Summary
Background
In resectable oesophageal squamous cell carcinoma (ESCC), the efficacy of camrelizumab combined with chemotherapy and apatinib followed by minimally invasive oesophagectomy is not clear. We aimed to fill this knowledge gap.
Methods
This investigator-initiated, single-arm, prospective, phase 2 trial was performed at the Second Affiliated Hospital of Zhejiang University, China. Patients (aged 18–75 years) who were histologically or cytologically diagnosed with ESCC were deemed suitable to participate in this trial. Patients received 2–3 cycles of neoadjuvant therapy with camrelizumab, nedaplatin, albumin paclitaxel, and apatinib; each cycle was repeated every 14 days. Surgery occurred 4–6 weeks after the last neoadjuvant treatment cycle. The primary outcome was the pathological complete response (PCR) rate of the tumour and lymph nodes. The changes in the peripheral blood immunoprofile among patients without PCR (ie, non-PCR [NPCR]) and with PCR were assessed by mass cytometry. This study was registered with ClinicalTrials.gov, NCT04666090.
Findings
42 patients were enrolled between November 23, 2020 and December 31, 2022. The disease control rate was 100.0% (95% CI, 91.6–100%), and the objective response rate was 83.3% (95% CI, 68.6–93.0%). Six (14.3%) patients experienced grade 3 adverse events. The most common were white blood cell count decrease (31.0%), alopecia (81.0%), asthenia (38.1%), and reactive cutaneous capillary endothelial proliferation (35.7%). 41 patients received minimally invasive oesophagectomy; all 41patients achieved R0 resection, and 18 (43.9%, 95% CI, 28.5–60.3%) patients achieved PCR. The median follow-up was 23 months and the 2-year survival rate was 85.9%. T-cell subsets in both the PCR and NPCR groups exhibited consistency in response to neoadjuvant therapy. In contrast, some of natural killer (NK) cells (NK-C03, NK-C11), B cells (B-C06) and monocytes (M-C05), exhibited significant differences between the PCR and NPCR groups before neoadjuvant therapy. M-C06 had a significant difference in the PCR group and NPCR group after neoadjuvant therapy. NK-C12 and B-C15 showed significant differences both before and after neoadjuvant therapy.
Interpretation
The application of camrelizumab, chemotherapy and apatinib in the neoadjuvant setting for locally advanced ESCC has shown promising antitumour activity and an acceptable safety profile in this single-arm study. In the neoadjuvant setting, NK cell, B cell, and monocyte subsets exhibited greater predictive power for immunotherapy responsiveness than T-cell subsets. Longer follow-up to assess survival outcomes and a phase 3 randomised trial are needed to further evaluate the proposed treatment.
Funding
The China Anti-Cancer Association and the “Leading Goose” Research and Development Project of Zhejiang Province.
Keywords: Oesophageal squamous cell carcinoma, Neoadjuvant therapy, Cancer immunotherapy, Minimally invasive oesophagectomy
Research in context.
Evidence before this study
Immunotherapy has demonstrated efficacy in antitumor activity on advanced oesophageal squamous cell carcinoma (ESCC). Immunotherapy combined with chemotherapy and antiangiogenesis has potential synergy in advanced ESCC. Camrelizumab combined with chemotherapy and apatinib has favourable antitumour activity and manageable safety in patients with advanced ESCC as a first-line treatment. For patients diagnosed with resectable ESCC, surgery is the primary therapeutic method. However, evidence of the efficacy of camrelizumab combined with chemotherapy and apatinib followed by minimally invasive oesophagectomy is still limited for these patients.
Added value of this study
This single-arm phase 2 trial, neoadjuvant therapy with camrelizumab, chemotherapy and apatinib in locally advanced ESCC, had a 43.9% (95% CI, 28.5–60.3%) pathological complete response (PCR) rate with a low incidence (14.3%) of grade 3 treatment-related adverse events (AEs) and no grade 4 or 5 AEs. The median postoperative follow-up was 23 months, and the 2-year survival rate was 85.9%. Prognostic biomarkers between the PCR and non-PCR (NPCR) groups were analysed. The results indicated that some of natural killer (NK) cells (NK-C03, NK-C11), B-C06, and M-C05 exhibited significant differences between the PCR and NPCR groups before neoadjuvant therapy. M-C06 had a significant difference in the PCR group and NPCR group after neoadjuvant therapy. NK-C12 and B-C15 showed significant differences both before and after neoadjuvant therapy.
Implications of all the available evidence
This study provides evidence of the efficacy and safety of camrelizumab, chemotherapy and apatinib in the neoadjuvant setting for resectable ESCC. Exploratory biomarker assessment showed that NK cell, B cell, and monocyte subsets exhibited greater predictive power for immunotherapy responsiveness than T-cell subsets. Longer follow-up to assess survival outcomes and a phase 3 randomised trial are needed to further evaluate the proposed treatment.
Introduction
Oesophageal cancer (EC) is the seventh most common cancer and is the sixth leading cause of cancer-related mortality worldwide.1 China is a country with a high incidence of EC, and the predominant pathological type is oesophageal squamous cell carcinoma (ESCC). Both the morbidity and mortality rates of ESCC in China account for approximately 50% of the global annual rates.2 Although multimodal treatments, such as surgery, radiotherapy, chemotherapy and other treatments, have been used in the past few decades, the survival rate of patients with ESCC remains relatively poor.3 The majority of patients with ESCC are diagnosed at an advanced stage since the clinical symptoms of early-stage ESCC are not obvious, which contributes to the poor survival of these patients.4
For patients diagnosed with resectable ESCC, surgery is the primary therapeutic method. However, direct surgery is not usually effective for patients with locally advanced ESCC. When performing surgery alone, surgeons have difficulty achieving R0 resection, which results in early postoperative tumour recurrence and subsequently a poor survival rate.5,6 Therefore, effective perioperative neoadjuvant treatment is necessary to reduce the risk of postoperative recurrence and improve the postoperative survival rate.7 The ideal perioperative neoadjuvant treatment should not increase postoperative complications and have few serious adverse events.
Preclinical studies have reported that immunotherapy combined with chemotherapy can provide a synergistic antitumour effect,8,9 which restrains tumour cell immune escape and enhances the immune response of the host.10 According to the National Comprehensive Cancer Network guidelines,11 immunotherapy is a recommended method for the treatment of advanced EC. Several studies have shown that camrelizumab (an anti-PD-1 antibody) plus chemotherapy provides effective treatment for advanced ESCC.12,13
Moreover, a previous study14 demonstrated that camrelizumab combined with chemotherapy and apatinib has favourable antitumour activity and manageable safety in patients with advanced ESCC as a first-line treatment, which indicates that this treatment strategy may have potential synergy. For patients with locally advanced ESCC, Wang et al.,15 in a phase 1b study of camrelizumab and apatinib plus chemotherapy followed by oesophagectomy, showed manageable safety. This phase 2 trial was designed to further observe and evaluate the efficacy and safety of camrelizumab combined with chemotherapy and apatinib for locally advanced ESCC. By using mass spectrometry (CyTOF) and bioinformatics pipelines, we comprehensively characterised the immune landscape in the peripheral blood of patients with ESCC before and after anti-PD-1 immunotherapy, aiming to explore the immune subsets correlated with the neoadjuvant immunotherapy response.
Methods
Study design and participants
This investigator-initiated, single-arm, prospective, phase 2 trial was performed at the Second Affiliated Hospital of Zhejiang University. Patients who were histologically or cytologically diagnosed with ESCC were deemed suitable to participate in this trial. The main inclusion criteria were (1) staged II-IVA according to the American Joint Committee on Cancer (AJCC) staging manual 8th edition; (2) age 18–75 years; (3) resectable and locally advanced disease as assessed by a thoracic surgeon; (4) consent given for participation in this trial; and (5) Eastern Cooperative Oncology Group (ECOG) performance score of 0–1. The main exclusion criteria were (1) prior chemotherapy or radiotherapy; (2) a history of other tumours within 5 years; (3) a history of active autoimmune disease; and (4) intolerance to oesophagectomy after respiratory and cardiologic function assessments.
Neoadjuvant procedure and outcome measurement
Before surgical resection, all patients received 2–3 cycles of neoadjuvant therapy with camrelizumab (200 mg intravenously on day 1), nedaplatin (50 mg/m2 intravenously on day 1), albumin paclitaxel (150 mg/m2 intravenously on day 1) and apatinib (250 mg orally on days 2–4); each cycle was repeated every 14 days.
Tumour assessments were performed by enhanced computed tomography of the chest and abdomen at staging and after 2 cycles of neoadjuvant therapy. The 3rd cycle of neoadjuvant therapy was repeated with patient consent unless the disease progressed or the patient refused since their reported dysphagia symptoms had not improved significantly. Prior to surgical treatment, the radiological response was evaluated by the radiologist according to Response Evaluation Criteria in Solid Tumours (RECIST) version 1.1. Based on the National Cancer Institute-Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 5.0, any adverse events that occurred 30 days after oesophagostomy or 90 days after the first dose of neoadjuvant treatment were recorded and graded by the investigators.
Surgical procedure and outcome measurement
Surgery was scheduled at 4–6 weeks after completion of the last neoadjuvant treatment cycles. Before surgery, the doctors of the department organised comprehensive discussions to establish the most suitable surgical method for each patient. During surgery, minimally invasive Ivor-Lewis (intrathoracic anastomosis) or McKeown (neck anastomosis) oesophagectomy, including two field extensive lymphadenectomies, was performed and the resection length was at least 5 cm from the tumour origin. After surgery, camrelizumab can be maintained for a maximum of 1 year. Chemotherapy or radiotherapy could be chosen by the patient based on the investigator's suggestion. Survival time was defined as the time from surgery to either the last follow-up (December 1, 2023) or the date of death as a result of any cause. Disease free survival time was defined as the time of the first date of disease recurrence or date of death due to any cause, whichever occurred first.
All resected tissues, including the primary tumour, lymph nodes, any abnormal-appearing tissue and surgical margin, were sent for paraffin embedding and pathological examination. The tumour bed could be identified combine with gastroscopy before the neoadjuvant therapy. It was cross-sectioned at 0.5 cm intervals and stained with hematoxylin and eosin. The tumour regression rate was divided into 4-tiered and confirmed by two independent pathologists. They used a previously reported method to measure the proportion of residual viable tumours16: grade 1: no evidence of residual viable tumours (pathological complete response, PCR); grade 2: 10% or fewer residual viable tumours (major pathological response, MPR); grade 3: 10%–50% residual viable tumours (partial response, PR); and grade 4: more than 50% residual viable tumours (stable disease, SD).
Sample preparation and CyTOF antibody staining
After patients were enrolled, 5 mL of peripheral blood was collected the day before each of the first two immunotherapy cycles. Granulocytes were removed from peripheral blood using a granulocyte depletion cocktail (Stemcell, 15,664). Peripheral blood mononuclear cells (PBMCs) were isolated using Ficoll (Solarbio, P8900) and cryopreserved in liquid nitrogen for subsequent analysis by CyTOF, which focusing on a panel of 42 immune molecules (Supplementary Table S1). After the tumour regression rate was confirmed by pathologists, we randomly selected portion of the neoadjuvant immunotherapy regimen, including 5 patients exhibiting PCR and 5 patients showing non-PCR (NPCR) (the tumour regression rates were 10%, 20%, 30%, 50% and 70% for these patients) (Fig. 2F), split into four groups: pre-NPCR (pre-therapy NPCR samples), post-NPCR (post-therapy NPCR samples), pre-PCR (pre-therapy PCR samples) and post-PCR (post-therapy PCR samples). After thawing the frozen mononuclear cells, the fractionated cells were washed twice with 1X PBS (Procell, PB180521) (PBS containing 0.1% bovine serum albumin) and then resuspended in staining buffer at a concentration of 1 × 106 cells/mL. For CyTOF antibody staining, PBMCs were incubated with a panel of metal-conjugated antibodies specific to various immune cell markers, including T cells, B cells, natural killer (NK) cells, and myeloid cell populations. The staining protocol followed the manufacturer's instructions.
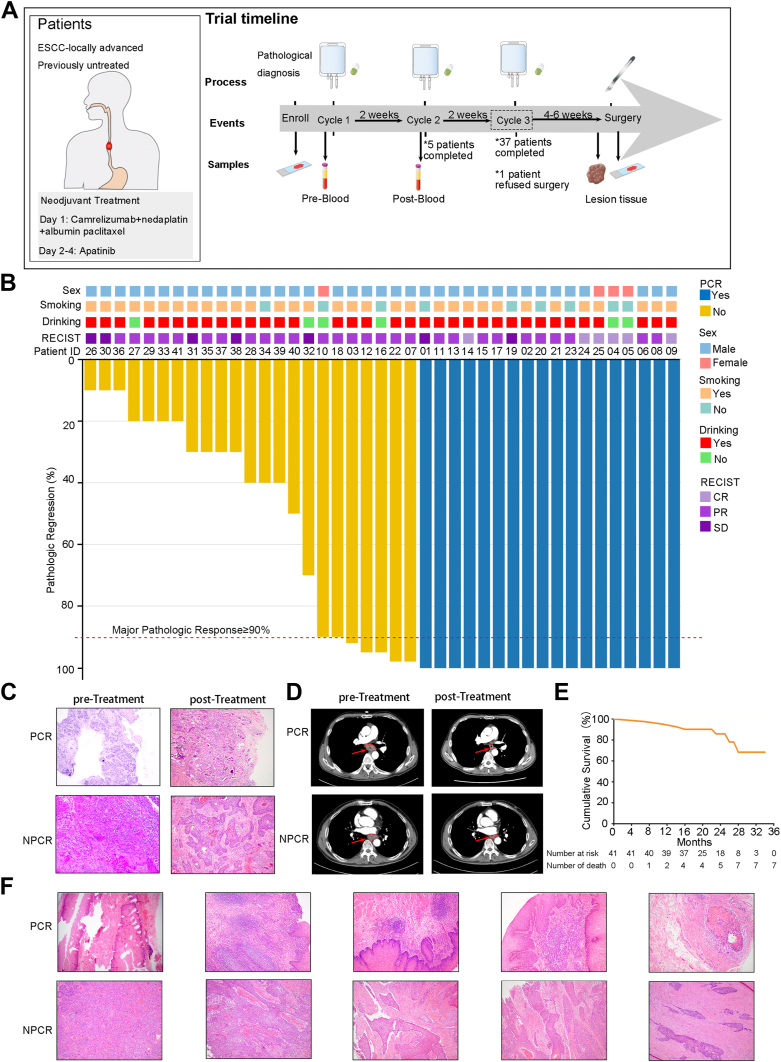
Fig. 2.
Study design and clinical response data of neoadjuvant treatment of resectable oesophageal squamous cell carcinoma (ESCC) (A) Trial timeline and sample collection in patients with oesophageal cancer. (B) Clinical metadata and analyses of each patient, including sex, smoking status, drinking status and radiological response metrics. Radiologic staging at the time of surgery was based on the Response Evaluation Criteria in Solid Tumours (RECIST) version 1.1, and the pathologic response was determined based on histologic assessment. (C) Hematoxylin and eosin-stained tumour specimen sections of pathological complete response (PCR) and non-PCR (NPCR) patients before and after neoadjuvant therapy. (D) Representative computed tomography scans of two patients with oesophageal cancer who underwent neoadjuvant therapy. The scans were taken before and after treatment in one patient with NPCR and one patient with PCR. (E) Kaplan–Meier curves of the cumulative survival rate for all patients (n = 41). (F) Hematoxylin and eosin staining was performed on surgically resected tumour specimen sections obtained from 5 patients with PCR and 5 patients with NPCR who underwent mass spectrometry.
CyTOF data acquisition
The stained PBMCs were acquired using a CyTOF mass cytometer (Fluidigm). Prior to acquisition, the instrument was tuned and normalised according to the manufacturer's guidelines. The acquisition parameters were set to collect 20,000 events per sample.
The acquired CyTOF data were exported as FCS files and analysed using R software (version R 4.3.1). The CyTOF data analysis package phonograph was employed for data preprocessing, quality control, and downstream analysis.
“PhenoGraph” algorithm implementation
To identify distinct immune cell populations within the flow cytometry data, we applied the “PhenoGraph” algorithm using R software (version R 4.3.1). The “PhenoGraph” algorithm utilises a graph-based clustering approach to group cells based on their phenotypic similarity. Specifically, the algorithm partitions the cells into clusters by constructing a k-nearest neighbour graph and iteratively optimising the clustering results. To characterise the identified cell clusters, we performed various downstream analyses. This included calculating the proportions of each cell type in different clusters, analysing marker expression patterns, and conducting other analyses. These analyses provided insights into the cellular composition and functional diversity within the PBMC samples. The definitions of immune cell subsets are available in the Supplementary Methods.
Study outcomes
The primary outcome of this study was the efficacy of the neoadjuvant treatment, which was evaluated by the PCR rate in the primary tumour and lymph nodes. The secondary outcomes of this study were feasibility and safety, which included adverse events assessed by the NCI-CTCAE version 5.0; surgical outcomes included the operative time, conversion rate, total postoperative hospital stay, blood loss, time to oral intake, major complications and mortality within 90 days.
Statistical analysis
A previous study reported that the PCR rate of neoadjuvant therapy for ESCC was 16%.17 Therefore, we assumed that neoadjuvant camrelizumab combined with chemotherapy and apatinib would achieve a higher PCR rate, as high as 36%. Approximately 38 participants needed to be recruited to detect such a difference when the type I error probability α was no more than 0.05 (one-sided) and the type II error β was 0.1. The dropout rate did not exceed 10% in this study. When appropriate, the data are expressed as proportions (percentages), means (standard deviations) or medians (ranges). 95% confidence intervals (CIs) were calculated using the exact binomial method. The Kaplan–Meier method was used to calculate the cumulative survival rate. When analysing mass cytometry data, we employed independent-samples t tests to compare differences between the PCR and NPCR groups, and paired-samples t tests were used to compare differences before and after neoadjuvant therapy. We applied SPSS version 23.0 (IBM, Armonk, NY) to analyse the data, and a value of p less than 0.05 was defined as significant.
Ethics statement
The protocol (available in the Supplementary Materials) was reviewed and approved by the Ethics Committee of the Second Affiliated Hospital of Zhejiang University (approval No. 2020-1007). This study was registered in ClinicalTrials.gov (Identifier: NCT04666090). Written informed consent forms were completed by all patients before enrolment.
Role of the funding source
This study was supported by the China Anti-Cancer Association (No. 2021001015 to Hong Shen) and the “Leading Goose” Research and Development Project of Zhejiang Province (No. 2023C03064 to Ming Wu). The funder of the study had no role in the study design, patient recruitment, data collection, data analysis, data interpretation, manuscript writing or the decision to submit the study for publication.
Results
Patient characteristics
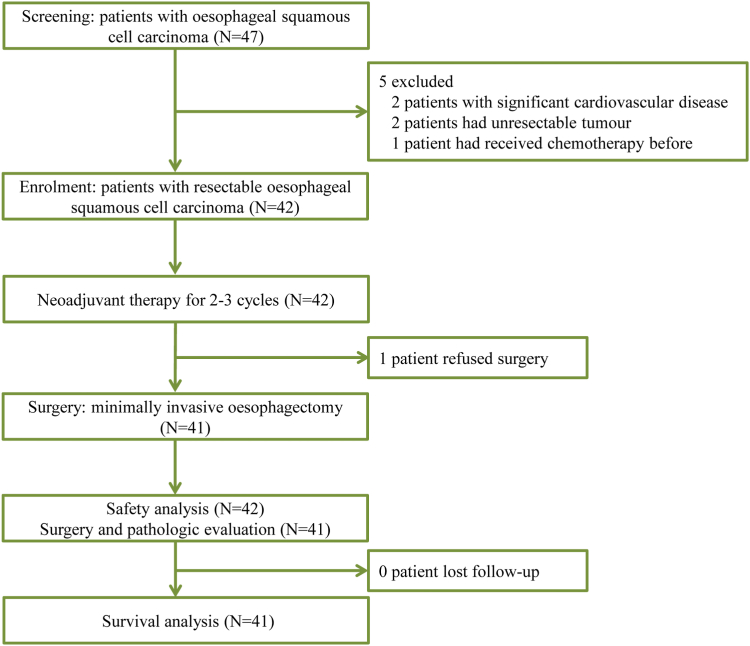
From November 23, 2020 to December 31, 2022, 47 patients were assessed for eligibility (Fig. 1). Five patients were excluded due to significant cardiovascular disease (n = 2), having unresectable tumours (n = 2) or receiving chemotherapy previously (n = 1). 42 patients were enrolled and treated in this trial. Only 1 patient finished 3 cycles of neoadjuvant therapy and completed the tumour assessment; however, this patient refused to receive surgical treatment and declined to participate in the study.
Fig. 1.
Trial profile.
Among the 42 patients, 5 (11.9%) patients completed 2 cycles of neoadjuvant therapy, and the other 37 (88.1%) patients completed 3 cycles of neoadjuvant therapy (Fig. 2A). The mean age was 64 years, and the majority of patients were male (90.5%). Most of the patients had a smoking history (78.6%) and alcohol-drinking history (85.7%). The tumours were located in the upper, middle and lower oesophagus in 2 (4.8%) patients, 28 (66.7%) patients, and 12 (28.6%) patients, respectively (Table 1).
Table 1.
Baseline demographics and characteristics of all enrolled patients (N = 42).
| Characteristics | |
|---|---|
| Age (years), mean (standard deviation) | 64 (6.6) |
| Sex, n (%) | |
| Male | 38 (90.5%) |
| Female | 4 (9.5%) |
| BMI (kg/m2), mean (standard deviation) | 21.9 (2.9) |
| Smoking history, n (%) | |
| Former or current | 33 (78.6%) |
| Never | 9 (21.4%) |
| Alcohol-drinking history, n (%) | |
| Former or current | 36 (85.7%) |
| Never | 6 (14.3%) |
| Comorbidities, n (%) | |
| No | 23 (54.8%) |
| Yes | 19 (44.2%) |
| Hypertension | 10 (23.8%) |
| Diabetes | 2 (4.8%) |
| Coronary heart disease | 7 (16.7%) |
| Cerebral infarction | 1 (2.4%) |
| ECOG performance status, n (%) | |
| 0 | 19 (45.2%) |
| 1 | 23 (54.8%) |
| Tumour location, n (%) | |
| Upper | 2 (4.8%) |
| Middle | 28 (66.7%) |
| Lower | 12 (28.6%) |
| Pulmonary function, mean (standard deviation) | |
| FVC (L) | 3.4 (0.7) |
| FEV1 (L) | 2.5 (0.6) |
| FEV1% | 90.5 (15.1) |
| DLCO% | 76.7 (14.3) |
| Cycles of neoadjuvant therapy, n (%) | |
| 2 | 5 (11.9%) |
| 3 | 37 (88.1%) |
| Objective response per RECIST v1.1, n (%, 95% CI) | |
| Complete response | 6 (14.3%, 5.4–28.5%) |
| Partial response | 29 (69.0%, 52.9–82.4%) |
| Stable disease | 7 (16.7%, 7.0–31.4%) |
| Objective response rate | 35 (83.3%, 68.6–93.0%) |
| Disease control rate | 42 (100.0%, 91.6–100%) |
BMI, body mass index; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 s; FEV1%, forced expiratory volume in 1 s as a percentage of predicted; DLCO%, diffusion capacity for carbon monoxide of the lung as a percentage of predicted; ECOG, Eastern Cooperative Oncology Group; RECIST, Response Evaluation Criteria in Solid Tumours; CI, confidence interval.
Efficacy
All 42 patients underwent an image evaluation according to the RECIST v1.1 criteria (Table 1). Six (14.3%) patients achieved a complete response, 29 (69.0%) patients achieved a partial response, and the other 7 (16.7%) patients had stable disease. No cases of progressive disease occurred during neoadjuvant treatment (Fig. 2B). The objective response rate and disease control rate were 83.3% and 100.0%, respectively. The pathologic response was evaluated in the 41 patients who received minimally invasive oesophagectomy (Table 3). Among the evaluable patients, 25 (61.0%, 95% CI, 44.5–75.8%) patients achieved MPR, including 18 (43.9%, 95% CI, 28.5–60.3%) patients with PCR, while the other 16 (39.0%, 95% CI, 24.2–55.5%) patients had an incomplete pathological response. The primary pathological changes observed in patients with PCR following neoadjuvant immunotherapy for ESCC were fibrous tissue hyperplasia, infiltration of chronic inflammatory cells, and the formation of lymphocyte aggregates, hyperplasia, and follicles (Fig. 2C). Fig. 2D displays computed tomography images illustrating the imaging characteristics of patients who achieved PCR and patients who did not achieve PCR before and after neoadjuvant therapy.
Table 3.
Surgical, pathological profile and adjuvant therapy of patients who underwent surgery (N = 41).
| Characteristics | |
|---|---|
| Interval between last neoadjuvant and surgery (day), median (range) | 33 (28–42) |
| Surgical perdure, n (%) | |
| Ivor-Lewis | 33 (80.5%) |
| McKeown | 8 (19.5%) |
| Cumulative operative time (min), mean (standard deviation) | 262 (48.4) |
| Blood loss (mL), median (range) | 50 (20–200) |
| Conversion, n (%) | 0 |
| Postoperative ICU stay (day), median (range) | 3 (1–12) |
| Unexpected admission to ICU, n (%) | 0 |
| Postoperative hospital stays (day), median (range) | 10 (7–35) |
| Time to oral intake (day), median (range) | 6 (6–29) |
| Major complications, n (%) | |
| Anastomotic leakage | 3 (7.3%) |
| Chylothorax | 2 (4.9%) |
| Atrial fibrillation | 10 (24.4%) |
| Atelectasis | 3 (7.3%) |
| Vocal-cord paralysis | 2 (4.9%) |
| Pneumonia | 17 (41.5%) |
| Reoperation | 1 (2.4%) |
| 90-day mortally | 0 |
| R0 resection, n (%) | 41 (100.0%) |
| Number of resected lymph nodes, mean (standard deviation) | 27.6 (10.4) |
| Number of resected lymph node stations, mean (standard deviation) | 7.4 (1.8) |
| Pathological response, n (%, 95% CI) | |
| MPR | 25 (61.0%, 44.5–75.8%) |
| PCR | 18 (43.9%, 28.5–60.3%) |
| IPR | 16 (39.0%, 24.2–55.5%) |
| PR | 2 (4.9%, 0.6–16.5%) |
| SD | 14 (34.1%, 20.1–50.6%) |
| Adjuvant therapy, n (%) | |
| No | 7 (17.1%) |
| Chemotherapy and camrelizumab | 9 (22.0%) |
| Camrelizumab | 25 (61.0%) |
ICU, intensive care unit; MPR, major pathological response; PCR, pathological complete response; IPR, incomplete pathological response; PR, partial response; SD, stable disease; CI, confidence interval.
Following surgery, 25 (61%) patients selected camrelizumab maintenance therapy, while 9 (22.0%) patients selected chemotherapy in combination with camrelizumab as adjuvant therapy (Table 3). At the cut-off day, the median postoperative follow-up was 23 months, and 41 patients had completed follow-up. A total of 6 (14.6%) patients experienced local tumour recurrence (4 patients) or distant metastasis (2 patients), and the 2-year disease free survival rate was 80.1%. A total of 7 patients died, four of whom died due to local tumour recurrence (2 patients) or distant metastasis (2 patients). The 2-year overall survival rate was 85.9% (Fig. 2E).
Safety
Treatment-related adverse events (TRAEs) during neoadjuvant chemoimmunotherapy are summarised in Table 2. The most common TRAEs were white blood cell decreased (31.0%), alopecia (81.0%), asthenia (38.1%), dizziness (21.4%), appetite decreased (28.6%), rash (26.2%) and reactive cutaneous capillary endothelial proliferation (35.7%). A total of six (14.3%) patients suffered from grade 3 TRAEs. One patient (2.4%) experienced a grade 3 white blood cell count decrease and a neutrophil count decrease. There were no grade 4 or 5 TRAEs reported. In addition, no TRAEs led to a dose reduction or discontinuation of treatment in the trial.
Table 2.
Adverse events during neoadjuvant chemoimmunotherapy (N = 42).
| Any grade, n (%) | Grade 1–2, n (%) | Grade 3, n (%) | |
|---|---|---|---|
| White blood cell decreased | 13 (31.0%) | 9 (21.4%) | 4 (9.5%) |
| Neutrophil count decreased | 7 (16.7%) | 5 (11.9%) | 2 (4.8%) |
| Platelet count decreased | 1 (2.4%) | 1 (2.4%) | 0 |
| Alopecia | 34 (81.0%) | 34 (81.0%) | 0 |
| Asthenia | 16 (38.1%) | 16 (38.1%) | 0 |
| Constipation | 6 (14.3%) | 6 (14.3%) | 0 |
| Diarrhea | 4 (9.6%) | 4 (9.6%) | 0 |
| Dizziness | 9 (21.4%) | 9 (21.4%) | 0 |
| Decreased appetite | 12 (28.6%) | 12 (28.6%) | 0 |
| Hypothyroidism | 1 (2.4%) | 1 (2.4%) | 0 |
| Lung infection | 6 (14.3%) | 5 (11.9%) | 1 (2.4%) |
| Nause | 7 (16.7%) | 7 (16.7%) | 0 |
| Rash | 11 (26.2%) | 11 (26.2%) | 0 |
| RCCEP | 15 (35.7%) | 15 (35.7%) | 0 |
| Vomiting | 7 (16.7%) | 7 (16.7%) | 0 |
| Gingival bleeding | 1 (2.4%) | 1 (2.4%) | 0 |
| Abdominal pain | 1 (2.4%) | 1 (2.4%) | 0 |
| Abdominal distension | 2 (4.8%) | 2 (4.8%) | 0 |
| Headache | 2 (4.8%) | 2 (4.8%) | 0 |
RCCEP, reactive cutaneous capillary endothelial proliferation.
There were no neoadjuvant treatment-related surgical delays, and the median interval between neoadjuvant treatment and surgery was 33 days. 33 (80.5%) patients and 8 (19.5%) patients underwent minimally invasive Ivor-Lewis and McKeown oesophagectomy, respectively. Additionally, no patients converted to open surgery. The mean operating time was 262 min, and the median blood loss was 50 mL. Regarding major postoperative complications, 3 (7.3%) patients suffered from anastomotic leakage and 2 (4.9%) patients had vocal cord paralysis. The median postoperative hospital stay was 10 days, and the median time to oral intake was 6 days. Pathological examinations revealed that all the patients achieved R0 resection, and the average number of lymph nodes harvested was 27. Moreover, there was no unexpected intensive care unit (ICU) admission or 90-day mortality (Table 3).
Immunotherapy response in patients with ESCC
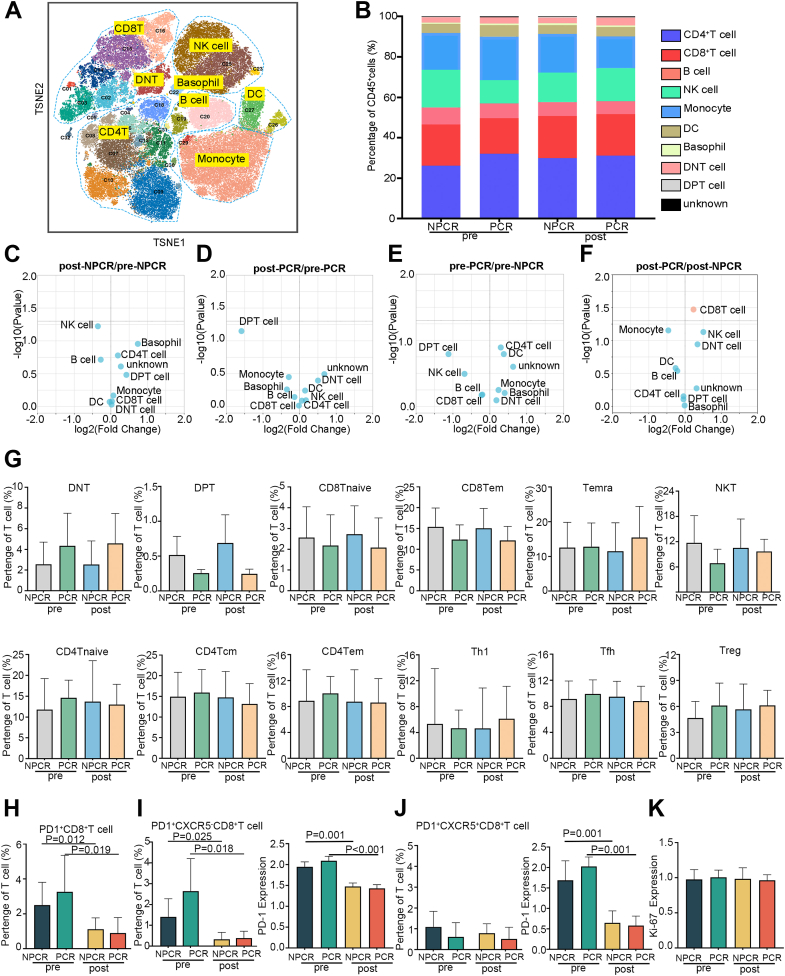
Analysis identified differences in cell frequency between patients with PCR and without PCR (ie, NPCR) both before immunotherapy and after immunotherapy (Fig. 3A and B). Following neoadjuvant therapy, patients with PCR exhibited an increase in the proportion of CD8+ T cells and NK cells, accompanied by a decrease in the monocyte proportion (Fig. 3C–F). Compared the frequency differences between these subpopulations across the groups, DNT cells were slightly more abundant in the PCR group than in the NPCR group, while DPT cells, CD8+ Tem cells, CD8+ T naive cells, and NKT cells were slightly less abundant in the PCR group, although these differences were not statistically significant (Fig. 3G). Compared to pretreatment levels, the administration of PD-1 monoclonal antibodies significantly reduced the number of PD1+ CD8+ T cells in both the PCR (p = 0.019) and NPCR (p = 0.012) groups (Fig. 3H). Based on CyTOF results, PD1+ CD8+ T cells in PBMCs of patients with ESCC were primarily categorised into two subgroups: PD1+ CXCR5- CD8+ T cells and PD1+ CXCR5+ CD8+ T cells. Both subpopulations in the PCR group (p = 0.018) and NPCR group (p = 0.025) significantly reduced for PD1+ CXCR5- CD8+ T cells, similar to the PD1+ CD8+ T cells. While, no significant differences were observed between the PCR and NPCR groups for PD1+ CXCR5+ CD8+ T cells (Fig. 3I and J). The proliferative capacity of PD1+CD8+ T cells remained unchanged after therapy in both the PCR and NPCR groups (Fig. 3K).
Fig. 3.
Immune cell dynamics in peripheral blood mononuclear cells from patients with oesophageal squamous cell carcinoma (ESCC) treated with neoadjuvant therapy (A) t-SNE plots of CD45+ cell subgroups in all samples (n = 20), coloured by cell subpopulation. (B) Comparison of peripheral blood mononuclear cells (PBMC) immune cell population frequencies in the pathological complete response (PCR) and non-PCR (NPCR) groups before and after treatment. Different colours indicate different immune cell subpopulations. (C, D, E and F) Paired differences in the abundance of major immune cell populations, before and after neoadjuvant therapy in the NPCR group (n = 5) (C), before and after neoadjuvant therapy in the PCR group (n = 5) (D), before neoadjuvant therapy between PCR and NPCR (n = 5) (E), and after neoadjuvant therapy between PCR and NPCR (n = 5) (F). Plot of log2-fold change versus negative log10 (nominal p value). The left side indicates low frequencies, and the right side indicates high frequencies. (G) Direct comparison of T-cell subset frequencies between the NPCR group (n = 5) and the PCR group (n = 5) before and after neoadjuvant therapy. (H) Direct comparison of PD1+CD8+ T-cell frequencies between the NPCR group (n = 5) and the PCR group (n = 5) before and after neoadjuvant therapy. (I) Direct comparison of PD1+CXCR5−CD8+ T-cell frequencies (left histogram gram) and PD1 (right histogram gram) expression between the NPCR group (n = 5) and the PCR group (n = 5) before and after neoadjuvant therapy. (J) Direct comparison of PD1+CXCR5+CD8+ T-cell frequencies (left histogram gram) and PD1 expression (right histogram gram) between the NPCR group (n = 5) and the PCR group (n = 5) before and after neoadjuvant therapy. (K) Direct comparison of Ki-67 expression in PD1+CD8+ T cells between the NPCR group (n = 5) and the PCR group (n = 5) before and after neoadjuvant therapy. Pre: pretreatment; post: posttreatment. Error bars represent standard deviations.
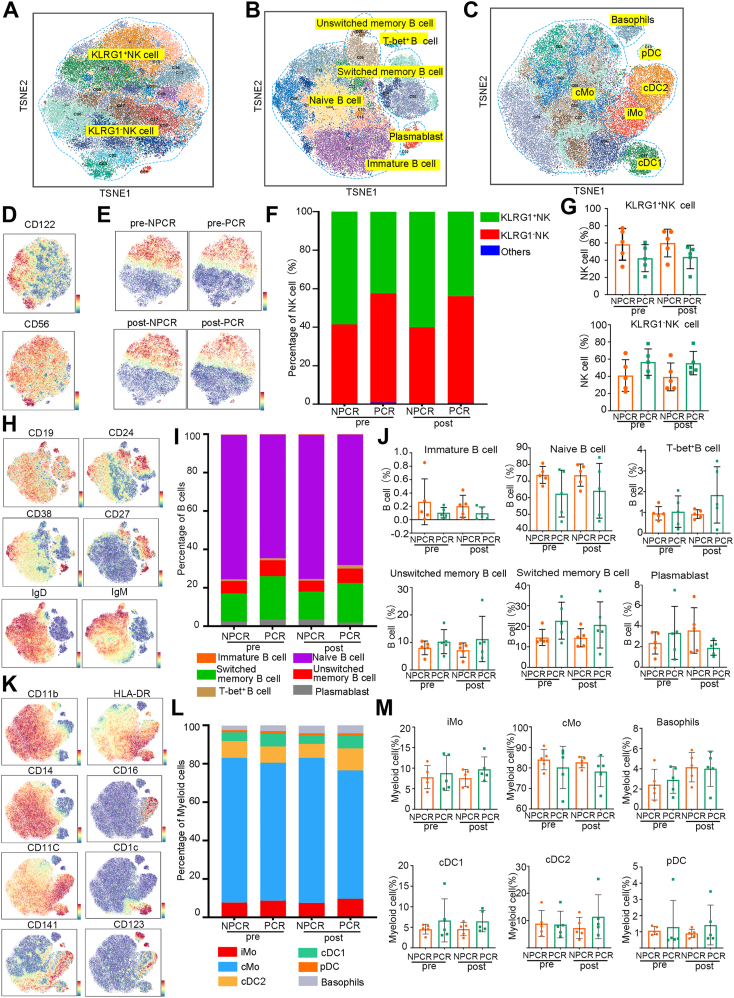
Identification of NK cell, B-cell and myeloid cell properties in neoadjuvant therapy
As T-cell subpopulations in both the PCR and NPCR groups exhibited consistency in response to immunotherapy, we investigated the potential of other immune cell subsets to predict the efficacy of immune neoadjuvant therapy. Initially, we defined subsets of NK cells (Fig. 4A), B cells (Fig. 4B), and myeloid cells (Fig. 4C) based on the expression of cell markers. We observed two major groups within NK cells (Fig. 4D) based on the expression of KLRG1: KLRG1+ and KLRG1- subsets (Fig. 4E). The NPCR group had a higher proportion of KLRG1+ cells, both before and after immunotherapy (Fig. 4F), but this difference was not statistically significant (Fig. 4G). We manually defined six B-cell subpopulations based on the expression of the surface markers (Fig. 4H). The proportion of each subgroup in the NPCR group before immunotherapy was consistent with that after immunotherapy, and the PCR group exhibited a similar pattern to the NPCR group (Fig. 4I). Subsequent statistical analysis of B-cell subpopulations did not show any statistically significant results (Fig. 4J). Based on the expression of surface markers, we identified six classical myeloid cell subpopulations (Fig. 4K). The cMo subgroup constituted the majority, comprising over 65% of all groups (Fig. 4L). Statistical analysis of these subpopulations did not reveal any significant differences (Fig. 4M).
Fig. 4.
Identification of natural killer (NK) cell, B-cell and myeloid cell properties in neoadjuvant therapy. (A, B and C) t-SNE plots of natural killer (NK) cells (A), B-cells (B) and myeloid cell clusters (C) in all samples (n = 20) by mass spectrometry using a PhenoGraph clustering algorithm. Coloured by cell clusters. (D) t-SNE plot of the NK cell markers CD122 and CD56. (E) t-SNE plot of KLRG1 expression in NK cells before and after treatment in the pathological complete response (PCR) and non-PCR (NPCR) groups. (F) Fractions of KLRG1+ NK cells and KLRG1- NK cells in the PCR and NPCR groups before and after treatment. (G) Direct comparison of KLRG1+ NK cells and KLRG1- NK cells between the NPCR group (n = 5) and the PCR group (n = 5) before and after neoadjuvant therapy. (H) t-SNE plot of the B-cell surface markers CD19, CD24, CD38, CD27, IgD and IgM. (I) Fractions of B-cell subsets in the PCR and NPCR groups before and after treatment. (J) Direct comparison of B-cell subsets between the NPCR group (n = 5) and the PCR group (n = 5) before and after neoadjuvant therapy. (K) t-SNE plot of the myeloid cell surface markers CD11b, HLA-DR, CD14, CD16, CD11c, CD1c, CD141 and CD123. (L) Fractions of myeloid cell subsets in the PCR and NPCR groups before and after treatment. (M) Direct comparison of myeloid cell subsets between the NPCR group (n = 5) and the PCR group (n = 5) before and after neoadjuvant therapy. Pre: pretreatment; post: posttreatment. Error bars represent standard deviations.
Exploration of cell clusters associated with neoadjuvant therapy responsiveness
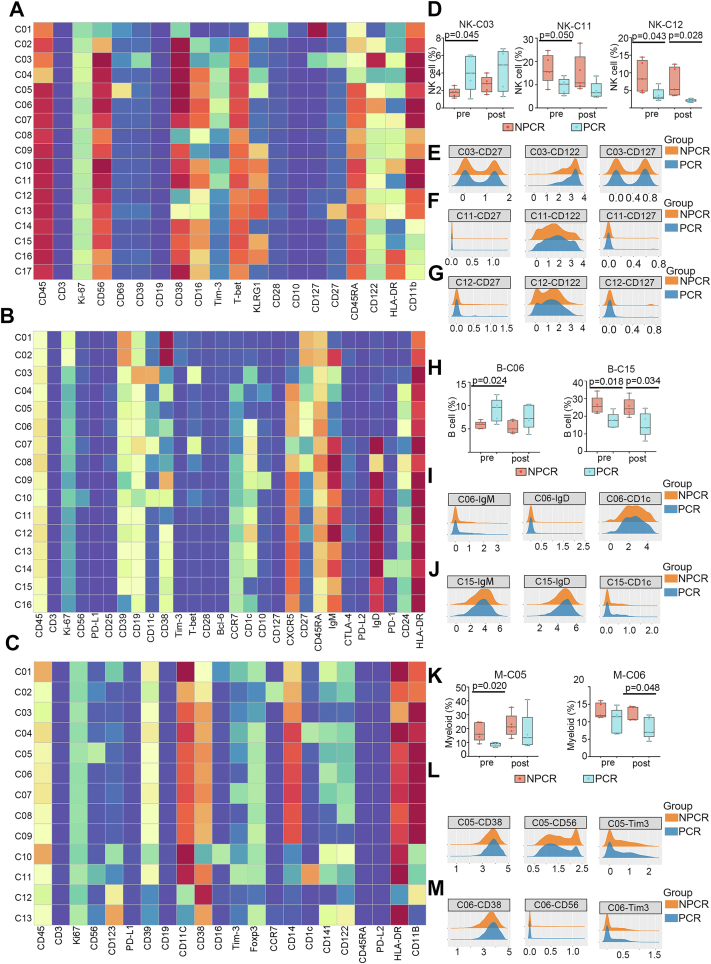
To explore immune populations that can effectively predict the efficacy of neoadjuvant therapy for ESCC, we analysed NK cells, B cells, and myeloid cells using unsupervised clustering algorithms (Fig. 5A, B, C). First, focusing on NK cells, we identified 3 clusters that exhibited significant differences (Fig. 5D). NK-C03 cells, categorised as naive NK cells based on the expression of the surface markers CD27, CD122, and CD127 (Fig. 5E), were significantly enriched in the PCR group before neoadjuvant therapy (p = 0.045) (Fig. 5D). On the other hand, NK-C11 cells, characterised as CD27−CD122+CD127- NK cells (Fig. 5F), showed significant enrichment in the NPCR group before neoadjuvant therapy (p = 0.05) (Fig. 5D). Another cluster of NK cells, NK-C12, defined as CD27−CD122+CD127+ (Fig. 5G), exhibited significant enrichment in the NPCR group both before (p = 0.043) and after treatment (p = 0.028) (Fig. 5D). Among the B-cell clusters, we identified two distinct clusters, B-C06 and B-C15 (Fig. 5H). The B-C06 cluster, characterised as an IgM−IgD-CD1c+ B cells (Fig. 5I), exhibited a significantly higher frequency in the PCR group than in the NPCR group before neoadjuvant therapy (p = 0.024) (Fig. 5H). In contrast, the B-C15 cluster, characterised as IgM+IgD+CD1c−B cells (Fig. 5J), exhibited higher levels in the PCR group than in the NPCR group, both before (p = 0.018) and after neoadjuvant therapy (p = 0.034) (Fig. 5H). Within the myeloid clusters, we identified two distinct subsets (Fig. 5K) that were classified as cMo: M-C05 (CD38+CD56+TIM-3+cMo) (Fig 5L) and M-C06 (CD38+CD56−TIM3- cMo) (Fig. 5M). M-C05 exhibited a higher frequency in the NPCR group than in the PCR group before neoadjuvant therapy (p = 0.02), while M-C06 showed significant enrichment in the NPCR group primarily after neoadjuvant therapy (p = 0.048) (Fig. 5K).
Fig. 5.
Exploration of cell clusters associated with neoadjuvant therapy responsiveness. (A, B and C) Heatmap of relative normalised protein expression of natural killer (NK) cell (A), B-cell (B) and myeloid cell (C) clusters in all samples (n = 20). (D) Direct comparison of NK-C03 cluster, NK-C11 cluster and NK-C12 cluster frequencies between the pathological complete response (PCR) group and non-PCR (NPCR) group before and after neoadjuvant therapy. (E, F and G) Density plot of CD27, CD122 and CD127 expression in the C03 cluster, C11 cluster and C12 cluster of NK cells. (H) Direct comparison of B-C06 cluster and B-C15 cluster frequencies between the NPCR group and the PCR group before and after neoadjuvant therapy. (I and J) Density plot of IgM, IgD and CD1c expression in the C06 cluster and C15 cluster of B cells. (K) Direct comparison of M-C05 cluster and M-C06 cluster frequencies between the NPCR group and the PCR group before and after neoadjuvant therapy. (L and M) Density plot of CD38, CD56 and Tim-3 expression in the C05 cluster and C06 cluster of myeloid cells. Pre: pretreatment; post: posttreatment. Whiskers represent min to max. Bounds of boxes represent the 25th and 75th percentiles, and centres represent medians.
Discussion
In this single-arm phase 2 trial, we demonstrated that the application of camrelizumab, chemotherapy in combination with apatinib, could achieve favourable antitumour efficacy, with considerable safety, low indications of postoperative comorbidity and no delay in surgery for patients with resectable ESCC.
Neoadjuvant therapy followed by surgical resection is the standard treatment for patients with resectable locally advanced ESCC. Effective neoadjuvant therapy can improve the R0 resection rate, eliminate micrometastases and prevent recurrence. Furthermore, it can improve the long-term survival rate for patients. The MPR rate, especially the PCR rate, is a crucial criterion for assessing the effectiveness of neoadjuvant therapy. In the phase 1b study of camrelizumab and apatinib plus chemotherapy reported by Wang et al.,15 the MPR rate was 51.7%, which was similar to the 61.0% (95% CI, 44.5–75.8%) MPR rate in our study. In other tumours, previous research has reported that achieving an MPR after neoadjuvant therapy was associated with better survival.18 During the initial study design, we assumed that camrelizumab and chemotherapy in combination with apatinib would achieve a higher PCR rate than chemotherapy alone.
Indeed, 43.9% (95% CI, 28.5–60.3%) of the patients who underwent surgery achieved PCR in our study, which was significantly higher than the PCR rate (16%) for chemotherapy alone that we used for our sample size calculation. The PCR rate in our study was similar to that in previous reports of neoadjuvant chemoradiotherapy combined with surgery; the PCR rates reported in the FFCD 9901 trial,19 CROSS trial,7 and NEOCRTEC5010 trial5 were 33.3%, 49%, and 43.2%, respectively. Furthermore, the 2-year survival rate was 85.9% in our study, which was slightly higher than the 75.1% for neoadjuvant chemoradiotherapy in the NEOCRTEC5010 trial.5 The objective response rate was 83.3% (95% CI, 68.6–93.0%), and the disease control rate was 100.0% (95% CI, 91.6–100%). These results indicated that our neoadjuvant therapy regimen showed superior antitumour efficacy, and there are several reasons to explain this phenomenon.
First, camrelizumab has shown promising antitumour effects in patients with advanced or metastatic ESCC.12,20 The ESCORT study reported by Huang et al.12 was a randomised, open-label, multicentre phase 3 trial comparing camrelizumab with chemotherapy as a second-line therapy for advanced or metastatic ESCC. Their results showed that camrelizumab could significantly increase clinical and overall survival benefits compared with chemotherapy. In addition, a subgroup analysis showed that camrelizumab treatment had a clinical benefit in each subgroup regardless of PD-L1 expression.
Second, intensive chemotherapy (once every 2 weeks) may lead to a higher dose intensity of paclitaxel than 3 weeks for each cycle,14 and camrelizumab had a synergistic effect with chemotherapy. A randomised, double-blind trial reported by Luo et al.13 showed that compared with chemotherapy, the addition of camrelizumab with chemotherapy, as the first-line therapy for patients with advanced or metastatic ESCC, could significantly improve disease-free survival and overall survival. Moreover, studies have shown that neoadjuvant camrelizumab with chemotherapy has excellent antitumour efficacy and demonstrated that these two therapeutic methods have synergistic effects when treating locally advanced ESCC.21,22
Third, it is possible that camrelizumab has a synergistic effect with apatinib. Apatinib is an antiangiogenic drug that can normalise tumour blood vessels and relieve immunosuppression by inhibiting vascular endothelial growth factor receptor (VEGFR).23 In patients with advanced ESCC, apatinib has shown efficacious activity during treatment.24 A single-arm, open-label and phase 2 study for patients with advanced ESCC by Meng et al.25 demonstrated that camrelizumab plus apatinib had promising antitumour activity and indicated a synergistic effect for these two drugs.
Although adding immunotherapy and antiangiogenic agents to chemotherapy can further improve antitumour effects, it may lead to more severe TRAEs that should be considered in clinical practice. However, the neoadjuvant combination of camrelizumab, chemotherapy and apatinib has demonstrated a favourable safety profile with good tolerability. All enrolled patients completed at least two cycles of neoadjuvant treatments without treatment-related surgical delays. The TRAEs in our study were consistent with previous studies of camrelizumab combined with chemotherapy, and no new safety-related AEs were identified. The most frequent AE of camrelizumab was reactive cutaneous capillary endothelial proliferation. However, the incidence of this AE was 35.7% in our study, which was lower than the incidence of 80% reported by other clinical studies of camrelizumab monotherapy.12,13,26 We speculated that the pathogenesis of reactive cutaneous capillary endothelial proliferation could involve antiangiogenic drugs. The relatively low incidence of reactive cutaneous capillary endothelial proliferation was also reported by another study for the combination of camrelizumab with apatinib.27 Furthermore, most of the AEs were grade 1–2 in this study. Only 14.3% of patients suffered from grade 3 TRAEs, and no grade 4 or 5 TRAEs occurred in our trial. The incidence of serious TRAEs was lower than that reported in the NEOCRTEC5010 study,5 which was approximately 60% for chemoradiotherapy. Collectively, these results indicated that camrelizumab, chemotherapy and apatinib were well tolerated in patients with ESCC.
For minimally invasive oesophagectomy, neoadjuvant treatment seems to have no influence on lymphadenectomy and postoperative complications. Radical lymphadenectomy can provide accurate pathologic staging, and it has been recommended that at least 15 lymph nodes should be harvested during oesophagectomy.28 In terms of lymphadenectomy, Biere et al.29 reported that approximately 20 lymph nodes were resected during minimally invasive oesophagectomy in a randomised controlled trial, which was similar to the average number of lymph nodes harvested in our study. For major postoperative complications, 7.3% of patients suffered from anastomotic leakage, and 4.9% of patients suffered from vocal cord paralysis. These results were consistent with previous studies of minimally invasive oesophagectomy.30 Moreover, there was no unexpected admission to the ICU, and no 90-day mortality occurred in the trial. Therefore, camrelizumab, chemotherapy and apatinib did not increase the difficulty of minimally invasive oesophagectomy or increase postoperative morbidity.
A total of 61.0% (95% CI, 44.5–75.8%) of patients in our study achieved MPR following surgical treatment, including 43.9% (95% CI, 28.5–60.3%) of them who attained PCR. One-third (39%, 95% CI, 24.2–55.5%) of the patients undergoing anti-PD1 neoadjuvant therapy did not exhibit an effective antitumour response. Given the superior antitumour efficacy achieved in immunotherapy for ESCC, it can be anticipated that the application of immunotherapy in the treatment of ESCC will continue to expand. However, along with this expansion, there will be an increasing proportion of patients who do not respond to this therapy or experience treatment relapse.
In this context, the identification of biomarkers for distinguishing between responders and nonresponders before treatment initiation would enable targeted administration of therapy to responsive patients while also offering alternative regimens for patients unlikely to respond, thus preventing disease progression.31 By employing CyTOF in conjunction with cluster analysis, we investigated the distinct immune profiles of patients either with or without PCR. Our findings revealed that neoadjuvant therapy induced changes in the composition of T-cell subsets in both the PCR and NPCR populations. Furthermore, we observed an indiscriminate suppressive effect on PD1+CD8+ T cells. Although camrelizumab primarily targets PD1 on T cells, using PD1+ CD8+ T cells as a predictive marker for treatment response is impractical. This suggests that T-cell characteristics may have limited predictive value for immunotherapy efficacy in patients with ESCC. In contrast, NK cells, B cells, and myeloid cells exhibited a smaller response to neoadjuvant therapy but showed some advantages in distinguishing between the NPCR and PCR groups. Therefore, further research on the immune features of these cell types may help identify more reliable biomarkers for predicting immunotherapy response.
There were three limitations to our study. First, this was an exploratory study, and some potential selection bias could not be eliminated since the number of enrolled patients was small. The chemotherapy cycle was not fixed during the trail, multiple comparison was not employed and we analysed only part of our neoadjuvant immunotherapy regimen. Therefore, further randomised controlled studies are needed to verify our findings. Second, longer follow-up studies are needed to test whether our neoadjuvant therapy regimen can confer survival benefits to patients with locally advanced ESCC. Third, we did not comprehensively investigate the underlying reasons behind the immunotherapy response disparity. Thus, more detailed investigations on these immune cell subsets are necessary.
In summary, these results provide clinical evidence for the application of camrelizumab, chemotherapy and apatinib in the neoadjuvant setting for locally advanced ESCC with promising antitumour activity and an acceptable safety profile. Longer follow-up to assess survival outcomes and a phase 3 randomised trial are now needed to formally evaluate the proposed treatment.
Contributors
MW, ZXW, SH, CQW and JZ contributed to study conception, design and protocol writing; ZXW, CQW, JZ, QW, RB, XFF and HH contributed to recruitment of patients; CW, HXP, ZXW and CQW contributed to data collection, analysis and interpretation. MW, ZXW and SH supervised the study. ZXW, CQW, JZ, and CW contributed to drafting the manuscript. ZXW, CQW, JZ, and CW accessed and verified the underlying data. All authors participated in revising the manuscript critically for important intellectual content. All the authors have read and approved the final manuscript.
Data sharing statement
The data collection form and extracted data can be made available upon request to the corresponding author Dr Hong Shen (Email: shenhong0023@zju.edu.cn) and Prof Ming Wu (Email: iwuming22@zju.edu.cn).
Declaration of interests
We declare no competing interests.
Acknowledgements
The authors would like to express their thanks to all the patients and their families.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2024.102579.
Contributor Information
Hong Shen, Email: shenhong0023@zju.edu.cn.
Ming Wu, Email: iwuming22@zju.edu.cn.
Appendix A. Supplementary data
References
- 1.Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Abnet C.C., Arnold M., Wei W.Q. Epidemiology of esophageal squamous cell carcinoma. Gastroenterology. 2018;154(2):360–373. doi: 10.1053/j.gastro.2017.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pennathur A., Gibson M.K., Jobe B.A., Luketich J.D. Oesophageal carcinoma. Lancet. 2013;381(9864):400–412. doi: 10.1016/S0140-6736(12)60643-6. [DOI] [PubMed] [Google Scholar]
- 4.Uhlenhopp D.J., Then E.O., Sunkara T., Gaduputi V. Epidemiology of esophageal cancer: update in global trends, etiology and risk factors. Clin J Gastroenterol. 2020;13(6):1010–1021. doi: 10.1007/s12328-020-01237-x. [DOI] [PubMed] [Google Scholar]
- 5.Yang H., Liu H., Chen Y., et al. Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (NEOCRTEC5010): a phase III multicenter, randomized, open-label clinical trial. J Clin Oncol. 2018;36(27):2796–2803. doi: 10.1200/JCO.2018.79.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cunningham D., Allum W.H., Stenning S.P., et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. New Engl J Med. 2006;355(1):11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 7.Shapiro J., van Lanschot J., Hulshof M., et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16(9):1090–1098. doi: 10.1016/S1470-2045(15)00040-6. [DOI] [PubMed] [Google Scholar]
- 8.Sun J.M., Shen L., Shah M.A., et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet. 2021;398(10302):759–771. doi: 10.1016/S0140-6736(21)01234-4. [DOI] [PubMed] [Google Scholar]
- 9.Gandhi L., Rodríguez-Abreu D., Gadgeel S., et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. New Engl J Med. 2018;378(22):2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 10.Emens L.A., Middleton G. The interplay of immunotherapy and chemotherapy: harnessing potential synergies. Cancer Immunol Res. 2015;3(5):436–443. doi: 10.1158/2326-6066.CIR-15-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Comprehensive Cancer Network . 2022. NCCN clinical practice guidelines in oncology: esophageal and esophagogastric junction cancers. Version 4.https://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf [Google Scholar]
- 12.Huang J., Xu J., Chen Y., et al. Camrelizumab versus investigator's choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): a multicentre, randomised, open-label, phase 3 study. Lancet Oncol. 2020;21(6):832–842. doi: 10.1016/S1470-2045(20)30110-8. [DOI] [PubMed] [Google Scholar]
- 13.Luo H., Lu J., Bai Y., et al. Effect of camrelizumab vs placebo added to chemotherapy on survival and progression-free survival in patients with advanced or metastatic esophageal squamous cell carcinoma: the ESCORT-1st randomized clinical trial. J Am Med Assoc. 2021;326(10):916–925. doi: 10.1001/jama.2021.12836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang B., Qi L., Wang X., et al. Phase II clinical trial using camrelizumab combined with apatinib and chemotherapy as the first-line treatment of advanced esophageal squamous cell carcinoma. Cancer Commun. 2020;40(12):711–720. doi: 10.1002/cac2.12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Z., Chen X., Li Y., et al. Phase Ib trial of camrelizumab combined with chemotherapy and apatinib for neoadjuvant treatment of locally advanced thoracic esophageal squamous cell carcinoma. J Natl Cancer Cent. 2022;2(2):98–105. [Google Scholar]
- 16.Saliba G., Detlefsen S., Carneiro F., et al. Tumor regression grading after neoadjuvant treatment of esophageal and gastroesophageal junction adenocarcinoma: results of an international Delphi consensus survey. Hum Pathol. 2021;108:60–67. doi: 10.1016/j.humpath.2020.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Klevebro F., Alexandersson V.D.G., Wang N., et al. A randomized clinical trial of neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for cancer of the oesophagus or gastro-oesophageal junction. Ann Oncol. 2016;27(4):660–667. doi: 10.1093/annonc/mdw010. [DOI] [PubMed] [Google Scholar]
- 18.Pataer A., Kalhor N., Correa A.M., et al. Histopathologic response criteria predict survival of patients with resected lung cancer after neoadjuvant chemotherapy. J Thorac Oncol. 2012;7(5):825–832. doi: 10.1097/JTO.0b013e318247504a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mariette C., Dahan L., Mornex F., et al. Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: final analysis of randomized controlled phase III trial FFCD 9901. J Clin Oncol. 2014;32(23):2416–2422. doi: 10.1200/JCO.2013.53.6532. [DOI] [PubMed] [Google Scholar]
- 20.Huang J., Xu B., Mo H., et al. Safety, activity, and biomarkers of SHR-1210, an anti-PD-1 antibody, for patients with advanced esophageal carcinoma. Clin Cancer Res. 2018;24(6):1296–1304. doi: 10.1158/1078-0432.CCR-17-2439. [DOI] [PubMed] [Google Scholar]
- 21.Liu J., Li J., Lin W., et al. Neoadjuvant camrelizumab plus chemotherapy for resectable, locally advanced esophageal squamous cell carcinoma (NIC-ESCC2019): a multicenter, phase 2 study. Int J Cancer. 2022;151(1):128–137. doi: 10.1002/ijc.33976. [DOI] [PubMed] [Google Scholar]
- 22.Yang W., Xing X., Yeung S.J., et al. Neoadjuvant programmed cell death 1 blockade combined with chemotherapy for resectable esophageal squamous cell carcinoma. J Immunother Cancer. 2022;10(1) doi: 10.1136/jitc-2021-003497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fukumura D., Kloepper J., Amoozgar Z., Duda D.G., Jain R.K. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol. 2018;15(5):325–340. doi: 10.1038/nrclinonc.2018.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J., Wang L. Efficacy and safety of apatinib treatment for advanced esophageal squamous cell carcinoma. Oncotargets Ther. 2017;10:3965–3969. doi: 10.2147/OTT.S132756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meng X., Wu T., Hong Y., et al. Camrelizumab plus apatinib as second-line treatment for advanced oesophageal squamous cell carcinoma (CAP 02): a single-arm, open-label, phase 2 trial. Lancet Gastroenterol. 2022;7(3):245–253. doi: 10.1016/S2468-1253(21)00378-2. [DOI] [PubMed] [Google Scholar]
- 26.Fang W., Yang Y., Ma Y., et al. Camrelizumab (SHR-1210) alone or in combination with gemcitabine plus cisplatin for nasopharyngeal carcinoma: results from two single-arm, phase 1 trials. Lancet Oncol. 2018;19(10):1338–1350. doi: 10.1016/S1470-2045(18)30495-9. [DOI] [PubMed] [Google Scholar]
- 27.Xu J., Shen J., Gu S., et al. Camrelizumab in combination with apatinib in patients with advanced hepatocellular carcinoma (RESCUE): a nonrandomized, open-label, phase II trial. Clin Cancer Res. 2021;27(4):1003–1011. doi: 10.1158/1078-0432.CCR-20-2571. [DOI] [PubMed] [Google Scholar]
- 28.Li H., Fang W., Yu Z., et al. Chinese expert consensus on mediastinal lymph node dissection in esophagectomy for esophageal cancer (2017 edition) J Thorac Dis. 2018;10(4):2481–2489. doi: 10.21037/jtd.2018.03.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Biere S.S., van Berge H.M., Maas K.W., et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet. 2012;379(9829):1887–1892. doi: 10.1016/S0140-6736(12)60516-9. [DOI] [PubMed] [Google Scholar]
- 30.Luketich J.D., Pennathur A., Awais O., et al. Outcomes after minimally invasive esophagectomy: review of over 1000 patients. Ann Surg. 2012;256(1):95–103. doi: 10.1097/SLA.0b013e3182590603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Topalian S.L., Forde P.M., Emens L.A., Yarchoan M., Smith K.N., Pardoll D.M. Neoadjuvant immune checkpoint blockade: a window of opportunity to advance cancer immunotherapy. Cancer Cell. 2023 doi: 10.1016/j.ccell.2023.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.