Abstract
With an increasing number of patients eligible for immune checkpoint inhibitors, the incidence of immune-related adverse events (irAEs) is on the rise. Dermatologic immune-related adverse events (D-irAEs) are the most common and earliest to manifest, often with important downstream consequences for the patient. Current guidelines lack clarity in terms of diagnostic criteria for D-irAEs. The goal of this project is to better define D-irAE for the purposes of identification, diagnosis, and future study of this important group of diseases.
The objectives of this project were to develop consensus guidance for an approach to D-irAEs including disease definitions and severity grading. Knowing that consensus among oncologists, dermatologists, and irAE subspecialists would be critical for usability, we formed a Dermatologic irAE Disease Definition Panel. The panel was composed of 34 experts, including oncologists, dermatologists, a rheumatologist, and an allergist/immunologist from 22 institutions across the USA and internationally. A modified Delphi consensus process was used, with two rounds of anonymous ratings by panelists and two virtual meetings to discuss areas of controversy. Panelists rated content for usability, appropriateness, and accuracy on 9-point scales in electronic surveys and provided free text comments. A working group aggregated survey responses and incorporated them into revised definitions. Consensus was based on numeric ratings using the RAND/UCLA Appropriateness Method with prespecified definitions.
Following revisions based on panelist feedback, all items received consensus in the second round of ratings. Consensus definitions were achieved for 10 core D-irAE diagnoses: ICI-vitiligo, ICI-lichen planus, ICI-psoriasis, ICI-exanthem, ICI-bullous pemphigoid, ICI-Grover’s, ICI-eczematous, ICI-eruptive atypical squamous proliferation, ICI-pruritus without rash, and ICI-erosive mucocutaneous. A standard evaluation for D-irAE was also found to reach consensus, with disease-specific exceptions detailed when necessary. Each disorder’s description includes further details on disease subtypes, symptoms, supportive exam findings, and three levels of diagnostic certainty (definite, probable, and possible).
These consensus-driven disease definitions standardize D-irAE classification in a useable framework for multiple disciplines and will be the foundation for future work. Given consensus on their accuracy and usability from a representative panel group, we anticipate that they can be used broadly across clinical and research settings.
Keywords: Immune Checkpoint Inhibitors, Inflammation, Immunotherapy, Guidelines as Topic
WHAT IS ALREADY KNOWN ON THIS TOPIC
Dermatologic immune-related adverse events (irAEs) are the earliest and most common irAE to occur from checkpoint inhibition.
D-irAEs have prognostic implications for patients and frequently disrupt cancer treatment.
Current classification schema for the different types of D-irAE are inexact, leading to missed opportunities for proper prognostication and optimal therapeutic decision-making.
WHAT THIS STUDY ADDS
The published diagnostic criteria for the 10 most common D-irAE subtypes enables a more precise diagnosis, as well as a shared understanding of standard evaluations and expectations when caring for a patient with a D-irAE.
HOW THIS MIGHT AFFECT RESEARCH, PRACTICE, OR POLICY
If employed in clinical trials and regular clinical care, the enhanced diagnostic specificity of D-irAEs, as defined by these consensus definitions, can guide personalized therapeutic strategies.
Furthermore, this consensus statement plays a crucial role in improving the understanding of typical D-irAE manifestations.
Their application can be expected to foster the development of more accurate predictive models, enhance prognostic capabilities and improve data capture in clinical trials.
Introduction
Immune checkpoint inhibitors (ICIs) are standard therapy for a growing number of advanced malignancies because of overall survival and durable response benefits.1–3 Importantly, they are increasingly being used in earlier stage disease. Twelve ICIs are currently approved by the US Food and Drug Administration (ipilimumab, pembrolizumab, nivolumab, cemiplimab, atezolizumab, durvalumab, avelumab, dostarlimab, relatlimab, tremelimumab, retifanlimab and toripalimab). It is estimated that 44% of patients with cancer in the USA are eligible for these immunomodulatory agents, which unleash T-cell activity against tumor cells.3 4
The immune-activating mechanism of ICIs often results in inflammatory toxicities, termed immune-related adverse events (irAEs); these are distinct from toxicities associated with traditional systemic cancer therapies and limit the therapeutic potential of ICIs.5 6 The majority of patients receiving ICI monotherapy and combination therapy experience irAEs, although most cases are grade 1 (mild) or 2 (moderate) according to Common Terminology Criteria for Adverse Events (CTCAE).7 While these agents can affect any organ, dermatologic irAEs (D-irAEs), also called cutaneous immune-related adverse events (CirAEs), are the most frequently reported and are often the earliest to manifest.7–11 D-irAEs encompass a broad spectrum of reactions, with maculopapular rash, pruritus, and vitiligo among the most commonly reported presentations, and bullous pemphigoid, lichenoid eruptions, psoriasis, and involvement of mucosal membranes, hair, and nails less commonly reported.1 7 12 13
The accurate diagnosis and proper management of D-irAEs, as well as development of prevention strategies, are challenging because of the lack of specific, standardized disease definitions and severity grading criteria that capture the heterogeneity of possible manifestations.14–16 For example, rash is typically reported as maculopapular, a non-specific eruption, which may be due to the lack of guidance in the CTCAE for classifying specific subtypes. Making an accurate diagnosis of rash subtype is critical for effective D-irAE treatment and for predicting rash associated morbidity/mortality risk; thus, diagnosing the D-irAE subtype may impact ICI management.8 17 18 Moreover, preventing the progression of toxicity may reduce the risk of ICI interruption, discontinuation, and use of systemic immunosuppression.1 5 13 19
Here, we report disease definitions for diagnosis and severity grading of D-irAEs. The definitions were developed through a Delphi consensus process similar to that which recently resulted in the first disease definitions for neurologic irAEs, in partnership with Project Data Sphere, an independent, not-for-profit initiative of the CEO Roundtable on Cancer.16 20 Guidance statements and descriptions are presented for dermatologic disease categories organized by most to least commonly reported. In addition to supporting diagnosis and clinical management, these definitions will improve the documentation of D-irAEs in ICI clinical trials, which often relies on non-dermatologists to assess and grade cutaneous toxicities. Precise definitions will also advance the clinical, histological, and immunophenotypical characterization of D-irAEs in clinical/translational research cohorts.21
Methods
A modified Delphi process was used with two iterations. A working group of oncodermatologists (SC, YS, NL) drafted a classification system with guidance statements and disease definitions to support the evaluation, diagnosis, and severity grading of D-irAEs. Core diagnoses for the draft were chosen based on the presentation and frequency of D-irAEs in epidemiologic and insurance claims data, in addition to clinical experience; the top 10 diagnoses were included.1 22 Notably, the intention of this framework is not to allow all clinicians to reach a definitive diagnosis in each case of D-irAE per se, but rather to allow each clinician, regardless of medical specialty or level of training, to reach a particular level of diagnostic certainty that may be appropriately limited. For example, certain diagnoses require evaluation by a board-certified dermatologist (BCD) to reach definite level of diagnosis.
The proposed system was reviewed by a panel of dermatologists, oncologists, and irAE subspecialists who were recruited via email based on their experience and expertise. Informed consent was implied with participation in the study, either by logging onto the virtual meetings or by filling out the survey for data collection purposes. This was detailed so that all participants understood participation with the study was considered implied consent. After participants were recruited, they were sent a round one survey asking for participants to grade all statements on usability. Group medians were categorized into ranges (1–3 not usable, 4–6 uncertain, 7–9 usable). Agreement was defined as <1/3 of ratings outside the 3-point range containing the median. Consensus was reached when the median rating fell in the 7–9 range with agreement. Afterward, a virtual meeting was held to discuss areas of disagreement. After compiling feedback, the core group of oncodermatologists made targeted changes to the proposed system and it was sent to participants as a round 2 survey, followed again by an optional virtual meeting. Items that reached consensus in round 1 and did not undergo substantial revisions were not re-rated. Final feedback was received which was used in the drafting of this manuscript and creation of the final proposed system. This Delphi process was exempted by the Massachusetts General Brigham Institutional Review Board (Protocol #2020P004146).
Results
The panel consisted of 34 clinical and subject matter experts who accepted the invitation to participate in the Delphi consensus process. Participants represented 22 different centers, including US medical centers in the Northeast (10), Midwest (2), South/Southeast (4), and West (3), as well as three international sites in Canada (2) and Ireland (1). Of the 34 participants, 26 (76%) completed the round 1 survey and 21 (62%) completed the round two survey. The 26-member panel who completed round 1 included dermatologists (16), medical oncologists (9), and an irAE subspecialist of a different medical specialty (1). The 20 participants who completed the round 2 survey included 12 dermatologists, 5 medical oncologists, and 3 other subspecialists. Data from all respondents in both rounds 1 and 2 were included in the final analysis.
The panel first identified the following unmet seven needs for D-irAE disease definitions (% of panel members identifying the issue): (1) adjudication of D-irAEs in clinical trials (88%); (2) classification of D-irAE phenotype for translational research (96%); (3) classification of patients for cohort studies (77%); (4) differentiating D-irAEs from alternative etiologies (77%); (5) grading D-irAE severity (81%); (6) identifying subclinical or mild disease (73%); (7) recognizing the spectrum of presentations (96%). Eight percent (n=2) of participants responded that there were ‘other’ unmet needs including: (1) definitions to allow for more accurate correlation of D-irAE and tumor response and (2) risk prediction (table 1).
Table 1.
Summary of median voting scores and percent agreement for general statements and items for the identification and management of D-irAEs
| General statements Round 1 |
Score | Measure |
| Please rate how accurately the lists of specific and non-specific morphologies capture the range of possible D-irAE presentations. | 7.7 (62%) | Accuracy |
| This list of core diagnoses was created from epidemiologic and insurance claims data. Please rate your agreement with the list of included D-irAE core diagnoses. | 7.7 (96%) | Agreement |
| Please rate the usability of this framework of increasing diagnostic specificity in your primary work setting. Consider whether you could practically apply this classification to your patients. | 7 (62%) | Usability |
| Please rate the overall appropriateness of the diagnostic workup. For the proposed tests, consider the value of the diagnostic information and whether the benefits outweigh any risks. | 8 (88%) | Appropriate |
| Timing and tempo: To be considered a D-irAE, symptoms must begin within 12 months of last infusion of the ICI therapy. Most D-irAEs, however, occur within 12 weeks of starting a new ICI. New onset D-irAEs beyond 6 months of starting a therapy are less common. Later onset D-irAEs generally develop insidiously whereas early onset D-irAEs are more likely to present acutely or subacutely. | 7.7 (65%) | Agreement |
| Exclusion of other etiologies: The diagnosis of all D-irAEs requires that other potential etiologies have been excluded by a work-up tailored to each patient. A careful history, baseline dermatologic exam, and ancillary data can help exclude or confirm pre-ICI dermatologic disease. Patients with known dermatologic disorders, particularly immune-mediated dermatologic conditions, are recommended to have a dermatologic examination prior to starting ICI therapy and be under a dermatologist’s or dermatology subspecialist’s care during ICI therapy, depending on the complexity of the patient’s dermatologic illness. | 9 (85%) | Agreement |
| Consideration of concurrent irAEs: Patients frequently have irAEs affecting multiple organ systems. The presence of a concurrent non-dermatologic irAE can be a clue that dermatologic symptoms represent an irAE. A dermatologic irAE can also prompt evaluation of other organ systems when known patterns of overlapping disease exist (ie, dermatomyositis). | 8.3 (85%) | Agreement |
| Improvement on holding drug and/or initiating corticosteroids: While improvement on holding ICI therapy or initiating corticosteroids is non-specific, this is expected in most patients with D-irAEs. Lack of improvement, particularly after several weeks of treatment with corticosteroids or another irAE therapy, should prompt re-consideration of diagnosis. | 7.7 (69%) | Agreement |
| For table 2, please rate how accurately the examples illustrate differing levels of severity for each dermatologic syndrome. | 8 (73%) | Accuracy |
| Autoantibodies: Some irAEs are associated with pathophysiologic antibodies. These antibodies may be known prior to ICI administration or be detected during evaluation of a D-irAE. These definitions do not distinguish whether there is an antibody present or not; they have instead been constructed to include criteria that ensure a relationship with immunotherapy such as onset after ICI and improvement with ICI cessation. Even if a patient has a known antibody prior to immunotherapy administration, the temporal association of irAE with an ICI suggests that the immunotherapy has contributed in some part to these symptoms. When naming a D-irAE in a patient with a known antibody, we recommend including the antibody as part of the diagnosis (for example, ‘Definite immune related bullous pemphigoid with BPAG 180 antibody’ or ‘probable immune-related bullous pemphigoid with positive 230 antibody’). Patients may additionally have abnormal antibodies after ICI therapy that are typically low titer. As many of these antibodies are non-specific and may be unrelated, a patient’s syndrome should be referenced back to known antibody syndromes before establishing a diagnosis with a given antibody. | 8 (81%) | Agreement |
| Paraneoplastic syndromes: Patients may have paraneoplastic syndromes exacerbated or triggered by ICI therapy. The distinction between a process that is driven by an underlying cancer and a process that is an irAE can have significant, often opposing, treatment implications. Similar to the approach to autoantibodies, these definitions have been constructed to include criteria that ensure a relationship with ICIs such as improvement after stopping the ICI. A patient may therefore initially have a ‘Possible’ or ‘Probable’ diagnosis before it becomes ‘Definite’ or an alternate diagnosis. The clinical decision for how to treat a patient is beyond the scope of these guidelines but will typically benefit from multidisciplinary collaboration. | 9 (96%) | Agreement |
| Please rate your agreement with the written statement on clinical trial adjudication. | 8 (96%) | Agreement |
| Round 2 | ||
| Please rate how accurately the lists of specific and non-specific morphologies capture the range of possible D-irAE presentations. | 8 (86%) | Accuracy |
| Please rate the usability of this framework of increasing diagnostic specificity in your primary work setting. Consider whether you could practically apply this classification to your patients. | 8 (81%) | Usability |
| (Revised statement) Timing and tempo: To be considered a D-irAE, symptoms must begin within 12 months of last infusion of the ICI therapy. Most D-irAEs, however, occur within 12 weeks of starting a new ICI. New onset D-irAEs beyond 6 months of starting a therapy are less common. | 8 (86%) | Agreement |
| (Revised statement) Improvement on holding drug and/or initiating corticosteroids: While not necessarily first line treatment, holding ICI therapy or initiating topical or systemic corticosteroids usually leads to improvement of D-irAEs. Lack of improvement, particularly after several weeks of treatment with topical or systemic corticosteroids or another irAE therapy, should prompt re-consideration of the D-irAE classification and diagnosis. | 7 (81%) | Agreement |
Each component could be scored on a scale of 1 to 9 (1–3, not usable; 4–6, uncertain; 7–9, usable). Agreement was defined as <1/3 of ratings outside the 3-point range containing the median. Green indicates consensus was reached, defined by when the median rating fell in the 7–9 range with agreement. Pink indicates consensus was not reached.
D-irAEs, dermatologic immune-related adverse events; ICIs, immune checkpoint inhibitors.
The first round included 63 items in the Delphi survey; 53 (84%) achieved consensus in round 1. Round 2 included 16 revised and six new components. All 22 components (100%) achieved consensus in round 2 (total of 69 consensus items). See table 2 for medians and range.
Table 2.
Summary of D-irAE disease definitions that achieved consensus in Delphi survey
| Core diagnoses Round 1 |
Subtypes | Symptoms | Examination findings | Labs/imaging | Diagnostic criteria | |
| (Accuracy) | (Accuracy) | (Accuracy) | (Appropriate) | (Accuracy) | (Usability) | |
| ICI-vitiligo | 9 (96%) | 8 (81%) | 9 (92%) | 9 (92%) | ||
| ICI-lichen planus | 9 (88%) | 9 (79%) | 9 (91%) | 8 (85%) | 8 (81%) | 8 (92%) |
| ICI-psoriasis | 9 (96%) | 8 (92%) | 8 (96%) | 8 (85%) | 8 (81%) | 8 (85%) |
| ICI-delayed type hypersensitivity (ICI-DTH) | 8 (67%) | 8 (71%) | 8 (71%) | 8 (92%) | 8 (77%) | 8 (73%) |
| ICI-bullous pemphigoid | 8 (92%) | 9 (100%) | 9 (96%) | 9 (88%) | 9 (84%) | 9 (81%) |
| ICI-Grover’s | 8 (88%) | 8 (92%) | 8 (96%) | 8 (88%) | 8 (76%) | 8 (81%) |
| ICI-eczematous | 8 (84%) | 8 (80%) | 8 (80%) | 8 (85%) | 8 (80%) | 8 (73%) |
| ICI-eruptive atypical squamous proliferation | 8 (83%) | 8 (80%) | 8 (79%) | 8 (81%) | ||
| ICI-erosive mucocutaneous | 8 (77%) | 8 (73%) | 8 (69%) | 8 (81%) | 8 (60%) | 8 (65%) |
| Round 2 | ||||||
| *ICI-pruritus without rash | 8 (90%) | 8 (95%) | 8 (90%) | 8 (81%) | 8 (86%) | 8 (86%) |
| ICI-exanthem (from ICI-DTH) | 8 (95%) | 8 (100%) | 8 (100%) | 8 (90%) | 8 (86%) | 8 (81%) |
| ICI-erosive mucocutaneous | 8 (90%) | 8 (95%) | 8 (85%) | 8 (100%) | 8 (81%) | 8 (81%) |
Each component could be scored on a scale of 1 to 9 (1–3 not usable; 4–6, uncertain; 7–9, usable). Agreement was defined as <1/3 of ratings outside the 3-point range containing the median. Green indicates consensus was reached, defined by when the median rating fell in the 7–9 range with agreement. Pink indicates consensus was not reached.
D-irAEs, dermatologic immune-related adverse events; ICI, immune checkpoint inhibitor.
Approach to D-irAE case definitions
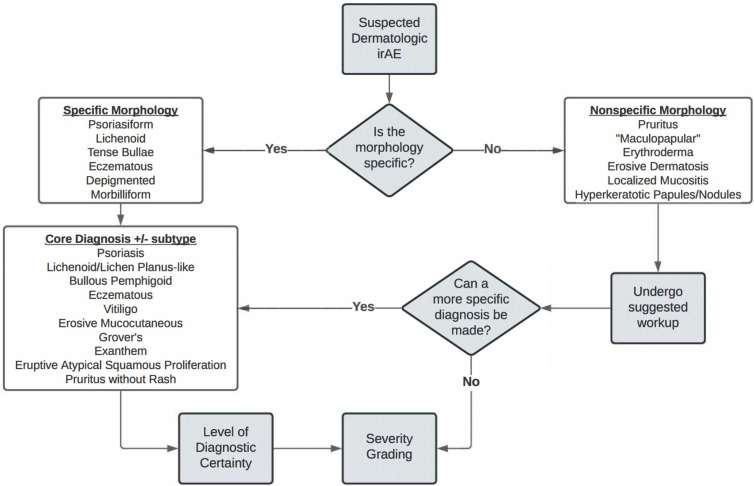
In the initial creation of the D-irAE framework, presenting morphology on physical examination was considered the first feature needed to classify D-irAE subtype (figure 1). This mimics clinical evaluation. When a patient presents with a possible D-irAE, the approach begins with identification of a specific morphology, when possible. Specific classifications include depigmented, lichenoid, psoriasiform, morbilliform, tense bullous, and eczematous. If one of these is present, an immediate move toward a core D-irAE diagnosis can be made. If one of the specific morphologies outlined above is not evident, or if the examiner is less familiar with these specific morphologic findings, one can move down the non-specific pathway and follow a suggested evaluation to reach a core diagnosis. If, despite the complete workup, a diagnosis is unable to be made, the patient’s eruption can remain ‘non-specific’. Both core diagnoses and ‘non-specific’ eruptions can undergo severity grading. The expert Delphi panel felt the list of specific and non-specific morphologies in the presented D-irAE framework accurately captured the range of the most common possible clinical presentations (median consensus score: 8, range 6–9). The panel felt that this list of diagnoses was consistent with what is generally seen in clinical practice (median: 8, range 5–9). Additionally, consensus was reached in considering usability of the framework in increasing diagnostic specificity (median: 8, range 3–9) (figure 1).
Figure 1.
Approach to diagnosis of dermatologic immune-related adverse events (D-irAEs). By following the algorithm, clinicians can use specific morphologic findings and suggested evaluation to reach a core D-irAE diagnosis with variable diagnostic certainty and severity grading. If a core diagnosis is not reached, a non-specific D-irAE can still be graded by severity.
Attribution of dermatologic syndrome to immune checkpoint inhibitor therapy
In considering different elements of the patient’s presentation that may help attribute a skin eruption to ICI therapy, the Delphi panel was asked to consider multiple factors that may play a role (online supplemental table S1).
jitc-2023-007675supp001.pdf (170.6KB, pdf)
Timing
While D-irAEs may present at a wide range of time points after ICI initiation, the panel felt by way of consensus (median: 8, range 5–9) that to be considered a D-irAE, symptoms must begin within 12 months of last infusion of the ICI therapy. Most D-irAEs, however, occur within 12 weeks of starting a new ICI. New onset D-irAEs beyond 6 months of starting therapy are less common for most morphologies.
Exclusion of other etiologies
The diagnosis of all D-irAEs requires that other potential etiologies have been excluded by an evaluation tailored to each patient. A history and baseline dermatologic examination can help exclude or confirm pre-ICI dermatologic disease. Patients with known dermatologic disorders, particularly immune-mediated dermatologic conditions, are recommended to have a dermatologic examination prior to starting ICI therapy and be under a dermatologist’s care during ICI therapy, depending on the complexity of the patient’s dermatologic illness (median: 9, range 6–9).
Consideration of concurrent non-dermatologic irAEs
Patients frequently have irAEs affecting multiple organ systems.23 Therefore, the presence of a concurrent non-dermatologic irAE increases the likelihood that dermatologic symptoms represent an irAE. A dermatologic irAE can also prompt evaluation of other organ systems, particularly when known patterns of overlapping disease exist (median: 8, range 6–9).
Improvement with holding drug and/or initiating corticosteroids
The panel agreed that, while not necessarily first-line treatment, holding ICI therapy or initiating topical or systemic immunosuppressive therapy usually leads to improvement of most D-irAEs. Lack of improvement, particularly after several weeks of treatment with topical or systemic corticosteroids or another irAE-targeted therapy, should prompt re-consideration of the D-irAE classification and diagnosis (median: 7, range 5–9).
Autoantibodies
Some irAEs are associated with pathophysiologic antibodies.24 25 These antibodies may be present prior to ICI administration, or be detected during evaluation of a D-irAE.25 The current definitions do not distinguish whether there is an antibody present or not or if the cutaneous eruption is occurring de novo or as a flare of pre-existing disease; they have instead been constructed to include criteria that suggest a relationship with immunotherapy (online supplemental table S2). A history of pre-existing dermatoses does appear to significantly increase the risk for development and increasing severity of D-irAEs. Even if a patient has a known antibody prior to immunotherapy administration, the temporal association of irAE with an ICI suggests that the immunotherapy has contributed in some part to these symptoms. When naming a D-irAE in a patient with a known antibody, we recommend including the antibody as part of the diagnosis (eg, ‘Definite immune related bullous pemphigoid with BPAG 180 antibody’ or ‘probable immune related bullous pemphigoid with positive BPAG 230 antibody’). Patients may additionally have abnormal antibodies after ICI therapy that are typically low titer and of unclear significance. As many of these antibodies are non-specific and may be unrelated, a patient’s syndrome should be referenced back to known antibody syndromes before establishing a diagnosis with a given antibody (median: 8, range 3–9).
Paraneoplastic syndromes
Patients may have paraneoplastic syndromes exacerbated or triggered by ICI therapy.26 27 The distinction between a process that is driven by an underlying cancer and a process that is an irAE can have significant, often opposing, treatment implications. Similar to the approach to autoantibodies, these definitions have been constructed to include criteria that suggest a relationship with ICIs (online supplemental table S2). A patient may therefore initially have a ‘Possible’ or ‘Probable’ diagnosis before it becomes ‘Definite’ or an alternate diagnosis. The clinical decision for how to treat a patient is beyond the scope of these guidelines but will typically benefit from multidisciplinary collaboration (median: 9, range 6–9).
Evaluation of all potential D-irAEs
The working group created a framework for a proposed evaluation for ICI-treated patients presenting with possible D-irAEs (Box 1). The evaluation may help move a non-specific eruption into a core diagnosis, or it may help determine whether the dermatologic findings are due to immunotherapy and with what degree of certainty. The Delphi panel reached a consensus that the proposed diagnostic algorithm is appropriate (median: 8, range 6–9).
Box 1. Recommended standard evaluation for D-irAEs.
STANDARD D-irAE WORKUP
Common:
Full skin examination by an experienced clinician
Full skin examination by a board-certified dermatologist
Skin biopsy for H&E
Laboratory evaluation to assess for evidence of systemic hypersensitivity reaction (CBC with differential, CMP, UA)
Possible:
Skin biopsy for direct immunofluorescence
ELISAs for antibody titers associated with autoimmune bullous disorders
ANA, ENA if photosensitivity component is noted
Uncommon/usually unnecessary:
For certain subtypes of D-irAEs, there are other specific evaluations that may be considered. These include:
Joint examination
Indirect immunofluorescence with salt-split skin
CBC, complete blood count; CMP, comprehensive metabolic panel; UA, urinalysis; ANA, antinuclear antibody; ENA, extractable nuclear antigens.
Notably, for certain diagnoses and unique morphologic presentations (noted below when applicable), a more specific evaluation is provided.
Core diagnosis disease definitions
For each of the core diagnoses (figure 2), the working group defined the classic morphologic findings, symptoms, and supportive examination findings associated with the condition. These include variants of disease and atypical presentations.
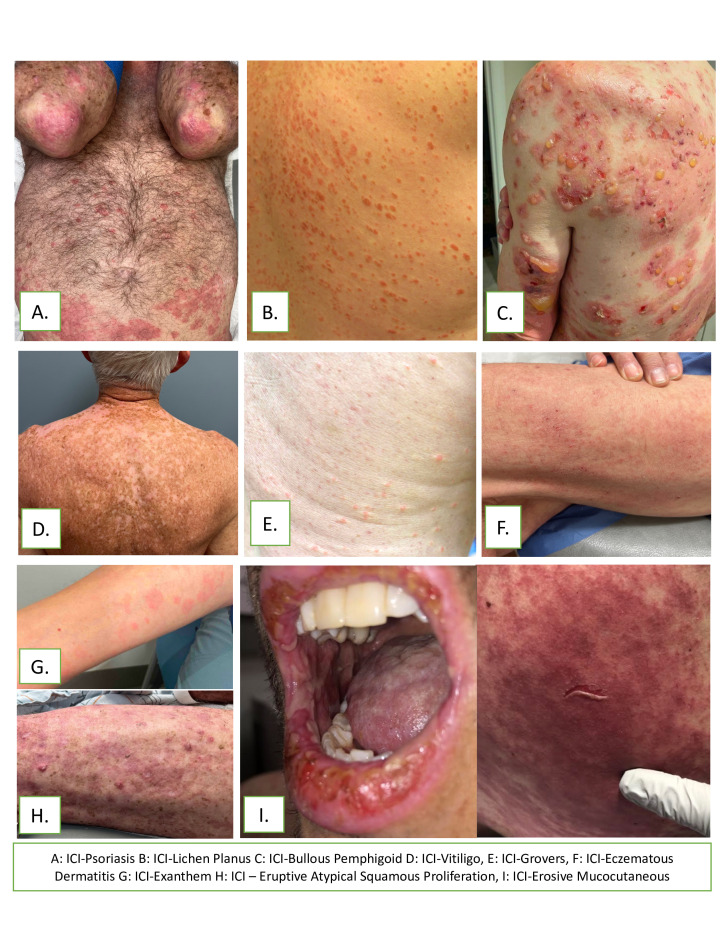
Figure 2.
Clinical photos of core dermatologic immune-related adverse events (D-irAE) diagnoses. (A) ICI-Psoriasis; (B) ICI-Lichen planus; (C) ICI-Bullous pemphigoid; (D) ICI-Vitiligo; (E) ICI-Grovers; (F) ICI-Eczematous dermatitis; (G) ICI-Exanthem; (H) ICI-Eruptive atypical squamous proliferation, (I) ICI-Erosive mucocutaneous. ICI, Immune checkpoint inhibitor.
Furthermore, for each core diagnosis, a proposed evaluation (in addition to the standard D-irAE evaluation) is presented when applicable.
Finally, each core diagnosis includes diagnostic criteria that will allow the evaluator to arrive at a definite, probable, or possible diagnosis of said D-irAE, given presence or absence of certain factors on examination or testing.
ICI-pruritus without rash
ICI-pruritus is a common D-irAE.1 22 Here, we define the specific diagnosis of ICI-pruritus without rash (box 1). If pruritus is seen in conjunction with a clinically apparent skin eruption, classification of said D-irAE should follow morphology of the eruption with pruritus as a supporting symptom.
ICI-pruritus may present as itch or burning; interference of sleep and activities of daily living (ADLs) may occur.28 The disease can be further characterized into two subtypes based on presentation: (1) localized and (2) generalized (online supplemental table S3).
The proposed evaluation for ICI-pruritus includes the standard D-irAE evaluation with the exception that skin biopsy is not commonly performed. Common diagnostic evaluation includes a full skin examination (FSE) including a test for dermatographism (firmly stroking the skin to assess for weal and flare response) to evaluate for histaminergic contribution to pruritus. Consideration of testing for bullous pemphigoid antibodies is advised, given prior literature demonstrating ICI-BP may be preceded solely by pruritus.29 Liver function tests, blood counts with differential, thyroid stimulated hormone, free thyroxine (T4), peripheral blood eosinophil count, IgE level, and iron studies should all be considered to evaluate for other underlying causes of or contributors to generalized pruritus.
Consensus by expert panel adjudication was reached for ICI-pruritus (median: 8, range 5–9), the symptoms (median: 8, range 5–9), the supportive exam findings (median: 8, range 5–9), labs/imaging (median: 8, range 3–9), the diagnostic criteria accuracy (median: 8, range 2–9), and usability (median: 8, range 5–9) (online supplemental table S4).
ICI-vitiligo
Vitiligo or vitiligo-like depigmentation is a specific D-irAE that is typically seen in the setting of melanoma therapy, although can occur less commonly with other tumor types.1 22 30 The diagnosis most commonly presents as sharply demarcated white (depigmented) macules or patches, which may appear pink after sun exposure, while the surrounding skin is typically normal or shows evidence of sun damage.31 Depigmentation may also occur at sites of prior inflammatory dermatoses. If the affected area is hair-bearing, the hair may turn white.31 A Wood’s lamp examination may be helpful to distinguish depigmentation from hypopigmentation as the latter is not as pronounced under illumination32 (online supplemental table S5).
Common diagnostic evaluation for ICI-vitiligo includes a FSE by a BCD or non-BCD and may include biopsies in cases without classic features or where there is morphologic overlap with other specific D-irAEs. If biopsies are obtained, although they are not required, they should be taken from the interface of pigmented and depigmented skin with notification of the pathologist to ensure the melanocytes are specifically confirmed to be absent. There are no labs that will rule in a diagnosis of ICI-vitiligo.
Consensus by expert panel adjudication was reached for the supportive examination findings (median: 9, range 6–9), labs/imaging (median: 8, range 3–9), diagnostic criteria accuracy (median: 9, range 5–9), and usability (median: 9, range 4–9) (online supplemental table S6).
ICI-lichen planus (ICI-LP)
Lichen planus, or lichenoid dermatitis, is among the most common specific inflammatory D-irAEs in patients receiving ICI therapy.1 The disease may present with or without significant itch and lesions may affect all body sites, including mucosal surfaces. ICI-LP can be further classified into six more common subtypes based on typical morphology: (1) hypertrophic, characterized by thick verrucous papules and plaques that favor the shins; (2) follicular, which involves hair on the body or scalp; scalp involvement can also cause scarring hair loss known as lichen planopilaris; (3) bullous, which occurs as a result of a florid interface dermatitis with subsequent loss of integrity of the basement membrane zone; bullae arise within existing plaques of LP and in uninvolved skin; (4) ulcerative, a variant that is usually seen on palms and soles; (5) pigmented, a variant that predominantly or entirely consists of hyperpigmented macules, usually seen on the face, arms, and upper torso; (6) mucosal, which consists of lacy, net-like, white patches and plaques with a violaceous base on the tongue or buccal mucosa; painful erosions and ulcers may also be seen, as well as atrophic, annular, and papular forms; lesions may also be seen on the conjunctivae, the vulva, vagina, glans penis, anus, tonsils, larynx, and throughout the gastrointestinal tract.33 Importantly, patients may present with multiple variants (eg, mucosal and bullous), making distinguishing severe LP from other diagnoses challenging34 (online supplemental table S7). Notably, this eruption has been associated with a significant survival benefit in the setting of ICIs.
The proposed workup for ICI-LP includes the standard D-irAE evaluation. Common diagnostic evaluation includes an FSE by a BCD and may include biopsies in cases without classic features or where there is morphologic overlap with other specific D-irAEs. Eosinophils in the infiltrate may or may not be present, although are commonly absent in sporadic LP. There are no definitive labs that will rule in a diagnosis of ICI-LP.
Consensus by expert panel adjudication was reached for the morphologic subtypes of ICI-LP (median: 9, range 3–9), the symptoms (median: 9, range 4–9), the supportive examination findings (median: 9, range 5–9), labs/imaging (median: 8, range 4–9), diagnostic criteria accuracy (median: 8, range 4–9), and usability (median: 8, range 3–9) (online supplemental table S8).
ICI-psoriasis
Psoriasis or psoriasiform dermatitis is a fairly common specific D-irAE,1 although the true incidence has likely been undercaptured; psoriasis is not a diagnosis found in the common terminology criteria for adverse event reporting guidelines used in clinical trials and by oncologists. Patients with a history of prior psoriasis develop flares of disease earlier than those who develop de novo disease after initiating ICI therapy; patients may also have a history of psoriatic arthritis or develop concomitant psoriatic arthritis.35
ICI-psoriasis may present with or without significant itch and may be associated with inflammatory joint symptoms.36 It can be further classified into six more common subtypes based on typical morphology: (1) plaque (psoriasis vulgaris), characterized by well-demarcated red or pink plaques with silvery/micaceous scale that favor extensor surfaces, scalp, scars, umbilicus, and gluteal cleft; (2) inverse, which presents as pink patches in inguinal, axillary, inframammary and gluteal folds, and may cause scaling and plaques on the genitalia; (3) guttate, which presents as diffuse pink or red papules (2–5 mm) with silvery scale, generally on the trunk or extremities; (4) palmoplantar, characterized by erythematous, scaling plaques over the palms and/or soles or significant hyperkeratosis with or without erythema; (5) pustular, which are patches and plaques of erythema with numerous pustules in areas forming lakes of pus; it may present with pustules limited to plaques, in an annular pattern or with sterile pustules and papules on the palms or soles; (6) erythrodermic, which consists of diffuse erythema and scaling over 80% body surface area, with active or prior plaques in classic locations, consistent nail findings and sparing of face.37 Patients may present with more than one subtype38 (online supplemental table S9).
The proposed workup for ICI-psoriasis includes the standard D-irAE evaluation. Additionally, workup may include joint examination, screening39 and consideration of imaging to rule in or out psoriatic arthritis (PsA); the presence of PsA would be supportive of a psoriasis diagnosis. There are no specific labs that will rule in a diagnosis of ICI-psoriasis, although laboratory evaluation can help rule out other etiologies.
Consensus by expert panel adjudication was reached for the morphologic subtypes of ICI-psoriasis (median: 9, range 6–9), the symptoms (median: 8, range 5–9), the supportive examination findings (median: 8, range 5–9), labs/imaging (median: 8, range 5–9), diagnostic criteria accuracy (median: 8, range 3–9), and usability (median: 8, range 1–9) (online supplemental table S10).
ICI-exanthem
ICI-exanthem is a common specific D-irAE that typically presents early in the ICI treatment course.40 Although there is some ambiguity in the name of this entity, the development of a type IV hypersensitivity (commonly manifested as an exanthematous/morbilliform drug rash) implies unique immunologic pathways that warrant a specific morphologic diagnosis.1 22
ICI-exanthem often presents with itching, but burning has been reported and some cases can be asymptomatic.40 ICI-exanthem can be classified into two morphologic subtypes based on the panel’s experience: (1) morbilliform, characterized by erythematous papules (3–4 mm) that may coalesce into plaques on the torso, extremities, and less commonly the face; (2) macular erythema, which involves geographic erythematous patches or thin scaly erythematous plaques. Later in the course of an ICI-exanthem, superficial desquamation is often present.
Notably, ICI-exanthem can precede more severe D-irAEs.40 As such, if patients who have been diagnosed with ICI-exanthem develop systemic symptoms, bullous lesions, or mucosal involvement, revision in D-irAE diagnostic category should be considered (online supplemental table S11).
The proposed workup for ICI-exanthem includes the standard D-irAE evaluation. Diagnostic evaluation for ICI-exanthem commonly includes an FSE by a BCD and may include biopsies in cases without classic features or where there is morphologic overlap with other specific D-irAEs. Laboratory studies may be done to rule out systemic hypersensitivity.
Consensus by expert panel adjudication was reached for the morphologic subtypes of ICI-exanthem (median: 8, range 5–9), the symptoms (median: 8, range 7–9), the supportive examination findings (median: 8, range 7–9), labs/imaging (median: 8, range 6–9), diagnostic criteria accuracy (median: 8, range 3–9), and usability (median: 8, range 3–9) (online supplemental table S12).
ICI-bullous pemphigoid (ICI-BP)
ICI-BP is an uncommon and challenging specific D-irAE that is usually seen in the context of PD-1 or PD-L1 inhibition.22 This disease process frequently results in discontinuation of ICI therapy, as well as the use of systemic immunosuppressants,41 although early diagnosis and targeted management can allow for continued ICI therapy in appropriate cases.42 ICI-BP cases tend to be more delayed in presentation than typical drug-induced BP, can manifest after a rash-free pruritic prodrome, may be ‘non-bullous’ with eczematous/urticarial plaques, or may present with bullae and other morphologies (eg, eczematous eruptions).41 These eruptions are important as they may have implications for tumor response and potentially survival.24
The morphologic findings of bullous pemphigoid as presented below reached consensus by expert panel adjudication. Specifically, the subtypes presented (median: 8, range 5–9), symptoms (median: 9, range 7–9), supportive examination findings (median: 9, range 5–9), labs/imaging (median: 9, range 5–9), diagnostic criteria accuracy (median: 9, range 3–9), and usability (median: 8, range 2–9) were all found to reach consensus (online supplemental tables S13, S14).
ICI-BP usually presents with, and may be preceded by, significant pruritus, and may be associated with mucosal symptoms/erosions.41 The disease can be further classified into four subtypes based on morphology: (1) classic BP, characterized by tense vesicles and bullae, open erosions with collarettes of scale, which may involve the oral mucosa in a minority of cases; some cases may be non-bullous with urticarial edematous plaques; (2) atypical-eczematous, which presents as scaly, moist plaques, with collarettes of scale and potential crust from dried serous drainage; (3) atypical-pruritus only, which does not have a clear rash but may have linear erosions from patient excoriation; (4) atypical-other, which could be any other type of skin eruption that may meet criteria based on biopsy and lab criteria.
Beyond the standard D-irAE workup, common diagnostic evaluation for ICI-BP includes two appropriately selected skin biopsies (one for direct immunofluorescence and one for standard H&E processing) and a serum test to determine bullous pemphigoid antibody titers. Possible evaluation may also include indirect immunofluorescence using the salt-split skin technique.
ICI-Grover’s disease
Grover’s disease is a D-irAE that may manifest as a flare of pre-existing disease or arise de novo.43 It may also be the presenting morphology of ICI-BP (type 4, atypical other, above).
ICI-Grover’s disease usually presents with significant but localized pruritus.43 The disease can be further classified into two subtypes based on morphology: (1) classic Grover’s, characterized by discrete red crusted 2–4 mm papules distributed on the trunk; lesions are typically excoriated; proximal extremities and neck can be affected, but palms, soles, and genitals are spared; (2) atypical-pustular or vesicular, which involves discrete pustules, papulopustules, or vesicles in a similar distribution to above.43
Consensus by expert panel adjudication was reached for the morphologic subtypes of ICI-Grover’s disease (median: 8, range 5–9), symptoms (median: 8, range 5–9), the supportive examination findings (median: 8, range 5–9), labs/imaging (median: 8, range 2–9), diagnostic criteria accuracy (median: 8, range 4–9), and usability (median: 8, range 4–9) (online supplemental tables S15, S16).
The proposed workup for ICI-Grover’s disease is the standard D-irAE evaluation. Common diagnostic evaluation includes an FSE and may include biopsies in cases without classic features or where there is morphologic overlap with other specific D-irAEs. There are no specific labs that will rule in a diagnosis of ICI-Grover’s disease.
ICI-eczematous dermatitis
Eczematous dermatitis is a D-irAE that may occur de novo or in patients with a history of atopy or other eczematous dermatitides.22 ICI-eczematous dermatitis can be further classified into five subtypes based on typical morphology: (1) atopic dermatitis-like, characterized by pink scaly patches and plaques, with a predilection for flexor surfaces; (2) nummular dermatitis; (3) contact-dermatitis-like; (4) erythrodermic; (5) dyshidrotic17 44 (online supplemental table S17).
The proposed workup for ICI-eczematous dermatitis includes the standard D-irAE evaluation. Additional possible workup would include a skin biopsy for direct immunofluorescence, labs for indirect immunofluorescence, and ELISAs for specific antibodies to rule out bullous pemphigoid. There are no specific labs that will rule in a diagnosis of ICI-eczematous dermatitis, although laboratory studies can rule out other diagnoses.
Consensus by expert panel adjudication was reached for the morphologic subtypes of ICI-eczematous dermatitis (median: 8, range 2–9), the symptoms (median: 8, range 4–9), the supportive examination findings (median: 8, range 4–9), labs/imaging (median: 8, range (5-9), the diagnostic criteria accuracy (median: 8, range 5–9), and usability (median: 8, range 5–9) (online supplemental table S18).
ICI-eruptive atypical squamous proliferation
The typical clinical presentation of ICI-eruptive atypical squamous proliferation includes multiple firm, rapidly growing, red, crusted papules and plaques, which can be intensely pruritic and eroded/ulcerated.45 Eruptive atypical squamous proliferation is frequently difficult to distinguish from the hypertrophic variant of LP and may represent a clinical continuum with hypertrophic LP (online supplemental table S19).
Lesions tend to involute spontaneously in contrast to cutaneous SCC, which continue to grow.46 Histologic overlap may make the diagnosis confusing and the pathologist should be made aware that both hypertrophic LP and eruptive atypical squamous proliferations are on the differential diagnosis.
The proposed workup for ICI-eruptive atypical squamous proliferation includes the standard D-irAE evaluation. Common diagnostic evaluation includes an FSE by a BCD and may include biopsies in cases without classic features or where there is morphologic overlap with other specific D-irAEs. There are no definitive specific labs that will rule in a diagnosis of ICI-eruptive atypical squamous proliferation.
Consensus by expert panel adjudication was reached for the supportive examination findings (median: 8, range 5–9), labs/imaging (median: 8, range 5–9), diagnostic criteria accuracy (median: 8, range 5–9), and usability (median: 8, range 4–9) (online supplemental table S20).
ICI-erosive mucocutaneous
Erosive mucocutaneous disease is a rare complication of ICI therapy and has been seen in isolation after ICI, or in some reports, after a second trigger/drug has been administered.47 There is considerable debate in the literature as to the prognostic implications of this category of disease, which highlights the importance of our ability to define it as a unique D-irAE. This presentation, given its severity, usually necessitates at least holding of the ICI, and often permanent discontinuation of ICI therapy. Additionally, systemic immunosuppression is often required.1 22
ICI-erosive mucocutaneous disease usually presents with skin pain that can be preceded by pruritus and more mild symptoms.47 The disease may be associated with mucosal symptoms/erosions and often spares the ocular mucosa. ICI-erosive mucocutaneous disease can be further classified into two subtypes based on morphology: (1) SJS/TEN-like, acute onset, characterized by quick tempo/onset (over maximum of 2 weeks without preceding rash) of erosive plaques with positive Nikolsky sign, with or without mucosal symptoms; (2) erosive LP-like, indolent onset, which involves slower tempo/onset (over two or more weeks, usually with a preceding eruption) of erosive plaques (online supplemental table S21).
Consensus by expert panel adjudication was reached for 3 of the categories for ICI-erosive mucocutaneous disease: subtypes (median: 8, range 3–9), symptoms (median: 8, range 3–9), and labs/imaging (median: 8, range 4–9). However, examination findings, diagnostic criteria accuracy and usability did not meet consensus in round 1. After further discussion in the consensus panel and editing of the definitions, the following categories did reach consensus in round 2: supportive examination findings (median: 8, range 5–9), diagnostic criteria accuracy (median: 8, range 3–9), and usability (median: 8, range 5–9) (online supplemental table S22).
Non-specific morphologies
Outside of the 10 core diagnoses discussed above, there exist other potential non-specific presentations that occur at higher frequencies. These non-specific presentations require an evaluation as described above in order to come to a core diagnosis, when possible. When a core diagnosis is not reachable, workup may at least reveal new data that will allow for more targeted therapy in addition to severity grading (online supplemental table S23). The non-specific morphologies include hyperkeratotic papules and plaques, isolated mucositis, or the previously often diagnosed ‘maculopapular rash.’ Importantly, we would encourage clinicians to avoid making a final diagnosis of these non-specific subtypes, but rather to use them as descriptive terms while undertaking the evaluation of patient with D-irAE.
Discussion
The above set of diagnostic criteria to assist in the evaluation of potential D-irAEs was developed by a multi-institutional, multidisciplinary panel using a Delphi consensus process, which was recently applied for the development of irAE-N consensus definitions.16 The accurate diagnosis of D-irAE subtype is critical for oncologists, dermatologists, and other specialists involved in the clinical care of patients who receive ICI as cancer therapy and experience an apparent cutaneous toxicity. The panel was established to include experts in both medical oncology and subspecialty care to ensure understandability and usefulness of the definitions. Specific diagnoses have different implications for oncologic prognosis,48 and when a core diagnosis is made, it can often be treated in a targeted fashion, with the goal of uncoupling the toxicity from the therapeutic effect of the ICI. While the treatment of each D-irAE subtype is beyond the scope of this Delphi process, the proper identification of each D-irAE subtype is critical in tailoring appropriate therapy that is both effective and the least detrimental to the oncology treatment plan for patients with cancer.
Different D-irAEs may also portend an elevated level of risk for the future development of other non-dermatologic irAEs. A recent study found that nearly half of patients who developed a D-irAE also had a non-dermatologic irAE, and in most cases, the D-irAE preceded other irAEs.49 Although the relationship between D-irAEs and other toxicities needs further study, it may be that patients with a D-irAE should be monitored more closely for the development of gastrointestinal, endocrine, and other irAEs, and this may allow for earlier detection and improved management of these toxicities.
In addition to the clinical setting, specific diagnoses of D-irAEs are critical for accurate data capture in clinical trials and for research involving data from trials. To date, the lack of specificity in the reporting of D-irAEs, especially in clinical trials, has created challenges in understanding the incidence of specific clinical morphologies and outcomes associated with these. The lack of specificity is due to a multitude of factors. An obvious challenge remains the absence of specific morphologies within the CTCAE. These have evolved to be more inclusive over the last several versions, making it possible to diagnose acneiform eruptions, among other specific toxicities. Even so, the limits of CTCAE are highlighted in that certain toxicities may be graded relatively lower despite severe morphologies, such as erosive mucocutaneous reactions or bullous pemphigoid, where less body surface area may be affected but erosions, mucosal damage or systemic symptoms may severely impact patient quality of life. Additionally, some presentations are still notably absent (psoriasis/psoriasiform eruptions) and thus are captured as non-specific rashes (most commonly ‘maculopapular rash’ in most clinical trial data. Additionally, clinicians need to have the knowledge to identify and label rash subtypes for specificity to be reported; to date, this has required dermatology training or involvement of dermatologists in clinical trials or clinical care. In the absence of someone familiar with the examination evaluating the full skin surface, eruptions are often collapsed as ‘maculopapular rash.’ While this diagnosis may mean the specific morphologic pattern of a morbilliform eruption to a BCD, it could be guttate psoriasis, lichenoid dermatitis, eczematous eruptions, or Grover’s disease to a non-BCD. Given this historical challenge with the use of the term ‘maculopapular rash’ to encompass all eruptions that have any macules or papules, the consensus panel settled on the term ICI-exanthem to capture the type IV delayed type hypersensitivity eruption that presents as a true maculopapular or morbilliform eruption. The use of the term maculopapular or its exclusion sparked considerable debate throughout the process, although ultimately consensus was reached. While maculopapular will still be undoubtedly used, ideally, it will be applied less frequently in cases where the specific D-irAE core diagnosis can be made. In addition to the relevance to clinical care and clinical trials, detailed and rigorous clinical phenotyping is essential for many areas of genomic and translational research. Having standardized clinical descriptions can allow for improved natural language text searching and have an impact on the characterization of paired blood and tissue samples. The rigorous classification may allow for the development of specific non-invasive blood biomarkers that have the potential to serve as predictive, diagnostic, and potentially even therapeutic targets that will personalize treatment. We hope the framework presented herein can assist clinicians as well as research personnel in the evaluation of a potential D-irAE related to a trial drug or in routine clinical practice, rendering a specific diagnosis whenever possible.
Importantly, while we have presented detailed diagnostic criteria for a limited set of 10 core diagnoses that are the most frequently encountered, we are aware of the wide breadth of potential cutaneous eruptions that occur in the setting of ICI therapy. It is important to emphasize that this framework is not meant to be exhaustive, but rather a tool to help the practicing researcher or clinician approach a potential D-irAE patient. Even if a core diagnosis is not reached, severity grading (online supplemental tables S24, S25) and appropriate evaluation will have been undertaken by following the framework proposed.
As we look to the future of patient care in oncology, D-irAEs will be an increasingly common occurrence given the increasing use of ICIs in the treatment of cancer. It is important for oncologists, dermatologists, and other specialists who may care for these patients to have a working knowledge of potential cutaneous adverse effects, including the nuances that exist.
Acknowledgments
We extend our sincere appreciation to Dr. Amanda Guidon for her invaluable contributions to the study design and development of materials for the project. The foundation of our research is built upon the Neuro irAE Consensus guidelines, a publication to which she led (https://pubmed.ncbi.nlm.nih.gov/34281989/). Her expertise and insights have been instrumental in shaping the direction and methodology of our study. We would also indebted for the tireless efforts of many on the team at Project Data Sphere past and present who have helped us bring this project to fruition, including Caitlin Grzeskowiak and Teillo Schaller.
Footnotes
@drsteventchen, @EugeneSemenovMD, @DrBetofMDPhD, @DrJNaidoo
Contributors: Authors SC, YS and NL created the first draft of the proposed D-irAE statements and diagnostic criteria. All authors participated substantially in the creation and revision involved in the Delphi consensus process, and all authors participated in critical review of the manuscript.
Funding: STC is supported by the Medical Dermatology Career Development Award from the Dermatology Foundation. YS is supported in part by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number K23AR080791. The work of NL was supported in part by NIH/NCI U54-CA225088. LCC funded by NIAMS K23AR075872; AM: This study was supported by the NIH/NCI Cancer Center Support Grant P30-CA008748
Competing interests: MS: Consulting with Amgen, AbbVie, Arcutis, Bausch Health, Boehringer Ingelheim, Bristol-Myers-Squibb, Eli Lilly Canada, Fresenius Kabi Canada, Galderma, Incyte, Janssen, L’Oreal Canada, LEO Pharmaceuticals, Merck, Novartis, Pierre Fabre, Pfizer, Sanofi, Sun Pharmaceuticals, UCB Canada.
LCC: Research funding- Bristol-Myers Squibb, Consulting- Amgen
AM: Research Funding: Amryt Pharma, Incyte Corporation, Kintara Therapeutics, Novartis, Novocure Consulting: ADC Therapeutics, Alira Health, AstraZeneca, Blueprint Medicines, Protagonist Therapeutics, OnQuality, and Janssen Royalties: UpToDate
CAN: CAN has received research grants from Boehringer Ingelheim and participated in an advisory board for work related to pustular psoriasis and palmoplantar pustulosis.
VH: Royalties from UpToDate
ML: Research funding: Onquality, Novartis, AZ, Lutris, Novocure; Consulting: Onquality, Novartis, AZ, Lutris, Novocure, La Roche Posay, Janssen
MC: PI for Blueprint, Cogent, Author for UpToDate
NRL: Consultant for or has received honoraria from Seattle Genetics, Sanofi, Bayer, Seattle Genetics, Sanofi, Silverback and Synox Therapeutics outside the submitted work.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s)
Ethics approval
This study involves human participants but Mass General Brigham IRB exempted the study given the minimal risk to participants who are not patients, but rather medical experts providing their opinion. Participants gave informed consent to participate in the study before taking part.
References
- 1. Wongvibulsin S, Pahalyants V, Kalinich M, et al. Epidemiology and risk factors for the development of cutaneous toxicities in patients treated with immune-Checkpoint inhibitors: A United States population-level analysis. J Am Acad Dermatol 2022;86:563–72. 10.1016/j.jaad.2021.03.094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gong J, Chehrazi-Raffle A, Reddi S, et al. Development of PD-1 and PD-L1 inhibitors as a form of cancer Immunotherapy: a comprehensive review of registration trials and future considerations. J Immunother Cancer 2018;6:8. 10.1186/s40425-018-0316-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Haslam A, Prasad V. Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor Immunotherapy drugs. JAMA Netw Open 2019;2:e192535. 10.1001/jamanetworkopen.2019.2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ellis SR, Vierra AT, Millsop JW, et al. Dermatologic toxicities to immune Checkpoint inhibitor therapy: A review of histopathologic features. J Am Acad Dermatol 2020;83:1130–43. 10.1016/j.jaad.2020.04.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kennedy LB, Salama AKS. A review of cancer Immunotherapy toxicity. CA Cancer J Clin 2020;70:86–104. 10.3322/caac.21596 [DOI] [PubMed] [Google Scholar]
- 6. Sibaud V. Dermatologic reactions to immune Checkpoint inhibitors: skin toxicities and Immunotherapy. Am J Clin Dermatol 2018;19:345–61. 10.1007/s40257-017-0336-3 [DOI] [PubMed] [Google Scholar]
- 7. Geisler AN, Phillips GS, Barrios DM, et al. Immune Checkpoint inhibitor-related Dermatologic adverse events. J Am Acad Dermatol 2020;83:1255–68. 10.1016/j.jaad.2020.03.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Puzanov I, Diab A, Abdallah K, et al. Managing toxicities associated with immune Checkpoint inhibitors: consensus recommendations from the society for Immunotherapy of cancer (SITC). J Immunother Cancer 2017;5:95. 10.1186/s40425-017-0300-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hassel JC, Heinzerling L, Aberle J, et al. Combined immune Checkpoint blockade (anti-PD-1/anti-CTLA-4): evaluation and management of adverse drug reactions. Cancer Treat Rev 2017;57:36–49. 10.1016/j.ctrv.2017.05.003 [DOI] [PubMed] [Google Scholar]
- 10. Sosa A, Lopez Cadena E, Simon Olive C, et al. Clinical assessment of immune-related adverse events. Ther Adv Med Oncol 2018;10:1758835918764628. 10.1177/1758835918764628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Deutsch A, Leboeuf NR, Lacouture ME, et al. Dermatologic adverse events of systemic anticancer therapies: cytotoxic chemotherapy, targeted therapy, and Immunotherapy. Am Soc Clin Oncol Educ Book 2020;40:485–500. 10.1200/EDBK_289911 [DOI] [PubMed] [Google Scholar]
- 12. Gault A, Anderson AE, Plummer R, et al. Cutaneous immune-related adverse events in patients with Melanoma treated with Checkpoint inhibitors. Br J Dermatol 2021;185:263–71. 10.1111/bjd.19750 [DOI] [PubMed] [Google Scholar]
- 13. Brahmer JR, Abu-Sbeih H, Ascierto PA, et al. Society for Immunotherapy of cancer (SITC) clinical practice guideline on immune Checkpoint inhibitor-related adverse events. J Immunother Cancer 2021;9:e002435. 10.1136/jitc-2021-002435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hsiehchen D, Watters MK, Lu R, et al. Variation in the assessment of immune-related adverse event occurrence, grade, and timing in patients receiving immune Checkpoint inhibitors. JAMA Netw Open 2019;2:e1911519. 10.1001/jamanetworkopen.2019.11519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hughes MS, Zheng H, Zubiri L, et al. Colitis after Checkpoint blockade: A retrospective cohort study of Melanoma patients requiring admission for symptom control. Cancer Med 2019;8:4986–99. 10.1002/cam4.2397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guidon AC, Burton LB, Chwalisz BK, et al. Consensus disease definitions for neurologic immune-related adverse events of immune Checkpoint inhibitors. J Immunother Cancer 2021;9:e002890. 10.1136/jitc-2021-002890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Coleman E, Ko C, Dai F, et al. Inflammatory eruptions associated with immune Checkpoint inhibitor therapy: A single-institution retrospective analysis with stratification of reactions by toxicity and implications for management. J Am Acad Dermatol 2019;80:990–7. 10.1016/j.jaad.2018.10.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Muntyanu A, Netchiporouk E, Gerstein W, et al. Cutaneous immune-related adverse events (irAEs) to immune Checkpoint inhibitors: A Dermatology perspective on management [Formula: see text]. J Cutan Med Surg 2021;25:59–76. 10.1177/1203475420943260 [DOI] [PubMed] [Google Scholar]
- 19. Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune Checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. J Clin Oncol 2018;36:1714–68. 10.1200/JCO.2017.77.6385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sphere PD. Immune-related adverse events. 2021. Available: https://www.projectdatasphere.org/research/programs/immune-related-adverse-events
- 21. Reynolds KL, Arora S, Elayavilli RK, et al. Immune-related adverse events associated with immune Checkpoint inhibitors: a call to action for collecting and sharing clinical trial and real-world data. J Immunother Cancer 2021;9:e002896. 10.1136/jitc-2021-002896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Le TK, Brown I, Goldberg R, et al. Cutaneous toxicities associated with immune Checkpoint inhibitors: an observational, Pharmacovigilance study. J Invest Dermatol 2022;142:2896–908. 10.1016/j.jid.2022.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shankar B, Zhang J, Naqash AR, et al. Multisystem immune-related adverse events associated with immune Checkpoint inhibitors for treatment of non-small cell lung cancer. JAMA Oncol 2020;6:1952–6. 10.1001/jamaoncol.2020.5012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nelson CA, Singer S, Chen T, et al. Bullous Pemphigoid after anti-programmed Death-1 therapy: A retrospective case-control study evaluating impact on tumor response and survival outcomes. J Am Acad Dermatol 2022;87:1400–2. 10.1016/j.jaad.2019.12.068 [DOI] [PubMed] [Google Scholar]
- 25. Bui A-TN, Singer S, Hirner J, et al. De Novo cutaneous connective tissue disease temporally associated with immune Checkpoint inhibitor therapy: A retrospective analysis. J Am Acad Dermatol 2021;84:864–9. 10.1016/j.jaad.2020.10.054 [DOI] [PubMed] [Google Scholar]
- 26. Manson G, Maria ATJ, Poizeau F, et al. Worsening and newly diagnosed Paraneoplastic syndromes following anti-PD-1 or anti-PD-L1 Immunotherapies, a descriptive study. J Immunother Cancer 2019;7:337. 10.1186/s40425-019-0821-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yatim A, Bohelay G, Grootenboer-Mignot S, et al. Paraneoplastic Pemphigus revealed by anti-programmed Death-1 Pembrolizumab therapy for cutaneous squamous cell carcinoma complicating Hidradenitis Suppurativa. Front Med (Lausanne) 2019;6:249. 10.3389/fmed.2019.00249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gandhi M, Oishi K, Zubal B, et al. Unanticipated toxicities from anticancer therapies: survivors' perspectives. Support Care Cancer 2010;18:1461–8. 10.1007/s00520-009-0769-1 [DOI] [PubMed] [Google Scholar]
- 29. Molina GE, Reynolds KL, Chen ST. Diagnostic and therapeutic differences between immune Checkpoint inhibitor-induced and idiopathic Bullous Pemphigoid: a cross-sectional study. Br J Dermatol 2020;183:1126–8. 10.1111/bjd.19313 [DOI] [PubMed] [Google Scholar]
- 30. Lo J, Hanania HL, Keiser MF, et al. Immune Checkpoint inhibitor-induced Vitiligo in cancer patients: characterization and management. Arch Dermatol Res 2023;315:1697–703. 10.1007/s00403-023-02577-7 [DOI] [PubMed] [Google Scholar]
- 31. Zottarelli F, Giorgione R, Gironi LC, et al. Vitiligo-like Depigmentation patterns in patients receiving Immunotherapy for metastatic Melanoma. Ital J Dermatol Venerol 2021;156:97–9. 10.23736/S2784-8671.18.06183-7 [DOI] [PubMed] [Google Scholar]
- 32. Bae JM, Lee RW. 365-nM Narrowband wood’s lamp for Vitiligo and Hypopigmentation disorders. J Am Acad Dermatol 2020;83:e283–4. 10.1016/j.jaad.2019.08.064 [DOI] [PubMed] [Google Scholar]
- 33. Gorouhi F, Davari P, Fazel N. Cutaneous and Mucosal Lichen Planus: a comprehensive review of clinical subtypes, risk factors, diagnosis, and prognosis. ScientificWorldJournal 2014;2014:742826. 10.1155/2014/742826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wat M, Mollanazar NK, Ellebrecht CT, et al. Lichen-Planus-Pemphigoides-like reaction to PD-1 Checkpoint blockade. J Cutan Pathol 2022;49:978–87. 10.1111/cup.14299 [DOI] [PubMed] [Google Scholar]
- 35. Yu Y, Zhou Y, Zhang X, et al. Immune Checkpoint inhibitors in the treatment of patients with cancer and preexisting psoriasis: A systematic review and meta-analysis of observational studies. Front Oncol 2022;12:934093. 10.3389/fonc.2022.934093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Verspohl SH, Holderried T, Behning C, et al. Prevalence, therapy and tumour response in patients with rheumatic immune-related adverse events following immune Checkpoint inhibitor therapy: a single-centre analysis. Ther Adv Musculoskelet Dis 2021;13. 10.1177/1759720X211006963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Boehncke W-H, Schön MP. Psoriasis. Lancet 2015;386:983–94. 10.1016/S0140-6736(14)61909-7 [DOI] [PubMed] [Google Scholar]
- 38. Bonigen J, Raynaud-Donzel C, Hureaux J, et al. Anti-Pd1-induced psoriasis: a study of 21 patients. J Eur Acad Dermatol Venereol 2017;31:e254–7. 10.1111/jdv.14011 [DOI] [PubMed] [Google Scholar]
- 39. Chang J, Litvinov IV, Ly C, et al. Utilization of the psoriasis epidemiology screening tool (PEST): A risk stratification strategy for early referral of Psoriatic arthritis patients to minimize irreversible erosive joint damage. J Cutan Med Surg 2022;26:600–3. 10.1177/12034754221128796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Weber JS, Kähler KC, Hauschild A. Management of immune-related adverse events and Kinetics of response with Ipilimumab. J Clin Oncol 2012;30:2691–7. 10.1200/JCO.2012.41.6750 [DOI] [PubMed] [Google Scholar]
- 41. Lopez AT, Khanna T, Antonov N, et al. A review of Bullous Pemphigoid associated with PD-1 and PD-L1 inhibitors. Int J Dermatol 2018;57:664–9. 10.1111/ijd.13984 [DOI] [PubMed] [Google Scholar]
- 42. Said JT, Talia J, Wei E, et al. Impact of biologic therapy on cancer outcomes in patients with immune Checkpoint inhibitor-induced Bullous Pemphigoid. J Am Acad Dermatol 2023;88:670–1. 10.1016/j.jaad.2022.06.1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chen W-S, Tetzlaff MT, Diwan H, et al. Suprabasal Acantholytic Dermatologic toxicities associated Checkpoint inhibitor therapy: A spectrum of immune reactions from Paraneoplastic Pemphigus-like to Grover-like lesions. J Cutan Pathol 2018;45:764–73. 10.1111/cup.13312 [DOI] [PubMed] [Google Scholar]
- 44. Hwang SJE, Carlos G, Wakade D, et al. Cutaneous adverse events (Aes) of anti-programmed cell death (PD)-1 therapy in patients with metastatic Melanoma: A single-institution cohort. J Am Acad Dermatol 2016;74:455–61. 10.1016/j.jaad.2015.10.029 [DOI] [PubMed] [Google Scholar]
- 45. Que SKT, Compton LA, Schmults CD. Eruptive squamous Atypia (also known as Eruptive Keratoacanthoma): definition of the disease entity and successful management via Intralesional 5-fluorouracil. J Am Acad Dermatol 2019;81:111–22. 10.1016/j.jaad.2018.10.014 [DOI] [PubMed] [Google Scholar]
- 46. Poole M, Schwartz RA, Lambert WC, et al. To treat or not to treat: PD-L1 inhibitor-induced Keratoacanthoma and squamous cell carcinoma. Arch Dermatol Res 2023;315:903–15. 10.1007/s00403-022-02468-3 [DOI] [PubMed] [Google Scholar]
- 47. Molina GE, Yu Z, Foreman RK, et al. Generalized Bullous Mucocutaneous eruption mimicking Stevens-Johnson syndrome in the setting of immune Checkpoint inhibition: A multicenter case series. J Am Acad Dermatol 2020;83:1475–7. 10.1016/j.jaad.2020.03.029 [DOI] [PubMed] [Google Scholar]
- 48. Tang K, Seo J, Tiu BC, et al. Association of cutaneous immune-related adverse events with increased survival in patients treated with anti-programmed cell death 1 and anti-programmed cell death ligand 1 therapy. JAMA Dermatol 2022;158:189–93. 10.1001/jamadermatol.2021.5476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Thompson LL, Krasnow NA, Chang MS, et al. Patterns of cutaneous and Noncutaneous immune-related adverse events among patients with advanced cancer. JAMA Dermatol 2021;157:577–82. 10.1001/jamadermatol.2021.0326 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2023-007675supp001.pdf (170.6KB, pdf)