Abstract
Background
Visual acuity and image stability are crucial for daily activities, particularly during head motion. The vestibulo-ocular reflex (VOR) and its suppression (VORS) support stable fixation of objects of interest. The VOR drives a reflexive eye movement to counter retinal slip of a stable target during head motion. In contrast, VORS inhibits this countermovement when the target stimulus is in motion. The VORS allows for object fixation when it aligns with the direction of the head’s movement, or when an object within or outside the peripheral vision needs to be focused upon.
Summary
Deficits of the VORS have been linked to age-related diseases such as balance deficits associated with an increased fall risk. Therefore, the accurate assessment of the VORS is of particular clinical relevance. However, current clinical assessment methods for VORS are mainly qualitative and not sufficiently standardised. Recent advances in digital health technology, such as smartphone-based videooculography, offer a promising alternative for assessing VORS in a more accessible, efficient, and quantitative manner. Moreover, integrating mobile eye-tracking technology with virtual reality environments allows for the implementation of controlled VORS assessments with different visual inputs. These assessment approaches allow the extraction of novel parameters with potential pathomechanistic and clinical relevance.
Key Messages
We argue that researchers and clinicians can obtain a more nuanced understanding of this ocular stabilisation reflex and its associated pathologies by harnessing digital health technology for VORS assessment. Further research is warranted to explore the technologies’ full potential and utility in clinical practice.
Keywords: Vestibulo-ocular reflex suppression, Vestibulo-ocular reflex, Virtual reality, Parkinson’s disease
Introduction
We rely heavily on sharp vision in our daily lives, especially when we move. When we turn our heads, the vestibulo-ocular reflex (VOR) causes our eyes to move at the same speed in the direction opposite to the head so that the image on the retina remains stable [1]. Conversely, VOR suppression (VORS) coordinates head-eye movements by inhibiting the VOR, aiding in tracking moving objects over large distances, like a ball during watching a tennis match [2]. During a gaze shift, the VOR and VORS interact: in the initial phase of a gaze shift, the VORS is higher than at the end. In the initial phase of gaze shift, the head and eyes usually move in the same direction, and the VOR must be suppressed. If the object is fixed with a rapid eye movement, the head moves, and the VOR gains use and requires less suppression (online suppl. Video 1; for all online suppl. material, see https://doi.org/10.1159/000537842) [3].
The VOR functions are based on a “3-neuron-arc” [1, 4, 5]. The Scarpa ganglion receives signals from the vestibular organ [6], which projects to the vestibular nucleus [7] and then to the nuclei of the oculomotor neurons (III, IV, and VI) [8]. The neuronal network of the VORS is not fully understood. However, the flocculus region of the cerebellum likely plays a key role [9]. The velocity gain serves as a well-established objective measure for assessing the VOR. It is calculated by dividing the speed of the eyes by the speed of the head. In an optimal scenario, the velocity gain attains an absolute value of 1.
A growing body of research suggests that the function of the VORS is relevant for daily life, worsens with ageing and age-associated diseases, and is closely and frequently associated with balance deficits and falls [10–13]. For example, a longitudinal observational study with 53 participants over 9 years found a positive correlation between the deterioration of VORS and Tinetti Balance Test performance [14]. Numerous studies point towards an association of reduced VORS and fall risk, underscoring these findings’ clinical relevance. For example, VORS reduction was more pronounced in elderly women with increased fall risk compared to those without increased fall risk [13]. Reduced VORS (defined as absolute value of velocity gain value during fixation >0.55) in older women was associated with an 18-fold increased risk of falls [15]. This association between reduced VORS and balance deficits and fall risk becomes particularly interesting in the light of already demonstrated associations between reduced VORS and “fall-prone conditions,” such as old age [10], Parkinson’s disease (PD) [16, 17], and progressive supranuclear palsy (PSP). The literature on VORS in PD is inconsistent as to whether PD is generally associated with reduced VORS [16, 18, 19]. A recent study by our group shows VORS deficits in PD patients. Moreover, data from this study [2] suggest that a reduction in horizontal VORS in persons with PD (PwPD) substantially increases future fall risk [17]. We investigated VORS with an eye camera in 19 PwPD, 16 persons with PSP, and 19 older adults when performing sinusoidal movements on a swivel chair. Both PwPD and persons with PSP had reduced VORS. However, only in PwPD, the horizontal VORS correlated with future falls over an observation period of 12 months, significantly and negatively, with a Spearman’s rho of 0.72 [17]. Thus, the VORS could also be suitable as a predictor for falls. Therefore, this viewpoint will focus on assessing VORS and how digital technologies can potentially help extract additional information from this assessment.
What Are the Current Assessment Principles of VORS?
In the current clinical routine and neurological examination, the VORS is, if at all, evaluated qualitatively. The examiner asks the patient to stretch the arms straight forward, fold the hands into each other, stretch the thumbs up, and fix the fingernails of the thumbs with the eyes. Then, the examiner observes the patient’s eyes, rarely with technical assistance, while turning the patient’s torso and head alternately to the left and right. In the case of reduced VORS, clinicians see a slow eye movement in the opposite direction to the body, followed by rapid correction saccades in the direction of the patient’s movement (shown in Fig. 1; online suppl. Video 2). These saccades may reach the fixation target precisely (exact) but may be insufficient in amplitude (hypometric) or exceed the fixation target (hypermetric) [20].
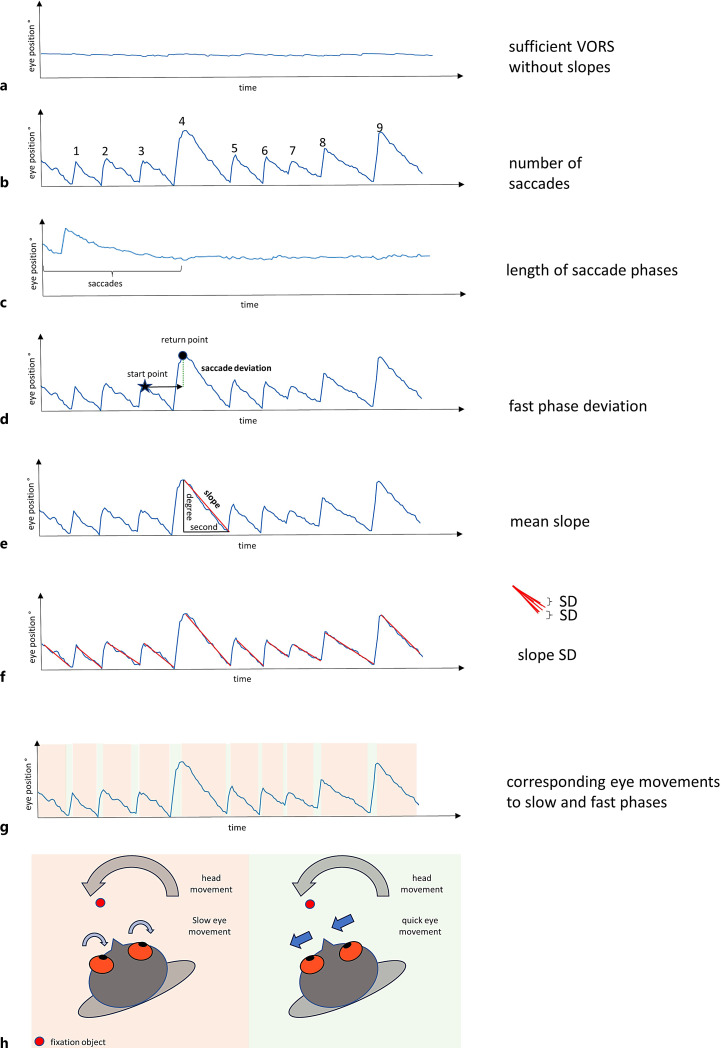
Fig. 1.
Potentially relevant vestibulo-ocular reflex suppression (VORS) parameters could be extracted with digital health technology, e.g., an eye tracker with VR. The blue line represents the eye movement in relation to the head. a The person fixes a point (e.g., a head-fixed target or a dot at the centre of the field of view in a VR setting), and the low movements express a successfully suppressed VOR. b–f show pathological eye movements (VORS deficits). b describes the number of saccades observed during a particular time phase. c describes the length of the phases with saccades in relation to the length of the whole examination. d describes how precisely the position before the start of the slow phase (start point) is reached by the subsequent fast phase. The position reached by the fast phase following the slow phase (return point) is determined, and the deviation of the start point from the return point can be calculated as fast phase deviation. e, f show how the mean and standard deviation (slope SD) from the mean could be calculated from the slow phase preceding the saccades. g, h show the eye movements caused by the slow (red box) and fast phases (green box) using the curves and a model.
Current State of Eye Movement Quantification with a Focus on VORS
Electrooculography [13, 15], scleral search coils [3, 9, 11, 12, 21, 22], and videooculography [2, 17, 23–25] have been used to determine the VORS quantitatively in research. Electrooculography measures corneoretinal potentials with electrodes usually placed on the inner and outer canthi medial and lateral to the eyes. Scleral search coils provide information about the eye position using a magnetic field that is generated through eye movement. The coils are usually placed on the episcleral region of the participant’s eye. Videooculography measures the position and movement of the eyes with high-resolution infrared cameras in front of the patient (e.g., embedded in glasses). A comparison of videooculography with search coils yielded high agreement between these methods [26]. Table 1 gives a non-exhaustive overview of studies using these methods to assess the VORS and the main results.
Table 1.
Studies using electrooculography, search coils, or videooculography to measure VORS
| Study | Cohorts investigated | Device used to assess VORS | Parameters analysed | Main results |
|---|---|---|---|---|
| Baloh et al. [10] (1993) | Healthy young and old adults | Electrooculography | Velocity gain | VORS is less effective in the old compared to the young cohort |
| Paige [11] (1994) | Healthy young and old adults | Search coil | Velocity gain | VORS is reduced at higher frequencies and less effective in the old compared to the young cohort |
| Demer [12] (1994) | Healthy young and old adults | Search coil | Velocity gain | VORS is less effective in the old compared to the young cohort |
| Belton et al. [9] (2000) | Rhesus monkeys | Search coil | Velocity gain | Cerebellar flocculus is involved in generation of VORS |
| Di Fabio et al. [13] (2001) | Healthy old adults | Electrooculography | Eye-trunk velocity slope | VORS is associated with an increased risk of falling |
| Di Fabio et al. [15] (2002) | Healthy old adults | Electrooculography | Velocity gain | VORS is associated with an increased risk of falling |
| Cullen et al. [22] (2004) | Macaque monkeys | Search coil | Velocity gain | VORS is reduced during gaze shifts, which is important for eye-head coordination |
| Barnes et al. [23] (2004) | Healthy adults | Videooculography | Velocity gain | VORS also occurs with imagined head-fixed targets |
| Kerber et al. [14] (2006) | Older adults with and without dizziness | Electrooculography | Velocity gain | VORS is associated with reduced balance and gait |
| Jacobson et al. [24] (2012) | Healthy adults | Infrared videooculography | Velocity gain | VORS also occurs with nonvisual stimuli |
| Daye et al. [3] (2015) | Healthy adults | Search coil | Position difference | VORS occurs during saccades |
| Johnston et al. [21] (2017) | Healthy adults, patients with neck pain | Search coil | Velocity gain | VORS is increased in patients with neck pain |
| Meyer [17] (2020) | Healthy adults, patients with PD, patients with PSP | Videooculography | Position gain, lag-corrected VORS score | VORS correlates positively with future falls in PD patients |
| Gandor et al. [25] (2020) | Healthy adults, patients with cerebellar disorders, patients with MSA | Videooculography, smartphone | Maximum slow phase peak velocity | VORS assessments with smartphones and videooculography show comparable similar results |
MSA, multiple system atrophy; PD, Parkinson’s disease; PSP, progressive supranuclear palsy; VORS, vestibulo-ocular reflex suppression.
The disadvantage of these methods is that they are time consuming, relatively complex to administer, and depend on a stationary system. Therefore, first attempts were made to use modern digital health technology [27] to measure eye movement. For example, we turned 20 healthy subjects (age 56 ± 15 years) and 19 patients with cerebellar disorders (age 70 ± 11 years) to the left and right on a swivel chair and filmed the eye movement using a smartphone to determine the VORS. These videos were then presented to experienced blinded investigators, who had to judge the VORS as either reduced or normal. The sensitivity of the video ratings to detect an impaired VORS was 99%, and its specificity was 92% [25]. Absolute values were compared with videooculography. In detail, saccades under visual suppression were compared with saccades in darkness by analysing nystagmus beats (reduction of 95 ± 7% in healthy controls and 26 ± 25% in patients with cerebellar disorders) and maximum slow phase velocity (reduction of 93 ± 8% in healthy controls and 42 ± 28% in patients with cerebellar disorders). Recent evidence furthermore suggests that smartphone technology is capable of precisely tracking and quantifying eye movements using extracted data [28, 29] and advances in artificial intelligence [29–31]. This raises the hope that consumer grade digital equipment can enable VORS evaluation with an acceptable level of accuracy.
Digital measurement methods can also capture aspects that could potentially impact the VORS but were previously not assessed under standardised conditions, such as background [13–15, 24]. For example, VOR and optokinetic nystagmus performance (a VOR-related eye movement caused by moving elements in front of the eyes) have been shown to be higher in conditions with a visual background than in darkness [32, 33]. It is tempting to speculate that this holds also true for the VORS, although we are not aware of any study that investigated this association. Digital measurements also allow a new dimension of eye movement assessment. For example, with the help of virtual reality (VR) glasses, the examiner can present a fixed dot in the centre of the field of vision, which the patient fixes while turning to the left and right. This approach can replace the head-fixed target approach currently often applied to assess the VORS. As a next level, moving backgrounds can be added.
How Has VORS Been Evaluated So Far, and What Can Digital Parameters Deliver?
The quantitative evaluation of the VORS is not yet standardised. For example, the velocity gain both with and without fixation of the eyes can be used to assess VORS performance. A lower velocity gain during fixation expressing VORS can be quantified [24]. Other options to assess the VORS are the evaluation of the position gain and the lag-corrected VORS gain [17]. Moreover, VORS assessment has been done during gaze shifts to determine the most pronounced VORS phases during these shifts [3].
The parameters used to date to capture the VORS are not only poorly standardised but also do not represent the full range of VORS performance. Novel eye tracking systems, ideally in combination with VR, will bring together the advantages of mobile, easy, and widely applicable assessment and detailed quantitative analysis of this important eye movement. Figure 1 gives an overview of potential new parameters for such analysis approaches based on example eye data we collected from a VR measurement, and we discuss in the next section why these parameters may be of interest.
Why Are Quantitative VORS Parameters, as They Can Be Measured with Digital Health Technology, Clinically Interesting?
We argue that the quantitative VORS parameters presented in Figure 1 could potentially provide clinical and daily relevant information for several reasons. The parameters a, b, and c in Figure 1 can provide information on whether VORS deficits are present and how “severe” they are. This reflects clinical examination practice and has been recently proven feasible [25].
The proportion of time in which fast and slow phases of VORS deficit-defining saccades occur (Fig. 1c) could be an expression of how pronounced the VORS deficit is or how well the person can suppress or “tame” the deficit. A low proportion of time in which saccades occur may indicate a low VORS deficit and a high proportion of time in which saccades occur may indicate a high VORS deficit.
Comparable amplitudes of successive saccades may indicate an adequate compensation of the VORS deficit because the counter-regulation always starts at similar times after the eyes have drifted away. Vice versa, different amplitudes between successive saccades could indicate either irregular “slipping away” or irregular/inaccurate correction with undershooting and/or overshooting counter-saccades. One way to objectify this deviation of the saccades is to compare the eye positions between the end positions of the two fast phases and determine a deviation (Fig. 1d). We argue that the evaluation of the fast component of VORS deficit-related saccades, in particular, has excellent potential to improve our understanding of the interaction of VORS deficit and mobility impairment as changes in saccade latency have already been demonstrated in PD patients with increased risk of falling, which have an increased latency of saccades, compared to PD patients without increased fall risk [34], and in PD patients, compared to healthy adults, during turns [35].
It is also conceivable that the mean velocity gain of the slow phase of the saccade due to a VORS deficit (Fig. 1e) has clinical relevance [24]. An increased velocity gain in the suppressed state could indicate that the person cannot keep a fixed target in the centre of the field of vision (even unintentionally). It is known from other forms of nystagmus that the frequency of nystagmus, which also results from the slow phase, is variable and can provide information about the severity of the disease [36, 37]. Furthermore, increased variability of this velocity gain (Fig. 1f) could provide pathomechanistically relevant information about the association between a VORS deficit and mobility deficits. A variability model already exists for the VOR, in which differences from trial to trial are explained by neuronal noise due to, e.g., the influence of other sensory systems [38]. So could the variability of the VORS be an expression of the ability to respond to, and compensate for, varying strengths of VOR signals.
Conclusion and Outlook
Recent advancements in digital technology, particularly mobile health technologies, offer new opportunities to assess VORS. Smartphone-based videooculography has shown promising results, with high sensitivity and specificity in detecting impaired VORS. Furthermore, VR and eye-tracking glasses provide a more standardised and versatile mobile approach for VORS assessment. These systems allow for different visual inputs, including various backgrounds, which can enhance the outcome of the evaluation and provide a more detailed analysis of VORS. This novel approach to VORS assessment combines the advantages of mobile, easy-to-use technology with quantitative analysis, enabling widespread application and detailed evaluation of this important eye movement impairment, considering entirely novel parameters that can be extracted from these data. In our view, the imminent implementation of such mobile digital health technology in medical research has the potential to enhance clinical evaluations, improve diagnostics, and facilitate targeted interventions for individuals with VORS deficits.
Acknowledgments
ChatGPT (version 3.5) has been used for language optimisation. We acknowledge financial support by Land Schleswig-Holstein within the funding programme Open Access Publikationsfonds.
Conflict of Interest Statement
Florin Gandor reports honoraria from AbbVie Pharma, Merz Pharma, BIAL Pharma, and Stada Pharma outside the submitted work. Patrik Theodor Nerdal reports being a participant at workshop supported by Natus.
Funding Sources
The authors declare that there was no financial support for this work.
Author Contributions
Patrik Theodor Nerdal drafted the manuscript and developed the illustrations. Walter Maetzler supervised the process and revised the manuscript. Florin Gandor, Maximilian Uwe Friedrich, Laurin Schappe, and Georg Ebersbach revised the manuscript.
Funding Statement
The authors declare that there was no financial support for this work.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
References
- 1. Bronstein AM, Patel M, Arshad Q. A brief review of the clinical anatomy of the vestibular-ocular connections: how much do we know? Eye. 2015;29(2):163–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Srulijes K, Mack DJ, Klenk J, Schwickert L, Ihlen EAF, Schwenk M, et al. Association between vestibulo-ocular reflex suppression, balance, gait, and fall risk in ageing and neurodegenerative disease: protocol of a one-year prospective follow-up study. BMC Neurol. 2015;15(1):192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Daye PM, Roberts DC, Zee DS, Optican LM. Vestibulo-ocular reflex suppression during head-fixed saccades reveals gaze feedback control. J Neurosci. 2015;35(3):1192–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cohen B. The vestibulo-ocular reflex arc. In: Kornhuber HH, editor. Vestibular system Part 1: basic mechanisms. Heidelberg: Springer-Verlag; 1974. Vol. 1; p. 477–540. [Google Scholar]
- 5. Halmagyi GM, Curthoys IS. A clinical sign of canal paresis. Arch Neurol. 1988;45(7):737–9. [DOI] [PubMed] [Google Scholar]
- 6. Travo C, Gaboyard-Niay S, Chabbert C. Plasticity of Scarpa’s ganglion neurons as a possible basis for functional restoration within vestibular endorgans. Front Neurol. 2012;3:3–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wijesinghe R, Camp A. The intrinsic plasticity of medial vestibular nucleus neurons during vestibular compensation: a systematic review and meta-analysis. Syst Rev. 2020;9(1):145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jang S, Lee M, Yeo S, Kwon H. Structural neural connectivity of the vestibular nuclei in the human brain: a diffusion tensor imagingS study. Neural Regen Res. 2018;13(4):727–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Belton T, Mccrea RA, Role RAM. Role of the cerebellar flocculus region in cancellation of the VOR during passive whole body rotation. J Neurophysiol. 2000;84(3):1599–613. [DOI] [PubMed] [Google Scholar]
- 10. Baloh RW, Jacobson KM, Socotch TM. The effect of aging on visual-vestibuloocular responses. Exp Brain Res. 1993;95(3):509–16. [DOI] [PubMed] [Google Scholar]
- 11. Paige GD. Senescence of human visual-vestibular interactions: smooth pursuit, optokinetic, and vestibular control of eye movements with aging. Exp Brain Res. 1994;98(2):355–72. [DOI] [PubMed] [Google Scholar]
- 12. Demer JL. Effect of aging on vertical visual tracking and visual-vestibular interaction. J Vestib Res. 1994;4(5):355–70. [PubMed] [Google Scholar]
- 13. Di Fabio RP, Emasithi A, Greany JF, Paul S. Suppression of the vertical vestibulo-ocular reflex in older persons at risk of falling. Acta Otolaryngol. 2001;121(6):707–14. [DOI] [PubMed] [Google Scholar]
- 14. Kerber KA, Ishiyama GP, Baloh RW. A longitudinal study of oculomotor function in normal older people. Neurobiol Aging. 2006;27(9):1346–53. [DOI] [PubMed] [Google Scholar]
- 15. Di Fabio RP, Greany JF, Emasithi A, Wyman JF. Eye-head coordination during postural perturbation as a predictor of falls in community-dwelling elderly women. Arch Phys Med Rehabil. 2002;83(7):942–51. [DOI] [PubMed] [Google Scholar]
- 16. Rascol O, Clanet M, Montastruc JL, Simonetta M, Soulier-Esteve MJ, Doyon B, et al. Abnormal ocular movements in Parkinson’s disease. Evidence for involvement of dopaminergic systems. Brain. 1989;112(Pt 5):1193–214. [DOI] [PubMed] [Google Scholar]
- 17. Meyer M. Assoziation des vestibulo-okulären Reflexes und dessen Suppression mit Sturzrisiko bei älteren Personen und neurodegenerativ erkrankten Personen. MD thesis. Tübingen: University of Tübingen; 2020. [Google Scholar]
- 18. Rascol O, Sabatini U, Fabre N, Senard JM, Simonetta-Moreau M, Montastruc JL, et al. Abnormal vestibuloocular reflex cancellation in multiple system atrophy and progressive supranuclear palsy but not in Parkinson’s disease. Mov Disord. 1995;10(2):163–70. [DOI] [PubMed] [Google Scholar]
- 19. Rascol OJ, Clanet M, Senard JM, Montastruc JL, Rascol A. Vestibulo-ocular reflex in Parkinson’s disease and multiple system atrophy. Adv Neurol. 1993;60:395–7. [PubMed] [Google Scholar]
- 20. Termsarasab P, Thammongkolchai T, Rucker JC, Frucht SJ. The diagnostic value of saccades in movement disorder patients: a practical guide and review. J Clin Mov Disord. 2015;2(1):14–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Johnston JL, Daye PM, Thomson GTD. Inaccurate saccades and enhanced vestibulo-ocular reflex suppression during combined eye-head movements in patients with chronic neck pain: possible implications for cervical vertigo. Front Neurol. 2017;8(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cullen KE, Huterer M, Braidwood DA, Sylvestre PA. Time course of vestibuloocular reflex suppression during gaze shifts. J Neurophysiol. 2004;92(6):3408–22. [DOI] [PubMed] [Google Scholar]
- 23. Barnes GR, Paige GD. Anticipatory VOR suppression induced by visual and nonvisual stimuli in humans. J Neurophysiol. 2004;92(3):1501–11. [DOI] [PubMed] [Google Scholar]
- 24. Jacobson GP, Piker EG, Do C, Mccaslin DL, Hood L. Suppression of the vestibulo-ocular reflex using visual and nonvisual stimuli. Am J Audiol. 2012;21(2):226–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gandor F, Tesch M, Neuhauser H, Gruber D, Heinze HJ, Ebersbach G, et al. Diagnostic accuracy of a smartphone bedside test to assess the fixation suppression of the vestibulo-ocular reflex: when nothing else matters. J Neurol. 2020;267(7):2159–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Agrawal Y, Schubert MC, Migliaccio AA, Zee DS, Schneider E, Lehnen N, et al. Evaluation of quantitative head impulse testing using search coils versus video-oculography in older individuals. Otol Neurotol. 2014;35(2):283–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vasudevan S, Saha A, Tarver ME, Patel B. Digital biomarkers: convergence of digital health technologies and biomarkers. NPJ Digit Med. 2022;5(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Azami H, Chang Z, Arnold SE, Sapiro G, Gupta AS. Detection of oculomotor dysmetria from mobile phone video of the horizontal saccades task using signal processing and machine learning approaches. IEEE Access. 2022;10:34022–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Friedrich MU, Schneider E, Buerklein M, Taeger J, Hartig J, Volkmann J, et al. Smartphone video nystagmography using convolutional neural networks: ConVNG. J Neurol. 2023;270(5):2518–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Parker TM, Farrell N, Otero-Millan J, Kheradmand A, Mcclenney A, Newman-Toker DE. Proof of concept for an “eyePhone” app to measure video head impulses. Digit Biomark. 2021;5(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yiu YH, Aboulatta M, Raiser T, Ophey L, Flanagin VL, zu Eulenburg P, et al. DeepVOG: open-source pupil segmentation and gaze estimation in neuroscience using deep learning. J Neurosci Methods. 2019;324:108307. [DOI] [PubMed] [Google Scholar]
- 32. Crognale MA, Schor CM. Contribution of chromatic mechanisms to the production of small-field optokinetic nystagmus (OKN) in normals and strabismics. Vis Res. 1996;36(11):1687–98. [DOI] [PubMed] [Google Scholar]
- 33. Waespe W, Henn V. Neuronal activity in the vestibular nuclei of the alert monkey during vestibular and optokinetic stimulation. Brain Res. 1977;27(5):523–38. [DOI] [PubMed] [Google Scholar]
- 34. Nemanich ST, Earhart GM. Freezing of gait is associated with increased saccade latency and variability in Parkinson’s disease. Clin Neurophysiol. 2016;127(6):2394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ambati VNP, Saucedo F, Murray NG, Powell DW, Reed-Jones RJ. Constraining eye movement in individuals with Parkinson’s disease during walking turns. Exp Brain Res. 2016;234(10):2957–65. [DOI] [PubMed] [Google Scholar]
- 36. Dietrich H, Pradhan C, Heidger F, Schniepp R, Wuehr M. Downbeat nystagmus becomes attenuated during walking compared to standing. J Neurol. 2022;269(12):6222–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Young AS, Lechner C, Bradshaw AP, MacDougall HG, Black DA, Halmagyi GM, et al. Capturing acute vertigo: a vestibular event monitor. Neurology. 2019;92(24):e2743–53. [DOI] [PubMed] [Google Scholar]
- 38. Nouri S, Karmali F. Variability in the vestibulo-ocular reflex and vestibular perception. Neuroscience. 2018;393:350–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.