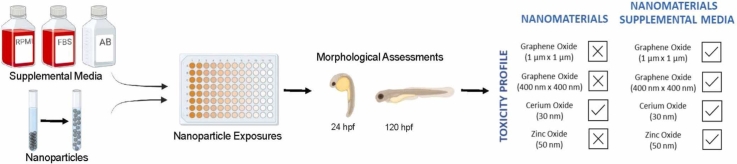
Abstract
Engineered nanomaterials (ENMs) are ubiquitous in contemporary applications, yet their environmental and human health impacts remain inadequately understood. This study addresses the challenge of identifying potential risks associated with ENM exposure by highlighting the significant variability in existing research methodologies. Without a systematic collection of toxicological data that encompasses standardized materials, relevant platforms, and assays, the task of identifying potential risks linked to ENM exposure becomes an intricate challenge. In vitro assessments often use media rich in ionic species, such as RPMI and fetal bovine serum (FBS). Zebrafish embryos, known to develop normally in low-ionic environments, were exposed to Cerium Oxide, Zinc Oxide, and Graphene Oxides in different media at varying concentrations. Here, we discovered that zebrafish embryos tolerated a mix of 80 % RPMI, 2 % FBS, and 1 % antibiotic cocktail. The results revealed that adverse effects observed in zebrafish with certain nanomaterials in Ultra-Pure (UP) water were mitigated in cell culture medium, emphasizing the importance of revisiting previously considered non-toxic materials in vitro. The zebrafish results underscore the importance of utilizing a multidimensional in vivo platform to gauge the biological activity of nanomaterials accurately.
Keywords: Zebrafish, Graphene Oxide, Zinc Oxide, Cerium Oxide, Cell culture media, embryonic development, engineered nanomaterials
Graphical Abstract
Highlights
-
•
Developing zebrafish exposed to ENMs in ultra-pure water produced adverse effects.
-
•
Embryonic zebrafish developed normally in cell culture media-like conditions.
-
•
Observed adverse effects from ENM exposure were mitigated in cell culture media.
1. Introduction
Engineered nanomaterials (ENMs) have unique and desirable properties, allowing superior industrial, biomedical, and consumer product performance [1]. There is also a need to know the potential human health effects of exposure to ENMs, as they are highly diverse and generally lack safety information. Previous comparative toxicity studies have shown varying results from similar ENMs [2], which could be due to the test environment. The choice of medium they are carried in can dramatically change their characteristics and how they interact within a biological system [2].
ENMs typically consist of nanoparticles (NPs) smaller than 100 nanometers. The toxicological responses caused by ENMs themselves may vary due to their size, oxidation state, and charge [3]. Most studies evaluating the toxicity of NPs use rodents, zebrafish, organoids, and cell models. Due to each model having different assay condition requirements, response profiles, and routes of exposure, it is difficult to compare toxicological outcomes among the models. For example, the toxicity of graphene oxides (GOs), a more cost-efficient alternative to graphene in medicine and biotechnology, have been extensively evaluated, with conflicting effects in cell culture. GOs induced increased cellular growth, no effect at all, or cellular damage, presumably attributed to differences in size, shape, and charge [3]. There were conflicting results when comparing cell culture and zebrafish exposure studies for other nanomaterials, like Cerium Oxide and Zinc Oxide. Some Cerium Oxide in vitro studies found that the nanomaterial led to oxidative stress and was cytotoxic; we previously found that Cerium Oxide exposures for zebrafish embryos did not cause any significant effects [4], [5]. On the other hand, both in vitro and in vivo studies found that Zinc Oxide caused significant effects, like mortality [5], [6].
Many in vitro studies have focused on cells or organoids and the responses elicited by ENM exposure. Cells proliferate in controlled physiochemical environments consisting of growth medium, supplements, and antibiotics [7], [8], [9]. Single cell types are highly efficient to gauge responses to ENM exposure with many replicates. The downside is that any one cell type in vitro will have an extremely limited range of potential response readouts compared to an organoid or in vivo model [8]. Comparing in vitro response readouts among multiple cell lines carries the caveat that many lines will require different environments such as type and concentration of various media, supplements, and antibiotics hence the often-varying effects of the same ENM across multiple in vitro models [10].
Developmental zebrafish has become a powerful model for assessing potential toxicants due to their sensitivity, small size, cost-efficiency, rapid throughput and homology to the human genome [5], [11], [12]. In vivo studies enable the gauging of ENM bioactivity in the whole animal, with a considerably larger repertoire of response readouts than would be possible from even hundreds of different in vitro assays. However, zebrafish embryos are commonly maintained in a buffered, low ionic strength embryo medium (EM), dissimilar to the general composition of cell culture media. Moreover, the precise composition of EM varies among laboratories, so these external condition variables will influence the biological readout of the assays. These extrinsic conditions amongst labs, assays, models, and others will be influential in reported ENM bioactivity.
The primary objective of this study was to gain a deeper understanding of how the composition of the medium affects the toxicological outcomes of exposure to ENMs. Initially, we examined whether zebrafish could develop normally in a culture environment for mammalian cells. If this proved well tolerated by the zebrafish, we hypothesized it would enable a more accurate comparison of ENM toxicity data obtained from in vitro cell-based assays and a high-throughput zebrafish system. Our findings revealed that a medium enriched with ionic substances, comprised of Roswell Park Memorial Institute medium (RPMI), fetal bovine serum (FBS), and an antibiotic mix (AB), did not adversely affect the development of zebrafish. These conditions mitigated the toxicity of graphene oxides of various sizes and Zinc Oxide (ZnO). In contrast, when exposed to graphene oxides (GOs) and ZnO in ultrapure (UP) water, embryonic zebrafish experienced elevated mortality rates and exhibited morphological abnormalities. The impact of the heightened ionic content highlights the necessity to reassess the estimates of ENM toxicity derived from cell cultures compared to zebrafish raised in conventional embryo medium.
2. Materials and methods
2.1. Sample preparation of RPMI, FBS, and AB for testing
Fetal Bovine Serum (FBS) and an antibiotic mix (AB) were purchased from Avantor VWR and stored at −10°C. Prior to the start of the experiment, FBS and AB were acclimated to room temperature for 10 minutes and then thawed at 30°C using a sand bath. Under a sterile laminar hood, the solutions were aliquoted into single use volumes (200 µL) for future use to prevent degradation. Table 1 depicts each solution’s constituents and the manufacturer. The ionic strength of RPMI was assumed to be 152 mM as previously determined by Kwon et al. [13].
Table 1.
Cell culture medium constituents used.
| Solution | Information |
|---|---|
| Antibiotic Mix (AB) | Quality Biological; Amphotericin B 0.25 µg/mL, Penicillin 100 units/mL, Streptomycin 100 µg/mL |
| Roswell Park Memorial Institute Medium (RPMI) | Quality Biological; Additional L-Glutamine (300 mg/L) and 10 mM HEPES buffer. |
| Fetal Bovine Serum (FBS) | Avantor; 100 % United States Origin |
2.2. Zebrafish husbandry and embryo collection
Adult Tropical 5D zebrafish were maintained in 14-hour light/10-hour dark cycles using protocols in compliance with the Institutional Animal Care and Use Committee (IACUP 5113; 2022) at Oregon State University’s Sinnhuber Aquatic Research Laboratory (Corvallis, OR). Approximately 400 adult zebrafish were kept in 50-gallon or 100-gallon tanks with 28°C filtered, recirculating water containing Instant Ocean salts (Spectrum Branks, Blacksburg, VA, USA) and fed with GEMMA micro feed from Skretting Inc. (Fontaine Les Vervins, France) [14].
A spawning funnel was set up the night before exposures, and embryos were collected from the funnel the following morning. Embryos were placed into glass Petri dishes and sorted for viability, and appropriate life-stage embryos were selected and stored in 28°C embryo medium (EM; 15 mM NaCl, 0.5 mM KCl, 1 mM MgSO4, 0.15 mM KH2PO4, 0.7 mM NaHCO3) [15].
2.3. ENMs: graphene oxides and metal oxides
The Nanomaterials Health Implications Research Consortium provided the ENMs used for this study. All ENMs were previously highly characterized using methods described by Bitounis et al., Parviz and Strano, Beltran-Huarac et al., and Sotiriou et al. [16], [17], [18], [19].
To create the graphene oxides used, Bitounis et al. followed a method provided by Parviz and Strano in a multiple step process to produce the ENMs. To synthesize GOs, the authors oxidized graphite under acidic conditions. Purification followed, adding endotoxin-free water to remove residual ions, acid, and any oxidized debris. Exfoliation by centrifugation was then used to reduce the size of large flakes and aggregates multiple times until the desired size was achieved and the precipitate was redispersed into endotoxin-free water. The graphene oxides were then characterized using field emission scanning electron microscopy, atomic force microscopy, and Raman Spectroscopy with further characterization for trace metal purity, endotoxic, and microbial load assessment [16], [17].
The metal oxides were created using flame spray synthesis, a method that involves using a metal oxide precursor dissolved in a highly flammable solvent. The solution is pumped out of a capillary alongside an oxygen stream where the solution is ignited, and the high heat causes the precursor to form the metal oxide. To characterize the CeO2, Beltran-Huarac et al. performed transmission electron microscopy, x-ray power diffraction, Brunauer-Emmet-Teller, inductively coupled plasma - mass spectrometry, x-ray photoelectron spectroscopy, Fourier transformed infrared spectroscopy [18]. For characterization of the ZnO, Sotiriou et al. used electron microscopy, x-ray diffraction, and x -ray photoelectron spectroscopy while measuring other characteristics such as photocatalytic activity, UV–vis transmission measurements [19].
Graphene Oxide 1 µm x 1 µm and Graphene Oxide 400 nm x 400 nm were both provided in suspension, suspended in endotoxin-free water. The metal oxides, CeO2 and ZnO, were both provided in powder form. The desired weight of each sample was dispersed in UP water.
Before exposures, the nanomaterials were dispersed in suspension using sonication held constant at room temperature (20–22°C). A Fisher Scientific Series 60 Sonic Dismembrator Model F60 accompanied by a water chiller-circulator was used, with respective sonication times included in Table 2.
Table 2.
Nanomaterials and Sonication times. The graphene oxides were supplied already suspended in solution (endotoxin-free water). The ENMs were sonicated for the respective times at constant 20–22°C.
| Nanomaterial | Stock Concentration (μg/mL) | Sonication time |
|---|---|---|
| Graphene Oxide 400 nm x 400 nm |
500 | 26 s |
| Graphene Oxide 1 µm x 1 µm |
310 | 2 min |
| Cerium Oxide 30 nm | n/a | 6 min |
| Zinc Oxide 50 nm | n/a | 16 min |
2.4. Embryonic exposures
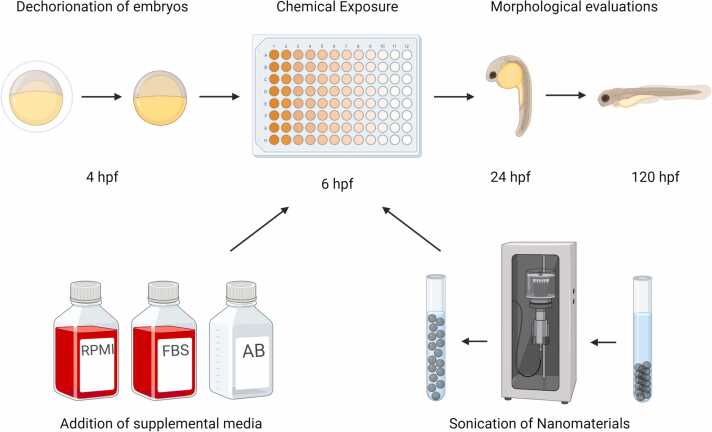
At 4 hours post-fertilization (hpf), the embryos were dechorionated using pronase (Sigma Aldrich) and an automated dechorionator using methods described in Mandrell et al. [20]. Dechorionated embryos were stored in a 90 mm glass petri dish containing EM. The various test solutions were made fresh, 2 hours pre-exposures. Solution preparation and plating were done in a laminar flow hood to minimize potential contamination. Test solutions (100 µL) were added to individual wells of round bottom tissue-culture treated plates (Corning™ Falcon™ 96-Well, cat#353227). Embryos were then manually singulated to wells using a flame-polished wide-bore glass Pasteur pipette. The plates were then sealed (ThermalSeal RTS™ Sealing Films, SKU Z734438–100EA) to minimize evaporation and contamination and placed on an orbital shaker overnight at 225 rpm at 28°C in the dark and removed at 24 hpf. The plates underwent 24 and 120 hpf assessments of mortality and malformations, where each concentration and control contained 32 animals.
The various solutions were evaluated separately to determine the maximum tolerable RPMI, FBS, and AB concentrations to mimic cell culture conditions that did not cause statistically significant mortality or malformations. RPMI was evaluated at 0, 20, 40, 60, 80, and 100 % with the addition of 1 % AB and UP. Separately, FBS was evaluated at 0, 2, 4, 6, 8, and 10 % with the addition of 1 % AB and UP. Controls for both consisted of 1 % AB in UP and UP only. Once determined, both RPMI and FBS were used at the maximum tolerable concentration to ensure that the constituents did not affect embryonic development. For ENM exposures, concentrations of 0, 5, 10.7, 23.2, and 50 µg/mL were used. Each ENM was sonicated before adding cell culture medium components or UP.
2.5. Embryonic endpoints and statistical analysis
Exposed embryos were evaluated at 24 and 120 hpf for mortality or morphological abnormalities based on 12 endpoints: mortality at 24 hpf, mortality at 120 hpf, delayed progression at 24 hpf, cranial, axis, edema, muscle, lower trunk, brain, skin, notochord, touch response [21]. Using a laboratory information management system (Zebrafish Acquisition and Analysis Program) (ZAAP), evaluation data was recorded as a binary presence or absence input for any mortality or morphological effect found in any of the 96 wells using endpoints mentioned above [21]. Custom R-scripts were used to analyze the data previously mentioned. Instances of mortality at 24 or 120 hpf and any effect observed by 120 hpf were depicted on composite plots, with each instance indicated by a circle. Responses across all endpoints were collapsed into a singular binary morphology endpoint named “any.effect” for a single embryo. Statistical significance was determined by computing a threshold for every chemical - endpoint pair compared to the background for all endpoints [21]. After evaluations were completed, all larvae were euthanized with buffered tricaine as per the IACUC-approved method.
3. Results and discussion
3.1. Embryonic zebrafish can develop in cell culture medium
Upon introducing embryonic zebrafish into cell culture medium, relevance to cell culture must be considered. RPMI is supplemented with FBS at 2–10 % for many mammalian cell lines. FBS is used in addition to RPMI as a supplement to maintain and prolong cellular viability [8], [9]. It contains growth factors, proteins, vitamins, hormones, and inorganic components essential to growth. The composition of FBS will vary across batches due to differences in conditions. Biological contamination is common due to expected optimal conditions for growth [9]. The use of antibiotics such as penicillin and streptomycin are key components that prevent the growth of bacteria. Cell culture models may use antibiotic supplements at 1 %. Higher concentrations have been shown to have cytotoxic effects. This study used an antibiotic cocktail of Amphotericin B 0.25 µg/mL, Penicillin 100 units/mL, and Streptomycin 100 µg/mL.
Embryonic zebrafish were used to assess the toxicity of the major constituents of cell culture medium: RPMI and FBS. Exposing the embryonic zebrafish to cell culture medium components (RPMI and FBS) individually allowed for assessing tolerable concentration ranges where no mortality or morphological effects were observed (Fig. 2). AB was added to each exposure to prevent bacterial growth. Effects commonly observed in both exposures consisted of mortality at 24 hpf and yolk sac edema. RPMI is a growth medium, and its nutrient availability may have resulted in reduced absorption of endogenous nutrients from the yolk sac. Zebrafish embryos do not require exogenous nutrients prior to commencement of self-feeding between 144 and 168 hpf. This allows zebrafish to develop in low-ionic solutions like UP [22]. The yolk sac nutrients are most readily absorbed via the skin and gills.
Fig. 1.
Exposure of embryonic zebrafish to cell culture medium and nanomaterials. Embryonic zebrafish were staged and dechorionated at 4 h post-fertilization (hpf) and placed in a 96 well plate at 6 hpf. The plates contained various test media and/or the ENMs were sonicated prior to being added to the plate. A total of 12 mortality and morphological effects were examined at 24 and 120 hpf. Figure created with BioRender.com.
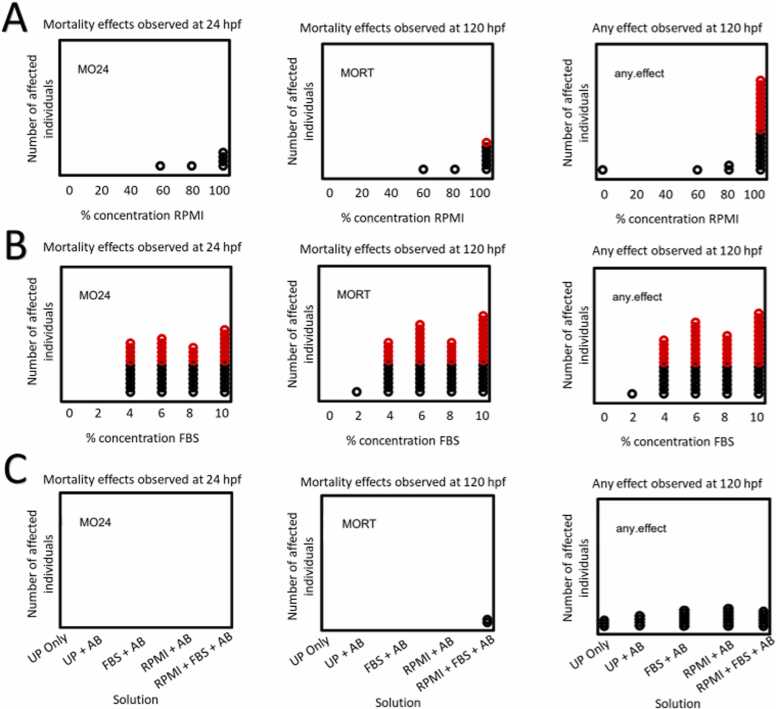
Fig. 2.
Impacts of cell culture medium on zebrafish development. Each circle in the figure represents one animal with an adverse outcome for the indicated endpoint, with statistically adverse outcomes (n = 32; p < 0.05) shown in red. “MO24” describes any observed mortality effects observed at 24 hpf. “MORT” represents any observed mortality effects observed at 120 hpf. “any.effect” represents any adverse effect observed including morphological and mortality at 120 hpf. (A) Embryonic exposures to 0, 20, 40, 60, 80, or 100 % RPMI with 1 % UP and AB. No significant effects were observed at 80 % RPMI with antibiotics at 1 %. (B) Embryonic exposures to FBS solution made of 0, 2, 4, 6, 8, or 10 % FBS with 1 % AB. (C) Embryonic exposures to various combinations of 80 % RPMI, 2 % FBS, and 1 % UP and AB.
Zebrafish embryos were dechorionated and exposed to varying concentrations of RPMI from 0 – 100 % in 20 % intervals with 1 % AB from 6 to 120 hpf (Fig. 2A). Mortality varied incidence across all concentrations of RPMI, though morphological effects were most prevalent in 100 % RPMI. While effects were observed for groups exposed to cell culture medium compared to UP water, none were significant (Fig. 2C). No statistically significant effects were associated with 80 % RPMI with 1 % AB in UP water, which was deemed appropriate for use.
To evaluate the tolerable concentration of FBS for zebrafish, embryos were exposed to 0 – 10 % FBS in 2 % intervals with the addition of 1 % AB from 6 to 120 hpf. Dechorionated embryos were exposed to varying concentrations of FBS with 1 % AB in UP water, as described in the methods. Significant mortality occurred at 24 hpf in concentrations of 4 % FBS and higher, with additional mortality by 120 hpf associated with FBS at 4 – 10 % (Fig. 2B). At 120 hpf, embryonic zebrafish exposed to 2 % FBS with 1 % AB in UP water showed no mortality or malformations.
To mimic cell culture medium, dechorionated embryos were exposed to the components combined at tolerable conditions. Various combinations of 80 % RPMI and 2 % FBS were used with 1 % UP water and AB (Fig. 2C). None of the components caused any significant adverse effects individually, nor when combined.
Cell culture studies have used up to 10 % FBS in culture media. For this study, embryonic zebrafish were exposed to concentrations ranging from 2 % to 10 % (Fig. 2B). FBS composition varies by manufacturer and lot number, which may impact FBS tolerance in zebrafish [8]. Any new lots of FBS used therefore required a range finding assessment to identify a tolerable concentration for the embryos.
By combining the tolerable concentrations of RPMI, FBS, AB, and UP, zebrafish were successfully reared in conditions that mimicked cell culture medium. Therefore, a more broadly comparable system was achieved between in vitro and in vivo assays. This should dramatically reduce the rate of false negatives in ENM toxicity evaluations using both cell culture and zebrafish embryos.
3.2. ENM toxicity is mitigated by cell culture medium constituents
With known tolerable concentrations of RPMI and FBS, ENM toxicity was assessed in the presence or absence of cell culture constituents in embryonic zebrafish. Dechorionated embryos were exposed to solutions containing 4 different ENMs from 6 – 120 hpf: Cerium Oxide (CeO2; 30 nm), Zinc Oxide (ZnO; 50 nm), and Graphene Oxides (GOs) of varying sizes (1 μm x 1 μm and 400 nm x 400 nm). The ENMs' toxicity in this study was independent of hydrodynamic size and charge. The ENMs were characterized by size, hydrodynamic radius, and zeta potential (Table S1) [16], [17], [18], [19]. These properties can affect overall toxicity due to interactions with the medium and model used.
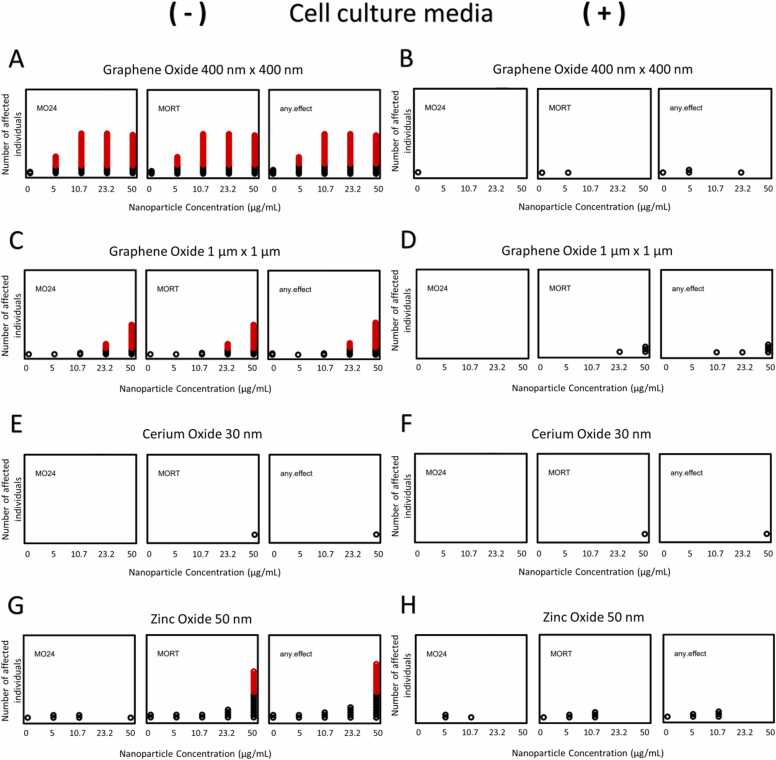
The overall toxicity of GOs depends on the size and oxidation state of the NPs [23]. In the absence of RPMI, FBS, and AB, GO exposures of both sizes resulted in mortality primarily at 24 hpf, similar to findings from Lopez et al. [23]. GO 400 nm x 400 nm showed significantly higher mortality at concentrations of 5 µg/mL and above. GO 400 nm x 400 nm dispersed in UP water was the most toxic of the ENMs assessed: all tested concentrations adversely impacted zebrafish development (Fig. 3A), consistent with previous studies [23]. We observed significant effects at 5 µg/mL GO 400 nm x 400 nm, whereas previous studies showed effects at 10.7 µg/mL [23]. GO 1 µm x 1 µm exposures showed significant mortality at 23.2 µg/mL and higher, whereas in previous studies, it elicited an adverse response beginning at 10.7 µg/mL (Fig. 3C) [23]. The most common effect observed in both GOs was mortality at 24 hpf, with few non-mortality related effects.
Fig. 3.
Embryonic zebrafish responses to ENMs in cell culture medium. Embryonic zebrafish were exposed to ENMs GO 400 nm x 400 nm, GO 1μm x 1 μm, CeO2, and ZnO where n = 32. Like Fig. 2, each circle represents an adverse outcome, with statistically significant outcomes (p-value < 0.05) shown in red. “MO24” is mortality at 24 hpf. “MORT” is mortality at 120 hpf. “any.effect” is cumulative morphology and mortality at 120 hpf. Fig. 3A, C, E, and G (left side of figure) show effects of ENMs in UP water with no addition of cell culture medium (-). Fig. 3B, D, F, and H (right side of figure) show effects of ENM exposures in the presence of cell culture medium. Nanoparticle concentrations used were 0, 5, 10.7, 23.2, and 50 µg/mL.
ZnO showed mortality at 24 hpf and 120 hpf across all concentrations, with the most significant effects observed in 50 µg/mL exposures at 120 hpf. Similar to previous studies, CeO2 had no observed effects at any concentrations used (Fig. 3E) [5].
With the addition of cell culture medium, no significant effects were observed in any exposures when combined with ENMs in this study (Fig. 3B, D, F, and H). All significant effects observed in ENM exposures without cell culture medium constituents (ENM and UP water only) were mitigated in embryo exposures mimicking cell culture conditions, which significantly reduced mortality and morphological effects. Overall, significant effects were reduced compared to ENM exposures in UP water only. CeO2 did not cause any significant adverse effects in any of the exposure assays.
From Table 3, The estimated effective concentration in which 50 % of animals are effected (EC50) for Graphene Oxide 400 nm x 400 nm dispersed in UP water was 5.14 µg/mL. There was no estimatable EC50 when cell culture medium was added to graphene oxide 400 nm x 400 nm. Graphene Oxide 1 µm x 1 µm dispersed in UP water had an estimated EC50 of 30.1 µg/mL, but an estimated EC50 could not be determined when cell culture medium was added. Cerium Oxide 30 nm dispersed in UP water only or with cell culture medium had no EC50. Zinc Oxide 50 nm dispersed in UP water had estimated EC50 of 170 µg/mL, but no estimated EC50 with cell culture medium. While some effects were observed in lower concentrations of GO 400 nm x 400 nm and ZnO in Figs. 3B and 3H, these were not significant.
Table 3.
Estimated EC50 values for the concentration of nanomaterial that will produce any effect in 50 % of exposed zebrafish. N/A denotes when an EC50 was not calculable.
| Nanomaterial | EC50 in UP (µg/mL) | EC50 in Cell Culture Medium (µg/mL) |
|---|---|---|
| Graphene Oxide 400 nm x 400 nm |
5.14 | N/A |
| Graphene Oxide 1 µm x 1 µm |
30.1 | N/A |
| Cerium Oxide 30 nm | N/A | N/A |
| Zinc Oxide 50 nm | 170 | N/A |
Notably, significant and detectable changes in toxicity were observed likely due to potential interactions between the ENMs and the cell culture medium. GOs are amphipathic, which allows them to interact with lipids more strongly in the cell culture medium than those in the embryo [23], [24]. Developmental toxicity was evident in embryonic zebrafish exposed to ENMs in UP water (without cell culture medium), but toxicity was mitigated in the presence of cell medium components (Fig. 3). The cell culture medium likely modified and/or reduced the interactions between embryonic zebrafish and ENMs.
The mitigation of toxicity using cell culture medium could be attributed to surface chemistry processes the result in the formation of a corona, or aggregation and sedimentation of the ENMs. ENM interactions are affected by the presence or absence of ionic species, such as salts or natural organic matter. In a study using sodium chloride (NaCl) to assess the influence of higher ionic solutions on titanium dioxide NPs, higher ionic strength caused the rate of sedimentation to increase with a decrease in toxicity of titanium dioxide NPs [25]. Cell culture medium contains inorganic salts such as NaCl, which could certainly impact ENM bioactivity in cell culture conditions.
The formation of a biocorona or natural organic corona, could sequester ENMs in solution. Biocoronas can form through the attachment of proteins, lipoproteins, metabolites, etc. to the surface of ENMs, creating either a soft or hard layer on the ENM surface. This can alter the physiochemical properties and ENM interactions in solutions, which includes reduced interactions with surrounding biological systems [2], [26], [27]. Cell culture medium contains various proteins, so the reduction in ENM toxicity in exposures containing cell culture medium could be attributed to the formation of biocoronas.
Studies focusing on adding natural and dissolved organic matter with nanoparticles in zebrafish have shown reduced bioaccumulation and toxicity. Xiao et al. exposed adult zebrafish to humic acid and fulvic acid with silver nanoparticles [27]. The silver NPs adsorbed humic and fulvic acid, and the physiochemical properties of the NP were altered by increasing the negative charge and overall size. This was thought to be caused by forming a “natural organic matter corona.” The acids both reduced the dissolution of silver NPs and were found to have reduced the uptake of particles in the zebrafish [27].
The aggregation and sedimentation of the ENMs within this study could have contributed to the absence of toxicity observed. Many models have been developed to understand how aggregation and sedimentation may occur, including both mathematical and computational models. The aggregation of particles is caused by the collision of free NPs, creating a cluster, or when the NP adsorbs organic or inorganic matter. Both processes could lead to sedimentation of NPs [28]. Mathematical models can be used to explain the process of sedimentation and resuspension of NPs in aquatic environments, as explained by Markus et al. [28].
Utilizing these mathematical models, computational models have been developed to further understand particle sedimentation. Hinderliter et al. developed the “In vitro Sedimentation, Diffusion, and Dosimetry model (ISDD)” [29]. The ISDD predictive model can help understand particokinetics and dosimetry of ENMs in common cell culture models, particularly in cases where constraints or limitations may exist. With regard to factors including fractal dimension, media height, particle size, particle density, particle surface area, agglomeration state, and characteristics, the model was able to calculate the transport rate of iron (III) oxide in vitro with varying amounts of particles per agglomerate. As the agglomerates grew, the sedimentation rate and extent increased while decreasing diffusion rates inefficiently packed agglomerates [28]. The aggregation of particles within cell culture medium likely influences ENM exposure toxicity assessments in cell culture conditions, which we observed in the current study.
The extrinsic interactions discussed thus far may more accurately represent how ENMs behave in vivo and in vitro. The addition of components that enhance ionic strength (NaCl) and introduce exogenous proteins could increase understanding of ENM toxicity, which can lead to more accurate toxicological assessments and better understanding of how we can take utilize ENMs and their applications. Reducing aggregation potential is necessary to understand the intrinsic properties of ENMs. ENM toxicity assessments should consider both extrinsic and intrinsic properties for a more comprehensive understanding of ENM hazard potential. Zebrafish has proven to be a valuable model that addresses the complexity of ENM effects on a whole system and mitigates the constraints inherent in cell culture approaches. Limitations within the zebrafish model exist as toxicological outcomes are generalized; therefore, additional studies are needed to evaluate and extrapolate the observed adverse effects of ENM exposures in cell culture and animal models to humans.
4. Conclusions
The study demonstrated that the toxic effects of nano zinc oxide (ZnO; 50 nm) and graphene oxides (GOs; sizes of 1 μm x 1 μm and 400 nm x 400 nm) were reduced by incorporating 80 % RPMI, 2 % FBS, and 1 % AB in UP water. It suggests that the chemical interactions between engineered nanomaterials (ENMs) and the applied medium could play a crucial role in mitigating toxicity toward cells and embryonic zebrafish. This reduction in ENM toxicity may be attributed to the medium's components, such as proteins and various solutes, which may adhere to the ENMs' surfaces. Utilizing the zebrafish model, the study explored different mediums to evaluate the bioavailability of ENMs more accurately within cell culture environments. There is a pressing need for further investigations to explore the toxicity of various ENMs within media compatible with low ionic solutions and enhance our understanding of ENM dynamics in cell culture settings. Comprehensive research is essential to ascertain the safety of nanomaterials across diverse conditions conclusively. Moreover, to thoroughly evaluate the risk associated with ENMs, it is critical to examine their behavioral impacts on embryonic zebrafish in scenarios both with and without the presence of cell culture medium.
Funding
This research was supported by the National Institutes of Health - National Institute of Environmental Health Sciences [U01 ES027294 and P30 ES030287].
CRediT authorship contribution statement
Robyn L. Tanguay: Writing – review & editing, Supervision, Funding acquisition, Conceptualization. Lisa Truong: Writing – review & editing, Validation, Supervision, Software, Project administration, Funding acquisition, Formal analysis, Conceptualization. Ryan Lopez: Writing – review & editing, Conceptualization. John V. Lam: Writing – review & editing, Writing – original draft, Visualization, Validation, Methodology, Investigation, Data curation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We would like to acknowledge Drs. Michael Simonich and Lindsey St. Mary for their assistance in the revision of this article and the NHIR Consortium for providing the ENMs used in this study.
Handling Editor: Dr. L.H. Lash
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.toxrep.2024.04.002.
Appendix A. Supplementary material
Supplementary material
Data Availability
Data will be made available on request.
References
- 1.RB Singh K., Nayak V., Sarkar T., Pratap Singh R. Cerium oxide nanoparticles: properties, biosynthesis and biomedical application. RSC Adv. 2020;10(45):27194–27214. doi: 10.1039/D0RA04736H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Casals E., Zeng M., Parra-Robert M., Fernández-Varo G., Morales-Ruiz M., Jiménez W., Puntes V., Casals G. Cerium oxide nanoparticles: advances in biodistribution, toxicity, and preclinical exploration. Small. 2020;16(20) doi: 10.1002/smll.201907322. [DOI] [PubMed] [Google Scholar]
- 3.Rhazouani A., Gamrani H., El Achaby M., Aziz K., Gebrati L., Uddin M.S., Aziz F. Synthesis and toxicity of graphene oxide nanoparticles: a literature review of in vitro and in vivo studies. BioMed. Res. Int. 2021;2021 doi: 10.1155/2021/5518999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eom H.-J., Choi J. Oxidative stress of CeO2 nanoparticles via p38-Nrf-2 signaling pathway in human bronchial epithelial cell, Beas-2B. Toxicol. Lett. 2009;187(2):77–83. doi: 10.1016/j.toxlet.2009.01.028. [DOI] [PubMed] [Google Scholar]
- 5.Wehmas L.C., Anders C., Chess J., Punnoose A., Pereira C.B., Greenwood J.A., Tanguay R.L. Comparative metal oxide nanoparticle toxicity using embryonic zebrafish. Toxicol. Rep. 2015;2:702–715. doi: 10.1016/j.toxrep.2015.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kao Y.Y., Chen Y.C., Cheng T.J., Chiung Y.M., Liu P.S. Zinc oxide nanoparticles interfere with zinc ion homeostasis to cause cytotoxicity. Toxicol. Sci. 2012;125(2):462–472. doi: 10.1093/toxsci/kfr319. [DOI] [PubMed] [Google Scholar]
- 7.Moore G.E., Gerner R.E., Franklin H.A. Culture of normal human leukocytes. JAMA. 1967;199(8):519–524. doi: 10.1001/jama.1967.03120080053007. [DOI] [PubMed] [Google Scholar]
- 8.van der Valk J., Brunner D., De Smet K., Fex Svenningsen Å., Honegger P., Knudsen L.E., Lindl T., Noraberg J., Price A., Scarino M.L., Gstraunthaler G. Optimization of chemically defined cell culture media – replacing fetal bovine serum in mammalian in vitro methods. Toxicol. Vitr. 2010;24(4):1053–1063. doi: 10.1016/j.tiv.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 9.Ryu A.H., Eckalbar W.L., Kreimer A., Yosef N., Ahituv N. Use antibiotics in cell culture with caution: genome-wide identification of antibiotic-induced changes in gene expression and regulation. Sci. Rep. 2017;7(1):7533. doi: 10.1038/s41598-017-07757-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arora M. Cell culture media: a review. Mater. Methods. 2022;3:175. doi: 10.13070/mm.en.3.175. 2013. [DOI] [Google Scholar]
- 11.Audira G., Lee J.-S., Siregar P., Malhotra N., Rolden M.J.M., Huang J.-C., Chen K.H.-C., Hsu H.-S., Hsu Y., Ger T.-R., Hsiao C.-D. Comparison of the chronic toxicities of graphene and graphene oxide toward adult zebrafish by using biochemical and phenomic approaches. Environ. Pollut. 2021;278 doi: 10.1016/j.envpol.2021.116907. [DOI] [PubMed] [Google Scholar]
- 12.Sawle A.D., Wit E., Whale G., Cossins A.R. An information-rich alternative, chemicals testing strategy using a high definition toxicogenomics and Zebrafish (Danio rerio) embryos. Toxicol. Sci. 2010;118(1):128–139. doi: 10.1093/toxsci/kfq237. [DOI] [PubMed] [Google Scholar]
- 13.Kwon D., Lee S.H., Kim J., Yoon T.H. Dispersion, fractionation and characterization of sub-100 nm P25 TiO2 nanoparticles in aqueous media. Toxicol. Environ. Health Sci. 2010;2:78–85. doi: 10.1007/BF03216516. [DOI] [Google Scholar]
- 14.Barton C.L., Johnson E.W., Tanguay R.L. Facility design and health management program at the sinnhuber aquatic research laboratory. Zebrafish. 2016;13 doi: 10.1089/zeb.2015.1232. 〈https://www.liebertpub.com/doi/10.1089/zeb.2015.1232〉 S1, S-39-S-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kimmel C.B., Ballard W.W., Kimmel S.R., Ullmann B., Schilling T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995;203(3):253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 16.Bitounis D., Parviz D., Cao X., Amadei C.A., Vecitis C.D., Sunderland E.M., Thrall B.D., Fang M., Strano M.S., Demokritou P. Synthesis and physicochemical transformations of size-sorted graphene oxide during simulated digestion and its toxicological assessment against an in vitro model of the human intestinal epithelium. Small (Weinh. Der Bergstr., Ger. ) 2020;16(21) doi: 10.1002/smll.201907640. e1907640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parviz D., Strano M. Endotoxin-free preparation of graphene oxide and graphene-based materials for biological applications. Curr. Protoc. Chem. Biol. 2018;10(4) doi: 10.1002/cpch.51. e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beltran-Huarac J., Zhang Z., Pyrgiotakis G., DeLoid G., Vaze N., Hussain S.M., Demokritou P. Development of reference metal and metal oxide engineered nanomaterials for nanotoxicology research using high throughput and precision flame spray synthesis approaches. NanoImpact. 2018;10:26–37. doi: 10.1016/j.impact.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sotiriou G.A., Watson C., Murdaugh K.M., Darrah T.H., Pyrgiotakis G., Elder A., Brain J.D., Demokritou P. Engineering safer-by-design, transparent, silica-coated ZnO nanorods with reduced DNA damage potential. Environ. Sci. Nano. 2014;1(2):144–153. doi: 10.1039/C3EN00062A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mandrell D., Truong L., Jephson C., Sarker M.R., Moore A., Lang C., Simonich M.T., Tanguay R.L. Automated zebrafish chorion removal and single embryo placement: optimizing throughput of zebrafish developmental toxicity screens. J. Lab. Autom. 2012;17(1):66–74. doi: 10.1177/2211068211432197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Truong L., Reif D.M., St Mary L., Geier M.C., Truong H.D., Tanguay R.L. Multidimensional in vivo hazard assessment using zebrafish. Toxicol. Sci. 2014;137(1):212–233. doi: 10.1093/toxsci/kft235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Truong L., Zaikova T., Richman E.K., Hutchison J.E., Tanguay R.L. Media ionic strength impacts embryonic responses to engineered nanoparticle exposure. Nanotoxicology. 2012;6(7):691–699. doi: 10.3109/17435390.2011.604440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lopez R.M., White J.R., Truong L., Tanguay R.L. Size- and oxidation-dependent toxicity of graphene oxide nanomaterials in embryonic zebrafish. Nanomaterials. 2022;12(7):1050. doi: 10.3390/nano12071050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim J., Cote L.J., Huang J. Two dimensional soft material: new faces of graphene oxide. Acc. Chem. Res. 2012;45(8):1356–1364. doi: 10.1021/ar300047s. [DOI] [PubMed] [Google Scholar]
- 25.Fang T., Yu L.P., Zhang W.C., Bao S.P. Effects of humic acid and ionic strength on TiO2 nanoparticles sublethal toxicity to zebrafish. Ecotoxicology. 2015;24(10):2054–2066. doi: 10.1007/s10646-015-1541-6. [DOI] [PubMed] [Google Scholar]
- 26.Mahmoudi M., Landry M.P., Moore A., et al. The protein corona from nanomedicine to environmental science. Nat. Rev. Mater. 2023;8:422–438. doi: 10.1038/s41578-023-00552-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiao B., Wang X., Yang J., Wang K., Zhang Y., Sun B., Zhang T., Zhu L. Bioaccumulation kinetics and tissue distribution of silver nanoparticles in zebrafish: The mechanisms and influence of natural organic matter. Ecotoxicol. Environ. Saf. 2020;194 doi: 10.1016/j.ecoenv.2020.110454. [DOI] [PubMed] [Google Scholar]
- 28.Markus A.A., Parsons J.R., Roex E.W.M., Voogt P. de, Laane R.W.P.M. Modeling aggregation and sedimentation of nanoparticles in the aquatic environment. Sci. Total Environ. 2015;506–507:323–329. doi: 10.1016/j.scitotenv.2014.11.056. [DOI] [PubMed] [Google Scholar]
- 29.Hinderliter P.M., Minard K.R., Orr G., Chrisler W.B., Thrall B.D., Pounds J.G., Teeguarden J.G. ISDD: a computational model of particle sedimentation, diffusion and target cell dosimetry for in vitro toxicity studies. Part. Fibre Toxicol. 2010;7(1):36. doi: 10.1186/1743-8977-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Data will be made available on request.