Simian virus 40 (SV40) and murine polyomavirus (PyV) serve as powerful tools to identify key cellular regulatory proteins and to discern the mechanisms by which these proteins exert their biological effects. Like all members of the polyomavirus group, SV40 and PyV have simple genomes (Fig. 1) that can be divided into an early region that is expressed prior to the onset of viral DNA replication and encodes the viral tumor antigens (T antigens) and a late region that encodes the viral capsid proteins (VP1, VP2, and VP3) (for a review, see reference 6). For the most part, SV40 and PyV co-opt cellular machines for viral DNA replication and transcription. In cell culture systems, the first 20 h postinfection is dedicated to driving the infected cells into the cell cycle so that cellular proteins needed for viral replication are expressed and in redirecting cell macromolecular synthesis systems to function in viral replication, transcription, and virion assembly. During this period the only viral proteins expressed are the T antigens, which play three critical roles in productive infection. The viral T antigens (i) alter and/or recruit specific host cell proteins to participate in virus production, (ii) block cellular antiviral defense systems, and (iii) participate directly in viral replication (e.g., large T antigens are DNA helicases and are thus required to replicate viral DNA).
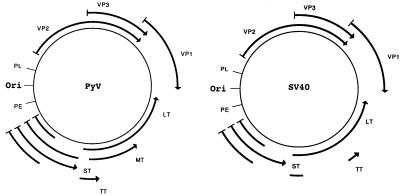
FIG. 1.
Genetic maps of SV40 and PyV. ori indicates position of the origin of DNA replication, PE is the early region promoter, PL is the late region promoter. Arrowed lines indicate coding sequences for viral proteins. Arrowhead indicates the carboxy terminus of the protein. LT, large T antigen; MT, middle T antigen; ST, small t antigen; and, TT, tiny t antigen.
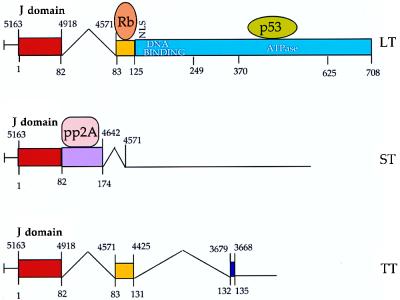
In the polyomaviruses, all the T antigens are encoded by a common precursor mRNA that is differentially spliced to yield multiple monocistronic mature mRNAs. SV40 expresses three such mRNAs, one each for large T antigen, small t antigen, and tiny t antigen, whereas PyV expresses four mRNAs, one each for large, middle, small, and tiny T antigens (Fig. 2). The splicing pattern is such that all T antigens encoded by a given virus have a common amino-terminal domain. The large, middle, and small T antigens are multifunctional proteins that carry a diverse array of biochemical activities (6, 27). Some of these activities reside in discrete functional domains, while others require interdomain interaction and/or the independent action of two or more domains. In cell culture, only the large T antigen (SV40) or the large and middle T antigens (PyV) are essential for productive infection and viral tumorigenicity. The small t antigens, while not absolutely essential, contribute to both infection and transformation. Little is known about the tiny T antigens (36, 54).
FIG. 2.
SV40 early region. Structure of the three early mRNAs is shown, along with a simplified domain map of the viral T antigens. Numbers above the line indicate SV40 genomic nucleotide position. Numbers below the line indicate amino acid position from the amino terminus of T antigen. Rb, retinoblastoma family-binding domain; p53, p53-binding region; pp2A, protein phosphatase 2A- binding domain.
T antigens mediate most of their biological effects by acting on specific cellular proteins (Table 1). For example, SV40 DNA replication requires the large T-antigen-mediated assembly of a preinitiation complex on the viral origin of replication (ori). T antigen binds ori directly through its DNA-binding domain, after which two T-antigen hexamers associate at ori in an ATP-dependent reaction. T antigen then recruits cellular proteins such as DNA polymerase α, primase, topoisomerase, and RPA to the complex via direct physical associations. Following initiation, each of the hexamers functions in the elongation reaction as a DNA helicase. Transcription control, specifically transactivation of the viral capsid genes and of specific cellular genes, is also mediated by the interaction of large T antigen with cellular transcription factors (9, 18). Finally, the transforming functions of the large, middle, and small t antigens are mediated by their direct physical association with cellular target proteins. For example, large T antigens bind the retinoblastoma protein family of tumor suppressors (pRb, p107, and p130), PyV middle T antigen associates with a number of components of the cellular signal transduction network during transformation, and small t antigen contributes to transformation by acting on the cellular protein phosphatase pp2A (Table 1). Thus, successful infection requires the ordered assembly and rearrangement of several different multiprotein complexes involved in DNA replication, gene expression, and virion assembly. Although the mechanism(s) used by the T antigens to effect these diverse processes has been obscure, recent evidence from a number of laboratories indicates that a class of proteins known as molecular chaperones may coordinate many aspects of polyomavirus infection.
TABLE 1.
Cellular proteins and protein complexes targeted by viral T antigensa
| LT | MT | ST |
|---|---|---|
| Transformation | ||
| pRb, p107, p130 | c-Src, c-Fyn, c-Yes | pp2A |
| p53b | pp2A | |
| CBP, p300, p400 | 14-3-3 | |
| mdm2 | Phosphatidylinositol3-kinase | |
| Insulin-like growth factor receptor | Phospholipase C-γ-1 | |
| SHC | ||
| Replication | ||
| DNA polymerase α | ||
| Primase | ||
| Single-stranded DNA binding protein RPA | ||
| Topoisomerase | ||
| Transcription | ||
| TATA binding protein | ||
| Sp1 | ||
| TEF-1 | ||
| RNA polymerase II (140K) | ||
| TFIIB |
Cellular proteins known to associate with SV40 large T antigen (LT); PyV middle T antigen (MT); and SV40 small t antigen (ST) (see references 6 and 27 for reviews). All of the proteins listed are thought to function as part of large multiprotein machines that function in DNA replication, transcription, and signal transduction.
PyV large T antigen does not associate with p53.
MOLECULAR CHAPERONES: ENGINEERS OF PROTEIN FUNCTION
Perhaps the best-studied molecular chaperones are the DnaK, DnaJ, and GrpE proteins, factors that were initially identified because mutations in the corresponding genes led to defects in phage λ replication (reviewed in reference 13). These chaperones possess unique activities, and homologs of each have been identified in all cell types from bacteria to humans.
DnaK is an ∼70-kDa ATPase whose synthesis is induced under heat shock or cellular stress conditions. Thus, proteins in the DnaK family are also known as Hsp70s (heat shock proteins with an apparent molecular weight of 70,000). DnaK is able to bind and release polypeptides concomitant with ATP binding and hydrolysis and possesses two distinct domains. The amino-terminal ∼44-kDa domain of DnaK is highly conserved among all DnaK family members, while the carboxyl ∼27-kDa domain is more variable and mediates substrate (i.e., polypeptide and possibly DnaJ) binding (10, 56).
The ATPase activity of DnaK is stimulated by DnaJ and GrpE. DnaJ is an ∼45-kDa molecular chaperone whose synthesis is also induced under stress conditions. DnaJ stimulates the rate-limiting hydrolysis of ATP of ADP on DnaK, thus locking polypeptide substrates into the chaperone (26). DnaJ family members have also been shown to bind directly some unfolded polypeptide substrates and folded proteins in order to exert specific chaperone-like activities (see below; reviewed in reference 16). The active interaction of DnaK and DnaJ chaperones is mediated by the J domain, a domain found in all DnaJ homologs, whose tertiary structure has been solved by nuclear magnetic resonance spectroscopy (17, 30, 34, 47). The J domain consists of four α-helices connected by loops and arranged such that helices 2 and 3 form a finger-like structure separated by the conserved sequence HPD (Fig. 3).
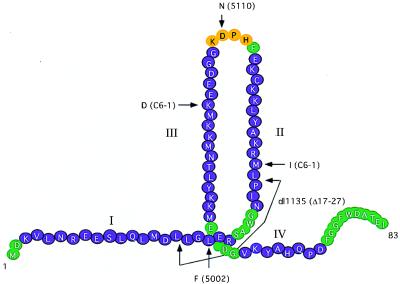
FIG. 3.
Depiction of the SV40 T-antigen J domain. I, II, III, and IV indicate α-helices as determined by sequence comparisons and secondary structure predictions. Positions of mutations in SV40 mutants 5110, dl1135, C6-1, C6-1R, and 5002 are shown. Although Spence and Pipas reported that 5002 was a double amino acid substitution mutant (42), subsequent studies have determined that the defective phenotype is due solely to the L19F change.
GrpE is a nucleotide exchange factor that stimulates ADP/ATP exchange on DnaK. While DnaJ stimulates the steady-state ATPase activity of DnaK by about 10-fold, GrpE elicits only a 2-fold increase in DnaK’s ATPase activity; however, the combined action of both chaperones can yield a >50-fold increase in the ATPase activity of DnaK (22, 26).
DnaK, DnaJ, and GrpE form a potent molecular machine capable of modulating a variety of cellular processes. DnaK, DnaJ, and GrpE facilitate bacteriophage λ DNA replication by disassembling a protein complex at the origin of replication: the ATP-catalyzed release of the λP and DnaJ proteins activates the DnaB helicase, an event required for the initiation of DNA replication (1, 57). During bacteriophage P1 plasmid replication, DnaJ binds to the inactive, dimeric replication protein, RepA. The addition of DnaK, GrpE, and ATP to the DnaJ- RepA complex dissolves the RepA dimer, forming active RepA monomers (51). A direct interaction between these chaperones and the heat shock-specific ς32 transcription factor has also been observed (12). In addition, the DnaK, DnaJ, and GrpE proteins have been shown to facilitate protein folding, repair thermally damaged proteins, and prevent protein aggregation both in vivo and in vitro (11, 21, 39, 41). Because they can prevent protein aggregation and may act as proteinaceous machines, some chaperones are required to promote protein transport across biological membranes (2). Finally, mutations in the genes encoding DnaK, DnaJ, and GrpE lead to defects in the degradation of misfolded or mutant proteins (reviewed in reference 15). Together, these results indicate that molecular chaperones can monitor the conformations of proteins, retain polypeptides in solution, directly facilitate protein folding and transport, and alter the activities of enzyme complexes.
POLYOMAVIRUS T ANTIGENS CONTAIN ACTIVE DNAJ DOMAINS
Kelley and Landry (19) and Cheetham et al. (4) independently reported that the J domain and the common amino-terminal sequences of polyomavirus T antigens share sequence identities. The J-domain sequence HPD (amino acids 42 to 44) in SV40 large and small T antigens is conserved among the T antigens of all sequenced polyomaviruses (31). The predicted secondary structures of this region of the T antigens and known J domains are nearly identical, including the placement of the HPD motif in a loop between two α-helices (Fig. 3) (3, 40, 43). Because the interaction of DnaJ chaperones with their DnaK partners requires the J domain, it was not surprising to discover, in retrospect, that SV40 T antigen associates with the mammalian cytosolic DnaK homolog, hsc70, and that this association is mediated through the T-antigen amino terminus (37, 38). Consistent with the notion that this interaction is mediated by the J domain, mutations in the HPD sequence abolish the interaction of hsc70 with large T antigen (3).
Recently, a number of reports have firmly established the presence of a functional J domain at the amino terminus of polyomavirus T antigens. First, an amino-terminal fragment of SV40 T antigen that includes the J domain stimulates the ATPase activity of both hsc70 and a cytosolic yeast hsc70, Ssa1p (43). Full-length large and small T antigens also stimulate Ssa1p ATPase activity, and this stimulation is partially blocked by antibodies directed to the amino terminus of T antigen and by mutations within the J domain. Second, full-length T antigen and the amino-terminal fragment of T antigen facilitate the ATP-dependent release of an unfolded polypeptide bound to Ssa1p, another hallmark of a productive DnaJ-DnaK interaction (43). The tiny t antigen encoded by PyV also stimulates hsc70 ATPase activity (36). Third, Kelley and Georgopolous have shown that the J domain of SV40 inserted into the corresponding domain of Escherichia coli DnaJ sustains bacterial growth and sensitivity to infection by bacteriophage λ (20); the J domains from the human polyomaviruses BK and JC also function in this system. Fourth, the J domain from the human HSJ-1 protein supports SV40 DNA replication when inserted into the corresponding region of T antigen (3, 45).
THE SV40 T ANTIGEN J DOMAIN IN VIRAL INFECTION AND TRANSFORMATION
Each polyomavirus T antigen has a J domain at the amino terminus, and in each case J-domain mutants are defective for some aspect of T-antigen function. Although T antigens with mutations in the J domain are frequently defective for DNA replication (29), it has proved difficult to identify the precise nature of the replication defect. This is because while J-domain mutants fail to replicate viral DNA in infected or transfected cells, the purified mutant T antigens exhibit all of the known biochemical activities necessary for replication and support various levels of SV40 DNA replication in vitro (7, 25, 50). Nonetheless, one replication-defective mutant, C6-1, contains two mutations (M30I and K51D) in the second and third helices of the J domain, respectively (Fig. 3) (14). Interestingly, a pseudorevertent of C6-1 known as C6-1R contains a mutation in helix 3 that restores viability, suggesting that the three helices in the J domain cooperate to effect viral DNA replication. On the contrary, one inviable mutant (5002) with two alterations (L19F and P28S) in the J domain does replicate viral DNA but is defective at a late stage of virion assembly (42) and some mutations in helix 1 and in the loop between helices 1 and 2 have no effect on DNA replication. Furthermore, T antigens not only act with specific cellular proteins but oligomerize. Thus, a mutant missing the entire J domain (Δ1-82) showed a defect in oligomerization (50), whereas a small deletion within the J domain (dl1135) shows normal oligomerization (7).
A final difficulty in examining mutations in the T-antigen J domain is that the penetrance displayed by the mutants is variable. For example, in SV40 the D44N mutant is defective for viral DNA replication in vivo. However, this mutant transforms established cell lines with nearly wild-type frequency. On the other hand, an amino-terminal fragment of T antigen transforms several cell types and, in this context, the D44N mutant is transformation defective (43). Since the J domain mediates the interaction of T antigen with hsc70, one possible explanation for this effect is that hsc70 binds T antigen through sequences near the carboxy terminus of T antigen in addition to the J domain. In this scenario, D44N in the full-length protein might bind a critical amount of hsc70 to function, whereas binding is defective in the absence of the carboxy terminus.
In-frame deletion mutants within the large T/small t common domain of SV40 T antigen are defective for the transformation of established cell lines (8, 32, 43, 55). Srinivasan et al. (43) reported that mutations in either the SV40 J domain or the T-antigen/Rb interaction motif (LXCXE motif) are transformation defective. Furthermore, these sequences must be in cis. This suggests that the J domain itself, or some cellular protein recruited by the J domain, such as hsc70, must act on the bound Rb to elicit transformation, a result consistent with reports that both the J domain and Rb-binding domains must be functional for T antigen to mediate increased degradation of p107 and p130 (45). More recently it has been shown that in SV40 and PyV, both the J domain and Rb-binding motifs are required to inactivate the tumor-suppressing functions of the Rb family (40, 44, 53).
The role of the SV40 J domain has also been examined in transgenic mice and further demonstrates that the J- and Rb-binding domains act in cis. When driven by the lymphotropic polyomavirus promoter, wild-type T antigen induces rapid tumorigenesis of the choroid plexus (5) but mutations in either the J domain (dl1135) or the Rb-binding domain (K1) prevent choroid plexus tumorigenesis (46). Furthermore, transgenic animals expressing both 1135 and K1 do not get tumors (49).
The J domain must also act in cis with some transforming function that maps to the carboxy-terminal half of T antigen (43). One possibility is that J-domain function is required for T antigen to block p53 tumor suppression functions. This would be consistent with the observations that (i) p53 binds to the carboxy-terminal half of T antigen, and (ii) a J domain mutant (dl1135) fails to block p53-mediated growth arrest (35). Another candidate for this transforming function is the CBP family of transcriptional adapter proteins (CBP, p300, and p400) (23). Transformation by the adenovirus E1A protein is mediated in part by E1A binding to this protein family. Transformation-defective mutants of E1A that fail to bind p300 are rescued by SV40 T antigen, and dl1135 is defective for this rescue (50), indicating that the J domain is required for the action of T antigen on the CBP family.
In contrast to the data presented above, there are two instances in which the J domain is not needed for some aspect of transformation. First, a T-antigen J-domain mutant (1135) is able to induce T-cell lymphomas in transgenic mice even though this same mutant is defective for tumorigenesis in other tissues such as the choroid plexus or B cells (46). Second, the T-antigen J domain is not essential to immortalize C57BL/6 mouse embryo fibroblasts (48). These results reemphasize that the penetrance displayed in vivo by J-domain mutants is variable.
T ANTIGENS TARGET MULTIPROTEIN COMPLEXES FOR CHAPERONE ACTION
The bacteriophage λ offers a paradigm as to how molecular chaperones might function in polyomavirus infection. During infection, λ DnaJ and DnaK free the DnaB helicase from its stable association with the multiprotein preinitiation complex at the viral origin of DNA replication. The energy needed to dissolve this association is provided by DnaK-mediated ATP hydrolysis and is used to alter the conformation of one or more members of the complex. However, there are important distinctions between λ and the polyomaviruses. First, the polyomaviruses encode their own DnaJ chaperones, the T antigens, and use them to recruit both hsc70 and the target cellular protein complexes. Second, the polyomaviruses use their chaperone machine for viral functions in addition to DNA replication, i.e., transcriptional control and virion assembly. However, one common theme that connects the disparate functions displayed by λ and polyomaviruses is the ensuing rearrangement of multiprotein complexes.
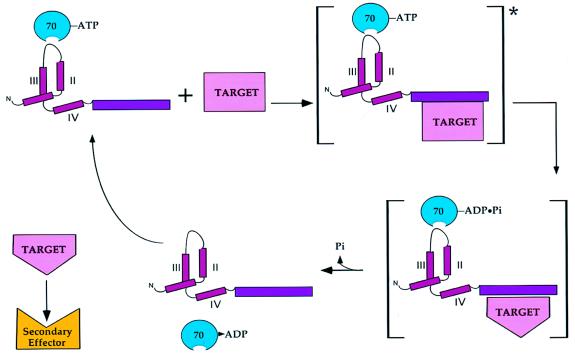
The fact that the J domain must act in cis with the Rb- and p53-binding domains to elicit transformation suggests a novel mechanism for T-antigen/chaperone action on tumor suppressors. The current model states that T antigen blocks tumor suppressor function by sequestering Rb proteins and p53 into stable, inactive complexes. The fact that chaperone action is needed in concert with the tumor suppressor-binding functions of T antigen suggests a more dynamic model (Fig. 4). In this model, T antigen recruits a target protein or protein complex into a ternary complex consisting of the target, T antigen, and hsc70. Energy, derived from ATP hydrolysis by hsc70, is then used to effect a conformational change on one or more components of the target complex, as occurs during λ DNA replication. For example, this energy might be used to release one or more proteins from an Rb-E2F complex to allow the E2F-dependent transcription of cellular genes that are required for viral replication.
FIG. 4.
Model for T-antigen chaperone action on a multiprotein complex. First, T antigen recruits ATP-bound hsc70 via the J domain and the target protein complex via a substrate-binding domain into an activated complex (asterisk). Next, energy derived from hsc70-mediated ATP hydrolysis induces a conformational change in one or more members of the target complex. This is followed by release of hsc70 and the altered target that can now act as an effector for downstream signaling events. II, III, and IV, J-domain α-helices.
Similarly, the rearrangement of large multiprotein complexes is a common feature of many cellular processes, including signal transduction, DNA replication, and transcriptional regulation, all of which are targets of T-antigen action (Table 1). Perhaps the T antigens act, in part, as scaffolds to recruit specific members of these complexes to associate with the chaperones. The chaperone machine would then be positioned to orchestrate the energy-dependent rearrangement of the multiprotein complex (Fig. 4).
FUTURE DIRECTIONS
Thus far, hsc70 is the only DnaK homolog found to associate with the T-antigen J domain. This does not exclude the possibility that known or new members of this family might be needed for viral infection, especially considering that mammals encode many DnaK and DnaJ homologs. For example, the incomplete penetrance of the SV40 D44N mutation (see above) might be due to the differential effects of this mutation on the interaction of T antigen with different DnaK homologs, one required for replication and another needed for transformation.
While it is clear that the action of T antigen on the Rb family requires a cis-acting J domain, there is one case in which a J-domain function can be supplied in trans. Small t antigen has been shown to transactivate the cyclin A gene, and transcriptional activation is blocked by mutations in the J domain (33). However, transcriptional activation is restored when a wild-type J domain is supplied in trans. Clearly we must decipher why some T-antigen chaperone functions must act in cis, while others may be supplied in trans.
The human papillomaviruses (HPV) and adenoviruses also encode transforming proteins that act on the Rb family and p53 tumor suppressors, but none of these proteins (E7, E6, E1A, or E1B 55K) possesses a J domain. If chaperone action is required for the modulation of tumor suppressor function, how do these proteins act? One intriguing possibility is that they may bind and recruit cellular DnaJ proteins, a hypothesis supported by the observation that the HPV18 E7 protein binds a human DnaJ homolog (28). Similarly, the replication of HPV11 DNA in vitro is greatly enhanced by the addition of exogenous hsp40 and hsc70, suggesting a role for chaperones in HPV replication (24).
While this research is in its infancy, it is already clear that molecular chaperones play a vital role in polyomavirus infection. Since work with polyomaviruses has shown that chaperone action is needed for several fundamental biological processes, i.e., DNA replication, transcription, and virion assembly, we suggest that many, if not all, viruses will either encode chaperones or recruit cellular chaperones. Such a hypothesis is readily testable and is already being investigated in numerous laboratories.
ACKNOWLEDGMENTS
This work was supported by grant CA40586 from the NIH to J.M.P. and by grant MCB9506002 from the NSF and a Junior Faculty Research Award from the American Cancer Society to J.L.B.
We thank Alison Slinskey and Jim Tremblay for help with the figures. We also thank Jim DeCaprio, Karl Munger, Terry Van Dyke, Tom Broker, and Louise Chow for allowing us to cite their unpublished observations.
REFERENCES
- 1.Alfano C, McMacken R. Heat shock protein-mediated disassembly of nucleoprotein structures is required for the initiation of bacteriophage λ replication. J Biol Chem. 1989;264:10709–10718. [PubMed] [Google Scholar]
- 2.Brodsky J L. Post-translational protein translocation: not all Hsp70s are created equal. Trends Biochem Sci. 1996;21:121–126. [PubMed] [Google Scholar]
- 3.Campbell K S, Mullane K P, Aksoy I A, Stubdal H, Zalvide J, Pipas J M, Silver P A, Roberts T M, Schaffhausen B S, DeCaprio J A. DnaJ/hsp40 chaperone domain of SV40 large T antigen promotes efficient viral DNA replication. Genes Dev. 1997;11:1098–1110. doi: 10.1101/gad.11.9.1098. [DOI] [PubMed] [Google Scholar]
- 4.Cheetham M E, Brion J P, Anderton B H. Human homologues of the bacterial heat-shock protein DnaJ are preferentially expressed in neurons. Biochem J. 1992;284:469–476. doi: 10.1042/bj2840469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen J, Tobin G J, Pipas J M, Van Dyke T. T antigen mutant activities in vivo: roles of p53 and pRB binding in tumorigenesis of the choroid plexus. Oncogene. 1992;7:1167–1175. [PubMed] [Google Scholar]
- 6.Cole C N. Polyomavirinae: the viruses and their replication. In: Fields B, et al., editors. Virology. 3rd ed. New York, N.Y: Lippincott-Raven; 1996. pp. 1997–2026. [Google Scholar]
- 7.Collins B S, Pipas J M. T antigens encoded by replication-defective SV40 mutants dl1135 and 5080. J Biol Chem. 1995;270:15377–15384. doi: 10.1074/jbc.270.25.15377. [DOI] [PubMed] [Google Scholar]
- 8.Conzen S D, Cole C N. The three transforming regions of SV40 T antigen are required for immortalization of primary mouse embryo fibroblasts. Oncogene. 1995;11:2295–2302. [PubMed] [Google Scholar]
- 9.Damania B, Alwine J C. TAF-like function of SV40 large T antigen. Genes Dev. 1996;10:1369–1381. doi: 10.1101/gad.10.11.1369. [DOI] [PubMed] [Google Scholar]
- 10.Flaherty K M, DeLuca-Flaherty C, McKay D B. Three dimensional structure of the ATPase fragment of a 70K heat shock cognate protein. Nature. 1990;346:623–628. doi: 10.1038/346623a0. [DOI] [PubMed] [Google Scholar]
- 11.Gaitanaris G A, Papavassiliou A G, Rubock P, Silverstein S J, Gottesman M E. Renaturation of denatured λ repressor requires heat shock proteins. Cell. 1990;61:1013–1020. doi: 10.1016/0092-8674(90)90066-n. [DOI] [PubMed] [Google Scholar]
- 12.Gamer J, Bujard H, Bukau B. Physical interaction between heat shock proteins DnaK, DnaJ, and GrpE and the bacterial heat shock transcription factor ς32. Cell. 1992;69:833–842. doi: 10.1016/0092-8674(92)90294-m. [DOI] [PubMed] [Google Scholar]
- 13.Georgopoulos C, Liberek K, Zylicz M, Ang D. Properties of the heat shock proteins of Escherichia coli and the autoregulation of the heat shock response. In: Morimoto R I, Tessieres A, Georgopoulos C, editors. The biology of heat shock proteins and molecular chaperones. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1994. pp. 209–249. [Google Scholar]
- 14.Gluzman Y, Ahrens B. SV40 early mutants that are defective for viral DNA synthesis but competent for transformation of cultured rat and simian cells. Virology. 1982;22:78–92. doi: 10.1016/0042-6822(82)90296-3. [DOI] [PubMed] [Google Scholar]
- 15.Gottesman S, Wickner S, Maurizi M R. Protein quality control: triage by chaperones and proteases. Genes Dev. 1997;11:815–823. doi: 10.1101/gad.11.7.815. [DOI] [PubMed] [Google Scholar]
- 16.Hartl F U. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–580. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- 17.Hill R B, Flanagan J M, Prestegard J H. 1H and 15N magnetic resonance assignments, secondary structure, and tertiary fold of Escherichia coli DnaJ(1–78) Biochemistry. 1995;34:5587–5596. doi: 10.1021/bi00016a033. [DOI] [PubMed] [Google Scholar]
- 18.Johnston S D, Yu X M, Mertz J E. The major transcriptional transactivation domain of simian virus 40 large T antigen associates nonconcurrently with multiple components of the transcriptional preinitiation complex. J Virol. 1996;70:1191–1202. doi: 10.1128/jvi.70.2.1191-1202.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelley W L, Landry S J. Chaperone power in a virus? Trends Biochem Sci. 1994;19:277–278. doi: 10.1016/0968-0004(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 20.Kelley W L, Georgopolous C. The T/t common exon of SV40, JCV, and BK polyomavirus T antigens can functionally replace the J-domain of the Escherichia coli DnaJ molecular chaperone. Proc Natl Acad Sci USA. 1997;94:3679–3684. doi: 10.1073/pnas.94.8.3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langer T, Lu C, Echols H, Flanagan J, Hayer M K, Hartl F U. Successive action of dnaK, dnaJ and GroEL along the pathway of chaperone-mediated folding. Nature. 1992;356:683–689. doi: 10.1038/356683a0. [DOI] [PubMed] [Google Scholar]
- 22.Liberek K, Marszalek J, Ang D, Georgopoulos C, Zylicz M. Escherichia coli dnaJ and grpE heat shock proteins jointly stimulate ATPase activity of dnaK. Proc Natl Acad Sci USA. 1991;88:2874–2878. doi: 10.1073/pnas.88.7.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lill N I, Tevethia M J, Eckner R, Livingston D M, Modjtahedi N. p300 family members associate with the carboxy terminus of simian virus 40 large tumor antigen. J Virol. 1997;71:129–137. doi: 10.1128/jvi.71.1.129-137.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu, J.-S., S.-R. Kuo, D. M. Cyr, T. R. Broker, and L. T. Chow. Personal communication.
- 25.Maulbecker C, Mohr I, Gluzman Y, Bartholomew J, Botchan M. A deletion in the simian virus 40 large T antigen impairs lytic replication in monkey cells in vivo but enhances DNA replication in vitro: new complementation function of T antigen. J Virol. 1992;66:2195–2207. doi: 10.1128/jvi.66.4.2195-2207.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCarty J S, Buchberger A, Reinstein J, Bukau B. The role of ATP in the functional cycle of the DnaK chaperone system. J Mol Biol. 1995;249:126–137. doi: 10.1006/jmbi.1995.0284. [DOI] [PubMed] [Google Scholar]
- 27.Messerschmitt A S, Dunant N, Ballmer-Hofer K. DNA tumor viruses and src family tyrosine kinases, an intimate relationship. Virology. 1997;227:271–280. doi: 10.1006/viro.1996.8316. [DOI] [PubMed] [Google Scholar]
- 28.Munger, K. Personal communication.
- 29.Peden K W C, Pipas J M. Simian virus 40 mutants with amino-acid substitutions near the amino-terminus of large T antigen. Virus Genes. 1992;6:107–118. doi: 10.1007/BF01703060. [DOI] [PubMed] [Google Scholar]
- 30.Pellecchia M, Szyperski T, Wall D, Georgopolous C, Wüthrich K. NMR structure of the J-domain and the Gly/Phe-rich region of the Escherichia coli DnaJ chaperone. J Mol Biol. 1996;260:236–250. doi: 10.1006/jmbi.1996.0395. [DOI] [PubMed] [Google Scholar]
- 31.Pipas J M. Common and unique features of T antigens encoded by the polyomavirus group. J Virol. 1992;66:3979–3985. doi: 10.1128/jvi.66.7.3979-3985.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pipas J M, Peden K W C, Nathans D. Mutational analysis of simian virus 40 T antigen: isolation and characterization of mutants with deletions in the T-antigen gene. Mol Cell Biol. 1983;3:203–213. doi: 10.1128/mcb.3.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Porras A, Bennett J, Howe A, Tokos K, Bouck N, Henglein B, Sathyamangalam S, Thimmapaya B, Rundell K. A novel simian virus 40 early-region domain mediates transactivation of the cyclin A promoter by small-t antigen and is required for transformation in small-t antigen-dependent assays. J Virol. 1996;70:6902–6908. doi: 10.1128/jvi.70.10.6902-6908.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qian Y Q, Patel D, Hartl F-U, McColl D J. Nuclear magnetic resonance solution structure of the human Hsp40 (HDJ-1) J-domain. J Mol Biol. 1996;260:224–235. doi: 10.1006/jmbi.1996.0394. [DOI] [PubMed] [Google Scholar]
- 35.Quartin R S, Cole C N, Pipas J M, Levine A J. The amino-terminal functions of the simian virus 40 large T-antigen are required to overcome wild-type p53-mediated growth arrest of cells. J Virol. 1994;68:1334–1341. doi: 10.1128/jvi.68.3.1334-1341.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riley M I, Yoo W, Mda N Y, Folk W R. Tiny T antigen: an autonomous polyomavirus T antigen amino-terminal domain. J Virol. 1997;71:6068–6074. doi: 10.1128/jvi.71.8.6068-6074.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sawai E T, Butel J S. Association of a cellular heat shock protein with simian virus 40 large T antigen in transformed cells. J Virol. 1989;63:3961–3973. doi: 10.1128/jvi.63.9.3961-3973.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sawai E T, Rasmussen G, Butel J S. Construction of SV40 deletion mutants and delimitation of the binding domain for heat shock protein to the amino terminus of large T-antigen. Virus Res. 1994;31:367–378. doi: 10.1016/0168-1702(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 39.Schröder H, Langer T, Hartl F-U, Bukau B. DnaK, DnaJ, and GrpE form a cellular chaperone machinery capable of repairing heat-induced protein damage. EMBO J. 1993;12:4137–4144. doi: 10.1002/j.1460-2075.1993.tb06097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sheng Q, Denis D, Ratnofsky M, Roberts T M, DeCaprio J A, Schaffhausen B. The DnaJ domain of polyomavirus large T antigen is required to regulate Rb family tumor suppressor function. J Virol. 1997;71:9410–9416. doi: 10.1128/jvi.71.12.9410-9416.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Skowyra D, Georgopoulos C, Zylicz M. The E. coli dnaK gene product, the hsp70 homolog, can reactivate heat-inactivated RNA polymerase in an ATP hydrolysis-dependent manner. Cell. 1990;62:939–944. doi: 10.1016/0092-8674(90)90268-j. [DOI] [PubMed] [Google Scholar]
- 42.Spence S L, Pipas J M. SV40 large T antigen functions at two distinct steps in viron assembly. Virology. 1994;204:200–209. doi: 10.1006/viro.1994.1524. [DOI] [PubMed] [Google Scholar]
- 43.Srinivasan A, McClellan A J, Vartikar J, Marks I, Cantalupo P, Li Y, Whyte P, Rundell K, Brodsky J L, Pipas J M. The amino-terminal transforming region of simian virus 40 large and small T antigens function as a J-domain. Mol Cell Biol. 1997;17:4761–4773. doi: 10.1128/mcb.17.8.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stubdal H, Zalvide J, Campbell K S, Schweitzer C, Roberts T M, DeCaprio J A. Inactivation of pRB-related proteins p130 and p107 mediated by the J domain of simian virus 40 large T antigen. Mol Cell Biol. 1997;17:4979–4990. doi: 10.1128/mcb.17.9.4979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stubdal H, Zalvide J, DeCaprio J A. Simian virus 40 large T antigen alters the phosphorylation state of the RB-related proteins p130 and p107. J Virol. 1996;70:2781–2788. doi: 10.1128/jvi.70.5.2781-2788.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Symonds H S, McCarthy S A, Chen J, Pipas J M, Van Dyke T. Use of transgenic mice reveals cell-specific transformation by a simian virus 40 T-antigen amino-terminal mutant. Mol Cell Biol. 1993;13:3255–3265. doi: 10.1128/mcb.13.6.3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Szyperski T, Pellecchia M, Wall D, Georgopolous C, Wüthrich K. NMR structure determination of the Escherichia coli DnaJ molecular chaperone: secondary structure and backbone fold of the N-terminal region (residues 2-108) containing the highly conserved J domain. Proc Natl Acad Sci USA. 1994;91:11343–11347. doi: 10.1073/pnas.91.24.11343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tevethia M J, Lacko H A, Kierstead T D, Thompson D L. Adding an Rb-binding site to an N-terminally truncated simian virus 40 T antigen restores growth to high cell density, and the T common region in trans provides anchorage-independent growth and rapid growth in low serum concentrations. J Virol. 1997;71:1888–1896. doi: 10.1128/jvi.71.3.1888-1896.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van Dyke, T. Personal communication.
- 50.Weisshart K, Bradley M K, Weiner B M, Schneider C, Moarefi I, Fanning E, Arthur A K. An N-terminal deletion mutant of simian virus (SV40) large T antigen oligomerizes incorrectly on SV40 DNA but retains the ability to bind to DNA polymerase alpha and replicate SV40 DNA in vitro. J Virol. 1996;70:3509–3516. doi: 10.1128/jvi.70.6.3509-3516.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wickner S, Hoskins J, McKenney K. Function of DnaJ and DnaK as chaperones in origin-specific DNA binding by RepA. Nature. 1991;350:165–167. doi: 10.1038/350165a0. [DOI] [PubMed] [Google Scholar]
- 52.Yaciuk P, Carter M C, Pipas J M, Moran E. Simian virus 40 large T antigen expresses a biological activity complementary to the p300-associated transforming function of the adenovirus E1A gene products. Mol Cell Biol. 1991;11:2116–2124. doi: 10.1128/mcb.11.4.2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zalvide J, Stubdal H, DeCaprio J A. The J domain of simian virus 40 large T antigen is required to functionally inactivate RB family proteins. Mol Cell Biol. 1998;18:1408–1415. doi: 10.1128/mcb.18.3.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zerrahn J, Knippschild U, Winkler T, Deppert W. Independent expression of the transforming amino-terminal domain of SV40 large T antigen from an alternatively spliced third SV40 early mRNA. EMBO J. 1993;12:4739–4746. doi: 10.1002/j.1460-2075.1993.tb06162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhu J, Rice P W, Gorsch L, Abate M, Cole C N. Transformation of a continuous rat embryo fibroblast cell line requires three separate domains of simian virus 40 large T antigen. J Virol. 1992;66:2780–2791. doi: 10.1128/jvi.66.5.2780-2791.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhu X, Zhao X, Burkholder W F, Gragerov A, Ogata C M, Gottesman M E, Hendrickson W A. Structural analysis of substrate binding by the molecular chaperone DnaK. Science. 1996;272:1606–1614. doi: 10.1126/science.272.5268.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zylicz M, Ang D, Liberek K, Georgopoulos C. Initiation of λ DNA replication with purified host and bacteriophage encoded proteins: the role of the dnaK, dnaJ, and grpE heat shock proteins. EMBO J. 1989;8:1601–1608. doi: 10.1002/j.1460-2075.1989.tb03544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]