Abstract
Subjects are often willing to pay a cost for information. In a procedure that promotes paradoxical choices, animals choose between a richer option followed by a cue that is rewarded 50% of the time (No Info) vs. a leaner option followed by one of two cues that signal certain outcomes: one always rewarded (100%) and the other never rewarded, 0% (Info). Since decisions involve comparing the subjective value of options after integrating all their features, preference for information may rely on cortico-amygdalar circuitry. To test this, male and female rats were prepared with bilateral inhibitory Designer Receptors Exclusively Activated by Designer Drugs (DREADDs) in the anterior cingulate cortex, orbitofrontal cortex, basolateral amygdala, or null virus (control). We inhibited these regions after stable preference was acquired. We found that inhibition of the anterior cingulate cortex destabilized choice preference in female rats without affecting latency to choose or response rate to cues. A logistic regression fit revealed that previous choice predicted current choice in all conditions, however previously rewarded Info trials strongly predicted preference in all conditions except in female rats following anterior cingulate cortex inhibition. The results reveal a causal, sex-dependent role for the anterior cingulate cortex in decisions involving information.
Keywords: chemogenetics, frontal cortex, information-seeking, value-based decision making
Introduction
The environment is full of unpredictable events, and information that reduces uncertainty about such events allows an organism to better predict and prepare for the future. However, several psychiatric conditions are characterized by a strong preference for information, or an intolerance of uncertainty, including autism spectrum disorder, substance use disorders, attention-deficit hyperactivity disorder (ADHD), generalized anxiety disorder, and obsessive-compulsive disorder (Tolin et al. 2003; Dugas et al. 2004; Boulter et al. 2014; Jenkinson et al. 2020; Mandali et al. 2021). Intolerance of uncertainty has been linked to “pathological doubt” during which the preference for information is dramatically increased (Tolin et al. 2003).
Obtaining information can be crucial for survival. However, if information cannot be used to modify action, a bias toward information can be considered paradoxical or even suboptimal because organisms should not invest resources to obtain information that does not affect the outcome of a choice. Imagine a situation in which an organism chooses between two sources of delayed reward; if rewards are signaled before each choice, it will be worth investing in that information to choose the best option. In contrast, if the outcomes are signaled after the choice, information is useless, since the organism cannot change its choice. The latter has been broadly studied at the behavioral level. In the so-called paradoxical or suboptimal choice task (Stagner and Zentall 2010; McDevitt et al. 2016; González et al. 2023), animals are presented with two alternatives, one providing a lower rate of reinforcement with different stimuli indicating the presence (S+) or absence (S−) of delayed food (i.e. Info) and another one (S3) providing a higher rate of reinforcement but with nondifferential stimuli signaling food (i.e. No Info) (see Fig. 1A). Birds consistently prefer the leaner but informative option despite the difference in reinforcement rates between alternatives (Stagner and Zentall 2010; Fortes et al. 2016; Macias et al. 2021). However, rats more often show high variability, with some experiments showing preference for the No Info option (Orduña and Trujano 2015; Orduña et al. 2016) and some showing preference for the Info option (Cunningham and Shahan 2019; Ajuwon et al. 2021). We propose that such individual differences allow us to test if animals use different strategies to make decisions, which can also shed light on how value is assigned.
Fig. 1.
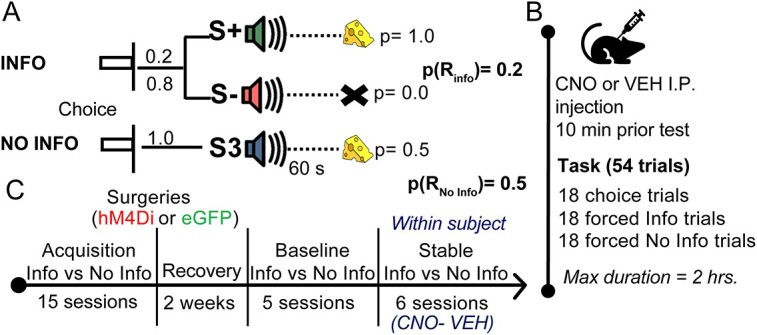
Task structure and experimental timeline. A) Rats choose between two levers (left or right). After pressing once, both levers retract and an auditory cue commences. If the rat chooses the “Info” alternative, on 20% of the trials, tone S+ plays for 60 s always ending with the delivery of one sugar pellet; the other 80% of the trials sound S− plays for 60 s always ending without food. If the rat chooses the “No Info” option, a third sound S3 plays for 60 s ending with the delivery of one sugar pellet in 50% of the trials. B) CNO or VEH was administered 10 min before starting the task. Each session consisted of 54 trials in which rats received 18 choice trials (both levers available to choose), 18 forced info trials (only the lever associated with the informative alternative was available), and 18 forced No Info trials (only the No Info lever was available). Animals were required to complete the session in 2 h. C) Rats were trained on the task over 15 sessions before they underwent bilateral viral infusion of inhibitory hM4Di mCherry DREADDs or null virus enhanced green fluorescent protein (eGFP) in ACC, BLA, or OFC. After 2 weeks of recovery, baseline performance was reestablished for five sessions before administrating CNO and VEH (order counterbalanced) for three consecutive sessions with a wash-out day between drugs.
The neural substrates of reinforcement uncertainty have been broadly researched, with evidence pointing to a distributed network that involves the prefrontal cortex (PFC) (Rushworth and Behrens 2008), striatum, hippocampus, basolateral amygdala (BLA) and mediodorsal thalamus (Winstanley and Floresco 2016; Soltani and Izquierdo 2019). Nevertheless, to our knowledge, there is no investigation of the specific brain regions using this behaviorally well-documented paradoxical choice procedure. Additionally, compared to primates (Iigaya et al. 2016; Iigaya et al. 2020), there is a paucity of rodent studies on the value of noninstrumental information and its neural substrates. When human subjects are assessed on choices between two cued alternatives— an informative vs. a noninformative one (but with no difference in overall reinforcement rate), subjects reliably prefer advanced information and blood-oxygen-level-dependent (BOLD) signal in ventromedial PFC tracks the value of the anticipation of reward (Iigaya et al. 2016, Iigaya et al. 2020). Neural correlates of uncertainty have been found in different subregions of the PFC in several species, among them, the orbitofrontal cortex (OFC) and anterior cingulate cortex (ACC) (Rushworth and Behrens 2008; Bromberg-Martin and Hikosaka 2009; Wallis 2012; Blanchard et al. 2015; Bromberg-Martin and Monosov 2020). Electrophysiological recording studies in rats and monkeys demonstrate that activity in OFC is associated with stimulus (cue) value and expected uncertainty or probabilistic risk (Jo and Jung 2016; Riceberg and Shapiro 2017; Namboodiri et al. 2019; Jenni et al. 2023). Similarly, studies have shown that ACC neurons signal value, uncertainty of predictions about rewards, or punishments (Monosov 2017; Jezzini et al. 2021) and track trial-by-trial outcomes of choice (Procyk et al. 2000; Shidara and Richmond 2002). On the other hand, in probabilistic-discounting tasks, when rodents are required to select between a large uncertain option versus a small certain option, inactivation of BLA decreases the likelihood of choosing the uncertain option (Ghods-Sharifi et al. 2009; Stopper and Floresco 2011; St Onge et al. 2012). This suggests the contribution of BLA may be biasing choice toward larger rewards, especially when the delivery of these rewards is uncertain. Furthermore, selectively disrupting PFC-to-BLA connections increases choice of the larger yet increasingly uncertain reward, indicating that communication between these two regions serves to modify choice biases (St Onge et al. 2012). Finally, lesions to either OFC or BLA (or their connection) results in slower learning about which option has the better payout, suggesting that these areas form a functional circuit for the adaptation of reward-maximizing strategies (Zeeb and Winstanley 2011; Zeeb and Winstanley 2013).
In this experiment, we examined the specific contributions of ACC, OFC, and BLA to this seemingly “suboptimal” choice phenomenon via chemogenetic manipulation. On the additional evidence that these regions participate in decision confidence under uncertainty (Lak et al. 2014;Stolyarova et al. 2019), we hypothesize that they may play vital, yet dissociable roles in decisions involving information value. To study this, we inactivated these regions during stable preference. We found that inhibition of ACC, but not OFC or BLA, destabilized choices involving information in females and reduced their ability to use previous information to guide current decisions.
Materials and methods
Animals
Sixty-six Long–Evans rats (Rattus Norvegicus), 36 females and 30 males, acquired from Envigo served as subjects. Subjects were between aged postnatal days (PND) 90 and 140 at the start of the experiment. Subjects were pair-housed before and single-housed after surgeries in transparent plastic tubs with wood shaving bedding in a vivarium maintained on a reverse 12 h light cycle. Experiments were conducted during the dark portion of the cycle at a minimum of 5 days per week. A progressive food restriction schedule was imposed prior to the beginning of the experiment to maintain rats at 85% of their initial free-feeding weights. Water was always available in their home cages. The procedures used in this experiment were conducted under approval and following the guidelines established by the Chancellor’s Animal Research Committee at UCLA.
Viral constructs
To express DREADDs on putative projection neurons in ACC, OFC, or BLA, an adeno-associated virus AAV8 driving the hM4Di-mCherry sequence under the CaMKIIa promoter was used (AAV8-CaMKIIa-hM4D(Gi)-mCherry, packaged by Addgene, viral prep #50477-AAV8), thus targeting pyramidal neurons. A virus without the hM4Di DREADD gene but containing the fluorescent tag enhanced Green Fluorescent Protein, eGFP (AAV8-CaMKIIa-EGFP, packaged by Addgene, viral prep #50469-AAV8) was infused into the ACC or BLA as a null virus control (there was no OFC null virus group). This null virus allowed us to control for nonspecific effects of surgical procedures (i.e. craniotomy, anesthesia), exposure to AAV8 and nonspecific effects of drug or injections.
Behavioral apparatus
This experiment was conducted using operant testing chambers, measuring 30 × 25 × 20 cm (L × W × H). Each chamber was housed in separate sound- and light-attenuating environmental isolation chests (ENV-008, Med Associates, Georgia, VT). The front and back walls and ceiling of the chambers were constructed of clear Plexiglas, the side walls were made of aluminum, and the floors were built of stainless-steel rods measuring 0.5 cm in diameter, spaced 1.5 cm center to center.
Each chamber had a pellet dispenser (ENV-203-45, Med Associates) and a cup-type pellet receptacle (ENV-200R1M, Med Associates). When activated, one sucrose pellet was delivered into the cup. The opening of the cup was equipped with an infrared beam and photodetector to record entries into the food niche. A 3.5 cm wide operant lever was positioned one cm to the left and right of the food niche on the metal wall.
A speaker (ENV-224DM) on the ceiling of the chamber delivered a siren (cycling between 1,500 and 1,900 Hz at a 0.5 s rate), a 1,000 Hz tone and a white noise, all were 8 dB above background to serve as the initial S+, S− and S3; and another siren (cycling between 4,000 and 3,500 Hz at a 0.5 s rate), a 3,000 Hz tone and a click train (4/s) 8 dB above background served as a second set of S+, S−, and S3 cues, counterbalanced across subjects. A diffuse incandescent light (ENV-227 M, Med Associates) was located on the bottom panel of the right-side chamber wall, 6 cm from the ceiling.
Surgical procedures
After completing the training phase, rats were anesthetized with isoflurane for bilateral infusion of ACC, OFC, or BLA inhibitory (Gi) DREADDS (AAV8-CaMKIIα-hM4D(Gi)-mCherry, Addgene, Cambridge, MA, viral prep #50477-AAV8) or eGFP (AAV8-CaMKIIa-EGFP, Addgene, Cambridge, MA, viral prep #50469-AAV8). Craniotomies were created, and a 26-gauge guide cannula (PlasticsOne, Roanoke, VA) with a dummy injector was lowered, after which the dummy injector was replaced with a 33-gauge internal injector (PlasticsOne, Roanoke, VA) was inserted. Animals were infused with two bilateral sites of injections in BLA (0.2 μL at 0.1 μL/min in AP: −2.5, ML: ±5.0, DV: −8.6, and 0.1 μL at 0.1 μL/min in AP: −2.5, ML: ±5.0, DV: −8.3; total volume per side = 0.3 μL), two bilateral sites in OFC (0.15 μL at 0.1 μL/min in AP: 4.0, ML: ±2.5, DV: −4.4, and 0.2 μL at 0.1 μL/min in AP: 3.7, ML: ±2.5, DV: −4.6; total volume per side = 0.35 μL) and one bilateral site in ACC (0.3 μL at 0.1 μL/min AP: +3.7, ML: ±0.8, DV: −2.4 for a total volume of 0.3 μL per side). All measurements were taken from bregma. After infusion, the cannula was left in place for 10 additional minutes to allow diffusion.
Drug treatment
Before testing, rats were given intraperitoneal (i.p.) injections of vehicle (VEH: 95% saline +5% DMSO) or clozapine-N-oxide, CNO (3 mg/kg CNO in 95% saline +5% DMSO) 10 min prior to beginning the behavioral task. The injection time prior to testing was shorter than some other work (Hart et al. 2020; Ye et al. 2023) to account for the longer duration of behavioral testing sessions as in a previous experiment (Stolyarova et al. 2019b). CNO and VEH were administered during Stable Info versus No Info condition in a within-subject design (Fig. 1B), where animals received three sessions of CNO (or VEH) followed by a washout day with no injection or training and then three sessions of VEH (or CNO) followed by another washout day, such that the order of drug administration was counterbalanced across animals.
Behavioral procedure
Pretraining
Before training, 10 sucrose pellets were given in the rat home cage to avoid food neophobia. On the first day, rats were trained to eat pellets from the pellet tray by delivering one pellet every 20 ± 15 s in the chamber (actual intertrial interval [ITI] values = 5, 10, 15, 20, 25, 30, and 35 s) for a total of 40 pellets. On days 2 and 3, rats were trained in an autoshaping procedure to lever press the left and right lever (lever presented in alternate order). Reinforcements were delivered following each lever press (i.e. a continuous reinforcement schedule) for a maximum of 40 pellets within a session, or after 30 min had elapsed.
Training of the paradoxical choice task
The training stage of the task was comprised of two types of trials: choice and forced trials (Fig. 1C). In choice trials, rats were required to choose between two levers available simultaneously. A choice trial started with the simultaneous insertion of both levers and the houselight turning on, the levers remained extended until a choice was made (no time limit). A choice was made by pressing a lever one time. After the choice was made, both levers retracted and the houselight turned off. If the lever associated with the Info option was pressed, 20% of the time, an auditory cue (S+) was presented for 60 s always ending with food; the other 80% of the time, another auditory cue (S−) was on for 60 s never ending with food. The total percentage of reinforced trials was 20%. If the rat chose the No Info alternative, a third auditory cue (S3) was presented for 60 s and ended with food on half of the trials. The percentage of reinforcement on this alternative was 50%. On forced trials, only one lever was presented at a time, following the same contingencies described above. All trials were separated by a 10 s ITI during which all stimuli were off. A single session constituted 54 trials, 18 choice trials, and 36 forced trials. The maximum duration of a session was set to 120 min. The assignment of sounds to S+, S−, or S3 and the lever side for each option was counterbalanced across animals but remained the same for individual animals throughout training. This condition lasted 15 sessions.
Stable preference
Approximately 2 weeks after surgery, rats received five training sessions as described above as a reminder of the task, used as a measure of baseline preference (Baseline Info vs. No Info, Fig. 1B). After this, rats received three sessions of the task with an i.p. injection of CNO and three sessions with an injection of VEH (order counterbalanced across animals). After the three-session round of injections, animals underwent a washout day in which they were not tested on the task. This condition lasted six sessions (8 days).
Histology
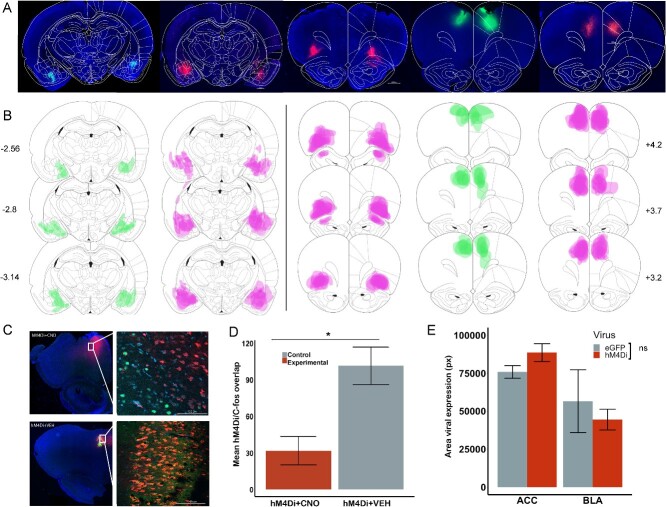
At the conclusion of the experiment, rats were euthanized by an overdose of sodium pentobarbital (Euthasol, 0.8 mL, i.p.; VetOne, Paris, France) and transcardially perfused with phosphate-buffered saline (PBS) followed by 10% buffered formalin acetate. Brains were extracted and post-fixed in this solution for 24 h followed by 30% sucrose cryoprotection. Tissue was sectioned in 40 μM thick slices and cover slipped with DAPI mounting medium (Prolong gold, Invitrogen, Carlsbad, CA) visualized using a BZ-X710 microscope (Keyence, Itasca, IL), and analyzed with BZ-X Viewer software (Fig. 2A).
Fig. 2.
Inhibitory DREADDs in ACC, BLA, and OFC; validation of ACC inhibition via c-fos immunohistochemistry. A) Representative placement of inhibitory hM4Di DREADDs and eGFP null virus at the anterior–posterior (AP) level + 3.7 for ACC and OFC, and − 2.8 for BLA relative to Bregma. Note, there were no eGFP for OFC. B) Reconstructions of placement of inhibitory hM4Di DREADDs (pink) and eGFP null virus (green) at AP level + 4.2, +3.7 and +3.2 for ACC, OFC, and AP −2.56, −2.8, and − 3.14 for BLA relative to Bregma. C) Representative image showing DAPI (blue), hM4Di-mCherry (red), c-fos immunoreactivity (green), and their overlap after injections of CNO and VEH in ACC at 2.5× and 20×. D) Mean cell count of four images per condition, hM4Di + CNO, hM4Di + VEH for ACC. E) Mean spread of viral expression across brain regions; pixel quantification was done using ImageJ software. Ns = nonsignificant, *P < 0.05, Mann–Whitney U test.
We have previously validated the efficacy of our CNO-activated inhibitory DREADDs ex vivo in slice (Stolyarova et al. 2019; Aguirre et al. 2023), in vivo using electrophysiological recordings (Ye et al. 2023), and behaviorally (Stolyarova et al. 2019b; Hart et al. 2020; Aguirre et al. 2023; Ye et al. 2023) in OFC, ACC, and/or BLA. A group of ACC animals (n = 4) received a CNO or VEH injection 30 min prior to the beginning of the perfusion. In this group of animals, a subset of the 40 μm coronal sections were also stained for c-Fos, following an adapted Abcam protocol for dry-mounted slides (Schneider Gasser et al. 2006). In this protocol, mounted tissue was marked with a hydrophobic pen, any medium was added with a pipette and removed using a vacuum. The tissue was incubated for 22 to 24 h at 4 °C in a solution of primary antibody (1:2,000 rabbit polyclonal to c-Fos, Abcam, Cambridge, MA) with 5% normal goat serum (Abcam, Cambridge, MA), and 0.1% TritonX-100 (Sigma, St. Louis, MO) in PBS. After the incubation, the tissue was washed with PBS three times during a 5 min period; then, the brain slides were incubated in a secondary antibody for 90 min protected from light at room temperature (0.1% TritonX-100, 5% normal goat serum, PBS solution with 1:500 goat anti-rabbit Alexa 488; Abcam, Cambridge, MA). Slides were washed again as described above. Then, tissue was incubated during 3 min with quenching reagent (1:1 ratio) to reduce background. Slides were washed with PBS and then cover-slipped with fluoroshield DAPI mounting medium (Abcam, Cambridge, MA). c-Fos immunoreactivity quantification images were visualized with a 20× objective with a 724 μm × 543 μm field of view using a confocal microscope (Model LSM 900, Zeiss, Germany). For each region, four images were taken from two or three coronal sections from both hemispheres at the same approximate AP coordinate (ACC +3.7 mm). To verify DREADD-mediated inhibition of pyramidal-neurons in ACC after CNO or VEH administration, we compared the number of c-fos-positive cells in the hM4Di-expressing regions following CNO vs. VEH injections. Cell counts were conducted using ImageJ software (Fig. 2C). We found greater overlap in the number of c-fos-positive cells in hM4Di + VEH than hM4Di + CNO cell areas (Mann–Whitney U test: W = 0, P = 0.009). We did not find a difference between the c-fos-positive cells in DAPI areas (DAPI + CNO vs. DAPI + VEH; Mann–Whitney U test: W = 8.5, P = 0.61) (Fig. 2D). DREADDs and eGFP expression were determined by matching histological sections to a standard rat brain atlas (Paxinos and Watson, 2006) and quantifying fluorescence using ImageJ (Rueden et al. 2017) where two independent raters measured the area of fluorescent pixels for each animal per hemisphere (Fig. 2E).
To approximate the amount of viral expression observed in the tissue across brain regions (ACC, OFC, and BLA) and virus (hM4Di and eGFP), we quantified the max area of fluorescent pixels of every animal presented in the reconstructions (Fig. 2B). We used an independent-samples t-test to compare viral spread (average fluorescent pixel area) for ACC hM4Di (MeanACC hM4Di = 88,542.87) vs. ACC eGFP (MeanACC eGFP = 75,795.5). The same analysis compared BLA hM4Di (MeanBLA hM4Di = 44,399.54) and BLA eGFP (MeanBLA eGFP = 56,519.33). No comparison with eGFP was conducted for OFC hM4Di (MeanOFC hM4Di = 100,132) given the absence of eGFP animals in OFC. However, there were no significant differences between ACC and OFC hM4Di expression (P = 0.26), and we show below that control groups were not different from each other and could be collapsed into one group. We found no significant differences in the viral expression between DREADDs hM4Di and control eGFP for ACC (t(18.52) = −1.77, P = 0.093, −95% confidence interval (CI) [27,850, 2,355]) or for BLA (t(2.46) = 0.56, P = 0.624, 95% CI [−66,729, 90,969]).
Data analysis
All analyses were performed via custom-written code in MATLAB (MathWorks, Inc., Natick, MA). There were three main conditions in our analyses: (i) preference for the Info alternative (Info choice divided by the total number of choices) (ii) latency to choose (the time from the beginning of the trial until a lever press was made), and (iii) response rate (RR; the number of entries into the food port) during the 60 s cue duration.
Preference data were analyzed with a series of mixed-effects general linear models (GLMs) (fitglme function; Statistics and Machine Learning Toolbox) first in omnibus analyses that included all factors (drug, virus, and sex), and all groups (ACC, BLA, OFC, and control). Analyses were further pursued pending significant interactions in the full model. All post hoc tests were corrected for the number of comparisons (with Bonferroni–Holm correction). Coding of variables for GLMs was as follows: 0 = females and 1 = males; 0 = control and 1 = hM4Di; and 0 = VEH and 1 = CNO.
For trial-by-trial data, we employed the bootstrap method to estimate the odds ratio (OR) and its CIs in logistic regression, providing a robust measure of the strength and direction of the association between the predictor variable and the outcome (Davison and Hinkley 2013). We generated 1,000 bootstrap samples from the original dataset, where each sample was of the same size as the original and was constructed by random sampling with replacement. For each bootstrap sample, we fitted a logistic regression model (logit function in MATLAB) and estimated the OR for the predictor variable. This process resulted in a distribution of 1,000 ORs, reflecting the sampling variability of the estimate. We then computed the 10th to 90th percentiles of this empirical distribution to obtain an 80% CI for the OR. We aimed to predict the probability of choice using three features: (i) latency to choose, (ii) previous choice, and (iii) Previous reward, depending on which option was chosen (Info or NoInfo trial). We used only free-choice data for the outcome variable and the previous choice and reward as either from free-choice or forced-choice data. Coding of variables for the logistic regression was as follows: 0 = females and 1 = males; 0 = NoInfo choice and 1 = Info choice; 0 = Reward | Info (reward given Info choice) and 1 = Reward | NoInfo (reward given NoInfo choice); and region as 1 = ACC, 2 = control, 3 = BLA, and 4 = OFC. Note that the β coefficients here correspond to log(odds ratio), which transforms odds ratio (>0) to a real number indicating how much one unit increase of the predictor contributes to log odds of the event occurring (vs. not occurring).
Statistical significance for GLM analyses was noted as P-values of less than 0.05, and P-values between 0.05 and 0.06 were noted as trending toward significance. Statistical significance for the results of the trial-by-trial analysis was noted as P-values of less than 0.01.
Results
Control group
Our control group was created by combining the animals that were infused with the eGFP virus and animals that were originally assigned to the eGFP group but wherein the histological analysis revealed either no-expression (n = 9) or unilateral expression (n = 4) compared with bilateral expression of eGFP (n = 9). To assess if these groups could be collapsed in further analyses, we performed a GLM for each phase of the experiment. Using the mean of the last three sessions of training, a GLM was conducted for mean preference using sex and control group (eGFP, unilateral, or no-expression) as between-subject factors and individual rat as a random factor (full model: γ ~ [1 + sex * control group + (1 | rat)]. We found no significant effect of control group (P = 0.54), sex (P = 0.335), or control group * sex interaction (P = 0.955). Similarly, a GLM was performed on preference change during the stable preference phase of the experiment, adding drug as a within-subject factor (full model: γ ~ [1 + drug * sex*control group + (1 + drug| rat)]. We found no significant predictors or interactions. Based on these results, the animals in the control groups were collapsed and treated as a single group for subsequent analysis and added to the “virus” factor as a fourth group (ACC hM4Di, BLA hM4Di, OFC hM4Di, and control).
Training: rats developed a consistent preference, responded quickly during choice trials, and responded more to the informative cue (S+)
Choice
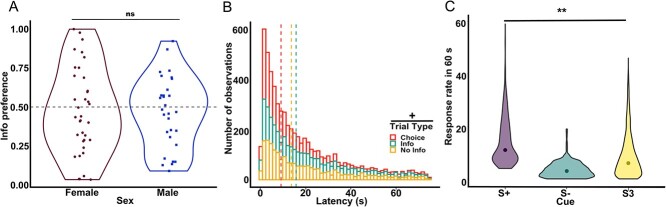
Training data for preference for the info option were first analyzed across all sessions, to evaluate learning of preference on the task. A GLM was conducted for preference for the Info option using sex as between-subject factor, session as within-subject factor, and individual rat as a random factor using the following formula: γ ~ [1 + sex*session + (1 + session| rat)]. We found a main effect of session [GLM: βsession = −0.008, t(868) = −2.09, P = 0.03] and sex [GLM: βsex = −0.12, t(868) = −2.34, P = 0.02] but no significant interaction [GLM: βsession*sex = 0.006, t(868) = 1.13, P = 0.26] (see Supplement 1). A main effect of session indicates the preference changed across training, wherein animals begin their preference for information around a probability of 0.5 (indifference) before switching to a preference for the Info or NoInfo alternative. While the main effect of sex illustrates that preference between male and female rats differed, the learning rates did not differ by sex given the nonsignificant interaction of sex by session.
To evaluate whether the observed preference remained consistent within each animal, we compared preference in no-drug conditions before any drug experience. To do so, the last three sessions of preference data for training were averaged and compared to the preference in the last three sessions of the baseline after surgery. A GLM comparing the averaged preference during training and baseline phase was performed, in which phase (training and baseline) was a within-subject factor and sex was a between-subject factor (full model: γ ~ [1 + phase * sex (1 + phase| rat)]. No significant effects (psex = 0.93, pphase = 0.26) or interactions (psex*phase = 0.73) were found, suggesting that preference remained stable across conditions once acquired (see Fig. 3A).
Fig. 3.
Preference was stable, and response latency and the RR exhibited the typical phenotype. A) Mean preference for the info alternative for the last three sessions of training, with individual rats represented as scatter plots. B) Total number of observations for latency to choose, on the last three sessions of training are shown for each trial type. Dashed lines indicate the median latency. A marginally significant difference was found between trial types, where responses in choice trials were faster than in forced (Info and NoInfo) trials. C) Violin plots of the distribution of total RR during the 60 s cue presentations are presented for each cue during the last three sessions of training. Dots indicate the median RR for each cue. A significant effect of cue was found. **P < 0.01, +P = 0.06, ns = nonsignificant.
Latencies
A GLM was conducted on median latencies in the last three sessions of training using trial type (forced Info, forced NoInfo, and choice) as within-subject factors, sex as a between-subject factor, and individual rat as a random factor using the following formula: γ ~ [1 + trial type * sex + (1 + trial type| rat)]. The results yielded a trend for an effect of trial type [GLM: βtrial type = 6.16, t(179) = 1.85, P = 0.06] wherein rats tended to be faster during choice trials (MedianChoice = 10.6 s, SEM ± 1.69) than either of the forced trials (MedianForced Info = 21.8 s, SEM ± 3.97, and MedianForced NoInfo = 18.1 s, SEM ± 3.81) (see Fig. 3B). This result replicates what previous literature has shown in this task in pigeons and starlings (González et al. 2023).
Response rate
The median RR during the 60 s cue was analyzed for the last three sessions of training. A GLM was conducted for median RR using sex as a between-subject factor, and individual rat and cue (S+, S− and S3) as random factors using the following formula: γ ~ [1 + sex + (1|rat:cue)]. Cue was introduced as a random factor to account for the fact that rats were presented with different frequencies of cues given the programmed contingencies (e.g. S+ present only 20% of all Info trials), but this difference also depended on the rat’s choice. For instance, an animal that chooses the No Info alternative exclusively during choice trials would have more presentations of cue S3 than any other cue. We found a main effect of cue [GLM: βcue = −2.11, t(185) = −2.89, P = 0.004], indicating that the RR was greater for the cue predicting food (MedianS+ = 11 presses/minute, SEM ± 0.34) than to the No Info cue (MedianS3 = 6 presses/minute, SEM ± 0.14), but the lowest RR was to the cue predicting absence of food (MedianS- = 3 presses/minute, SEM ± 0.06). This is also in line with previous work in which the lowest RR was reported for S− and typically a greater RR for S+ than S3 (Hinnenkamp et al. 2017; Gonzalez and Blaisdell 2021).
Info preference: inhibition of ACC destabilized preference in female but not male rats
Choice
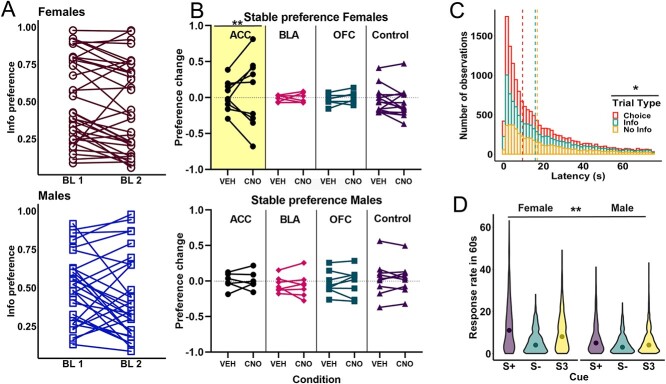
Stable preference was analyzed by averaging the three sessions of baseline, and then comparing this choice preference with the three sessions of CNO and three sessions of VEH for each subject. An initial analysis on actual preference was performed (see Supplement 2) during baseline (BL), CNO, and VEH conditions for each group (ACC, BLA, OFC, and control) and sex. The formula of the GLM was γ ~ [1 + group * drug * sex + (1 + drug| rat)]. We found no effect of any factor (P > 0.41) nor interactions (P > 0.26), probably due to the high variability in initial preference within each group, and therefore, any potential changes in preference depended on each rat’s baseline. Thus, we compared changes in stable preference by calculating the absolute difference between preference in baseline-to-CNO and baseline-to-VEH conditions (see Fig. 4A). A GLM was conducted for absolute preference change using drug as a within-subject factor, virus and sex as between-subject factors, and individual rat as random factor using the following formula: γ ~ [1 + virus * drug * sex + (1 + drug| rat)]. We found a significant interaction of drug*virus [GLM: βdrug*virus = 0.065, t(124) = 4.88, P = 3.24e-06] and sex*drug*virus [GLM: βsex*drug*virus = −0.065, t(124) = −3.15, P = 0.002]. Thus, we were justified to conduct follow-up analyses with individual group comparisons: γ ~ [1 + virus * drug * sex + (1 + drug| rat)]. For the comparison of ACC hM4Di vs. control, we found a significant interaction of drug*virus [GLM: βdrug*virus = 0.074, t(68) = 5.58, P = 4.61e-07] and drug*virus*sex interaction [GLM: βsex*virus*drug = −0.077, t(68) = −3.70, P = 0.0004]. Comparisons of OFC hM4Di vs. control and BLA hM4Di vs. control were not significant. Post hoc analysis using Bonferroni correction and sex as a covariate for the ACC hM4Di group resulted in a significant effect of drug [t(29) = 3.66, P = 0.002]. In contrast, there was no significant effect of drug in the control group [t(41) = 1.59, P = 0.239]. Overall, these results indicate that ACC inhibition causes a destabilization of preference in female rats.
Fig. 4.
Inhibition of ACC destabilized info preference in female but not male rats, while latencies and the RR were unaltered by inhibition. A) Baseline info preference for females (top) and males (bottom) over two baselines. The first baseline (BL1) was obtained after surgery, and the second baseline (BL2) was obtained at the end of the experiment. B) Mean change in preference for the info alternative between the last three sessions of baseline after surgery minus the preference during VEH and CNO administration for females (top) and males (bottom). Note that a positive change indicates a shift in preference toward the info option, whereas a negative change indicates a preference shift toward the NoInfo option. Values around 0 indicate no change in preference. C) Total number of observations of latency to choose across drug conditions is shown for each trial type. Dashed lines indicate the median latency. A significant difference between trial types was found, where choice trials were faster than forced (Info and NoInfo) trials. D) Violin plots of the distribution of total RR during the 60 s cue presentations are presented for each cue for all drug conditions for females (left) and males (right). Dots indicate the median RR for each cue. Females responded more than males and a significant effect of cue was found. **P < 0.01, *P < 0.05.
Latencies
Latency to choose was analyzed by calculating the median for CNO and VEH sessions (see Fig. 4B). A GLM was conducted for median latency using drug and trial type as within-subject factor, virus and sex as between-subject factors, and individual rat as a random factor using the following formula: γ ~ [1 + trial type * virus * drug * sex + (1 + trial type + drug| rat)]. We found a main effect of trial type [GLM: βtrial type = 5.61, t(380) = 2.19, P = 0.028] indicating that rats were faster responding during choice trials (MedianChoice = 8.4 s, SEM ± 0.4) than forced trials (MedianForced Info = 14.8 s, SEM ± 0.66, and MedianForced NoInfo = 15.1 s, SEM ± 0.62) (see Supplement 4.A). Note that we did not find any difference by sex or virus, suggesting that the change in preference observed in the female rats following ACC inhibition did not affect decision speed.
Response Rate
The RR during the 60 s cue duration was also analyzed by calculating the median during CNO and VEH sessions (see Fig. 4C). A GLM was conducted for the median RR using drug as within-subject factor, virus and sex as between-subject factors, and individual rat and cue (S+, S−, and S3) as random factors using the following formula for the full model: γ ~ [1 + cue * virus * drug * sex + (1 + drug| rat:cue)]. As in training, cue was defined as a random factor given that there were different probabilities in experiencing each cue based on an individual rat’s choices. We found a main effect of sex [GLM: βsex = −7.87, t(379) = −2.17, P = 0.03], with females showing higher RRs (MedianFemale = 7 presses/min, SEM ± 0.06) than males (MedianMale = 4 presses/min, SEM ± 0.46) (see Supplement 4.B).
Previously rewarded information choices are not as predictive of current choices in ACC-inhibited females
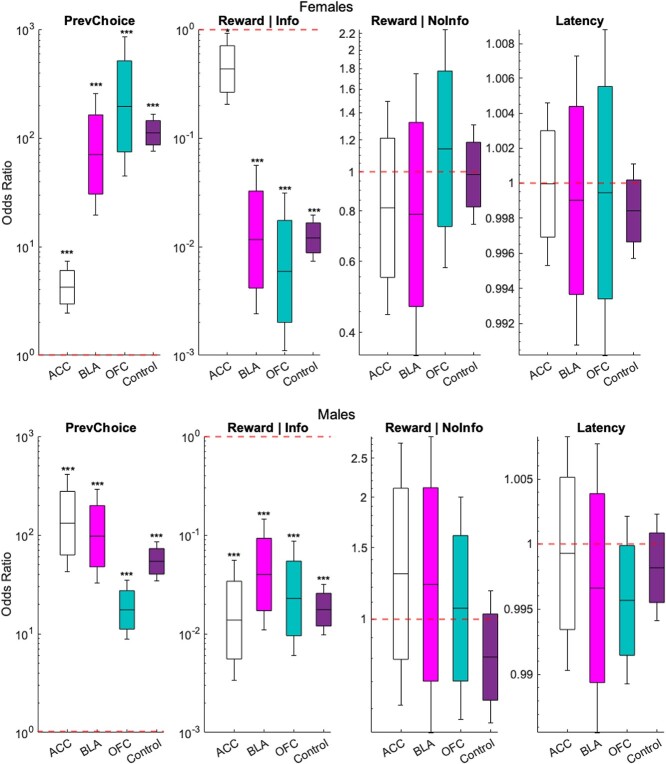
To further understand the trial-by-trial nature of the effect of ACC inhibition on choice behavior, we fit a logistic regression, a type of GLM for binary classification, to each rat’s data that belonged to one of the experimental groups (ACC hM4Di-CNO, BLA hM4Di-CNO, OFC hM4Di-CNO), or control group-CNO for females and males, with bootstrapping (see Data analysis). Using the three features described in the analysis section, we aimed to predict the probability of current choice. We computed the odd ratio (OR) and 95% and 80% CI for each condition’s regression fit: If an OR was greater than 1, it represented an increase in the odds of the outcome happening given a one-unit increase in the predictor. If the OR was less than 1, it represented a decrease in its odds, given a one-unit increase in the predictor. And finally, an OR of exactly 1 indicated that the predictor did not affect the probability of the outcome.
The model resulted in a significant predictor of previous choice to current choice for both females and males across all groups [tACC-fem(392) = 5.19, P = 2.05e-07; tBLA-fem(2,385) = 6.55, P = 5.66e-11; tOFC-fem(249) = 7.05, P = 1.77e-12; tcontrol-fem(331) = 23.64, P = 1.23e-123; tACC-male(314) = 8.48, P = 2.22e-17; tBLA-male(1,277) = 8.26, P = 1.39e-16; tOFC-male(266) = 8.31, P = 8.80e-17; tcontrol-male(334) = 17.12, P = 9.02e-66]. Previous Reward | Info was also a significant predictor of current choice for females and males across groups [tACC-fem(392) = −2.28, P = 0.02; tBLA-fem(2,385) = −5.61, P = 1.92e-08; tOFC-fem(249) = −6.05, P = 1.42e-09; tcontrol-fem(331) = −17.85, P = 2.49e-71; tACC-male(314) = −6.11, P = 9.86e-10; tBLA-male(1,277) = −5.08, P = 3.68e-07; tOFC-male(266) = −5.56, P = 2.63e-08; tcontrol-male(334) = −13.59, P = 4.35e-42]. However, previous Reward | No Info and Latency were not significant predictors of current choice (P > 0.19) (Fig. 5). Given that we found that the effect of previous reward depended on which option was chosen in the trial, we calculated the percentage of switching if rats received a reward for the Info option (that is, switching to the No Info, given that they selected the Info option and received a reward): 72.15% for females and 86.95% for males across all groups. This pattern was not observed when rats received a reward after choosing the No Info option (17.85% for females and 49.84% for males across conditions). This result indicates adoption of a win–stay strategy following No Info choices but a win–switch strategy following Info choice, generally across all groups. Note that this is not the case when we ignore reward, for choice alone (PrevChoice, Fig. 5): the percentage of switch in males is 18.38% and 27.38% for Info and No Info choices, respectively, and 25.47% and 27.42% in females for Info and No Info choices, respectively.
Fig. 5.
Rewarded info trials are less predictive of current choice following ACC inhibition in females. Odd Ratios of different trial predictors. Each panel represents a feature of the model (previous choice, previous reward in an info choice, previous reward in a NoInfo choice, and latency) for female (top) and male (bottom) animals following CNO administration in four different groups: ACC hM4Di, BLA hM4Di, OFC hM4Di, and control. Boxes, error bars, and lines represent 80% CI, 95% CI, and mean OR, respectively. Red dashed line y = 1 indicates no significant prediction of current choice. *P < 0.05, **P < 0.001, ***P < 0.0001.
We next assessed the influence of brain region condition (ACC hM4Di-CNO, BLA hM4Di-CNO, OFC hM4Di-CNO, or control group-CNO) and sex (males and females) on choice trial outcomes using trial-by-trial data using GLM. In the full model this included (i) previous choice, (ii) previous reward given an Info chohice, or Reward | Info, (iii) previous reward given a NoInfo choice, or Reward | NoInfo, (iv) previous latency, (v) sex, and (vi) brain region condition (Region) as predictors. The formula for this omnibus analysis was γ ~ [1 + PrevChoice + RewardInfo + RewardNoInfo + PrevLatency + Region + sex + PrevChoice*Region + PrevChoice*sex + RewardInfo*Region + RewardInfo*sex + RewardNoInfo*Region + RewardNoInfo*sex + Latency*Region + Latency*sex + Region*sex]. Corroborating the findings above, we found that previous choice and previous Reward | Info were significant predictors of current choice: [GLM: βPrevChoice = 3.09, t(5,534) = 9.57, P = 1.03e-21 and βRewardInfo = −2.12, t(5,534) = −4.93, P = 8.14e-07], with previous choice enhancing the likelihood of the same choice (win–stay) and previous Reward | Info promoting instead a win–switch strategy. We also found sex as a significant predictor of choice [GLM: βsex = 0.65, t(5,534) = 2.53, P = 0.01; males > females]. Three significant interactions emerged from this analysis: previous choice × region [GLM: βPrevChoice × Region = 0.42, t(5,534) = 2.89, P = 3.8e-03] previous Reward | Info × region [GLM: βRewardInfo × Region = −0.72, t(5,534) = −3.68, P = 2.2e-04] and region × sex [GLM: βRegion × sex = −0.24, t(5,534) = −2.54, P = 0.01]. Expectedly, trial latency and Reward | NoInfo trials were not significant predictors of current choice (Fig. 5).
Given the interaction with sex, we were justified to analyze males and females separately. For males, there was only a significant interaction with previous choice × region (GLM: βPrevChoice × Region = −0.55, t(2,185) = −2.99, P = 0.003], whereas for females, the significant interactions were for both previous choice × region (GLM: βPrevChoice × Region = 1.77, t(3,345) = 7.27, P = 3.5e-13] and Reward | Info trial × region [GLM: βRewardInfo × Region = −2.05, t(3,345) = −6.96, P = 3.4e-12]. Following Bonferroni–Holm correction for number of comparisons, post hoc analyses revealed that previous choice (PrevChoice) was a significant predictor of current choice in both sexes and in all brain regions (i.e. under all conditions, P-values < 1.23e-11). Interestingly, Reward | Info choice (RewardInfo) was a significant predictor in all conditions (P-values < 9.63e-73), except in ACC hM4Di-CNO females (P > 0.01). These results indicate that female rats are more stochastic in their decisions following ACC inhibition, on rewarded Info trials. We further discuss the interpretation of these results below.
Discussion
Despite the well-characterized roles of OFC and ACC in decision-making (Izquierdo 2017; Bromberg-Martin and Monosov 2020; Sosa et al. 2021), few studies find clear dissociations between these regions in rodents. For example, both OFC and ACC are important in confidence report as measured by temporal wagering (Lak et al. 2014; Stolyarova et al. 2019) and are also involved in stimulus-based reversal learning (Ye et al. 2023). In the present study, we found a dissociation between ACC and OFC that may shed light on their individual contributions in decision-making about noninstrumental information and point to a hierarchy of functions within rodent frontal cortex. Specifically, we found that ACC inhibition rendered female animals’ decisions about information more stochastic, also corroborated by the logistic regression model, which showed that previous rewarded Info trials were not good predictors of future choices when ACC was offline. The pattern of results on latencies and the RR also indicates that the effect of inhibition on decision-making is not due to performance decrements (i.e. we found unchanged latencies and RRs). Thus, it could be that such “performance monitoring” is a feature of rodent ACC, more than OFC. Indeed, ACC has been linked more to the representation of reward opportunities across the environment at different timescales while also keeping track of animals’ previous actions (Kolling et al. 2016; Wittmann et al. 2016; Spitmaan et al. 2020), suggestive of a special metacognitive role (van Veen et al. 2004; Stolyarova et al. 2019b; Kane et al. 2022; Takeuchi et al. 2022).
There were also some surprising results of this experiment. We had expected BLA to exert a more prominent role in Info choices given its role in value updating and decision-making under uncertainty (Ghods-Sharifi et al. 2009; St Onge et al. 2012; Winstanley and Floresco 2016; Soltani and Izquierdo 2019), but that was not the case here. It is possible that BLA may be more important in either the initial learning and/or the updating of cues associated with the different alternatives (i.e. following reversals or selective reinforcer devaluation), so a more thorough investigation of that possibility is warranted. It was also curious that all animals generally adopted a win–switch strategy following a rewarded Info trial (Fig. 5). We, like many others, have reported a more adaptive win–stay strategy in both action- and cue-based learning tasks (Ito and Doya 2009; Harris et al. 2021; Aguirre et al. 2023). Unlike those tasks, the present experiment features a unique trial structure where trials are experienced in trios (two forced and one choice, presented pseudorandomly). Because of this trial structure, we think rats may adopt a different strategy. Indeed, rats can be encouraged to use win–shift strategies when in tasks that require them to alternate between arms, similar to foraging in mazes (Olton and Schlosberg 1978; Gaffan and Davies 1981). However, since these studies were conducted using only male rats, how female rats learn and switch strategies is still an open question.
Our results presented here also replicate the vast literature investigating the behavior behind the preference for the informative, yet “suboptimal” alternative. As other researchers have previously reported, the preference for the informative option is more variable in rats than pigeons or starlings when that option results in less food (Stagner and Zentall 2010; Vasconcelos et al. 2015). This indicates that perhaps there are different sensitivities to information and/or reinforcement history between species. Regarding latencies to make a choice, we replicate what has been observed in several studies: animals respond faster on “true” choice trials than forced-choice trials. Ecologists have discussed this counterintuitive result by suggesting that animals did not evolve to make simultaneous but rather sequential choices in nature, such that latencies during forced choice trials can be used to predict preference (Kacelnik et al. 2010). Finally, the RR during the cue presentation followed the typical pattern reported previously: animals respond more in the presence of the always and partially reinforced cue and do not respond to the nonreinforced cue (Hinnenkamp et al. 2017; Gonzalez and Blaisdell 2021). It is important to highlight that animals are not required to respond during the duration of the cue; however, they typically do, suggesting a Pavlovian association established between the cue and the outcome. This association we believe is central to the preference, in which a stable preference emerges when an association between the reinforced cue (S+) and the outcome and the nonreinforced cue (S−) and the outcome is established (González et al. 2023).
A similar variation of the task presented in this study was used to assess preference for non-instrumental information in humans and monkeys. In those studies, subjects are given a choice between two alternatives: one provides informative cues that indicate the trial’s outcome, and another provides noninformative cues that do not indicate the trial’s outcome. Similar to our task, the information provided by the cues does not influence or change the outcome. However, humans and monkeys were also informed about the quantity (money for humans and juice for monkeys) of the outcome in a given trial. The results showed that macaque monkeys and humans prefer information and that they are willing to sacrifice water/money to obtain immediate information about the outcomes. One group previously found that OFC neurons encode variables that are relevant in learning and decision-making but do not integrate these variables into a single value (Blanchard et al. 2015; Aguirre et al. 2023). This result is consistent with our findings in which OFC inactivation resulted in an attenuated use of previous choice information revealed by the logistic regression, suggesting problems in retrieving the memory of previous choice, but this did not translate into a real change in preference. ACC may have a higher-level role in sustaining information-seeking (Hunt et al. 2018; White et al. 2019). For example, activity ramps up in this region in anticipation of information becoming available to resolve uncertainty before reward delivery (White et al. 2019). Our results support the monitoring role of ACC in decisions about information, where inhibition altered the integration of value, destabilizing preference.
We found that the ACC inhibition destabilized preference, but only in female animals. We did not find changes in latency to choose or RRs, indicating that the observed differences were not due to motor impairment or due to changes in the association between cues and outcomes. Therefore, the change in preference with ACC inhibition is unlikely due to problems in accessing overall value of each option. Previous studies have determined that the ACC does not support simple effort or the hedonic value of a given alternative (i.e. one option). Instead, it computes the value across options, that is, it tracks the overall “better” choice (Hart et al. 2017; Hart et al. 2020). Here, we found that the change in preference following ACC inhibition was not in any particular direction. If animals with ACC offline had issues in accessing the overall value of the best option, in this case the No Info option, we would have observed an increase in preference for the Info option only. In contrast, we found that preference became more stochastic. Previous research has shown that ACC needs to be engaged when animals are asked to stick to a strategy (i.e. win–stay) and animals instead show high variability of responses, thus more stochastic behavior, when ACC is offline (Tervo et al. 2014; Tervo et al. 2021). The preference change may also indicate a reduced ability to link previous actions (in this case, the previous lever press) to the current trial. However, this is unlikely because the motor response to the lever press did not change (i.e. latencies were unaffected), nor did the assigned value of the cues (i.e. RR to cues also did not change). We propose instead that ACC inhibition results in an impairment in accessing the value of information. Previous studies using this paradigm indicate that the contrast between both informative cues (i.e. the difference in information between the S+ and the S−) is essential for the development of a preference for information. Other research has already suggested that the ACC is important for information-seeking behavior; however, these studies reported this using electrophysiological recording and neuroimaging in monkeys and humans, respectively (Kennerley and Wallis 2009; Monosov 2017; Bromberg-Martin and Monosov 2020). Our study provides the first causal evidence of the importance of ACC in decision-making involving information.
Sex differences in the involvement of ACC in value-based decision making have been reported before. In a recent study, Cox et al. (2023) found that ACC inhibition disrupted the relationship between the value of each alternative and motivation to engage in the task in female mice. However, they did not find changes in preference as we found in our study. This group also reported that the ACC-to-dorsomedial striatal neuron pathway represents negative outcomes more strongly in female than males rats, suggesting differential sensitivity to negative feedback (Cox et al. 2023). Similarly, in tasks involving decision-making under risk, it has been determined that female rats are more risk-averse (Orsini et al. 2016), indicating that female and male rats use different strategies to make decisions (Orsini et al. 2021). In our task, similar to Cox et al. (2023), we did not find differences in preference between female and male rats before inhibition. However, the destabilization of preference might indicate that females rely more strongly on the ACC to keep track of reward statistics.
Similar to the results in rodents, groups studying human subjects have uncovered sex differences in strategy in decision-making (Chowdhury et al. 2019; Chen et al. 2021), usually in tasks involving risk (van den Bos et al. 2013). These sex differences are important to understand because, on the one hand, there is a higher prevalence of depression- or anxiety-related disorders in women (Cyranowski et al. 2000); and on the other hand, there is increasing evidence that neuropsychiatric conditions such as ADHD, bipolar disorder, and autism show different onset, symptom severity, and prognosis dependent on sex (Grissom and Reyes 2019; Hwang et al. 2020). This evidence suggests differential involvement of circuits involved in decision-making by sex, and our study contributes to the effort in finding the neural mechanisms behind these potential differences.
Supplementary Material
Acknowledgments
We thank Dr Pamela Mattar for her help with the protocol and technique for c-fos IHC and members of the Izquierdo lab for early feedback and suggestions on these experiments.
Contributor Information
Valeria V González, Department of Psychology, University of California-Los Angeles, 502 Portola Plaza, Los Angeles, CA 90095, United States.
Yifan Zhang, Department of Computer Science, University of Southern California, Salvatori Computer Science Center, 941 Bloom Walk, Los Angeles, CA 90089, United States.
Sonya A Ashikyan, Department of Psychology, University of California-Los Angeles, 502 Portola Plaza, Los Angeles, CA 90095, United States.
Anne Rickard, Department of Psychology, University of California-Los Angeles, 502 Portola Plaza, Los Angeles, CA 90095, United States.
Ibrahim Yassine, Department of Psychology, University of California-Los Angeles, 502 Portola Plaza, Los Angeles, CA 90095, United States.
Juan Luis Romero-Sosa, Department of Psychology, University of California-Los Angeles, 502 Portola Plaza, Los Angeles, CA 90095, United States.
Aaron P Blaisdell, Department of Psychology, University of California-Los Angeles, 502 Portola Plaza, Los Angeles, CA 90095, United States; The Brain Research Institute, University of California-Los Angeles, 695 Charles E Young Dr S, Los Angeles, CA 90095, United States; Integrative Center for Learning and Memory, University of California-Los Angeles, 695 Charles E Young Dr S, Los Angeles, CA 90095, United States.
Alicia Izquierdo, Department of Psychology, University of California-Los Angeles, 502 Portola Plaza, Los Angeles, CA 90095, United States; The Brain Research Institute, University of California-Los Angeles, 695 Charles E Young Dr S, Los Angeles, CA 90095, United States; Integrative Center for Learning and Memory, University of California-Los Angeles, 695 Charles E Young Dr S, Los Angeles, CA 90095, United States; Integrative Center for Addictions, University of California-Los Angeles, 695 Charles E Young Dr S, Los Angeles, CA 90095, United States.
Author contributions
Valeria V. Gonzalez (Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Software, Visualization, Writing—original draft, Writing—review & editing), Yifan Zhang (Formal analysis, Software), Sonya A. Ashikyan (Data curation, Investigation, Writing—original draft), Anne Rickard (Data curation, Investigation), Ibrahim Yassine (Data curation, Investigation), Juan Luis Romero-Sosa (Investigation, Writing—review & editing), Aaron P. Blaisdell (Funding acquisition, Resources, Writing—review & editing), and Alicia Izquierdo (Conceptualization, Data curation, Funding acquisition, Methodology, Resources, Software, Supervision, Writing—original draft, Writing—review & editing).
Funding
This work was supported by R01 DA047870 (A.I.), BCS-1844144 (A.P.B.) and UC’s Chancellor Postdoctoral Fellowship (V.V.G.).
Conflict of interest statement: None declared.
References
- Aguirre CG, Woo JH, Sosa JLR, Munier JJ, Perez J, Goldfarb M, Das K, Gomez M, Ye T, Pannu J, et al. Dissociable contributions of basolateral amygdala and ventrolateral orbitofrontal cortex to flexible learning under uncertainty. J Neurosci. 2024:44(2):e0622232023. 10.1523/JNEUROSCI.0622-23.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajuwon V, Ojeda A, Murphy RA, et al. Paradoxical choice and the reinforcing value of information. Anim Cogn. 2023:26:623–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard TC, Hayden BY, Bromberg-Martin ES. Orbitofrontal cortex uses distinct codes for different choice attributes in decisions motivated by curiosity. Neuron. 2015:85(3):602–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulter C, Freeston M, South M, Rodgers J. Intolerance of uncertainty as a framework for understanding anxiety in children and adolescents with autism spectrum disorders. J Autism Dev Disord. 2014:44(6):1391–1402. [DOI] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Hikosaka O. Midbrain dopamine neurons signal preference for advance information about upcoming rewards. Neuron. 2009:63(1):119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Monosov IE. Neural circuitry of information seeking. Curr Opin Behav Sci. 2020:35:62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CS, Knep E, Han A, Ebitz RB, Grissom NM. Sex differences in learning from exploration. elife. 2021:10:e69748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury TG, Wallin-Miller KG, Rear AA, Park J, Diaz V, Simon NW, Moghaddam B. Sex differences in reward- and punishment-guided actions. Cogn Affect Behav Neurosci. 2019:19(6):1404–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J, Minerva AR, Fleming WT, Zimmerman CA, Hayes C, Zorowitz S, Bandi A, Ornelas S, McMannon B, Parker NF, et al. A neural substrate of sex-dependent modulation of motivation. Nat Neurosci. 2023:26(2):274–284. [DOI] [PubMed] [Google Scholar]
- Cunningham PJ, Shahan TA. Rats engage in suboptimal choice when the delay to food is sufficiently long. J Exp Psychol Anim Learn Cogn. 2019:45(3):301–310. [DOI] [PubMed] [Google Scholar]
- Cyranowski JM, Frank E, Young E, Shear MK. Adolescent onset of the gender difference in lifetime rates of major depression: a theoretical model. Arch Gen Psychiatry. 2000:57(1):21–27. [DOI] [PubMed] [Google Scholar]
- Davison AC, Hinkley DV. Bootstrap methods and their application. Cambridge: Cambridge University Press; 2013. [Google Scholar]
- Dugas MJ, Buhr K, Ladouceur R. The role of intolerance of uncertainty in Etiology and maintenance. Generalized anxiety disorder: advances in research and practice. New York, NY, US: The Guilford Press; 2004, pp. 143–163. [Google Scholar]
- Fortes I, Vasconcelos M, Machado A. Testing the boundaries of "paradoxical" predictions: pigeons do disregard bad news. J Exp Psychol Anim Learn Cogn. 2016:42(4):336–346. [DOI] [PubMed] [Google Scholar]
- Gaffan EA, Davies J. The role of exploration in win-shift and win-stay performance on a radial maze. Learn Motiv. 1981:12(3):282–299. [Google Scholar]
- Ghods-Sharifi S, St Onge JR, Floresco SB. Fundamental contribution by the basolateral amygdala to different forms of decision making. J Neurosci. 2009:29(16):5251–5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez VV, Blaisdell AP. The role of inhibition in the suboptimal choice task. J Exp Psychol Anim Learn Cogn. 2021:47(4):429–444. [DOI] [PubMed] [Google Scholar]
- González VV, Izquierdo A, Blaisdell AP. Theoretical mechanisms of paradoxical choices involving information. Comp Cogn Behav Rev. 2023:18:11–31. [Google Scholar]
- Grissom NM, Reyes TM. Let's call the whole thing off: evaluating gender and sex differences in executive function. Neuropsychopharmacology. 2019:44(1):86–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris C, Aguirre C, Kolli S, Das K, Izquierdo A, Soltani A. Unique features of stimulus-based probabilistic reversal learning. Behav Neurosci. 2021:135(4):550–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart EE, Gerson JO, Zoken Y, Garcia M, Izquierdo A. Anterior cingulate cortex supports effort allocation towards a qualitatively preferred option. Eur J Neurosci. 2017:46(1):1682–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart EE, Blair GJ, O'Dell TJ, Blair HT, Izquierdo A. Chemogenetic modulation and single-photon calcium imaging in anterior cingulate cortex reveal a mechanism for effort-based decisions. J Neurosci. 2020:40(29):5628–5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnenkamp JE, Shahan TA, Madden GJ. How suboptimal is suboptimal choice? J Exp Anal Behav. 2017:107(1):136–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt LT, Malalasekera WMN, Berker AO, Miranda B, Farmer SF, Behrens TEJ, Kennerley SW. Triple dissociation of attention and decision computations across prefrontal cortex. Nat Neurosci. 2018:21(10):1471–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang YIJ, Arnold S, Srasuebkul P, Trollor J. Understanding anxiety in adults on the autism spectrum: an investigation of its relationship with intolerance of uncertainty, sensory sensitivities and repetitive behaviours. Autism. 2020:24(2):411–422. [DOI] [PubMed] [Google Scholar]
- Iigaya K, Story GW, Kurth-Nelson Z, Dolan RJ, Dayan P. The modulation of savouring by prediction error and its effects on choice. elife. 2016:5:e13747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iigaya K, Hauser TU, Kurth-Nelson Z, O'Doherty JP, Dayan P, Dolan RJ. The value of what's to come: neural mechanisms coupling prediction error and the utility of anticipation. Sci Adv. 2020:6(25):eaba3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Doya K. Validation of decision-making models and analysis of decision variables in the rat basal ganglia. J Neurosci. 2009:29(31):9861–9874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A. Functional heterogeneity within rat orbitofrontal cortex in reward learning and decision making. J Neurosci. 2017:37(44):10529–10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson R, Milne E, Thompson A. The relationship between intolerance of uncertainty and anxiety in autism: a systematic literature review and meta-analysis. Autism. 2020:24(8):1933–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenni NL, Symonds N, Floresco SB. Medial orbitofrontal cortical regulation of different aspects of Pavlovian and instrumental reward seeking. Psychopharmacology. 2023:240(3):441–459. [DOI] [PubMed] [Google Scholar]
- Jezzini A, Bromberg-Martin ES, Trambaiolli LR, Haber SN, Monosov IE. A prefrontal network integrates preferences for advance information about uncertain rewards and punishments. Neuron. 2021:109(14):2339–2352.e2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo S, Jung MW. Differential coding of uncertain reward in rat insular and orbitofrontal cortex. Sci Rep. 2016:6(1):24085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacelnik A, Vasconcelos M, Monteiro T, Aw J. Darwin’s “tug-of-war” vs. starlings’ “horse-racing”: how adaptations for sequential encounters drive simultaneous choice. Behav Ecol Sociobiol. 2010:65(3):547–558. [Google Scholar]
- Kane GA, James MH, Shenhav A, Daw ND, Cohen JD, Aston-Jones G. Rat anterior cingulate cortex continuously signals decision variables in a patch foraging task. J Neurosci. 2022:42(29):5730–5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennerley SW, Wallis JD. Encoding of reward and space during a working memory task in the orbitofrontal cortex and anterior cingulate sulcus. J Neurophysiol. 2009:102(6):3352–3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolling N, Behrens T, Wittmann MK, Rushworth M. Multiple signals in anterior cingulate cortex. Curr Opin Neurobiol. 2016:37:36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lak A, Costa GM, Romberg E, Koulakov AA, Mainen ZF, Kepecs A. Orbitofrontal cortex is required for optimal waiting based on decision confidence. Neuron. 2014:84(1):190–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macias A, Gonzalez VV, Machado A, Vasconcelos M. The functional equivalence of two variants of the suboptimal choice task: choice proportion and response latency as measures of value. Anim Cogn. 2021:24(1):85–98. [DOI] [PubMed] [Google Scholar]
- Mandali A, Sethi A, Cercignani M, Harrison NA, Voon V. Shifting uncertainty intolerance: methylphenidate and attention-deficit hyperactivity disorder. Transl Psychiatry. 2021:11(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt MA, Dunn RM, Spetch ML, Ludvig EA. When good news leads to bad choices. J Exp Anal Behav. 2016:105(1):23–40. [DOI] [PubMed] [Google Scholar]
- Monosov IE. Anterior cingulate is a source of valence-specific information about value and uncertainty. Nat Commun. 2017:8(1):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namboodiri VMK, Otis JM, Heeswijk K, Voets ES, Alghorazi RA, Rodriguez-Romaguera J, Mihalas S, Stuber GD. Single-cell activity tracking reveals that orbitofrontal neurons acquire and maintain a long-term memory to guide behavioral adaptation. Nat Neurosci. 2019:22(7):1110–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olton DS, Schlosberg P. Food-searching strategies in young rats: win-shift predominates over win-stay. J Comp Physiol Psychol. 1978:92(4):609–618. [Google Scholar]
- Orduña V, Trujano RE. Rats are optimal in a choice task in which pigeons are not. Behav Process. 2015:119:22–27. [DOI] [PubMed] [Google Scholar]
- Orduña V, Trujano RE, López P, Rojas-Leguizamón M. Optimal behavior by rats in a choice task is associated to a persistent conditioned inhibition effect. Behav Process. 2016:130:65–70. [DOI] [PubMed] [Google Scholar]
- Orsini CA, Willis ML, Gilbert RJ, Bizon JL, Setlow B. Sex differences in a rat model of risky decision making. Behav Neurosci. 2016:130(1):50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini CA, Blaes SL, Hernandez CM, Betzhold SM, Perera H, Wheeler AR, Ten Eyck TW, Garman TS, Bizon JL, Setlow B. Regulation of risky decision making by gonadal hormones in males and females. Neuropsychopharmacology. 2021:46(3):603–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Charles W. The rat brain in stereotaxic coordinates: hard cover edition. Elsevier; 2006. [Google Scholar]
- Procyk E, Tanaka YL, Joseph JP. Anterior cingulate activity during routine and non-routine sequential behaviors in macaques. Nat Neurosci. 2000:3(5):502–508. [DOI] [PubMed] [Google Scholar]
- Riceberg JS, Shapiro ML. Orbitofrontal cortex signals expected outcomes with predictive codes when stable contingencies promote the integration of reward history. J Neurosci. 2017:37(8):2010–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueden CT, Schindelin J, Hiner MC, DeZonia BE, Walter AE, Arena ET, Eliceiri KW. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinformatics. 2017:18(1):529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MFS, Behrens TEJ. Choice, uncertainty and value in prefrontal and cingulate cortex. Nat Neurosci. 2008:11(4):389–397. [DOI] [PubMed] [Google Scholar]
- Schneider Gasser EM, Straub CJ, Panzanelli P, Weinmann O, Sassoe-Pognetto M, Fritschy JM. Immunofluorescence in brain sections: simultaneous detection of presynaptic and postsynaptic proteins in identified neurons. Nat Protoc. 2006:1(4):1887–1897. [DOI] [PubMed] [Google Scholar]
- Shidara M, Richmond BJ. Anterior cingulate: single neuronal signals related to degree of reward expectancy. Science. 2002:296(5573):1709–1711. [DOI] [PubMed] [Google Scholar]
- Soltani A, Izquierdo A. Adaptive learning under expected and unexpected uncertainty. Nat Rev Neurosci. 2019:20(10):635–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosa JLR, Buonomano D, Izquierdo A. The orbitofrontal cortex in temporal cognition. Behav Neurosci. 2021:135(2):154–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitmaan M, Seo H, Lee D, Soltani A. Multiple timescales of neural dynamics and integration of task-relevant signals across cortex. Proc Natl Acad Sci USA. 2020:117(36):22522–22531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Onge JR, Stopper CM, Zahm DS, Floresco SB. Separate prefrontal-subcortical circuits mediate different components of risk-based decision making. J Neurosci. 2012:32(8):2886–2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagner JP, Zentall TR. Suboptimal choice behavior by pigeons. Psychon Bull Rev. 2010:17(3):412–416. [DOI] [PubMed] [Google Scholar]
- Stolyarova A, Rakhshan M, Hart EE, O'Dell TJ, Peters MAK, Lau H, Soltani A, Izquierdo A. Contributions of anterior cingulate cortex and basolateral amygdala to decision confidence and learning under uncertainty. Nat Commun. 2019:10(1):4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopper CM, Floresco SB. Contributions of the nucleus accumbens and its subregions to different aspects of risk-based decision making. Cogn Affect Behav Neurosci. 2011:11(1):97–112. [DOI] [PubMed] [Google Scholar]
- Takeuchi D, Roy D, Muralidhar S, Kawai T, Bari A, Lovett C, Sullivan HA, Wickersham IR, Tonegawa S. Cingulate-motor circuits update rule representations for sequential choice decisions. Nat Commun. 2022:13(1):4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tervo DGR, Proskurin M, Manakov M, Kabra M, Vollmer A, Branson K, Karpova AY. Behavioral variability through stochastic choice and its gating by anterior cingulate cortex. Cell. 2014:159(1):21–32. [DOI] [PubMed] [Google Scholar]
- Tervo DGR, Kuleshova E, Manakov M, Proskurin M, Karlsson M, Lustig A, Behnam R, Karpova AY. The anterior cingulate cortex directs exploration of alternative strategies. Neuron. 2021:109(11):1876–1887 e1876. [DOI] [PubMed] [Google Scholar]
- Tolin DF, Abramowitz JS, Brigidi BD, Foa EB. Intolerance of uncertainty in obsessive-compulsive disorder. J Anxiety Disord. 2003:17(2):233–242. [DOI] [PubMed] [Google Scholar]
- Vasconcelos M, Monteiro T, Kacelnik A. Irrational choice and the value of information. Sci Rep. 2015:5(1):13874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos R, Homberg J, Visser L. A critical review of sex differences in decision-making tasks: focus on the Iowa gambling task. Behav Brain Res. 2013:238:95–108. [DOI] [PubMed] [Google Scholar]
- Veen V, Holroyd CB, Cohen JD, Stenger VA, Carter CS. Errors without conflict: implications for performance monitoring theories of anterior cingulate cortex. Brain Cogn. 2004:56(2):267–276. [DOI] [PubMed] [Google Scholar]
- Wallis JD. Cross-species studies of orbitofrontal cortex and value-based decision-making. Nat Neurosci. 2012:15(1):13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JK, Bromberg-Martin ES, Heilbronner SR, Zhang K, Pai J, Haber SN, Monosov IE. A neural network for information seeking. Nat Commun. 2019:10(1):5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Floresco SB. Deciphering decision making: variation in animal models of effort- and uncertainty-based choice reveals distinct neural circuitries underlying Core cognitive processes. J Neurosci. 2016:36(48):12069–12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann MK, Kolling N, Akaishi R, Chau BK, Brown JW, Nelissen N, Rushworth MF. Predictive decision making driven by multiple time-linked reward representations in the anterior cingulate cortex. Nat Commun. 2016:7(1):12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye T, Romero-Sosa JL, Rickard A, Aguirre CG, Wikenheiser AM, Blair HT, Izquierdo A. Theta oscillations in anterior cingulate cortex and orbitofrontal cortex differentially modulate accuracy and speed in flexible reward learning. Oxf Open Neurosci. 2023:2:kvad005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeb FD, Winstanley CA. Lesions of the basolateral amygdala and orbitofrontal cortex differentially affect acquisition and performance of a rodent gambling task. J Neurosci. 2011:31(6):2197–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeb FD, Winstanley CA. Functional disconnection of the orbitofrontal cortex and basolateral amygdala impairs acquisition of a rat gambling task and disrupts animals' ability to alter decision-making behavior after reinforcer devaluation. J Neurosci. 2013:33(15):6434–6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.