Abstract
Background
Technological devices such as smartphones, wearables and virtual assistants enable health data collection, serving as digital alternatives to conventional biomarkers. We aimed to provide a systematic overview of emerging literature on ‘digital biomarkers,’ covering definitions, features and citations in biomedical research.
Methods
We analysed all articles in PubMed that used ‘digital biomarker(s)’ in title or abstract, considering any study involving humans and any review, editorial, perspective or opinion-based articles up to 8 March 2023. We systematically extracted characteristics of publications and research studies, and any definitions and features of ‘digital biomarkers’ mentioned. We described the most influential literature on digital biomarkers and their definitions using thematic categorisations of definitions considering the Food and Drug Administration Biomarkers, EndpointS and other Tools framework (ie, data type, data collection method, purpose of biomarker), analysing structural similarity of definitions by performing text and citation analyses.
Results
We identified 415 articles using ‘digital biomarker’ between 2014 and 2023 (median 2021). The majority (283 articles; 68%) were primary research. Notably, 287 articles (69%) did not provide a definition of digital biomarkers. Among the 128 articles with definitions, there were 127 different ones. Of these, 78 considered data collection, 56 data type, 50 purpose and 23 included all three components. Those 128 articles with a definition had a median of 6 citations, with the top 10 each presenting distinct definitions.
Conclusions
The definitions of digital biomarkers vary significantly, indicating a lack of consensus in this emerging field. Our overview highlights key defining characteristics, which could guide the development of a more harmonised accepted definition.
Keywords: Medical Informatics
Introduction
Biomarkers are defined as a set of characteristics that are objectively measured and used as indicators of normal biological processes, pathogenic processes or biological responses that appear due to exposure or therapeutic interventions.1 This comprises physiological, molecular, histologic and radiographic measurements.2 The US Food and Drug Administration (FDA) subclassifies susceptible/risk, diagnostic, monitoring, prognostic, predictive, response and safety biomarkers.1 They highlight that a full biomarker description must include the source or matrix, the measurable characteristic(s) and the methods used to measure the biomarker.1 The digitalisation of our world impacting daily living and healthcare broadens the spectrum of the possible source and methods used to measure biomarkers and introduces a novel dimension of measurable characteristics. This allows digital devices used daily, such as smartphones, wearable devices, sensors and smart home devices, to provide a new category of biomarkers, often called ‘digital biomarkers’. In recent years, digital biomarkers became increasingly present in routine care and in research in many areas of medicine, such as cardiology, oncology or COVID-19. For example, smartphone recorded cough sounds have been used as a digital biomarker to detect asthma and respiratory infections in clinical trials,3 4 or deep learning was applied to data from a three-axis accelerometer to predict sleep/wake patterns.4 5 Moreover, such digital biomarkers have spread in the field of neurology, which has a large unmet need for non-invasive and objective biomarkers reflecting cognitive and motor functions that are traditionally assessed with specific tests performed by neurologists.6 Beyond monitoring health and disease status, predicting the occurrence and development of diseases would be promising applications of such novel approaches.7
Thus, digital biomarkers have the potential to offer valuable insights on the health of patients. They usually have high temporal resolution (up to (quasi-)continuous), are usually objective (and not subject to interobserver variability) and can have high external validity as they may be applied in the patient’s routine environment (as opposed to, eg, the clinic or a research environment).8
Many everyday digital tools used mainly for entertainment/leisure purposes (eg, fitness trackers) are increasingly considered as a source of helpful information that may be transformed into digital biomarkers. Yet, with all this diversity in application and complex interaction with rapidly evolving technology, it becomes necessary to provide a clear and precise definition of the fundamental underlying concepts to facilitate research and decision-making with and on these novel approaches.
One of the first definitions of this novel type of biomarker was provided by Dorsey et al, who defined digital biomarkers as ‘the use of a biosensor to collect objective data on a biological (eg, blood glucose, serum sodium), anatomical (eg, mole size) or physiological (eg, heart rate, blood pressure) parameter obtained using sensors followed by algorithms to transform these data into interpretable outcome measures, helping to address many of the shortcomings in current measures.’ Furthermore, they stated that these new measures ‘include portable (eg, smartphones), wearable, and implantable devices, and are by their nature largely independent of raters.’9 A later definition given in 2020 by the European Medicines Agency (EMA) was based on ‘digital measures’ (‘measured through digital tools’) and did not include the requirement of algorithms as a defining feature: ‘a digital biomarker is an objective, quantifiable measure of physiology and/or behaviour used as an indicator of biological, pathological process or response to an exposure or an intervention that is derived from a digital measure. […]’)10
Others gave broader definitions including further defining features, for example, defining digital biomarkers as ‘objective, quantifiable, quantitative, physiological and behavioural data that are collected and measured by means of digital devices such as portables, wearables, implantables or digestibles. The data collected are used to explain, influence and/or predict health-related outcomes’.2 6 11
Overall, such a disagreement between definitions used by regulators and in articles published in high-impact biomedical journals raised concerns that no clear consensus exists among researchers and users of this novel approach and terminology, increasing the risk for miscommunication. There are numerous examples where differences in definitions have been recognised as critical cause of inefficiencies and delay in health research and avoidable controversy, uncertainty and potential harm in clinical care and public health.12–15 The Biomarkers, EndpointS and other Tools (BEST) framework developed by the FDA and US National Institutes of Health with ‘the goals of improving communication, aligning expectations, and improving scientific understanding’ highlights that ‘unclear definitions and inconsistent use of key terms can hinder the evaluation and interpretation of scientific evidence and may pose significant obstacles to medical product development programmes’.1We aimed to provide a systematic overview of the emerging literature on digital biomarkers and characterisation of the definitions of digital biomarkers that are provided in biomedical journal articles by performing a systematic mapping and citation analysis of all articles that prominently used the term ‘digital biomarker’. We sought to determine differences in characteristics of common definitions to provide a foundation for subsequent activities to develop clearer and consistent definitions that ensure improved application of digital biomarkers in research and healthcare decision-making.
Methods
Design
We analysed all articles published at any time in PubMed that prominently used the term ‘digital biomarker’, that is, either in title or abstract.
We systematically explored definitions of digital biomarkers that are provided and/or referred to in the biomedical literature, that is, journal articles that are indexed in PubMed, in a mapping review without a formal assessment of included studies.16 We structured our review report to the ‘Preferred Reporting Items for Systematic Reviews and Meta-Analyses’ guidance, where applicable.17 We did not use a prespecified protocol.
Eligibility criteria, information source and search strategy
We searched PubMed and included all articles mentioning ‘digital biomarker’ or ‘digital biomarkers’ in their title or abstract (by searching PubMed for ‘digital biomarker*(tiab)’; date of last search: 8 March 2023). We excluded animal research.
Study selection
One reviewer (AKMA) screened titles, abstracts and full texts for eligibility. Confirmation by a second reviewer (JH or LGH) was planned for situations where the reviewer was unsure, but this case never occurred given the clear and objective selection criteria.
Data extraction
We developed a spreadsheet to structure the data extraction process. One reviewer (AKMA) extracted data with confirmation by a second reviewer (JH or LGH) in case of any uncertainty.
We extracted from every article: author(s), publication year, title, journal, corresponding author, and country of correspondence, article type (ie, primary research, review or other type (eg, editorial, comment, opinion-based letter)). Of primary research articles, we additionally extracted definitions of digital biomarkers that are provided and/or referred to (based on a semantic search for indicators of definition such as ‘digital biomarkers are’, ‘… are defined as’, ‘… can be defined’, ‘the definition of … is’), medical context, and whether the article is about the development and/or validation of a digital biomarker. The number of global citations was obtained by using metadata from OpenAlex18; accessed via the Local Citation Network19 (as of 26 June 2023).
Data analysis and categorisation of definition components
We considered the BEST framework to derive components of definitions for digital biomarkers.1 We analysed the identified digital biomarker definitions by assessing if they contained descriptions that fall within three key components, that is, the (1) type of data that is measured (eg, whether data were measured objectively, continuously or quantitatively), (2) data collection method (eg, whether sensors, computers, portables, wearables, implantables or digestibles were used to collect data) and (3) purpose of the digital biomarker (eg, whether a biomarker was used as measure of disease progression or to predict health-related outcomes). We defined definitions as duplicates when they used the same sequence of words. We illustrate the frequency of various terminologies used in all provided definitions with a word cloud.20 We analysed the structural similarity of definitions that were provided without a reference by performing hierarchical clustering on the distance-matrix containing pairwise ‘Indel’-distances, that is, ‘the minimum number of insertions and deletions required to change one (definition) into the other’.21 Since we aimed at exploring how digital biomarkers are defined in the biomedical literature, we did not critically assess the included articles and studies. For the analysis of citations, we calculated the quotient of number of global citations (retrieved by the Local Citation Network19) and years since publication per article. To create a citation network of citing and cited relationships between the articles, we used the Local Citation Network with the OpenAlex scholarly index.19 22
We used descriptive statistics by reporting numbers and percentages. For all analyses, we used R (V.4.2.2) or Python (V.3.11.4).
Results
We identified 415 articles that had ‘digital biomarker’ in their title or abstract (online supplemental S1). The first article was published in 2014 (median publication year 2021; figure 1; online supplemental S2). Most articles described primary studies (n=283; 68%) and were published in digital medicine specialty journals, including Digital Biomarkers (n=35; 8%), Journal of Medical Internet Research (n=21; 5%) or npj Digital Medicine (n=19; 4%; table 1). Of the 415 articles, 128 (31%) provided at least 1 definition of a digital biomarker.
Figure 1.
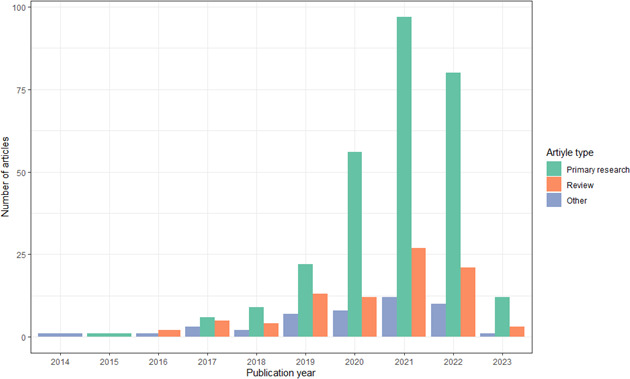
The annual number of published article types referring to digital biomarkers as of 8 March 2023 (n=415).
Table 1.
Characteristics of all 415 articles in PubMed using ‘digital biomarker’ in title or abstract
| All articles (n=415) | Articles with a definition of digital biomarker (n=128) | |
| n (%) | n (%) | |
| Publication year: median, range | 2020, 2014–2023 | 2021, 2015–2023 |
| Type of articles | ||
| Primary research | 283 (68.2) | 59 (46.1) |
| Development of a digital biomarker* | – | 53 (41.4) |
| Medical context | ||
| Neurology | – | 25 (19.5) |
| Cardiology | – | 3 (2.3) |
| Endocrinology | – | 3 (2.3) |
| Geriatrics | – | 3 (2.3) |
| Psychiatry | – | 3 (2.3) |
| Sleep medicine | – | 3 (2.3) |
| Infectiology | – | 2 (1.6) |
| Oncology | – | 2 (1.6) |
| Psychology | – | 2 (1.6) |
| Rheumatology | – | 2 (1.6) |
| Addiction medicine | – | 1 (0.8) |
| Not specified | – | 7 (5.5) |
| Disease specific | ||
| Dementia/MCI/CI | – | 16 (12.5) |
| Parkinson’s disease | – | 5 (3.9) |
| Diabetes | – | 3 (2.3) |
| Alcohol use disorder | – | 2 (1.6) |
| Arthritis | 2 (1.6) | |
| COVID-19 | – | 2 (1.6) |
| Multiple sclerosis | – | 2 (1.6) |
| Not specified | – | 14 (10.9) |
| Others* | – | 8 (6.2) |
| Validation of a digital biomarker† | – | 35 (27.3) |
| Reviews | 87 (21.0) | 50 (39.1) |
| Editorials, opinions, perspectives, etc | 45 (10.8) | 19 (14.8) |
| Journals | ||
| Digital Biomarkers | 35 (8.4) | 15 (11.7) |
| Journal of Medical Internet Research | 21 (5.1) | 5 (3.9) |
| npj Digital Medicine | 19 (4.6) | 8 (6.3) |
| Sensors (Basel, Switzerland) | 18 (4.3) | 2 (1.6) |
| Frontiers in Digital Health | 16 (3.8) | 9 (7.0) |
| JMIR mHealth and uHealth | 14 (3.4) | 7 (5.5) |
| Scientific Reports | 12 (2.9) | – |
| Frontiers in Psychiatry | 10 (2.4) | 6 (4.7) |
| Other | 270 (65.0)† | 76 (59.4)‡ |
| Affiliated country of corresponding authors§ | ||
| USA | – | 69 (53.9) |
| Switzerland | – | 22 (17.2) |
| Germany | – | 16 (12.5) |
| UK | – | 16 (12.6) |
| Canada | – | 11 (8.6) |
| France | – | 10 (7.8) |
| Other | – | 90 (70.3) |
All extracted data are provided in online supplemental S2.
*Fewer than 2 articles.
†Fewer than 10 articles.
‡Fewer than 5 articles.
§More than 1 category possible.
.MCI, mild cognitive impairment.
bmjhci-2023-100914supp001.pdf (392.5KB, pdf)
Characteristics of articles providing a definition of digital biomarker
The 128 articles with a definition of digital biomarker were published between 2015 and 2023 (median: 2021). Of them, 59 articles were primary studies, 50 were reviews and 19 were other types of articles (table 1).
Almost all primary studies described the development of one or more digital biomarkers (53 of 59 articles), and many described a validation process of biomarkers (35 of 59 articles). The most frequent medical field of the primary research articles that described the development of one or more digital biomarkers was neurology (25 of 53), while the spectrum of medical fields was overall very wide (table 1). The most frequent diseases were dementia and related disorders (16 of 53 articles, ie, (mild) cognitive impairment or Alzheimer’s disease), Parkinson’s disease (5 of 53 articles) and diabetes (3 of 53 articles), with numerous other conditions addressed in one or two studies (eg, atrial fibrillation, cervical cancer, depression, heart failure and muscular dystrophy; online supplemental S2).
The corresponding authors were mostly from the USA (69 of 128 articles), Switzerland (22 of 128 articles), Germany (16 of 128 articles) and the UK (16 of 128 articles; table 1).
The articles were cited a median of 6 times (range 0–517, IQR 2–20, overall 2,705); on average two times per year (range 0–86, IQR 1–5; online supplemental S2). We show the citation network (ie, citing and cited relationships within the sample of these 128 articles) online (https://LocalCitationNetwork.github.io/?fromJSON=Digital-Biomarker-Definitions.json).
Definitions of digital biomarkers
Overall, 128 articles reported between 1 and 7 definitions (median 1, IQR 1–2). In 91 articles, at least 1 reference was provided for these definitions made by the authors (median 1, range 1–13, IQR 1–2, overall 274 references); for 37 articles with 51 definitions, no reference was provided (online supplemental S2).
The mostly used references to support the definitions were Coravos et al4 (referenced by 51 of 91 articles); Dorsey et al9 (11 articles); Califf23 (9 articles); Piau et al24 (9 articles); Babrak et al6 (8 articles) and Coravos et al25 (8 articles). All these articles were among the 415 articles analysed here. The original definitions in these top-cited articles can be found in table 2. Other references were used by less than five articles.
Table 2.
The top cited definitions of Digital Biomarkers within the 415 articles
| Authors (year); reference | Number of articles citing this definition in the 415 articles | Definition (original quote) |
| Coravos et al4 | 51 | ‘We describe an emerging class of biomarker, the “digital biomarker”, which has important implications for both clinical trials and clinical care. “Digital” refers to the method of collection as using sensors and computational tools, generally across multiple layers of hardware and software. The measurements are often made outside the physical confines of the clinical environment using home-based connected products including wearable, implantable, and ingestible devices, and sensors. Digital biomarkers span a broad range of diagnostic and prognostic measurements.’ |
| Dorsey et al9 | 11 | ‘Digital biomarkers—the use of a biosensor to collect objective data on a biological (eg, blood glucose, serum sodium), anatomical (eg, mole size), or physiological (eg, heart rate, blood pressure) parameter followed by the use of algorithms to transform these data into interpretable outcome measures can help address many of the shortcomings in current measures. These new measures, which include portable (eg, smartphones), wearable, and implantable devices, are by their nature largely independent of raters. They are, therefore, not prone to rater bias. The goal of digital biomarkers is to maximize the ecological validity and temporal and spatial resolution of capturing motor and nonmotor phenomena that are expected to change over time.’ |
| Piau et al24 | 9 | ‘Digital biomarkers are defined here as objective, quantifiable, physiological, and behavioral data that are collected and measured by means of digital devices, such as embedded environmental sensors, portables, wearables, implantables, or digestibles. Digital biomarkers allow objective, ecologically valid, long-term follow-up with frequent or continuous assessment that can be minimally obtrusive or function in the background of everyday activity.’ Klicken oder tippen Sie hier, um Text einzugeben. |
| Babrak et al6 | 8 | ‘Digital biomarkers are objective, quantifiable, physiological, and behavioral measures that are collected by means of digital devices that are portable, wearable, implantable, or digestible. These data are often used to explain, influence, and/or predict health-related outcomes. Digital biomarkers fall within the scope of traditional biomarkers in relation to addressing health related questions, with use of a digital and portable technology that adds new dimensions, unique features, and challenges. digital biomarkers are usually less or non-invasive, modular, and often cheaper to measure. They can produce qualitative and quantitative measurements, but most importantly, they provide easier and cheaper access to continuous and longitudinal measurements.’ |
| Califf23 | 8 | ‘… digital biomarkers derived from sensors and mobile technologies. …these data are in large part derived from new sources including smartphones and wearable electronic devices and facilitated by novel technologies that allow for the streaming and storage of complex data, standards for evaluating these biomarkers are just now developing.’ |
| Coravos et al25 | 8 | ‘A digital biomarker could be any of the seven BEST biomarker types. The term digital refers to the method of collection as using sensors and computational tools, generally across multiple layers (eg, a full stack) of hardware and software.’ |
In total, the 128 articles reported 202 definitions; 75 of which were duplicates. Hence, we identified 127 unique definitions across the 128 articles.
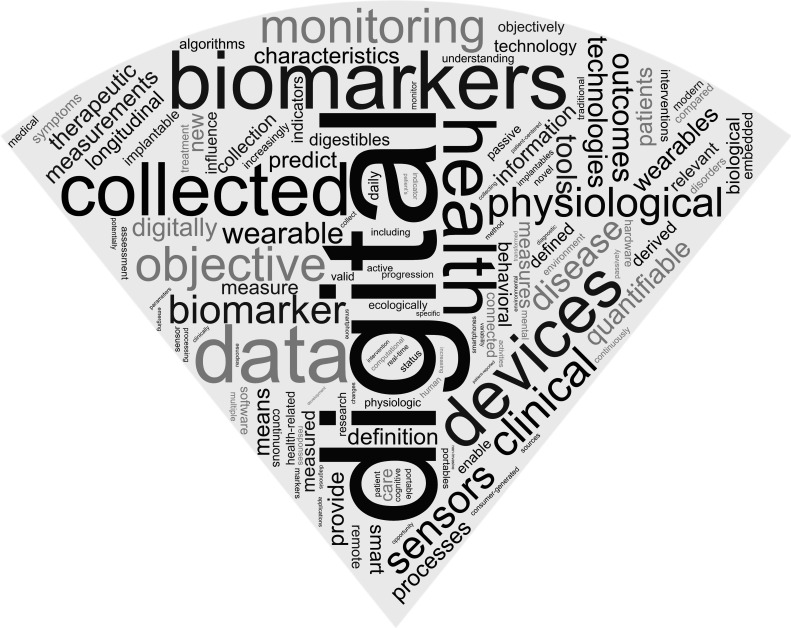
The 10 most frequently used terms that most of the 127 unique definitions contained were ‘digital’ (125 of 127 definitions; 98%), ‘biomarkers’ (109 of 127 definitions; 85%), ‘data’ (62 of 127 definitions; 48%), ‘collected’ (55 of 127 definitions; 43%), ‘devices’ (50 of 127 definitions; 39%), ‘health’ (42 of 127 definitions; 33%), ‘physiological’ (37 of 127 definitions; 29%), ‘objective’ (37 of 127 definitions; 29%), ‘wearable’ (34 of 127 definitions; 26%) and ‘behavioural’ (33 from 127 definitions; 25%; figure 2).
Figure 2.
Word cloud with the most frequently used terms in the analysed digital biomarker(s) definitions.
Of the 127 unique definitions, 56 definitions refer to the type of data that are collected, 78 definitions contain information on the data collection method, and 50 definitions provide information on the purpose of the digital biomarker. Only 23 of 127 definitions involve all 3 components and 26 contain none of these components (table 3; online supplemental S3; online supplemental S2).
Table 3.
Definitions of digital biomarkers that include three key components: type of data, data collection method and purpose of a digital biomarker (n=23)
| Authors (year), reference | Definition (original quote) | Three key components classification: (1) type of data, (2) data collection method and (3) intended use/purpose |
| Andrade et al32 | ‘Digital biomarkers may have a place as an objective, accurate, and low-cost patient metric to support risk stratification and clinical planning. Digital biomarkers use digital information to objectively measure biological and pathological processes and have the potential to overcome some of the above-mentioned limitations of conventional prognostic tools. Digital data, in particular data from accelerometers and other wearable sensors, are a non-invasive, passively collected low-cost source of individual information. Further exploration of clinical uses for these data may improve clinical decision-making with minimal risk and cost.’ |
|
| Babrak et al6 | ‘Digital biomarkers are objective, quantifiable, physiological, and behavioral measures that are collected by means of digital devices that are portable, wearable, implantable, or digestible. These data are often used to explain, influence, and/or predict health-related outcomes. Digital biomarkers fall within the scope of traditional biomarkers in relation to addressing health related questions, with use of a digital and portable technology that adds new dimensions, unique features, and challenges. digital biomarkers are usually less or non-invasive, modular, and often cheaper to measure. They can produce qualitative and quantitative measurements, but most importantly, they provide easier and cheaper access to continuous and longitudinal measurements.’ |
|
| Bartolome and Prioleau33 | ‘Digital biomarkers refer to objective, quantifiable physiological, and behavioral measures that are collected by means of digital devices, such as wearable devices, for the purpose of outcomes explaining, influencing, or predicting health. However, unlike traditional biomarkers that provide a “snapshot view” based on limited measurements collected over time, digital biomarkers are often derived from longitudinal and continuous measurements, and thus can capture dynamic changes in health and related outcomes.’ |
|
| Bijlani et al, Nam et al, Parziale and Mascalzoni, Phillips et al, and Wright and Jones34–38 | ‘Digital biomarkers are consumer-generated physiological and behavioral measures collected through connected digital tools that can be used to explain, influence and/or predict health-related outcomes. Health-related outcomes can vary from explaining disease to predicting drug response to influencing fitness behaviors. In our definition of digital biomarkers, we exclude patient-reported measures (eg, survey data), genetic information, and data collected through traditional medical devices and equipment. These data types, though still a key component of research and clinical care that may be stored digitally, are not digitally measured or truly dependent on software.’ |
|
| Dillenseger et al39 | ‘… digital biomarkers—digital health technologies— to explain, influence and/or predict health-related outcomes. Digital biomarkers stem is quite broad, and range from wearables that collect patients’ activity during digitalized functional tests to digitalized diagnostic procedures and software-supported magnetic resonance imaging evaluation. With the increasing digitalization of healthcare, medicine now gains access to a new type of biomarker. So-called digital biomarkers enable the translation of up-to-date new data sources into informative, actionable knowledge. Digital biomarkers are basically collected by digital tools. Digital biomarkers mean objective, quantifiable physiological and behavioral data that are measured and collected by digital devices. The data collected by, for example, portables, wearables, implantables or digestibles are typically used to generate, influence and/or predict health-related outcomes, and thus represent deep digital phenotyping, collecting clinically meaningful and objective digital data.’ |
|
| Dorsey et al9 | ‘Digital biomarkers—the use of a biosensor to collect objective data on a biological (eg, blood glucose, serum sodium), anatomical (eg, mole size), or physiological (eg, heart rate, blood pressure) parameter followed by the use of algorithms to transform these data into interpretable outcome measures can help address many of the shortcomings in current measures. These new measures, which include portable (eg, smartphones), wearable, and implantable devices, are by their nature largely independent of raters. They are, therefore, not prone to rater bias. The goal of digital biomarkers is to maximize the ecological validity and temporal and spatial resolution of capturing motor and nonmotor phenomena that are expected to change over time.’ |
|
| Gielis et al40 | ‘Complementary to their biological counterparts, digital biomarkers are “user-generated physiological and behavioral measures collected through connected digital devices to explain, influence and/or predict health-related outcomes.’ |
|
| Harms et al41 | ‘Digital biomarkers are defined as objective, quantifiable physiological and behavioral data that are collected and measured by means of digital devices. Their use has revolutionized clinical research by enabling high-frequency, longitudinal, and sensitive measurements. Digital biomarkers are that the latter are collected via digital devices and can be collected outside of traditional clinical settings. The digital devices collecting these biomarkers can include wearables, implantables, ingestible devices, and smartphones and tablets. Examples of digital biomarkers are objective consumer-grade data such as voice, temperature, activity, gait, blood oxygen, heart rate, touch, and augmented reality, all collected via mobile and wearable technologies. As opposed to standard clinical measures, digital biomarkers enable high-frequency, longitudinal, and objective measurements, largely independent of the clinical rater. Digital biomarkers can continuously monitor patients to assess therapy response and disease progression without the need for clinical assessment. Moreover, they often exhibit higher sensitivity than traditional clinically used methods, enabling early predictive diagnostics by identifying patients at risk of overt clinical disease.’ |
|
| Hartl et al42 | ‘Digital biomarkers are defined as physiological and behavioral measures collected via digital devices (such as portables, wearables, implantables and digestibles) that characterize, influence, or predict health-related outcomes. Digital biomarkers offer several potential advantages compared to traditional clinical assessments. Digital biomarker products are usually the result of the combination of multiple individual hardware (sensors) and software (operating systems and algorithms) components. Digital biomarkers as clinical endpoints provide objective and quantitative measures yet still require broader clinical use and health authority acceptance.’ |
|
| Hartl et al42 | ‘Digital biomarkers: Physiological and behavioral measures collected by means of digital devices such as portables, wearables, implantables, or digestibles that characterize, influence, or predict health-related outcomes.’ |
|
| Katsaros et al43 | ‘Digital biomarkers are objective measurements of physiological, pathologic, or anatomic characteristics continuously collected outside the clinical environment via home-based connected devices. Passively collecting data from patients’ mobile or wearable devices potentially offers a convenient and unobtrusive method to prospectively identify psychosocial burden and deliver tailored social support to the right patients at the right time.’ |
|
| Motahari-Nezhad et al44 | ‘Sensors and digital devices have revolutionized the measurement, collection, and storage of behavioral and physiological data, leading to the new term digital biomarkers. Digital biomarkers are measured across multiple layers of the hardware (eg, sensors) and software of medical devices that capture signals (behavioral and physiological data) from patients. Digital biomarkers can increase diagnostic and therapeutic precision in the modern health care system by remotely and continuously measuring reliable clinical data and allowing continuous monitoring and evaluation. Captured by wearable, implantable, and digestible devices and sensors, digital biomarkers can be used at home to provide clinical data, collecting data that is not possible in the clinical setting. This information can improve physicians’ and patients’ decisions, personalize the treatment, and predict diseases’ current and future status.’ |
|
| Nam et al35 | ‘In terms of IoT, the digital biomarker represents digitized data acquired from patients via IoT devices. Therefore, the digital biomarker can be defined as a biomarker that is objectively and quantitatively measured using digital devices and be used to explain or predict health-related outcomes. Digital biomarker is measured using the digital tools that include portable, wearable, implantable or digestible devices, and exclude data obtained via patient-reported measurements or traditional devices and equipment. In a broad sense, digital biomarker include all human data that can be measured using digital tool.’ |
|
| Palanica et al45 | ‘Digital biomarkers are digitally collected data, such as heart rate from a wearable device, that are transformed through mathematical models into indicators of health outcomes like prediabetes. Some digital biomarkers have been found to outperform traditional clinical methods, for example, for arrhythmia detection, because of their ability to continuously monitor patients outside of the clinic. The most successful digital biomarkers have been developed based on supervised, unsupervised, and semi-supervised machine learning models.’ |
|
| Petersen et al46 | ‘The use of remotely collected data that monitors health and behavior is an emerging area of research. Such data could be considered digital biomarkers objective information that can be used to predict changes in health status and the use of digital biomarkers offers a more efficient method of identifying such markers as the use of devices continuously collecting data increases. One critical requirement in the development of digital biomarkers is connecting these novel measurements to health outcomes.’ |
|
| Phillips et al37 | ‘Digital biomarker technologies, which fall into the category of ‘wearables and biosensing devices’, use consumer-generated physiological and behavioral measures collected through connected digital tools that can be used to explain, influence, and/or predict health-related outcomes. These technologies may focus on measurements for consumer use only, or clinical measurements that are transmitted to clinicians for health care decision-making. They may passively monitor ongoing activities (such as steps taken) or be used to actively collect specific measurements (such as blood glucose).’ |
|
| Piau et al47 | ‘Digital biomarker definition. Objective, quantifiable, physiological, and/or behavioral data that are collected and measured by means of digital devices such as embedded environmental sensors, portables, wearables, implantables, or digestibles, and which opens up opportunities for the remote collection and processing of ecologically valid, real-life, continuous, long-term, health-related data.’ |
|
| Sahandi Far et al48 | ‘Digital biomarkers (DB), as captured using sensors embedded in modern smart devices, are a promising technology for home-based sign and symptom monitoring in Parkinson disease (PD). The emergence of new technologies has led to a variety of sensors (ie, acceleration, gyroscope, GPS, etc) embedded in smart devices for daily use (ie, smartphone, smartwatch). Such sensor data, alongside other digital information recorded passively or when executing prespecified tasks, may provide valuable insight into health-related information. Such applications are now commonly referred to as digital biomarkers (DB). DB being collected frequently over a long period of time can provide an objective, ecologically valid, and more detailed understanding of the inter- and intra-individual variability in disease manifestation in daily life.’ |
|
| Seyhan and Carini49 | ‘Digital biomarkers (BMs) can have several applications beyond clinical trials in diagnostics—to identify patients affected by a disease or to guide treatment. Digital BMs present a big opportunity to measure clinical endpoints in a remote, objective, and unbiased manner. Digital BMs are defined as an objective, quantifiable physiological and behavioral data that are collected and measured by means of digital devices. The data collected is typically used to explain, influence and/or predict health-related outcomes.’ |
|
| Shandhi et al50 | ‘Multiple studies suggest the utility of digital biomarkers, objective and quantifiable digitally collected physiological and behavioral data (eg, resting heart rate (RHR), step count, sleep duration, and respiratory rate), collected by consumer devices along with patient-reported symptoms to monitor the progression of respiratory and influenza-like illnesses.’ |
|
| Tavabi et al51 | ‘Digital biomarkers are physiological and behavioral measures collected from participants through digital tools that can be used to explain, influence, or predict health-related outcomes.’ |
|
| van den Brink et al52 | ‘Wearable technologies, including smartphones and smartwatches, are increasingly utilized in the healthcare domain for the development of so-called digital biomarkers. This novel type of biomarker is characterized by being measured non-invasively, continuously, and under real-world conditions using digital technology, allowing for a more holistic and personal insight into someone’s health. Therefore, digital biomarkers enable accessible health and behavioral feedback to the user and are particularly suited for driving the healthcare transition towards prevention, empowering people in the self-management of health and disease. Furthermore, digital biomarkers can provide users with more frequent and detailed contextual information and continuously update personal lifestyle recommendations.’ |
|
| Zetterström et al53 | ‘We define a DB as patient-generated physiological and behavioural measures collected through sensors and other connected digital tools that can be used to monitor, predict and/or influence health-related outcomes.’ |
|
There were almost no structural similarities between the 51 identified definitions in 37 articles without a reference (for those with a reference, similarities such as paraphrasing are expected; online supplemental S4).
Discussion
We systematically searched and characterised the biomedical literature that used the term digital biomarker and analysed the provided definitions of the concept. We identified 415 articles using ‘digital biomarker’ in title and/or abstract that were published between 2014 and 2023. Of them, 128 articles provided 127 different definitions. By comparing the defining features, we aimed to better understand what those who use this term in the context of biomedical research or healthcare mean by ‘digital biomarker’ and which components are deemed the essence of it.26
The first definition of a digital biomarker is from 2015.27 Within 8 years, more than 127 definitions have been used, with none of them clearly being the most widely used; indicating a high heterogeneity of the concept of digital biomarkers. The definitions often cover different aspects of definitional components that are traditionally used to describe more conventional biomarkers. Authors have created their own concepts and gave an identity to this type of biomarker. The variation in these definitions and the fact that only 23 of them provide a full description containing all components of FDA’s BEST framework, shows how broad the current understanding of this fundamental concept is.
Digital biomarkers emerged as a concept in medical and technological domains, although with a diverse terminology across different academic journals. In the medical field, digital biomarkers are often referred to as biomarkers of health or disease obtained through digital health technologies. In the technical field, these biomarkers are viewed as data-driven indicators collected from sensors, wearables and other portable digital technologies that provide an assessment of the health status. These diverse terminologies and definitions reflect the interdisciplinary nature of digital biomarkers with their application in a broad spectrum of biomedicine which underlines the importance of unified concepts to enhance the communications and cross-disciplinary collaborations on this evolving field.
Regulatory perspectives
The EMA has defined digital biomarkers in 2020 in their draft guidance ‘Questions and answers: Qualification of digital technology-based methodologies to support approval of medicinal products’, stating their ‘clinical meaning is established by a reliable relationship to an existing, validated endpoint’.10 EMA draws a clear line to electronic clinical outcome assessments (eCOA), whose ‘clinical meaning is established de novo’. According to EMA’s terminology, both digital biomarkers and eCOA are derived from ‘digital measures’ and can be used as ‘digital endpoints’.10
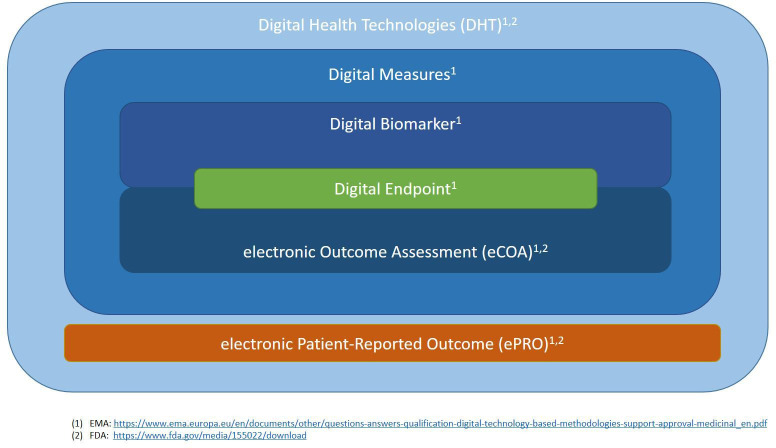
On the other hand, the term ‘digital biomarker’ cannot be found in the FDA draft guidance ‘Digital Health Technologies for Remote Data Acquisition in Clinical Investigations’, which instead features eCOA as examples of digital health technologies.28 Figure 3 contains our semantic interpretation of the terminology used by EMA and FDA.
Figure 3.
Semantic overview of terminology used by EMA and FDA. Digital health technologies obtain digital measures, which include digital biomarkers and electronic clinical outcome assessment (eCOA). Digital biomarkers and eCOAs both can provide digital endpoints. EMA, European Medicines Agency; FDA, Food and Drug Administration.
This distinction can rarely be observed in the medical literature—we found this term in 8 of the 415 articles analysed and a PubMed search for ‘electronic clinical outcome assessment*’ returned also only 8 articles mentioning it in title or abstract (as of 31 August 2023), compared with the 415 for our search term ‘digital biomarker*’. As Vasudevan et al stated in 2022: ‘There are currently multiple definitions of the term digital biomarker reported in the scientific literature, and some seem to conflate established definitions of a biomarker and a clinical outcomes assessment (COA)’.11
This divergency in the terminology of digital biomarkers between the academic literature and the regulators’ language raises challenges and ambiguity. Consequently, a more cohesive and comprehensive framework within the digital biomarker field is needed to strengthen the clarity and continue growing the potential that this data could bring for health.
The development of a substantive and unified definition of digital biomarkers would be an important step in shaping a conceptual framework for the development, assessment and reporting of digital biomarkers. Our results may inform this process by using the existing understanding of digital biomarkers systematically analysed in this study as a basis. To achieve a common and more unified understanding of what digital biomarkers are—and are not—a Delphi study could be useful.29 30 Such a study would aim to combine multiple views and expectations on the existing definitions of digital biomarkers and their components until a consensus is reached. Ideally, that would be achieved by an international panel with expert’s representative of all relevant stakeholders covering a range of medical fields (eg, cardiology, neurology), professional backgrounds (eg, clinical care/rehabilitation/nursing, software developers, device manufacturer, editors, guideline developers), and professional perspectives (eg, academia, regulatory, industry/technology, publishing) and involving patients.
Limitations
There are some limitations to our study.
First, we used a limited search only in a single database using the single term of ‘digital biomarker*’, which may have overlooked some other relevant studies. PubMed was chosen as literature database given its outstanding role, reflecting the most impactful journals in biomedicine.31 We focused on this single term because we assume it to be the most central and widely used term describing the concept of ‘digital biomarker’. It is very unlikely that the definitions would be much more uniform in potentially overlooked studies or would we have included other potential concepts, and it is quite possible that many more different definitions would emerge, especially from digital biomarker developments contained in technical literature databases (such as IEEE Explore or ACM Digital Library). Therefore, we may have even underestimated the large number of different definitions.
Second, the screening and data extraction were performed by a single reviewer only. This may have resulted in some studies that were overlooked and some misclassifications, but it is unlikely that our main interpretation would change. Third, we developed a simple framework with three key elements of definitions based on a well-established framework (BEST), but the categorisation of elements is subjective to some degree. However, we employed a structured analysis that confirmed the observed heterogeneity across definitions.
Conclusions
Clear and unambiguous communication and research reporting is essential for the effective implementation of scientific innovations and developments. This requires clear definitions and consistent use and understanding of key terms and concepts. A lack of clarity and consistency can lead to research waste, delay or even misdirection of promising developments and potential. Digital biomarkers offer the opportunity to collect objective, meaningful, patient-relevant data cost-effectively with unprecedented granularity. An exact understanding of what they are and how they are described in biomedical literature is essential to let them shape the future of clinical research and enable them to provide most useful evidence for research and care. Our study can inform the development of a harmonised and more widely accepted definition, for example, with a Delphi study.
bmjhci-2023-100914supp002.xlsx (459.7KB, xlsx)
Acknowledgments
We thank Saido Haji Abukar (Medical Bachelor student at ETH Zurich) for her support with the study selection and data extraction.
Footnotes
Contributors: All authors made substantial contributions to the conception and design of the work; all authors have drafted the work or substantively revised it; all authors have approved the submitted version; all authors have agreed both to be personally accountable for the author’s own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved and the resolution documented in the literature.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: RC2NB (Research Center for Clinical Neuroimmunology and Neuroscience Basel) is supported by Foundation Clinical Neuroimmunology and Neuroscience Basel. One of the main projects of RC2NB is the development and evaluation of a digital biomarkers which is supported by grants from Novartis, Roche and Innosuisse (Swiss Innovation Agency). All authors declare no competing interests.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.FDA-NIH Biomarker Working Group . BEST (Biomarkers, EndpointS, and Other Tools) Resource. Silver Spring (MD): Food and Drug Administration (US), 2016. [PubMed] [Google Scholar]
- 2.Motahari-Nezhad H, Péntek M, Gulácsi L, et al. Outcomes of digital biomarker-based interventions: protocol for a systematic review of systematic reviews. JMIR Res Protoc 2021;10:e28204. 10.2196/28204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moschovis PP, Sampayo EM, Cook A, et al. The diagnosis of respiratory disease in children using a phone-based cough and symptom analysis algorithm: the Smartphone recordings of cough sounds 2 (SMARTCOUGH-C 2) trial design. Contemp Clin Trials 2021;101:106278. 10.1016/j.cct.2021.106278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coravos A, Khozin S, Mandl KD. Developing and adopting safe and effective digital biomarkers to improve patient outcomes. NPJ Digit Med 2019;2:14. 10.1038/s41746-019-0090-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolz R, Munro J, Guerrero R, et al. [P3–200]: predicting sleep/wake patterns from 3‐axis accelerometry using deep learning. Alzheimer’s &Amp; Dementia 2017;13. 10.1016/j.jalz.2017.06.1412 [DOI] [Google Scholar]
- 6.Babrak LM, Menetski J, Rebhan M, et al. Traditional and digital biomarkers: two worlds apart Digit Biomark 2019;3:92–102. 10.1159/000502000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buegler M, Harms R, Balasa M, et al. Digital biomarker-based individualized prognosis for people at risk of dementia. Alzheimers Dement (Amst) 2020;12:e12073. 10.1002/dad2.12073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woelfle T, Bourguignon L, Lorscheider J, et al. Wearable sensor technologies to assess motor functions in people with multiple sclerosis: systematic scoping review and perspective. J Med Internet Res 2023;25:e44428. 10.2196/44428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dorsey ER, Papapetropoulos S, Xiong M, et al. The first frontier: digital biomarkers for neurodegenerative disorders. Digit Biomark 2017;1:6–13. 10.1159/000477383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.European Medicines Agency . Questions and answers: qualification of Digital technology-based Methodologies to support approval of medicinal products 2020. 2022. Available: https://www.ema.europa.eu/en/documents/other/questions-answers-qualification-digital-technology-based-methodologies-support-approval-medicinal_en.pdf
- 11.Vasudevan S, Saha A, Tarver ME, et al. Digital biomarkers: convergence of digital health technologies and biomarkers. NPJ Digit Med 2022;5:36. 10.1038/s41746-022-00583-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cook DJ, Reeve BK, Guyatt GH, et al. Stress ulcer prophylaxis in critically ill patients. resolving discordant meta-analyses. JAMA 1996;275:308–14. [PubMed] [Google Scholar]
- 13.Oliveira ML, Lucchetta RC, Bonetti A de F, et al. Efficacy outcomes reported in trials of multiple sclerosis: a systematic Scoping review. Mult Scler Relat Disord 2020;45:102435. 10.1016/j.msard.2020.102435 [DOI] [PubMed] [Google Scholar]
- 14.Soriano JB, Murthy S, Marshall JC, et al. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis 2022;22:e102–7. 10.1016/S1473-3099(21)00703-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verghis R, Blackwood B, McDowell C, et al. Heterogeneity of surrogate outcome measures used in critical care studies: a systematic review. Clin Trials 2023;20:307–18. 10.1177/17407745231151842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grant MJ, Booth A. A typology of reviews: an analysis of 14 review types and associated methodologies. Health Info Libr J 2009;26:91–108. 10.1111/j.1471-1842.2009.00848.x [DOI] [PubMed] [Google Scholar]
- 17.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. J Clin Epidemiol 2021;134:178–89. 10.1016/j.jclinepi.2021.03.001 [DOI] [PubMed] [Google Scholar]
- 18.OpenAlex . Openalex 2023. Available: https://openalex.org/ [Accessed 16 Jun 2023].
- 19.Woelfle T. Local citation network 2019. Available: https://LocalCitationNetwork.github.io [Accessed 16 Jun 2023].
- 20.Zygomatic . Wordclouds.Com 2023. Available: https://www.wordclouds.com/ [Accessed 25 Apr 2023].
- 21.Bachmann M. RapidFuzz 3.2.0: Indel 2021, Available: https://maxbachmann.github.io/RapidFuzz/Usage/distance/Indel.html [Accessed 3 Aug 2023].
- 22.Priem J, Piwowar H, Orr R. Openalex: a fully-open index of scholarly works, authors, venues, institutions, and concepts 2022. n.d. Available: https://arxiv.org/abs/2205.01833
- 23.Califf RM. Biomarker definitions and their applications. Exp Biol Med (Maywood) 2018;243:213–21. 10.1177/1535370217750088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piau A, Wild K, Mattek N, et al. Current state of digital biomarker technologies for real-life, home-based monitoring of cognitive function for mild cognitive impairment to mild Alzheimer disease and implications for clinical care. J Med Internet Res 2019;21:e12785. 10.2196/12785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coravos A, Goldsack JC, Karlin DR, et al. Digital medicine: a primer on measurement. Digit Biomark 2019;3:31–71. 10.1159/000500413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta A, Mackereth S. Definitions: the stanford encyclopedia of philosophy 2023, Available: https://plato.stanford.edu/entries/definitions/ [Accessed 31 Jan 2024].
- 27.Gerbelot R, Koenig A, Goyer C, et al. A Wireless patch for sleep respiratory disorders applications. Annu Int Conf IEEE Eng Med Biol Soc 2015;2015:2279–82. 10.1109/EMBC.2015.7318847 [DOI] [PubMed] [Google Scholar]
- 28.Food and Drug Administration (US) . Digital health Technologies for remote data acquisition in clinical investigations. FDA; 2021. Available: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/digital-health-technologies-remote-data-acquisition-clinical-investigations [Accessed 20 Jul 2021]. [Google Scholar]
- 29.Okoli C, Pawlowski SD. The Delphi method as a research tool: an example, design considerations and applications. Information & Management 2004;42:15–29. 10.1016/j.im.2003.11.002 [DOI] [Google Scholar]
- 30.Khodyakov D, Grant S, Kroger J, et al. Disciplinary trends in the use of the Delphi method: a bibliometric analysis. PLoS One 2023;18:e0289009. 10.1371/journal.pone.0289009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National Cancer Institute at the National Institutes of Health . NCI dictionary of cancer terms: biomedicine 2024. Available: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/biomedicine [Accessed 5 Feb 2025].
- 32.Andrade AQ, Lim R, Kelly T-L, et al. Wrist accelerometer temporal analysis as a prognostic tool for aged care residents: a sub-study of the remindar trial. J Am Geriatr Soc 2023;71:1124–33. 10.1111/jgs.18181 [DOI] [PubMed] [Google Scholar]
- 33.Bartolome A, Prioleau T. A computational framework for discovering digital biomarkers of glycemic control. NPJ Digit Med 2022;5:111. 10.1038/s41746-022-00656-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bijlani N, Nilforooshan R, Kouchaki S. An unsupervised data-driven anomaly detection approach for adverse health conditions in people living with dementia: cohort study. JMIR Aging 2022;5:e38211. 10.2196/38211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nam KH, Kim DH, Choi BK, et al. Internet of things, digital biomarker, and artificial intelligence in spine: current and future perspectives. Neurospine 2019;16:705–11. 10.14245/ns.1938388.194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parziale A, Mascalzoni D. Digital biomarkers in psychiatric research: data protection qualifications in a complex ecosystem. Front Psychiatry 2022;13:873392. 10.3389/fpsyt.2022.873392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phillips KA, Douglas MP, Trosman JR, et al. What goes around comes around": lessons learned from economic evaluations of personalized medicine applied to digital medicine. Value Health 2017;20:47–53. 10.1016/j.jval.2016.08.736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wright JM, Jones GB. Harnessing the digital exhaust: incorporating wellness into the pharma model. Digit Biomark 2018;2:31–46. 10.1159/000488132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dillenseger A, Weidemann ML, Trentzsch K, et al. Digital biomarkers in multiple sclerosis. Brain Sci 2021;11:11:1519. 10.3390/brainsci11111519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gielis K, Vanden Abeele M-E, De Croon R, et al. Dissecting digital card games to yield digital biomarkers for the assessment of mild cognitive impairment: methodological approach and exploratory study. JMIR Serious Games 2021;9:e18359. 10.2196/18359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harms RL, Ferrari A, Meier IB, et al. Digital biomarkers and sex impacts in Alzheimer’s disease management - potential utility for innovative 3p medicine approach. EPMA J 2022;13:299–313. 10.1007/s13167-022-00284-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hartl D, de Luca V, Kostikova A, et al. Translational precision medicine: an industry perspective. J Transl Med 2021;19:245. 10.1186/s12967-021-02910-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Katsaros D, Hawthorne J, Patel J, et al. Optimizing social support in oncology with digital platforms. JMIR Cancer 2022;8:e36258. 10.2196/36258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Motahari-Nezhad H, Fgaier M, Mahdi Abid M, et al. Digital biomarker-based studies: scoping review of systematic reviews. JMIR Mhealth Uhealth 2022;10:e35722. 10.2196/35722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Palanica A, Docktor MJ, Lieberman M, et al. The need for artificial intelligence in digital therapeutics. Digit Biomark 2020;4:21–5. 10.1159/000506861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petersen CL, Weeks WB, Norin O, et al. Development and implementation of a person-centered, technology-enhanced care model for managing chronic conditions: cohort study. JMIR Mhealth Uhealth 2019;7:e11082. 10.2196/11082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Piau A, Rumeau P, Nourhashemi F, et al. Information and communication technologies, a promising way to support pharmacotherapy for the behavioral and psychological symptoms of dementia. Front Pharmacol 2019;10:1122. 10.3389/fphar.2019.01122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sahandi Far M, Eickhoff SB, Goni M, et al. Exploring test-retest reliability and longitudinal stability of digital biomarkers for Parkinson disease in the M-power data set: cohort study. J Med Internet Res 2021;23:e26608. 10.2196/26608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seyhan AA, Carini C. Are innovation and new technologies in precision medicine paving a new era in patients centric care J Transl Med 2019;17:114. 10.1186/s12967-019-1864-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shandhi MMH, Cho PJ, Roghanizad AR, et al. A method for intelligent allocation of diagnostic testing by leveraging data from commercial wearable devices: a case study on COVID-19. NPJ Digit Med 2022;5:130. 10.1038/s41746-022-00672-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tavabi N, Stück D, Signorini A, et al. Cognitive digital biomarkers from automated transcription of spoken language. J Prev Alzheimers Dis 2022;9:791–800. 10.14283/jpad.2022.66 [DOI] [PubMed] [Google Scholar]
- 52.van den Brink WJ, van den Broek TJ, Palmisano S, et al. Digital biomarkers for personalized nutrition: predicting meal moments and interstitial glucose with non-invasive, wearable technologies. Nutrients 2022;14:4465. 10.3390/nu14214465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zetterström A, Hämäläinen MD, Karlberg E, et al. Maximum time between tests: a digital biomarker to detect therapy compliance and assess schedule quality in measurement-based eHealth systems for alcohol use disorder. Alcohol Alcohol 2019;54:70–2. 10.1093/alcalc/agy086 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjhci-2023-100914supp001.pdf (392.5KB, pdf)
bmjhci-2023-100914supp002.xlsx (459.7KB, xlsx)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplemental information.