Abstract
Objectives
To assess incidence, severity and predictors of COVID-19, including protective post-vaccination levels of antibodies to the receptor-binding domain of SARS-CoV-2 spike protein (anti-RBD), informing further vaccine strategies for patients with immune-mediated inflammatory diseases (IMIDs) on immunosuppressive medication.
Methods
IMIDs on immunosuppressives and healthy controls (HC) receiving SARS-CoV-2 vaccines were included in this prospective observational study. COVID-19 and outcome were registered and anti-RBD antibodies measured 2–5 weeks post-immunisation.
Results
Between 15 February 2021 and 15 February 2023, 1729 IMIDs and 350 HC provided blood samples and self-reported COVID-19. The incidence of COVID-19 was 66% in patients and 67% in HC, with re-infection occurring in 12% of patients. Severe COVID-19 was recorded in 22 (2%) patients and no HC. No COVID-19-related deaths occurred. Vaccine-induced immunity gave higher risk of COVID-19 (HR 5.89 (95% CI 4.45 to 7.80)) than hybrid immunity. Post-immunisation anti-RBD levels <6000 binding antibody units/mL were associated with an increased risk of COVID-19 following three (HR 1.37 (95% CI 1.08 to 1.74)) and four doses (HR 1.28 (95% CI 1.02 to 1.62)), and of COVID-19 re-infection (HR 4.47 (95% CI 1.87 to 10.67)).
Conclusion
Vaccinated patients with IMID have a low risk of severe COVID-19. Hybrid immunity lowers the risk of infection. High post-immunisation anti-RBD levels protect against COVID-19. These results suggest that knowledge on COVID-19 history, and assessment of antibody levels post-immunisation can help individualise vaccination programme series in high-risk individuals.
Trial registration number
Keywords: COVID-19, vaccination, autoimmunity, biological therapy
WHAT IS ALREADY KNOWN ON THIS TOPIC
Development of vaccines and vaccination strategies against SARS-CoV-2 have been important to patients with immune-mediated inflammatory diseases (IMIDs), but evidence is still lacking across diagnoses and medications regarding the incidence of severe COVID-19 during long-term follow-up throughout a multidose vaccine programme and exposure to different viral variants.
Breakthrough infections induce a robust immunological response in both vaccinated patients with IMID and healthy individuals, but it is still unknown how this translates into clinical protection.
The role of post-vaccination antibody levels in protection against COVID-19 is still an ongoing debate.
WHAT THIS STUDY ADDS
Vaccinated patients with IMID have a reassuringly low incidence of severe disease and death caused by SARS-CoV-2 despite a high occurrence of COVID-19 during long-term follow-up through several vaccine doses and different variants of concern.
Patients with post-immunisation levels of antibodies to the receptor-binding domain of SARS CoV-2 spike protein (anti-RBD) above a specific cut-off are better protected against COVID-19, compared with patients with weaker humoral responses to vaccines or breakthrough infection.
Hybrid immunity protects better than vaccination alone against infection.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Knowledge on COVID-19 history, and assessment of anti-RBD antibody levels post-immunisation can help individualise vaccination programme series in high-risk individuals.
These data may also assure patients with IMID and healthcare providers and help to normalise life in this phase of the pandemic.
Introduction
The rapid development of vaccines and vaccination strategies against SARS-CoV-2 have been of particular importance to patients with immune-mediated inflammatory diseases (IMIDs) on immunosuppressive therapies. This patient group may still be at increased risk of severe COVID-19, hospitalisation and death from COVID-19.1–6 Vaccine responses are attenuated due to immunosuppression, and levels of antibodies to the receptor-binding domain of SARS CoV-2 spike protein (anti-RBD) decline more rapidly following vaccination in immunosuppressed patients than is the case in healthy individuals.7–12 Risk factors for severe COVID-19 includes higher age, male sex and comorbidities, as well as certain immunosuppressive therapies, like anti-CD20 therapy.2 3 13 14 However, in patients with IMID on immunosuppressive therapies, evidence is lacking across diagnoses and medications regarding the incidence of severe COVID-19 during long-term follow-up after vaccination, throughout a multidose vaccine programme and exposure to different variants of concern.
Breakthrough infections induce a robust immunological response in both vaccinated patients with IMID and healthy individuals, but it is still unknown how this translates into clinical protection. In healthy individuals, clinical protection against severe disease has been estimated to last for at least a year post-omicron infection.9 15 16 If and how clinical COVID-19 should be accommodated into future vaccine programmes for patients with IMID is controversial.
The role of anti-RBD antibody levels in protection against COVID-19 is still an ongoing debate. The anti-RBD antibody level sufficient to protect against clinical COVID-19, and a possible association between antibody levels, and severe COVID-19 have not been established.
Repeated vaccination has been shown to improve immune responses in patients with IMID and to close the gap between this patient group and the healthy population in terms of humoral immune responses.8 Identification of patients at risk of severe COVID-19 is important to guide future vaccination policy, and to avoid unnecessary vaccination in patients who are adequately protected. It may be helpful to individualise recommendations based on previous vaccine responses and prior COVID-19, as well as diagnosis, age and medication.
The aim of this prospective observational cohort study was to determine the incidence, outcome and predictors of COVID-19 in patients with IMID with vaccine or hybrid immunisation, with a particular focus on identifying protective anti-RBD antibody levels.
Methods
Study design and participants
Nor-vaC is an ongoing longitudinal observational study conducted at two large centres for patients with IMID in Norway: the Division of Rheumatology, Diakonhjemmet Hospital, and the Department of Gastroenterology, Akershus University Hospital. Patients (aged ≥18 years) with rheumatoid arthritis, psoriatic arthritis, spondyloarthritis, Crohn’s disease or ulcerative colitis on immunosuppressive medication, and intending to undergo SARS-CoV-2 vaccination, were identified from hospital records and invited to participate (online supplemental tables 1 and 2). In total, 2250 patients were recruited into the study prior to initiation of the national vaccination programme in February 2021. Healthcare workers were recruited as controls.
rmdopen-2023-003545supp001.pdf (623.8KB, pdf)
Participants received vaccination as recommended by the national vaccination programme administered by the Norwegian Institute of Public Health.9 Primary vaccination in patients with IMID consisted of three doses followed by a fourth (booster) dose at least 12 weeks after the third dose. A fifth vaccine dose was recommended to patients with IMID from August 2022. According to the national vaccination programme, boosters were administered as either a half-dose of mRNA-1273 or as a full dose of BNT162b2. Healthy controls received two vaccine doses as primary vaccination, and a third (booster) dose at least 12 weeks after the second dose.
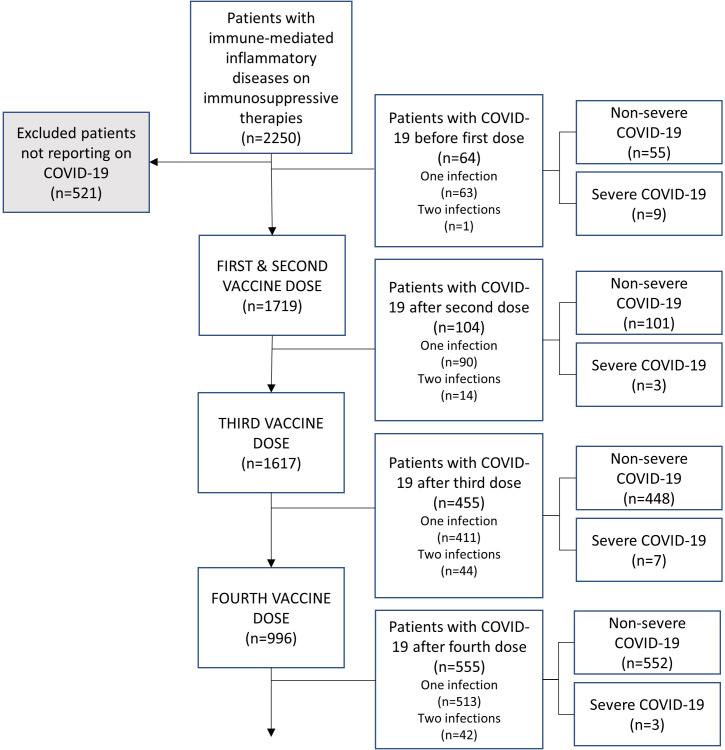
In the present analyses, we included patients that responded to questionnaires on COVID-19 status or were registered as hospitalised due to COVID-19 (figure 1).
Figure 1.
Study population.
The study is registered at Clinicaltrials.gov, NCT04798625.
Procedures
Participating patients with IMID and healthy controls provided serum samples on a regular basis 2–5 weeks after each vaccination and after a positive PCR or rapid antigen test for COVID-19. Anti-RBD antibody levels were assessed using an in-house bead-based method validated against a microneutralisation assay at the Department of Immunology at Oslo University Hospital.17 Throughout the study, anti-RBD levels were reported back to study participants.
Demographic data were collected at baseline, and information on immunosuppressive medication was self-reported both at baseline and at the time of vaccination or infection through questionnaires. Comorbidities listed in the Charlson Comorbidity Index (online supplemental table 3) were collected through questionnaires and hospital records.18 Gender data were defined by national ID numbers, indicating male or female sex. Patients received monthly reminders through questionnaires to report any COVID-19 infection verified by either a PCR test or a rapid antigen test. Patients were also asked for information regarding symptom severity and symptom duration longer or shorter than 2 weeks.
Data from Diakonhjemmet Hospital were collected using Nettskjema; a self-administered electronic questionnaire tool hosted by University of Oslo (https://www.uio.no/english/services/it/adm-services/nettskjema/) and stored in the research portal Services for Sensitive Data (https://www.uio.no/english/services/it/research/sensitive-data/index.html). Data from Akershus University Hospital were collected using Viedoc, V.4 (Sweden).
Data on administered vaccine types were provided from the national database of COVID-19 vaccinations, the Norwegian Immunisation Registry (SYSVAK). Data on hospital admissions and cause of death were collected from hospital records, the Norwegian Patient Registry and the Norwegian Cause of Death Registry. Only patients who were admitted to the hospital for at least 24 hours were included. The International Classification of Diseases, Tenth Revision codes were used to find patients admitted due to COVID-19 with the combination ‘viral pneumonia’ and ‘COVID-19’, or ‘COVID-19’ in any order and in combination with any other diagnoses.
The main outcomes were COVID-19 (defined as PCR or antigen test verified infection), severe COVID-19 (defined as hospital admission or death due to COVID-19) and COVID-19 re-infection. Hybrid immunity, meaning immunity provided by a combination of vaccine series and COVID-19, was in the present Cox analyses defined as patients with registered COVID-19 following three vaccine doses, and compared with infection-naïve patients with four vaccine doses.
Statistical analyses
Descriptive statistics were used to summarise baseline characteristics of patients and controls, as well as patients reporting persisting symptoms of COVID-19 over 2 weeks.
To examine predictors of COVID-19, and COVID-19 re-infection, we performed Cox regression analyses with period of exposure starting 2 weeks after each vaccine dose (second, third and fourth) or COVID-19, running until next vaccine dose or infection. Patients were included if they had provided blood samples following the vaccine in question (online supplemental figure 1).
Adjustments were made for age and sex with calendar month as a time-varying covariate to adjust for differences in viral pressure and shielding recommendations. Predefined possible predictors of COVID-19, and COVID-19 re-infection were age, sex, any comorbidity, IMID diagnosis, immunosuppressive medication and anti-RDB antibody levels assessed 2–5 weeks after vaccination or infection.
To identify protective anti-RBD antibody levels against COVID-19, and COVID-19 re-infection in the patient population, we examined different cut-off levels. Cox regression analyses were performed from above or below 50, 100, 200 and for each 1000 binding antibody units (BAU)/mL up to 25 000 BAU/mL following a second, third and fourth vaccine dose, as well as a third dose plus COVID-19 (hybrid immunity). As for the other predictors, analyses were adjusted for age and sex, plus calendar month as a time-varying covariate. To assess the sensitivity of the results to the number of covariate adjustments, an analysis was performed adjusting only for calendar month. The Cox regression model adjusted for calendar month is shown in online supplemental figure 2. Comorbidities were defined as one or more comorbidities (online supplemental table 3). The risk of severe COVID-19 following different vaccine doses and hybrid immunity was compared with Fisher’s exact test, and median antibody levels and age were compared with Mann-Whitney U test. All analyses were performed using STATA V.16.0.
Patient and public involvement
The research question was identified in collaboration with the user representative in the project group who has also been involved in the planning and conduction of the study. The user representative has also played an important role in the dissemination of the study results.
Results
General characteristics
Between 15 February 2021 and 15 February 2023, 1729 patients with IMID (648 rheumatoid arthritis, 272 psoriatic arthritis, 289 spondyloarthritis, 305 Crohn’s disease, 215 ulcerative colitis) reported on their COVID-19 history, provided blood samples and were included in the present analyses (figure 1). Median age of patients was 54 years (IQR 42–64), 971 (56%) were women, 758 (44%) were men. Median age of 350 healthy controls was 43 years (IQR 32–54), 277 (79%) were women and 73 (21%) were men. Baseline characteristics are shown in table 1.
Table 1.
Characteristics of patients and healthy controls
| All patients (n=1729) | Healthy controls (n=350) |
||||
| Any COVID-19 (n=1140) | |||||
| Severe COVID-19 (n=22) |
COVID-19 re-infection (n=139) |
||||
| Demographics | |||||
| Age, years | 54 (42–64) | 52 (41–62) | 61 (48–74) | 51 (37–59) | 43 (32–54) |
| Female | 971 (56%) | 670 (59%) | 10 (45%) | 88 (63%) | 277 (79%) |
| Male | 758 (44%) | 470 (41%) | 12 (55%) | 51 (37%) | 73 (21%) |
| Any comorbidity* | 717 (41%) | 491 (43%) | 20 (91%) | 68 (49%) | – |
| Diagnosis | |||||
| Rheumatoid arthritis | 648 (37%) | 425 (37%) | 11 (50%) | 56 (40%) | – |
| Psoriatic arthritis | 272 (16%) | 184 (16%) | 2 (9%) | 20 (14%) | – |
| Spondyloarthritis | 289 (17%) | 206 (18%) | 3 (14%) | 17 (13%) | – |
| Crohn’s disease | 305 (18%) | 181 (16%) | 2 (9%) | 25 (18%) | – |
| Ulcerative colitis | 215 (12%) | 144 (13%) | 4 (18%) | 21 (15%) | – |
| Medication | |||||
| TNFi monotherapy† | 701 (41%) | 484 (42%) | 7 (32%) | 63 (45%) | – |
| TNFi combination therapy‡ | 379 (22%) | 257 (22%) | 4 (18%) | 26 (19%) | – |
| Methotrexate | 345 (20%) | 217 (19%) | 3 (14%) | 27 (19%) | – |
| Rituximab | 75 (4%) | 56 (5%) | 2 (9%) | 9 (6%) | – |
| Interleukin inhibitor§ | 76 (4%) | 33 (3%) | 1 (5%) | 3 (2%) | – |
| Janus kinase inhibitor¶ | 50 (3%) | 34 (3%) | 2 (9%) | 5 (4%) | – |
| Vedolizumab | 58 (3%) | 29 (3%) | 0 | 4 (3%) | – |
| Other** | 45 (3%) | 30 (3%) | 3 (14%) | 2 (1%) | – |
| Second vaccine | |||||
| BNT162b2 | 1294 (75%) | 853 (75%) | 19 (86%) | 100 (72%) | 225 (64%) |
| mRNA-1273 | 425 (24%) | 277 (24%) | 1 (5%) | 38 (27%) | 124 (35%) |
| None | 10 (1 %) | 11 (1%) | 2 (9%) | 1 (1%) | 1 (0.2%) |
| Third vaccine | |||||
| BNT162b2 | 965 (56%) | 611 (54%) | 11 (50%) | 66 (47%) | 297 (85%) |
| mRNA-1273 | 633 (37%) | 433 (38%) | 4 (18%) | 53 (38%) | 13 (4%) |
| None | 131 (7%) | 96 (8%) | 7 (32%) | 20 (14%) | 40 (11%) |
| Fourth vaccine | |||||
| BNT162b2 | 481 (28%) | 264 (23%) | 2 (9%) | 31 (22%) | 1 (0.2%) |
| mRNA-1273 | 506 (29%) | 321 (28%) | 1 (5%) | 30 (22%) | 1 (0.2%) |
| None | 742 (43%) | 555 (49 %) | 19 (86%) | 78 (56 %) | 348 (99%) |
Data are median (IQR) or n (%).
*Comorbidities listed in Charlson Comorbidity Index (online supplemental table 3).
†TNFi: infliximab, etanercept, adalimumab, golimumab, certolizumab pegol.
‡Combination therapy: methotrexate, sulfasalazine, leflunomide, azathioprine, prednisolone.
§Interleukin inhibitors: ustekinumab, secukinumab, tocilizumab.
¶Janus kinase inhibitors: tofacitinib, baricitinib, upadacitinib, filgotinib.
**Other: abatacept, sulfasalazine, leflunomide, azathioprine, prednisolone.
TNFi, tumour necrosis factor inhibitors.
Incidence and predictors of COVID-19
In total, 1140 (66%) of 1729 patients and 236 (67%) of 350 healthy controls reported having COVID-19 at least once. During the observation period, 139 (12%) patients vs 32 (14%) healthy controls reported undergoing COVID-19 twice. Sex, any comorbidity, IMID diagnosis or immunosuppressive medication were not associated with COVID-19 (table 2). The majority of infections (969 (85%) of 1140) among patients occurred after 1 January 2022, when the omicron variant became predominant in Norway. Timing of infections in the entire cohort is illustrated in online supplemental figure 3A. Of the 1140 patients with COVID-19, 64 (6%) had undergone infection prior to vaccination (figure 2). Symptom duration of COVID-19 over 2 weeks were reported by 201 (18%) patients and 29 (12%) healthy controls.
Table 2.
Risk factors for COVID-19 following vaccination and hybrid immunity
| Following second dose Patients included, n=1392 Events of COVID-19, n=61 |
Following third dose Patients included, n=1092 Events of COVID-19, n=277 |
Following fourth dose Patients included, n=639 Events of COVID-19, n=297 |
Hybrid immunity* Patients included, n=212 Events of COVID-19, n=29 |
|||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Anti-RBD antibody levels (BAU/mL) post-immunisation | ||||||||
| <2000† | 1.30 (0.77 to 2.19) | 0.33 | 1.20 (0.87 to 1.65) | 0.26 | 1.05 (0.77 to 1.44) | 0.74 | N/A | – |
| <4000† | 0.85 (0.51 to 1.43) | 0.55 | 1.21 (0.93 to 1.57) | 0.16 | 1.17 (0.90 to 1.51) | 0.24 | N/A | – |
| <6000† | 0.70 (0.40 to 1.22) | 0.20 | 1.37 (1.08 to 1.74) | 0.011 | 1.28 (1.02 to 1.62) | 0.036 | 4.47 (1.87 to 10.67) | 0.0010 |
| <8000† | 0.62 (0.30 to 1.27) | 0.19 | 1.47 (1.14 to 1.90) | 0.0033 | 1.44 (1.13 to 1.83) | 0.0033 | 3.84 (1.67 to 9.87) | 0.0020 |
| <10 000† | 1.24 (0.37 to 4.15) | 0.73 | 1.68 (1.24 to 2.28) | 0.00087 | 1.59 (1.22 to 2.07) | 0.00061 | 2.99 (1.33 to 6.73) | 0.0080 |
| <12 000† | 1.18 (0.26 to 5.36) | 0.83 | 1.97 (1.36 to 2.86) | 0.00031 | 1.50 (1.12 to 2.02) | 0.0066 | 2.40 (1.09 to 5.28) | 0.030 |
| Characteristics | ||||||||
| Age‡ | 0.99 (0.97 to 1.01) | 0.27 | 0.98 (0.97 to 0.99) | <0.0001 | 0.98 (0.98 to 0.99) | 0.00024 | 1.00 (0.97 to 1.04) | 0.78 |
| Female sex§ | 0.79 (0.47 to 1.31) | 0.36 | 1.28 (1.00 to 1.63) | 0.048 | 1.11 (0.87 to 1.40) | 0.40 | 1.35 (0.60 to 3.07) | 0.47 |
| Any comorbidity† | 1.21 (0.69 to 2.13) | 0.50 | 1.03 (0.80 to 1.33) | 0.81 | 0.94 (0.74 to 1.19) | 0.59 | 1.02 (0.45 to 2.31) | 0.97 |
| Diagnosis† | ||||||||
| Rheumatoid arthritis | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | ||||
| Psoriatic arthritis | 0.43 (0.05 to 3.73) | 0.44 | 1.73 (0.77 to 3.88) | 0.18 | 1.09 (0.48 to 2.49) | 0.83 | 0.66 (0.20 to 2.19) | 0.50 |
| Spondyloarthritis | 0.92 (0.20 to 4.21) | 0.91 | 1.64 (0.66 to 4.05) | 0.28 | 1.26 (0.51 to 3.09) | 0.61 | 0.71 (0.21 to 2.46) | 0.59 |
| Crohn’s disease | 0.56 (0.08 to 3.76) | 0.55 | 0.85 (0.34 to 2.11) | 0.73 | 1.36 (0.57 to 3.21) | 0.49 | 0.58 (0.17 to 1.98) | 0.38 |
| Ulcerative colitis | 0.99 (0.16 to 6.01) | 0.99 | 1.14 (0.44 to 2.92) | 0.78 | 1.50 (0.55 to 4.08) | 0.42 | 0.42 (0.11 to 1.57) | 0.20 |
| Medication† | ||||||||
| TNFi monotherapy¶ | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | ||||
| TNFi, combination therapy** | 1.05 (0.55 to 2.00) | 0.89 | 0.81 (0.59 to 1.11) | 0.198 | 0.86 (0.62 to 1.18) | 0.34 | 0.88 (0.33 to 2.37) | 0.80 |
| Methotrexate | 0.59 (0.23 to 1.51) | 0.27 | 1.19 (0.83 to 1.71) | 0.35 | 0.87 (0.63 to 1.21) | 0.42 | 2.01 (0.64 to 6.24) | 0.23 |
| Rituximab | 3.02 (0.84 to 10.80) | 0.090 | 1.53 (0.76 to 3.09) | 0.23 | 0.91 (0.56 to 1.48) | 0.71 | 2.85 (0.56 to 14.53) | 0.97 |
| Interleukin inhibitors†† | N/A | – | 0.61 (0.30 to 1.25) | 0.18 | 0.42 (0.21 to 0.83) | 0.013 | N/A | – |
| Janus kinase inhibitors | 0.95 (0.22 to 4.07) | 0.95 | 1.63 (0.89 to 2.97) | 0.11 | 0.72 (0.37 to 1.39) | 0.32 | N/A | – |
| Vedolizumab | 2.57 (0.75 to 8.79) | 0.13 | 0.72 (0.37 to 1.38) | 0.32 | 0.25 (0.10 to 0.61) | 0.0025 | N/A | – |
| Other*** | 1.53 (0.34 to 6.93) | 0.58 | 1.00 (0.40 to 2.48) | 1.00 | 0.91 (0.44 to 1.88) | 0.80 | N/A | – |
*Three vaccine doses followed by COVID-19.
†Adjusted for age, sex and calendar month.
‡Adjusted for sex and calendar month.
§Adjusted for age and calendar month.
¶TNFi: infliximab, etanercept, adalimumab, golimumab, certolizumab pegol.
**Combination therapy: methotrexate, sulfasalazine, leflunomide, azathioprine, prednisolone <15 mg.
††Interleukin inhibitors: ustekinumab, secukinumab, tocilizumab.
***Other: abatacept, sulfasalazine, leflunomide, azathioprine, prednisolone <15 mg.
BAU, binding antibody units; N/A, not available; RBD, receptor binding domain; ref, reference; TNFi, tumour necrosis factor inhibitors.
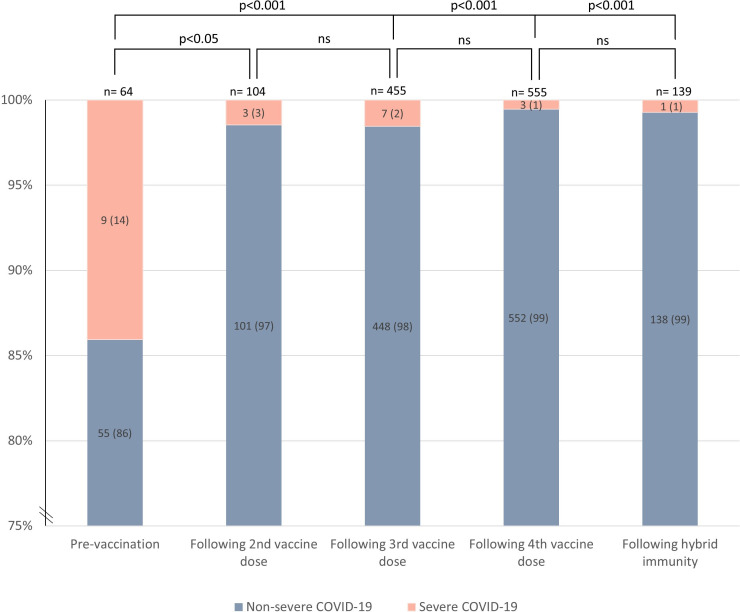
Figure 2.
Distribution of non-severe and severe COVID-19 (requiring hospitalisation) across vaccine doses and hybrid immunity (vaccine series and COVID-19) assessed by Fisher’s exact test. Numbers in parentheses are percentages. ns, not significant.
Severe COVID-19
Severe COVID-19 occurred in 22 (2%) of 1140 patients compared with none of healthy controls, and 9 (41%) of 22 occurred prior to vaccination (figure 2). Patients with severe COVID-19 were older than patients with non-severe disease (median age 61 (IQR 48–74) vs 52 (41–62) years) (p=0.04) (table 1). The risk of severe COVID-19 was lower post-vaccination versus prevaccination (figure 2). Prevaccination, 9 of 64 patients (14%) with COVID-19 had severe disease, compared with 3 of 104 (3%) following the second vaccine dose (p<0.05), 7 of 455 (2%) following the third vaccine dose (p<0.001), 3 of 555 (1%) following the fourth vaccine dose (p<0.001) and 1 of 139 (1%) following hybrid immunity (p<0.001) (figure 2). In total, 20 (91%) of 22 patients with severe COVID-19 reported any comorbidity (online supplemental table 4). Four patients required intensive care (3 rheumatoid arthritis, 1 psoriatic arthritis); two prevaccination, one after two vaccine doses and one after four vaccine doses (online supplemental table 4). Two patients were on treatment with prednisolone monotherapy, one used methotrexate and one tumour necrosis factor inhibitor combination therapy (online supplemental table 4). No COVID-19-related deaths were reported.
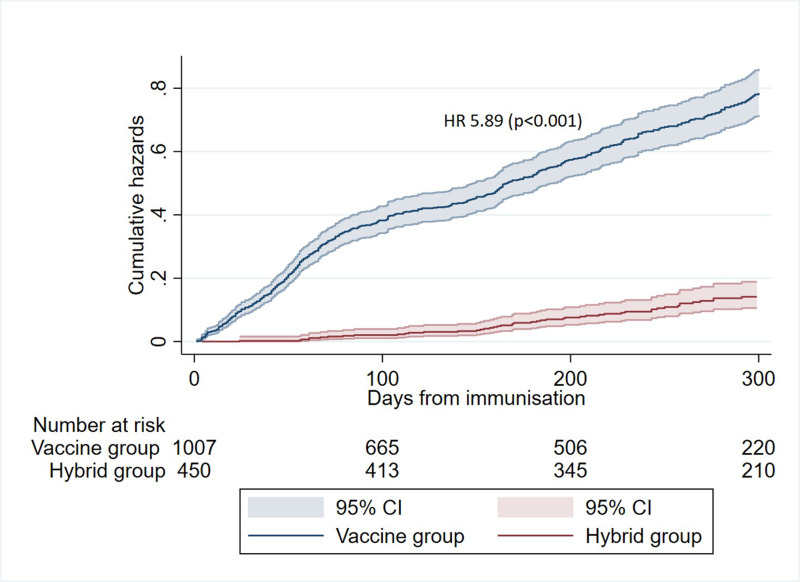
Hybrid immunity
Patients with vaccine-induced immunity with four vaccine doses had higher risk of infection compared with patients with hybrid immunity (three vaccine doses plus COVID-19) (HR 5.89 (95% CI 4.45 to 7.80), p<0.001) (figure 3). One (0.72%) of 139 patients with hybrid immunity was admitted to hospital due to COVID-19, compared with 12 (1.20%) of 1001 patients with only vaccine-induced immunity (figure 2). Timing of infections in the groups with hybrid versus vaccine-induced immunity is shown in online supplemental figure 3B.
Figure 3.
Protection provided by hybrid immunity (three vaccine doses and COVID-19) compared with immunity by four vaccine doses. Risk of COVID-19 in vaccine group (blue) versus hybrid group (red). The vaccine group comprised of infection-naïve patients with four vaccine doses and the hybrid group of patients with three vaccine doses followed by COVID-19. Patients were censored if vaccinated before infection.
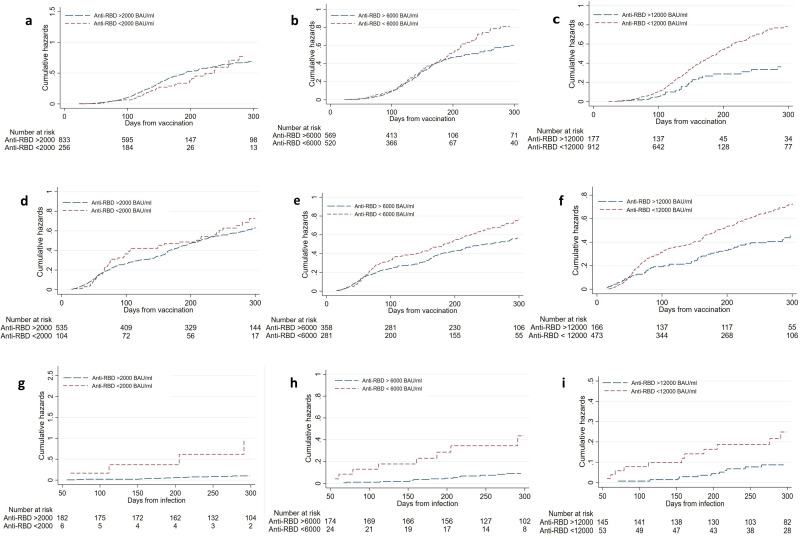
Role of post-vaccination anti-RBD antibody levels
Patients with post-vaccination anti-RBD antibody levels <6000 BAU/mL had a higher risk of COVID-19 until the next immunisation after three (HR 1.37 (95% CI 1.08 to 1.74), p=0.011) and four vaccinations (HR 1.28 (1.02 to 1.62), p=0.036) (table 2, figure 4A–F). Likewise, post-infection anti-RBD antibody levels <6000 BAU/mL increased the risk of COVID-19 re-infection (HR 4.47 (95% CI 1.87 to 10.67), p=0.0010) (figure 4G–I, online supplemental table 5). The number of patients excluded due to missing data is reported in online supplemental figure 1.
Figure 4.
Protective post-vaccination anti-RBD antibody levels following a third (A–C) and fourth (D–F) vaccine dose, and following hybrid immunity (G–I). Patients below anti-RBD antibody threshold in red, patients above threshold in blue. RBD, receptor-binding domain.
The median post-vaccination anti-RBD antibody level in vaccinated patients with severe infection (n=11) was 444 BAU/mL (IQR 31–1634). This was lower as compared with levels in the whole population following the second (2114 BAU/mL (IQR 732–5749), p<0.001), third (5897 BAU/mL (1939–9761), p<0.001) and fourth vaccine dose (7924 BAU/mL (3785–15 049), p<0.001), and following hybrid immunity (23 505 BAU/mL (11 423–37 007), p<0.001).
Discussion
In this large observational study of patients with IMID on immunosuppressive medication followed through the different stages of the pandemic vaccination programme, we demonstrate that although vaccinated patients had a high incidence of COVID-19 comparable to healthy controls, the risk of severe COVID-19 was low. Hybrid immunity was superior to vaccine-induced immunity in preventing COVID-19. Post-immunisation anti-RBD antibody levels >6000 BAU/mL were protective of infection following both vaccine-induced and hybrid immunity.
Both unvaccinated and vaccinated patients with IMID on immunosuppressive therapies have previously been shown to be at greater risk of hospitalisation and death due to COVID-19 compared with healthy individuals.1 3 19 However, data on the long-term protection against severe COVID-19 after vaccinations in this patient group are lacking.20 In this study, only 13 out of 1719 patients were admitted to hospital due to COVID-19 after receiving two or more vaccine doses. Risk of severe disease was higher prevaccination than post-vaccination. We cannot exclude that this is partly related to other factors than vaccination status, including the pathogenicity of the different viral variants and changes in care for immunosuppressed patients with COVID-19.
Due to the low number of patients with severe COVID-19, we were unable to perform statistical analyses on predictive factors. However, the majority of patients with severe disease had anti-RBD antibody levels in the lower quartile. Patients who were aware of their low anti-RBD antibody levels may have shielded during periods of high transmission, but the low incidence of severe COVID-19 and the absence of COVID-19-related deaths in this large cohort still suggest that most patients with low post-vaccination anti-RBD antibody levels did not have a severe COVID-19 disease course. This underlines the importance of cellular immunity in viral control and prevention of severe disease.21 Cellular immunity has previously been shown to be less affected by immunosuppressive medication.10 Severe COVID-19 occurred in 2% of patients compared with none of the healthy controls, indicating a higher risk in patients with IMID on immunosuppressive therapies compared with healthy individuals. However, the control group in our study were younger than the patients with IMID, and the higher incidence of severe COVID-19 in patients could partly be explained by older age and comorbidities.
To our knowledge, this is the first study in the omicron era to describe precise post-immunisation anti-RBD antibody levels that give real-life clinical protection against COVID-19 in patients with IMID on immunosuppressive therapies. Prior literature describing the healthy population has shown that lower antibody levels are associated with increased risk of COVID-19 with both the wild-type and the omicron-variant.22 23 One omicron-era study on patients with inflammatory bowel disease could not identify a protective antibody level post-vaccination, only post-infection.24 Here, we found that post-vaccination antibody levels >6000 BAU/mL both following vaccination and infection resulted in better protection against symptomatic COVID-19. The protective antibody level of 6000 BAU/mL defined in our study cannot be directly transferred to other assays, as there is yet no recognised universal standardisation of anti-RBD antibody measurements. However, the cut-off defined in our study population was close to the median anti-RBD antibody level found in the overall cohort. It is thus reasonable to assume that a similar cut-off would be found approximately at the median level in other large IMID cohorts analysed using other platforms. In patients with only two vaccine doses, we could not identify a protective anti-RBD antibody level against clinical COVID-19. Importantly, the viral pressure in Norway was low in the observation period after distributing the second vaccine dose due to national shielding recommendations and only a few cases of COVID-19 (n=104) were registered in this time period. One could argue that measuring antibody levels is outdated as the virus continuously evolves. However, neutralising antibodies are shown to correlate with overall antibodies to multiple variants of the virus, and we believe measuring antibody levels is still of relevance in high-risk individuals post-vaccination to plan timing of the next booster dose.17
Evaluating the clinical protection offered by hybrid immunity is of importance in planning future vaccination strategies. We have previously shown in the same cohort that hybrid immunity induces a superior humoral response to that induced by vaccines alone.9 In the current study, we report that hybrid immunity results in higher anti-RBD antibody levels, and protects against COVID-19 re-infection. This finding, which is in line with prior studies in healthy individuals, is of significant clinical importance.25–27 As the SARS-CoV-2 virus evolves, a concern is that vaccination based on the original strain, will determine which antibodies are elicited when exposed to emerging variants (immune imprinting).28 However, hybrid immunity has been shown to enhance both B-cell and T-cell immunity compared with vaccination alone.29 30 Here, we demonstrate the clinical effectiveness of hybrid immunity compared with vaccine-induced immunity in protecting against COVID-19. Government-endorsed testing for COVID-19 has been abandoned in many countries, but as the clinical significance of hybrid immunity becomes clearer, there may be growing evidence to recommend individual testing if symptoms indicative of COVID-19 are present in this patient group.
Strengths of this study include a comprehensive and consistent follow-up of a large cohort of patients with IMID on immunosuppressive therapies over 2 years during different stages of the pandemic vaccination programme with regular blood sampling and questionnaires, through varying viral pressure, different dominating variants of concern and different national recommendations for shielding. This enabled monthly registration of COVID-19 and duration of symptoms, as well as standardised collection of serum samples following both vaccination and breakthrough infection. National registries ensured high-quality and validated data on severe COVID-19 and vaccination.
This study also has some limitations. We have been unable to adjust for shielding behaviour, and this may have influenced the rate of infections in particularly vulnerable patients. However, our finding that patients have the same incidence of COVID-19 overall as exposed healthcare workers is in line with previous results and may indicate no major difference in shielding behaviour between the two populations during the study period.31 According to a Dutch study, there was no difference in the incidence of breakthrough infections between healthy controls and patients with IMID.32 These findings support that missing data on infections in our material are unlikely to impact the comparison between these two groups. Another limitation is that we do not have national registration data on COVID-19 as this registration ceased from 24 January 2022. Data on non-severe COVID-19 are thus self-reported. Although requests for reporting infections were sent out monthly, we cannot exclude reporting bias between patients and healthy controls. Most asymptomatic infections have probably not been reported in both groups. Furthermore, in our analyses on protective antibody levels following hybrid immunity the number of re-infections is limited, and a larger sample size could possibly identify a lower threshold of protection. Nor-vaC was designed to address vaccine responses, and patients were included into the study from February 2021. We cannot fully address COVID-19 risk and outcomes in unvaccinated patients with IMID. Some patients with IMID may have died from severe COVID-19 during the first year of the pandemic prior to the start of the current study. Furthermore, due to few patients with severe COVID-19, we were not able to adjust for various confounders or explore predictors of this outcome.
In conclusion, vaccinated patients with IMID have a reassuringly low incidence of severe disease and death caused by SARS-CoV-2 despite a high occurrence of COVID-19 during long-term follow-up. As hybrid immunity protects better than vaccination alone against infection, clinical COVID-19 should be accommodated into vaccine programmes for this patient group.
A prior study from this cohort has shown that patients with IMID have a superior humoral response following hybrid immunity compared with vaccination alone.9 The present analyses demonstrate how this is translated into strong clinical protection for the following months, supporting to postpone vaccination at least 4–6 months following infection. This implies the need for testing if patients with IMID experience clinical symptoms of COVID-19.
Patients with anti-RBD antibody levels above a specific cut-off are better protected against COVID-19, compared with patients with weaker humoral responses. Evaluation of post-vaccination antibody responses could be considered in order to individualise the vaccination regimen in high-risk individuals.
Acknowledgments
We thank the patients and healthcare workers who have participated in the Norwegian study of vaccine response to COVID-19. We are grateful for the time and effort they have invested in the project. We thank the patient representatives in the study group, Kristin Isabella Kirkengen Espe and Roger Thoresen. Many people have contributed to the study design and implementation of the study at Diakonhjemmet Hospital, Akershus University Hospital and Oslo University Hospital. We thank Fridtjof Lund-Johansen and his group members Adity Chopra and Lisa Tietze for providing the serology data described in this study. We thank all study personnel, laboratory personnel and other staff involved at the clinical departments, particularly Synnøve Aure, Margareth Sveinsson, May Britt Solem and Kjetil Bergsmark.
Footnotes
@siljews
JTV, GLG, KKJ and SWS contributed equally.
Contributors: HSØ, JS and ATT verified all the data in the study. HSØ, GLG, KKJ and SWS made the final decision to submit the manuscript for publication. HSØ, KHB, IJ, ATT, IEC, LAM, SAP, JTV, GLG, KKJ and SWS conceived and designed the study. HSØ, GLG, KKJ and SWS drafted the manuscript. SM, TKK, GG, JJ, EAH, LAM, JTV, GLG, KKJ and SWS supervised the study. HSØ, KHB, IJ, ATT, GBK, SAP, JTV, GLG, KKJ and SWS contributed to acquisition of data. HSØ and JS contributed to statistical analysis. JS, ATT, GBK, EAH, LAM, JTV, GLG and KKJ contributed with administrative, technical or material support. EAH, LAM, JTV, GLG and KKJ obtained funding for this study. All authors contributed to interpretation and analysis of the data. All authors critically revised the manuscript and approved the final submitted version. All authors confirm they had full access to data in the study and accept responsibility for the decision to submit for publication. HSØ is the guarantor and accepts full responsibility for the work and/or the conduct of the study, had access to the data, and controlled the decision to publish
Funding: Nor-vaC was an investigator-initiated study with no initial funding. During its conduct, study grants were received from The Coalition for Epidemic Preparedness Innovations (CEPI); a KG Jebsen Foundation (grant 19); Dr Trygve Gythfeldt og frues forskningsfond; Karin Fossum Foundation; the Research Foundation at Diakonhjemmet Hospital; Oslo University Hospital; University of Oslo; the South-Eastern Norway Regional Health Authority. No commercial entity was involved in this study.
Disclaimer: The funders of the study had no role in design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript and decision to submit the manuscript for publication. The funders had no right to veto publication or to control the decision regarding which journal the paper was submitted. All drafts of the paper were prepared by the authors. All authors approved the final submitted version.
Competing interests: KHB reports funding from Akershus University Hospital and speakers bureau for Janssen-Cilag. TKK reports grants from AbbVie, Amgen, Bristol-Myers Squibb, Merck Sharp & Dohme, Novartis, Pfizer, Union Chimique Belge; consulting fees from AbbVie, Biogen, Celltrion, Eli Lilly, Gilead, Mylan, Novartis, Pfizer, Sandoz, Sanofi and speakers bureau from Amgen, Celltrion, Egis, Evapharma, Ewopharma, Hikma, Oktal, Sandoz and Sanofi. GG reports speakers bureau from Pfizer, Bayer, Sanofi, Thermo Fisher, GSK and Vitusapotek, and participation on advisory board for Moderna, AstraZeneca, Janssen and Seqirus. JJ reports grants from Boehringer Ingelheim; speakers bureau from AbbVie, Bristol-Myers Squibb, Galapagos, Gilead, Janssen, Pfizer, Roche, Sandoz and Takeda; and participation on advisory board for AbbVie, Bristol-Myers Squibb, Galapagos, Gilead, Janssen, Lilly, Pfizer, Roche, Sandoz and Takeda. EAH reports consultant fees from AbbVie, Boehringer Ingelheim, Eli Lilly and Gilead; and speakers bureau from Pfizer and UCB. LAM reports funding from KG Jebsen foundation, support for infrastructure and biobanking from the University of Oslo and Oslo University Hospital, grants from the Coalition of Epidemic Preparedness Innovations and speakers bureau from Novartis, and Cellgene. JTV reports grants from the Coalition of Epidemic Preparedness Innovations. GLG reports funding from the South-Eastern Norway Regional Health Authority, The Coalition for Epidemic Preparedness Innovations, RCN Covid (312693), a KG Jebsen Foundation (grant 19), Dr Trygve Gythfeldt og frues forskningsfond, Karen Fossum Foundation, the Research Foundation at Diakonhjemmet Hospital and Oslo University Hospital; speakers bureau from AbbVie, Galapagos, Pfizer and Union Chimique Belge; and participation on advisory board of AbbVie, Galapagos, Pfizer and Union Chimique Belge. KKJ reports speakers bureau from Bristol-Myers Squibb and Roche. SWS reported participation on advisory board AstraZeneca. All other authors declare no competing interests.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. A de-identified patient dataset can be made available to researchers on reasonable request. The data will only be made available after submission of a project plan outlining the reason for the request and any proposed analyses and will have to be approved by the Nor-vaC steering group. Project proposals can be submitted to the corresponding author. Data sharing will have to follow appropriate regulations.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study is approved by the Norwegian Regional Ethical Committees, and by appropriate institutional review boards (reference numbers 235424 and 135924). Participants gave informed consent to participate in the study before taking part.
References
- 1. MacKenna B, Kennedy NA, Mehrkar A, et al. Risk of severe COVID-19 outcomes associated with immune-mediated inflammatory diseases and immune-modifying therapies: a nationwide cohort study in the opensafely platform. Lancet Rheumatol 2022;4:e490–506. 10.1016/S2665-9913(22)00098-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Md Yusof MY, Arnold J, Saleem B, et al. Breakthrough SARS-CoV-2 infections and prediction of moderate-to-severe outcomes during rituximab therapy in patients with rheumatic and musculoskeletal diseases in the UK: a single-centre cohort study. Lancet Rheumatol 2023;5:e88–98. 10.1016/S2665-9913(23)00004-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Machado PM, Schäfer M, Mahil SK, et al. Characteristics associated with poor COVID-19 outcomes in people with psoriasis, psoriatic arthritis and axial spondyloarthritis: data from the COVID-19 psoprotect and global rheumatology alliance physician-reported registries. Ann Rheum Dis 2023;82:698–709. 10.1136/ard-2022-223499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chevalier K, Genin M, Jean TP, et al. Covaid: identification of factors associated with severe COVID-19 in patients with inflammatory rheumatism or autoimmune diseases. Front Med (Lausanne) 2023;10:1152587. 10.3389/fmed.2023.1152587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li H, Wallace ZS, Sparks JA, et al. Risk of COVID-19 among unvaccinated and vaccinated patients with rheumatoid arthritis: a general population study. Arthritis Care Res (Hoboken) 2023;75:956–66. 10.1002/acr.25028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sparks JA, Wallace ZS, Seet AM, et al. Associations of baseline use of biologic or targeted synthetic DMARDs with COVID-19 severity in rheumatoid arthritis: results from the COVID-19 global rheumatology alliance physician registry. Ann Rheum Dis 2021;80:1137–46. 10.1136/annrheumdis-2021-220418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Syversen SW, Jyssum I, Tveter AT, et al. Immunogenicity and safety of standard and third-dose SARS–Cov-2 vaccination in patients receiving immunosuppressive therapy. Arthritis Rheumatol 2022;74:1321–32. 10.1002/art.42153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Syversen SW, Jyssum I, Tveter AT, et al. Immunogenicity and safety of a three-dose SARS-Cov-2 vaccination strategy in patients with immune-mediated inflammatory diseases on immunosuppressive therapy. RMD Open 2022;8:e002417. 10.1136/rmdopen-2022-002417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bjørlykke KH, Ørbo HS, Tveter AT, et al. Four SARS-CoV-2 vaccine doses or hybrid immunity in patients on immunosuppressive therapies: a Norwegian cohort study. Lancet Rheumatol 2023;5:e36–46. 10.1016/S2665-9913(22)00330-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jyssum I, Kared H, Tran TT, et al. Humoral and cellular immune responses to two and three doses of SARS-CoV-2 vaccines in rituximab-treated patients with rheumatoid arthritis: a prospective, cohort study. Lancet Rheumatol 2022;4:e177–87. 10.1016/S2665-9913(21)00394-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alexander JL, Liu Z, Muñoz Sandoval D, et al. COVID-19 vaccine-induced antibody and T-cell responses in immunosuppressed patients with inflammatory bowel disease after the third vaccine dose (VIP): a multicentre, prospective, case-control study. Lancet Gastroenterol Hepatol 2022;7:1005–15. 10.1016/S2468-1253(22)00274-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Christensen IE, Jyssum I, Tveter AT, et al. The persistence of anti-spike antibodies following two SARS-Cov-2 vaccine doses in patients on immunosuppressive therapy compared to healthy controls-a prospective cohort study. BMC Med 2022;20:378. 10.1186/s12916-022-02587-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stalman EW, Wieske L, van Dam KPJ, et al. Breakthrough infections with the SARS-Cov-2 Omicron (B.1.1.529) variant in patients with immune-mediated inflammatory diseases. Ann Rheum Dis 2022;81:1757–66. 10.1136/ard-2022-222904 [DOI] [PubMed] [Google Scholar]
- 14. Hyams C, Challen R, Marlow R, et al. Severity of Omicron (B.1.1.529) and Delta (B.1.617.2) SARS-CoV-2 infection among hospitalised adults: a prospective cohort study in Bristol, United Kingdom. Lancet Reg Health Eur 2023;25:100556. 10.1016/j.lanepe.2022.100556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kared H, Wolf A-S, Alirezaylavasani A, et al. Immune responses in Omicron SARS-Cov-2 breakthrough infection in vaccinated adults. Nat Commun 2022;13:4165. 10.1038/s41467-022-31888-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bobrovitz N, Ware H, Ma X, et al. Protective effectiveness of previous SARS-CoV-2 infection and hybrid immunity against the Omicron variant and severe disease: a systematic review and meta-regression. Lancet Infect Dis 2023;23:556–67. 10.1016/S1473-3099(22)00801-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tran TT, Vaage EB, Mehta A, et al. Titers of antibodies against ancestral SARS-CoV-2 correlate with levels of neutralizing antibodies to multiple variants. NPJ Vaccines 2022;7:174. 10.1038/s41541-022-00586-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 19. Hasseli R, Richter JG, Hoyer BF, et al. Characteristics and outcomes of SARS-CoV-2 breakthrough infections among double-vaccinated and triple-vaccinated patients with inflammatory rheumatic diseases. RMD Open 2023;9:e002998. 10.1136/rmdopen-2023-002998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shoham S, Batista C, Ben Amor Y, et al. Vaccines and therapeutics for immunocompromised patients with COVID-19. EClinicalMedicine 2023;59:101965. 10.1016/j.eclinm.2023.101965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moss P. The T cell immune response against SARS-Cov-2. Nat Immunol 2022;23:186–93. 10.1038/s41590-021-01122-w [DOI] [PubMed] [Google Scholar]
- 22. Asamoah-Boaheng M, Goldfarb DM, Karim ME, et al. The relationship between anti-spike SARS-CoV-2 antibody levels and risk of breakthrough COVID-19 among fully vaccinated adults. J Infect Dis 2023;227:339–43. 10.1093/infdis/jiac403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yamamoto S, Mizoue T, Ohmagari N. Analysis of previous infection, vaccinations, and anti–SARS-CoV-2 antibody titers and protection against infection with the SARS-CoV-2 Omicron BA.5 variant. JAMA Netw Open 2023;6:e233370. 10.1001/jamanetworkopen.2023.3370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kennedy NA, Janjua M, Chanchlani N, et al. Vaccine escape, increased breakthrough and reinfection in Infliximab-treated patients with IBD during the Omicron wave of the SARS-CoV-2 pandemic. Gut 2023;72:295–305. 10.1136/gutjnl-2022-327570 [DOI] [PubMed] [Google Scholar]
- 25. Stein C, Nassereldine H, Sorensen RJD, et al. Past SARS-CoV-2 infection protection against re-infection: a systematic review and meta-analysis. The Lancet 2023;401:833–42. 10.1016/S0140-6736(22)02465-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nordström P, Ballin M, Nordström A. Risk of SARS-CoV-2 reinfection and COVID-19 hospitalisation in individuals with natural and hybrid immunity: a retrospective, total population cohort study in Sweden. Lancet Infect Dis 2022;22:781–90. 10.1016/S1473-3099(22)00143-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lin D-Y, Gu Y, Xu Y, et al. Association of primary and booster vaccination and prior infection with SARS-CoV-2 infection and severe COVID-19 outcomes. JAMA 2022;328:1415–26. 10.1001/jama.2022.17876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chemaitelly H, Ayoub HH, Tang P, et al. Long-term COVID-19 booster effectiveness by infection history and clinical vulnerability and immune imprinting: a retrospective population-based cohort study. Lancet Infect Dis 2023;23:816–27. 10.1016/S1473-3099(23)00058-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Barateau V, Peyrot L, Saade C, et al. Prior SARS-CoV-2 infection enhances and reshapes spike protein–specific memory induced by vaccination. Sci Transl Med 2023;15:eade0550. 10.1126/scitranslmed.ade0550 [DOI] [PubMed] [Google Scholar]
- 30. Shenoy P, Ahmed S, Paul A, et al. Hybrid immunity versus vaccine-induced immunity against SARS-CoV-2 in patients with autoimmune rheumatic diseases. Lancet Rheumatol 2022;4:e80–2. 10.1016/S2665-9913(21)00356-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Boekel L, Besten YR, Hooijberg F, et al. SARS-CoV-2 breakthrough infections in patients with immune-mediated inflammatory diseases during the Omicron dominant period. Lancet Rheumatol 2022;4:e747–50. 10.1016/S2665-9913(22)00221-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Boekel L, Stalman EW, Wieske L, et al. Breakthrough SARS-CoV-2 infections with the Delta (B.1.617.2) variant in vaccinated patients with immune-mediated inflammatory diseases using immunosuppressants: a substudy of two prospective cohort studies. Lancet Rheumatol 2022;4:e417–29. 10.1016/S2665-9913(22)00102-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2023-003545supp001.pdf (623.8KB, pdf)
Data Availability Statement
Data are available on reasonable request. A de-identified patient dataset can be made available to researchers on reasonable request. The data will only be made available after submission of a project plan outlining the reason for the request and any proposed analyses and will have to be approved by the Nor-vaC steering group. Project proposals can be submitted to the corresponding author. Data sharing will have to follow appropriate regulations.