Abstract
Objectives
Different SARS-CoV-2 variants can differentially affect the prevalence of Post Covid-19 Condition (PCC). This prospective study assesses prevalence and severity of symptoms three months after an Omicron infection, compared to Delta, test-negative and population controls. This study also assesses symptomology after reinfection and breakthrough infections.
Methods
After a positive SARS-CoV-2 test, cases were classified as Omicron or Delta based on ≥ 85% surveillance prevalence. Three months after enrolment, participants indicated point prevalence for 41 symptoms and severity, using validated questionnaires for four symptoms. PCC prevalence was estimated as the difference in prevalence of at least one significantly elevated symptom, identified by permutation test, in cases compared to population controls.
Results
At three months follow-up, five symptoms and severe dyspnea were significantly elevated in Omicron cases (n = 4138) compared to test-negative (n = 1672) and population controls (n = 2762). PCC prevalence was 10·4% for Omicron cases and 17·7% for Delta cases (n = 6855). In Omicron cases, severe fatigue and dyspnea were more prevalent in reinfected than primary infected, while severity of symptoms did not significantly differ between cases with a booster or primary vaccination course.
Conclusions
Prevalence of PCC is 41% lower after Omicron than Delta at three months. Reinfection seems associated with more severe long-term symptoms compared to first infection.
Keywords: Long COVID, Post Covid-19 condition, COVID-19, SARS-CoV-2, Omicron, Delta, Long-term symptoms, Prevalence, Symptoms, Reinfection
1. Introduction
Worldwide 750 million SARS-CoV-2 infections have occurred up to March 2023, and numerous publications report that for some symptoms persist for months [[1], [2], [3]]. This condition is referred to as Post Covid-19 Condition (PCC), and can have a significant impact on individuals and health care [3]. A case definition of PCC by the WHO stipulated difficulty in everyday life functioning [4]. However, prevalence and severity of symptoms associated with PCC may vary with different variants of concern (VOC). Indeed, a systematic review has shown that PCC prevalence is lowest after the Omicron variant though most studies are heterogenous due to differences in control group inclusion and follow-up time, resulting in differing prevalence estimates [5]. Compared to B.1.617.2 (Delta), B.1.1.529 (Omicron) has already been characterized by higher transmissibility, lower pathogenicity and shorter acute phase [6]. Additionally, Omicron is better at immune escape than Delta which also raises the question to what extent vaccination protects against PCC-related symptoms after Omicron breakthrough infections and to what extent a previous infection may protect against PCC-related symptoms after an Omicron reinfection [7].
For pre-Omicron VOC, the prevalence and severity of these sequela are already documented for several countries including the Netherlands [2,[8], [9], [10]]. In the Netherlands, over 8·5 million SARS-CoV-2 infections were reported up to March 2023, of those nearly 4·2 million infections occurred from December 2021 on during the Omicron VOC emergence [11].
This study aimed to assess PCC symptom prevalence and severity after Omicron compared to Delta, test-negative controls and population controls. Moreover we assessed the effect of the booster against developing PCC-related symptoms after Omicron breakthrough infections and the effect of a reinfection compared to a first infection.
2. Methods
2.1. Design, participants and inclusion
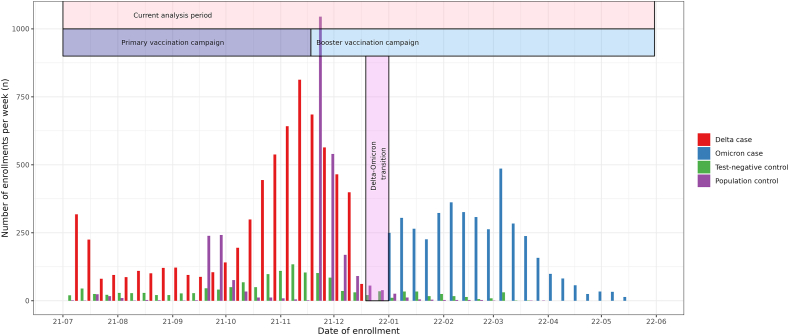
Data were collected in the context of the Dutch prospective LongCOVID-study. Study design details are described in the previously published study protocol [12]. This paper reports a follow-up study from our previous findings on long term prevalence and severity of symptoms 3 months after Alpha and Delta SARS-CoV-2 infection [8]. In brief, here we report on Omicron cases aged 18 or older three months after testing positive for SARS-CoV-2 and were enrolled between January 3rd and May 31st, 2022 (Fig. 1). Cases variants of infection were not determined by genotyping; instead, the Omicron period is defined by ≥ 85% proportion of Omicron in the Dutch pathogen surveillance [13]. Likewise Delta cases enrolled between July 5th, 2021 and December 19th, 2021. Cases between December 19th, 2021 and January 3rd were excluded as neither variant was dominant. Cases were recruited within seven days following a positive PCR or an antigen SARS-CoV-2 test from testing facilities. Test-negative controls who reported symptoms as testing reason and population controls without previous suspected or confirmed COVID-19 were included to control for background prevalence of symptoms. Population controls from the Netherlands were randomly invited by direct mailing. Controls were included if they enrolled between July 5th, 2021 and May 31st, 2022. Participants received questionnaires at baseline (T0) and after three months follow-up (T3).
Fig. 1.
Timeline of, the current analysis period, the Dutch COVID-19 vaccination program and inclusion and classification of participants in the current study.
2.2. Outcomes and covariates
The primary outcome was the prevalence of PCC, which we defined – slightly modified from our previous study – as the difference in prevalence of at least one significantly elevated symptom in Omicron cases compared to the prevalence in the population control group after three months [8]. Likewise we assessed the prevalence of PCC in Delta cases. As secondary outcomes we assessed the prevalence of symptoms with a clinically relevant severity, using validated questionnaires with population norm scores. These included severe fatigue measured with the subscale fatigue of the Checklist Individual Strength (CIS, cut-off score ≥35) [14,15]; severe self-reported cognitive problems on the Cognitive Failure Questionnaire (CFQ, cut-off score ≥44) [16,17]; severe pain on the bodily subscale (cut-off score ≤55), and social and physical functioning of the RAND SF-36 Health Status Inventory [18]; and severe dyspnea on the modified Medical Research Council (mMRC, cut-off score ≥1) scale [19]. We compared the prevalence of severe fatigue, severe cognitive problems, severe pain and severe dyspnea for Omicron cases, Delta cases, test-negative controls and population controls. Vaccination and reinfection methods are described in the Supplementary Methods.
Information on demographics, vaccination status, general health status, use of health care and medication, and comorbidities (adapted from the TiC–P) were collected at baseline [20].
2.3. Statistical analyses
Statistical procedures were based on a predefined, published study protocol [12]. Briefly, the primary analysis was a complete case analysis with only participants completing both T0 and T3. Baseline data were compared with Chi-squared tests and with Mann-Whitney U for age and number of symptoms. Four sensitivity analyses were used to substitute for missing data on symptoms at T3: multiple imputation, last observation carry forward, best case and worst case scenario (See Supplementary Methods). Prevalence of 41 symptoms and prevalence of the severe symptoms were compared between Omicron cases, Delta cases and both control groups by permutation tests which were stratified for predefined confounders age, sex, level of education and number of comorbidities. Significantly elevated symptoms in Omicron cases compared to both control groups were defined by a two-sided 5% significance level with Benjamini-Hochberg adjusted p-values [21]. Prevalence of at least one significantly elevated symptom at T3 was then assessed for Omicron and Delta cases and both control groups. PCC prevalence was estimated as the difference in prevalence of at least one significantly elevated symptom in the cases compared to the population controls. Likewise, comparisons were made for Omicron cases with a booster and with only a completed primary vaccination course and for Omicron cases with a first infection and a reinfection. Lastly, to evaluate the sensitivity of the PCC definition we compared the severity scores between cases and population controls who did not fulfil our definition.
Analyses were performed with R version 4·2·0 (packages listed in Supplementary methods).
Ethics approval
The research protocol was shared with the Medical Ethics Review Committee Utrecht, and an official waiver for ethical approval (reference number: MvdL/mb/21/500208) was obtained given the non-invasive nature of data collection. All participants gave informed consent before inclusion in the study.
3. Results
Baseline characteristics for cases and both control groups that completed both T0 and T3 questionnaires (complete case) are shown in Table 1. In total 4138 Omicron cases, 6855 Delta cases, 1672 test-negative controls and 2726 population controls were included, see also flowchart Fig. S1. By estimation, around 2.5% Delta and Omicron cases had a variant misclassification due to the 85% cut-off and the short 2 week transition period. Differences between controls and cases in vaccination status are mostly due to differing inclusion times.
Table 1.
Demographics and acute illness at baseline.
| Complete case | Omicron cases | Delta cases | Test-negative controls | Population controls |
|---|---|---|---|---|
| N | 4138 | 6855 | 1672 | 2726 |
| Age, median [IQR] | 55·8 [43·8; 65·9] | 52·1 [40·0; 62·8] *** | 57·3 [44·0; 66·0] | 53·2 [41·9; 60·7] *** |
| Sex, % (n) | * | *** | ||
| Female | 62·0 (2567) | 62·9 (4315) | 64·4 (1076) | 68·6 (1871) |
| Male | 37·7 (1561) | 36·8 (2526) | 35·1 (587) | 31·2 (850) |
| Other | 0·1 (4) | 0·1 (9) | 0·4 (6) | 0·0 (1) |
| Pregnancy, % (n) | 2·2 (19) | 2·4 (45) | 2·1 (7) | 4·1 (27) * |
| BMI, mean (SD) | 25·91 (4·68) | 25·76 (4·60) | 26·00 (4·86) | 25·84 (4·65) |
| Smoking, % (n) | *** | *** | ||
| Current smoker | 3·9 (160) | 4·4 (301) | 7·4 (123) | 5·7 (156) |
| Former smoker | 27·0 (1117) | 25·6 (1754) | 30·1 (503) | 21·2 (577) |
| Never smoker | 67·0 (2771) | 67·3 (4614) | 59·7 (999) | 71·0 (1935) |
| Level of education, % (n) | ** | *** | *** | |
| Low | 3·2 (132) | 3·4 (235) | 2·2 (37) | 5·2 (142) |
| Medium | 32·0 (1324) | 35·0 (2402) | 26·6 (445) | 38·5 (1049) |
| High | 64·8 (2682) | 61·5 (4218) | 71·2 (1190) | 56·3 (1535) |
| History with COVID-19, % (n) | 10·9 (453) | 9·0 (617) ** | 0·0 (0) *** | 0·0 (0) *** |
| Nr of comorbidities, % (n) | *** | *** | *** | |
| 0 | 44·2 (1827) | 47·1 (3231) | 40·0 (668) | 54·0 (1471) |
| 1-2 | 42·7 (1766) | 42·3 (2902) | 42·9 (718) | 36·5 (996) |
| >2 | 13·2 (545) | 10·5 (722) | 17·1 (286) | 9·5 (259) |
| Respiratory disease, % (n) | 17·4 (720) | 16·6 (1137) | 21·5 (359) *** | 11·3 (309) *** |
| Hypertension, % (n) | 14·1 (584) | 12·0 (826) ** | 14·8 (248) | 10·8 (294) *** |
| Diabetes, % (n) | 3·7 (155) | 2·9 (202) * | 3·8 (63) | 3·4 (93) |
| Cardiovasculair disease, % (n) | 2·5 (102) | 2·0 (135) | 2·8 (47) | 1·4 (38) ** |
| Use of healthcare, % (n) | 6·2 (257) | 10·6 (727) *** | 12·0 (200) *** | 5·5 (151) |
| Medication use, % (n) | 74·5 (3083) | 77·2 (5292) ** | 68·7 (1148) *** | 22·2 (605) *** |
| Admitted to hospital, % (n) | 0·2 (7) | 0·1 (5) | 0·1 (1) | 0·5 (6) |
| Vaccination status at T0, % (n) | *** | *** | *** | |
| Boostered | 76·1 (2970) | 0·3 (18) | 11·0 (160) | 1·9 (46) |
| Complete primary course | 21·8 (853) | 93·4 (5274) | 83·1 (1206) | 94·9 (2346) |
| Partially vaccinated | 0·5 (21) | 2·1 (118) | 3·3 (48) | 1·5 (37) |
| Unvaccinated | 1·5 (60) | 4·2 (238) | 2·5 (37) | 1·7 (43) |
| Number of symptoms at T0, median [IQR] | 8 [5; 12] | 9 [6; 13]*** | 5 [3; 8]*** | 0 [0; 2] *** |
| Recruitment period | 03-01-2022 - 31-05-2022 | 05-07-2021 - 19-12-2021 | 05-07-2021 - 31-05-2022 | 05-07-2021 - 31-05-2022 |
| Variant dominant at T0, % (n) | ||||
| Delta | 0·0 (0) | 100 (6855) | 83·0 (1250) | 94·6 (2551) |
| Delta-Omicron | 0·0 (0) | 0·0 (0) | 3·8 (57) | 3·2 (87) |
| Omicron | 100 (4138) | 0·0 (0) | 13·2 (199) | 2·2 (58) |
Note:Age and number of symptoms at T0 were compared with Mann-Whitney U test, BMI with t-test, and all other baseline demographics with the Chi-square test.
The significance levels are reported at p-value: * < 0.05; ** < 0.01; *** < 0.001 compared to Omicron cases.
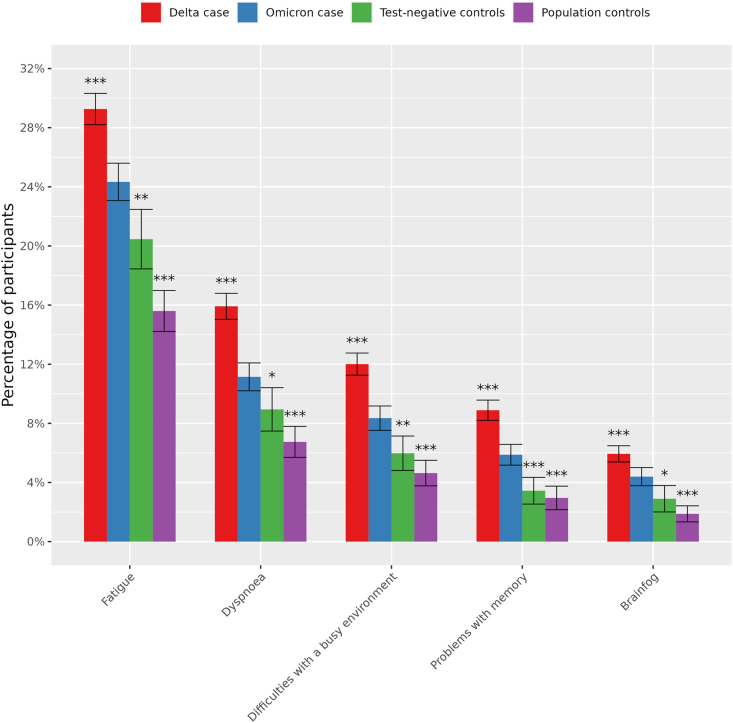
Fig. 2 shows that fatigue (24·3%; p.BH = 0·0077), dyspnea (11·1%; p.BH = 0·025), difficulties with a busy environment (8·4%; p.BH = 0·0077), problems with memory (5·9%; p.BH = 0·00092) and brainfog (2·9%; p.BH = 0·014) were significantly elevated in Omicron cases compared to both control groups (shown p.BH-values here are compared to test-negative controls, all p.BH < 0·0001 compared to population controls) after three months follow-up in the complete case scenario. Yet, the prevalence of all five symptoms was significantly lower in Omicron cases compared to Delta cases. Prevalence of all 41 symptoms are available in Supplementary Table S1.
Fig. 2.
Standardised prevalence (95% confidence intervals) of the 5 symptoms at T3 that were significantly elevated (p.BH < 0,05) between Omicron cases and both control groups and their prevalence in Delta cases using complete case analysis without substituting for missing values at T3. Symptoms are ranked by prevalence in Omicron cases. *BH.adjusted p-value < 0.05; **BH.adjusted p-value < 0.01; ***BH.adjusted p-value < 0.001 compared to Omicron cases. Symptoms are self-reported and quantified according to the mean standardized prevalence.
Fatigue and dyspnea were generally reported in both the acute phase and at T3 while difficulty with a busy environment, problems with memory and brainfog were generally reported more at T3 only (Fig. S3).
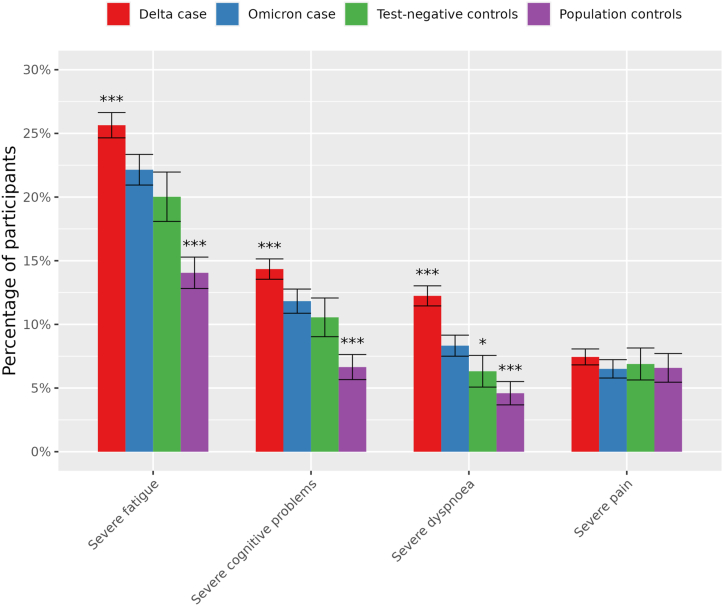
Severe dyspnea had a significantly higher prevalence in Omicron cases (8·3%) compared to test-negative and population control groups (6·3% and 4·2%, p.BH 0·02 and < 0·001); Fig. 3). Severe fatigue and severe cognitive problems were significantly more prevalent in Omicron cases (11·8%) compared to population controls (6,6%, p.BH < 0·001) but not compared to test-negative controls (10·6%, p.BH = 0·14). Finally, severe fatigue (25·6% vs 22·1%), severe cognitive problems (14·3% vs 11·8%) and severe dyspnea (12·2% vs 8·3%) were significantly more prevalent in Delta compared to Omicron cases (all p.BH < 0·0001).
Fig. 3.
Standardised prevalence (95% confidence intervals) of severity score cut-off values in cases and both control groups using complete case analysis without substituting for missing values at T3. Severe fatigue: Checklist Individual Strength (CIS), subscale fatigue ≥35, severe cognitive problems: Cognitive Failure Questionnaire (CFQ) ≥44, severe dyspnoea: modified Medical Research Council dyspnoea scale mMRC ≥1, severe pain: SF-36 subscale bodily pain ≤55. *BH.adjusted p-value < 0.05; **BH.adjusted p-value < 0.01; ***BH.adjusted p-value < 0.001 compared to Omicron cases.
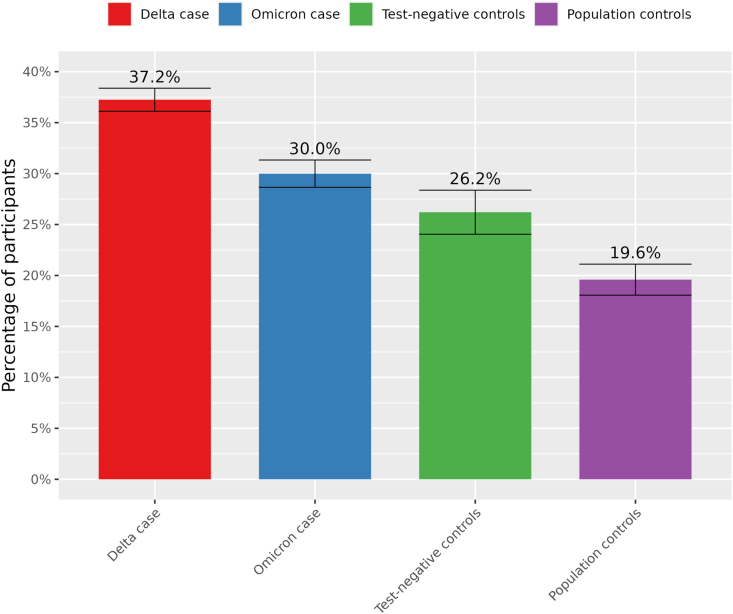
In the complete case scenario, the overall prevalence of the five significantly elevated symptoms was found to be significantly lower for Omicron cases (30·0%; CI: 28.6%–31.3%) compared to Delta cases (37·2%; CI: 36.1%–37.2%; p < 0.0001) but higher compared to test-negative controls (26·2%; CI: 24.0%–28.4%; p = 0.0011) and population controls (19·6%; CI: 18.1%–21.1% p < 0.0001) at T3 (Fig. 4). Prevalence of PCC was therefore estimated at 10·4% in Omicron cases, compared to 17·7% in Delta cases. Differences between Omicron cases and Delta cases and control groups were also noticeable in the multiple imputation, carry forward and worst case scenario when substituting for missing values (Supplementary Fig. S2). In the best case scenario differences between Omicron cases and controls were no longer significant. We found no significant differences between boostered and primary course Omicron cases for severity of symptoms and prevalence of at least one PCC-associated symptom (Supplementary Figs. S4–5). A significantly higher prevalence of at least one PCC-associated symptom and severe fatigue and dyspnoea was found for reinfected Omicron cases compared to a first infection (Supplementary Figs. S6–7). Omicron and Delta cases that fulfilled the case definition had lower scores for social and physical functioning than those that did not fulfil the definition (Supplementary Fig. S8).
Fig. 4.
Standardized prevalence (95% confidence intervals) of at least one of the five significantly elevated symptoms at T3 in Delta and Omicron cases compared to test-negative and population controls.
Cases that did not fulfil our PCC case definition had significantly worse scores for CIS-fatigue, CFQ and SF36-pain than population controls not fulfilling the definition, but the absolute differences were less than 1 point on all scales (Supplementary Table S4).
4. Discussion
In this prospective cohort study we found that three months after Omicron, symptoms that were significantly elevated compared to both control groups were fatigue, dyspnea, difficulties with a busy environment, problems with memory and brainfog. Prevalence of PCC – i.e. the difference in prevalence of at least one of these symptoms in cases compared to the population controls – was 41% lower for Omicron cases compared to Delta cases (10.4% vs 17.7%, respectively).
Severity of symptoms was also lower after Omicron than after Delta for fatigue, cognitive impairment and dyspnea. Still these symptoms were reported significantly more often as severe in Omicron cases compared to population controls, whereas only severe dyspnea was increased compared to test-negative controls. Indeed, as also reported by others, long-term outcomes for Omicron SARS-CoV-2 seem more comparable with other respiratory pathogens than for Delta [22]. Nevertheless, the prevalence of severe fatigue and severe cognitive problems after Omicron exceeds that of unexposed population controls. Previous research in the LongCOVID study on three month follow-up of prevalence and severity of symptoms following an Alpha or Delta infection found a total of 13 significantly elevated symptoms [8]. In the Omicron analysis only 5 symptoms exceeded background prevalence in both control groups. Most notably, the COVID-19 characteristic symptoms loss of smell and taste are 5·5 and 4·2 times higher in prevalence at T3 for Delta than Omicron cases (Supplementary Table 1). A possible explanation could be that Omicron generally has less involvement of the lower respiratory tract and a milder acute phase than Delta [6]. These findings are in line with a Danish and UK study that have shown lower odds for PCC with Omicron compared to Delta [23,24]. Interestingly, a Norwegian study did not find differences in long-term symptoms when the variants co-circulated [25]. This discrepancy between studies may have to do with different levels of immunity in the population when comparing Omicron and Delta infections in different time periods or differences in subvariants analysed. In the current analyses, we focussed on adults aged 18 and older, without further stratification of age-specific effects. Notably though, lower risks of PCC following Omicron compared to other VOCs have also been reported for adolescents [26].
Cases with a reinfection seemed to have a higher prevalence for PCC with a 1·2 times higher prevalence of PCC-related symptoms compared to a first SARS-CoV-2 infection with the Omicron variant. Over 750 million people have been infected, which infers an increasing likelihood of reinfection occurring with (sub)variants better at escaping immunity. Natural immunity, and also hybrid immunity with vaccination, against subsequent infection has additionally been shown to wane over time [27]. Despite observing minimal differences in comorbidities and medication use, it cannot be excluded that reinfected cases had unmeasured or low systemic impaired health possibly putting them at (slightly) higher risk both for reinfection and PCC. This would imply that the probability of developing symptomatic COVID-19 could be due to different health status rather than that the reinfection itself would be associated with a higher risk of developing PCC. This would be in line with a previous study that has shown that hybrid immunity from prior infection and vaccination did not abrogate risk of long-term symptoms [28]. Additional research on PCC and reinfection is needed to better understand this relationship.
Waning immunity may also infer a diminishing vaccine protection against PCC after an Omicron infection. Omicron cases with a primary course received their last vaccination a median 142 days prior to infection while boostered cases had a median time difference of 60 days. Still the booster compared to a primary vaccination course seemed at most modestly protective for PCC: fatigue and cognitive problems were not significantly less frequently severe in cases with a booster, and the study may lack the power to detect smaller differences [12]. Studies have shown a partial protection of the primary course compared to unvaccinated cases for pre-Omicron variants [8,29,30] and for Omicron [31]. Research on the effect of the booster is limited but one study shows a lower association with PCC for three-dose vaccinated Omicron cases compared to two-dosed [24]. Generally, there is evidence to suggests that a booster provides an albeit temporary protection against infection with Omicron [32]. Indirect effects by preventing infection and transmission compounded with a modest direct effect may still yield a more than modest reduction in PCC incidence. Still, our findings suggest that booster induced immunity has either waned or offers limited direct protection against long-term symptoms following an Omicron breakthrough infection. Altogether, the increasing probability of an infection being a reinfection, the waning of vaccine-induced immunity and the high infection rate caused by Omicron may have resulted in a larger number of PCC patients though the risk per infection may be lower.
4.1. Strengths and limitations
A strength of this prospective cohort study is the inclusion of large numbers of Delta and Omicron cases as well as two control groups to be able to estimate the prevalence of PCC corrected for the background prevalence of symptoms in the population and symptoms likely due to other respiratory infections. Reporting of reinfections and vaccination status made it possible to investigate their association with the prevalence of PCC after SARS-CoV-2 infection. Moreover, recruiting at test sites rather than hospitals resulted in a cohort that is representative of the general population, though likely limiting inclusion of asymptomatic cases.
This study also has limitations. Firstly, the T3 survey had a response rate of 70%. It is possible that 30% missed due to lack of symptoms or, oppositely, becoming severely ill. Therefore we substituted for missing values by multiple alternative imputation scenarios, which showed robustness of our finding that Omicron was less severe than Delta. Secondly, background prevalence was largely established on controls recruited during the Delta period which may have a different background prevalence than the Omicron period due to restrictions and seasonal effects. However, most controls included during the Delta period enrolled close to the Omicron period with 40 day median difference. Supplementary Table 1 additionally shows that between T0 and T3 background prevalence fluctuates little. Moreover, severe fatigue from our control groups (20·0% for test-negative and 14·0% for the population control) was similar to a large Dutch population cohort (18%, n = 78363) [33]. Besides a different VOC, other explanatory factors could exist for the difference in PCC, such as differential levels of existing immunity, and different season, since Delta cases were included in summer and autumn of 2021 and Omicron during winter and spring of 2021/2022. Applying permutation tests that compared strata on age, sex, education and comorbidities is expected to minimize impact on differences between study groups. The self-reporting of symptoms as applied in this study reflects the participants perception and thus the impact on their daily life, rather than objectifying reported symptoms. To be able to assess clinically relevant scores for fatigue, dyspnoea, cognitive impairment and pain we used well-validated questionnaires. Additionally, social and physical functioning scores were lower for cases with PCC-associated symptoms compared to those without, in line with other studies that assessed severity of post-infectious symptoms [34]. Finally, our case definition of PCC may not have been sensitive enough to capture all post-covid symptoms, since cases that did not fulfil our case definition had worse scores for CIS-fatigue, CFQ and SF36-pain than population controls not fulfilling the case definition. However, the absolute differences in mean scores were minimal, which suggests that our case definition still captured the vast majority of PCC.
Role of the funding source
The study is executed by the National Institute for Public Health by order of the Ministry of Health. The study is not the result of a competitive grant. The Dutch Ministry of Health, Welfare and Sport does not have a role in the design of this study, its execution, analyses and interpretation of results.
Data availability statement
Supporting clinical documents including the study protocol and statistical analysis plan will be available immediately following publication of this Article for at least 1 year. Researchers who provide a methodologically sound proposal will within the applicable privacy legislation be allowed to access to the de-identified individual participant data that underlie the results reported in this article. Proposals should be sent to the corresponding author. These proposals will be reviewed and approved by the investigators on the basis of scientific merit. To gain access, data requestors will need to sign a data access agreement. Data associated with the study has not been deposited in a publicly available repository. Data will be made available on request as illustrated above.
CRediT authorship contribution statement
Siméon de Bruijn: Writing – original draft, Methodology, Formal analysis, Data curation, Conceptualization. Albert Jan van Hoek: Writing – review & editing, Validation, Supervision, Methodology, Investigation, Funding acquisition, Data curation, Conceptualization. Elizabeth N. Mutubuki: Writing – review & editing, Methodology, Data curation, Conceptualization. Hans Knoop: Writing – review & editing, Methodology. Jaap Slootweg: Writing – review & editing, Visualization, Software, Methodology, Formal analysis. Anna D. Tulen: Writing – review & editing. Eelco Franz: Writing – review & editing, Supervision, Resources, Funding acquisition, Conceptualization. Cees C. van den Wijngaard: Writing – review & editing, Validation, Supervision, Project administration, Methodology, Funding acquisition, Formal analysis, Data curation, Conceptualization. Tessa van der Maaden: Writing – review & editing, Visualization, Validation, Methodology, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank Caroline van den Ende for structural updating of literature on PCC.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e28941.
List of abbreviations
- - CIS
Checklist Individual Strength
- - CFQ
Cognitive Failure Questionnaire
- - mMRC
Modified Medical Research Council dyspnea scale
- - PCC
Post-Covid-19 Condition
- - PCR
polymerase chain reaction
- - SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- - SF-36
SF-36 item Health Survey
- - TiC–P:
Treatment Inventory of Costs in Patients with psychiatric disorders
- - VOC
Variant of Concern
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.World Health Organization WHO coronavirus (COVID-19) dashboard. 2021. https://covid19.who.int/ Access date 4 May 2022. 4 May 2022]; Available from:
- 2.Groff D., et al. Short-term and long-term rates of postacute sequelae of SARS-CoV-2 infection: a systematic review. JAMA Netw. Open. 2021;4(10) doi: 10.1001/jamanetworkopen.2021.28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parums D.V. Editorial: long COVID, or post-COVID syndrome, and the global impact on health care. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 2021:27. doi: 10.12659/MSM.933446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soriano J.B., et al. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect. Dis. 2021 doi: 10.1016/S1473-3099(21)00703-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernández-de-Las-Peñas C., et al. Long-COVID symptoms in individuals infected with different SARS-CoV-2 variants of concern: a systematic review of the literature. Viruses. 2022;14(12) doi: 10.3390/v14122629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Menni C., et al. Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of omicron and delta variant dominance: a prospective observational study from the ZOE COVID Study. Lancet. 2022;399(10335):1618–1624. doi: 10.1016/S0140-6736(22)00327-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bowe B., Xie Y., Al-Aly Z. Acute and postacute sequelae associated with SARS-CoV-2 reinfection. Nat. Med. 2022;28(11):2398–2405. doi: 10.1038/s41591-022-02051-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Maaden T., et al. Prevalence and severity of symptoms 3 Months after infection with SARS-CoV-2 compared to test-negative and population controls in The Netherlands. J. Infect. Dis. 2022:1537–6613. doi: 10.1093/infdis/jiac474. (Electronic) [DOI] [PubMed] [Google Scholar]
- 9.Ballering, A.V., S.K.R. van Zon, T.C. Olde Hartman, and J.G.M. Rosmalen, Persistence of Somatic Symptoms after COVID-19 in the Netherlands: an Observational Cohort Study. (1474-547X (Electronic)) doi. [DOI] [PMC free article] [PubMed]
- 10.Chen C., et al. Global prevalence of post COVID-19 condition or long covid: a meta-analysis and systematic review. J. Infect. Dis. 2022;226(9):1593–1607. doi: 10.1093/infdis/jiac136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rijksinstituut voor Volksgezondheid en Milieu (RIVM) Covid-19 aantallen per gemeente per publicatiedatum. 2022 https://data.rivm.nl/covid-19/ 31-10-2022]; Available from: [Google Scholar]
- 12.Mutubuki E.N., et al. Prevalence and determinants of persistent symptoms after infection with SARS-CoV-2: protocol for an observational cohort study (LongCOVID-study) BMJ Open. 2022;12(7) doi: 10.1136/bmjopen-2022-062439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rijksinstituut voor Volksgezondheid en Milieu (RIVM) Covid-19 rapportage van SARS-CoV-2 varianten in Nederland via de aselecte steekproef van RT-PCR positieve monsters in de nationale kiemsurveillance. 2022. https://data.rivm.nl/covid-19/ [cited 2022 11-02-2022]; Available from:
- 14.Worm-Smeitink M., et al. The assessment of fatigue: psychometric qualities and norms for the Checklist individual strength. J. Psychosom. Res. 2017;98:40–46. doi: 10.1016/j.jpsychores.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Vercoulen J.H., et al. Dimensional assessment of chronic fatigue syndrome. J. Psychosom. Res. 1994;38(5):383–392. doi: 10.1016/0022-3999(94)90099-x. [DOI] [PubMed] [Google Scholar]
- 16.Ponds R., Van Boxtel M., Jolles J. De cognitive failure questionnaire als maat voor subjectief cognitief functioneren. Tijdschr. Neuropsychol. 2006;(2):37–45. [Google Scholar]
- 17.Broadbent D.E., Cooper P.F., FitzGerald P., Parkes K.R. The cognitive failures questionnaire (CFQ) and its correlates. Br. J. Clin. Psychol. 1982;21(1):1–16. doi: 10.1111/j.2044-8260.1982.tb01421.x. [DOI] [PubMed] [Google Scholar]
- 18.van der Zee K.I., Sanderman R. Research Institute SHARE; 2012. Het meten van de algemene gezondheidstoestand met de RAND-36, een handleiding: umcg/Rijksuniversiteit groningen. [Google Scholar]
- 19.Mahler D.A., Wells C.K. Evaluation of clinical methods for rating dyspnea. Chest. 1988;93(3):580–586. doi: 10.1378/chest.93.3.580. [DOI] [PubMed] [Google Scholar]
- 20.Bouwmans C., et al. Feasibility, reliability and validity of a questionnaire on healthcare consumption and productivity loss in patients with a psychiatric disorder (TiC-P) BMC Health Serv. Res. 2013;13:217. doi: 10.1186/1472-6963-13-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hochberg Y., Benjamini Y. More powerful procedures for multiple significance testing. Stat. Med. 1990;9(7):811–818. doi: 10.1002/sim.4780090710. [DOI] [PubMed] [Google Scholar]
- 22.Nehme M., et al. Prevalence of post-coronavirus disease condition 12 Weeks after omicron infection compared with negative controls and association with vaccination status. Clin. Infect. Dis. 2022;76(9):1567–1575. doi: 10.1093/cid/ciac947. [DOI] [PubMed] [Google Scholar]
- 23.Antonelli M., et al. Risk of long COVID associated with delta versus omicron variants of SARS-CoV-2. Lancet. 2022;399(10343):2263–2264. doi: 10.1016/S0140-6736(22)00941-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spiliopoulos L., et al. Post-acute symptoms four months after SARS-CoV-2 infection during the Omicron period: a nationwide Danish questionnaire study. medRxiv. 2022;(22280990):2022.10.12. doi: 10.1101/2022.10.12.22280990. [DOI] [Google Scholar]
- 25.Magnusson, K., et al., Post-covid medical complaints after SARS-CoV-2 Omicron vs Delta variants - a prospective cohort study. medRxiv, 2022: p. 2022.05.23.22275445 doi: 10.1101/2022.05.23.22275445.
- 26.Buonsenso D., et al. Risk of long Covid in children infected with Omicron or pre-Omicron SARS-CoV-2 variants. Acta Paediatr. 2023;112(6):1284–1286. doi: 10.1111/apa.16764. [DOI] [PubMed] [Google Scholar]
- 27.Goldberg, Y., et al., Protection and Waning of Natural and Hybrid Immunity to SARS-CoV-2. (1533-4406 (Electronic)) doi. [DOI] [PMC free article] [PubMed]
- 28.Al-Aly Z., Bowe B., Xie Y. 2022. Outcomes of SARS-CoV-2 Reinfection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao, P., J.A.-O. Liu, and M.A.-O. Liu, Effect of COVID-19 Vaccines on Reducing the Risk of Long COVID in the Real World: A Systematic Review and Meta-Analysis. LID - 10.3390/ijerph191912422 [doi] LID - 12422. (1660-4601 (Electronic)) doi. [DOI] [PMC free article] [PubMed]
- 30.Al-Aly Z., Bowe B., Xie Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat. Med. 2022 doi: 10.1038/s41591-022-01840-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ballouz T., et al. Post COVID-19 condition after Wildtype, Delta, and Omicron variant SARS-CoV-2 infection and vaccination: pooled analysis of two population-based cohorts. medRxiv. 2022:2022.09. doi: 10.1101/2022.09.25.22280333. 25.22280333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ioannou G.N., et al. Effectiveness of mRNA COVID-19 vaccine boosters against infection, hospitalization, and death: a target trial emulation in the omicron (B.1.1.529) variant era. Ann. Intern. Med. 2022 doi: 10.7326/m22-1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goërtz Y.M.J., et al. Fatigue in patients with chronic disease: results from the population-based Lifelines Cohort Study. Sci. Rep. 2021;11(1) doi: 10.1038/s41598-021-00337-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ursinus J., et al. Prevalence of persistent symptoms after treatment for lyme borreliosis: a prospective observational cohort study. The Lancet Regional Health – Europe. 2021:6. doi: 10.1016/j.lanepe.2021.100142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Supporting clinical documents including the study protocol and statistical analysis plan will be available immediately following publication of this Article for at least 1 year. Researchers who provide a methodologically sound proposal will within the applicable privacy legislation be allowed to access to the de-identified individual participant data that underlie the results reported in this article. Proposals should be sent to the corresponding author. These proposals will be reviewed and approved by the investigators on the basis of scientific merit. To gain access, data requestors will need to sign a data access agreement. Data associated with the study has not been deposited in a publicly available repository. Data will be made available on request as illustrated above.