Abstract
The recent surge in the discovery of hidden diversity within rheophilic taxa, particularly in West and East Africa, prompted a closer examination of the extent to which the current taxonomy may obscure the diversity of riffle-dwelling suckermouth catfishes in the genus Chiloglanis in southern Africa. Currently, the region comprises eight valid species within this genus. Seven of them have relatively narrow geographic distribution ranges except for C.neumanni, which is considered to be widely distributed, occurring from the Buzi River system in the south, and its northern limit being the eastward draining river systems in Tanzania. Recent surveys of the middle Zambezi River system revealed Chiloglanis specimens that were distinguishable from the known species of the genus from southern Africa. Integration of molecular and morphological data indicated that these specimens from the Mukwadzi River represent a new species to science, herein described as Chiloglaniscarnatus Mutizwa, Bragança & Chakona, sp. nov. This species is readily distinguished from its southern African congeners by the possession of a distinctive extended dermal tissue covering the base of the dorsal fin and the possession of ten mandibular teeth (vs 8, 12, or 14 in the other taxa). Results from this study add to the growing evidence of a high level of undocumented diversity within riffle-dwelling taxa in southern Africa.
Key words: Diversity, freshwater, integrative taxonomy, rheophilic taxa, southern Africa
Introduction
Rheophilic habitats are characterised by fast flowing water and rocky substratum, which provide a wide range of specialised niches for distinct aquatic taxa adapted to these environments (Thompson 2013; Hrbek et al. 2018). Delimitation of species boundaries in rheophilic taxa using only morphological traits has previously presented challenges due to their superficially similar morphology, which is shaped by exposure to similar environmental drivers (Seegers 2008). However, integrative taxonomy as well as recent collections in under-sampled areas within the African continent have changed the previous perception that rheophilic habitats were depauperate (Schmidt et al. 2015, 2016, 2017, 2023; Thomson et al. 2015; Schmidt and Barrientos 2019; Kashindye et al. 2021; Mazungula and Chakona 2021; Day et al. 2023). These studies, which implemented integrative taxonomic approaches, have allowed the discovery of hidden diversity, particularly within the catfish genera Chiloglanis Peters, 1868 and Amphilius Günther, 1864, from different regions of the continent. An emerging pattern shows that species that were previously perceived to have broad geographic ranges represent species complexes comprising distinct lineages confined to specific river basins (Chakona et al. 2018; Mutizwa et al. 2021). Recently, a careful examination of the once broadly distributed catfish species, C.occidentalis Pellegrin, 1933 and C.micropogon Poll, 1952 from West Africa and A.natalensis Boulenger, 1917 from southern Africa, resulted in the description of 15 new species (Schmidt et al. 2017, 2023; Mazungula and Chakona 2021). Following these findings, rheophilic habitats have been identified as a new frontier for the discovery of hidden diversity of freshwater fishes in southern Africa and other poorly explored regions on the continent (Morris et al. 2016; Schmidt et al. 2016; Chakona et al. 2018).
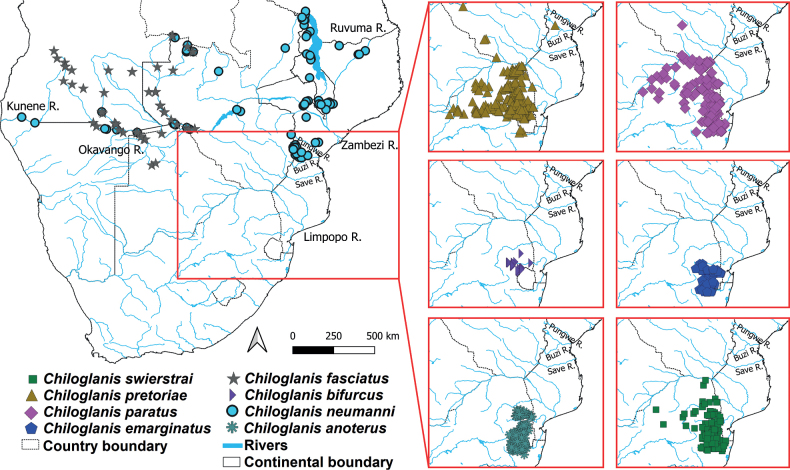
The Mochokidae is the most species-rich freshwater catfish family that is endemic to Africa (Vigliotta 2008). Currently, this family has 228 valid species that are distributed across several river systems in sub-Saharan Africa, with the highest diversity occurring in the Congo River (Seegers 2008; Vigliotta 2008; Fricke et al. 2024). The Mochokidae is sister to a clade containing families Auchenoglanididae, Claroteidae, Malapteruridae, and Schilbeidae (Sullivan et al. 2006; Schedel et al. 2022). The genera within Mochokidae have been split into two subfamilies: the first is Chiloglanidinae, characterised by lips and barbels that are modified into an oral disc (suckermouth), a structure that is absent in the second subfamily Mochokinae. Chiloglanidinae contains the genera Chiloglanis Peters, 1868, Atopodontus Friel & Vigliotta, 2008, Atopochilus Sauvage, 1879, and Euchilichthys Boulenger, 1900, whereas Mochokinae includes the genera Mochokus Joannis, 1835, Mochokiella Howes, 1980, Acanthocleithron Nichols & Griscom, 1917, Microsynodontis Boulenger, 1903, and Synodontis Cuvier, 1816. Some of the intergeneric (e.g., the monophyly of Mochokinae) and the intrageneric (e.g., the monophyly of Synodontis) relationships within Mochokidae are not well supported and require broader species sampling to resolve (Sullivan et al. 2006; Vigliotta 2008; Day et al. 2013; Pinton et al. 2013; Schedel et al. 2022). Currently, in southern Africa Chiloglanis has eight recognised species: C.bifurcus Jubb & Le Roux, 1969, C.emarginatus Jubb & Le Roux, 1969, C.anoterus Crass, 1960, C.paratus Crass, 1960, C.fasciatus Pellegrin, 1936, C.pretoriae Van der Horst, 1931, C.swierstrai Van der Horst, 1931, and C.neumanni Boulenger, 1911. Except for C.neumanni, all these species are narrow range endemics (Fig. 1). For example, C.bifurcus is confined to a relatively small geographical range, occurring between 900 and 1200 metres above sea level in the Inkomati River system (Roux and Hoffman 2017a).
Figure 1.
Distribution of Chiloglanis species in southern Africa based on data from the National Research Foundation-South African Institute for Aquatic Biodiversity extracted from the GIBF database (https://www.gbif.org).
Uncertainties about the identity of the broadly distributed C.neumanni in southern Africa have persisted for decades. This species was described from the Bubu River, a tributary of the Great Ruaha River basin in Tanzania, and was considered to be distributed across several eastern, central, and southern African river systems (Daget et al. 1986; Bell-Cross and Minshull 1988). However, following extensive surveys of river systems in east Africa and comprehensive examination of specimens from this region, Seegers (1996) did not record C.neumanni from localities outside the Great Ruaha River system, indicating that this species was not as widely distributed as previously thought. Although the name C.neumanni has persisted in subsequent literature from southern Africa, ichthyologists have consistently made remarks that the suckermouth catfishes of this region required detailed taxonomic investigation to determine their identity (Marshall 2011). In recent years, there has been general consensus among southern African ichthyologists that the species currently referred to as C.neumanni in this region actually represents an undescribed species or even a species complex, including several undescribed species. This assertion is based on the extensive geographic distance between southern Africa and the Bubu River, as well as the emerging patterns of undescribed diversity within other species with similar distribution ranges as C.neumanni. For example, studies of A.uranoscopus (Pfeffer, 1889) and Zaireichthysrotundiceps (Hilgendorf, 1905) led to the resurrection of two synonyms and the description of nine new species (Thomson and Page 2010; Eccles et al. 2011). Indeed, a genetic study by Chakona et al. (2018) identified six unique lineages within the genus Chiloglanis from the Eastern Zimbabwe Highlands ecoregion, a result that is consistent with Marshall’s (2011) postulation that the continued use of the name C.neumanni in southern Africa potentially obscures the actual diversity of this group of fishes in this region. A total of four new species to science are currently being described from the Eastern Zimbabwe Highlands ecoregion, with two of them being endemic to this region (Chakona et al., pers. obs.).
During surveys of the southern tributaries of the middle Zambezi River system in 2016 and 2019, morphologically distinct suckermouth catfishes were collected from the Mukwadzi River that drains the western margin of the Great Dyke in Zimbabwe. These specimens could not be attributed to any of the currently described species or recently identified lineages of Chiloglanis from this region. The present work represents the first in a series of studies that aim to resolve the taxonomy of suckermouth catfishes of southern Africa. This study applied integrative taxonomic approaches combining genetic and morphological data to determine the taxonomic distinctiveness of the recently collected specimens from the middle Zambezi River. The significance and implications of incomplete documentation of the diversity of rheophilic species in a region where their unique habitats are under threat from multiple environmental impacts are discussed.
Materials and methods
Collections
Specimens were collected from two sites in the Mukwadzi River, a tributary of the Manyame River, a south bank affluent of the middle Zambezi River, during surveys in 2016 and 2019. Samples were collected using a battery-powered Samus 725GN backpack electric fisher with a block net placed downstream to capture dislodged animals in the fast-flowing current. The specimens were photographed to document the live colour pattern then euthanized with clove oil. Muscle tissue from the right side of the specimens was cut out and preserved in 99% ethanol for molecular analysis. Voucher specimens for morphological studies were fixed in 10% formalin in the field and subsequently transferred to 70% ethanol for long term preservation. Additional tissue samples and voucher specimens used in the present study were obtained from the National Fish Collection at the National Research Foundation-South African Institute for Aquatic Biodiversity (NRF-SAIAB) in Makhanda (Tables 1, 2).
Table 1.
List of 80 COI sequences used in the present study including six new sequences of the specimens from the Mukwadzi River. The new sequences (in bold) include the hologenetype identified by an asterisk (*) and paragenetypes identified by a plus (+).
| Species name | River system | GPS coordinates (Latitude, Longitude) | COI sequence ID |
|---|---|---|---|
| Atopochilussavorgnani | Congo | – | MK073983 |
| Congo | – | MK073984 | |
| Chiloglanisanoterus | Mlumati | -25.7567, 31.4386 | LN610269 |
| Mlumati | -25.7692, 31.3367 | LN610270 | |
| Mlumati | -25.7692, 31.3367 | LN610271 | |
| Mlumati | -25.8672, 31.3347 | LN610272 | |
| Chiloglanisbifurcus | Mlumati | – | MH432062 |
| Mlumati | – | SB8458 | |
| Mlumati | – | SB8462 | |
| Chiloglanisfasciatus | Okavango | -13.5943, 16.8805 | ANGFW077-12 |
| Okavango | -12.6713, 16.1114 | ANGFW131-12 | |
| Okavango | -12.6713, 16.1114 | ANGFW132-12 | |
| Okavango | -12.6713, 16.1114 | ANGFW133-12 | |
| Okavango | -12.6713, 16.1114 | ANGFW134-12 | |
| Chiloglanisparatus | Phongolo | – | MPUMA025 |
| Phongolo | – | SB8459 | |
| Chiloglanispretoriae | Limpopo | -23.9904, 31.8258 | LN610341 |
| Chiloglanis sp. ‘dwarf’ | Honde | -18.4337, 32.8969 | MH432047 |
| Honde | -18.5992, 32.729 | MH432054 | |
| Makanga | -18.5438, 32.8013 | MH432044 | |
| Mupenga | -18.5725, 32.8038 | MH432042 | |
| Mupenga | -18.5725, 32.8038 | MH432048 | |
| Mutarazi | -18.5324, 32.8075 | MH432018 | |
| Chiloglanis sp. ‘dwarf’ | Mutarazi | -18.5324, 32.8075 | MH432019 |
| Mutarazi | -18.5324, 32.8075 | MH432032 | |
| Nyamhingura | -18.3696, 32.9354 | MH432025 | |
| Nyamhingura | -18.3696, 32.9354 | MH432026 | |
| Nyamhingura | -18.3696, 32.9354 | MH432027 | |
| Phalombe | -15.81, 35.646 | MAFW097 | |
| Pungwe | -18.3955, 32.9707 | MH432030 | |
| Pungwe | -18.3955, 32.9707 | MH432031 | |
| Pungwe | -18.45, 32.8968 | MH432046 | |
| Pungwe | -18.45, 32.8968 | MH432057 | |
| Pungwe | -18.3955, 32.9707 | MH432061 | |
| Ruo | -16.0403, 35.6633 | MAFW029 | |
| Chiloglanis sp. ‘Shire’ | Shire | -15.061, 35.219 | MAFW119 |
| Chiloglaniscarnatus sp. nov. | Manyame | -17.4249, 30.5854 | PP156890* |
| Manyame | -17.4249, 30.5854 | PP156891 + | |
| Manyame | -17.4249, 30.5854 | PP156892 + | |
| Manyame | -17.4249, 30.5854 | PP156893 + | |
| Manyame | -17.4249, 30.5854 | PP156894 + | |
| Manyame | -17.4249, 30.5854 | PP156895 + | |
| Chiloglanis sp. ‘Nyangombe’ | Chidya | -18.2653, 32.5903 | MH432020 |
| Chidya | -18.2653, 32.5903 | MH432021 | |
| Chidya | -18.2653, 32.5903 | MH432022 | |
| Chidya | -18.2653, 32.5903 | MH432033 | |
| Chiloglanis sp. ‘Pungwe’ | Chiyengwa | -18.6878, 32.922 | MH432040 |
| Honde | -18.5992, 32.729 | MH432049 | |
| Pungwe | -18.3955, 32.9707 | MH432028 | |
| Pungwe | -18.3955, 32.9707 | MH432029 | |
| Chiloglanis sp. ‘roughskin’ | Buzi | -19.932, 33.826 | SAFW910 |
| Chiyengwa | -18.6878, 32.922 | MH432045 | |
| Chiyengwa | -18.6878, 32.922 | MH432051 | |
| Honde | -18.5438, 32.8044 | MH432036 | |
| Makanga | -18.5438, 32.8013 | MH432043 | |
| Mupenga | -18.5725, 32.8038 | MH432038 | |
| Mupenga | -18.5725, 32.8038 | MH432039 | |
| Mupenga | -18.5725, 32.8038 | MH432041 | |
| Mupenga | -18.5725, 32.8038 | MH432060 | |
| Ngarura | -18.5474, 32.8718 | MH432052 | |
| Ngarura | -18.5474, 32.8718 | MH432053 | |
| Ngarura | -18.5474, 32.8718 | MH432059 | |
| Nyamukombe | -18.3821, 33.0327 | MH432034 | |
| Nyamukombe | -18.3821, 33.0327 | MH432035 | |
| Nyamukombe | -18.3821, 33.0327 | MH432058 | |
| Nyamukwara | -18.6918, 32.9236 | MH432055 | |
| Nyamukwara | -18.6918, 32.9236 | MH432056 | |
| Pungwe | -18.4414, 32.8875 | MH432050 | |
| Rwera | -18.5434, 32.8044 | MH432037 | |
| Chiloglanis sp. ‘Zambezi’ | Zambezi | -15.656, 30.953 | SAFW893 |
| Nyangombe | -18.0829, 32.5819 | MH432023 | |
| Nyangombe | -18.0829, 32.5819 | MH432024 | |
| Okavango | -14.9397, 17.7188 | ANGFW015-12 | |
| Okavango | -13.5943, 16.8805 | ANGFW078-12 | |
| Okavango | -14.6497, 16.9066 | ANGFW211-12 | |
| Chiloglanisswierstrai | Phongolo | – | SB8457 |
| Phongolo | – | SB8460 | |
| Phongolo | – | SB8461 | |
| Euchilichthysboulengeri | Dipumu | -6.0045, 22.3905 | HM418085 |
| Euchilichthysroyauxi | Epulu | – | KT192823 |
Table 2.
List of 184 specimens examined in this study including 19 specimens collected from the Mukwadzi River.
| Species | Type status | Catalogue No. | No. specimens | River system | Latitude, Longitude |
|---|---|---|---|---|---|
| Chiloglanisanoterus | Holotype | SAIAB 186246 | 1 | Phongola | -27.5, 30.4667 |
| Chiloglanisbifurcus | Holotype | SAIAB 120160 | 1 | Incomati | -25.4333, 30.7167 |
| Paratype | SAIAB 120161 | 6 | Incomati | -25.4333, 30.7167 | |
| Paratype | SAIAB 120529 | 3 | Incomati | -25.3833, 30.35 | |
| Chiloglanisemarginatus | Holotype | SAIAB 120117 | 1 | Incomati | -25.9833, 30.6833 |
| Paratype | SAIAB 120118 | 9 | Incomati | -25.85, 30.2 | |
| Chiloglanisfasciatus | _ | SAIAB 204928 | 6 | Okavango | -14.3872, 16.2876 |
| _ | SAIAB 204916 | 4 | Okavango | -14.387, 16.2873 | |
| Chiloglaniscarnatus sp. nov. | Holotype | SAIAB 236631 | 1 | Manyame | -17.4249, 30.5854 |
| Paratype | SAIAB 211349 | 13 | Manyame | -17.4244, 30.5845 | |
| Paratype | SAIAB 211346 | 5 | Manyame | -17.4249, 30.5854 | |
| Chiloglanisparatus | Holotype | SAIAB 186248 | 1 | Phongola | -27.3833, 31.5 |
| Paratype | SAIAB 120050 | 1 | Incomati | _ | |
| Chiloglanisswierstrai | Paratype | SAIAB 30013 | 1 | Phongola | -25.6667, 27.8333 |
| Paratype | SAIAB 21805 | 5 | Phongola | -27.4333, 31.5167 | |
| Holotype | SAIAB 186247 | 1 | Phongola | -27.4167, 31.1833 | |
| Chiloglanispretoriae | _ | SAIAB 82972 | 10 | Limpopo | -23.0105, 30.4785 |
| _ | SAIAB 70603 | 3 | Incomati | -25.8478, 27.7836 | |
| _ | SAIAB 70822 | 3 | Limpopo | -25.3883, 28.3117 | |
| Chiloglanisneumanni | Lectotype | BMNH190575249 | 1 | Bubu | _ |
| Paralectotype | BMNH190575250 | 1 | Bubu | _ | |
| Paralectotype | BMNH190575250 | 1 | Bubu | _ | |
| Chiloglanis sp. ‘rough skin’ | _ | SAIAB 201075 | 4 | Pungwe | -18.4414, 32.8875 |
| _ | SAIAB 201095 | 2 | Chiyengwa | -18.6878, 32.922 | |
| _ | AC14CL10 | 11 | Mupenga | -18.5725, 32.8038 | |
| _ | SAIAB 200955 | 5 | Ngarura | -18.5474, 32.8718 | |
| _ | SAIAB 200933 | 9 | Nyamukombe | -18.3821, 33.0327 | |
| _ | SAIAB 201035 | 15 | Rwera | -18.5434, 32.8044 | |
| _ | SAIAB 201047 | 3 | Nyamukombe | -18.3821, 33.0327 | |
| _ | SAIAB 201088 | 8 | Nyamukwara | -18.6918, 32.9236 | |
| _ | SAIAB 201026 | 8 | Honde | -18.5438, 32.8044 | |
| Chiloglanis sp. ‘dwarf’ | _ | AC14CL10 | 10 | Mupenga | -18.5725, 32.8038 |
| _ | SAIAB 200940 | 3 | Pungwe | -18.45, 32.8968 | |
| _ | SAIAB 200923 | 1 | Pungwe | -18.3955, 32.9707 | |
| _ | SAIAB 205087 | 5 | Mutarazi | -18.5324, 32.8075 | |
| _ | SAIAB 205074 | 3 | Nyamhingura | -18.3696, 32.9354 | |
| _ | AC13BL04 | 3 | Pungwe | -18.3955, 32.9707 | |
| Chiloglanis sp. ‘Pungwe’ | _ | AC13BL04 | 2 | Pungwe | -18.3955, 32.9707 |
| _ | SAIAB 201095 | 1 | Chiyengwa | -18.6878, 32.922 | |
| _ | SAIAB 201067 | 1 | Honde | -18.5992, 32.729 | |
| Chiloglanis sp. ‘Nyangombe’ | _ | SAIAB 210408 | 6 | Chidya | -18.2653, 32.5903 |
| Chiloglanis sp. ‘Zambezi’ | _ | SAIAB 200517 | 2 | Nyangombe | -18.0829, 32.5819 |
| _ | SAIAB 81243 | 2 | Lower Zambezi | -15.656, 30.953 | |
| _ | SAIAB 186643 | 1 | Okavango | -14.9397, 17.7188 | |
| _ | SAIAB 186709 | 1 | Okavango | -13.5943, 16.8805 |
DNA extraction, amplification, and sequencing
A total of six new COI sequences of Chiloglaniscarnatus sp. nov. were generated for this study. Preparation and sequencing of genetic material was done in the Aquatic Genomics Research Platform at the NRF-SAIAB. Genomic DNA was extracted from preserved tissues using the salting-out method (Sunnucks and Hales 1996). The mitochondrial DNA cytochrome c oxidase subunit I (COI) gene was amplified by polymerase chain reaction (PCR) using the universal fish DNA barcoding primer set FishF1 and FishR1 (Ward et al. 2005). PCRs were performed with a Veriti 96 well thermal cycler (Applied Biosystems, USA) and each reaction mixture (25 µL) contained 50–100 ng) of template DNA, 6.5 µL of water, 0.5 µL of each primer (10 µM), and 12.5 µL Taq DNA polymerase 2× master mix red (Amplicon PCR enzymes and reagents, Denmark). The PCR amplification profile had an initial denaturation step of 3 min at 94 °C followed by 38 cycles of 30 sec at 94 °C, annealing at 55 °C for 30 sec, and extension at 72 °C for 50 sec, and final extension at 72 °C for 7 min. The amplicons were purified using an Exonuclease I-Shrimp Alkaline Phosphate (Exo/SAP, Thermo Fisher Scientific, USA) protocol (Werle et al. 1994), sequenced using standard fluorescent BigDye v. 3.1 (Applied Biosystems, USA) terminator chemistry in the forward direction, and analysed on a 3500 Genetic Analyser (Applied Biosystems, USA) at the NRF-SAIAB. Additional sequences were obtained from the public databases GenBank (https://www.ncbi.nlm.nih.gov/genbank/) and Barcode of Life Data Systems (BOLD) (http://www.boldsystems.org/) (Table 1).
Phylogenetic analyses
Phylogenetic analyses included genetic sequences generated from Chiloglaniscarnatus sp. nov., six of the seven nominal species from southern Africa, six candidate species of Chiloglanis identified by Chakona et al. (2018) (Chiloglanis sp. ‘roughskin’, Chiloglanis sp. ‘Zambezi’, Chiloglanis sp. ‘Nyangombe’, Chiloglanis sp. ‘Pungwe’, Chiloglanis sp. ‘Shire’, Chiloglanis sp. ‘dwarf’), and three outgroup species (Euchilichthysboulengeri Nichols & LaMonte, 1934; Euchilichthysroyauxi Boulenger, 1902; Atopochilussavorgnani Sauvage, 1879) (Table 1). Genetic material for C.neumanni from its type locality and C.emarginatus could not be accessed before finalising this study. Mitochondrial DNA sequences were edited, aligned, and trimmed in MEGA-X (Kumar et al. 2016). The sequences were translated into amino acid sequences in MEGA-X to check for stop codons and gaps to ensure that they were copies of functional mitochondrial protein coding sequences. Haplotype groups were identified using DNASP 6 (Rozas et al. 2017). The most suitable model for nucleotide substitution was selected using the Akaike Information Criterion (AIC) (Akaike 1974) as implemented in the program jModelTest 0.1.1 (Darriba et al. 2012). Bayesian phylogenetic inference was performed in MrBayes 3.2.6 (Ronquist et al. 2012) using the TIM3+I+G evolutionary model identified using jModeltest. The phylogenetic tree and posterior probabilities were inferred using four Markov chain Monte Carlo (MCMC) chains which were run for 2 × 106 generations with tree sampling every 1000 generations. The program Tracer 1.7 (Rambaut et al. 2018) was used to analyse the quality of the trace files generated by MrBayes and to determine the number of trees to be discarded as burn-in. The first 25% of the sampled trees for each analysis was discarded as burn-in, and the remaining trees were used to calculate a majority rule consensus tree. Maximum likelihood (ML) analysis of the same dataset was performed in RAxML v. 8 (Stamatakis 2014) through the graphical user interface raxmlGUI v. 2 (Silvestro and Michalak 2012). A total of 10 ML searches were performed in raxmlGUI and support values for the ML tree nodes were estimated by 1000 non-parametric bootstrap inferences (Felsenstein 1985). Bootstrap values equal to or higher that 70% (Hillis and Bull 1993), and posterior probability values at 0.95 or higher (Alfaro and Holder 2006), were considered strong support.
Molecular species delimitation
Four molecular species delimitation methods were used to delineate candidate species within the suckermouth catfishes of southern Africa using the same dataset used for the phylogenetic analysis. The first two methods, Automatic Barcode Gap Discovery (ABGD; Puillandre et al. 2012) and Assemble Species by Automatic Partitioning (ASAP; Puillandre et al. 2021) infer the barcode gap from the data to partition sequences into proposed candidate species. These methods were performed on their respective webservers (https://bioinfo.mnhn.fr/abi/public/abgd/ and https://bioinfo.mnhn.fr/abi/public/asap/asapweb.html). The intraspecific diversity priors were set at Pmin = 0.001 and Pmax = 0.1) for both methods. The Kimura (K80) TS/TV distance model was used and the remaining settings were left at their default parameters. The second pair of species delimitation methods included the Bayesian implementation of the Poisson Tree Processes (bPTP) (Zhang et al. 2013) and the General Mixed Yule Coalescent (GMYC) (Pons et al. 2006; Fujisawa and Barraclough 2013). Both GMYC and bPTP require a phylogenetic tree as input and from this tree they estimate rates of branching events to infer which parts of the tree are likely to follow a speciation model (interspecific variation) and which parts follow a coalescent model (intraspecific variation). The bPTP was performed on the web server (http://species.h-its.org/ptp/) using the same tree generated for phylogenetic reconstruction and a MCMC run for 1 × 106 generations with 10% burn-in. For the GMYC analysis a fully resolved ultrametric tree was inferred in Bayesian evolutionary analysis by sampling trees (BEAST) 2.4.6 (Bouckaert et al. 2014) using a strict clock and Yule model and the MCMC was ran for 1 × 107 generations with tree sampling every 1000 generations. The program Tracer 1.7.2 was used to analyse the quality of the trace files generated by BEAST. TreeAnnotator (Helfrich et al. 2018) was used to summarise the trees sampled by BEAST into a single maximum credibility tree with a burn-in of 25%. The species’ limits by threshold Statistics (splits) package (http://r-forge.r-project.org/projects/splits) in R 3.5.0 (R Core Team 2018) was used to identify the candidate species from the maximum credibility tree produced by TreeAnnotator. Model corrected intraspecific and interspecific genetic distances of the molecular taxonomic units identified by the species delimitation methods were calculated in PAUP* 4.0a163 (Swofford 2003).
Morphological analyses
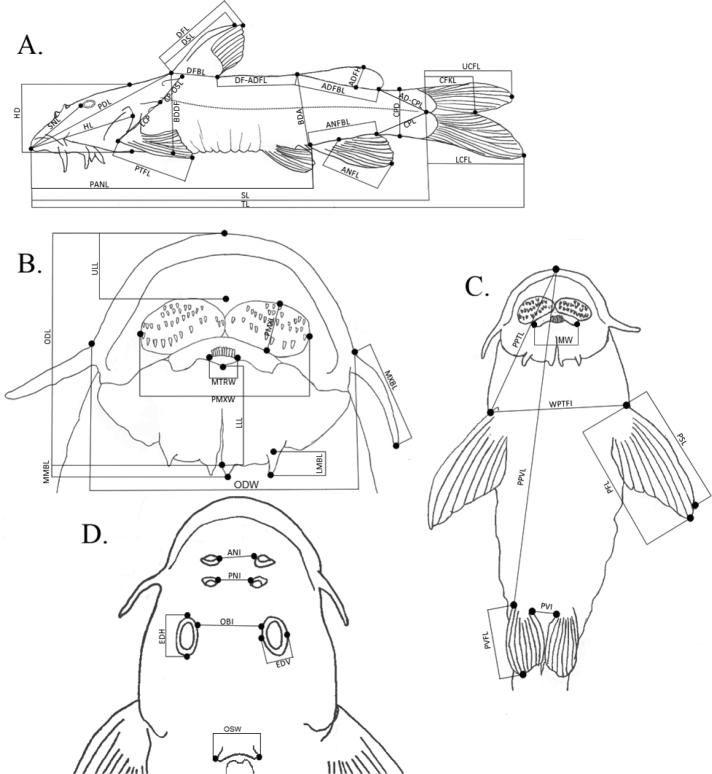
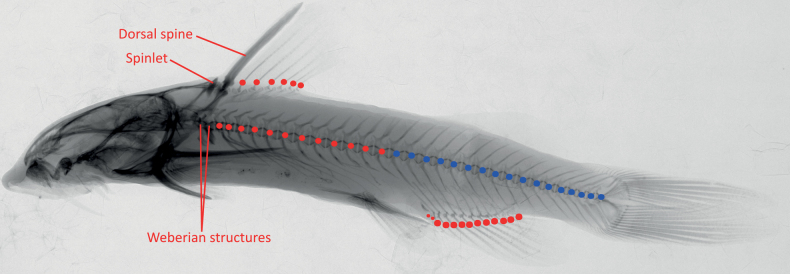
A total of 19 specimens of Chiloglaniscarnatus sp. nov. collected from the Mukwadzi River were examined in the present study. Comparative material included the lectotype of C.neumanni, holotypes of six valid species from southern Africa and five candidate species identified by Chakona et al. (2018) (Table 2). Because type material for C.fasciatus could not be accessed before finalising this study, 10 conspecific specimens collected from close to the type locality of this species and identified using the species description by Pellegrin (1936) and the key in Skelton (2001) were used as topotypes. The syntypes of C.pretoriae were severely deformed, thus only their meristic counts were included for comparison in this study, but 16 specimens collected from near the type locality of C.pretoriae and identified using the key in Skelton (2001) were used as topotypes for this species. Formulae and terminology of morphometric and meristic characters followed Schmidt et al. (2015), Friel and Vigliotta (2008), and Skelton and White (1990). A total of 49 morphometric characters were measured to the nearest 0.1 mm using digital Vernier callipers following Friel and Vigliotta (2008) (Table 3, Fig. 2A–D). External meristic counts were performed under a stereo microscope. Vertebrae counts were made from radiographs taken at the NRF-SAIAB using an Inspex 20i Digital X-ray Imaging System (Kodex Inc., New Jersey, USA). Radiographs for the lectotype and paralectotypes of C.neumanni were taken at the Royal Museum for Central Africa, Belgium (MRAC) using a VisiX-MedexLoncin (www.medex.be). A total of nine meristic characters were examined: number of mandibular teeth, pre-maxillary teeth, pectoral-fin rays, pelvic-fin rays, dorsal-fin rays, anal-fin rays, abdominal vertebrae, caudal vertebrae, and total vertebrae (Fig. 3). Following Roberts (1989), vertebrae counts excluded the Weberian structures and began from the fifth vertebra which was identified by a pair of large but slender ribs, and included the hypural complex which was counted as one vertebra. The abdominal vertebrae were defined as the vertebrae that occurred anterior to the first anal fin ray pterygiophore. Caudal vertebrae were defined as those that occurred posterior to the first anal fin ray pterygiophore and included the hypural complex which was counted as one vertebra (Roberts 1989) (Fig. 3). The genital papillae were examined to determine the sex of the specimens following Friel and Vigliotta (2008). Morphological measurements were standardised by transforming body measurements into percentages of the standard length (SL) and head measurements into percentages of the head length (HL). Principal component analyses (PCA) were performed in PAST v. 3.12 (Hammer et al. 2001) using the covariance matrix for the morphometric data in order to identify morphological characters that contributed the most to distinguishing Chiloglaniscarnatus sp. nov. from the other Chiloglanis species from southern Africa.
Table 3.
Morphological characters examined in the present study.
| Morphological characters | Abbreviation |
|---|---|
| Adipose fin to caudal peduncle length | AD-CPL |
| Adipose-fin base length | ADFBL |
| Adipose-fin height | ADFH |
| Anal-fin base length | ANFBL |
| Anal-fin length along longest ray | ANFL |
| Anterior nare interspace | ANI |
| Body depth at anus | BDA |
| Body depth at dorsal-fin insertion | BDDF |
| Caudal fork length | CFKL |
| Caudal peduncle depth | CPD |
| Caudal peduncle length | CPL |
| Dorsal fin to adipose fin length | DF-ADFL |
| Dorsal-fin base length | DFBL |
| Dorsal-fin length along longest ray | DFL |
| Dorsal-spine length | DSL |
| Eye diameter (horizontal axis) | EDH |
| Eye diameter (vertical axis) | EDV |
| Head depth | HD |
| Head length to opercular membrane margin | HL |
| Lateral mandibular barbel length | LMBL |
| Length of post-cleithral process | LCP |
| Lower caudal-fin lobe length | LCFL |
| Lower lip length | LLL |
| Mandibular tooth row width | MTRW |
| Maxillary barbel length | MXBL |
| Medial mandibular barbel length | MMBL |
| Mouth width | MW |
| Occipital shield width | OSW |
| Oral disc length | ODL |
| Oral disc width | ODW |
| Orbital interspace | OBI |
| Pectoral-fin length | PFL |
| Pectoral-spine length | PSL |
| Pelvic-fin interspace | PVI |
| Pelvic-fin length | PVFL |
| Post-cleithral process to occipital shield length | CP-OSL |
| Posterior nares interspace | PNI |
| Pre-anal length | PANL |
| Pre-dorsal length | PDL |
| Pre-maxillary tooth-patch length | PMXL |
| Pre-maxillary tooth-patch width | PMXW |
| Pre-pectoral length | PPTL |
| Pre-pelvic length | PPVL |
| Snout length | SNL |
| Standard length | SL |
| Total length | TL |
| Upper caudal-fin lobe length | UCFL |
| Upper lip length | ULL |
| Width at pectoral-fin insertion | WPTFI |
| Abdominal vertebrae | – |
| Anal fin-ray count | – |
| Caudal vertebrae | – |
| Dorsal fin-ray count | – |
| Mandibular tooth count | – |
| Pectoral fin-ray count | – |
| Pelvic fin-ray count | – |
| Pre-maxillary tooth count | – |
| Total vertebrae | – |
Figure 2.
Illustrations depicting linear measurements recorded from Chiloglanis specimens A lateral view B ventral view of the Oral disc C ventral view D dorsal view of the head. Abbreviations: AD-CPL-adipose fin to caudal peduncle length, ADFBL-adipose-fin base length, ADFH-adipose-fin height, ANFBL-anal-fin base length, ANFL-anal-fin length along longest ray, ANI-anterior nares interspace, BDA-body depth at anus, BDDF-body depth at dorsal-fin insertion, CFKL-caudal fork length, CPD-caudal peduncle depth, CPL-caudal peduncle length, CP-OSL- post-cleithral process to occipital shield length, DF-ADFL-dorsal fin to adipose fin length, DFBL-dorsal-fin base length, DFL-dorsal-fin length along longest ray, DSL-dorsal-spine length, EDH-eye diameter (horizontal axis), EDV-eye diameter (vertical axis), HD-head depth, HL-head length to opercular membrane margin, LCFL-Lower caudal-fin lobe length, LCP-length of post-cleithral process, LLL-lower lip length, LMBL-Lateral mandibular barbel length, MMBL-Medial mandibular barbel length, MTRW-mandibular tooth row width, MXBL-maxillary barbel length, MW-mouth width, OBI-orbital interspace, ODL-oral disc length, ODW-oral disc width, OSW-occipital shield width, PANL-pre-anal length, PDL-pre-dorsal length, PMXL-pre-maxillary tooth-patch length, PMXW- pre-maxillary tooth patch width, PNI-posterior nares interspace, PPTL-pre-pectoral length, PPVL-pre-pelvic length, PSL-pectoral-spine length, PFL-pectoral-fin length, PVFL-pelvic-fin length, PVI-pelvic-fin interspace, SL-standard length, SNL-snout length, TL-total length, UCFL–Upper caudal-fin lobe length, ULL-upper lip length, WPTFI-width at pectoral-fin insertion.
Figure 3.
Illustration showing how fin rays and vertebrae were counted using x-ray radiographs. The red dots along the vertebra represent the abdominal vertebrae and the blue dots represent the caudal vertebrae.
Results
Phylogenetic analyses
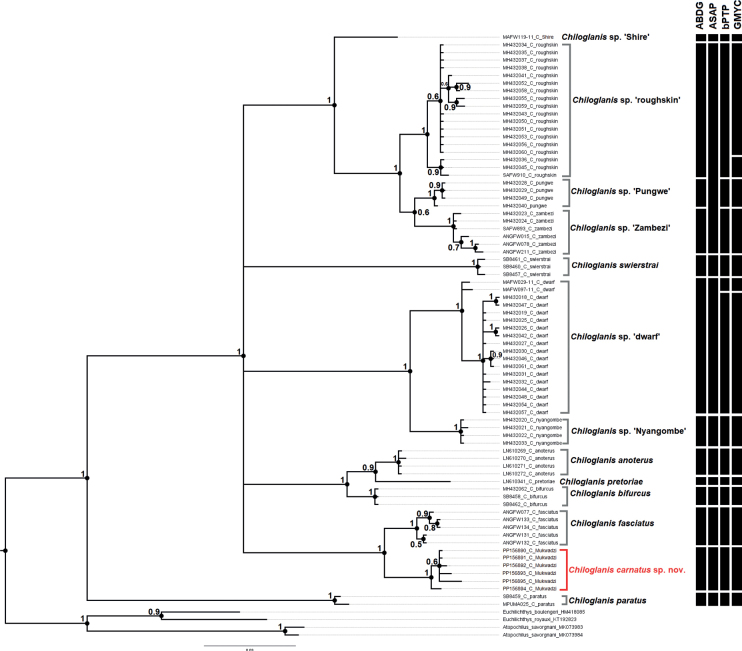
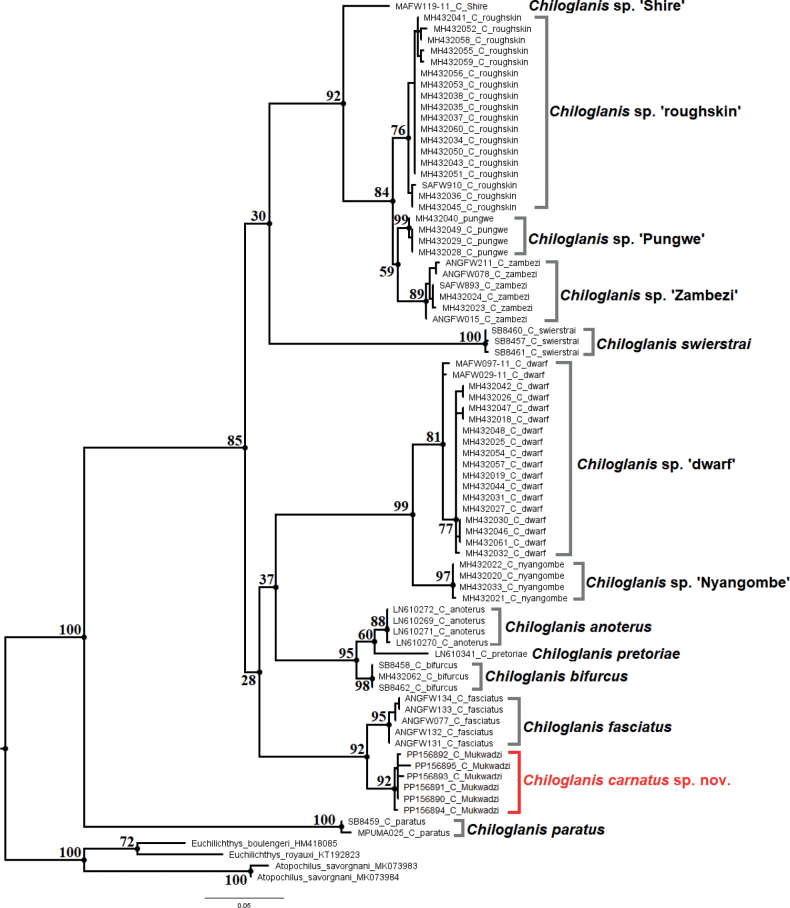
The COI alignment of 80 sequences had 534 base pairs and 176 variable sites. A total of 47 unique haplotypes were identified. Although the Bayesian phylogenetic tree was not fully resolved, it showed genetic structuring that supported the monophyly of suckermouth catfishes from southern Africa (Fig. 4). Chiloglaniscarnatus sp. nov. was recovered as an exclusive group that is genetically divergent (2.8–15.0% genetic distances) from other Chiloglanis species and lineages from southern Africa (Figs 4, 5; Table 4). With the exception of C.pretoriae and Chiloglanis sp. ‘Shire’, all recovered clades were well-supported (posterior probability > 0.95). Genetic divergences within valid and candidate species ranged from 0–1.5% and interspecific divergences ranged from 1.3–15.7% (Table 4). Chiloglanisparatus from the Phongolo River was recovered as the most basal clade that is sister species to all the southern African suckermouth catfishes included in the present study. The relationships among the remaining taxa were not well resolved as they were recovered within five polytomous clades with weak support between them (Fig. 4). The first clade contained Chiloglaniscarnatus sp. nov. from the Manyame River and C.fasciatus from the Okavango River. The second clade contained C.anoterus and C.bifurcus from the Incomati River system as well as C.pretoriae from the Limpopo River system. The third clade contained Chiloglanis sp. ‘Nyangombe’ from the Nyangombe River and Chiloglanis sp. ‘dwarf’ from the Pungwe and Ruo rivers. The fourth clade contained Chiloglanisswierstrai from the Limpopo River system. The fifth clade contained the Chiloglanis sp. ‘Zambezi’, Chiloglanis sp. ‘Pungwe’, Chiloglanis sp. ‘roughskin’, and Chiloglanis sp. ‘Shire’ lineages. The Chiloglanis sp. ‘roughskin’ lineage occurs in the Buzi and Pungwe rivers, whereas Chiloglanis sp. ‘Pungwe’ is endemic to the Pungwe River. Chiloglanis sp. ‘Shire’ and Chiloglanis sp. ‘Zambezi’ lineages were found in the lower Zambezi River system with the latter lineage also occurring in the Okavango River. The phylogenetic tree inferred using the ML approach had similar topology to the Bayesian inference tree (Fig. 5).
Figure 4.
Bayesian inference tree of the species and lineages of the genus Chiloglanis found in southern African. The numbers at the nodes represent the Bayesian posterior probabilities. The black bars represent candidate species proposed by four molecular species delimitation methods: Automatic Barcode Gap Discovery (ABGD), Automatic Partitioning (ASAP), Bayesian implementation of the Poisson Tree Processes (bPTP), and General Mixed Yule Coalescent (GMYC).
Figure 5.
Maximum likelihood tree of the species and lineages of the genus Chiloglanis found in southern African. The numbers at the nodes represent the bootstrap values.
Table 4.
Ranges of cytochrome oxidase I (COI) genetic distances (%) between the Chiloglanis species included in the present study.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Chiloglanis sp. ‘dwarf’ | 0–1.5 | |||||||||||||||
| 2 | Chiloglanis sp. ‘Nyangombe’ | 3.6–4.5 | 0–0.2 | ||||||||||||||
| 3 | Chiloglanis sp. ‘Zambezi’ | 10.7–11.4 | 9.0–9.7 | 0–0.9 | |||||||||||||
| 4 | Chiloglanis sp. ‘Pungwe’ | 11.0–11.8 | 9.9–10.3 | 2.1–3.0 | 0–0.2 | ||||||||||||
| 5 | Chiloglanis sp. ‘roughskin’ | 10.5–11.6 | 9.4–10.3 | 2.2–3.9 | 1.3–2.6 | 0–1.3 | |||||||||||
| 6 | Chiloglanis sp. ‘Shire’ | 11.4–11.9 | 10.1–10.3 | 5.1–5.6 | 5.0–5.2 | 4.1–5.2 | _ | ||||||||||
| 7 | Chiloglaniscarnatus sp. nov. | 12.0–13.7 | 12.9–13.9 | 10.7–12.0 | 11.0–12.0 | 10.1–11.2 | 10.3–11.0 | 0–1.1 | |||||||||
| 8 | Chiloglanisanoterus | 11.0–11.4 | 11.0–11.2 | 9.5–10.1 | 9.7–10.7 | 9.6–9.9 | 9.7 | 9.7–11.4 | 0–0.2 | ||||||||
| 9 | Chiloglanispretoriae | 9.9–10.3 | 10.1–10.3 | 10.7–11.0 | 10.7–10.8 | 11.0–11.4 | 10.1 | 10.7–11.4 | 3.4–3.6 | _ | |||||||
| 10 | Chiloglanisfasciatus | 10.9–12.0 | 12.0–12.4 | 10.9–11.4 | 11.2–11.6 | 10.3–10.9 | 10.3 | 2.8–3.9 | 9.0–9.2 | 9.6–9.7 | 0–0.6 | ||||||
| 11 | Chiloglanisswierstrai | 12.4–13.9 | 13.7–14.2 | 11.4–11.8 | 11.4–11.8 | 11.2–11.8 | 10.1–11.0 | 12.4–13.3 | 11.4–11.8 | 12.4–12.7 | 9.0–12.5 | 0.2–0.4 | |||||
| 12 | Chiloglanisbifurcus | 9.9–10.3 | 10.5–10.7 | 9.9–10.3 | 10.5–10.7 | 10.3–10.7 | 9.4 | 9.8–10.5 | 2.4–2.6 | 4.1 | 9.0–9.2 | 11.8–12.2 | 0 | ||||
| 13 | Chiloglanisparatus | 15.0–15.5 | 14.4–14.8 | 14.2–14.8 | 14.8–15.2 | 15.0–15.7 | 13.9–14.0 | 14.0–15.0 | 13.3–13.7 | 13.7–13.9 | 13.7–14.4 | 15.4–15.7 | 13.1–13.5 | 0.6 | |||
| 14 | Atopochilussavorgnani | 15.7–16.1 | 15.5–16.3 | 15.0–15.9 | 15.5–16.1 | 15.0–15.5 | 14.2–14.4 | 15.5–16.9 | 15.0–15.7 | 14.8–15.0 | 15.0–15.5 | 16.5–16.9 | 15.2–15.7 | 15.0–15.5 | 1.1 | ||
| 15 | Euchilichthysboulengeri | 15.2–15.4 | 15.0–15.2 | 15.2–15.4 | 14.4 | 14.0–14.2 | 14.2 | 15.4–16.1 | 14.6–15.4 | 14.8 | 15.0–15.4 | 13.7–13.9 | 14.6–15.4 | 13.3–13.5 | 11.2–11.4 | _ | |
| 16 | Euchilichthysroyauxi | 16.5–17.0 | 16.3–16.5 | 16.3–16.9 | 15.7 | 15.4–16.1 | 14.8 | 15.7–16.5 | 16.3–16.5 | 16.5 | 15.2–15.5 | 15.4–15.5 | 16.3 | 13.5–3.9 | 10.7 | 6.6 | _ |
Molecular species delimitation
All four molecular species delimitation methods identified Chiloglaniscarnatus sp. nov., Chiloglanis sp. ‘Shire’, Chiloglanis sp. ‘Nyangombe’, C.swierstrai, C.anoterus, C.pretoriae, C.bifurcus, C.fasciatus, and C.paratus as unique molecular taxonomic units (Fig. 4). The Assemble Species by Automatic Partitioning method recovered the least number of candidate species, this method grouped Chiloglanis sp. ‘roughskin’, Chiloglanis sp. ‘Pungwe’, and Chiloglanis sp. ‘Zambezi’ into a single molecular taxonomic unit. The General Mixed Yule Coalescent method recovered the highest number of molecular taxonomic units. This method identified additional molecular taxonomic units within Chiloglanis sp. ‘roughskin’ and Chiloglanis sp. ‘dwarf’. The Automatic Barcode Gap Discovery and bPTP inferred similar molecular taxonomic units with the exception of Chiloglanis sp. ‘dwarf’ which was split into two molecular taxonomic units by the latter method.
Morphological analyses
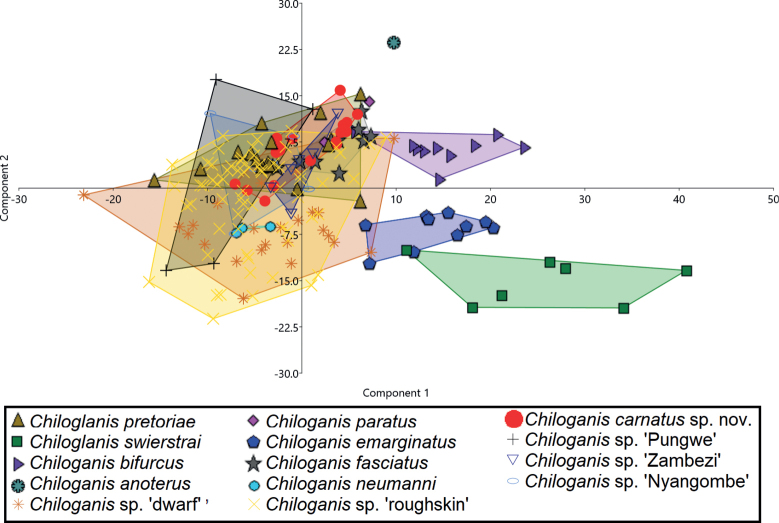
Principal component analysis (PCA) of the morphometric characters showed that Chiloglaniscarnatus sp. nov. is separated from C.swierstrai and C.anoterus along principal component 1 (PCI) (Fig. 6). This separation was associated with maxillary barbel length (Table 6). Chiloglaniscarnatus sp. nov. (20.3–28.8%HL) has relatively shorter maxillary barbels compared to C.swierstrai (44.2–66.8%HL) and Chiloglanis sp. ‘Zambezi’ (31.3–37.0%HL, Table 5, Fig. 7A, B). Chiloglaniscarnatus sp. nov. is separated from C.swierstrai, C.anoterus, and C.neumanni along principal component 2 (PCII) (Fig. 6). Separation along PCII is associated with the oral disc width (Table 6). Chiloglaniscarnatus sp. nov. has a relatively smaller oral disc width (51.1–64.6%HL) compared to C.anoterus (69.1%HL, Table 5, Fig. 7C).
Figure 6.
Scatter plot of the first two principal components of the morphometric characters of Chiloglanis species and lineages from southern African.
Table 6.
The PCA loadings for the first two principal components for the measured characters of Chiloglanis species and lineages from southern Africa.
| Principal component | 1 | 2 |
|---|---|---|
| Eigenvalue | 90.91 | 70.84 |
| % variance | 22.46 | 17.50 |
| Adipose fin to caudal peduncle length | -0.02 | -0.02 |
| Adipose-fin base length | 0.05 | -0.01 |
| Adipose-fin height | -0.04 | 0.00 |
| Anal-fin base length | 0.03 | -0.05 |
| Anal-fin length along longest ray | -0.03 | -0.06 |
| Body depth at anus | -0.02 | -0.04 |
| Body depth at dorsal-fin insertion | -0.09 | -0.06 |
| Caudal peduncle depth | -0.05 | -0.02 |
| Caudal peduncle length | 0.08 | -0.10 |
| Dorsal-fin to adipose fin length | -0.02 | -0.02 |
| Dorsal-fin base length | -0.17 | 0.08 |
| Dorsal-spine length | -0.06 | 0.04 |
| Pre-anal length | -0.16 | 0.17 |
| Pre-dorsal length | -0.10 | 0.11 |
| Pre-pectoral length | -0.08 | 0.10 |
| Pre-pelvic length | -0.12 | 0.15 |
| Pectoral-spine length | 0.04 | 0.01 |
| Pectoral-fin length | 0.04 | 0.03 |
| Pelvic-fin length | 0.00 | 0.02 |
| Width at pectoral-fin insertion | -0.04 | 0.06 |
| Pelvic-fin interspace | 0.00 | 0.02 |
| Head length | -0.18 | 0.07 |
| Anterior nares interspace | 0.14 | -0.08 |
| Eye diameter (vertical axis) | 0.11 | -0.03 |
| Lower lip length | 0.02 | 0.37 |
| Mandibular tooth row width | -0.16 | -0.05 |
| Maxillary barbel length | 0.60 | -0.41 |
| Mouth width | 0.35 | 0.21 |
| Orbital interspace | -0.18 | -0.14 |
| Oral disc length | 0.22 | 0.34 |
| Oral disc width | 0.39 | 0.41 |
| Pre-maxillary tooth-patch length | 0.00 | 0.09 |
| Pre-maxillary tooth-patch width | 0.23 | 0.24 |
| Posterior nares interspace | 0.13 | -0.13 |
| Snout length | -0.05 | 0.35 |
| Upper lip length | -0.09 | 0.15 |
Table 5.
Summary of morphological characters examined in the present study. All values except standard length (SL) and Head length (HL) are given as percentages of the HL or SL. For the meristics the mode is given alongside the range of the counts in parentheses where the counts varied.
| Species | Chiloglaniscarnatus sp. nov. | Chiloglanispretoriae | Chiloglanisanoterus | Chiloglanisbifurcus | Chiloglanisemarginatus | Chiloglanisfasciatus | Chiloglanisneumanni | Chiloglanisparatus | Chiloglanisswierstrai | Chiloglanis sp. ‘dwarf’ | Chiloglanis sp. ‘Nyangombe’ | Chiloglanis sp. ‘Pungwe’ | Chiloglanis sp. ‘roughskin’ | Chiloglanis sp. ‘Zambezi’ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of specimens | 19 | 16 | 1 | 10 | 10 | 10 | 3 | 2 | 7 | 25 | 6 | 4 | 65 | 6 |
| Total length | 45.3–62.2 | 31.7–67.1 | 80.1 | 68.7–84.9 | 50.2–66.6 | 37.7–53.3 | 0–42.7 | 44–51.9 | 45.2–65.7 | 31.4–51.1 | 33.1–48.2 | 34.4–62.9 | 39.8–87.6 | 55.2–62.6 |
| Standard length | 35.5–48.9 | 26.5–54.6 | 61.7 | 51.4–63.9 | 40.3–55.6 | 30.3–41.7 | 33.4–39.8 | 35.8–42.4 | 34.9–51.9 | 26.0–41.6 | 26.0–38.5 | 24.6–48.6 | 31.6–66.6 | 43.7–50.6 |
| Head length | 12.1–15.6 | 8.8–19.4 | 20.3 | 15.7–19.5 | 12.3–15.7 | 10.0–13.7 | 10.1–12.6 | 11.1–13.8 | 9.2–13.6 | 8.0–12.8 | 8.9–13.1 | 6.6–15.6 | 10.3–22.8 | 13.9–16.8 |
| % Standard length | ||||||||||||||
| Pre-pectoral length | 26.9–30.0 | 26.3–32.7 | 30.9 | 27.4–31.1 | 24.5–27.3 | 29.5–32.4 | 26.1–27.8 | 28.4–30.0 | 25.0–27.0 | 24.3–33.1 | 29.6–32.3 | 30.2–35.9 | 25.7–32.4 | 25.8–31.4 |
| Pre-dorsal length | 39.9–43.7 | 40.7–48.7 | 38.6 | 38.9–42.9 | 37.7–42.7 | 40.4–44.9 | 38.6–41.5 | 39.2–42.3 | 34.5–36.7 | 36.6–50.5 | 39.0–46.8 | 35.4–44.7 | 36.0–44.1 | 36.2–46.3 |
| Pre-pelvic length | 56.0–59.3 | 53.6–58.4 | 59.7 | 52.5–58.1 | 51.3–56.8 | 56.1–59.7 | 55.6–57.5 | 55.2–58.6 | 49.1–54.8 | 50.7–58.6 | 51.7–64.2 | 57.2–65.1 | 53.6–61.6 | 55.3–60.7 |
| Pre-anal length | 67.6–73.3 | 66.4–72.8 | 73.3 | 63.4–69.3 | 63.0–68.2 | 67.7–73.3 | 70.7–72.4 | 67.6–71.3 | 64.1–69.5 | 64.1–75.3 | 65.2–80.1 | 56.7–80.2 | 64.3–77.6 | 58.7–76.5 |
| Dorsal fin to adipose fin length | 18.2–22.6 | 18.1–25.8 | 25.5 | 21.4–29.1 | 20.2–24.4 | 21.4–24.1 | 21.6–26.9 | 23.9–28 | 18.7–26.8 | 21.5–28.6 | 20.2–28.4 | 21.9–29.1 | 19.2–30.2 | 20.5–21.6 |
| Pectoral-spine length | 15.0–19.8 | 13.5–19.8 | 11.5 | 17.8–22.4 | 17.2–22 | 17.5–21.5 | 16.8–22.4 | 19.7–22.7 | 19.0–23.1 | 13.7–20 | 13.7–20.2 | 18.2–19.9 | 13.8–26.6 | 14.7–22.0 |
| Pectoral-fin length | 19.3–23.6 | 14.1–22.6 | 19 | 22.9–26.6 | 19.5–24.3 | 21.4–25.1 | 21.9–24.5 | 22.6–26.4 | 23.2–25.5 | 15.4–24.3 | 18.2–21.1 | 22.1–25.9 | 17.6–27.4 | 20.5–26.1 |
| Width at pectoral-fin insertion | 23.0–25.3 | 24.4–29.8 | 24.3 | 24.7–27.9 | 23.9–26.5 | 23.7–26.1 | 21.4–24.9 | 23.8–25.8 | 17.6–21.5 | 21.7–25.7 | 22.3–26.1 | 23.9–27.0 | 21.0–27.6 | 23.6–25.7 |
| Pelvic-fin length | 10.8–14.2 | 12.2–15.9 | 12.9 | 14.6–17.2 | 10.8–14.6 | 11.6–14.6 | 13.6–14 | 13.0–13.1 | 12.3–15.4 | 11.7–15.3 | 11.7–13.3 | 13.8–16.0 | 10.2–17.5 | 13.4–15.7 |
| Pelvic-fin interspace | 3.0–5.1 | 2.1–4.7 | 4.3 | 4.4–6.2 | 2.8–4.7 | 2.5–4.7 | 3.0–3.9 | 4.4–4.7 | 2.7–4.9 | 1.8–6.0 | 2.0–4.2 | 2.7–5.4 | 2.6–8.2 | 3.8–5.0 |
| Body depth at dorsal-fin insertion | 15.5–20.7 | 16.0–21.0 | 19.1 | 16.2–21.8 | 17.3–22.4 | 15.4–19.3 | 17.7–22.1 | 15.2–18.1 | 12.2–19.2 | 16.8–22.7 | 15.7–19.3 | 17.2–20.8 | 17.2–25.3 | 17.3–20.2 |
| Body depth at anus | 13.9–17.6 | 15.3–18.7 | 18 | 16.7–21.2 | 15.5–18.6 | 11.9–14.5 | 13.5–16.1 | 12.9–15.4 | 11.3–14.6 | 14.3–19.9 | 13.1–16.2 | 13.8–17.8 | 12.5–20.4 | 14.0–15.6 |
| Dorsal-spine length | 13.2–18.0 | 13.3–20.9 | 11.3 | 13–17.5 | 14.0–17.4 | 15.8–20.5 | 17.1–20.4 | 18.4–21.3 | 14.0–15.0 | 11.6–20.1 | 14.5–17.9 | 13.8–20.6 | 12.5–25.4 | 11.9–17.8 |
| Dorsal-fin base length | 10.7–14.1 | 12.8–18.0 | 8.6 | 9.5–13.2 | 8.8–12.9 | 10.4–13.7 | 8.5–10.2 | 12.8–14.6 | 7.5–9.6 | 11.5–23.7 | 12.8–16.6 | 20.6–28 | 11.8–30.6 | 15.4–24.7 |
| Adipose fin to caudal peduncle length | 12.9–17.0 | 13.3–17.7 | 15.2 | 14.9–18.5 | 13.1–17.0 | 13.1–16.8 | 11.9–14.1 | 13.6–15.6 | 14.0–15.9 | 14.4–19.8 | 15.1–20.9 | 11.3–16.1 | 13.3–21.6 | 15.7–17.2 |
| Adipose-fin base length | 17.0–23.3 | 16.2–25.2 | 16.5 | 9.2–13.6 | 14.6–19.6 | 12.4–17.8 | 15.5–17.4 | 13.7–15.4 | 17.0–22.5 | 13.2–17 | 11.3–17.3 | 13.2–19.6 | 10.4–19.3 | 14.9–17.4 |
| Adipose-fin height | 4.1–6.8 | 4.2–5.8 | 3.5 | 2.9–4.6 | 2.3–5.0 | 3.3–5.2 | 2.7–3.1 | 3.0–3.9 | 3.5–5.2 | 2.8–5.4 | 3.2–5.3 | 3.3–5.6 | 3.3–8.7 | 5.0–6.2 |
| Anal-fin length along longest ray | 11.7–17.9 | 13.7–18.3 | 15.5 | 14.1–17.8 | 11.6–14.6 | 11.5–16.6 | 19.2–20.9 | 12.8–14.6 | 10.1–15.5 | 11.8–18.7 | 13.0–17.2 | 11.0–17.5 | 10.9–20.5 | 13.2–16.9 |
| Anal-fin base length | 10.5–13.5 | 11.7–15.4 | 12.9 | 11.5–15.3 | 10.9–14.9 | 8.9–11.4 | 11.1–12.1 | 10.6–11.0 | 11.7–15 | 10.6–16.4 | 11.9–15.5 | 11.3–19.4 | 8.2–15.6 | 11.8–14.2 |
| Caudal peduncle depth | 11.3–13.2 | 11–13.8 | 12.2 | 11.1–14.1 | 10.2–11.9 | 7.5–8.8 | 9.5–9.9 | 9.6–9.9 | 7.2–8.7 | 10.9–12.6 | 10.0–11.9 | 9.6–12.8 | 9.8–14.9 | 10.0–11.1 |
| Caudal peduncle length | 15.9–19.7 | 15.8–22.6 | 18.6 | 19.9–22.7 | 19.1–23.7 | 18.8–21.7 | 16.0–18.8 | 17.7–18.9 | 19.6–22.0 | 17.4–24.5 | 20.3–22.9 | 13.6–15.8 | 13.9–21.9 | 16.2–17.9 |
| Head length | 30.5–34.9 | 33.3–38.6 | 32.9 | 29.5–31.3 | 26.5–30.7 | 30.4–35.2 | 30.2–31.7 | 31.0–32.6 | 24.8–28.0 | 27.8–34.9 | 32.8–39.4 | 25.9–33.7 | 28.3–36.3 | 31.4–34.1 |
| % Head length | ||||||||||||||
| Eye diameter (vertical axis) | 9.9–13.8 | 11.6–18.3 | 10.6 | 12.1–16.6 | 11.9–16.5 | 9.4–13.1 | 9.1–14.9 | 10.6–12.5 | 13.2–18.6 | 11.8–16.1 | 12.6–13.9 | 10.9–20.4 | 7.4–15.3 | 11.5–15.1 |
| Orbital interspace | 21.5–28.7 | 22.6–28.9 | 23.4 | 19.5–24.6 | 18.3–24.4 | 18.5–25.4 | 25.7–30.2 | 22.2–23.9 | 15.3–22.7 | 23.3–38.5 | 20.9–25.2 | 23.8–38.4 | 18.0–38.9 | 22.7–30.4 |
| Anterior nares interspace | 9.5–15.5 | 12.4–16.6 | 11.9 | 19.5–21.2 | 16.5–22.4 | 11.5–17.6 | 13.9–19.8 | 13.3–15.5 | 15.7–22.4 | 10.9–18.4 | 13.0–14.7 | 13.7–23.0 | 11.0–21.6 | 13.5–16.7 |
| Posterior nares interspace | 10.3–15.5 | 11–15.5 | 11.8 | 15.3–21.9 | 13.8–20.9 | 9.9–16.1 | 14.9–22.2 | 9.3–10.9 | 12.6–18.2 | 12.9–16.7 | 12.6–14.7 | 8.9–13.0 | 7.5–18.4 | 10.2–13.0 |
| Snout length | 54.0–66.2 | 55.7–65.7 | 65 | 58.2–64.8 | 49.5–59.9 | 58.9–69.3 | 51.5–56.2 | 56.9–59.0 | 51.5–57.5 | 52.3–66.1 | 51.4–67.3 | 54.7–84 | 51.1–68.0 | 53.4–66.2 |
| Pre-maxillary tooth-patch length | 8.2–12.3 | 8.2–13.9 | 11.7 | 7.0–9.3 | 6.3–8.2 | 7.5–9.5 | 9.9–12.9 | 9.2–11.8 | 8.5–11.6 | 6.8–10.3 | 6.1–9.9 | 7.5–11.5 | 5.4–14.3 | 9.9–12.1 |
| Pre-maxillary tooth-patch width | 36.8–47.9 | 30.7–46.8 | 51.5 | 44.1–50.2 | 39.9–46.2 | 40.3–46.7 | 36.4–38.9 | 41.3–44.2 | 39.5–47.1 | 35.9–45.3 | 39.6–47.8 | 31.7–44.5 | 29.7–50.1 | 37.2–45.1 |
| Mandibular tooth row width | 4.6–8.1 | 16.0–25.6 | 10.5 | 10.4–17.3 | 9.6–13.5 | 4.8–6.6 | 9.9–13.5 | 7.2–7.7 | 10.0–16.6 | 13.6–25.0 | 19.0–25.5 | 17.6–27.8 | 11.9–22.2 | 20.5–25.4 |
| Maxillary barbel length | 20.3–28.8 | 21.3–36.8 | 22.7 | 23.8–41.8 | 29.1–41.8 | 26.4–31.2 | 21.8–30.2 | 24.3–27.5 | 44.2–66.8 | 17.4–34.3 | 20.1–28.1 | 19.7–32.1 | 20.2–45.7 | 31.3–37.0 |
| Upper lip length | 11.1–16.2 | 11.7–18.8 | 16.7 | 8.4–12.3 | 6.6–10.6 | 8.8–15.5 | 9.1–10.0 | 12.3–12.4 | 7.0–10.5 | 6.9–14.3 | 8.4–13.5 | 11.3–19.5 | 10.2–19.5 | 12.5–17.2 |
| Lower lip length | 18.3–26.6 | 22.4–27.7 | 25.1 | 17.7–25.6 | 23.5–28.8 | 18.8–24.9 | 19.8–27.0 | 22.8–27.6 | 19.2–26.0 | 12.6–22.9 | 20.7–29.3 | 19.7–27.8 | 9.6–16.8 | 20.7–24.7 |
| Mouth width | 23.9–33.8 | 24.8–32.6 | 39.6 | 30.9–39.1 | 25.7–36.2 | 25.6–32.1 | 20.6–22.8 | 28.0–35.9 | 27.9–34.0 | 20.4–33.4 | 27.1–33.8 | 25.2–37.8 | 17.5–32.8 | 24.7–31.1 |
| Oral disc width | 51.1–64.6 | 48.4–70.3 | 69.1 | 59.3–69.3 | 58.9–66.3 | 51.4–64.1 | 47.6–53.5 | 60.8–64.8 | 51.8–63.0 | 44.4–57.3 | 45.8–56.1 | 63.4–69.7 | 35.7–69.2 | 54.4–63.3 |
| Oral disc length | 48.6–57 | 46.0–61.1 | 63.8 | 53.7–61.3 | 43.3–54.7 | 48.4–59.7 | 41.6–53.2 | 55.7–57.8 | 48.6–62.4 | 40.7–53.9 | 41.9–58.2 | 46.5–57.4 | 38.6–57.1 | 47.9–57.3 |
| Meristics | ||||||||||||||
| Mandibular tooth count | 10 | 12 | 12 | 8 | 8 (6–8) | 8 | 8 | 12 | 11 (11–14) | _ | _ | _ | _ | _ |
| Pre-maxillary tooth count | 60 (43–69) | 51–59 | 86 | 54 (50–64) | 36–54 | 64 (51–65) | 55–60 | 39–51 | 50 (34–59) | _ | _ | _ | _ | _ |
| Pectoral-fin ray count | 8 (6–8) | 8 | 8 | 8 (7–8) | 7 | 8 | 8 | 8 | 8 | _ | _ | _ | _ | _ |
| Pelvic-fin ray count | 7 (6–7) | 7 | 7 | 7 | 7 (7–8) | 7 | 7 | 7 | 7 | _ | _ | _ | _ | _ |
| Dorsal-fin ray count | 6 (5–7) | 6 | 5 | 5 (5–6) | 6 (5–6) | 6 (5–6) | 5 | 5 | 5 (5–6) | _ | _ | _ | _ | _ |
| Anal-fin ray count | 12 (12–13) | 10 | 13 | 12 (9–13) | 10 (10–12) | 9 (8–12) | 10 (10–12) | 9 | 12 (9–13) | _ | _ | _ | _ | _ |
| X-rays | ||||||||||||||
| Number of specimens | 9 | 2 | 1 | 4 | 6 | 7 | 5 | 1 | 6 | _ | _ | _ | _ | _ |
| Abdominal vertebrae | 13 (11–13) | 12–13 | 13 | 11 (10–11) | 10 (10–12) | 13 (12–13) | 13 (12–13) | 12 | 12 (11–13) | _ | _ | _ | _ | _ |
| Caudal vertebrae | 17 (16–18) | 18–19 | 17 | 20 (18–20) | 19 (17–19) | 16 (16–17) | 16 (16–18) | 16 | 20 (16–20) | _ | _ | _ | _ | _ |
| Total vertebrae | 29 (29–30) | 28 | 30 | 30 (29–30) | 29 (28–30) | 29 (28–29) | 28 | 27 | 32 (31–32) | _ | _ | _ | _ | _ |
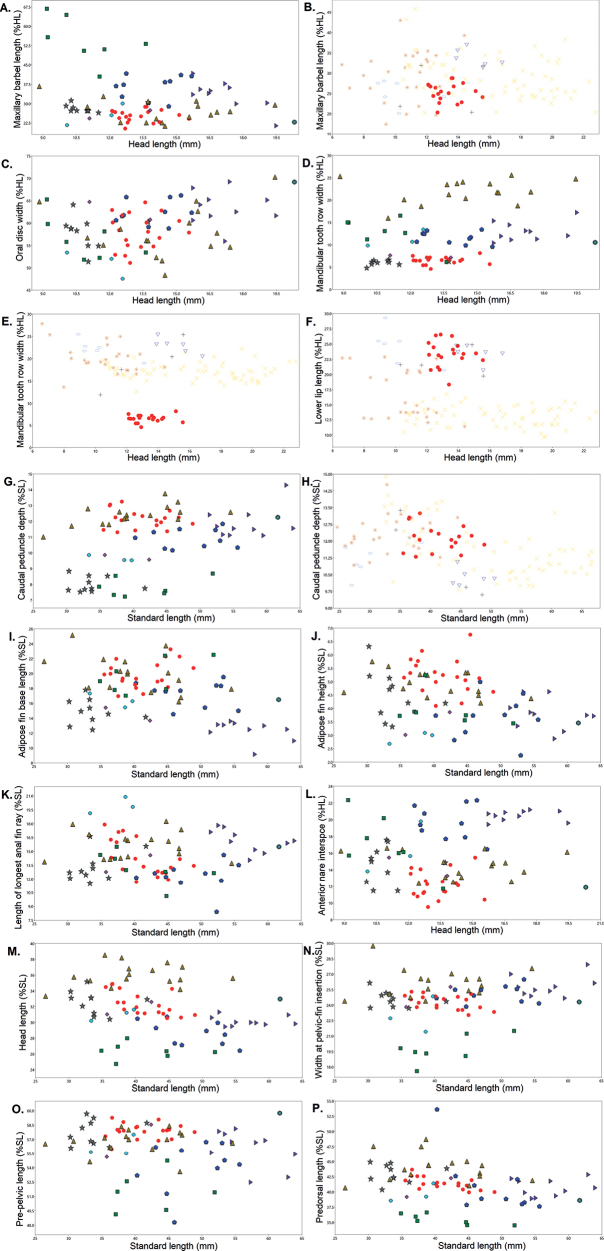
Figure 7.
Scatterplots of the morphometric characters of the Chiloglanis species and lineages from southern African. Key: Chiloglaniscarnatus sp. nov. (red circle), C.pretoriae (brown triangle), C.swierstrai (dark green square), C.bifurcus (purple right-pointing triangle), C.anoterus (green heavy asterisk), C.paratus (pink diamond), C.emarginatus (Blue pentagon), C.fasciatus (grey star), C.neumanni (light blue circle), Chiloglanis sp. ‘dwarf’ (orange eight spoked asterisk), Chiloglanis sp. ‘roughskin’ (yellow multiplication sign), Chiloglanis sp. ‘Pungwe’ (black plus sign), Chiloglanis sp. ‘Zambezi’ (blue down-pointing hollow triangle), Chiloglanis sp. ‘Nyangombe’ (light blue hollow circle).
Additional scatterplots were generated to explore the characters that further distinguish the Chiloglaniscarnatus sp. nov. specimens. The Chiloglaniscarnatus sp. nov. specimens have a narrower mandibular tooth row width (4.6–8.1%HL) compared to C.pretoriae (16.0–25.6%HL), C.swierstrai (10.0–16.6%HL), C.neumanni (9.9–13.5%HL), C.emarginatus (9.6–13.5%HL), C.bifurcus (10.4–17.3%HL), C.anoterus (10.5%HL), Chiloglanis sp. ‘dwarf’ (13.6–25.0%HL), Chiloglanis sp. ‘Nyangombe’ (19.0–25.5%HL), Chiloglanis sp. ‘Pungwe’ (17.6–27.8%HL), Chiloglanis sp. ‘roughskin’ (11.9–22.2%HL), and Chiloglanis sp. ‘Zambezi’ (20.5–25.4%HL; Fig. 7D, E). Chiloglaniscarnatus sp. nov. has an oral disc with relatively longer lower lips (18.3–26.6%HL) compared to Chiloglanis sp. ‘roughskin’ (9.6–16.8%HL, Fig. 7F). Chiloglaniscarnatus sp. nov. has a relatively deeper caudal peduncle (11.3–13.2%SL) compared to C.neumanni (9.5–9.9%SL), C.paratus (9.6–9.9%SL), C.fasciatus (7.5–8.8%SL), C.swierstrai (7.2–8.7%SL), and Chiloglanis sp. ‘Zambezi’ (10.0–11.1%SL, Fig. 7G, H). A longer adipose-fin base length distinguishes Chiloglaniscarnatus sp. nov. (17.0–23.3%SL) from C.bifurcus 9.2–13.6%SL) (Fig. 7I). Larger adipose fin height (4.1–6.8%SL) and shorter anal fin rays (11.7–17.9%SL) further distinguish Chiloglaniscarnatus sp. nov. from C.neumanni (adipose fin height: 2.7–3.1%SL; anal fin ray length: 19.2–20.9%SL; Fig. 7J, K). A shorter distance between the anterior nares of Chiloglaniscarnatus sp. nov. (9.5–15.5%HL) separates it from C.bifurcus (19.5–21.2%HL), C.emarginatus (16.5–22.4%HL), and C.swierstrai (15.7–22.4%HL, Fig. 7L). Chiloglaniscarnatus sp. nov. has a relatively longer head (30.5–34.9%SL vs 24.8–28.0%SL), relatively wider body at pectoral-fin insertion (23.0–25.3%SL vs 17.6–21.5%SL), and relatively longer pre-pelvic (56.0–59.3%SL vs 49.1–54.8%SL) and pre-dorsal distances (39.9–43.7%SL vs 34.5–36.7%SL) that readily separated them from C.swierstrai (Fig. 7M–P).
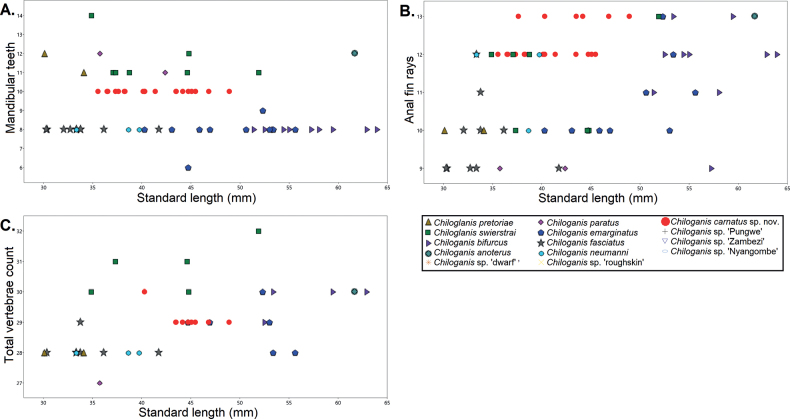
Comparison of meristic characters revealed consistent differences between Chiloglaniscarnatus sp. nov. specimens and the other species from southern Africa. Chiloglaniscarnatus sp. nov. specimens have ten closely packed mandibular teeth that separate them from C.bifurcus, C.emarginatus, C.fasciatus, and C.neumanni that have eight mandibular teeth as well as from C.anoterus, C.pretoriae, C.paratus, and C.swierstrai that have > 10 mandibular teeth (Fig. 8A). A higher number of anal-fin rays separates Chiloglaniscarnatus sp. nov. specimens (12–13) from C.paratus (9) and C.pretoriae (10) (Fig. 8B). Chiloglaniscarnatus sp. nov. specimens have a higher number of total vertebrae (29–30) compared to C.neumanni (28), C.pretoriae (28), and C.paratus (27) (Fig. 8C).
Figure 8.
Scatterplots of the meristic characters of the Chiloglanis species from southern African.
The integrated approach used in this study provided genetic and morphological characters that clearly and consistently distinguish Chiloglaniscarnatus sp. nov. from the known species and lineages from this region. This study has thus provided evidence that supports the description of the Chiloglaniscarnatus sp. nov. as a new species.
Taxonomic account
. Chiloglanis carnatus
Mutizwa, Bragança & Chakona sp. nov.
8CFDB994-A475-5916-8EA9-F0D21DE7495F
https://zoobank.org/E1F0912C-986F-450F-9B90-400D86F5F3BC
Material examined.
Holotype. Zimbabwe • ♂, stored in 70% ethanol, 46.8 mm SL, Fig. 9A–E; Mukwadzi River near bridge on the road to Mutorashanga, Manyame River sub-catchment, middle Zambezi River system, Mashonaland West Province, 17.42485°S, 30.58542°E; 30 Jun. 2016; A. Chakona, W. Kadye and T. Bere; SAIAB 236631; genseq-1 COIPP156890. Paratypes. Zimbabwe • 5 ♀, stored in 70% ethanol, 36.5–45.5 mm SL; near bridge on the road to Mutorashanga, Mukwadzi River, Manyame River sub-catchment, middle Zambezi River system, Mashonaland West Province, 17.42485°S, 30.58542°E; 30 Jun. 2016; A. Chakona, W. Kadye and T. Bere; SAIAB 211346; genseq-2 COIPP156891 to PP156895. Zimbabwe • 6 ♀, 35.5–45.1 mm SL, 7 ♂, 36.5–48.9 mm SL, stored in 70% ethanol; near bridge on the road to Mutorashanga, Mukwadzi River, Manyame River system, middle Zambezi Basin, Mashonaland West Province, 17.42444°S, 30.58453°E; 11 Apr. 2019; A. Chakona, W. Kadye and T. Bere; SAIAB 211349.
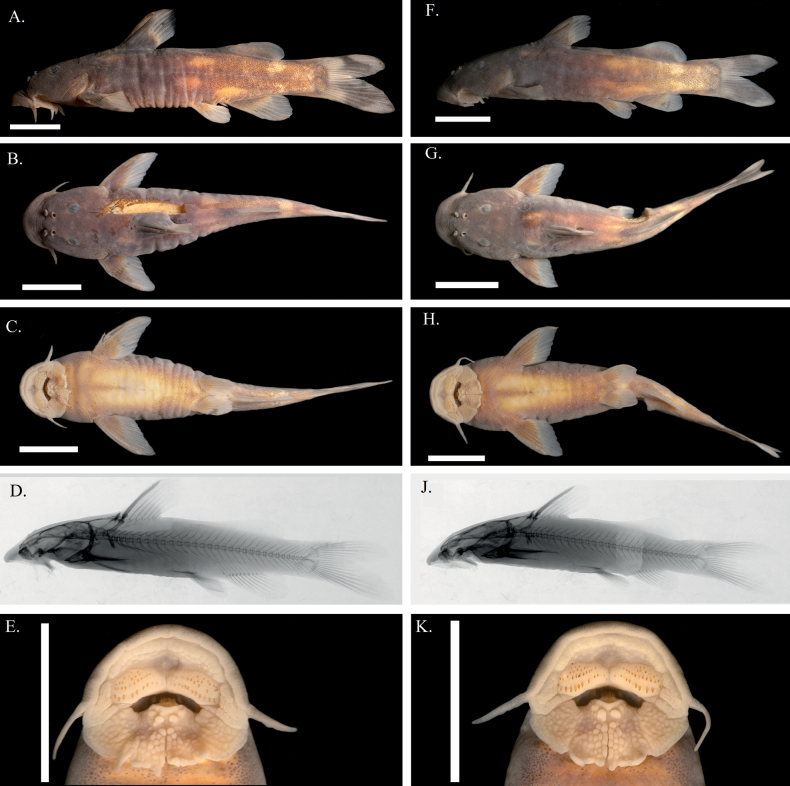
Figure 9.
Holotype of Chiloglaniscarnatus sp. nov., SAIAB 236631 male (A–E) and female paratype specimen SAIAB 211346 (F–K). Scale bars: 1 cm.
Diagnosis.
Chiloglaniscarnatus sp. nov. is readily distinguished from its congeners in southern Africa (i.e. C.anoterus, C.bifurcus, C.emarginatus, C.fasciatus, C.paratus, C.pretoriae and C.swierstrai) by the presence of a dorsal fin that has a basal portion covered by a fleshy skin, a character which is absent in the other species. Chiloglaniscarnatus possesses ten closely packed mandibular teeth, that further distinguishes it from C.fasciatus that has eight closely packed mandibular teeth; C.bifurcus and C.emarginatus that have eight widely spaced mandibular teeth; C.anoterus, C.paratus, and C.pretoriae that have 12 closely packed mandibular teeth; and C.swierstrai that has 14 closely packed mandibular teeth. Chiloglaniscarnatus possesses a deeply forked caudal fin that readily separates it from C.pretoriae and C.emarginatus that have emarginate caudal fins, and from C.anoterus that has a caudal fin with extended median rays in males and emarginate in females. Chiloglaniscarnatus possesses a caudal fin with an upper lobe that is shorter than the lower lobe. This distinguishes it from C.bifurcus that has a caudal fin with an upper lobe that is longer than the lower lobe. Chiloglaniscarnatus has an oral disc with a well-developed mid-ventral cleft that distinguishes it from C.swierstrai that possesses an oral disc without a mid-ventral cleft. Chiloglaniscarnatus possesses a smooth skin with a few tubercles occasionally found on the head that separates it from C.fasciatus that has its entire dorsal and lateral body surfaces mostly covered by small tubercles. Chiloglaniscarnatus has a dorsal spine with crenate anterior and posterior margins that distinguish it from C.paratus that has a dorsal spine with a serrated posterior margin.
Description.
Morphometric proportions and meristics are summarised in Table 7. Holotype meristic counts are given in parentheses.
Table 7.
Summary of morphological characters for Chiloglaniscarnatus sp. nov. All values except standard length (SL) and Head length (HL) are given as percentages of the HL or SL.
| Holotype | Paratypes | ||||
|---|---|---|---|---|---|
| Male | Males | Females | |||
| Number of specimens | 7 | 11 | |||
| Range | Mean | Range | Mean | ||
| Total length | 58.2 | 45.3–62.2 | 49.8 | 45.3–56.1 | 52.2 |
| Standard length | 46.8 | 36.5–48.9 | 39.6 | 35.5–45.5 | 41.8 |
| Head length | 14.3 | 12.1–15.1 | 13.0 | 12.3–15.6 | 13.5 |
| % Standard length | |||||
| Pre-pectoral length | 28.1 | 26.9–30.0 | 28.9 | 27.1–29.1 | 28.3 |
| Pre-dorsal length | 40.2 | 40.0–42.6 | 41.6 | 39.9–43.7 | 41.3 |
| Pre-pelvic length | 58.4 | 56.0–58.8 | 57.8 | 56.9–59.3 | 57.9 |
| Pre-anal length | 71.1 | 67.6–70.8 | 69.1 | 67.9–73.3 | 70.6 |
| Dorsal fin to adipose fin length | 20.9 | 18.4–22.2 | 20.6 | 18.2–22.6 | 20.6 |
| Pectoral-spine length | 18.6 | 15.6–19.8 | 17.7 | 15.0–18.6 | 16.5 |
| Pectoral-fin length | 20.9 | 20.9–23.6 | 22.4 | 19.3–22.2 | 20.9 |
| Width at pectoral-fin insertion | 23.8 | 23.3–25.2 | 24.3 | 23.0–25.3 | 24.3 |
| Pelvic-fin length | 12.2 | 13.3–14.2 | 13.7 | 10.8–14.1 | 12.3 |
| Pelvic-fin interspace | 4.6 | 3.3–4.6 | 4.0 | 3.0–5.1 | 3.9 |
| Body depth at dorsal-fin insertion | 18.9 | 15.5–20.7 | 18.0 | 16.2–20.1 | 17.8 |
| Body depth at anus | 17.6 | 15.3–16.9 | 15.8 | 13.9–17.0 | 15.9 |
| Dorsal-spine length | 15.7 | 13.6–18.0 | 16.1 | 13.2–17.7 | 15.9 |
| Dorsal-fin length along longest ray | 17.9 | 15.2–20.7 | 18.5 | 16.2–20.0 | 17.4 |
| Dorsal-fin base length | 11.0 | 12.1–14.1 | 13.1 | 10.7–13.8 | 12.3 |
| Adipose fin to caudal peduncle length | 13.5 | 12.9–17.0 | 15.0 | 10.3–16.4 | 13.8 |
| Adipose-fin base length | 22.3 | 17.0–22.0 | 19.6 | 17.2–23.3 | 19.8 |
| Adipose-fin height | 5.1 | 4.1–6.1 | 5.2 | 4.2–6.8 | 5.3 |
| Anal-fin length along longest ray | 14.2 | 13.1–17.2 | 15.7 | 11.7–17.9 | 13.4 |
| Anal-fin base length | 12.1 | 11.8–15.3 | 13.2 | 11.1–13.4 | 12.5 |
| Caudal peduncle depth | 12.2 | 11.3–13.2 | 12.1 | 11.4–13.1 | 12.1 |
| Caudal peduncle length | 16.8 | 16.0–19.2 | 18.3 | 15.9–19.7 | 17.5 |
| Caudal fork length | 12.3 | 9.8–14.5 | 11.4 | 9.2–14.4 | 11.7 |
| Head length | 30.6 | 30.9–34.8 | 32.9 | 30.5–34.9 | 32.2 |
| % Head length | |||||
| Head depth | 57.4 | 43.9–57.6 | 51.2 | 48.2–57.3 | 51.2 |
| Eye diameter (vertical axis) | 11.9 | 10.6–13.2 | 11.7 | 9.9–13.8 | 11.9 |
| Eye diameter (horizontal axis) | 15.7 | 13.0–16.4 | 14.1 | 12.9–16.8 | 15.0 |
| Orbital interspace | 25.1 | 22.3–28.7 | 24.1 | 21.5–26.8 | 24.5 |
| Anterior nares interspace | 12.1 | 9.5–15.5 | 12.1 | 10.4–14.6 | 12.2 |
| Posterior nares interspace | 12.6 | 11.0–15.5 | 13.5 | 10.3–15.4 | 12.7 |
| Snout length | 61.1 | 54.3–63.8 | 58.7 | 54.0–66.2 | 60.7 |
| Pre-maxillary tooth-patch length | 9.9 | 8.2–11.0 | 9.9 | 8.8–12.3 | 10.4 |
| Pre-maxillary tooth-patch width | 44.3 | 36.8–44.7 | 41.1 | 38.4–47.9 | 42.1 |
| Mandibular tooth row width | 6.7 | 4.6–8.1 | 6.4 | 5.4–7.1 | 6.4 |
| Maxillary barbel length | 27.6 | 20.3–27.2 | 25 | 22.3–28.8 | 25.3 |
| Upper lip length | 15.1 | 11.1–14.5 | 13.1 | 11.3–16.2 | 13.9 |
| Lower lip length | 23.4 | 18.3–25.2 | 22.7 | 20.7–26.6 | 23.8 |
| Mouth width | 29.2 | 25.3–30.8 | 27.3 | 23.9–33.8 | 28.1 |
| Oral disc width | 62.8 | 51.1–62.9 | 57.2 | 52.9–64.6 | 58.2 |
| Oral disc length | 54.3 | 48.6–57.0 | 53.1 | 50.2–56.4 | 53.3 |
| Postcleithral process to occipital shield | 37.8 | 29.5–36.3 | 33.1 | 32.2–38.3 | 35.5 |
| Length of postcleithral process | 29.6 | 23.4–28.9 | 25.5 | 22.9–27.8 | 25.9 |
| Occipital shield width | 23.6 | 14.6–19.5 | 16.9 | 14.9–24.2 | 18.8 |
| Lower caudal-fin lobe length | 13.4 | 9.3–13.0 | 10.6 | 10.0–12.7 | 11.2 |
| Upper caudal-fin lobe length | 10.8 | 8.7–12.1 | 9.8 | 9.1–11.7 | 10.4 |
| Medial mandibular barbel length | 0.6 | 0.2–0.6 | 0.4 | 0.4–0.9 | 0.6 |
| Lateral mandibular barbel length | 1.3 | 1.0–1.8 | 1.4 | 1.1–1.8 | 1.4 |
| Meristics | Range | Mode | Range | Mode | |
| Mandibular tooth count | 10 | 8–10 | 10 | 8–10 | 10 |
| Pre-maxillary tooth count | 59 | 43–69 | _ | 49–68 | 60 |
| Pectoral fin-ray count | 8 | 7–8 | 8 | 6–8 | 8 |
| Pelvic fin-ray count | 7 | 7 | 7 | 6–7 | 7 |
| Dorsal fin-ray count | 6 | 6 | 6 | 5–7 | 6 |
| Anal fin-ray count | 13 | 12–13 | 12 | 12–13 | 12 |
| Abdominal vertebrae | 12 | 12 | _ | 11–13 | 13 |
| Caudal vertebrae | 17 | 17 | _ | 16–18 | 16 |
| Total vertebrae | 29 | 29 | _ | 29–30 | 29 |
Body shape. Anterior portion of body slightly compressed dorsally, becoming laterally compressed from pelvic fin insertion to caudal peduncle. Body greatest depth at dorsal-fin insertion. Pre-dorsal profile convex, sharply slopping from snout to posterior nostril, gently from nostril to dorsal-fin origin. Post-dorsal profile about straight from dorsal-fin base to adipose-fin origin, becoming gently concave from adipose-fin origin to caudal fin. Ventral profile gently convex from region just posterior to oral disc to anal-fin origin, becoming gently concave from anal-fin origin to caudal fin.
Head. slightly depressed dorsally. Oval eye dorsally positioned, ~ 1/2 distance between snout and gill opening. Interorbital distance greater than distance between nostrils. Anterior and posterior nostrils closer to the eye than snout. Distance between anterior nostrils slightly greater than distance between posterior nostrils. Posterior nostril medially positioned relative to orbit. Anterior nostril with posterior flap; posterior nostril with anterior flap. Occipital-nuchal shield not visible through skin. Gill opening above pectoral fin insertion.
Oral disc. Mouth inferior; large upper and lower lips combined to form oral disc (see Fig. 9E, K). Oral disc width greater than length. Upper and lower lips with pronounced roundish papillae, largest papillae concentrated around mid-ventral cleft of lower lip. Three pairs of barbels. Maxillary barbel unbranched, originating from lateral region of oral disc, extending to posterior region of oral disc. Lateral mandibular barbel longer than medial mandibular barbel, both incorporated into lower lip. Shallow cavity above lower lip.
Dentation. Pre-maxillary teeth arranged in three or four rows; variable number of teeth (43–69). Up to 5+5 closely packed mandibular teeth; central teeth projecting higher than outer teeth forming a gentle arc; replacement tooth row emerges anteriorly to the functional row.
Fins. Dorsal-fin ray count 5–7 (6), originating in anterior 1/3 of body, posterior to pectoral-fin origin. Dorsal fin basal portion covered by a fleshy skin prominent in large adult males and females with ~ ¾ of the dorsal spine and the first two rays also covered by fleshy skin (Fig. 10). Dorsal spine length ~ 80% of longest dorsal fin ray length. Dorsal spine with dentate anterior and posterior margins. Pectoral-fin ray count 6–8 (8), origin anterior to gill opening; pectoral spine anterior margin smooth; dentate posterior margin; pectoral spine length ~ 80% of pectoral fin length. Adipose fin origin preceded by anterior tissue flange; rounded (Fig. 10). Caudal fin forked, lower lobe longer than upper lobe. Anal-fin ray count 12 or 13 (13), origin posterior to origin of adipose fin; terminating just before end of adipose-fin; rounded. Pelvic-fin ray count 6 or 7 (7), origin posterior to midpoint between end of dorsal-fin and adipose-fin origin; rounded.
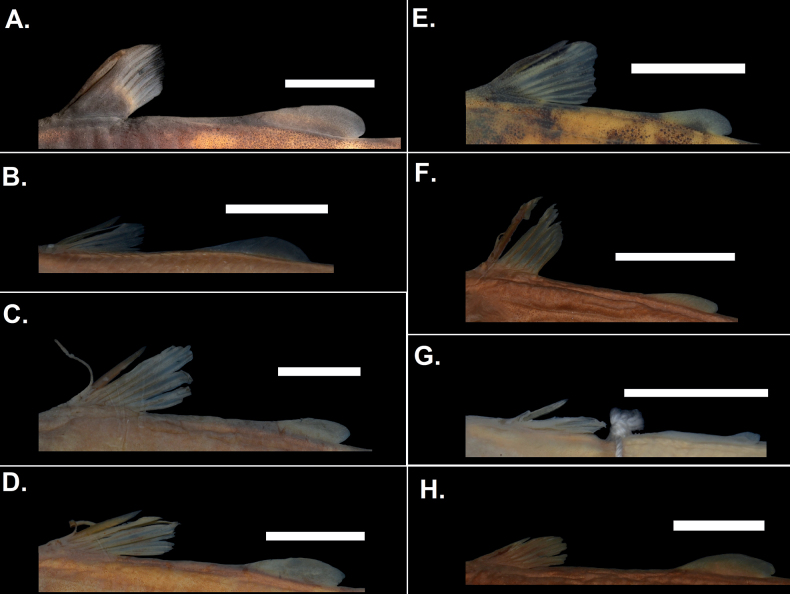
Figure 10.
Comparison of the dorsal and adipose fins of Chiloglaniscarnatus sp. nov. and the type specimens of the valid southern African species AChiloglaniscarnatus sp. nov. (SAIAB 236631) specimens have an extended dermal tissue covering the base of the dorsal fin that distinguishes them from BC.swierstrai (SAIAB 186247) CC.bifurcus (SAIAB 120160) DC.emarginatus (SAIAB 120117) EC.fasciatus (SAIAB 204928) FC.paratus (SAIAB 186248) GC.pretoriae (SAIAB 30011) HC.anoterus (SAIAB 186246). Scale bars: 1 cm.
Skin. Skin smooth with occasional tubercles present, concentrated on dorsal and lateral surface of head. Lateral line complete; originating anterior to dorsal fin at same horizontal level of orbit and sloping ventrally until it lies mid-laterally along body.
Sexual dimorphism. Urogenital opening situated adjacent to origin of anal fin. Urogenital papillae sexually dimorphic; elongated in males; reduced and separated from anus by shallow invagination in females.
Colouration. Overall body background colouration brown with yellowish ventral surface. Anterior portion of body dark brown becoming paler towards posterior. Small dark melanophores scattered across entire dorsal and lateral sides. Six yellowish brown blotches on lateral surface of body; two vertically arranged posterior to end of adipose fin; one above origin of anal fin; two above pelvic fin origin; and one below dorsal fin origin. Basal 1/3 of fins pale to dark brown with medium and posterior portion of fins gradually becoming translucent. Dark blotch cuts vertically across caudal peduncle lobes.
Vertebral counts. Total vertebrae 29 or 30 (29), abdominal vertebrae 11–13 (12), caudal vertebrae 16–18 (17).
Etymology.
The specific epithet carnatus means fleshy, referring to the dermal tissue covering the base of the dorsal fin of some of the larger specimens of this species and the general robust body structure of this species compared to its regional congeners.
Distribution.
Chiloglaniscarnatus was collected from two sites in the Mukwadzi River near the bridge on the Mutorashanga Road. The Mukwadzi River is a perennial river that originates from wetlands (dambos) on the eastern side of the Great Dyke. This river flows in a north-western direction cutting through the Great Dyke before it joins the Manyame River. The Great Dyke is a major intrusion of mafic and ultramafic rocks that have vast ore deposits, including gold, silver, chromium, platinum, nickel, and asbestos. The rich mineral deposits have resulted in the establishment of many mines along the Great Dyke. The sites where C.carnatus was collected were in a communal area surrounded by rural communities on the western slope of the Great Dyke. The substratum at the sites was composed of bedrock, cobbles and gravel, and the riparian vegetation was dominated by Syzygium Gaertner, 1788 and Phragmites Adanson, 1763. At these sites C.carnatus co-occurred with native fish species that include Labeocylindricus Peters, 1852, Opsaridiumzambezense (Peters, 1852), Enteromiustrimaculatus (Peters, 1852), Tilapiasparrmanii Smith, 1840, Clariasgariepinus (Burchell, 1822), and Labeobarbusmarequensis (Smith, 1841) as well as the non-native species Serranochromisjallae (Boulenger, 1896) and Micropterussalmoides (Lacepède, 1802).
Discussion
This study integrated molecular and morphological data to evaluate the taxonomic distinctiveness of specimens of suckermouth catfishes that were collected from the middle Zambezi River system in Zimbabwe. Based on substantial genetic differentiation as well as consistent meristic, morphometric, and qualitative differences from its southern African congers, a new species of Chiloglanis is described. This is the first description more than five decades after the last comprehensive review of Chiloglanis species from southern Africa (see Jubb and Le Roux 1969). This study adds to the growing body of literature that demonstrates the value of integrative taxonomic approaches in the discovery and description of new species within this region (Maake et al. 2014; Morris et al. 2016; Riddin et al. 2016; Kambikambi et al. 2021; Mazungula and Chakona 2021). As evidenced from this study and work by Chakona et al. (2018), additional species of suckermouth catfishes from southern Africa remain to be formally described. It is anticipated that ongoing taxonomic studies on this group of fishes will result in the description of at least ten new species from this region. These species were all previously included under a single species, C.neumanni, but this study and ongoing work by researchers from the NRF-SAIAB indicates that this species does not occur in southern Africa. Updated taxonomic information of Chiloglanis species from this region will improve our understanding of biogeographic and phylogeographic patterns as well as drainage evolution in the region.
The dentition of species in the genus Chiloglanis, like that of most members of the family Mochokidae, is highly specialised (Roberts 1989). Chiloglaniscarnatus possesses ten closely packed mandibular teeth, a number not found in any other Chiloglanis species in southern Africa. Variation in the number of mandibular teeth in individual specimens can be observed due to tooth loss from the functional row, delayed exposure of some teeth in the replacement row, or early advancement of some replacement row teeth (Roberts 1989). However, by examining both the functional and replacement rows, it was possible to determine the diagnostic number of teeth for this species. Outside southern Africa, the presence of ten mandibular teeth has been reported in west African species such as C.kolenteSchmidt et al., 2017, C.kabaensisSchmidt et al., 2017, C.nzerekoreSchmidt et al., 2017, C.occidentalis Pellegrin, 1933, and C.normani Pellegrin, 1933 (Paugy et al. 2003; Schmidt et al. 2017). In addition to dentation, there were several morphometric characters associated with the oral disc (e.g., maxillary barbel length, oral disc width, lower lip length and mandibular tooth row width) that distinguish C.carnatus from congenerics in southern Africa. Considering the importance of the oral disc in the ecology of the species in this genus, these differences warrant further study, particularly assessing potential differences in trophic ecology.
Rheophilic habitats form ‘islands’ with suitable environmental conditions for specialised taxa such as those in the genus Chiloglanis. The disjunct distribution of these habitats within a river may play an important role in promoting genetic and morphological diversity within rheophilic taxa. Some rheophilic species have very narrow distribution ranges such that significant differences have been found in the fish communities occurring at different rapids within the same river system (Hrbek et al. 2018). In southern Africa high genetic and morphological diversity within C.anoterus has been reported from geographically isolated populations in the upper sections of the Phongolo and Inkomati river systems, highlighting the importance of the rheophilic habitats in headwater streams (Morris et al. 2016). The close association of Chiloglanis species with rheophilic habitats probably promotes diversification; however, this has yet to be explicitly tested within this region. The discovery of the C.carnatus from a small section of the Mukwadzi River as well as other undescribed species within southern Africa (Chakona et al. 2018) emphasises the need for accelerating inventory of the diversity found in rheophilic habitats as these may harbour a considerable number of species which are still unknown to science.
A number of southern African freshwater fish species in the genera Enteromius Cope, 1867, Nothobranchius Peters, 1868, Pseudobarbus Smith, 1841, Sandelia Castelnau, 1861, Galaxias Cuvier, 1816, and Oreochromis Günther, 1889 are threatened with extinction due to their narrow geographic ranges, the introduction of invasive species, and habitat degradation (Marshall and Tweddle 2007; Jordaan and Chakona 2017; Roux and Hoffman 2017b; Nagy and Watters 2019). Among the Chiloglanis species from southern Africa, C.bifurcus and C.emarginatus are under threat with the former classified as Critically Endangered and the latter as Vulnerable in the IUCN Red List of threatened species (Roux and Hoffman 2017a, 2018). Chiloglanisbifurcus is a narrow-range endemic species that is confined to the upper sections of the Inkomati River system, whereas C.emarginatus’ range in the Phongolo River system has declined substantially over the past decades (Roux and Hoffman 2017a, 2018). Habitat loss though flow regulation, pollution, and sedimentation has been attributed as the main driver of population decline in both these species (Roux and Hoffman 2017a, 2018). Chiloglaniscarnatus was collected from two sites in the Mukwadzi River. The section downstream of these sites as well as other tributaries of the Mukwadzi River are heavily impacted by anthropogenic activities. There are at least 13 small impoundments in the Mukwadzi River before its confluence with the Manyame River. Largemouth bass (Micropterussalmoides) and the nembwe (Serranochromisjallae) were also introduced into this river system, and this combination of flow modification, water abstraction, and non-native species is likely to negatively impact populations of native species (Gratwicke and Marshall 2001; Gratwicke et al. 2003; Kadye et al. 2013; Kadye and Booth 2020). In addition to the modification of this river and the non-native species, the rich mineral resources found within the Great Dyke attract formal and informal mining operations which also threaten the species living within these rivers through increased sedimentation/siltation which may cause habitat loss. Although little is known about the distribution of C.carnatus beyond the sites sampled in this study, multiple anthropogenic activities in the Mukwadzi River catchment raise concerns about the conservation status of this species.
The description of C.carnatus contributes towards clarifying the taxonomic uncertainty surrounding species of the genus Chiloglanis found within the geographic range formerly attributed to C.neumanni within southern Africa. The discovery of C.carnatus follows the common pattern found among recent taxonomic studies within the region whereby comprehensive sampling across poorly explored regions and the use of integrated taxonomic approaches has identified unique diversity within species previously thought to have wide distribution ranges (Bragança et al. 2020; Kambikambi et al. 2021; Mazungula and Chakona 2021). This pattern is likely to be consistent across southern Africa suggesting underestimation of the region’s biodiversity. In particular, species such as those from the genus Chiloglanis are likely to be more diverse since they occur in disjunct distributions in rheophilic habitats, which are likely to be associated with allopatric speciation. This study also raises the awareness of the potential unique riverine diversity of the rivers that flow through the Great Dyke, an important geological feature where 20 endemic plant species that are adapted to the unique serpentine soils have been recorded (Wild 1965). Further exploration of the aquatic fauna of this poorly surveyed region is likely to uncover additional new species for science.
Supplementary Material
Acknowledgements
We would like to thank the NRF-SAIAB personnel including Paul Skelton, Roger Bills, Maditaba Meltaf, Nkosinathi Mazungula, Nonkoliso Mgibntaka, Amanda Gura, Zinzi Somana, Siphamandla Mceleli, Gwynneth Matcher, and Taryn Bodil for the support during this study.
Citation
Mutizwa TI, Kadye WT, Bragança PHN, Bere T, Chakona A (2024) Hidden in the riffles: A new suckermouth catfish (Mochokidae, Chiloglanis) from the middle Zambezi River system, Zimbabwe. ZooKeys 1197: 57–91. https://doi.org/10.3897/zookeys.1197.114679
Additional information
Conflict of interest
The authors have declared that no competing interests exist.
Ethical statement
Ethical clearance for the approaches used for sample collection and processing was approved by the National Research Foundation-South African Institute for Aquatic Biodiversity (NRF-SAIAB) Animal Ethics Committee (Ref#: 2014/03 and REF#: 25/4/1/7/5_2022-02).
Funding
This research was supported by the Rhodes University Sandisa Imbewu Grant, the NRF-Research Development Grant (CSRP190416431023), NRF-SAIAB Refresh project (FBIP-211006643719) and NRF-SAIAB Topotypes project (IBIP-BS 13100251309).
Author contributions
Conceptualization: WK, PB, TB, AC. Data curation: TIM. Formal analysis: TIM. Funding acquisition: WK, AC. Investigation: TIM. Methodology: PB, AC, WK, TIM. Project administration: AC, WK. Resources: AC, TB. Supervision: AC, WK, PB. Visualization: PB, TIM. Writing – original draft: TIM. Writing – review and editing: TIM, PB, AC, TB, WK.
Author ORCIDs
Tadiwa I. Mutizwa https://orcid.org/0000-0003-4017-1720
Wilbert T. Kadye https://orcid.org/0000-0002-5273-8360
Pedro H. N. Bragança https://orcid.org/0000-0002-8357-7010
Taurai Bere https://orcid.org/0000-0002-8603-5137
Albert Chakona https://orcid.org/0000-0001-6844-7501
Data availability
All of the data that support the findings of this study are available in the main text.
References
- Adanson M. (1763) Familles des Plantes. Vincent, Paris, 640 pp. 10.5962/bhl.title.271 [DOI] [Google Scholar]
- Akaike H. (1974) A new look at the statistical model identification. IEEE Transactions on Automatic Control 19(6): 716–723. 10.1109/TAC.1974.1100705 [DOI] [Google Scholar]
- Alfaro ME, Holder MT. (2006) The posterior and the prior in Bayesian phylogenetics. Annual Review of Ecology, Evolution, and Systematics 37(1): 19–42. 10.1146/annurev.ecolsys.37.091305.110021 [DOI] [Google Scholar]
- Bell-Cross G, Minshull JL. (1988) The Fishes of Zimbabwe. National Museums and Monuments of Zimbabwe, Harare, 294 pp. [Google Scholar]
- Bouckaert R, Heled J, Kühnert D, Vaughan T, Wu CH, Xie D, Suchard MA, Rambaut A, Drummond AJ. (2014) BEAST 2: a software platform for Bayesian evolutionary analysis. PLoS Computational Biology 10(4): e1003537. 10.1371/journal.pcbi.1003537 [DOI] [PMC free article] [PubMed]
- Boulenger GA. (1896) Liste des poisons recueillis par le R. P. Louis Jalla a Kazungula, Haut Zambese. Bollettino dei Musei di Zoologia ed Anatomia 11(260): 1–2. [Google Scholar]
- Boulenger GA. (1900) Matériaux pour la Faune du Congo. Poissons Nouveaux du Congo. Sixième Partie. Annales du musée du congo publiées par ordre du secrétaire d’état, Bruxelles, 520–529.
- Boulenger GA. (1902) Additions à la faune ichthyologique de bassin du Congo. Matériaux pour la faune du Congo. Annales du Musee du Congo (Ser. Zoology) 2: 19–57. [Google Scholar]
- Boulenger GA. (1903) On the fishes collected by Mr. G. L. Bates in southern Cameroon. Proceedings of the Zoological Society of London 1(3): 21–29. 10.1111/j.1469-7998.1903.tb08256.x [DOI] [Google Scholar]
- Boulenger GA. (1911) Catalogue of the Fresh-Water Fishes of Africa in the British Museum (Natural History), London, 481–482.
- Bragança PHN, Smith TG, Vreven EJWMN, Chakona A. (2020) Integrative taxonomy reveals hidden diversity in the southern African darters genus Nannocharax Günther 1867 (Characiformes: Distichodontidae). Journal of Fish Biology 97(6): 1713–1723. 10.1111/jfb.14535 [DOI] [PubMed] [Google Scholar]
- Burchell WJ. (1822) Travels in the Interior of Southern Africa. Longman, London, 280 pp. 10.5962/bhl.title.100911 [DOI] [Google Scholar]
- Castelnau FL. (1861) Mémoire sur les poissons de l’Afrique australe. Paris 1–3: 1–78. 10.5962/bhl.title.3819 [DOI]
- Chakona A, Kadye WT, Bere T, Mazungula DN, Vreven EJWMN. (2018) Evidence of hidden diversity and taxonomic conflicts in five stream fishes from the Eastern Zimbabwe Highlands freshwater ecoregion. ZooKeys 768: 69–95. 10.3897/zookeys.768.21944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope ED. (1867) Supplement on some new species of American and African fishes. Transactions of the American Philosophical Society 13(3): 400–407. [Google Scholar]
- Crass RS. (1960) Notes on the freshwater fishes of Natal with descriptions of four new species. Annals of the Natal Museum 14(3): 446–456. [Google Scholar]
- Cuvier G. (1816) Le Règne Animal distribué d’après son organisation pour servir de base à l’histoire naturelle des animaux et d’introduction à l’anatomie comparée. Les reptiles, les poissons, les mollusques et les annélides. A. Belin, Paris, Edition 1 v 2: 1–532.
- Daget J, Gosse JP, Van den Audenaerde DT. (1986) CLOFFA 2 Check-List of the Freshwater Fishes of Africa. ORSTOM, Paris, 111 pp. [Google Scholar]
- Darriba D, Taboada GL, Doallo R, Posada D. (2012) JModelTest 2: More models, new heuristics and parallel computing. Nature Methods 9(8): e772. 10.1038/nmeth.2109 [DOI] [PMC free article] [PubMed]
- Day JJ, Peart CR, Brown KJ, Friel JP, Bills RI, Moritz T. (2013) Continental diversification of an African catfish radiation (Mochokidae: Synodontis). Systematic Biology 62(3): 351–365. 10.1093/sysbio/syt001 [DOI] [PubMed] [Google Scholar]
- Day JJ, Steell EM, Vigliotta TR, Withey LA, Bills R, Friel JP, Genner MJ, Stiassny MLJ. (2023) Exceptional levels of species discovery ameliorate inferences of the biogeography and diversification of an Afrotropical catfish family. Molecular Phylogenetics and Evolution 107754: e107754. 10.1016/j.ympev.2023.107754 [DOI] [PubMed]
- Eccles DH, Tweddle D, Skelton PH. (2011) Eight new species in the dwarf catfish genus Zaireichthys (Siluriformes: Amphiliidae). Smithiana. Bulletin 13(4): 3–28. [Google Scholar]
- Felsenstein J. (1985) Confidence limits on phylogenies: An approach using the bootstrap. Evolution; International Journal of Organic Evolution 39(4): e783. 10.2307/2408678 [DOI] [PubMed]
- Fricke R, Eschmeyer WN, Van der Laan R [Eds] (2024) Eschmeyer’s catalog of fishes: genera, species, references. http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp [Accessed 20 January 2024]
- Friel JP, Vigliotta TR. (2008) Atopodontusadriaensi, a new genus and species of African suckermouth catfish from the Ogooué and Nyanga River systems of Gabon (Siluriformes: Mochokidae). Proceedings of the Academy of Natural Sciences of Philadelphia 157(1): 13–23. 10.1635/0097-3157(2008)157[13:AAANGA]2.0.CO;2 [DOI]
- Fujisawa T, Barraclough TG. (2013) Delimiting species using single-locus data and the Generalized Mixed Yule Coalescent approach: A revised method and evaluation on simulated data sets. Systematic Biology 62(5): 707–724. 10.1093/sysbio/syt033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaertner J. (1788) Syzygium. De Fructibus et Seminibus Plantarum 1: e166. 10.5962/bhl.title.102753 [DOI]
- Gratwicke B, Marshall BE. (2001) The relationship between the exotic predators Micropterussalmoides and Serranochromisrobustus and native stream fishes in Zimbabwe. Journal of Fish Biology 58(1): 68–75. 10.1111/j.1095-8649.2001.tb00499.x [DOI] [Google Scholar]
- Gratwicke B, Marshall BE, Nhiwatiwa T. (2003) The distribution and relative abundance of stream fishes in the upper Manyame River, Zimbabwe, in relation to land use, pollution and exotic predators. African Journal of Aquatic Science 28(1): 25–34. 10.2989/16085914.2003.9626596 [DOI] [Google Scholar]
- Günther A. (1864) Catalogue of the Physostomi, containing the families Siluridae, Characinidae, Haplochitonidae, Sternoptychidae, Scopelidae, Stomiatidae in the collection of the British Museum. Catalogue of the fishes in the British Museum 5: 315–316. [Google Scholar]
- Günther DA. (1889) On Some Fishes from the Kilimanjaro District. Proceedings of the Zoological Society of London 57 No. 1. Blackwell Publishing Ltd., Oxford, 70–72. 10.1111/j.1469-7998.1889.tb06752.x [DOI]
- Hammer Ø, Harper DAT, Ryan PD. (2001) PAST: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica 4(1): 1–9. [Google Scholar]
- Helfrich P, Rieb E, Abrami G, Lücking A, Mehler A. (2018) TreeAnnotator: versatile visual annotation of hierarchical text relations. Proceedings of the eleventh international conference on language resources and evaluation (LREC 2018).
- Hilgendorf F. (1905) Fische von Deutsch und Englisch Ost-Afrika. Gesammelt von Oscar Neumann 1893–1895. Zoologische Jahrbucher. Systematik 22: 405–420. [Google Scholar]
- Hillis DM, Bull JJ. (1993) An empirical test of bootstraping as a method for assessing confidence in phylogenetic analysis. Systematic Biology 42(2): 182–192. 10.1093/sysbio/42.2.182 [DOI] [Google Scholar]
- Howes GJ. (1980) A New Catfish from Sierra Leone. Bulletin of the British Museum, Natural History. Zoology 38: 165–170. 10.5962/p.12613 [DOI] [Google Scholar]
- Hrbek T, Meliciano NV, Zuanon J, Farias IP. (2018) Remarkable geographic structuring of rheophilic fishes of the lower Araguaia River. Frontiers in Genetics 9(8): 1–12. 10.3389/fgene.2018.00295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joannis L de. (1835) Observations sur les poissons du Nil, et description de plusieurs espèces 24 nouvelles. Magasin de zoologie, 53 pp.
- Jordaan M, Chakona A. (2017) Pseudobarbusburchelli. The IUCN Red List of Threatened Species 2017: e.T107649398A100170338. 10.2305/IUCN.UK.2017-3.RLTS.T107649398A100170338.en [Accessed on 17 October 2023] [DOI]
- Jubb RA, Le Roux P. (1969) Revision of the Chiloglanis (Pisces: Mochokidae) of Southern Africa and descriptions of two new species. Annals of the Cape Provincial Museums 8(2): 13–23. [Natural History] [Google Scholar]
- Kadye WT, Booth AJ. (2020) Environmental niche patterns of native and non-native fishes within an invaded African river system. Journal of Fish Biology 96(5): 1269–1277. 10.1111/jfb.13988 [DOI] [PubMed] [Google Scholar]
- Kadye WT, Chakona A, Marufu LT, Samukange T. (2013) The impact of non-native rainbow trout within Afro-montane streams in eastern Zimbabwe. Hydrobiologia 720(1): 75–88. 10.1007/s10750-013-1624-4 [DOI] [Google Scholar]
- Kambikambi MJ, Kadye WT, Chakona A. (2021) Allopatric differentiation in the Enteromiusanoplus complex in South Africa, with the revalidation of Enteromiuscernuus and Enteromiusoraniensis, and description of a new species, Enteromiusmandelai (Teleostei: Cyprinidae). Journal of Fish Biology 99(3): 931–954. 10.1111/jfb.14780 [DOI] [PubMed] [Google Scholar]
- Kashindye BB, Manda BK, Friel JP, Chakona A, Vreven EJWMN. (2021) A new species of African suckermouth catfish (Teleostei: Mochokidae), from the Upper Congo basin. Ichthyological Exploration of Freshwaters 9902(11): 1–14. [Google Scholar]
- Kumar S, Stecher G, Tamura K. (2016) MEGA7: Molecular evolutionary genetics analysis Version 7.0 for bigger datasets. Molecular Biology and Evolution 33(7): 1870–1874. 10.1093/molbev/msw054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacepède BGE. (1802) Histoire Naturelle des Poissons 4. Chez Plassan Imprimeur-Libraire, Paris, 324–329.
- Maake PA, Gon O, Swartz ER. (2014) Descriptions of three new species of Marcusenius Gill, 1862 (Teleostei: Mormyridae) from South Africa and Mozambique. Zootaxa 3780(3): 455–480. 10.11646/zootaxa.3780.3.2 [DOI] [PubMed] [Google Scholar]
- Marshall BE, Tweddle D. (2007) . Oreochromismortimeri. The IUCN Red List of Threatened Species 2007: e.T63337A12659594. 10.2305/IUCN.UK.2007.RLTS.T63337A12659594.en [Accessed 17 October 2023] [DOI]
- Marshall BE. (2011) The Fishes of Zimbabwe and Their Biology. Smithania Monograph 3. South African Institute for Aquatic Biodiversity (private), Grahamstown, South Africa, 169–175.
- Mazungula DN, Chakona A. (2021) An integrative taxonomic review of the Natal mountain catfish, Amphiliusnatalensis Boulenger 1917 (Siluriformes, Amphiliidae), with description of four new species. Journal of Fish Biology 99(1): 1–21. 10.1111/jfb.14714 [DOI] [PubMed] [Google Scholar]
- Morris J, Ford AGP, Ali JR, Peart CR, Bills R, Day JJ. (2016) High levels of genetic structure and striking phenotypic variability in a sexually dimorphic suckermouth catfish from the African Highveld. Biological Journal of the Linnean Society. Linnean Society of London 117(3): 528–546. 10.1111/bij.12650 [DOI] [Google Scholar]
- Mutizwa TI, Kadye WT, Chakona A. (2021) Deep genetic and morphological divergence in the Hippopotamyrusansorgii species complex (Teleostei: Mormyridae) in southern Africa. Journal of Fish Biology 99(2): 1–14. 10.1111/jfb.14743 [DOI] [PubMed] [Google Scholar]
- Nagy B, Watters B. (2019) Nothobranchiusmkuziensis. The IUCN Red List of Threatened Species 2019: e.T131471491A131471537. 10.2305/IUCN.UK.2019-3.RLTS.T131471491A131471537.en [Accessed 17 October 2023] [DOI]
- Nichols JT, Griscom L. (1917) Freshwater fishes of the Congo Basin obtained by the American Museum Congo Expedition, 1909–1915. Bulletin of the American Museum of Natural History 37: 64–83. [Google Scholar]
- Nichols JT, La Monte FR, Callewaert R. (1934) More new fishes from the Kasai District of the Belgian Congo. American Museum novitates 723: 1–6. [Google Scholar]
- Paugy D, Lévêque C, Teugels GG. (2003) Poissons d’eaux douces et saumâtres de l’Afrique de l’Ouest, édition complète. (IRD-MNHN-M, Vol. 815). Paris-Turvuren.
- Pellegrin J. (1933) Voyage de Ch. Alluaud et PA Chappuis en Afrique occidentale Française (Dec. 1930-Mars 1931). Poissons. Archiv für Hydrobiologie 26: 101–120. [Google Scholar]
- Pellegrin J. (1936) Contribution à l’ichthyologie de l’Angola. Arquivos do Museu Bocage 7: 45–62. [Google Scholar]
- Peters WCH. (1852) Diagnosen von neuen Flussfischen aus Mossambique. Bericht Über Die Zur Bekanntmachung Geeigneten Verhandlungen Der Königlichen Preussischen Akademie Der Wissenschaften Zu Berlin, 681–685.
- Peters WCH. (1868) Ueber eine von dem Baron Carl von der Decken entdeckte neue Gattung von Welsen, Chiloglanisdeckenii, und einige andere Süsswasserfische aus Ostafrika. Monatsberichte der Königlichen Preussischen Akademie der Wissenschaften zu Berlin 1868: 598–602. [Google Scholar]
- Pinton A, Agnèse JF, Paugy D, Otero O. (2013) A large-scale phylogeny of Synodontis (Mochokidae, Siluriformes) reveals the influence of geological events on continental diversity during the Cenozoic. Molecular Phylogenetics and Evolution 66(3): 1027–1040. 10.1016/j.ympev.2012.12.009 [DOI] [PubMed] [Google Scholar]
- Pfeffer GJ. (1889) Übersicht der von Herrn Dr. Franz Stuhlmann in Ägypten, auf Sansibar und dem gegenüberliegenden Festlande gesammelten Reptilien, Amphibien, Fische, Mollusken und Krebse. Jahrbuch Der Hamburgischen Wissenschaftlichen Anstalten 6(4): 1–36. [Google Scholar]
- Poll M. (1952) Poissons de rivières de la région des lacs Tanganika et Kivu recuellis par G. Marlier. Revue de Zoologie et de Botanique Africaines 46(3–4): 221–236. [Google Scholar]
- Pons J, Barraclough TG, Gomez-Zurita J, Cardoso A, Duran DP, Hazell S, Hazell S, Kamoun S, Sumlin WD, Vogler AP. (2006) Sequence-based species delimitation for the DNA taxonomy of undescribed insects. Systematic Biology 55(4): 595–609. 10.1080/10635150600852011 [DOI] [PubMed] [Google Scholar]
- Puillandre N, Lambert A, Brouillet S, Achaz G. (2012) ABGD, Automatic Barcode Gap Discovery for primary species delimitation. Molecular Ecology 21(8): 1864–1877. 10.1111/j.1365-294X.2011.05239.x [DOI] [PubMed] [Google Scholar]
- Puillandre N, Brouillet S, Achaz G. (2021) ASAP: assemble species by automatic partitioning. Molecular Ecology Resources 21(2): 609–620. 10.1111/1755-0998.13281 [DOI] [PubMed] [Google Scholar]
- R Core Team (2018) R: A language and environment for statistical computing. R Foundation for Statistical 308 Computing, Vienna. https://www.R-project.org/
- Rambaut AR, Drummond AJ, Dong X, Baele G, Suchard MA. (2018) Posterior Summarization in Bayesian Phylogenetics Using Tracer 1.7. Systematic Biology 67(5): 901–904. 10.1093/sysbio/syy032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddin MA, Bills R, Villet MH. (2016) Phylogeographic, morphometric and taxonomic re-evaluation of the river sardine, Mesobolabrevianalis (Boulenger, 1908) (Teleostei, Cyprinidae, Chedrini). ZooKeys 641: 121–150. 10.3897/zookeys.641.10434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts TR. (1989) Systematic revision and description of new species of suckermouth catfishes (Chiloglanis, Mochokidae) from Cameroun. Proceedings of the California Academy of Sciences 46(6): 151–178. [Google Scholar]
- Ronquist F, Teslenko M, Van Der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. (2012) Mrbayes 3.2: Efficient bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61(3): 539–542. 10.1093/sysbio/sys029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux F, Hoffman A. (2017a) Chiloglanisbifurcus. The IUCN Red List of Threatened Species 2017: e.T4632A100193958. 10.2305/IUCN.UK.2017-3.RLTS. T4632A1001 93958.en [DOI]
- Roux F, Hoffman A. (2017b) Enteromiustreurensis. The IUCN Red List of Threatened Species 2017: e.T2572A100159826. 10.2305/IUCN.UK.2017-3.RLTS.T2572A100159826.en [Accessed 17 October 2023] [DOI]
- Roux F, Hoffman A. (2018) Chiloglanisemarginatus. The IUCN Red List of Threatened Species 2018: e.T63366A100194297. 10.2305/IUCN.UK.2018-1.RLTS.T63366A100194297.en [DOI]
- Rozas J, Ferrer-Mata A, Sánchez-DelBarrio JC, Guirao-Rico S, Librado P, Ramos-Onsins SE, Sánchez-Gracia A. (2017) DnaSP 6: DNA Sequence Polymorphism Analysis of Large Data Sets. Molecular Biology and Evolution 34(12): 3299–3302. 10.1093/molbev/msx248 [DOI] [PubMed] [Google Scholar]
- Sauvage HE. (1879) Notice sur la faune ichthyologique de l’Ogôoué. Bulletin de la Société philomathique de Paris (7th Série) 3: 90–103.
- Schedel FDB, Chakona A, Sidlauskas BL, Popoola MO, Wingi NU, Neumann D, Vreven EJWMN, Schliewen UK. (2022) New phylogenetic insights into the African catfish families Mochokidae and Austroglanididae. Journal of Fish Biology 100(5): 1171–1186. 10.1111/jfb.15014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt RC, Bart HL, Nyingi WD. (2015) Two new species of African suckermouth catfishes, genus Chiloglanis (Siluriformes: Mochokidae), from Kenya with remarks on other taxa from the area. Zootaxa 4044(1): 45–64. 10.11646/zootaxa.4044.1.2 [DOI] [PubMed] [Google Scholar]
- Schmidt RC, Bart Jr HL, Pezold F. (2016) High levels of endemism in suckermouth catfishes (Mochokidae: Chiloglanis) from the Upper Guinean forests of West Africa. Molecular Phylogenetics and Evolution 100: 199–205. 10.1016/j.ympev.2016.04.018 [DOI] [PubMed] [Google Scholar]
- Schmidt RC, Bart Jr HL, Pezold F, Friel JP. (2017) A Biodiversity hotspot heats up: nine new species of suckermouth catfishes (Mochokidae: Chiloglanis) from Upper Guinean Forest streams in West Africa. Copeia 105(2): 301–338. 10.1643/CI-16-474 [DOI] [Google Scholar]
- Schmidt RC, Barrientos C. (2019) A new species of suckermouth catfish (Mochokidae: Chiloglanis) from the Rio Mongo in Equatorial Guinea. Zootaxa 4652(3): 507–519. 10.11646/zootaxa.4652.3.7 [DOI] [PubMed] [Google Scholar]
- Schmidt RC, Bragança PH, Friel JP, Pezold F, Tweddle D, Bart HL. (2023) Two New Species of Suckermouth Catfishes (Mochokidae: Chiloglanis) from Upper Guinean Forest Streams in West Africa. Ichthyology & Herpetology 111(3): 376–389. 10.1643/i2022067 [DOI] [Google Scholar]
- Seegers L. (2008) The catfishes of Africa: a handbook for identification and maintenance. Aqualog.
- Seegers L. (1996) . The fishes of the Lake Rukwa drainage. Annales-Musee Royal de l’Afrique Centrale. Sciences Zoologiques (Belgium) ISSN 0770-4666, 213–236.
- Silvestro D, Michalak I. (2012) RaxmlGUI: A graphical front-end for RAxML. Organisms, Diversity & Evolution 12(4): 335–337. 10.1007/s13127-011-0056-0 [DOI] [Google Scholar]
- Skelton PH. (2001) A Complete Guide To The Freshwater Fishes Of Southern Africa. Struik, Cape Town, South Africa, 241–242.
- Skelton PH, White PN. (1990) Two new species of Synodontis (Pisces: Siluroidei: Mochokidae) from southern Africa. Ichthyological Exploration of Freshwaters 1(3): 277–287. [Google Scholar]
- Smith A. (1840) Pisces. In Illustrations of the zoology of South Africa; consisting chiefly of figures and descriptions of the objects of natural history collected during an expedition into the interior of South Africa in 1834–36. Journal of the Society for the Bibliography of Natural History 2(6): 187–189. [Google Scholar]
- Smith A. (1841) Pisces. In Illustrations of the zoology of South Africa; consisting chiefly of figures and descriptions of the objects of natural history collected during an expedition into the interior of South Africa in 1834–36. Journal of the Society for the Bibliography of Natural History 2(6): 187–189. [Google Scholar]
- Stamatakis A. (2014) RAxML Version 8: A tool for Phylogenetic Analysis and Post-Analysis of Large Phylogenies. 10.1093/bioinformatics/btu033 [DOI] [PMC free article] [PubMed]
- Swofford DL. (2003) PAUP*. Phylogenetic Analysis Using Parsimony (*and other Methods) Version 4. Sinauer Associates, Sunderland.
- Sullivan JP, Lundberg JG, Hardman MA. (2006) A phylogenetic analysis of the major groups of catfishes (Teleostei: Siluriformes) using rag1 and rag2 nuclear gene sequences. Molecular Phylogenetics and Evolution 41(3): 636–662. 10.1016/j.ympev.2006.05.044 [DOI] [PubMed] [Google Scholar]
- Sunnucks P, Hales DF. (1996) Numerous transposed sequences of mitochondrial cytochrome oxidase I-II in aphids of the genus Sitobio (Hemiptera: Aphidae). Molecular Biology and Evolution 13(3): 510–524. 10.1093/oxfordjournals.molbev.a025612 [DOI] [PubMed] [Google Scholar]
- Thompson DM. (2013) Pool-Riffle. In Treatise on Geomorphology 9: 364–378. 10.1016/B978-0-12-374739-6.00246-3 [DOI] [Google Scholar]
- Thomson AW, Page LM. (2010) Taxonomic revision of the Amphiliusuranoscopus group (Teleostei: Siluriformes) in Kenya, with the description of a new species from the Athi River. Bulletin of the Florida Museum of Natural History 49(2): 45–66. [Google Scholar]
- Thomson AW, Page LM, Hilber SA. (2015) Revision of the Amphiliusjacksonii complex (Siluriformes: Amphiliidae), with the descriptions of five new species. Zootaxa 3986(1): 61–87. 10.11646/zootaxa.3986.1.3 [DOI] [PubMed] [Google Scholar]
- Van der Horst CJ. (1931) Some South African siluroid fishes. Annals of the Transvaal Museum XIV(3): 245–250.
- Vigliotta TR. (2008) A phylogenetic study of the African catfish family Mochokidae (Osteichthyes, Ostariophysi, Siluriformes), with a key to genera. Proceedings of the Academy of Natural Sciences of Philadelphia 157(1): 73–136. 10.1635/0097-3157(2008)157[73:APSOTA]2.0.CO;2 [DOI]
- Ward RD, Zemlak TS, Innes BH, Last PR, Hebert PDN. (2005) DNA barcoding Australia’s fish species. Philosophical Transactions of the Royal Society of London, Series B, Biological Sciences 360(1462): 1847–1857. 10.1098/rstb.2005.1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werle E, Schneider C, Renner M, Volker M, Fiehn W. (1994) Convenient single-step, one tube purification of PCR products for direct sequencing. Nucleic Acids Research 22(20): 4354–4355. 10.1093/nar/22.20.4354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild H. (1965) The flora of the Great Dyke of Southern Rhodesia with special reference to the sepentine soils. Kirkia 5(1): 49–86. [Google Scholar]
- Zhang J, Kapli P, Pavlidis P, Stamatakis A. (2013) A general species delimitation method with applications to phylogenetic placements. Bioinformatics 29(22): 2869–2876. 10.1093/bioinformatics/btt499 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All of the data that support the findings of this study are available in the main text.