Abstract
Background:
Patients with irritable bowel syndrome (IBS) show lower resilience than healthy controls (HCs), associated with greater symptom severity and worse quality of life. However, little is known about affected markers of resilience or the influence of sex. Furthermore, as resilience is complex, a comprehensive assessment, with multiple resilience measures, is needed. Therefore, we aimed to evaluate perceived and relative resilience and their neural correlates in men and women with IBS.
Methods:
In 402 individuals (232 IBS [73.3% women] and 170 HCs [61.2% women]), perceived resilience was assessed by the Connor-Davidson Resilience Scale (CDRISC) and Brief Resilience Scale (BRS); relative resilience was assessed by the standardized residual of the Short Form-12 mental component summary score predicted by the Adverse Childhood Experiences score. Non-rotated partial least squares analysis of region-to-region resting-state connectivity data was used to define resilience-related signatures in HCs. Disease and sex-related differences within these signatures were investigated.
Key Results:
Scores on all resilience measures were lower in IBS than in HCs (p’s<0.05). In all three resilience-related signatures, patients with IBS showed reduced connectivity largely involving the central autonomic network (p’s<0.001). Men with IBS showed lower CDRISC scores than women with IBS, and greater reductions in CDRISC-related connectivity, associated with worse symptom severity (p<0.05).
Conclusions & Inferences:
Individuals with IBS show reduced perceived and relative resilience, with reduced connectivity suggesting impaired homeostasis maintenance. Men with IBS may show additional impairment in specific aspects of resilience. Treatments aimed at improving resilience may benefit patients with IBS, especially men with IBS.
Keywords: functional magnetic resonance imaging, irritable bowel syndrome, psychological resilience, sex differences
Graphical Abstract
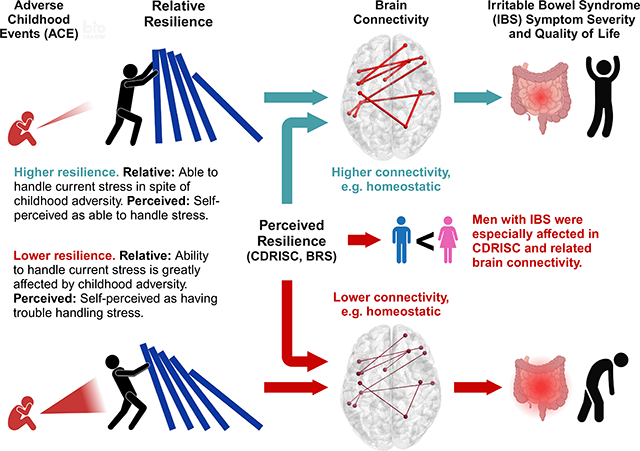
Compared to healthy controls, individuals with IBS show reduced self-perceived resilience, relative resilience (ability to positively handle stress relative to a history of childhood adversity), and resilience-related brain connectivity, which is associated with worse symptom severity and quality of life. Some findings were stronger in men than women with IBS.
1. Introduction
Irritable bowel syndrome (IBS) is a female predominant, stress-sensitive disorder of gut brain interactions, characterized by abdominal pain and alterations in stool form and/or frequency.1,2 Stress plays an important role in IBS, as stressful life events in early life and adulthood increase the odds of having IBS and stress is a common trigger of IBS symptom flares.3,4 Several studies have shown lower resilience, which is the ability to recover and adapt positively to stress and adversity, in patients with IBS compared to healthy controls (HCs).3–5 Reduced resilience in IBS is associated with an enhanced cortisol response, greater symptom severity, worse health-related quality of life (QOL), and compromised corticolimbic inhibition.3,4 Further, patients with IBS show alterations in major resting-state brain networks, including executive function, salience, and emotion regulation networks, contributing to increased responsiveness to sensory stimuli and stressors.6 Treatments aimed at improving resilience may benefit patients with IBS.
Resilience is a dynamic process that is influenced in part by genetic sex, gonadal steroids, and epigenetic regulation of stress response.7 Chronic stress, including adverse childhood experiences, can increase the vulnerability of developing chronic, stress-related illness and negative health behaviors.8 Behavioral and intervention studies that reduce stress and improve coping have indicated the importance of executive functions (especially those relating to self-control and cognitive reappraisal), emotional regulation (the ability to modulate and process emotions), and autonomic function (homeostasis) in resilience.9–11 Consistent with this, neuroimaging studies have implicated alterations within the neural networks associated with the central executive network (CEN),12 inferior frontal and cingulate regions13, prefrontal control regions,14–16 and the central autonomic network (CAN)17 in resilience.
As resilience is a complex entity, different methods to assess resilience have been developed, each with its own strengths and limitations.18 The Connor-Davidson Resilience Scale (CDRISC) and Brief Resilience Scale (BRS) are considered measures of perceived resilience, as these questionnaires focus on perceptions of resiliency rather than manifested resilience.19,20 However, the CDRISC and BRS differ in complexity, with the BRS solely focused on ‘bounce-back ability’, while the CDRISC, as a multi-dimensional scale, focuses on internal and external resources that aid in recovery from stress. Another type of resilience is relative resilience, which attempts to assess manifested resilience by considering the level of positive functioning relative to the adversity burden.18 Research confirms that these different measures of resilience and scales capture different aspects of resilience important to wellbeing.18,21 One strategy to deal with this heterogeneity in resilience assessment is to use multiple resilience measures, rather than a single measure, enabling a more comprehensive picture of the specific type and neural correlates of resilience affected in IBS.
Further, sex should be considered an important variable in resilience research, as it influences the impact of stressors,7,22 types of adversity experienced,23 and neural correlates of resilience-related factors and processes.24,25 Additionally, there are well-known sex differences in IBS (e.g. it is a female-predominant disorder with sex differences in symptom presentation and response to treatment).26,27 Despite this, the potential influence of sex on resilience in IBS has not been well studied.
Therefore, this study aimed to investigate perceived and relative resilience and their neural correlates, as well as their relationships with symptom severity and disease-specific QOL, in individuals with IBS, with an evaluation of the influence of sex. We hypothesized that the CAN, CEN, and emotion-regulation network (ERN) would be commonly involved in perceived and relative resilience in HCs, and that individuals with IBS would show reduced resilience-related connectivity involving these networks, associated with worse symptom severity and disease-specific QOL, potentially with sex differences.
2. Materials and Methods
2.1. Participants
Participants were comprised of men and women with IBS and HCs (aged 18–60 years) who completed a resting-state magnetic resonance imaging scan and questionnaires in clinical research studies conducted at the G. Oppenheimer Center for Neurobiology of Stress and Resilience at the University of California, Los Angeles (UCLA) from 2012 to 2019. All participants with IBS were evaluated by a clinician with expertise in IBS to determine fulfillment of Rome III or IV diagnostic criteria, depending on the time of recruitment.2,28 All bowel habit types were included. HCs were comprised of healthy individuals without a history of IBS. The following exclusion criteria were applied to both HCs and participants with IBS: eating disorders; gastric, abdominal, or colon surgery; diabetes; major organ (kidney, heart, lung) disease or neurological condition (traumatic brain injury, seizures); cancer; major surgery within 6 months of scanning; alcohol or drug misuse; impaired ability to follow instructions; clinical trial participation within 4 weeks; MRI contraindications; excessive physical exercise; and ongoing major psychiatric diagnoses.
De-identified data were obtained from studies approved by the UCLA Institutional Review Board (Nos. 11–002604, 16–000187, 11–002828, 16–000187, 10–001515, 11–000069, 15–001591, 12–001802). Written informed consent was obtained from all participants.
2.2. Questionnaire Assessments
Perceived resilience was assessed using two common resilience questionnaires, the CDRISC and BRS, which differ in complexity. The CDRISC is a 25-item, multi-dimensional survey focused on internal and external resources that aid in recovery from stress.19 The CDRISC has 5 domains, comprising persistence, emotional-cognitive, adaptability, control-meaning, and meaning.19 The total CDRISC score ranges 0–100, with higher scores indicating higher resilience. The BRS is a 6-item unidimensional survey solely focused on the ability to “bounce back” from stress and adversity.20 The total BRS score ranges from 0–30, with higher scores indicating higher resilience in terms of bounce-back ability.
Relative resilience was assessed as the standardized residual of the Short Form-12 (SF-12) mental component score (MCS) predicted by the Adverse Childhood Experiences (ACE) total score.29,30 Positive scores indicate better than expected mental health functioning (MCS score) given the adversity burden (ACE score). The SF-12 is a widely used, 12-item generic health-related QOL survey, with two summary scores; of these, the MCS is commonly used as an indicator of psychological functioning, with lower scores indicating worse mental wellbeing.29 The ACE is an 18-item survey on early life adverse advents (sexual, physical and emotional abuse, household substance abuse, parental separation or divorce, mental illness in household, incarcerated household member, and parent treated violently) occurring before the age of 18 years.30 In the present dataset, MCS was negatively correlated with the ACE total score (r=−0.20, p<0.001), i.e., worse mental wellbeing was associated with a higher number of adverse childhood experiences. Therefore, it was considered appropriate for the assessment of relative resilience. Higher relative resilience scores indicate greater resilience.
All participants completed the Hospital Anxiety and Depression (HAD) scale, which assesses anxiety and depressive symptoms in the prior month; higher scores indicate greater anxiety/depression symptoms.31 Participants with IBS also completed validated questionnaires for IBS, the IBS-QOL and IBS Symptom Severity Scale (IBS-SSS).32,33 The IBS-QOL assesses disease-specific QOL in the prior month; scores range 1–100, with higher scores indicating less disruption of life due to IBS.32 The IBS-SSS assesses the severity and frequency of IBS symptoms, including abdominal pain and stool pattern 33. IBS-SSS scores range 1–500; higher scores indicate worse symptom severity, with scores 175–300 considered as moderate and >300 as severe.
2.3. Neuroimaging
Participants completed a resting-state scan (10-min in duration; repetition time, 2000 ms; echo time, 28 ms; flip angle, 9°; voxel size, 3.4×3.4×4.0 mm), collected with eyes closed, on a 3T scanner (Siemens, Erlangen, Germany). A T1-weighted scan was also completed, using various acquisition protocols (Supplementary Table S1). Images underwent quality control by visual inspection and MRIQC.34 Images were preprocessed using fMRIprep.35 Briefly, resting-state images were aligned to the T1-weighted image, normalized to MNI space, and denoised by regressing out noise components on Automatic Removal of Motion Artifacts (AROMA)-independent component analysis (ICA), as well as white/cerebrospinal fluid signals (orthogonalized to ICA components).36 Subsequently, denoised images were parcellated into 179 cortical and subcortical regions based on the Destrieux and Harvard-Oxford atlases.37 Each region was assigned to one of nine networks based on previous literature on the key nodes of major networks (Supplementary Table S2). Pairwise region-to-region connectivity was calculated as the Fisher z-transformed correlation between region timecourses.
2.4. Statistical analysis
Data are reported as the mean ± standard error. Patient characteristics were evaluated using general linear modeling, with sex and diagnosis, and their interaction, as fixed factors. General linear modeling was also used to compare resilience data, controlling for age. Sex differences within each group (HC, IBS) were evaluated in a linear contrast analysis. Relationships among resilience measures, symptom severity (IBS-SSS), and IBS-QOL were evaluated by the Pearson correlation coefficient. General linear modeling and correlation analyses were performed using SPSS version 28 (IBS Corp., Albany, NY). P-values <0.05 were considered statistically significant.
We first determined the neural signature (i.e. neural correlate) of each resilience measure in HCs using non-rotated partial least squares (PLS) correlation analysis;38 reliability of the relationship between region-to-region connectivity and each resilience measure was determined by 5000 bootstrap samples. PLS was implemented using plscmd (https://www.rotman-baycrest.on.ca). Subsequently, the union of the most robust connections (99.8th percentile, all p<0.001) from PLS correlation analyses performed in female HCs, male HCs, and all HCs was used to define resilience-related signature for each measure. This strategy was used to ensure that sex-specific and non-specific (i.e. common) features would be represented. The three resulting resilience signatures (i.e. BRS, CDRISC, and relative resilience signatures) had 73–80 connections each, which were plotted using circos.39
Subsequently, diagnosis, sex, and sex*diagnosis effects in connectivity within each of the three resilience-related signatures were evaluated by non-rotated PLS analysis using a priori contrasts. Analyses were performed with adjustment for age by residualizing the data. The reliability of group differences was determined by 5000 bootstrap samples; connections with a p-value <0.05 (|bootstrap ratio|>1.96) were considered statistically significant. Relationships between the strength of connectivity in affected connections in IBS and symptom severity and IBS-QOL were evaluated by the Pearson correlation coefficient. P-values <0.05 were considered statistically significant.
3. Results
3.1. Participant characteristics
The study population was comprised of 402 participants (Male HC: n=66; Female HC: n=104; Male IBS: n=62; Female IBS: n=170). Participant characteristics are shown in Table 1. There was a significant diagnosis*sex interaction for age (p=0.04), with a significantly lower mean age in women with IBS (27.7±0.8 years) than in men with IBS (31.2±1.3 years) and male and female HCs (30.1±1.3 and 31.0±1.0 years, respectively). There were significant race/ethnicity differences, with more Hispanic (34% vs 21%, p=0.006) and non-white participants (59% vs 44%, p=0.01) in HCs than in IBS. ACE (1.7±0.1 vs 1.1±0.1, p=0.006), HAD-anxiety (7.6±0.3 vs 3.8±0.3, p<0.001), and HAD-depression scores (3.6±0.2 vs 1.8±0.2, p<0.001) were significantly higher in participants with IBS than in HCs, without sex differences. Among participants with IBS, symptom severity (IBS-SSS) and IBS-QOL were significantly worse in women than in men (IBS-SSS: 230.1±6.8 vs 195.6±11.4, respectively, p=0.01; IBS-QOL: 63.2±1.6 vs 72.4±2.7, respectively, p=0.004).
Table 1.
Participant characteristics
| HC | IBS | GLM p-values | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Sex diff p-value |
Male | Female | Sex diff p-value |
Dx | Sex | Dx*Sex | |
|
| |||||||||
| N | 66 | 104 | 62 | 170 | |||||
| Hispanic (%) † | 28.8 | 37.5 | 0.32 | 14.5 | 24.3 | 0.11 | 0.006 | 0.12 | |
| Race (%) †‡ | 0.22 | 0.71 | 0.01 | 0.58 | |||||
| Asian | 19.7 | 28.8 | 25.8 | 21.4 | |||||
| Black | 6.1 | 8.7 | 6.5 | 7.5 | |||||
| White | 51.5 | 33.7 | 58.1 | 54.3 | |||||
| Mixed | 10.6 | 10.6 | 6.5 | 8.7 | |||||
| Other | 12.1 | 18.3 | 3.2 | 8.1 | 0.06 | 0.76 | |||
| Age (yrs) | 30.1±1.3 | 31.0±1.0 | 0.55 | 31.2±1.3 | 27.7±0.8 | 0.02 | 0.33 | 0.26 | 0.04 |
| ACE (0–10) | 0.9±0.2 | 1.4±0.2 | 0.06 | 1.7±0.2 | 1.8±0.1 | 0.72 | 0.006 | 0.10 | 0.26 |
| HAD-Anxiety (0–21) | 3.4±0.5 | 4.2±0.4 | 0.19 | 7.6±0.5 | 7.7±0.3 | 0.86 | <0.001 | 0.29 | 0.41 |
| HAD-Depression (0–21) | 1.7±0.4 | 1.8±0.3 | 0.88 | 3.8±0.4 | 3.3±0.2 | 0.17 | <0.001 | 0.41 | 0.29 |
| Bowel Habit (C:D:U:M) | 8:40:7:7 | 68:47:13:45 | <0.001 | ||||||
| IBS-SSS (0–500) | 195.6±11.4 | 230.1±6.8 | 0.01 | ||||||
| IBS-QOL (0–100) | 72.4±2.7a | 63.2±1.6b | 0.004 | ||||||
Data are presented as mean±standard error, unless otherwise indicated. Range of scores on questionnaires is indicated in parentheses.
Fisher’s exact test
(other includes American Indian, Hawaiian, and decline to state)
n=58
n=167
HC, healthy control; IBS, irritable bowel syndrome; Dx, diagnosis; GLM, general linear modelling; ACE, Adverse Childhood Experiences; HAD, Hospital Anxiety and Depression scale; IBS-SSS, IBS Symptom Severity Scale; IBS-QOL, IBS-Quality of Life questionnaire; C:D:U:M, constipation, diarrhea, unspecified, mixed
3.2. Diagnosis and sex differences in resilience measures
Scores were significantly lower in participants with IBS than in HCs for all measures of resilience, including the BRS (20.2±0.2 vs 23.2±0.4, respectively, p<0.001), CDRISC (total score: 71.4±1.0 vs 77.8±1.0, respectively, p<0.001), and relative resilience (−0.4±0.1 vs 0.4±0.1, respectively, p<0.001) (Table 2; Figure 1). In addition, scores on all CDRISC domains were lower in participants with IBS than in HCs. Among HCs, there were no significant sex differences in the three measures of resilience. However, compared to women with IBS, men with IBS had significantly lower CDRISC total scores (68.7±1.7 vs 74.0±1.0, respectively, p=0.007), as well as persistence, control-meaning, and meaning domain scores. In contrast, the BRS score was not significantly different between men and women with IBS (19.9±0.6 vs 20.4±0.4, respectively, p=0.44), and although relative resilience tended to be lower in men with IBS than in women with IBS (−0.48±0.12 vs −0.24±0.07, respectively), the difference failed to reach significance (p=0.08).
Table 2.
Detailed resilience data
| HC | IBS | GLM p-values | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Sex diff p-value |
Male | Female | Sex diff p-value |
Dx | Sex | Dx*Sex | |
|
| |||||||||
| N | 66 | 104 | 62 | 170 | |||||
| BRS (0–30) | 23.4±0.6 | 22.9±0.5 | 0.56 | 19.9±0.6 | 20.4±0.4 | 0.44 | <0.001 | 0.92 | 0.34 |
| CDRISC | |||||||||
| Total (0–100) | 78.0±1.6 | 77.7±1.3 | 0.92 | 68.7±1.7 | 74.0±1.0 | 0.007 | <0.001 | 0.07 | 0.053 |
| Persistence (0–32) | 26.1±0.6 | 25.7±0.5 | 0.58 | 23.0±0.6 | 25.1±0.4 | 0.001 | <0.001 | 0.07 | 0.01 |
| Emotional-Cognitive (0–28) | 20.8±0.5 | 20.3±0.4 | 0.42 | 18.9±0.5 | 19.4±0.3 | 0.47 | .002 | 0.93 | 0.28 |
| Adaptability (0–20) | 16.8±0.4 | 16.8±0.3 | 0.95 | 14.9±0.4 | 15.8±0.2 | 0.053 | <0.001 | 0.20 | 0.17 |
| Control-Meaning (0–12) | 9.2±0.3 | 9.6±0.2 | 0.25 | 8.0±0.3 | 8.9±0.2 | 0.004 | <0.001 | 0.005 | 0.26 |
| Meaning (0–8) | 5.0±0.3 | 5.3±0.2 | 0.44 | 4.0±0.3 | 4.9±0.2 | 0.01 | .006 | 0.02 | 0.23 |
| Relative resilience | 0.36±.11 | 0.44±.09 | 0.61 | −0.48±.12 | −0.24±.07 | 0.08 | <0.001 | 0.12 | 0.40 |
Data are presented as mean±standard error. Range of scores on questionnaires is indicated in parentheses. HC, healthy control; IBS, irritable bowel syndrome; CDRISC, Connor-Davidson Resilience Scale; BRS, Brief Resilience Scale
Figure 1.

Group differences in scores on each resilience measure. Significant differences (p<0.05) are indicated by an *.
3.3. Correlations between resilience measures and IBS symptoms and QOL
Correlations between resilience measures showed significant moderate-to-strong positive coefficients, accounting for 10%−52% of the variance, with the strongest correlations between the two measures of perceived resilience (BRS and CDRISC) (Table 3). Among all participants with IBS, CDRISC, BRS, and relative resilience were significantly positively correlated with the IBS-QOL score (i.e. greater perceived/relative resilience was associated with better disease-specific QOL), with low-to-moderate correlation coefficients, accounting for 4%−11% of the variance. Negative correlations between resilience measures and the IBS-SSS score (i.e. greater resilience was associated with lower IBS symptom severity) were not as strong as those with the IBS-QOL score, accounting for 1%−2% of the variance and reaching significance for the BRS and relative resilience (p’s <0.05), but not for the CDRISC (p=0.09). The same pattern of results generally applied in men and women with IBS, with few exceptions (Table 4).
Table 3.
Correlations (r) between resilience measures according to sex and diagnosis
| CDRISC-BRS | CDRISC-relative resilience | BRS-relative resilience | |
|---|---|---|---|
|
| |||
| Female HC | 0.63* | 0.57* | 0.57* |
| Male HC | 0.72* | 0.42* | 0.31* |
| Female IBS | 0.66* | 0.48* | 0.45* |
| Male IBS | 0.58* | 0.56* | 0.48* |
p<0.05
HC, healthy control; IBS, irritable bowel syndrome; CDRISC, Connor-Davidson Resilience Scale; BRS, Brief Resilience Scale
Table 4.
Correlations (r) between resilience and clinical measures
| BRS | CDRISC | Relative resilience | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| IBS-SSS | IBS-QOL | IBS-SSS | IBS-QOL | IBS-SSS | IBS-QOL | |
|
| ||||||
| All IBS (n=232) | −0.18* | 0.33* | −0.11 | 0.21* | −0.15* | 0.32* |
| Female IBS (n=170) | −0.24* | 0.36* | −0.13 | 0.27* | −0.15 | 0.33* |
| Male IBS (n=62) | −0.03 | 0.29* | −0.20 | 0.17 | −0.27* | 0.42* |
p<0.05
IBS, irritable bowel syndrome; CDRISC, Connor-Davidson Resilience Scale; BRS, Brief Resilience Scale; IBS-SSS, IBS symptom severity; IBS-QOL, IBS-specific quality of life
3.4. Resilience-related brain connectivity signatures in HCs
Resilience-related signatures, defined in HCs, are shown in Figure 2. At the connection-level, overlap among the signatures was relatively limited. Specifically, although the CDRISC-related signature showed overlap with BRS-related (8 connections) and relative resilience-related signatures (6 connections), the BRS-related and relative resilience-related signatures had few connections in common (3 connections), and only one connection was common to all three resilience-related signatures (between the right rectus gyrus and left orbital H-shaped gyrus within the CAN). However, at the network-level, common features among the resilience-related signatures were apparent; most notably, all three resilience-related networks had extensive CAN involvement. General differences among the signatures were also apparent at the network-level; namely, BRS had the most extensive default mode network (DMN) component, CDRISC had the most extensive CEN component, and relative resilience had the most extensive ERN component.
Figure 2.
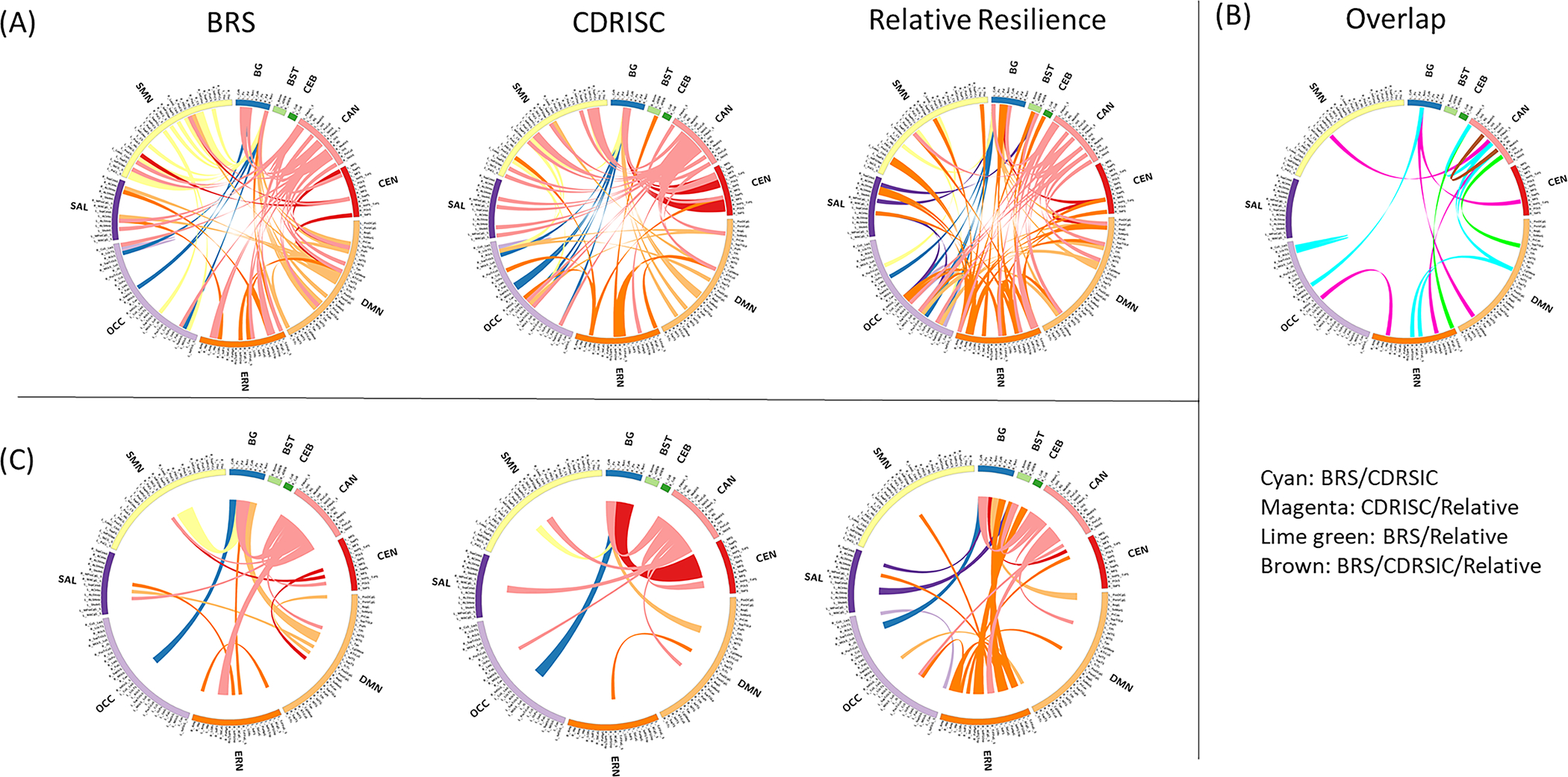
Neural correlates of each resilience measure in healthy controls at the connection-level (A and B) and network-level (C). (A) Lines represent connections positively correlated with each resilience measure (p<0.001). (B) Connections positively correlated with at least two resilience measures are shown (i.e. overlap between resilience measures). (C) The proportions of involved connections between networks are represented by line width to highlight main features of the neural correlates of each resilience measure.
3.5. Resilience-related brain connectivity affected by IBS and sex
3.5.1. Main effect of diagnosis
For all three resilience measures, participants with IBS showed significant reductions in connectivity strength in numerous connections compared to that in HCs; no connections showed increased connectivity in IBS vs HCs (Figure 3A; Supplementary Table S3). Within the BRS-related signature, connectivity affected by IBS largely involved the CAN and DMN. Within the CDRISC-related signature, connectivity affected by IBS largely involved the CAN, salience network (SAL), and sensorimotor network (SMN). Within the relative resilience-related signature, connectivity affected by IBS largely involved the CAN and ERN.
Figure 3.
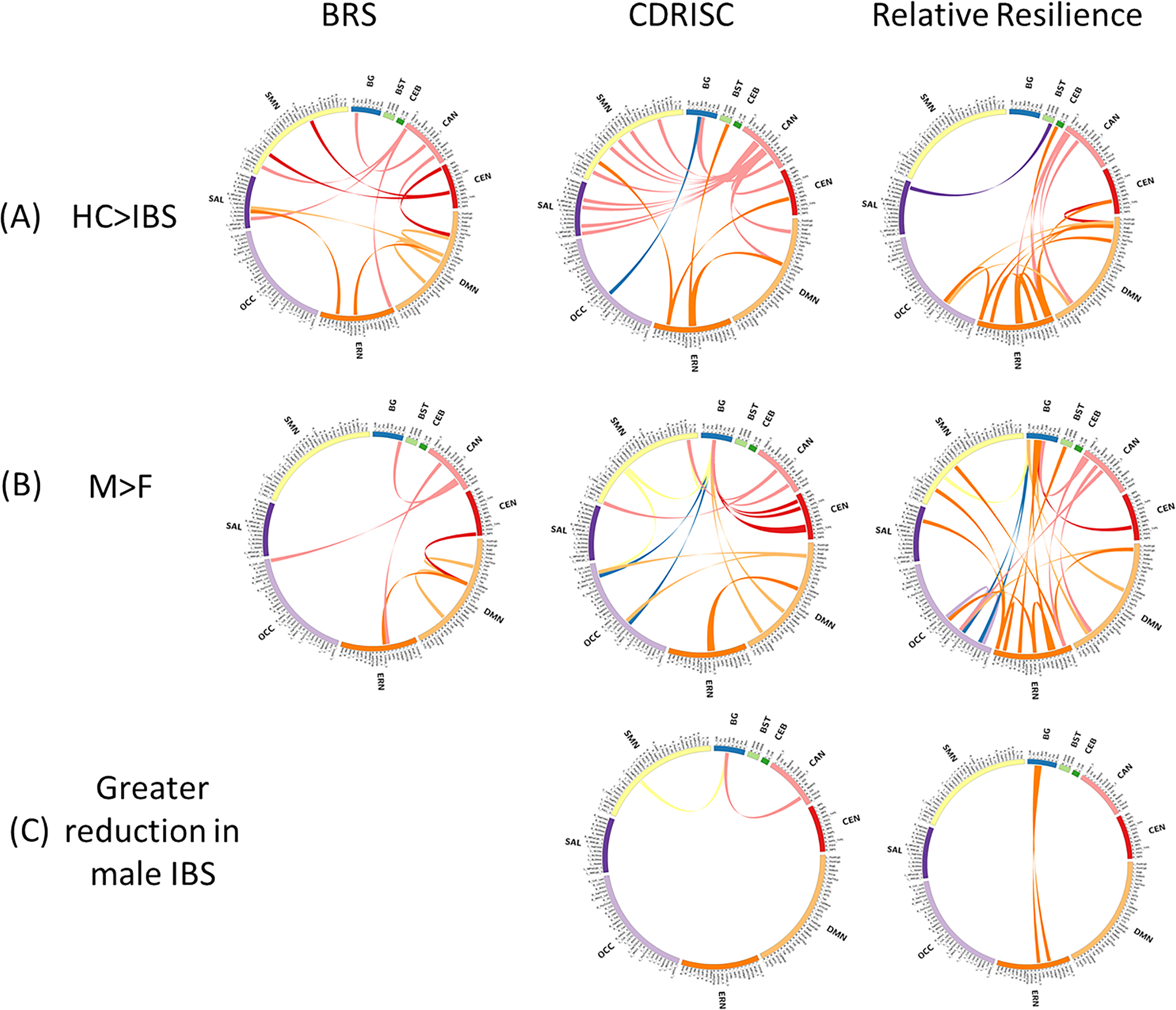
Resilience-related brain connectivity affected by IBS and sex on non-rotated partial least squares analysis. (A) Connectivity showing a significant diagnosis main effect. Lines represent resilience-related connections with reduced strength in participants with IBS than in HCs (p<0.05). (B) Connectivity showing a significant sex main effect. Lines represent resilience-related connections with reduced strength in women than in men (p<0.05) (C) Connectivity showing a significant diagnosis*sex effect. Lines represent resilience-related connections reduced to a greater extent in men with IBS than in women with IBS relative to same-sex HCs (p<0.05). IBS, irritable bowel syndrome; HC, healthy control
3.5.2. Main effect of sex
All three resilience-related signatures showed numerous sex differences in connectivity, reflecting greater connectivity in men than in women regardless of diagnosis; no connections showed increased connectivity in women vs men (Figure 3B; Supplementary Table S3). Overall, the BRS-related network showed the fewest number of connections with a sex difference, mainly involving CAN and DMN connectivity with regions in other networks. Sex differences in the CDRISC-related signature largely involved basal ganglia and DMN connectivity with regions in other networks. Overall, the relative resilience-related signature showed the most connections with a sex difference, largely involving basal ganglia and ERN connectivity with regions in other networks, as well as within the ERN.
3.5.3. Diagnosis*sex interaction
Several connections within CDRISC- and relative-resilience signatures showed a significant diagnosis*sex interaction, reflecting greater reductions in men with IBS than in women with IBS relative to that in same-sex HCs (Figure 3C; Supplementary Table S3). Within the CDRISC-related signature, men with IBS showed greater reduction in connectivity between the left nucleus accumbens and left superior frontal sulcus (in the SMN) and right orbital sulcus (in the CAN) than women with IBS relative to that in same-sex HCs. Within the relative resilience-related signature, men with IBS showed greater reduction in connectivity between the left pallidum and left subcallosal gyrus (in the ERN) and between the left nucleus accumbens and left transverse frontopolar cortex (in the ERN) than women with IBS relative to that in same-sex HCs. No connections in the BRS-related signature showed a significant diagnosis*sex interaction.
3.5.4. Associations between affected connectivity and clinical measures
Significant associations between reduced BRS-related connectivity and worse IBS-SSS (5 connections involving the DMN, ERN, and CAN) and worse IBS-QOL (4 connections involving the CEN, SMN, DMN, SAL, and ERN) were observed (Figure 4; Supplementary Table S4). Significant associations between reduced CD-RISC connectivity and worse IBS-SSS (3 connections involving the CAN, SMN, basal ganglia, DMN, and ERN) were observed. Unlike the other resilience measures, relative resilience-related connectivity was not correlated with IBS-SSS or IBS-QOL scores among all participants with IBS; however, reduced connectivity in one relative resilience-related connection within the ERN was significantly correlated with worse IBS-SSS among men with IBS (Supplementary Figure S1).
Figure 4.

Associations between affected connectivity and clinical measures. Lines represent resilience-related connections that are reduced in IBS (from Figure 3A,C) and show clinical relevance; that is, the reduction in connectivity is significantly correlated with worse symptom severity, assessed by the IBS-SSS, and/or worse disease-specific quality of life, assessed by the IBS-QOL (p<0.05). IBS-SSS, Irritable Bowel Syndrome Symptom Severity Scale; IBS-QOL, IBS- Quality of Life questionnaire
4. Discussion
We aimed to test the general hypothesis that individuals with IBS show reduced resilience-related connectivity associated with worse symptom severity and disease-specific QOL, potentially with sex differences. The main findings of this study were that, compared to HCs, participants with IBS showed reduced resilience scores for all types of resilience, as well as numerous reductions in resilience-related brain connectivity, some of which correlated with IBS symptom severity and disease-specific QOL. Further, sex differences were observed for CDRISC (at behavioral and neural levels) and relative resilience (at the neural level), but not BRS, with men with IBS more affected than women with IBS.
4.1. Resilience in IBS
As in previous IBS resilience studies, scores on the BRS and CDRISC were lower in participants with IBS than in HCs 3,4. Moreover, all domains of the CDRISC were affected. The BRS was designed to assess the most basic meaning of the word ‘resilience’, which was considered as the ability to bounce back/recover from stress.20 In contrast, the CDRISC captures not only one’s ability to adapt and bounce back, but also one’s attitudes (e.g. enjoy a challenge) and external resources (e.g. social support) known to help with recovery from stress.19 As measures of perceived resilience, these two questionnaires focus on perceptions of resilience, which may better reflect self-efficacy for coping with future adversity (especially for the CDRISC), rather than psychological adaptation from past adversity, as assessed by relative resilience.18 The present study adds to the literature on resilience in IBS by demonstrating that relative resilience is also reduced in IBS. Thus, impacted resilience in IBS not only involves less favorable self-perceptions of one’s ability to handle stressors via internal and external resources, but also clearly involves diminished adaptation to early experiences of adversity. Overall, these results further confirm resilience as a potential treatment target in brain-gut IBS therapies.
Although all resilience measures showed moderate positive correlations with better IBS-related QOL, the correlations of higher resilience with lower symptom severity based on the IBS-SSS were generally weaker, and may be less than expected. However, these results are consistent with previous investigations of resilience in a large sample of patients with IBS, in which associations between measures of resilience and IBS-QOL were more robust than those between resilience and symptoms.3 The IBS-SSS measures the severity of current symptoms (past 10 days) while the IBS-QOL is a more global measure and assesses the QOL over the past month. We consider it unsurprising that resilience is better correlated with the chronic global disease state than with a relatively short-term assessment of gastrointestinal symptom severity. Although low resilience may generally impact the illness considered in its entirety, including the burden of symptoms, more than the severity of symptoms, treatments known to improve resilience, such as mindfulness-based stress reduction, cognitive behavioral therapy, and gut-directed hypnotherapy,40–42 have been shown to improve IBS symptom severity in addition to QOL.
4.2. Neural correlates of different types of resilience
The present study also provides new information on the neural correlates of both perceived and relative resilience in healthy individuals, and the impact of IBS. As may be expected, the neural signatures related to the two measures of perceived resilience (CDRISC and BRS) showed the most overlap, largely involving the CAN, ERN, and DMN. Additionally, the relative resilience-related signature overlapped more with the CDRISC-related signature than the BRS-related signature, which may be due to the above-mentioned differences in the complexity of measures. The generally more extensive ERN component for relative resilience than for perceived resilience measures may reflect the importance of maintained ERN integrity for positive psychological adaptation to early life adversity.43
All three resilience-related signatures had extensive involvement of the CAN, which is the major cortical site involved in generating the autonomic nervous system response to perturbations of homeostasis and exteroceptive challenges.44 Further, the one connection that was robustly positively correlated with all three resilience measures involved the right rectus gyrus and left orbital H-shaped gyrus, both of which are within the orbitofrontal cortex (OFC) and the CAN. This common connection suggests that functional connectivity integrity among regions in the OFC is important to the general concept of resilience, consistent with previous neuroimaging studies of resilience45 and the known roles of the OFC in behavioral flexibility and autonomic modulation. The OFC flexibly regulates and coordinates adaptive behavioral and autonomic responses; without the OFC, there is an uncoupling of these elements, which may contribute to distress and maladaptive behavior.46
The OFC achieves coordination between behavioral and autonomic responses via its distributed connections. The medial and lateral OFC are connected with the medial and lateral hypothalamus, respectively.47 Further, the OFC interacts with regions in the ERN (amygdala, anterior cingulate cortex), SAL (anterior insula), and CEN.48–50 Imaging studies have implicated the OFC in the post-trauma recovery period and resilience.51,52 Additionally, greater connectivity between the OFC and parahippocampal gyrus (in the ERN) has been previously shown to be correlated with increased resilience as assessed by the BRS.45 Consistent with this, CAN-ERN connectivity correlated with resilience measures, especially for BRS and relative resilience, in the present study.
4.3. Impacted resilience-related connectivity in IBS
All three resilience measures showed reduced connectivity in participants with IBS compared to that in HCs, including connections involving the CAN, especially with regions in the DMN, ERN, and SAL.
Affected connections with clinical relevance (reduced connectivity was associated with worse IBS symptom severity and/or IBS-QOL) mainly involved the DMN. The DMN plays a role in parasympathetic regulation (unlike other networks that interact with the CAN), concordant with its involvement in self-referential processes.53,54 These results suggest that resilience-related connectivity involved in autonomic modulation, especially parasympathetic regulation, is impacted in IBS, consistent with previous literature indicating autonomic dysfunction in IBS, albeit with sex differences, as discussed below.55–58
Another notable clinically relevant connection was between the left lateral orbital sulcus (in the ERN) and the vertical ramus of the left lateral sulcus (in the SAL). This connection was highly robust, with reduced connectivity correlating with worse IBS-QOL among all participants with IBS and men with IBS and with worse IBS symptom severity in women with IBS (Supplementary Figure S1). The SAL network plays a key role in evaluating the salience of existing or predicted threats to homeostasis and interacts with other networks to assist in generating appropriate behavioral responses to salient stimuli.59 The lateral orbitofrontal cortex in the ERN is important for adaptive behavior, involved in anticipating choices and updating information.60 Thus, increased connectivity between these regions may buffer the impact of heightened emotional arousal responses reported in IBS6 by relegating resources to better enable adaptive behaviors.
4.4. Influence of sex
Although HCs did not show sex differences in any of the three resilience measures, men with IBS showed significantly lower scores on the CDRISC, and marginally lower scores on relative resilience, compared to those in women with IBS. In contrast, no sex differences in the BRS were observed in any comparison (among all participants, within HCs, and within participants with IBS). Further, CDRISC-related connectivity between the left nucleus accumbens and the right orbital sulcus (in the CAN) and left superior frontal sulcus (in the sensorimotor network) was especially reduced in men with IBS, which may be related to greater autonomic disruptions (increased cardiosympathetic/parasympathetic balance) in men with IBS than in women with IBS.56,61 Overall, the present results suggest that men with IBS are especially affected in specific aspects of resilience that are assessed on the CDRISC, which may indicate a reduced range of resources important for resilience. Thus, fostering a range of resources supporting resilience may be especially important in male patients, although further studies are needed.
Several studies have examined sex differences in different types of resilience, with mixed results. Some studies have found higher perceived resilience in men than in women,62 while others suggest greater manifest resilience in women (e.g. less mortality under adversity).63 A previous study that examined both relative and perceived resilience found higher perceived resilience and lower relative resilience in men than in women.18 In contrast, we did not find any sex differences in resilience among HCs in the present study. These two studies differed in study populations, comprising individuals in waiting rooms at an urban hospital in Atlanta18 vs. HCs from UCLA and the greater Los Angeles community (in the present study), as well as in the measures used for perceived (various versions of the CDRISC, and in the present study, the BRS) and relative resilience (measures of depressive and posttraumatic stress symptoms relative to the Childhood Trauma Questionnaire total score18 vs. the SF-12 MCS score relative to the ACE score used in the present study). As discussed below, better standardization of relative resilience may aid in reducing such discrepancies.
A general influence of sex, regardless of diagnosis, was observed in some domains of the CDRISC and all three resilience-related neural signatures, with the BRS showing the fewest connections, and relative resilience showing the most connections, with an overall sex difference. In particular, basal ganglia connectivity showed sex differences, with connectivity greater in males than in females, in all three resilience-related neural signatures. This is consistent with a tractography study indicating stronger fiber connections between basal ganglia and numerous frontal and cingulate regions in men than in women.64
4.5. Measuring relative resilience
Assessing relative resilience by considering the level of positive functioning relative to the adversity burden is growing in popularity. Relative resilience may be assessed as a categorical variable, classifying individuals as resilient (positive functioning with adversity exposure), reactive (impaired functioning with adversity exposure), vulnerable (impaired functioning without adversity exposure), and unexposed and well (positive functioning without adversity exposure). However, with categorical groupings, nuances are lost. Thus, a method to assess relative resilience as a continuous variable, taking the standardized residual of a measure of functional status predicted by a measure of adversity exposure (i.e. the difference between actual and predicted functioning scores given the adversity burden) was developed.65 The main strength of this type of resilience assessment is that it provides an indicator of manifested resilience; however, it is based on a statistical model and is thus impacted by sample characteristics. By assessing relative resilience as a continuous variable, the present study was able to show that reduced resilience in IBS is not limited to perceived resilience. Further, our measure of relative resilience was associated with a neural signature with a more extensive ERN component than common measures of perceived resilience.
Various questionnaires on psychological functioning, including questionnaires on internalizing symptoms 66, depressive and posttraumatic stress symptoms,18 QOL,67 adolescent/childhood psychopathology,68 and generalized psychological distress,65 have been used in studies on relative resilience using this approach. Additionally, standardized (CDC-Kaiser ACE scale,69 Childhood Trauma Questionnaire18,67) and unstandardized assessments of ACEs66,68 and the Traumatic Life Events Questionnaire have been used in calculating relative resilience.65 In the present study, SF-12 MCS and ACE scores were used to calculate relative resilience by the residual approach. To our knowledge, no study has used the SF-12 MCS score in the calculation of relative resilience. However, several properties support its use in the assessment of relative resilience. Specifically, the MCS score is widely used an indicator of psychological functioning in the general population and has been shown to be negatively associated with the ACE score in various populations,70–72 as well as in the current study, rendering the regression of ACE on MCS meaningful. As the assessment of relative resilience by the residuals approach is relatively innovative, efforts to standardize its calculation could mitigate some of its limitations and render comparisons across studies easier.
4.6. Limitations
The present study had some limitations which warrant further investigation in future studies. Although a strength of the present study is the utility of multiple measures of resilience that tap into various psychological and behavioral domains, the lack of standardization in the assessment of relative resilience discussed above could lead to differing interpretations. Further, the study sample included fewer men than women, especially among those with IBS. Thus, further studies with larger, more balanced samples are needed to confirm these findings. In addition, the study sample included a combined population of Rome III- and IV-positive patients (n=104 and n=128, respectively); however, these patients were comparable in ACE, IBS-SSS, IBS-QOL, HAD anxiety and depression, and CDRISC (p’s>0.05). Although Rome IV-positive patients had higher BRS scores (19.5±05 vs 21.0±0.4, p=.03) and relative resilience (−0.53±0.11 vs −0.12±0.08, p=.003) than Rome III-positive patients, the scores in both IBS groups were still significantly lower than those in HCs. Further, for CDRISC and relative resilience, but not BRS, correlations between resilience and IBS-SSS/IBS-QOL were more robust in Rome III-positive patients than in Rome IV-positive patients which may be due to the more restrictive diagnostic Rome IV criteria which selects for patients with more severe symptoms (Supplementary Table S5).73 However, potential differences in relationships between resilience and symptoms according to diagnosis criteria should further evaluated in larger-scale studies. Another limitation is that we considered only ACE and not adulthood stressors in assessing relative resilience; however, ACE is widely used for the assessment of early childhood adversity and has been previously used in the assessment of relative resilience.65
4.7. Future directions
Although we maintain that a multi-assessment approach provides important information regarding potential targets, there are difficulties in incorporating multiple assessments into study/clinical protocols, such as increasing the patient burden in terms of time. A previous study found more robust associations between resilience and IBS symptom severity and QOL with the BRS than with the CDRISC.3 Further, the BRS, not the CDRISC, was associated with stress responsiveness as assessed by the cortisol response to adrenocorticotropic hormone stimulation.3 Similarly, in the present study, relationships between worse IBS symptom severity and IBS-QOL, lower resilience scores, and impaired resilience-related connectivity were more clearly shown for the BRS than for the CDRISC and relative resilience. These results suggest the BRS as the most clinically relevant assessment of resilience in IBS. However, relative resilience showed similar correlations with IBS symptom severity and IBS-QOL as those with the BRS, and given its novel status in IBS research, further research is warranted. In particular, the impact of various types of resilience on affected stress responses (e.g. cortisol responses to stressors) and treatments for IBS such as mindfulness-based stress reduction, cognitive behavioral therapy, and gut-directed hypnotherapy,40–42 should be elucidated in future studies.
4.8. Conclusions
Individuals with IBS show reductions in both perceived and relative resilience, which reflects psychological adaptation to adversity, along with reduced connectivity in resilience-related neural correlates. In particular, BRS-related connections supporting homeostasis maintenance were affected in IBS. Men with IBS may show additional impairment in specific aspects of resilience, along with greater reductions in connections supporting autonomic regulation. Treatments aimed at improving resilience may benefit patients with IBS, which in turn may reduce symptom severity and improve health-related QOL. Further studies, particularly with larger numbers of men, are needed.
Supplementary Material
Table S1. T1-weighted acquisition parameters
Table S2. Region abbreviation and network key
Table S3. Main effects of diagnosis and sex, and their interaction, in resilience-related connectivity (corresponds to Figure 3)
Table S4. Clinically relevant reductions in resilience-related connectivity in all patients, men with IBS, and women with IBS (corresponds to Figure 4)
Figure S1. Sex-specific correlations between reduced resilience-related connectivity (from Figure 3A,C) and worse IBS symptom severity, assessed by the IBS-SSS, and disease-specific quality of life, assessed by the IBS-QOL.
Acknowledgements:
We thank Cathy Liu for designing the graphical abstract. Stock images from BioRender.com were used in the graphical abstract with permission.
Funding:
This research was funding by NIH grants U54DK064539 to EAM, LC; K23DK106528 to AG; R03DK121025 to AG; ULTR001881 to AG; R01AT007137 to KT; U01DK082370 to EAM; and R01DK048351 to EAM.
Footnotes
Disclosures: AG is a research consultant for YAMAHA. EAM is a member of the scientific advisory boards of Danone, Axial Therapeutics, Amare, Mahana Therapeutics, Pendulum, Bloom Biosciences, and APC Microbiome Ireland. LC serves as an advisory board member or consultant for Ardelyx, Arena Pharmaceuticals, Bausch Health, Immunic, Ironwood Pharmaceuticals, Inc., Mauna Kea Technologies, and Trellus; and receives grant support from AnX Robotica, Arena Pharmaceuticals, and Ironwood Pharmaceuticals. LAK, KT, BDN, and JSL declare no conflict of interest.
Data availability:
De-identified and raw neuroimaging data are available through the NIH-funded Pain and Interoception Imaging Network (PAIN) data repository (https://www.painrepository.org/repositories). Access to the data is based on membership according to a collaborative principle.
References
- 1.Drossman DA. Functional Gastrointestinal Disorders: History, Pathophysiology, Clinical Features and Rome IV. Gastroenterology. 2016. [DOI] [PubMed] [Google Scholar]
- 2.Mearin F, Lacy BE, Chang L, et al. Bowel Disorders. Gastroenterology. 2016. [DOI] [PubMed] [Google Scholar]
- 3.Park SH, Naliboff BD, Shih W, et al. Resilience is decreased in irritable bowel syndrome and associated with symptoms and cortisol response. Neurogastroenterol Motil. 2018;30(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parker CH, Naliboff BD, Shih W, et al. The Role of Resilience in Irritable Bowel Syndrome, Other Chronic Gastrointestinal Conditions, and the General Population. Clin Gastroenterol Hepatol. 2021;19(12):2541–2550 e2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ben-Ezra M, Hamama-Raz Y, Palgi S, Palgi Y. Cognitive appraisal and psychological distress among patients with irritable bowel syndrome. Isr J Psychiatry Relat Sci. 2015;52(1):54–59. [PubMed] [Google Scholar]
- 6.Mayer EA, Labus JS, Tillisch K, Cole SW, Baldi P. Towards a systems view of IBS. Nat Rev Gastroenterol Hepatol. 2015;12(10):592–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hodes GE, Epperson CN. Sex Differences in Vulnerability and Resilience to Stress Across the Life Span. Biol Psychiatry. 2019;86(6):421–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franke HA. Toxic Stress: Effects, Prevention and Treatment. Children (Basel). 2014;1(3):390–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kent M, Mardian AS, Regalado-Hustead ML, et al. Adaptive Homeostatic Strategies of Resilient Intrinsic Self-Regulation in Extremes (RISE): A Randomized Controlled Trial of a Novel Behavioral Treatment for Chronic Pain. Front Psychol. 2021;12:613341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Obradovic J, Portilla XA, Ballard PJ. Biological Sensitivity to Family Income: Differential Effects on Early Executive Functioning. Child Dev. 2016;87(2):374–384. [DOI] [PubMed] [Google Scholar]
- 11.Wu L, Zhang X, Wang J, et al. The associations of executive functions with resilience in early adulthood: A prospective longitudinal study. J Affect Disord. 2021;282:1048–1054. [DOI] [PubMed] [Google Scholar]
- 12.Miller GE, Chen E, Armstrong CC, et al. Functional connectivity in central executive network protects youth against cardiometabolic risks linked with neighborhood violence. Proc Natl Acad Sci U S A. 2018;115(47):12063–12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Wit S, Wierenga LM, Oranje B, et al. Brain development in adolescents at ultra-high risk for psychosis: Longitudinal changes related to resilience. Neuroimage Clin. 2016;12:542–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schweizer S, Walsh ND, Stretton J, Dunn VJ, Goodyer IM, Dalgleish T. Enhanced emotion regulation capacity and its neural substrates in those exposed to moderate childhood adversity. Soc Cogn Affect Neurosci. 2016;11(2):272–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodman AM, Jenness JL, Weissman DG, Pine DS, McLaughlin KA. Neurobiological Markers of Resilience to Depression Following Childhood Maltreatment: The Role of Neural Circuits Supporting the Cognitive Control of Emotion. Biol Psychiatry. 2019;86(6):464–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59(6):1037–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carnevali L, Koenig J, Sgoifo A, Ottaviani C. Autonomic and Brain Morphological Predictors of Stress Resilience. Front Neurosci. 2018;12:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishimi K, Choi KW, Cerutti J, Powers A, Bradley B, Dunn EC. Measures of adult psychological resilience following early-life adversity: how congruent are different measures? Psychol Med. 2021;51(15):2637–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Connor KM, Davidson JR. Development of a new resilience scale: the Connor-Davidson Resilience Scale (CD-RISC). Depress Anxiety. 2003;18(2):76–82. [DOI] [PubMed] [Google Scholar]
- 20.Smith BW, Dalen J, Wiggins K, Tooley E, Christopher P, Bernard J. The brief resilience scale: assessing the ability to bounce back. Int J Behav Med. 2008;15(3):194–200. [DOI] [PubMed] [Google Scholar]
- 21.Ye Y, Wu C, Yang C. The differences between the Connor–Davidson Resilience Scale and the Brief Resilience Scale When Assessing Resilience: Confirmatory Factor Analysis and Predictive Effects. Preprints. 2022;2022010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amerio A, Bertuccio P, Santi F, et al. Gender Differences in COVID-19 Lockdown Impact on Mental Health of Undergraduate Students. Front Psychiatry. 2021;12:813130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haahr-Pedersen I, Perera C, Hyland P, et al. Females have more complex patterns of childhood adversity: implications for mental, social, and emotional outcomes in adulthood. Eur J Psychotraumatol. 2020;11(1):1708618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldfarb EV, Seo D, Sinha R. Sex differences in neural stress responses and correlation with subjective stress and stress regulation. Neurobiol Stress. 2019;11:100177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kilpatrick LA, Istrin JJ, Gupta A, et al. Sex commonalities and differences in the relationship between resilient personality and the intrinsic connectivity of the salience and default mode networks. Biol Psychol. 2015;112:107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kilpatrick LA, Tillisch K. Irritable Bowel Syndrome. 2012. [Google Scholar]
- 27.Chang L, Heitkemper MM. Gender differences in irritable bowel syndrome. Gastroenterology. 2002;123(5):1686–1701. [DOI] [PubMed] [Google Scholar]
- 28.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130(5):1480–1491. [DOI] [PubMed] [Google Scholar]
- 29.Turner-Bowker D, Hogue SJ. Short Form 12 Health Survey (SF-12). In: Michalos AC, ed. Encyclopedia of Quality of Life and Well-Being Research. Dordrecht: Springer Netherlands; 2014:5954–5957. [Google Scholar]
- 30.Felitti VJ, Anda RF, Nordenberg D, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14(4):245–258. [DOI] [PubMed] [Google Scholar]
- 31.Snaith RP. The Hospital Anxiety And Depression Scale. Health Qual Life Outcomes. 2003;1:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patrick DL, Drossman DA, Frederick IO, DiCesare J, Puder KL. Quality of life in persons with irritable bowel syndrome: development and validation of a new measure. Dig Dis Sci. 1998;43(2):400–411. [DOI] [PubMed] [Google Scholar]
- 33.Spiegel B, Bolus R, Harris LA, et al. Measuring irritable bowel syndrome patient-reported outcomes with an abdominal pain numeric rating scale. Aliment Pharmacol Ther. 2009;30(11–12):1159–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Esteban O, Birman D, Schaer M, Koyejo OO, Poldrack RA, Gorgolewski KJ. MRIQC: Advancing the automatic prediction of image quality in MRI from unseen sites. PLoS One. 2017;12(9):e0184661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Esteban O, Markiewicz CJ, Blair RW, et al. fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat Methods. 2019;16(1):111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pruim RHR, Mennes M, van Rooij D, Llera A, Buitelaar JK, Beckmann CF. ICA-AROMA: A robust ICA-based strategy for removing motion artifacts from fMRI data. Neuroimage. 2015;112:267–277. [DOI] [PubMed] [Google Scholar]
- 37.Destrieux C, Fischl B, Dale A, Halgren E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage. 2010;53(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McIntosh AR, Lobaugh NJ. Partial least squares analysis of neuroimaging data: applications and advances. Neuroimage. 2004;23 Suppl 1:S250–263. [DOI] [PubMed] [Google Scholar]
- 39.Krzywinski M, Schein J, Birol I, et al. Circos: an information aesthetic for comparative genomics. Genome Res. 2009;19(9):1639–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lackner JM, Jaccard J, Group IBSOSR. Factors Associated With Efficacy of Cognitive Behavior Therapy vs Education for Patients With Irritable Bowel Syndrome. Clin Gastroenterol Hepatol. 2019;17(8):1500–1508 e1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gaylord SA, Palsson OS, Garland EL, et al. Mindfulness training reduces the severity of irritable bowel syndrome in women: results of a randomized controlled trial. Am J Gastroenterol. 2011;106(9):1678–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peter J, Fournier C, Keip B, et al. Intestinal Microbiome in Irritable Bowel Syndrome before and after Gut-Directed Hypnotherapy. Int J Mol Sci. 2018;19(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gupta A, Mayer EA, Acosta JR, et al. Early adverse life events are associated with altered brain network architecture in a sex- dependent manner. Neurobiol Stress. 2017;7:16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lamotte G, Shouman K, Benarroch EE. Stress and central autonomic network. Auton Neurosci. 2021;235:102870. [DOI] [PubMed] [Google Scholar]
- 45.Son SJ, Park B, Choi JW, et al. Psychological Resilience Enhances the Orbitofrontal Network in the Elderly With Mild Cognitive Impairment. Front Psychiatry. 2019;10:615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reekie YL, Braesicke K, Man MS, Roberts AC. Uncoupling of behavioral and autonomic responses after lesions of the primate orbitofrontal cortex. Proc Natl Acad Sci U S A. 2008;105(28):9787–9792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hirose S, Osada T, Ogawa A, et al. Lateral-Medial Dissociation in Orbitofrontal Cortex-Hypothalamus Connectivity. Front Hum Neurosci. 2016;10:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Price JL. Connections of orbital cortex. In: Zald D, Rauch S, eds. The Orbitofrontal Cortex. Oxford University Press; 2006:0. [Google Scholar]
- 49.Saper CB. The central autonomic nervous system: conscious visceral perception and autonomic pattern generation. Annu Rev Neurosci. 2002;25:433–469. [DOI] [PubMed] [Google Scholar]
- 50.Critchley HD, Nagai Y, Gray MA, Mathias CJ. Dissecting axes of autonomic control in humans: Insights from neuroimaging. Auton Neurosci. 2011;161(1–2):34–42. [DOI] [PubMed] [Google Scholar]
- 51.Roeckner AR, Oliver KI, Lebois LAM, van Rooij SJH, Stevens JS. Neural contributors to trauma resilience: a review of longitudinal neuroimaging studies. Transl Psychiatry. 2021;11(1):508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dolcos S, Hu Y, Iordan AD, Moore M, Dolcos F. Optimism and the brain: trait optimism mediates the protective role of the orbitofrontal cortex gray matter volume against anxiety. Soc Cogn Affect Neurosci. 2016;11(2):263–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beissner F, Meissner K, Bar KJ, Napadow V. The autonomic brain: an activation likelihood estimation meta-analysis for central processing of autonomic function. J Neurosci. 2013;33(25):10503–10511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. [DOI] [PubMed] [Google Scholar]
- 55.Kano M, Yoshizawa M, Kono K, et al. Parasympathetic activity correlates with subjective and brain responses to rectal distension in healthy subjects but not in non-constipated patients with irritable bowel syndrome. Sci Rep. 2019;9(1):7358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tillisch K, Mayer EA, Labus JS, Stains J, Chang L, Naliboff BD. Sex specific alterations in autonomic function among patients with irritable bowel syndrome. Gut. 2005;54(10):1396–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Manabe N, Tanaka T, Hata J, Kusunoki H, Haruma K. Pathophysiology underlying irritable bowel syndrome--from the viewpoint of dysfunction of autonomic nervous system activity. J Smooth Muscle Res. 2009;45(1):15–23. [DOI] [PubMed] [Google Scholar]
- 58.Stress Fukudo S. and visceral pain: focusing on irritable bowel syndrome. Pain. 2013;154 Suppl 1:S63–S70. [DOI] [PubMed] [Google Scholar]
- 59.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214(5–6):655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nogueira R, Abolafia JM, Drugowitsch J, Balaguer-Ballester E, Sanchez-Vives MV, Moreno-Bote R. Lateral orbitofrontal cortex anticipates choices and integrates prior with current information. Nat Commun. 2017;8:14823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Labus JS, Naliboff BN, Fallon J, et al. Sex differences in brain activity during aversive visceral stimulation and its expectation in patients with chronic abdominal pain: a network analysis. Neuroimage. 2008;41(3):1032–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Campbell-Sills L, Forde DR, Stein MB. Demographic and childhood environmental predictors of resilience in a community sample. J Psychiatr Res. 2009;43(12):1007–1012. [DOI] [PubMed] [Google Scholar]
- 63.Cullen MR, Baiocchi M, Eggleston K, Loftus P, Fuchs V. The weaker sex? Vulnerable men and women’s resilience to socio-economic disadvantage. SSM Popul Health. 2016;2:512–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lei X, Han Z, Chen C, Bai L, Xue G, Dong Q. Sex Differences in Fiber Connection between the Striatum and Subcortical and Cortical Regions. Front Comput Neurosci. 2016;10:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sheerin CM, Stratton KJ, Amstadter AB, Education Clinical Center Mirecc Workgroup T, McDonald SD. Exploring resilience models in a sample of combat-exposed military service members and veterans: a comparison and commentary. Eur J Psychotraumatol. 2018;9(1):1486121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Amstadter AB, Moscati A, Oxon MA, Maes HH, Myers JM, Kendler KS. Personality, cognitive/psychological traits and psychiatric resilience: A multivariate twin study. Pers Individ Dif. 2016;91:74–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Denckla CA, Consedine NS, Spies G, et al. Associations between neurocognitive functioning and social and occupational resilience among South African women exposed to childhood trauma. Eur J Psychotraumatol. 2017;8(1):1394146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cahill S, Hager R, Chandola T. The validity of the residuals approach to measuring resilience to adverse childhood experiences. Child Adolesc Psychiatry Ment Health. 2022;16(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen Y, Huang CC, Yang M, Wang J. Relationship Between Adverse Childhood Experiences and Resilience in College Students in China. J Fam Violence. 2022:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Subramaniam M, Abdin E, Vaingankar JA, et al. Association of adverse childhood experiences with diabetes in adulthood: results of a cross-sectional epidemiological survey in Singapore. BMJ Open. 2021;11(3):e045167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rosenberg SD, Lu W, Mueser KT, Jankowski MK, Cournos F. Correlates of adverse childhood events among adults with schizophrenia spectrum disorders. Psychiatr Serv. 2007;58(2):245–253. [DOI] [PubMed] [Google Scholar]
- 72.Bonomi AE, Cannon EA, Anderson ML, Rivara FP, Thompson RS. Association between self-reported health and physical and/or sexual abuse experienced before age 18. Child Abuse Negl. 2008;32(7):693–701. [DOI] [PubMed] [Google Scholar]
- 73.Black CJ, Yiannakou Y, Houghton LA, Ford AC. Epidemiological, Clinical, and Psychological Characteristics of Individuals with Self-reported Irritable Bowel Syndrome Based on the Rome IV vs Rome III Criteria. Clin Gastroenterol Hepatol. 202018(2):392–398. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. T1-weighted acquisition parameters
Table S2. Region abbreviation and network key
Table S3. Main effects of diagnosis and sex, and their interaction, in resilience-related connectivity (corresponds to Figure 3)
Table S4. Clinically relevant reductions in resilience-related connectivity in all patients, men with IBS, and women with IBS (corresponds to Figure 4)
Figure S1. Sex-specific correlations between reduced resilience-related connectivity (from Figure 3A,C) and worse IBS symptom severity, assessed by the IBS-SSS, and disease-specific quality of life, assessed by the IBS-QOL.
Data Availability Statement
De-identified and raw neuroimaging data are available through the NIH-funded Pain and Interoception Imaging Network (PAIN) data repository (https://www.painrepository.org/repositories). Access to the data is based on membership according to a collaborative principle.


