Abstract
Varicella-zoster virus (VZV) encodes five gene products that do not have homologs in herpes simplex virus. One of these genes, VZV open reading frame 32 (ORF32), is predicted to encode a protein of 16 kDa. VZV ORF32 protein was shown to be phosphorylated and located in the cytosol of virus-infected cells. Antibody to ORF32 protein immunoprecipitated 16- and 18-kDa phosphoproteins from VZV-infected cells. Since VZV encodes two protein kinases that might phosphorylate ORF32 protein, immunoprecipitations were performed with cells infected with VZV mutants unable to express either of the viral protein kinases. Cells infected with VZV unable to express the ORF66 protein kinase contained both the 16- and 18-kDa ORF32 phosphoproteins; however, cells infected with the VZV ORF47 protein kinase mutant showed only the 16-kDa ORF32 phosphoprotein. Treatment of [35S]methionine-labeled proteins with calf intestine alkaline phosphatase resulted in a decrease in size of the ORF32 proteins from 16 and 18 kDa to 15 and 17 kDa, respectively. VZV unable to express ORF32 protein replicated in human melanoma cells to titers similar to those seen with parental virus; however, VZV unable to express ORF32 was impaired for replication in U20S osteosarcoma cells. Thus, VZV ORF32 protein is posttranslationally modified by the ORF47 protein kinase. Since the VZV ORF47 protein kinase has recently been shown to be critical for replication in human fetal skin and lymphocytes, its ability to modify the ORF32 protein suggests that the latter protein may have a role for VZV replication in human tissues.
Varicella-zoster virus, a member of the alphaherpesvirus subfamily, is the etiologic agent of chickenpox and herpes zoster. The other human member of this subfamily, herpes simplex virus (HSV), causes orofacial lesions and genital herpes. While these two viruses cause very different diseases, their genomes show a remarkable degree of similarity. The two genomes have similar structural organizations, and over 90% of the genes in VZV have homologs in HSV.
Five of the 69 unique genes in VZV, open reading frames 1 (ORF1), -2, -13, -32, and -57, do not have HSV homologs (3). VZV ORF1 encodes a membrane protein that is dispensable for replication in vitro (2). VZV ORF13 encodes the viral thymidylate synthetase, which also is not required for replication in cell culture (1). The other three VZV genes, ORF2, -32, and -57, have not been studied.
VZV ORF32 is predicted to encode a 143-amino-acid protein located in the unique long (UL) region of the genome (4). The ORF32 protein is predicted to be very hydrophilic (7) and contains a large number of acidic amino acids. Mapping of transcripts in this region shows a 3.0-kb RNA transcribed in a rightward direction that overlaps ORF32 (18).
While VZV ORF32 does not have a homolog in HSV, ORF32 does have sequence homology with two other herpesvirus proteins. Equine herpesvirus type 1 (EHV-1) gene 34 (23) and EHV-4 gene 34 (22) are positional homologs of VZV ORF32. In a 39-amino-acid region, ORF32 has 70% amino acid similarity to the two EHV proteins; within this region, VZV ORF32 shares 9 identical amino acids, IPRVFPDTP, with EHV-4 gene 34. The function of these EHV proteins is unknown at present.
Here we show that ORF32 encodes 16- and 18-kDa phosphoproteins that are expressed in the cytosol of virus-infected cells. By constructing an ORF32 deletion mutant, we show that these proteins are dispensable for virus replication. In virus-infected cells lacking the VZV ORF47 protein kinase, the 18-kDa protein is not detected. Thus, the ORF32 protein is posttranslationally modified by the ORF47 protein kinase.
MATERIALS AND METHODS
Cells and viruses.
MeWo (human melanoma) cells were used for transfections and preparation of virus stocks. U2OS and SAOS2 human osteosarcoma cells were obtained from the American Type Culture Collection. Recombinant viruses were derived from cosmids corresponding to the attenuated Oka strain of VZV. VZV with stop codons in ORF47 (ROka47S), ORF66 (ROka66S), or ORF 47 and ORF66 (ROka47S/66SA) have been previously described (5, 6).
Plasmids and cosmids.
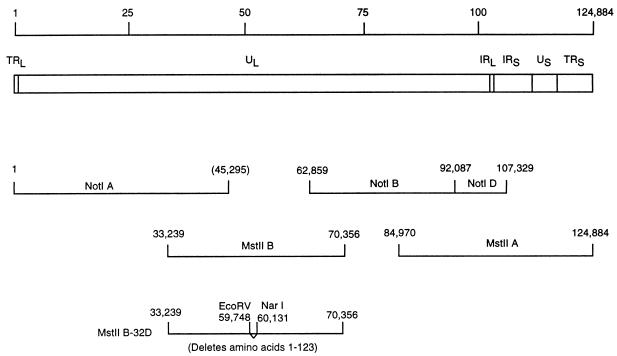
VZV cosmids NotI A, NotI BD, MstII A, and MstII B contain the entire VZV genome (Fig. 1). To produce VZV with a deletion in ORF32, the MstII B cosmid was cut with NotI, which cuts at VZV nucleotides 45295 and 62859, and the 17.6-kb fragment containing ORF32 was inserted into the NotI site of pBluescript-SK+ (Stratagene, La Jolla, Calif.). The resulting plasmid was cut with AgeI and AatII (which cut at VZV nucleotides 59238 and 60699, respectively), and the nearly 1.5-kb fragment released from it was cloned into the AgeI and AatII sites of plasmid Litmus 29 (New England Biolabs, Beverly, Mass.). The resulting plasmid was cut with EcoRV and NarI (which cut at VZV nucleotides 59748 and 60131, respectively), treated with T4 DNA polymerase to produce blunt ends, and ligated to itself. Two independent clones were selected, and the sequence of the plasmids was determined to verify that the junction of the ORF32 deletion had the expected sequence. The two clones were then cut with AgeI and AatII, and the mutated DNAs were inserted into the NotI plasmid. The latter were then cut with NotI, and the ORF32 mutant DNA was inserted into cosmid MstII B to produce cosmids MstII B-32DA and MstII B-32DB (Fig. 1). These cosmids have a deletion that begins 18 nucleotides before the start codon of ORF32 and ends 60 nucleotides before the stop codon of ORF32.
FIG. 1.
Construction of recombinant VZV with a deletion in ORF32. The VZV genome is 124,884 bp in length (line 1) and contains UL, unique short (US), terminal repeat (TR), and internal repeat (IR) DNA sequences (line 2). NotI and MstII restriction fragments used to generate parental VZV are shown (lines 3 and 4). Cosmid MstII B-32D (line 5) has a deletion beginning before the start codon and ending at codon 123 of ORF32. Two identical, independent clones of cosmids MstII B-32D, termed MstII B-32DA and MstII B-32DB, were used to construct VZV with ORF32 deleted.
To translate ORF32 in vitro, the entire coding region of ORF32 was amplified by PCR with primers that contained an XbaI site and the first seven codons of ORF32 (GATCGTCTAGATATGGAATCGTCTAACATTAACG), as well as an EcoRI site and the last seven codons of ORF32 followed by the stop codon (GATCGAGAATTCTTAATCGGTGTCAGAATCTTCATC). After digestion with XbaI and EcoRI, the PCR product was cloned into plasmid pGEM-2 (Promega Corp., Madison, Wis.).
Transfections.
To produce VZV with a deletion in ORF32, MeWo cells were transfected with 1 μg of cosmid NotI A, NotI BD, MstII B-32DA, or MstII B-32DB; 0.5 μg of cosmid MstIIA; 50 ng of plasmid pCMV62; and 2 μg of sheared salmon sperm DNA. Transfection of MeWo cells was performed as previously described (1).
Southern blots and growth characteristics of recombinant VZV.
VZV DNA was purified from nucleocapsids, cut with EcoRI or BamHI, fractionated on agarose gels, transferred to nylon membranes, and hybridized with a [32P]dCTP-labeled probe derived from the four DNA cosmids spanning the entire VZV genome. VZV DNA was also cut with AatII and AgeI and hybridized to a probe containing VZV ORF32. Growth curves for recombinant VZV were determined as described previously (1).
Generation of ORF32 fusion protein.
The coding region of VZV ORF32, from codons 11 to 143, was amplified from VZV cosmid DNA by PCR with two primers. The first primer contained a BamHI site followed by VZV nucleotides 59796 to 59816 (CGCGGATCCCAACCGTCGTCTATCGCACAT), and the second primer contained an EcoRI site followed by VZV nucleotides 60174 to 60194 (CGCGAATTCATCGGTGTCAGAATCTTCATC). The amplified DNA was digested with BamHI and EcoRI and inserted into plasmid pGEX-2T (Pharmacia, Piscataway, N.J.), which contains a portion of the glutathione S-transferase (GST) gene. The junction of the ORF32 DNA and pGEX-2T DNA was confirmed by sequencing. Escherichia coli cells containing the plasmid expressing the GST-ORF32 fusion protein were lysed by sonication, and GST-ORF32 fusion protein was purified with glutathione-Sepharose.
Antibodies, immunoprecipitation, phosphatase treatment, and cell fractionation studies.
Rabbits were immunized three times with 150 μg of GST-ORF32 fusion protein, and antiserum was obtained and absorbed four times with lysates from uninfected MeWo cells.
VZV-infected and uninfected cells were radiolabeled with [35S]methionine or 32Pi and lysed. The supernatant was incubated with rabbit antibody to ORF32 protein or monoclonal antibody to VZV gE (Chemicon, Temecula, Calif.) followed by protein A-Sepharose. Immune complexes were fractionated on sodium dodecyl sulfate-polyacrylamide gels. In some cases, immune complexes were treated with 10 U of calf intestine alkaline phosphatase (New England Biolabs, Beverly, Mass.) for 30 min at 37°C, an additional 10 U of enzyme was added for 30 min, and the proteins were fractionated on gels.
Membrane and cytosolic fractions from VZV-infected MeWo cells were prepared as previously described (2). For immunofluorescence studies, cells were fixed in 50% methanol–50% acetone, incubated with rabbit antibody to ORF32 protein, and fluorescein isothiocyanate-labeled goat anti-rabbit antibody was added.
RESULTS
VZV ORF32 encodes 16- and 18-kDa cytoplasmic proteins.
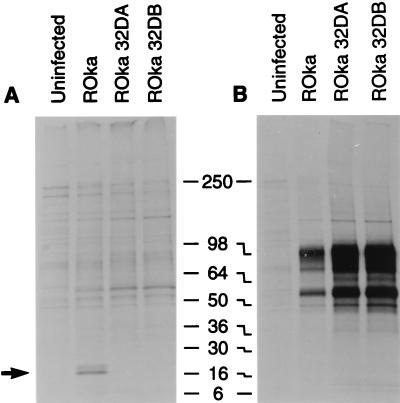
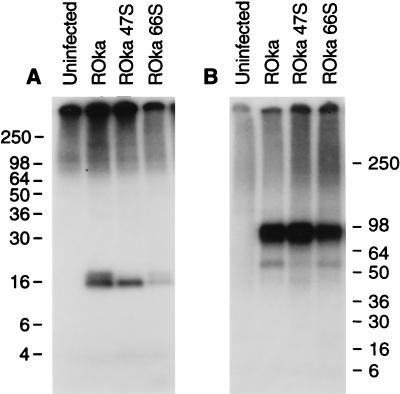
To verify that ORF32 is expressed in VZV-infected cells, rabbit antibody was made to a GST-ORF32 fusion protein. Immunoprecipitations of lysates from [35S]methionine-labeled cells obtained with antisera to VZV ORF32 protein showed 16- and 18-kDa proteins from recombinant Oka (ROka) VZV-infected cells (Fig. 2A). Similar proteins were not present in uninfected cells. Immunoprecipitation with monoclonal antibody to VZV gE served as a control for infection (Fig. 2B).
FIG. 2.
Immunoprecipitation of ORF32 protein from VZV-infected cells. Antibody to ORF32 immunoprecipitates 16- and 18-kDa proteins in lysates from VZV ROka-infected cells (arrow), but not ROka32DA- or ROka32DB-infected cells (A). Cells infected with VZV ROka, ROka32DA, or ROka32DB express proteins that react with monoclonal antibody to VZV gE (B). Numbers refer to molecular masses of proteins in kilodaltons.
To further verify that ORF32 encodes a protein, the ORF32 gene was transcribed in vitro with T7 RNA polymerase and translated with rabbit reticulocyte lysate. A polypeptide with a size of 16 kDa was detected (Fig. 3, lane IVT) which is similar in size to the smaller protein detected in VZV-infected cells.
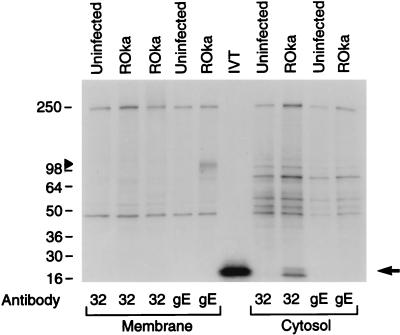
FIG. 3.
VZV ORF32 is expressed in the cytosol of VZV-infected cells. Uninfected or VZV ROka-infected cells were radiolabeled with [35S]methionine, and membrane (lanes 1 to 5) and cytosolic (lanes 7 to 10) fractions were prepared. An aliquot of each fraction was immunoprecipitated with antibody to ORF32 (lanes 1 to 3 and 7 to 8) or VZV gE (lanes 4 to 5 and 9 to 10). VZV ORF32 protein is detected only in the cytosolic fraction of cells infected with VZV (lane 8, arrow), while gE is present in the membrane fraction (lane 5, arrowhead). Lane 6 shows a 16-kDa protein obtained by in vitro translation (IVT) of the ORF32 gene with T7 polymerase. Numbers refer to molecular masses of proteins in kilodaltons.
To determine where the ORF32 protein is located in virus-infected cells, cytosolic and membrane fractions were prepared from radiolabeled VZV-infected cells. Immunoprecipitation with antibody to ORF32 protein showed that the protein is located in the cytosolic fraction of infected cells (Fig. 3), but not in the membrane fraction. As a control for separation of the cellular fractions, VZV gE localized to the membrane (Fig. 3), but not the cytosolic fraction. Immunofluorescence studies with antibody to ORF32 protein showed that the protein was present in the cytoplasm of virus-infected cells (17).
VZV ORF32 protein is phosphorylated.
To determine whether ORF32 encodes a phosphoprotein, VZV-infected cells were radiolabeled with 32Pi and immunoprecipitations were performed. Antibody to ORF32 protein immunoprecipitated 16- and 18-kDa phosphoproteins from VZV-infected cells (Fig. 4A). gE is also phosphorylated (25); antibody to gE detected 90- to 95-kDa phosphoproteins in VZV-infected cells (Fig. 4B).
FIG. 4.
VZV ORF32 protein is phosphorylated. Cells infected with VZV ROka or VZV ROka47S/66S were radiolabeled with 32Pi, and lysates were immunoprecipitated with antibody to ORF32 protein (A) or VZV gE (B). Numbers refer to molecular masses of proteins in kilodaltons.
The ORF47 protein kinase is responsible for posttranslational modification of ORF32.
VZV encodes two protein kinases, the ORF47 and ORF66 proteins (5, 6). To determine if either of the VZV protein kinases might be associated with the phosphorylation of ORF32 protein, melanoma cells were infected with a VZV mutant that does not express either of the protein kinases and radiolabeled with 32Pi, and ORF32 protein was immunoprecipitated. Immunoprecipitation of VZV ORF32 from cells infected with a VZV mutant that is unable to express both the ORF47 and ORF66 protein kinases (6) resulted in detection of the 16-kDa ORF32 phosphoprotein, but not the larger 18-kDa ORF32 protein (Fig. 4A). Immunoprecipitation of another aliquot of radiolabeled cells with monoclonal antibody to VZV gE verified that the extent of infection in cells with the double protein kinase mutant was comparable to that with the parental virus (Fig. 4B).
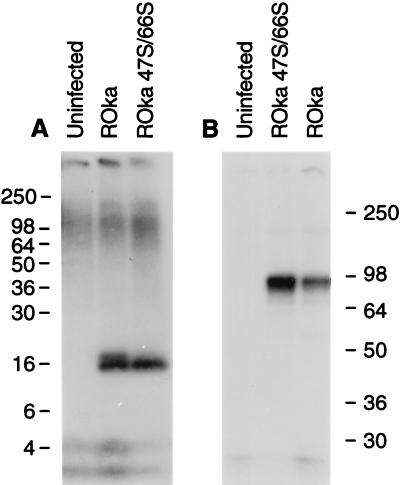
To determine which of the VZV-encoded protein kinases is responsible for posttranslational modification associated with the larger 18-kDa ORF32 protein, melanoma cells were infected with VZV mutants that do not express the individual VZV ORF47 or ORF66 protein kinases and immunoprecipitations were performed. Both the 16- and 18-kDa ORF32 phosphoproteins were present in lysates from cells infected with the ORF66 mutant; however, only the smaller 16-kDa protein was present in cells infected with the ORF47 mutant (Fig. 5A). Immunoprecipitation of lysates with antibody to VZV gE indicated that each of the lysates contained similar levels of gE (Fig. 5B), confirming that the lack of detection of the larger 18-kDa ORF32 protein was not due to a lower level of virus infection in VZV ORF47-infected cells.
FIG. 5.
VZV ORF47 is required for posttranslational processing associated with phosphorylation of ORF32 protein. Cells were infected with VZV ROka, ROka47S, or ROka66S, and radiolabeled with 32Pi, and lysates were immunoprecipitated with antibody to ORF32 protein (A) or VZV gE (B). Numbers refer to molecular masses of proteins in kilodaltons.
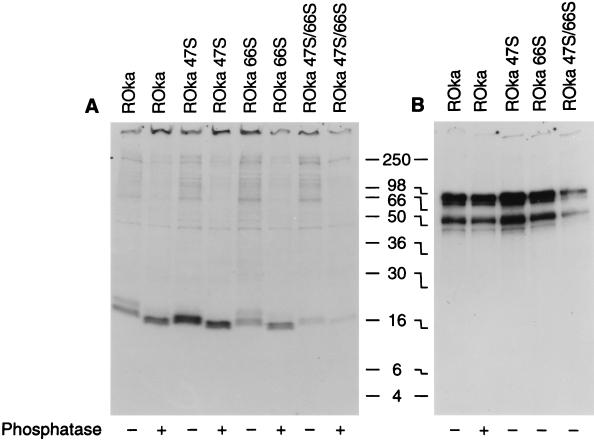
To further evaluate the posttranslational processing of ORF32, cells were infected with VZV ROka, ROka47S, or ROka66S and radiolabeled with [35S]methionine, and ORF32 protein was immunoprecipitated. Some of the immunoprecipitates were treated with calf intestine alkaline phosphatase and then fractionated on polyacrylamide gels. Treatment of immunoprecipitates from VZV ROka- or VZV ROka66S-infected cells with alkaline phosphatase reduced the size of the 16- and 18-kDa bands to 15 and 17 kDa, respectively (Fig. 6A). Incubation of the 16-kDa protein immunoprecipitated from VZV ROka47S-infected cells with alkaline phosphatase yielded a 15-kDa band. Monoclonal antibody to VZV gE was used to immunoprecipitate gE from another aliquot of the lysates (Fig. 6B), showing that each of the lysates was derived from VZV-infected cells.
FIG. 6.
Dephosphorylation reduces the size of ORF32 protein. Cells infected with VZV ROka and ORF47 or ORF66 mutants were radiolabeled with [35S]methionine, and lysates were immunoprecipitated with antibody to ORF32 protein (A) or gE (B). Lysates in some lanes were treated with calf intestine alkaline phosphatase (indicated by + below the autoradiograph), while lysates in other lanes were not treated (indicated by −) before being loaded onto the gel.
VZV ORF32 is not required for growth of VZV in cell culture.
To determine whether ORF32 is necessary for growth of the virus in vitro, a VZV ORF32 deletion mutant was constructed. Melanoma cells were transfected with cosmids that have a deletion in ORF32 (MstII B-32DA or MstII B-32DB) along with cosmids NotI A, NotI BD, and MstII A. Cytopathic effects, indistinguishable from those seen with parental VZV, were present in cells transfected with the ORF32 deletion cosmids, and two clones of each of the resulting viruses, ROka32DA and ROka32DB, were propagated in melanoma cells.
Virion DNA was prepared from cells infected with VZV ROka and the ORF32 deletion mutants, and Southern blots were performed to verify that the genomes had the expected configurations. Digestion of DNA from VZV ROka, ROka32DA, and ROka32DB with EcoRI or BamHI showed restriction fragments that were identical to those seen with the parental virus (17). Digestion of virion DNA with AatII and AgeI showed that the AatII-AgeI fragment in VZV ROka was diminished in size by 0.4 kb in the ORF32 mutants due to the deletion in ORF32 (17).
To verify that cells infected with the ORF32 deletion mutants were unable to express ORF32 protein, infected cells were radiolabeled and lysates were immunoprecipitated with antibody to the protein. While cells infected with ROka expressed 16- and 18-kDa proteins that reacted with antibody to ORF32 protein, ROka32DA- and ROka32DB-infected cells did not produce similar proteins (Fig. 2A, lanes 3 and 4). To ensure that the absence of expression of ORF32 protein was not due to the lack of VZV gene expression, immunoprecipitations were performed with cells infected with the ORF32 deletion mutants with antibody to gE. Cells infected with the mutants each expressed VZV gE (Fig. 2B).
Inability to express VZV ORF32 impairs the growth of virus in U20S osteosarcoma cells.
To determine whether the absence of ORF32 affects the growth of VZV in vitro, MeWo cells were infected with parental (ROka) and ORF32 deletion mutants, and plaque sizes were measured. The mean diameter of plaques (± the standard deviation) after infection of MeWo cells with cells containing VZV ROka (0.81 ± 0.18 mm) was similar to the size of plaques from cells infected with VZV ROka32DA (0.85 ± 0.18 mm). The growth of the ORF32 mutant virus was also examined in two osteosarcoma cell lines: U20S cells that express a wild-type p53 and retinoblastoma susceptibility gene product (pRB) and SAOS2 cells that have inactive p53 and pRb (24). The mean plaque size in SAOS2 osteosarcoma cells infected with ROka (0.37 ± 0.07 mm) was also similar to that of ROka32DA (0.35 ± 0.07 mm). In contrast, U20S osteosarcoma cells infected with the ORF32 mutants produced smaller plaques than VZV ROka. The mean plaque size in U20S osteosarcoma cells of VZV ROka was 0.36 ± 0.09 mm, while the plaque size for VZV ROka32DA was 0.20 ± 0.09 mm (P < 0.01). The plaque size of the ORF32 mutants in human fibroblasts and schwannoma and neuroblastoma cells was similar to that of the parental virus (data not shown).
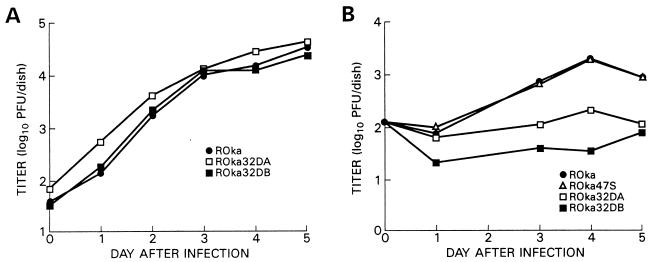
To further evaluate whether ORF32 impairs the growth of VZV, melanoma and U20S osteosarcoma cells were infected with parental virus or ORF32 deletion mutants, and the titer of virus was determined at different time points. VZV ORF32 deletion mutants grew to titers similar to those seen in cells infected with the parental virus in melanoma cells (Fig. 7A), but to lower titers than parental virus in U20S osteosarcoma cells (Fig. 7B).
FIG. 7.
Growth of VZV ORF32 deletion mutants in cell culture. MeWo (A) or osteosarcoma (B) cells were inoculated with VZV-infected cells, and at various times after infection, the cells were harvested and the titer of virus in MeWo cells was determined.
Since ORF47 is responsible for the posttranslational modification associated with the 18-kDa ORF32 phosphoprotein, a VZV mutant unable to express ORF47 protein (ROka47SA) might have growth properties resembling those of a VZV ORF32 deletion mutant. However, infection of U20S osteosarcoma cells with ROka47SA resulted in much larger plaques than those of ROka32DA (0.43 ± 0.10 and 0.20 ± 0.09 mm, respectively; P < 0.01), but only slightly larger than those of ROka (0.36 ± 0.09 mm). Infection of U2OS osteosarcoma cells with ROka47SA resulted in titers of virus similar to those obtained with the parental virus, unlike the lower titers seen with the ORF32 deletion mutants (Fig. 7B). Thus, the absence of the 18-kDa ORF32 phosphoprotein is not responsible for the impaired growth of VZV ORF32 deletion mutants in U20S osteosarcoma cells.
DISCUSSION
These experiments show that VZV ORF32 encodes phosphoproteins with sizes of 16 and 18 kDa localized in the cytosol of virus-infected cells and that the ORF32 protein is posttranslationally modified by the VZV ORF47 protein kinase. While the ORF47 protein kinase is conserved among each of the human herpesviruses (14, 16, 19), none of the other human herpesviruses has a homolog of the VZV ORF32 protein.
VZV ORF32 has positional and sequence homology with two animal herpesvirus proteins, EHV-1 gene 34 and EHV-4 gene 34. These three proteins are similar in length and have low isoelectric points (3.9 to 4.8), and at least 15% of each of the proteins is comprised of acidic amino acids. The VZV protein has 34% overall amino acid identity with EHV-1 gene 34 protein and 29% amino acid identity with the EHV-4 gene 34 protein. All three proteins are very hydrophilic, and none of them is predicted to have a sufficiently long hydrophobic region that would allow the protein to be stably inserted into membranes.
VZV ORF32 is predicted to encode a 16-kDa protein based on its amino acid sequence (4). Both in vitro-translated ORF32 protein and one of the two proteins immunoprecipitated from VZV-infected cells were 16 kDa. Using a VZV mutant unable to express ORF47, we found that the 16-kDa ORF32 phosphoprotein was expressed, but the 18-kDa phosphoprotein was absent. The smaller 16-kDa ORF32 phosphoprotein is probably phosphorylated by a cellular kinase, since deletion of the only two kinases predicted to be encoded by the VZV genome, ORF47 and ORF66, did not affect phosphorylation of the 16-kDa protein. Thus, both ORF47 and cellular kinases are probably required for the full maturation of ORF32 protein.
VZV ORF47 has previously been shown to encode a protein kinase that phosphorylates serine and, to a lesser extent, threonine residues (12, 13, 21). Immunoprecipitation of ORF47 protein (12), or coimmunoprecipitation of ORF47 protein with ORF62 protein (13), followed by incubation in a protein kinase assay resulted in phosphorylation of the ORF47 and ORF62 proteins, respectively. In contrast, immunoprecipitation of radiolabeled viral phosphoproteins from cells infected with an ORF47 mutant virus failed to show any difference in the phosphorylation of ORF62 protein (5). These results suggested that while ORF47 protein is capable of phosphorylating ORF62 protein, ORF47 protein is not necessary for phosphorylation of ORF62 in virus-infected cells. Analysis of other VZV phosphoproteins, including ORF4, ORF63, and ORF68 proteins, indicated that the ORF47 protein was not required for their posttranslational processing (5). Therefore, ORF32 protein is the first VZV protein that has been shown to require ORF47 protein for posttranslational processing in VZV-infected cells.
Calf intestine alkaline phosphatase dephosphorylates proteins containing phosphoserines and phosphothreonines (20). VZV ORF32 is predicted to contain 14 serine and 9 threonine residues. Several of these residues cluster near acidic amino acids, particularly at the carboxy terminus of the protein. A similar clustering of serines and threonines near acidic residues has been noted for HSV ICP22 (15), the substrate of the HSV UL13 protein kinase, which is the homolog of ORF47 protein kinase.
The protein kinases located in the UL region of the herpesvirus genomes are highly conserved (8) and are thought to have regulatory functions that are important for replication of herpesviruses. Moffat and colleagues have shown that VZV replicates in human fetal lymphocytes and causes necrosis of the epidermal and dermal layers of human fetal skin in the SCID-hu mouse (11). Unlike VZV, HSV does not replicate in human fetal lymphocytes and results in necrosis of only the superficial epidermal layer of human skin in the SCID-hu mouse (10). While prior studies with a VZV ORF47 mutant showed that the UL kinase was not required for growth of the virus in cell culture (5), recent experiments using the SCID-hu mouse model indicate that ORF47 protein is required for VZV replication in human fetal lymphocytes and skin in the mouse (9). Since ORF32 does not have a homolog in HSV, the ability of ORF47 to posttranslationally modify ORF32 suggests that ORF32 may play a role in the ability of VZV to replicate to high titers in human fetal lymphocytes and skin. Future experiments testing the ability of the VZV ORF32 deletion mutant to replicate in human tissues implanted into SCID mice may yield new insights into the role of this protein in vivo and the differences in the diseases associated with VZV and HSV.
ACKNOWLEDGMENTS
Sanjay Reddy and Edward Cox contributed equally to this report.
We thank David Alling for assistance with statistics and Stephen Straus for review of the manuscript.
Weily Soong is a Howard Hughes Medical Institute-National Institutes of Health Research Scholar.
REFERENCES
- 1.Cohen J I, Seidel K E. Generation of varicella-zoster virus (VZV) and viral mutants from cosmid DNAs: VZV thymidylate synthetase is not essential for replication in vitro. Proc Natl Acad Sci USA. 1993;90:7376–7380. doi: 10.1073/pnas.90.15.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen J I, Seidel K E. Varicella-zoster virus open reading frame 1 encodes a membrane protein that is dispensable for growth of VZV in vitro. Virology. 1995;206:835–842. doi: 10.1006/viro.1995.1006. [DOI] [PubMed] [Google Scholar]
- 3.Cohen J I, Straus S E. Varicella-zoster virus and its replication. In: Fields B N, Knipe D M, Howley P M, Chanock R M, Melnick J L, Monath T P, Roizman B, Straus S E, editors. Fields virology. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2525–2545. [Google Scholar]
- 4.Davison A J, Scott J E. The complete DNA sequence of varicella-zoster virus. J Gen Virol. 1986;67:1759–1816. doi: 10.1099/0022-1317-67-9-1759. [DOI] [PubMed] [Google Scholar]
- 5.Heineman T C, Cohen J I. The varicella-zoster virus (VZV) open reading frame 47 (ORF47) protein kinase is dispensable for viral replication and is not required for phosphorylation of ORF63 protein, the VZV homolog of herpes simplex virus ICP22. J Virol. 1995;69:7367–7370. doi: 10.1128/jvi.69.11.7367-7370.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heineman T C, Seidel K, Cohen J I. The varicella-zoster virus ORF66 protein induces kinase activity and is dispensable for viral replication. J Virol. 1996;70:7312–7317. doi: 10.1128/jvi.70.10.7312-7317.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kyte J, Doolittle R F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 8.Leader D P. Viral protein kinases and protein phosphatases. Pharmacol Ther. 1993;59:343–389. doi: 10.1016/0163-7258(93)90075-o. [DOI] [PubMed] [Google Scholar]
- 9.Moffat J, Zerboni L, Sommer M, Heineman T, Cohen J, Kaneshima H, Arvin A. Abstracts of the 23rd International Herpesvirus Workshop. York, United Kingdom. 1998. VZV ORF47 protein kinase is required for growth in human T-cells and skin tissues in the SCID-hu mouse, abstr. 181; p. 157. [Google Scholar]
- 10.Moffat J F, Zerboni L, Kinchington P R, Grose C, Kaneshima H, Arvin A M. Attenuation of the vaccine Oka strain of varicella-zoster virus and role of glycoprotein C in alphaherpesvirus virulence demonstrated in the SCID-hu mice. J Virol. 1998;72:965–974. doi: 10.1128/jvi.72.2.965-974.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moffat J F, Stein M D, Kaneshima H, Arvin A M. Tropism of varicella-zoster virus for human CD4+ and CD8+ T lymphocytes and epidermal cells in SCID-hu mice. J Virol. 1995;69:5236–5242. doi: 10.1128/jvi.69.9.5236-5242.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ng T I, Grose C. Serine protein kinase associated with varicella-zoster virus ORF 47. Virology. 1992;191:9–18. doi: 10.1016/0042-6822(92)90161-h. [DOI] [PubMed] [Google Scholar]
- 13.Ng T I, Keenan L, Kinchington P R, Grose C. Phosphorylation of varicella-zoster virus open reading frame (ORF) 62 regulatory product by viral ORF 47-associated protein kinase. J Virol. 1994;68:1350–1359. doi: 10.1128/jvi.68.3.1350-1359.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicholas J. Determination and analysis of the complete nucleotide sequence of human herpesvirus 7. J Virol. 1996;70:5975–5989. doi: 10.1128/jvi.70.9.5975-5989.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Purves F C, Roizman B. The UL13 gene of herpes simplex virus 1 encodes the functions for posttranslational processing associated with phosphorylation of the regulatory protein α22. Proc Natl Acad Sci USA. 1992;89:7310–7314. doi: 10.1073/pnas.89.16.7310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Purves F C, Ogle W O, Roizman B. Processing of the herpes simplex virus regulatory protein α22 mediated by the UL13 protein kinase determines the accumulation of a subset of α and γ mRNAs and proteins in infected cells. Proc Natl Acad Sci USA. 1993;90:6701–6705. doi: 10.1073/pnas.90.14.6701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reddy, S. M., E. Cox, I. Iofin, W. Soong, and J. I. Cohen. Unpublished data.
- 18.Reinhold W C, Straus S E, Ostrove J M. Directionality and further mapping of varicella-zoster virus transcripts. Virus Res. 1988;9:249–261. doi: 10.1016/0168-1702(88)90034-2. [DOI] [PubMed] [Google Scholar]
- 19.Russo J J, Bohenzky R A, Chien M-C, Chen J, Yan M, Maddalena D, Parry J P, Peruzzi D, Edelman I S, Chang Y, Moore P S. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus. Proc Natl Acad Sci USA. 1996;93:14862–14867. doi: 10.1073/pnas.93.25.14862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shenolikar S. Detection of phosphorylation by enzymatic techniques. In: Coligan J E, Dunn B M, Ploegh H L, Speicher D W, Wingfield P T, editors. Current protocols in protein science. New York, N.Y: John Wiley & Sons, Inc.; 1995. pp. 13.5.1–13.5.8. [Google Scholar]
- 21.Stevenson D, Colman K L, Davison A J. Characterization of the putative protein kinases specified by varicella-zoster virus genes 47 and 66. J Gen Virol. 1994;75:317–326. doi: 10.1099/0022-1317-75-2-317. [DOI] [PubMed] [Google Scholar]
- 22.Telford E A R, Watson M S, Perry J, Cullinane A A, Davison A J. The DNA sequence of equine herpesvirus-4. J Gen Virol. 1998;79:1197–1203. doi: 10.1099/0022-1317-79-5-1197. [DOI] [PubMed] [Google Scholar]
- 23.Telford E A R, Watson M S, McBride K, Davison A J. The DNA sequence of equine herpesvirus-1. Virology. 1992;189:304–316. doi: 10.1016/0042-6822(92)90706-u. [DOI] [PubMed] [Google Scholar]
- 24.Yao F, Schaffer P A. An activity specified by the osteosarcoma line U2OS can substitute functionally for ICP0, a major regulatory protein of herpes simplex virus type 1. J Virol. 1995;69:6249–6258. doi: 10.1128/jvi.69.10.6249-6258.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yao Z, Jackson W, Grose C. Identification of the phosphorylation sequence in the cytoplasmic tail of the varicella-zoster virus Fc receptor glycoprotein gpI. J Virol. 1993;67:4464–4473. doi: 10.1128/jvi.67.8.4464-4473.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]