrast to wild-type CD4. All these forms of CD4 were cross-linked with OKT-4 MAb, which recognizes an epitope in extracellular domain 4 of the CD4 receptor. The pattern of protein tyrosine phosphorylation after stimulation with OKT-4 MAb was comparable for each of the CD4 constructs (Fig. 5B); however, wild-type CD4, which associates with p56lck, displayed an increased in p56lck phosphorylation. Similar patterns of protein tyrosine phosphorylation were observed when the CD4 receptor was cross-linked with MAbs that recognize different epitopes of the CD4 receptor (Leu3a and 13B8-2; data not shown). No changes in tyrosine phosphorylation were observed after cross-linking with UV-psoralen-inactivated HIV (data not shown). Wild-type CD4, the construct with the mutation at the dicysteine residues, and constructs truncated at residues 402 or 418 exhibited different patterns of p56lck kinase activity in unstimulated cells (Fig. 5C). Cross-linking of CD4 with OKT-4 MAb led to a rapid and potent autophosphorylation of p56lck in wild-type CD4. Mutated receptors that do not associate with p56lck presented either no modification (402 truncated mutant) or abolition (dicysteine and 418 truncated mutants) of initial p56lck kinase activity. These results indicate that the apoptotic phenotype is associated with neither a particular pattern of tyrosine phosphorylation nor p56lck signaling.
FIG. 5.
CD4 signaling in cells expressing wild-type (wt) or mutant CD4 after cross-linking with OKT-4 anti-CD4 MAb. (A) Association of wild-type or mutant CD4 with p56lck. Equal amounts of protein lysate from CD4 wild-type and CD4 mutants, M407T, dileucine (diL), dicysteine (diC), and 402 truncated were immunoprecipitated (IP) with OKT-4 anti-CD4 MAb. Western blots of immune complexes were probed with antiserum specific for p56lck. Vector was used as a negative control. lck of mouse origin containing the SH3-SH2 domains (amino acids 54 to 226) was used as a positive control. (B) Antiphosphotyrosine (4G10) immunoblots of wild-type and mutant CD4 cellular lysates (40 μg of protein/lane) unstimulated (upper panel) or stimulated (lower panel) with anti-CD4 MAb (OKT-4). Cross-linking was performed as follows. Cells were incubated with anti-CD4 at 4°C for 30 min, washed with cold PBS, and then cross-linked with a secondary antibody [GAM-IgG F(ab′)2] at 37°C for 2 min and lysed. The molecular masses of the markers, in kilodaltons are indicated. (C) In vitro kinase activity of autophosphorylated p56lck in wild-type or mutant CD4 unstimulated (upper panel) or stimulated after cross-linking with OKT-4 MAb as previously described (lower panel).
Initial signaling mediated through the CD4 cytoplasmic tail is sufficient to predispose HIV-infected cells to apoptotic cell death.
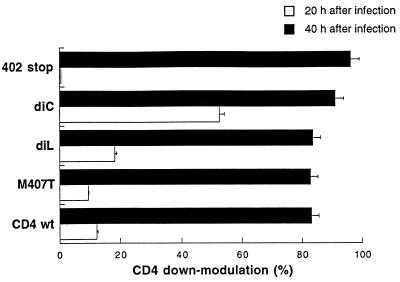
Because signaling mediated through the CD4 receptor is critical to transduce an apoptotic signal in HIV-infected cells, we hypothesized that less efficient down-modulation of the CD4 receptor may enhance the process, prolonging the presence of CD4 receptors at the cell surface. Nef, which functions early in the viral life cycle, has been shown to down-modulate the expression of the CD4 receptor at the cell surface during HIV infection. We analyzed CD4 down-modulation during HIV infection of cells expressing wild-type CD4 or mutated variants of CD4. We also determined whether the dileucine motif and other critical hydrophobic residues such as methionine 407, which have been shown to be required in Nef-induced CD4 down-regulation, might be critical during an acute infection.
Expression of the CD4 receptor was evaluated by flow cytometry at 0, 20, and 40 h after infection with HIV-1LAI (MOI, 0.1) (Fig. 6). CD4 constructs with mutations at the dileucine motif and residue 407 were down-regulated as efficiently as the wild type. Therefore, motifs in the cytoplasmic tail critical for Nef-mediated endocytosis did not affect CD4 down-modulation during an acute HIV infection. The CD4 construct with the mutation at the dicysteine motif, which does not associate with p56lck but retains the full-length cytoplasmic tail, was down-regulated more rapidly than the wild type. At 20 h after infection, more than 50% of the surface CD4 was endocytosed, confirming that interaction of CD4 and p56lck contributes to maintaining the presence of CD4 at the cell surface; however, the level of HIV-induced apoptosis was not reduced in these cells. The CD4 truncated at residue 402 was less down-regulated at 20 h, which may be a result of the resistance of CD4 to both Vpu-induced degradation and Nef-induced down-regulation when the cytoplasmic tail is deleted (15, 53). By 40 h, wild-type and mutated CD4 receptors had similar levels of CD4 down-regulation. The extent of apoptosis, therefore, was not related to the rate of cell surface CD4 down-modulation. These results suggest that initial signaling through the CD4 cytoplasmic tail and HIV-1 infection are sufficient to generate the apoptotic potential of the cell.
FIG. 6.
Down-modulation of CD4 in A2.01 cells expressing wild-type (wt) or mutant CD4 infected with HIVLAI. Expression of the CD4 receptor was determined by flow cytometry at 0, 20, and 40 h after infection with HIVLAI (MOI, 0.1). Samples were stained by direct immunofluorescence with FITC-labelled anti-CD4 MAb OKT-4. For down-regulation of CD4 surface expression at 20 and 40 h, a representative of three independent experiments is shown. diL, dileucine; diC, dicysteine.
Signaling through the CD4 receptor modulates HIV-induced apoptosis.
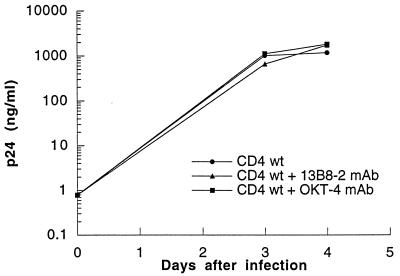
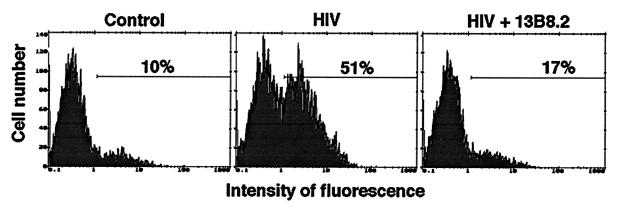
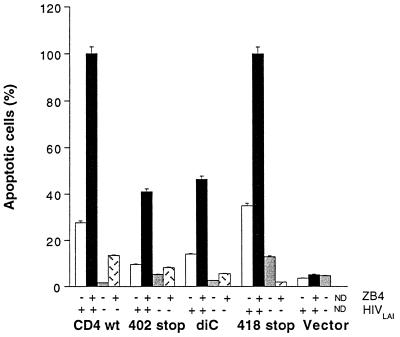
The CD4 cytoplasmic tail has been shown to be important for HIV-induced apoptosis. Therefore, we were interested to find whether interference with CD4 signaling could modify the apoptotic outcome of HIV-infected cells. Dimerization is sufficient for activation of many receptors. The CDR3-like region in domain 1 of the CD4 receptor is critical for CD4 dimerization and may play an important role in CD4 signaling (11, 30, 48). Therefore, we examined whether the anti-CD4 MAb 13B8-2, which binds the CDR3-like region in domain 1, might affect the apoptotic process by interfering with CD4 homodimer formation and signal transduction. We also analyzed if the anti-CD4 MAb OKT-4, which binds a different epitope in domain 4 of the CD4 molecule, interfered with apoptosis. Cells expressing wild-type or mutated CD4 receptors were infected with HIVLAI (MOI, 0.1) in the presence or absence of 13B8-2 MAb (10 μg/ml) or OKT-4 MAb (10 to 20 μg/ml). Viral production measured by the p24 antigen ELISA was comparable in HIV-infected cells cultured in the presence or absence of these MAbs. The growth rate of HIVLAI in cells expressing wild-type CD4 (Fig. 7) and in cells expressing mutated CD4 receptors (data not shown) were similar. The anti-CD4 MAb 13B8-2 inhibited HIV-induced apoptosis without significantly inhibiting viral production in cells expressing wild-type CD4 (Table 1). Cells expressing the CD4 mutated at the dicysteine motif were unstable after several passages, and background levels of apoptosis up to 20% were sometimes observed in uninfected cells. HIV-induced apoptosis was efficiently prevented in cells expressing CD4 mutations at residue 407, the dileucine motif, or the dicysteine motif or truncated at residue 418 that do not associate with p56lck. This effect was specific to 13B8-2 MAb, since apoptosis was not prevented (wild-type CD4 or constructs mutated at the dileucine motif or residue 407) or partially prevented (CD4 mutant mutated at the dicysteine motif or truncated at residue 418) when HIV-infected cells were cultured in the presence of OKT-4 MAb. Importantly, 13B8-2 MAb was able to prevent HIV-induced apoptosis without inhibiting HIV replication, in primary CD4+ T cells infected with HIV-1LAI (MOI, 0.1) (Fig. 8).
FIG. 7.
Virus production assessed by p24 antigen level in cells expressing wild-type (wt) CD4 infected with HIVLAI. Cells expressing wild-type CD4 were infected with HIVLAI (MOI, 0.1) in the presence or absence of 13B8-2 MAb (10 μg/ml) or OKT-4 MAb (10 to 20 μg/ml).
TABLE 1.
The anti-CD4 MAb 13B8-2 inhibits HIV-mediated apoptosis in A2.01 cells expressing the CD4 receptor
| CD4 | % of apoptotic cells at day 4 after infectiona
|
p24 (ng/ml) at day 4 after infection
|
||||
|---|---|---|---|---|---|---|
| HIV | HIV + 13B8-2 | HIV + OKT-4 | HIV | HIV + 13B8-2 | HIV + OKT-4 | |
| Wild type | 21.8 | 11.8 | 20.9 | 1,180 | 1,380 | 2,890 |
| M407T | 22.4 | 9.7 | 24.7 | 1,170 | 3,080 | 2,410 |
| dileucine | 19 | 7.4 | 21.9 | 2,100 | 2,610 | 1,590 |
| dicysteine | 11 | 0.5 | 8 | 2,750 | 1,060 | 790 |
| 402 truncated | 4.4 | 2.5 | 5.6 | 1,140 | 2,560 | 1,060 |
| 418 truncated | 11.8 | 2.8 | 6.2 | 2,400 | 1,270 | 1,780 |
Apoptosis was assessed by cell cycle analysis by flow cytometry with propidium iodide. Percentages of apoptotic cells were obtained by subtracting background apoptosis for uninfected cell lines. Levels of apoptosis in uninfected cells ranged between 4 to 8% (except for cells expressing the dicysteine, mutant where background was up to 20%).
FIG. 8.
The anti-CD4 MAb 13B8-2 inhibits HIV-induced apoptosis in primary CD4+ T cells. Purified CD4+ T cells were cultured in fresh medium containing rIL-2 (final concentration, 40 U/ml) and stimulated with phytohemagglutinin (3 μg/ml; Sigma). After 72 h of stimulation, CD4+ T cells were infected with HIV-1LAI (MOI, 0.1) and cultured in fresh medium containing rIL-2 (final concentration, 40 U/ml) in the presence or absence of 13B8-2 MAb (10 μg/ml). Apoptosis assessed by fluorochrome-labelled annexin-V binding on day 4 after infection is represented.
Thus, specific signals transduced through the CD4 receptor may modify the apoptotic outcome of HIV-infected cells, substantiating the notion that the initial CD4 signaling is determining in the subsequent propensity of cells to undergo HIV-induced apoptosis.
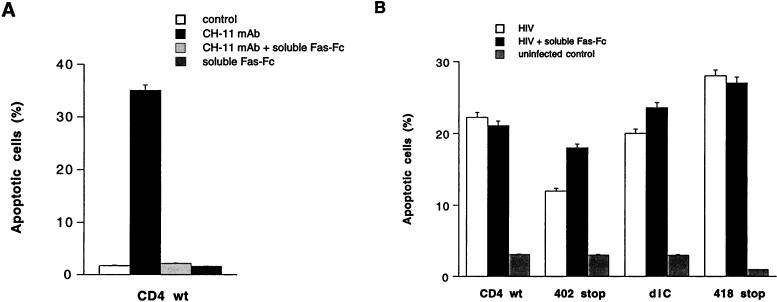
HIV-induced apoptosis is not a consequence of Fas-Fas ligand interaction but may be modulated by Fas signaling.
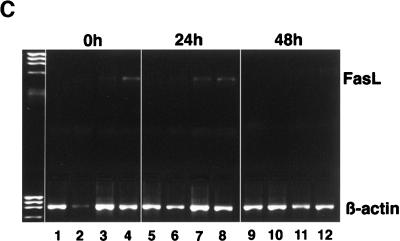
Fas signaling is involved in the induction of apoptosis during HIV infection of primary CD4+ T cells. We speculated that HIV signaling through the CD4 cytoplasmic tail may modulate Fas-mediated apoptosis by accelerating the process of cell death through up-regulation of Fas ligand expression (57). Cells expressing either wild-type CD4 or mutants of the CD4 receptor constitutively expressed Fas (97%). To study the role of Fas signaling in HIV-induced apoptosis, three different anti-Fas antibodies were used: (i) clone CH-11, (ii) soluble Fas-Fc, and (iii) clone ZB4. The prototypic MAb CH-11 (IgM) is referred to as agonistic anti-Fas antibody since it induces Fas signaling and apoptosis. The antagonistic anti-Fas chimeric antibody Fas-Fc prevents Fas-Fas ligand interaction acting as a competitive inhibitor. MAb ZB4 (IgG1) is referred to as antagonistic anti-Fas antibody since it prevents signaling mediated by CH-11 MAb and consequently prevents apoptosis (26). We verified first that uninfected and HIV-infected A2.01 cell lines (wild-type and mutant CD4) were sensitive to Fas-mediated apoptosis by using the prototypic CH-11 MAb (Fig. 9A). Then, to study the role of Fas-Fas ligand interaction in the apoptotic process, cells expressing wild-type or mutant CD4 were infected with HIV while Fas-Fas ligand interaction was blocked with the antagonistic decoy Fas chimeric antibody (Fas-Fc, 10 μg/ml). The extent of apoptosis was monitored by light microscopy and flow cytometry (fluorochrome-labeled annexin-V binding). Soluble Fas-Fc, which efficiently abrogated apoptosis mediated by agonistic Fas IgM antibody clone CH-11 (Fig. 9A), did not prevent apoptosis in HIV-infected cells (Fig. 9B). To further confirm that Fas-Fas ligand interaction was not the mechanism involved in HIV-induced apoptosis, we analyzed the expression of Fas ligand at the cell surface and Fas ligand mRNA upon HIV-1LAI infection. Cell surface expression of Fas ligand, determined by flow cytometry (biotin-conjugated anti-FasL MAb clone NOK-1 in the presence of metalloproteinase inhibitor) 0 and 48 h after infection, was not detectable in cells expressing wild-type or mutated CD4 receptors and was not up-regulated upon HIV-1LAI infection (data not shown). Fas ligand mRNA expression, analyzed by RT-PCR at 0, 24, and 48 h after infection, was detected only in A2.01 cells expressing the dicysteine and the 418 truncated mutant and was not upregulated upon HIV infection (Fig. 9C). This low level of basal Fas ligand mRNA expression in cells expressing the dicysteine and truncated 418 mutants may contribute to the lower stability of CD4 on these cells after several passages in comparison to that of cells expressing CD4 wild-type or mutant M407T, dileucine, or 402 stop.
FIG. 9.
Cell death in A2.01 cells expressing wild-type (wt) or mutant CD4 is not mediated by Fas-Fas ligand interaction. (A) Susceptibility of A2.01 cells expressing wild-type CD4 to the agonistic anti-Fas antibody (clone CH-11). Apoptosis was determined in cells cultured for 4 h with CH-11 MAb (0.2 μg/ml) in the absence or presence of the antagonistic decoy Fas antibody (Fas-Fc, 10 μg/ml). (B) Apoptosis upon HIV-1 infection in wild-type and mutant CD4 while preventing Fas-Fas ligand interaction with the antagonistic decoy Fas antibody (Fas-Fc 10 μg/ml). Infected cells were cultured with soluble Fas-Fc for 3 days, and apoptosis was assessed by fluorochrome-labelled annexin-V binding on day 3 after infection. HIV-infected cells cultured in the absence or presence of soluble Fas-Fc and uninfected cells are represented. The results presented are representative of two individual experiments. diC, dicysteine. (C) Fas ligand mRNA expression in wild-type CD4 and CD4 mutants infected with HIVLAI. RNAs prepared from 2 × 106 cells at 0, 24, and 48 h after infection were reverse transcribed into cDNAs, and the products were amplified by PCR with FasL and β-actin primers. Results for wild-type (lanes 1, 5, and 9), 402 truncated (lanes 2, 6 and 10), diC (lane 3, 7 and 11) and 418 truncated (lanes 4, 8 and 12) CD4 are shown.
Lastly, to study if Fas signaling may be modulated in HIV-infected cells, we determined the extent of apoptosis in the different cell lines infected with HIV-1LAI in the presence or absence of the antagonistic anti-Fas antibody clone ZB4. ZB4 antibody inhibited cell death in either HIV-infected cells or uninfected cells cultured for 24 h in the presence of CH-11 antibody (0.2 μg/ml); the level of inhibition of Fas-mediated apoptosis was modulated over time, with complete inhibition at 4 h but partial inhibition at 24 h (data not shown). Apoptosis was increased considerably when HIV-infected cells were cultured for 72 h in the presence of ZB4 antibody (0.2 μg/ml) compared to cells cultured without ZB4 antibody (Fig. 10). As reported by others, in uninfected cells this antibody did not induce apoptotic cell death; however, in HIV-infected cells this anti-Fas antibody considerably potentiated apoptosis. Therefore, the outcome of Fas signaling with ZB4 MAb was different in noninfected and HIV-infected cells. Thus, HIV-induced apoptosis in lymphoblastoid T-cell line A2.01 does not involve Fas-Fas ligand interaction, despite the presence of a functional Fas pathway, but remains sensitive to Fas modulation as exemplified by potentiation of cell death by ZB4 in HIV-infected cells.
FIG. 10.
Apoptosis upon HIV-1 infection in cells expressing wild-type (wt) or mutant CD4 while preventing Fas-Fas ligand interaction with the IgG1 anti-Fas antibody ZB4 (0.2 μg/ml). Apoptosis in HIV-infected and uninfected cells was measured each day under light microscopy. Apoptosis on day 4 after infection, after 72 h of treatment with ZB4 MAb, is shown. The results presented are representative of two independent experiments. diC, dicysteine.
DISCUSSION
Our group previously demonstrated that the CD4 cytoplasmic tail is required for HIV-induced apoptosis (21). Here, we investigated the role played by CD4 signaling in HIV-induced apoptosis and determinated whether Fas-Fas ligand interaction and Fas pathway were involved in this process. To investigate the role played by the CD4 cytoplasmic tail in the process of HIV-induced apoptosis, wild-type and mutated CD4 receptors were introduced into the A2.01 lymphoblastoid T-cell line, a variant of the A3.01 T-cell line that lacks CD4 expression (28, 29). The cytoplasmic tail was essential in delivering a signal for apoptosis during HIV-1 infection, independent of the CD4-p56lck association. More specifically, the sequence located between amino acid residues 402 and 418 of the cytoplasmic tail was shown to be required for HIV-induced apoptosis in acutely infected cells. The growth rates of HIV were comparable in an acute infection of cells expressing wild-type or mutated CD4 receptors. Thus, the potential for transducing an apoptotic signal was not related to differences in viral production.
The role of NF-κB in mediating apoptosis in cells expressing wild-type or mutant CD4 receptor was addressed, since NF-κB activation has been reported to either protect or induce apoptotic cell death (4, 7, 34, 52). In our acute-infection model, the propensity of cells expressing different CD4 molecules to undergo apoptosis was not related to differences in basal NF-κB activity and HIV-induced apoptosis did not lead to NF-κB activation.
Cell death was also unrelated to the expression of gene products from the Bcl-2 family (Bcl-2, Bcl-XL, and Bax) that modulate apoptosis (47). Cross-linking of the CD4 receptor by anti-CD4 MAb or HIV envelope protein gp120 has been shown to reduce the expression of Bcl-2 in CD4+ T cells (36). However, A2.01 lymphoblastoid T cells expressing wild-type and mutated CD4 receptors lacked endogenous Bax (death promoting) and presented no striking difference in the levels of Bcl-2 or Bcl-XL (survival factor) during HIV infection (results not shown). The association of p56lck with CD4, mediated through cysteine motifs in the cytoplasmic tail of CD4 and the N-terminal region of p56lck (69), is critical for CD4-mediated signaling in T-cell activation (31, 63, 73). However, some reports support a signaling role for the CD4 receptor without involving the p56lck protein (46, 51). In our study, the induction of apoptosis required signaling through the CD4 cytoplasmic tail; however, the apoptotic process was independent of the association of the protein tyrosine kinase p56lck with the cytoplasmic tail of CD4 and did not require activation of p56lck signaling. The stability of CD4 expression at the cell surface was lower for the dicysteine and the 418 truncated mutants, and this instability increased with passaging. This lower stability was reflected by the variability in the levels of apoptosis observed for these mutants in the data presented in Table 1 in comparison to Fig. 3 and 10, with lower and comparable levels of apoptosis in cells expressing wild-type CD4 respectively. However, despite the disruption of the CD4-p56lck association, these cells underwent apoptosis, demonstrating that p56lck was not critical for HIV-induced apoptosis. CD4 expression was stable for wild-type CD4 and the other mutants (M407T, dileucine, and 402 truncated) that could associate with p56lck. We have previously shown that expression of p56lck in C8166-45 or MT-2 cells, both deficient in p56lck, increased HIV-induced apoptosis and syncytium formation (21); the role of p56lck in these studies may have been to maintain CD4 to the cell surface for a longer period (58). Our results indicate that another signaling pathway, independent of lck, is participating in HIV-induced apoptosis and that residues in the cytoplasmic tail proximal to the p56lck binding domain are critical to transduce the apoptotic signal.
To determine if prolonging the presence of the CD4 receptor at the cell surface would increase HIV-induced apoptosis, we examined CD4 down-regulation during HIV infection in cells expressing wild-type and mutant CD4 receptors. Constructs with mutations at the dileucine motif and residue 407 were of particular interest since expression of Nef has been shown to mediate endocytosis of CD4 from the cell surface through a dileucine motif in the cytoplasmic tail (1, 33, 41). The hydrophobic residue 407 has also been shown to contribute to CD4 down-modulation by Nef (41, 60). However, under our conditions of acute HIV-1 infection and in contrast to studies of transiently coexpressed Nef and CD4, CD4 down-modulation was unaffected by the expression of these mutant receptors compared with wild-type CD4. This suggests that cooperative effects of Vpu and Env may be sufficient to compensate for the lack of Nef in effecting CD4 down-modulation. Therefore, the dileucine motif and residue 407 are not critical for CD4 down-regulation during an acute HIV-1 infection of lymphoblastoid T cells, despite the apparent function of Nef in the early phase of virus infection (14, 16, 17). The CD4 receptor mutated at the dicysteine motif was down-regulated more rapidly than was wild-type CD4 after HIV-1 infection; however, this did not interfere with the induction of apoptosis, suggesting that initial signaling through the CD4 cytoplasmic tail is a primary determinant for the subsequent induction of apoptosis.
Jacotot et al. (43) have reported that the CD4 cytoplasmic tail is dispensable for HIV-induced apoptosis; however, in these experiments, CD4 expression was not comparable to wild-type CD4 and was heterogeneous in cells expressing the truncated CD4 receptor. The conditions of infection were different since these authors used a lower MOI. Nevertheless, apoptosis was still delayed in HIV-infected cells expressing the truncated CD4 cytoplasmic tail. Lastly, apoptosis was monitored by analyzing DNA fragmentation and the presence of nucleosomal histone which detect cells in the late phases of apoptosis. Here, apoptosis was assessed by three different methods: fluorochrome labeled annexin-V binding (an early step), cell cycle analysis and light microscopy, all of which gave concordant results which confirmed that the CD4 cytoplasmic tail is critical for HIV-induced apoptosis in an acute infection.
Our data suggest that HIV infection could generate a preapoptotic state in HIV-infected cells by lowering the signal threshold that primes for apoptosis. Additional signals mediated through the CD4 receptor and the Fas/CD95 molecule were able to modulate the apoptotic outcome of HIV infection. Anti-CD4 antibody 13B8-2 inhibited HIV-induced apoptosis in productively infected A2.01 lymphoblastoid T-cell line, independently of its antiviral effect. Moreover, this phenotype was confirmed in primary CD4+ T cells. In contrast to the results of Benkirane et al. (8), HIV production was unaffected by 13B8-2 MAb treatment. The reason for this discrepancy in the ability of 13B8-2 MAb to inhibit HIV-1 replication may be the conditions of infection used. We used a 200- to 1,000-fold higher multiplicity of infection (MOI of 0.1 to 0.5) and allowed fusion of virions to the cell membrane to proceed at 37°C. Under such conditions, the effect of 13B8-2 MAb treatment on HIV production was not apparent, despite preventing HIV-induced apoptosis. These results suggest that 13B8-2 interferes with the transduction of an apoptotic signal during acute HIV infection. Moreover, this effect was specific to MAb 13B8-2, since OKT-4, which also binds CD4, did not affect HIV-induced apoptosis. Hypothetically, 13B8-2 might rescue infected cells from apoptosis by interfering with the dimerization process of the CD4 receptor and CD4 signaling that triggers apoptosis. Several studies have shown that negative charges on the CDR3-like region of CD4 domain 1 may be critical for CD4-mediated signal transduction and may represent a major site for CD4 dimerization (9–11, 30, 48). Indeed, dimerization of cell surface receptors may be a general mechanism for the regulation of signal transduction (37). However, it is also possible that 13B8-2 partially affects gp120-CD4 association and interferes with membrane fusion; conflicting data have been reported for the CDR3 region, either confirming (13) or refuting (12) a role for the CDR3 region in the fusion process. The mechanism by which 13B8.2 MAb inhibits apoptosis is under investigation. Our collaborators have found that an uncharacterized protein which binds to the CD4 cytoplasmic tail, independently of the cysteines at position 420 and 422, could be responsible in mediating the signal transduction event associated with inhibition of HIV replication (at low MOI) (22).
In this experimental model of HIV-induced apoptosis, we examined whether the mechanism by which HIV infection primes for apoptotic cell death was related to the expression of death factors that regulate apoptosis. Among the numerous factors that regulate cell death in mammalian cells (for a review, see reference 56), the Fas-Fas ligand system has been shown to be involved in T-cell apoptosis in HIV-1-infected patients (6, 24, 25, 45). We investigated in detail the role of Fas-Fas ligand in HIV-induced apoptosis in wild-type and mutated CD4 receptors. Neither the antagonistic decoy Fas antibody (Fas-Fc) nor the IgG1 anti-Fas antibody clone ZB4 prevented apoptosis induced by HIV infection. In agreement with a recent report (32), this suggests that HIV-induced apoptosis is not mediated by Fas-Fas ligand interaction in lymphoblastoid T-cell lines. Intracellular signaling events mediated by anti-Fas MAb ZB4 have been shown to prevent apoptosis induced by Fas-agonistic antibody clone CH-11. However, in acute HIV infection, anti-Fas MAb ZB4 potentiated apoptotic cell death, suggesting that cross-linking of Fas by ZB4 may be more efficient in delivering a death signal in HIV-infected cells. Fadeel et al. (26) have reported that anti-Fas IgG1 antibodies recognizing the same epitope of Fas/CD95 may mediate different biological effects in vitro. Moreover, in a recent report, Tanaka et al. (65) have shown that signaling through the soluble form of Fas ligand and signaling through the insoluble membrane-bound Fas ligand have different outcomes. In this study, the different outcome of ZB4 signaling in uninfected and HIV-infected cells may be related to a lower threshold for apoptosis or to the recruitment of a more efficient death-inducing signaling complex at the plasma membrane in HIV-infected cells. Thus, ZB4 signaling may either inhibit (Fas antagonist) or promote (HIV infection as reported here) apoptosis depending on the cellular conditions. The induction of apoptosis triggered by treatment with the anti-Fas MAb ZB4, although very potent at increasing the extent of apoptosis in HIV-infected A2.01 cells, could not potentiate the induction of apoptosis in primary CD4+ T cells (data not shown). This implies that other compensatory mechanisms are operating in primary cells or that the signal transduction pathway responsible for the increased susceptibility to apoptosis is not operational in the primary T cells.
In summary, our studies demonstrate the role of the CD4 cytoplasmic tail in transducing a signal to mediate HIV-induced apoptosis, independent of CD4-p56lck signaling. CD4 signaling was critical and discriminatory since anti-CD4 antibody 13B8-2 but not OKT-4 could abrogate apoptosis in spite of intense viral replication. This suggests that signaling through the CDR-3 region of the CD4 receptor is important and that dimerization of the CD4 receptor may be required in this process. Moreover, initial signaling mediated through the CD4 receptor was a primary determinant for the induction of apoptosis; apoptosis was not modulated by a prolongated presence of the CD4 receptor at the cell surface, and early interference with CD4 signaling using anti-CD4 antibody 13B8-2 was able to prevent apoptosis in HIV-infected cells. The propensity to undergo apoptosis was independent of Fas-Fas ligand interaction. However, a role for Fas signaling could still be invoked by the potentiation of apoptosis when HIV-infected cells were treated with the anti-Fas antibody clone ZB4.
ACKNOWLEDGMENTS
We thank John Reed and Shinichi Kitada for Western analysis for the Bcl-2 family protein, Jeanne Aufderheide and Philip Dao for the p24 assays, and Sara Albanil for the high-titer stocks of HIV-1LAI. We acknowledge the assistance of Marjan Hezareh in performing EMSAs and Judy Norberg and Michele Lutz in the flow cytometry analyses. We thank John Guatelli and Aymeric de Parseval for comments and critical review of the manuscript.
This work was supported by grants from the Center for AIDS Research and from the National Institutes of Health (AI 29164 and CA 67394-02 to J.C.; AI 27670, AI 38858, and AI 36214 (to D.D.R.) and the Research Center for AIDS and HIV Infection of the San Diego Veterans Affairs Medical Center. J.E. was sponsored by a Human Science Frontier Program fellowship.
REFERENCES
- 1.Aiken C, Konner J, Landau N R, Lenburg M E, Trono D. Nef induces CD4 endocytosis: requirement for a critical motif in the membrane- proximal CD4 cytoplasmic domain. Cell. 1994;76:853–864. doi: 10.1016/0092-8674(94)90360-3. [DOI] [PubMed] [Google Scholar]
- 2.Alnemri E S, Livingston D J, Nicholson D W, Salvesen G, Thornberry N A, Wong W W, Yuan J. Human ICE/CDE-3 protease nomenclature. Cell. 1996;87:171. doi: 10.1016/s0092-8674(00)81334-3. [DOI] [PubMed] [Google Scholar]
- 3.Ayyavoo V, Mahboubi A, Mahalingam S, Ramalingan R, Kudchodkar S, Williams W V, Green D R, Weiner D B. HIV-1 Vpr suppresses immune activation and apoptosis through regulation of nuclear factor kB. Nat Med. 1997;3:1117–1123. doi: 10.1038/nm1097-1117. [DOI] [PubMed] [Google Scholar]
- 4.Baeuerle P A, Baltimore D. NF-kB: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 5.Banda N K, Bernier J, Kurahara D K, Kurrle R, Haigwood N, Sekaly R P, Finkel T H. Crosslinking CD4 by human immunodeficiency virus gp120 primes T cells for activation-induced apoptosis. J Exp Med. 1992;176:1099–1106. doi: 10.1084/jem.176.4.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baumler C B, Bohler T, Herr I, Benner A, Krammer P H, Debatin K M. Activation of the CD95 (APO-1/Fas) system in T cells from human immunodeficiency virus type 1 infected children. Blood. 1996;88:1741–1746. [PubMed] [Google Scholar]
- 7.Beg A A, Sha W C, Bronson R T, Ghosh S, Baltimore D. Embryonic lethality and liver degradation in mice lacking the RelA component of NF-kB. Nature (London) 1995;376:167–170. doi: 10.1038/376167a0. [DOI] [PubMed] [Google Scholar]
- 8.Benkirane M, Corbeau P, Housset V, Devaux C. An antibody that binds the immunoglobulin CDR3-like region of the CD4 molecule inhibits provirus transcription in HIV-infected cells. EMBO J. 1993;12:4909–4921. doi: 10.1002/j.1460-2075.1993.tb06185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benkirane M, Hirn M, Carriere D, Devaux C. Functional epitope analysis of the human CD4 molecule: antibodies that inhibit human immunodeficiency virus type 1 gene expression bind to the immunoglobulin CDR3-like region of CD4. J Virol. 1995;69:6898–6903. doi: 10.1128/jvi.69.11.6898-6903.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benkirane M, Schmid-Antomarchi H, Littman D R, Hirn M, Rossi B, Devaux C. The cytoplasmic tail of CD4 is required for inhibition of human immunodeficiency virus type 1 replication by antibodies that bind to the immunoglobulin CDR3-like region in domain 1 of CD4. J Virol. 1995;69:6904–6910. doi: 10.1128/jvi.69.11.6904-6910.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Briant L, Signoret N, Gaubin M, Robert-Hebmann V, Zhang X, Murali R, Greene M I, Piatier-Tonneau D, Devaux C. Transduction of an activation signal that follows HIV-1 binding to CD4 and CD4 dimerization involves the immunoglobulin CDR3-like region in domain 1 of CD4. J Biol Chem. 1997;272:19441–19450. doi: 10.1074/jbc.272.31.19441. [DOI] [PubMed] [Google Scholar]
- 12.Broder C C, Berger E A. CD4 molecules with a diversity of mutations encompassing the CDR3 region efficiently support human immunodeficiency virus type 1 envelope glycoprotein-mediated cell fusion. J Virol. 1993;67:913–926. doi: 10.1128/jvi.67.2.913-926.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Camerini D, Seed B. A CD4 domain important for HIV-mediated syncytium lies outside the virus binding site. Cell. 1990;60:747–754. doi: 10.1016/0092-8674(90)90089-w. [DOI] [PubMed] [Google Scholar]
- 14.Chen B K, Gandhi R T, Baltimore D. CD4 down-modulation during infection of human T cells with human immunodeficiency virus type 1 involves independent activities of vpu, env, and nef. J Virol. 1996;70:6044–6053. doi: 10.1128/jvi.70.9.6044-6053.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen M-Y, Maldarelli F, Martin M A, Strebel K. Human immunodeficiency virus type 1 Vpu protein induces degradation of CD4 in vitro: the cytoplasmic domain of CD4 contributes to Vpu sensitivity. J Virol. 1993;67:3877–3884. doi: 10.1128/jvi.67.7.3877-3884.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chowers M Y, Pandori M W, Spina C A, Richman D D, Guatelli J C. The growth advantage conferred by HIV-1 nef is determined at the level of viral DNA formation and is independent of CD4 downregulation. Virology. 1995;212:451–457. doi: 10.1006/viro.1995.1502. [DOI] [PubMed] [Google Scholar]
- 17.Chowers M Y, Spina C A, Kwoh T J, Fitch N J, Richman D D, Guatelli J C. Optimal infectivity in vitro of human immunodeficiency virus type 1 requires an intact nef gene. J Virol. 1994;68:2906–2914. doi: 10.1128/jvi.68.5.2906-2914.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cinek T, Hilgert I, Horejsí V. An alternative way of CD4 and CD8 association with protein kinases of the Src family. Immunogenetics. 1995;41:110–116. doi: 10.1007/BF00182321. [DOI] [PubMed] [Google Scholar]
- 19.Coffin J M. HIV population dynamics in vivo: implications for genetic variation, pathogenesis, and therapy. Science. 1995;267:483–489. doi: 10.1126/science.7824947. [DOI] [PubMed] [Google Scholar]
- 20.Corbeil J, Richman D D. Productive infection and subsequent interaction of CD4-gp120 at the cellular membrane is required for HIV-induced apoptosis of CD4+ T cells. J Gen Virol. 1995;76:681–690. doi: 10.1099/0022-1317-76-3-681. [DOI] [PubMed] [Google Scholar]
- 21.Corbeil J, Tremblay M, Richman D D. HIV-induced apoptosis requires the CD4 receptor cytoplasmic tail and is accelerated by interaction of CD4 with p56lck. J Exp Med. 1996;183:39–48. doi: 10.1084/jem.183.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coudronnière N, Corbeil J, Robert-Hebmann V, Mesnard J-M, Devaux C. The lck protein tyrosine kinase is not involved in antibody-mediated CD4 (CDR loop) signal transduction that inhibits HIV-1 transcription. Eur J Immunol. 1998;28:1445–1457. doi: 10.1002/(SICI)1521-4141(199805)28:05<1445::AID-IMMU1445>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 23.Darzynkiewicz Z, Li X, Gong J. Assays of cell viability: discrimination of cells dying by apoptosis. Methods Cell Biol. 1994;41:15–38. doi: 10.1016/s0091-679x(08)61707-0. [DOI] [PubMed] [Google Scholar]
- 24.Estaquier J, Idziorek T, Zou W, Emilie D, Farber C M, Bourez J M, Ameisen J-C. Th1/Th2 cytokines and T-cell death: preventive effect of IL-12 on activation-induced and CD95 (Fas/Apo-1)-mediated apoptosis of CD4+ T cells from HIV-infected persons. J Exp Med. 1995;182:1759–1767. doi: 10.1084/jem.182.6.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Estaquier J, Tanaka M, Suda T, Nagata S, Golstein P, Ameisen J-C. Fas-mediated apoptosis of CD4+ and CD8+ T cells from human immunodeficiency virus-infected persons: differential in vitro preventive effect of cytokines and protease antagonists. Blood. 1996;87:4959–4966. [PubMed] [Google Scholar]
- 26.Fadeel B, Thorpe C J, Chioldi F. Anti-Fas IgG1 antibodies recognizing the same epitope of Fas/APO-1 mediate different biological effects in vitro. Int Immunol. 1997;69:6898–6903. doi: 10.1093/intimm/9.2.201. [DOI] [PubMed] [Google Scholar]
- 27.Finkel T H, Tudor-Williams G, Banda N K, Cotton M F, Curiel T, Monks C, Baba T W, Ruprecht R M, Kupfer A. Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV- and SIV-infected lymph nodes. Nat Med. 1995;1:129–134. doi: 10.1038/nm0295-129. [DOI] [PubMed] [Google Scholar]
- 28.Folks T, Benn S, Rabson A, Theodore T, Hoggan M D, Martin M, Lightfoote M, Sell K. Characterization of a continuous T-cell line susceptible to the cytopathic effects of the acquired immunodeficiency syndrome (AIDS)-associated retrovirus. Proc Natl Acad Sci USA. 1985;82:4539–4543. doi: 10.1073/pnas.82.13.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Folks T, Powell D M, Lightfoote M, Benn S, Martin M A, Fauci A. Induction of HTLV-III/LAV from a nonvirus producing T-cell line: implications for latency. Science. 1985;231:600–602. doi: 10.1126/science.3003906. [DOI] [PubMed] [Google Scholar]
- 30.Friedman T M, Reddy A P, Wassell R, Jameson B A, Korngold R. Identification of a human CD4-CDR3-like surface involved in CD4+ T cell function. J Biol Chem. 1996;271:22635–22640. doi: 10.1074/jbc.271.37.22635. [DOI] [PubMed] [Google Scholar]
- 31.Glaichenhaus N, Shastri N, Littman D R, Turner J M. Requirement for association of p56lck with CD4 in antigen-specific signal transduction in T cells. Cell. 1991;64:511–520. doi: 10.1016/0092-8674(91)90235-q. [DOI] [PubMed] [Google Scholar]
- 32.Glynn J M, McElligott D L, Mosier D E. Apoptosis induced by HIV infection in H9 T cells is blocked by ICE-family protease inhibition but not by a Fas (CD95) antagonist. J Immunol. 1996;157:2754–2758. [PubMed] [Google Scholar]
- 33.Gratton S, Yao X-J, Venkatesan S, Cohen E A, Sekaly R-P. Molecular analysis of the cytoplasmic domain of CD4. J Immunol. 1996;157:3305–3311. [PubMed] [Google Scholar]
- 34.Grimm S, Bauer M K A, Baeuerle P A, Schulze-Osthoff K. Bcl-2 down-regulates the activity of transcription factor NF-kB induced upon apoptosis. J Cell Biol. 1996;134:13–23. doi: 10.1083/jcb.134.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Groux H, Torpier G, Monte D, Mouton Y, Capron A, Ameisen J-C. Activation-induced death by apoptosis in CD4+ T cells from HIV-infected asymptomatic individuals. J Exp Med. 1992;175:331–340. doi: 10.1084/jem.175.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hashimoto F, Oyaizu N, Kalyanaraman S, Pahwa S. Modulation of Bcl-2 protein by CD4 cross-linking: a possible mechanism for lymphocyte apoptosis in human immunodeficiency virus infection and for rescue of apoptosis by interleukin-2. Blood. 1997;90:745–753. [PubMed] [Google Scholar]
- 37.Heldin C-H. Dimerization of cell surface receptors in signal transduction. Cell. 1995;80:213–223. doi: 10.1016/0092-8674(95)90404-2. [DOI] [PubMed] [Google Scholar]
- 38.Henkart P A. ICE family proteases: mediators of all apoptotic cell death. Immunity. 1996;4:195–201. doi: 10.1016/s1074-7613(00)80428-8. [DOI] [PubMed] [Google Scholar]
- 39.Ho D D. Dynamics of HIV-1 replication in vivo. J Clin Invest. 1997;99:2565–2567. doi: 10.1172/JCI119443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ho D D, Neumann A U, Perelson A S, Chen W, Leonard J M, Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature (London) 1995;373:123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 41.Hua J, Cullen B R. Human immunodeficiency virus types 1 and 2 and simian immunodeficiency virus nef use distinct but overlapping target sites for downregulation of cell surface CD4. J Virol. 1997;71:6742–6748. doi: 10.1128/jvi.71.9.6742-6748.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Idziorek T, Estaquier J, de Bels F, Ameisen J-C. YOPRO-1 permits cytofluorometric analysis of programmed cell death (apoptosis) without interfering with cell viability. J Immunol Methods. 1995;185:249–258. doi: 10.1016/0022-1759(95)00172-7. [DOI] [PubMed] [Google Scholar]
- 43.Jacotot E, Krust B, Callebaut C, Laurent-Crowford A G, Blanco J, Hovanessian A G. HIV-1 envelope glycoproteins-mediated apoptosis is regulated by CD4 dependent and independent mechanisms. Apoptosis. 1997;2:1997. doi: 10.1023/a:1026435625144. [DOI] [PubMed] [Google Scholar]
- 44.Kärber G. Beiträge zur kollektiven Behandlung pharmakologisher Reihenversuche. Arch Exp Pathol Pharmakol. 1931;162:480–483. [Google Scholar]
- 45.Katsikis P D, Wunderlich E S, Smith C A, Hertzenberg L A, Hertzenberg L A. Fas antigen stimulation induces marked apoptosis of T lymphocytes in HIV-infected individuals. J Exp Med. 1995;181:2029–2036. doi: 10.1084/jem.181.6.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Killeen N, Littman D R. Helper T-cell development in the absence of CD4-p56lck association. Nature. 1993;364:729–732. doi: 10.1038/364729a0. [DOI] [PubMed] [Google Scholar]
- 47.Kroemer G. The proto-oncogene Bcl-2 and its role in regulating apoptosis. Nat Med. 1997;3:614–620. doi: 10.1038/nm0697-614. [DOI] [PubMed] [Google Scholar]
- 48.Langedijk J P M, Puijk W C, Paul van Hoorn W, Meloens R H. Location of CD4 dimerization site explains critical role of CDR3-like region in HIV-1 infection and T-cell activation and implies a model for complex of coreceptor-MHC. J Biol Chem. 1993;268:16875–16878. [PubMed] [Google Scholar]
- 49.Laurent-Crawford A G, Krust B, Muller S, Riviere Y, Rey-Cuille M-A, Bechet J-M, Montagnier L, Hovanessian A G. The cytopathic effect of HIV is associated with apoptosis. Virology. 1991;185:829–839. doi: 10.1016/0042-6822(91)90554-o. [DOI] [PubMed] [Google Scholar]
- 50.Laurent-Crawford A G, Krust B, Riviere Y, Desgranges C, Muller S, Kieny M-P, Dauguet C, Hovanessian A G. Membrane expression of HIV envelope glycoproteins triggers apoptosis in CD4 cells. AIDS Res Hum Retroviruses. 1993;9:761–773. doi: 10.1089/aid.1993.9.761. [DOI] [PubMed] [Google Scholar]
- 51.Lemasson I, Briant L, Hague B, Coudronniere N, Heron L, David C, Rebouissou C, Kindt T, Devaux C. An antibody that binds domain 1 of CD4 inhibits replication of HIV-1, but not HTLV-I, in a CD4-positive/p56lck-negative HTLV-I-transformed cell line. J Immunol. 1996;156:859–865. [PubMed] [Google Scholar]
- 52.Liu Z-G, Hsu H, Goeddel D V. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-kB activation prevents cell death. Cell. 1996;87:565–576. doi: 10.1016/s0092-8674(00)81375-6. [DOI] [PubMed] [Google Scholar]
- 53.Mangasarian A, Foti M, Aiken C, Chin D, Carpentier J-L. The HIV Nef protein acts as a connector with sorting pathways in the golgi and at the plasma membrane. Immunity. 1997;6:67–77. doi: 10.1016/s1074-7613(00)80243-5. [DOI] [PubMed] [Google Scholar]
- 54.McGuire K L, Iacobelli M. Involvement of Rel, Fos and Jun proteins in binding activity to the IL-2 promoter CD28 response element/AP-1 sequence in human T cells. J Immunol. 1997;159:1319–1327. [PubMed] [Google Scholar]
- 55.Muro-Cacho C A, Pantaleo G, Fauci A S. Analysis of apoptosis in lymph nodes of HIV-infected persons. J Immunol. 1995;154:5555–5566. [PubMed] [Google Scholar]
- 56.Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 57.Oyaizu N, Adachi Y, Hashimoto F, McCloskey T W, Hosaka N, Kayagaki N, Yagita H, Pahwa S. Monocytes express Fas ligand upon CD4 cross-linking and induce CD4+ T cell apoptosis: a possible mechanism of bystander cell death in HIV infection. J Immunol. 1997;158:2456–2463. [PubMed] [Google Scholar]
- 58.Pelchen-Matthews A, Boulet I, Littman D R, Fagard R, Marsh M. The protein tyrosine kinase p56lck inhibits CD4 endocytosis by preventing entry of CD4 into coated pits. J Cell Biol. 1992;117:279–290. doi: 10.1083/jcb.117.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rogel M E, Wu L I, Emerman M. The human immunodeficiency virus type 1 vpr gene prevents cell proliferation during chronic infection. J Virol. 1995;69:882–888. doi: 10.1128/jvi.69.2.882-888.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Salghetti S, Mariani R, Skowronski J. Human immunodeficiency virus type 1 nef and p56lck protein-tyrosine kinase interact with a common element in CD4 cytoplasmic tail. Proc Natl Acad Sci USA. 1995;92:349–353. doi: 10.1073/pnas.92.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Spina C A, Kwoh T J, Chowers M Y, Guatelli J C, Richman D D. The importance of nef in the induction of human immunodeficiency virus type 1 replication from primary quiescent CD4 lymphocytes. J Exp Med. 1994;179:115–123. doi: 10.1084/jem.179.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stewart S A, Poon B, Jowett J B M, Chen I S Y. Human immunodeficiency virus type 1 Vpr induces apoptosis following cell cycle arrest. J Virol. 1997;71:5579–5592. doi: 10.1128/jvi.71.7.5579-5592.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Straus D B, Weiss A. Genetic evidence for the involvement of the lck tyrosine kinase in signal transduction through the T cell antigen receptor. Cell. 1992;70:585–593. doi: 10.1016/0092-8674(92)90428-f. [DOI] [PubMed] [Google Scholar]
- 64.Susin S A, Zamzami N, Castedo M, Daugas E, Wang H G, Geley S, Fassy F, Reed J C, Kroemer G. The central executioner of apoptosis: multiple connections between protease activation and mitochondria in Fas/APO-1/CD95- and ceramide-induced apoptosis. J Exp Med. 1997;186:25–37. doi: 10.1084/jem.186.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tanaka M, Itai T, Adachi M, Nagata S. Downregulation of Fas ligand by shedding. Nat Med. 1998;4:31–36. doi: 10.1038/nm0198-031. [DOI] [PubMed] [Google Scholar]
- 66.Telford W G, King L E, Fraker P J. Comparative evaluation of several DNA binding dyes in the detection of apoptosis-associated chromatin degradation by flow cytometry. Cytometry. 1992;13:137–142. doi: 10.1002/cyto.990130205. [DOI] [PubMed] [Google Scholar]
- 67.Terai C, Kornbluth R S, Pauza C D, Richman D D, Carson D A. Apoptosis as a mechanism of cell death in cultured T lymphoblasts acutely infected with HIV-1. J Clin Invest. 1991;87:1710–1715. doi: 10.1172/JCI115188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thompson C B. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 69.Turner J M, Brodsky M H, Irving B A, Levin S D, Perlmutter R M, Littman D R. Interaction of the unique N-terminal region of tyrosine kinase p56lck with cytoplasmic domains of CD4 and CD8 is mediated by cysteine motifs. Cell. 1990;60:755–765. doi: 10.1016/0092-8674(90)90090-2. [DOI] [PubMed] [Google Scholar]
- 70.Veillette A, Bookman M A, Horak E M, Bolen J B. The CD4 and CD8 T cell surface antigens are associated with the internal membrane tyrosine-protein kinase p56lck. Cell. 1988;55:301–308. doi: 10.1016/0092-8674(88)90053-0. [DOI] [PubMed] [Google Scholar]
- 71.Veillette A, Bookman M A, Horak E M, Samelson L E, Bolen J B. Signal transduction through the CD4 receptors involves the activation of the internal membrane tyrosine-protein kinase p56lck. Nature (London) 1989;338:257–259. doi: 10.1038/338257a0. [DOI] [PubMed] [Google Scholar]
- 72.Wei X, Ghosh S K, Taylor M E, Johnson V A, Emini E A, Deutsch P, Lifson J D, Bonhoeffer S, Nowak M A, Hahn B H, Saag M S, Shaw G M. Viral dynamics in human immunodeficiency virus type 1 infection. Nature (London) 1995;373:117–122. doi: 10.1038/373117a0. [DOI] [PubMed] [Google Scholar]
- 73.Weiss A, Littman D R. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76:263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 74.Westendorp M O, Frank R, Ochsenbauer C, Stricker K, Dhein J, Walcazak H, Debatin K-M, Krammer P H. Sensitization of T cells to CD95-mediated apoptosis by HIV-1 tat and gp120. Nature (London) 1995;375:497–500. doi: 10.1038/375497a0. [DOI] [PubMed] [Google Scholar]
- 75.Xu H, Littman D R. A kinase-independent function of lck in potentiating antigen-specific T cell activation. Cell. 1993;1993:633–643. doi: 10.1016/0092-8674(93)90511-n. [DOI] [PubMed] [Google Scholar]
- 76.Yee J-K, Miyanohara A, LaPorte P, Bouic K, Burns J C, Friedmann T. A general method for the generation of high-titer, pantropic retroviral vectors: highly efficient infection of primary hepatocytes. Proc Natl Acad Sci USA. 1994;91:9564–9568. doi: 10.1073/pnas.91.20.9564. [DOI] [PMC free article] [PubMed] [Google Scholar]