Abstract
Objectives
To compare the characteristics of patients with type 2 diabetes mellitus in general practice and those included in randomised controlled trials on which clinical practice guidelines are based.
Design
Cross-sectional comparative study.
Setting
We asked 45 general practitioners from three French Departments to identify the 15 patients with type 2 diabetes mellitus they most recently saw in consultation. In parallel, we selected randomised controlled trials included in the Cochrane systematic review on which the clinical practice guidelines for type 2 diabetes mellitus were based.
Participants
We included 675 patients with type 2 diabetes mellitus, and data were collected from 23 randomised controlled trials, corresponding to 36 059 patients.
Outcome measures
Characteristics of general-practice patients were extracted from medical records by a unique observer. The same baseline characteristics of patients included in randomised controlled trials from the Cochrane systematic review were extracted and meta-analysed. We assessed standardised differences between these two series of baseline characteristics. A difference greater than 0.10 in absolute value was considered meaningful.
Results
General-practice patients were older than randomised controlled trial patients (mean (SD) 68.8 (1.1) vs 59.9 years (standardised difference 0.8)) and had a higher body mass index (mean (SD) 31.5 (6.9) vs 28.2 kg/m2 (standardised difference 0.5)) but smoked less (11.0% vs 29.3% (standardised difference −0.6)). They more frequently used antihypertensive drugs (82.1% vs 37.5% (standardised difference 1.2)) but less frequently had a myocardial infarction (7.6% vs 23.1% (standardised difference −1.1)).
Conclusions
Patients with type 2 diabetes mellitus cared for in general practice differ in a number of important aspects from patients included in randomised controlled trials on which clinical practice guidelines are based. This situation hampers the applicability of these guidelines. Future randomised trials should include patients who better fit the ‘average’ general-practice patient with type 2 diabetes mellitus to help improve the translation of study findings in daily practice.
Keywords: primary care, randomized controlled trial, general diabetes
STRENGTH AND LIMITATIOINS OF THIS STUDY.
Selected randomised controlled trials were published between 1995 and 2012 and patient care has evolved since then; however, actual recommendations are still based on these trials.
Some data were missing in trial reports, notably for cardiovascular history and drugs, which could have led to some overestimation or underestimation of the associated standardised differences.
We considered baseline characteristics of patients actually included in randomised controlled trials rather than selection criteria, which enabled us to estimate standardised differences.
General practitioners were from various areas and work organisations, and patients were randomly selected, which ensured generalisability of our results and limitation of bias."
Introduction
General practitioners are reluctant to apply the results of clinical trials to their patients.1 One of the main reasons is the lack of representativeness of patients included in these trials.2 In 2007, two studies, one of patients with asthma and the other of patients with hypertension, showed that a very small proportion of patients from general practice met the selection criteria of the randomised trials.3 4 More recently, in 2019, a third study showed the same kind of results for patients with type 2 diabetes mellitus.5 Indeed, older patients or those with multimorbidities are frequently excluded from such trials.6 However, primary care patients are frequently multimorbid.7 They also have less severe disease and more undifferentiated symptoms than patients included in randomised controlled trials.8
A common characteristic of the three studies is that they focused on the eligibility criteria of patients included in randomised controlled trials rather than the characteristics of patients actually included in these trials. The trials were also conducted 10–15 years ago, but the report of the hypertensive study acknowledged that “[m]ore recent trials showed participants’ profiles that better reflected those of the patients under treatment in a general practice”.4 Therefore, the representativeness question should be re-investigated, as well because the generalisability of systematic reviews, which are based on randomised controlled trials, to primary care patients is rarely discussed.9
Type 2 diabetes mellitus is one of the most common conditions managed in primary care. The latest clinical practice guidelines for the treatment strategy for glycaemic control in type 2 diabetes mellitus are mainly based on the 2013 Hemmingsen et al review.10 Therefore, we selected type 2 diabetes mellitus as an illustrating disease to assess how primary care patients may differ from patients included in the randomised controlled trials on which the associated clinical practice guidelines are based.
Methods
Study design and information sources
The present study was a comparative study. We used a sample of patients with type 2 diabetes mellitus that were included in a cross-sectional study in which general practitioners were invited to identify eligible patients. We also used theoretical values for patient characteristics derived from the Hemmingsen et al Cochrane review.10
Selection of general practitioners and patients
Eligible general practitioners were voluntary physicians practising on the date of the study from two different departments in France. We contacted by email eligible general practitioners who were practising as training supervisors from three different departments in France. These departments were selected for their diversity of practice area (rural or urban) and work organisation (only general practitioners, multidisciplinary health clinic, working alone). The patient study population consisted of adults aged 18 years or older with a diagnosis of type 2 diabetes mellitus in their medical records. We selected the last 15 eligible patients seen in consultation by each general practitioner. Information posted in the general practitioner’s waiting room was available for patients. We excluded patients who refused the use of their medical data had to tell their general practitioner and patients under 18 years of age.
Selection of the studies from the Cochrane review
Studies were eligible if they were randomised controlled trials included in the most recent Cochrane systematic review published in 2013 by Hemmingsen et al.10 We excluded reports of non-randomised trials, randomised controlled trials not written in English or reports of trials that did not describe patient baseline characteristics.
Data collection
We extracted data related to general practitioners, general-practice patients and randomised controlled trial patients by using three standardised spreadsheets that were developed and tested by the three authors.
General practitioner characteristics
For each general practitioner, we collected information on sex, age, distance from the hospital, practice area (rural, semi-rural, urban) and work organisation (multidisciplinary healthcare clinic, only physicians, working alone, other).
General-practice patient characteristics
From February to May 2022, data were collected from individual medical records by using a data extraction form. Prior to the visit, general practitioners had been asked to identify the 15 eligible patients with type 2 diabetes mellitus most recently seen. The following variables were collected: sex, age, cardiovascular risk factors (smoking status, weight and body mass index (BMI), serum cholesterol and triglycerides levels, blood pressure), diabetes duration, glycosylated haemoglobin level, serum creatinine level, cardiovascular history, glucose-lowering medications (oral glucose-lowering drug and insulin) and cardiovascular medications (antihypertensive drugs, lipid-lowering drugs and aspirin).
Randomised controlled trial patient characteristics
From trial reports, one of us (AD) collected selection criteria and patient baseline characteristics. The collected variables were the same as those collected for general-practice patients.
Statistical analysis
No sample size calculation was performed because no quantitative hypothesis was formulated. However, we asked each general practitioner to identify 15 patients and initially expected to have 50 general practitioners agreeing to participate in the study.
For general-practice patients, continuous data extracted from the medical fields are expressed as mean (SD) and binary variables as numbers (percentage). For patients included in randomised controlled trials, we performed a random meta-analysis for each baseline characteristic to assess a mean value (online supplemental appendix 1). This value was then considered a theoretical one to which summary statistics estimated from the sample of primary care patient baseline characteristics were compared. Hence, we assessed standardised differences, with a standardised difference of >0.10 (meaning a difference in means of more than 0.1 SD) denoting a meaningful difference.
bmjopen-2023-077582supp001.pdf (44.2KB, pdf)
Patients and public involvement
None.
Results
Characteristics of the general practitioners
Of the 71 general practitioners contacted by email, 45 agreed to participate and included 675 patients. One general practitioner refused to participate and 25 others did not answer. The mean (SD) age was 42.1 (8.0) years, and more than half of the general practitioners worked in a multidisciplinary clinic (table 1).
Table 1.
Characteristics of the general practitioners (n=45)
| Age (years), mean (SD) | 42.1 (8.0) |
| Female sex | 19 (42.2) |
| Distance from hospital (km), mean (SD) | 12.8 (10.3) |
| Location of the practice | |
| Urban | 13 (28.9) |
| Rural | 15 (33.3) |
| Semi-rural | 17 (37.8) |
| Work organisation | |
| Multidisciplinary healthcare clinic | 26 (57.8) |
| Only physicians | 17 (37.8) |
| Working alone | 1 (2.2) |
| Municipal health centre | 1 (2.2) |
Data are n (%) unless otherwise indicated.
Selection of the randomised controlled trials
Among the 28 trials included in the Cochrane systematic review, 23 were selected for this study, corresponding to 36 059 patients. Two reports were unavailable, one was written in Russian and another in Chinese, and the last one reported no patient baseline characteristics. References and characteristics of the 23 trials are in online supplemental appendices 2 and 3, table 1.
Comparison of baseline characteristics of primary care patients and patients included in randomised controlled trials
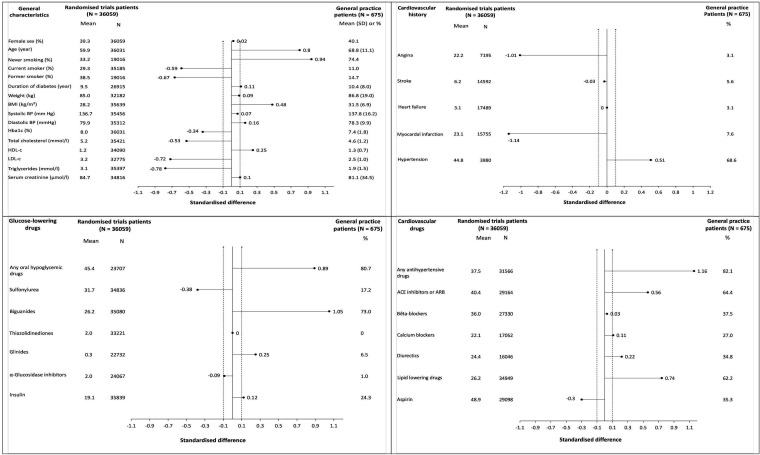
General-practice patients were older than randomised controlled trial patients (mean (SD) 68.8 (1.1) vs 59.9 years (standardised difference 0.8)), they had higher mean BMI (31.5 (6.9) kg/m2 vs 28.2 kg/m2 (standardised difference 0.5)) but smoked less (11.0% vs 29.3% (standardised difference −0.6)). They also had less history of myocardial infarction (7.6% vs 23.1% (standardised difference −1.1)). General-practice patients more frequently took oral hypoglycaemic drugs (82.1% vs 37.5% (standardised difference 1.2)) and anti-hypertensive drugs (82.1% vs 37.5% (standardised difference 1.2)) than randomised controlled trial patients (figure 1).
Figure 1.
Comparison of the characteristics of general practice patients and randomised trials patients (legends). Each subfigure displays the comparison of general practice patients to patients included in randomised trials. For the former, summary statistics (means and SD, or percentages) are reported on the right; for the former, a mean value (considered as a theoretical value) was obtained through meta-analyses of baseline characteristics of the patients included in the selected randomised trials. For each characteristic, a standardised difference between general practice and randomised patients was estimated and plotted. The dotted lines in −0.1 and 0.1 SD define the limits of a non-meaningful standardised difference. ACE inhibitor, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blockers; BMI, body mass index; BP, blood pressure; Hba1c, glycated haemoglobin; HDL-c, high-density lipoprotein cholesterol; LDL-c, low-density lipoprotein cholesterol.
Discussion
We found substantial differences in characteristics between general-practice type 2 diabetes mellitus patients and those included in the randomised controlled trials on which the associated clinical guidelines are based. General-practice patients were older, had higher BMI, more frequently took anti-hypertensive and lipid-lowering drugs but had less cardiovascular history than randomised controlled trial patients.
A strength of this study is that it considered baseline characteristics of patients actually included in randomised controlled trials rather than selection criteria. Doing so allowed us to estimate standardised differences, revealed to be very high for some variables, thus illustrating huge discrepancies between general-practice patients and patients included in randomised controlled trials. Another strength is that the general practitioners selected for this study were from various areas and work organisations, which ensured good generalisability of our results. Finally, we collected data from the last 15 patients seen in consultation with the general practitioner, thus leading to a randomly selected sample, which limits bias. However, the study has some limitations. First, the Hemmingsen et al review was published in 2013 and the randomised controlled trials included were published between 1995 and 2012. Also, patient care may have evolved. For example, cardiovascular risk in patients with type 2 diabetes mellitus has decreased with the use of angiotensin-converting enzyme inhibitors11 and the introduction of statins.12 This approach could explain why general-practice patients seen in consultation in 2022 were more likely to use angiotensin-converting enzyme inhibitors or lipid-lowering medications than participants of the randomised controlled trials. However, recommendations for control of glycosylated haemoglobin levels in patients with type 2 diabetes mellitus are still based on the results of these trials and to our knowledge, there has been no recent randomised trial. Furthermore, more recent studies of diabetes mellitus, especially testing new antidiabetic therapies, also found a low representativeness of patients included in the trials (in contrast to our study, they focused on eligibility criteria rather than inclusion characteristics).5 13 Second, our sample of general practitioners was a convenience sample rather than a random sample of general practitioners, as were the patients included in the study because we chose people who agreed to participate. This study population could be quite different from the ‘general population’ in terms of sociodemographic and other factors. A solution may have been to use population data sets or representative samples often taken from electronic health records as a control group; however, people in population data sets may not necessarily consult their general practitioner and therefore may not be representative of the patients consulting a general practitioner, as shown in the ecology of medical care by Green et al.14 Finally, some data were missing in trial reports, notably for cardiovascular history and drugs, which could have led to some overestimation or underestimation of the associated standardised differences. However, except for a history of angina, hypertension and stroke, mean values derived from meta-analyses of baseline patient characteristics of randomised controlled trials were based on more than 15 000 patients.
Conclusions
Randomised controlled trials of type 2 diabetes mellitus on which recommendations are based included participants that were not fully representative of general-practice patients. There is a need for studies including a wider range of patients with type 2 diabetes mellitus such as older patients or those with polypharmacy to ensure a better generalisability of the results.
Supplementary Material
Footnotes
BG and CD-D contributed equally.
Contributors: AD, CD-D and BG conceived the study. AD participated in data collection. AD extracted the data. BG and AD analysed data. AD wrote the first draft of the manuscript, which was revised by CD-D and BG. All coauthors read and approved the final version of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. De-identified participant data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The Ethics Committee of the CHRU of Tours approved the study protocol (no. 2022 005, dated 24 March 2022). The French committee for data handling (CNIL) approved the study (no. 2022_006, dated 27 January 2022). Information posted in the general practitioner’s waiting room was available for patients.
References
- 1.Fahey T. Applying the results of clinical trials to patients to general practice: perceived problems, strengths, assumptions, and challenges for the future. Br J Gen Pract J R Coll Gen Pract 1998;48:1173–8. [PMC free article] [PubMed] [Google Scholar]
- 2.Rothwell PM. External validity of randomised controlled trials: “to whom do the results of this trial apply The Lancet 2005;365:82–93. 10.1016/S0140-6736(04)17670-8 [DOI] [PubMed] [Google Scholar]
- 3.Travers J, Marsh S, Williams M, et al. External validity of randomised controlled trials in asthma: to whom do the results of the trials apply Thorax 2007;62:219–23. 10.1136/thx.2006.066837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uijen AA, Bakx JC, Mokkink HGA, et al. Hypertension patients participating in trials differ in many aspects from patients treated in general practices. J Clin Epidemiol 2007;60:330–5. 10.1016/j.jclinepi.2006.05.015 [DOI] [PubMed] [Google Scholar]
- 5.Canivell S, Mata-Cases M, Vlacho B, et al. How many patients with type 2 diabetes meet the inclusion criteria of the cardiovascular outcome trials with Sglt2 inhibitors? estimations from a population database in a Mediterranean area. J Diabetes Res 2019;2019:2018374. 10.1155/2019/2018374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bugeja G, Kumar A, Banerjee AK. Exclusion of elderly people from clinical research: a descriptive study of published reports. BMJ 1997;315:1059. 10.1136/bmj.315.7115.1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnett K, Mercer SW, Norbury M, et al. Epidemiology of Multimorbidity and implications for health care, research, and medical education: a cross-sectional study. The Lancet 2012;380:37–43. 10.1016/S0140-6736(12)60240-2 [DOI] [PubMed] [Google Scholar]
- 8.Sonis J. Applicability of clinical trial results to primary care. JAMA 1998;280:1746–a. 10.1001/jama.280.20.1746-a [DOI] [PubMed] [Google Scholar]
- 9.Missiou A, Tatsioni A. Systematic reviews do not comment on applicability for primary care. J Clin Epidemiol 2015;68:1152–60. 10.1016/j.jclinepi.2015.06.003 [DOI] [PubMed] [Google Scholar]
- 10.Hemmingsen B, Lund SS, Gluud C, et al. Targeting intensive Glycaemic control versus targeting conventional Glycaemic control for type 2 diabetes mellitus. Cochrane Database Syst Rev 2013.:CD008143. 10.1002/14651858.CD008143.pub3 [DOI] [PubMed] [Google Scholar]
- 11.Effects of Ramipril on cardiovascular and Microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE Substudy. Lancet 2000;355:253–9. 10.1016/S0140-6736(99)12323-7 [DOI] [PubMed] [Google Scholar]
- 12.Cholesterol Treatment Trialists’ (CTT) Collaborators Cholesterol treatment trialists’ (CTT) collaborators. efficacy of cholesterol-lowering therapy in 18 686 people with diabetes in 14 randomised trials of Statins: a meta-analysis. Lancet 2008;371:117–25. 10.1016/S0140-6736(08)60104-X [DOI] [PubMed] [Google Scholar]
- 13.Sen A, Goldstein A, Chakrabarti S, et al. The Representativeness of eligible patients in type 2 diabetes trials: a case study using GIST 2.0. J Am Med Inform Assoc 2018;25:239–47. 10.1093/jamia/ocx091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Green LA, Fryer GE, Yawn BP, et al. The Ecology of medical care Revisited. N Engl J Med 2001;344:2021–5. 10.1056/NEJM200106283442611 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-077582supp001.pdf (44.2KB, pdf)
Data Availability Statement
Data are available upon reasonable request. De-identified participant data are available upon reasonable request.