Introduction
Globally, 26 million people suffer from heart failure (HF).1 In the United States, approximately 6.7 million adults over the age of 20 suffer from HF, and its prevalence is expected to reach more than 8 million by 2030.2,3 The total medical costs associated with HF are projected to rise from $30.7 billion to $69.8 billion by 2030, which is equivalent to $244 for every American adult.2,3 Hospital admission, as well as its associated costs, is often the result of patients seeking care for escalating symptoms such as dyspnea.4,5 However, even with advancements in the treatment of HF, management of symptoms by patients (i.e., self-care management) and clinicians, a critical aspect of HF treatment, is difficult.6
Symptom burden, which includes the burden of physical and psychological symptoms, is a significant problem for patients with HF.7 Symptom burden refers to the number of subjectively quantifiable symptoms that adversely affect patient health and lead to multiple negative, physical, and emotional consequences.8 Physical and psychological symptoms, including dyspnea, fatigue, pain, orthopnea, edema, loss of appetite, anxiety, and depression are widely reported among patients with HF.9 These symptoms can significantly interfere with a patient’s ability to perform daily activities. Furthermore, in a dose-dependent manner, untreated symptoms are associated with an increased likelihood of clinical events, such as emergency department visits, hospitalizations, and mortality.10 Therefore, appropriate management of symptom burden by patients with HF should not be underestimated.10
Self-care management (SCM) is a cornerstone of treatment for HF patients.11 Riegel and Dickson define HF self-management (one of the three sets of activities undertaken in self-care) as activities that patients take to respond to symptoms of HF exacerbations as they arise.11–13 The revised situation-specific theory of HF self-care also posits two other sets of activities, self-care maintenance and symptom monitoring.14 Patients who engage in adequate self-care live longer, experience fewer hospitalizations, and possess a better quality of life.15–17 Nonetheless, self-care is often poor among patients with HF.18 Furthermore, culture has an impact on self-care in general. Traditional beliefs and ideas, including fatalism, cultural norms, and normative thinking can play a significant role in self-care practices.19
Fatalism is an individual’s belief that events are predetermined and inevitable, and that individuals have little or no control over them.20 As a result, fatalistic individuals are more likely to accept what happens in their lives without attempting to change it or engaging in behaviors that could prevent or manage an illness.21 For example, fatalistic individuals with diabetes have poor medication adherence and self-care behaviors compared to those who are not fatalistic.22,23 Fatalistic individuals more commonly have major misperceptions about the risks they face for heart disease and cancer.24 Several investigators have shown that higher levels of fatalism are associated with worse patient outcomes.25,26 Those with fatalistic attitudes believe they have little control over their health outcomes, resulting in reduced motivation to engage in self-care activities.22 Clinician efforts to increase patient motivation to engage in self-care have had variable success, likely because of incomplete understanding of the factors affecting self-care management.27,28 Research is needed to improve our understanding of factors associated with self-care so that more effective interventions can be designed.
Clinicians and researchers have assumed that higher symptom burden would be strongly associated with better SCM in patients with HF, because having a higher symptom burden would prompt one to engage in better self-care; however, this association is not always present or the data about the association are conflicting.29–31 One hypothesis for these conflicting or non-significant findings is that there may be a relationship between symptom burden and SCM, but that it is mediated by a third variable. Fatalism is an appropriate candidate for this mediator.22 Investigators indicate that increased fatalism can detrimentally affect self-care and lead to adverse health outcomes.23,32 Thus, our aim was to determine whether fatalism mediates an association between symptom burden and SCM.
Methods
Design, Sample, and Setting
We conducted a cross-sectional secondary analysis of baseline data derived from a prospective randomized controlled trial, evaluating the impact of a 6-month dietary intervention on HF symptoms, health-related quality of life, and clinical outcomes.33 This study has been fully described elsewhere,33 and a brief description follows. The participants were recruited from outpatient clinics and hospitals in Kentucky. Eligible patients were adults who could read and speak English, had a diagnosis of chronic symptomatic HF with New York Heart Association (NYHA) functional class II-IV.
We used baseline data, which were collected before intervention, for analysis. Patients completed questionnaires in their homes or other place they chose. A research assistant was with the patient to assist them if they had any questions. Patients with complete data on all variables needed for the analysis (n=95) were included in the mediation analysis, whereas 120 patients with HF were included in the original study. There were no differences in demographic variables between participants included in the original study and those included in the current study.
Participants were excluded from the primary study if they had any of the following: 1) body mass index below 17 kg/m2 or above 46 kg/m2; 2) underlying medical condition that caused systemic inflammation; 3) diminished appetite or difficulty absorbing food; 4) consumed dietary supplements containing lycopene or omega-3 fatty acids; 5) allergies to rice bran oil; 6) listed for a heart transplant; or 7) cognitive impairment (either diagnosed in the medical record or determined through screening).
Procedure
This study was approved by the institutional review board of the University of Kentucky. A trained research nurse verified the eligibility of participants. Those who agreed to participate signed the consent form and provided evidence of informed consent using the teach-back method. The Montreal Cognitive Assessment was used to screen patients for cognitive function following their consent.34 Participants with scores of sixteen or lower on the Montreal Cognitive Assessment were excluded from the study. Baseline questionnaires were completed by patients after they provided consent.
Measures
Symptom Burden
The Memorial Symptom Assessment Scale (MSAS-HF) was used to assess symptom burden. The MSAS-HF consists of 32 items derived from Portenoy’s Memorial Symptom Assessment Scale, originally intended to assess the symptoms among patients with cancer.35 The MSAS-HF is a comprehensive instrument that provides multidimensional information regarding a wide range of symptoms experienced by patients with HF.36 The participants rate 32 possible symptoms they may have experienced during the past seven days. Depending on the presence of symptoms, the respondents were asked to rate the frequency of symptoms on a scale of 1 to 4 (occasionally to almost constantly), their severity on a scale of 1 to 4 (mild to very severe), and degree of distress experienced on a scale of 0 to 4 (not at all to very severe). A higher number signifies a higher burden from frequency, severity, and distress.35 Symptom burden scores are derived by adding the mean frequency, severity, and distress scores.9 Each subscale and the overall MSAS-HF scale has previously been demonstrated to be valid and reliable in patients with HF.36
Fatalism
Fatalism was measured using a valid and reliable instrument called the Cardiovascular Disease (CVD) Fatalism Instrument.37,38 The CVD Fatalism Instrument was modified from the General Health Fatalism Instrument with the permission of the original author and then psychometrically tested.37,38 Several items on the original scale were revised to make them disease-specific specifically to address heart disease. For instance, the statement from the initial scale, “If someone is meant to get a serious disease, they will get it no matter what they do,” was revised to “If someone is meant to get heart disease, they will get it no matter what they do.” The general items on the original scale, such as “My health is a matter of luck” and “I often feel helpless when experiencing problems” remained unchanged.38 The CVD Fatalism instrument consists of 20 items with Likert scale response options ranging from 1 (strongly disagree) to 5 (strongly agree). The scale ranges from 20 to 100, with higher scores indicating greater fatalism.38 The validity and reliability of this scale have been documented in patients with HF.39
Self-Care Management
Self-care management was evaluated using the management subscale of Self-Care of Heart Failure Index (SCHFI) version 6.0, which consists of 22 items with well-established reliability and validity in HF studies.40,41 The six items of the SCM Scale are designed to assess patients’ abilities to recognize symptoms, to respond appropriately (e.g., seek medical treatment, decrease fluid intake, and take diuretics), and evaluate their response to treatment. The HF SCM subscale is scored using a Likert scale of 1 to 4 responses:1 (never or rarely), 2 (sometimes), 3 (frequently), and 4 (always or daily).41 Scores were calculated by adding each item and converting them to a 100-point scale, where higher scores indicate better SCM. An assessment score of less than 70 indicates low levels of self-care.42
Demographic and Clinical Characteristics
We collected demographic information (e.g., age, sex, race/ethnicity, education, employment, and marital status) using a standard questionnaire. Trained research assistants conducted thorough patient interviews to determine NYHA classification. Patients were assigned to NYHA categories (classes II-IV) based on the extent to which their physical activity was restricted by their symptoms.43,44
Data analysis
The study variables were descriptively analyzed, using means, standard deviations, and frequency distributions. Version 28 IBM SPSS was used to conduct statistical analyses. We used the Hayes’ PROCESS macro (Model 4) to test the mediation effect of fatalism on the association of symptom burden with SCM.45 Using the PROCESS macro, simultaneous multiple regressions were run to test the total, direct and indirect effects. As shown in Figure 1, in the PROCESS macro-output, “a” represents the coefficient for the direct effect of the independent variable (i.e., symptom burden) on the mediator (i.e., fatalism), and “b” represents the coefficient for the direct effect of the mediator (i.e., fatalism) on outcome variable (i.e., SCM). Initially, we examined the direct effect (c’) of symptom burden on the dependent variable (i.e., SCM). Coefficient c’ represents the direct effect of the independent variable on the dependent variable. To determine the mediation effect, we examine the coefficient “a*b”, which represents the effect of the independent variable on the dependent variable through the mediator.46 We used ordinary least squares (OLS) regression with 5000 bootstrapped samples. The covariates were not included in the first mediation analysis. A subsequent mediation analysis was conducted while controlling for age, sex, and NYHA class. Studies have shown that these covariates are associated with the symptom burden, SCM, and fatalism.47–49 Furthermore, before performing the analysis, we checked for the possibility of a moderation effect of fatalism in the association between symptom burden and SCM and found that no moderation effect was evident.
Figure 1.
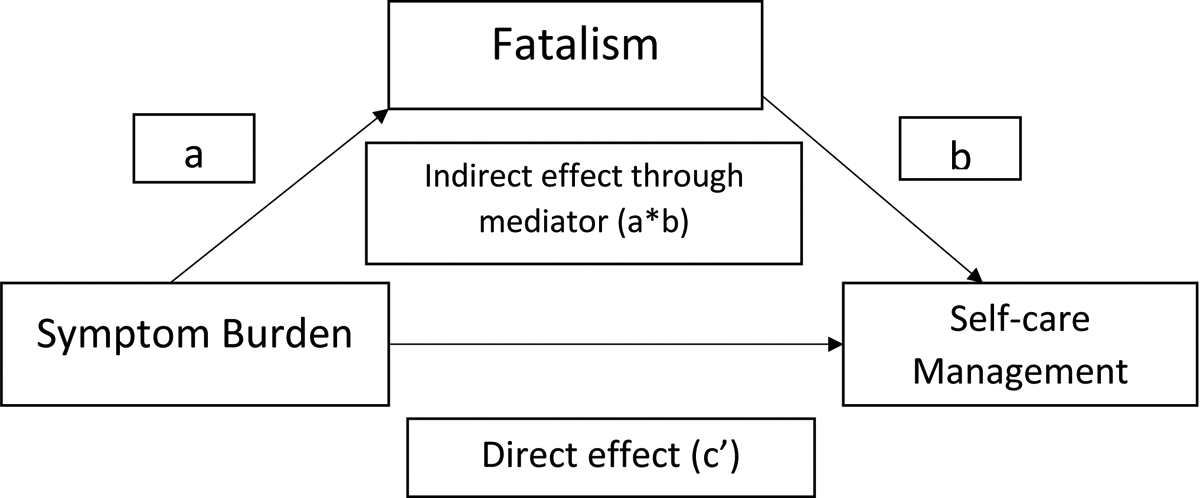
Conceptual diagram of the mediation model: symptom burden to self-care management via fatalism (based on Hayes model 4)
“a” represents the coefficient for the direct effect of the independent variable (i.e., symptom burden) on the mediator (i.e., fatalism), and “b” represents the coefficient for the direct effect of the mediator (i.e., fatalism) on outcome variable (i.e., self-care management). Coefficient c’ represents the direct effect of the independent variable on the dependent variable. Coefficient “a*b”, which represents the effect of the independent variable on the dependent variable through the mediator.
Results
Sample Characteristics
Table 1 presents the characteristics of the sample. The mean age of patients was 62 ± 12 years. Most patients (60%) were males, white (70%), married (49%), had received at least a high school education (89%), were retired due to illness (32%), and or were categorized as NYHA functional classes III and IV (70%). The mean score for SCM was 62.94 (SD=19.76), indicating inadequate self-care in this sample. The mean score for symptom burden was 43.27 (SD=31.81), indicating moderate to high burden among the sample participants. The mean score for fatalism was 48.5 (SD=11.07). There are no cut points for this instrument, but the possible range is 20 – 100 and the range in this sample was 23 – 76 with a median of 49 (25th percentile = 41, 75th percentile = 56), suggesting a moderate level of fatalism in this sample based on the sample mean.
Table 1.
Sample Characteristics (N=95)
| Characteristics | Mean ± SD or n (%) |
|---|---|
| Age, years | 62 ± 12 |
| Gender | |
| Male | 57 (60) |
| Female | 38 (40) |
| Race | |
| White | 67 (70.5) |
| Black | 22 (23.2) |
| Other | 6 (6.4) |
| Marital status | |
| Single | 17 (17.9) |
| Married | 47 (49.5) |
| Divorced/separated | 21 (22.1) |
| Widowed | 6 (6.3) |
| Living with good friend/partner | 4 (4.2) |
| Education level, years | 13 ± 2.6 |
| Employment | |
| Employed full- or part-time | 13 (13.7) |
| Unemployed by choice | 1 (1.1) |
| Sick Leave/Disability | 26 (27.4) |
| Homemaker | 2 (2.1) |
| Retired due to illness | 30 (31.6) |
| Retired not due to illness | 22 (23.2) |
| Unemployed/ Laid off | 1 (1.1) |
| NYHA | |
| I/II | 29 (30) |
| III/IV | 66 (70) |
| Self-care Management subscale (SCHFI) | 62.94 ± 19.76 |
| Symptom Burden (MSAS-HF) | 43.97 ± 31.81 |
| Fatalism scale (CVD Fatalism Instrument) | 48.5 ± 11.07 |
Abbreviations: CVD: Cardiovascular Disease, MSAS-HF: Memorial Symptom Assessment Scale-Heart Failure, NYHA: New York Heart Association, SCHFI: Self-Care of Heart Failure Index, SD: Standard Deviation.
Association of Symptom Burden, Fatalism, and Self-care Management
The results of the two mediation analyses are shown in Figures 2 and 3. Our first mediation analysis was not adjusted for covariates (Figure 2). Symptom burden was significantly associated with fatalism (a= 0.004; p<0.015), in that greater symptom burden was associated with higher levels of fatalistic beliefs. Fatalism was significantly associated with SCM (b = −9.132; p<0.007) in that patient with higher levels of fatalism had poorer SCM. Through this indirect pathway, higher symptom burden was associated with poorer SCM.
Figure 2.
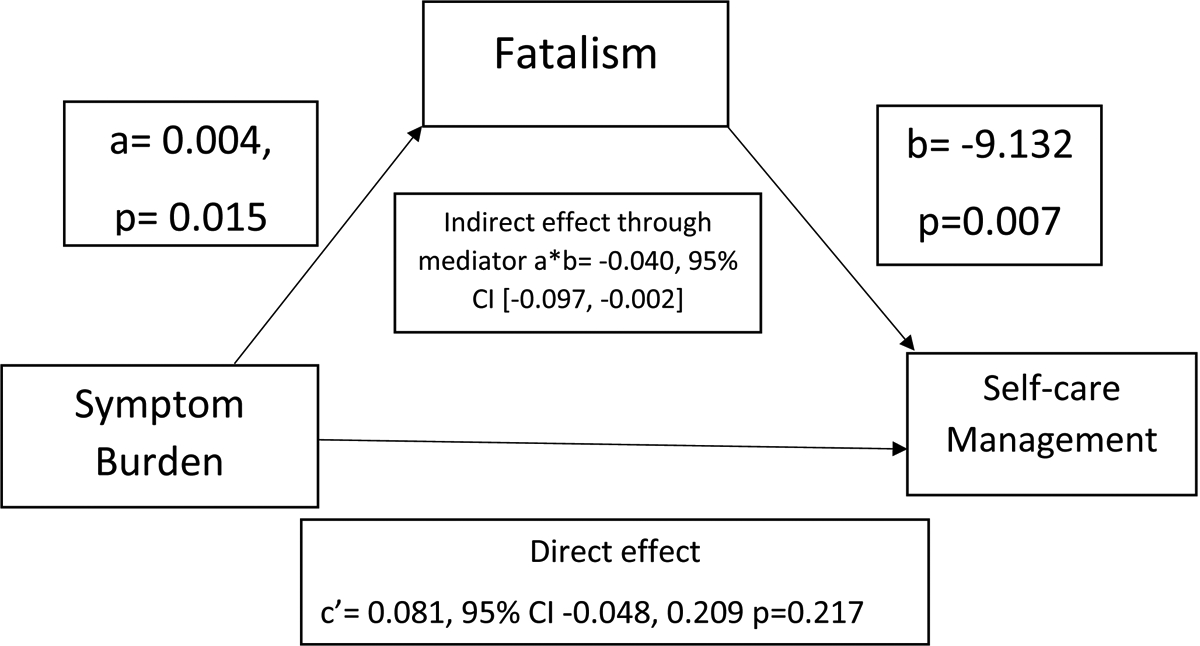
Mediation by fatalism of the association between symptom burden and self-care management without covariates
Coefficients for the model; CI= Confidence Interval
Figure 3.
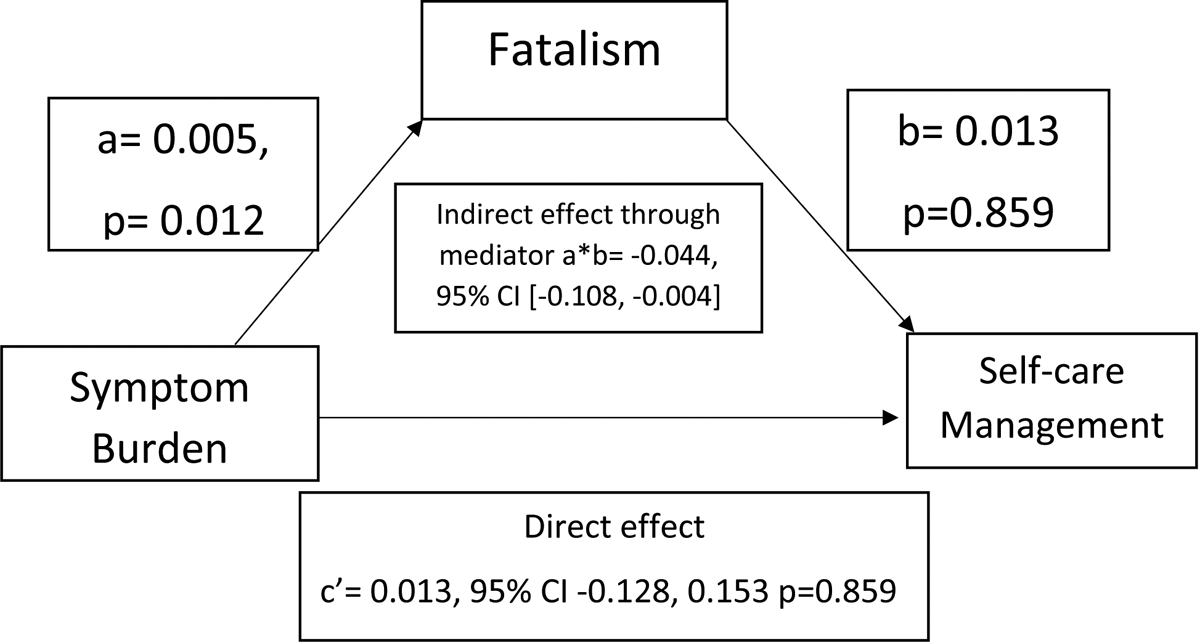
Mediation by fatalism of the association between symptom burden and self-care management controlling for covariates
Coefficients for the model; CI= Confidence Interval
Covariates: Age, sex, and NYHA (New York Heart Association) class
There was a significant indirect association of symptom burden with SCM through fatalism, indicating that fatalism was a mediating factor (a*b= −0.040, 95 % confidence interval (CI) [−0.097, −0.002]). In addition, no significant direct relationship was found between symptom burden and SCM in the presence of fatalism (c’ = 0.081; 95% CI = [−0.048, 0.209]; p = 0.217). Fatalism mediated the relationship between symptom burden and SCM. Patients with higher symptom burden were more fatalistic, and greater fatalism was associated with worse SCM. Table 2 presents a summary of the mediation analysis and Figure 2 illustrates each path.
Table 2.
Summary of Mediation Analysis with and without Covariates
| Relationship | Direct Effect coefficient (p value) | Confidence Interval for Direct Effect | Indirect Effect coefficient | Confidence Interval for Indirect Effect | Conclusion |
|---|---|---|---|---|---|
| Mediation without Covariates (Figure 2) | |||||
| Symptom Burden->Fatalism->Self-care Management | 0.081 (0.217) | Lower Bound −0.048 Upper Bound 0.209 |
−0.040 | Lower Bound −0.097 Upper Bound −0.002 |
Mediation Present |
| Mediation with Covariates ( Figure 3 ) | |||||
| Symptom Burden->Fatalism->Self-care Management | 0.013 (0.859) |
Lower Bound −0.128 Upper Bound 0.153 |
−0.044 | Lower Bound −0.108 Upper Bound −0.004 |
Mediation Present |
The second mediation model adjusting for covariates (i.e., age, sex, and NYHA class) is presented in Table 2 and Figure 3. There was a significant indirect (mediator) association of fatalism between symptom burden and SCM. (a*b effect= −0.044, 95% CI: −0.108 to −0.004). There is no definitive evidence that symptom burden is directly associated with SCM in the presence of fatalism (c’ = 0.013; 95% CI= [−0.128 to 0.153]; p = 0.859). Figure 3 illustrates each path and summarizes the mediation analysis.
Discussion
In this study, we examined the mediating effect of fatalism on the relationship between symptom burden and SCM among adults with heart failure. Our findings demonstrated that fatalism acts as a mediator between symptom burden and SCM. Patients with higher symptom burden had more fatalistic beliefs about their health, which led to less engagement in self-care activities. These findings are important because the discovery of mediators provides information about the mechanisms underlying the associations between variables. Using these findings, we can build strategies and interventions to improve patient outcomes.
Fatalistic beliefs are associated with passive coping styles and poorer health outcomes.50 Fatalistic beliefs result in lower odds of engaging in preventive behaviors such as regular exercise, quitting smoking, and consuming a sufficient amount of fruits and vegetables.51 Urizar and Sears,50 reported a greater prevalence of fatalism among patients with more severe cardiovascular disease. This finding suggests that fatalistic beliefs may be more prevalent among individuals with HF, who are likely to have a higher symptom burden. In addition to inhibiting positive health behaviors, fatalism can be experienced in response to poor health or chronic illness.52
A higher level of fatalism has been associated with an increased risk of recurrence and mortality from all causes, possibly due to of a lack of empowerment.53 Han Shi et.al.,25 found that some participants maintained a fatalistic attitude concerning their future with HF based on their experience of futility in the past, as well as acceptance of possibility of death at any time. In addition, repeated exacerbations of symptoms may demotivate patients from pursuing self-care due to fatalism and a sense of futility.25 The results of our study are consistent with the findings of Dickson et al.54 and others who found that SCM was influenced by spirituality and fatalism. The participants expressed a belief in God being in control, used prayer, and looked to a higher power for guidance and direction, which has elements of fatalism.54 In some religions, both Christian and non-Christian, practitioners of the faith hold views that can be considered fatalistic when they place their health in God’s hands. For example, Galdas et al, found that faith, spirituality, and fatalistic beliefs were often considered of greater importance than healthcare professionals’ recommendations, influencing patients’ acceptance of illnesses, perceptions of illnesses, and beliefs about managing their condition by changing their lifestyles.55 The prevalence of fatalistic beliefs affects patients’ acceptance of illness and perceptions of symptom burden, resulting in a diminished focus on self-care.50,55
Symptom burden profoundly affects patients’ ability to engage in SCM, which is crucial for improving HF patients’ health outcomes.5 Investigators found that fatigue and breathlessness increase task difficulty, affecting both SCM and daily living activities.56 Fatalism is a particularly relevant mediator in this relationship, as it captures the psychological aspect of coping with a chronic condition.57 Health care providers can identify potential barriers to effective SCM and design interventions that address these beliefs by understanding the mediating role of fatalism, which provides insight into patients’ thought processes and decisions regarding their health.
The influence of traditional beliefs and ideas, including fatalism, on SCM as a whole has not been examined in many studies. Our study suggests that fatalistic beliefs in a higher power play an important role in developing self-care approaches, as well as influencing the way individuals manage illnesses. The findings of our study are consistent with those in the published literature examining traditional beliefs and ideas, including fatalism and cultural norms in health and health behaviors. This suggests that fatalism influences the behaviors of individuals who suffer from chronic illnesses and cardiovascular diseases.58,59
Implications
Promoting an internal locus of control (i.e., belief that one’s behavior are guided by their own decisions) and increasing patients’ levels of perceived control (i.e., an individual’s perception that their actions can directly influence their own outcomes) can be effective strategies to address fatalism and engagement in self-care.60–62 We found that fatalism mediates the relationship between symptom burden and SCM in adults with HF. Despite high levels of symptom burden, patients who hold fatalistic beliefs about their health are less likely to engage in self-care activities.51 However, promoting an internal locus of control can help counteract fatalistic beliefs and encourage patients to manage their own health.63,64 It may be possible to motivate patients to engage in self-care behaviors by promoting the belief that their actions and behaviors can directly impact their health outcomes. Patient education, goal setting, and motivational interviewing may be effective strategies to promote internal locus of control and increased levels of perception control.61,65 Promoting internal locus of control can result in improved health outcomes in patients with chronic conditions.66 As a result, interventions aimed at improving SCM in adults with HF should emphasize the promotion of an internal locus of control and increased levels of perceived control to combat fatalistic beliefs and encourage active participation in self-care activities.
Limitations
Our study has some limitations that need to be noted. First, the study sample consisted primarily of white individuals recruited from one southern state in the United States, which limits the generalizability of this study. Second, because this was a secondary analysis, variables (such as HF etiology) that were not collected in the primary study were not accounted for in our statistical analysis. We did not control for variables (other than NYHA status, age, and sex), which could have affected outcomes. Such variables include comorbidity burden, which likely increases fatalism and symptom burden, but which has a variable effect on SCM. Third, we only used baseline data in this study, our results were cross-sectional, and causality cannot be inferred directly, although mediation analysis implies causality. Mediation analysis revealed that the predictor variable affects the outcome variable through the intermediate variable, suggesting a causal pathway.46 Future investigators should explore the differences in symptom burden, fatalism, and SCM status over time, especially after the implementation of interventions aimed at promoting better SCM.
Conclusion
We aimed to provide insight into the relationship between symptom burden, fatalism, and SCM among adults with HF. We found that fatalistic attitudes play an important mediating role in the association between symptom burden and SCM, highlighting the importance of addressing both physical symptoms as well as the emotional and psychological aspects of care for patients with HF.
Acknowledgment:
We would like to extend our sincere gratitude to the National Institute of Health, the National Institute for Nursing Research (1R01NR016824/1R01NR013430), and the National Center for Research Resources (UL1RR033173) for their generous grant support for the original study titled “Nutrition Intervention to Decrease Symptoms in Patients with Heart Failure.”
Funding and Declaration of Interest:
There are no conflicts of interest or funding to be disclosed by the authors.
Abbreviation
- CVD
Cardiovascular Disease
- HF
Heart failure
- MSAS-HF
Memorial Symptom Assessment Scale-Heart Failure
- NYHA
New York Heart Association
- SCM
Self-Care Management
- SCHFI
Self-Care of Heart Failure Index
References
- 1.Ponikowski P, Anker SD, AlHabib KF, et al. Heart failure: preventing disease and death worldwide. ESC Heart Fail. 2014;1(1):4–25. [DOI] [PubMed] [Google Scholar]
- 2.Heidenreich PA, Albert NM, Allen LA, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6(3):606–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsao CW, Aday AW, Almarzooq ZI, et al. Heart Disease and Stroke Statistics-2023 Update: A Report From the American Heart Association. Circulation. 2023;147(8):e93–e621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fida N, Piña IL. Trends in heart failure hospitalizations. Curr Heart Fail Rep. 2012;9(4):346–353. [DOI] [PubMed] [Google Scholar]
- 5.Alpert CM, Smith MA, Hummel SL, Hummel EK. Symptom burden in heart failure: assessment, impact on outcomes, and management. Heart Fail Rev. 2017;22(1):25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inamdar AA, Inamdar AC. Heart Failure: Diagnosis, Management and Utilization. J Clin Med. 2016;5(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamatani Y, Iguchi M, Ikeyama Y, et al. Comprehensive symptom assessment using Integrated Palliative care Outcome Scale in hospitalized heart failure patients. ESC Heart Fail. 2022;9(3):1963–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gapstur RL. Symptom burden: a concept analysis and implications for oncology nurses. Oncol Nurs Forum. 2007;34(3):673–680. [DOI] [PubMed] [Google Scholar]
- 9.Zambroski CH, Moser DK, Bhat G, Ziegler C. Impact of symptom prevalence and symptom burden on quality of life in patients with heart failure. Eur J Cardiovasc Nurs. 2005;4(3):198–206. [DOI] [PubMed] [Google Scholar]
- 10.Alpert CM, Smith MA, Hummel SL, Hummel EK. Symptom burden in heart failure: assessment, impact on outcomes, and management. Heart failure reviews. 2017;22(1):25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Durante A, Paturzo M, Mottola A, Alvaro R, Vaughan Dickson V, Vellone E. Caregiver Contribution to Self-care in Patients With Heart Failure: A Qualitative Descriptive Study. J Cardiovasc Nurs. 2019;34(2):E28–e35. [DOI] [PubMed] [Google Scholar]
- 12.Riegel B, Dickson VV. A situation-specific theory of heart failure self-care. Journal of cardiovascular Nursing. 2008;23(3):190–196. [DOI] [PubMed] [Google Scholar]
- 13.Riegel B, Dickson VV, Vellone E. The Situation-Specific Theory of Heart Failure Self-care: An Update on the Problem, Person, and Environmental Factors Influencing Heart Failure Self-care. J Cardiovasc Nurs. 2022;37(6):515–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riegel B, Dickson VV, Faulkner KM. The Situation-Specific Theory of Heart Failure Self-Care: Revised and Updated. J Cardiovasc Nurs. 2016;31(3):226–235. [DOI] [PubMed] [Google Scholar]
- 15.Jonkman NH, Westland H, Groenwold RH, et al. Do Self-Management Interventions Work in Patients With Heart Failure? An Individual Patient Data Meta-Analysis. Circulation. 2016;133(12):1189–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee CS, Bidwell JT, Paturzo M, et al. Patterns of self-care and clinical events in a cohort of adults with heart failure: 1 year follow-up. Heart Lung. 2018;47(1):40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaarsma T, Hill L, Bayes-Genis A, et al. Self-care of heart failure patients: practical management recommendations from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2021;23(1):157–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cross SH, Mehra MR, Bhatt DL, et al. Rural-urban differences in cardiovascular mortality in the US, 1999–2017. JAMA. 2020;323(18):1852–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Osokpo O, Riegel B. Cultural factors influencing self-care by persons with cardiovascular disease: an integrative review. International journal of nursing studies. 2021;116:103383. [DOI] [PubMed] [Google Scholar]
- 20.Abraído-Lanza AE, Viladrich A, Flórez KR, Céspedes A, Aguirre AN, De La Cruz AA. Commentary: fatalismo reconsidered: a cautionary note for health-related research and practice with Latino populations. Ethn Dis. 2007;17(1):153–158. [PMC free article] [PubMed] [Google Scholar]
- 21.Morgan PD, Tyler ID, Fogel J. Fatalism revisited. Semin Oncol Nurs. 2008;24(4):237–245. [DOI] [PubMed] [Google Scholar]
- 22.Walker RJ, Smalls BL, Hernandez-Tejada MA, Campbell JA, Davis KS, Egede LE. Effect of diabetes fatalism on medication adherence and self-care behaviors in adults with diabetes. Gen Hosp Psychiatry. 2012;34(6):598–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abbott L, Slate E, Graven L, Lemacks J, Grant J. Fatalism, Social Support and Self-Management Perceptions among Rural African Americans Living with Diabetes and Pre-Diabetes. Nurs Rep. 2021;11(2):242–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klein WM, Ferrer RA, Graff KA, Kaufman AR, Han PK. Perceived ambiguity, fatalism, and believing cancer is more prevalent than heart disease. Am J Prev Med. 2014;46(4):e45–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chew HSJ, Sim KLD, Cao X, Chair SY. Motivation, Challenges and Self-Regulation in Heart Failure Self-Care: a Theory-Driven Qualitative Study. Int J Behav Med. 2019;26(5):474–485. [DOI] [PubMed] [Google Scholar]
- 26.Wingham J, Harding G, Britten N, Dalal H. Heart failure patients’ attitudes, beliefs, expectations and experiences of self-management strategies: a qualitative synthesis. Chronic Illn. 2014;10(2):135–154. [DOI] [PubMed] [Google Scholar]
- 27.Sterling MR, Barbaranelli C, Riegel B, et al. The Influence of Preparedness, Mutuality, and Self-efficacy on Home Care Workers’ Contribution to Self-care in Heart Failure: A Structural Equation Modeling Analysis. J Cardiovasc Nurs. 2022;37(2):146–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee CS, Westland H, Faulkner KM, et al. The effectiveness of self-care interventions in chronic illness: A meta-analysis of randomized controlled trials. Int J Nurs Stud. 2022;134:104322. [DOI] [PubMed] [Google Scholar]
- 29.Auld JP, Mudd JO, Gelow JM, Hiatt SO, Lee CS. Self-care Moderates the Relationship Between Symptoms and Health-Related Quality of Life in Heart Failure. J Cardiovasc Nurs. 2018;33(3):217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee KS, Jeon E-S, Park J-H, et al. Symptom detection and the relationship with self-care in heart failure. European Journal of Cardiovascular Nursing. 2022;21(8):821–829. [DOI] [PubMed] [Google Scholar]
- 31.Deng J, Radina E, Fu MR, et al. Self‐Care Status, Symptom Burden, and Reported Infections in Individuals With Lower‐Extremity Primary Lymphedema. Journal of Nursing Scholarship. 2015;47(2):126–134. [DOI] [PubMed] [Google Scholar]
- 32.Asuzu CC, Walker RJ, Williams JS, Egede LE. Pathways for the relationship between diabetes distress, depression, fatalism and glycemic control in adults with type 2 diabetes. Journal of Diabetes and its Complications. 2017;31(1):169–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lennie TA, Moser DK, Biddle MJ, et al. Nutrition intervention to decrease symptoms in patients with advanced heart failure. Res Nurs Health. 2013;36(2):120–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kang JM, Cho YS, Park S, et al. Montreal cognitive assessment reflects cognitive reserve. BMC Geriatr. 2018;18(1):261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Portenoy RK, Thaler HT, Kornblith AB, et al. The Memorial Symptom Assessment Scale: an instrument for the evaluation of symptom prevalence, characteristics and distress. European Journal of Cancer. 1994;30(9):1326–1336. [DOI] [PubMed] [Google Scholar]
- 36.Zambroski C, Lennie T, Chung M, Heo S, Smoot T, Ziegler C. Use of the memorial symptom assessment scale-heart failure in heart failure patients. Paper presented at: Circulation 2004. [Google Scholar]
- 37.Shen L, Condit CM, Wright L. The psychometric property and validation of a fatalism scale. Psychol Health. 2009;24(5):597–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adegboyega A, Chung ML, Moser DK, Mudd-Martin G. Psychometric Testing of a Cardiovascular Disease Fatalism Instrument Among Adults With Cardiovascular Disease Risks. J Nurs Meas. 2021;29(1):153–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mudd-Martin G, Rayens MK, Lennie TA, et al. Fatalism moderates the relationship between family history of cardiovascular disease and engagement in health-promoting behaviors among at-risk rural Kentuckians. J Rural Health. 2015;31(2):206–216. [DOI] [PubMed] [Google Scholar]
- 40.Riegel B, Carlson B, Moser DK, Sebern M, Hicks FD, Roland V. Psychometric testing of the self-care of heart failure index. J Card Fail. 2004;10(4):350–360. [DOI] [PubMed] [Google Scholar]
- 41.Vellone E, Riegel B, Cocchieri A, et al. Psychometric testing of the Self-Care of Heart Failure Index Version 6.2. Res Nurs Health. 2013;36(5):500–511. [DOI] [PubMed] [Google Scholar]
- 42.Riegel B, Lee CS, Dickson VV, Carlson B. An update on the self-care of heart failure index. J Cardiovasc Nurs. 2009;24(6):485–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;79(17):e263–e421. [DOI] [PubMed] [Google Scholar]
- 44.Bennett JA, Riegel B, Bittner V, Nichols J. Validity and reliability of the NYHA classes for measuring research outcomes in patients with cardiac disease. Heart Lung. 2002;31(4):262–270. [DOI] [PubMed] [Google Scholar]
- 45.Abu-Bader S, Jones TV. Statistical mediation analysis using the sobel test and hayes SPSS process macro. International Journal of Quantitative and Qualitative Research Methods. 2021. [Google Scholar]
- 46.Hayes AF. Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. Guilford publications; 2017. [Google Scholar]
- 47.Haedtke CA, Moser DK, Pressler SJ, Chung ML, Wingate S, Goodlin SJ. Influence of depression and gender on symptom burden among patients with advanced heart failure: Insight from the pain assessment, incidence and nature in heart failure study. Heart Lung. 2019;48(3):201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wagner LS, Wagner TH. The effect of age on the use of health and self-care information: confronting the stereotype. Gerontologist. 2003;43(3):318–324. [DOI] [PubMed] [Google Scholar]
- 49.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128(16):1810–1852. [DOI] [PubMed] [Google Scholar]
- 50.Urizar GG Jr., Sears SF Jr. Psychosocial and cultural influences on cardiovascular health and quality of life among Hispanic cardiac patients in South Florida. J Behav Med. 2006;29(3):255–268. [DOI] [PubMed] [Google Scholar]
- 51.Niederdeppe J, Levy AG. Fatalistic beliefs about cancer prevention and three prevention behaviors. Cancer Epidemiol Biomarkers Prev. 2007;16(5):998–1003. [DOI] [PubMed] [Google Scholar]
- 52.Franklin MD, Schlundt DG, McClellan LH, et al. Religious fatalism and its association with health behaviors and outcomes. Am J Health Behav. 2007;31(6):563–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chew HSJ, Lopez V. Empowered to Self-Care: A Photovoice Study in Patients With Heart Failure. J Transcult Nurs. 2018;29(5):410–419. [DOI] [PubMed] [Google Scholar]
- 54.Dickson VV, McCarthy MM, Howe A, Schipper J, Katz SM. Sociocultural influences on heart failure self-care among an ethnic minority black population. J Cardiovasc Nurs. 2013;28(2):111–118. [DOI] [PubMed] [Google Scholar]
- 55.Galdas PM, Oliffe JL, Wong ST, Ratner PA, Johnson JL, Kelly MT. Canadian Punjabi Sikh men’s experiences of lifestyle changes following myocardial infarction: cultural connections. Ethn Health. 2012;17(3):253–266. [DOI] [PubMed] [Google Scholar]
- 56.Austin RC, Schoonhoven L, Richardson A, Kalra PR, May CR. Qualitative interviews results from heart failure survey respondents on the interaction between symptoms and burden of self-care work. J Clin Nurs. 2022. [DOI] [PubMed] [Google Scholar]
- 57.Gonzalez JS, Tanenbaum ML, Commissariat PV. Psychosocial factors in medication adherence and diabetes self-management: Implications for research and practice. Am Psychol. 2016;71(7):539–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Osokpo O, Riegel B. Cultural factors influencing self-care by persons with cardiovascular disease: An integrative review. Int J Nurs Stud. 2021;116:103383. [DOI] [PubMed] [Google Scholar]
- 59.Airhihenbuwa CO, Ford CL, Iwelunmor JI. Why culture matters in health interventions: lessons from HIV/AIDS stigma and NCDs. Health Educ Behav. 2014;41(1):78–84. [DOI] [PubMed] [Google Scholar]
- 60.Montague MC, Nichols SA, Dutta AP. Self-management in African American women with diabetes. Diabetes Educ. 2005;31(5):700–711. [DOI] [PubMed] [Google Scholar]
- 61.Heo S, Lennie TA, Pressler SJ, Dunbar SB, Chung ML, Moser DK. Factors associated with perceived control and the relationship to quality of life in patients with heart failure. Eur J Cardiovasc Nurs. 2015;14(2):137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Biddle MJ, Moser DK, Pelter MM, Robinson S, Dracup K. Predictors of Adherence to Self-Care in Rural Patients With Heart Failure. J Rural Health. 2020;36(1):120–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kretchy IA, Owusu-Daaku FT, Danquah S. Locus of control and anti-hypertensive medication adherence in Ghana. Pan Afr Med J. 2014;17 Suppl 1(Suppl 1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pourhoseinzadeh MM, Gheibizadeh MP, Moradikalboland MPCBCP. The Relationship between Health Locus of Control and Health Behaviors in Emergency Medicine Personnel. Int J Community Based Nurs Midwifery. 2017;5(4):397–407. [PMC free article] [PubMed] [Google Scholar]
- 65.Clements L, Frazier SK, Lennie TA, Chung ML, Moser DK. Improvement in Heart Failure Self-Care and Patient Readmissions with Caregiver Education: A Randomized Controlled Trial. West J Nurs Res. 2022:1939459221141296. [DOI] [PubMed] [Google Scholar]
- 66.Büyükkaya Besen D, Günüşen N, Arda Sürücü H, Koşar C. Predictor effect of Locus Of Control (LOC) on self-care activities and metabolic control in individuals with type 2 diabetes. PeerJ. 2016;4:e2722. [DOI] [PMC free article] [PubMed] [Google Scholar]


