Abstract
Background
KRAS mutations in metastatic colorectal cancer (mCRC) are used as predictive biomarkers to select therapy with EGFR monoclonal antibodies (mAbs). Other factors may be significant determinants of benefit.
Methods
Individual patient data from randomised trials with a head-to-head comparison between EGFR mAb versus no EGFR mAb (chemotherapy alone or best supportive care) in mCRC, across all lines of therapy, were pooled. Overall survival (OS) and progression-free survival (PFS) were compared between groups. Treatment effects within the predefined KRAS biomarker subsets were estimated by adjusted hazard ratio (HRadj) and 95% confidence interval (CI). EGFR mAb efficacy was measured within the KRAS wild-type subgroup according to BRAF and NRAS mutation status. In both KRAS wild-type and mutant subgroups, additional factors that could impact EGFR mAb efficacy were explored including the type of chemotherapy, line of therapy, age, sex, tumour sidedness and site of metastasis.
Results
5675 patients from 8 studies were included, all with known mCRC KRAS mutation status. OS (HRadj 0.90, 95% CI 0.84–0.98, p = 0.01) and PFS benefit (HRadj 0.73, 95% CI 0.68–0.79, p < 0.001) from EGFR mAbs was observed in the KRAS wild-type group. PFS benefit was seen in patients treated with fluorouracil (HRadj 0.75, 95% CI 0.68–0.82) but not with capecitabine-containing regimens (HRadj 1.04, 95% CI 0.86–1.26) (pinteraction = 0.002). Sidedness also interacted with EGFR mAb efficacy, with survival benefit restricted to left-sided disease (pinteraction = 0.038). PFS benefits differed according to age, with benefits greater in those under 70 (pinteraction = 0.001). The survival benefit was not demonstrated in those patients with mutations found in the KRAS, NRAS or BRAF genes. The presence of liver metastases interacted with EGFR mAb efficacy in patients with KRAS mutant mCRC (pinteraction = 0.004).
Conclusion
The benefit provided by EGFR mAbs in KRAS WT mCRC is associated with left-sided primary tumour location, younger patient age and absence of NRAS or BRAF mutations. Survival benefit is observed with fluorouracil but not capecitabine. Exploratory results support further research in KRAS mutant mCRC without liver metastases.
Subject terms: Prognostic markers, Colon cancer, Targeted therapies
Introduction
The epidermal growth factor receptor (EGFR) was identified as a potential therapeutic target in the fight against cancer more than 20 years ago [1, 2]. Subsequently, anti-EGFR monoclonal antibodies (mAbs) were developed, particularly cetuximab and panitumumab, and these have changed the treatment landscape for many patients with metastatic colorectal cancer (mCRC) [3]. Improved outcomes with EGFR mAbs have been reported in first, second- and third-line therapy for mCRC [4–8].
Despite the overall benefit, the use of anti-EGFR mAbs in mCRC is associated with treatment-related toxicity and a lack of response in a significant proportion of patients. Moreover, the financial expense of EGFR mAbs is a significant consideration [9]. The cost-effectiveness and therapeutic index of EGFR mAbs have been improved through the identification of molecular biomarkers to predict which patients are more likely to benefit and to determine which patients should not be selected for such treatment. Studies initially demonstrated that benefit derived from the addition of EGFR mAb has limited to patients with tumours wild-type (WT) at KRAS exon 2 and subsequently to “extended RAS” WT tumours, which do not harbour activating mutations at KRAS exons 2,3 and 4 or NRAS exons 2, 3 and 4 [4–6, 8, 10]. Cetuximab and panitumumab were incorporated into international guidelines recommending their use in patients with RAS WT mCRC in conjunction with or after chemotherapy [11, 12].
Some studies, however, have failed to demonstrate survival benefits with the application of EGFR mAbs in patients with KRAS WT mCRC [13, 14]. Inconsistent findings across the trials have been attributed to the nature of the concurrent chemotherapy backbone, patient selection factors and to chance. The true magnitude of the benefit remains unclear. We set out to explore the effect of KRAS mutations on the efficacy of EGFR mAbs in the treatment of mCRC.
Methods
Individual patient data from randomised trials, collected in the ARCAD database and identified in Project Data Sphere (PDS), with head-to-head comparison between EGFR mAb, administered either alone or with chemotherapy, versus the same treatment without the EGFR mAb (i.e., chemotherapy alone or BSC alone) in mCRC, across all lines of therapy (first, second and later), were pooled. Studies that tested VEGF mAbs only were not considered. For studies that included both EGFR and VEGF mAbs, the treatment arms with VEGF mAbs and a combination of EGFR and VEGF mAbs were excluded. Due to not being adopted in practice, the treatment arms with intermittent use of chemotherapy were also excluded. Only individual mCRC patients with known KRAS mutation status were included in the pooled analysis. Overall survival (OS) and progression-free survival (PFS) were compared within the KRAS WT and KRAS mutant (MT) cohorts by the Cox model, stratified by studies and adjusted by age, gender, and performance status. Treatment effects were estimated by adjusted hazard ratio (HRadj) and 95% confidence interval (CI).
The primary study objective was to determine the effect of EGFR mAbs on survival outcomes when used in the treatment of mCRC according to KRAS mutation status. KRAS was selected as the biomarker of interest as this was the first to be reported to be associated with EGFR mAb benefit. The effect of additional biomarkers, principally BRAF and NRAS mutations, particularly within the KRAS WT subgroup, was also explored. We sought to determine if the survival effect of anti-EGFR mAb is influenced by; BRAF and NRAS mutation status, sidedness (left v right), age, sex, type of chemotherapy backbone (oxaliplatin or irinotecan) and type of fluoropyrimdine (fluorouracil or capecitabine). We also evaluated the effect of the line of therapy (first, second or third (last) line). The site of metastatic disease (liver, lung, lymph nodes) and prior surgical interventions (surgery to excise primary, surgery to excise metastases) were also explored. P-values for survival comparisons < 0.01 were considered statistically significant to account for multiple comparisons. Interaction P-values of < 0.05 were considered significant.
Results
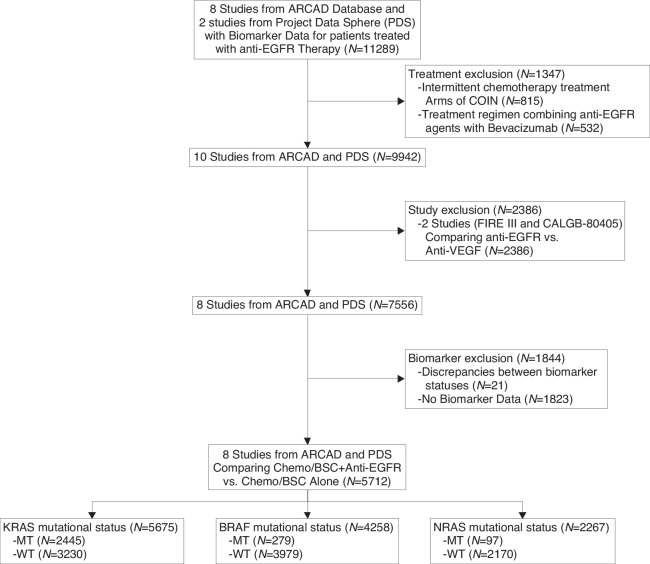
Ten studies from the ARCAD database and PDS included anti-EGFR therapies. Two studies were excluded because they contained bevacizumab in the comparator arm. Among the remaining 8 trials that qualified for inclusion, 815 patients who received intermittent chemotherapy (one treatment arm from the COIN trial) were excluded. An additional 532 patients who received a combination of anti-EGFR agents with bevacizumab were excluded. Furthermore, 1844 patients were excluded where no biomarker data was available. 5675 patients with data available on the KRAS mutation status of the CRC were included and analysed. The consort flow diagram (Fig. 1) details the study and patient selection process.
Fig. 1. Consort diagram.
ARCAD Aide et Recherche en Cancérologie Digestive, EGFR epidermal growth factor receptor, VEGF vascular endothelial growth factor, MT mutant, WT wild-type.
The included study titles and the number of patients that each study contributed to this analysis are detailed in Supplemental Table 1. Supplemental Table 2 summarises data reported in the original publications of these trials, where only 2 of the 8 included studies demonstrated statistically significant improvement in overall survival in the patients with KRAS WT tumours and none reported benefit in the patients with KRAS MT tumours.
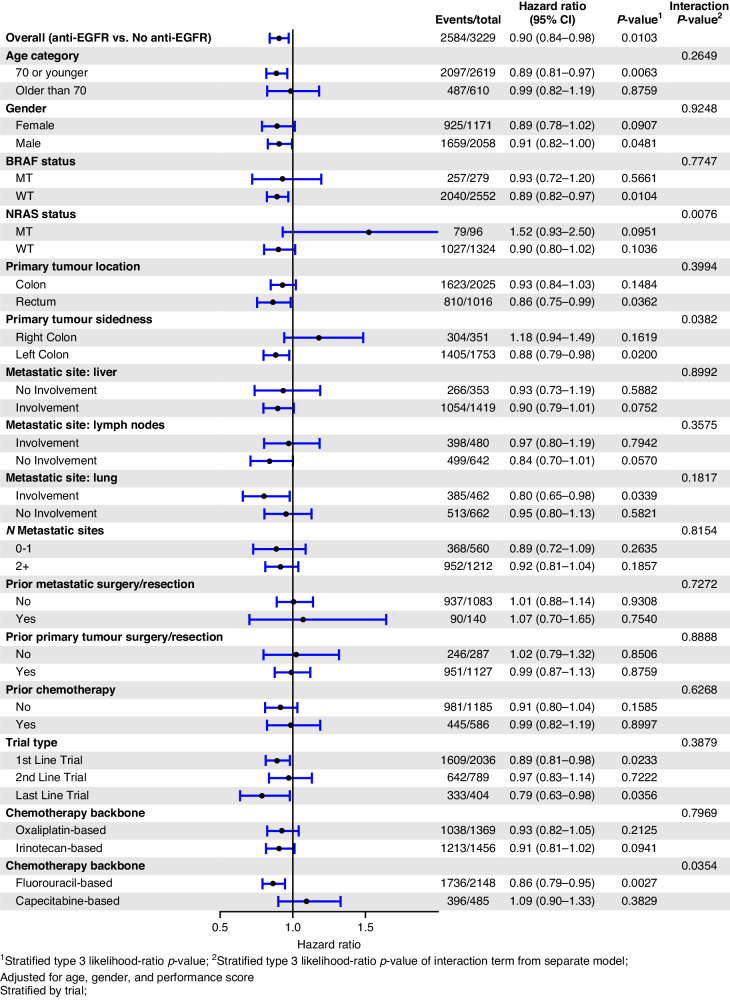
3230 patients had KRAS WT tumours, and 1601 of these patients were treated with EGFR mAbs. 2445 patients had KRAS MT tumours, and 1244 of these patients received EGFR mAbs. Overall survival was prolonged in those patients with KRAS WT mCRC who were treated with an EGFR mAb, but the benefit bordered the predefined significance level (HRadj 0.90, 95% CI 0.84–0.98, p = 0.01). PFS was significantly improved with EGFR mAbs in the KRAS WT group (HRadj 0.73, 95% CI 0.68–0.79, p < 0.001). Sidedness was a significant determinant of benefit, with EGFR mAb use associated with PFS prolongation in left-side tumours but not right-sided disease (left-sided PFS HRadj 0.74, 95% CI 0.67–i0.83, p < 0.001; right-sided PFS HRadj 1.03, 95% CI 0.81–1.32, p = 0.798, pinteraction 0.021).
Where NRAS and BRAF mutation status were known, the presence of mutations in these genes was found to affect the EGFR mAb survival benefit. Patients with KRAS WT mCRC who had NRAS MT did not benefit from EGFR mAbs (OS HRadj 1.52, 95% CI 0.93–2.50, p = 0.095, pinteraction = 0.008; PFS HRadj 1.61, 95% CI 1.00–2.61, p = 0.048, pinteraction = 0.001). The interaction test for overall survival was not significant according to BRAF mutation status (OS HRadj 0.93, 95% CI 0.72–1.20, p = 0.566, pinteraction = 0.775) but it did interact with PFS benefit (PFS HRadj 0.93, 95% CI 0.72–1.21, p = 0.608, pinteraction = 0.014).
When BSC was the control arm (the ‘later line’ trials), EGFR mAb therapy provided OS and PFS benefit in patients with KRAS WT cancers (OS HRadj 0.79, p = 0.036 and PFS HRadj 0.41, p < 0.001). The PFS benefit observed in these ‘later line’ trials was greater than the PFS benefit seen in the first- and second-line trials (pinteraction < 0.001) for patients with KRAS WT mCRC.
When fluoropyrimidine-based treatment was the control arm, the choice of fluoropyrimidine made a difference, as the survival benefit was only observed in patients treated with fluorouracil (OS HRadj 0.86, 95% CI 0.79 – 0.95, p = 0.003, and PFS HRadj 0.75, 95% CI 0.68–0.82, p < 0.001) and not in those treated with capecitabine (OS HRadj 1.09, 95% CI 0.90-1.33 and PFS HRadj 1.04, 95% CI 0.86–1.26). The difference between fluorouracil and capecitabine satisfied the interaction test for OS (pinteraction = 0.035) and PFS (pinteraction = 0.002). The benefit associated with EGFR mAb use in patients with KRAS WT mCRC was seen when either irinotecan or oxaliplatin were used in the partnering chemotherapy combination, with no significant difference between the two.
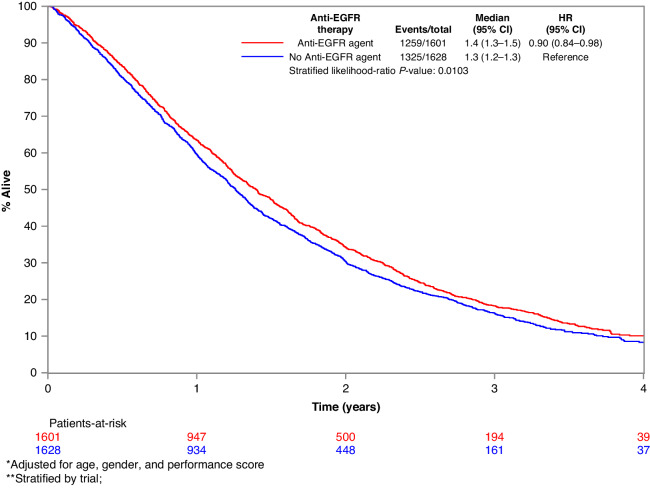
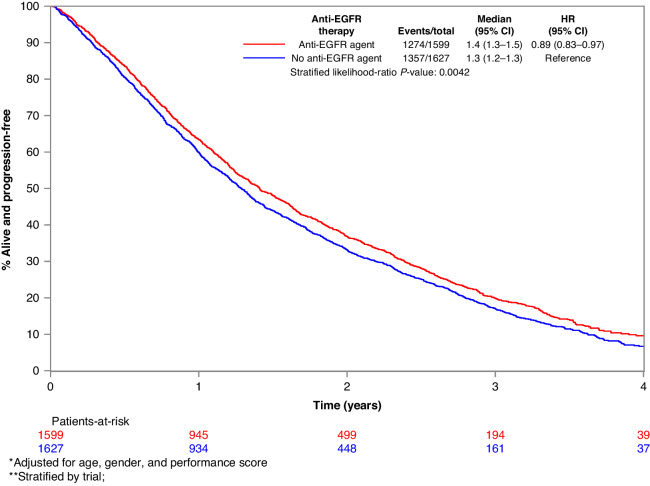
EGFR mAb associated PFS benefit was larger in those with KRAS WT tumours if they were aged 70 or younger (HRadj 0.68, p < 0.001) compared to those over 70 (HRadj 0.93, p = 0.465). Other explored variables, including gender, site of metastatic disease, the extent of metastatic disease, previous resection of the bowel cancer primary, and the number of lines of therapy did not have a significant influence on the benefit associated with EGFR mAb in patients in KRAS WT mCRC. The forest plot of OS by variables of interest and the associated Kaplan–Meier survival curves for OS in the KRAS WT subgroup are depicted in Figs. 2 and 3, respectively. The forest plot of PFS according to the variables of interest, and the associated Kaplan–Meier PFS curves for PFS in the KRAS WT subgroup are depicted in Figs. 4 and 5, respectively.
Fig. 2. Forest plot of OS in the KRAS wild-type subgroup.
Forest plot of overall survival for KRAS wild-type subgroup of patients treated with EGFR mAb + chemo/BSC versus chemo/BSC alone. EGFR epidermal growth factor receptor.
Fig. 3. Kaplan–Meier OS curves in the KRAS WT subgroup.
Kaplan–Meier overall survival curves for KRAS wild-type subgroup of patients treated with EGFR mAb + chemo/BSC versus chemo/BSC alone. EGFR epidermal growth factor receptor.
Fig. 4. Forest plot—PFS in KRAS wild-type subgroup.
Forest plot of progression-free survival for KRAS wild-type subgroup of patients treated with EGFR mAb + chemo/BSC versus chemo/BSC alone. EGFR epidermal growth factor receptor.
Fig. 5. Kaplan–Meier PFS curves in the KRAS WT subgroup.
Kaplan–Meier progression-free survival curves for KRAS wild-type subgroup of patients treated with EGFR mAb + chemo/BSC versus chemo/BSC alone. EGFR epidermal growth factor receptor.
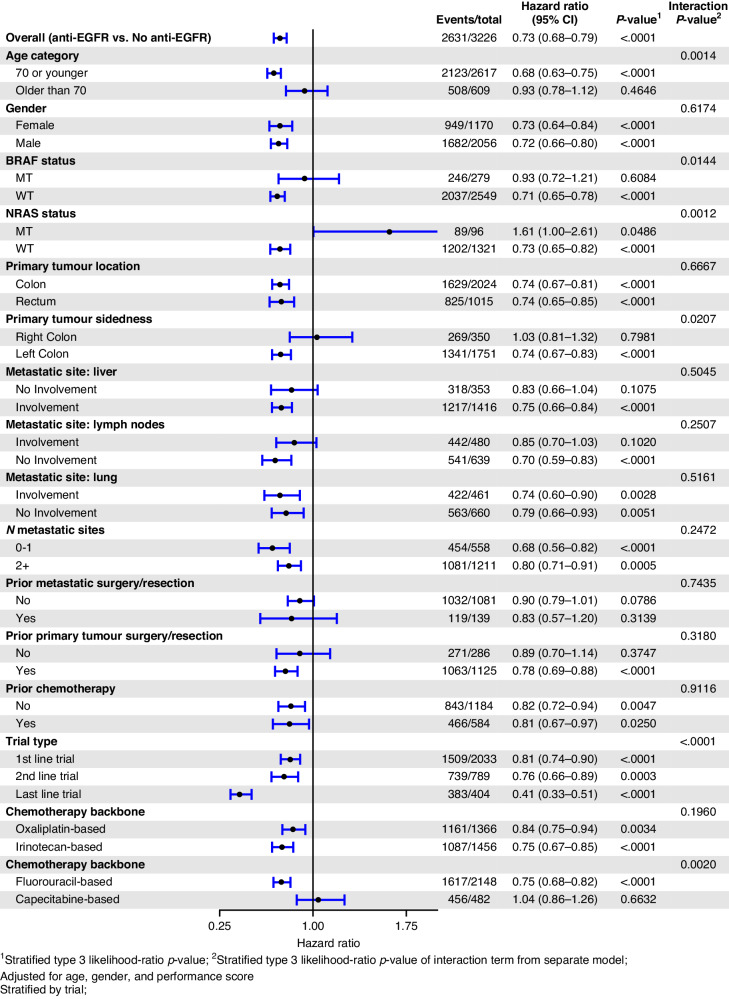
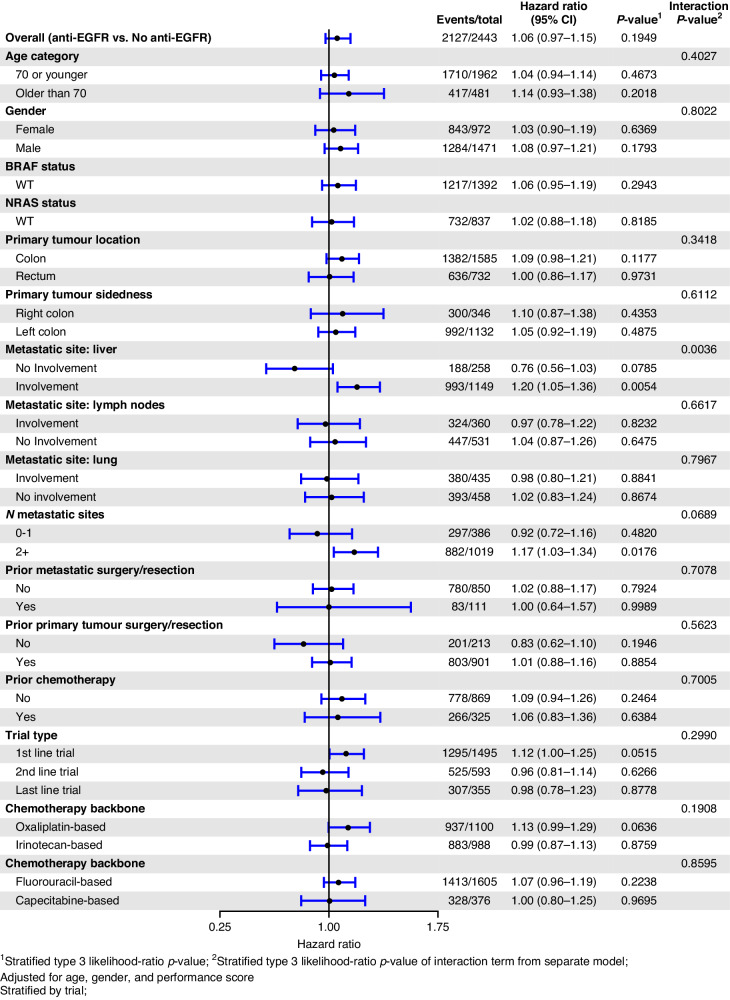
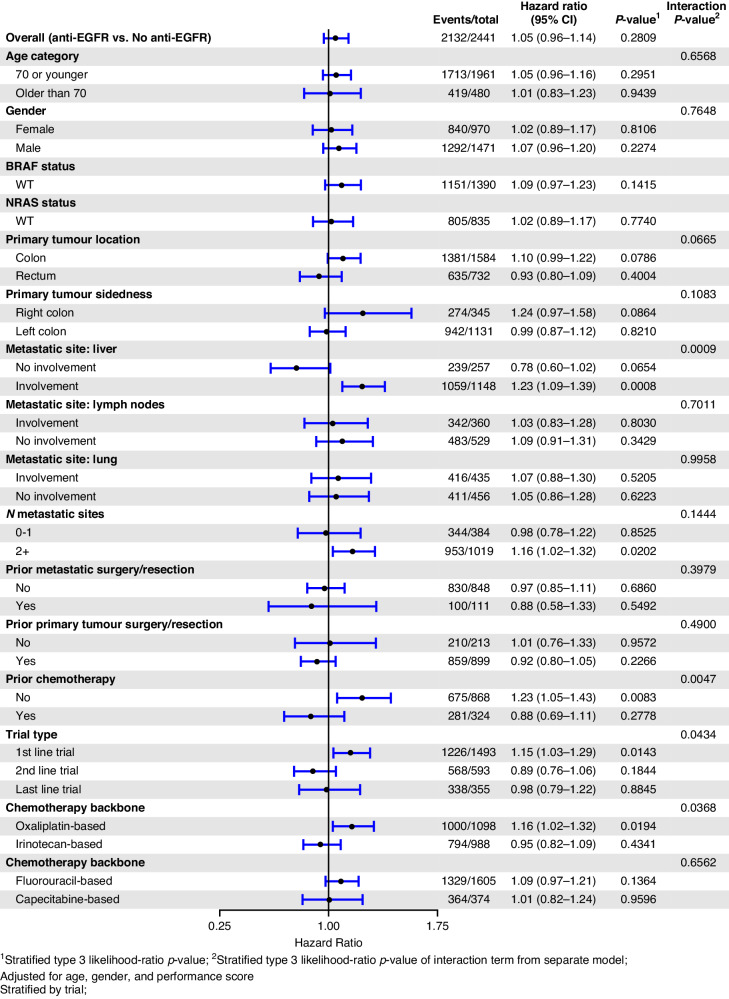
For the KRAS MT subgroup, the use of EGFR mAb did not improve OS (HR 1.06, 95% CI 0.97–1.15) or PFS (HR 1.05, 95% CI 0.96–1.14). Exploratory analyses showed a detrimental treatment effect of EGFR mAbs in KRAS mutant mCRC with liver metastasis (OS: HRadj 1.20, p = 0.005, pinteraction = 0.004; PFS: HRadj 1.23, p < 0.001, pinteraction < 0.001). In the KRAS MT cohort, the OS HR for those without liver metastases was 0.76 (p = 0.078) and PFS HR was 0.78 (p = 0.065). The forest plots of OS and PFS in the KRAS MT cohort according to the variables of interest are depicted in Figs. 6 and 7, respectively.
Fig. 6. Forest plot—overall survival in the KRAS mutant subgroup.
Forest plot of overall survival for KRAS mutant subgroup of patients treated with EGFR mAb + chemo/BSC versus chemo/BSC alone. EGFR epidermal growth factor receptor.
Fig. 7. Forest plot—PFS in the KRAS MT subgroup.
Forest plot of progression-free survival for KRAS mutant subgroup of patients treated with EGFR mAb + chemo/BSC versus chemo/BSC alone. EGFR epidermal growth factor receptor.
Discussion
This is the largest IPD analysis to explore the predictive value of KRAS mutation status in mCRC. EGFR mAbs prolong OS and PFS in KRAS WT mCRC. KRAS mutation status has been adopted globally as a predictive biomarker for EGFR mAb efficacy [15]. Our findings support current clinical practice guidelines that restrict the use of EGFR mAbs to patients with mCRC which is KRAS WT. In contrast, those patients with mCRC that carry KRAS mutations do not benefit from EGFR mAb. We observed a lack of benefit using EGFR mAbs in NRAS mutant mCRC. Moreover, there was a trend toward inferior survival outcomes when EGFR mAb was used in the treatment of NRAS mutant mCRC. For BRAF mutant mCRC, we did not observe a benefit with EGFR mAb use, but the results did not suggest harm. In addition, the interaction test for BRAF mutation status was only significant for PFS and not OS. Further analysis of the impact of the BRAF status of mCRC, through later lines of therapy and by tumour sidedness, is warranted.
We explored multiple variables to identify potential patient subgroups within the KRAS MT cohort that may benefit from EGFR mAb therapy. We observed a non-statistically significant survival benefit for those patients without liver metastases. However, the use of EGFR mAbs was associated with a detrimental effect in patients with KRAS MT mCRC with liver involvement. These findings are intriguing and suggest that the pattern of cancer spread may influence the biological effects of anti-EGFR mAb therapy in the setting of KRAS MT mCRC, in either a positive or negative direction. These findings are best considered hypothesis-generating, especially as there is no treatment effect overall in the KRAS MT group.
Tumour-sidedness is a variable that has been incorporated into clinical practice, with left-sided location favoring EGFR mAb use compared to right-sided tumours [16, 17]. Our analysis supports this clinical practice, as we observed differences in EGFR mAb efficacy based on tumour sidedness. The survival benefit was statistically significant in the left-sided cancer cohort, but we could not demonstrate a benefit when patients with right-sided cancers received EGFR mAbs. When adding a monoclonal antibody to first-line chemotherapy, clinicians choose between bevacizumab and EGFR mAb. The side of cancer origin is an important factor in treatment decision consideration in current clinical practice. We did not consider studies that included bevacizumab in our meta-analysis.
The scale of our IPD analysis represents the largest dataset to examine a potential interaction between the type of chemotherapy and EGFR mAb effect. We observed a significant difference according to the type of fluoropyrimidine used. For patients with KRAS WT mCRC, EGFR mAb benefit was observed in those who received fluorouracil but there was no demonstrable benefit in those treated with capecitabine. A difference in EGFR mAb efficacy according to the type of fluoropyrimdine used in the chemotherapy backbone has been reported previously, with infusional fluorouracil associated with better outcomes when used with EGFR mAb than either capecitabine or bolus fluorouracil regimens [18, 19]. Differences in the toxicity profile of these chemotherapies plus EGFR mAb combinations, leading to dose reduction and treatment interruption, may explain these differences. Another purported biologically plausible mechanism is the ability of EGFR mAbs to reduce cell cycling through the promotion of G1 arrest. This reduces metabolic activation of capecitabine within cells leading to a reduced cytotoxic effect. It should be noted that only one study, the COIN study [13], used capecitabine in preference to fluorouracil in the chemotherapy doublet backbone. Another potential chemotherapy difference is the fluoropyrimdine doublet partnering drug, irinotecan versus oxaliplatin. Irinotecan-based chemotherapy regimens have been associated with greater EGFR mAb efficacy when compared to oxaliplatin-backbone regimens [18, 20]. We did not identify differences when comparing oxaliplatin-based chemotherapy regimens to regimens that use irinotecan.
Our results indicate a decline in benefit from EGFR mAb use with advancing age, specifically in those aged over 70. Reduced benefit with advanced age has been observed in previous studies of chemotherapy for mCRC. Potential reasons include increasing treatment toxicity with age, lower treatment dose delivery, and the effect of co-morbidities and competing risk factors for survival. Our findings highlight the need to consider the risks of toxicity and take into account co-morbidities when selecting the use of an EGFR mAb in patients aged over 70. One way of improving the safety profile of EGFR mAb-based therapy in the elderly is to consider reducing the intensiveness of the chemotherapy, using single-agent fluoropyrimidine instead of doublet chemotherapy, as demonstrated in the phase II PANDA study [21].
The meta-analysis supports the use of EGFR mAbs in the treatment of mCRC without mutations in KRAS, particularly for left-sided colorectal cancer and where the tumour does not harbour NRAS or BRAF mutations. The relative magnitude of benefit is greatest in later lines of therapy with single-agent EGFR mAb when compared to BSC. In earlier lines of therapy, when the EGFR mAb is combined with chemotherapy, fluorouracil-containing doublet chemotherapy regimens should be preferred to capecitabine-containing regimens. These findings could be considered in management guidelines to aid appropriate treatment decisions in clinical practice.
Supplementary information
Acknowledgements
Supported by La Fondation A.R.C.A.D., Aide et Recherche en Cancérologie Digestive and Australian National Health and Medical Research Council (NHMRC) Project Grant 1085364.
Author contributions
Authors Karapetis and Sorich initially developed the study concept. All authors reviewed the study proposal and contributed to the study design. Authors Shi, Pederson and Liu collated the individual patient data from the relevant and included studies, through the ARCAD database, and performed the statistical analyses. Interpretation of the analyses and completion of the manuscript was led by author Karapetis, with all authors contributing to the final analyses and report. The authors did not receive any third-party assistance in conducting the study or in writing their findings.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Data availability
The data sharing of individual patient data from each participating trial will be subject to the policy and procedures of the institutions and groups who conducted the original study.
Competing interests
Karapetis CS declares an advisory board role for Roche, Amgen and Merck Serono. Liu H declares no potential competing interests. Sorich M declares research grants received from Pfizer. Pederson LD declares no potential competing interests. Van Cutsem E declares participation to advisory boards for Abbvie, ALX, Amgen, Array, Astellas, AstraZeneca, Bayer, Beigene, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi, GSK, Incyte, Ipsen, Lilly, Merck Sharp & Dohme, Merck KGaA, Mirati, Novartis, Nordic, Pierre Fabre, Pfizer, Roche, Seattle Genetics, Servier, Takeda, Terumo, Taiho, Zymeworks. He also declares research grants from Amgen, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Ipsen, Lilly, Merck Sharp & Dohme, Merck KGaA, Novartis, Roche, Servier paid to his institution. Maughan T declares that he has consulted for AstraZeneca, Nordic Pharma and Ground Truth Laboratories. Douillard JY declares no potential competing interests. O’Callaghan CJ declares no potential competing interests. Jonker D declares no potential competing interests. Bokemeyer C declares advisory board involvement for Astra Zeneca, Bayer, BioNTech, BMS, Jansen Cilag, Merck Serono, Oncology Drug Consult CRO, and Sanofi Aventis. He also declares compensation as an invited speaker for AOK Germany, Med Update and Roche Pharma. Sobrero A declares advisory board and speaker at satellites for Roche, Merck, Amgen, Bayer, Sanofi, Servier, BMS, MSD. Cremolini C declares an advisory board role with Merck Serono, MSD, Nordic Pharma, Roche, Pierre Fabre and Takeda, and she has been an invited speaker with compensation for Amgen, Bayer, Merck Serono, MSD, Pierre Fabre and Servier. She also declares that she is a local principal investigator on trials sponsored by Bayer, Hutchinson, Merck, Roche, Seagen and Servier. Chibaudel B declares no potential competing interests. Zalcberg J declares leadership roles with ICON Group, Lipotek and Praxis. He declares stock ownership of BioMarin, Opthea, Amarin, Concert Pharmaceuticals, Frequency Therapeutics, Gilead, Madrigal Pharmaceuticals, uniQure, Zogenix, Orphazyme, Moderna Therapeutics, TWST, Novavax and Teladoc. He declares honoraria received from Gilead Sciences, MSD Oncology and Viatris. He declares a consulting or advisory role for MSD, CEND, Deciphera, Revolution Medicine, Specialised Therapeutics, FivepHusion, Genorbio, 1Global, Novotech, Alloplex Biotherapeutics Inc, NOUS Consulting and Oncology Republic. He has received paid travel and accommodation expenses from MSD Oncology, ICON Group and PRAXIS. Adams R declares conference attendance fees paid by Amgen. Buyse M declares stock ownership in IDDI (International Drug Development Institute, Belgium). Peeters M16 declares no potential competing interests. Yoshino T declares honoraria received from Chugai Pharma, Takeda Pharma, Merck, Bayer Yakuhin, Ono Pharmaceutical and MSD K.K; Consulting Fee from Sumitomo Corp.; Research Grant/Funding from Amgen, Chugai, Daiichi Sankyo, Eisai, FALCO Biosystems, Genomedia Inc., Molecular Health, MSD, Nippon Boehringer Ingelheim, Ono, Pfizer, Roche Diagnostics, Sanofi, Sysmex and Taiho de Gramont A declares no potential competing interests. Shi Q declares consulting/advisory role from Yiviva Inc., Boehringer Ingelheim Pharmaceuticals, Inc., Regeneron Pharmaceuticals, Inc., Hoosier Cancer Research Network, Kronos Bio, and Mirati Therapeutics Inc.; Honorarium/speaker role from Chugai Pharmaceutical Co., Ltd.
Ethics approval and consent to participate
The ARCAD database collaboration research protocol was approved by the Mayo Clinic Institution Review Board. All patients had consented to take part in the randomised controlled trials from which the data was collected and stored in the ARCAD database.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41416-024-02604-y.
References
- 1.Baselga J, Norton L, Masui H, Pandiella A, Coplan K, Miller WH, Jr, et al. Antitumor effects of doxorubicin in combination with anti-epidermal growth factor receptor monoclonal antibodies. J Natl Cancer Inst. 1993;85:1327–33. doi: 10.1093/jnci/85.16.1327. [DOI] [PubMed] [Google Scholar]
- 2.Kim ES, Khuri FR, Herbst RS. Epidermal growth factor receptor biology (IMC-C225) Curr Opin Oncol. 2001;13:506–13. doi: 10.1097/00001622-200111000-00014. [DOI] [PubMed] [Google Scholar]
- 3.Baselga J. The EGFR as a target for anticancer therapy–focus on cetuximab. Eur J Cancer. 2001;37:S16–22. doi: 10.1016/S0959-8049(01)00233-7. [DOI] [PubMed] [Google Scholar]
- 4.Van Cutsem E, Kohne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N. Engl J Med. 2009;360:1408–17. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 5.Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol. 2010;28:4697–705. doi: 10.1200/JCO.2009.27.4860. [DOI] [PubMed] [Google Scholar]
- 6.Peeters M, Price TJ, Cervantes A, Sobrero AF, Ducreux M, Hotko Y, et al. Final results from a randomized phase 3 study of FOLFIRI {+/−} panitumumab for second-line treatment of metastatic colorectal cancer. Ann Oncol. 2014;25:107–16. doi: 10.1093/annonc/mdt523. [DOI] [PubMed] [Google Scholar]
- 7.Jonker DJ, O’Callaghan CJ, Karapetis CS, Zalcberg JR, Tu D, Au HJ, et al. Cetuximab for the treatment of colorectal cancer. N. Engl J Med. 2007;357:2040–8. doi: 10.1056/NEJMoa071834. [DOI] [PubMed] [Google Scholar]
- 8.Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, Freeman DJ, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626–34. doi: 10.1200/JCO.2007.14.7116. [DOI] [PubMed] [Google Scholar]
- 9.Mittmann N, Au HJ, Tu D, O’Callaghan CJ, Isogai PK, Karapetis CS, et al. Prospective cost-effectiveness analysis of cetuximab in metastatic colorectal cancer: evaluation of National Cancer Institute of Canada Clinical Trials Group CO.17 trial. J Natl Cancer Inst. 2009;101:1182–92. doi: 10.1093/jnci/djp232. [DOI] [PubMed] [Google Scholar]
- 10.Karapetis CS, Khambata-Ford S, Jonker DJ, O’Callaghan CJ, Tu D, Tebbutt NC, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N. Engl J Med. 2008;359:1757–65. doi: 10.1056/NEJMoa0804385. [DOI] [PubMed] [Google Scholar]
- 11.Yoshino T, Arnold D, Taniguchi H, Pentheroudakis G, Yamazaki K, Xu RH, et al. Pan-Asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer: a JSMO-ESMO initiative endorsed by CSCO, KACO, MOS, SSO and TOS. Ann Oncol. 2018;29:44–70. doi: 10.1093/annonc/mdx738. [DOI] [PubMed] [Google Scholar]
- 12.Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, et al. Colon cancer, version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:329–59. doi: 10.6004/jnccn.2021.0012. [DOI] [PubMed] [Google Scholar]
- 13.Maughan TS, Adams RA, Smith CG, Meade AM, Seymour MT, Wilson RH, et al. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet. 2011;377:2103–14. doi: 10.1016/S0140-6736(11)60613-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seymour MT, Brown SR, Middleton G, Maughan T, Richman S, Gwyther S, et al. Panitumumab and irinotecan versus irinotecan alone for patients with KRAS wild-type, fluorouracil-resistant advanced colorectal cancer (PICCOLO): a prospectively stratified randomised trial. Lancet Oncol. 2013;14:749–59. doi: 10.1016/S1470-2045(13)70163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taniguchi H, Yamazaki K, Yoshino T, Muro K, Yatabe Y, Watanabe T, et al. Japanese Society of Medical Oncology Clinical Guidelines: RAS (KRAS/NRAS) mutation testing in colorectal cancer patients. Cancer Sci. 2015;106:324–7. doi: 10.1111/cas.12595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sunakawa Y, Ichikawa W, Tsuji A, Denda T, Segawa Y, Negoro Y, et al. Prognostic impact of primary tumor location on clinical outcomes of metastatic colorectal cancer treated with cetuximab plus oxaliplatin-based chemotherapy: a subgroup analysis of the JACCRO CC-05/06 trials. Clin Colorectal Cancer. 2017;16:e171–e80. doi: 10.1016/j.clcc.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 17.Kim D, Kim SY, Lee JS, Hong YS, Kim JE, Kim KP, et al. Primary tumor location predicts poor clinical outcome with cetuximab in RAS wild-type metastatic colorectal cancer. BMC Gastroenterol. 2017;17:121. doi: 10.1186/s12876-017-0694-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan DL, Pavlakis N, Shapiro J, Price TJ, Karapetis CS, Tebbutt NC, et al. Does the chemotherapy backbone impact on the efficacy of targeted agents in metastatic colorectal cancer? A systematic review and meta-analysis of the literature. PLoS ONE. 2015;10:e0135599. doi: 10.1371/journal.pone.0135599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vale CL, Tierney JF, Fisher D, Adams RA, Kaplan R, Maughan TS, et al. Does anti-EGFR therapy improve outcome in advanced colorectal cancer? A systematic review and meta-analysis. Cancer Treat Rev. 2012;38:618–25. doi: 10.1016/j.ctrv.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Loupakis F, Cremolini C, Salvatore L, Schirripa M, Lonardi S, Vaccaro V, et al. Clinical impact of anti-epidermal growth factor receptor monoclonal antibodies in first-line treatment of metastatic colorectal cancer: meta-analytical estimation and implications for therapeutic strategies. Cancer. 2012;118:1523–32. doi: 10.1002/cncr.26460. [DOI] [PubMed] [Google Scholar]
- 21.Lonardi S, Rasola C, Lobefaro R, Rossini D, Formica V, Scartozzi M, et al. Initial panitumumab plus fluorouracil, leucovorin, and oxaliplatin or plus fluorouracil and leucovorin in elderly patients with RAS and BRAF wild-type metastatic colorectal cancer: The PANDA Trial by the GONO Foundation. J Clin Oncol. 2023;41:5263–73. doi: 10.1200/JCO.23.00506. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sharing of individual patient data from each participating trial will be subject to the policy and procedures of the institutions and groups who conducted the original study.